Abstract
N-carbamylglutamate (NCG), an analogue of N-acetyl-L-glutamate (NAG), can increase arginine synthesis in mammals and improve the reproductive performance. However, the effect of NCG on poultry laying performance is still unclear. This study investigated the effect of dietary NCG on development of chicken ovarian follicles. The dosage and timing for NCG administration were evaluated for its effect on follicular development. Results showed that supplementation with 1% NCG in the diet for 14 D led to accelerated development of growing follicles (over 60 μm in oocyte diameter) and significantly increased feed intake and feed efficiency. Plasma amino acids (AA) analysis showed that feeding with 1% NCG significantly increased of plasma AA levels. RNA-seq analysis revealed that NCG supplementation upregulated expression of genes related to angiogenesis and cell proliferation, but downregulated expression of apoptosis-related genes. Meanwhile, RT-qPCR and Western blot analysis validated the RNA-seq results. Moreover, NCG enhanced plasma NO level; upregulated expression of PKG-I, Raf1, and p-p38; and increased angiogenesis of the ovaries. In conclusion, dietary NCG (1% for 14 D) can promote development of ovarian follicles by increasing angiogenesis in ovaries of the chicken.
Key words: N-carbamylglutamate, chicken, follicles, angiogenesis, NO
INTRODUCTION
As an important nutritional source of high-quality proteins, poultry eggs contain all essential amino acids (AA) that can be easily absorbed. Though the egg production was greatly elevated by breeding high-yield poultry layers, their reproductive performance largely depends on exogenous nutrient supply and intrinsic ovarian development. However, less than 5% primordial follicles can develop into preovulatory follicles, indicating that the laying performance of hens has a huge potential of improvement through enhanced ovarian development by exogenous optimal manipulations.
Balanced supply and efficient parturition of the nutrients via blood circulation are prerequisite for gonadal development and high reproductive performance. Protein undernutrition by partial albumen removal from the eggs during embryonic development diminished reproductive capacity of hens (Willems et al., 2016). Energy and protein dilution in pullet diets of broiler breeder may have detrimental effects on the performance of offspring (Moraes et al., 2019). Furthermore, progress of follicle development was attenuated although there were fewer developing follicles than the primordial follicles in malnutritional ewe fetuses (Lea et al., 2006). Although it is known that nutritional conditions determine animal reproductive function, to date very few studies have focused on the functions of specific nutrients with defined chemical structures to follicular development. As a semi-necessary AA in mammals, arginine (Arg) plays multiple roles in metabolism pathways in the ovary and can improve the litter size. After feeding with Arg during pregnancy in sows, embryonic development was enhanced, the number of live-birth was increased by 22% and the weight of live litters was elevated by 24% (Mateo et al., 2007; Li et al., 2014). Furthermore, supplemented with Arg for Longyan laying ducks can improve small yellow follicles development (Xia et al., 2017). In addition, Arg supplementation improved litter size involving upregulation of nitric oxide synthase 3 (NOS3) and vascular endothelial growth factor (VEGF) in placental surface vessels during late pregnancy of the sows (Wu et al., 2012a).
The ovary is rich in blood vessels to supply nutrients, hormones, and cytokines for follicles development and maturation. There is a close relation between follicular development and vascular network in the ovary. In hens, the expression of angiogenic gene VEGF and its receptor in the healthy prehierarchical follicles (6–8 mm, before selection) were elevated relative to atretic follicles (Kim et al., 2016). Promotion of angiogenesis in theca layers facilitated yolk deposition in chicken prehierarchical follicles (Lin et al., 2019). In the cow, vasculature in the healthy follicles was significantly greater than that in atretic follicles (Moonmanee et al., 2013). Moreover, a defective endothelial nitric oxide synthase/nitric oxide system and vasculature led to reduced bovine oocyte competence and fertility (Tessaro et al., 2011). NO also played a major role in the injury of the intestinal tract induced by selenium deficiency in chickens (Yu et al., 2015). N-carbamylglutamate (NCG), an analogue of N-acetyl-L-glutamate (NAG), was able to replace NAG involved in urea cycle where more endogenous Arg was produced (Frank et al., 2007; Wu et al., 2010). With the catalysis of the nitric oxide synthase, Arg can be converted to NO and citrulline. NCG was able to increase NO concentration, enhance the gene expression of NOS3 and VEGFA in pregnant sows, thus, increase litter size (Wu et al., 2012a; Cai et al., 2018). NO is known for its pro-angiogenic functions. Therefore, NCG may also promote follicular development by increasing ovarian angiogenesis. In addition, supplement with NCG in the diet can activate the PI3K/Akt/mTOR signaling pathway by increasing the content of Arg, thereby, promoting successful implantation of the embryo and maintaining pregnancy (Zeng et al., 2012). These studies indicate that NCG may exert regulating role in the reproductive performance by increasing NO production.
Since rich blood supply is essential for the rapid ovarian growth and follicular development in poultries, we assume the pro-angiogenic effect of NCG may promote ovarian development, then increase laying performance in laying hens. Therefore, this study investigated the effect of NCG on follicular development by morphological observation and RNA-seq technology to reveal the differential transcriptomics profiles. The predominant genes related with NCG action were further validated by RT-qPCR method. The results may provide a foundation for dietary supplement of NCG to increase laying performance in laying hens.
MATERIALS AND METHODS
Experimental Design and Animal Raising
Fertilized Hyline chicken eggs were incubated at 38.5°C and 60% humidity. After hatching, 20 chicks were randomly divided into control group (basal diet) and 3 treatment groups (basal diet supplemented with 0.01%, 0.1%, and 1% NCG, respectively) for 2 wk. The 1% NCG dosage was selected according to the ovaries/BW ratio, cortex follicle number, and oocyte diameter. Next, 30 new hatchlings were randomly divided into 2 groups, control group (basal diet) and NCG-treatment group (basal diet supplemented with 1% NCG) for 7, 14, or 21 D. The ovaries of 5 chickens were taken for measurement of the oocyte diameter. The best feeding time was selected according to the oocyte diameter. The optimal dosage of NCG (1%) and feeding time (14 D) were chosen as the treatment diet in the following experiments. A total of 58 chickens were randomly divided into 2 groups (29/group): control group (basal diet) and treatment group (basal diet supplemented with 1% NCG) for 14 D. BW and feed of chickens were weighed on day 0 ∼ 7 and 8 ∼ 14 in each repetition. At the 14th experimental day, ovaries were obtained. In each group, 15 ovaries were randomly selected and divided into 3 repetitions with 5 ovaries in each repetition, another 5 ovaries were selected for morphological observation and the remaining 9 ovaries were used to Western blot.
The nutrient level and composition of the basal diets were listed in Table 1 and chickens had ad libitum access to feed and water. Temperature was maintained at approximately 32°C and the humidity maintained at about 60%. The photoperiods were 22 h light: 2 h dark (0 ∼ 3 D), 21 h light: 3 h dark (4 ∼ 7 D), and 20 h light: 4 h dark (8 ∼ 14 D), respectively. This study was carried out in accordance with the Guiding Principles for the Care and Use of Laboratory Animals of Zhejiang University. The experimental protocols were approved by the Committee on the Ethics of Animal Experiments of Zhejiang University (No. ZJU2015-156-12).
