Abstract
The objective of this study was to evaluate the effects of dietary linoleic acid (LA) on growth performance, antioxidant capacity, and lipid metabolism in pigeon squabs by supplementing LA in their parental diets. A completely randomized design that consisted of a control group, 1% dietary LA addition group (LA1%), 2% dietary LA addition group (LA2%), and 4% dietary LA addition group (LA4%) was used. Six squabs from each treatment were randomly sampled at the day of hatch and days 7, 14, and 21 after hatch. The results showed that parental dietary LA had no significant influence (P > 0.05) on body weight (BW) gain or relative organ weights (% of BW) in squabs. The activities of superoxide dismutase, catalase, and glutathione peroxidase in the LA1% were significantly increased (P < 0.05) compared with those in the control group. The malondialdehyde content in the LA1% was significantly lower (P < 0.05) than that in the control group. The levels of serum triglyceride in the LA1% and LA2% were significantly decreased (P < 0.05) compared with those in the control group, whereas the serum high-density lipoprotein cholesterol level in the LA1% and LA2% and the free fatty acid level in the LA4% were significantly higher (P < 0.05) than those of the control group. The activities of lipoprotein lipase, hepatic lipase, and hormone-sensitive lipase in the LA1% were significantly higher (P < 0.05) than those in the control group. The 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in the LA1% and the hormone-sensitive lipase activity in the LA4% were significantly decreased (P < 0.05) compared with those in the control group. The mRNA expression of carnitine palmitoyltransferase 1, acyl-CoA 1, and peroxisome proliferator-activated receptor α was significantly upregulated (P < 0.05) in the LA1% compared with that in the control group. The Oil Red O staining area in the LA1% and LA2% was significantly reduced compared with that in the control group. The results indicated that although supplemental LA had negligible effects on growth and development in pigeon squabs, parental dietary LA at a concentration of 1% could have beneficial effects on maintaining squabs healthy as reflected by improved antioxidant capacity and lipid metabolism.
Key words: linoleic acid, growth performance, lipid metabolism, pigeon, parental dietary
Introduction
Known for delicious nutrient meat, domestic pigeons (Columba livia) are reared as a kind of commercial meat-type poultry in China (Xu et al., 2019) and because the growth rate of pigeon squabs is rather high (Sales and Janssens, 2003). This superior characteristic of rapid growth can be partly attributed to the fact that pigeons are altrices and that pigeon squabs are initially fed with crop milk (Vandeputte-Poma, 1980), which is composed of desquamated epithelial cells of the crop mucosa and is secreted by both female and male parent pigeons (Bharathi et al., 1993). Deficient in carbohydrate, the crop milk mainly contains protein and lipids that provide squabs with energy (Davies, 1939). In consistent with the finding of Shetty and Hegde (1991), our laboratory previously analyzed the composition of fatty acids in crop milk and confirmed that linoleic acid (LA) was the most abundant polyunsaturated fatty acid (PUFA) (Zhang et al., 2016). Those findings suggested that LA might play a vital role in growth and development in pigeon squabs.
The most PUFA are long-straight-chain fatty acids with multiple double bonds and 18–22 carbon atoms in length (Oshodi et al., 1995). Previous research elucidated that PUFA such as LA, arachidonic acid, docosahexaenoic acid (DHA), and eicosapentaenoic acid (EPA) were crucial for body functions including the immune system, as well as for the formation and functioning of the cytomembrane (Malcicka et al., 2018). In humans, replacement of saturated fatty acid intake by LA reduced the risk of coronary artery disease (Wood et al., 1987, Mensink and Katan, 1992). Kalmijn et al. (1997) suggested that DHA intake was inversely associated with cognitive impairment in elderly men. And there was a strong inverse relationship between the score for the lifetime severity of schizophrenia and the ratio of LA, arachidonic acid, DHA, and EPA to other fats in the diet. The PUFA can be classified into ω-3 PUFA and ω-6 PUFA depending on the position of the first double bond. A growing body of research that focused on ω-6 PUFA further indicated that ω-6 PUFA played an important role in cell reproduction, physiology, and signaling (Belury, 2002, Elyassimi et al., 2008, De Veth et al., 2009). Linoleic acid, as an ω-6 PUFA, was an essential nutrient in diverse organisms (Malcicka et al., 2018). It has been long thought that because most animals lacked the ability to synthesize LA de novo, they required a dietary source of this fatty acid (Renobales et al., 2010, Aboshi et al., 2013, Shimizu et al., 2014). Thus, LA might be effective as an additive for farm animals. Many studies have shown that LA and its isomer participated in numerous functions in animals, such as growth performance in catfish and meat geese (Bogut et al., 2002, Tan et al., 2009, Zhang et al., 2016), antioxidant capacity in rats and laying hens (Kim et al., 2005, Qi et al., 2011), and lipid metabolism in broiler chicks and laying ducks (Du and Ahn, 2003, Wang et al., 2015). However, there are limited studies on the efficacy of dietary LA in the nutrition of altricial birds such as pigeons.
Thus, the objective of this study was to evaluate the effects of parental dietary LA on growth performance, antioxidant capacity, and lipid metabolism by determining BW gain, relative organ weights (% of body weight [BW]), antioxidant indices, serum lipid levels, activities of lipid metabolism–related enzymes, mRNA expression of lipid metabolism–related genes, and liver morphology in domestic pigeons (C. livia).
Materials and methods
All experimental protocols involving animals were approved by the Animal Care and Welfare Committee of Animal Science College and the Scientific Ethical Committee of the Zhejiang University (no. ZJU2013105002) (Hangzhou, China).
