Abstract
Wheat bran, while a nutritious and economic feed ingredient, contents high levels of non-starch polysaccharides which entraps nutrients and interferes digestion and absorption. To study the influence of fermented wheat bran by xylanase-producing Bacillus cereus on growth performance and intestinal microflora of broiler chickens, a total of 180 broilers (21-day-old, mixed of male and female) were randomly divided into 3 treatments, with 6 replicates in each treatment and 10 broilers in each replicate: 1) control check (CK), corn-soybean meal-based diet; 2) wheat bran group (WB), 5% of the corn were replaced with wheat bran; and 3) fermented wheat bran group (FWB), 5% of the corn were replaced with fermented wheat bran. Growth performance was determined in the period of 21- to 42-day-old. Intestinal digestive enzyme activities and microbiota diversity were analyzed on day 42. No differences were observed on growth performance among treatments (P > 0.05). The activity of amylase in the duodenum of FWB was 1.56 times higher than CK (P < 0.05). The Chao1 index of microbiota in cecum of FWB increased 24.26% compared with CK (P < 0.01). The amount of Bifidobacteriaceae in cecum of WB was 29.1 times and 15.8 times higher than CK and FWB (P < 0.05) respectively. Principal co-ordinates analysis in cecum revealed the dissimilarity microbiota among treatments. In summary, the use of fermented wheat bran to partially replace corn (5%) in diets had no adverse effect on growth performance and triggered beneficial effects such as increasing duodenal amylase activity and intestinal microflora abundance in broiler chickens. These observations support that solid-state fermentation by xylanase-producing Bacillus cereus is feasible approach to pre-treat wheat bran for feedstuff industry.
Key words: Bacillus cereus, broiler, microbiota, solid-state fermentation, wheat bran
INTRODUCTION
Byproducts such as wheat bran derived from food processing are traditional feedstuff materials. Although they contain multiple nutrients including vitamins and anti-oxidative compounds, the entrapment of these nutrients by hemicellulose xylan and other non-starch polysaccharides (NSP) blocks animal's digestion and absorption (Hemery et al., 2007; Olukosi and Adeola, 2008). NSP can cause signals of satiety after the formation of chyme through water swelling in gut (Leo et al., 2012), and affect the composition of intestinal microorganism (Koropatkin et al., 2012). Recent advances in breaking these NSP by enzymes or probiotics rekindles interests in using wheat bran and other food processing byproducts in animal diets (Apprich et al., 2014; Koegelenberg and Chimphango, 2017; Gallardo et al., 2018). Challenges of formulating wheat bran into diets come from the anti-nutrition effects of the enzymatic digestion products of NSP. Efficient methods that would help to deplete xylose and xylan in wheat bran are of particular interest in the poultry industry.
Xylan, a main component of hemicellulose in the cell wall of wheat bran, releases xylose after digested by xylanase (Prückler et al., 2014). Previous studies have shown that dietary supplementation of xylanase can effectively degrade xylan in poultry diets, not only releasing nutrients, but reducing viscosity of chyme and colonization of pathogenic microorganisms (Choct et al., 1995; Bedford, 1996; Bedford and Morgan, 1996). However, the cost of purified enzymes and the meticulous digestion conditions highly limited the filed application of lab-scale wheat bran pre-treating procedures.
In the current study, B.cereus W-3 was used to prepare fermented wheat bran. Feed trial was conducted to evaluate the effects of dietary inclusion of fermented wheat bran on growth performance and intestinal microbiota alterations in broiler chickens. The aim was to certify the application of B.cereus fermented wheat bran in broiler's diets, and provide new research ideas and techniques for the effective use of wheat bran in feed industry.
MATERIALS AND METHODS
Materials and Reagents
Longhai Industrial Co. Ltd in Shanxi, China offered all broilers. Corn, soybean meal, wheat bran, soybean oil, and fat powder were purchased from Lihua Feed Factory in Shanxi, China; and other materials of diets came from Yangling Do Better-Victory Biotechnology Co. Ltd in Shaanxi, China. Products like pectin, starch, xylan, 3,5-dinitrosalicylic acid (DNS) and inorganic reagents were all ordered from Sigma-Aldrich Trading Co. Ltd in Missouri, USA.
Screening and Identification of Microorganism
Liquid media for bacterial culture contained the following components: beef extract 5 g, peptone 10 g, NaCl 5 g, starch 10 g (another 10 g of agar was required for solid media), distilled water 1 L, and adjusted pH to 6.5 to 7.0 before autoclaved.
Soil samples which were used for the isolation of strains were supplied by University of Little Rock in America. The prepared inoculum was incubated from single colony of Bacillus isolates purified with dilution separation method on the solid media. Then the bacteria solution for solid-state fermentation (SSF) of each colony was obtained by incubated overnight at 37°C and adjusted OD600 to 1.0 with liquid media. The SSF was conducted in 100 mL autoclaved flasks containing 20 g of wheat bran and added 5 mL bacteria solution. After thoroughly mixed and incubated at 37°C for 48 h, DNS colorimetric method (Miller, 1959) was used to measure the amount of reducing sugar produced by enzymes with the purpose to screen out the strain with the highest fermentation ability. Pectin, starch and xylan were used as substrates to evaluate the activity of pectinase, amylase and xylanase produced during SSF respectively in order to ascertain the optimal fermentation time.
For the explicit taxonomic position of this strain, 16S rDNA sequence was obtained and compared with 16S rRNA sequences database by the Basic Local Alignment Search Tool in NCBI (https://blast.ncbi.nlm.nih.gov/Blast.cgi). The genetic distance and phylogenetic tree were estimated by using MEGA 7.0 software.
Diets Processing and Feeding
All protocols were approved by the animal care committee of Shanxi Agricultural University. The intestine of broiler develops gradually in 0 to 21 d, and it is easy for broilers to get sick with the insufficient endogenous digestive system. Besides the larger feed consumption during 21 to 42 d compared with 0 to 21 d can bring greater economic benefits. Therefore, the feeding trial did not include the first 21 d. 200 of broilers (14-day-old, mixed of male and female) were bought and adaptively raised with normal diets for 7 d at feeding center of Shanxi Agricultural University. On day 21, 180 healthy broilers were selected and randomly divided into 3 treatments with 6 replicates in each treatment and 10 broilers in each replicate (Body weight was 7.82 ± 0.076 kg per replicate). Broilers in control check (CK) were fed with normal diets. While broilers in wheat bran group (WB) and fermented wheat bran group (FWB) were fed with diets where 5% corn was replaced with wheat bran and fermented wheat bran, respectively. The materials and nutritional components of different diets were shown in Table 1. After incubated in a 37°C shaker overnight, SSF was carried out with 100 OD W-3 solution and 100 mL sterile water in per 1 kg wheat bran at 37°C shakers for 72 h. Fermented wheat bran was made every 3 d to keep fresh. The routine nutrient of wheat bran and fermented wheat bran was analyzed and the result was shown in Table 2. Each replicate was fed and weighted separately. The contrast feeding test sustained from 21 to 42 d with free intake of diets and water overall process, and all broilers were hungered for 12 h at beginning and end of feeding to avoid the impact of chyme on experiments.