Table 1.
Composition and nutrient level of the basal diets.
| Ingredients | Content (%) |
|---|---|
| Corn | 65.5 |
| Bran | 2.5 |
| Soybean meal | 29 |
| Premix1 | 3 |
Supplied per kilogram of premix: dl-a-tocopherol acetate ≥280 mg; sodium menadiene bisulfite, 30 ∼ 96 mg; thiamine nitrate ≥38 mg; vitamin B2 ≥ 60 mg; pyridoxine hydrochloride ≥60 mg; vitamin B12 ≥ 0.2 mg; nicotinamide ≥420 mg; D-calcium pantothenate ≥420 mg; folic acid ≥12.0 mg; D-biotin ≥3.0 mg; choline chiorlde ≥5.6 g; vitamin A acetate, 160,000 ∼ 200,000 IU; vitamin D3, 4.4 ∼ 100 mg; Cu, 140 ∼ 420 mg; Fe, 1.6 × 103 ∼ 1.3 × 104; Mn, 1.2 × 103 ∼ 3.0 × 103; Zn, 1.2 × 103 ∼ 2.4 × 103; Se, 2.0 ∼ 6.0 mg; I, 6.0 ∼ 18 mg; methionine, 2.6 ∼ 5.2%; P, 1.8 ∼ 5.0%; Ca, 5.0 ∼ 20%; NaCl, 4.0 ∼ 9.0%; H2 O2 ≤ 10.
Sample Collection
At the time of the experiment (9:00 at the 14th experimental day), BW of chickens was measured and blood was withdrawn by cardiac puncture for plasma preparation. After that, chickens were sacrificed post-anesthesia, ovaries were obtained, and documented the weight, then washed them in cold PBS (pH 7.4) 3 times to make them as clean as possible for morphological and biochemical analysis.
Morphological Observation
The fixed ovaries were dehydrated with grade ethanol, then cleared in xylene and embedded in paraffin. The embedded samples were sectioned at 5 μm thickness for hematoxylin and eosin (HE) staining, immunohistochemistry (IHC), terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL), and immunofluorescence (IF) staining. HE, IHC, and IF staining were performed following standard procedures (He et al., 2013; Liu et al., 2018). A total of 5 serial sections across the largest cross-section of the ovaries were selected for follicles counting and measurement of oocyte diameter. The primary antibodies used for the IF/IHC were as follows: mouse anti-proliferating cell nuclear antigen (PCNA) (1:1000, Abcam, Cambridge, UK), rabbit anti-VEGF (1:500, Hangzhou HuaAn Biotechnology Co., Hangzhou, China), mouse anti-BAX (1:100, ab5714), and mouse anti-CD31 (provided by Dr. Xun Tan). DAB was used and the images were captured using an Eclipse 80i microscope (Nikon, Tokyo, Japan). The fluorescence images of the slides were visualized using an IX70 fluorescence microscope (OLYMPUS, Tokyo, Japan). The apoptosis of ovarian cells was detected with a TUNEL Brightgreen Apoptosis Detection Kit (Vazyme Biotech Co., Nanjing, China). Fluorescence images of the slides were observed using an IX70 fluorescence microscope. In each section, 5 fields were selected randomly to count the number of TUNEL-positive cells (green fluorescence). The percentage of the green labeled cells vs. the total cell number represented the apoptosis rate.
Analysis of Plasma AA
Plasma samples were weighted (about 0.5 g) and acid hydrolyzed with 4.0 mL 6 M HCl in vacuum-sealed hydrolysis vials at 110°C for 22 h. The content of AA was analyzed using an Amino acid analyzer (L-8900 Hitachi-hitech, Japan).
RNA Isolation and RNA-seq Analysis
Total RNA was extracted from ovaries using Trizol reagent (Takara, Shiga, Japan) according to the manufacturer's procedure. mRNA was purified with poly-T oligo-attached magnetic beads. Then the RNA was fragmented into small pieces under an elevated temperature condition. Small RNA pieces were reverse-transcribed to cDNA to construct a cDNA library. AMPure XP system (Beckman Coulter, Beverly, USA) was used to screen 250 ∼ 300 bp cDNA fragments. Phusion High-Fidelity DNA polymerase, Universal PCR primers, and Index (X) Primer were used to perform PCR amplification. Finally, PCR products were purified with AMPure XP system and the quality of the library was assessed with the Agilent Bioanalyzer 2,100 system. In total, 6 RNA-seq libraries (3 control and 3 NCG groups) were constructed. Clean data (clean reads) was obtained by removing contained adaptor contamination, low-quality bases, and undetermined bases. Then, sequence quality was assessed by Q20, Q30, and GC content of the clean reads. All downstream analyses were based on clean data of high quality. Hisat2 (v2.0.5) was used as the mapping tool to build the index of the reference genome and aligned the paired-end clean reads to the reference genome. Index of the reference genome was built using Hisat2 (v2.0.5) and paired-end clean reads were aligned to the reference genome (reference genome download link, ftp://ftp.ensembl.org/pub/release-83/fasta/gallus_gallus/dna/).
qPCR Analysis
Total RNA was extracted from follicles using a Trizol reagent (Vazyme, Nanjing, China). RNA concentrations were measured using a NanoDrop 2000c (Thermo Scientific, Waltham, USA). The cDNA was generated from 2 μg total RNA using a HiScript II 1st Strand cDNA Synthesis Kit (Vazyme, Nanjing, China) according to the manufacturer's protocol. qPCR was performed in triplicate using a SYBR Premix Ex TaqTM (Vazyme, Nanjing) in ABI 7500 HT Real-Time PCR machine (Applied Biosystems, Foster City, CA, USA). The qPCR conditions were as follows: 95°C for 10 min and then 40 cycles of 95°C for 30 s, 64°C for 34 s, and 72°C for 30 s. The 2−ΔΔCt formula method was used to analyze relative mRNA expression calibrated with β-actin as the reference gene. Primers were listed in Table 2.
Table 2.
Sequences of the primers for PCR.
| Gene name | Accession no. | Primer sequence (5′-3′) | Product size (bp) |
|---|---|---|---|
| CCND1 | NM_205,381.1 | F: CCTCAAGAAAAGCCGGTTGC | 86 |
| R: CTGCGGTCAGAGGAATCGTT | |||
| CDK2 | NM_0,011,99857.1 | F: TCCGTATCTTCCGCACGTTG | 183 |
| R: GCTTGTTGGGATCGTAGTGC | |||
| Caspase-3 | NM_204,725.1 | F: CAGCTGAAGGCTCCTGGTTT | 98 |
| R: GCCACTCTGCGATTTACACG | |||
| VEGFA | NM_0,011,10355.1 | F: GTCGTACATATTCAGGCCATC | 197 |
| R: GATTCTTTGGTCTGCAGTCAC | |||
| NOS3 | JQ434761.1 | F: GAACCCCCAAGACCTACGTGC | 180 |
| R: CCTGCCCCATGGTCATTCCTC | |||
| β-actin | NM_205,518 | F: ACACCCACACCCCTGTGATGAA | 136 |
| R: TGCTGCTGACACCTTCACCATTC |
Measurements of NO Level
The plasma samples were used to determine NO concentration with a NO assay kit (Nanjing Jiancheng Bioeng Ins, Nanjing, China).
