Abstract
Histomoniasis, caused by the protozoan parasite Histomonas meleagridis, is a disease to which turkeys are especially susceptible. Currently, no chemoprophylaxis compounds are available to mitigate this disease. Boric acid (BA) exhibits antifungal, antiseptic, and antiviral properties and has been used in the treatment of yeast infections. Based on these characteristics, an experiment was conducted to evaluate whether BA might be an efficacious prophylaxis against challenge with wild-type H. meleagridis (WTH). On day-of-hatch, poults were randomly assigned to either a basal control diet or a BA diet (basal diet + 0.2% BA). Groups consisted of a nonchallenged control (NC; basal diet), 0.2% BA + challenge (BA; 0.2% BA diet), and a positive-challenged control (PC; basal diet). On day 21, challenged groups were intracloacally inoculated with 2 × 105 WTH cells/turkey, and lesions were evaluated on day 14 postchallenge. Individual body weights were recorded on day 0, 21, and 35 to calculate the prechallenge and postchallenge body weight gain (BWG). The BA group resulted in lower prechallenge day 0 to day 21 BWG (P = 0.0001) than the NC group. Postchallenge day 21 to day 35, BWG was also lower (P = 0.0503) in the BA group than the PC group. No differences between the BA and PC groups were detected for mortalities associated with histomoniasis. Moreover, liver and cecal lesions were not statistically different between the BA and PC groups. Taken together, these data suggest that BA was not efficacious in the prevention or reduction of histomoniasis disease severity when provided at 0.2% dietary concentration under these experimental conditions.
Key words: blackhead, boric acid, boron, histomoniasis, Histomonas meleagridis
Introduction
Histomoniasis, also commonly known as blackhead, is a disease of turkeys associated with high mortality (Callait et al., 2002). Considered critically and economically impactful to both turkeys and chickens, histomoniasis is a serious concern facing the poultry industry (Duffy et al., 2005, Lotfi et al., 2014). Histomonas meleagridis, the etiological agent of histomoniasis, penetrates the cecal epithelial lining, replicates, enters the bloodstream, and parasitizes the liver (Clarkson, 1963, Hess and McDougald, 2013). Research on this organism waned in the 1960s following the introduction of nitroimidazoles, nitrofurans, and arsenical compounds for prophylaxis and treatment of outbreaks; these compounds have since been banned because of regulatory action (Van der Heijden et al., 2005, Hess et al., 2006). In 2015, the arsenic-based drug nitarsone (Histostat), the last remaining FDA-approved drug for prevention of histomoniasis, was withdrawn from the market because of concerns about inorganic arsenic residues in treated poultry (Regmi et al., 2016). Unfortunately, no alternatives to the previously used drugs have been identified, whereas in vitro and in vivo studies continue to yield variable results against H. meleagridis (Thøfner et al., 2012).
Boron is an essential element to humans, animals, and plants (Eren et al., 2012). However, the NRC (1994) has no recommended level of boron for poultry daily intake. Application of boric acid (BA), a boron compound, to the litter is used in the prevention of darkling beetles within the poultry industry (Sander et al., 1991, Dufour et al., 1992). Treatment of litter with BA at a rate of 0.4 to 0.9 kg/9.3 m2 did not significantly negatively impact feed conversion or decrease body weight (Dufour et al., 1992). Previous studies have suggested the important biological role that boron may have on the biochemical mechanisms influencing mineral metabolism and normal growth (Kurtoğlu et al., 2005, Çinar et al., 2015). Dietary supplementation of boron is considered economical in that a 100 mg/kg diet was estimated to cost 0.5 USD per ton of prepared feed (Bozkurt and Küçükyilmaz, 2015). Boron supplementation within the diet (240 ppm, 0.024%) was not detrimental to broiler performance when administered from day-of-hatch to 21 D, although boron levels within breast muscle and liver tissues increased in proportion to boron dietary concentration (Rossi et al., 1993). Boron (20 mg/kg) supplementation in a basal diet had no impact on body weight or feed consumption in chickens; results did not suggest growth promotion or metabolic mineral regulation (Küçükyilmaz et al., 2017). However, the acute oral mean lethal dose of BA in 1-day-old chicks was determined to be 2.95 ± 0.35 g/kg of body weight, resulting in the classification as a slightly toxic chemical (Sander et al., 1991).
