Abstract
Objective:
Increased rates of carbapenem-resistant Acinetobacter baumannii has forced clinicians to rely upon last-line agents, such as the polymyxins, or empiric, un-optimized combination therapy. Therefore, the objectives of this study were to: (1) evaluate the in vitro pharmacodynamics of meropenem and polymyxin B (PMB) combinations against A. baumannii; (2) utilise a mechanism-based mathematical model to quantify bacterial killing; and (3) develop a genetic algorithm (GA) to define optimal dosing strategies of meropenem and PMB.
Methods:
A. baumannii (N16870; MICmeropenem = 16mg/L, MICPMB = 0.5mg/L) was studied in the hollow-fibre infection model (initial inoculum 108 cfu/mL) over 14 days against meropenem and PMB combinations. A mechanism-based model (MBM) of the data and population pharmacokinetics of each drug were used to develop a GA to define the optimal regimen parameters.
Results:
Monotherapies resulted in regrowth to ~1010 cfu/mL by 24 h while combination regimens employing high intensity PMB exposure achieved complete bacterial eradication (0 cfu/mL) by 336 h. The MBM demonstrated a SC50 (PMB concentration for 50% of maximum synergy on meropenem killing) of 0.0927mg/L for PMB-susceptible subpopulations versus 3.40mg/L for PMB-resistant subpopulations. The GA had a preference for meropenem regimens that improved the %T>MIC via longer infusion times and shorter dosing intervals. The GA predicted that treating 90% of simulated subjects harbouring a 108 cfu/mL starting inoculum to a point of 100 cfu/mL would require a regimen of meropenem 19.6 g/day 2 h prolonged infusion (2hPI) q5h + PMB 5.17 mg/kg/day 2hPI q6h (where the 0h meropenem and PMB doses should be ‘loaded’ with 80.5% and 42.2% of the daily dose, respectively).
Conclusion:
This study provides a methodology leveraging in vitro experimental data, a mathematical pharmacodynamic model, and population pharmacokinetics provides a possible avenue to optimize treatment regimens beyond the use of the “traditional” indices of antibiotic action.
Introduction
Of the regularly used antibacterial agents, the majority were originally marketed between the 1950s and 1990s.[1] Because of this, many commonly used antibacterial agents were not subjected to the more rigorous, contemporary pharmacokinetic/pharmacodynamic evaluations to define optimal dosage regimens. Thus, there is a large, unmet scientific need to study antimicrobial agents, such as the polymyxins or carbapenems, more intensively in order to better translate improved treatment strategies into a form that can help high-risk patient populations.
Carbapenem-resistant Acinetobacter baumannii (CRAB) has been defined as a critical-priority pathogen and placed among the top tier of bacteria requiring research for clinical solutions, according to the World Health Organization.[2–5] Unfortunately, as resistance has increased, agents capable of treating extensively drug resistant A. baumannii have left the polymyxins as a last-line, critically important class of antibiotics against CRAB.[6–9]
Previous in vitro studies have shown a utility in using carbapenems in combination with polymyxins against CRAB in order to revitalise carbapenem activity and to prevent the development of polymyxin resistance.[10–15] Paul and colleagues tested this hypothesis for significance at the clinical level in the AIDA trial, a randomised controlled trial, which found that combination of colistin + meropenem was not superior to monotherapy with colistin.[16] Though this trial represents a massive and important step forward in our analyses of combination antimicrobials in critically ill patients, it left several questions fundamentally unanswered. First, the applicability of these results to polymyxin B (PMB), which, unlike colistin, is administered in its active form and has more favourable pharmacokinetics, and, second, whether or not subjects were treated with the optimal meropenem dose (all subjects received 2g 3 h prolonged infusion Q8H) and had confirmed concentrations in the target range. In regards to the pharmacokinetic differences, approximately half (51%) of subjects in the AIDA trial were treated for pneumonia, meaning that suboptimal epithelial lining fluid concentrations of colistin may have been an additional factor in favouring monotherapy. Specifically, the poor lung availability of colistin has been hypothesized as being due to a risk of binding to mucin in the lungs, which may lower the free concentration of colistin.[17]
Dose selection, as opposed to dose optimisation, is typically achieved using empiric relationships between drug concentration and bacterial killing.[18, 19] However, this approach provides a limited exploration of regimen structures, especially for drugs given in combination. A genetic algorithm (GA) is a class of computational tools used for optimisation, which provides significant flexibility over the items the user wishes to optimise as well as the factors used to determine treatment success.[20, 21]
Therefore, the main objectives of this study were to generate additional data on the pharmacodynamics of meropenem plus PMB against the previously published CRAB isolate; develop an updated mechanistic model that describes the intrinsic activity of both agents and the synergy of the two together; and implement a GA to define an optimal, proof-of-concept dosing regimen for administering both drugs together. [14]
Methods
Bacterial Isolates, Antibiotics and Media
A. baumannii (N16870, MICPMB = 0.5 mg/L, MICMeropenem = 16 mg/L) was used for all experiments.[14] Meropenem (AKSci, Union City, CA) and polymyxin B ([PMB], Sigma Aldrich, St. Louis, MO) were utilised. Bacteria were grown in Muller-Hinton Broth (Difco, Detroit, MI) supplemented with calcium (25 mg/L) and magnesium (12.5 mg/L). MICs were determined by broth microdilution in duplicate per CLSI.[22]
Hollow-Fibre Infection Model
Cellulosic cartridges (C3008; FiberCell Systems Inc, Fredrick, MD) were inoculated with N16870 to achieve a starting concentration of 108 cfu/mL.[13, 23] The bacteria were treated for 14 days, with samples being collected to obtain total counts, population analysis profiles, and drug concentration.
