Abstract
Cobb 400, male broilers (n=4,752) were fed one of 12 diets, with 12 pens/diet and 33 birds/pen. Treatments consisted of 3 levels of phytate P (0.24, 0.345, or 0.45%) and 4 doses of phytase (0, 500, 1,000, or 2,000 phytase units (FTU)/kg). Diets were formulated with reduced Ca (0.22%), available P (0.20%), energy (80 to 120 kcal/kg), and amino acids (1 to 5%) when compared with breed requirements. Prediction equations suggested feeding dietary phytate P > 0.275, 0.295, or 0.319% reduced feed intake (FI) and body weight gain (BWG) and increased feed conversion ratio, respectively, from day 0 to 21. Supplementing phytase at 561, 1,285, or >2,000 FTU/kg resulted in the maximum FI, BWG, or feed efficiency, respectively. From day 0 to 42, maximum BWG or feed efficiency were achieved at phytate P concentrations <0.281 or 0.25%, respectively. Supplementing phytase at 449 or 2,000 FTU/kg maximized BWG or feed efficiency, respectively. Tibia ash weight, percent or Ca concentration were maximized at phytate P concentrations <0.24, 0.296, or 0.24%, respectively and phytase supplementation at 822 or >2,000 FTU/kg maximized tibia ash weight or percent respectively. In the absence of phytase, phytate (IP6) concentration in the gizzard was greatest in birds fed 0.45% phytate P and phytase supplementation between 1,132 to 1,285 FTU/kg resulted in the lowest IP6 concentration in the gizzard. There was no effect of dietary phytate P on the concentration of phytate esters (IP5 or IP4) in the gizzard, which were minimized at 1,208 FTU/kg of phytase. In the absence of phytase, the concentration of phytate ester (IP3) or inositol in the gizzard was greatest in birds fed 0.345% phytate P and phytase supplementation at ∼500 FTU/kg minimized IP3, whereas 2,000 FTU/kg maximized inositol, except in birds fed 0.45% phytate P, which was maximized at 202 FTU/kg of phytase. Prediction equations can be useful to determine the influence of phytase and phytate P on broiler performance, phytate degradation and bone ash.
Key words: Bone ash, broiler, inositol, phytate
INTRODUCTION
There is a considerable amount of data in the literature supporting anti-nutritional effects of dietary phytate on growth performance and nutrient utilization. In a recent review article, Woyengo and Nyachoti (2013) suggested the negative effect of phytate on body weight gain (BWG) is particularly relevant as the dietary concentration of phytate increases by 0.5 percentage points (from 0.78 to 1.57% phytate). However, greater negative effects of dietary phytate on BWG (losses of 28 to 44%) were noted in broilers, laying chicks or pigs when fed phytate-free diets compared with diets containing 1.65 to 2.0% phytate (Woyengo and Nyachoti, 2013). Some of this work was conducted using sodium phytate and synthetic-type diets, rather than phytate from natural feed ingredients from plants. Due to its chemical properties and solubility throughout the intestinal tract, sodium phytate may not fully mimic the anti-nutritional effects of phytate in plant-based feed ingredients (Onyango et al., 2009). However, others have reported phytate from rice bran significantly reduced BWG and increased feed conversion ratio (FCR) of broilers fed higher concentrations of phytate in P-adequate or P-deficient diets (Cabahug et al., 1999; Liu et al., 2008).
Phytase, is an enzyme capable of breaking down phytate into lower phytate esters and inositol (Walk et al., 2018). This process allows the previously bound P to be available to the animal while also improving Ca, amino acid and energy digestibility and utilization (Cowieson et al., 2006). Through the use of phytase, and the increased availability of nutrients that would otherwise be bound to phytate, the P, Ca, amino acid, and energy content of the diet may be reduced without a negative impact on animal growth performance or bone ash. However, as the concentration of phytate increases in the diet, the magnitude of the response or the anti-nutritional effect of phytate on nutrients is greater (Cowieson et al., 2006). To achieve rapid and nearly complete phytate destruction, particularly at higher concentrations of phytate P or in diets severely limited in Ca, P, energy, and amino acids, increasing doses of phytase may be necessary. For example, in a low-P diet containing 0.22% phytate P, 500 phytase units (FTU)/kg of phytase was able to increase BWG with no further benefit reported at 1,000 FTU/kg phytase. However, in the same experiment, as the phytate P content of the diet increased to 0.44%, phytase supplementation at 1,000 FTU/kg was required to minimize the anti-nutritional effect of phytate and improve BWG comparable to the 0.22% phytate P diet with phytase (Liu et al., 2008). Therefore, the objective of this experiment was to use prediction equations to determine the impact of increasing phytate P, from rice bran, and increasing doses of phytase supplemented into diets containing sub-optimal levels of nutrients, on broiler growth performance, bone ash, plasma urea N and hydrolysis of phytate (IP6) to phytate esters (IP5, IP4, and IP3) and inositol in the gizzard. We hypothesized an increase in phytase dose was required to overcome the anti-nutritional properties of the greater phytate P concentration and result in an improvement in growth of broilers fed diets formulated with sub-optimal levels of nutrients.
MATERIALS AND METHODS
All experimental procedures complied with Indian ethical standards for use of vertebrate animals in research.
Animals and Husbandry
Cobb 400 male broilers (n=4752) were obtained at day of hatch and placed in floor pens on clean rice husk at a stocking density of 14.4 chicks/m2. There were 33 birds/pen and 12 replicate pens/diet. Birds were vaccinated against Newcastle Disease virus and Infectious Bursal Disease virus per label recommendations. For the entire duration of the experiment (42 day), birds were maintained on a lighting program of 23L:1D and allowed ad libitum access to feed and water.
Dietary Treatments
Experimental diets were fed in mash form and based on corn, soybean meal, rice DDGs and 12% polished rice or rice bran to change the phytate P concentration of the diets (Table 1). Dietary treatments consisted of three levels of phytate P (0.24, 0.345, or 0.45%) and four concentrations of phytase (0, 500, 1,000 or 2,000 FTU/kg) arranged as a 3 × 4 factorial. The standard (0.24%) and high (0.45%) phytate P diets were mixed as 2 separate basal diets and then split 50:50 and mixed to create the moderate (0.345%) phytate P diet. Each of the 3 phytate P diets were then split into 4 batches to include the phytase concentrations creating 12 treatments total. All diets were formulated with a reduction of Ca (0.22%), available P (0.20%), energy (80 to 120 kcal/kg) and amino acids (1 to 5%) when compared with the requirements from the VenCobb 400 Broiler Management Guide (Cobb-Vantress Inc., Siloam Spring, AR). Corn was exchanged with phytase where appropriate to equal 100%. The phytase was a modified Escherichia coli 6-phytase expressed in Trichoderma reesei with an expected activity of 5,000 FTU/g (Quantum Blue, AB Vista, Marlborough UK). One phytase unit is defined as the amount of enzyme required to release 1 µ mol of inorganic P/min from sodium phytate at 37°C and pH 5.5. All diets contained a xylanase at 16,000 xylanase units (BXU)/kg (Econase XT, AB Vista, Marlborough UK).
Table 1.
