Abstract
During incubation, embryonic growth and development are dependent on nutrients deposited in the egg. The content of the yolk can be transferred to the embryo in 2 ways: directly into the intestine via the yolk stalk or through the highly vascularized yolk sac membrane. It has been suggested that, as a result of genetic selection and improved management, the increase in posthatch growth rate and concurrently the increase in metabolic rate of broiler chickens during the last 50 yr has also increased embryonic metabolism. A higher metabolic rate during incubation would imply a lower residual yolk weight and possibly lower energy reserve for the hatchling. This might affect posthatch development and performance. This review examined scientific publications published between 1930 and 2018 to compare residual yolk weight at hatch, metabolic heat production, and yolk utilization throughout incubation. This review aimed to investigate 1) whether or not residual yolk weight and composition has been changed during the 88-yr period considered and 2) which abiotic and biotic factors affect yolk utilization in poultry during incubation and the early posthatch period. It can be concluded that 1) residual yolk weight and the total solid amount of the residual yolk at hatch seem to be decreased in the recent decades. It cannot be concluded whether the (lack of) differences between old and modern strains are due to genetic selection, changed management and incubation conditions, or moment of sampling (immediately after hatch or at pulling). It is remarkable that with the genetic progress and improved management and incubation conditions over the last 88 yr, effects on yolk utilization efficiency and embryonic metabolic heat production are limited; 2) factors specially affecting residual yolk weight at hatch include egg size and incubation temperature, whereas breeder age has more influence on nutrient composition of the residual yolk.
Key words: poultry, incubation, yolk sac utilization, metabolic heat production, efficiency
Introduction
For almost a century, genetic selection and improved management have led to significant progress in productive traits in different poultry species (Joseph and Moran, 2005, Barott, 1937, Buzala et al., 2014). For example, laying hens nowadays produce more than 320 eggs during their 52-wk production cycle and male broiler chickens can achieve a body weight of 3 kg or even higher within 42 D (Druyan, 2010). This genetic selection for different performance traits, such as eggs or meat, has largely changed posthatch growth and development (Buzala et al., 2015). Nowadays, broiler chickens need half as many days to reach slaughter weight as compared with 1956 (Druyan, 2010), which means that the prenatal period, which is still approximately 21 D, has become a larger part of the production cycle of the broiler chicken (approximately 35%) (de Oliveira et al., 2009, Hamidu et al., 2007, Speier et al., 2012). This implies that growth and development of the broiler chicken during the prenatal period has become more important than that some decades ago for 2 reasons: 1) simply because the embryonic phase has become a larger part of the total lifespan of a chicken and 2) due to the faster postnatal growth rate chickens should be prepared for that in the embryonic phase, meaning that organ development (e.g., bone, muscles, heart) should be optimal at the moment of hatching. The genetic selection for layers has been different to that for broiler chickens, with particular emphasis on shell strength (Shafey, 2002), which in turn has reduced eggshell conductance (Shafey, 2002, Nangsuay et al., 2015a). Eggshell conductance determines oxygen availability within the egg and thus influences embryonic development and chicken quality.
Growth and development of the avian embryo are dependent on the nutrients deposited in the egg (Yadgary and Uni, 2012). The highest density of solids can be found within the egg yolk (48%; Romanoff and Romanoff, 1949). Egg yolk lipids are the primarily source of energy during the second half of incubation and early posthatch period; more than 90% of the total energy produced by the embryo and hatchling originates from oxidation of egg yolk lipids (Noble and Ogunyemi, 1989, Speake et al., 1998, Sato et al., 2006). Yolk size and composition are therefore important factors that contribute to the energy available for maintenance and embryonic growth and development (Noble and Cocchi, 1990). In addition, utilization of the available yolk is related to the metabolic rate of the embryo, as well as the size and composition of the residual yolk at hatch (Nangsuay et al., 2013). It can be hypothesized that alterations in yolk utilization during incubation and/or in the early posthatch period, and consequently on hatchling quality and development in the first week of rearing, might have an effect on growth performance, health, and welfare in later life. For this reason, it is important to understand the mechanisms underlying yolk utilization and whether or not yolk utilization and/or residual yolk weight can be influenced by, for example, genetics or management factors during incubation and/or the early posthatch period. Therefore, the aim of this review is 1) to investigate whether residual yolk utilization, residual yolk weight, and metabolic heat production (MHP) are affected by genetic selection and/or management changes in the recent decades and 2) to discuss biotic and abiotic factors affecting yolk utilization and residual yolk weight in poultry during incubation and the early posthatch period. First, yolk utilization during incubation and the early posthatch period will be described. Thereafter, factors affecting embryonic MHP and residual yolk sac weight at hatch will be discussed. Because most of the existing literature on yolk utilization and residual yolk weight is on broiler and layer chicken (Gallus gallus domesticus) studies, this review will mainly focus on these species of birds, but, where available, we will also refer to studies performed on ducks or turkeys.
Yolk sac utilization
Yolk Sac Utilization During Incubation
At oviposition, the egg consists macroscopically of 3 main components: eggshell, yolk, and albumen (Romanoff, 1960). The egg yolk contains approximately 49% water, 33% fat, 17% protein, and traces of carbohydrates and minerals (Romanoff and Romanoff, 1949). Compared with the yolk, the albumen is less dense as it consists largely of water (88–90%), with the remaining being solids: 10% proteins, 1% carbohydrates, and traces of lipids (Romanoff and Romanoff, 1949). The yolk is mainly important to provide nutrients to the embryo and its extraembryonic membranes during incubation, as yolk lipids contribute to approximately 90% of the embryonic energy production used for maintenance, body growth, and development (Romanoff, 1960, Speake et al., 1998).
The size and the composition of the yolk change throughout incubation (Romanoff, 1967). The water content and thus the weight of the yolk was found to increase approximately 4% during the first week of incubation as a result of the influx of water from the albumen to create the subembryonic fluid (Romanoff, 1967, Willems et al., 2014). After this small initial peak, Romanoff (1967) found that the water content of the yolk sac gradually decreased from approximately 53 to 30% at hatch. More recent studies (Nangsuay et al., 2013, Nangsuay et al., 2015a) found that the water content of the residual yolk at hatch was just above 50%, which is much higher than Romanoff (1967) concluded and comparable with the initial water content of the egg yolk. The reason for the difference found between the studies is unknown but might be related to differences in yolk utilization between strains or to the lower weight losses (mainly water) that are currently achieved throughout the incubation process.
The changes in the solid part of the yolk sac seem to be related to the nutrient requirements of the developing embryo, the ability to absorb nutrients from the yolk sac, and the influx of albumen. During the first week of incubation, glucose is used as the main energy source as the yolk sac membrane (YSM) is not sufficiently developed to transport yolk nutrients from the yolk sac to the embryonic circulation (Yadgary et al., 2010). In addition, the chorioallantoic membrane (CAM), which functions as a respiratory organ, is not sufficiently developed to provide enough O2 for complete fatty acid oxidation (Moran, 2007). Because the embryo is still small and the nutrient utilization is relatively low within the first week of incubation, the amount of the yolk solids do not change largely (Δ = −0.44 mg; Romanoff, 1967). After embryonic day (ED) 10, the YSM and the CAM are completely developed, and the embryo is able to utilize the yolk content at a higher rate (Speake et al., 1998). This is also expressed by a larger reduction in the solid part of the egg yolk in the second week of incubation (Δ = −1.38 mg; Romanoff, 1967).
Especially during the last week of incubation, there is a rapid absorption of solids from the yolk (Δ = −2.99 mg) and particularly of the lipids, which is associated with accumulation of lipids within embryonic tissues (Noble and Cocchi, 1990) and a high lipid oxidation (Peebles et al., 2000). Until ED13, the net lipid loss from the yolk of an average egg is approximately 200 to 300 mg per day. Between ED15 and ED21, the net lipid loss from the yolk increases substantially, and during the last 2 D of incubation, yolk lipids are even removed at a rate of nearly 1 g per day (Noble and Cocchi, 1990). There are further indications that there is a preference for the uptake of lipids from the yolk compared with other nutrient components during this period (Noble and Cocchi, 1990). It should be realized that the aforementioned calculations are based on studies conducted more than 50 yr ago, and it can be questioned whether these values still hold for the current breeds and strains. This will be discussed in the following.
Besides the absorption of yolk solids, there is also an influx of solids within the yolk in the last week of incubation as a result of the albumen flow into the amniotic cavity, where after it is swallowed by the embryo and especially albumen proteins seem to appear in the yolk sac (Vieira and Moran, 1999, Willems et al., 2014). At the same time, part of the albumen seems to be absorbed directly into the yolk sac by the use of the yolk stalk (see below; Transfer Route 2: the Yolk Stalk). It needs to be emphasized that when yolk utilization and/or residual yolk weight at hatch is evaluated, the influx of water and solids from albumen into the yolk sac needs to be taken into account as well (Uni et al., 2012).
In addition to lipids, amino acids and carbohydrates are utilized from the yolk during incubation as well, but to a lesser extent. Amino acids are selectively absorbed from the yolk and albumen and are mainly used for protein synthesis in tissues, ammonia metabolism, nitrogen transport between tissues, nucleotide synthesis, support of the immune system and as precursors for gluconeogenesis (Barri et al., 2011). Carbohydrates are particularly important for the embryo during periods when oxygen availability is limited, which occurs during the differentiation phase of development (week 1) and during the hatch phase (week 3) (Christensen et al., 1993, Pulikanti et al., 2010). Because of the low initial concentrations of carbohydrates within the egg, gluconeogenesis is an important process throughout incubation (de Oliveira et al., 2008).
Before nutrients from the yolk sac can be used for energy or growth within specific embryonic tissues, they need to be transferred to the blood circulatory system first. This appears to be possible by 2 routes: through the highly vascularized YSM or directly into the intestines via the yolk stalk (Noy and Sklan, 1998). Both routes will be discussed in the following.
Transfer Route 1: the Yolk Sac Membrane
The YSM is an extraembryonic membrane responsible for the transport of nutrients from the yolk sac to the embryonic circulation (Noble and Cocchi, 1990). The YSM is divided into 2 structural areas: an outer mesodermal layer of flattened cells, which performs a supportive role, and an inner endodermal layer of cylindrical epithelial cells, which is responsible for yolk absorption (Speake et al., 1998). During the first week of incubation, the inner endodermal layer of the YSM increases in area and spreads over the surface of the yolk. At approximately ED10, the whole yolk is surrounded by the YSM and contains a structure comparable with the small intestine, including folds and a surface of microfolds (Speake et al., 1998, Yadgary et al., 2013). The YSM consists of an absorptive area and a nonabsorptive area, together forming the total YSM area (Yadgary et al., 2013).