Containing antifungal, antiseptic, and antiviral properties, BA has been used to treat yeast infections (Hernandez-Patlan et al., 2018a). Within an in vitro model, BA decreased concentration of Salmonella Enteritidis within the intestinal compartment (Hernandez-Patlan et al., 2018a). However, during an in vivo study, a concentration of 0.1% BA within a basal diet had no significant reduction in Salmonella Enteritidis (Hernandez-Patlan et al., 2018b). Bacterial flora are important in development of histomoniasis, contributing to the interest in BA as a potential chemoprophylactic compound against the disease. The in vitro growth rate of Trichomonas vaginalis, protozoan causative agent of trichomoniasis in humans, was reduced with low BA concentrations (0.2%) and exhibited lethality to trichomonads at higher concentrations (≤0.4%), independent of environmental acidification (Brittingham and Wilson, 2014). Considering these experiments, the antifungal properties and the potential cost-effectiveness, we hypothesized that BA might be efficacious in the prevention against H. meleagridis at the selected dietary concentration of 0.2%. The results of this study are presented in this Research Note.
Materials and methods
Animal Source and Diet
A total of 120 D of hatch female turkey poults were obtained from a local commercial hatchery. Poults were neck-tagged individually and randomly allocated to floor pens at the University of Arkansas Poultry Health Laboratory. Early poult mortalities unrelated to histomoniasis were recorded, and the altered group numbers are reported in the experiment. All animal handling procedures were in compliance with the Institutional Animal Care and Use Committee (IACUC protocol #18113) of the University of Arkansas. A corn–soy–based starter feed that met or exceeded nutrient requirements for poultry (NRC, 1994) and water were provided ad libitum. Boric acid (Product #B6768; Sigma-Aldrich, St. Louis, MO) was incorporated into the basal diet at a concentration of 0.2% for the treatment group receiving the BA diet and was fed ad libitum beginning on day-of-hatch. Poults were randomly assigned to treatment groups consisting of a nonchallenged control (NC; n = 34; basal diet), 0.2% BA + challenge (0.2% BA; n = 27; 0.2% BA diet), and positive-challenged control (PC; n = 20; basal diet). All poults were individually weighed on day 0, 21, and 35 for calculation of prechallenge and postchallenge body weight gain (BWG).
Histomonas Meleagridis
A virulent wild-type H. meleagridis (WTH) was obtained and cultivated based upon previously published methods (Van der Heijden et al., 2005, Van der Heijden and Landman, 2007). Viable WTH cells/mL were enumerated with a hemocytometer. On day 21, each poult in the 0.2% BA and PC groups received a total dose of 2 × 105 WTH cells administered intracloacally with an animal gavage needle. Inoculation occurred twice with a 1 h period between each inoculation.
Lesion Scoring System
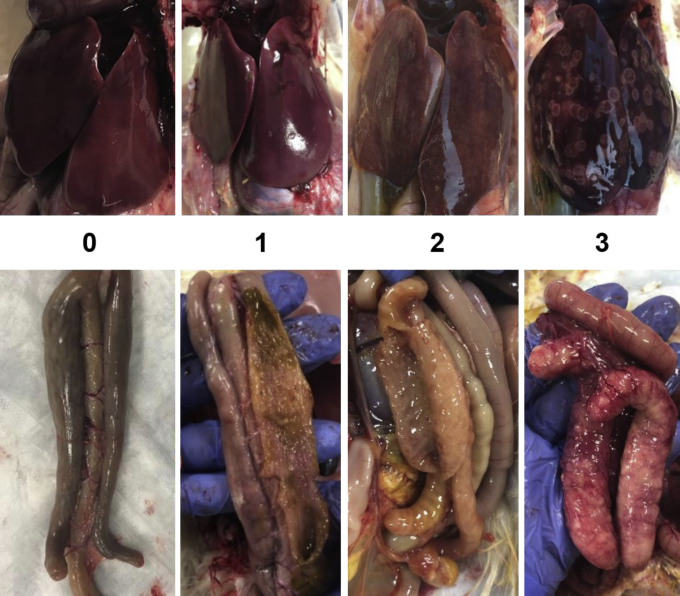
Liver and cecal lesions associated with histomoniasis were tabulated from all mortalities. On day 14 postchallenge, all remaining poults were evaluated postmortem for liver and cecal lesions. The individuals determining the lesion scores were blinded to the treatments. Classic lesions associated with histomoniasis were evaluated on a scale of “0” to “3”, with “3” being the most severe (Figure 1). According to this scale, a liver score of “0” indicates no detectable H. meleagridis-related lesions; “1” indicates detectible lesions that are not clinically relevant (not significant ongoing pathology); “2” indicates intermediate lesions suggesting significant pathology but not imminent mortality; “3” indicates confluent or nearly confluent lesions deemed likely to be fatal. The cecae were observed and palpated from the serosal surface (the mucosa was not evaluated). According to this scale, a cecal score of “0” indicates no detectable H. meleagridis–related lesions observed from serosal inspection and palpation; “1” indicates thickening (not clinically significant) of the cecae; “2” indicates clinically meaningful cecal wall thickening without cecal cores; “3” indicates classic typhlitis with thickened cecal walls, inflammation, and cecal cores.