Western Blot
The ovaries were homogenized using 500 μL ice-cold RIPA supplemented with 1 mM phenylmethanesulfonyl fluoride (Beyotime, Shanghai, China) and proteinase inhibitors (Beijing Solarbio Science & Technology Co., Beijing, China). Total protein concentrations were determined using a BCA protein assay kit (Nanjing Jiancheng Bioeng Ins, Nanjing, China). A total of 24 μg protein was separated with SDS-PAGE and transferred to polyvinylidene difluoride (PVDF) membrane (0.22 μm, Millipore, Bedford, USA). After blocking with 5% skim milk, the PVDF membrane was incubated with corresponding primary antibodies including rabbit anti-VEGF (1:500), rabbit anti-p-p38, rabbit anti-Raf1, rabbit anti-PKG-I (1:500, Hangzhou HuaAn Biotechnology Co., Hangzhou, China), rabbit anti‐CCND1 (1:200), rabbit anti‐CDK2 (1:200, Boster Bioengineering Co., Wuhan, China), mouse anti-PCNA (1:1000), or mouse anti‐β‐actin (1:1000, Abcam, Cambridge, UK) at 4°C overnight. Then the PVDF membrane was incubated with the secondary antibody at room temperature for 1 h. Immunological signals were detected by an enhanced chemiluminescence kit (Bio-Rad, Hercules, USA) using a ChemiScope 3400 Mini machine (Clinx, Shanghai, China). The band intensities were quantified with Quantity One Software and the results were normalized to β-actin.
Statistical Analysis
All experiments were repeated at least 3 times. The data were expressed as the mean ± SEM and analyzed by One-way ANOVA with the post hoc Dunnett's test and the independent samples t-test using SPSS 16.0 software (SPSS Inc., Chicago, USA). Results were considered statistically significant when P < 0.05.
RESULTS
Effects of NCG on Follicular Development
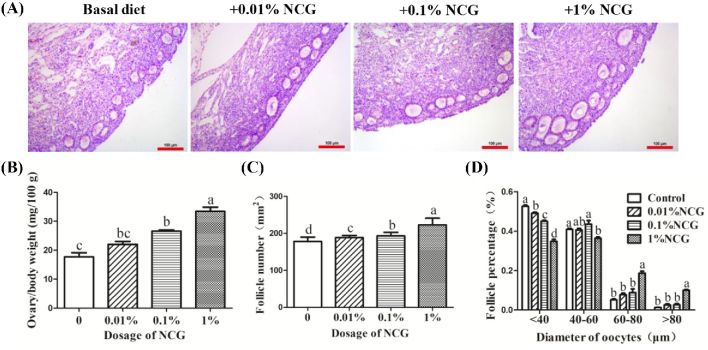
The HE staining of ovarian tissues after supply with different doses of NCG for 14 D showed that NCG promoted follicular development in a dose-dependent pattern (Figure 1A). Diet with NCG can promote the ratio of ovaries/BW, cortex follicle density, and oocyte diameter. Compared with the control group, the ratio of ovary/BW in the 2 groups (0.1% and 1% NCG groups) was increased by 50.2% and 88.8%, respectively (Figure 1B). The follicles density within the cortex in 0.1% and 1% NCG groups were also increased significantly by 8.3% and 24.9% (Figure 1C). The number of follicles (with the oocyte diameter >60 μm) in 1% NCG group was significantly higher than other 3 groups (Figure 1D). Based on these results, the dosage of 1% NCG was adopted in the next experiments.
Figure 1.
Effect of different doses of N-carbamylglutamate (NCG) on follicular development. (A) hematoxylin and eosin (HE) staining was used to evaluate the different doses of NCG on follicular development. Scale bar: 100 μm. (B) The ratio of ovary/BW after NCG administration. (C) The number of follicles in the cortex after NCG administration. (D) The number of follicles with different oocyte diameter. Different lowercase letters indicated significant difference (P < 0.05).
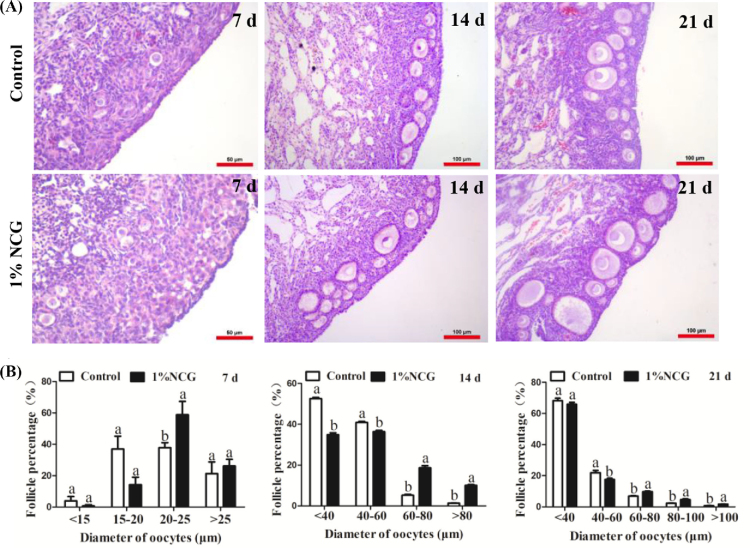
Effects of NCG on Follicular Diameter at Different Feeding Times
There was no significant change in the number of large follicles (with the oocyte diameter >25 μm) after NCG administration for 7 D. However, at day 14 after NCG supplement, the number of follicles (with the oocyte diameter >60 μm) was significantly increased (Figure 2B). Moreover, larger follicle number (with the oocyte diameter 60 ∼ 100 μm or >100 μm) was further increased after NCG feeding at day 21 (Figure 2).
Figure 2.
Effect of N-carbamylglutamate (NCG) on changes in follicular diameter at different feeding time. (A) Ovarian morphology by hematoxylin and eosin (HE) staining after NCG administration at 7 D, 14 D, and 21 D. (B) The number of follicles with different diameter of oocytes. Different lowercase letters indicated significant difference (P < 0.05).
Effects of NCG on Feed Intake, Feed Conversion Ratio, and Plasma AA Levels
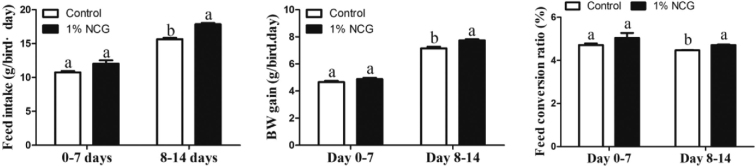
After supplement with 1% NCG, the feed intake, BW gain and feed conversion ratio were increased on days 0 ∼ 14, especially during days 8 ∼ 14 (Figure 3). In addition, supplement with 1% NCG for 14 D, chicken plasma AA were measured. As shown in Table 3, diet with 1% NCG can significantly increase the content of AA including Asp, Thr, Ser, Glu, Gly, Ala, Cys, Val, Met, Ile, Leu, Tyr, Phe, Lys, His, Arg, and Pro when compared with control group (P < 0.05).
Figure 3.
Effect of N-carbamylglutamate (NCG) on feed intake, BW gain, and feed conversion ratio (P < 0.05).