Animals and Diets
A total of 240 pairs of parental white King pigeons under the same breeding cycle (60 wk of age) were obtained from a commercial pigeon farm (Wenzhou, China). Each pair was housed in a man-made aviary equipped with a nest and a perch. The birds were randomly assigned to 4 treatments of 60 pairs each, which consisted of 6 replications of 10 pairs of pigeons. The 4 treatment groups were as follows: (1) control group, (2) 1% LA addition group (LA1%), (3) 2% LA addition group (LA2%), and (4) 4% LA addition group (LA4%). The LA (>99.0% purity) was obtained from a commercial supplier (Hebei Baiwei Biotechnology Co., Ltd., Handan, China). All experimental diets were isonitrogenous and isocaloric. The LA source was substituted for rapeseed oil on an equal-weight basis to equalize the total fat level among diets according to Qi et al. (2011). The ingredients, nutrient levels, and analyzed LA content of experimental diets for parent pigeons are shown in Table 1. The fatty acid contents of rapeseed oil and each diet analyzed according to Xie et al. (2013) are shown in Supplementary Table S1 and Supplementary Table S2, respectively. The parent pigeons were fed twice daily (7:00 am and 3:00 pm), and water was given ad libitum throughout the study.
Table 1.
Ingredient compositions and nutrient levels of experimental diets1 for parental pigeons2 (on as-fed basis).
| Items | Control | LA1% | LA2% | LA4% |
|---|---|---|---|---|
| Ingredients of a whole-grain form feed (%) | ||||
| Corn | 56.27 | 56.27 | 56.27 | 56.27 |
| Pea | 28.12 | 28.12 | 28.12 | 28.12 |
| Wheat | 5.63 | 5.63 | 5.63 | 5.63 |
| Sorghum | 5.63 | 5.63 | 5.63 | 5.63 |
| Rapeseed oil | 4.35 | 3.26 | 2.17 | 0 |
| Linoleic acid | 0 | 1.09 | 2.18 | 4.35 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 |
| Calculated nutrients3 (%) | ||||
| Metabolizable energy (MJ/kg)4 | 13.84 | 13.84 | 13.84 | 13.84 |
| Crude protein | 12.63 | 12.63 | 12.63 | 12.63 |
| Analyzed linoleic acid | 1.82 | 2.75 | 3.68 | 5.53 |
| Ingredients of grit meal (%) | ||||
| Limestone | 52.93 | 52.93 | 52.93 | 52.93 |
| Shell meal | 28.10 | 28.10 | 28.10 | 28.10 |
| Yellow mud | 14.05 | 14.05 | 14.05 | 14.05 |
| Salt | 1.41 | 1.41 | 1.41 | 1.41 |
| Ferrous sulfate (monohydrate) | 0.23 | 0.23 | 0.23 | 0.23 |
| Premix5 | 3.28 | 3.28 | 3.28 | 3.28 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 |
| Calculated nutrients2 (%) | ||||
| Calcium | 27.79 | 27.79 | 27.79 | 27.79 |
| Phosphorus | 0.01 | 0.01 | 0.01 | 0.01 |
| Sodium | 0.03 | 0.03 | 0.03 | 0.03 |
| Chlorine | 0.59 | 0.59 | 0.59 | 0.59 |
| Ferrum | 0.30 | 0.30 | 0.30 | 0.30 |
Control, control group; LA1%, 1% linoleic acid addition group; LA2%, 2% linoleic acid addition group; LA4%, 4% linoleic acid addition group.
All feeds were fed in a whole-grain form at 07:00 and 15:00 h every day, and grit meal was offered to the birds on a continuous basis.
Nutrient values were calculated from tables of feed composition and nutritive values in China (28th edition, 2017).
Metabolizable energy values determined in pigeons were calculated from those reported for chickens in accordance with a previous study (the study by Hullar et al., 1999), which observed that the metabolizable energy values of feed in pigeons did not differ significantly from those in chickens.
The premix provided the following per kg of diet: vitamin A, 5,000 IU; vitamin E, 50 IU; vitamin D3, 2,000 IU; copper sulfate, 15 mg; manganese sulfate, 45 mg; zinc sulfate, 90 mg.
All parent pigeons laid 2 eggs in a clutch, and they were supplied with experimental diets immediately on the day after laying the second egg. The eggs were picked out and transferred to an incubator for an 18-day artificial incubation. On the 18th D, 480 artificially hatched pigeon squabs with similar BW (14.76 ± 0.80 g) were selected from this commercial pigeon farm. They were randomly pair matched and allocated to nests of the experimental parental pigeons. Those parent pigeons were continually fed with the experimental diets for another 21 D. Pigeon squabs were fed with crop milk secreted by parent pigeons. The fatty acid contents of crop milk are shown in Supplementary Table S3. An ambient temperature of 18–26°C, a relative humidity of 60–70%, and an illumination procedure of 12 h of light and 12 h of darkness (12L:12D) was maintained throughout the study period.
Sample Collection
On the day of hatch (DOH), 7 D after hatch (D7), 14 D after hatch (D14), and 21 D after hatch (D21), 6 squabs per treatment (one squab for each replication) were selected for sampling and subsequent analyses randomly. Before weighing and slaughter, the D7, D14, and D21 squabs were fasted for 12 h. The DOH squabs were weighed and slaughtered within 2 h after hatch but before feeding. All squabs were killed by cervical dislocation. The organs were weighed individually including the heart, liver, spleen, kidney, pancreas, proventriculus, gizzard, and small intestine with digesta in the segments. The relative organ weights (% of BW) were shown as a percentage of organ weight to the BW.
Besides, livers samples from D21 squabs were collected, frozen in liquid nitrogen, and then immediately stored at –80°C for subsequent analyses (i.e., antioxidant index, lipid metabolism–related enzyme activity and gene expression, morphological examination). Blood samples from 21-day squabs were collected from the brachial vein and drawn into Eppendorf tubes (10 mL). After allowing the whole blood to clot, the serum was centrifuged for 10 min (3,000 g). Pure serum samples were aspirated using a pipette, stored in 1.5-ml Eppendorf tubes at –80°C, and thawed at 4°C before analyses.