Table 1.
Dietary ingredient and nutrient composition based on air dry.1
| Diet materials composition Energy density | CK | WB | FWB |
|---|---|---|---|
| Ingredient (g/kg) | |||
| Corn | 673.6 | 619.2 | 615.4 |
| Soybean meal | 260.4 | 252.8 | 256.2 |
| Wheat bran | − | 50.0 | − |
| Fermented wheat bran | − | − | 50.0 |
| Soybean oil | 30.7 | 30.7 | 30.7 |
| Fatty powder | − | 11.9 | 12.5 |
| Stone powder | 13.3 | 13.4 | 13.4 |
| CaHPO4 | 10.8 | 10.5 | 10.5 |
| Lysine | 3.3 | 3.4 | 3.3 |
| Methionine | 1.2 | 1.3 | 1.3 |
| Threonine | 0.6 | 0.7 | 0.6 |
| Salt | 2.5 | 2.5 | 2.5 |
| Choline | 2.0 | 2.0 | 2.0 |
| Mineral mixture2 | 1.0 | 1.0 | 1.0 |
| Vitamin premix3 | 0.3 | 0.3 | 0.3 |
| Anti-oxidant | 0.3 | 0.3 | 0.3 |
| Calculated nutritional content (%) | |||
| Metabolizable energy (kcal/kg) | 3100 | 3100 | 3100 |
| Crude protein | 17.50 | 17.50 | 17.50 |
| Calcium | 0.80 | 0.80 | 0.80 |
| Total phosphorus | 0.52 | 0.54 | 0.54 |
| Absorbed phosphorus | 0.30 | 0.30 | 0.30 |
| Salt | 0.28 | 0.28 | 0.28 |
| Lysine | 1.10 | 1.10 | 1.10 |
| Methionine | 0.40 | 0.40 | 0.40 |
| Methionine and cystine | 0.71 | 0.71 | 0.71 |
| Threonine | 0.75 | 0.75 | 0.75 |
| Tryptophan | 0.21 | 0.21 | 0.21 |
CK: Control check; WB: Wheat bran group; FWB: fermented wheat bran group.
The mineral mixture provides following per kg of diets: Cu (as copper sulfate), 8 mg; Fe (as ferrous sulfate), 80 mg; Zn (as zinc sulfate), 80 mg; Mn (as manganese sulfate), 100 mg; I (as calcium iodide), 0.35 mg; Se (as sodium selenite), 0.30 mg.
The vitamin premix provides following per kg of diets: vitamin A, 18,000 IU; vitamin D3, 2,000 IU; vitamin E, 20 IU; vitamin K, 0.5 mg; thiamine, 2 mg; riboflavin, 8 mg; vitamin B3, 10 mg; vitamin B5, 35.0 mg; vitamin B6, 3.5 mg; biotin, 0.18 mg, folic acid, 0.55 mg; vitamin B12, 0.01 mg.
Table 2.
Routine nutrient of wheat bran and fermented wheat bran.
| Item | Wheat bran | Fermented wheat bran | SEM | P value |
|---|---|---|---|---|
| Dry matter1, % | 92.29 | 92.20 | 0.050 | 0.268 |
| Crude protein, %DM | 13.95 | 14.95 | 0.757 | 0.449 |
| Starch, %DM | 5.76 | 5.67 | 0.223 | 0.792 |
| Crude fiber, %DM | 10.15 | 9.51 | 0.334 | 0.246 |
| Natural detergent fiber, %DM | 46.15 | 43.64 | 0.776 | 0.084 |
| Acid detergent fiber, %DM | 10.47 | 10.51 | 0.904 | 0.978 |
| Hemicellulose, %DM | 35.67 | 33.13 | 0.766 | 0.078 |
Dry matter was calculated based on air dry.
Data Measurement
Performance
Body weight and feed consumption of each replicate were recorded in the morning of 21 and 42 d. Average weight gain (AWG), average feed intake (AFI), and feed conversion rate (FCR) of each replicate were calculated accordingly.
Gathering Intestinal Contents
One broiler was randomly selected from each replicate, and duodenum, jejunum, ileum, and cecum were ligated after humanely sacrificing and dissecting. Luminal contents of each intestinal segment were collected into 3 EP tubes for enzyme activity analysis, microbiota diversity analysis, and samples' back-up, respectively.
Enzyme Activity of Intestinal Contents
Immediately after the collection of intestinal luminal samples, protease inhibitor and sterile water were added, and enzyme solutions were obtained by mixing and centrifugation. A total of 18 samples with 3 treatments and 6 samples in each treatment were obtained. DNS colorimetric method was used to measure the amount of reducing sugar produced by enzymes using pectin, starch, and xylan as substrates, respectively, with the purpose to evaluate the activity of enzyme solution. The standard curve of absorption-reducing sugar was prepared with the same method using glucose standard solution as the substrate, and the enzyme activity was expressed by the amount of reducing sugar produced in one minute (ng/min).
Diversity of Intestinal Microbiota
The digestive juice changes rapidly from acid to neutral in duodenum, where a lot of digestive enzymes are secreted (protease, lipase, and carbohydrase for example). These are challenges for microorganisms to survive and pass duodenum successfully. Cecum is the main place for chyme fermentation where microorganisms play a crucial role. Accordingly, the microbiota diversity and evolutionary variance of duodenum and cecum in all treatments were analyzed. High-throughput sequencing was carried out to obtain the total 16S rDNA sequences of intestinal flora. After sampling of contents in different intestinal segments of all treatments, bacterial genomic DNA of these contents were extracted and universal primer pair 338F (5′-ACT CCT ACG GG AGG CAG CAG-3′) and 806R (5′-GGA CTA CHV GGG TWT CTA AT-3′) were used to amplify V3-V4 regions of genomic 16S rDNA, following the examination and collection of PCR products by 2% agarose gel electrophoresis. With quantitative detection by QuantiFluor™-ST blue fluorescence quantitative system (Promega, Madison, USA), sequences of 16S rDNA fragment were obtained and summed up with Miseq platform (Illumina, San Diego, USA).
