Abstract
SC9-2 is a recombinant Marek's disease virus (MDV) strain lacking the meq oncogene. Previous study demonstrated that SC9-2 virus provides good protection against challenge with a very virulent MDV rMd5, but it induces immunosuppressive effects in specific pathogen-free (SPF) chickens. In the present study, SC9-2 was serially passaged on chicken embryo fibroblast (CEF) cell cultures. The pathogenicity and immune efficacy of SC9-2/10th and SC9-2/40th against rMd5 were evaluated. Animal experimental results showed that SC9-2/10th and SC9-2/40th showed no lethality or tumorigenicity in SPF chickens. Body weight of chickens inoculated with SC9-2/40th were significantly higher than that of the chickens inoculated with SC9-2/10th but lower than that of the uninoculated controls. The severity of bursa and thymus atrophy (BTA) and spleen enlargement in SC9-2/40th-inoculated chickens were also weaker than the SC9-2/10th-inoculated ones but stronger than the uninoculated controls. Chickens inoculated with SC9-2/40th and SC9-2/10th showed similar antibody levels induced by H9N2 subtype avian influenza virus/Newcastle disease virus inactivated vaccines, both of which were lower than the uninoculated controls. Replication of SC9-2/40th was significantly lower than SC9-2/10th in feather follicle epithelium (FFE) of infected chickens. The immune protection index of SC9-2/40th was also lower than that of SC9-2/10th, but the difference was not significantly, and both of which were significant higher than that of the commercial MDV vaccine CVI988/Rispens. The results of our studies demonstrated that SC9-2/40th showed weaker severity of BTA, spleen enlargement, and body weight loss and lower replication level in FFE than SC9-2/10th in SPF chickens. However, SC9-2/40th was able to confer better immune protection as compared with CVI988/Rispens vaccination in SPF chickens. In conclusion, serially attenuation of SC9-2 in CEFs reduced the lymphoid organ atrophy and replication in SPF chickens, and the immune protective efficacy of attenuated viruses was still superior than CVI988/Rispens.
Key words: Marek's disease virus, meq gene, cell culture passage, attenuation, immune efficacy
Introduction
Marek's disease virus (MDV) is one of the most contagious alphaherpesviruses and causes Marek's disease (MD), which is characterized by the development of T-cell lymphoma and the lymphocytic infiltration of peripheral nerves, skin, and visceral organs in chickens (Churchil and Biggs, 1967, Schat and Nair, 2008). Marek's disease viruses are classified into 3 serotypes, but only gallid herpesvirus type 2 (GaHV-2) is oncogenic, causing tumors in chickens, wherease the other 2 serotypes are nononcogenic (Witter and Schat, 2003, Witter et al., 2005). The 3 MDV serotypes possess similar genomic organization but differential gene compositions (Lee et al., 2000, Kingham et al., 2001, Spatz and Schat, 2011, Su et al., 2012). Viral IL-8, meq, and pp38 genes, which play an important role in pathogenesis, are unique to GaHV-2 (Cui et al., 1991, Cui et al., 2004, Parcells et al., 2001, Reddy et al., 2002, Lupiani et al., 2004, Brown et al., 2006). Meq is a 339-amino acid long protein encoded within the EcoR I Q fragment of GaHV-2 and has been shown to be consistently expressed in all MDV tumors and latent cells (Qian et al., 1995, Liu et al., 1997).
It has been demonstrated that meq-null MDV, constructed by knocking out of both copies of meq gene based on cosmid or bacterial artificial chromosome (BAC) clone of MDV, lost its tumorigenicity and provided perfect protective efficacy against challenge with MDV in chickens (Lee et al., 2008, Li et al., 2011, Su et al., 2015). However, the meq-null MDV induced lymphoid organ bursa and thymus atrophy (BTA) and body weight loss in specific pathogen-free (SPF) chickens (Lee et al., 2012). GX0101 is a field MDV strain isolated in China and has a unique genomic structure with a reticuloendotheliosis virus (REV) long terminal repeat (LTR) insert (Cui et al., 2010, Su et al., 2013). SC9-2 is a meq-null MDV strain constructed by knocking out both copies of meq gene and the BAC bone of GX0101 BAC clone using homologous recombination technique (Su et al., 2016). Our previous studies also demonstrated that SC9-2 virus provided protection superior to CVI988/Rispens, but it induced immunosuppressive effects in SPF chickens (Su et al., 2016). Published report suggests that attenuation of virulent MDV by cell culture passage on chicken kidney cells, duck embryo fibroblast, or chicken embryo fibroblast (CEF) is an effective way to decrease the viral pathogenicity (Churchill et al., 1969, Dudnikova et al., 2009, Lee et al., 2012). To eliminate the immunosuppressive effects of SC9-2, serially passages were carried out on CEF cell cultures in the present study. The pathogenesis of SC9-2/10th and SC9-2/40th viruses, as well as their protective efficacy against challenge with very virulent (vv) MDV rMd5, were then evaluated in SPF chickens.
Materials and methods
Ethics Statement
The study protocol and all animal studies were approved by the Shandong Agricultural University Animal Care and Use Committee (SACUC Permission number: AVM01736-1) and performed in accordance with the “Guidelines for Experimental Animals” of the Ministry of Science and Technology (Beijing, China). Any bird deemed to have reached the humane endpoint was culled.
