Abstract
Post-transplant cyclophosphamide (PTCy) has become a promising option after allo-SCT, but infections may be more common than in traditional protocols. We herein report 117 consecutive adults who received PTCy-based alloSCT in our hospital: HaploSCT (34%), MRD (19%), and VUD (47%), respectively. The 18-month incidence of severe bacterial, viral, and IFI was 56%, 69%, and 8.7%, without differences between donor type, except for CMV infection and viral hemorrhagic cystitis, which had a higher incidence in the haploSCT cohort (58% vs. 43% and 30% vs. 8% on day +90, p < 0.05). Late infections by conventional respiratory viruses were common in all groups [33/87 (38%)]. The 2-year survival was 72% and did not differ by donor type. IRM at day 30, day 100, and 18 months was 1.7%, 4.4%, and 12%, without differences by donor type (p = 0.7). The primary cause of IRM was bacterial infection (42%). Grade 2–4 acute GvHD was the only independent predictor of IRM. Donor type had no impact on IRM or on survival. In our study, severe infections were common in all donor types using PTCy, with higher rates of early post-engraftment CMV-I and viral HC in haploSCT recipients, although lethal infections were uncommon and similar in all donor types.
Subject terms: Stem-cell therapies, Haematological cancer, Graft-versus-host disease, Infectious diseases
Introduction
Infections are a major cause of morbidity and the primary cause of mortality in 35–45% of deaths after allogeneic hematopoietic stem cell transplantation (alloSCT) [1–6]. Historically, the use of unrelated donors has been associated with an increased risk of severe infectious complications, with more severe infections in non-HLA fully matched transplants [7–10]. Improving immune reconstitution while mitigating graft-versus-host disease (GvHD) has been identified as the key albeit elusive factor for reducing the occurrence of severe opportunistic infections [11].
With the advent of the 20th century, Luzkik et al. [12] pioneered the use of high dose post-transplant cyclophosphamide (PTCy) in the haploidentical SCT (haploSCT) setting, with a surprisingly low incidence of severe forms of both acute and chronic GvHD and similar NRM and survival than alloSCT with other traditional stem cell donor types [13, 14]. At our institution, haploSCT was introduced as the preferred alternative donor source in 2013 using a chemotherapy-only myeloablative conditioning regimen and PTCy plus tacrolimus as GvHD prophylaxis [15]. PTCy was later integrated into HLA-matched related donor (MRD) and HLA-matched or 1-allele mismatched volunteer unrelated donor (VUD) transplant protocols, with positive initial results [16–18], and in 2019 our group published the initial results of incorporatingPTCy as GVHD prophylaxis outside the haploidentical setting [19]. Despite encouraging results, opportunistic infections without severe GVHD were a major concern, being the main cause of death for non-haploSCT (7 of 19 deaths) and haploSCT (3/4 cases of NRM) recipients in our preliminary studies [15, 19].
To further explore this topic, we herein describe the incidence, risk factors, and impact of severe infections on long-term outcomes in alloSCT incorporating PTCy for GvHD prophylaxis, with the primary objective of exploring the potential impact of the donor type (haploSCT vs. MDR/VUD) on these infections.
Patients and methods
Inclusion criteria and transplant characteristics
This retrospective cohort includes all consecutive adult patients who received a first alloSCT with PTCy-based GVHD prophylaxis in our institution between June 2013 and January 2020. Patients were treated according to institutional programs in accordance with ethical standards. Written consent for transplant procedures and for the use of medical records for research was obtained from all participants. Data were collected by manual review of the electronic medical records. Main patient characteristics are shown in Table 1.
Table 1.
Patient characteristics and main transplantation outcomes.
| HaploSCT (n = 40) | MRD/VUD/MMUD (n = 77) |
P value | |
|---|---|---|---|
| Median follow-up for survivors, range (days) | 851 (83–2432) | 526 (42–1488) | 0.001 |
| Median age, range | 47 (21–71) | 54 (20–72) | 0.15 |
| Gender | |||
| Female | 19 (47) | 35 (45) | 0.5 |
| HLA match | |||
| Identical sibling | – | 22 (29) | 0.001 |
| 10/10 match unrelated donor | – | 23 (30) | |
| 9/10 mismatch related or unrelated donor | – | 32 (41) | |
| Underlying disease | |||
| AL/MDS | 28 (70) | 39 (52) | 0.2 |
| MPN | 1 (2) | 10 (13) | |
| Lymphoid malignancies | 9 (23) | 20 (26) | |
| Others | 2 (5) | 8 (11) | |
| Disease status at SCT | 0.1 | ||
| CR (first or second) | 29 (72) | 41 (53) | |
| Others | 11 (28) | 36 (47) | |
| Disease risk index | 0.4 | ||
| High/very high | 15 (38) | 31 (37) | |
| Previous SCT | 0.8 | ||
| Yes | 6 (15) | 10 (13) | |
| CMV serology | |||
| Donor positive/Recipient positive | 22 (55) | 33 (43) | |
| Donor negative/Recipient positive | 8 (20) | 23 (30) | 0.4 |
| Donor negative/Recipient negative | 5 (12.5) | 15 (19.5) | |
| Conditioning regimen | |||
| Reduced-intensity regimen | 1 (2) | 56 (73) | 0.001 |
| Myeloablative regimen | 39 (100) | 21 (27) | |
| Median CD34 + dose/kg | 5 (1.2–6) | 5.5 (1.6–8) | 0.17 |
| Acute GvHD | |||
| Grades II–IV | 7/35 (20) | 22/71 (31) | 0.3 |
| Number of patients at risk for cGvHD at day + 100 | 30 | 47 | |
| Chronic GvHD | 6 (20) | 7 (15) | 0.2 |
MRD HLA-identical matched related donor, VUD HLA-matched volunteer unrelated donor, MMUR 1-allelel mismatched volunteer unrelated donor, AL acute leukemia, MDS myelodysplastic syndrome, MPN myeloproliferative neoplasm, SCT stem cell transplantation, CR complete response, GvHD graft-versus-host disease, cGvHD chronic GvHD.
Conditioning regimen and GvHD prophylaxis
All patients in the haploSCT group underwent transplantation following our institutional myeloablative conditioning (MAC) Thiotepa–Fludarabine–Bululphan (TBF) protocol, which has been previously described in detail [15]. Similar conditioning regimens were used in the MRD/VUD cohorts, although formally classified as reduced-conditioning regimens (RIC) based on low-dose thiotepa combined with the classical Fludarabina–Busulphan or Fludarabine–Melphalan RIC regimens [20–22], as previously reported [19]. In the MAC setting, fludarabine (90 mg/m2 IV) plus fractionated total body irradiation (TBI) at a total dose of 8 to 13.5-Gy or 4 days of busulphan were administered to patients with acute leukemia (AL) or myelodysplastic syndrome (MDS) age <51 years [23].