Antioxidant Index and Serum Lipid Level Measurement
The livers were thawed and chopped into small pieces on ice. The 10% (w/v) homogenates were prepared in 10 mmol phosphate buffer (pH 7.4) and centrifuged at 10,000 g for 15 min at 4°C. The supernatant was collected and stored at –80°C to measure antioxidant indices. The antioxidant capacity of the liver sample was analyzed by the same method in triplicate. Total superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GSH-Px), and malondialdehyde (MDA) were measured spectrophotometerically (UV-2000, Unico Instruments Co., Ltd., Shanghai, China) using commercial kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). All the procedures were carried out as per the manufacturer's instructions. The activities/concentrations were expressed as units per milligram of protein.
Total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), very low-density lipoprotein cholesterol (vLDL-C), and free fatty acid (FFA) in serum were determined using commercially available test kits (Beijing Sino-uk Institute of Biological Technology, Beijing, China). The aforementioned indices were measured using an automatic biochemical analyzer (Hitachi 7600-020, Hitachi, Co., Tokyo, Japan).
Lipid Metabolism–Related Enzyme Activity Analyses
Enzyme activities were measured using the supernatant of homogenized tissue. The homogenates were made using the same methods mentioned previously. Activities of acetyl-CoA carboxylase (ACC), fatty acid synthase (FAS), and hormone-sensitive lipase (HSL) were assayed by absorbance changes at a wavelength of 450 nm using commercial kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) as per the instructions of the manufacturer. Activities of lipoprotein lipase (LPL) and hepatic lipase (HL) were assayed by absorbance changes at a wavelength of 550 nm using commercial kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) as per the instructions of the manufacturer. The activity of 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGR) was assayed by absorbance changes at a wavelength of 340 nm using commercial kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) as per the instructions of the manufacturer. The activities were expressed as units per gram or milligram of protein.
Lipid Metabolism–Related Gene Expression
Total RNA was isolated from each tissue sample (approximately 100 mg) by the TRIzol procedure (Invitrogen, Carlsbad, CA) as per the manufacturer's instructions. Total RNA was quantified by both native RNA electrophoresis on 1.0% agarose gel and the UV absorbance ratio at 260 and 280 nm and stored at –80°C. The complementary DNA was synthesized from 2 μg of total RNA using Moloney-murine leukemia virus (M-MLV) reverse transcriptase (Takara, Dalian, China) at 42°C for 60 min with the oligo dT-adaptor primer following the protocol of the manufacturer.
The abundance of mRNA was determined on a StepOnePlus Real-Time PCR system (ABI 7500, Applied Biosystems, Foster City, CA). Gene-specific primers (Table 2) for FAS, ACC, carnitine palmitoyltransferase 1 (CPT1), acyl-CoA 1 (ACO), peroxisome proliferator-activated receptor α (PPARα), peroxisome proliferator-activated receptor γ (PPARγ), and the endogenous reference gene (β-actin) were designed according to the published mRNA sequence. The PCR reaction used the SYBR Premix PCR kit (TaKaRa, Dalian, China). The PCR program was as follows: 95°C for 1 min, followed by 45 cycles at 95°C for 10 s and 62°C for 25 s. The standard curve was determined using pooled samples. Efficiency of the real-time PCR primers for all the examined genes was calculated from standard curves. Each sample was performed in triplicate, and no template control was included. Specificity of the amplification was verified at the end of PCR run by melting curve analysis. Specificity of the product was also confirmed by running samples on a 1.2% agarose gel, excising for purification using a DNA purification kit (TaKaRa, Dalian, China) and DNA sequencing (Sangon Biotech Co., Ltd., Shanghai, China). The Ct value for β-actin < 0.5 between all tissue types and time points was therefore considered to be an appropriate endogenous control. Average gene expression relative to the endogenous control for each sample was calculated using the 2−ΔΔCt method (Schmittgen, 2001). The calibrator for each gene in the experiment was the average ΔCt value of the control group.
Table 2.
Primers used for quantitative real-time PCR analyses of gene expression in domestic pigeons.
| Genes | Primer | Sequence (5’→3′) | Length of the amplicon (bp) | Accession number1 |
|---|---|---|---|---|
| ACC | ACC-F | CTCATGGTCTTCGCCAACTGGA | 87 | XM_013367232.1 |
| ACC-R | CACGATGTAGGCACCGAACTT | |||
| FAS | FAS-F | AAACTGAAGGCTGCTGATAAGT | 184 | XM_005515764.1 |
| FAS-R | CCTCCAATAAGGTGCGGTGAT | |||
| CPT1 | CPT1a-F | TCGTCTTGCCATGACTGGTG | 143 | XM_013369225.1 |
| CPT1a-R | GCTGTGGTGTCTGACTCGTT | |||
| ACO | ACO-F | GGCATTGAGGAGTGTCGGA | 244 | XM_005503118.2 |
| ACO-R | GCACAGTCACAGATGGAGCA | |||
| PPARα | PPARα-F | AGAATAAGGAAGCCGAAGTTC | 104 | XM_021297326.1 |
| PPARα-R | GGAGAAGCCAGGGATAGATTTG | |||
| PPARγ | PPARγ-F | CCAGCGACATCGACCAGTT | 145 | XM_021288013.1 |
| PPARγ-R | GGTGATTTGTCTGTCGTCTTTCC | |||
| β-actin | β-actin-F | CCCATCTACGAAGGCTACGC | 150 | AB618546.1 |
| β-actin-R | CTTGATGTCACGCACAATTTC |
Abbreviations: ACC, acetyl-CoA carboxylase; ACO, acyl-CoA 1; CPT1, carnitine palmitoyltransferase 1; FAS, fatty acid synthase; PPARα, peroxisome proliferator-activated receptor α; PPARγ, peroxisome proliferator-activated receptor γ.
Accession number is from the National Center for Biotechnology Information (https://www.ncbi.nlm.nih.gov/).
