Abstract
The current research aimed to estimate the effect of dietary supplementation with glycinin isolated from soybeans on the growth performance, carcass traits, and selected blood metabolites of broiler chicks. A total of 200 1-wk-old broiler chicks were administered diets without glycinin (control treatment) or diets supplemented with 3 concentrations of soy glycinin (0.5, 1.0, or 1.5 g/kg of feed) for 6 wk. At the end of the feeding period, body weight was significantly higher in broiler chicks with glycinin supplementation (P < 0.05 or 0.01). The best values for body weight and body weight gain were recorded in the groups fed diets supplemented with 0.5 and 1.0 g glycinin/kg feed. Feed conversion was significantly (P < 0.05) improved in broilers in the glycinin-supplemented groups during the 1 to 6 and 3 to 6 wk growth periods. The highest value of breast yield was observed in broiler chicks supplemented with glycinin at a concentration of 1.0 g/kg of feed. Water-holding capacity increased with increasing concentrations of glycinin in the feed, up to 1.0%. Serum creatinine and urea concentrations decreased gradually (P < 0.01) as the concentration of glycinin in the feed increased. Broiler chicks receiving increasing concentrations of glycinin exhibited significantly (P < 0.01) lower levels of serum triglycerides, total cholesterol, high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol. All meat samples from broiler chicks supplemented with glycinin had significantly higher catalase activities. These data suggest that feeding broiler chicks diets supplemented with soy glycinin (0.5 to 1.5 g/kg of feed) can improve feed conversion, enhance body weight gain, and lower abdominal fat.
Keywords: soy glycinin, broiler, growth, abdominal fat, cholesterol
INTRODUCTION
Feed additives have been widely utilized to promote animal growth performance and to improve general health and they are commonly applied in the poultry industry (Abd El-Hack et al., 2015; Alagawany et al., 2019; Taha et al., 2019). Soybean seed proteins are a class of high-molecular weight proteins, mainly consisting of globulins. They can be subdivided into 2 main forms: 11S globulin (glycinin) and 7S globulin (β-conglycinin). Glycinin, with a molecular mass of 360 kDa, is composed of 6 constituent subunits (A1a B2, A2 B1a, AB, A5 A4 B3, A3 B4, and A1b B2), each consisting of an acidic (20 kDa) and a basic (34 kDa) polypeptide, linked by a disulfide bond. The β-conglycinin subunit (150 to 200 kDa) is a trimeric glycosylated protein (Sitohy et al., 2012). Although soybeans contain a considerable amount of protein, in this protein mixture, glycinin is complexed with other proteins and has been shown to be void of biological activity in such a state. Being a basic protein, it is usually present in the form of an association or aggregation with negatively charged proteins (e.g., conglycinin). Therefore, there is marked difference between glycinin in its original state and isolated glycinin (Osman et al., 2016a,b). The antimicrobial activities of these two soy protein fractions have been well investigated and documented (Osman et al., 2013; Mahgoub et al., 2016; Osman et al., 2016a,b). Current research is increasingly targeting the discovery of new additives capable of ensuring the adequate growth and development of farm animals by protecting them from diseases (Abd El-Hack et al., 2019; Gado et al., 2019). Antibiotics are frequently used in the poultry industry worldwide to protect the animals from disease and improve their growth performance. However, the repeated use of antibiotics in poultry diets has adverse effects, including the emergence of drug resistant pathogens, the accumulation of antibiotic residues in animal products, and as expected, reductions in beneficial intestinal microflora (Barton, 2000). These potential hazards have led to some restrictive regulations on the use of antibiotics in poultry and other animal industries in many countries. As a result, current studies are increasingly focusing on discovering potentially less hazardous alternatives to the frequently used antibiotics for maintaining health and performance under the prevalent commercial conditions.
One possible alternative to antibiotics is the use of probiotics, which may have beneficial effects on poultry health (Mountzouris et al., 2007). Several studies have reported the beneficial effects of phytogenic additives and probiotics on poultry growth performance and productivity (Abd El-Hack and Alagawany, 2015), nutrient retention, gut health (Awad et al., 2010), intestinal microflora (Mountzouris et al., 2010), susceptibility to diseases, and immune function (Abd El-Hack et al., 2016). Glycinin has previously been shown to be an antibacterial agent. The current study hypothesized that glycinin supplementation in the broiler chicken diet may led to improved health conditions and thus, more efficient poultry performance. Based on its well-established antibacterial activity, glycinin may reduce the susceptibility of poultry to diseases, enhance immune function, and improve growth performance. Medicinal plants have been investigated for their potential use as growth bio-stimulators and as possible substitutes for antibiotics. Medicinal plants are currently being used as feed additives to maintain the balance of the existing intestinal flora through their antibacterial role and to increase the secretion of specific enzymes (Trombetta et al., 2005). The aim of the current research was to evaluate the effectiveness of soy glycinin, as a potential substitute for antibiotics, in promoting growth performance, carcass traits, and meat quality of broiler chicks. The study also analyzed changes in the biochemical blood profile of broiler chicks supplemented with glycinin, to understand the underlying mechanisms for any profitable changes in broiler chick body size and quality.
MATERIALS AND METHODS
Glycinin (11S Globulin)
Soybean (Glycine max L.) seeds were purchased from a local market in the city of Zagazig, Sharkia, Egypt. Soybean seeds were ground to pass through a 1 mm2 sieve and the resulting powder was defatted using a mixed solvent of chloroform: methanol (3:1, v/v) for 8 h. A soybean protein isolate was separated by acid precipitation at the isoelectric point (pH 4.5), using a previously described procedure (Sitohy and Osman, 2010). The soybean protein isolate was used for the isolation of glycinin, according to the method described by Nagano et al. (1992).
Broiler Chicks, Experimental Design, and Experimental Feed
This study was performed at the Poultry Research Farm, Faculty of Agriculture, Zagazig University, Zagazig, Egypt. Experimental procedures were performed after the approval of the Local Experimental Animal Care Committee and the ethical institutional committee of Zagazig University.