Table 3.
Effect of NCG feeding on plasma AA levels in chickens.
| Control |
1% NCG |
|||
|---|---|---|---|---|
| AA (g/100 g Plasma) | Mean | SEM | Mean | SEM |
| Asp | 0.075b | 0.0011 | 0.095a | 0.0042 |
| Thr | 0.038b | 0.0015 | 0.048a | 0.0012 |
| Ser | 0.041b | 0.0010 | 0.052a | 0.0018 |
| Glu | 0.138b | 0.0027 | 0.171a | 0.0036 |
| Gly | 0.023b | 0.0005 | 0.031a | 0.0014 |
| Ala | 0.041b | 0.0009 | 0.050a | 0.0014 |
| Cys | 0.026b | 0.0010 | 0.037a | 0.0007 |
| Val | 0.039b | 0.0007 | 0.046a | 0.0014 |
| Met | 0.017b | 0.0005 | 0.023a | 0.0011 |
| Ile | 0.030b | 0.0014 | 0.040a | 0.0005 |
| Leu | 0.067b | 0.0023 | 0.081a | 0.0012 |
| Tyr | 0.029b | 0.0012 | 0.039a | 0.0009 |
| Phe | 0.029b | 0.0007 | 0.040a | 0.0007 |
| Lys | 0.045b | 0.0015 | 0.056a | 0.0011 |
| His | 0.018b | 0.0005 | 0.022a | 0.0007 |
| Arg | 0.043b | 0.0009 | 0.057a | 0.0020 |
| Pro | 0.022b | 0.0005 | 0.026a | 0.0008 |
Means within a row with different letters differ (P < 0.05).
Gene Expression Model and Gene Ontology Terms
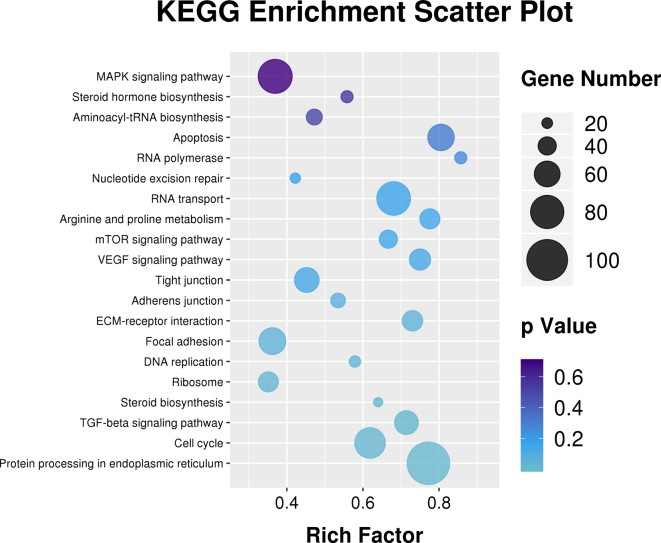
In order to explore the effects of NCG on chicken ovarian development, RNA-seq was employed to analyze the transcript profiles of the control group and treatment group. After removing the low-quality data (including adaptor reads, undetermined reads, and low-quality reads), a total of 320,758,046 (85.79%) clean reads with a Q30-value > 92.00% were retained from 373,890,666 raw reads (Supplementary Table 1). The clustering results showed that the gene expression patterns were highly consistent among 3 duplicate samples in either control or NCG groups, indicating an excellent recurrence and stabilization of data for RNA-seq (Supplementary Figure 1). Analysis of the pathways enriched by differentially expressed genes in control and NCG group according to the KEGG databases showed that cell cycle, DNA replication, VEGF, and apoptosis signaling pathway were all enriched (Figure 4).
Figure 4.
KEGG analysis of differentially expressed genes between control group and N-carbamylglutamate (NCG) group. The size of dot indicated the number of differential genes. The Y-axis and X-axis indicated functional pathways and rich factor.
Effect of NCG on Ovarian Angiogenesis
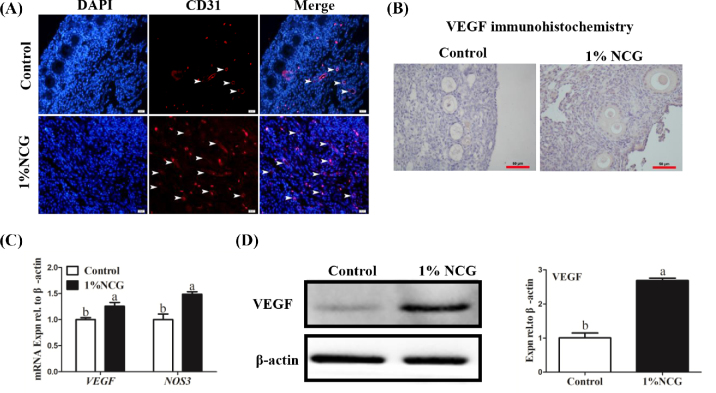
Among all the differently expressed genes analyzed by RNA-seq, we screened some genes involved in angiogenesis, cell proliferation, and apoptosis (Table 4). Compared with the control group, VEGFA, VEGFC, RAF1, mitogen-activated protein kinase 11, cyclin dependent kinase 2 (CDK2), cyclin dependent kinase 3, transforming growth factor beta were upregulated, whereas caspase-3, caspase-7, and H-RAS were downregulated in the NCG group. Next, cluster of differentiation 31 (CD31) was detected IF staining in order to observe the effects of NCG on ovarian angiogenesis. Compared with the control group, the area of CD31-positive staining was remarkably increased in the NCG group (Figure 5A). The location of blood vessels was mainly distributed in the ovarian medulla. Meanwhile, the number of VEGF-positive staining in the ovary was increased in the 1% NCG group (Figure 5B). Furthermore, by RT-qPCR and RNA-seq analysis, VEGF and NOS3 mRNAs (angiogenesis-related) were upregulated significantly in 1% NCG group, compared with the control group (P < 0.05, Figure 5C and Table 4). In addition, expression of VEGF protein was also elevated (Figure 5D). Those results indicated that NCG might promote ovarian angiogenesis by increasing expression of angiogenesis-related genes.
Table 4.