Calculations and Statistic Analysis
After preliminary arrangement of data with Microsoft Excel 2017, one-way ANOVA in SPSS 24.0 was used to analyze the dissimilarity among treatments, where after Duncan multiple comparative test was proceeded for the data with significant imparity. Values of P < 0.05 were considered statistically remarkable. The intestinal microbiota diversity analysis was carried out on the Majorbio I-Sanger Cloud Platform (https://www.i-sanger.com).
RESULTS
Screening Xylanase-producing Microorganism
30 strains were isolated that grew robustly on plates and measured the activity of pectinase, amylase and xylanase of these strains grown in liquid medium. After comprehensive analysis, strain W-3 showed highest activity in xylanase and was chosen to conduct SSF of wheat bran. Fig. 1 summarizes the enzymatic activities of W-3 strain at different time points during SSF. After 96 h of fermentation, pectinase, amylase, and xylanase had the highest activity at 1.297, 2.018, and 1.923 ng/min, respectively. There were also a few other strains that performed SSF. However, they did not produce higher enzymatic activities than W-3 or their enzymatic activities did not peak synchronously (date not shown). Thus, following studies were conducted using W-3 strain.
Figure 1.
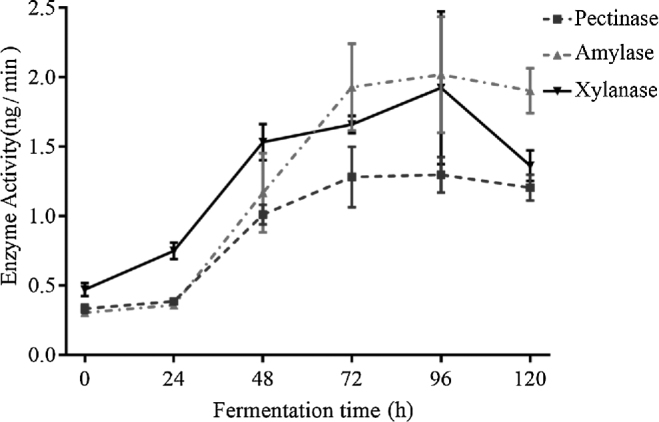
Activity of pectinase, amylase and xylanase secreted by Bacillus cereus W-3 in different fermentation time. Bars represent standard deviation in each time point.
Identification of W-3 Strain
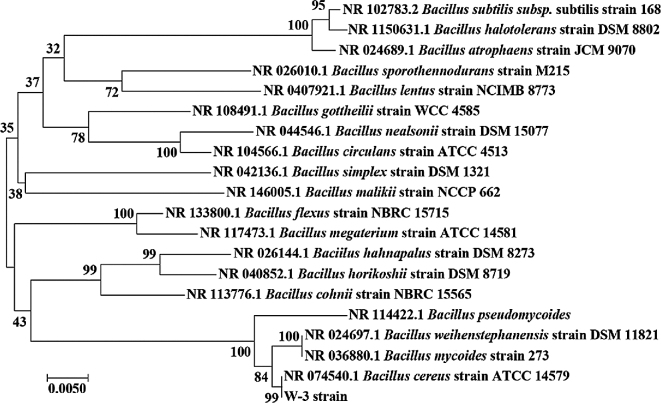
For identification, the 16S rDNA sequence of W-3 strain was obtained and a phylogenic analysis was conducted as was shown in Fig. 2. Indeed, W-3 presented the closest evolutionary relationship with Bacillus cereus. Recent studies have proposed some unique beneficial effects of B.cereus as probiotics (Duraisamy et al., 2018). Thus, B.cereus W-3 strain was used to ferment wheat bran (as shown in Fig. 1) to substitute a portion of corn in diets of broilers.
Figure 2.
Phylogenetic analysis of Bacillus cereus W-3. The evolutionary history is inferred with the Neighbor-Joining method. The percentage of replicate trees in which the associated taxa cluster together in the bootstrap test of 1,000 replicates are shown next to the branches. The tree is drawn to scale, with branch lengths to infer the evolutionary distances, which are computed using the Maximum Composite Likelihood method.
Substitution of Corn with Fermented Wheat Bran
For the corn-based diet, 5% of corn was substituted by wheat bran or fermented wheat bran, and the three experimental diets were fed to evaluate the effects on AFI, AWG, and FCR in the period of 21 to 42 d (Table 3). Although directly adding wheat bran had a trend to decrease feed intake and weight gain, no differences were found among all treatments in AFI, AWG, and FCR. Besides, no side or toxic effects such as vomiting or diarrhea were observed in broilers throughout the study.
Table 3.
Effect of different diets on performance of broilers during 21 and 42 d.1
| Index2 | CK | WB | FWB |
|---|---|---|---|
| AFI (kg) | 2.85 ± 0.050 | 2.63 ± 0.085 | 2.77 ± 0.075 |
| AWG (kg) | 1.47 ± 0.057 | 1.36 ± 0.066 | 1.50 ± 0.051 |
| FCR | 1.94 ± 0.044 | 1.93 ± 0.042 | 1.84 ± 0.014 |
CK: Control check; WB: Wheat bran group; FWB: fermented wheat bran group. AFI: average feed intake; AWG: average weight gain; FCR: feed conversion ratio.
All results are shown with mean ± standard error.
Effect of Fermented Wheat Bran on Intestinal Enzymes
To appraise if fermented wheat bran had any beneficial effect, intestinal enzyme activities were measured and shown in Fig. 3. In contrast with CK, the amylase activity of FWB had a conspicuous increase from 0.44 to 1.14 ng/min (P < 0.05), and the xylanase activity of FWB was promoted from 0.52 to 0.84 ng/min in duodenum (P = 0.071). No differences were observed on digestive enzymes in other segments of all treatments (P > 0.05).
Figure 3.