To adjust for the growing nutritional demands of the embryo, the YSM is in a continuous stage of change (Romanoff, 1960). For example, the change in total YSM weight (absorptive + nonabsorptive area) is associated with structural changes in especially the absorptive area of the YSM; a larger area is needed to absorb and transfer nutrients as the embryo develops (Yadgary et al., 2011). Between ED5 and ED10, the YSM weight increases from approximately 0.19 to 1.44 g (Yadgary et al., 2013), which is probably related to the growth of the YSM around the yolk (Yadgary et al., 2011). Between ED10 and ED15, there is a strong increase in YSM weight from 1.44 to 6.44 g, related to an abundant presence of microfolds. Between ED15 and ED21, the weight of the YSM decreases again from 6.44 to approximately 3.84 g. Until approximately 2 to 3 D before hatch, there is an increase in the absorptive area of the YSM, probably due to changes in the germ layer and the proliferation of the absorptive cells (Yadgary et al., 2013). However, in the last 2 to 3 D before hatch, the absorptive area of the YSM decreases in size, despite the growing nutritional demands of the embryo (Yadgary et al., 2011). This size reduction of the YSM is likely to be facilitated by apoptotic processes in the absorptive cells, due to the internalization of the yolk sac in the abdominal cavity of the embryo just before hatch (Romanoff, 1960, Yadgary et al., 2010). The decrease of the absorptive area, however, does not affect the rate of lipid uptake between ED17 and ED20, suggesting that the efficiency of the YSM toward lipid absorption, digestion, and secretion of enzymes increases during this period (Yadgary et al., 2013). Just before hatch, usually at approximately ED19, the blood circulation of the YSM decreases in functionality because of death of the arteries of the YSM as a result of the contraction of the umbilical vessels (Romanoff, 1952). This is probably related to the internalization of the yolk sac in the abdominal cavity of the embryo (Romanoff, 1960).
The YSM is highly vascularized, which is important to transport nutrients to the embryonic circulation (Romanoff, 1960). Yolk lipid composition is not comparable with embryonic lipid composition, which is probably related to preference in the absorption and utilization of specific lipids by the YSM (Speake et al., 1998, Yalçın et al., 2012) and the need of especially long-chain polyunsaturated fatty acids for membrane development and/or specific embryonic tissue (Noble and Cocchi, 1990). The uptake of yolk lipids via the YSM occurs through nonspecific phagocytosis. Lipolytic enzymes, generated by the yolk sac, break down the yolk contents (Dzoma and Dorrestein, 2001), and the products of this enzymatic activity are absorbed, hydrolysed, and formed into very-low-density lipoprotein particles that can be released to the embryonic circulation via the extensive network of blood vessels (Romanoff, 1960, Noble and Cocchi, 1990, Speake et al., 1998).
Transfer Route 2: the Yolk Stalk
The contribution of the yolk stalk to nutrient uptake during incubation is still a point of discussion. The yolk stalk is a narrow tube connecting the yolk sac and the jejunum of the embryo (Romanoff, 1960, Dzoma and Dorrestein, 2001) and can already be recognized at ED3 (Patten, 1920). In the beginning of the incubation period, the opening from the intestine to the yolk sac is a short narrow slit. At ED6, the yolk stalk is enclosed within the outer or somatic stalk, which is the neck of the amnion. The yolk stalk grows to a maximum length of 3.0 to 4.0 mm between ED16 and ED20 (Virchow, 1891). In the lumen of the yolk stalk, the connective tissue layer produces villus-like folds (Barri, 2008). The yolk stalk consists of an outer and an inner layer; the outer layer or somatic stalk connects the embryo and amnion, and the inner layer, or yolk stalk, connects the embryo and the yolk sac (Romanoff, 1960). At hatch, the yolk stalk has an opening into the small intestine at the proximal end (Noy et al., 1996).
It has been suggested that no direct passage of yolk contents through the yolk stalk into the intestines takes place because this passage is prevented by a loop of tissue, which is probably the intestinal loop attached to the yolk stalk (Speake et al., 1998). This loop probably blocks the movement of yolk from the yolk sac to the intestines; the exact mechanism is, however, not further described. However, other studies showed that the yolk stalk might be important for the transit of yolk contents directly into the intestines (Esteban et al., 1991, Noy and Sklan, 1998, Yadgary et al., 2011). This apparent contradiction between studies might be related to the time of measurement; particularly after ED18 and in early postnatal life, the yolk stalk may play an important role in transportation of yolk content into the intestines. It appears that the transfer of the yolk content to the embryo shifts from only the YSM to mostly the yolk stalk, starting at approximately ED18. This is based on the fact that from that moment onward, large amounts of yolk content are found in the intestines (Speier et al., 2012) and gene expression of nutrient transporters and digestive enzymes in the YSM is downregulated (Speier et al., 2012, Yadgary et al., 2013). After hatch, it has been clearly shown that the yolk stalk is important for the transport of nutrients. When the yolk stalk was tied in early posthatched chickens, the highest amount of yolk contents remained in the yolk sac (Esteban et al., 1991). In addition, an open passage along the yolk stalk was observed up to 3 D after hatch (Noy et al., 1996). This suggests that yolk contents could pass into the intestines via the lumen of the yolk stalk in at least newly hatched to 3-day-old chickens (Esteban et al., 1991). Although the contribution of the yolk stalk to facilitate yolk utilization toward the embryo and chicken seems to be confirmed by several studies, the rate of utilization and the possibility to selectively absorb specific nutrients by the yolk stalk are yet unknown.
Yolk Sac Utilization During the Posthatch Period
Only part of the yolk sac content is utilized during incubation. At approximately ED19, the residual yolk is internalized in the abdominal cavity of the embryo (Romanoff, 1960) and provides nutrients to the chicken up to 5 D after hatch (Lamot, 2017). The exact contribution of the residual yolk toward the nutritional intake of the posthatch chicken is unclear as studies demonstrated ambiguous results. It was estimated that in fed chickens the residual yolk contributed only for 10 to 11% toward the total dietary energy and protein intake from 0 to 3 D of age (Wijtten, 2011), while another study estimated this to be approximately 30% (Murakami et al., 1992). The latter study found that chickens with a deutectomy (removal of the complete yolk sac) at 6 h after hatch had a significantly lower body weight at day 7 (estimated Δ = −33%) after hatch than chickens without deutectomy. A comparable result was found by Turro-Vincent et al. (1994); body weight gain at 4 and 7 D after hatch was lower in deutectomized chickens than in control chickens with an intact yolk sac (Δ = −40%). These studies suggest that the residual yolk might have an important nutritional role after hatch and contributes to body weight gain in the first days after hatch. It appears that the relative contribution of the residual yolk to the dietary energy and protein intake of the posthatch chicken depends on the moment, amount, and composition of the exogenous feed consumed (Lamot, 2017). This is supported by studies showing that delayed access to feed after hatch (48–72 h) resulted in higher residual yolk weights at 96 h after hatch than in immediately fed chickens (Noy et al., 1996, El-Husseiny et al., 2008). This suggests that immediately fed chickens have a higher yolk utilization than delayed-fed chickens, which might be related to a higher intestinal activity, which is probably due to peristaltic movements (Noy et al., 1996). However, other studies comparing immediate or delayed posthatch feed intake up to 72 h did not find differences in yolk utilization or residual yolk weights (Gonzales et al., 2003, Van den Brand et al., 2010). The reason for these ambiguous results among studies about effects of early feed and water provision on utilization of the residual yolk is unclear. Potential reasons might be differences in incubation temperature (Ipek et al., 2014), brooding temperature (Akşit et al., 2010), or breeder age (Van der Pol et al., 2013), but much is unknown about how these factors affect posthatch yolk utilization. Owing to a lack of available literature, it is also unknown how embryonic yolk utilization or residual yolk weight at hatch is related to posthatch performance, health, and welfare, and this remains an interesting field of study.
To conclude, to define optimal yolk utilization during incubation, a good understanding of the underlying mechanisms taking place in the yolk sac, YSM, yolk stalk, and intestines is needed. After hatch, the residual yolk functions as the primary nutrient supply for chickens when feed is unavailable, but the exact contribution of the residual yolk toward the nutritional intake, or more specifically the protein or lipid intake, is unclear, as well as the interaction with other factors, such as the moment of first feeding.
Factors affecting residual yolk weight at hatch
Because genetic selection and changed management conditions during the last decades have resulted in strongly increased postnatal growth in broiler chickens (Yalçin et al., 2017), it has been suggested that the metabolic rate of the developing modern broiler chicken embryo has been increased throughout incubation as well (Bruzual et al., 2000). A higher metabolic rate would result in a higher utilization of yolk content during incubation and probably implies a higher embryonic MHP and a lower residual yolk weight and thus lower energy reserves at the moment of hatch. When chickens hatch with a lower amount of residual yolk and/or different residual yolk composition, this might affect development and performance in later life (Murakami et al., 1992). It is therefore important to know whether or not modern broiler breeds have a relatively lower amount of residual yolk and yolk nutrients at hatch than available (broiler) breeds in the past. Because of the lack of sufficient studies for other poultry breeds and species, we focus on broiler chickens only.
To examine possible changes in yolk utilization during incubation, it is necessary to determine residual yolk weight at hatch. For the current review, we selected scientific articles published between 1930 and June 2018 in which the residual yolk weight at hatch was provided. Furthermore, data on breed, strain, breeder age, egg storage duration, incubator or eggshell temperature (EST), egg weight, chicken weight at hatch, and yolk-free body mass (YFBM) were registered when available. This was done to identify whether or not modern broiler breeds differ in the amount of residual yolk at hatch compared with broiler breeds in the past and to investigate to which extent residual yolk weight at hatch is affected by (a)biotic factors, such as breeder flock age, egg composition, egg size, egg storage duration and condition, and incubation conditions (HSUS, 2008, Zuidhof et al., 2014). These factors are probably interrelated; for example, initial egg composition, and especially the distribution of yolk and albumen, is determined by breed, strain, breeder flock age, and egg weight (Tona et al., 2003a, Wolanski et al., 2006, Nangsuay et al., 2015a).
When figures were not provided, chicken weight was calculated as (YFBM + residual yolk weight) and YFBM as (chicken weight − residual yolk weight). Yolk-free body mass and residual yolk weight were also calculated as the percentage of chicken weight and residual yolk weight. Data were obtained as averages per treatment group. A treatment group was defined as any group of animals exposed to a particularly treatment (treatments could be, e.g., strain, breeder age, egg weight, storage duration, incubation temperature) within a study; the total number of studies was 42. Table 1 demonstrates effects of year of publication, storage duration, and incubation temperature on chicken weight, YFBM, and residual yolk weight at hatch. These results will be discussed in the following.
Table 1.