Unmodified hematopoietic progenitor cells, mostly peripheral blood stem cells (PBSC) were infused on Day 0 (target cell dose 5 × 106/kg CD34 + cells). Three patients (2.6%) received bone marrow stem cells. PTCy was given days +3 and +4 at a dose of 50 mg/kg IV once daily, followed by tacrolimus (0.03 mg/kg as a 24 h IV infusion or orally to maintain a target trough serum level of 8 ng/mL, range 5–15 ng/mL) starting on day +5.
Sirolimus was used instead of tacrolimus in case of prior renal failure (loading oral dose of 4 mgr on day +5 followed by 2 mgr daily with dose modifications to maintain a target trough serum level of 7 ng/mL, range 5–12 ng/mL). Post-transplant growth factors were not routinely used.
Supportive care and anti-infectious prophylaxis
Patients were nursed in HEPA-filtered rooms during the early post-SCT aplastic period. Most patients received quinolone prophylaxis during neutropenia and/or until the start of broad-spectrum antibiotics. Piperacillin-tazobactam or cefepime alone were used as empirical therapy for febrile neutropenia, unless previous infection with resistant bacteria occurred; in this case, an appropriate antibacterial combination was used. Antiviral prophylaxis consisted of low-dose acyclovir (400 mg twice a day i.v. or 800 mg orally), which was maintained (in combination with cotrimoxazole) for a minimum of 1 year after SCT or until immunosuppressive therapy was stopped. A mold active antifungal agent (posaconazole, voriconazole, or other systemic antifungal drugs) was used whenever the patient was given high dose steroids for the treatment of GvHD, while fluconazole was used during the pre-engraftment period. Serum galactomannan (2 times per week) was included in the monitoring strategy during this period. Serial blood monitoring using quantitative PCR for cytomegalovirus (CMV) and Epstein-Barr virus (EBV) infection was done 1–2 times a week until day +100 or indefinitely for those with active GvHD, as described elsewhere [24]. Pre-emptive anti-CMV therapy was started when a level of DNAemia of >1000 IU/ml was found in one blood sample or two consecutive samples had a level of >500 IU/mL. Patients with EBV DNAemia of >1000 copies/mL on at least two consecutive samples were treated with rituximab, as previously described [24].
Transplant and infection-related definitions
Periods of infectious risk were defined as day 0 to day +30 (pre-engraftment), days +31 to +100 (early post-engraftment) and beyond day +100 (late post-engraftment). Neutrophil engraftment was the first of three consecutive days of ANC > 500 cells/mm3 following post-transplant nadir, and platelet engraftment the first of three measurements showing >20,000 platelets/mm3 without platelet transfusion in previous 7 days.
Acute GvHD (aGvHD) and chronic GvHD (cGvHD) were defined per published criteria [25, 26]. Cause of death was determined following the algorithm suggested by Copelan et al. [27].
Infection-related mortality (IRM) was defined as death attributable to a recent severe infection by the primary physician(s) and the coordinator of the study (I.G.) and/or when a lethal infection was identified at autopsy. Any bacterial, viral, or invasive fungal infection (IFI) requiring intravenous treatment, or leading to or prolonging a hospitalization were considered as being severe, as were all CMV and EBV infections. In case of common skin contaminants, bloodstream infection (BSI) was diagnosed if ≥2 consecutive blood cultures were positive for the same species. Infection data were collected retrospectively until the patient’s death or last follow-up, using standardized definitions of severe infections after SCT based on the most recent guidelines (https://www.ebmt.org/working-parties/infectious-diseases-working-party-idwp). Other severe viral infections considered in this study were: (i) disseminated varicella-zoster virus (VZV) infection; (ii) Human Herpesvirus 6 (HHV-6) encephalitis, diagnosed by positive PCR from cerebrospinal fluid; (iii) Adenovirus (ADV) disease, diagnosed when adenovirus was identified in samples from an affected organ(s) by immunohistochemistry; (iv) pneumonia due to a conventional respiratory virus (CRV); and (v) BK polyomavirus-related hemorrhagic cystitis (BKPV-HC).
Statistical analysis
The primary objective of the study was to analyze and compare the cumulative incidence (C.I.) of IRM among donor sources (haploSCT vs. MRD/VUD), whereas secondary endpoints were the description of the major types of severe infections. In addition, other conventional post-transplant outcomes were described and compared.
Descriptive statistics were used to show the patients’ general characteristics The Kaplan–Meier method was used to estimate actuarial overall survival (OS) and progression-free survival (PFS). Estimates of neutrophil (>0.5 × 109/l) and platelet (>20 × 109/L) recovery, bacterial infections, IFI, severe viral infections, non-relapse mortality (NRM), GVHD, and disease relapse were calculated using cumulative incidence curves, to account for competing risks. Crosstabs and Student’s t-test were used to identify baseline characteristics associated with IRM and OS.
Factors with P value <0.1 in univariate analysis were entered into a multivariate proportional hazards Cox regression analysis [28]. P values <0.05 were considered statistically significant, and the hazard ratios (HRs) and their 95% confidence intervals (95% CIs) were calculated. All analyses were performed using SPSS version 18.0 (SPSS Inc, Chicago, IL, USA) or the CMPRSK package in R 2.4.1.
Results
Patient characteristics
We included 117 consecutive patients in the study. As shown in Table 1, our cohort contained many high-risk patients. Donors were MRD, fully matched VUD, 1-allele-mismatched VUD (MMUD), and haploidentical donors in 22 (19%), 23 (20%), 32 (27%), and 40 (34%) cases, respectively. There were no significant differences in baseline characteristics between the different transplant cohorts. Eighty-six patients (74%) were CMV seropositive. The median follow-up in survivors was 1056 days (range: 83–2432) in HaploSCT recipients and 526 days (range: 42–1488) in the non-haploidentical cohort, respectively. Thus, follow-up was censored at 18 months (543 days) for all incidence and survival analyses.
Overall outcomes
Most patients (94%) achieved neutrophil engraftment, with seven cases developing graft failure. In addition, one patient from each group had an early IRM during aplasia. In the 108 patients with sustained donor engraftment, the median time to neutrophil and platelet recovery was day +20 (range: 12–56) and day +26 (range: 12–171) in haploSCT recipients, while in the non-haplo cohort the median times to neutrophil and platelet recovery were 23 (range: 12–36) and 22 days (range: 10–249), respectively (non-significant differences). The incidence of grade 2–4 aGvHD at day +120 was low in both transplant groups [20.3% (95% C.I: 13–28%) and 29% (95% C.I: 19–39%), respectively, p = 0.2], with a trend for a higher incidence in MMUD transplants [42.6% (95% C.I: 32–53) p = 0.1].