Calculated and analyzed nutrient content of the basal diets.
| Feeding phase |
Starter diets |
Grower diets |
||
|---|---|---|---|---|
| Phytate P | Standard | High | Standard | High |
| Ingredient, % of diet (as-fed basis) | ||||
| Corn | 51.58 | 51.30 | 59.07 | 58.78 |
| Soybean meal, 48% | 27.62 | 25.60 | 20.40 | 18.39 |
| Rice dried distillers grains w/solubles, 47% | 5.00 | 5.00 | 5.00 | 5.00 |
| Polished rice | 12.00 | 12.00 | ||
| De-oiled rice bran | 12.00 | 12.00 | ||
| Soybean oil | 0.52 | 3.31 | 0.99 | 3.78 |
| Salt | 0.42 | 0.43 | 0.37 | 0.37 |
| Limestone | 1.00 | 0.55 | 1.04 | 0.60 |
| Dicalcium phosphate1 | 0.95 | 0.85 | 0.67 | 0.57 |
| Lysine-HCl | 0.20 | 0.23 | 0.14 | 0.18 |
| DL-methionine | 0.19 | 0.20 | 0.12 | 0.13 |
| Threonine | 0.02 | 0.03 | 0.01 | |
| Premix2 | 0.15 | 0.15 | 0.15 | 0.15 |
| Inert (corn/phytase)3 | 0.04 | 0.04 | 0.04 | 0.04 |
| Xylanase4 | 0.01 | 0.01 | 0.01 | 0.01 |
| Chromium | 0.30 | 0.30 | ||
| Nutrient composition, % | ||||
| Crude protein | 21.35 | 21.35 | 18.35 | 18.35 |
| ME, kcal/kg | 2,955.00 | 2,955.00 | 3,060.00 | 3,060.00 |
| Dry matter | 87.49 | 87.70 | 87.69 | 87.90 |
| Calcium | 0.72 | 0.72 | 0.66 | 0.66 |
| Total phosphorus | 0.54 | 0.74 | 0.46 | 0.66 |
| Available phosphorus | 0.25 | 0.25 | 0.20 | 0.20 |
| Phytate phosphorus | 0.24 | 0.45 | 0.23 | 0.44 |
| Total methionine + cysteine | 0.93 | 0.94 | 0.78 | 0.79 |
| Total lysine | 1.25 | 1.26 | 1.00 | 1.01 |
| Digestible methionine + cysteine | 0.83 | 0.83 | 0.69 | 0.69 |
| Digestible lysine | 1.13 | 1.13 | 0.90 | 0.90 |
| Sodium | 0.18 | 0.18 | 0.16 | 0.16 |
Dicalcium phosphate supplied 17% P and 21% Ca.
Supplied per kilogram of diet: iron (ferrous sulfate), 34 mg; manganese (manganese sulfate), 38 mg; zinc (zinc sulfate), 34 mg; copper (basic copper chloride), 6 mg; iodine (calcium iodate), 0.8 mg; selenium (sodium selenite), 113 μ g; vitamin A, 9.4 MIU; vitamin D3 2.1 MIU; vitamin E, 22.5 mg; vitamin B12, 11 μ g; riboflavin, 3.8 mg; niacin, 25 mg; d-pantothenic acid, 11 mg; vitamin K, 1.5 mg; folic acid, 0.8 mg; vitamin B6, 1.9 mg; thiamine, 1.5 mg; and biotin, 60 μ g.
Corn was added in place of phytase in the diets without phytase supplementation. The phytase used was Quantum Blue (AB Vista, Marlborough, UK) with an expected activity of 5,000 phytase units/g.
The xylanase used was Econase XT (AB Vista, Marlborough, UK) with an expected activity of 160,000 xylanase units/g.
Response Variables
Birds were weighed by pen prior to placement (day 0), day 21 and 42 to determine mean BW and calculate mean BWG. Feed addition and feed remaining were measured at day 0, feed changes (day 21), and the conclusion of the trial (day 42) to calculate feed intake (FI). Body weight gain and FI were used to calculate FCR. Mortality was recorded daily. Any culled or dead birds were weighed. Treatment FI and thus FCR were adjusted according to the number of bird days/pen, where bird day is defined as the number of days each bird survived.
On day 21, 8 birds of average BW/pen were anaesthetized by exposure to CO2 gas for approximately 30 s and euthanized by cervical dislocation for gizzard digesta collection. Digesta was obtained from the entire gizzard, pooled/pen and immediately frozen on dry ice. Digesta was dried at 70°C for 24 h and ground to pass a 1 mm screen. The digesta was analyzed for phytate (IP6) and phytate esters (IP5, IP4, and IP3) and inositol according to methods described by Lee et al., (2018). Briefly, digesta samples were extracted at room temperature in 5 mL of 100 mM NaF, 20 mM Na2 EDTA and adjusted to pH 10 with NaOH. The extracted samples were filtered and aliquots (20 µ L) were injected on to a Carbo Pac PA200 HPLC column (Dionex, UK). Peaks were detected at 290 nm using a Jasco UV-2077 Plus UV detector and integrated in ChromNav (Jasco) software. Inositol was determined by HPLC pulsed amperometry on a Dionex DX-600 HPLC system using a Carbo Pac MA1 column and an ED50 electrochemical detector (Dionex, UK) configured with a gold electrode and operating a standard Dionex carbohydrate waveform. The experimental diets were analyzed for amino acids (method 982.30), crude protein (method 984.13 A-D), Ca (method 975.03 B(b)), and P (method 968.08) according to AOAC (2006) at the University of Missouri Agricultural Experiment Station (Columbia, MO). Phytase activity recovered in the diets was determined according to modified methods of Engelen et al., (2001). Xylanase activity recovered in the diets were determined using birch xylan as a substrate at pH 5.3 and 50°C. The method is based on the end-point determination of reducing sugars using a DNS-based colorimetric system. The color produced is proportional to enzyme activity. Xylanase units are expressed as nanomoles/second of xylose reducing sugar equivalents (BXU/g). Phytate content of the ingredients and the experimental diets was determined using the K-PHYT kit from Megazyme (Bray, Ireland) and phytate P content was calculated as 28.2% of the total phytate.
On day 21, one bird representing the mean BW of the pen was selected and euthanized to determine tibia breaking strength and tibia ash. Both tibiae were collected, freed of soft tissue, and dried at 100°C for 3 h before soaking in petroleum ether for 48 h. Right tibiae were used to determine bone breaking strength using the 3-point method with a universal testing machine (EZ Test, Shimadzu, Japan). The bone was rested on 2 points with a gap of 50 mm and pressure was applied with a pressure sensitive load cell (10 kg) at the center of the two points, which coincided with the center of the bone at a speed of 5 cm/min. Both tibiae from each bird were ashed together at 600 ± 20°C for 2 h for determination of bone ash.
On day 21, 2 birds/pen were selected and approximately 1.5 ml blood was drawn from the brachial vein of each bird into heparin coated 2 ml centrifuge tubes. Blood samples were centrifuged at 500 × g for 15 min at room temperature (26°C) to collect plasma. The urea concentration in plasma was measure by a commercial kit (product code 120,241, Erba Mannheim, Transasia Bio-medicals Ltd, Baddi, India), using kinetic enzymatic methods. Briefly, the method is based on the principle of conversion of urea to L-glutamate in presence of urease and glutamate dehydrogenase enzymes, whose absorbance was measured at 340 nm.
