Abstract
Galactooligosaccharides (GOS) delivered in ovo improve intestinal health of broiler chickens. This study aimed to demonstrate the impact of in ovo stimulation with GOS prebiotic on day 12 of egg incubation on performance and welfare traits in broiler chickens. The incubating eggs were divided into 3 groups, based on the substance injected in ovo: 3.5 mg of GOS dissolved in 0.2 mL physiological saline (GOS), 0.2 mL physiological saline (S), or uninjected controls (C). Constant heat stress (HS) was induced on days 32 to 42 post-hatch by increasing environmental temperature to 30°C. Thermoneutral (TN) animals were kept at 25°C. The performance (body weight [BW], daily feed intake [DFI], daily weight gain [DWG], and feed conversion rate [FCR]) were measured and mortality was scored for starter (days 0 to 13), grower (days 14 to 27), and finisher (days 28 to 42) feeding phases. Rectal temperature was scored on days 32 to 42. Food-pad dermatitis (FPD) was scored post-mortem (day 42). GOS increased (P < 0.01) BW on day 42 (2.892 kg in GOS vs. 2.758 kg in C). Heat stress significantly reduced (P < 0.01) final BW (2.516 kg in TN vs. 3.110 kg in HS). During finisher phase, DFI was significantly higher in GOS vs. C (173.2 g vs. 165.7 g; P < 0.05). FCR calculated for the entire rearing period (days 0 to 42) ranged from 1.701 in C to 1.653 in GOS (P < 0.05). GOS improved FCR in HS animals during finisher phase (P < 0.05). Rectal temperature of GOS chickens under HS reached 42.5°C 1 day earlier than C and S (P < 0.05), which suggests that those birds recovered earlier from the high environmental temperature. Heat stress increased (P < 0.05) mortality about 5 times compared to TN during finisher phase (from 1.59% in TN to 7.69% in HS). GOS decreased FPD in TN conditions by 20% (no lesions in 81% in GOS vs. 60% in C). GOS delivered in ovo mitigated negative effects of HS on performance and welfare in broiler chickens.
Key words: broiler performance, food-pad dermatitis, galactooligosaccharides, heat stress, in ovo stimulation
INTRODUCTION
Heat stress (HS) in chickens induced by exposure to elevated ambient temperatures has a strong and immediate effect on performance and welfare (Lara and Rostagno, 2013). Highly selected fast-growing broiler chickens are more susceptible to heat than egg layers (Sandercock et al., 2006). Broiler chickens are particularly sensitive to heat in the last period of rearing, when their circulatory system is inefficient in relation to body weight (BW) (Drain et al., 2007). The direct effects of the heat exposure on broiler chickens is reduced feed intake (FI) and BW gain, which negatively affects overall performance (Donkoh, 1989). Reduced FI is followed by high water consumption, which results in prevalence of foot-pad dermatitis (FPD) (Swiatkiewicz et al., 2017). Excessive hyperthermia often accompanied by increased stocking density is a major cause of a sudden death syndrome (Imaeda, 2000) and high mortality rates during transportation in the summer months (Warriss et al., 2005). Altogether, exposing broiler chickens to high ambient temperatures is followed not only by economic losses, but also by compromised animal welfare, expressed by high mortality rates and occurrence of severe foot lesions.
There are different approaches to mitigate the problem of heat in poultry, including adjustment in ventilation infrastructure, admixture of genetic factor of more resilient animals, epigenetic conditioning and dietary interventions (Renaudeau et al., 2012). The latter one very often includes in-feed prebiotic and probiotics (Sugiharto et al., 2017). The rationale for such treatment is to improve intestinal health, which is one of the key factors influencing the vulnerability of the chickens to heat. One of the first physiological symptoms of HS is dysbiosis of intestinal microflora, which brings immediate pressure to gut integrity. Dysbiosis in microflora composition, reduction in mucus layer (Burkholder et al., 2008), and alteration of tight junctions in intestinal epithelia (Varasteh et al., 2015) indicate a compromised gut barrier function and integrity. Due to the compromised barrier function of the gut, intestinal bacteria and their toxins access internal milieu and trigger acute pro-inflammatory immune responses. This condition is referred to as endotoxemia and leads straight to the heat stroke (Leon and Helwig, 2010). The approach to prevent such detrimental symptoms of HS is to promote eubiotic microflora and improve intestinal health of the host.
Gastrointestinal tract (GIT) of the newly hatched chicks is rapidly colonized after hatching, when chicks get in contact with environment. In natural hatching, the first microbial inoculum is passed from the hen to the offspring with droppings on the eggshell or in the litter. Commercial hatcheries apply biosafety measures to prevent microbial contamination during the process of artificial hatching. One of the critical control points to manage hygienic conditions within the hatchery is decontamination of the eggs with chemical agents (Samberg and Meroz, 1995). In this manner, the natural mechanism through which newly hatched chicks receive the first microbial inoculum is disrupted (Olsen et al., 2017). As a consequence, the neonatal intestine is likely to get colonized with random microbial strains, including extraintestinal pathogens, e.g., zoonotic avian pathogenic Escherichia coli (Mellata, 2013).
Typical management practice in commercial hatcher-ies requires a hatching window, during which animals are deprived of food or water. Hatching window is the period from the hatching of the first chick to the arrival of the whole batch at the farm and it usually takes between 48 and 72 h. To ensure that the chicks' intestine is protected over that time, the natural promoters of the beneficial microbial profile (i.e., prebiotics, probiotics, and synbiotics) should thus be applied before hatching. In ovo technology allows for efficient stimulation of the chicken intestinal microflora during perinatal period. It is carried out by injection of a prebiotic or synbiotic into the egg's air cell on day 12 of egg incubation (Siwek et al., 2018). Such in ovo injection can be automated without losses in eggs hatchability (Bednarczyk et al., 2011). It provides a potent stimulus to the embryonic gut, and chicks obtained from injected eggs have already established a beneficial bacteria profile at hatching (Bednarczyk et al., 2016).