Among the 77 evaluable cases, the 1 year C.I. of overall cGvHD was 19.7% (95% C.I: 10–27) and 11% (95% C.I: 2–20) in haplo and non-haplo SCT recipients, while the incidence of moderate-severe forms of cGvHD was 3.8% and 6%, respectively (p = 0.4). No significant differences were found in the 18-month NRM between both groups (13% vs. 19.8%, p = 0.5). Again, MMUD transplants had a higher NRM at 18 months [38% (95% C.I: 18–48), p = 0.05].
The 18-month C.I. of relapse was 18% (95% CI 11–29%) and 29% (95% CI 17–41%) in haploSCT and non-haploSCT recipients (p = 0.5), while the OS was 79.7% (95% CI 67–90%) and 75% (95% CI 65–75%), respectively. The main cause of NRM was an opportunistic infection in both cohorts (12/19 cases of NRM).
Severe infections
Using microbiological and clinical criteria, 262 severe infections occurred in 98 of 117 patients (84%), with a median of 2 events/patient (range: 0–7). Major pathogens and their distribution in different post-transplant time periods are summarized in Table 2. Thirty-nine percent were of bacterial, 4% fungal, and 57% viral origin. Median time to infection was 14 days for bacterial, 58 days for fungal, and 43 days for viral infection, respectively.
Table 2.
Etiologies of the documented infections by time period.
| Pre-engraftment (≤30days) | Intermediate and Late (>30days) | |||
|---|---|---|---|---|
| Only frequencies are shown in parenthesis | HaploSCT (n = 40) |
MRD/VUD/MMUD (n = 77) |
HaploSCT (n = 38) |
MRD/VUD/MMUD (n = 70) |
| Patients with ≥1 severe infection | 18 (45) | 31 (40) | 6 (16) | 22 (31) |
| Bacterial infections | 23 | 37 | 10 | 33 |
| Staphylococcus spp | 7 | 17 | 2 | 4 |
| Coagulase negative | 7 | 15 | 2 | 4 |
| Enterococcus spp | 3 | 3 | − | 4 |
| Streptococcus spp | 3 | 5 | 3 | 5 |
| S. pneumoniae | − | − | 2 | 4 |
| Gram-negative bacteria | 9 | 10 | 4 | 13 |
| C. difficile colitis | 1 | 1 | 1 | 5 |
| Other | − | 1 | − | 2 |
| IFI | 2 | 3 | 1 | 4 |
| IPA | 2 | 2 | 1 | 2 |
| Other | – | 1 | − | 2 |
| Viral infections | 14 | 6 | 49 | 73 |
| CMV | ||||
| Reactivation | 7 | 4 | 17 | 29 |
| Disease | − | − | 1 | − |
| EBV | − | − | 3 | − |
| Reactivation | − | − | 3 | − |
| PTLPD | − | − | − | − |
| HSV or VZV | − | – | 4 | 6 |
| HHV-6 encephalitis | 1 | − | − | 2 |
| Viral haemorrhagic cystitis | 4 | 1 | 8 | 5 |
| Community‐acquired respiratory virus | 2 | 1 | 14 | 27 |
| Others (highlight) | − | − | 2 | 4 |
MRD HLA-identical matched related donor, VUD HLA-matched volunteer unrelated donor, MMUR 1-allelel mismatched volunteer unrelated dono, CMV cytomegalovirus, EBV Epstein Barr virus, PTLPD post-transplant lymphoproliferative disorder, HVS Herpes simplex virus, VZV Varicella-zoster virus, HHV-6 Human herpesvirus 6, LRTI low respiratory tract infection.
Early severe infections (<day +30)
Overall, 49 patients (42%) had 60 early post-SCT or pre-engraftment blood-stream infections (PE-BSI) [median time to the first PE-BSI: 12 days (range: 0–30)]. Coagulase-negative staphylococci were responsible for 37% of PE-BSI episodes followed by Gram-negative bacteria (GNB) species (32%) and Streptococcus spp. (13%). The most represented GNB was Escherichia coli (13%). Donor type did not influence the rate of PE-BSIs, and the day +30 incidence of PE-BSI was 45% (95%CI: 26–61%) in haploSCT and 40.5% (95%CI: 29–52%) in non-haploSCT recipients, respectively (p = 0.7). In addition, the development of PE-BSI had no impact on survival when analyzed as a time-dependent covariate (see Table 3).
Table 3.
Univariate and multivariate analysis of the overall survival at 18 months.
| Variables | 18-month OS | |||
|---|---|---|---|---|
| Probability (95% C.I.) |
Univ. P value | Multivariate P | HR (95% C.I.) |
|
| Recipient age, in years | ||||
| • ≤40 (n = 23) | 78% (70–86) | 0.1 | 0.2 | |
| • >40 (n = 94) | 59% (48–70) | |||
| Disease risk Index | ||||
| • Low-Intermediate (n = 71) | 79.3% (69–90) | 0.08 | 0.07 | |
| • High-very high (n = 46) | 67.4% (53–81) | |||
| CD34 + cell count | ||||
| • ≥5 × 10e6/kg (n = 88) | 70.3% (60–81) | 0.1 | 0.09 | |
| • <5 × 10e6/kg (n = 28) | 88.2% (75–94) | |||
| 2–4 acute GvHDa | ||||
| • No (n = 77) | 81.6% (71–91) | 0.06 | 0.02 | 1.6 (1.2–2) |
| • Yes (n = 29) | 69.8% (54–85) | |||
| CMV reactivationa | ||||
| • No (n = 56) | 81% (69–93) | 0.06 | 0.1 | |
| • Yes (n = 56) | 68.7% (56–79) | |||
| Invasive fungal infectiona | ||||
| • No (n = 107) | 78.3% (70–84) | 0.001 | 0.01 | 3.1 (2.5–3.6) |
| • Yes (n = 10) | 34.3% (10–50) | |||
Other variables tested in the univariate analyses included: recipient and donor sex, conditioning regimen, use of TBI, donor type, development of pre-engraftment bacteremia*, C. difficile colitis*, hemorrhagic cystitis* and moderate-severe cGvHD*. All these variables had a P value > 0.5 in univariate analysis and are thus not included in the table.