Average (minimum-maximum) initial egg weight and chicken weight, yolk-free body mass (YFBM), and residual yolk weight at hatch of broiler chickens based on studies between 1930 and 2018.
| Factor of influence | N1 | Chicken weight, g | YFBM, g | YFBM, % | Residual yolk, g | Residual yolk, % |
|---|---|---|---|---|---|---|
| Year of study | ||||||
| All studies | 126 | 43.9 (33.6–53.4) | 38.1 (30.3–44.8) | 86.8 (72.8–94.4) | 5.6 (2.3–12.1) | 12.8 (6.4–27.2) |
| ≤2000 | 2 | 43.4 (39.8–47.0) | 37.1 (35.1–39.1) | 85.7 (83.2–88.3) | 6.3 (4.7–7.9) | 14.3 (11.7–16.8) |
| >2000 | 124 | 43.9 (33.6–53.4) | 38.1 (30.3–44.8) | 86.9 (72.8–94.4) | 5.6 (2.3–12.1) | 12.8 (6.4–27.2) |
| Storage duration | ||||||
| ≤7 D | 57 | 43.4 (33.6–51.4) | 37.8 (30.8–43.0) | 87.1 (76.7–94.4) | 5.6 (2.3–10.1) | 12.8 (6.4–23.3) |
| >7 D | 20 | 42.6 (36.9–46.7) | 37.5 (32.6–41.4) | 88.0 (80.8–91.6) | 4.7 (3.0–8.3) | 10.9 (8.0–17.9) |
| Incubation temperature/EST | ||||||
| <37.0°C | 2 | 40.3 (39.5–41.0) | 34.8 (34.5–35.0) | 86.4 (85.4–87.3) | 4.8 (4.4–5.1) | 11.8 (10.7–12.9) |
| 37.0°C–38.2°C | 73 | 44.5 (33.6–53.4) | 39.0 (30.3–44.8) | 87.5 (79.3–94.4) | 5.5 (2.3–9.6) | 12.3 (6.4–20.7) |
| >38.2°C | 14 | 42.0 (37.2–47.7) | 34.7 (32.2–37.4) | 83.0 (72.8–91.1) | 7.2 (3.1–12.1) | 16.8 (8.2–27.2) |
Abbreviation: EST, egg shell temperature.
Number of treatment groups. A treatment group was defined as any group of animals exposed to a particular treatment (treatments could be, e.g., strain, breeder age, egg weight, storage duration, incubation temperature) within a study. The total number of studies was 42.
Effect of Generation (Modern vs. Past Broiler Breeders)
The boundary between modern and past broiler breeds was more or less arbitrarily set at the year 2000, resulting in 2 treatment groups before and 124 treatment groups after 2000 (Table 1). Two factors were important to set the boundary at the year 2000: 1) approximately at that year, first studies were published that used EST instead of machine or air temperature; 2) incubator types switched from more sealed off to open ventilation incubators (French, personal communication). It should be noted that there was a large variation within all studies for breed, storage duration and conditions, breeder age, egg weight, and so forth. Furthermore, the moment of sampling relative to the hatch moment was not indicated in most studies. In more recent studies (>2010), hatchlings were often sampled on a fixed time, shortly after they emerged from the eggshell, on their biological age (Molenaar et al., 2010, Maatjens et al., 2014, Maatjens et al., 2016, Nangsuay et al., 2015a, Nangsuay et al., 2015b, Nangsuay et al., 2016, Nangsuay et al., 2017), but in older studies, sampling was usually performed at pulling time, on the chickens' chronological age, where the individual hatch time was not taken into account. Measuring residual yolk weight shortly after hatch on a fixed time will probably reduce the variation within treatment groups and will probably result in a larger residual yolk size than measuring residual yolk weight at pulling time. This means that obtained results need to be interpreted carefully. Review of the studies indicated a wide range of reported values for chicken weight, YFBM, and residual yolk weight of broiler hatchlings. For example, residual yolk weight ranged between 6.4 and 27.2% within the 124 treatment groups of the modern broiler breeder (>2000; Table 1). As mentioned previously, the residual yolk weight is composed of both water and nutrients and weight alone may not truly represent the nutritional value of the residual yolk. Therefore, we decided to evaluate the weight of the residual yolk together with the amount of solids to ensure a fair comparison between old and modern breeder strains.
It seems that the water-to-solid ratio of the residual yolk has been changed over the years. Data of Romanoff (1967), based on 13 studies performed between 1923 and 1962, showed that the residual yolk weight after 21 D of incubation was on average 7.3 g and the dry matter (DM) concentration, 70.2%. The strains used were most likely layer chickens. The large variation found in size and solid concentration among the different studies was shown by other data presented by Romanoff (1967), where a residual yolk of 9.79 g was found with a DM concentration of 55.2%. More recent studies consistently found a DM concentration below 50%. Nangsuay et al. (2015a) found that residual yolk weight of Lohmann Brown layer chickens was 6.38 g, with a DM concentration of 47.4% at hatch (21.0 D of incubation). The residual yolk weight was slightly lower in Ross broiler chickens with 6.14 g (P = 0.046), but the DM concentration was higher with 49.8% (P < 0.001) measured at hatch (20.7 D of incubation). Another study of Nangsuay et al. (2013) investigated the effect of breeder age and egg size in broiler chickens on residual yolk weight and DM concentration at hatch (21.4 D of incubation). They found that the residual yolk weight was only affected by egg weight; a heavier egg resulted in a larger residual yolk weight (3.91 vs. 2.67 g; P < 0.001). Furthermore, DM concentration was only affected by flock age; an old flock (53 wk old) showed a higher DM concentration than a young flock (29 wk old) (48.9 vs. 45.5%, respectively; P < 0.001).
Effect of Egg Size and Breeder Age
During the last decades, differential genetic selection for eggs or meat has led to heavier eggs in broiler breeders than in layer breeders (Sahan et al., 2014). In addition, if broiler and layer eggs from the same egg size are compared, broiler eggs contain more yolk and less albumen than layer eggs (Ulmer-Franco et al., 2010). However, when eggs of the same breed and breeder age are heavier, this is mainly due to an increase in the amount of albumen (Everaert et al., 2008), resulting in a proportionate decrease in yolk weight and an increase in albumen weight with egg weight (Ho et al., 2011, Zuidhof et al., 2014).
Effects of breed on egg weight and egg composition are partly inter-related with effects of breeder age. Egg weight increases with age and normally reaches a plateau at the end of the laying cycle (Silversides and Scott, 2001). This is shown not only in broiler breeders but also in laying hens, ducks, and turkeys (Table 2). With aging of the breeder, yolk and albumen contents are disproportionately changed, as the proportion of yolk increases at the expense of the albumen and eggshell (O'Dea et al., 2004). It can be questioned whether there are any effects of the difference in egg size and egg composition, regardless of breeder age, on residual yolk weight and composition at hatch. Remarkably, this aspect is hardly investigated. Nangsuay et al. (2011) found that chickens of different breeder flock ages (29 and 53 wk) had comparable residual yolk weight at hatch (approximately 3.8 g) when the egg weight was identical, even when the fresh yolk weight differed up to 4.5 g (16.2 vs. 20.7 g for 29- and 53-wk-old broiler breeders, respectively). This may indicate that yolk utilization during incubation was higher in eggs of older flocks, resulting in a higher embryonic MHP. However, the influx of albumen and the solid composition of the residual yolk should be taken into account as well to investigate true yolk utilization, as Nangsuay et al. (2013) showed that DM concentration of the residual yolk was higher in old (53 wk) than in young (29 wk) breeder flocks. Regardless of breeder flock age, Nangsuay et al., 2011, Nangsuay et al., 2015a found that chickens originating from larger eggs had a larger residual yolk weight than chickens originating from smaller eggs, but without differences in DM concentration. This might lead to the conclusion that residual yolk weight at hatch appears to be determined by egg size rather than by breeder flock age and that residual yolk composition is more determined by breeder flock age than by egg size.
Table 2.
Effects of breeder flock ages on egg weight and egg composition in different breeds and strains.
| Breed | Strain | Flock age (wk) | Egg weight (g) | Shell (%)1 | Yolk (%)1 | Albumen (%)1 | Reference |
|---|---|---|---|---|---|---|---|
| Layer | Isa White | 25 | 52.49 | 10.75 | 23.61 | 65.64 | Silversides and Scott (2001) |
| Layer | Isa White | 31 | 55.97 | 10.36 | 25.65 | 63.99 | |
| Layer | Isa White | 49 | 60.49 | 9.92 | 27.30 | 62.78 | |
| Layer | Isa White | 59 | 61.71 | 9.52 | 28.16 | 62.32 | |
| Layer | Isa Brown | 25 | 56.44 | 10.61 | 22.39 | 67.01 | Silversides and Scott (2001) |
| Layer | Isa Brown | 31 | 58.50 | 10.49 | 24.17 | 65.34 | |
| Layer | Isa Brown | 49 | 63.39 | 10.24 | 24.95 | 64.81 | |
| Layer | Isa Brown | 59 | 63.65 | 10.03 | 25.66 | 64.32 | |
| Broiler | Cobb 500 | 29 | 53.8 | 8.8 | 27.8 | 63.3 | Ulmer-Franco et al. (2010) |
| Broiler | Cobb 500 | 59 | 71.3 | 8.6 | 31.3 | 58.5 | |
| Pekin duck | Star 53 | 28 | 75.66 | 9.97 | 28.80 | 61.24 | Onbaşilar et al. (2014) |
| Pekin duck | Star 53 | 34 | 83.61 | 9.72 | 29.54 | 60.74 | |
| Pekin duck | Star 53 | 40 | 87.62 | 9.97 | 31.78 | 58.25 | |
| Broiler | Ross 308 | 32 | 53.38 | 12.21 | 31.37 | 55.13 | Yalçin et al. (2008) |
| Broiler | Ross 308 | 42 | 61.35 | 11.28 | 34.01 | 54.58 | |
| Broiler | Ross 308 | 65 | 64.61 | 11.64 | 37.12 | 52.21 | |
| Broiler | Arbor Acres | 26 | 52.3 | 11.8 | 27.4 | 60.8 | Peebles et al. (2000) |
| Broiler | Arbor Acres | 31 | 59.4 | 11.9 | 28.2 | 59.9 | |
| Broiler | Arbor Acres | 35 | 62.3 | 10.8 | 30.9 | 58.3 | |
| Broiler | Arbor Acres | 41 | 64.7 | 12.6 | 30.3 | 57.1 | |
| Broiler | Arbor Acres | 47 | 67.3 | 10.6 | 31.0 | 58.4 | |
| Turkey | Hybrid/Nicholas | 30 | 75.09 | 9.16 | 26.09 | 64.69 | Hamidu et al. (2011) |
| Turkey | Hybrid/Nicholas | 34 | 88.30 | 9.41 | 26.83 | 63.63 | |
| Turkey | Hybrid/Nicholas | 55 | 97.16 | 8.55 | 31.00 | 60.26 | |
| Turkey | Hybrid/Nicholas | 60 | 98.91 | 8.27 | 32.30 | 59.21 |
Relative to egg weight.