Cell Cultures and Viruses
Specific pathogen-free chickens and chicken embryos for preparation of CEF cells were from SPAFAS Co. (Jinan, China). Chicken embryo fibroblast cell cultures were used for virus propagation and continuous cell culture passages. The meq-null MDV strain SC9-2 was preserved in our lab and stored in liquid nitrogen (Su, et al., 2016). Recombinant Md5 virus (rMd5) was generated from cosmids derived from the vv MDV Md5 strain as previously described (Reddy et al., 2002). The commercial MDV vaccine CVI988/Rispens was obtained from Beijing Lingyu Company (Rispens et al., 1972a, Rispens et al., 1972b).
Attenuation of SC9-2 Virus
The CEF-passed SC9-2 construct at passage 9 was serially passaged in CEF cell cultures. Marek's disease virus SC9-2 was inoculated in the CEF monolayer cell culture at a multiplicity of infection of 0.3. When plaque formation was advanced 4∼5 D postinfection (D.p.i.), cells were washed with 2 mL PBS, digesting with 1 mL of 0.25% trypsin, and then resuspended with Dulbeco's modified Eagle medium supplemented with 5% fetal bovine serum. Cell containing virus were then inoculated in new monolayers of CEF for the passage of MDV SC9-2. Samples of infected cells were cryopreserved starting at 10th until 40th, and these viruses were designated as SC9-2/10th or SC9-2/40th. The titer of MDV was measured by counting the plaques on the CEFs. Briefly, the virus was serially diluted, inoculated on the CEFs, and incubated for 5 to 7 D at 37°C in a humidified atmosphere containing 5% CO2. Plaques on the CEFs were counted, and the virus titer was finally calculated.
Experimental Design 1
To compare the pathogenic properties of SC9-2/10th with SC9-2/40th, 148 1-day-old SPF chicks were randomly divided into 4 equal groups (37 in each group) and reared separately in isolators. When the chicks were 1-day-old, in each group, chicks were inoculated intra-abdominally (i.a.) with 2000 PFU of SC9-2/10th, SC9-2/40th, or GX0101 viruses, whereas control ones were inoculated with uninfected CEF cultures.
Effect of SC9-2/10th and SC9-2/40th on Immune Organs
At 14 D.p.i., 12 chickens per group were used to evaluate lymphoid organ BTA. The whole body weights of each chicken were measured prior to euthanasia, and all thymus lobes, bursa and spleen from each chicken were weighed after collection. The relative weights of the thymus, bursa, and spleen to the whole body were determined.
Effect of SC9-2/10th and SC9-2/40th on Body Weight
Body weight measurements of the chickens in the different groups were performed at 7, 14, 21, and 28 D.p.i to evaluate the effect of SC9-2/10th or SC9-2/40th virus on growth rate.
Kinetics of Replication of SC9-2/10th and SC9-2/40th in Feather Follicle Epithelium
Feather Follicle Epithelium (FFE) samples were collected from 5 chickens of each group on 7, 14, 21, and 28 D.p.i., and DNA from FFE were prepared using standard procedures as previously described (Abdul-Careem et al., 2006). The MDV-specific primers were designed to be specific for the unique molecular marker of REV LTR in GX0101. Absolute quantification of virus copies of SC9-2/10th or SC9-2/40th in chickens was carried out using the real-time quantitative PCR as previously described (Su et al., 2015). The chicken ovo gene (GeneBnk NO. Y00407) was cloned into the pMD18 T vector to construct the plasmid pMD18-T-ovo. The plasmid GX0101 BAC or pMD18-T-ovo was diluted to 109 copies per 2 uL and serially diluted to 10 copies per 2 uL by 10 times gradient dilution. The Ct value of GX0101 BAC and pMD18-T-ovo from 109 to 101 copies were detected, and the standard curve was established on the ABI PRISM 7,500 sequence detection system. Each reaction system contains 0.5 uM MDV-specific primers, 0.2 uM LTR probe, 10 uL 2 × TaqMan Gene Expression Master Mix buffer, and 2 uL of DNA (approximately 200 ng). The reaction volume was brought up to 20 uL by the addition of water. An ABI PRISM 7,500 sequence detection system (Applied Biosystems) was used to amplify and detect the reaction products under the following conditions: 50°C for 2 min and 95°C for 10 min, followed by 40 cycles of 94°C (15 s), and 60°C (1 min).
Antibody Responses to NDV and AIV-H9 Inactivated Vaccines
To compare the immunosuppressive effect of SC9-2/10th or SC9-2/40th virus on the antibody response to vaccinations, all chickens from each treatment were vaccinated with NDV and AIV-H9 inactivated vaccines as previously described after 6 D.p.i. (Sun et al., 2007). On 28, 35, and 42 D postvaccination, sera from chickens of each group were randomly collected to measure hemagglutination inhibition antibody titers to NDV and AIV-H9. First, 2-fold series dilution of the collected sera were reacted with 4-hemagglutining unit virus of NDV or AIV-H9 respectively. The 1% red blood cell of chicken suspension was then added as an indicator of the hemagglutination inhibition titers.