Cum Inc. cumulative incidence, K–M Kaplan–Meier probability, HR Hazard ratio, 95% C.I. 95% confidence interval, GvHD Graft versus host disease, CMV cytomegalovirus.
aPost-transplant variables were analyzed as time-dependent covariates.
Post-engraftment bacterial infections (beyond day +30)
Twenty-eight patients (24%) had at least one severe bacterial infection beyond day +30, which occurred at a median of 124 days (range: 34–915) after SCT, with 5 (18%) of them having more than one episode. The 18-month incidence of post-engraftment bacterial infections was 58.1% (95% CI: 43–71) in haploSCT recipients and 56% (95% CI: 42–70) in the non-haploSCT cohorts (p = 0.9).
Fifteen episodes of bacterial infection occurred after day +100. A GNB was isolated in 40% of the 43 total episodes of late bacterial infections and six cases were due to Streptococcus pneumoniae. Of note, the rates of these infections did not differ by the presence or absence of grade 2–4aGvHD nor cGvHD (details not shown). Throughout the whole study period, we identified nine (8.7%) MDR organisms, mostly MDR-GNB (ESBL producer Enterobacteriaceae in six cases and MDR-P.aeuruginosa in three). Eight patients (7%) developed C. difficile-associated infection, with a median onset of 138 days (range: 6–777) post transplant.
Invasive fungal infections (IFI)
Three and seven patients (7.5% and 9%) in the haploSCT and non-haploSCT cohorts were diagnosed with an IFI, leading to an overall 100-day and 18-month incidence of 5.3% (95%CI: 1–9) and 8.7% (95% CI: 3–14), respectively. Most of the cases occurred in the first 100 days after SCT, with a median onset of 58 days (range: 12–609). Invasive aspergillosis (IA) was the most common IFI in both study groups, accounting for 70% of cases.
CMV and other viral infections
Fifty-six patients (48%) developed CMV infection/reactivation (CMV-I) after alloSCT, with a 30-day, 100-day and 18-month incidence of 8.6% (95% CI: 3.3–13%), 46.7% (95% CI: 37–55%) and 49% (95% CI: 38–57%), respectively. The median onset of CMV-I was 41 days (range: 9–198). CMV disease occurred in only 1 patient Recipients of a HaploSCT had a higher incidence of CMV-I at 18 months than patients who received transplant from a MRD/VUD/MMUD donor [(incidence of 61% (95% CI: 41–74%) vs. 44% (95% CI: 31–54%) (p = 0.03)].
As shown in Fig. 1, differences were especially appreciable between days +30 and +90 (45% vs. 36%, p = 0.01). When only CMV seropositive recipients were analyzed, the incidence of CMV-I at 90 days was 75.6% (95% CI: 58–92%) vs. 53% (95% CI: 40–66%), respectively (p = 0.009).
Fig. 1. Cumulative Incidence of CMV infection.
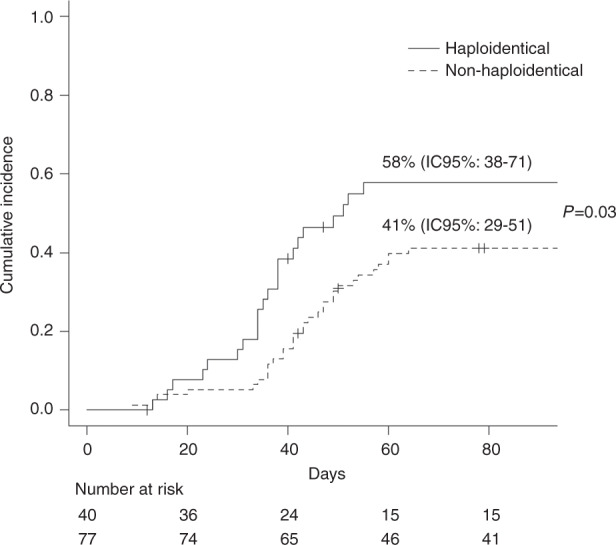
Cumulative incidence of CMV infection/reactivation (CMV-I) between days +30 and +90 post transplant in recipients of a HaploSCT vs patients who received transplant from a MRD/VUD/MMUD donor.
The rates of other severe viral infections are detailed in Table 2. Seventeen patients (14.5%) had a viral infection-related hemorrhagic cystitis (HC) at a median of 47 days after SCT (range: 10–222). Fourteen (82.5%) were BKPV-HC, while adenovirus was implicated in three cases. The rate of viral HC was higher in haploSCT recipients than in the non-haploSCT groups (27.5% vs. 7.8%, p = 0.01). EBV infection was documented in three patients, but without any case of lymphoproliferative disease. HHV-6 encephalitis occurred in three patients (2.6%) at a median of 66 days (range: 25–212) post-SCT.
After CMV-I, the most common group of viral infections diagnosed were lower respiratory tract infections (traqueobronchitis with or without pneumonia) by conventional respiratory viruses (CRV), which occurred in 38 patients (32.5%). Respiratory syncytial virus (n = 11) was the most common CRV. As expected, infections by CRV occurred mostly late after SCT, with a median onset of day +258 (range: 1–1425) and only three cases (7%) occurring before day +100. Only two patients died from these infections (pneumonia due to influenza A and SARS-CoV-2 on days +334 and +208 post-SCT, respectively). As shown in Table 2, CRV infections did not differ between haploSCT and non-haploSCT recipients.
Infection-related mortality (IRM) and long-term outcomes
Infection was the primary cause of death in 36% of patients who died and a contributing cause in an additional 24%. Median time to IRM was 149 days (range: 12–1266), with 42% of these deaths occurring in the first 100 days. IRM did not differ between transplant groups. The 18-month incidence of IRM was 7.9% (95% CI: 5–13%) in haploSCT recipients and 13.4% (95% CI: 4–19%) in the non-haploSCT cohorts, respectively (p = 0.7). Bacterial infections were the main cause of IRM (42%), followed by viral (25%) and IFI (17%).
At last follow-up, 84 patients were alive (72%) and 79 of them (67.5%) in complete remission with an 18-month OS and PFS of 71.3% (95% CI: 62–81%) and 67.4% (95% CI: 59–75), respectively, and again no differences by donor type (p = 0.8; details not shown) (see Fig. 2). In univariate and multivariate analysis only occurrence of grade 2–4 aGvHD increased the risk of IRM (p = 0.01, HR 3). With respect to OS (Table 3), grade 2–4 aGvHD and development of an IFI were significantly associated with higher mortality in multivariate analysis.
Fig. 2. Overall survival.