In-feed delivered galactooligosaccharides (GOS) have been proved to exert beneficial effects on intestinal health in broiler chickens under HS (Varasteh et al., 2015). We have recently demonstrated that GOS used for in ovo stimulation improves gene expression signatures of innate immunity (cytokine gene expression), barrier function (mucin and host defense peptides gene expression), and intestinal integrity (tight junctions gene expression) in jejunum and cecum of the broiler chickens (Slawinska et al., 2019a). GOS also increased expression of Bifidobacteria spp. in jejunum and cecum, which confirmed its bifidogenic effects upon delivery in ovo (Slawinska et al., 2019a). When combined with the HS, stimulation with GOS in ovo proved to mitigate heat-induced immune responses and oxidative stress in the spleen of broiler chickens (Slawinska et al., 2019b). Finally, GOS delivered in ovo modulated positive profile of the fatty acids in pectoral muscle of the heat-stressed chickens (Tavaniello et al., 2019). Hereby, we formulated a hypothesis that the beneficial effects of in ovo stimulation with GOS could mitigate the overall detrimental effects of HS in broiler chickens. The goal of this study was to analyze the effects of GOS delivered in ovo on performance and welfare traits in broiler chickens subjected to HS.
MATERIALS AND METHODS
In Ovo Treatment
Fertilized eggs of broiler chickens (Ross 308) were incubated in a commercial hatchery. On the day 12 of egg incubation, a single dose of 3.5 mg GOS/egg (GOS) dissolved in 0.2 mL physiological saline (1,000 eggs/group) or 0.2 mL physiological saline (S) (0.9% NaCl) was injected in ovo into air chamber (1,000 eggs/group). Control eggs (C) (1,000 eggs/group) remained uninjected. The hole in the eggshell was sealed with natural glue to prevent moisture loss from the embryo. Directly after in ovo treatment, the eggs were placed back in the incubators. GOS prebiotic used in this study (trade name: Bi2 tos, Clasado Biosciences Ltd., Jersey, UK) is manufactured by enzymatic transgalactosylation of the milk lactose by the whole cells of Bifidobacterium bifidum 41171 (Tzortzis et al., 2005). Further information on the in ovo treatment can be found elsewhere (Slawinska et al., 2019a).
Hatchability
Hatchability was measured as proportion of hatched chicks to the number of fertile eggs (candling was done on day 12 of eggs incubation, prior to in ovo injection). To obtain high results of hatchability after in ovo injection, we have developed a standardized protocol and for each bioactive solution we optimize only dosage of the prebiotic. For GOS, the dose optimization trial has been reported by Bednarczyk et al. (2016). It included testing the 3 doses of GOS for in ovo injection on day 12 of eggs incubation: 0.18, 0.88, 3.5, and 7.0 mg/embryo (500 injected embryos each). The dose of 3.5 mg/embryo GOS, which we also used in this study, was the highest prebiotic dose that increased meconial Bifidobacterium spp. and Lactobacillus spp. at hatching while maintaining high hatchability rates (∼90% of in ovo-injected eggs).
Animal Procedures
After hatching, all the chicks were vaccinated according to the current commercial practice (coccidiosis, Infectious Bronchitis Virus, Marek's disease virus, Newcastle and Gumboro disease). A total of 900 male chicks (300 chicks/treatment) were divided into 6 groups (150 birds/group) and reared in floor pens: 3 groups (6 pens/group, 25 birds/pen) were reared in thermoneutral condition (TN) and 3 groups (6 pens/group, 25 birds/pen) were reared under HS condition. Heat stress was induced on day 32 by increasing environmental temperature to 30°C and lasted for 10 consecutive days to mimic a chronic HS. Birds were fed a commercial basal diet following a phase-feeding program according to age with starter (days 0 to 13), grower (days 14 to 27), and finisher (days 28 to 42) diets. Composition of the diets is presented in Table 1.
Table 1.
Composition of the diets used in the study.
| Item | Starter (days 0–13) | Grower 1 (days 14–27) | Finisher (days 28–42) |
|---|---|---|---|
| Dietary components (kg) | |||
| Corn | 42.17 | 34.96 | 12.73 |
| White corn | 0.00 | 0.00 | 15.00 |
| Wheat | 10.00 | 20.00 | 25.01 |
| Sorghum | 0.00 | 0.00 | 5.00 |
| Soybean meal | 23.11 | 20.63 | 17.60 |
| Expanded soybean | 10.00 | 10.00 | 13.00 |
| Sunflower | 3.00 | 3.00 | 3.00 |
| Corn gluten | 4.00 | 3.00 | 0.00 |
| Soybean oil | 3.08 | 4.43 | 5.48 |
| Dicalcium phosphate | 1.52 | 1.20 | 0.57 |
| Calcium carbonate | 0.91 | 0.65 | 0.52 |
| Sodium bicarbonate | 0.15 | 0.10 | 0.15 |
| Salt | 0.27 | 0.27 | 0.25 |
| Choline chloride | 0.10 | 0.10 | 0.10 |
| Lysine sulfate | 0.59 | 0.55 | 0.46 |
| DL-methionine | 0.27 | 0.29 | 0.30 |
| Threonine | 0.15 | 0.14 | 0.14 |
| Enzyme–Roxazyme G2G | 0.08 | 0.08 | 0.08 |
| Phytase 0.1% | 0.10 | 0.10 | 0.10 |
| Vitamins–minerals Premix1 | 0.50 | 0.50 | 0.50 |
| Calculated nutrients content | |||
| Dry matter, % | 88.57 | 88.65 | 88.64 |
| Protein, % | 22.70 | 21.49 | 19.74 |
| Lipid, % | 7.06 | 8.24 | 9.74 |
| Fiber, % | 3.08 | 3.04 | 3.07 |
| Ash, % | 5.85 | 5.17 | 4.49 |
| Lys, % | 1.38 | 1.29 | 1.21 |
| Met, % | 0.67 | 0.62 | 0.59 |
| Met+Cys, % | 1.03 | 0.97 | 0.91 |
| Calcium, % | 0.91 | 0.80 | 0.59 |
| Phosphate, % | 0.63 | 0.57 | 0.46 |
| Metabolizable energy (kcal/kg) | 3.076 | 3.168 | 3.264 |
Provided the following per kg of diet: vitamin A (retinyl acetate), 13,000 IU; vitamin D3 (cholecalciferol), 4,000 IU; vitamin E (DL-α tocopheryl acetate), 80 IU; vitamin K (menadione sodium bisulfite), 3 mg; riboflavin, 6.0 mg; pantothenic acid, 6.0 mg; niacin, 20 mg; pyridoxine, 2 mg; folic acid, 0.5 mg; biotin, 0.10 mg; thiamine, 2.5 mg; vitamin B12, 20 μg; Mn, 100 mg; Zn, 85 mg; Fe, 30 mg; Cu, 10 mg; I, 1.5 mg; Se, 0.2 mg; ethoxyquin, 100 mg.