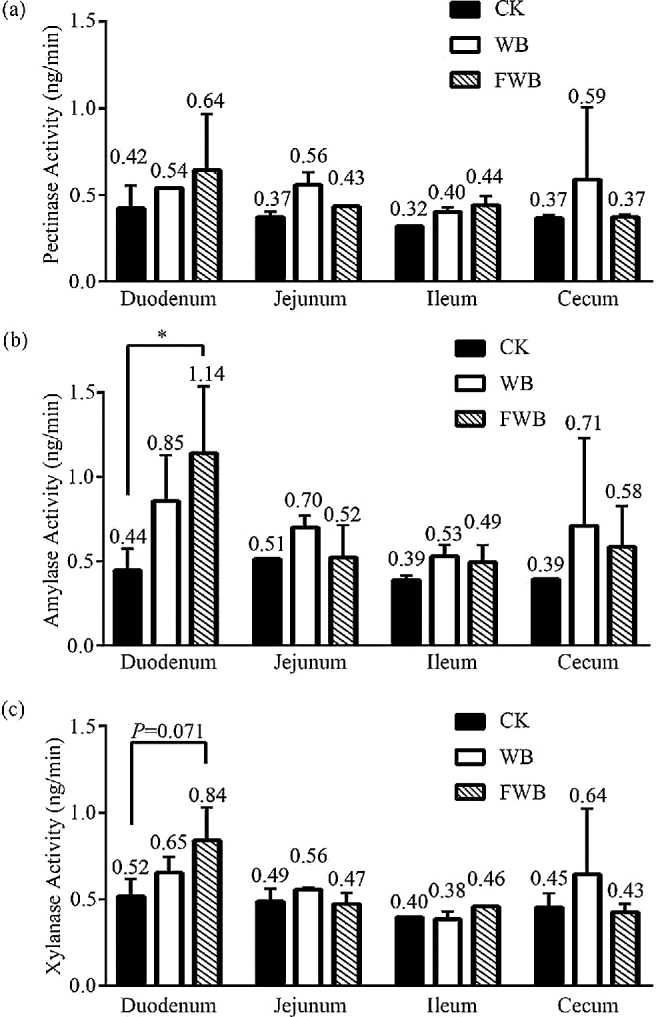
Effect of wheat bran on intestinal enzyme activity of pectinase (a), amylase (b), and xylanase (c). The enzyme activity is expressed with the amount of reducing sugar produced by enzyme per minute. * shows significant difference which P < 0.05. CK: control check; WB: wheat bran group; FWB: fermented wheat bran group.
Effect of Fermented Wheat Bran on Intestinal Microbiota
After assembling and filtering, 967,054 high-quality 16S rDNA sequences were generated from 18 samples. All samples came to an equal sequencing depth (23,810 reads per sample) with subsampling, and 2664 operational taxonomic units at 97% identity were obtained. The diversity of samples had entered into stable period when the number of reads close to 2,000, which indicated a near-complete sampling of the microbiota with 23,810 reads.
Alpha Diversity Analysis
With no differences found in duodenum (P > 0.05), only the result of alpha analysis of cecum was listed in Table 4. Compared with CK, the Sobs index and Chao1 index of FWB remarkably enhanced from 391.67 to 461.33 (P < 0.05) and from 469.37 to 583.24 (P < 0.01), respectively.
Table 4.
Alpha diversity analysis for cecum microbiota in different treatments.1
| Index2 | CK | WB | FWB |
|---|---|---|---|
| Sobs | 391.67 ± 12.91b | 397.67 ± 52.17a,b | 461.33 ± 13.01a |
| Chao1 | 469.37 ± 13.20B | 469.57 ± 66.89A,B | 583.24 ± 8.96A |
| Shannon | 4.15 ± 0.11 | 3.89 ± 0.25 | 4.26 ± 0.31 |
| Simpson | 0.04 ± 0.01 | 0.06 ± 0.02 | 0.06 ± 0.03 |
CK: Control check; WB: Wheat bran group; FWB: fermented wheat bran group.
The results are shown with mean ± standard error and P values are analyzed with student's t-test. Sobs index shows the observed richness. Chao1 index reflect the abundance. Shannon index is positively and Simpson index is negatively related to the richness and uniformity of microbiota in samples
means in the same row with no common superscripts are significantly different (P < 0.05).
means in the same row with no common superscripts are highly significantly different (P < 0.01).
After analysis of microbiota structure in cecum, a total of 7 family levels where the contents were more than 0.05% were shown in Fig. 4. No differences were observed on Ruminococcaceae, Lachnospiraceae, Bacteroidaceae, Rikenellaceae, Porphyromonadaceaea, and norank_o_Gastranaerophilales in different treatments (P > 0.05). The Bifidobacteriaceae settled in the cecum of WB (14.83%) notably increased contrasted with CK (0.51%, P < 0.05) and FWB (0.94%, P < 0.05).
Figure 4.
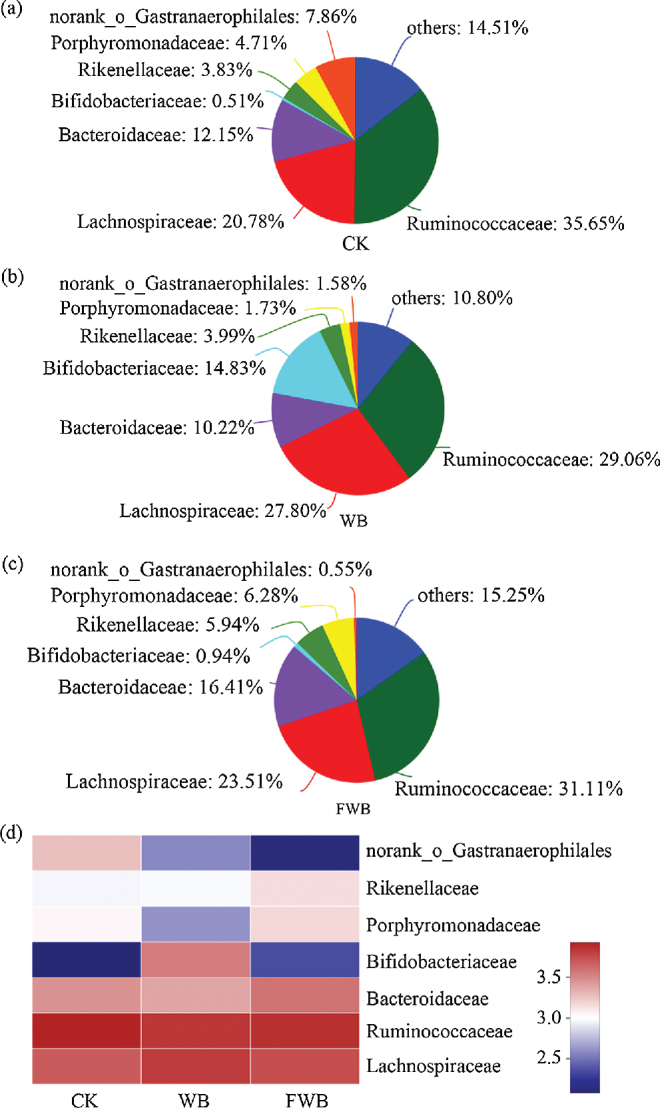
The cecum microbiota structure in family level of control check (CK, a), wheat bran group (WB, b), fermented wheat bran group (FWB, c), and Heatmap (d). All family levels which proportions are less than 0.05% are merged into others. The percentage of each family level is mean value in each treatment. The color of heatmap is determined by logarithm of the number of populations in each family level.