Compare the Pathogenic Properties of SC9-2/10th and SC9-2/40th
After inoculation with viruses, all chickens were evaluated daily for symptoms of MD and were euthanized and examined for gross lesions, when they showed clinical evidence of MD. All surviving chickens were sacrificed for necropsy to evaluate the presence of gross lesions after 13 wk of observation. Cumulative mortality and gross tumor rate were used for comparing the pathogenicity of SC9-2/10th and SC9-2/40th.
Experimental Design 2
To evaluate the vaccine efficacy of SC9-2/10th, SC9-2/40th as compared with that of the commercial CVI988/Rispens in SPF chickens when challenged with rMd5, 100 one-day-old SPF chickens were randomly divided into 4 equal groups (25 in each group) and reared separately in isolators. On day 1, 2000 PFU of SC9-2/10th or SC9-2/40th were inoculated i.a. into each chicken in groups 1 or 2, and chickens in group 3 received 2,000 PFU of CVI988/Rispens. No vaccine was inoculated for group 4. Five days later, each chicken in groups 1, 2, 3, and 4 was challenged i.a. with 1,000 PFU of vv MDV strain rMd5. Chickens, which inoculated with uninfected CEF cultures in the experiment 1, were the control ones as group 5. During 90 D after the challenges, all dead chickens were recorded and necropsied. At the end of the experiment, all surviving chickens were killed and necropsied. Vaccinal immunity to MD was expressed as a protective index calculated as the percentage of gross MD in nonvaccinated challenged control chickens minus the percentage of gross MD in vaccinated, challenged chickens divided by the percentage of gross MD in nonvaccinated challenged control chickens × 100.
Statistics Analysis
Statistical analysis was performed with the GraphPad Prism (version 5.0). Differences between groups were examined for statistical significance by one-way ANOVA with Tukey's multiple comparison tests.
Results
Effect of SC9-2/10th and SC9-2/40th on Immune Organ
As shown in Table 1, the relative thymus and bursa weight of chickens in SC9-2/40th group were significantly higher than those of chickens infected with SC9-2/10th or GX0101 on 14 D.p.i (P < 0.05), but the relative spleen weight of chickens from the SC9-2/40th group were significantly lower (P < 0.05). When compared with the chickens in the control group, the relative thymus and bursa weight of chickens were lower, and the relative spleen was higher in chickens of all virus inoculated group (P < 0.05).
Table 1.
Comparisons of the relative immune organ weights of the chickens challenged with different viruses (n = 12).
| Virus | Bursa wt/Body wt1 | Thymus wt/Body wt1 | Spleen wt/Body wt1 |
|---|---|---|---|
| SC9-2/40th | 0.27 ± 0.05a | 0.29 ± 0.08a | 0.22 ± 0.05a |
| SC9-2/10th | 0.22 ± 0.05b | 0.20 ± 0.06b | 0.26 ± 0.05b |
| GX0101 | 0.15 ± 0.09c | 0.12 ± 0.09c | 0.32 ± 0.04c |
| Control | 0.36 ± 0.10d | 0.45 ± 0.14d | 0.14 ± 0.04d |
a-dThe numbers in the table indicate the mean ± standard deviation. The different superscript letters represent significant differences (P < 0.05).
Immune organ weight/body weight × 100.
Effect of SC9-2/10th and SC9-2/40th on Body Weight
Compared with the uninoculated controls, GX0101 virus infection had an obvious inhibitory effect on the growth rate of the infected chickens (Figure 1). The body weights of chickens in the SC9-2/40th inoculation group were significantly higher than both of the SC9-2/10th inoculation group and the GX0101 inoculation group (P < 0.05). However, the body weights of chickens infected with SC9-2/40th were lower than the uninoculated controls (P < 0.05).
Figure 1.
The body weights of chickens in each group. Body weights of the chickens in different groups were measured at 7, 14, 21, and 28 D postinfection with SC9-2/10th or SC9-2/40th to evaluate the effect of virus infection on growth rates.
Virus Copies of SC9-2/10th and SC9-2/40th in FFE
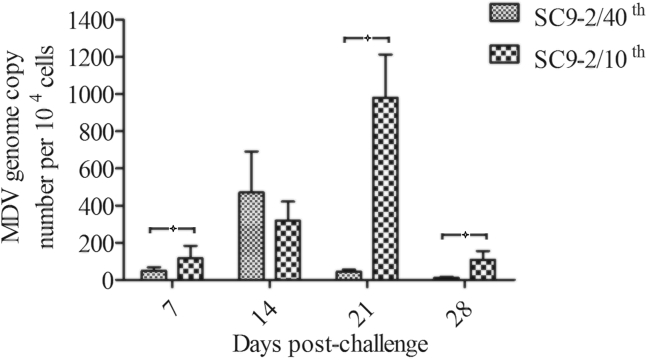
Virus copies in FFE were assessed with quantitative PCR at different time points postinoculation with MDVs (Figure 2). As shown, the genome copy numbers of the SC9-2/40th virus from FFE of infected chickens peaked at 14 D.p.i. and that of the SC9-2/10th virus peaked at 21 D.p.i. The genome copy numbers of SC9-2/40th virus were lower than those of the SC9-2/10th virus (P < 0.05), except at 14 D.p.i.
Figure 2.
