Abstract
Previous work has identified an effect of hatch time on chick femur mineralization. This experiment assessed the impact of hatch time and a 24-h post-hatch unfed time period on chick bone mineralization and yolk mineral utilization. In early hatching chicks, yolk Mg, Zn, K, P, Fe, and Cu decreased by 40 to 50% over the 24-h post-hatch unfed time period, whereas yolk Ca and Na decreased by 25 to 40% (P = 0.026). Yolk Sr was intermediate, decreasing by 37%. Late hatching chicks which had been hatched for no more than 30 h had a higher femur bone ash percentage compared to early hatching chicks which had spent over a 30-hour sojourn unfed in the incubator (P = 0.013). These results indicate that removing chicks from the incubator within 30 h of their hatch is likely to benefit their femoral mineralization.
Key words: hatch time, bone ash, yolk, chick, mineral
Introduction
Production improvements in modern meat chickens via genetic selection and advanced husbandry practices have resulted in rapidly growing birds. Between 1957 and 2005, the growth rate of meat chickens increased by more than 400% (Zuidhof et al. 2014). In 2015, the target for male Cobb 500 birds was 3 kg liveweight at 6 wk of age (Cobb-Vantress, 2015), which is a typical lifespan for these birds. In commercial hatchery operations, standard practice is to transfer eggs to a hatcher incubator at embryonic day (ED) 18 and then remove all chicks from the hatcher incubator (commonly referred to as take-off) at ED21.5. Incubation therefore represents one-third of production time from the start of incubation to slaughter at 6 wk of age (Muir and Groves, 2018). Consequently, events that occur during incubation and, in the immediate post-hatch period, can have an impact on chick quality and production throughout their 6-wk grow out.
One day before hatching, chicks draw the remnant yolk sac and the membrane surrounding it into their abdominal cavity, forming the residual yolk sac (Moran, 2007). This is the only source of nutrition for newly hatched chicks until exogenous food is provided (Mitchell and Kettlewell, 2009). Nutritional requirements of the chick immediately after hatching are poorly understood (De Jong et al., 2017). Traditionally, standard practice has been completely reliant on the yolk sac for the provision of nutrients until placement of birds on farm (Mitchell and Kettlewell, 2009). The emergence of post-hatch care incubation systems (Van de Ven et al., 2009, Molenaar et al., 2014, Van Roovert-Reijrink, 2014) and neonatal supplements (Batal and Parsons, 2002) demonstrates the growing interest in providing young chicks with access to exogenous sources of nutrition as soon as possible after their emergence from the egg.
In conventional hatching systems, the chick hatch window begins around ED19.5 (Tona et al., 2003, Groves and Muir, 2017) and continues until chick takes off from the hatcher, typically at ED21.5, resulting in a proportion of chicks spending up to 48 h in the hatcher tray without food and water. This unfed and unwatered time period is notionally referred to as their sojourn. Hence, a batch of recently hatched chicks will consist of birds with comparatively shorter or longer sojourns, depending on when they emerged from the egg (Careghi et al., 2005, Groves and Muir, 2017, Muir and Groves, 2019). Hatchery procedures such as chick grading and vaccination, followed by their transport to the grower farm, can extend the period a chick is without food and water for up to a further 24 h (Tona et al., 2003, Powell et al., 2016). Hence, the earliest hatching chicks may spend up to 72 h from their hatch until they have access to food and water on the grower farm. Careghi et al. (2005) demonstrated that a 48-h delay in feeding reduced the relative growth of chicks compared to chicks that had immediate access to food. Powell et al. (2016) identified impaired muscle growth when access to food was delayed by 24 h after hatching, and these observations have been confirmed by a meta-analysis (De Jong et al., 2017), where the absence of food and water for 24 h or longer was seen to have a negative effect on body weight gain and food intake. Some studies indicate that the advantage gained by early access to food is limited to the first few weeks after hatching (Hollemans et al., 2018).
Reduced locomotor ability as a result of leg weakness is an important production and welfare issue in modern meat chicken production (Bessei, 2006, Knowles et al., 2008). During embryonic and early chick growth in the absence of hatching supplements, the chick is sourcing all of its nutrients from the yolk sac (Uni et al., 2012). This also coincides with the period of highest cartilage and bone development in the bird (Angel, 2007; Van der Pol et al., 2014). However, it is not known whether the residual yolk sac contains the balanced source of minerals required for optimal bone mineralization in the early post-hatch period. In this regard, Muir and Groves (2018) demonstrated that early hatching chicks, that had hatched by 492 h (20.5 D) of incubation, which then had a 24-h delay in access to food and water, had significantly lower femoral bone ash, compared to early hatching chicks that were provided with food and water shortly after hatching.
Although the characteristics of the profile of mineral absorption from the yolk during incubation have been explored (Johnston and Comar, 1955, Richards and Packard, 1996, Yair and Uni, 2011, Hopcroft et al., 2019), our understanding of the absorption of minerals from the residual yolk sac in newly hatched chicks, and how this impacts their bone development, is limited.
Therefore, the aims of this experiment were to assess the impact of chick hatch time and the length of the chick's sojourn in the hatcher tray on chick traits, including chick weight, chick length, femoral bone ash, serum Ca and P, residual yolk weight, and yolk mineral reserves. The latter will also furnish benchmark values for residual yolk mineral levels in early or late hatching chicks, including the effect of the duration of their sojourn. This knowledge will offer opportunities to improve chick management during the immediate post-hatch stages of meat chicken production.
Materials and methods
All experimental procedures were approved by the University of Sydney Animal Ethics Committee (protocol number 2014/729) and strictly complied with the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes as prepared by the National Health and Medical Research Council (2013).
Incubation
Eight hundred sixty-four Cobb 500 meat chicken eggs, that had been laid by 53-week-old breeder birds, were obtained from a commercial hatchery (Cordina Hatchery, Greystanes, NSW) and stored in a room held at 17°C for 5 D.