Morphological Examination
Tissue samples (approximately 0.5 cm3) were obtained from the liver and fixed in 10% neutral buffered formalin solution for histological analyses. Those samples were dehydrated, cleared, and embedded in paraffin. Serial sections (5 μm) were placed on glass slides and stained with hematoxylin and eosin. Frozen sections of the liver (5 μm) were stained with Oil Red O for evaluation of hepatic fatty change. The Oil Red O staining area was measured using Image-pro plus 6.0 (Media Cybernetics, Inc., Rockville, MD).
Statistical Analyses
Data were statistically analyzed by one-way ANOVA using SPSS 24.0 for Windows (SPSS Inc., Chicago, IL). When significant differences were found (P < 0.05), Tukey post hoc tests were performed.
Results
Body Weight
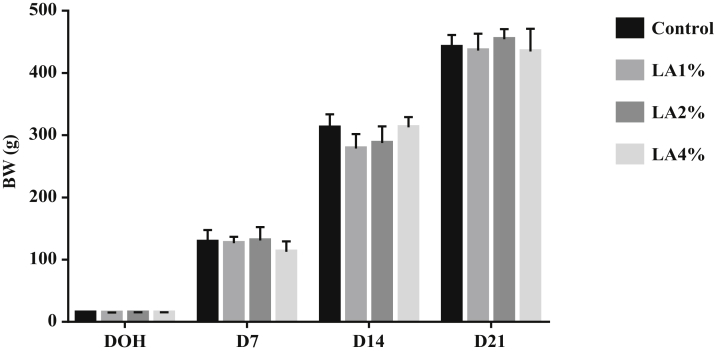
The effects of parental dietary LA on BW of pigeon squabs are shown in Figure 1. Parental dietary supplementation with linoleic acid had no significant effect (P > 0.05) on BW on the DOH, D7, D14, and D21 among the 4 groups.
Figure 1.
Effects of parental dietary linoleic acid on body weight (BW) in domestic pigeon squabs on the day of hatch (DOH), 7 D after hatch (D7), 14 D after hatch (D14), and 21 D after hatch (D21). Values are means ± SEM of 6 squabs. Control, control group; LA1%, 1% linoleic acid addition group; LA2%, 2% linoleic acid addition group; LA4%, 4% linoleic acid addition group
Organ Development
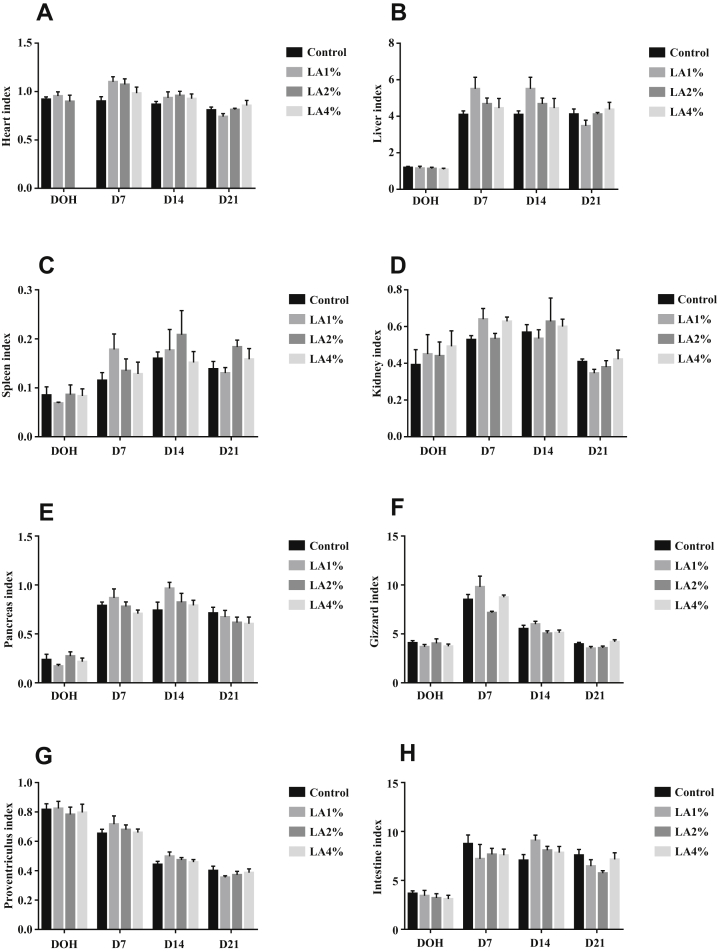
The effects of parental dietary LA on organ development in pigeon squabs are shown in Figure 2. There was no significance (P > 0.05) in the indices of the heart, liver, spleen, kidney, pancreas, gizzard, proventriculus, or small intestine on the DOH, D7, D14, and D21 among the 4 groups.
Figure 2.
Effects of parental dietary linoleic acid on relative organ weights (% of body weight) in domestic pigeon squabs on the day of hatch (DOH), day 7 after hatch (D7), day 14 after hatch (D14), and day 21 after hatch (D21). (A) Heart, (B) liver, (C) spleen, (D) kidney, (E) pancreas, (F) gizzard, (G) proventriculus, and (H) intestine. Relative organ weights (% of body weight) are determined as absolute weight normalized to body weight. Control, control group; LA1%, 1% linoleic acid addition group; LA2%, 2% linoleic acid addition group; LA4%, 4% linoleic acid addition group. Values are means ± SEM of 6 squabs. a,b Means within the same day sharing no common superscripts differ significantly (Tukey test, P < 0.05).
Hepatic Antioxidant Capacity
The effects of parental dietary LA on hepatic antioxidant capacity in pigeon squabs are shown in Table 3. The activities of SOD, CAT, and GSH-Px in the LA1% were significantly increased (P < 0.05) compared with those in the control group. The MDA content in the LA1% was significantly lower (P < 0.05) than that of the control group. And there was no significance (P > 0.05) in the activities of SOD, CAT, or GSH-Px or MDA content among the control group, LA2%, and LA4%. The response of the activities of SOD and CAT and MDA content to parental dietary addition of LA was quadratic (P < 0.05). The activities of SOD, CAT, and GSH-Px were maximized by 1% LA addition, and the MDA content was minimized by 1% LA addition. The GSH-Px activity displayed a linear (P = 0.039) and quadratic trend (P = 0.019), with the maximum response in the LA1%.