Effect of Egg Storage Duration Before Incubation
Commercial hatcheries set broiler hatch eggs normally after 3 to 5 D of storage to minimize negative effects of egg storage on hatchability and chicken quality at hatch (Reijrink et al., 2010). However, especially layer or parent stock hatcheries sometimes need to prolong the storage duration (Tona et al., 2003b, Fasenko, 2007), dependent on the supply of hatching eggs, hatchery capacity, and market demand for day-old chickens (Fasenko, 2007). Egg storage beyond 7 D is associated with a longer incubation duration (Yassin et al., 2009), lower hatchability (Tona et al., 2004, Silva et al., 2008), lower chicken quality at hatch (Silva et al., 2008, Yassin et al., 2009), lower subsequent growth performance, and a higher posthatch mortality (Tona et al., 2004). Table 1 shows that broiler chickens originating from eggs that were stored beyond 7 D did not seem to differ in chicken weight or YFBM at hatch but had a 0.9-g smaller residual yolk weight at hatch than chickens originating from eggs stored for less than 7 D, which may be explained by a longer incubation duration. These results are based on a comparison of 20 (>7 D of storage) vs. 57 (<7 D of storage) treatment groups in different studies. However, this is not confirmed by individual studies on effects of storage duration on chicken quality, including yolk utilization. Reijrink et al. (2010) found that chickens from eggs stored for 4 D compared with 14 D had a higher YFBM of 0.5 g at hatch, but a comparable residual yolk weight (5.8 vs. 6.1 g, for eggs stored for 4 or 14 D, respectively) at 12 h after emergence from the eggshell. Silva et al. (2008) (4, 9, or 14 D of storage) and Yalçin et al. (2016) (3 or 14 D of storage) also found no effect of storage duration on residual yolk weight at hatch, nor on chicken weight and YFBM at pull time. This suggests that prolonged storage duration appears to affect embryonic chicken development and postnatal performance negatively but does not change residual yolk weight at hatch. Whether or not prolonged egg storage changes yolk utilization and/or efficiency of nutrient use requires further investigation.
Effects of Incubation Temperature
During incubation, embryos act mainly as poikilothermic (Tazawa et al., 1988), which means that their metabolic rate, yolk utilization, and embryonic growth during incubation are temperature dependent (Lourens et al., 2006a). Several studies examined effects of incubation temperature on embryonic development (Leksrisompong et al., 2007, Piestun et al., 2008, Molenaar et al., 2010, Maatjens et al., 2014) and found that a higher (>37.8°C) incubation temperature or EST from ED7 to hatch reduced embryonic development, which was demonstrated by shorter chicken length, lower chicken weight, lower YFBM, and higher residual yolk weight at hatch (Metcalfe et al., 1981, Leksrisompong et al., 2007, Molenaar et al., 2010; Table 1). A limited number of studies (Maatjens et al., 2014, Maatjens et al., 2016) investigated effects of a lower incubation temperature (<37.8°C) on embryonic development. Remarkably, an incubation temperature of 36.7°C from ED19 onward (Maatjens et al., 2014) or an EST of 35.6°C or 36.7°C from ED15 onward (Maatjens et al., 2016) did not affect residual yolk weight at hatch compared with an incubation temperature of 37.8°C, although incubation duration was longer (up to 21 h). The lower YFBM and higher residual yolk weight at hatch, due to a higher incubation temperature after ED7, is probably related to a disbalance between metabolic rate and oxygen availability as explained by, for example, Nangsuay et al. (2017). However, lowering the incubation temperature to 37.8°C appears to restore the balance between metabolic rate and oxygen availability, meaning that a further decrease in incubation temperature did not influence yolk utilization.
Based on the consistent literature about effects of incubation temperature on YFBM and residual yolk weight at hatch, it can be concluded that it appears plausible that part of the variation among studies related to embryonic yolk utilization and residual yolk weight at hatch can be explained by incubation temperature and particularly when a higher incubation temperature than 37.8°C is applied in the last week of incubation.
Effects of Posthatch Brooding Temperature
A proper thermal environment after hatch seems to be important for chickens to use nutrients for development in the posthatch period (Scott and Washburn, 1985). Brooding temperatures of approximately 32°C resulted in higher body weight gain than cool brooding temperatures (approximately 26°C; Mikec et al., 2006, Van der Pol et al., 2013). Effects of brooding temperature in the first week after hatch on particular residual yolk weight or utilization are limited. Mikec et al. (2006) exposed chickens to 27°C, 32°C, or 35°C between day 1 and 3 after hatch and to 25°C, 30°C, or 33°C, respectively, at day 4 and 5 after hatch. They found that approximately 60% of the yolk sac content was utilized on the first posthatch day. On the second day, 40% of the first day resorption, 35% on the third day, and 25% on the fourth day. No differences in residual yolk weights were found between brooding temperature groups at day 1 to 5 after hatch. However, body weight gain until day 5 after hatch was higher in the 32°C to 30°C treatment group than in the 27°C to 25°C treatment group, which might be due to differences in feed intake, which is probably positively influenced by brooding temperature (Moraes et al., 2002). The latter study used brooding temperatures of 20°C, 25°C, or 35°C for the first week of age and found no differences in residual yolk weight between the different brooding temperatures. These results suggest that yolk utilization in the early posthatch period appears to be independent of brooding temperature, which does not mean that body weight gain is not affected by the first week brooding temperature.
Factors affecting metabolic rate and embryonic heat production
Because egg nutrients are the only available nutritional source for the developing embryo, variation in egg composition, caused by breed, strain, breeder age, egg size, or breeder nutrition, can possibly influence yolk utilization and therefore embryonic development (French and Tullett, 1990). The biochemical processes to convert egg nutrients to energy requires O2 and produces CO2, water, and heat, and these “by-products” will in general be increased in accordance with a high embryonic metabolic rate and growth rate (Etches, 1996). When considering the utilization of energy sources, it is essential to quantify the metabolic rate or MHP throughout the incubation process. The MHP of an embryo is determined by the total amount of energy used from the eggs and the efficiency of converting this energy into chicken body tissue (Ar et al., 1987, Pearson et al., 1991).
Effect of Generation (Modern vs. Past Broiler Breeders)
Studies, conducted between 1937 and 2018 in which embryonic O2 consumption and/or CO2 production was determined, were used to identify any trends or responses in MHP due to genetic selection or changed management conditions. Selected studies used different experimental techniques, breeds and strains, breeder ages, and egg sizes. To facilitate direct comparisons, data from available studies were directly obtained from the studies or recalculated from available data. If both O2 consumption and CO2 production were determined, heat production was calculated, using the formula of Romijn and Lokhorst (1961). If only O2 consumption or CO2 production was determined, heat production was calculated using the same formula, using a respiration quotient of 0.78 (Romanoff, 1967). If no data, but only figures about O2 production, CO2 production, and/or heat production, were published, values were estimated as accurately as possible.
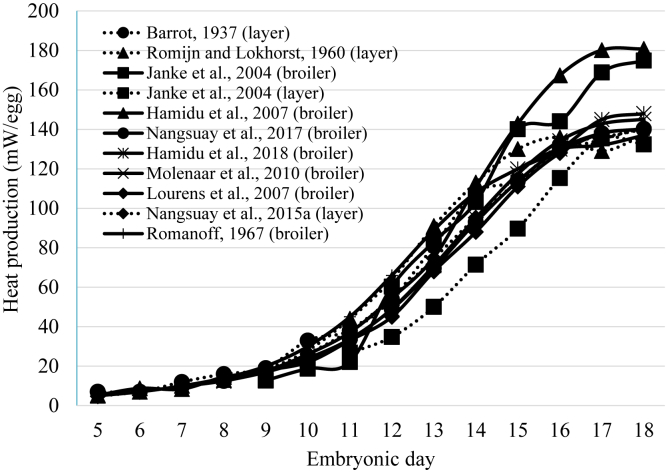
Figure 1 shows embryonic MHP at several consecutive days of 11 studies, conducted between 1937 and 2018. Metabolic heat production before ED5 and after ED18 was not used because before ED5, MHP is very low and difficult to determine accurately, and after ED18, internal pipping and lung ventilation starts, which results in a huge increase and variation in MHP. Studies differed in MHP up to 17% at ED18 throughout the years: 143 mW/egg (Tazawa, 1973); 137 mW/egg (Tazawa et al., 1988); 145 mW/egg (Tazawa et al., 1992); 128 mW/egg (Pearson et al., 1996); 141 mW/egg (Dzialowski et al., 2002); 151 mW/egg (Black and Burggren, 2004); 140 mW/egg (Janke et al., 2004); 144 mW/egg (O'Dea et al., 2004); 137 mW/egg (Lourens et al., 2006b); 125 mW/egg (Sato et al., 2006); 140 mW/egg (Druyan, 2010); 148 mW/egg (Nangsuay et al., 2013). As these studies used eggs from different flock ages and different egg weights were included, it might be better to express the MHP per gram of egg to facilitate direct comparison between studies. However, when expressing the MHP per gram of egg, results remained similar to results shown in Figure 1 and are therefore not presented.
Figure 1.
Embryonic metabolic heat production in broiler and layer strains from different studies published between 1937 and 2018.
From Figure 1 and the other literature sources indicated previously, it can be concluded that embryonic MHP in the last decades only showed a small increase, despite the tremendous progress in postnatal performance (egg or meat production).
Effect of Strain and Breeder Age
Variation in MHP among studies is found to be considerable, and one of the contributing factors is probably the difference between broiler and layer strains. Broiler strains have a higher embryonic metabolic rate than laying strains (Janke et al., 2004, Sato et al., 2006). This difference in metabolic rate and lipid metabolism between broiler and layer embryos is probably related to the yolk weight in fresh eggs (Nangsuay et al., 2015a) and rate of yolk utilization (Druyan, 2010) between broiler and layer eggs, which in turn is related to the difference in eggshell conductance. As broiler breeders produce heavier eggs with a larger yolk than layer breeders, a broiler egg contains more energy and, therefore, can metabolize more nutrients (Mortola and Al Awam, 2010, Nangsuay et al., 2015a), resulting in higher embryonic MHP. The results of these studies indicate that metabolic rate and MHP of embryos depend on the yolk weight. This is also demonstrated by Nangsuay et al. (2013), who found a strong correlation between fresh yolk weight and energy utilization (R2 = 0.88). In addition, the larger yolk weight of broiler eggs than that of layer eggs might have an influence on the absorptive area of the YSM (Yadgary et al., 2013) and the vascularization network of the YSM (Adair et al. 1990), resulting in a higher yolk absorption and utilization and consequently in a higher MHP.