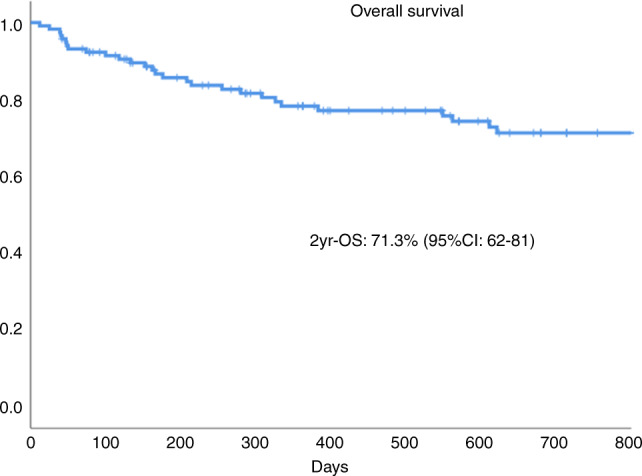
Overall survival at 2 years.
Discussion
In the present analysis, we describe a complete picture of infectious complications in patients receiving an alloSCT with PTCy prophylaxis and found that the rates of severe bacterial and fungal infections and the incidence of IRM were similar between the different donors studied. However, this was not the case for several types of post-engraftment viral infections, which were more common in haploSCT. Additional interesting findings should be further discussed.
First of all, our results support the lack of significant differences in the overall outcomes using different stem cell donors when high-dose PTCy is used, with low rates of both moderate-to-severe acute and chronic GvHD [16–18, 29]. A possible exception is the trend for higher GvHD and NRM in the MMUD group, which requires further study due to the small patient numbers to date.
Despite the theoretical better infectious profile of PTCy-based strategies compared to T-cell depleted SCT [30–32], we found that infections were a primary or contributing cause of death in around a half of the patients who died in the follow-up period. Of note, IRM was linked to aGvHD, despite the low incidence of severe forms using PTCy.
In terms of bacterial infections, one of the main findings of this study was the similar incidence of PE-BSIs among the different stem cell donors (45% in haploSCT and 40.5% in the non-haploSCT cohorts, p = 0.7), rates which are comparable to those reported using different GvHD prophylaxis strategies and conditioning regimen intensities [33–37].
Overall, this is a positive observation since PTCy could potentially increase the mucosal barrier injury-linked infections due to more intense and prolonged damage to the gastro-intestinal mucosa combined with a more prolonged duration of neutropenia and monocytopenia, leading to a higher pre-engraftment (before day +30) IRM [38–40]. With respect to post-engraftment infections, there was a low incidence of late bacterial infections (24% of evaluable cases), probably explained by the low incidence of severe forms of both acute and chronic GvHD. In our report, a total of 8.7% MDR-bloodstream infections were identified, a lower rate compared to other studies [41–46]. In addition, the 7% rate of C. difficile-associated infection is in the lower range of the previously reported rates of 9–25% [47–50].
We found an 8.7% incidence of IFI at 1.5 years, within the current objective of having an incidence <10% after alloSCT [51, 52], with the majority of cases occurring after engraftment but before day +100 post transplant. This low incidence of IFI can be explained by the low incidence of GvHD and the reduction in the use of prolonged steroid treatment.
Regarding viral infections, their incidence was high in early post-engraftment phase and more frequent in haploSCT recipients. Both CMV-I and viral HC were especially high in haploSCT recipients between days +30 to +90 post transplant, with incidences of 45% and 27%, respectively, comparable to other haploSCT studies [32, 40, 53, 54]. Beyond day +100 rates of CMV and HC infection were similar between the different stem cell donor groups. Interestingly, we confirmed the low incidence of CMV disease and the lack of EBV-related lymphoproliferative disease, as recently reported by Kanakry et al. [55]. HaploSCT recipients have historically been at increased risk of viral infections because only half of the donor’s HLA is expressed by the recipient’s antigen presenting cells, potentially leading to delayed HLA-restricted immune effector functions if immunodominant HLA loci are missing in the new donor-recipient HLA environment [56].
The lower incidence of infections observed in our study after day +100 [57, 58]may reflect an effective restoration of antimicrobial immunity during the post transplant period, favored in part by the low rate of both acute and chronic GvHD.
Prior studies have reported an incidence of IRM of 9% to 20% in patients receiving PTCy-based alloSCT platforms [30, 32, 40], although most studies have focused on haploSCT since this has been the most common setting for the use of PTCy. Our finding of a 9% IRM at 18 months, without differences between haploSCT and MRS/VUD/MMUD is thus promising.
The current study shares the limitations inherent to all retrospective analyses of complex clinical scenarios, including potential selection bias, as we cannot exclude the possibility that some non-severe infections were not captured because of incomplete reporting. However, all patients were followed at the same institution; therefore it is unlikely that clinically-relevant infectious complications were systematically missed; moreover, the diagnostic procedures and prophylactic measures were similar for all patients, thus contributing to the homogeneity in the diagnosis of the infectious events. In addition, as far as we know there are no previous studies providing detailed information on infectious morbidity and mortality after PTCy over an extended period of time and comparing the results between different stem cell donors.