The eggs were incubated as outlined in the study by Hopcroft et al. (2019) and were from the same cohort as eggs reported on in that work. Briefly, each egg was identified by a unique number pencilled onto the shell, weighed, and then randomly placed into one of 6 288-egg-capacity incubators (E2A - Multiquip Pty Limited, Austral, New South Wales, Australia) (144 eggs per incubator distributed on 4 trays of 36 eggs), for setting (i.e., the period from ED0 until ED17.5, or 420 h of incubation [HOI]). During the setting phase, the eggshell temperature (EST) of one egg on each tray in each incubator was monitored using Remote Intelligent Multisensors (TSIC716 Advanced Sensor Technology; Netic Pty Ltd., Ryde, NSW, Australia). Depending on the EST readings, the incubator temperature was manually adjusted to maintain an EST of 37.8°C throughout the first 17.5 D of the setting phase. Egg shell temperature was consistently within 0.2°C of 37.8°C. Relative humidity was maintained between 50 and 60% throughout by evaporation. Each tray of eggs was automatically turned 90° each hour.
Eggs were candled on ED16.5 (396 HOI), at which time 116 nonviable eggs were removed. On ED17.5 (420 HOI), each egg was weighed and transferred into one of 8 hatching trays that were each divided into 60 individually identified hatching cells, allowing individual identification of hatched chicks. The hatcher trays were placed in a randomized fashion into one Aussieset incubator (Bellsouth Pty Ltd., Victoria, Australia) at an initial air temperature of 37.2°C and 60% relative humidity. Air temperature was dropped to 36.0°C by ED21 and relative humidity raised to 65%. The difference between ED0 and ED17.5 (420 HOI) egg weight was used to calculate the percentage weight loss of individual eggs during the setting phase.
Hatch Time
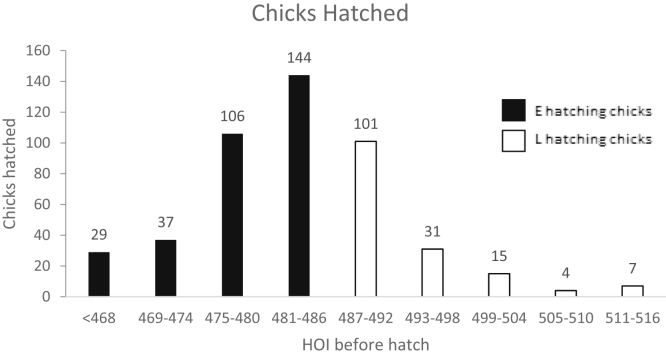
Observation of individual chick hatch time was determined on 9 occasions by opening the incubator, pulling out each tray, and recording which chicks had hatched. The first observation was made at ED19.5 (468 HOI), and subsequent observations were undertaken every subsequent 6 h (i.e., ED19.75, 20, 20.25, 20.5, 20.75, 21, 21.25; 474, 480, 486, 492, 498, 504, 510 HOI) with a final observation at ED21.5 (516 HOI). From this information, each chick was assigned to its hatch window (Figure 1). Chicks were only considered hatched once they had cleared the shell and their down was dry.
Figure 1.
Number of newly hatched chicks, every 6 h, between 468 and 516 h of incubation. HOI, hours of incubation; E, chicks hatching by 486 HOI; L, chicks hatching between 487 and 516 HOI.
Chick Sampling
For the purposes of this experiment, birds hatching ≤ ED 20.25 (486 HOI) were classified as early hatching chicks (E), and birds hatching >20.25-21.5 ED (>486–516 HOI) were classified as late hatching chicks (L). Chicks were selected for collection of biological samples at 2 time points: ED20.5 (492 HOI) and ED21.5 (516 HOI). The combination of hatch time (E or L) and the length of the sojourn due to the 2 different sample times (either <30 h or 30 + h sojourn) generated 3 treatment groups of chicks for sampling. They are as follows: E chicks sampled at ED20.5 (492 HOI), that is, early hatching chicks that had a sojourn time of less than 30 h (identified as E < 30, n = 24); E chicks sampled at ED21.5 (516 HOI), that is, early hatching chicks that had a sojourn time between 30 and 48 h (identified as E30+, n = 46); and L chicks sampled at ED21.5 (516 HOI), that is, late hatching chicks that had a sojourn time of under 30 h (identified as L < 30, n = 29). As all remaining chicks were taken off from the hatcher incubator at ED21.5 (as is typical of commercial hatchery procedures, and in accordance with the animal ethics approval for this experiment), a group of late hatching chicks with a sojourn time of more than 30 h was not available.
Chicks were selected so that the chicks in a sample group had hatch times proportional to the general population. Within this structure, chicks were selected randomly. Only chicks which had hatched by ED20.25 (486 HOI) were selected for sampling at ED20.5. Before sampling, it was decided that chicks which had just hatched, that is, from the ED20.5 (492 HOI) hatch group, would not be sampled at ED20.5 (492 HOI).
Collection of biological samples from chicks followed the procedures described by Muir and Groves (2018). Chicks to be sampled were identified and then removed from the incubator and weighed, and blood was collected from the jugular vein using a 1 mL syringe and ½ inch, 27-gauge needle, into SST tube and allowed to clot before being centrifuged to retrieve serum. Each chick was then humanely euthanized, and their length (from the top of the toenail on the middle toe to the tip of the beak) was recorded. The residual yolk sac (including yolk sac membrane) was removed, weighed, and frozen for later mineral analysis. The sex of each bird was recorded, and the right leg was removed for femoral bone analysis. After the second sample time at ED21.5, any remaining chicks were removed from the incubator and weighed.
Laboratory Procedures
Bone Ash
Femur samples were cleaned of any loose tissue with a paper towel. The femur was weighed, to give total bone weight. The femur was then held in a drying oven at 105°C for 24 h, following which the dry bone weight was recorded. Next, the femur was placed into a muffle furnace at 200°C, and the temperature increased by 100°C increments with corresponding time pause, until 600°C. After 8 h, samples were removed from the furnace and allowed to cool in a desiccator and ash weights were recorded (Muir and Groves, 2018).