Beta Diversity Analysis
Hierarchical cluster tree (Fig. 5) of intestinal microbiota was obtained through R language analysis by the unweighted pair-group method with arithmetic mean and Unweighted UniFrac distance. Duodenum and cecum were divided into 2 distinct floras in this tree and the replicates in the same treatments were also clustered well with a close branch length.
Figure 5.
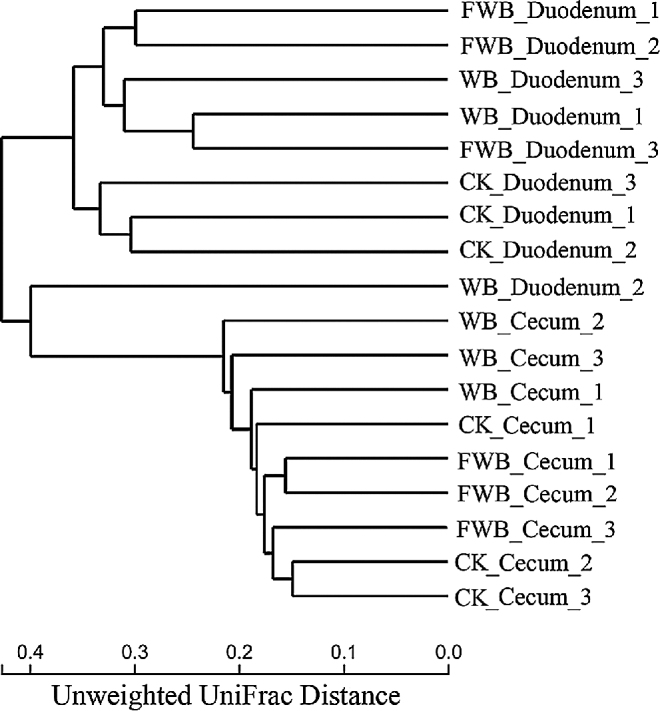
Hierarchical clustering tree based on Unweighted UniFrac distance. The length of branch represents the distance among samples. Replicates are shown with numbers at the end of each samples. CK: control check; WB: wheat bran group; FWB: fermented wheat bran group.
The further principal co-ordinates analysis was carried out with Unweighted UniFrac distance to evaluate the variety in microbiota composition of duodenum and cecum. It was obvious from Fig. 6 that CK and FWB had a high degree of polymerization both in duodenum and cecum, and the distribution areas of these treatments are different. Points of replicate samples in WB had low degree of polymerization contrasted with CK and FWB.
Figure 6.
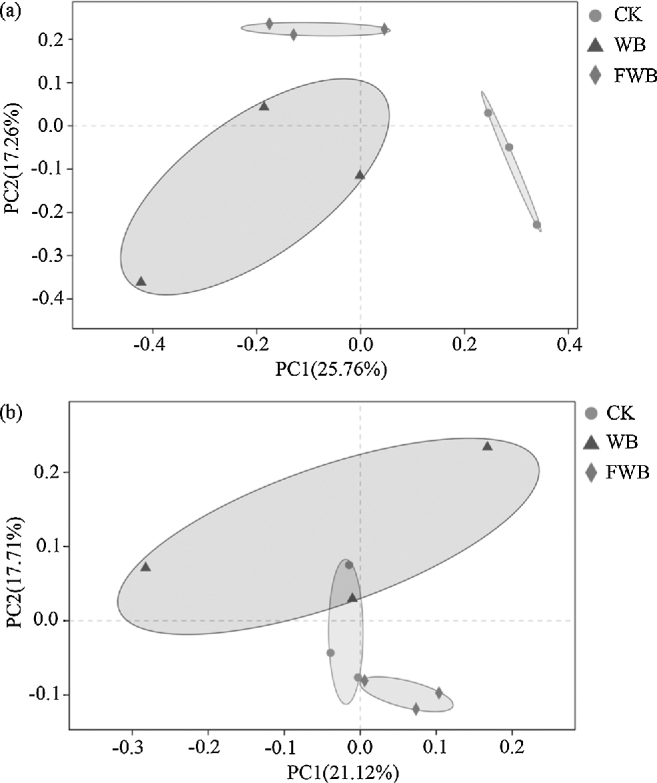
Principal co-ordinates analysis (PCoA) analysis of microbiota in duodenum (a) and cecum (b). Horizontal and vertical axes are the 2 main factors affecting the diversity clustering based on Unweighted UniFrac distance. The percentage in brackets of each axe represents the factor's contribution to the separation of samples. Smaller distance between 2 points means closer microbiota diversity of these samples. The fitting region of each treatment is expressed with ellipse on the figure. CK: control check; WB: wheat bran group; FWB: fermented wheat bran group.
DISCUSSION
Wheat bran can be used as raw material in poultry's diets with the abundant dietary fiber, NSP, and lower price (Wanzenböck et al., 2017). However, broilers do not have sufficient endogenous digestive enzymes for degradation of wheat bran (Yuan et al., 2017). It has been reported that the wheat bran NSP could increase intestinal viscosity (Choct et al., 1996; Murphy et al., 2009; Leo et al., 2012), reduce intestinal motility, block nutrient absorption (Cozannet et al., 2017; Smeets et al., 2018), and limit growth potential in broilers. SSF is frequently used in the pre-treatment of feedstuff in order to break down NSP. In this study, replacing 5% corn with B.cereus fermented wheat bran did not affect the growth performance of broilers. Suggesting that SSF using xylanase-producing microorganisms can degrade NSP and be used in the pre-treatment of feedstuff, which has similar standpoint to previous studies (Latorre et al., 2015; Supriyati et al., 2015; Ye et al., 2017). Compared with other methods to degrade NSP in diets, such as adding exogenous enzymes (Cardoso et al., 2018), appending microbial agents (Wealleans et al., 2017), and low temperature freezing (Khadem et al., 2016), SSF not only ensures the nutrient level of diets, but optimizes the intestinal microbiota and further improves the digestion and absorption capacity of broiler chickens.