Simulated Human Dosing Regimens
Regimens were simulated in the HFIM for meropenem (t½ = 2.5 h, fu= 98%) and PMB (t½ = 8 h, fu = 42%) based on a population pharmacokinetic studies in critically ill patients. For meropenem, pharmacokinetic profiles were based on data generated from 27 critically ill patients who received doses ranging between 2 and 9g/day.[24, 25] For PMB, pharmacokinetic profiles were based on data generated from 24 adult critically ill patients who received physician-selected, intravenous PMB dosage regimens ranging from 0.45 to 3.38 mg/kg/day.[24] Because of a much longer half-life for PMB compared to meropenem, PMB was administered as multiple boluses using a supplementation scheme as previously reported.[14]
Regimen I (fCss,avg = 0.75 mg/L): polymyxin B loading dose 1.11 mg/kg for 1 dose followed by 0.72 mg/kg q12h starting 12 h later (fAUCss = 18.0 mg•h/L at steady-state) + meropenem 4g 3 h prolonged infusion Q8h
Regimen II (fCss,avg = 2.5 mg/L): polymyxin B loading dose 3.71 mg/kg for 1 dose followed by 2.40 mg/kg q12h starting 12 h later (fAUCss = 59.8 mg•h/L at steady-state) + meropenem 4g 3 h prolonged infusion Q8h
Regimen III (fCss,avg = 5.0 mg/L): polymyxin B loading dose 7.43 mg/kg for 1 dose followed by 4.80 mg/kg q12h starting 12 h later (fAUCss = 120 mg•h/L at steady-state) + meropenem 4g 3 h prolonged infusion Q8h
Regimen IV (fCss,avg = 10 mg/L): polymyxin B loading dose (14.8 mg/kg for 1 dose followed by 9.60 mg/kg q12h starting 12 h later (fAUCss = 239 mg•h/L at steady-state) + meropenem 4g 3 h prolonged infusion Q8h
Mechanism-based Mathematical Model
The data was modelled in S-ADAPT utilizing S-ADAPT_TRAN for pre- and post-processing.[26–28] The finalised model structure consisted of subpopulations that were meropenem-PMB susceptible-susceptible, susceptible-resistant, and resistant-susceptible, where each subpopulation had its own growth rate and killing constants (Figure S1). Bacterial replication was modelled such that the probability of bacterial doubling would decrease as the total bacterial population approached the system’s carrying capacity. Each of the subpopulations was divided into two sub-compartments: a vegetative and a replicating compartment, where the cell division process was assumed to be rapid (i.e, kdiv = 50h−1). The proposed model of synergy was unidirectional (PMB affecting meropenem), and was implemented in this manner based on the preponderance of models already in the literature that involve the combination of a membrane permeablising agent with a beta-lactam.[15, 29–31]
Genetic Algorithm
Population simulations were generated only accounting for pharmacokinetic variability (PMB population PK based on Sandri and Landersdorfer et al. and meropenem population PK based on Mattioli et al.).[24, 25] Because of inherent increases in inter-individual variability present in critically ill patients, both clearance and distributional parameters for PMB and meropenem used in simulations have substantial inter-individual variability (e.g, IIV CV% in the range of 40-60%) than in the healthy volunteer data supplied in the package insert.[25, 32, 33]
Results
Observed pharmacokinetics were found to be in good agreement with the predicted pharmacokinetics for both meropenem (determined in the previous study by Lenhard et al., R2 = 0.982, slope = 0.750, intercept = −1.81 mg/L) and polymyxin B (determined in this study, R2 = 0.830, slope = 1.05, intercept = −1.18 mg/L).[14] For meropenem, the slope of 0.75 indicates the observed concentrations were less than those predicted. The HFIM studies successfully provided a range of bactericidal activity as intended. Regimen I, the lowest polymyxin B (PMB) dose tested, showed an initial killing to a nadir of the total bacterial population of 462 cfu/mL followed by regrowth to the system carrying capacity of > 1010 cfu/mL. Contrastingly, Regimen II-IV, which had doses of PMB higher than those previously published, produced bactericidal activity with sustained reduction of total bacterial counts of >99.9% over 14 d. The initial rate of bacterial killing (determined by simple linear regression from t = 0 h to the nadir of counts), showed a rapid, dose-proportional killing of −3.90 * 106 cfu/mL/h, −8.63 * 106 cfu/mL/h, −7.65 * 106 cfu/mL/h, and −9.65 * 106 cfu/mL/h for Regimens I-IV, respectively. Population analysis profiles quantified the proliferation of subpopulations resistant to both PMB and meropenem for Regimen I. Counts on the antibiotic-containing agar were largely suppressed in pre-treatment conditions (0 h), but appear starting at 144 h and continue throughout the experiment. (Figure 1A–D)
Figure 1: Hollow-Fibre Infection Model of Dose-escalated Polymyxin B + Meropenem against CRAB.