The dry bone weight was subtracted from the whole bone weight to give the bone water weight for each bird. The ash weight was subtracted from the dry bone weight to determine the amount of nonmineral substance in the bone, called bone ‘organic’ weight. The ash weight itself was taken as the bone mineral weight. The ash weight was also calculated as a percentage of the dry bone weight to give the bone ash percentage.
Serum Analysis
Calcium and phosphorus concentration was determined in serum as described by Groves and Muir (2014). These assays were conducted by The University of Sydney, Veterinary Teaching Hospital, Camden. After clotting, blood samples were centrifuged at 1,455 × g for 10 min at 4°C and the serum was retrieved. Total Ca was estimated using the metallochromagen Arsenazo III reagent (Catalogue No. TR29226, Thermo Fisher Scientific Inc., Middletown, VA, USA) which forms a colored chromophore with Ca ions at pH 6.75, measured at 650 nm. Inorganic P concentration was measured using an inorganic P reagent (direct UV method without reduction) producing unreduced phosphomolybdate and measured at 340 nm (Catalogue No. TR30026; Thermo Fisher Scientific Inc., Middleton, Virginia, USA). Colorimetric assessments were conducted using a Konelab 20 XTi (Thermo Electron, Waltham, Massachusetts, USA) clinical chemistry analyzer.
Yolk Mineral Analysis
To assess the concentration of Ca, Cu, Fe, K, Mg, Mn, Na, P, Sr, and Zn in the residual yolk sac, the samples were handled as described by Hopcroft et al., (2019). Briefly, each individual yolk sac was homogenized, weighed, and freeze-dried and the dried yolk weight was recorded. Five hundred milligrams of each freeze-dried yolk sample was mixed with 5 mL of nitric acid, covered with a watch glass, and heated at 50°C for 2 h, then at 90°C for 30 min, before being cooled to room temperature. Two milliliters of hydrogen peroxide was added, and the sample was reheated to 90°C for 10 min and then 120°C for 30 min. Finally, each sample was diluted to 30 mL with distilled water before being analyzed via inductively coupled plasma optical emission spectroscopy at the University of New South Wales (iCAP 6000 Series; Thermo Electron Corporation, Waltham, MA, USA) according to manufacturer's instructions. The concentration of each mineral in the yolk was then adjusted to the absolute amount of mineral multiplying it by the dried yolk weight. Finally, dry yolk weight was subtracted from fresh yolk weight to give an approximate measure of the water content of the residual yolk sac.
Statistical Analysis
Data were analyzed with the SAS Enterprise Guide 6.1 software package (SAS Institute Inc., Cary, NC, USA).
Dividing hatched chicks into E and L groups allowed retrospective identification of eggs which produced E and L chicks. The egg weight at ED0 and ED17.5, the percentage of egg weight loss from ED0 until ED17.5, and ED21.5 chick weights were subjected to a Student's t-test using hatch group E or L as the treatment factor.
Experimentally collected biological data from sampled chicks were subjected to a one-way ANOVA using the groups E < 30, E30+, and L < 30. Tukey's HSD post hoc test was applied to separate significantly different means at P < 0.05.
To determine and compare the approximate change in sampled chick yolk and bone data over a 24-h-unfed sojourn, individual values from E30+ birds were first subtracted from the corresponding E < 30 average value (e.g., the femur mineral weight values for each E30+ bird were subtracted from the reported average E < 30 femur mineral weight result). These values were then divided by the E < 30 average value to give individual E30 + chicks an approximate percentage change of that value after a 24-h-unfed sojourn in the hatcher.
The calculated change values were then subjected to one-way ANOVAs using the different parameters as treatment groups; that is, the change in yolk solids, water content, dry weight, and absolute mineral weights were compared against each other, and the whole bone, water, organic, and mineral weights were compared against each other. Tukey's HSD post hoc test was applied to separate significantly different means at P < 0.05.
Results
Incubation
E eggs weighed 66.15 (±0.23) g at ED0, and 59.14 (±0.23) g at ED17.5 (420 HOI) (Table 1). This was lower than the weight of L eggs at the same time points (67.07 (±0.33)) (P = 0.02) and 60.24 (±0.32) g (P = 0.005), respectively. The average percentage egg weight loss between ED0 and ED17.5 for E eggs was 10.6% and for L eggs, 10.2% (P = 0.06; Table 1).
Table 1.
Egg weights and egg weight loss during incubation (±SE).
| Sample group | E | L | P |
|---|---|---|---|
| Initial egg weight (g) | 66.15 (±0.23) | 67.07 (±0.33) | 0.02 |
| Egg weight at embryonic day 17.5 (g) | 59.14 (±0.23) | 60.24 (±0.32) | 0.005 |
| Egg weight loss during incubation (%) | 10.6 | 10.2 | 0.06 |
Abbreviations: E, chicks hatching by 486 HOI; L, chicks hatching between 487 and 516 HOI.
Hatch Time
The number of chicks that hatched within each 6 h window, starting from ED19.5 (468 HOI), is shown in Figure 1. Twenty-nine chicks had hatched by ED19.5 (468 HOI), but 83% of chicks hatched between ED19.75 and 20.5 (474-492 HOI). Chicks were then divided according to the period in which they hatched; birds hatching by ED20.25 (486 HOI) were identified as the early hatching group (E), and birds hatching after ED20.25 and by ED21.5 (516 HOI) were the late hatching group (L). Sixty-seven percent of hatched chicks hatched E, that is, ≤ ED20.25, and 33% hatched L, that is, > ED20.25 – ED21.5.
Chick Sampling
The results from chick sampling are shown in Table 2. E < 30 and L < 30 birds were heavier at their respective sample times than E30 + birds (45.73; 45.97 and 43.28 g, respectively, P = 0.002). Yolk-free body weight did not differ (P = 0.426) between the 3 treatment groups. At 19.12 cm long, E < 30 birds were shorter (P = 0.007) than E30+ (19.77 cm) and L < 30 (19.67 cm) birds.