Performance and Welfare Parameters
Performance parameters (BW; FI) were recorded on pen basis on days 0, 13, 27, and 42 of age. Daily weight gain (DWG), daily feed intake (DFI), and feed conversion rate (FCR) were calculated. FCR was estimated as the ratio of FI to DWG. Welfare traits included rectal temperature, mortality and incidences of FPD. Mortality was recorded daily on days 0 to 42 and expressed as percentage. Survivability was considered percentage of viable chickens. Rectal temperature was taken daily from 2 individuals per cage on days 32 to 42 (during HS) in both HS and TN groups. Incidence and severity of FPD was estimated on day 42 using a 3-point scale (0—no lesions, 1—mild lesions <0.8 cm, and 2—severe lesions) (Ekstrand et al., 1997). European broiler index (EBI) was calculated based on the following formula:
The experiment was ended on day 42 by slaughtering animals in a commercial slaughterhouse. The experimental procedures were approved by the Ministry of Health in Rome, Italy (no. 503/2016-PR).
Statistics
Performance and mortality data were evaluated by analysis of variance by 2-way ANOVA, in a 3 × 2 factorial design that included in ovo injection (GOS, S, or C) and ambient temperature condition (HS or TN) as factors. Pen was considered as biological replicate (n = 6). Post hoc comparisons between groups were performed with Tukey's HSD test. Differences were considered significant at P < 0.05. FPD were analyzed by chi-square test. Analyses were performed in SAS software (SAS Institute Inc., Cary, NC, USA). Figures were created in GraphPad Prism 7.00 for Windows (GraphPad Software, La Jolla California USA, www.graphpad.com).
RESULTS AND DISCUSSION
Many environmental factors in poultry farming, such as feeding, vaccination program or biosecurity measures are, to some extent, quite straightforward to standardize. Others, like intestinal microflora, are sometimes hard to predict and influence. The intestinal microflora contains the diverse populations of microorganisms that live along GIT in a close relation with the host. Research done in gnotobiotic animals show that the physiology and immunology of the host strongly depend on presence and composition of the microflora (Mitsuhiro and Jun-ichi, 1994; Volf et al., 2017). Dysbiosis is potentially dangerous during HS. Therefore, intestinal microflora cannot be neglected in modern farming practices. In this paper, we have used a GOS delivered in ovo to modulate intestinal microflora and influence performance and welfare traits of broiler chickens under HS.
Hatchability
Hatchability is one of the most important parameters that allow to estimate successful in ovo intervention. In this study, hatchability of the in ovo-injected eggs (90.2% in GOS and 89.2% in S) did not differ from the uninjected control eggs (90.8% in C). Standardized protocols for in ovo delivery of prebiotics on day 12 of egg incubation allows for high hatchability scores, because the bioactive solution is deposited in the air cell, from which the prebiotic diffuses into the bloodstream (Siwek et al., 2018). In ovo injection performed this way does not penetrate the inner parts of the egg, which could potentially disturb the embryo viability and decrease the hatchability.
Growth Performance
Table 2 presents effects of in ovo delivery (C vs. S. vs. GOS) and ambient temperature (TN vs. HS) on overall performance results of the broiler chickens in 3 feeding phases (starter, grower, and finisher). Body weight of the newly hatched chicks were significantly lower in S and GOS vs. C (45.3 and 45.2 g vs. 49.3 g; P < 0.01). Such negative effects of in ovo treatment were transient and did not last during starter and grower feeding phases (P > 0.05). On the contrary, there was a significant increase in BW on day 42 in GOS vs. C (2.892 kg in GOS vs. 2.758 kg in C; P < 0.01). We have demonstrated earlier that GOS delivered in ovo (alone or in synbiotic) triggers increase in the total activity of the pancreatic digestive enzymes—amylase, lipase, and trypsin (Pruszynska-Oszmalek et al., 2015). Improved digestive ability could explain improved growth performance of GOS.
Table 2.
Effects of in ovo treatment and ambient temperature on growth, feed intake, and feed conversion ratio in broiler chickens during starter, grower, and finisher feeding phases.