The fundamental method to improve digestive ability is to enhance the activity of digestive enzymes in intestines, so as to speed up the degradation of nutrients in diets. Researches showed that intestinal enzyme activity in broilers was mainly affected by diets composition (Kheravii et al., 2018), bacteria agents (Li et al., 2015), and ambient temperature (Osman and Tanios, 1983). The addition of exogenous enzymes can promote feed digestion and absorption efficiency of nutrient in poultry's diets (Ramesh and Devegowda, 2004; Zhang et al., 2014; Yin et al., 2018). In this study, the inclusion of fermented wheat bran in diets enhanced the activity of xylanase and amylase in duodenum, where xylanase and amylase have the highest proportions of all intestinal segments. These results imply that the fermentation of wheat bran can improve intestinal enzymes activity and digestive capacity of broilers.
Wheat bran contains fibers that could help to optimize the composition and activity of intestinal microorganism, and produce beneficial effects on the growth of broilers (D'Hoe et al., 2018). NSP in wheat bran has the ability to change the distribution of nutrients after the formation of chyme in the intestinal tract, meanwhile undigested nutrient contents and velocity of chyme could further affect the diversity of intestinal microbiota (Koropatkin et al., 2012). Alpha diversity analysis in this study showed that the microbiota richness in cecum evidently increased in FWB, but no obvious dissimilarity was found on the diversity of microbiota in cecum. Based on the previous study (David et al., 2014), a probable reason may be that the fermented wheat bran added in diets changes the microbiota structure in cecum where it causes the increase of several dominant species and the elimination of some weak species, which has similar effects with probiotics (Gao et al., 2017).
In the literature, promised responses have been recorded in using B.cereus as a probiotic to treat intestinal diseases (Scharek-Tedin et al., 2013) and improve feed efficiency (Gil de los Santo et al., 2005). B.cereus can also inhibit the proliferation of pathogenic bacteria, and subsequently leading to the optimization of intestinal microbiota (Taras et al., 2005; Wang et al., 2018). While a few strains that have close homology to B.cereus are free of toxigenic genes (Cui et al., 2019), it is noteworthy that some strains isolated from the B.cereus group have genes that could cause vomiting in animals (Mousumi et al., 2011). Therefore, the toxicity test is of great importance for the effective application of B.cereus. In the current study, no health problems were noticed in broilers fed with W-3-fermented wheat bran, indicating W-3 is free of toxicity. However, currently there is no direct evidence to prove that the W-3 strain does not contain any toxicity gene. Thus, future studies will need to ensure the safety of W-3 before field application.
Growth performance of broilers is closely related to the microbiota diversity in cecum, where the chyme is largely fermented (Meimandipour et al., 2009). Higher richness of microbiota in cecum usually represents higher growth performance in broilers (Stanley et al., 2013). Dietary fiber from wheat or wheat bran is usually used as nutrient during fermentation in cecum to promote the colonization of intestinal probiotics (Courtin et al., 2008). So, large amount of undigested NSP in diets of WB formed chyme and were fermented in cecum, which promoted the colonization of Bifidobacteriaceae. This is consistent with reported study (Kermanshahi et al., 2018). Furthermore, the amount of Bifidobacteriaceae colonized in cecum remained unchanged after dietary inclusion of fermented wheat bran. This inference demonstrated that the in vitro fermentation with xylanase-producing B.cereus successfully achieved the goal to degrade the NSP and to improve the digestibility of broilers on wheat bran.
After calculation, the cost of fermented feed decreased 1.22% compared with the normal feed when 5% of corn was replaced with fermented wheat bran, and each further 1% of replacement would reduce 0.24% of the cost. Therefore, the following study will continue on the optimization of strain dose, fermentation time and dietary additive amount of fermented wheat bran for economic benefit.
Probiotics can inhibit the growth of pathogenic bacteria and improve the overall structure of cecum microbiota, which is beneficial to the intestinal health of broilers (Hsu et al., 2004). Although beta diversity analysis proved that both wheat bran and fermented wheat bran added in diets have effects on changing intestinal microbiota, it is still unclear, and need to be further clarified, that whether cecum Bifidobacteriaceae colonization is benefit to broilers.
In conclusion, the xylanase-producing B.cereus strain W-3 was shown as effective in fermenting wheat bran. The use of fermented wheat bran to replace corn (5%) in broiler diets had no influence on growth performance, but increased digestive enzyme activities and the richness of intestinal microbiota. Wheat bran promoted the colonization of Bifidobacteriaceae in cecum. Intestinal microbiota structure was improved by both wheat bran and fermented wheat bran.
ACKNOWLEDGEMENTS
This research was supported by grants from Research Project Supported by Shanxi Scholarship Council of China (2017–067) and the Natural Science Foundation of Shanxi Province (201601D1040). We thank Fusheng Tang in University of Arkansas at Little Rock for his numerous valuable suggestions during the manuscript preparation. Also, we thank Shanghai Majorbio Bio-pharm Technology Co. Ltd in Shanghai of China for supporting free analysis online.