Table 2.
Biological observations of sampled chicks (±SE).
| Sample group | E < 30 | E30+ | L < 30 | P |
|---|---|---|---|---|
| Initial egg weight (g) | 65.84 (±0.57) | 65.98 (±0.63) | 66.92 (±0.81) | 0.526 |
| Chick weight(g) | 45.73a (±0.6) | 43.28b (±0.51) | 45.97a (±0.72) | 0.002 |
| Yolk-free body weight (g) | 39.08 (±0.46) | 39.44 (±0.46) | 40.15 (±0.61) | 0.426 |
| Chick length (cm) | 19.12b (±0.11) | 19.77a (±0.09) | 19.67a (±0.2) | 0.007 |
| Femur whole bone weight (mg) | 259 (±5.2) | 263 (±4.7) | 273 (±6.2) | 0.678 |
| Femur water weight (mg) | 190 (±4.1) | 195 (±0.3.6) | 202 (±4.7) | 0.632 |
| Femur organic weight (mg) | 52.9 (±1.1) | 53.4 (±1.0) | 55.1 (±1.3) | 0.794 |
| Femur mineral weight (mg) | 16.1 (±0.2) | 15.5 (±0.3) | 16.1 (±0.3) | 0.695 |
| Femur bone ash % | 24.8a,b (±2.5) | 22.9b (±3.3) | 25.5a (±3.5) | 0.013 |
| Serum Ca (mmol/L) | 2.32 (±0.03) | 2.35 (±0.02) | 2.27 (±0.03) | 0.088 |
| Serum P (mmol/L) | 1.82a (±0.05) | 1.75a,b (±0.03) | 1.65b (±0.05) | 0.041 |
| Yolk weight (g) | 6.77a (±0.23) | 3.85c (±0.18) | 5.82b (±0.24) | <0.001 |
| Yolk water content (g) | 3.51a (±0.11) | 2.1c (±0.09) | 3.05b (±0.11) | <0.001 |
| Yolk solids (g) | 3.26a (±0.13) | 1.75c (±0.1) | 2.77b (±0.14) | <0.001 |
| PCV | 31.6 (±0.78) | 30.0 (±0.5) | 29.9 (±0.68) | 0.174 |
a,b,cMeans within a row with different letter superscripts differ significantly (P < 0.05).
Abbreviations: E < 30, chicks that hatched by ≤ 486 h of incubation, with a sojourn in the hatcher tray of less than 30 h; E30+, chicks that hatched by ≤ 486 h of incubation, with a sojourn in the hatcher tray of between 30 and 48 h; L < 30, chicks that hatched from >486 until 516 h of incubation, with a sojourn in the hatcher tray of less than 30 h.
Femur whole bone, water, organic, and mineral weights did not significantly differ between treatments (P = 0.678, 0.632, 0.794, and 0.695, respectively, Table 2). Numerically, whole bone, water, and organic weights were lightest for E < 30, heaviest for L < 30, and intermediate for E30+. For femur mineral weight, E30+ was the lightest and E < 30 and L < 30 equally heavier.
L < 30 birds had higher femoral bone ash at sampling (25.5%) compared to E30+ (22.9%) birds, with E < 30 birds intermediate (24.8%) (P = 0.013; Table 2).
The rate of change between each bone weight measurement did not differ significantly (P = 0.545). Numerically, bone water content increased by 2.5%, whole bone weight increased by 1.9%, bone organic component increased by 1.0%, and bone mineral content decreased by 3.5%.
Serum Ca was not significantly different between the 3 treatments at sampling (P = 0.088), but serum Ca tended to be lower in L < 30 birds compared to E < 30 and E30 + birds. Serum P was highest in E < 30 birds and lowest in L < 30 birds (P = 0.041). Yolk weight, yolk water content, and the weight of the yolk solids were highest in E < 30 birds, intermediate in L < 30 birds, and lowest in E30 + birds (P < 0.001 in all instances).
The concentrations of Ca, Cu, Mn, and Sr in the yolk were higher in E30 + chicks than in E < 30 and L < 30 birds (P < 0.025) (Table 3). The yolk concentration of Na was higher in E30 + and L < 30 compared to the E < 30 chicks (P < 0.001). Iron and K concentrations in the yolk tended to be higher in E30 + birds compared to E < 30 and L < 30 (P = 0.152, 0.234, respectively). Yolk Mg, P, and Zn concentrations were similar in all treatment groups. The total amount of the selected minerals in the yolk of the 3 treatment groups are displayed in Table 4. Total yolk Ca and Na were higher in E < 30 and L < 30 compared to E30 + chicks (P < 0.001), while total yolk Cu, Fe, and K were highest in E < 30, intermediate in L < 30, and lowest in E30 + chicks (P < 0.001). The total amounts of Mg, P, Sr, and Zn were lower in E30 + compared to those in both E < 30 and L < 30 chicks (P < 0.001) but were present in similar quantities in the latter 2 treatments. The amount of Mn present in the yolk tended to be higher in L < 30 birds (P = 0.157), with E < 30 slightly higher then E30 + chicks.
Table 4.
Mean mineral total content in residual yolk (±SE).
| Mineral | E < 30 | E30+ | L < 30 | P |
|---|---|---|---|---|
| Ca (mg) | 42.5a (±1.5) | 31.6b (±1.3) | 40.3a (±1.7) | <0.001 |
| Cu (μg) | 4.1a (±0.21) | 2.3c (±0.11) | 3.2b (±0.18) | <0.001 |
| Fe (mg) | 0.076a (±0.008) | 0.057c (±0.011) | 0.063b (±0.006) | <0.001 |
| K (mg) | 4.49a (±0.238) | 2.43c (±0.09) | 3.71b (±0.253) | <0.001 |
| Mg (mg) | 1.14a (±0.068) | 0.58b (±0.039) | 0.98a (±0.078) | <0.001 |
| Mn (μg) | 7.8 (±0.62) | 7.5 (±0.58) | 9.3 (±0.72) | 0.072 |
| Na (mg) | 4.0a (±0.21) | 2.86b (±0.17) | 4.12a (±0.28) | <0.001 |
| P (mg) | 20.49a (±1.07) | 11.33b (±0.85) | 17.21a (±1.24) | <0.001 |
| Sr (μg) | 23.4a (±1.26) | 14.8b (±0.86) | 19.8a (±1.12) | <0.001 |
| Zn (mg) | 0.13a (±0.007) | 0.067b (±0.005) | 0.107a (±0.009) | <0.001 |
a,b,cMeans within a row with different letter superscripts differ significantly (P < 0.001).