In addition to differences between breeds, embryonic MHP might be affected by other biotic and abiotic factors as well. Two important biotic factors are strain (within breed) and breeder age. Comparing the metabolic rate of 2 modern broiler strains (Cobb 500 and Ross 308), it was shown that Ross 308 embryos had a slightly higher embryonic MHP at ED15 until ED18 than Cobb 500 embryos, even though they did not differ in initial yolk weight or energy content in the yolk and albumen. This higher embryonic MHP might be explained by a higher embryonic O2 availability due to a higher eggshell conductance in Ross 308 than in Cobb 500 eggs (Tona et al., 2010, Nangsuay et al., 2015b). As previously mentioned, breeder age influences egg composition, particularly yolk and albumen content. The studies shown in Figure 1 used eggs from different breeder ages, meaning that differences in MHP might be caused by differences in egg composition due to differences in breeder age. Several studies have shown that embryos originating from larger eggs from older breeders (>50 wk) had a higher embryonic MHP of approximately 26 mW/egg at ED18 than embryos originating from smaller eggs from young breeders (<40 wk) (Lourens et al., 2006b, Mortola and Al Awam, 2010, Nangsuay et al., 2013). Both larger eggs and eggs of older flocks do have larger yolks, and thus, more nutrients, than smaller eggs and eggs of younger flocks, and consequently more energy is available to be metabolized in larger eggs (Lourens et al., 2006b, Nangsuay et al., 2013).
Effect of Storage Duration
Storage duration is an abiotic factor that can influence embryonic MHP (Hamidu et al., 2018, Haque et al., 1996, Romijn and Lokhorst, 1960, Segura et al., 2006, Fasenko, 2007, Uddin and Hamidu, 2014), as embryos from long-stored eggs (>7 D) had a lower metabolic rate during incubation over an 18-D period (Segura et al., 2006) than short-stored eggs (<7 D). For example, over the incubation period between day 0 and 18, embryos originating from eggs stored for 4 D produced on average more metabolic heat than embryos originating from eggs stored for 15 D (35.2 vs. 32.4 mW, respectively) (Segura et al., 2006). These findings are supported by Uddin and Hamidu (2014), who found a (not always significant) higher MHP from ED10 until ED19 in embryos originating from eggs stored for 4 D than in embryos originating from eggs stored for 14 D.
Effect of Incubation Temperature
A second abiotic factor affecting MHP and yolk utilization is incubation temperature (Lourens et al., 2007). Several studies found a higher embryonic MHP in eggs that were incubated at a higher EST (38.9°C) during the second week of incubation (Lourens et al., 2007, Molenaar et al., 2010) than at a control EST of 37.8°C. Higher incubation temperatures lead to higher metabolic rate, and as a result, more O2 is needed. During the last week of incubation, the demand of O2 exceeds the diffusion capacity of the egg shell pore system and the CAM, and as a consequence, metabolic rate is reduced. This results in a lower MHP at a high EST (38.9°C) compared with a control EST (37.8°C) in the last week of incubation (until ED18), in contrast to what is seen in the second week of incubation (Lourens et al., 2007). Overall, it has been shown that heat production can be different on some specific days (particularly the last few days before internal pipping), but total heat production is often equal between embryos incubated at 38.9°C compared with at 37.8°C.
Yolk utilization efficiency
The aforementioned results suggest that both the weight and DM concentration of the residual yolk at hatch have been decreased in the recent decades. This result can probably not be assigned to genetic selection only because management and incubation factors might also play a role, as discussed previously. For example, egg size and composition have been changed in the last decades with a trend toward a larger egg with a larger yolk, although the percentage of yolk has become lower, which is confirmed in the study of Collins et al. (2014). They compared 2 meat-type chickens, the Athens Canadian Random Bred and the Cobb 500 at 43 wk of age, and found that eggs of the Cobb strain were 13 g heavier, but the relative amount of solids was 0.9% lower than those of the ACRB strain. The weight of the residual yolk at hatch was not indicated, but the solid concentration of the residual yolk was higher in the Cobb than in the ACRB strain (P < 0.0001), whereas the YFBM expressed as a percentage of the initial egg weight was very similar (61.8 and 61.6% for Cobb and ACRB, respectively). The incubation temperature may have been a confounding factor in this study because both strains, and therefore different egg weights, were incubated in the same incubator and it is known that large compared with small eggs will experience a higher temperature under the same conditions, affecting the way nutrients are utilized (Lourens et al., 2006b, Molenaar et al., 2010, Nangsuay et al., 2016). The results of the study by Collins et al. (2014) may indicate that not only absorption of egg nutrients but possibly also the efficiency of nutrient utilization may have been changed over the years with embryos of modern broiler strains being more efficient, which is also found in the posthatch period (Havenstein et al., 2003). The latter can be further explored by evaluating the gross changes of the solids within the egg throughout the incubation period.
Because there are only a few studies performed related to this topic, calculations were made from data of Romanoff (1967) (strains not specified, but probably layers, based on 12 earlier studies) and from broiler and layer data of Molenaar et al. (2010); Ross 308, Nangsuay et al. (2013); Ross 308, Nangsuay et al. (2016); Ross 308, Cobb, Nangsuay et al. (2015a); Ross 308, Lohmann Brown, and Nangsuay et al. (2015b); Ross 308, Cobb (Table 3). The total amount of solids in the egg was used to calculate the relative amount of solids that was found within the yolk-free body (YFB) and residual yolk at hatch, as well as the external loss of the initial egg solids. The efficiency to transfer solids from the egg toward the YFB was expressed as a percentage and calculated by dividing the solids retained within the YFB by the solids used throughout incubation (solids in total egg - solids in residual yolk) (adapted from Molenaar et al., 2010; Table 3).
Table 3.
Egg weight, solid content of egg, yolk-free body mass (YFBM), residual yolk (RY), external losses and relative content of YFBM, RY, and external loss to initial solid content of eggs of six different studies comparing strain, incubation conditions, flock age, and sampling time of the chickens.
| Study | Treatment | Strain | Sampling time | Egg weight, g3 | Solid content, g |
YFBM, % | RY, % | External loss, % | Efficiency, %6 | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Egg4 | YFBM | RY | External loss5 | |||||||||
| Romanoff, 1967 | None | Not known | Pulling time | 60.0 | 14.07 | 5.81 | 5.4 | 2.86 | 41.3 | 38.4 | 20.3 | 67.0 |
| Molenaar et al., 2010 | EST1 of 37.8°C, >ED7 | Hybro | 12 h after hatch | 62.4 | 14.69 | 7.90 | 3.05 | 3.74 | 53.8 | 20.8 | 25.5 | 67.9 |
| Molenaar et al., 2010 | EST1 of 38.9°C, >ED7 | Hybro | 12 h after hatch | 62.4 | 14.59 | 7.23 | 3.72 | 3.64 | 49.6 | 25.5 | 24.9 | 66.5 |
| Molenaar et al., 2010 | O22 of 17%, ED7-ED19 | Hybro | 12 h after hatch | 62.4 | 14.64 | 6.91 | 4.11 | 3.62 | 47.2 | 28.1 | 24.7 | 65.6 |
| Molenaar et al., 2010 | O22 of 21%, ED7-ED19 | Hybro | 12 h after hatch | 62.3 | 14.64 | 7.70 | 3.13 | 3.81 | 52.6 | 21.4 | 26.0 | 66.9 |
| Molenaar et al., 2010 | O22 of 25%, ED7-ED19 | Hybro | 12 h after hatch | 62.5 | 14.64 | 8.09 | 2.91 | 3.64 | 55.3 | 19.9 | 24.9 | 69.0 |
| Molenaar et al., 2010 | 12 h after hatch | Hybro | 12 h after hatch | 62.4 | 14.64 | 7.57 | 3.38 | 3.69 | 51.7 | 23.1 | 25.2 | 67.2 |
| Molenaar et al., 2010 | 48 h after hatch | Hybro | 48 h after hatch | 62.4 | 14.64 | 8.20 | 1.42 | 5.02 | 56.0 | 9.7 | 34.3 | 62.0 |
| Nangsuay et al., 2013 | Small eggs | Ross 308 | pulling | 58.4 | 13.19 | 8.43 | 1.26 | 3.50 | 63.9 | 9.6 | 26.6 | 70.6 |
| Nangsuay et al., 2013 | Large eggs | Ross 308 | pulling | 65.8 | 14.49 | 8.86 | 1.85 | 3.79 | 61.1 | 12.7 | 26.1 | 70.0 |
| Nangsuay et al., 2013 | Young flock | Ross 308 | pulling | 62.0 | 13.38 | 8.27 | 1.58 | 3.53 | 61.8 | 11.8 | 26.4 | 70.1 |
| Nangsuay et al., 2013 | Old flock | Ross 308 | pulling | 62.2 | 14.39 | 9.04 | 1.52 | 3.83 | 62.8 | 10.6 | 26.6 | 70.2 |
| Nangsuay et al., 2015a | Layer strain | Lohmann Brown | 6 h after hatch | 63.0 | 12.93 | 6.76 | 3.02 | 3.15 | 52.3 | 23.4 | 24.3 | 68.2 |
| Nangsuay et al., 2015a | Broiler strain | Ross 308 | 6 h after hatch | 63.2 | 14.31 | 7.90 | 3.06 | 3.35 | 55.2 | 21.4 | 23.4 | 70.2 |
| Nangsuay et al., 2015b | Broiler strain Cobb | Cobb 500 | 3 h after hatch | 62.6 | 13.82 | 7.15 | 3.29 | 3.38 | 51.7 | 23.8 | 24.5 | 67.9 |
| Nangsuay et al., 2015b | Broiler strain Ross | Ross 308 | 3 h after hatch | 62.2 | 14.06 | 7.18 | 3.10 | 3.78 | 51.1 | 22.0 | 26.9 | 65.5 |
| Nangsuay et al., 2016 | Young flock | Cobb 500 + Ross 308 | 3 h after hatch | 61.1 | 12.93 | 7.15 | 3.26 | 2.52 | 55.3 | 25.2 | 19.5 | 73.9 |
| Nangsuay et al., 2016 | Old flock | Cobb 500 + Ross 308 | 3 h after hatch | 61.6 | 14.05 | 7.40 | 3.90 | 2.75 | 52.7 | 27.8 | 19.6 | 72.9 |
| Nangsuay et al., 2016 | Broiler strain Cobb | Cobb 500 | 3 h after hatch | 61.3 | 13.52 | 7.20 | 3.80 | 2.52 | 53.3 | 28.1 | 18.6 | 74.1 |
| Nangsuay et al., 2016 | Broiler strain Ross | Ross 308 | 3 h after hatch | 61.4 | 13.46 | 7.34 | 3.36 | 2.76 | 54.5 | 25.0 | 20.5 | 72.7 |
Abbreviation: YFB, yolk-free body.
EST: eggshell temperature.
O2: oxygen concentration.
Fresh egg weight, including eggshell.
Solid content in both egg albumen and yolk, excluding eggshell.
External loss was calculated as (solid content total egg)–(solid content YFB)–(solid content RY).
Efficiency of solid transfer from egg toward YFB was calculated as ([solid content YFB]/[solid content total egg-solid content residual yolk])*100%.