| Treatment (Tr) |
Temperature (T) |
Significance |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Trait | C1 | S2 | GOS3 | TN4 | HS5 | SEM | Tr | T | Tr × T |
| Starter (days 0–13) | |||||||||
| Chick body weight (g, day 0) | 49.3a | 45.3b | 45.2b | 46.4 | 46.8 | 0.94 | ** | NS | NS |
| Body weight (kg) | 0.428 | 0.412 | 0.428 | 0.426 | 0.419 | 0.006 | NS | NS | NS |
| Daily weight gain (g/bird/d) | 28.9 | 28.1 | 29.4 | 29.1 | 28.6 | 0.44 | NS | NS | NS |
| Daily feed intake (g/bird/d) | 37.0 | 36.2 | 37.1 | 37.0 | 36.5 | 0.46 | NS | NS | NS |
| FCR | 1.281 | 1.291 | 1.261 | 1.273 | 1.282 | 0.01 | NS | NS | NS |
| Mortality (%) | 2.28 | 0.65 | 0.98 | 1.30 | 1.30 | 0.02 | NS | NS | NS |
| Grower (days 14–27) | |||||||||
| Body weight (kg) | 1.56 | 1.53 | 1.58 | 1.56 | 1.55 | 0.016 | NS | NS | NS |
| Daily weight gain (g/bird/d) | 80.4 | 79.6 | 81.1 | 80.3 | 80.5 | 0.97 | NS | NS | NS |
| Daily feed intake (g/bird/d) | 116.8 | 113.6 | 116.9 | 116.1 | 115.4 | 1.04 | 0.054 | NS | NS |
| FCR | 1.452 | 1.428 | 1.442 | 1.447 | 1.436 | 0.02 | NS | NS | NS |
| Mortality (%) | 0.67 | 2.62 | 1.95 | 2.19 | 1.30 | 0.03 | NS | NS | NS |
| Finisher (days 28–42) | |||||||||
| Body weight (kg) | 2.76b | 2.79b | 2.89a | 3.11 | 2.52 | 0.029 | ** | ** | NS |
| Daily weight gain (g/bird/d) | 80.4b | 84.7a,b | 88.0a | 103.1 | 65.7 | 1.44 | ** | ** | NS |
| Daily feed intake (g/bird/d) | 165.7b | 168.7a,b | 173.2a | 185.3 | 153.2 | 2.05 | * | ** | NS |
| FCR | 2.148a | 2.054a,b | 2.016b | 1.799 | 2.347 | 0.03 | * | ** | * |
| Mortality (%) | 6.34 | 3.04 | 4.54 | 1.59 | 7.69 | 0.05 | NS | ** | NS |
| Total (days 0–42) | |||||||||
| Body weight (kg) | 2.76b | 2.79b | 2.89a | 3.11 | 2.52 | 0.029 | ** | ** | NS |
| Feed intake (kg) | 4.56 | 4.50 | 4.62 | 4.81 | 4.31 | 0.02 | NS | ** | NS |
| Daily weight gain (g/bird/d) | 64.3b | 64.8a,b | 66.8a | 71.79 | 58.77 | 0.66 | * | ** | NS |
| Daily feed intake (g/bird/d) | 108.6 | 107.3 | 110.01 | 114.6 | 102.6 | 0.99 | NS | ** | NS |
| FCR | 1.701a | 1.664a,b | 1.653b | 1.597 | 1.749 | 0.01 | * | ** | NS |
| Mortality (%) | 8.98 | 6.10 | 7.39 | 4.93 | 10.01 | 0.05 | NS | NS | NS |
| European Broiler Index | 350.0 | 368.6 | 377.4 | 427.6 | 303.1 | 11.7 | NS | ** | NS |
C = Control (untreated); 2 S = in ovo injected with physiological saline (mock-treated); 3 GOS (galactooligosaccharides) = in ovo injected with GOS (prebiotic-treated); 4 TN—thermoneutral conditions; 5 HS—heat stress conditions (on days 32–42); Significance: NS = P > 0.05; *P < 0.05; **P < 0.01
Means within a row lacking a common superscript differ (P < 0.05).
FCR, Feed conversion rate
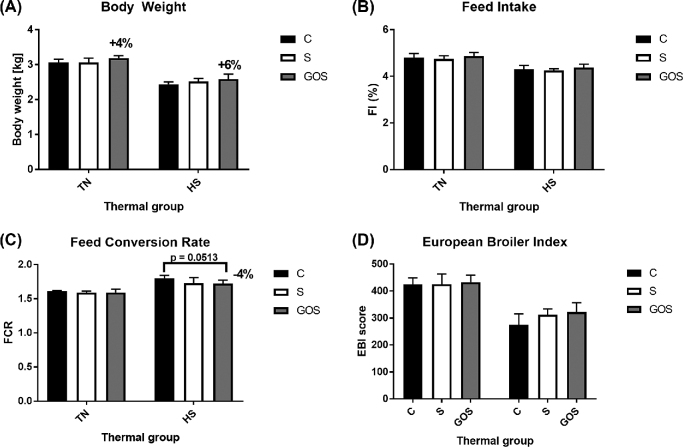
Thermal challenge was applied for the last 10 days of finisher feeding phase. Heat stress significantly reduced final BW (2.516 kg in TN vs. 3.110 kg in HS; P < 0.01). The DWG during the finisher feeding phase under HS conditions was reduced by as much as 37.4 g in HS vs. TN (P < 0.01). Loss in growth efficiency during HS could be explained by 32.13 g reduction in DFI in HS vs. TN (P < 0.01). We did not find statistical evidence whether GOS injected in ovo improved BW in HS chickens (P > 0.05 interaction between in ovo treatment and thermal challenge), but there was numerical improvement in BW on day 42 in both thermal conditions in GOS vs. C (120 g = 4% in TN and 150 g = 6% in HS; Figure 1).
Figure 1.
Performance parameters of broiler chickens injected in ovo with galactooligosaccharides in response to heat stress (day 42).
Dampened growth performance during heat is not surprising. Growth rate in broiler chickens mainly depends on the level of feed consumed. As described below, feed consumption and efficiency dropped rapidly in response to HS. It can be explained by the fact that digestion, absorption, and metabolism of the nutrients increase metabolic heat production. In high ambient temperature, animal needs to minimize the production rate of metabolic heat and therefore it reduces FI. Lei et al. (2013) demonstrated that heat-induced anorexia in broiler chickens is possibly mediated by the appetite-regulating peptides, such as ghrelin or cholecystokinin, secreted by the GIT. In addition, there is a difference in the level of metabolic heat production after consuming different nutrients. Protein-based diets induce more metabolic heat production in comparison to fat-based diet, because heat leads to increased protein catabolism (Renaudeau et al., 2012). In turn, endocrine alterations caused by heat, such as increased plasma corticosterone, leads to reduced lipolysis and enhanced fat accumulation, which decreases metabolic heat production (Lara and Rostagno 2013). Aside from limiting the rate of metabolic heat production through decreased FI, heat-stressed animals also increase energy expenditure for dissipating the heat. Chickens can decrease their body temperature through evaporation of water from lungs or air sac (respiratory or evaporative water loss), one of the dominant processes for holding body temperature in the normothermic zone. Those mechanisms of maintaining euthermia are energy-consuming (Weathers, 1981). Therefore, not only the animals reduce FI during HS but also increase energy use to cool down. Taken together, those mechanisms could have led to decreased growth performance in Ross broiler chickens under chronic HS.