A total of two-hundred 1-wk-old broiler chicks, weighing an average of 117.13 ± 1.34 g, were used in a completely randomized design experiment with 4 treatments, each with 50 broiler chicks (groups of 10 birds in 5 replicates). Each bird group was kept in a galvanized wire cage (40 × 50 × 100 cm). The 4 dietary treatments consisted of the basal diet plus 0.0, 0.5, 1.0, or 1.5 g glycinin/kg feed, with the first treatment serving as the control. All groups of broiler chicks were fed the experimental diet for 6 wk. The experimental diets were fed in 2 phases: the starter phase (weeks 1 to 3) and the finisher phase (weeks 3 to 6). Feed and water were offered ad libitum throughout the experimental period. The feed met the broiler chicks' requirements according to the guidelines of the National Research Council (NRC, 1994). Vaccinations and medical programs were implemented according to the age of broiler chicks, under the supervision of a veterinarian. The formulation and composition of the commercial broiler chick basal diet was calculated according to NRC (1994) guidelines (Table 1).
Table 1.
Chemical composition of basal diets (as fed)
| Basal diets |
||
|---|---|---|
| Ingredients (%) | Starter (weeks 1 to 3) | Finisher (weeks 3 to 6) |
| Yellow corn | 57.13 | 60.53 |
| Gluten meal | 6.50 | 6.10 |
| Soybean meal | 31.65 | 27.15 |
| Limestone | 1.24 | 1.15 |
| Dicalcium phosphate | 1.70 | 1.50 |
| NaCl | 0.30 | 0.30 |
| Soybean oil | 1.00 | 2.85 |
| Vit-min premix* | 0.30 | 0.30 |
| L-Lysine | 0.13 | 0.10 |
| DL-methionine | 0.05 | 0.02 |
| Total | 100 | 100 |
| Calculated analysis** | ||
| Metabolizable energy (MJ) | 12.33 | 12.94 |
| Crude protein % | 23.00 | 21.00 |
| Crude fibers % | 3.56 | 3.31 |
| Phosphorous (Available) % | 0.45 | 0.40 |
| Calcium % | 1.00 | 0.90 |
| Methionine + cysteine % | 0.83 | 0.74 |
| Lysine % | 1.20 | 1.05 |
Growth vitamin and mineral premix. Each 2.5 kg consists of: vitamin A, 12,000,000 IU; vitamin D3, 2,000,000 IU; vitamin E, 10 g; vitamin K3, 2 g; vitamin B1, 1,000 mg; vitamin B2, 49 g; vitamin B6, 105 g; vitamin B12, 10 mg; pantothenic acid, 10 g; niacin, 20 g, folic acid, 1,000 mg; biotin, 50 g; choline chloride, 500 mg, Fe, 30 g; Mn, 40 g; Cu, 3 g; Co, 200 mg; Si, 100 mg; and Zn, 45 g.
Evaluation of Traits and Measurements
Broiler chicks were individually weighed every 7 D. Mortality was observed and recorded. Average daily feed intake, body weight gain (BWG), and feed conversion ratio (FCR) were calculated based on previous data. Feed waste was recorded daily and was used to estimate feed consumption. At the end of the 6-wk experiment, 16 broiler chicks (4 in each group) were sampled randomly for carcass evaluations and were weighed and manually slaughtered. The weights of the carcasses, liver, gizzard, heart, thigh, breast, and abdominal fat were recorded and expressed as g/kg of slaughter weight, as follows:
Blood samples were collected from sacrificed broiler chicks in clean sterile tubes and left to coagulate. They were then centrifuged at 3,500 rpm (2,328.24 × g) for 15 min to separate the serum. Serum samples were stored in Eppendorf tubes at −20°C until analysis. The following parameters were determined spectrophotometrically in serum using commercial diagnostic kits provided by Bio Diagnostic Co. (Giza, Egypt): total protein (TP), albumin (ALB), aspartate aminotransferase, alanine aminotransferase (ALT), creatinine, urea, total cholesterol (TC) and high-density lipoprotein (HDL), cholesterol, and triglycerides (TG s).
Activities of Antioxidant Enzymes in Meat Samples
Immediately after slaughter, 24 fresh meat samples (6 samples/treatment group) were taken from the thigh (peroneus longus muscle) and breast (pectoralis major muscle), and visible fat was removed. Five grams of meat from the thigh and 5 g of meat from the breast were homogenized together. Sample homogenates (10% w/v) in 0.05 M phosphate buffer (pH 7) were centrifuged at 12,000 × g for 60 min at 4°C to separate the supernatant, which was subsequently used to measure catalase (cat no. CA 25 17) and superoxide dismutase (SOD, cat no. SD 25 21) activities using kits purchased from Bio Diagnostic Co., according to the manufacturer's method (Qwele et al., 2013).
The activities of catalase and SOD were calculated as follows:
and
where blank 1 is a mixture of the working solution and working enzyme; blank 2 is a mixture of the meat homogenate with a working solution; and blank 3 consists of dilution buffer, deionized water, and meat homogenate.
Meat Characteristics
Sample Preparation
Broiler chicks were slaughtered, plucked, dressed, and washed before being cut into pieces. A total of 24 fresh meat samples (6 samples/treatment group) were taken from the thigh (peroneus longus muscle) and breast (pectoralis major muscle). In all 500 g samples from the thigh and 500 g samples from the breast were cut into 3 to 4 cm thick steaks and minced in the laboratory using a meat grinder (1,600 watts; Moulinex, Lyon, France). The basic formulation of chicken meat was (kg/g) 1,000 g of meat, 2% salt, 2.5% spice mixture (50 g of black pepper, 30 g of cloves), and 3 g of onion juice. Chicken patties (50 g) were grilled in an air oven before brushing the meat with a small amount of corn oil, and the meat was grilled under moderate heat (190 to 200°C) for 20 to 30 min, according to the method described by Nath et al. (1996).
Cooking Loss
The weight of the patties was measured and used for calculating the cooking yields for each treatment, as the percentage of cooked weight to raw weight. The difference between the individual raw and cooked patties was taken as the percent cooking loss. The amount of moisture retained in the cooked product per 100 g of raw sample represented the moisture retention level (Kumar and Sharma, 2004).