Figure 1.
Histomoniasis lesion scoring system developed at the University of Arkansas Poultry. Health Laboratory. Classic lesions associated with histomoniasis for liver and cecae were evaluated on a scale of “0” to “3.” A score of “0” indicates no detectible lesions; “1” indicates lesions not clinically relevant; “2” indicates intermediate lesions suggesting significant pathology but not imminent mortality; “3” indicates confluent or nearly confluent lesions deemed likely to be fatal.
Statistical Analysis
BWG data were analyzed using JMP Pro 14 software, with significant differences between groups determined using a Student 2-tailed t test with P ≤ 0.05 considered significant. Differences in mortalities associated with histomoniasis were analyzed using a chi-square test. Lesion score data were analyzed using the Proc Mixed Procedure in SAS 9.4 software with significance between mean lesion score values considered at P ≤ 0.05 in comparison to the PC group. Using a chi-square test, each subgrouping of scores (“0” to “3”) were further compared between the 0.2% BA and PC groups to analyze differences between lesion scores.
Results
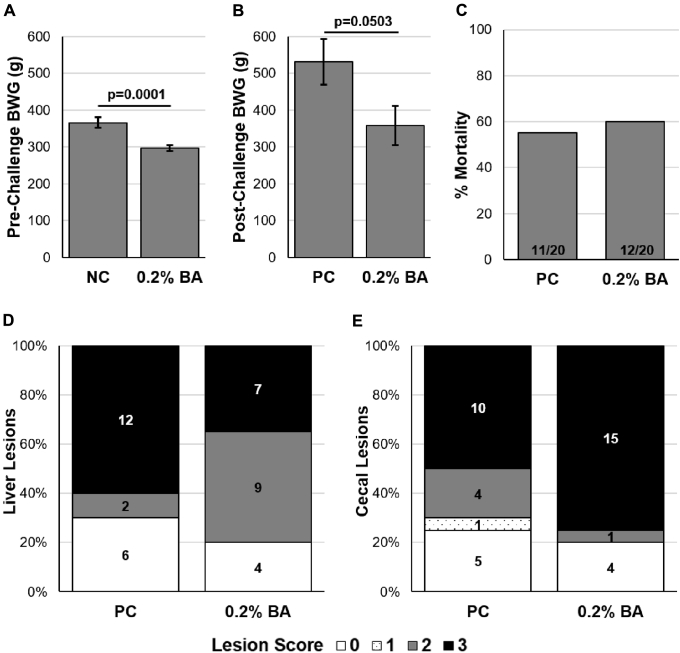
Prechallenge BWG from day 0 to day 21 was significantly lower (P = 0.0001) in the 0.2% BA group as compared with poults fed the basal diet in the NC group (Figure 2A). Postchallenge BWG from day 21 to day 35 in the 0.2% BA group was lower (P = 0.0503) than the PC group (Figure 2B). No differences were detected in mortalities associated with histomoniasis in the 0.2% BA group as compared with the PC group (Figure 2C). Moreover, neither liver nor cecal mean lesion scores were reduced in the 0.2% BA group as compared with the PC following WTH-challenge (Figure 2D and E, respectively). With the chi-square test, liver lesion scores of “2” were more prevalent (P ≤ 0.05) in the 0.2% BA group as compared with the PC group.
Figure 2.