Feed Intake and Efficiency
In ovo treatment had suggestive impact on DFI during grower phase (P ≈ 0.05) and significant impact on DFI and FCR during finisher phase (P < 0.05). There were no significant or numerical differences between treatment groups in feed performance in the starter feeding phase (P > 0.05). During finisher phase, DFI was significantly higher in GOS vs. C (173.2 g vs. 165.7 g; P < 0.05). As described earlier, such increase in DFI in the last period of rearing was efficiently used to enhance growth performance of the chickens. Single in ovo delivery of GOS on day 12 of eggs incubation resulted in improved FCR of broilers during finisher phase from 2.148 in C to 2.016 in GOS (P < 0.05). Overall FCR calculated for entire rearing period (days 0 to 42) was also improved by GOS, from 1.701 in C to 1.653 in GOS (P < 0.05). As widely expected, 10 days of thermal challenge deteriorated feed efficiency in finisher phase. DFI in finisher phase (days 28 to 42) was reduced by heat (P < 0.01) from 185.3 g in TN to 153.2 g in HS. Growth performance in those groups was even poorer, which resulted in FCR rising from 1.799 in TN to 2.347 in HS (P < 0.01). Applying thermal challenge during finisher phase also resulted in poorer FCR calculated for the entire rearing phase (days 0 to 42), which increased from 1.597 in TN to 1.749 in HS (P < 0.01). However, in this study we have demonstrated that in ovo delivery of GOS significantly improved FCR in HS animals during finisher phase (interaction between in ovo treatment and thermal challenge P <0.05). When we looked at the mean values of the entire rearing period (days 0 to 42; Figure 1), the numerical improvement of FCR in GOS vs. C in HS chickens was 0.08 FCR (+4% FCR in GOS vs. C) at statistically suggestive level (P = 0.0513). These results indicate that GOS delivery in ovo mitigated heat-induced decrease in growth performance and feed efficiency in broiler chickens.
In this study, we have pinpointed a correlation between intestinal health, feed efficiency, and response to heat based on the performance data. The mechanisms and various effects of in ovo stimulation with prebiotics (including GOS) have been described by Siwek et al. (2018). The GOS prebiotic delivered in ovo on day 12 of eggs incubation aims to selectively stimulate growth of the indigenous bacteria, which colonize the embryonic GIT and provide a life-long boost to intestinal health in broiler chickens. Slawinska et al. (2019a) has demonstrated that GOS delivered in ovo expressed bifidogenic effects in cecum, and also enhanced gut barrier function and intestinal physiology genes by modulating gene expression signatures in chicken GIT mucosa. One of the major aspects of improved intestinal health in response to heat enhanced gut integrity, which reduces risk of heat-induced endotoxemia. By maintaining strong barrier function of the gut, the tissues are protected from massive inflammatory responses to microbial toxins released from the gut. The anti-inflammatory effects of GOS delivered in ovo expressed in heat-stressed broiler chickens have been already demonstrated by Slawinska et al. (2019b).
European Broiler Index
Growth performance (expressed by DWG), feed efficiency (expressed by FCR), and health status of the stock (measured by % survivability) were used to calculate EBI, which is a unified index to assess the economic efficiency of broiler chickens (Marcu et al., 2013). Average EBI values were significantly influenced by heat, resulting in a nearly 30% decrease (from 427.59 in TN to 303.09 in HS) (P < 0.01). Influence of the thermal challenge on EBI value results from a difference in the growth (DWG) and feed performance (FCR) as well as mortality rate in different ambient temperatures. In this study, HS decreased DWG and increased FCR and mortality, which influence the loss of broiler efficiency during HS.
Body Temperature
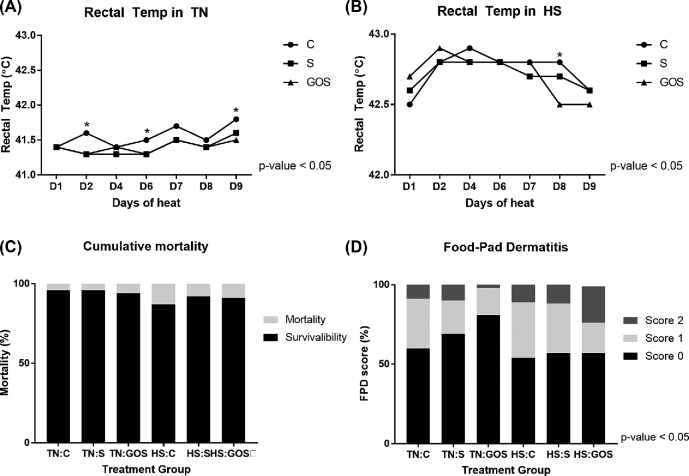
Body temperature is one of the factors that reflect the physiological response to heat and associated inflammation. Figure 2A and B shows the body temperature during the last 10 D of rearing (the measurements were taken from day 32 to 41) in TN (Figure 2A) and HS (Figure 2B). In TN, the average body temperature was fluctuating around 41.5°C, but we observed that C had the highest body temperature compared to S and GOS, especially on days 2 and 9 of measurements (P < 0.05). In TN conditions, the differences in the body temperature in control and treated groups result from the changes in the host physiology. The influence of the gut microflora on the body temperature is not fully understood. Kluger et al. (1990) found that rats that were given antibiotics to deplete their intestinal microflora had decreased body temperature, which was quite opposite to our findings. Chevalier et al. (2015) determined that the gut microflora adopts to cold treatment and modulates host's physiological and metabolic responses. Increased body temperature in C group, as found in this study, could be an indication of inflammatory status. However, this hypothesis was not confirmed by the data; blood level of immunoglobulins or serum amyloid A was comparable between GOS, S, and C groups (M. Bertocchi, personal communication).
Figure 2.
Effects of galactooligosaccharides delivered in ovo and heat stress induced during last 10 D of rearing on rectal temperature (days 32 to 42), mortality, and incidences of food-pad dermatitis in broiler chickens (day 42).