Abbreviations: E < 30, chicks that hatched by ≤ 486 h of incubation, with a sojourn in the hatcher tray of less than 30 h; E30+, chicks that hatched by ≤ 486 h of incubation, with a sojourn in the hatcher tray of between 30 and 48 h; L < 30, chicks that hatched from >486 until 516 h of incubation, with a sojourn in the hatcher tray of less than 30 h.
Table 3.
Mean mineral concentration in residual yolk (±SE).
| Mineral (mg/kg) | E < 30 | E30+ | L < 30 | P |
|---|---|---|---|---|
| Ca | 13562b (±777) | 19849a (±1,124) | 15137b (±664) | <0.001 |
| Cu | 1.27b (±0.04) | 1.37a (±0.05) | 1.19b (±0.04) | 0.011 |
| Fe | 22.8 (±1.8) | 31 (±3.7) | 23.9 (±2.6) | 0.152 |
| K | 1,392 (±56) | 1,487 (±57) | 1,353 (±61) | 0.234 |
| Mg | 350 (±17) | 345 (±18.6) | 345 (±15.9) | 0.981 |
| Mn | 2.44b (±0.22) | 4.70a (±0.52) | 3.14b (±0.73) | <0.001 |
| Na | 1260b (±71) | 1653a (±45) | 1491a (±65) | <0.001 |
| P | 6,233 (±177) | 6,288 (±229) | 6,081 (±177) | 0.784 |
| Sr | 7.36b (±0.427) | 9.04a (±0.552) | 7.38b (±0.398) | 0.025 |
| Zn | 40 (±1.85) | 38.4 (±2) | 37.9 (±2.41) | 0.814 |
a,b,cMeans within a row with different letter superscripts differ significantly (P < 0.05).
Abbreviations: E < 30, chicks that hatched by ≤ 486 h of incubation, with a sojourn in the hatcher tray of less than 30 h; E30+, chicks that hatched by ≤ 486 h of incubation, with a sojourn in the hatcher tray of between 30 and 48 h; L < 30, chicks that hatched from >486 until 516 h of incubation, with a sojourn in the hatcher tray of less than 30 h.
Statistical analysis revealed that yolk components could be separated into 4 groups with similar rates of change during a 24-h sojourn for E birds—with some components spanning multiple groups (Table 5, P < 0.001). Total yolk weight, yolk solids weight, yolk water weights, and absolute yolk Mg, Zn, K, P, Fe, Cu, and Sr weights decreased by 40-50% from E < 30 to E30+. Total yolk weight, yolk water weight, and total yolk Sr and Na decreased by 30 to 45% from E < 30 to E30+. Yolk water and total yolk Sr, Na, and Ca weights decreased by 25 to 40% from E < 30 to E30+. Total yolk Mn decreased by 3% from E < 30 to E30+.
Table 5.
Mean change1 in total yolk minerals from E < 30 to E30 + chicks over 24-h sojourn (±SE).
| Yolk component | Reduction over 24 h (%) |
|---|---|
| Mg | 49 (±0.23)a |
| Zn | 48 (±0.27)a |
| Yolk solids | 46 (±0.20)a |
| K | 46 (±0.14)a |
| P | 45 (±0.28)a,b |
| Fe | 45 (±0.24)a,b |
| Cu | 44 (±0.17)a,b |
| Total yolk | 43 (±0.19)a,b |
| Yolk water | 40 (±0.17)a,b,c |
| Sr | 37 (±0.25)a,b,c |
| Na | 29 (±0.29)b,c |
| Ca | 26 (±0.20)c |
| Mn | 3 (±0.20)d |
| P | <0.001 |
a-dMeans with different letter superscripts differ significantly (P < 0.001).
Abbreviations: E < 30, chicks that hatched by ≤486 hours of incubation, with a sojourn in the hatcher tray of less than 30 hours; E30+, chicks that hatched by ≤486 hours of incubation, with a sojourn in the hatcher tray of between 30 and 48 hours.
Analysis of variance was performed on the difference between individual E30 + chick yolk values and the corresponding average E < 30 value, as a percentage of the corresponding average E < 30 value. For example, E30 + chicks had on average 40% less yolk water than the average E < 30 chick. Yolk components were used as treatment groups.
Discussion
As seen in this experiment, Cobb chicks can hatch over a 48-h period. When managing these birds during the early post-hatch period, it is valuable to understand some of the physiological differences that exist as a consequence of their hatch time and sojourn. This experiment is one of the first to explore some of the physiological differences, including bone mineralization and yolk mineral reserves, between chicks that hatched at different times and therefore spent differing sojourn times in the hatcher tray after they had hatched.
It should be noted that this work is based on the same trial as reported on in Hopcroft et al. (2019), in which eggs were monitored and sampled for yolk minerals during incubation.
Using the standard incubation EST of 37.8°C, the distribution of Cobb chick hatch times throughout the hatch window observed in this trial is consistent with previous studies (Tona et al. 2003; Muir and Groves, 2018, Muir and Groves, 2019), with more than 80% of chicks hatching by 492 HOI (ED20.5). In conventional incubation practice, these birds will not receive food or water for a minimum of 24 h, a delay which can negatively impact chick weight, relative growth, and breast muscle development (Careghi et al., 2005, Powell et al., 2016, De Jong et al., 2017).