Body weight gain (BWG) at (A) prechallenge from day 0 to day 21 and (B) postchallenge from day 21 to day 35. BWG data expressed as mean ± SEM and were analyzed using Student 2-tailed t test in JMP Pro 14. (C) Percentage of mortalities associated with histomoniasis postchallenge. No difference was detected when analyzed with a chi-square test. Cumulative lesion scores associated with histomoniasis from day 10 to day 14 postchallenge for (D) liver and (E) cecae. A lesion score of “0” is indicative of a healthy bird with no disease whereas a score of “3” indicates classically severe histomoniasis. No differences between mean lesion scores were detected with SAS Proc Mixed Procedure for either liver or cecal lesions. Numbers within columns indicate the total poults per evaluated lesion score. Poults in challenged groups received 2 × 105Histomonas meleagridis cells/turkey on day 21 via intracloacal administration. Abbreviations: NC, nonchallenged control; BA, 0.2% boric acid diet + challenge; PC, positive-challenged control. Boric acid (Product #B6768, Sigma-Aldrich, St. Louis, MO).
Discussion
The significantly lower BWG resulting from this selected dietary concentration of BA suggests that higher levels of BA would clearly not be acceptable for commercial use (Figure 2A and B). Lowered BWG is consistent with previous literature indicating that toxic levels of BA can induce increased feed conversion and decreased body weight (Dufour et al., 1992). Efficacy of alternative chemoprophylaxis compounds against related protozoa is suggested because of the close relationship of H. meleagridis to other amoebae and flagellates (Hu and McDougald, 2004). However, selective toxicity against protozoa is crucial to ensure that the chemoprophylaxis compound is harmful to the parasite without causing irreversible damage to the host. This research note suggests that dietary inclusion of BA alone is not an encouraging candidate for prevention of histomoniasis in turkeys.
References
- Bozkurt M., Küçükyilmaz K. The role of boron in poultry nutrition Part II: compositional and mechanical properties of bone and egg quality. Worlds Poult. Sci. J. 2015;71:483–492. [Google Scholar]
- Brittingham A., Wilson W.A. The antimicrobial effect of boric acid on Trichomonas vaginalis. Sex. Transm. Dis. 2014;41:718–722. doi: 10.1097/OLQ.0000000000000203. [DOI] [PubMed] [Google Scholar]
- Callait M., Granier C., Chauve C., Zenner L. In vitro activity of therapeutic drugs against Histomonas meleagridis (Smith, 1895) Poult. Sci. 2002;81:1122–1127. doi: 10.1093/ps/81.8.1122. [DOI] [PubMed] [Google Scholar]
- Çinar M., Küçükyilmaz K., Bozkurt M., Çatli A., Bintaş E., Akşit H., Konak R., Yamaner Ç., Seyrek K. Effects of dietary boron and phytase supplementation on growth performance and mineral profile of broiler chickens fed on diets adequate or deficient in calcium and phosphorus. Br. Poult. Sci. 2015;56:576–589. doi: 10.1080/00071668.2015.1079699. [DOI] [PubMed] [Google Scholar]
- Clarkson M. Immunological responses to Histomonas meleagridis in the Turkey and fowl. Immunology. 1963;6:156–168. [PMC free article] [PubMed] [Google Scholar]
- Duffy C., Sims M., Power R. Evaluation of dietary NatustatTM for control of Histomonas meleagridis in male turkeys on infected litter. Avian Dis. 2005;49:423–425. doi: 10.1637/7344-022105R2.1. [DOI] [PubMed] [Google Scholar]
- Dufour L., Sander J.E., Wyatt R.D., Rowland G.N., Page R. Experimental exposure of broiler chickens to boric acid to assess clinical signs and lesions of toxicosis. Avian Dis. 1992;36:1007–1011. [PubMed] [Google Scholar]
- Eren M., Uyanik F., Guclu B.K., Cinar M. Effects of dietary boric acid and borax supplementation on growth performance and some biochemical parameters in broilers. Revue Méd. Vét. 2012;163:546–551. [Google Scholar]
- Van der Heijden H.M.J.F., Landman W.J.M. Improved culture of Histomonas meleagridis in a modification of Dwyer medium. Avian Dis. 2007;51:986–988. doi: 10.1637/8018-051007-REVIEWR.1. [DOI] [PubMed] [Google Scholar]