Differentially expressed genes involved in angiogenesis, proliferation, and apoptosis of chicken ovaries.
| Gene ID | log2 FC | Gene symbol | Description |
|---|---|---|---|
| Upregulated genes | |||
| ENSGALG00000008612 | 1.0855 | MAPK11 | Serine/threonine/dual specificity protein kinase |
| ENSGALG00000007130 | 0.56057 | cRac1A | Small GTPase superfamily, ARF/SAR type |
| ENSGALG00000010290 | 0.690431388 | VEGFA | Vascular endothelial growth factor A |
| ENSGALG00000000619 | 1.229192093 | ANGPTL4 | Angiopoietin like 4 |
| ENSGALG00000003943 | 0.61655 | PRKCA | Tyrosine-protein kinase, catalytic domain |
| ENSGALG00000002583 | 0.94258 | PIK3CD | Phosphoinositide 3-kinase, accessory (PIK) domain |
| ENSGALG00000000169 | 0.26220019 | PCNA | Proliferating cell nuclear antigen |
| ENSGALG00000010978 | 0.733954755 | ANGPTL3 | Angiopoietin like 3 |
| ENSGALG00000027798 | 1.07208274 | CDK2 | Cyclin dependent kinase 2 |
| ENSGALG00000010847 | 0.60128 | VEGFC | Vascular endothelial growth factor C |
| ENSGALG00000010346 | 0.394403223 | TGFB3 | TGFB3 |
| ENSGALG00000000883 | 0.29463 | MAPKAPK2 | Tyrosine-protein kinase, catalytic domain |
| ENSGALG00000006014 | 0.73038 | PRKCB | Protein kinase C beta |
| ENSGALG00000027946 | 0.25803602 | NOS3 | Nitric oxide synthase 3 |
| ENSGALG00000001501 | 0.35888 | MAPK1 | Mitogen-activated protein (MAP) kinase, ERK1/2 |
| ENSGALG00000008462 | 0.388697039 | CDK3 | Cyclin dependent kinase 3 |
| ENSGALG00000000826 | 1.5124 | MAPK13 | Mitogen-activated protein (MAP) kinase, p38 |
| ENSGALG00000011620 | 0.42019 | AKT1 | Pleckstrin homology domain |
| ENSGALG00000005065 | 0.42708 | PLA2G4A | Lysophospholipase, catalytic domain |
| ENSGALG00000004998 | 0.27178 | RAF1 | Raf-like Ras-binding |
| ENSGALG00000004290 | 0.469614321 | ANGPTL1 | Angiopoietin like 1 |
| ENSGALG00000007555 | 0.3221956 | CCND1 | Cyclin D1 |
| ENSGALG00000003085 | 0.81682609 | CDK1 | Cyclin dependent kinase 1 |
| ENSGALG00000009062 | 0.304761106 | CDK14 | Cyclin dependent kinase 14 |
| ENSGALG00000006038 | 0.21550712 | TGFBR3 | Transforming growth factor beta receptor 3 |
| Downregulated genes | |||
| ENSGALG00000008933 | −0.32531148 | CASP7 | Peptidase C14, caspase domain |
| ENSGALG00000008934 | −0.62104 | PIK3CA | Phosphoinositide 3-kinase, accessory (PIK) domain |
| ENSGALG00000002859 | −0.39812 | RAC3 | Mitochondrial Rho-like |
| ENSGALG00000014790 | −0.287482558 | CDK7 | Cyclin dependent kinase 7 |
| ENSGALG00000016171 | −0.77253 | FAK | Ubiquitin-related domain |
| ENSGALG00000011442 | −0.117947066 | TGFBR2 | Transforming growth factor beta receptor 2 |
| ENSGALG00000010638 | 0.525196291 | CASP3 | Peptidase C14, caspase domain |
| ENSGALG00000001267 | −0.51013 | MAP2K2 | Mitogen-activated protein kinase 2 |
| ENSGALG00000014786 | −0.69634 | PIK3R1 | Rho GTPase activation protein |
| ENSGALG00000006885 | −1.003 | H-RAS | Small GTPase superfamily, Ras type |
| ENSGALG00000012885 | −0.5992686 | BCL2 | BCL2, apoptosis regulator |
Figure 5.
Effect of N-carbamylglutamate (NCG) on angiogenesis in the ovary. (A) Immunofluorescent staining of CD31. White arrowheads: blood vessels. Scale bar: 50 μm. (B) Immunohistochemistry of vascular endothelial growth factor (VEGF) in the ovaries. Scale bar: 50 μm. (C) Change in VEGF and NOS3 mRNA expression. (D) Relative expression of VEGF protein. Different lowercase letters indicated significant difference (P < 0.05).
Effect of NCG on Proliferation of Ovarian Somatic Cells
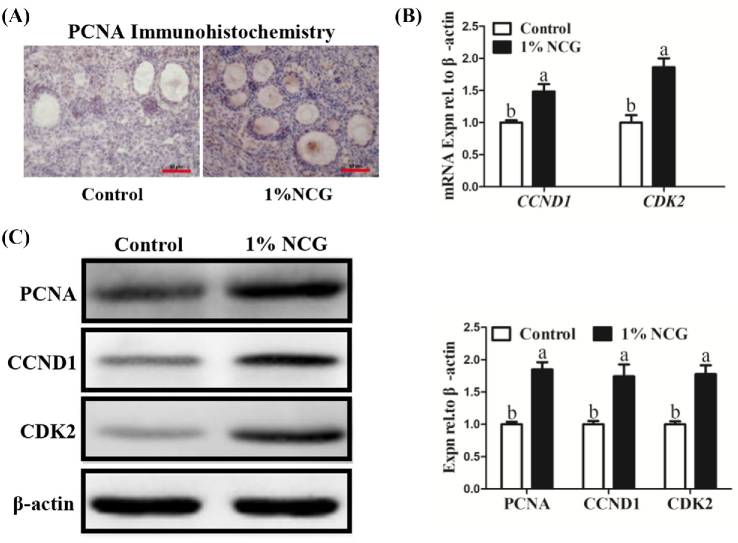
IHC staining of PCNA in the ovarian tissues showed NCG administration increased expression of PCNA protein (Figure 6A). Besides, expression of proliferation-associated gene cyclin D1 (CCND1) and CDK2 were elevated significantly in NCG group, which was consistent with the RNA-seq results (Figure 6B and Table 4). At the same time, the result of Western blot indicated that expression of proliferation-associated proteins (PCNA, CCND1, and CDK2) was increased significantly in NCG group compared with the control group (Figure 6C).
Figure 6.
Effect of N-carbamylglutamate (NCG) on proliferation of ovarian somatic cells. (A) Immunohistochemistry of proliferating cell nuclear antigen (PCNA) in the ovaries. Scale bar: 50 μm. (B) Expression of proliferation-associated gene CCND1 and CDK2 changes. (C) Relative expression of PCNA, CCND1, and CDK2. Different lowercase letters indicated significant difference (P < 0.05).
Effect of NCG on Apoptosis of Ovarian Somatic Cells
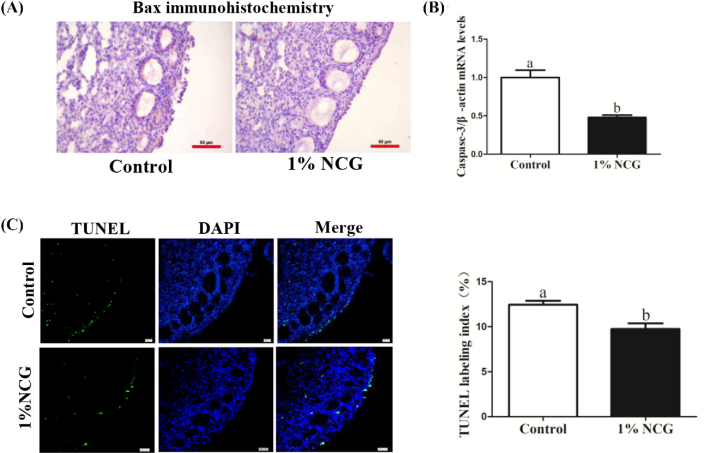
Cell apoptosis related protein of BCL2 associated X (Bax) was detected by IHC staining and caspase-3 gene was detected by RT-qPCR. The result showed that NCG treatment reduced expression of Bax in the ovaries (Figure 7A). Simultaneously, expression of caspase-3 gene was decreased remarkably in the NCG group, which was in accordant with RNA-seq (Figure 7B, Table 4). In addition, a significant difference between control and 1% NCG group was observed with TUNEL assay. TUNEL index was 12.4% in the control group, whereas it was 9.7% in 1% NCG group (Figure 7C). Those data indicated that diet with NCG for 14 D significantly inhibited cell apoptosis in the ovaries.