Water-Holding Capacity
Water-holding capacity (WHC) was estimated using a previously described method (Tomotake et al., 2002). Representative samples of poultry meat (5 g) were placed in 50 mL centrifuge tubes and weighed. Subsequently, distilled water was added in small increments to each tube while stirring with a glass rod. After thoroughly wetting the samples, the mixture was centrifuged (4,000 rpm, 10 min) and the supernatant was removed by decantation. Water-holding capacity was calculated according to the following equation:
where W0 is the sample weight (g), W1 is the tube weight + the sample weight (g), and W2 is the tube weight + the sediment weight (g).
Sensory Evaluation
All samples (8 patties/treatment) were assessed for their general appearance, color, flavor, and juiciness, at a temperature range of 30 to 35°C, by a panel of 8 experienced judges. An 8-point descriptive scale was used, where 8 indicated extremely desirable taste and 1 indicated extremely poor taste. Tap water was provided to panelists between testing samples to eliminate the effect of each previous sample. The results were calculated as the mean ± SD for each treatment.
Statistical Analysis
A completely randomized design using the generalized linear model procedures of SPSS (IBM, Armonk, NY, USA; Nie et al., 1970) was used to analyze the variance (ANOVA) among samples. The Student–Newman–Keuls test was used to determine the differences among means. Statistical significances were based upon P < 0.05.
RESULTS
Growth Performance
The data shown in Table 2 show the effects of supplementing broiler chicks with different concentrations of glycinin for 6 wk on growth performance. Live body weight (LBW) was significantly (P < 0.05) higher in glycinin-treated groups than in the control group. Adding glycinin to the diet at 0.5 or 1 g/kg of feed significantly increased the body weight of broiler chicks by approximately 8 to 9%. There were no significant differences in body weight between the different concentrations of glycinin (0.5, 1.0, and 1.5 g/kg of feed), indicating that the lowest added concentration (0.5 g/kg of feed) was sufficient for inducing the maximum effect. Additionally, BWG values were significantly (P < 0.05 or P < 0.01) affected by the addition of glycinin, during the 3 to 6 wk period, but not the 1 to 3 wk period. It is worth noting that the highest values of LBW and BWG were recorded in groups fed diets supplemented with 0.5 and 1.0 g glycinin/kg feed, while the lowest LBW and BWG values were obtained from the control group (i.e., broiler chicks with no glycinin supplementation).
Table 2.
Growth performance of broiler chicks supplemented with different concentrations of glycinin
| Live body weight (g) |
Body weight gain (g) |
Feed intake (g/day) |
Feed conversion (g feed/g gain) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Items | 1 wk | 3 wk | 6 wk | 1 to 3 wk | 3 to 6 wk | 1 to 6 wk | 1 to 3 wk | 3 to 6 wk | 1 to 6 wk | 1 to 3 wk | 3 to 6 wk | 1 to 6 wk |
| Glycinin (g/kg of feed) | ||||||||||||
| 0.0 | 117.00 | 755.00 | 1945.06c | 45.57 | 59.51b | 53.77b | 66.3a | 143.10 | 104.70 | 1.45 | 2.4a | 1.95a |
| 0.5 | 116.75 | 778.39 | 2077.81a | 47.26 | 64.98a | 57.68a | 62.1b | 148.70 | 105.40 | 1.31 | 2.29a,b | 1.83b |
| 1.0 | 118.25 | 732.86 | 2063.44a | 43.90 | 66.53a | 57.21a | 60.1b | 140.60 | 100.35 | 1.37 | 2.11b | 1.75b |
| 1.5 | 118.50 | 736.07 | 2021.56a,b | 44.11 | 64.28a | 55.97a,b | 62.7b | 147.10 | 104.90 | 1.42 | 2.29a,b | 1.87a,b |
| SEM | 0.34 | 8.18 | 18.16 | 0.60 | 1.23 | 0.65 | 0.80 | 1.74 | 0.95 | 0.04 | 0.04 | 0.03 |
| P value | 0.235 | 0.145 | 0.034 | 0.134 | 0.003 | 0.035 | 0.017 | 0.384 | 0.210 | 0.057 | 0.019 | 0.040 |
SEM, standard error of the mean; wk, week.
Means in the same column not followed by superscript letters or followed by the same superscript letter are not significantly different (P > 0.05).
Feed intake was significantly (P < 0.05) affected by glycinin addition, solely during the first period (1 to 3 wk). Albeit not significant, the addition of glycinin resulted in a trend toward a positive effect on feed intake at concentrations of 0.5 and 1.5 g/kg of feed. Feed conversion was significantly (P < 0.05) affected by glycinin supplementation during the 3 to 6 and 1 to 6 wk periods. The highest FCR was observed with 1.0 g glycinin/kg feed, while the lowest FCR was observed in the control group.
Blood Biochemical Parameters
The effects of glycinin supplementation on serum biochemical parameters of broiler chicks are listed in Table 3. Glycinin treatments significantly affected (P < 0.05) serum TP and globulin concentrations and ALB:globulin ratio. Broiler chicks fed the control diet (without glycinin supplementation) had higher concentrations of serum TP and globulin compared to the other treatment groups.
Table 3.
Plasma total protein and its fractions in broiler chicks supplemented with different concentrations of glycinin
| Items | Total protein (g/dL) | Albumin (g/dL) | Globulin (g/dL) | A/G* ratio |
|---|---|---|---|---|
| Glycinin (g/kg of feed) | ||||
| 0.0 | 6.58a | 3.20 | 3.47a | 0.96b |
| 0.5 | 6.37b | 3.33 | 3.03b | 1.10a |
| 1.0 | 6.30b | 3.33 | 2.97b | 1.12a |
| 1.5 | 6.27b | 3.27 | 3.00b | 1.09a |
| SEM | 0.04 | 0.03 | 0.05 | 0.02 |
| P value | 0.016 | 0.096 | 0.002 | 0.002 |
SEM, standard error of the mean; *A/G, albumin/globulin ratio.
Means in the same column not followed by superscript letters or followed by the same superscript letter are not significantly different (P > 0.05).