Data seem to show that the total amount of solids in the initial egg was quite similar between the strains in the study by Romanoff (1967) and the prime and older flocks of the broiler strains in the studies of Molenaar et al. (2010) and Nangsuay et al., 2013, Nangsuay et al., 2015a, Nangsuay et al., 2015b, Nangsuay et al., 2016. However, layer strains and young broiler breeder flocks showed a lower total amount of solids in the initial egg (Nangsuay et al., 2013, Nangsuay et al., 2015b, Nangsuay et al., 2016). Based on the studies presented in Table 3, it appears that the solid content of the residual yold at hatch has been decreased in the last decades but that external losses (including meconium and MHP) have been slightly increased. Looking to the overall efficiency to transfer solids from the egg toward the YFB, variation among studies is considerable, but on average, there seems to be an increase. This confirms the hypothesis of Collins et al. (2014) that modern chicken strains are able to utilize their nutrients more efficiently throughout incubation than strains used in the past. The results might further implicate that the extent to which MHP is increased within modern broiler strains is influenced by the efficiency in which egg nutrients are utilized.
Conclusion
It can be concluded that 1) residual yolk weight and the total solid amount of the residual yolk at hatch seem to be decreased over a period of 88 yr. Metabolic heat production during incubation tends to be slightly increased in the last decades. This increase is influenced by several factors, such as strain, flock age, egg size, and incubation conditions. The extent of the increase in MHP seems to be related to a slight increase in the efficiency of yolk solid utilization as well. It should be noticed that variation among studies for these variables is considerable and that data on strains in the past are largely lacking. Therefore, it cannot be concluded whether the (lack of) differences between old and modern strains are due to genetic selection, changed management and incubation conditions, or moment of sampling (immediately after hatch or at pulling). It is remarkable that with the genetic progress and improved management and incubation conditions over the last 88 yr, which have had considerable effects on posthatch performance, effects on yolk utilization efficiency and embryonic MHP are limited; 2) factors affecting residual yolk weight at hatch are particularly egg size and incubation temperature, whereas breeder age is affecting especially the nutrient composition of the residual yolk. Egg storage duration and posthatch brooding temperature seem to play a minor role. However, there is a paucity of reliable published data concerning (factors affecting) yolk utilization and residual yolk weight at hatch and the relationship with posthatch performance.
Acknowledgments
The financial support of the European Poultry Breeders association for conducting this study is gratefully acknowledged.
The authors declare they do not have any conflict of interest.
References
- Adair T.H., Gay W.J., Montani J.P. Growth regulation of the vascular system: evidence for a metabolic hypothesis. Am. J. Physiol. 1990;259:393–404. doi: 10.1152/ajpregu.1990.259.3.R393. [DOI] [PubMed] [Google Scholar]
- Akşit M., Yalçin S., Yenisey C., Özdemir D. Brooding temperatures for chicks acclimated to heat during incubation: effects on post-hatch intestinal development and body weight under heat stress. Br. Poult. Sci. 2010;51:444–452. doi: 10.1080/00071668.2010.495746. [DOI] [PubMed] [Google Scholar]
- Ar A., Arieli B., Belinsky A., Yom-Tov Y. Energy in avian eggs and hatchlings: utilization and transfer. J. Exp. Zool. Suppl. 1987;1:151–164. [PubMed] [Google Scholar]
- Barri A. Virginia Polytech. Institut. State Univ.; Virginia, USA: 2008. Effects of Incubation Temperature and Transportation Stress on Yolk Utilization, Small Intestine Development, and Post-hatch Performance of High-Yield Broiler Chicks. PhD thesis. [Google Scholar]
- Barri A., Honacker C.F., Sottosanti J.R., Hulet R.M., McElroy A.P. Effect of incubation temperature on nutrient transporters and small intestine morphology in broiler chickens. Poult. Sci. 2011;90:118–125. doi: 10.3382/ps.2010-00908. [DOI] [PubMed] [Google Scholar]
- Barott H.G. Effect of temperature, humidity and other factors on hatch of hen’s eggs and on energy metabolism of chick embryos. USDA Technic. Bull. 1937;553:1–45. [Google Scholar]
- Black J.L., Burggren W.W. Acclimation to hypothermic incubation in developing chicken embryos (Gallus domesticus): I. Developmental effects and chronic and acute metabolic adjustments. J. Exp. Biol. 2004;207:1543–1552. doi: 10.1242/jeb.00909. [DOI] [PubMed] [Google Scholar]
- Bruzual J., Peak S., Brake J., Peebles E. Effects of relative humidity during the last five days of incubation and brooding temperature on performance of broiler chicks from young broiler breeders. Poult. Sci. 2000;79:1385–1391. doi: 10.1093/ps/79.10.1385. [DOI] [PubMed] [Google Scholar]
- Buzala M., Adamski M., Janicki B. Characteristics of performance traits and the quality of meat and fat in Polish oat geese. World Poult. Sci. J. 2014;70:531–542. [Google Scholar]
- Buzala M., Janicki B., Czarnecki R. Consequences of different growth rates in broiler breeder and layer hens on embryogenesis, metabolism and metabolic rate: a review. Poult. Sci. 2015;94:728–733. doi: 10.3382/ps/pev015. [DOI] [PubMed] [Google Scholar]
- Christensen V.L., Donaldson W.E., Nestor K.E. Embryonic viability and metabolism in turkey lines selected for egg production or growth. Poult. Sci. 1993;72:829–838. [Google Scholar]
- Collins K.E., McLendon B.L., Wilson J.L. Egg characteristics and hatch performance of Athens Canadian Random Bred 1955 meat-type chickens and 2013 Cobb 500 broilers. Poult. Sci. 2014;93:2151–2157. doi: 10.3382/ps.2014-03895. [DOI] [PubMed] [Google Scholar]
- de Oliveira J.E., Uni Z., Ferket P.R. Important metabolic pathways in poultry embryos prior to hatch. World Poult. Sci. J. 2008;64:488–499. [Google Scholar]
- de Oliveira J.E., Druyan S., Uni Z., Ashwell C.M., Ferket P.R. Prehatch intestinal maturation of turkey embryos demonstrated through gene expression patterns. Poult. Sci. 2009;88:2600–2609. doi: 10.3382/ps.2008-00548. [DOI] [PubMed] [Google Scholar]
- Druyan S. The effects of genetic line (broiler vs. layers) on embryo development. Poult. Sci. 2010;89:1457–1467. doi: 10.3382/ps.2009-00304. [DOI] [PubMed] [Google Scholar]
- Dzialowski E.M., von Plettenberg D., Elmonoufy N.A., Burggren W.W. Chronic hypoxia alters the physiological and morphological trajectories of developing chicken embryos. Comp. Biochem. Physiol. Part A: Mol. Integr. Physiol. 2002;131:713–724. doi: 10.1016/s1095-6433(02)00009-0. [DOI] [PubMed] [Google Scholar]
- Dzoma B.M., Dorrestein G.M. Yolk sac retention in the ostrich (Struthio camelus): histopathologic, anatomic, and physiologic considerations. J. Av. Med. Surg. 2001;15:81–89. [Google Scholar]
- El-Husseiny O., El-Wafa S.A., El-Komy H. Influence of fasting or early feeding on broiler performance. Int. J. Poult. Sci. 2008;7:263–271. [Google Scholar]
- Esteban S., Rayo J.M., Moreno M., Sastre M., Rial R.V., Tur J.A. A role played by the vitelline diverticulum in the yolk sac resorption in young post-hatched chickens. J. Comp. Physiol. B. 1991;160:645–648. [Google Scholar]
- Etches R.J. CAB International; Wallingford, U.K: 1996. Reproduction in Poultry. [Google Scholar]
- Everaert N., Willemsen H., De Smit L., Witter A., Baerdemaeker J., Decuypere E. Comparison of a modern broiler and layer strain during embryonic development and the hatching process. Br. Poult. Sci. 2008;49:574–582. doi: 10.1080/00071660802357025. [DOI] [PubMed] [Google Scholar]
- Fasenko G.M. Egg storage and the embryo. Poult. Sci. 2007;86:1020–1024. doi: 10.1093/ps/86.5.1020. [DOI] [PubMed] [Google Scholar]
- French N.A., Tullett S.G. Variation in the eggs of poultry species. In: Tullett S.G., editor. Avian Incubation. Butterworth & Co Ltd; Kent, U.K: 1990. pp. 59–77. [Google Scholar]
- Gonzales E., Kondo N., Saldanha E.S., Loddy M.M., Careghi C., Decuypere E. Performance and physiological parameters of broiler chickens subjected to fasting on the neonatal period. Poult. Sci. 2003;82:1250–1256. doi: 10.1093/ps/82.8.1250. [DOI] [PubMed] [Google Scholar]
- Hamidu J.A., Fasenko G.M., Feddes J.J.R., O’Dea E.E., Ouelette C.A., Wineland M., Christensen V.L. The effect of broiler breeder genetic strain and parent flock age on eggshell conductance and embryonic metabolism. Poult. Sci. 2007;86:2420–2432. doi: 10.3382/ps.2007-00265. [DOI] [PubMed] [Google Scholar]
- Hamidu J.A., Fasenko G.M., Guan L., Barreda D.R., Feddes J.J.R. Influence of parent flock age on embryonic metabolism in modern turkey strains. Poult. Sci. 2011;90:426–434. doi: 10.3382/ps.2010-00967. [DOI] [PubMed] [Google Scholar]
- Hamidu J.A., Torres C.A., Johnson-Dahl M.L., Korver D.R. Physiological response of broiler embryos to different incubator temperature profiles and maternal flock age during incubation. 1. Embryonic metabolism and day-old chick quality. Poult. Sci. 2018;97:2934–2946. doi: 10.3382/ps/pey089. [DOI] [PubMed] [Google Scholar]
- Haque M., Pearson J., Hou P.L., Tazawa H. Effects of pre-incubation egg storage on embryonic functions and growth. Resp. Physiol. 1996;103:89–98. doi: 10.1016/0034-5687(95)00062-3. [DOI] [PubMed] [Google Scholar]
- Havenstein G.B., Ferket P.R., Qureshi M.A. Growth, livability, and feed conversion of 1957 versus 2001 broilers when fed representative 1957 and 2001 broiler diets. Poult. Sci. 2003;82:1500–1508. doi: 10.1093/ps/82.10.1500. [DOI] [PubMed] [Google Scholar]