Figure 7.
Effect of N-carbamylglutamate (NCG) on apoptosis of ovarian somatic cells. (A) Immunohistochemistry of Bax in the ovaries. Scale bar: 50 μm. (B) Expression of apoptosis-associated gene caspase-3 changes. (C) Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) index of ovary tissues. Scale bar: 50 μm. Different lowercase letters indicated significant difference (P < 0.05).
Effect of NCG on Plasma NO Concentration and Expression of PKG-I, Raf1, and Phosphorylation of p38
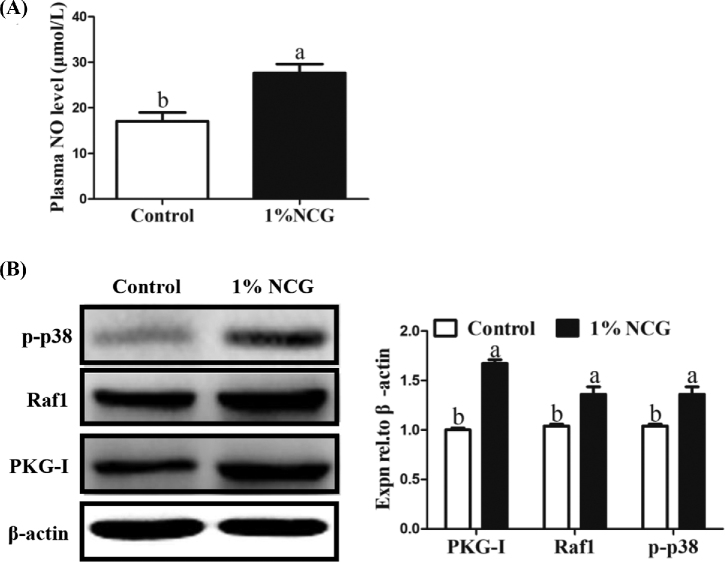
Plasma NO concentration was detected and the result was shown in Figure 8A. Plasma NO was remarkably increased in NCG group compared with the control group (P < 0.05). The proteins of cyclic guanosine monophosphate-dependent protein kinase I (PKG-I), Raf1, and phosphorylated p38 (p-p38) were detected by Western blot and results displayed that expression of PKG-I, Raf1, and p-p38 proteins was all upregulated significantly in 1% NCG group for 14 D (Figure 8B).
Figure 8.
Effect of N-carbamylglutamate (NCG) on changes in plasma NO levels and expression of PKG-I, Raf1, p-p38. (A) Plasma NO levels. (B) Expression of PKG-I, Raf1, and p-p38 proteins. Different lowercase letters indicated significant difference (P < 0.05).
DISCUSSION
In recent years, NCG has been found to improve the reproductive performance in mammals (Zhang et al., 2014). In the intrauterine-growth-retarded Hu suckling lambs, supply with NCG for 21 D led to beneficial effects on growth, gut integrity, immune function, and antioxidant capacity (Zhang et al., 2018). In addition, NCG is able to increase endogenous Arg and muscle leucine production and improve muscle protein synthesis in finishing pigs by activating the mTOR signaling pathway (Ye et al., 2017). Diet with 0.12% or 0.16% NCG can increase the feed conversion ratio and concentration of plasma Arg of mirror carp (Wang et al., 2019). However, any possible effects of NCG on reproductive performance in poultry has been overlooked. In this study, the effect of NCG on follicular development of laying hens was explored. These results showed that, supplementation in the diet with 1% NCG for 14 D resulted in enhanced follicles development. Therefore, addition of NCG in the diet could promote follicles growth in the laying hens.
The growing follicles usually contain a single oocyte, 1 or more layers of granulosa cells, and an outer ring of theca cells. There is a privileged dialogue between the oocyte and its surrounding follicular cells through paracrine action or gap junction. For example, KIT ligand expressed by granulosa/cumulus cell can bind KIT receptor on oocytes to promote formation of primordial follicles in the chicken (Guo et al., 2019). In contrast, growth differentiation factor 9 and bone morphogenetic protein 15 secreted by oocytes can bind the receptors on granulosa cells, and promote the proliferation and differentiation of granulosa/cumulus cell. A previous study reported that NCG treatment increased the number of bovine granulosa cells (Feng et al., 2018). Our study indicated that 1% NCG supplementation increased expression of PCNA, CCND1, and CDK2; and decreased expression of Bax and caspase-3. Meanwhile, TUNEL index in the NCG group was lower than the control group. Therefore, NCG promoted the development of follicles by promoting proliferation and inhibiting apoptosis of the ovarian cells.
Angiogenesis is initiated when the endothelial cells suffer from angiogenic stimuli and lose quiescence. There are many endogenous and exogenous factors that can regulate angiogenesis in the ovarian follicles, including VEGF, angiopoietin, and fibroblast growth factor. VEGF plays an important role in the development of follicles. The percentage of apoptotic cells was significantly decreased when granular cells were cultured in vitro with VEGF (Kosaka et al., 2007). The medium and large antral follicles were absent in the ovaries of marmoset monkeys after injected with VEGF trap (Wulff et al., 2002). Angiogenesis is critical for ovarian development and functions. This study revealed that NCG markedly enhanced VEGF expression. Furthermore, the results of CD31 IHC and IF staining indicated that the number of blood vessels was increased significantly in the 1% NCG group. Simultaneously, we found plasma NO level was increased significantly in the NCG group (P < 0.05). Meanwhile, the results of RNA-seq and Western blot demonstrated the expressions of PKG-I, Raf1 and p-p38 proteins were also upregulated significantly in the NCG group (P < 0.05).
NO can promote angiogenesis and play an important role in ischemic remodeling, mural cell recruitment, and blood flow reserve (Yu et al., 2005). Soluble guanylyl cyclase catalyzes production of cyclic guanosine monophosphate by NO stimulation after VEGF binds with VEGFR2. In turn, cyclic guanosine monophosphate leads to the activation of PKG-I, and the latter improves phosphorylation of mitogen-activated protein kinase cascades (ERK1/2 and p38), which promotes angiogenesis through increasing proliferation, migration, and differentiation of the endothelial cells (Koika et al., 2010). VEGF is able to increase proliferation of human umbilical vein endothelial cells and activate Raf1. This progress depends on NO and its downstream effector, PKG (Hood and Granger, 1998). Thus, increased NO level by NCG may promote ovarian angiogenesis through upgrading expression of PKG-I, Raf1, and p-p38.