Data in Table 4 show that there was no significant effect of the treatments on liver enzyme values. Serum creatinine and urea concentrations (P < 0.01) decreased linearly as the concentration of glycinin in the feed increased. Urea is a microbial product known to have negative effects on health in humans, birds, and animals. The lowest serum concentrations of creatinine and urea were recorded in broiler chicks fed diets with glycinin at a concentration of 1.5 g/kg of feed.
Table 4.
Liver and kidney function parameters of broiler chicks supplemented with different concentrations of glycinin.
| Items | Aspartate aminotransferase (IU/L) | Alanine aminotransferase (IU/L) | Creatinine (mg/dl) | Urea (mg/dl) |
|---|---|---|---|---|
| Glycinin (g/kg of feed) | ||||
| 0.0 | 124.00 | 57.57 | 0.65a | 48.35a |
| 0.5 | 123.67 | 59.67 | 0.43b | 42.33b |
| 1.0 | 122.67 | 58.67 | 0.19c | 36.00c |
| 1.5 | 120.33 | 56.00 | 0.17c | 27.33d |
| SEM | 0.54 | 0.54 | 0.06 | 2.40 |
| P value | 0.131 | 0.063 | 0.00 | 0.00 |
SEM, standard error of the mean.
Means in the same column not followed by superscript letters or followed by the same superscript letter are not significantly different (P > 0.05).
In the present work, TC, HDL-cholesterol, low-density lipoprotein (LDL)-cholesterol, and TGs, gradually but significantly (P < 0.01), decreased with increasing levels of glycinin supplementation. This change in lipid profile distribution may be due to increased biosynthesis and accumulation of cholesterol in the liver (Table 5).
Table 5.
Plasma lipids and their fractions in broiler chicks supplemented with different concentrations of glycinin
| Items | Triglycerides (mg/dL) | Cholesterol (mg/dl) | LDL (mg/dl) | HDL (mg/dL) |
|---|---|---|---|---|
| Glycinin (g/kg of feed) | ||||
| 0.0 | 87.03a | 104.11a | 55.59a | 33.89a |
| 0.5 | 54.00b | 91.67b | 50.54b | 30.33b |
| 1.0 | 34.00c | 86.00b | 52.53c | 27.67c |
| 1.5 | 28.33c | 72.33c | 41.99d | 24.67d |
| SEM | 7.30 | 3.50 | 7.87 | 0.99 |
| P value | 0.00 | 0.00 | 0.00 | 0.00 |
LDL, low-density lipoprotein; HDL, high-density lipoprotein; SEM, standard error of the mean.
Means in the same column not followed by superscript letters or followed by the same superscript letter are not significantly different (P > 0.05).
Carcass Characteristics
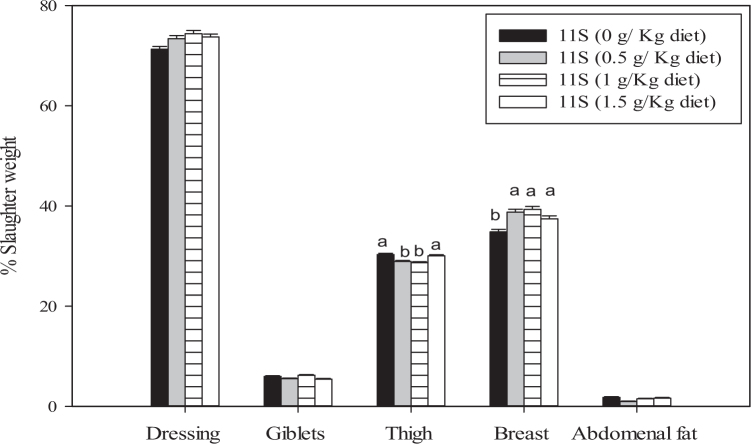
The data in Figure 1 show the influence of dietary supplementation with different concentrations of glycinin on carcass traits at the end of the experimental period. The percentages of the total carcass comprising breast and thigh were significantly (P < 0.01) affected by dietary treatments. The highest breast yield value was observed in broiler chicks supplemented with glycinin at a concentration of 1.0 g/kg of feed. However, the highest value of thigh yield was recorded in broiler chicks fed the control diet. Dressing percentage values tended to increase as the glycinin supplementation concentration increased, but this effect was not significant. Another positive effect of glycinin supplementation was a trend toward reduced abdominal fat in all treatment groups, compared to the control group, but this effect was also not statistically significant. As such, the addition of glycinin to broiler diets at different concentrations resulted in a nonsignificant trend towards increased dressing percentage and a reduced amount of abdominal fat.
Figure 1.
Carcass characteristics of broiler chicks supplemented with different concentrations of glycinin (0.0, 0.5, 1.0, or 1.5 g/kg of feed) in the diet from 1 to 6 wk of age. Values are mean ± standard error of 3 replicates.
Water-Holding Capacity and Cooking Loss of Poultry Meat
As Figure 2 indicates, WHC increased with increasing concentrations of glycinin in the feed, up to 1.0%. Further increases in glycinin concentration lowered WHC. This change in WHC was reflected in the extent of cooking loss of poultry meat, with higher WHC resulting in lower levels of cooking loss. The highest WHC of poultry meat (∼30%) was observed at 1 g glycinin/kg feed and this amount of supplementation was associated with the lowest level of cooking loss (∼8%). As such, supplementing broiler chicks with glycinin at 1 g/kg of feed is highly recommended to improve meat quality.
Figure 2.