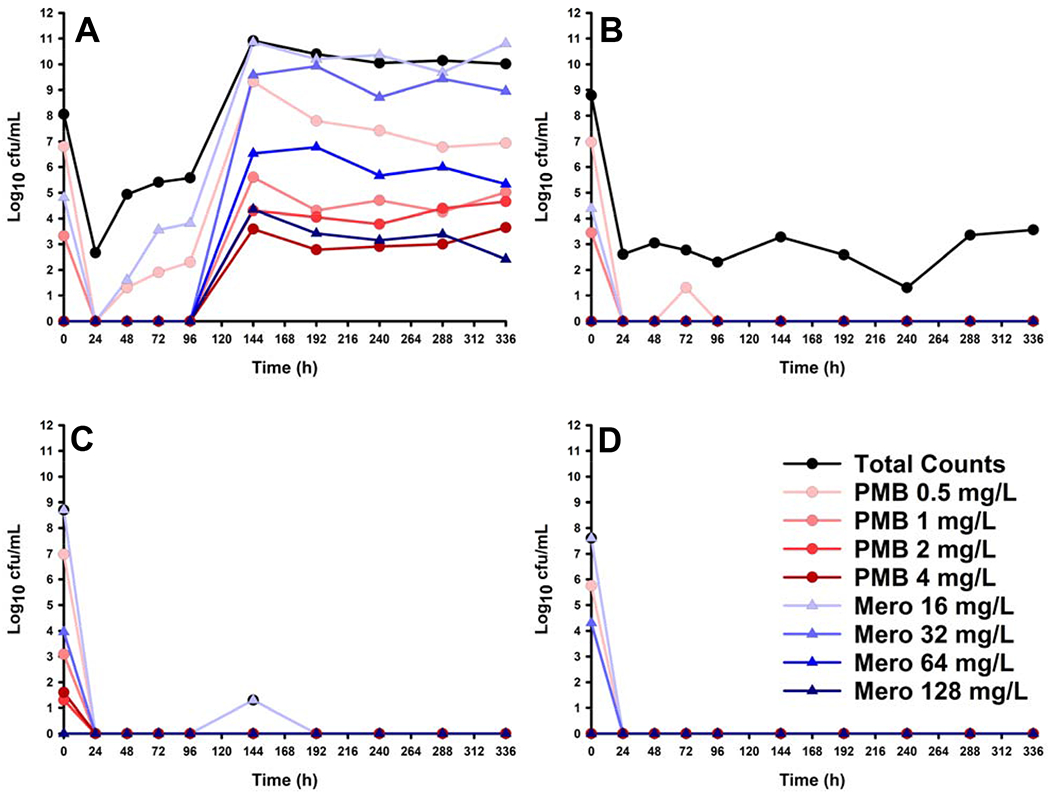
Total bacterial counts were determined by plating HFIM samples on drug-free Muller-Hinton agar (black circles). Additionally, population analysis profiles were determined by plating HFIM samples on either polymyxin B-containing (blue-shaded triangles) or meropenem-containing (red-shaded circles) Muller-Hinton agar. (A) Regimen I, polymyxin B loading dose 1.11 mg/kg for 1 dose followed by 0.72 mg/kg q12h starting 12 h later; (B) Regimen II, polymyxin B loading dose 3.71 mg/kg for 1 dose followed by 2.40 mg/kg q12h starting 12 h later; (C) Regimen III, polymyxin B loading dose 7.43 mg/kg for 1 dose followed by 4.80 mg/kg q12h starting 12 h later; (D) Regimen IV, polymyxin B loading dose (14.8 mg/kg for 1 dose followed by 9.60 mg/kg q12h starting 12 h later. For all of the above regimens, meropenem was administered as follows: 4g 3hPI Q8H
The finalised MBM was able to describe all HFIM data from the present study (Figure 2) and the previously reported study (Figure S2).[14] Evaluation of the distribution of i/pWRES and NPDE plots (Figure S3), in addition to the precision of parameter estimates (Table S1), showed that the model was able to describe the data sufficiently well.
Figure 2: Model Fit of Dose-escalated Polymyxin B + Meropenem against CRAB.
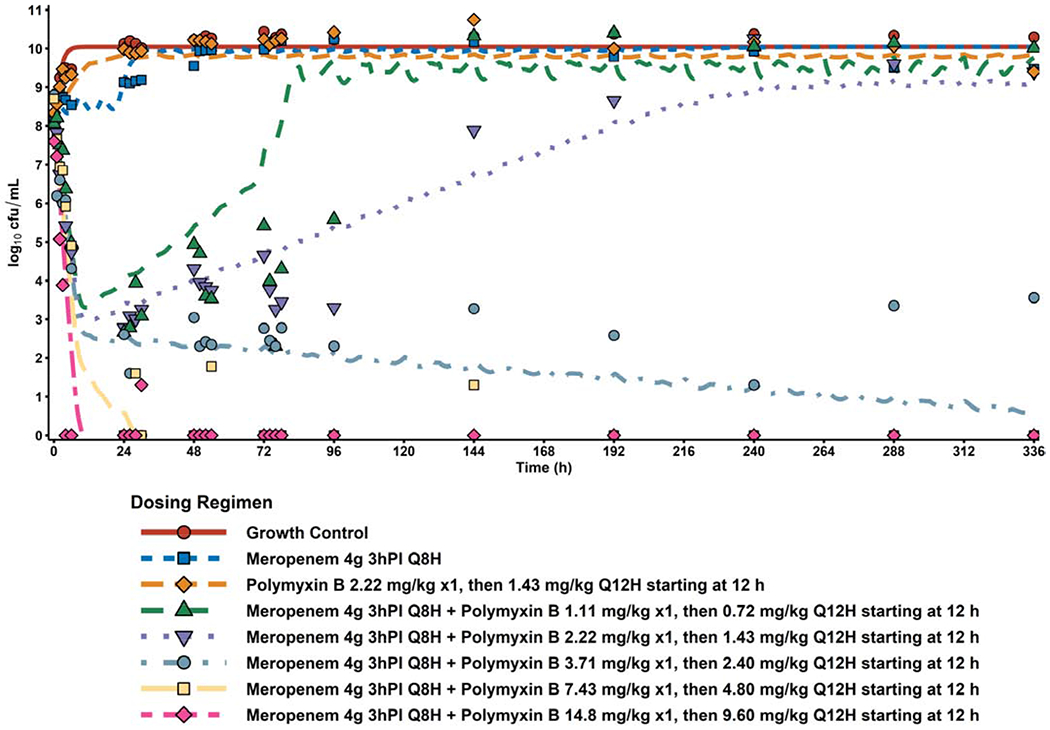
The post hoc fits for the full array of dose-escalated polymyxin B + 4 g meropenem 3h prolonged infusion q8h (lines) overlaid with observed data from the HFIM (points). Additionally, negative controls (growth control, polymyxin B monotherapy, and 4 g meropenem monotherapy) from the previous study by Lenhard et al. are also shown as reference on the finalized model’s performance.[20]
Subpopulations were described as three distinct groups of cells with unique growth rates and killing parameters for each antimicrobial. These subpopulations represented small fractions of the starting inoculum, with mutation frequencies estimated as being −8.50 and −5.64 for the SR (i.e., meropenem-susceptible, PMB-resistant) and RS (i.e., meropenem-resistant, PMB-susceptible) subpopulations, respectively (full parameter estimates available in Table S1). These estimates on the fraction of resistant subpopulations, in combination with the observed changes in population analysis profiles, indicate an increase in resistance for experimental arms experiencing regrowth. Additionally, it was found that the model performed significantly better when the growth rate was allowed to change in response to PMB presence. A turnover process driven by PMB exposure modified the change in growth rate and increased meropenem sensitivity. For PMB-susceptible and -resistant subpopulations, the turnover process had 50% of maximal effect at PMB concentrations of 0.0927 and 3.40 mg/L, respectively.