Table 3.
Effects of parental dietary linoleic acid on hepatic antioxidant indices in domestic pigeon squabs.1
| Antioxidant indices | SOD (U/mg) | CAT (U/mg) | GSH-Px (U/mg) | MDA (nmol/mg) |
|---|---|---|---|---|
| Control | 5.87b | 3.78b | 7.06b | 0.36a |
| LA1% | 13.67a | 8.70a | 8.42a | 0.30b |
| LA2% | 6.70b | 4.33b | 7.83a,b | 0.31a,b |
| LA4% | 6.58b | 4.05b | 7.64b | 0.35a |
| P value | 0.000 | 0.000 | 0.002 | 0.008 |
| SEM | 0.44 | 0.35 | 0.26 | 0.02 |
| Contrast | ||||
| Linear | 0.052 | 0.052 | 0.039 | 0.602 |
| Quadratic | 0.000 | 0.000 | 0.019 | 0.001 |
a–bMeans (n = 6) within a row with no common superscripts differ significantly (P < 0.05).
Abbreviations: CAT, catalase; GSH-Px, glutathione peroxidase; MDA, malondialdehyde; SOD, superoxide dismutase.
Control = control group; LA1% = 1% linoleic acid addition group; LA2% = 2% linoleic acid addition group; LA4% = 4% linoleic acid addition group.
Serum Lipid Levels
The effects of parental dietary LA on serum lipid levels in pigeon squabs are shown in Table 4. The levels of TG in the LA1% and LA2% were significantly decreased (P < 0.05) compared with those in the control group, but there was no significance (P > 0.05) in the TG level between the control group and LA4%. The levels of HDL-C in the LA1% and LA2% were significantly higher (P < 0.05) than those in the control group, but there was no significance (P > 0.05) in the HDL-C level between the control group and LA4%. The levels of TG and HDL-C in response to addition of LA were quadratic (P < 0.05), and the levels of TG and HDL-C were maximized in the LA1% and LA2%. The FFA level in the LA4% was significantly increased (P < 0.05) compared with that in the control group, but there was no significance (P > 0.05) in the FFA level among the control group, LA1%, and LA2%. The FFA level in response to addition of LA was linear (P < 0.05). And no significance (P > 0.05) was observed in the level of TC, LDL-C, or vLDL-C.
Table 4.
Effects of parental dietary linoleic acid on the serum lipid level in domestic pigeon squabs.1
| Lipid level | TG (mmol/L) | TC (mmol/L) | HDL-C (mmol/L) | LDL-C (mmol/L) | vLDL-C (μmol/L) | FFA (μmol/L) |
|---|---|---|---|---|---|---|
| Control | 3.81a | 6.90 | 2.99b | 2.46 | 161.63 | 356.73b |
| LA1% | 2.02b | 7.48 | 4.03a | 3.13 | 146.40 | 377.52a,b |
| LA2% | 2.11b | 7.30 | 3.73a | 3.06 | 151.59 | 428.50a,b |
| LA4% | 2.81a,b | 5.72 | 3.03b | 2.15 | 162.94 | 464.12a |
| P value | 0.003 | 0.127 | 0.000 | 0.139 | 0.468 | 0.012 |
| SEM | 0.42 | 0.76 | 0.22 | 0.50 | 12.42 | 31.70 |
| Contrast | ||||||
| Linear | 0.060 | 0.141 | 0.798 | 0.364 | 0.908 | 0.001 |
| Quadratic | 0.001 | 0.060 | 0.000 | 0.033 | 0.131 | 0.744 |
a–bMeans (n = 6) within a row with no common superscripts differ significantly (P < 0.05).
Abbreviations: FFA, free fatty acid; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TG, triglyceride; TC, total cholesterol; vLDL-C, very low-density lipoprotein cholesterol.
Control = control group; LA1% = 1% linoleic acid addition group; LA2% = 2% linoleic acid addition group; LA4% = 4% linoleic acid addition group.
Lipid Metabolism–Related Enzyme Activities
The effects of parental dietary LA on activities of hepatic lipid metabolism–related enzymes in pigeon squabs are shown in Table 5. The activities of LPL, HL, and HSL in the LA1% were significantly higher (P < 0.05) than those in the control group. The HMGR activity in the LA1% and the HSL activity in the LA4% were significantly decreased (P < 0.05) compared with those in the control group. There was no significance (P > 0.05) in the activities of LPL, HL, or HMGR among the control group, LA2% and LA4%, and there was no significance (P > 0.05) in HSL activity between the control group and LA2%. The activities of LPL, HL, and HMGR in response to addition of LA were quadratic (P < 0.05). The activities of LPL and HL were maximized in the LA1%, and the HMGR activity was minimized in the LA1%. The HSL activity displayed a linear (P < 0.05) and quadratic trend (P < 0.05), with the maximum response in the LA1%. Besides, no significance was (P > 0.05) observed in the activities of ACC or FAS among the 4 groups.
Table 5.
Effects of parental dietary linoleic acid on activities of hepatic lipid metabolism–related enzymes in domestic pigeon squabs.1
| Enzyme | LPL (U/g) | HL (U/mg) | ACC (U/mg) | FAS (ng/mg) | HSL (U/g) | HMGR (umol/min.mg) |
|---|---|---|---|---|---|---|
| Control | 0.29b | 0.18b | 3.59 | 1.01 | 7.88b | 0.34a |
| LA1% | 0.47a | 0.23a | 3.79 | 0.99 | 11.11a | 0.24b |
| LA2% | 0.33b | 0.20a,b | 3.39 | 0.85 | 7.91b | 0.31a |
| LA4% | 0.35b | 0.19b | 3.41 | 1.08 | 5.90c | 0.30a |
| P value | 0.000 | 0.019 | 0.463 | 0.440 | 0.000 | 0.000 |
| SEM | 0.02 | 0.01 | 0.28 | 0.14 | 0.62 | 0.01 |
| Contrast | ||||||
| Linear | 0.219 | 0.630 | 0.302 | 0.846 | 0.000 | 0.139 |
| Quadratic | 0.000 | 0.008 | 0.648 | 0.230 | 0.000 | 0.003 |
a–cMeans (n = 6) within a row with no common superscripts differ significantly (P < 0.05).