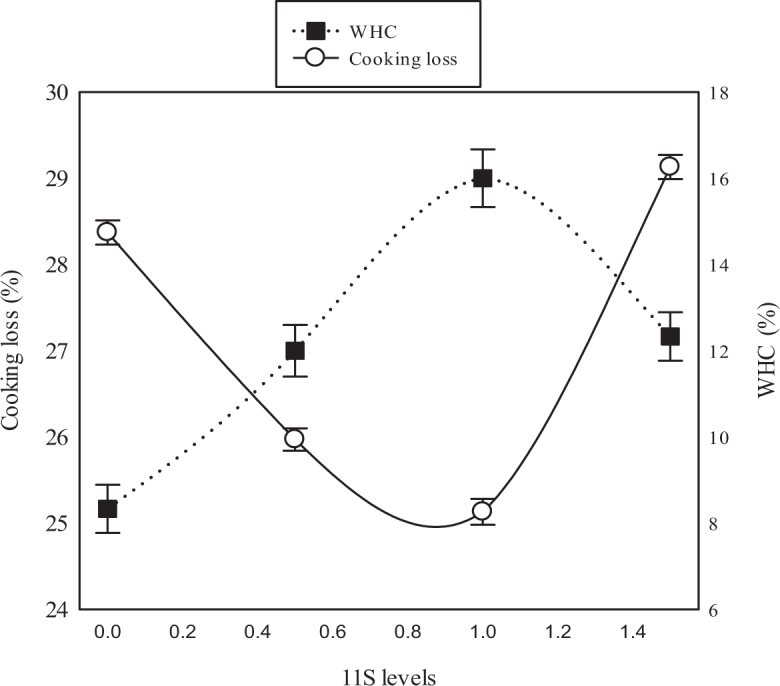
Cooking loss and water-holding capacity of meat from broiler chicks supplemented with different concentrations of glycinin (0, 0.5, 1, or 1.5 g/kg of feed) in the diet from 1 to 6 wk of age. Values are mean ± standard error of three replicates.
Activities of Antioxidant Enzymes in Meat
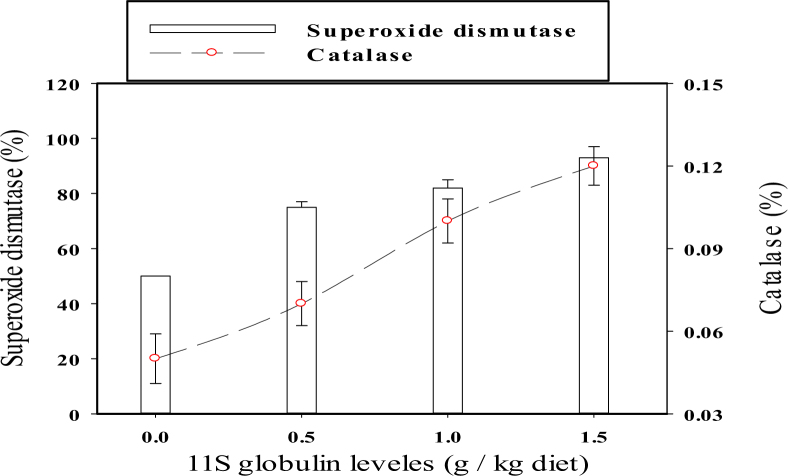
The activities of antioxidant enzymes, such as SOD and catalase, in meat from broiler chicks supplemented with different concentrations (0.0, 0.5, 1.0, and 1.5 g/kg diet) of glycinin, are presented in Figure 3.
Figure 3.
Activities of enzymatic antioxidants, catalase and superoxide dismutase, in meat from broiler chicks supplemented with different concentrations of glycinin (0.0, 0.5, 1.0, or 1.5 g/kg of feed) in the diet from 1 to 6 wk of age. Values are mean ± standard error of three replicates.
Catalase is an antioxidant enzyme that is widely distributed in all animal tissues. All the meat samples from broiler chicks supplemented with glycinin had significantly higher catalase activities than meat from control chicks. Catalase activities were 0.07, 0.10, and 0.12% in meat from broiler chicks supplemented with glycinin at 0.5, 1.0, and 1.5 g/kg of feed, respectively, compared to meat from chicks on the control diet, which showed a catalase activity of 0.05%. Meat from broiler chicks supplemented with glycinin also had significantly higher SOD activities, with 75, 82, and 93% at 0.5, 1.0, and 1.5 g/kg of feed, respectively, compared to meat from broiler chicks fed the control diet (50%).
Sensory Quality of Meat
Table 6 shows the sensory properties of poultry meat after feeding broiler chicks diets supplemented with glycinin for 6 wk. It was clear that the overall appearance of the meat was not significantly changed by dietary supplementation with glycinin. Meat color was slightly, but significantly, affected by dietary supplementation with glycinin. However, meat flavor was not significantly improved by dietary supplementation with glycinin. Variations in tenderness, juiciness, and texture between the different treatment groups were very slight and were not significant. Hence, overall sensory quality was not significantly affected by dietary supplementation with glycinin at 0.5 and 1.0 g/kg of feed. A slight, but significant reduction in overall sensory quality was only observed at the higher level of supplementation (1.5 g glycinin/kg of feed).
Table 6.
Sensory evaluation of meat from broiler chicks supplemented with different concentrations of glycinin
| Glycinin levels | Appearance | Color | Flavor | Tenderness | Juiciness | Texture | Overall |
|---|---|---|---|---|---|---|---|
| 0.00 g/kg of feed | 6.92a | 7.00a | 6.50a | 6.58a | 6.58a | 6.75a | 7.42a |
| 0.50 g/kg of feed | 6.50b | 6.58b | 6.50a | 6.50a | 6.08b | 6.50b | 6.75b |
| 1.00 g/kg of feed | 6.92a | 6.92a | 6.75a | 6.67a | 6.67a | 6.83a | 7.33a |
| 1.50 g/kg of feed | 6.00b | 6.38b | 6.23b | 6.46b | 6.00b | 6.31 | 6.77b |
| P value | 0.48 | 0.67 | 0.83 | 0.61 | 0.39 | 0.81 | 0.70 |
Note: If P ≤ 0.05, there are significant differences between treatments.
DISCUSSION
The molecular masses of glycinin subunits are 21 and 34 kDa, corresponding to the basic and acidic subunits, respectively (Sitohy et al., 2012). The antimicrobial activities of glycinin have recently been investigated (Osman et al., 2013; Osman et al., 2016b; Mahgoub et al., 2016; Sitohy M, Osman, 2018), with 11S showing antimicrobial action against pathogenic and spoilage bacteria equivalent to or greater than penicillin, by virtue of its cationic and hydrophobic nature (Osman et al., 2013).