For each of the clinical situations (i.e., a regimen to produce bacterial eradication in 50%, 75%, or 90% of simulated patients) and each of the microbiologic conditions (102, 104, 106 or 108 cfu/mL starting inoculum), the GA successfully converged and produced a regimen that results 336 h bacterial counts near the eradication target (+/− 2 log10 cfu/mL). (Table 1, Figure 3, and Figures S4–5).
Table 1:
Optimal Dosing Regimen Simulation from Genetic Algorithm
| % Simulated Patients with Approximate Eradication | Inoculum Sizea | Antibiotic | Optimal Daily Dose | Optimal Infusion Time | Optimal Number of Daily Doses | Optimal Loading Dose |
|---|---|---|---|---|---|---|
| g/day, mg/kg/dayb | h | # | %c | |||
| 90% of Simulated Patients with Approximate Eradication | 108 | Meropenem | 19.6 | 2.0 | 5 | 80.5% |
| Polymyxin B | 5.17 | 2.0 | 4 | 42.2% | ||
| 106 | Meropenem | 18.3 | 3.0 | 4 | 10.2% | |
| Polymyxin B | 4.84 | 2.0 | 4 | 42.7% | ||
| 104 | Meropenem | 6.22 | 1.5 | 1 | - | |
| Polymyxin B | 1.51 | 2.0 | 2 | 18.3% | ||
| 102 | Meropenem | 4.87 | 0.5 | 1 | - | |
| Polymyxin B | 1.77 | 2.0 | 3 | 65.7% | ||
| 75% of Simulated Patients with Approximate Eradication | 108 | Meropenem | 18.1 | 2.0 | 5 | 16.2% |
| Polymyxin B | 4.50 | 1.5 | 1 | - | ||
| 106 | Meropenem | 18.3 | 2.0 | 4 | 58.0% | |
| Polymyxin B | 4.17 | 1.5 | 4 | 50.4% | ||
| 50% of Simulated Patients with Approximate Eradication | 108 | Meropenem | 14.7 | 1.5 | 4 | 62.0% |
| Polymyxin B | 4.01 | 1.5 | 4 | 46.9% | ||
| 106 | Meropenem | 13.2 | 2.5 | 4 | 45.7% | |
| Polymyxin B | 3.63 | 1.5 | 4 | 33.3% | ||
High inoculum doses were optimised for 108cfu/mL starting inoculum, whereas low inoculum doses were optimised for a 106 cfu/mL starting inoculum.
Meropenem doses given as g, Polymyxin B doses given as mg/kg
For the first day, the first dose was given as x% of the total daily dose, the remainder of the daily dose was evenly split among the remaining doses. Daily dose was evenly split among the number of daily doses for days 2-14
Figure 3: Simulation of the Genetic Algorithm-optimised Regimens for Coverage of 90% of Simulated Patients.