Thermoregulation in animals requires a thermal gradient to dissipate the heat. It means that the body temperature must be higher than the ambient temperature for effective cooling of the organism (Collier et al., 2019). Heat stress induced by elevated ambient temperature led to increased body temperature in all groups; the highest body temperature was found in GOS on day 2 and in C on day 3. However, GOS reached physiological body temperature (42.5°C) 1 day earlier than C and S (P < 0.05), which suggests that those birds recovered earlier from the high environmental temperature. Cooper and Washburn (1998) reported a strong negative correlation between body temperature during HS and traits of economic importance such as growth performance and feed efficiency. In this study, we also confirmed that HS increased body temperature and decreased performance of broiler chickens. However, explaining the effects of GOS and HS on the mechanisms of the thermoregulation would require investigating gut-brain axis and endocrine responses.
Mortality
Mortality is one of the basic welfare parameters that indicate the health status of the flock. Figure 2C shows mortality rates by experimental group and feeding phase. In this study, thermal challenge increased mortality among the broiler chickens by five times during finisher phase (from 1.59% in TN to 7.69% in HS; P < 0.05). In the entire rearing period (days 0 to 42), mortality due to heat was doubled (from 4.93% in TN to 10.1% in HS; P > 0.05) (Table 2). Both acute and chronic HS triggers mortality in broiler chickens, which is often associated with heart failure (Olkowski, 2007), ascites (Deeb et al., 2002) or cardiopulmonary disorders (Sandercock et al., 2006). Broiler chickens are not adjusted to handle high temperatures due to their body composition. Fast-growing broiler chickens can be twice as large as slow-growing ones, but the proportionate increase in size of their organs has not been achieved through selective breeding. Increased mortality of broiler chickens during heat is a major economic and welfare concern. The heat can be detrimental in form of heat waves in moderate climates, more frequent due to climatic changes (acute HS). Chronic HS often occurs in tropical regions, where it deteriorates broiler chicken production. Furthermore, there is a particular risk of losing animals at market age during transit, when they rely solely on the microenvironment of the transport infrastructure (Mitchell and Kettlewell, 1998).
There are different approaches proposed to minimize such losses. One is to increase adaptation of the individuals to the elevated temperatures by thermal conditioning of the incubating eggs (Moraes et al., 2003). Another is genetic breeding of the featherless mutants or admixture of the genotypes of heat-resistant breeds (e.g., Fayoumi or Naked-neck) (Bekele et al., 2010). In this study we proposed an approach in which the intestinal health was targeted for better adaptation to the HS. Intestinal epithelia take part in digestion and absorption of the nutrients as well as provide environmental niche for mucosal microflora that adheres to the gut walls. Together with the mucous layer, intestinal epithelia create physical barrier between gut content and the milieu of the body. Gut barrier function relies to a large extent on intestinal health and it is sensitive to heat. Heat stress immediately disrupts tight junctions and leaks intestinal content into the milieu of the body, triggering systemic inflammatory responses (Tellez et al., 2017). Acute inflammation together with spiraling hyperthermia leads to heat stroke and sudden deaths. We have determined that GOS delivered in ovo increases physical and immunological barriers of the gut by increased expression of the genes encoding tight junctions, mucin and host defense peptides in broiler chickens (Slawinska et al., 2019a). We have also demonstrated that GOS dampened systemic inflammatory responses in hyperthermic animals (Slawinska et al., 2019b). In this manner, we may conclude that in ovo nutritional strategy using GOS may be useful to improve welfare standards in broiler chickens. However, it should be accompanied with other measures to prevent deteriorating HS effects.
Food-Pad Dermatitis
Foot lesions, such as FPD, are currently considered leading welfare issue in poultry farming. The origin of FPD is quite complex, as it is believed to result from several factors with particular regard to litter moisture, nutrition, and genetic susceptibility (Shepherd and Fairchild, 2010). In this study, we have determined that GOS delivered in ovo decreased prevalence of FPD in TN conditions by 20% (no lesions in 81% in GOS vs. 60% in C) (Figure 2D). The necrotic lesions that occur in FPD result from skin inflammation. Low-level inflammatory responses are characteristic to the dysbiotic individuals. In our earlier studies, we have demonstrated that GOS (or GOS-based synbiotic) delivered in ovo dampens pro-inflammatory gene expression signatures in chicken gut-associated lymphoid tissue (i.e., cecal tonsils) (Slawinska et al., 2016; Dunislawska et al., 2017). Another possible mechanism of decreased skin inflammation in GOS is improved gut barrier function upon in ovo delivery of GOS (Slawinska et al., 2019a). The beneficial, anti-inflammatory effects of GOS delivered in ovo were not maintained during HS. Reversely, the incidences of the most severe foot lesions were most prevalent in GOS vs. C (23% in GOS vs. 11% in C). We did not measure water consumption during our study, but we suppose that the increased FI in GOS during HS could have been accompanied by elevated water consumption, which in turn caused higher litter moisture. We recommend that in ovo stimulation with GOS should be complemented with either nutrition or environmental intervention that could bring the litter moisture down during heat.
CONCLUSIONS
In this study, we have demonstrated beneficial effects of GOS delivered in ovo on day 12 of egg incubation in broiler chickens challenged with heat. In TN conditions, GOS increased overall growth performance and feed efficiency and improved FPD score. GOS delivered in ovo significantly reduced harmful effects of hyperthermia on feed efficiency during finisher feeding phase. Regarding welfare traits, stimulation with GOS dampened body temperature during TN and HS and numerically improved survivability during HS. In summary, improved intestinal health in broiler chickens is a key factor in mitigating negative effects of the environmental stressors. Early modulation of the intestinal microbiota using in ovo stimulation with GOS may represent an efficient and cost-effective method to improve a number of traits, including performance and welfare.
ACKNOWLEDGEMENTS
The research was supported by OVOBIOTIC project (grant no. RBSI14WZCL) from Ministry of Education, Universities and Research (MIUR) in Rome, Italy.