Calculations and Statistical Analyses
Data were analyzed as a completely randomized 3 × 4 factorial using the fit model platform of JMP Pro 14.0 (SAS Institute, Cary, NC). Outliers were determined as 3 times the root mean square error plus or minus the mean of response. Plotting the growth performance, bone ash, plasma urea N, or gizzard inositol concentration data using a normal quantile plot indicated the means were normally distributed. Mortality and gizzard phytate and phytate ester concentration were not normally distributed. Therefore, data were transformed using Box-Cox transformations and refit using best λ prior to statistical analysis. Prediction equations were determined using transformed data. Figures are presented as the untransformed means. For all parameters, prediction equations were conducted testing the linear and non-linear effects of phytase log dose, phytate P and the interactions as continuous variables. The full model equation was: y = a + bx + cx2 + dv + ev2 + fxv + gx2v + hxv2 + ix2v2, where y = response variable, a = intercept, b - i = coefficients, x = calculated phytate P in the experimental diets, and v = calculated log dose of phytase in the experimental diets. The log dose for 0 FTU/kg was estimated using 50 FTU/kg (log dose=1.699), which is equivalent to the background phytase activity recovered in diets containing no phytase. Parameters estimates that were not significant in the model and were not included in a significant interaction were removed from the model and the estimates recalculated. Pen served as the experimental unit for all parameters measured. Significance was accepted at P ≤ 0.05.
RESULTS AND DISCUSSION
Phytate P content of the main feed ingredients was determined prior to feed formulation to ensure the expected phytate P levels in the diet were achieved. The phytate P content of the main cereal ingredients was 0.12, 0.21, 0.25, 0.38, and 1.96% for polished rice, corn, rice distillers dried grains with solubles, soybean meal, and de-oiled rice bran, respectively. Phytate P, nutrients analyzed in the diets and enzyme activity recovered in the experimental diets were similar to formulated values (Table 2).
Table 2.
Analyzed phytate phosphorus content of the main ingredients used to formulate the diets, analyzed nutrient content of the experimental diets and enzyme activities recovered in the experimental diets.
| Starter basal diets |
Grower basal diets |
|||||
|---|---|---|---|---|---|---|
| Item | Standard phytate P | Moderate phytate P | High phytate P | Standard phytate P | Moderate phytate P | High phytate P |
| Analyzed nutrient composition of the experimental diets, % | ||||||
| Crude protein | 21.54 | 21.67 | 21.84 | 19.30 | 18.73 | 19.52 |
| Total phosphorus | 0.52 | 0.62 | 0.75 | 0.45 | 0.59 | 0.74 |
| Total calcium | 0.67 | 0.63 | 0.56 | 0.65 | 0.53 | 0.55 |
| Total lysine | 1.34 | 1.29 | 1.34 | |||
| Phytate phosphorus | 0.23 | 0.33 | 0.42 | 0.22 | 0.32 | 0.45 |
| Phytase activity recovered in the experimental diets, phytase units/kg | ||||||
| 0 | < 50 | < 50 | < 50 | < 50 | < 50 | < 50 |
| 500 | 400 | 329 | 466 | 504 | 543 | 604 |
| 1,000 | 917 | 911 | 1,030 | 847 | 934 | 946 |
| 2,000 | 2,200 | 1,850 | 1,970 | 1,480 | 1,790 | 1,780 |
| Xylanase activity recovered in the experimental diets, xylanase units/kg | ||||||
| 0 | 14,000 | 15,800 | 15,500 | 18,200 | 18,000 | 18,700 |
| 500 | 14,000 | 18,700 | 16,300 | 18,200 | 18,400 | 18,300 |
| 1,000 | 16,900 | 15,400 | 16,600 | 16,800 | 18,600 | 15,700 |
| 2,000 | 16,600 | 15,400 | 17,200 | 14,200 | 15,600 | 16,700 |
Animal Performance
Livability from hatch to day 21 was 98.5% and was poorly predicted (adjusted R2=0.08) by linear or non-linear effects of phytate P, phytase dose or the interactions (data not shown). The prediction equations for growth performance are presented in Table 3. From day 0 to 21, predicted FI (Figure 1A) or BWG (Figure 1B) non-linearly decreased at dietary phytate P concentrations greater than 0.275 or 0.294%, respectively, regardless of the dose of phytase. There was a non-linear increase in FI or BWG as phytase log dose increased in the diet, with maximum FI achieved at 561 FTU/kg and maximum BWG achieved at 1,285 FTU/kg, regardless of the phytate P concentration in the diet. There was a non-linear influence of dietary phytate P concentration on FCR, with the lowest (better) FCR achieved at phytate P concentrations less than or equal to 0.319% (Figure 1C). Phytase supplementation log linearly improved FCR and the lowest FCR could not be predicted beyond 2,000 FTU/kg.
Table 3.
Predicted effect of graded concentrations of phytate P (x) and log doses of phytase (v) on broiler growth performance from hatch to day 21 or 42 and tibia ash, minerals and bone breaking strength (BBS) on day 21.
| Model |
||||
|---|---|---|---|---|
| Item | Equation | RMSE | Adjusted R2 | P-value |
| Hatch to day 21 | ||||
| Feed intake, g | y = 754.7 + 921.8x − 1,670x2} + 204.2v − 37.05v2 | 37.5 | 0.33 | <0.0001 |
| Body weight gain, g | y = 409 + 1,369x − 2324x2 + 172.7 − 27.72v2 | 29.7 | 0.55 | <0.0001 |
| Feed conversion, g:g | y = 1.593 − 1.117x + 1.772x2 − 0.033v | 0.02 | 0.66 | <0.0001 |
| Hatch to day 42 | ||||
| Feed intake, g | y = 3,587 + 601.6v − 124.3v2 | 147 | 0.03 | 0.0372 |
| Body weight gain, g | y = 1,706 + 2,559x − 4,570x2 + 384.3v − 72.37v2 | 98.3 | 0.27 | <0.0001 |
| Feed conversion, g:g | y = 1.855 − 0.888x + 1.767x2 − 0.024v | 0.03 | 0.58 | <0.0001 |
| Day 21 | ||||
| Tibia ash weight, g | y = 1.063 − 1.572x + 1.204v − 0.206v2 | 0.17 | 0.53 | <0.0001 |
| Tibia ash, % | y = 33.32 + 78.79x − 133.3x2 + 1.512v | 1.69 | 0.47 | <0.0001 |
| Tibia Ca, % | y = 33.22 + 13.31x − 33.42x2 | 0.79 | 0.54 | <0.0001 |
| Tibia P, % | y = 32.73 − 118.0x + 199.0x2 − 7.475v + 55.38v2 − 90.92x2v | 1.06 | 0.18 | <0.0001 |
| BBS, N | y = 133.6 − 456.7x + 599.1x2 + 6.800v | 15.3 | 0.13 | <0.0001 |
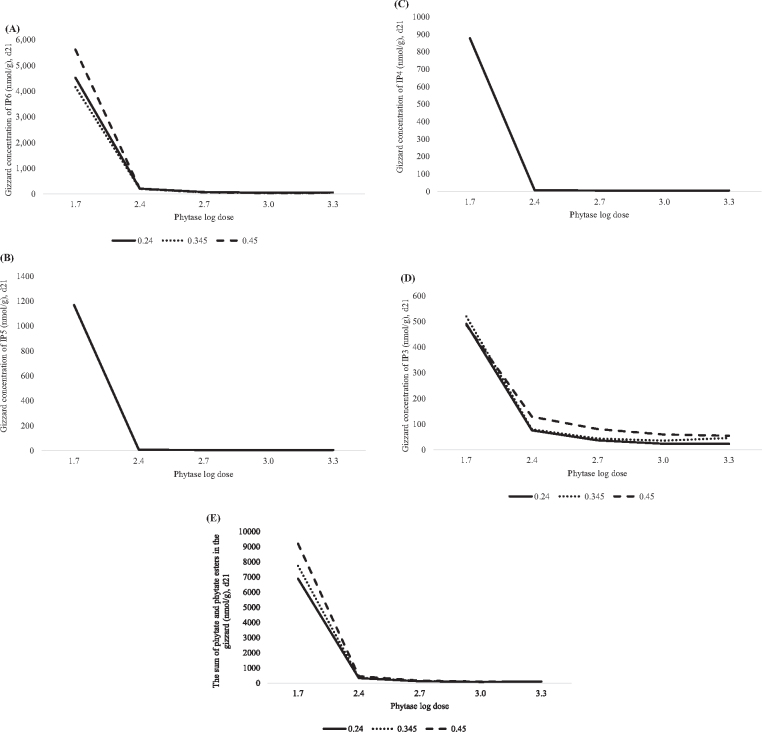
Figure 1.