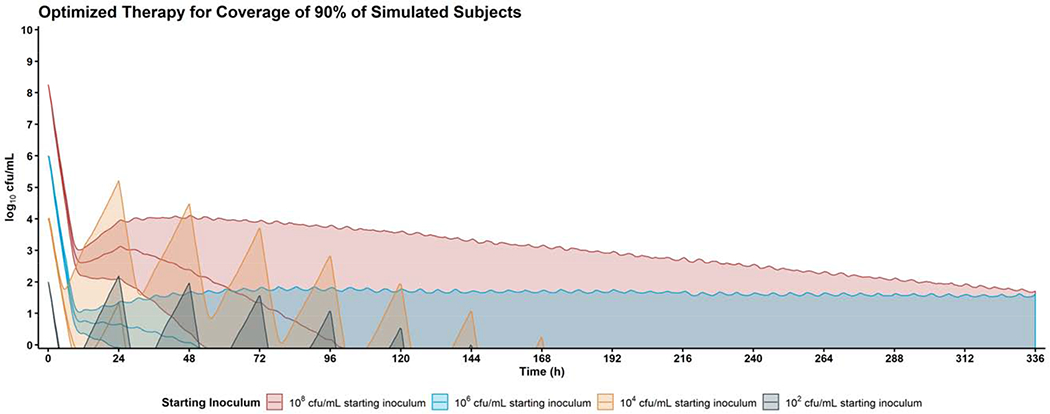
The genetic algorithm was able to identify an optimised combination of meropenem + polymyxin B for the treatment of high (red) and low (blue) inoculum infections. Shaded regions represent the 90% prediction interval of the treatment based on previously published population pharmacokinetics in critically ill patients.[30, 46] To produce eradication in 90% of simulated subjects with a high inoculum (108 cfu/mL) infection, the optimised regimen was: Meropenem 17.8 g/day 2.5 h prolonged infusion q6h (with 22.2% of the total first day’s dose given at 0 h) + Polymyxin B 5.33 mg/kg/day 2 h prolonged infusion q8h (with 51.1% of the total first day’s dose given at 0 h). To produce eradication in 90% of simulated subjects with a low inoculum (106 cfu/mL) infection, the optimised regimen was: Meropenem 16.9 g/day 2.5 h prolonged infusion q5h (with 65.1% of the total first day’s dose given at 0 h) + Polymyxin B 4.74 mg/kg/day 2.5 h prolonged infusion q6h (with 33.6% of the total first day’s dose given at 0 h).
Discussion
The proliferation of drug resistant A. baumannii in the clinical setting has resulted in an increased need to use combination therapy. The main finding of this study was the successful generation of six optimised regimens which were designed to be able to improve the probability of achieving bacterial counts of 0 cfu/mL at either a 106 or 108 cfu/mL inoculum and in either 50%, 75%, or 90% of subjects.
To define effects of meropenem plus PMB, the MBM-based approach incorporated information regarding drug action and current hypotheses regarding adaptive resistance and synergy. Principally, the final model can be divided into two main sub-components: (1) subpopulation-based synergy sub-model; (2) mechanistic synergy sub-model that described the action of PMB to increase meropenem activity through enhanced membrane permeability. (Supplement Figure S1) The model developed in this study was accurately able to estimate all parameters, and by implementing a mechanistic description of the observed data it was able to interpolate bacterial killing in un-tested regimens.
Population pharmacokinetic studies in critically ill subjects were used to drive the MBM. The choice of a critically ill patient population was highly important given the nosocomial nature of CRAB infections and the high inter-individual pharmacokinetic variability in the critically ill patient population.[24, 25] When performing population simulations of patients receiving both meropenem and PMB, the high inter-individual variability of both drugs in critically ill subjects becomes an important, efficacy-limiting factor.
To optimize a dosing regimen to treat high inoculum infections that would cover 90% of simulated subjects, the doses identified by the GA were much larger than package insert. (Figure 3) In this situation, meropenem 17.8 g/day q6h with a 2.5 h prolonged infusion would be required. The GA suggested that 22.2% of the total daily dose of meropenem be administered at 0 h. However, because the proposed loading dose is near the 25% value of a non-loaded dose, the results indicate that no loading dose would be needed. Conversely, the proposed concomitant PMB regimen determined that 51.1% of the total daily dose be given at 0h for optimal killing.
Consequences of this large variability are depicted by the results from the GA, which shows that, in order to provide sufficient antibacterial effect in 50%, 75%, or 90% of patients, both meropenem and PMB would require doses above their current FDA-approved and guideline-recommended limits.[17] Ultimately, these augmented dose regimens mean that, in the absence of a personalised-treatment approach, blind dosing strategies designed to cover a given proportion of the patient population are likely to be clinically inappropriate measures when treating CRAB.
Multiple optimised regimens were successfully produced in this study (each targeting a given inoculum), but meropenem dosages well above package insert were required to provide sufficient bacterial killing for 50% of simulated subjects (14.7 and 13.2 g/day for high and low inoculum infections, respectively). With appropriate therapeutic drug management strategies, studies have shown that the daily dose of meropenem can be as high as 11 g for patients with significantly altered clearance/volume in order to produce the desired target concentrations while maintaining safety.[34, 35] Though the augmented doses of PMB are likely to not be viable in a clinical situation, augmented meropenem could still be possible against CRAB or other highly resistant bacterial species.[36–38]
This work, though focusing on meropenem and PMB, was designed to be broadly implemented using any drug combination where there is a model of drug effect (either empiric or mechanistic). In cases where a mechanistic strategy to describe pharmacodynamics may often be too time consuming and technically infeasible, an empiric model may be used to describe the pharmacodynamic effect of multiple drugs at a terminal time-point (examples can be found in a very helpful and classic review by Greco et al.).[39] Additionally, the choice in objective function for the GA (supplied in supplement) is overly simplistic: it only focuses on bacterial killing to achieve a log(cfu/mL) = 0. This work is currently limited by the use of a single isolate in development of the optimized regimens. Future studies could focus on the use of a semi-mechanistic modelling approach that incorporates pharmacodynamic covariates in order to better extend these regimen optimization procedures to a wider array of bacterial isolates. [40–42] This study was additionally limited by the aggressive target endpoint for optimization, which produced regimens that are not feasible for clinical use. Future studies could, however, optimize treatment based on a target greater than 100 cfu/mL or using an altogether different biomarker (e.g., resistant subpopulation counts instead of total counts), which may improve the ability to discern clinically relevant combination regimens. The ultimate target of killing, however, was relatively strict (eradication by 14 days) compared to current standard approaches used in drug development (stasis vs −1 log reduction vs −2 log reduction by 24 h), which could be modified in future studies. The inoculum effect explored in the optimization procedure is presently limited by the data that underlies the pharmacodynamic model; specifically, only one inoculum (i.e., 108 cfu/mL) was used to generate the data. (Additional discussion of this limitation can be found in the supplemental methodology). Moreover, using a GA to optimise treatment would also factor in each drug’s toxicity, which should be carefully weighted based on severity and in relation to the efficacy endpoint. For example, the nephrotoxicity concerns from polymyxin therapy would be more strongly dose limiting than the potential seizures caused by high dose meropenem.