E hatching chicks arose from smaller eggs at set compared to L hatching eggs. Furthermore, the eggs of E hatching chicks tended to lose more weight by ED17.5. This information could be used to predict chick hatch time. Hence, eggs could be weighed and sorted before placement in hatchers to place larger and smaller eggs in groups facilitating narrower hatch windows and shorter chick sojourn, the benefits of which have been explored in this experiment.
As chicks hatch at different times, the timing of sample collection in this experiment was allocated to allow for comparison between birds that have hatched E with a shorter sojourn (E < 30), birds that hatch early with a longer sojourn (E30+), and birds that hatch late with a short sojourn (L < 30). A point worth clarifying is that despite chicks spending either <30 h or 30+ h sojourn after hatching and before sampling, the time between sample points was 24 h, thus comparison between E < 30 and E30 + chicks is actually evaluating the effect of a 24 h sojourn period on birds from these groups. In this work, a take-off time of ED21.5 (516 HOI) has been used as representative of common practice in commercial hatcheries. Given the common take-off time, in this trial, it was impossible for an L hatching chick to have a long (>30 h) sojourn, and hence, that treatment was not able to be evaluated in this experiment.
The 3 sample groups included in this study allowed for comparison of the effect of chick hatch time and the duration of their sojourn on some physiological traits in the early post-hatch period. Comparing E < 30 birds at ED20.5 with L < 30 birds at ED21.5 establishes how chick hatch time may impact chick quality. The impact of a 24-h sojourn on early hatching chick qualities can be evaluated by comparing the characteristics of E < 30 chicks with E30+, which both hatched early but had either a short (<30 h) or long (≥30 h) sojourn. Finally, comparing E30 + to L < 30 chicks evaluates the differences between early and late hatching chicks at the common take-off time of ED21.5 (516 HOI).
Femoral bone ash percentage of E30 + birds was significantly lower than that of L < 30 birds (P = 0.013). This indicates that either, or both, chick hatch time and length of time in the incubator without food results in reduced femur bone mineralization during this critical period of skeletal development (Angel, 2007). When the post hoc test error rate is set at 10% rather than 5%, early hatching chicks with a short sojourn (E < 30) demonstrated femoral bone ash percentages that were the same as L < 30 chicks and higher than that of E30 + chicks. This suggests that chick femoral mineralization at hatch is more similar for chicks of similar sojourn times compared to chicks within the same hatch group but with differing (short or long) sojourn times.
Interestingly, equivalent E < 30 and E30 + chicks from the study by Muir and Groves (2018) had clearly similar bone ash, and chicks did not experience a decline in bone ash during the sojourn, as may be interpreted in the present study. The bone ash of the equivalent E < 30 and L < 30 chicks also differed (Muir and Groves, 2018). Presenting the bone compartment weights in the previous trial would enable a better understanding of the different outcomes. For instance, E hatching chicks might have had constant rates of organic and inorganic bone development between sample points, maintaining a constant bone ash percentage, but had slow overall growth.
Critically, however, in both experiments, the E30 + chicks, representative of most chicks leaving the hatchery, had a lower bone ash percentage compared to the chicks that hatched later (L < 30) at take-off from the incubator at 516 HOI (ED21.5).
Chick length was similar between E30 + and L < 30 birds. As E < 30 birds are shorter than both E30 + and L < 30 groups, E birds grow in length while unfed in the incubator, and L birds continue to grow while in ovo and during their short sojourn. Presumably, an increase in chick length is facilitated by longitudinal bone growth, which is a parameter that should be specifically evaluated in future studies. Assuming that bones continue to grow in length during the later stages of incubation, and/or immediately after hatching, this may be occurring at the expense of optimal bone mineralization, or even be triggering resorption of already established bone mineral. Muir and Groves (2018) reported an increase in the length of E30 + chicks, irrespective of if they were fed or not, but shorter late hatching chicks. Crucially, alongside the increased length of the E30+ (fed) chicks was their significant increase in bone ash, as opposed to E30+ (unfed) birds (Muir and Groves, 2018), suggesting that more nutrients than provided from the remnant yolk sac were required in early hatching chicks for increased bone mineralization immediately after hatching. This is an important finding, as the first week after hatch has been identified as the most important period for bone ossification, which is linked to reduced incidence of skeletal abnormalities later in life (Angel, 2007).
The levels of minerals in the serum may reflect the birds' need for Ca and P to maintain homeostasis as a priority over bone mineralization. In this experiment, there were no differences in serum Ca between the hatch and sojourn times. Serum P, however, was markedly reduced in the L < 30 compared to E < 30 and E30 + chicks, despite the 2 former groups having similar bone ash. More research investigating the relationship between serum mineral levels and the rate of bone deposition is required.
Lighter yolk weights of E30 + chicks, when compared to E < 30 and L < 30 chicks, and similar yolk-free body weights in each group, illustrate that chicks are drawing on their yolk reserves to support their development after hatching before having access to food. This finding supports the findings from Muir and Groves (2018), which additionally found that E30+(fed) chicks increased in yolk-free body weight over 24 h, compared to unfed E30+, E < 30, and L < 30 chicks. This again may support the importance of providing prompt access to food (Careghi et al., 2005, Powell et al., 2016, De Jong et al., 2017, Muir and Groves, 2018).
Yolk mineral values are provided as both concentrations and total amount within the yolk for the 3 treatment groups. To the best of our knowledge, this is the first publication reporting chick residual yolk mineral values that differentiate between chick hatch time. With increased interest in the post-hatch stage of production, these data highlight the need to consider hatch time as a differentiating factor between chicks. It also provides benchmark values for commercial application and further research.
The total amount of most yolk minerals reported in this experiment (Cu, Fe, K, Mg, P, Zn) decreased at a rate that was similar to reductions in whole yolk and yolk solids between E < 30 and E30+. Transport of whole yolk after hatching can occur through the yolk sac membrane into the vascular system, or through the vitelline diverticulum into the gastrointestinal tract (Lambson, 1970, Esteban et al., 1991, Noy and Sklan, 1996). A possible interpretation of the similar rates of decrease between most yolk components is that yolk is being absorbed as a whole, rather than individual components being selectively absorbed. Previous work has shown that Cu, Fe, P, and Zn for embryonic development during incubation is located in the yolk at point of lay, from where they decrease at relatively constant rates to whole yolk throughout incubation (Richards, 1997, Yair and Uni, 2011, Hopcroft et al., 2019).