REFERENCES
- Apprich S., Tirpanalan Ö., Hell J., Reisinger M., Böhmdorfer S., Siebenhandl-Ehn S., Novalin S., Kneifel W. Wheat bran-based biorefinery 2: valorization of products. LWT - Food Sci. Technol. 2014;56:222–231. [Google Scholar]
- Bedford M.R. The effect of enzymes on digestion1. J. Appl. Poult. Res. 1996;5:370–378. [Google Scholar]
- Bedford M.R., Morgan A.J. The use of enzymes in poultry diets. Worlds Poult. Sci. J. 1996;52:61–68. [Google Scholar]
- Cardoso V., Fernandes E.A., Santos H.M.M., Maçãs B., Lordelo M.M., Telo da Gama L., Ferreira L.M.A., Fontes C.M.G.A., Ribeiro T. Variation in levels of non-starch polysaccharides and endogenous endo-1,4-β-xylanases affects the nutritive value of wheat for poultry. Br. Poult. Sci. 2018;59:218–226. doi: 10.1080/00071668.2018.1423674. [DOI] [PubMed] [Google Scholar]
- Choct M., Hughes R.J., Trimble R.P., Angkanaporn K., Annison G. Non-starch polysaccharide-degrading enzymes increase the performance of broiler chickens fed wheat of low apparent metabolizable energy. J. Nutr. 1995;125:485–492. doi: 10.1093/jn/125.3.485. [DOI] [PubMed] [Google Scholar]
- Choct M., Hughes R.J., Wang J., Bedford M.R., Morgan A.J., Annison G. Increased small intestinal fermentation is partly responsible for the anti-nutritive activity of non-starch polysaccharides in chickens. Br. Poult. Sci. 1996;37:609–621. doi: 10.1080/00071669608417891. [DOI] [PubMed] [Google Scholar]
- Courtin C., Broekaert W., Swennen K., Lescroart O., Onagbesan O., Buyse J., Decuypere E., Van de Wiele T., Marzorati M., Verstraete W., Huyghebaert G., Delcour J. Dietary inclusion of wheat bran arabinoxylooligosaccharides induces beneficial nutritional effects in chickens. Cereal Chem. J. 2008;85:607–613. [Google Scholar]
- Cozannet P., Kidd M.T., Montanhini Neto R., Geraert P.-A. Next-generation non-starch polysaccharide-degrading, multi-carbohydrase complex rich in xylanase and arabinofuranosidase to enhance broiler feed digestibility. Poult. Sci. 2017;96:2743–2750. doi: 10.3382/ps/pex084. [DOI] [PubMed] [Google Scholar]
- Cui Y., Märtlbauer E., Dietrich R., Luo H., Ding S., Zhu K. Multifaceted toxin profile, an approach toward a better understanding of probiotic Bacillus cereus. Crit. Rev. Toxicol. 2019 doi: 10.1080/10408444.2019.1609410. [DOI] [PubMed] [Google Scholar]
- D'Hoe K., Conterno L., Fava F., Falony G., Vieira-Silva S., Vermeiren J., Tuohy K., Raes J. Prebiotic wheat bran fractions induce specific microbiota changes. Front. Micrbiol. 2018;9:1–15. doi: 10.3389/fmicb.2018.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David L.A., Maurice C.F., Carmody R.N., Gootenberg D.B., Button J.E., Wolfe B.E., Ling A.V., Devlin A.S., Varma Y., Fischbach M.A., Biddinger S.B., Dutton R.J., Turnbaugh P.J. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duraisamy S., Balakrishnan S., Jayachandran J., Husain F., Kumarasamy A. Effect of Bacillus cereus peptide conjugated with nanoporous silica on inactivation of Listeria monocytogenes in apple juice, as an ecofriendly preservative. Environ. Sci. Pollut. Res. 2018;25:29345–29355. doi: 10.1007/s11356-018-2882-5. [DOI] [PubMed] [Google Scholar]
- Gallardo C., Dadalt J.C., Trindade Neto M.A. Nitrogen retention, energy, and amino acid digestibility of wheat bran, without or with multicarbohydrase and phytase supplementation, fed to broiler chickens1. J. Anim. Sci. 2018;96:2371–2379. doi: 10.1093/jas/sky062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao P., Ma C., Sun Z., Wang L., Huang S., Su X., Xu J., Zhang H. Feed-additive probiotics accelerate yet antibiotics delay intestinal microbiota maturation in broiler chicken. Microbiome. 2017;5:91. doi: 10.1186/s40168-017-0315-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil de los Santos J, Storch R.,O.B., Gil-Turnes C. Bacillus cereus var. toyoii and Saccharomyces boulardii increased feed efficiency in broilers infected with Salmonella enteritidis. Br. Poult. Sci. 2005;46:494–497. doi: 10.1080/00071660500181461. [DOI] [PubMed] [Google Scholar]
- Hemery Y., Rouau X., Lullien-Pellerin V., Barron C., Abecassis J. Dry processes to develop wheat fractions and products with enhanced nutritional quality. J. Cereal Sci. 2007;46:327–347. [Google Scholar]
- Hsu C., Liao J., Chung Y., Hsieh C., Chan Y. Xylooligosaccharides and fructooligosaccharides affect the intestinal microbiota and precancerous colonic lesion development in rats. J. Nutr. 2004;134:1523–1528. doi: 10.1093/jn/134.6.1523. [DOI] [PubMed] [Google Scholar]
- Kermanshahi H., Shakouri M.D., Daneshmand A. Effects of non-starch polysaccharides in semi-purified diets on performance, serum metabolites, gastrointestinal morphology, and microbial population of male broiler chickens. Livest. Sci. 2018;214:93–97. [Google Scholar]
- Khadem A., Lourenço M., Delezie E., Maertens L., Goderis A., Mombaerts R., Höfte M., Eeckhaut V., Van Immerseel F., Janssens G.P.J. Does release of encapsulated nutrients have an important role in the efficacy of xylanase in broilers? Poult. Sci. 2016;95:1066–1076. doi: 10.3382/ps/pew002. [DOI] [PubMed] [Google Scholar]
- Kheravii S.K., Swick R.A., Choct M., Wu S.-B. Upregulation of genes encoding digestive enzymes and nutrient transporters in the digestive system of broiler chickens by dietary supplementation of fiber and inclusion of coarse particle size corn. BMC Genomics. 2018;19:208. doi: 10.1186/s12864-018-4592-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koegelenberg D., Chimphango A.F.A. Effects of wheat-bran arabinoxylan as partial flour replacer on bread properties. Food Chem. 2017;221:1606–1613. doi: 10.1016/j.foodchem.2016.10.130. [DOI] [PubMed] [Google Scholar]