In summary, this study provides a data-driven, model-informed process to evaluate the effectiveness of combination drug therapies and to determine the optimal treatment strategy. Controlling and enhancing key regimen features such as dose, infusion rate, number of daily doses, and % of daily dose loaded at the initiation of therapy will be important considerations in the precision use of multiple agents to combat microbial resistance. Additionally, this study showed that the synergistic use of meropenem and polymyxin B combination therapy may be insufficient to adequately treat most patients, but that the use of more aggressive therapeutic monitoring strategies to control patient serum concentrations may still allow the combination to be useful to select individuals (e.g., low inoculum infectious such as bacteraemia).
Supplementary Material
Acknowledgements
Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award numbers R01AI111990 and 1R01AI148560. JL is an Australian National Health and Medical Research Council (NHMRC) Principal Research Fellow. NMS wishes to acknowledge David Hilts, for his support and guidance on all the many things that made this project worth doing.
Transparency Declaration:
Portions of this work were presented at the 2019 American Conference on Pharmacometrics (ACoP 10) by Dr. Smith. Dr. Li reports other from Qpex Biopharma, USA, personal fees from Genentech, WuHan Healcare Pharmaceuticals, DocMode Health Technologies for seminars, grants from NIAID/NIH, outside the submitted work; In addition, Dr. Li has a patent Polymyxin Derivatives as Antimicrobial compounds (WO/2015/149131) with royalties paid to Qpex Biopharma. Dr. Tsuji reports grants from NIH/NIAID, during the conduct of the study. All other authors declare that they have no conflicts of interest related to the work presented herein.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- [1].Gould K, Antibiotics: from prehistory to the present day, Journal of Antimicrobial Chemotherapy 71(3) (2016) 572–575. [DOI] [PubMed] [Google Scholar]
- [2].Tacconelli E, Carrara E, Savoldi A, Harbarth S, Mendelson M, Monnet DL, Pulcini C, Kahlmeter G, Kluytmans J, Carmeli Y, Ouellette M, Outterson K, Patel J, Cavaleri M, Cox EM, Houchens CR, Grayson ML, Hansen P, Singh N, Theuretzbacher U, Magrini N, Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis, Lancet Infect Dis 18(3) (2018) 318–327. [DOI] [PubMed] [Google Scholar]
- [3].C. Centers for Disease, Antibiotic Resistance Threats in the United States, 2013., (2013.). [Google Scholar]
- [4].C. Centers for Disease, Prevention, Guidance for control of infections with carbapenem-resistant or carbapenemase-producing Enterobacteriaceae in acute care facilities, MMWR Morb Mortal Wkly Rep 58(10) (2009) 256–60. [PubMed] [Google Scholar]
- [5].C. Centers for Disease, Prevention, Vital signs: carbapenem-resistant Enterobacteriaceae, MMWR Morb Mortal Wkly Rep 62(9) (2013) 165–70. [PMC free article] [PubMed] [Google Scholar]
- [6].Li J, Nation RL, Old Polymyxins Are Back: Is Resistance Close?, Clinical Infectious Diseases 43(5) (2006) 663–664. [DOI] [PubMed] [Google Scholar]
- [7].Li J, Nation RL, Turnidge JD, Milne RW, Coulthard K, Rayner CR, Paterson DL, Colistin: the re-emerging antibiotic for multidrug-resistant Gram-negative bacterial infections, Lancet Infect Dis 6(9) (2006) 589–601. [DOI] [PubMed] [Google Scholar]
- [8].Nation RL, Li J, Cars O, Couet W, Dudley MN, Kaye KS, Mouton JW, Paterson DL, Tam VH, Theuretzbacher U, Tsuji BT, Turnidge JD, Consistent global approach on reporting of colistin doses to promote safe and effective use, Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 58(1) (2014) 139–41. [DOI] [PubMed] [Google Scholar]
- [9].World Health Organization, Critically Important Antimicrobials for Human Medicine, 4th ed., WHO, online, 2016. [Google Scholar]
- [10].Clock SA, Tabibi S, Alba L, Kubin CJ, Whittier S, Saiman L, In vitro activity of doripenem alone and in multi-agent combinations against extensively drug-resistant Acinetobacter baumannii and Klebsiella pneumoniae, Diagn Microbiol Infect Dis 76(3) (2013) 343–6. [DOI] [PubMed] [Google Scholar]
- [11].March GA, Bratos MA, A meta-analysis of in vitro antibiotic synergy against Acinetobacter baumannii, Journal of microbiological methods 119 (2015) 31–6. [DOI] [PubMed] [Google Scholar]
- [12].Ni W, Shao X, Di X, Cui J, Wang R, Liu Y, In vitro synergy of polymyxins with other antibiotics for Acinetobacter baumannii: a systematic review and meta-analysis, International journal of antimicrobial agents 45(1) (2015) 8–18. [DOI] [PubMed] [Google Scholar]