- Ho D.H., Reed W.L., Burggren W.W. Egg yolk environment differentially influences physiological and morphological development of broiler and layer chicken embryos. J. Exp. Biol. 2011;214:619–628. doi: 10.1242/jeb.046714. [DOI] [PubMed] [Google Scholar]
- HSUS . Human Soc.; U.S.A: 2008. An HSUS Report: Welfare Issues with Transport of Day-Old Chicks. [Google Scholar]
- Ipek A., Sahan U., Baycan S., Sözcü A. The effects of different eggshell temperatures on embryonic development, hatchability, chick quality, and first-week broiler performance. Poult. Sci. 2014;93:464–472. doi: 10.3382/ps.2013-03336. [DOI] [PubMed] [Google Scholar]
- Janke O., Tzschentke B., Boerjan M. Comparative investigations of heat production and body temperature in embryos of modern chicken breeds. Av. Poult. Biol. Rev. 2004;15:191–196. [Google Scholar]
- Joseph N.S., Moran E.T. Characteristics of eggs, embryos, and chicks from broiler breeder hens selected for growth or meat yield. J. Appl. Poult. Res. 2005;14:275–280. [Google Scholar]
- Lamot D. Wageningen University; Wageningen, the Netherlands: 2017. First Week Nutrition for Broiler Chickens. PhD thesis. [Google Scholar]
- Leksrisompong N., Romero-Sanchez H., Plumstead P., Brannan K., Brake J. Broiler incubation. 1. Effect of elevated temperature during late incubation on body weight and organs of chicks. Poult. Sci. 2007;86:2685–2691. doi: 10.3382/ps.2007-00170. [DOI] [PubMed] [Google Scholar]
- Lourens A., van den Brand H., Heetkamp M.J.W., Meijerhof R., Kemp B. Metabolic responses of chick embryos to short-term temperature fluctuations. Poult. Sci. 2006;85:1081–1086. doi: 10.1093/ps/85.6.1081. [DOI] [PubMed] [Google Scholar]
- Lourens A., Molenaar R., van den Brand H., Heetkamp M.J.W., Meijerhof R., Kemp B. Effect of egg size on heat production and the transition of energy from egg to hatchling. Poult. Sci. 2006;85:770–776. doi: 10.1093/ps/85.4.770. [DOI] [PubMed] [Google Scholar]
- Lourens A., van den Brand H., Heetkamp M.J.W., Meijerhof R., Kemp B. Effects of eggshell temperature and oxygen concentration on embryo growth and metabolism during incubation. Poult. Sci. 2007;86:2194–2199. doi: 10.1093/ps/86.10.2194. [DOI] [PubMed] [Google Scholar]
- Maatjens C.M., Reijrink I.A.M., Molenaar R., van der Pol C.W., Kemp B., van den Brand H. Temperature and CO2 during the hatching phase. I. Effects on chick quality and organ development. Poult. Sci. 2014;93:645–654. doi: 10.3382/ps.2013-03490. [DOI] [PubMed] [Google Scholar]
- Maatjens C.M., van Roovert-Reijrink I.A.M., Engel B., van der Pol C.W., Kemp B., van den Brand H. Temperature during the last week of incubation. I. Effects on hatching pattern and broiler chicken embryonic organ development. Poult. Sci. 2016;95:956–965. doi: 10.3382/ps/pev447. [DOI] [PubMed] [Google Scholar]
- Metcalfe J., McCutcheon I.E., Francisco D.L., Metzenberg A.B., Welch J.E. Oxygen availability and growth of the chick embryo. Resp. Physiol. 1981;46:81–88. doi: 10.1016/0034-5687(81)90091-8. [DOI] [PubMed] [Google Scholar]
- Mikec M., Biđin Z., Valentić A., Savić V., Zelenika T.A., Raguž-Đurić R., Novak I.L., Balenovic M. Influence of environmental and nutritional stressors on yolk sac utilization, development of chicken gastrointestinal system and its immune status. World Poult. Sci. J. 2006;62:31–40. [Google Scholar]
- Molenaar R., Meijerhof R., van den Anker I., Heetkamp M.J.W., van den Borne J.J.G.C., Kemp B., van den Brand H. Effect of eggshell temperature and oxygen concentration on survival rate and nutrient utilization in chicken embryos. Poult. Sci. 2010;89:2010–2021. doi: 10.3382/ps.2010-00787. [DOI] [PubMed] [Google Scholar]
- Moraes V., Malheiros R., Furlan R.L., Bruno L., Malheiros E., Macari M. Effect of environmental temperature during the first week of brooding period on broiler chick body weight, viscera and bone development. Br. J. Poult. Sci. 2002;4 doi: 10.1590/S1516-635X2002000100003. [DOI] [Google Scholar]
- Moran E.T. Nutrition of the developing embryo and hatchling. Poult. Sci. 2007;86:1043–1049. doi: 10.1093/ps/86.5.1043. [DOI] [PubMed] [Google Scholar]
- Mortola J.P., Al Awam K. Growth of the chicken embryo: implications of egg size. Comp. Biochem. Physiol. Part A: Mol. Integr. Physiol. 2010;156:373–379. doi: 10.1016/j.cbpa.2010.03.011. [DOI] [PubMed] [Google Scholar]
- Murakami H., Akiba Y., Horiguchi M. Growth and utilization of nutrients in the newly-hatched chick with or without removal of residual yolk. Growth Dev. Aging. 1992;56:75–84. [PubMed] [Google Scholar]
- Nangsuay A., Ruangpanit Y., Meijerhof R., Attamankune S. Yolk absorption and embryo development of small and large eggs originating from young and old breeder hens. Poult. Sci. 2011;90:2648–2655. doi: 10.3382/ps.2011-01415. [DOI] [PubMed] [Google Scholar]
- Nangsuay A., Meijerhof R., Ruangpanit Y., Kemp B., van den Brand H. Energy utilization and heat production of embryos from eggs originating from young and old broiler breeder flocks. Poult. Sci. 2013;92:474–482. doi: 10.3382/ps.2012-02643. [DOI] [PubMed] [Google Scholar]
- Nangsuay A., Molenaar R., Meijerhof R., van den Anker I., Heetkamp M.J.W., Kemp B., van den Brand H. Differences in egg nutrient availability, development, and nutrient metabolism of broiler and layer embryos. Poult. Sci. 2015;94:415–423. doi: 10.3382/ps/pev007. [DOI] [PubMed] [Google Scholar]
- Nangsuay A., Meijerhof R., van den Anker I., Heetkamp M.J.W., Kemp B., van den Brand H. Development and nutrient metabolism of embryos from two modern broiler strains. Poult. Sci. 2015;94:2546–2554. doi: 10.3382/ps/pev234. [DOI] [PubMed] [Google Scholar]
- Nangsuay A., Meijerhof R., van den Anker I., Heetkamp M.J.W., de Souza Morita V., Kemp B., van den Brand H. Effects of breeder age, broiler strain, and eggshell temperature on development and physiological status of embryos and hatchlings. Poult. Sci. 2016;95:1666–1679. doi: 10.3382/ps/pew080. [DOI] [PubMed] [Google Scholar]
- Nangsuay A., Meijerhof R., van den Anker I., Heetkamp M.J.W., Kemp B., van den Brand H. Energy utilization and heat production of embryos from eggs originating from young and old broiler breeder flocks. Poult. Sci. 2017;92:474–482. doi: 10.3382/ps.2012-02643. [DOI] [PubMed] [Google Scholar]
- Noble R.C., Cocchi M. Lipid metabolism and the neonatal chicken. Prog. Lipid Res. 1990;29:107–140. doi: 10.1016/0163-7827(90)90014-c. [DOI] [PubMed] [Google Scholar]
- Noble R.C., Ogunyemi D. Lipid changes in the residual yolk and liver of the chick immediately after hatching. Biol. Neonate. 1989;56:228–236. doi: 10.1159/000243127. [DOI] [PubMed] [Google Scholar]
- Noy Y., Sklan D. Yolk utilization in the newly hatched poult. Br. Poult. Sci. 1998;39:446–451. doi: 10.1080/00071669889042. [DOI] [PubMed] [Google Scholar]
- Noy Y., Uni Z., Sklan D. Routes of yolk utilisation in the newly-hatched chick. Br. Poult. Sci. 1996;37:987–995. doi: 10.1080/00071669608417929. [DOI] [PubMed] [Google Scholar]
- O'Dea E.E., Fasenko G.M., Feddes J.J.R., Robinson F.E., Segura J.C., Ouellette C.A., van Middelkoop J.H. Investigating the eggshell conductance and embryonic metabolism of modern and unselected domestic avian genetic strains at two flock ages. Poult. Sci. 2004;83:2059–2070. doi: 10.1093/ps/83.12.2059. [DOI] [PubMed] [Google Scholar]
- Onbaşılar E., Erdem E., Hacan Ö, Yalçın S. Effects of breeder age on mineral contents and weight of yolk sac, embryo development, and hatchability in Pekin ducks. Poult. Sci. 2014;93:473–478. doi: 10.3382/ps.2013-03355. [DOI] [PubMed] [Google Scholar]
- Patten B.M. P. Blakiston’s Son and Co; Philidelphia, U.S.A: 1920. Extra-embryonic Membranes. The Early Embryology of the Chick. [Google Scholar]
- Pearson S.D., Ackerman R.A., Seagrave R.C. The energetics of embryonic growth and development. I. Oxygen consumption, biomass growth, and heat production. J. Theoret. Biol. 1991;152:223–240. doi: 10.1016/s0022-5193(05)80454-0. [DOI] [PubMed] [Google Scholar]
- Pearson J., Haque M., Hou P.L., Tazawa H. Developmental patterns of O2 consumption, heart rate and O2 pulse in unturned eggs. Resp. Physiol. 1996;103:83–87. doi: 10.1016/0034-5687(95)00061-5. [DOI] [PubMed] [Google Scholar]
- Peebles E.D., Zumwalt C.D., Doyle S.M., Gerard P., Latour M.A., Boyle C.R., Smith T.W. Effects of breeder age and dietary fat source and level on broiler hatching egg characteristics. Poult. Sci. 2000;79:698–704. doi: 10.1093/ps/79.5.698. [DOI] [PubMed] [Google Scholar]
- Piestun Y., Shinder D., Ruzal M., Halevy O., Yahav S. The effect of thermal manipulations during the development of the thyroid and adrenal axes on in-hatch and post-hatch thermoregulation. J. Therm. Biol. 2008;33:413–418. [Google Scholar]
- Pulikanti R., Peebles E.D., Keirs R.W., Bennett L.W., Keralapurath M.M., Gerard P.D. Pipping muscle and liver metabolic profile changes and relationships in broiler embryos on days 15 and 19 of incubation. Poult. Sci. 2010;89:860–865. doi: 10.3382/ps.2009-00531. [DOI] [PubMed] [Google Scholar]
- Reijrink I.A.M., Berghmans D., Meijerhof R., Kemp B., van den Brand H. Influence of egg storage time and preincubation warming profile on embryonic development, hatchability, and chick quality. Poult. Sci. 2010;89:1225–1238. doi: 10.3382/ps.2009-00182. [DOI] [PubMed] [Google Scholar]
- Romanoff A.L. Membrane growth and function. Ann. New York Acad. Sci. 1952;55:288–301. doi: 10.1111/j.1749-6632.1952.tb26545.x. [DOI] [PubMed] [Google Scholar]