Predicted effect of graded concentrations of phytate P (x) and log doses of phytase (v) on broiler growth performance from hatch to day 21. A. Feed intake (FI) in grams was predicted with the equation: y = 754.7 + 921.8x − 1,670x2 + 204.2v − 37.05v2, adjusted R2=0.33, RMSE=37.5, P < 0.0001. Maximum FI was achieved at 561 phytase units (FTU)/kg of phytase, regardless of dietary phytate P concentration or ≤0.275% phytate P, regardless of phytase concentration in the diet. B. Body weight gain (BWG) in grams was predicted with the equation: y = 409 + 1,369x − 2,324x2 + 172.7 − 27.72v2, adjusted R2=0.55, RMSE=29.7, P < 0.0001. Maximum BWG was achieved at 1,285 FTU/kg of phytase, regardless of the dietary phytate P concentration or ≤0.294% phytate P, regardless of phytase concentration in the diet. C. Feed conversion ratio (FCR) was predicted with the equation: y = 1.593 − 1.117x + 1.772x2 − 0.033v, adjusted R2=0.66, RMSE=0.02, P < 0.0001. Optimum (lowest) FCR was achieved at 2,000 FTU/kg of phytase, regardless of the phytate P concentration or ≤0.319% phytate P, regardless of the phytase concentration in the diet.
Overall (hatch to day 42), livability was 96.3% and not influenced by phytate P, phytase or the interaction (data not shown). Feed intake was poorly (adjusted R2=0.03) predicted by a non-linear effect of phytase log dose (Table 3). Body weight gain and FCR were influenced by a non-linear effect of dietary phytate P (Table 3), whereas BWG decreased at phytate P concentrations greater than or equal to 0.281% (Figure 2A) and FCR increased (worse) at dietary phytate P concentrations greater than or equal to 0.25% (Figure 2B). Phytase supplementation improved BWG in a non-linear-log fashion and FCR in a log-linear manner with maximum BWG achieved at 449 FTU/kg and minimum (better) FCR unable to predict at phytase concentrations greater than 2,000 FTU/kg, regardless of phytate P concentration in the diet.
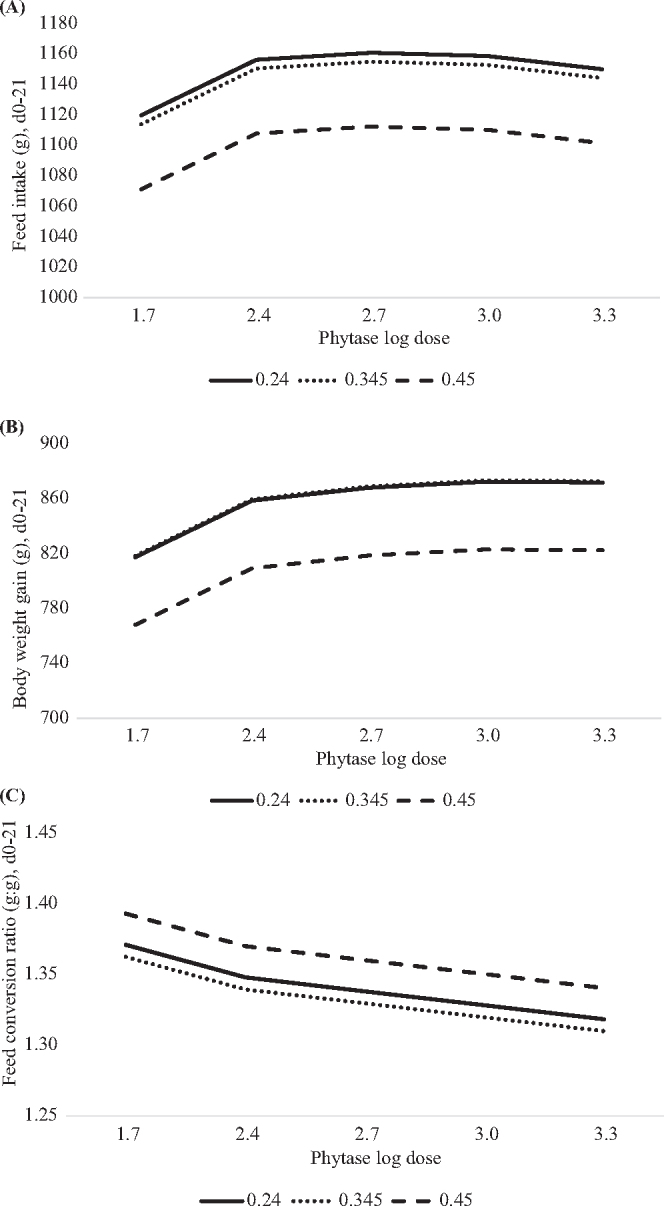
Figure 2.
Predicted effect of graded concentrations of phytate P (x) and log doses of phytase (v) on broiler growth performance from hatch to day 42. Feed intake (FI) in grams was not predicted due to the poor fit of the model. A. Body weight gain (BWG) in grams was predicted with the equation: y = 1,706 + 2,559x − 4,570x2 + 384.3v − 72.37v2, adjusted R2=0.27, RMSE=98.3, P < 0.0001. Maximum BWG was achieved at 449 phytase units (FTU)/kg of phytase, regardless of the dietary phytate P concentration or ≤0.281% phytate P, regardless of phytase concentration in the diet. B. Feed conversion ratio (FCR) was predicted with the equation: y = 1.855 − 0.888x + 1.767x2 − 0.024v, adjusted R2=0.58, RMSE=0.03, P < 0.0001. Optimum (lowest) FCR was achieved at 2,000 FTU/kg of phytase, regardless of the phytate P concentration or ≤0.25% phytate P, regardless of the phytase concentration in the diet.
The prediction equations suggested optimum growth performance was achieved at different doses of dietary phytase and the response to phytase was different depending on the age of the broilers. These results are in agreement with Babatunde et al. (2019) who suggested age, phytase dose, and length of phytase feeding will impact phytase efficacy on BWG or P digestibility. In the current trial, 561, 1,285 and >2,000 FTU/kg were required to optimize FI, BWG, or FCR, respectively, from hatch to day 21. Whereas overall (hatch to day 42), 449 or >2,000 FTU/kg were required to optimize BWG or FCR, respectively. Previous authors have also reported greater doses of phytase were required in younger birds compared with older birds to optimize BWG (Babatunde et al., 2019) or amino acid digestibility (Li et al., 2015) and this may be associated with limited intake, particularly when fed nutrient deficient diets as in the current experiment. Interestingly, there was a log-linear effect of phytase dose on FCR, regardless of the phytate P content of the diet or the age of the birds. Superdoses of phytase (≥1,500 FTU/kg) have been previously reported to result in significant improvements in FCR, in the presence (Walk et al., 2014) or absence of improvements in BWG (Walk et al., 2013; Broch et al., 2018), with approximately 2.5 points in FCR achieved for each 500 FTU/kg above that of the standard dose (Aftab et al., 2018). These improvements in FCR were associated with the mitigation of the anti-nutritional effects of phytate and overall improvement in nutrient utilization and efficiency.