The effect of supplementing the diet of broiler chicks with glycinin at different concentrations for 6 wk on growth performance was investigated in this study. The effects on growth performance between the 3 concentrations of glycinin used (0.5, 1.0, and 1.5 g/kg of feed) were not significantly different, indicating that the lowest concentration of glycinin tested (0.5 g/kg of feed) was sufficient for inducing the maximum effect. The present findings generally agree with those of Hernandez et al. (2004), who reported that dietary supplementation with phytogenic additives enhances the secretion of digestive enzymes and consequently, nutrient digestibility, thus promoted the growth of broiler chicks. Additionally, Mukhtar (2011) reported that supplementing broiler chicks' diets with 600 mg of G1 clove oil/kg of feed resulted in an increase in body weight over both control and antibiotic-treated groups. The same author confirmed that dietary supplementation with 600 mg of G1 clove oil/kg of feed increased BWG by approximately 2.24% compared to the control group. Furthermore, Lee et al. (2003) showed that supplementing the diets of broiler chicks with essential oils improved their growth performance. Plant extracts and phytobiotics extracted from various plant parts (e.g., leaves, roots, tubers) from different sources, e.g., fruits of herbs and spices, have been recommended as excellent growth enhancers in the poultry industry (Wallace et al., 2010). This beneficial effect may be due to the synergistic actions of the various active molecules within the extracts, which enhance feed utilization efficiency and thus, have positive effects on growth and production (Hashemi and Davoodi, 2010). The inclusion of herbs in poultry feed is thought to affect the metabolism of poultry by combating stress and curtailing the activity of pathogenic bacteria in the gastrointestinal tract, preventing the spread of pathogens and improving the production and activity of digestive enzymes (Sanjyal and Sapkota, 2012). Alternatively, Elagib et al. (2013) reported that the incorporation of 3% garlic (Allium sativum) powder (250 g) in the diet, caused a significant increase in feed intake, BWG, and FCR and improved the growth performance of broilers.
The disturbance in lipid profile found in our study may be due to increased biosynthesis and accumulation of cholesterol in the liver, as reported in several previous studies (Lanjewar et al., 2008). Supplementing broilers with tulsi (Ocimum tenuiflorum) leaf powder (0.5%) and selenium (0.3 ppm) has been reported to significantly decrease lipid peroxidation levels and increase plasma glutathione concentrations (Reddy, 2006). However, supplementing broilers with a combination of aloe vera (Aloe vera) and turmeric (Curcuma longa) was reported to have no significant effect on serum glucose, HDL, LDL, TC, or triglyceride concentrations.
The addition of glycinin to broiler diets at different concentrations resulted in a non-significant trend towards increased dressing percentage and a reduced amount of abdominal fat. This is similar to the results of Emadi and Kermanshahi (2006), who reported that supplementing broilers with phytogenic additives (0.75% turmeric rhizome powder) led to improved carcass quality, leaner meat, significantly reduced abdominal fat pads by up to 57%, and an improved ratio of heart weight to LBW. Furthermore, it has been reported that turmeric powder supplementation in broiler feed leads to higher dressing percentage (approximately 57%) and increased liver, spleen, and whole giblets weight (Durrani et al., 2006). Singh et al. (2007) reported that supplementing broilers with a mix of amla and turmeric powder, at a concentration of 5 g/kg in broiler feed, resulted in enhanced dressing percentage and a lower rate of mortality in broiler chicks. The concentration of water in meat is a very important determinant of its quality and acceptability. Generally, approximately 2 to 6% of the water content is removed from poultry muscle after death and more water is removed during cooking. This phenomenon is greatly controlled by structural elements of the meat (Puolanne, 2017). The results obtained in the present study indicated the enhancement of WHC, suggesting that supplementing broiler chicks with glycinin may have affected the structural components of the muscle. Furthermore, a higher WHC can consequently improve the quality of cooked poultry meat. This result confirmed the potential of poultry meat to retain moisture throughout processing, thus enhancing product quality for both consumers and processors (Bowker, 2017).
High oxidative stability of muscle-based foods is important, as it helps prevent or delay products becoming rancid. Increased antioxidant status in the living animal and subsequently, in the raw products from the animal, is considerably beneficial for both the consumer and the processing industry (Zhang et al., 2005).
Endogenous antioxidant enzymes, especially catalase, have been reported to potentially delay the onset of oxidative rancidity in stored meat (Pradhan et al., 2000). SOD, one of the most important enzymes in the antioxidant defense system, repairs cells and reduces the damage incurred to them by superoxides, by converting them to more stable products, thus terminating free radical chain reactions. The consumption of glycinin by broiler chicks significantly increased SOD activity, demonstrating the ability of glycinin to protect the animals' body/cells against damage by quenching free radicals. This observation may indicate that glycinin provided an efficient protective mechanism against superoxide anion radicals relative to the amino acid content. Some amino acids, such as histidine, tyrosine, methionine, and cysteine, have been reported to have antioxidant activity. Specifically, histidine has been reported to have strong radical-scavenging activity through the decomposition of its imidazole (Xie et al., 2008). Supplementing broiler chicks' diets with glycinin at concentrations of 0.5 to 1.0 g/kg of feed can be recommended, as at these glycinin concentrations, there was no reduction in the sensory quality of the poultry meat product.
Based on our results and on the previous studies discussed above, we conclude that soy glycinin supplementation (at 0.5, 1.0, and 1.5 g/kg of feed) can positively affect the growth performance of broiler chicks. In addition, chicks fed diets supplemented with soy glycinin had lower abdominal fat and lower serum concentrations of cholesterol, creatinine, and urea. Thus, fortification of poultry diets with low concentrations of soy glycinin can be effectively used to ensure more hygienic conditions and thus, result in favorable growth performance. Glycinin may be suitable as a replacement for antibiotics and may reduce the current antibiotic dependency, which has deleterious effects and can lead to the emergence of drug-resistance pathogenic bacterial strains.