Virus copies of MDV SC9-2/10th or SC9-2/40th in SPF chickens. Virus copies of SC9-2/10th or SC9-2/40th virus in chickens as determined by the viral genome copy numbers in the feather follicle epithelium with real-time qPCR of the REV LTR fragment.  indicated that the viral genome copy numbers were different between the 2 groups. Abbreviations: SPF, specific pathogen-free; MDV, Marek's disease virus; REV, reticuloendotheliosis virus; LTR, long terminal repeat.
indicated that the viral genome copy numbers were different between the 2 groups. Abbreviations: SPF, specific pathogen-free; MDV, Marek's disease virus; REV, reticuloendotheliosis virus; LTR, long terminal repeat.
Comparison of the Effect of SC9-2/10th and SC9-2/40th on Antibody Responses
As shown in Table 2, the GX0101-infected chickens exhibited weaker humoral immune responses to the inactivated NDV and AIV vaccines as compared with those of the control ones at 28, 35, and 42 D postimmunization (P < 0.05). The SC9-2/40th-infected chickens showed similar antibody levels as compared with those of the SC9-2/10th inoculation ones, and both of which were higher than those of the GX0101-infected ones (P > 0.05, Table 2). However, the SC9-2/40th-infected chickens showed lower antibody levels compared with the uninoculated controls (P < 0.05).
Table 2.
Influences of SC9-2/10th and SC9-2/40th viruses infection on HI antibody titers to NDV and AIV-H9 after vaccination (log2).
| Virus | 28 dpv1 |
35 dpv |
42 dpv |
|||
|---|---|---|---|---|---|---|
| AIV-H9 | NDV | AIV-H9 | NDV | AIV-H9 | NDV | |
| SC9-2/40th | 9.18 ± 1.0a | 4.82 ± 1.18a | 9.32 ± 0.57a | 5.14 ± 1.36a | 9.85 ± 1.23a | 5.45 ± 1.46a |
| SC9-2/10th | 9.00 ± 1.5a | 4.53 ± 1.33a | 9.27 ± 0.65a | 5.00 ± 0.85a | 9.63 ± 1.21a | 5.54 ± 1.51a |
| GX0101 | 7.67 ± 1.0b | 3.44 ± 1.24b | 8.11 ± 1.36b | 4.22 ± 1.09b | 8.3 ± 1.34b | 4.30 ± 1.16b |
| Control | 9.81 ± 1.25c | 5.57 ± 1.54c | 10.23 ± 0.94c | 6.19 ± 1.25c | 10.57 ± 1.36c | 6.42 ± 0.98c |
a-cThe numbers in the table indicate the mean ± standard deviation. The different letters represent significant differences (P < 0.05). The same letters indicate the differences were not significant (P > 0.05).
Abbreviation: HI, hemagglutination inhibition.
dpv, days postvaccination.
Pathogenicity of SC9-2/10th and SC9-2/40th Virus in SPF Chickens
During 13 wk postchallenge with MDVs, no chicken in the SC9-2/10th or SC9-2/40th virus inoculation group died, and no MDV-specific clinical symptom or tumor was observed (Table 3). On the other hand, 64% of chickens died in the GX0101 challenge group, and 20% of chickens developed tumors among the liver, kidney, and other organs.
Table 3.
Pathogenicity of SC9-2/10th or SC9-2/40th virus and protective efficacy of that against rMd5 in SPF chickens.
| Virus | Challenge | MD mort. | MD lesi. | PIα |
|---|---|---|---|---|
| SC9-2/10th | - | 0/25 (0) | 0/25 (0) | - |
| SC9-2/40th | - | 0/25 (0) | 0/25 (0) | - |
| GX0101 | - | 16/25 (64) | 25/25 (100) | - |
| SC9-2/10th | rMd5 | 1/25 (4) | 1/25 (4) | 96a |
| SC9-2/40th | rMd5 | 2/25 (8) | 2/25 (8) | 92a |
| CVI988/Rispens | rMd5 | 8/25 (32) | 8/25 (32) | 68b |
| - | rMd5 | 25/25 (100) | 25/25 (100) | - |
| Control | - | 0/25 (0) | 0/25 (0) | - |
a,bIndicates significant difference (P < 0.05) in PI among the 2 experimental groups.
MD mort: MD mortality/%, MD lesi: MD lesions/%.
Abbreviations: SPF, specific pathogen-free; MD, Marek's disease.
αPI = protection index.
Protective Efficacy of SC9-2/10th and SC9-2/40th Virus Against Challenge With rMd5 in SPF Chickens
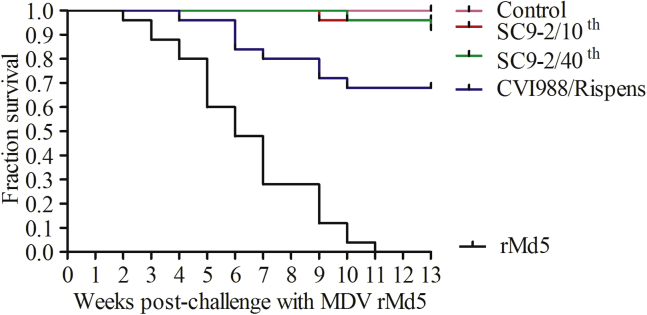
The protection efficacy of SC9-2/10th, SC9-2/40th, and CVI988/Rispens against challenge with the MDV rMd5 were compared in SPF chickens. As shown in Table 3, the unvaccinated chickens challenged with rMd5 had 100% mortality. The mortality rate of SC9-2/10th and SC9-2/40th vaccinated birds were 4 and 8%, respectively. In addition, the CVI988/Rispens vaccinated, and rMd5 challenged chickens showed 32% MD lesions and mortality. Therefore, the SC9-2/10th and SC9-2/40th virus protected 96 and 92% of the chickens against challenge with rMd5, respectively, whereas CVI988/Rispens protected 68% of the animals. Based on MD incidences, the protection index of CVI988/Rispens was 68 following viral challenge with rMd5 virus, whereas those of the SC9-2/10th and SC9-2/40th were 96 and 92, respectively (Figure 3).