In conclusion, our analysis found that severe infections were the main cause of NRM after alloSCT with PTCy, although their incidence was low in both donor groups. We did not observe remarkable differences in rates, microorganisms involved or diagnosis period of infection except in case of CMV and viral HC which were more frequently diagnosed early post-engraftment in the haploidentical cohort. Our data supports yet another promising role of using PTCy as GvHD prophylaxis, although efforts toward reducing the rates of CMV-I and viral HC, especially in haploSCT recipients, are clearly needed.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Sierra Jorge, Martino Rodrigo
References
- 1.Kim SH, Kee SY, Lee DG, Choi SM, Park SH, Kwon JC, et al. Infectious complications following allogeneic stem cell transplantation: reduced-intensity vs myeloablative conditioning regimens. Transpl Infect Dis. 2013;15:49–59. doi: 10.1111/tid.12003. [DOI] [PubMed] [Google Scholar]
- 2.Yoo JH, Lee DG, Choi SM, Choi JH, Park YH, Kim YJ, et al. Infectious complications and outcomes after allogeneic hematopoietic stem cell transplantation in Korea. Bone Marrow Transpl. 2004;34:497–504. doi: 10.1038/sj.bmt.1704636. [DOI] [PubMed] [Google Scholar]
- 3.Ninin E, Milpied N, Moreau P, Andre-Richet B, Morineau N, Mahe B, et al. Longitudinal study of bacterial, viral, and fungal infections in adult recipients of bone marrow transplants. Clin Infect Dis. 2001;33:41–47. doi: 10.1086/320871. [DOI] [PubMed] [Google Scholar]
- 4.Mitchell AE, Derrington P, Turner P, Hunt LP, Oakhill A, Marks DI. Gram-negative bacteraemia (GNB) after 428 unrelated donor bone marrow transplants (UD-BMT): risk factors, prophylaxis, therapy and outcome. Bone Marrow Transpl. 2004;33:303–10. doi: 10.1038/sj.bmt.1704338. [DOI] [PubMed] [Google Scholar]
- 5.Junghanss C, Marr KA. Infectious risks and outcomes after stem cell transplantation: are nonmyeloablative transplants changing the picture? Curr Opin Infect Dis. 2002;15:347–53. doi: 10.1097/00001432-200208000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Leather HL, Wingard JR. Infections following hematopoietic stem cell transplantation. Infect Dis Clin North Am. 2001;15:483–520. doi: 10.1016/s0891-5520(05)70157-4. [DOI] [PubMed] [Google Scholar]
- 7.van Kraaij MG, Verdonck LF, Rozenberg-Arska M, Dekker AW. Early infections in adults undergoing matched related and matched unrelated/mismatched donor stem cell transplantation: a comparison of incidence. Bone Marrow Transpl. 2002;30:303–9. doi: 10.1038/sj.bmt.1703643. [DOI] [PubMed] [Google Scholar]
- 8.Williamson EC, Millar MR, Steward CG, Cornish JM, Foot AB, Oakhill A, et al. Infections in adults undergoing unrelated donor bone marrow transplantation. Br J Haematol. 1999;104:560–8. doi: 10.1046/j.1365-2141.1999.01229.x. [DOI] [PubMed] [Google Scholar]
- 9.Small TN, Papadopoulos EB, Boulad F, Black P, CastroMalaspina H, Childs BH, et al. Comparison of immune reconstitution after unrelated and related T-cell-depleted bone marrow transplantation: effect of patient age and donor leukocyte infusions. Blood. 1999;93:467–80. [PubMed] [Google Scholar]
- 10.Bjorklund A, Aschan J, Labopin M, Remberger M, Ringden O, Winiarski J, et al. Risk factors for fatal infectious complications developing late after allogeneic stem cell transplantation. Bone Marrow Transplant. 2007;40:1055–62. doi: 10.1038/sj.bmt.1705856. [DOI] [PubMed] [Google Scholar]
- 11.Atilla E, Atilla PA, Bozdağ SC, Demirer T. A review of infectious complications after haploidentical hematopoietic stem cell transplantations. Infection. 2017;45:403–11. doi: 10.1007/s15010-017-1016-1. [DOI] [PubMed] [Google Scholar]
- 12.Luznik L, O’Donnell PV, Symons HJ, Chen AR, Leffell MS, Zahurak M, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transpl. 2008;14:641–50.. doi: 10.1016/j.bbmt.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bashey A, Zhang X, Jackson K, Brown S, Ridgeway M, Solh M, et al. Comparison of outcomes of hematopoietic cell transplants from T-replete haploidentical donors using post-transplantation cyclophosphamide with 10 of 10 HLA-A, -B, -C, -DRB1, and -DQB1 allelematched unrelated donors and hla-identical sibling donors: a multivariable analysis including disease risk index. Biol Blood Marrow Transpl. 2016;22:125–33.. doi: 10.1016/j.bbmt.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 14.Sun Y, Beohou E, Labopin M, Volin L, Milpied N, Yakoub-Agha I, et al. Unmanipulated haploidentical versus matched unrelated donor allogeneic stem cell transplantation in adult patients with acute myelogenous leukemia in first remission: a retrospective pair-matched comparative study of the Beijing approach with the EBMT data base. Haematologica. 2016;101:e352–4. doi: 10.3324/haematol.2015.140509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Esquirol A, Querol S, Garcia-Cadenas I, Novelli S, Garrido A, Saavedra S, et al. When an HLA identical donor is not available in adults with hematological neoplasms: single-center comparison of single-unit cord blood transplantation and haploidentical-related PBSC transplantation with PTCy using a standardized conditioning platform (thiotepa-busulfan-fludarabine) Ann Hematol. 2020;99:157–65. doi: 10.1007/s00277-019-03870-0. [DOI] [PubMed] [Google Scholar]
- 16.Ruggeri A, Labopin M, Bacigalupo A, Afanasyev B, Cornelissen JJ, Elmaagacli A, et al. Posttransplant cyclophosphamide for GVHD prophylaxis in HLA matched sibling or matched-unrelated donor transplant for patients with acute leukemia, on behalf of ALWP-EBMT. J Hematol Oncol. 2018;11:40. doi: 10.1186/s13045-018-0586-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shah MV, Saliba RM, Rondon G, Chen J, Soebbing D, Rus I, et al. Pilot study using post-transplant cyclophosphamide (PTCy), tacrolimus and mycophenolate GVHD prophylaxis for olderpatientsreceiving 10/10 HLA-matched unrelated donor hematopoietic stem cell transplantation. Bone Marrow Transpl. 2019;54:601–6. doi: 10.1038/s41409-018-0367-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prem S, Atenafu EG, Al-Shaibani Z, Loach D, Law A, Lam W, et al. Low rates of acute and chronic GVHD with ATG and PTCy in matched and mismatched unrelated donor peripheral bloodstem cell transplants. Eur J Haematol. 2019;102:486–93. doi: 10.1111/ejh.13230. [DOI] [PubMed] [Google Scholar]