Abbreviations: ACC, acetyl-CoA carboxylase; FAS, fatty acid synthase; HL, hepatic lipase; HMGR, 3-hydroxy-3-methylglutaryl coenzyme A reductase; HSL, hormone-sensitive lipase; LPL, lipoprotein lipase.
Control = control group; LA1% = 1% linoleic acid addition group; LA2% = 2% linoleic acid addition group; LA4% = 4% linoleic acid addition group.
Lipid Metabolism–Related Gene Expression
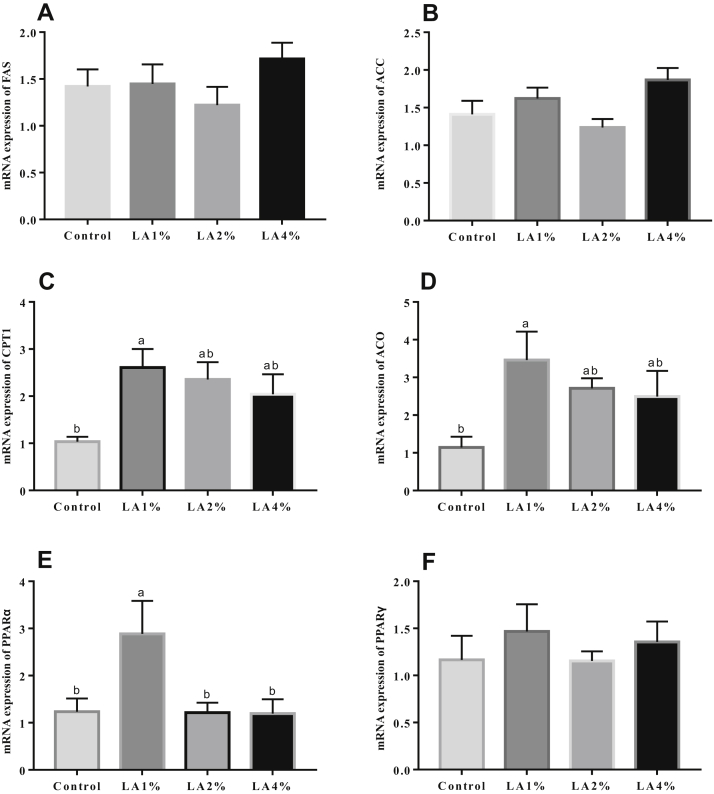
The effects of parental dietary LA on the mRNA expression of hepatic lipid metabolism–related genes in pigeon squabs are shown in Figure 3. No significance (P > 0.05) was observed in the mRNA expression of FAS, ACC, or PPARγ among the 4 groups. The mRNA expression of CPT1, ACO, and PPARα was significantly upregulated (P < 0.05) in the LA1% compared with the control group, but there was no significance (P > 0.05) in these 3 gene expression levels among the control group, LA2%, and LA4%.
Figure 3.
Effects of parental dietary linoleic acid on the mRNA expression of hepatic lipid metabolism–related genes in domestic pigeon squabs. (A) mRNA expression of fatty acid synthase (FAS), (B) mRNA expression of acetyl-CoA carboxylase (ACC), (C) mRNA expression of carnitine palmitoyltransferase 1 (CPT1), (D) mRNA expression of acyl-CoA (ACO), (E) mRNA expression of peroxisome proliferator-activated receptor α (PPARα), and (F) mRNA expression of peroxisome proliferator-activated receptor γ (PPARγ). Control, control group; LA1%, 1% linoleic acid addition group; LA2%, 2% linoleic acid addition group; LA4%, 4% linoleic acid addition group. Values are means ± SEM of 6 squabs. a,b Means sharing no common superscripts differ significantly (Tukey test, P < 0.05).
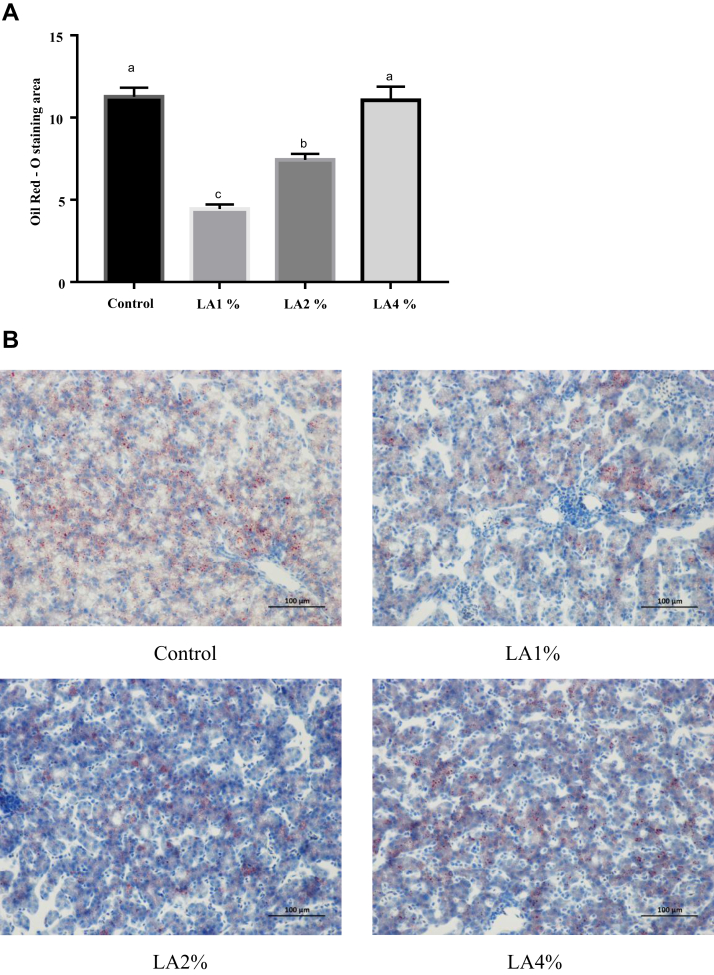
Liver Morphology and Lipid Accumulation
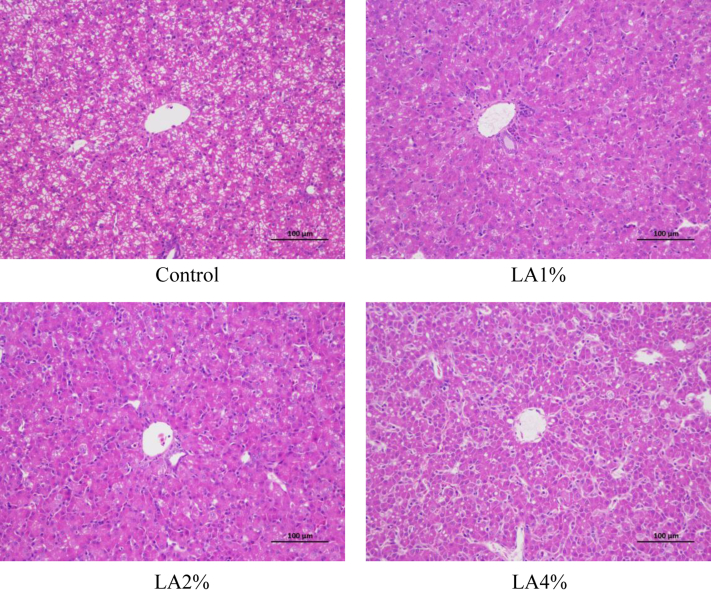
The effects of parental dietary LA on liver morphology and lipid accumulation in pigeon squabs are shown in Figure 4 and Figure 5, respectively. No damage was observed in hepatic central veins or hepatic cells among the 4 groups. Nevertheless, the Oil Red O staining area in the LA1% was significantly reduced (P < 0.05) compared with that in the other groups, and the staining area in the LA2% was also significantly decreased (P < 0.05) compared with that in the control group and LA4%. There was no significance (P > 0.05) in the Oil Red O staining area between the control group and LA4%.
Figure 4.
Hematoxylin and eosin staining showing effects of parental dietary linoleic acid on hepatic morphological changes in domestic pigeon squabs. Control, control group; LA1%, 1% linoleic acid addition group; LA2%, 2% linoleic acid addition group; LA4%, 4% linoleic acid addition group. Bar = 100 μm.
Figure 5.
Oil Red O staining showing effects of parental dietary linoleic acid on hepatic lipid accumulation in domestic pigeon squabs. Control, control group; LA1%, 1% linoleic acid addition group; LA2%, 2% linoleic acid addition group; LA4%, 4% linoleic acid addition group. (A) Oil Red O staining area was measured using Image-pro plus 6.0. Values are means ± SEM of 6 squabs. a,b Means sharing no common superscripts differ significantly (Tukey test, P < 0.05). (B) Representative Oil Red O staining of livers in the 4 experimental groups. Bar = 100 μm.
Discussion