Figure 3.
A plot of survival patterns of chickens vaccinated with SC9-2/10th, SC9-2/40th, and CVI988/Rispens followed by challenge with rMd5. Chickens were inoculated with 2000 PFU of the indicated viruses at 1-day-old, followed by challenge with rMd5 5 D later and were then maintained in separated isolators for 13 wk. Abbreviation: MDV, Marek's disease virus.
Discussion
Marek's disease is one of the major tumor disease in poultry. Although MD has been effectively controlled by vaccines, virulence of field isolates of MDV has continued to evolve toward greater virulence and even vv plus MDV under the immune pressure of vaccines (Read et al., 2015, Ralapanawe et al., 2016). The increased virulence of field MDV led to the upgrading of vaccines from a no pathogenic herpesvirus of turkey to the attenuated CVI988/Rispens. However, it has been shown that the widely held “gold-standard” vaccine CVI988/Rispens does not confer adequate protection against MDV (Kreager, 1998, Yu et al., 2013, Zhang et al., 2015, Cui et al., 2016). Unfortunately, more than 10 strains of candidate recombinant vaccine viruses have been investigated, but none showed a better protection against MD than the CVI988/Rispens virus (Witter and Kreager, 2004).
The meq-null recombinant MDV SC9-2 was demonstrated to provide superior protection against MDV than CVI988/Rispens but induced lymphoid organ BTA and body weight loss in SPF chickens in our previous studies (Su et al., 2016). Considering that attenuation of MDV is an important tool used in generating new candidate vaccines, SC9-2 virus was serially passaged in CEF cell cultures for 40 passages in the present study. Our study found that both the severity of BTA and spleen enlargement in SC9-2/40th inoculated chickens were significantly weaker than the SC9-2/10th inoculated ones, whereas the body weight of chickens inoculated with SC9-2/40th were significantly higher. Chickens inoculated with SC9-2/40th and SC9-2/10th showed similar antibody levels induced by AIV/NDV inactivated vaccines, both of which were lower than uninoculated controls. This clearly suggests that although SC9-2/40th virus still showed slightly immunosuppressive effect, it was much weaker as compared with the parental GX0101 in SPF chickens. The effect of attenuated virus on the antibody responses induced by inactivated vaccines should also be taken into account when MDV are serially attenuated and to be used as safe vaccines. In addition, replication of SC9-2/40th was significantly lower than SC9-2/10th in FFE of the infected chickens. It is probably that the horizontal transmission capacity of SC9-2/40th was weaker than SC9-2/10th. Previous studies have shown that attenuation of MDV by serial passages decreased virus titer in vivo and reduced propensity for contact spread (Schat et al., 1985). It has been reported that the meq-deleted strain rMd5ΔMeq, constructed using cosmid clone technology, lost the ability to replicate in lymphocytes after serially passaged in duck embryo fibroblast cell cultures for 40 passages (Lee et al., 2012). The capacity for the virus to induce lymphoid organ BTA and body weight loss in MDV maternal antibody negative chickens were also lost. Data in their paper suggest that there is an association between viral replication in vivo and lymphoid organ BTA, which is consistent to our results. Although the SC9-2/40th virus showed decreased lymphoid organ BTA and viral replication as compared with those of SC9-2/10th, it still induced the lymphoid organ BTA as compared with uninoculated control in chickens. The reason for the difference between SC9-2/40th and passage 40 of rMd5Δmeq in terms of the immunosuppressive efficacy and viral replication may be the parental virus GX0101, which is a vv virus with a high transmission capacity but weaker pathogenicity than rMd5. Our published report suggests that the REV LTR insert in the genome significantly increased the replication and horizontal transmission of GX0101 virus (Sun et al., 2010). The LTR deleted virus GX0101ΔLTR-induced slighter thymic atrophy as compared with the parental virus GX0101. Previous studies on RM1, which also contain an REV LTR, demonstrated that the recombinant virus causes more severe thymic atrophy than the parental virus JM strain, which is consistent to our studies (Witter et al., 1997). Therefore, there is also a close relationship between LTR and thymic atrophy induced by the recombinant viruses. Destruction of B and T cells account for early-MDV-immunosuppression in the lymphoid organs, resulting BTA, but not for the late-MDV-immunosuppression (Faiz et al., 2016). The mechanisms involved and the factors influencing the late-MDV-immunosuppression involving the efficacy of other vaccines are poorly understood (Faiz et al., 2017). The reason for the relative constant antibodies induced by inactivated vaccines remains to be determined.