ACKNOWLEDGMENTS
This research was funded by the Deanship of Scientific Research at Princess Nourah bint Abdulrahman University through the Fast-track Research Funding Program.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
REFERENCES
- Abd El-Hack M.E., Alagawany M. Performance, egg quality, blood profile, immune function, and antioxidant enzyme activities in laying hens fed diets with thyme powder. J. Anim. Feed Sci. 2015;24:127–133. [Google Scholar]
- Abd El-Hack M.E., Alagawany M., Farag M.R., Tiwari R., Karthik K., Dhama K. Nutritional, healthical and therapeutic efficacy of black cumin (Nigella sativa) in animals, poultry and humans. Int. J. Pharmacol. 2016;12:232–248. [Google Scholar]
- Abd El-Hack M.E., Abdelnour S.A., Abd El-Moneim A.E., Arif M., Khafaga A.F., Shaheen H., Samak D., Swelum A.A. Putative impacts of phytogenic additives to ameliorate lead toxicity in animal feed. Environ. Sci. Pollut. Res. 2019;26:23209–23218. doi: 10.1007/s11356-019-05805-8. [DOI] [PubMed] [Google Scholar]
- Abd El-Hack M.E., Mahgoub S.A., Alagawany M., Dhama K. Influences of dietary supplementation of antimicrobial cold pressed oils mixture on growth performance and intestinal microflora of growing Japanese quails. Int. J. Pharmacol. 2015;11:689–696. [Google Scholar]
- Alagawany M., Elnesr S.S., Farag M.R., Abd El-Hack M.E., Khafaga A.F., Taha A.E., Tiwari R., Yatoo M.I., Bhatt P., Marappan G., Dhama K. Licorice (glycyrrhiza glabra) herb as an eco-friendly additive to promote poultry health – current knowledge and prospects. Animals. 2019;9:536. doi: 10.3390/ani9080536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awad W., Ghareeb K., Böhm J. Effect of addition of a probiotic microorganism to broiler diet on intestinal mucosal architecture and electrophysiological parameters. J. Anim. Phyisol. Anim. Nutr. 2010;94:486–494. doi: 10.1111/j.1439-0396.2009.00933.x. [DOI] [PubMed] [Google Scholar]
- Barton MD. Antibiotic use in animal feed and its impact on human health. Nutr. Res. Rev. 2000;13:279–299. doi: 10.1079/095442200108729106. [DOI] [PubMed] [Google Scholar]
- Bowker B. Poultry Quality Evaluation. Woodhead Publishing; 2017. Developments in our understanding of water-holding capacity; pp. 77–113. [Google Scholar]
- Durrani F., Ismail M., Sultan A., Suhail S., Chand N., Durrani Z. Effect of different levels of feed added turmeric (Curcuma longa) on the performance of broiler chicks. J. Agri. Biol. Sci. 2006;1:9–11. [Google Scholar]
- Elagib H., El-Amin W., Elamin K.M., Malik H. Effect of dietary garlic (Allium sativum) supplementation as feed additive on broiler performance and blood profile. J. Anim. Sci. Adv. 2013;3:58–64. [Google Scholar]
- Emadi M., Kermanshahi H. Effect of turmeric rhizome powder on performance and carcass characteristics of broiler chickens. Int. J. Poult. Sci. 2006;5:1069–1072. [Google Scholar]
- Gado A.R., Ellakany H.F., Elbestawy A.R., Abd El-Hack M.E., Khafaga A.F., Taha A.E., Arif M., Mahgoub S.A. Herbal medicine additives as powerful agents to control and prevent avian influenza virus in poultry– a review. Ann. Anim. Sci. 2019 doi: 10.2478/aoas-2019-0043.0. [DOI] [Google Scholar]
- Hashemi S., Davoodi H. Phytogenics as new class of feed additive in poultry industry. J. Anim. Sci. Adv. 2010;9:2295–2304. [Google Scholar]
- Hernandez F., Madrid J., Garcia V., Orengo J., Megias M. Influence of two plant extracts on broilers performance, digestibility, and digestive organ size. Poult. Sci. 2004;83:169–174. doi: 10.1093/ps/83.2.169. [DOI] [PubMed] [Google Scholar]
- Kumar M., Sharma B. Quality and storage stability of low-fat pork patties containing barley flour as-fat substitute. J. Food Sci. Tech. Mys. 2004;41:496–502. [Google Scholar]
- Lanjewar R., Zanzad A., Ramteke B., Deshmukh G. Effect of dietary supplementation of tulsi (O. sanctum) leaf powder on the growth performance and serum lipid profile in broilers. Indian J. Anim. Nut. 2008;25:395–397. [Google Scholar]
- Lee K.W., Everts H., Kappert H., Frehner M., Losa R., Beynen A. Effects of dietary essential oil components on growth performance, digestive enzymes and lipid metabolism in female broiler chickens. British Poult. Sci. 2003;44:450–457. doi: 10.1080/0007166031000085508. [DOI] [PubMed] [Google Scholar]
- Mahgoub S.A., Osman A.O., Sitohy M.Z. Impeding Bacillus spore germination in vitro and in milk by soy glycinin during long cold storage. J. Gen. Appl. Microbiol. 2016;62:52–59. doi: 10.2323/jgam.62.52. [DOI] [PubMed] [Google Scholar]
- Mountzouris K., Tsitrsikos P., Palamidi I., Arvaniti A., Mohnl M., Schatzmayr G., Fegeros K. Effects of probiotic inclusion levels in broiler nutrition on growth performance, nutrient digestibility, plasma immunoglobulins, and cecal microflora composition. Poult. Sci. 2010;89:58–67. doi: 10.3382/ps.2009-00308. [DOI] [PubMed] [Google Scholar]
- Mountzouris K., Tsirtsikos P., Kalamara E., Nitsch S., Schatzmayr G., Fegeros K. Evaluation of the efficacy of a probiotic containing Lactobacillus, Bifidobacterium, Enterococcus, and Pediococcus strains in promoting broiler performance and modulating cecal microflora composition and metabolic activities. Poult. Sci. 2007;86:309–317. doi: 10.1093/ps/86.2.309. [DOI] [PubMed] [Google Scholar]