- [13].Lenhard JR, Smith NM, Bulman ZP, Tao X, Thamlikitkul V, Shin BS, Nation RL, Li J, Bulitta JB, Tsuji BT, High Dose Ampicillin/Sulbactam Combinations Combat Polymyxin-Resistant Acinetobacter baumannii in a Hollow-Fiber Infection Model, Antimicrob Agents Chemother (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lenhard JR, Bulitta JB, Connell TD, King-Lyons N, Landersdorfer CB, Cheah SE, Thamlikitkul V, Shin BS, Rao G, Holden PN, Walsh TJ, Forrest A, Nation RL, Li J, Tsuji BT, High-intensity meropenem combinations with polymyxin B: new strategies to overcome carbapenem resistance in Acinetobacter baumannii, The Journal of antimicrobial chemotherapy 72(1) (2017) 153–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Rao GG, Ly NS, Bulitta JB, Soon RL, San Roman MD, Holden PN, Landersdorfer CB, Nation RL, Li J, Forrest A, Tsuji BT, Polymyxin B in combination with doripenem against heteroresistant Acinetobacter baumannii: pharmacodynamics of new dosing strategies, The Journal of antimicrobial chemotherapy (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Paul M, Daikos GL, Durante-Mangoni E, Yahav D, Carmeli Y, Benattar YD, Skiada A, Andini R, Eliakim-Raz N, Nutman A, Zusman O, Antoniadou A, Pafundi PC, Adler A, Dickstein Y, Pavleas I, Zampino R, Daitch V, Bitterman R, Zayyad H, Koppel F, Levi I, Babich T, Friberg LE, Mouton JW, Theuretzbacher U, Leibovici L, Colistin alone versus colistin plus meropenem for treatment of severe infections caused by carbapenem-resistant Gram-negative bacteria: an open-label, randomised controlled trial, Lancet Infect Dis 18(4) (2018) 391–400. [DOI] [PubMed] [Google Scholar]
- [17].Tsuji BT, Pogue JM, Zavascki AP, Paul M, Daikos GL, Forrest A, Giacobbe DR, Viscoli C, Giamarellou H, Karaiskos I, Kaye D, Mouton JW, Tam VH, Thamlikitkul V, Wunderink RG, Li J, Nation RL, Kaye KS, International Consensus Guidelines for the Optimal Use of the Polymyxins: Endorsed by the American College of Clinical Pharmacy (ACCP), European Society of Clinical Microbiology and Infectious Diseases (ESCMID), Infectious Diseases Society of America (IDSA), International Society for Anti-infective Pharmacology (ISAP), Society of Critical Care Medicine (SCCM), and Society of Infectious Diseases Pharmacists (SIDP), Pharmacotherapy 39(1) (2019) 10–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Onufrak NJ, Forrest A, Gonzalez D, Pharmacokinetic and Pharmacodynamic Principles of Anti-infective Dosing, Clinical therapeutics 38(9) (2016) 1930–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Nielsen EI, Cars O, Friberg LE, Pharmacokinetic/pharmacodynamic (PK/PD) indices of antibiotics predicted by a semimechanistic PKPD model: a step toward model-based dose optimization, Antimicrob Agents Chemother 55(10) (2011) 4619–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Weiss A, Nowak-Sliwinska P, Current Trends in Multidrug Optimization: An Alley of Future Successful Treatment of Complex Disorders, SLAS TECHNOLOGY: Translating Life Sciences Innovation 22(3) (2016) 254–275. [DOI] [PubMed] [Google Scholar]
- [21].Paterson IK, Hoyle A, Ochoa G, Baker-Austin C, Taylor NG, Optimising Antibiotic Usage to Treat Bacterial Infections, Sci Rep 6 (2016) 37853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Clinical and Laboratory Standards Institute, Performance Standards for Antimicrobial Susceptibility Testing: Twenty-fifth Informational Supplement, Clinical and Laboratory Standards Institute, Wayne, PA, USA, 2015. [Google Scholar]
- [23].Smith NM, Bulman ZP, Sieron AO, Bulitta JB, Holden PN, Nation RL, Li J, Wright GD, Tsuji BT, Pharmacodynamics of dose-escalated ‘front-loading’ polymyxin B regimens against polymyxin-resistant mcr-1-harbouring Escherichia coli, The Journal of antimicrobial chemotherapy 72(8) (2017) 2297–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sandri AM, Landersdorfer CB, Jacob J, Boniatti MM, Dalarosa MG, Falci DR, Behle TF, Bordinhao RC, Wang J, Forrest A, Nation RL, Li J, Zavascki AP, Population pharmacokinetics of intravenous polymyxin B in critically ill patients: implications for selection of dosage regimens, Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 57(4) (2013) 524–31. [DOI] [PubMed] [Google Scholar]
- [25].Mattioli F, Fucile C, Del Bono V, Marini V, Parisini A, Molin A, Zuccoli ML, Milano G, Danesi R, Marchese A, Polillo M, Viscoli C, Pelosi P, Martelli A, Di Paolo A, Population pharmacokinetics and probability of target attainment of meropenem in critically ill patients, European journal of clinical pharmacology 72(7) (2016) 839–48. [DOI] [PubMed] [Google Scholar]
- [26].Bauer RJ, S-ADAPT/MCPEM User’s Guide, 1.57 ed.2011. [Google Scholar]