We also evaluated the protection efficacy of SC9-2/40th or SC9-2/10th against challenge with vv rMd5 strain. Our results showed that SC9-2/40th provided protection superior to CVI988/Rispens in SPF chickens (Table 3). The protection index of SC9-2/40th was slightly lower than that of SC9-2/10th, but the difference was not significant. The results indicate that attenuation of SC9-2 virus for 40 passages on CEF has no influence on its protective efficacy. Whether the protective efficacy could be influenced after further passages needs further research. CVI988/Rispens virus has been attenuated by cell culture passage to generate a safe vaccine, which had been used worldwide for controlling MD in the field (Rispens et al., 1972a, Rispens et al., 1972b). However, direct attenuation of virulent viruses by cell culture passage is indeed a long process and remains much uncertainty (Spatz et al., 2008). Our and other studies supported that deletion of major pathogenic gene in MDV using a gene knockout system combined with attenuation of MDV in cell culture may be an effective tool for generating new candidate vaccines.
In conclusion, serial passage of SC9-2 on CEF cells decreased the severity of BTA, spleen enlargement, and body weight loss caused by the virus, whereas there was no change in the protective efficacy. Passage 40 of SC9-2 provided protection superior to CVI988/Rispens in the SPF chickens. The immunosuppressive effect of SC9-2 may be eliminated by further attenuation of this virus on cell cultures to generate a safe and effective vaccine against MD.
Acknowledgments
The study was supported by grants of the National Key Research and Development Program of China (2017YFD0500700), the Key Program of NSFC-Henan Joint Found (U1604232), the National Natural Science Foundation of China (31402235), and the China Postdoctoral Science Foundation Funded Project (2018T110703, 2016M592234).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Contributor Information
Shuai Su, Email: ssu6307@163.com.
Ruiai Chen, Email: chensa727@126.com.
References
- Abdul-Careem M.F., Hunter B.D., Sarson A.J., Mayameei A., Zhou H., Sharif S. Marek's disease virus-induced transient paralysis is associated with cytokine gene expression in the nervous system. Viral Immunol. 2006;19:167–176. doi: 10.1089/vim.2006.19.167. [DOI] [PubMed] [Google Scholar]
- Brown A.C., Baigent S.J., Smith L.P., Chattoo J.P., Petherbridge L.J., Hawes P., Allday M.J., Nair V. Interaction of MEQ protein and C-terminalbinding protein is critical for induction of lymphomas by Marek’s disease virus. Proc. Natl. Acad. Sci. U. S. A. 2006;103:1687–1692. doi: 10.1073/pnas.0507595103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchil A.E., Biggs P.M. Agent of Marek's disease in tissue culture. Nature. 1967;215:528–530. doi: 10.1038/215528a0. [DOI] [PubMed] [Google Scholar]
- Churchill A.E., Chubb R.C., Baxendale W. The attenuation, with loss of oncogenicity, of the herpes-type virus of Marek's disease (strain HPRS-16) on passage in cell culture. J. Gen. Virol. 1969;4:557–564. doi: 10.1099/0022-1317-4-4-557. [DOI] [PubMed] [Google Scholar]
- Cui N., Su S., Sun P., Zhang Y., Han N., Cui Z. Isolation and pathogenic analysis of virulent Marek's disease virus field strain in China. Poult. Sci. 2016;95:1521–1528. doi: 10.3382/ps/pew073. [DOI] [PubMed] [Google Scholar]
- Cui X., Lee L., Reed W.M., Kung H.J., Reddy S.M. Marek’s disease virus-encoded vIL-8 gene is involved in early cytolytic infection but dispensable for establishment of latency. J. Virol. 2004;78:4753–4760. doi: 10.1128/JVI.78.9.4753-4760.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Z.Z., Lee L.F., Liu J.L., Kung H.J. Structural analysis and transcriptional mapping of the Marek’s disease virus gene encoding pp38, an antigen associated with transformed cells. J. Virol. 1991;65:6509–6515. doi: 10.1128/jvi.65.12.6509-6515.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Z., Zhuang G., Xu X., Sun A., Su S. Molecular and biological characterization of a Marek's disease virus field strain with reticuloendotheliosis virus LTR insert. Virus Genes. 2010;40:236–243. doi: 10.1007/s11262-009-0437-z. [DOI] [PubMed] [Google Scholar]
- Dudnikova E., Vlasov A., Norkina S., Kireev D., Witter R.L. Factors influencing the attenuation of serotype 1 Marek's disease virus byserial cell culture passage and evaluation of attenuated strains for protection and replication. Avian Dis. 2009;53:63–72. doi: 10.1637/8411-071908-Reg.1. [DOI] [PubMed] [Google Scholar]