Pigeon squabs have a superior high relative growth rate (Sales and Janssens, 2003). In the present study, squabs' BW at D7 was nearly 8 times to that at the DOH, and the BW at D14 was almost 20 times to that at the DOH, which was consistent with the experiment by Zhang et al. (2017). However, dietary LA had no significant effect on BW or the growth trend in this study. Similarly, previous studies showed that dietary LA or materials rich in LA did not influence the growth performance in piglets, laying hens, or meat rabbits (Uwayjan et al., 1983, Li, 2011, Zhang, 2018). In contrast, addition of 1% LA to a standard fish feed had beneficial effects on fish growth indicators (Bogut et al., 2002). A thorough understanding of the organ growth trajectory is rather helpful to elucidate the possible mechanism of the growth and development in squabs (Zhang et al., 2017). In the present study, relative organ weights (% of BW) had no significant change in response to LA addition, which was in accordance with the effects of LA supplementation on BW of squabs. Unfortunately, knowledge of the influence of dietary LA on the pigeon growth and development appears limited, and we have been unable to find any other study to confirm this result. Nevertheless, LA may be an important active substrate to keep squabs healthy owing to its high concentration in crop milk. Thus, the effects of parental dietary LA on antioxidant capacity and lipid metabolism in pigeon squabs were further evaluated in the present study.
Both oxygen free radical and lipid peroxidation reactions play important roles in animal body metabolism and functions, including maintenance of normal physiological dynamic balance, immune responses, and biochemical reactions (Babior et al., 2003). Once the balance is disturbed, a range of free radical chain reactions and metabolic disorders are induced (Djordjević, 2004). As detrimental by-products of basic cellular metabolism in aerobic organisms, free radicals (e.g., superoxide anion radical, hydrogen peroxide, singlet oxygen, and hydroxyl radical) combine with membrane PUFA, inducing free radical chain reactions which result in damage to the cytomembrane structure and functions, causing various diseases (Cheeseman and Slater, 1993, Apel and Hirt, 2004, Miller et al., 2010). Antioxidant enzymes such as SOD, CAT, and GSH-Px play critical roles in scavenging oxygen free radicals (Jaeschke, 1995, Fantel, 1996). In the present study, we observed that the activities of total SOD, CAT, and GSH-Px were significantly increased in liver homogenates in the LA1%, suggesting that the abilities of scavenging superoxide anions and hydroxyl radicals were enhanced in pigeon squabs. On the other hand, MDA content significantly decreased in the LA1%. Because MDA is a hydroxyl radical–initiated lipid peroxidation product (Marnett, 1999, Skoie et al., 2019), reducing production of MDA also prevents further damage to the cytomembrane. Similarly, Kim et al. (2005) found that the dietary isomer of LA in rats effectively lowered hepatic thiobarbituric acid–reactive substances and reduced lipid peroxidation by increasing oxidative stability. In combination with increased activities of antioxidant enzymes by dietary LA, it could be indicated that dietary LA at a concentration of 1% could be beneficial to health by improving antioxidant status in pigeon squabs.