- 19.García-Cadenas I, Awol R, Esquirol A, Saavedra S, Bosch-Vilaseca A, Novelli S, et al. Incorporating posttransplant cyclophosphamide-based prophylaxis as standard-of-care outside the haploidentical setting: challenges and review of the literature. Bone Marrow Transpl. 2020;55:1041–9. [DOI] [PubMed]
- 20.Martino R, Iacobelli S, Brand R, Jansen T, van Biezen A, Finke J, et al. Retrospective comparison of reduced-intensity conditioning and conventional high-dose conditioning for allogeneic hematopoietic stem cell transplantation using HLA-identical sibling donors in myelodysplasticsyndromes. Myelodysplastic Syndrome subcommittee of the Chronic Leukemia Working Party of the European Blood and Marrow Transplantation Group. Blood. 2006;108:836–46.. doi: 10.1182/blood-2005-11-4503. [DOI] [PubMed] [Google Scholar]
- 21.Parody R, Lopez-Corral L, Godino OL, Cadenas IG, Martinez AP, Vazquez L, et al. GVHD prophylaxis with sirolimus-tacrolimus may overcome the deleterious effect on survival of HLA mismatch after reduced-intensity conditioning alloSCT. Bone Marrow Transpl. 2015;50:121–6. doi: 10.1038/bmt.2014.220. [DOI] [PubMed] [Google Scholar]
- 22.Piñana JL, Valcárcel D, Fernández-Avilés F, Martino R, Rovira M, Barba P, et al. MTX or mycophenolatemofetil with CsA as GVHD prophylaxis after reduced-intensity conditioning PBSCT from HLA-identical siblings. Bone Marrow Transpl. 2010;45:1449–56.. doi: 10.1038/bmt.2009.362. [DOI] [PubMed] [Google Scholar]
- 23.Kanakry CG, O’Donnell PV, Furlong T, de Lima MJ, Wei W, Medeot M, et al. Multi-institutional study of post-transplantation cyclophosphamide as single-agent graft-versus-host disease prophylaxis after allogeneic bone marrow transplantation using myeloablativebusulfan and fludarabine conditioning. J Clin Oncol. 2014;32:3497–505.. doi: 10.1200/JCO.2013.54.0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Piñana JL, Martino R, Barba P, Margall N, Roig MC, Valcárcel D, et al. Cytomegalovirus infection and disease after reduced intensity conditioning allogeneic stem cell transplantation: single-centre experience. Bone Marrow Transpl. 2010;45:534–42. doi: 10.1038/bmt.2009.180. [DOI] [PubMed] [Google Scholar]
- 25.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. Consensus conference on acute GVHD grading. Bone Marrow Transpl Bone Marrow Transpl. 1995;15:825–8. [PubMed] [Google Scholar]
- 26.Lee StephanieJ, Wolff D, Kitko C, Koreth J, Inamoto Y, et al. Measuring therapeutic response in chronic graft-versus-host disease. national institutes of health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: IV. the 2014 response Criteria Working Group. Biol Blood Marrow Transpl. 2015;21:984–99. doi: 10.1016/j.bbmt.2015.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Copelan E, Casper JT, Carter SL, van Burik JAH, Hurd D, Mendizabal AM, et al. A scheme for defining cause of death and its application in the T cell depletion trial. Biol Blood Marrow Transpl. 2007;13:1469–76. doi: 10.1016/j.bbmt.2007.08.047. [DOI] [PubMed] [Google Scholar]
- 28.Fine JP, Gray RJ. A proportional hazards model for the sub-distribution of acompeting risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 29.Ruggeri A, Labopin M, Bacigalupo A, Afanasyev B, Cornelissen JJ, Elmaagacli A, et al. Post-transplant cyclophosphamide for graft-versus-host disease prophylaxis in HLA matched sibling or matched unrelated donor transplant for patients with acute leukemia, on behalf of ALWP-EBMT. A J Hematol Oncol. 2018;11:40. doi: 10.1186/s13045-018-0586-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fayard A, Daguenet E, Blaise D, Chevallier P, Labussière H, Berceanu A, et al. Evaluation of infectious complications after haploidentical hematopoietic stem cell transplantation with post-transplant cyclophosphamide following reduced-intensity and myeloablative conditioning: a study on behalf of the Francophone Society of Stem Cell Transplantation and Cellular Therapy (SFGM-TC) Bone Marrow Transpl. 2019;54:1586–94. doi: 10.1038/s41409-019-0475-7. [DOI] [PubMed] [Google Scholar]
- 31.Mohty R, Brissot E, Battipaglia G, Ruggeri A, Dulery R, et al. Infectious complications after post-transplantation cyclophosphamide and anti-thymocyte globulin-based haploidentical stem cell transplantation. Br J Haematol. 2019;187:e64–e68. doi: 10.1111/bjh.16189. [DOI] [PubMed] [Google Scholar]
- 32.Crocchiolo R, Bramanti S, Vai A, Sarina B, Mineri R, Casari E, et al. Infections after T-replete haploidentical transplantation and high-dose cyclophosphamide as graft-versus-host disease prophylaxis. Transpl Infect Dis. 2015;17:242–9. doi: 10.1111/tid.12365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dandoy CE, Kim S, Chen M, Ahn KW, Ardura MI, Brown V, et al. Incidence, risk factors, and outcomes of patients who develop mucosal barrier injury-laboratory confirmed bloodstream infections in the first 100 days after allogeneic hematopoietic stem cell transplant. JAMA Netw Open. 2020;3:e1918668. doi: 10.1001/jamanetworkopen.2019.18668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dandoy CE, Ardura MI, Papanicolaou GA, Auletta JJ. Bacterial bloodstream infections in the allogeneic hematopoietic cell transplant patient: new considerations for a persistent nemesis. Bone Marrow Transpl. 2017;52:1091–106. doi: 10.1038/bmt.2017.14. [DOI] [PubMed] [Google Scholar]
- 35.Mikulska M, Raiola AM, Galaverna F, Balletto E, Borghesi ML, Varaldo R, et al. Preengraftment bloodstream infections after allogeneic hematopoietic cell transplantation: impact of T cell-replete transplantation from a haploidentical donor. Biol Blood Marrow Transpl. 2018;24:109–18. doi: 10.1016/j.bbmt.2017.08.024. [DOI] [PubMed] [Google Scholar]
- 36.Martino R, Bautista G, Parody R, García I, Esquirol A, Rovira M, et al. Severe infections after single umbilical cord blood transplantation in adults with or without the co-infusion of CD34+ cells from a third-party donor: results of a multicenter study from the Grupo Español de Trasplante Hematopoyético (GETH) Transpl Infect Dis. 2015;17:221–33.. doi: 10.1111/tid.12361. [DOI] [PubMed] [Google Scholar]
- 37.Parody R, Martino R, Rovira M, Vazquez L, Vázquez MJ, de la Cámara R, et al. Severe infections after unrelated donor allogeneic hematopoietic stem cell transplantation in adults: comparison of cord blood transplantation with peripheral blood and bone marrow transplantation. Biol Blood Marrow Transpl. 2006;12:734–48.. doi: 10.1016/j.bbmt.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 38.Almyroudis NG, Fuller A, Jakubowski A, Sepkowitz K, Jaffe D, Small T, et al. Pre- and post-engraftment bloodstream infection rates and associated mortality in allogeneic hematopoietic stem cell transplant recipients. Transpl Infect Dis. 2005;7:11–7. doi: 10.1111/j.1399-3062.2005.00088.x. [DOI] [PubMed] [Google Scholar]