Increasing the dietary phytate P concentration, with rice bran, was predicted to significantly decrease FI and BWG and increase FCR in a non-linear manner in broilers from hatch to day 21 or 42. Previous authors have also reported phytate from rice bran significantly reduced BWG and increased FCR of broilers fed increasing concentrations of phytate in P-adequate or P-deficient diets (Cabahug et al., 1999; Liu et al., 2008; Santos et al., 2014). Woyengo and Nyachoti (2013) suggested the negative effect of phytate on BWG is particularly relevant as the dietary concentration of phytate increases by 0.5 percentage points (from 0.78 to 1.57% phytate). In the current experiment, young broilers appeared able to handle a diet with up to 0.29 to 0.31% phytate P (1.03 to 1.10% dietary phytate) before resulting in losses in gain or FCR, regardless of the phytase concentration in the diet. Whereas overall, birds appeared to be more sensitive to the phytate P concentration in the diet, particularly FCR, which was optimized at less than 0.25% phytate P (<0.89% phytate). Phytate is a significant anti-nutrient, as indicated by the reduction in FI, gain and loss in feed efficiency in the current experiment. Long term feeding of dietary phytate P at concentrations greater than 0.25%, particularly in nutrient restricted diets, may create undue stress on nutrient transporters and endogenous enzymes (Liu et al., 2008) and nutrient uptake (Cowieson et al., 2006), which resulted in reduced feed efficiency at lower concentrations of dietary phytate P over the lifetime of the bird.
Bone Parameters and Plasma Urea N
The prediction equations for tibia ash weight, percent and mineral concentration are presented in Table 3. Tibia ash weight was predicted to linearly decrease as phytate P concentration in the diet increased to greater than or equal to 0.24%, regardless of the phytase concentration in the diet (Figure 3A). Phytase supplementation improved tibia ash weight in a non-linear-log manner with the greatest ash weight predicted at 822 FTU/kg. Tibia ash percent was predicted to decrease in a non-linear manner as dietary phytate P content increased to greater than or equal to 0.296%, regardless of the phytase concentration in the diet (Figure 3B). Phytase supplementation was predicted to log-linearly increase tibia ash percent with maximum concentration unable to be predicted at phytase concentrations beyond 2,000 FTU/kg. Tibia Ca concentration was predicted to decrease in a non-linear manner as dietary phytate P concentration increased greater than 0.24%, regardless of the phytase concentration in the diet (Figure 3C). Bone breaking strength (adjusted R2=0.18) and bone P concentration (adjusted R2=0.13) were poorly predicted by linear and non-linear effects of phytate P, log dose of phytase or the interactions (Table 3). The predicted linear or non-linear effect of phytate P, log dose of phytase or the interactions on plasma urea N could not be modeled (data not shown).
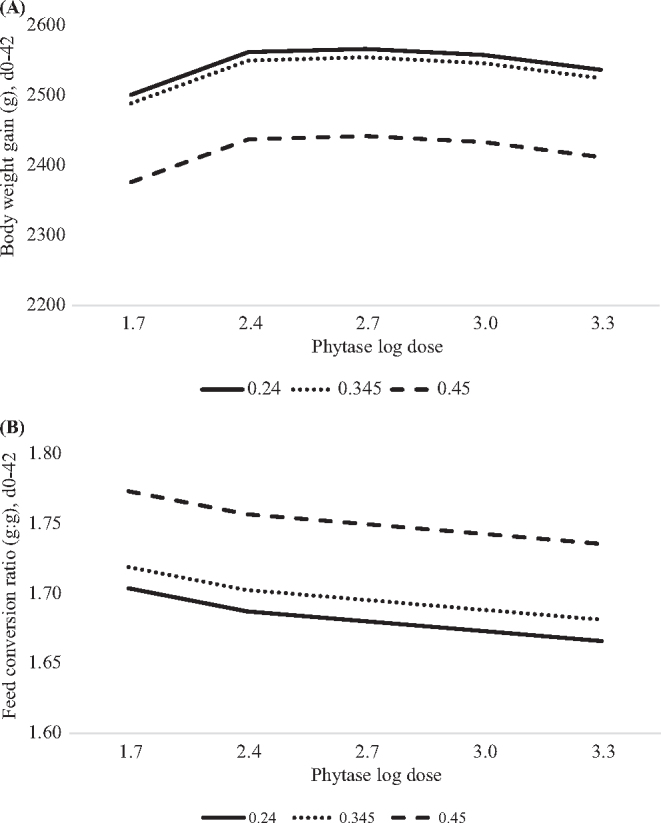
Figure 3.
Predicted effect of graded concentrations of phytate P (x) and log doses of phytase (v) on broiler tibia parameters at day 21. A. Tibia ash weight in grams was predicted with the equation: y = 1.063 − 1.572x + 1.204v − 0.206v2, adjusted R2=0.53, RMSE=0.17, P < 0.0001. Maximum tibia ash weight was achieved at 822 phytase units (FTU)/kg of phytase, regardless of dietary phytate P concentration or ≤0.24% phytate P, regardless of phytase concentration in the diet. B. Tibia ash concentration (%) was predicted with the equation: y = 33.32 + 78.79x − 133.3x2 + 1.512v, adjusted R2=0.47, RMSE=1.69, P < 0.0001. Maximum tibia ash concentration was achieved at 2,000 FTU/kg of phytase, regardless of the dietary phytate P concentration or ≤0.296% phytate P, regardless of phytase concentration in the diet. C. Tibia calcium concentration (%) was predicted with the equation: y = 33.22 + 13.31x − 33.42x2, adjusted R2=0.54, RMSE=0.79, P < 0.0001. Maximum tibia calcium concentration was achieved at ≤0.24% phytate P, regardless of phytase dose in the diet. Tibia phosphorus concentration and bone breaking strength were not determined due to the poor fit of the model.
Augspurger and Baker (2004) reported increases in tibia ash percent and weight with phytase supplementation up to 10,000 FTU/kg. In the current experiment, tibia ash weight was maximized at 822 FTU/kg whereas tibia ash percent log-linearly increased with phytase supplementation up to and greater than 2,000 FTU/kg. Bone ash weight is thought to be a more sensitive indicator of bone mineralization, but can be variable due to day, phytase level and method of tissue or fat extraction (Hall et al., 2003). In the current experiment, the prediction equations only accounted for about 50% of the variability and therefore, other factors, such as random variability or differences in the Ca or P content in the bone matrix, could explain some of this variability; particularly as tibia ash weight and Ca concentration were predicted to decrease in a linear and non-linear manner, respectively, as the concentration of dietary phytate P increased beyond 0.24% (0.85% phytate). Viveros et al. (2002) reported bone Ca concentration was significantly greater in birds fed diets deficient in non-phytate P, with no effect of 500 FTU/kg on bone Ca. Phytate is a strong chelator of dietary Ca (Nelson et al., 1968), particularly as pH increases in the small intestine. In the current trial, feeding increasing levels of phytate P, in Ca- and P-deficient diets, would have increased the capacity for phytate to bind to Ca thereby reducing the availability of dietary Ca for the bird. Bone ash percent was predicted to decrease in a non-linear manner as dietary phytate P increased to greater than 0.296% (1.05% phytate). Previous authors have reported significant reductions in toe ash percent of broilers fed increasing concentrations of dietary phytate P, in adequate and reduced P diets, and phytase supplementation up to 400 FTU/kg maximized toe ash (Cabahug et al., 1999). Santos et al. (2014) reported no effect of increasing dietary phytate (with rice bran) on bone ash weight in 21-day-old broilers; however, phytase supplementation at 500 FTU/kg significantly improved bone ash weight, regardless of the dietary phytate P content. The differences in phytate or phytase response on bone ash between the experiments may be related to bone evaluated (tibia vs. toe), age of broilers, method of tissue or fat extraction, and nutrient sufficiency of the diets.