As an analogue of NAG, NCG, can increase endogenous Arg production and improve reproductive performance in mammals. Dietary supplementation with NCG improved the expressions of AA transporters Slc6a19, Slc7a9, Slc1a1, and increased intestinal absorptive function in the weaned piglets (Yang et al., 2013). Furthermore, oral supply of glutamate and NCG in combination manifested better favorable effects on intestinal epithelium cell proliferation than glutamate alone (Wu et al., 2012b). In addition, NCG could promote intestinal mucosal immunity in piglets after Escherichia coli challenge, enhance the antioxidant capacity of rat jejunum under oxidative stress (Zhang et al., 2013; Xiao et al., 2016). Moreover, dietary NCG supplementation improve growth performance in yellow-feather broilers through modified homeostasis of Arg metabolism (Hu et al., 2019). These studies indicate NCG is able to enhance intestinal absorption of Arg and then increase NO concentration by improving the structure of the intestine, meliorating the gut microbiome, or increasing the level of the AA transporters in the poultry.
In conclusion, supplementation of 1% NCG increased the feed intake and feed conversion ratio in the chicken. Meanwhile, NCG administration raised plasma Arg and NO concentration, elevated VEGF, PKG-I, Raf1, and p-p38 expression, and eventually promoted ovarian angiogenesis. Simultaneously, the proliferation of ovarian somatic cells was promoted, whereas the apoptosis was inhibited. Ultimately, dietary NCG improved follicular development through enhanced ovarian angiogenesis in the chicken.
NCG administration raised plasma Arg and NO concentrations, elevated VEGF, PKG-I, Raf1, and p-p38 expression, and eventually promoted ovarian angiogenesis. Simultaneously, the proliferation of ovarian somatic cells was promoted, whereas the apoptosis was inhibited. Therefore, dietary NCG administration improved follicular development through enhanced ovarian angiogenesis in the chicken.
ACKNOWLEDGEMENTS
This study was supported by the National Natural Science Foundation of China (Nos. 31772693, 31972635 and 31472160). The authors are grateful to Drs. Jian Li, Xun Tan, and Xingting Liu (Zhejiang University) for help in the experiments.
Footnotes
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.3382/ps/pez545.
Table S1. The raw reads and clean reads obtained by RNA-seq.
Figure S1. The clustering analysis results of gene expression model between control groups (CK_1, CK_2, CK_3) and NCG groups (NCG_1, NCG_2, NCG_3) libraries in chicken ovaries.
Supplementary Material
REFERENCES
- Cai S., Zhu J., Zeng X., Ye Q., Ye C., Mao X., Zhang S., Qiao S., Zeng X. Maternal N-carbamylglutamate supply during early pregnancy enhanced pregnancy outcomes in sows through modulations of targeted genes and metabolism pathways. J. Agric. Food Chem. 2018;66:5845–5852. doi: 10.1021/acs.jafc.8b01637. [DOI] [PubMed] [Google Scholar]
- Feng T., Schutz L.F., Morrell B.C., Perego M.C., Spicer L.J. Effects of N-carbamylglutamate and L-arginine on steroidogenesis and gene expression in bovine granulosa cells. Anim. Reprod. Sci. 2018;188:85–92. doi: 10.1016/j.anireprosci.2017.11.012. [DOI] [PubMed] [Google Scholar]
- Frank J.W., Escobar J., Nguyen H.V., Jobgen S.C., Jobgen W.S., Davis T.A., Wu G. Oral N-carbamylglutamate supplementation increases protein synthesis in skeletal muscle of piglets. J. Nutr. 2007;137:315–319. doi: 10.1093/jn/137.2.315. [DOI] [PubMed] [Google Scholar]
- Guo C., Liu G., Zhao D., Mi Y., Zhang C., Li J. Interaction of follicle-stimulating hormone and stem cell factor to promote primordial follicle assembly in the chicken. Front. Endocrinol. (Lausanne) 2019;10:91. doi: 10.3389/fendo.2019.00091. 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B., Mi Y., Zhang C. Gonadotropins regulate ovarian germ cell mitosis/meiosis decision in the embryonic chicken. Mol. Cell. Endocrinol. 2013;370:32–41. doi: 10.1016/j.mce.2013.02.010. [DOI] [PubMed] [Google Scholar]
- Hood J., Granger H.J. Protein kinase G mediates vascular endothelial growth factor-induced Raf-1 activation and proliferation in human endothelial cells. J. Biol. Chem. 1998;273:23504–23508. doi: 10.1074/jbc.273.36.23504. [DOI] [PubMed] [Google Scholar]
- Hu Y., Shao D., Wang Q., Xiao Y., Zhao X., Shen Y., Zhang S., Tong H., Shi S. Effects of dietary N-carbamylglutamate supplementation on growth performance, tissue development and blood parameters of yellow-feather broilers. Poult. Sci. 2019;98:2241–2249. doi: 10.3382/ps/pey591. [DOI] [PubMed] [Google Scholar]
- Kim D., Lee J., Johnson A.L. Vascular endothelial growth factor and angiopoietins during hen ovarian follicle development. Gen. Comp. Endocrinol. 2016;232:25–31. doi: 10.1016/j.ygcen.2015.11.017. [DOI] [PubMed] [Google Scholar]
- Koika V., Zhou Z., Vasileiadis I., Roussos C., Finetti F., Monti M., Morbidelli L., Papapetropoulos A. PKG-I inhibition attenuates vascular endothelial growth factor-stimulated angiogenesis. Vascul. Pharmacol. 2010;53:215–222. doi: 10.1016/j.vph.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Kosaka N., Sudo N., Miyamoto A., Shimizu T. Vascular endothelial growth factor (VEGF) suppresses ovarian granulosa cell apoptosis in vitro. Biochem. Biophys. Res. Commun. 2007;363:733–737. doi: 10.1016/j.bbrc.2007.09.061. [DOI] [PubMed] [Google Scholar]
- Lea R.G., Andrade L.P., Rae M.T., Hannah L.T., Kyle C.E., Murray J.F., Rhind S.M., Miller D.W. Effects of maternal undernutrition during early pregnancy on apoptosis regulators in the ovine fetal ovary. Reproduction. 2006;131:113–124. doi: 10.1530/rep.1.00844. [DOI] [PubMed] [Google Scholar]
- Li X., Bazer F.W., Johnson G.A., Burghardt R.C., Frank J.W., Dai Z., Wang J., Wu Z., Shinzato I., Wu G. Dietary supplementation with L-arginine between days 14 and 25 of gestation enhances embryonic development and survival in gilts. Amino Acids. 2014;46:375–384. doi: 10.1007/s00726-013-1626-6. [DOI] [PubMed] [Google Scholar]
- Lin X., Ma Y., Qian T., Yao J., Mi Y., Zhang C. Basic fibroblast growth factor promotes prehierarchical follicle growth and yolk deposition in the chicken. Theriogenology. 2019;139:90–97. doi: 10.1016/j.theriogenology.2019.07.025. [DOI] [PubMed] [Google Scholar]