- [27].Bulitta JB, Bingolbali A, Shin BS, Landersdorfer CB, Development of a new pre- and post-processing tool (SADAPT-TRAN) for nonlinear mixed-effects modeling in S-ADAPT, AAPS J 13(2) (2011) 201–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Bulitta JB, Landersdorfer CB, Performance and robustness of the Monte Carlo importance sampling algorithm using parallelized S-ADAPT for basic and complex mechanistic models, AAPS J 13(2) (2011) 212–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Deris ZZ, Yu HH, Davis K, Soon RL, Jacob J, Ku CK, Poudyal A, Bergen PJ, Tsuji BT, Bulitta JB, Forrest A, Paterson DL, Velkov T, Li J, Nation RL, The combination of colistin and doripenem is synergistic against Klebsiella pneumoniae at multiple inocula and suppresses colistin resistance in an in vitro pharmacokinetic/pharmacodynamic model, Antimicrob Agents Chemother 56(10) (2012) 5103–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ly NS, Bulitta JB, Rao GG, Landersdorfer CB, Holden PN, Forrest A, Bergen PJ, Nation RL, Li J, Tsuji BT, Colistin and doripenem combinations against Pseudomonas aeruginosa: profiling the time course of synergistic killing and prevention of resistance!, The Journal of antimicrobial chemotherapy 70(5) (2015) 1434–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Landersdorfer CB, Yadav R, Rogers KE, Kim TH, Shin BS, Boyce JD, Nation RL, Bulitta JB, Combating Carbapenem-Resistant <span class="named-content genus-species" id="named-content-1">Acinetobacter baumannii</span> by an Optimized Imipenem-plus-Tobramycin Dosage Regimen: Prospective Validation via Hollow-Fiber Infection and Mathematical Modeling, Antimicrobial Agents and Chemotherapy 62(4) (2018) e02053–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Sandri AM, Landersdorfer CB, Jacob J, Boniatti MM, Dalarosa MG, Falci DR, Behle TF, Saitovitch D, Wang J, Forrest A, Nation RL, Zavascki AP, Li J, Pharmacokinetics of polymyxin B in patients on continuous venovenous haemodialysis, The Journal of antimicrobial chemotherapy 68(3) (2013) 674–7. [DOI] [PubMed] [Google Scholar]
- [33].A. Pharmaceuticals, MERREM IV, in: F.a.D. Administration; (Ed.) online, 1996. [Google Scholar]
- [34].Cojutti P, Sartor A, Righi E, Scarparo C, Bassetti M, Pea F, Population Pharmacokinetics of High-Dose Continuous-Infusion Meropenem and Considerations for Use in the Treatment of Infections Due to KPC-Producing Klebsiella pneumoniae, Antimicrob Agents Chemother 61(10) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Taccone FS, Cotton F, Roisin S, Vincent JL, Jacobs F, Optimal meropenem concentrations to treat multidrug-resistant Pseudomonas aeruginosa septic shock, Antimicrob Agents Chemother 56(4) (2012) 2129–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Pea F, Della Siega P, Cojutti P, Sartor A, Crapis M, Scarparo C, Bassetti M, Might real-time pharmacokinetic/pharmacodynamic optimisation of high-dose continuous-infusion meropenem improve clinical cure in infections caused by KPC-producing Klebsiella pneumoniae?, International journal of antimicrobial agents 49(2) (2017) 255–258. [DOI] [PubMed] [Google Scholar]
- [37].Stewart A, Graves B, Hajkowicz K, Ta K, Paterson DL, The Use of Therapeutic Drug Monitoring to Optimize Treatment of Carbapenem-Resistant Enterobacter Osteomyelitis, Microbial drug resistance (Larchmont, N.Y.) 21(6) (2015) 631–5. [DOI] [PubMed] [Google Scholar]
- [38].Fournier A, Eggimann P, Pagani JL, Revelly JP, Decosterd LA, Marchetti O, Pannatier A, Voirol P, Que YA, Impact of the introduction of real-time therapeutic drug monitoring on empirical doses of carbapenems in critically ill burn patients, Burns : journal of the International Society for Burn Injuries 41(5) (2015) 956–68. [DOI] [PubMed] [Google Scholar]
- [39].Greco WR, Bravo G, Parsons JC, The search for synergy: a critical review from a response surface perspective, Pharmacological reviews 47(2) (1995) 331–85. [PubMed] [Google Scholar]
- [40].Tsuji BT, Bulitta JB, Brown T, Forrest A, Kelchlin PA, Holden PN, Peloquin CA, Skerlos L, Hanna D, Pharmacodynamics of early, high-dose linezolid against vancomycin-resistant enterococci with elevated MICs and pre-existing genetic mutations, The Journal of antimicrobial chemotherapy 67(9) (2012) 2182–90. [DOI] [PubMed] [Google Scholar]
- [41].van Hasselt JGC, Gupta A, Hussein Z, Beijnen JH, Schellens JHM, Huitema ADR, Population pharmacokinetic-pharmacodynamic analysis for eribulin mesilate-associated neutropenia, Br J Clin Pharmacol 76(3) (2013) 412–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kloft C, Wallin J, Henningsson A, Chatelut E, Karlsson MO, Population Pharmacokinetic-Pharmacodynamic Model for Neutropenia with Patient Subgroup Identification: Comparison across Anticancer Drugs, Clinical Cancer Research 12(18) (2006) 5481–5490. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