Phytate, Phytate Esters, and Inositol
The prediction equations for the transformed concentrations of IP6, IP5, IP4, IP3 or inositol in the gizzard are presented in Table 4. The predicted concentration of IP6 in the gizzard was influenced in a non-linear manner by phytate P and phytase log dose. In the absence of phytase, the maximum concentration of IP6 was predicted at 4,524, 4,164, or 5,618 nmol/g in birds fed 0.24, 0.345, or 0.45% phytate P, respectively. The predicted minimum IP6 concentration in the gizzard was achieved with 1,132, 1,285, or 1,208 FTU/kg of phytase at 0.24, 0.345, or 0.45% dietary phytate P, respectively (Figure 4A). There was no effect of phytate P concentration on the concentration of IP5 or IP4 in the gizzard. The predicted concentration of IP5 (Figure 4B) or IP4 (Figure 4C) in the gizzard decreased in a non-linear-log manner with a minimum concentration achieved with 1,028 FTU/kg, regardless of the phytate P concentration of the diet. The predicted concentration of IP3 in the gizzard was influenced in a non-linear manner by phytate P and phytase log dose. In the absence of phytase, the predicted concentration of IP3 in the gizzard was 491, 519, or 486 nmol/g in birds fed 0.24, 0.345, or 0.45% phytate P, respectively (Figure 4D). The predicted minimum IP3 concentration in the gizzard was achieved with 1,462, 966, or 1,828 FTU/kg at 0.24, 0.345 or 0.45% dietary phytate P, respectively. The sum of IP esters in the gizzard was influenced by phytate P and phytase log dose. In the absence of phytase, the predicted sum of IPs in the gizzard increased in a linear manner at 6,924, 7,771, or 9,238 nmol/g in birds fed 0.24, 0.345, or 0.45% phytate P, respectively (Figure 4C). The predicted minimum concentration of the sum of IPs in the gizzard was achieved at 1,096, 1,170, or 1,245 FTU/kg with 0.24, 0.345, or 0.45% dietary phytate P, respectively. Finally, the predicted concentration of inositol in the gizzard was influenced by a non-linear effect of phytate P and phytase log dose. The predicted maximum inositol concentration in the gizzard was achieved at >2,000 FTU/kg in broilers fed diets containing 0.24 or 0.345% phytate P or 202 FTU/kg in broilers fed 0.45% phytate P (Figure 5). In the absence of phytase, the predicted concentration of inositol in the gizzard was 19.6, 28.1, or 25.9 µ mol/g, respectively.
Table 4.
Predicted effect of graded concentrations of phytate P (x) and log doses of phytase (v) on the concentration of phytate (IP6) or phytate esters (IP5, IP4, or IP3) in the gizzard of 21-day-old broilers.
| Model |
|||||
|---|---|---|---|---|---|
| Item, nmol/g | Equation | λ | RMSE | Adjusted R2 | P-value |
| IP61 | y = 8,829 − 2,4618x + 38,375x2 − 5,888v + 1,030v2 + 18,225xv − 28,144x2v − 3,389xv2 + 5,162x2v2 | 0.195 | 30.9 | 0.99 | <0.0001 |
| IP51 | y = 566 − 281v + 46.6v2 | −0.647 | 1.77 | 1.00 | <0.0001 |
| IP41 | y = 521 − 251v + 41.7v2 | −0.689 | 1.60 | 1.00 | <0.0001 |
| IP31 | y = − 914 + 15,421v − 24,186v2 + 1,186x − 284v2 − 13,409xv + 20,950x2v + 2746xv2 − 4,240x2v2 | 0.362 | 24.7 | 0.95 | <0.0001 |
| ∑ IP6-IP31 | y = 9,371 + 414x + 1,658x2 − 5709v + 955v2 − 436xv | 0.194 | 47.1 | 1.00 | <0.0001 |
| Inositol, μ mol/g | y = − 291 + 2,179x − 3546x^2} + 237v − 52.9v2 − 1,721xv + 2,902x2v + 385xv2 − 648x2v2 | 1.69 | 0.87 | <0.0001 | |
Means (x) were transformed and using the Box-Cox platform in JMP v. 14. Equations and RMSE were determined using the refit transformed data and best λ.
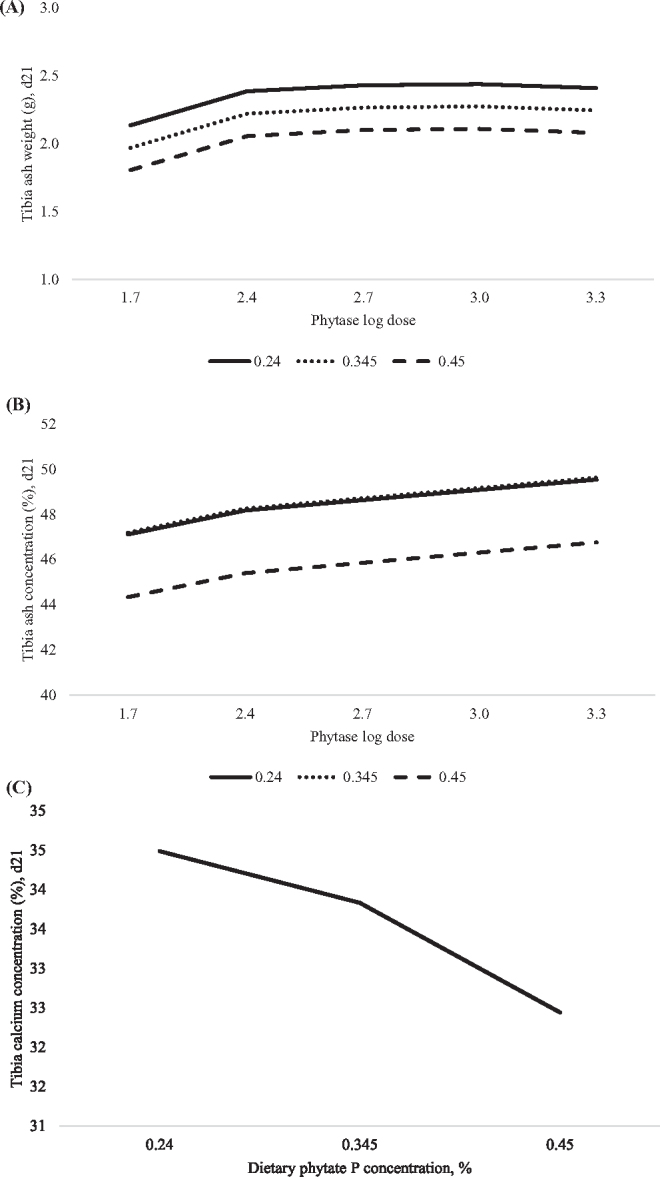
Figure 4.