REFERENCES
- Bednarczyk M., Stadnicka K., Kozlowska I., Abiuso C., Tavaniello S., Dankowiakowska A., Slawinska A., Maiorano G. Influence of different prebiotics and mode of their administration on broiler chicken performance. Animal. 2016;10:1271–1279. doi: 10.1017/S1751731116000173. [DOI] [PubMed] [Google Scholar]
- Bednarczyk M., Urbanowski M., Gulewicz P., Kasperczyk K., Maiorano G., Szwaczkowski T. Field and in vitro study on prebiotic effect of raffinose family oligosaccharides in chickens. B. Vet. I Pulawy. 2011;55:465–469. [Google Scholar]
- Bekele F., Ådnøy T., Gjøen H.M., Kathle J., Abebe G. Production performance of dual purpose crosses of two indigenous with two exotic chicken breeds in sub-tropical environment. Int. J. Poult. Sci. 2010;9:702–710. [Google Scholar]
- Burkholder K.M., Thompson K.L., Einstein M.E., Applegate T.J., Patterson J.A. Influence of stressors on normal intestinal microbiota, intestinal morphology, and susceptibility to Salmonella enteritidis colonization in broilers. Poult. Sci. 2008;87:1734–1741. doi: 10.3382/ps.2008-00107. [DOI] [PubMed] [Google Scholar]
- Chevalier C., Stojanović O., Colin D.J., Suarez-Zamorano N., Tarallo V., Veyrat-Durebex C., Rigo D., Fabbiano S., Stevanović A., Hagemann S., Montet X., Seimbille Y., Zamboni N., Hapfelmeier S., Trajkovski M. Gut microbiota orchestrates energy homeostasis during cold. Cell. 2015;163:1360–1374. doi: 10.1016/j.cell.2015.11.004. [DOI] [PubMed] [Google Scholar]
- Collier R.J., Baumgard L.H., Zimbelman R.B., Xiao Y. Heat stress: physiology of acclimation and adaptation. Anim. Front. 2019;9:12–19. doi: 10.1093/af/vfy031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper M., Washburn K. The relationships of body temperature to weight gain, feed consumption, and feed utilization in broilers under heat stress. Poult. Sci. 1998;77:237–242. doi: 10.1093/ps/77.2.237. [DOI] [PubMed] [Google Scholar]
- Deeb N., Shlosberg A., Cahaner A. Genotype-by-environment interaction with broiler genotypes differing in growth rate. 4. Association between responses to heat stress and to cold-induced ascites. Poult. Sci. 2002;81:1454–1462. doi: 10.1093/ps/81.10.1454. [DOI] [PubMed] [Google Scholar]
- Donkoh A. Ambient temperature: a factor affecting performance and physiological response of broiler chickens. Int. J. Biometeorol. 1989;33:259–265. doi: 10.1007/BF01051087. [DOI] [PubMed] [Google Scholar]
- Drain M.E., Whiting T.L., Rasali D.P., D'Angiolo V.A. Warm weather transport of broiler chickens in Manitoba. I. Farm management factors associated with death loss in transit to slaughter. Can. Vet. J. 2007;48:76–80. [PMC free article] [PubMed] [Google Scholar]
- Dunislawska A., Slawinska A., Stadnicka K., Bednarczyk M., Gulewicz P., Jozefiak D., Siwek M. Synbiotics for broiler chickens – in vitro design and evaluation of the influence on host and selected microbiota populations following in ovo delivery. PLoS One. 2017;12 doi: 10.1371/journal.pone.0168587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrand C., Algers B., Svedberg J. Rearing conditions and foot-pad dermatitis in Swedish broiler chickens. Prev. Vet. Med. 1997;31:167–174. [PubMed] [Google Scholar]
- Imaeda N. Influence of the stocking density and rearing season on incidence of sudden death syndrome in broiler chickens. Poult. Sci. 2000;79:201–204. doi: 10.1093/ps/79.2.201. [DOI] [PubMed] [Google Scholar]
- Kluger M.J., Conn C.A., Franklin B.R.E.N.D.A., Freter R.O.L.F., Abrams G.D. Effect of gastrointestinal flora on body temperature of rats and mice. Am. J. Physiol. 1990;258:552–557. doi: 10.1152/ajpregu.1990.258.2.R552. [DOI] [PubMed] [Google Scholar]
- Lara L., Rostagno M. Impact of heat stress on poultry production. Animals. 2013;3:356–369. doi: 10.3390/ani3020356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei L., Hepeng L., Xianlei L., Hongchao J., Hai L., Sheikhahmadi A., Yufeng W., Zhigang S. Effects of acute heat stress on gene expression of brain–gut neuropeptides in broiler chickens (Gallus gallus domesticus) J. Anim. Sci. 2013;91:5194–5201. doi: 10.2527/jas.2013-6538. [DOI] [PubMed] [Google Scholar]
- Leon L.R., Helwig B.G. Heat stroke: role of the systemic inflammatory response. J. Appl. Physiol. 2010;109:1980–1988. doi: 10.1152/japplphysiol.00301.2010. [DOI] [PubMed] [Google Scholar]
- Marcu A., Vacaru-Opriş I., Dumitrescu G., Ciochina L.P., Marcu A., Nicula M., Peţ I., Dronca D., Kelciov B. The influence of the genotype on economic efficiency of broiler chickens growth. Sci. Pap. Anim. Scie. Biotech. 2013;46:339–346. [Google Scholar]
- Mellata M. Human and avian extraintestinal pathogenic Escherichia coli: infections, zoonotic risks, and antibiotic resistance trends. Foodborne Pathog. Dis. 2013;10:916–932. doi: 10.1089/fpd.2013.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell M., Kettlewell P. Physiological stress and welfare of broiler chickens in transit: solutions not problems! Poult. Sci. 1998;77:1803–1814. doi: 10.1093/ps/77.12.1803. [DOI] [PubMed] [Google Scholar]
- Mitsuhiro F., Jun-ichi O. Nutritional and physiological characteristics in germ-free chickens. Comp. Biochem. Physiol. A Physiol. 1994;109:547–556. [PubMed] [Google Scholar]