- 39.Chang J, Hsiao M, Blodget E, Akhtari MJ. Increased risk of 100-day and 1-year infection-related mortality and complications in haploidentical stem cell transplantation. Blood Med. 2019;10:135–43. doi: 10.2147/JBM.S201073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oltolini C, Greco R, Galli L, Clerici D, Lorentino F, Xue E, et al. Infections after allogenic transplant with post-transplant cyclophosphamide: impact of donor HLA matching. Biol Blood Marrow Transpl. 2020;S1083-8791:30045–8. doi: 10.1016/j.bbmt.2020.01.013. [DOI] [PubMed] [Google Scholar]
- 41.Patriarca F, Cigana C, Massimo D, Lazzarotto D, Geromin A, Isola M, et al. Risk factors and outcomes of infections by multidrug-resistant gram-negative bacteria in patients undergoing hematopoietic stem cell transplantation. Biol Blood Marrow Transpl. 2017;23:333–9. doi: 10.1016/j.bbmt.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 42.Puerta-Alcalde P, Cardozo C, Marco F, Suárez-Lledó M, Moreno E, Morata L, et al. Changing epidemiology of bloodstream infection in a 25-years hematopoietic stem cell transplant program: current challenges and pitfalls on empiric antibiotic treatment impacting outcomes. Bone Marrow Transpl. 2020;55:603–12. doi: 10.1038/s41409-019-0701-3. [DOI] [PubMed] [Google Scholar]
- 43.Bilinski J, Robak K, Peric Z, Marchel H, Karakulska-Prystupiuk E, Halaburda K. Impact of gut colonization by antibiotic-resistant bacteria on the outcomes of allogeneic hematopoietic stem cell transplantation: a retrospective, single-center study. Biol Blood Marrow Transpl. 2016;22:1087–93. doi: 10.1016/j.bbmt.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 44.Mikulska M, Bono V, Raiola A, Bruno B, Gualandi F, Occhini D, et al. Blood stream infections in allogeneic hematopoietic stem cell transplant recipients: reemergence of gram-negative rods and increasing antibiotic resistance. Biol Blood Marrow Transpl. 2009;15:47–53. doi: 10.1016/j.bbmt.2008.10.024. [DOI] [PubMed] [Google Scholar]
- 45.Liu C, Frank DN, Horch M, Chau S, Ir D, Horch EA, et al. Associations between acute gastrointestinal GvHD and the baseline gut microbiota of allogeneic hematopoietic stem cell transplant recipients and donors. Bone Marrow Transpl. 2017;52:1643–50. doi: 10.1038/bmt.2017.200. [DOI] [PubMed] [Google Scholar]
- 46.Köhler N, Zeiser R. Intestinal microbiota influence immune tolerance post allogeneic hematopoietic cell transplantation and intestinal GVHD. Front Immunol. 2019;9:3179. doi: 10.3389/fimmu.2018.03179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Willems L, Porcher R, Lafaurie M, Casin I, Robin M, Xhaard A, et al. Clostridium difficile infection after allogeneic hematopoietic stem cell transplantation: incidence, risk factors, and outcome. Biol Blood Marrow Transpl. 2012;18:1295–301. doi: 10.1016/j.bbmt.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 48.Alonso CD, Treadway SB, Hanna DB, Huff CA, Neofytos D, Carroll KC, et al. Epidemiology and outcomes of Clostridium difficile infections in hematopoietic stem cell transplant recipients. Clin Infect Dis. 2012;54:1053–63.. doi: 10.1093/cid/cir1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Santiago M, Eysenbach L, Allegretti J, Aroniadis O, Brandt LJ, Fischer M, et al. Microbiome predictors of dysbiosis and VRE decolonization in patients with recurrent C. difficile infections in a multi-center retrospective study. Microbiol. 2019;5:1–18. doi: 10.3934/microbiol.2019.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alonso CD, Marr KA. Clostridium difficile infection among hematopoietic stem cell transplant recipients: beyond colitis. Curr Opin Infect Dis. 2013;26:326–31.. doi: 10.1097/QCO.0b013e3283630c4c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kontoyiannis DP, Marr KA, Park BJ, Alexander BD, Anaissie EJ, Walsh TJ, et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001-6: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin Infect Dis. 2010;50:1091–100. doi: 10.1086/651263. [DOI] [PubMed] [Google Scholar]
- 52.Liu YC, Chien SH, Fan NW, Hu MH, Gau JP, Liu CJ, et al. Incidence and risk factors of probable and proven invasive fungal infection in adult patients receiving allogeneic hematopoietic stem cell transplantation. J Microbiol Immunol Infect. 2016;49:567–74. doi: 10.1016/j.jmii.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 53.Ruggeri A, Roth-Guepin G, Battipaglia G, Mamez AC, Malard F, Gomez A, et al. Incidence and risk factors for hemorrhagic cystitis in unmanipulated haploidentical transplant recipients. Transpl Infect Dis. 2015;17:822–30.. doi: 10.1111/tid.12455. [DOI] [PubMed] [Google Scholar]
- 54.Copelan OR, Sanikommu SR, Trivedi JS, Butler C, Ai J, Ragon BK, et al. Higher incidence of hemorrhagic cystitis following haploidentical related donor transplantation compared with matched related donor transplantation. Biol Blood Marrow Transpl. 2019;25:785–90. doi: 10.1016/j.bbmt.2018.12.142. [DOI] [PubMed] [Google Scholar]
- 55.Kanakry JA, Kasamon YL, Bolaños-Meade J, Borrello IM, Brodsky RA, Fuchs EJ, et al. Absence of post-transplantation lymphoproliferative disorder after allogeneic blood or marrow transplantation using post-transplantation cyclophosphamide as graft-versus-host disease prophylaxis. Biol Blood Marrow Transpl. 2013;19:1514–7. doi: 10.1016/j.bbmt.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chang YJ, Zhao XY, Huang XJ. Immune reconstitution after haploidentical hematopoietic stem cell transplantation. Biol Blood Marrow Transpl. 2014;20:440. doi: 10.1016/j.bbmt.2013.11.028. [DOI] [PubMed] [Google Scholar]
- 57.Marinelli T, Wee LYA, Rowe E, Chhetri R, Friel O, Higgins G, et al. Respiratory viruses cause late morbidity in recipients of hematopoietic stem cell transplantation. Biol Blood Marrow Transpl. 2020;26:782–8. doi: 10.1016/j.bbmt.2019.12.724. [DOI] [PubMed] [Google Scholar]
- 58.Piñana JL, Gómez MD, Pérez A, Madrid S, Balaguer-Roselló A, Giménez E, et al. Community-acquired respiratory virus lower respiratory tract disease in allogeneic stem cell transplantation recipient: risk factors and mortality from pulmonary virus-bacterial mixed infections. Transpl Infect Dis. 2018;20:e12926. doi: 10.1111/tid.12926. [DOI] [PMC free article] [PubMed] [Google Scholar]