The predicted effect of graded concentrations of phytate P (x) and log doses of phytase (v) on phytate (IP6) and phytate ester (IP5, IP4, and IP3) concentration and in the gizzard of 21-day-old broilers. A. Gizzard IP6 concentration was predicted with the equation: y = 8,829 − 24,618x + 38,375x2 − 5,888v + 1,030v2 + 18,225xv − 28,144x2v − 3,389xv2 + 5,162x2v2, adjusted R2=0.99, RMSE=30.9, P < 0.0001. In the absence of phytase, the predicted concentration of IP6 in the gizzard was 4,524, 4,164, or 5,618 nmol/g in birds fed 0.24, 0.345, or 0.45% phytate P, respectively. The predicted minimum IP6 concentration in the gizzard was achieved with 1,132, 1,285, or 1,208 phytase units (FTU)/kg of phytase at 0.24, 0.345 or 0.45% dietary phytate P, respectively. B. Gizzard IP5 concentration was predicted with the equation: y = 566 − 281v + 46.6v2, adjusted R2=1.00, RMSE=1.77, P < 0.0001. The predicted minimum concentration of IP5 in the gizzard was achieved with 1,028 FTU/kg, regardless of the phytate P concentration of the diet. C. Gizzard IP4 concentration was predicted with the equation: y = 521 − 251v + 41.7v2, adjusted R2=1.00, RMSE=1.60, P < 0.0001. The predicted minimum IP4 concentration in the gizzard was achieved with 1,028 FTU/kg, regardless of the phytate P concentration in the diet. D. Gizzard IP3 concentration predicted with the equation: y = − 914 + 15421v − 24,186v2 + 1186x − 284v2 − 13409xv + 20,950x2v + 2746xv2 − 4240x2v2, adjusted R2=0.95, RMSE=24.7, P < 0.0001. In the absence of phytase, the predicted concentration of IP3 in the gizzard was 491, 519, or 486 nmol/g in birds fed 0.24, 0.345, or 0.45% phytate P, respectively. The predicted minimum IP3 concentration in the gizzard was achieved with 1,462, 966 or 1,828 FTU/kg at 0.24, 0.345 or 0.45% dietary phytate P, respectively. e. The concentration of the sum of all IPs in the gizzard was predicted with the equation: y = 9,371 + 414x + 1,658x2 − 5,709v + 955v2 − 436xv, adjusted R2=1.00, RMSE=47.1, P < 0.0001. In the absence of phytase, the predicted sum of IPs in the gizzard was 6,924, 7,771 or 9,238 nmol/g in birds fed 0.24, 0.345 or 0.45% phytate P, respectively. The predicted minimum concentration of the sum of IPs in the gizzard was achieved with 1,096, 1,170 or 1,245 FTU/kg at 0.24, 0.345 or 0.45% dietary phytate P, respectively.
Figure 5.
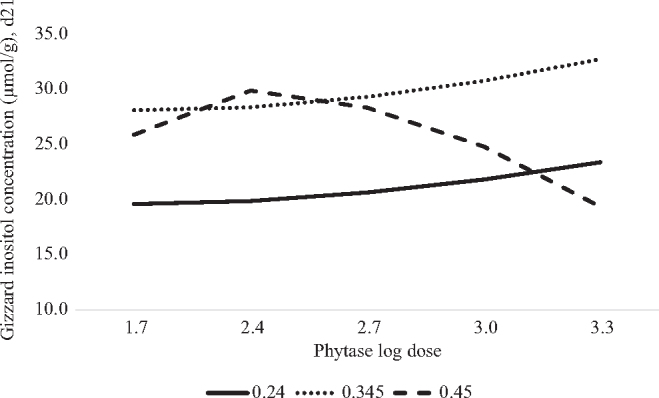
Predicted effect of graded concentrations of phytate P (x) and log doses of phytase (v) on gizzard inositol concentration (µ mol/g) of 21-day-old broilers. Inositol concentration was predicted with the equation: y = − 291 + 2,179x − 3,546x2 + 237v − 52.9v2 − 1,721xv + 2,902x2v + 385xv2 − 648x2v2, adjusted R2=0.87, RMSE=1.69, P < 0.0001. The predicted maximum inositol concentration in the gizzard was achieved at 2,000 phytase units (FTU)/kg in broilers fed diets containing 0.24 or 0.345% phytate P or 202 FTU/kg in broilers fed 0.45% phytate P. In the absence of phytase, the predicted concentration of inositol in the gizzard was 19.6, 28.1, or 25.9 µ mol/g, respectively.
Phytate (IP6) degradation by endogenous microbial or luminal phytases in broilers fed low Ca and available P diets can range from 62 to 89% in the ileum (Rodehutscord and Rosenfelder, 2016). However, IP6 degradation in the crop of birds fed diets without microbial phytase was found to be very low, at only 9% (Zeller et al., 2015). In the current trial, increasing dietary phytate P increased the concentration of IP6 in the gizzard in a non-linear manner, with an 8% decrease and then a 19% increase in the IP6 concentration in the gizzard of birds fed the 0.345 or 0.45% phytate P diets, respectively, when compared with birds fed the 0.24% phytate P diets. This change in IP6 concentration in the gizzard is most likely an effect of increasing the concentration of IP6 in the diet and intake, which decreased in a non-linear manner as dietary phytate P increased above 0.275%. Supplementation of phytase around 1,200 FTU/kg minimize the IP6 concentration in the gizzard, regardless of the phytate P content of the diet. Previous authors have also reported significant decreases in IP6 concentration in the crop or ileum, at 56 or 93% IP6 hydrolysis, respectively, in broilers fed 1,500 FTU/kg (Sommerfeld et al., 2018) or gizzard, at 99.5% IP6 hydrolysis, in broilers fed 2,000 FTU/kg (Walk and Olukosi, 2019).
Phytase hydrolyzes IP6 into the lower phytate esters, IP5, IP4, IP3 and IP2 and this was noted in the current trial, with non-linear reductions in IP5 or IP4 concentration as phytase dose increased to ∼1,000 FTU/kg, regardless of the concentration of dietary phytate P. Sommerfeld et al. (2018) and Walk and Olukosi (2019) reported significant decreases in IP5 content of the crop or gizzard as phytase dose increased in the diet, with an increase or no effect of phytase dose on IP4 concentration in the crop or gizzard, respectively. The lack of an effect of dietary phytate P on IP5 or IP4 concentration in the gizzard, in the absence of phytase, would be expected, as these are products of IP6 hydrolysis by phytase and are at concentrations much lower than that of IP6 (75 to 81% of the total IP6) in diets without phytase. Finally, while at much lower concentrations than the other measured phytate esters, IP3 concentration in the gizzard was influenced in a non-linear manner by phytate P and phytase log dose in the diets. In general, IP3 content in the gizzard was greatest in birds fed 0.345% phytate P and 966 FTU/kg were required to minimize the concentration of IP3 in birds fed these diets, whereas in the 0.24 or 0.45% phytate P diets, 1,462 or 1,828 FTU/kg, respectively, was required to minimize the IP3 concentration. These results were not expected and without characterization of the specific IP3 isomers or further characterization of IP2 or IP1, it is difficult to determine the origin of the IP3 products. However, the concentration of inositol in the gizzard may shed some light on the phytate breakdown pattern noted in the current trial, as birds fed diets containing 0.45% phytate P had the greatest concentration of inositol in the gizzard at 202 FTU/kg, which then decreased as phytase dose increased; whereas greater than 2,000 FTU/kg was predicted to maximize inositol concentration in the gizzard of birds fed 0.24 or 0.345% phytate P. Previous authors have reported significant increases in inositol concentration in the crop, gizzard, or ileum of birds fed increasing doses of phytase or inositol (Walk et al., 2014; Sommerfeld et al., 2018; Walk and Olukosi, 2019). Could the high dietary phytate P or the end products of IP3 degradation, in the high phytate P diets, interfere or inhibit phytate degradation to inositol in the gizzard? Further characterization of the lower phytate esters, such as IP2 or IP1 and the isomers are required to elucidate the predicted responses noted in the current trial.