- Faiz N., Cortes A.L., Guy J.S., Fletcher O.J., West M., Montiel E., Gimeno I.M. Early infection with Marek's disease virus can jeopardize protection conferred by laryngotracheitis vaccines: a method to study MDV-induced immunosuppression. Avian Pathol. 2016;45:606–615. doi: 10.1080/03079457.2016.1191618. [DOI] [PubMed] [Google Scholar]
- Faiz N.M., Cortes A.L., Guy J.S., Fletcher O.J., Cimino T., Gimeno I.M. Evaluation of factors influencing the development of late Marek's disease virus-induced immunosuppression: virus pathotype and host sex. Avian Pathol. 2017;46:376–385. doi: 10.1080/03079457.2017.1290214. [DOI] [PubMed] [Google Scholar]
- Kingham B.F., Zelník V., Kopácek J., Majerciak V., Ney E., Schmidt C.J. The genome of herpesvirus of turkeys: comparative analysis with Marek's disease viruses. J. Gen. Virol. 2001;82:1123–1135. doi: 10.1099/0022-1317-82-5-1123. [DOI] [PubMed] [Google Scholar]
- Kreager K. Chicken industry strategies for control of tumor virus infections. Poult. Sci. 1998;77:1213–1216. doi: 10.1093/ps/77.8.1213. [DOI] [PubMed] [Google Scholar]
- Lee L.F., Heidari M., Zhang H., Lupiani B., Reddy S.M., Fadly A. Cell culture attenuation eliminates rMd5ΔMeq-induced bursal and thymic atrophy and renders the mutant virus as an effective and safe vaccine against Marek's disease. Vaccine. 2012;30:5151–5158. doi: 10.1016/j.vaccine.2012.05.043. [DOI] [PubMed] [Google Scholar]
- Lee L.F., Lupiani B., Silva R.F., Kung H.J., Reddy S.M. Recombinant Marek's disease virus (MDV) lacking the Meq oncogene confers protection against challenge with a very virulent plus strain of MDV. Vaccine. 2008;26:1887–1892. doi: 10.1016/j.vaccine.2008.01.046. [DOI] [PubMed] [Google Scholar]
- Lee L.F., Wu P., Sui D., Ren D., Kamil J., Kung H.J., Witter R.L. The complete unique long sequence and the overall genomic organization of the GA strain of Marek’s disease virus. Proc. Natl. Acad. Sci. U. S. A. 2000;97:6091–6096. doi: 10.1073/pnas.97.11.6091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Sun A., Su S., Zhao P., Cui Z., Zhu H. Deletion of the Meq gene significantly decreases immunosuppression in chickens caused by pathogenic Marek's disease virus. Virol. J. 2011;5:2. doi: 10.1186/1743-422X-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J.L., Lee L.F., Ye Y., Qian Z., Kung H.J. Nucleolar and nuclear localization properties of a herpesvirus bZIP oncoprotein. MEQ. J. Virol. 1997;71:3188–3196. doi: 10.1128/jvi.71.4.3188-3196.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupiani B., Lee L.F., Cui X., Gimeno I., Anderson A., Morgan R.W., Silva R.F., Witter R.L., Kung H.J., Reddy S.M. Marek’s disease virus-encoded Meq gene is involved in transformation of lymphocytes but is dispensable for replication. Proc. Natl. Acad. Sci. U. S. A. 2004;101:11815–11820. doi: 10.1073/pnas.0404508101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parcells M.S., Lin S.F., Dienglewicz R.L., Majerciak V., Robinson D.R., Chen H.C., Wu Z., Dubyak G.R., Brunovskis P., Hunt H.D., Lee L.F., Kung H.J. Marek’s disease virus (MDV) encodes an interleukin-8 homolog (vIL-8): characterization of the vIL-8 protein and a vIL-8 deletion mutant MDV. J. Virol. 2001;75:5159–5173. doi: 10.1128/JVI.75.11.5159-5173.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Z., Brunovskis P., Rauscher F., III., Lee L., Kung H.J. Transactivation activity of Meq, a Marek’s disease herpesvirus bZIP protein persistently expressed in latently infected transformed T cells. J. Virol. 1995;69:4037–4044. doi: 10.1128/jvi.69.7.4037-4044.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralapanawe S., Walkden-Brown S.W., Islam A.F., Renz K.G. Effects of Rispens CVI988 vaccination followed by challenge with Marek's disease viruses of differing virulence on the replication kinetics and shedding of the vaccine and challenge viruses. Vet. Microbiol. 2016;183:21–29. doi: 10.1016/j.vetmic.2015.11.025. [DOI] [PubMed] [Google Scholar]
- Read A.F., Baigent S.J., Powers C., Kgosana L.B., Blackwell L., Smith L.P., Kennedy D.A., Walkden-Brown S.W., Nair V.K. Imperfect vaccination can Enhance the transmission of highly virulent pathogens. Plos Biol. 2015;13:e1002198. doi: 10.1371/journal.pbio.1002198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy S.M., Lupiani B., Gimeno I.M., Silva R.F., Lee L.F., Witter R.L. Rescue of a pathogenic Marek’s disease virus with overlapping cosmid DNAs: use of a pp38 mutant to validate the technology for the study of gene function. Proc. Natl. Acad. Sci. U. S. A. 2002;99:7054–7059. doi: 10.1073/pnas.092152699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rispens B.H., van H., Vloten, Mastenbroek N., Maas J.L., Schat K.A. Control of Marek’s disease in The Netherlands. I. Isolation of an avirulent Marek’s disease virus (strain CVI988) and its use in laboratory vaccination trials. Avian Dis. 1972;16:108–125. [PubMed] [Google Scholar]