The quantity of Ca and Na (and, to a lesser extent, Sr) in the yolk did not decrease at the same rate as was observed with the other minerals and yolk solids. Calcium and Mg have been shown to be mobilized from the eggshell during incubation and transported into the chick's vascular system for metabolic use during incubation (Yair et al., 2015). It is well established that during incubation, Ca, highly concentrated in the serum, moves into the yolk via the yolk sac membrane (Gabrielli and Accili, 2010) and that this also occurs after hatching (Noy and Sklan, 1996). Hopcroft et al., (2019) found that Ca and Sr levels in the yolk during incubation were strongly positively correlated to each other and rose to equal or higher total levels at ED18.5 compared to at ED0, whereas Mg did not increase to ED0 levels during incubation. Considering the post-hatch serum Ca and P, and yolk mineral results reported in the current experiment, it seems likely that the lower rate of change of Ca, and to a lesser extent Sr, during the 24-hour sojourn compared to other minerals is due to deposition of mineral from the vascular system via the yolk sac membrane into the yolk.
Residual yolk Mn tended to only slightly decrease from E < 30 to E30 + chicks (P = 0.072). Values were spread over a wide range, indicating potential difficulty in accurately measuring this mineral. If the low reduction of Mn from the yolk is real, a possible explanation is that Mn is not being selectively absorbed by the chick. This challenges the hypothesis put forward in this paper that the yolk is being absorbed as a whole, without differentiation. An alternate hypothesis is that, similar to Ca and Sr, Mn can be redeposited into the yolk from the vascular system via the yolk sac membrane (YSM). To further investigate this, Mn concentration in serum and the YSM could be measured. Manganese is required for chondroitin sulfate production (Leach et al. 1969), which in turn is required for bone mineralization.
Yolk mineral absorption, serum mineral levels, and bone mineralization should be explored further in the context of chick hatch time and the duration of the chick sojourn to optimize post-hatch interventions for optimal chick quality. The results of this experiment will serve as a foundation for future work in the post-hatch care area.
Conclusion
From this study, it would appear that the longer, unfed sojourn of E chicks compromises bone mineralization, whereas E or L hatch alone did not. Previous work demonstrates that provision of food after hatching can mitigate this impact. Hence, there is a potential cost–benefit advantage to be gained through the use of post-hatch care systems and products. These should be evaluated on a case-by-case basis to determine the suitability of individual solutions to particular poultry production models.
Acknowledgments
The authors would like to acknowledge Poultry Hub Australia for providing a scholarship for Ryan Hopcroft, Agrifutures Chicken meat program for funding the operating costs of the project, and the Poultry Research Foundation team (Joy Gill, Melinda Hayter, Kate Dehon, Duwei Chen and Kylie Warr) at The University of Sydney and, Susan Ball of Zootechny, for their help.
References
- Angel R. Metabolic disorders: limitations to growth and mineral deposition into broiler skeleton after hatch and leg problems. J. Appl. Poult. Res. 2007;16:138–149. [Google Scholar]
- Batal A.B., Parsons C.L. Effect of fasting versus feeding Oasis after hatching on nutrient utilization in chicks. Poult. Sci. 2002;81:853–859. doi: 10.1093/ps/81.6.853. [DOI] [PubMed] [Google Scholar]
- Bessei W. Welfare of broilers: a review. Worlds Poult. Sci. J. 2006;62:455–466. [Google Scholar]
- Careghi C., Tona K., Onagbesan O., Buyse J., Decuypere E., Bruggeman I. The spread of hatch and interaction with delayed feed access after hatch on broiler performance until seven days of age. Poult. Sci. 2005;84:1314–1320. doi: 10.1093/ps/84.8.1314. [DOI] [PubMed] [Google Scholar]
- Cobb-Vantress Broiler performance and nutrition supplement. 2015. http://cobb-vantress.com/docs/default-source/cobb-500-guides/Cobb500_Broiler_Performance_And_Nutrition_Supplement.pdf
- De Jong I.C., van Riel J., Bracke M.B.M., van den Brand H. A ‘meta-analysis’ of effects of post-hatch food and water deprivation on development, performance and welfare of chickens. PLos One. 2017;12:e0189350. doi: 10.1371/journal.pone.0189350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban S., Rayo J., Moreno M., Sastre M., Rial R., Tur J. A role played by the vitelline diverticulum in the yolk sac resorption in young post hatched chickens. J. Comp. Physiol. B. 1991;160:645–648. [Google Scholar]
- Gabrielli M.G., Accili D. The chick chorioallantoic membrane: a model of molecular, structural, and functional adaptation to transepithelial ion transport and barrier function during embryonic development. J. Biomed. Biotechnol. 2010;2010:1–12. doi: 10.1155/2010/940741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves P.J., Muir W.I. A meta-analysis of experiments linking incubation conditions with subsequent leg weakness in broiler chickens. PLoS One. 2014;9:e102682. doi: 10.1371/journal.pone.0102682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves P.J., Muir W.I. Early hatching predisposes Cobb broiler chickens to tibial dyschondroplasia. Animal. 2017;11:112–120. doi: 10.1017/S1751731116001105. [DOI] [PubMed] [Google Scholar]