- Mukhtar M.A. The effect of dietary clove oil on broiler performance. Aust. J. Basic Appl. Sci. 2011;5:49–51. [Google Scholar]
- Nagano T., Hirotsuka M., Mori H., Kohyama K., Nishinari K. Dynamic viscoelastic study on the gelation of 7 S globulin from soybeans. J. Agric. Food Chem. 1992;40:941–944. [Google Scholar]
- Nath R., Mahapatra C., Kondaiah N., Singh J. Quality of chicken patties as influenced by microwave and conventional oven cooking. J. Food Sci. Tech. 1996;33:162–164. [Google Scholar]
- Nie N.H., Bent D.H., Hull C.H. McGraw-Hill; New York, NY: 1970. SPSS: Statistical Package for the Social Sciences. [Google Scholar]
- NRC . Natl. Acad. Press; Washington, DC: 1994. Nutrition Requirements of Poultry. [Google Scholar]
- Osman A.O., Mahgoub S.A., Sitohy M.Z. Preservative action of 11S (glycinin) and 7S (β-conglycinin) soy globulin on bovine raw milk stored either at 4 or 25 C. J. Dairy Res. 2013;80:174–183. doi: 10.1017/S0022029913000095. [DOI] [PubMed] [Google Scholar]
- Osman A., Abbas E., Mahgoub S., Sitohy M. Inhibition of Penicillium digitatum in vitro and in postharvest orange fruit by a soy protein fraction containing mainly β-conglycinin. J. Gen. Plant Pathol. 2016;82:293–301. [Google Scholar]
- Osman A., Daidamony G., Sitohy M., Khalifa M., Enan G. Soybean glycinin basic subunit inhibits methicillin resistant-vancomycin intermediate Staphylococcus aureus (MRSA-VISA) in vitro. Int. J. App. Res. Nat. Prod. 2016;9:17–26. [Google Scholar]
- Pradhan A., Rhee K., Hernández P. Stability of catalase and its potential role in lipid oxidation in meat. Meat Sci. 2000;54:385–390. doi: 10.1016/s0309-1740(99)00114-x. [DOI] [PubMed] [Google Scholar]
- Puolanne E. Developments in our understanding of water-holding capacity in meat. new aspects of meat quality: from genes to ethics. Food Sci. Tech. Nut. 2017:167–190. [Google Scholar]
- Qwele K., Hugo A., Oyedemi S., Moyo B., Masika P., Muchenje V. Chemical composition, fatty acid content and antioxidant potential of meat from goats supplemented with Moringa (Moringa oleifera) leaves, sunflower cake and grass hay. Meat Sci. 2013;93:455–462. doi: 10.1016/j.meatsci.2012.11.009. [DOI] [PubMed] [Google Scholar]
- Reddy L. Tamil Nadu Veterinary and Animal Sciences University (TANUVAS); 2006. Effect of dietary supplementation of Tulasi (Ocimum sanctum) and selenium on antioxidative enzymes status in broilers.http://krishikosh.egranth.ac.in/handle/1/88699 M.Sc. Thesis. [Google Scholar]
- Sanjyal S., Sapkota S. Supplementation of broilers diet with different sources of growth promoters. Nepal J. Sci. Technol. 2012;12:41–50. [Google Scholar]
- Singh G., Maurya S., Catalan C.A. A comparison of chemical, antioxidant and antimicrobial studies of cinnamon leaf and bark volatile oils, oleoresins and their constituents. Food Chem. Toxicol. 2007;45:1650–1661. doi: 10.1016/j.fct.2007.02.031. [DOI] [PubMed] [Google Scholar]
- Sitohy M., Osman A. Antimicrobial activity of native and esterified legume proteins against Gram-negative and Gram-positive bacteria. Food Chem. 2010;120:66–73. [Google Scholar]
- Sitohy M., Osman A. Bioactive compounds in soybean proteins and its applications in food systems. In: Negm A.M., Abu-hashim M.M., editors. Sustainability of Agricultural Environment in Egypt: Part I. The Handbook of Environmental Chemistry. Vol. 76. Springer; Cham: 2018. pp. 147–160. [Google Scholar]
- Sitohy M.Z., Mahgoub S.A., Osman A.O. In vitro and in situ antimicrobial action and mechanism of glycinin and its basic subunit. Int. J. Food Microbiol. 2012;154:19–29. doi: 10.1016/j.ijfoodmicro.2011.12.004. [DOI] [PubMed] [Google Scholar]
- Taha A.E., Hassan S.S., Shewita R.S., El-seidy A.A., Abd El-Hack M.E., Hussein E.O.S., Saadeldin I.M., Swelum A.A., El-Edel M.A. Effects of supplementing broiler diets with coriander seed powder on growth performance, blood hematology, ileum microflora, and economic efficiency. J. Anim. Physiol. Anim. Nutr. 2019;103:1474–1483. doi: 10.1111/jpn.13165. [DOI] [PubMed] [Google Scholar]
- Tomotake H., Shimaoka I., Kayashita J., Nakajoh M., Kato N. Physicochemical and functional properties of buckwheat protein product. J. Agric. Food Chem. 2002;50:2125–2129. doi: 10.1021/jf011248q. [DOI] [PubMed] [Google Scholar]
- Trombetta D., Castelli F., Sarpietro M.G., Venuti V., Cristani M., Daniele C., Saija A., Mazzanti G., Bisignano G. Mechanisms of antibacterial action of three monoterpenes. Antimicrob. Agent. Chemo. 2005;49:2474–2478. doi: 10.1128/AAC.49.6.2474-2478.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace R., Oleszek W., Franz C., Hahn I., Baser K.H., Mathe A., Teichmann K. Dietary plant bioactives for poultry health and productivity. British Poult. Sci. 2010;51:461–487. doi: 10.1080/00071668.2010.506908. [DOI] [PubMed] [Google Scholar]
- Xie Z., Huang J., Xu X., Jin Z. Antioxidant activity of peptides isolated from alfalfa leaf protein hydrolysate. Food Chem. 2008;111:370–376. doi: 10.1016/j.foodchem.2008.03.078. [DOI] [PubMed] [Google Scholar]
- Zhang S.X., Farouk M.M., Young O.A. Functional stability of frozen normal and high pH beef. Meat Sci. 2005;69:765–772. doi: 10.1016/j.meatsci.2004.11.009. [DOI] [PubMed] [Google Scholar]