- Romanoff A.L. Macmillan Co.; New York, U.S.A: 1960. The Avian Embryo: Structural and Function Development. [Google Scholar]
- Romanoff A.L. Wiley; New York, U.S.A: 1967. Biochemistry of the Avian Embryo. [Google Scholar]
- Romanoff A.L., Romanoff A.J. John Wiley and Sons; New York, U.S.A: 1949. The Avian Egg. [Google Scholar]
- Romijn C., Lokhorst W. Foetal heat production in the fowl. J. Physiol. 1960;150:239–249. doi: 10.1113/jphysiol.1960.sp006384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romijn C., Lokhorst W. Some aspects of energy metabolism in birds. In: Brouwer E., van Es A.J.H., editors. Proceedings of the Second Symposium on Energy Metabolism. Methods and Results of Experiments with Animals. EAAP publication No. 10, EAAP; Rome, Italy: 1961. pp. 49–58. [Google Scholar]
- Sahan U., Ipek A., Söszü A. Yolk sac fatty acid composition, yolk absorption, embryo development, and chick quality during incubation in eggs from young and old broiler breeders. Poult. Sci. 2014;93:2069–2077. doi: 10.3382/ps.2013-03850. [DOI] [PubMed] [Google Scholar]
- Sato M., Tachibana T., Furuse M. Heat production and lipid metabolism in broiler and layer chickens during embryonic development. Comp. Biochem. Physiol. Part A: Mol. Integr. Physiol. 2006;143:382–388. doi: 10.1016/j.cbpa.2005.12.019. [DOI] [PubMed] [Google Scholar]
- Scott T., Washburn K. Evaluation of growth, hormonal, and hematological responses of neonatal chickens to reduced temperature brooding. Poult. Sci. 1985;64:777–784. doi: 10.3382/ps.0640777. [DOI] [PubMed] [Google Scholar]
- Segura J.C., Ouellette C.A., Feddes J.J.R., Fasenko G.M., Zuidhof M. Development of a metabolic calorimeter system to measure heat production of domestic avian embryos during incubation. Can. Biosyst. Engineer. 2006;48:1–4. [Google Scholar]
- Shafey T.M. Effects of egg size and eggshell conductance on hatchability traits of meat and layer breeders flocks. Asian-Australas. J. Anim. Sci. 2002;15:1–6. [Google Scholar]
- Silva F.H.A., Faria D.E., Torres K.A.A., Faria Filho D.E., Coelho A.A.D., Savino V.J.M. Influence of egg pre-storage heating period and storage length on the digestive tract of newly-hatched broiler chicks. Braz. J. Poult. Sci. 2008;10:23–28. [Google Scholar]
- Silversides F., Scott T. Effect of storage and later age on quality of eggs from two lines of hens. Poult. Sci. 2001;80:1240–1245. doi: 10.1093/ps/80.8.1240. [DOI] [PubMed] [Google Scholar]
- Speake B.K., Murray A.M., Noble R.C. Transport and transformations of yolk lipids during development of the avian embryo. Prog. Lipid Res. 1998;37:1–32. doi: 10.1016/s0163-7827(97)00012-x. [DOI] [PubMed] [Google Scholar]
- Speier J.S., Yadgary L., Uni Z., Wong E.A. Gene expression of nutrient transporters and digestive enzymes in the yolk sac membrane and small intestine of the developing embryonic chick. Poult. Sci. 2012;91:1941–1949. doi: 10.3382/ps.2011-02092. [DOI] [PubMed] [Google Scholar]
- Tazawa H. Hypothermal effect on the gas exchange in chicken embryo. Respir. Physiol. 1973;17:21–31. doi: 10.1016/0034-5687(73)90107-2. [DOI] [PubMed] [Google Scholar]
- Tazawa H., Wakayama H., Turner J., Paganelli C. Metabolic compensation for gradual cooling in developing chick embryos. Comp. Biochem. Physiol. 1988;89:125–129. [Google Scholar]
- Tazawa H., Hashimoto Y., Nakazawa S., Whittow G. Metabolic responses of chicken embryos and hatchlings to altered O2 environments. Resp. Physiol. 1992;88:37–50. doi: 10.1016/0034-5687(92)90027-t. [DOI] [PubMed] [Google Scholar]
- Tona K., Malheiros R., Bamelis F., Careghi C., Moraes V., Onagbesan O. Effects of storage time on incubating egg gas pressure, thyroid hormones, and corticosterone levels in embryos and on their hatching parameters. Poult. Sci. 2003;82:840–845. doi: 10.1093/ps/82.5.840. [DOI] [PubMed] [Google Scholar]
- Tona K., Bamelis F., De Ketelaere B., Bruggeman V., Moraes V., Buyse J. Effects of egg storage time on spread of hatch, chick quality, and chick juvenile growth. Poult. Sci. 2003;82:736–741. doi: 10.1093/ps/82.5.736. [DOI] [PubMed] [Google Scholar]
- Tona K., Onagbesan O., De Ketelaere B., Decuypere E., Bruggeman V. Effects of age of broiler breeders and egg storage on egg quality, hatchability, chick quality, chick weight, and chick posthatch growth to forty-two days. J. Appl. Poult. Res. 2004;13:10–18. [Google Scholar]
- Tona K., Onagbesan O., Kamers B., Everaert N., Bruggeman V., Decuypere E. Comparison of Cobb and Ross strains in embryo physiology and chick juvenile growth. Poult. Sci. 2010;89:1677–1683. doi: 10.3382/ps.2009-00386. [DOI] [PubMed] [Google Scholar]
- Turro-Vincent I., Nitsan Z., Picard M., Dunnington E., Siegel P. Removal of residual yolk at hatch influences food choice and feeding activity in lines of chickens selected for high or low juvenile body weight. Reprod. Nutr. Dev. 1994;34:449–460. doi: 10.1051/rnd:19940506. [DOI] [PubMed] [Google Scholar]
- Uddin Z., Hamidu J.A. Prolong egg storage affects broiler breeder embryonic metabolism and chick quality. J. Anim. Sci. Advanc. 2014;4:973–977. [Google Scholar]
- Ulmer-Franco A.M., Fasenko G.M., Christopher E.E.O. Hatching egg characteristics, chick quality, and broiler performance at 2 breeder flock ages and from 3 egg weights. Poult. Sci. 2010;89:2735–2742. doi: 10.3382/ps.2009-00403. [DOI] [PubMed] [Google Scholar]
- Uni Z., Yadgary L., Yair R. Nutritional limitations during poultry embryonic development. J. Appl. Poult. Res. 2012;21:175–184. [Google Scholar]
- Van den Brand H., Molenaar R., van der Star I., Meijerhof R. Early feeding affects resistance against cold exposure in young broiler chickens. Poult. Sci. 2010;89:716–720. doi: 10.3382/ps.2009-00432. [DOI] [PubMed] [Google Scholar]
- Van der Pol C.W., van Roovert-Reijrink I.A.M., Maatjens C.M., van den Brand H., Molenaar R. Effect of relative humidity during incubation at a set eggshell temperature and brooding temperature posthatch on embryonic mortality and chick quality. Poult. Sci. 2013;92:2145–2155. doi: 10.3382/ps.2013-03006. [DOI] [PubMed] [Google Scholar]
- Vieira S.L., Moran E.T. Effects of egg of origin and chick post-hatch nutrition on broiler live performance and meat yields. World Poult. Sci. J. 1999;55:125–142. [Google Scholar]
- Virchow H. Hirschwald; Berlin, Germany: 1891. Der Dottersack des Huhnes. [Google Scholar]
- Wijtten P.J.A. Wageningen University; Wageningen, the Netherlands: 2011. Nutrition Driven Small Intestinal Development and Performance of Weaned Pigs and Young Broilers. PhD thesis. [Google Scholar]
- Willems E., Decuypere E., Buyse J., Everaert N. Importance of albumen during embryonic development in avian species, with emphasis on domestic chicken. World Poult. Sci. J. 2014;70:503–518. [Google Scholar]
- Wolanski N., Renema R., Robinson F., Carney V., Fancher B. Relationship between chick confirmation and quality measures with early growth traits in males of eight selected pure or commercial broiler breeder strains. Poult. Sci. 2006;85:1490–1497. doi: 10.1093/ps/85.8.1490. [DOI] [PubMed] [Google Scholar]
- Yadgary L., Uni Z. Yolk sac carbohydrate levels and gene expression of key gluconeogenic and glycogenic enzymes during chick embryonic development. Poult. Sci. 2012;91:444–453. doi: 10.3382/ps.2011-01669. [DOI] [PubMed] [Google Scholar]
- Yadgary L., Cahaner A., Kedar O., Uni Z. Yolk sac nutrient composition and fat uptake in late-term embryos in eggs from young and old broiler breeder hens. Poult. Sci. 2010;89:2441–2452. doi: 10.3382/ps.2010-00681. [DOI] [PubMed] [Google Scholar]
- Yadgary L., Yair R., Uni Z. The chick embryo yolk sac membrane expresses nutrient transporter and digestive enzyme genes. Poult. Sci. 2011;90:410–416. doi: 10.3382/ps.2010-01075. [DOI] [PubMed] [Google Scholar]
- Yadgary L., Kedar O., Adepeju O., Uni Z. Changes in yolk sac membrane absorptive area and fat digestion during chick embryonic development. Poult. Sci. 2013;92:1634–1640. doi: 10.3382/ps.2012-02886. [DOI] [PubMed] [Google Scholar]
- Yalçin S., Çabuk M., Bruggeman V., Babacanoğlu E., Buyse J., Decuypere E., Siegel P.B. Acclimation to heat during incubation. 1. Embryonic morphological traits, blood biochemistry, and hatching performance. Poult. Sci. 2008;87:1219–1228. doi: 10.3382/ps.2007-00435. [DOI] [PubMed] [Google Scholar]
- Yalçın S., Bağdatlıoğlu N., Yenisey Ç., Siegel P.B., Özkan S., Akşit M. Effect of manipulation of incubation temperature on fatty acid profiles and antioxidant enzyme activities in meat-type chicken embryos. Poult. Sci. 2012;91:3260–3270. doi: 10.3382/ps.2012-02145. [DOI] [PubMed] [Google Scholar]
- Yalçin S., Gursel I., Bilgen G., Izzetoglu G.T., Horuluoglu B.H., Glucluer G. Egg storage duration and hatch window affect gene expression of nutrient transporters and intestine morphological parameters of early hatched broiler chicks. Animal. 2016;10:805–811. doi: 10.1017/S175173111500261X. [DOI] [PubMed] [Google Scholar]
- Yalçin S., Gursel I., Bilgen G., Horuluoglu B.H., Gucluer G., Izzetoglu G.T. Effect of egg storage duration and brooding temperatures on chick growth, intestine morphology and nutrient transporters. Animal. 2017;11:1791–1797. doi: 10.1017/S1751731117000404. [DOI] [PubMed] [Google Scholar]
- Yassin H., Velthuis A.G., Boerjan M., van Riel J. Field study on broilers' first-week mortality. Poult. Sci. 2009;88:798–804. doi: 10.3382/ps.2008-00292. [DOI] [PubMed] [Google Scholar]
- Zuidhof M., Schneider B., Carney V., Korver D., Robinson F. Growth, efficiency, and yield of commercial broilers from 1957, 1978, and 2005. Poult. Sci. 2014;93:2970–2982. doi: 10.3382/ps.2014-04291. [DOI] [PMC free article] [PubMed] [Google Scholar]