- Hollemans M., De Vries S., Lammers A., Clouard C. Effects of early nutrition and transport of 1-day-old chickens on production performance and fear response. Poult. Sci. 2018;97:2534–2542. doi: 10.3382/ps/pey106. [DOI] [PubMed] [Google Scholar]
- Hopcroft R.L., Cowieson A.J., Muir W.I., Groves P.J. Changes to mineral levels in the yolk of meat chicken embryos during incubation. Poult. Sci. 2019;98:1511–1516. doi: 10.3382/ps/pey423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston P.M., Comar C.L. Distribution and contribution of calcium from the albumen, yolk and shell to the developing chick embryo. Am. J. Physiol. 1955;183:365–370. doi: 10.1152/ajplegacy.1955.183.3.365. [DOI] [PubMed] [Google Scholar]
- Knowles T.G., Kestin S.C., Haslam S.M., Brown S.N., Green L.E., Butterworth A., Pope S.J., Pfeiffer D., Nicol C.J. Leg disorders in broiler chickens: prevalence, risk factors and prevention. PLoS One. 2008;3:e1545. doi: 10.1371/journal.pone.0001545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambson R.O. An electron microscopic study of the entodermal cells of the yolk sac of the chick during incubation and after hatching. Am. J. Anat. 1970;129:1–20. doi: 10.1002/aja.1001290102. [DOI] [PubMed] [Google Scholar]
- Leach R.M., Muenster A.M., Wien E.M. Studies on the role of manganese in bone formation: II. Effect upon chondroitin sulfate synthesis in chick epiphyseal cartilage. Arch. Biochem. Biophys. 1969;133:22–28. doi: 10.1016/0003-9861(69)90483-4. [DOI] [PubMed] [Google Scholar]
- Mitchell M.A., Kettlewell P.J. Proceedings of the 8th European Symposium on Poultry Welfare. The World’s Poultry Science Association (WPSA); Cervia, Italy: 2009. Welfare of poultry during transport – a review; pp. 90–100. [Google Scholar]
- Molenaar R., Gooding T., Lamot D., Wijtten P.J.A., van der Pol C.W., Maatjens C.M., van Roovert-Reijrink I.A.M. Incubation and brooding conditions essential for the optimization of neonatal nutrition. Proc.Aust. Poult. Sci.Symp. 2014;25:17–20. [Google Scholar]
- Moran E.T., Jr. Nutrition of the developing embryo and hatching. Poult. Sci. 2007;86:1043–1049. doi: 10.1093/ps/86.5.1043. [DOI] [PubMed] [Google Scholar]
- Muir W.I., Groves P.J. Incubation and hatch management: consequences for bone mineralization in Cobb 500 meat chickens. Animal. 2018;12:794–801. doi: 10.1017/S1751731117001938. [DOI] [PubMed] [Google Scholar]
- Muir W.I., Groves P.J. The leg strength of two commercial strains of meat chicken subjected to different incubation profiles. Animal. 2019;13:1489–1497. doi: 10.1017/S1751731118002999. [DOI] [PubMed] [Google Scholar]
- National Health and Medical Research Council . Australian code for the care and use of animals for scientific purposes, 8th edition. National Health and Medical Research Council; Canberra: 2013. [Google Scholar]
- Noy Y., Sklan D. Uptake capacity for glucose, methionine and oleic acid in the proximal small intestine of posthatch chicks. Poult. Sci. 1996;75:998–1002. doi: 10.3382/ps.0750998. [DOI] [PubMed] [Google Scholar]
- Powell D.J., Velleman S.G., Cowieson A.J., Singh M., Muir W.I. Influence of chick hatch time and access to feed on broiler muscle development. Poult. Sci. 2016;95:1433–1448. doi: 10.3382/ps/pew047. [DOI] [PubMed] [Google Scholar]
- Richards M.P. Trace mineral metabolism in the avian embryo. Poult. Sci. 1997;76:152–164. doi: 10.1093/ps/76.1.152. [DOI] [PubMed] [Google Scholar]
- Richards M.P., Packard M.J. Mineral metabolism in avian embryos. Poult. Avian Biol. Rev. 1996;7:143–161. [Google Scholar]
- Tona K., Bamelia F., De Ketelaere B., Bruggeman V., Morales V.M.B., Buyse J., Onagbesan O., Decuypere E. Effects of egg storage time on spread of hatch, chick quality, and chick juvenile growth. Poult. Sci. 2003;82:736–741. doi: 10.1093/ps/82.5.736. [DOI] [PubMed] [Google Scholar]
- Uni Z., Yadgary L., Yair R. Nutritional limitations during poultry embryonic development. J. Appl. Poult. Res. 2012;21:175–184. [Google Scholar]
- Van de Ven L.J.F., van Wagenberg A.V., Groot Koerkamp P.W.G., Kemp B., van den Brand H. Effects of combined hatchling and brooding system on hatchability, chick weight and mortality of broilers. Poult. Sci. 2009;88:2273–2279. doi: 10.3382/ps.2009-00112. [DOI] [PubMed] [Google Scholar]
- Van der Pol C.W., van Roovert-Reijrink I.A.M., Maatjens C.M., van den Anker I., Kemp B., van den Brand H. Effect of eggshell temperature throughout incubation on broiler hatchling leg bone development. Poult. Sci. 2014;93:2878–2883. doi: 10.3382/ps.2014-04210. [DOI] [PubMed] [Google Scholar]
- Van Roovert-Reijrink I.A.M. The most important benefits of early feeding after hatch. Internat. Hatch. Prac. 2014;28:12–13. [Google Scholar]
- Yair R., Shahar R., Uni Z. In ovo feeding with minerals and vitamin D3 improves bone properties in hatchlings and mature broilers. Poult. Sci. 2015;94:2695–2707. doi: 10.3382/ps/pev252. [DOI] [PubMed] [Google Scholar]
- Yair R., Uni Z. Content and uptake of minerals in the yolk of broiler embryos during incubation and effect of nutrient enrichment. Poult. Sci. 2011;90:1523–1531. doi: 10.3382/ps.2010-01283. [DOI] [PubMed] [Google Scholar]
- Zuidhof M.J., Schneider B.L., Carney V.L., Korver D.R., Robinson F.E. Growth, efficiency and yield of commercial broilers from 1957, 1978 and 2005. Poult. Sci. 2014;93:2970–2982. doi: 10.3382/ps.2014-04291. [DOI] [PMC free article] [PubMed] [Google Scholar]