- Moraes V., Malheiros R., Bruggeman V., Collin A., Tona K., Van As P., Onagbesan O., Buyse J., Decuypere E., Macari M. Effect of thermal conditioning during embryonic development on aspects of physiological responses of broilers to heat stress. J. Therm. Biol. 2003;28:133–140. [Google Scholar]
- Olkowski A. Pathophysiology of heart failure in broiler chickens: structural, biochemical, and molecular characteristics. Poult. Sci. 2007;86:999–1005. doi: 10.1093/ps/86.5.999. [DOI] [PubMed] [Google Scholar]
- Olsen R., Kudirkiene E., Thøfner I., Pors S., Karlskov-Mortensen P., Li L., Papasolomontos S., Angastiniotou C., Christensen J. Impact of egg disinfection of hatching eggs on the eggshell microbiome and bacterial load. Poult. Sci. 2017;96:3901–3911. doi: 10.3382/ps/pex182. [DOI] [PubMed] [Google Scholar]
- Pruszynska-Oszmalek E., Kolodziejski P.A., Stadnicka K., Sassek M., Chalupka D., Kuston B., Nogowski L., Mackowiak P., Maiorano G., Jankowski J., Bednarczyk M. In ovo injection of prebiotics and synbiotics affects the digestive potency of the pancreas in growing chickens. Poult. Sci. 2015;94:1909–1916. doi: 10.3382/ps/pev162. [DOI] [PubMed] [Google Scholar]
- Renaudeau D., Collin A., Yahav S., De Basilio V., Gourdine J., Collier R.J. Adaptation to hot climate and strategies to alleviate heat stress in livestock production. Animal. 2012;6:707–728. doi: 10.1017/S1751731111002448. [DOI] [PubMed] [Google Scholar]
- Samberg Y., Meroz M. Application of disinfectants in poultry hatcheries. Revue Scientifique et Technique-Office International des Epizooties. 1995;14:365. doi: 10.20506/rst.14.2.849. [DOI] [PubMed] [Google Scholar]
- Sandercock D., Hunter R., Mitchell M., Hocking P. Thermoregulatory capacity and muscle membrane integrity are compromised in broilers compared with layers at the same age or body weight. Brit. Poult. Sci. 2006;47:322–329. doi: 10.1080/00071660600732346. [DOI] [PubMed] [Google Scholar]
- Shepherd E., Fairchild B. Footpad dermatitis in poultry. Poult. Sci. 2010;89:2043–2051. doi: 10.3382/ps.2010-00770. [DOI] [PubMed] [Google Scholar]
- Siwek M., Slawinska A., Stadnicka K., Bogucka J., Dunislawska A., Bednarczyk M. Prebiotics and synbiotics – in ovo delivery for improved lifespan condition in chicken. BMC Vet. Res. 2018;14:402. doi: 10.1186/s12917-018-1738-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slawinska A., Plowiec A., Siwek M., Jaroszewski M., Bednarczyk M. Long-term transcriptomic effects of prebiotics and synbiotics delivered in ovo in broiler chickens. PLoS One. 2016;11 doi: 10.1371/journal.pone.0168899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slawinska A., Dunislawska A., Plowiec A., Radomska M., Lachmanska J., Siwek M., Tavaniello S., Maiorano G. Modulation of microbial communities and mucosal gene expression in chicken intestines after galactooligosaccharides delivery in ovo. PLoS One. 2019;14 doi: 10.1371/journal.pone.0212318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slawinska A., Mendes S., Dunislawska A., Siwek M., Zampiga M., Sirri F., Meluzzi A., Tavaniello S., Maiorano G. Avian model to mitigate gut-derived immune response and oxidative stress during heat. Biosystems. 2019;178:10–15. doi: 10.1016/j.biosystems.2019.01.007. [DOI] [PubMed] [Google Scholar]
- Sugiharto S., Yudiarti T., Isroli I., Widiastuti E., Kusumanti E. Dietary supplementation of probiotics in poultry exposed to heat stress–a review. Ann. Anim. Sci. 2017;17:591–604. [Google Scholar]
- Swiatkiewicz S., Arczewska-Wlosek A., Jozefiak D. The nutrition of poultry as a factor affecting litter quality and foot pad dermatitis–an updated review. J. Anim. Physiol. Anim. Nutr. 2017;101:e14–e20. doi: 10.1111/jpn.12630. [DOI] [PubMed] [Google Scholar]
- Tavaniello S., Slawinska A., Prioriello D., Petrecca V., Bertocchi M., Sirri F., Salvatori G., Maiorano G. Effect of galactooligosaccharides delivered in ovo on meat quality traits of broiler chickens exposed to heat stress. Poult. Sci. 2019 doi: 10.3382/ps/pez556. (submitted). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellez G., Jr, Tellez-Isaias G., Dridi S. Heat stress and gut health in broilers: role of tight junction proteins. Adv. Food Technol. Nutr. Sci. Open J. 2017;3:e1–e4. [Google Scholar]
- Tzortzis G., Goulas A.K., Gibson G.R. Synthesis of prebiotic galactooligosaccharides using whole cells of a novel strain, Bifidobacterium bifidum NCIMB 41171. Appl. Microbiol. Biotechnol. 2005;68:412–416. doi: 10.1007/s00253-005-1919-0. [DOI] [PubMed] [Google Scholar]
- Varasteh S., Braber S., Akbari P., Garssen J., Fink-Gremmels J. Differences in susceptibility to heat stress along the chicken intestine and the protective effects of galacto-oligosaccharides. PLoS One. 2015;10 doi: 10.1371/journal.pone.0138975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volf J., Polansky O., Sekelova Z., Velge P., Schouler C., Kaspers B., Rychlik I. Gene expression in the chicken caecum is dependent on microbiota composition. Vet. Res. 2017;48:85. doi: 10.1186/s13567-017-0493-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warriss P., Pagazaurtundua A., Brown S. Relationship between maximum daily temperature and mortality of broiler chickens during transport and lairage. Brit. Poult. Sci. 2005;46:647–651. doi: 10.1080/00071660500393868. [DOI] [PubMed] [Google Scholar]
- Weathers W.W. Physiological thermoregulation in heat-stressed birds: consequences of body size. Physiol. Zool. 1981;54:345–361. [Google Scholar]