- Rispens B.H., van H., Vloten, Mastenbroek N., Maas J.L., Schat K.A. Control of Marek’s disease in The Netherlands. II. Field trials on vaccination with an avirulent strain (CVI988) of Marek’s disease virus. Avian Dis. 1972;16:126–138. [PubMed] [Google Scholar]
- Schat K.A., Calnek B.W., Fabricant J., Graham D.L. Pathogenesis of infection with attenuated Marek's disease virus strains. Avian Pathol. 1985;14:127–146. doi: 10.1080/03079458508436213. [DOI] [PubMed] [Google Scholar]
- Schat K.A., Nair V. Marek’s disease. In: Saif Y.M., editor. Diseases of Poultry 12th Edn. Blackwell Publishing Professional; Ames: 2008. pp. 452–514. [Google Scholar]
- Spatz S.J., Schat K.A. Comparative genomic sequence analysis of the Marek's disease vaccine strain SB-1. Virus Genes. 2011;42:331–338. doi: 10.1007/s11262-011-0573-0. [DOI] [PubMed] [Google Scholar]
- Spatz S.J., Rue C., Schumacher D., Osterrieder N. Clustering of mutations within the inverted repeat regions of a serially passaged attenuated gallid herpesvirus type 2 strain. Virus Genes. 2008;37:69–80. doi: 10.1007/s11262-008-0242-0. [DOI] [PubMed] [Google Scholar]
- Su S., Cui N., Cui Z., Zhao P., Li Y., Ding J., Dong X. Complete genome sequence of a recombinant Marek's disease virus field strain with one reticuloendotheliosis virus long terminal repeat insert. J. Virol. 2012;86:13818–13819. doi: 10.1128/JVI.02583-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su S., Cui N., Li J., Sun P., Li H., Li Y., Cui Z. Deletion of the BAC sequences from recombinant meq-null Marek's disease (MD) virus increases immunosuppression while maintaining protective efficacy against MD. Poult. Sci. 2016;95:1504–1512. doi: 10.3382/ps/pew067. [DOI] [PubMed] [Google Scholar]
- Su S., Cui N., Sun A., Li Y., Ding J., Chen Z., Zhao P., Cui Z. Sequence analysis of the whole genome of a recombinant Marek's disease virus strain, GX0101, with a reticuloendotheliosis virus LTR insert. Arch. Virol. 2013;158:2007–2014. doi: 10.1007/s00705-013-1671-1. [DOI] [PubMed] [Google Scholar]
- Su S., Cui N., Zhou Y., Chen Z., Li Y., Ding J., Wang Y., Duan L., Cui Z. A recombinant field strain of Marek's disease (MD) virus with reticuloendotheliosis virus long terminal repeat insert lacking the meq gene as a vaccine against MD. Vaccine. 2015;33:596–603. doi: 10.1016/j.vaccine.2014.12.057. [DOI] [PubMed] [Google Scholar]
- Sun A.J., Xu X.Y., Petherbridge L., Zhao Y.G., Nair V., Cui Z.Z. Functional evaluation of the role of reticuloendotheliosis virus long terminal repeat (LTR) integrated into the genome of a field strain of Marek’s disease virus. Virology. 2010;397:270–276. doi: 10.1016/j.virol.2009.11.017. [DOI] [PubMed] [Google Scholar]
- Sun S.H., Cui Z.Z., Sun A.J. Comparisons of preventive effects of maternal antibody on immunosuppression induced by homologous and heterologous reticuloendotheliosis viruses. Acta Veterinaria Zootechnica Sinica. 2007;38:488–492. [Google Scholar]
- Witter R.L., Calnek B.W., Buscaglia C., Gimeno I.M., Schat K.A. Classification of Marek’s disease viruses according to pathotype: philosophy and methodology. Avian Pathol. 2005;34:75–90. doi: 10.1080/03079450500059255. [DOI] [PubMed] [Google Scholar]
- Witter R.L., Li D., Jones D., Lee L.F., Kung H.J. Retroviral insertional mutagenesis of a herpesvirus: a Marek's disease virus mutant attenuated for oncogenicity but not for immunosuppression or in vivo replication. Avian Dis. 1997;41:407–421. [PubMed] [Google Scholar]
- Witter R.L., Kreager K.S. Serotypviruses modified by backpassage or insertional mutagenesis: approaching the threshold of vaccine efficacy in Marek's disease. Avian Dis. 2004;48:768–782. doi: 10.1637/7203-050304R. [DOI] [PubMed] [Google Scholar]
- Witter R.L., Schat K.A. In: Diseases of Poultry 11th Edn. Saif Y.M., editor. Iowa State Press; Ames: 2003. pp. 407–467. [Google Scholar]
- Yu Z.H., Teng M., Luo J., Wang X.W., Ding K., Yu L.L., Su J.W., Chi J.Q., Zhao P., Hu B., Zhang G.P., Liu J.X. Molecular characteristics and evolutionary analysis of field Marek's disease virus prevalent in vaccinated chicken flocks in recent years in China. Virus Genes. 2013;47:282–291. doi: 10.1007/s11262-013-0942-y. [DOI] [PubMed] [Google Scholar]
- Zhang Y.P., Li Z.J., Bao K.Y., Lv H.C., Gao Y.L., Gao H.L., Qi X.L., Cui H.Y., Wang Y.Q., Ren X.G., Wang X.M., Liu C.J. Pathogenic characteristics of Marek's disease virus field strains prevalent in China and the effectiveness of existing vaccines against them. Vet. Microbiol. 2015;177:62–68. doi: 10.1016/j.vetmic.2014.12.020. [DOI] [PubMed] [Google Scholar]