In conclusion, prediction equations can be useful to determine the influence of dietary phytate P and phytase and their interaction on growth performance, bone ash, and phytate degradation patterns in the gizzard. Dietary phytate P appears to have a greater anti-nutrient impact on FCR, at lower dietary concentrations, when compared with BWG. Feeding increasing doses of phytase linearly improved FCR, in diets formulated to contain sub-optimal nutrient levels, regardless of the phytate P content of the diet, through the destruction of phytate and lower phytate esters in the gizzard.
REFERENCES
- Aftab U., Bedford M.R., Creswell D. Prospects of improving efficiency of feed utilization in broiler. World Poult. Sci. J. 2018;74:427–442. [Google Scholar]
- AOAC . 18th ed. AOAC; Arlington, VA: 2006. Official methods of analysis of AOAC international. [Google Scholar]
- Augpsurger N.R., Baker D.H. High dietary phytase levels maximize phytate-phosphorus utilization but do not affect protein utilization in chicks fed phosphorus- or amino acid-deficient diets. J. Anim. Sci. 2004;82:1100–1107. doi: 10.2527/2004.8241100x. [DOI] [PubMed] [Google Scholar]
- Babatunde O.O., Cowieson A.J., Wilson J.W., Adeola O. Influence of age and duration of feeding low-phosphorus diet on phytase efficacy in broiler chickens during the starter phase. Poult. Sci. 2019;98:2588–2597. doi: 10.3382/ps/pez014. [DOI] [PubMed] [Google Scholar]
- Broch J., Nunes R.V., Eyng C., Pesti G.M., de Souza C., Sangalli G.G., Fascina V., Teixeira L. High levels of dietary phytase improves broiler performance. Anim. Feed Sci. Technol. 2018;244:56–65. [Google Scholar]
- Cabahug S., Ravindran V., Selle P.H., Bryden W.L. Response of broiler chickens to microbial phytase supplementation as influenced by dietary phytic acid and non-phytate phosphorus contents. I. Effects on bird performance and toe ash. Br. Poult. Sci. 1999;40:660–666. doi: 10.1080/00071669987052. [DOI] [PubMed] [Google Scholar]
- Cowieson A.J., Acamovic T., Bedford M.R. Phytic acid and phytase: impliaations for protein utilization by poultry. Poult. Sci. 2006;85:878–885. doi: 10.1093/ps/85.5.878. [DOI] [PubMed] [Google Scholar]
- Engelen A.J., van der Heeft F.C., Randsdorp P.H.G., Somers W.A.C. Determination of phytase activity in feed by colorimetric enzymatic method: collaborative interlaboratory study. J. AOAC. Int. 2001;84:629–633. [PubMed] [Google Scholar]
- Hall L.E., Shirley R.B., Bakalli R.I., Aggrey S.E., Pesti G.M., Edwards H.M., Jr Power of two methods for the estimation of bone ash of broilers. Poult. Sci. 2003;82:414–418. doi: 10.1093/ps/82.3.414. [DOI] [PubMed] [Google Scholar]
- Lee S.A., Dunne J., Febery E., Brearley C.A., Mottram T., Bedford M.R. Exogenous phytase and xylanase exhibit opposing effects on real-time gizzard pH in broilers. Br. Poult. Sci. 2018;59:568–578. doi: 10.1080/00071668.2018.1496403. [DOI] [PubMed] [Google Scholar]
- Li W., Angel R., Kim S.-W., Jimenez-Moreno E., Proszkowiec-Weglarz M., Plumstead P.W. Age and adaptation to Ca and P deficiencies: 2. Impacts on amino acid digestibility and phytase efficacy in broilers. Poult. Sci. 2015;94:2917–2931. doi: 10.3382/ps/pev273. [DOI] [PubMed] [Google Scholar]
- Liu N., Ru Y.J., Li F.D., Cowieson A.J. Effect of diet containing phytate and phytase on the activity and messenger ribonucleic acid expression of carbohydrase and transporter in chickens. J. Anim. Sci. 2008;86:3432–3439. doi: 10.2527/jas.2008-1234. [DOI] [PubMed] [Google Scholar]
- Nelson T.S., McGillivray J.J., Shieh T.R., Wodzinski R.J., Ware J.H. Effect of phytate on the calcium requirement of chicks. Poult. Sci. 1968;47:1985–1989. doi: 10.3382/ps.0471985. [DOI] [PubMed] [Google Scholar]
- Onyango E.M., Asem E.K., Adeola O. Phytic acid increases mucin and endogenous losses from the gastrointestinal tract of chickens. Br. J. Nutr. 2009;101:836–842. doi: 10.1017/S0007114508047740. [DOI] [PubMed] [Google Scholar]
- Rodehutscord M., Rosenfelder P. Update on phytate degradation pattern in the gastrointestinal tract of pigs and broiler chickens. In: Walk C.L., editor. Phytate destruction – consequences for precision animal nutrition. Wageningen Academic Publishers; The Netherlands: 2016. pp. 15–32. [DOI] [Google Scholar]
- Santos T.T., Walk C.L., Srinongkote S. Influence of phytate level on broiler performance and the efficacy of 2 microbial phytases from 0 to 21 days of age. J. Appl. Poult. Res. 2014;23:181–187. [Google Scholar]
- Sommerfeld V., Kunzel S., Schollenberger M., Kuhn I., Rodehutscord M. Influence of phytase or myo-inositol supplements on performance and phytate degradation products in the crop, ileum, and blood of broiler chickens. Poult. Sci. 2018;97:920–929. doi: 10.3382/ps/pex390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viveros A., Brenes A., Arija I., Centeno C. Effects of microbial phytase supplementation on mineral utilization and serum enzyme activities in broiler chicks fed different levels of phosphorus. Poult. Sci. 2002;81:1172–1183. doi: 10.1093/ps/81.8.1172. [DOI] [PubMed] [Google Scholar]
- Walk C.L., Olukosi O.A. Influence of graded concentrations of phytase in high-phytate diets on growth performance, apparent ileal amino acid digestibility, and phytate concentration in broilers from hatch to day 28 post-hatch. Poult. Sci. 2019 doi: 10.3382/ps/pez106. [DOI] [PubMed] [Google Scholar]
- Walk C.L., Bedford M.R., Olukosi O.A. Effect of phytase on growth performance, phytate degradation and gene expression of myo-inositol transporters in the small intestine, liver and kidney of 21-day old broilers. Poult. Sci. 2018;97:1155–1162. doi: 10.3382/ps/pex392. [DOI] [PubMed] [Google Scholar]
- Walk C.L., Santos T.T., Bedford M.R. Influence of superdoses of a novel microbial phytase on growth performance, tibia ash, and gizzard phytate and inositol in young broilers. Poult. Sci. 2014;93:1172–1177. doi: 10.3382/ps.2013-03571. [DOI] [PubMed] [Google Scholar]
- Walk C.L., Bedford M.R., Santos T.T., Paiva D., Bradley J.R., Wladecki H., Honaker C., McElroy A.P. Extra-phosphoric effects of superdoses of a novel microbial phytase. Poult. Sci. 2013;92:719–725. doi: 10.3382/ps.2012-02727. [DOI] [PubMed] [Google Scholar]
- Woyengo T.A., Nyachoti C.M. Review: anti-nutritional effects of phytic acid in diets for pigs and poultry – current knowledge and directions for future research. Can. J. Anim. Sci. 2013;93:9–21. [Google Scholar]
- Zeller E., Schollenberger M., Kuhn I., Rodehutscord M. Hydrolysis of phytate and formation of inositol phosphate isomers without or with supplemented phytases in difference segments of the digestive tract of broilers. J. Nutr. Sci. 2015;4:1–12. doi: 10.1017/jns.2014.62. [DOI] [PMC free article] [PubMed] [Google Scholar]