- Liu X., Lin X., Mi Y., Li J., Zhang C. Grape seed proanthocyanidin extract prevents ovarian aging by inhibiting oxidative stress in the hens. Oxid. Med. Cell. Longev. 2018;2018:1–16. doi: 10.1155/2018/9390810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateo R.D., Wu G., Bazer F.W., Park J.C., Shinzato I., Kim S.W. Dietary l-arginine supplementation enhances the reproductive performance of gilts. J. Nutr. 2007;137:652–656. doi: 10.1093/jn/137.3.652. [DOI] [PubMed] [Google Scholar]
- Moonmanee T., Navanukraw C., Uriyapongson S., Kraisoon A., Aiumlamai S., Guntaprom S., Rittirod T., Borowicz P.P., Redmer D.A. Relationships among vasculature, mitotic activity, and endothelial nitric oxide synthase (eNOS) in bovine antral follicles of the first follicular wave. Domest. Anim. Endocrinol. 2013;45:11–21. doi: 10.1016/j.domaniend.2013.03.002. [DOI] [PubMed] [Google Scholar]
- Moraes T.G.V., Pishnamazi A., Wenger I.I., Renema R.A., Zuidhof M.J. Energy and protein dilution in broiler breeder pullet diets reduced offspring body weight and yield. Poult. Sci. 2019;98:2555–2561. doi: 10.3382/ps/pey603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessaro I., Luciano A.M., Franciosi F., Lodde V., Corbani D., Modina S.C. The endothelial nitric oxide synthase/nitric oxide system is involved in the defective quality of bovine oocytes from low mid-antral follicle count ovaries. J. Anim. Sci. 2011;89:2389–2396. doi: 10.2527/jas.2010-3714. [DOI] [PubMed] [Google Scholar]
- Wang L., Li J., Wang C., Zhao Z., Luo L., Du X., Xu Q. Effect of N-carbamoylglutamate supplementation on the growth performance, antioxidant status and immune response of mirror carp (Cyprinus carpio) fed an arginine-deficient diet. Fish Shellfish Immunol. 2019;84:280–289. doi: 10.1016/j.fsi.2018.10.001. [DOI] [PubMed] [Google Scholar]
- Willems E., Guerrero-Bosagna C., Decuypere E., Janssens S., Buyse J., Buys N., Jensen P., Everaert N. Differential expression of genes and DNA methylation associated with prenatal protein undernutrition by albumen removal in an avian model. Sci. Rep. 2016;6 doi: 10.1038/srep20837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Ruan Z., Gao Y., Yin Y., Zhou X., Wang L., Geng M., Hou Y., Wu G. Dietary supplementation with L-arginine or N-carbamylglutamate enhances intestinal growth and heat shock protein-70 expression in weanling pigs fed a corn- and soybean meal-based diet. Amino Acids. 2010;39:831–839. doi: 10.1007/s00726-010-0538-y. [DOI] [PubMed] [Google Scholar]
- Wu X., Yin Y.L., Liu Y.Q., Liu X.D., Liu Z.Q., Li T.J., Huang R.L., Ruan Z., Deng Z.Y. Effect of dietary arginine and N-carbamoylglutamate supplementation on reproduction and gene expression of eNOS, VEGFA and PlGF1 in placenta in late pregnancy of sows. Anim. Reprod. Sci. 2012;132:187–192. doi: 10.1016/j.anireprosci.2012.05.002. [DOI] [PubMed] [Google Scholar]
- Wu X., Zhang Y., Liu Z., Li T.J., Yin Y.L. Effects of oral supplementation with glutamate or combination of glutamate and N-carbamylglutamate on intestinal mucosa morphology and epithelium cell proliferation in weanling piglets. J. Anim. Sci. 2012;90:337–339. doi: 10.2527/jas.53752. [DOI] [PubMed] [Google Scholar]
- Wulff C., Wilson H., Wiegand S.J., Rudge J.S., Fraser H.M. Prevention of thecal angiogenesis, antral follicular growth, and ovulation in the primate by treatment with vascular endothelial growth factor Trap R1R2. Endocrinology. 2002;143:2797–2807. doi: 10.1210/endo.143.7.8886. [DOI] [PubMed] [Google Scholar]
- Xia W., Fouad A.M., Chen W., Ruan D., Wang S., Fan Q., Wang Y., Cui Y., Zheng C. Estimation of dietary arginine requirements for Longyan laying ducks. Poult. Sci. 2017;96:144–150. doi: 10.3382/ps/pew205. [DOI] [PubMed] [Google Scholar]
- Xiao L., Cao W., Liu G., Fang T., Wu X., Jia G., Chen X., Zhao H., Wang J., Wu C., Cai J. Arginine, N-carbamylglutamate, and glutamine exert protective effects against oxidative stress in rat intestine. Anim. Nutr. 2016;2:242–248. doi: 10.1016/j.aninu.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H.S., Fu D.Z., Kong K.F., Wang W.C., Yang X.J., Nyachoti C.M., Yin Y.L. Dietary supplementation with N-carbamylglutamate increases the expression of intestinal amino acid transporters in weaned Huanjiang mini-pig piglets. J. Anim. Sci. 2013;91:2740–2748. doi: 10.2527/jas.2012-5795. [DOI] [PubMed] [Google Scholar]
- Ye C., Zeng X., Zhu J., Liu Y., Ye Q., Qiao S., Zeng X. Dietary N-carbamylglutamate supplementation in a reduced protein diet affects carcass traits and the profile of muscle amino acids and fatty acids in finishing pigs. J. Agric. Food Chem. 2017;65:5751–5758. doi: 10.1021/acs.jafc.7b02301. [DOI] [PubMed] [Google Scholar]
- Yu J., deMuinck E.D., Zhuang Z., Drinane M., Kauser K., Rubanyi G.M., Qian H.S., Murata T., Escalante B., Sessa W.C. Endothelial nitric oxide synthase is critical for ischemic remodeling, mural cell recruitment, and blood flow reserve. Proc. Natl. Acad. Sci. USA. 2005;102:10999–11004. doi: 10.1073/pnas.0501444102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Yao H., Gao X., Zhang Z., Wang J.-F., Xu S.-W. The role of nitric oxide and oxidative stress in intestinal damage induced by selenium deficiency in chickens. Biol. Trace Elem. Res. 2015;163:144–153. doi: 10.1007/s12011-014-0164-8. [DOI] [PubMed] [Google Scholar]
- Zeng X., Huang Z., Mao X., Wang J., Wu G., Qiao S. N-carbamylglutamate enhances pregnancy outcome in rats through activation of the PI3K/PKB/mTOR signaling pathway. PLoS One. 2012;7 doi: 10.1371/journal.pone.0041192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Che L.Q., Lin Y., Zhuo Y., Fang Z.F., Xu S.Y., Song J., Wang Y.S., Liu Y., Wang P., Wu D. Effect of dietary N-carbamylglutamate levels on reproductive performance of gilts. Reprod. Domest. Anim. 2014;49:740–745. doi: 10.1111/rda.12358. [DOI] [PubMed] [Google Scholar]
- Zhang F., Zeng X., Yang F., Huang Z., Liu H., Ma X., Qiao S. Dietary N-carbamylglutamate supplementation boosts intestinal mucosal immunity in Escherichia coli challenged piglets. PLoS One. 2013;8 doi: 10.1371/journal.pone.0066280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Zhao F., Peng A., Dong L., Wang M., Yu L., Loor J.J., Wang H. Effects of dietary l-arginine and N-carbamylglutamate supplementation on intestinal integrity, immune function, and oxidative status in intrauterine-growth-retarded suckling lambs. J. Agric. Food Chem. 2018;66:4145–4154. doi: 10.1021/acs.jafc.8b00726. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.