- Koropatkin N.M., Cameron E.A., Martens E.C. How glycan metabolism shapes the human gut microbiota. Nat. Rev. Microbiol. 2012;10:323–335. doi: 10.1038/nrmicro2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latorre J.D., Hernandez-Velasco X., Kuttappan V.A., Wolfenden R.E., Vicente J.L., Wolfenden A.D., Bielke L.R., Prado-Rebolledo O.F., Morales E., Hargis B.M., Tellez G. Selection of Bacillus spp. for cellulase and xylanase production as direct-fed microbials to reduce digesta viscosity and clostridium perfringens proliferation using an in vitro digestive model in different poultry diets. Front. Vet. Sci. 2015;2:1–8. doi: 10.3389/fvets.2015.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leo S., Frankie P., Kathryn O.S., Jenny W. Wheat bran: Its composition and benefits to health, a european perspective. Int. J. Food Sci. Nutr. 2012;63:1001–1013. doi: 10.3109/09637486.2012.687366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Li Z., Wei Z., Liu T., Zou X., Liao Y., Luo Y. Long-term effects of oral tea polyphenols and Lactobacillus brevis M8 on biochemical parameters, digestive enzymes, and cytokines expression in broilers. J. Zhejiang Univ. Sci. B. 2015;16:1019–1026. doi: 10.1631/jzus.B1500160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meimandipour A., Shuhaimi M., Hair-Bejo M., Azhar K., Kabeir B.M., Rasti B., Yazid A.M. In vitro fermentation of broiler cecal content: The role of Lactobacilli and pH value on the composition of microbiota and end products fermentation. Lett. Appl. Microbiol. 2009;49:415–420. doi: 10.1111/j.1472-765X.2009.02674.x. [DOI] [PubMed] [Google Scholar]
- Miller G.A.I.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959;31:426–428. [Google Scholar]
- Mousumi B., Gopinath B.N., Thandavarayan R. Phenotypic & genetic characterization of Bacillus cereus isolated from the acute diarrhoeal patients. Indian J. Med. Res. 2011;133:88–95. [PMC free article] [PubMed] [Google Scholar]
- Murphy T.C., Mccracken J.K., McCann M.E.E., George J., Bedford M.R. Broiler performance and in vivo viscosity as influenced by a range of xylanases, varying in ability to effect wheat in vitro viscosity. Br. Poult. Sci. 2009;50:716–724. doi: 10.1080/00071660903389950. [DOI] [PubMed] [Google Scholar]
- Olukosi O., Adeola O. Whole body nutrient accretion, growth performance and total tract nutrient retention responses of broilers to supplementation of xylanase and phytase individually or in combination in wheat-soybean meal based diets. J. Poult. Sci. 2008;45:192–198. [Google Scholar]
- Osman A.M., Tanios N.I. The effect of heat on the intestinal and pancreatic levels of amylase and maltase of laying hens and broilers. Comp. Biochem. Physiol. A Comp. Physiol. 1983;75:563–567. doi: 10.1016/0300-9629(83)90421-8. [DOI] [PubMed] [Google Scholar]
- Prückler M., Siebenhandl-Ehn S., Apprich S., Höltinger S., Haas C., Schmid E., Kneifel W. Wheat bran-based biorefinery 1: Composition of wheat bran and strategies of functionalization. LWT - Food Sci. Technol. 2014;56:211–221. [Google Scholar]
- Ramesh K., Devegowda G. Proceeding of 22nd World's Poultry Congress, Istanbul, Turkey. 2004. Effect of feeding varying levels of double zero rapeseed meal with and without enzyme supplementation on performance of broilers. [Google Scholar]
- Scharek-Tedin L., Pieper R., Vahjen W., Tedin K., Neumann K., Zentek J. Bacillus cereus var. Toyoi modulates the immune reaction and reduces the occurrence of diarrhea in piglets challenged with Salmonella Typhimurium DT1041. J. Anim. Sci. 2013;91:5696–5704. doi: 10.2527/jas.2013-6382. [DOI] [PubMed] [Google Scholar]
- Smeets N., Nuyens F., Van Campenhout L., Delezie E., Niewold T. Interactions between the concentration of non-starch polysaccharides in wheat and the addition of an enzyme mixture in a broiler digestibility and performance trial. Poult. Sci. 2018;97:2064–2070. doi: 10.3382/ps/pey038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley D., Geier M.S., Denman S.E., Haring V.R., Crowley T.M., Hughes R.J., Moore R.J. Identification of chicken intestinal microbiota correlated with the efficiency of energy extraction from feed. Vet. Microbiol. 2013;164:85–92. doi: 10.1016/j.vetmic.2013.01.030. [DOI] [PubMed] [Google Scholar]
- Supriyati T. Haryati, Susanti T., Susana I.W.R. Nutritional value of rice bran fermented by Bacillus amyloliquefaciens and humic substances and its utilization as a feed ingredient for broiler chickens. Asian-Australas. J. Anim. Sci. 2015;28:231–238. doi: 10.5713/ajas.14.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taras D., Vahjen W., Macha M., Simon O. Response of performance characteristics and fecal consistency to long-lasting dietary supplementation with the probiotic strain Bacillus cereus var. toyoi to sows and piglets. Arch. Anim. Nutr. 2005;59:405–417. doi: 10.1080/17450390500353168. [DOI] [PubMed] [Google Scholar]
- Wang Y., Zhang J., Wang Y., Wang K., Wei H., Shen L. Isolation and characterization of the Bacillus cereus BC7 strain, which is capable of zearalenone removal and intestinal flora modulation in mice. Toxicon. 2018;155:9–20. doi: 10.1016/j.toxicon.2018.09.005. [DOI] [PubMed] [Google Scholar]
- Wanzenböck E., Apprich S., Tirpanalan Ö., Zitz U., Kracher D., Schedle K., Kneifel W. Wheat bran biodegradation by edible Pleurotus fungi - a sustainable perspective for food and feed. LWT-Food Sci. Technol. 2017;86:123–131. [Google Scholar]
- Wealleans A.L., Walsh M.C., Romero L.F., Ravindran V. Comparative effects of two multi-enzyme combinations and a Bacillus probiotic on growth performance, digestibility of energy and nutrients, disappearance of non-starch polysaccharides, and gut microflora in broiler chickens. Poult. Sci. 2017;96:4287–4297. doi: 10.3382/ps/pex226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye M., Sun L., Yang R., Wang Z., Qi K. The optimization of fermentation conditions for producing cellulase of Bacillus amyloliquefaciens and its application to goose feed. R. Soc. Open Sci. 2017;4 doi: 10.1098/rsos.171012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin D., Yin X., Wang X., Lei Z., Wang M., Guo Y., Aggrey S.E., Nie W., Yuan J. Supplementation of amylase combined with glucoamylase or protease changes intestinal microbiota diversity and benefits for broilers fed a diet of newly harvested corn. J. Anim. Sci. Biotechnol. 2018;9:24. doi: 10.1186/s40104-018-0238-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L., Wang M., Zhang X., Wang Z. Effects of protease and non-starch polysaccharide enzyme on performance, digestive function, activity and gene expression of endogenous enzyme of broilers. PLoS One. 2017;12 doi: 10.1371/journal.pone.0173941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Xu J., Lei L., Jiang Y., Gao F., Zhou G.H. Effects of xylanase supplementation on growth performance, nutrient digestibility and non-starch polysaccharide degradation in different sections of the gastrointestinal tract of broilers fed wheat-based diets. Asian-Australas. J. Anim. Sci. 2014;27:855–861. doi: 10.5713/ajas.2014.14006. [DOI] [PMC free article] [PubMed] [Google Scholar]