- Van der Heijden H.M.J.F., McDougald L.R., Landman W.J.M. High yield of parasites and prolonged in vitro culture of Histomonas meleagridis. Avian Pathol. 2005;34:505–508. doi: 10.1080/03079450500368474. [DOI] [PubMed] [Google Scholar]
- Hernandez-Patlan D., Solis-Cruz B., Méndez-Albores A., Latorre J.D., Hernandez-Velasco X., Tellez G., López-Arellano R. Comparison of PrestoBlue® and plating method to evaluate antimicrobial activity of ascorbic acid, boric acid and curcumin in an in vitro gastrointestinal model. J. Appl. Microbiol. 2018;124:423–430. doi: 10.1111/jam.13659. [DOI] [PubMed] [Google Scholar]
- Hernandez-Patlan D., Solis-Cruz B., Pontin K.P., Latorre J.D., Baxter M.F., Hernandez- Velasco X., Merino-Guzman R., Méndez-Albores A., Hargis B.M., Lopez-Arellano R., others Evaluation of a solid dispersion of curcumin with polyvinylpyrrolidone and boric acid against Salmonella Enteritidis infection and intestinal permeability in broiler chickens: a pilot study. Front. Microbiol. 2018;9:1289. doi: 10.3389/fmicb.2018.01289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess M., Kolbe T., Grabensteiner E., Prosl H. Clonal cultures of Histomonas meleagridis, Tetratrichomonas gallinarum and a Blastocystis sp. established through micromanipulation. Parasitology. 2006;133:547–554. doi: 10.1017/S0031182006000758. [DOI] [PubMed] [Google Scholar]
- Hess M., McDougald L. Histomoniasis (blackhead) and other protozoan diseases of the intestinal tract. In: Swayne E., Glisson J.R., McDougald L.R., Nolan L.K., Suarez D.L., Nair V.L., editors. Diseases of Poultry. 13th ed. Wiley- Blackwell; Ames, IA: 2013. pp. 1172–1178. [Google Scholar]
- Hu J., McDougald L. The efficacy of some drugs with known antiprotozoal activity against Histomonas meleagridis in chickens. Vet. Parasitol. 2004;121:233–238. doi: 10.1016/j.vetpar.2004.02.022. [DOI] [PubMed] [Google Scholar]
- Küçükyilmaz K., Bozkurt M., Çinar M., Tüzün A.E. Evaluation of the boron and phytase, alone or in combination, in broiler diets. J. Poult. Sci. 2017;54:26–33. doi: 10.2141/jpsa.0150181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtoğlu F., Kurtoğlu V., Çelik I., Keçeci T., Nizamlioğlu M. Effects of dietary boron supplementation on some biochemical parameters, peripheral blood lymphocytes, splenic plasma cells and bone characteristics of broiler chicks given diets with adequate or inadequate cholecalciferol (vitamin D3) content. Br. Poult. Sci. 2005;46:87–96. doi: 10.1080/00071660400024001. [DOI] [PubMed] [Google Scholar]
- Lotfi A., Hauck R., Olias P., Hafez H.M. Pathogenesis of histomonosis in experimentally infected specific-pathogen-free (SPF) layer-type chickens and SPF meat-type chickens. Avian Dis. 2014;58:427–432. doi: 10.1637/10782-012814-Reg.1. [DOI] [PubMed] [Google Scholar]
- NRC . 9th rev. Natl. Acad. Press; Washington, DC: 1994. Nutrient Requirements of Poultry. [Google Scholar]
- Regmi P.R., Shaw A.L., Hungerford L.L., Messenheimer J.R., Zhou T., Pillai P., Omer A., Gilbert J.M. Regulatory considerations for the approval of drugs against histomoniasis (blackhead disease) in turkeys, chickens, and game birds in the United States. Avian Dis. 2016;60:725–730. doi: 10.1637/11451-061516-Review.1. [DOI] [PubMed] [Google Scholar]
- Rossi A., Miles R., Damron B., Flunker L. Effects of dietary boron supplementation on broilers. Poult. Sci. 1993;72:2124–2130. doi: 10.3382/ps.0722124. [DOI] [PubMed] [Google Scholar]
- Sander J.E., Dufour L., Wyatt R.D., Bush P.B., Page R.K. Acute toxicity of boric acid and boron tissue residues after chronic exposure in broiler chickens. Avian Dis. 1991;35:745–749. [PubMed] [Google Scholar]
- Thøfner I.C.N., Liebhart D., Hess M., Schou T.W., Hess C., Ivarsen E., Fretté X., Christensen L.P., Grevsen K., Engberg R.M., others Antihistomonal effects of artemisinin and Artemisia annua extracts in vitro could not be confirmed by in vivo experiments in turkeys and chickens. Avian Pathol. 2012;41:487–496. doi: 10.1080/03079457.2012.714459. [DOI] [PubMed] [Google Scholar]