Because TG and cholesterol are transported as lipoprotein particles and FFA from the origin to the target through blood circulation (Wnuk et al., 2013), serum concentrations of TG, TC, LDL-C, HDL-C, vLDL-C, and FFA are parameters used to measure serum lipid levels and are good indicators of health status (Ma et al., 2014). Diets containing unsaturated fatty acids have been reported to reduce serum TG levels (Liu et al., 2011). In our present study, the levels of TG in the LA1% and LA2% were significantly decreased compared with those in the control group, which was consistent with the previous study. High-density lipoprotein cholesterol is known as the “good” cholesterol that removes cholesterol from bloodstream and carries it back to the liver for recycling (Du et al., 2016). In our present study, the HDL-C level in the LA1% and LA2% was significantly increased, suggesting that parental dietary supplementation with 1∼2% LA was beneficial to serum lipid levels and hence improvement of body health in squabs. It has been known that almost all fat that accumulates in the body is derived from the diet or synthesized in the liver (Peng et al., 2018). As expected, reduced lipid droplets in hepatic morphology were also observed in these 2 groups. However, the FFA level in the LA4% was significant higher than that in the control group. Because high FFA stimulated production of reactive oxygen species (Inoguchi et al., 2000), which would affect antioxidant capacity in animals, it might indicate that excessive addition of LA would damage body health in pigeon squabs.
The balance between TG production and degradation determines the circulating TG level. Lipogenesis-related enzymes such as FAS, ACC, HMGR, and PPARγ and lipolysis-related enzymes such as LPL, HL, HSL, ACO, CPT1, and PPARα have long been known to affect lipid homeostasis (Joseph et al., 2002, Ma et al., 2014, Xu et al., 2018) and mainly play central roles in TG metabolism. Unlike mammalian species, de novo fatty acid synthesis occurs exclusively in the liver in domestic birds (Badinga et al., 2003). In this study, no significance was observed either in activity or in mRNA expression of ACC and FAS in the liver among the 4 treatment groups, implying that parental dietary LA supplementation did not influence the process of fatty acid synthesis. Consistently, Wang et al. (2015) also elucidated that different supplemental levels of LA had no effects on the relative expression levels of FAS and ACC genes. However, the activity of HMGR, which is a rate-limiting enzyme in the synthesis of cholesterol (Honda et al., 2010), was decreased in the LA1% group. On account of no significance in the level of serum TC observed in this study, we conjectured that cholesterol metabolism was not only regulated by HMGR but also controlled by other key metabolites such as insulin, glucagon, or cortisol.
Because parental dietary LA has negligible effects on lipogenesis, the decrease in TG levels should be attributed to the degradation of TG. Three key enzymes involved in circulating lipid metabolism are HSL, LPL, and HL. As the key neutral fat enzyme, HSL mainly hydrolyzes monoacylglycerol, diacylglycerol, and TG into FFA (Niu et al., 2010). Lipoprotein lipase also has a major role in TG-rich lipoprotein metabolism by catalyzing the hydrolysis of TG in chylomicrons (Kersten, 2014). Regarding HL, which is synthesized in the liver, it is known to play a role in catalyzing the hydrolysis of the smaller remnants and TG in vLDL (Perret et al., 2002). Our results showed that parental supplementation with 1% LA significantly increased the activities of HSL, LPL, and HL in squabs, which accelerated lipolysis and in turn decreased lipid droplet accumulation in the liver of pigeon squabs. Moreover, β-oxidation of fatty acids is one of the major processes in lipolysis, and key enzymes or transcription factors participating in this process are ACO, CPT1, and PPARα. Acyl-CoA 1 catalyzes the first step of β-oxidation of a variety of substrates broken down in the peroxisome (Liu et al., 2011). As a rate-limiting enzyme, CPT1 plays an important role in transporting fatty acids through the inner membrane of mitochondria (Jogl et al., 2010). Because fatty acids are ligands for PPARα, PPARα stimulates hepatic fatty acid oxidation (Patsouris et al., 2004). In our present study, the mRNA expression of ACO, CPT1, and PPARα was significantly higher in the LA1% than in the control group, which indicated that β-oxidation was enhanced and therefore lipolysis was reinforced. These results were in line with the increased activities of lipolysis-related enzymes.
Interestingly, in this study, parental addition of 1% LA improved antioxidant capacity and strengthened lipolysis in squabs, whereas parental addition of 2% and 4% LA had negligible effects on antioxidant capacity, and addition of 4% LA even had detrimental impacts on lipid metabolism. The reason might be due to the impact on the balance between ω-6 PUFA and ω-3 PUFA (e.g., DHA and EPA) with excessive addition of LA, the balance of which may have been disrupted owing to competition between ω-6 and ω-3 PUFA for the enzymes involved in fatty acid elongation and desaturation (Khang et al., 2007, Gupta et al., 2016). However, knowledge of the influence of LA supplementation on pigeons' antioxidant capacity and lipid metabolism appears limited, and we have been unable to find any other study to confirm this result. Further studies are required to verify this conjecture.
In conclusion, the results obtained from this study indicated that although supplemental LA had negligible effects on growth and development in pigeon squabs, parental dietary supplementation with 1% LA could have beneficial effects on maintaining squabs healthy, as reflected by improved antioxidant capacity and lipid metabolism.
Acknowledgments
The research was supported by the Fundamental Research Funds for the Central Universities (no. 2019QNA6028), Zhejiang Provincial Major Scientific and Technological Special Project for New Variety Breeding of Livestock and Poultry during “the 13th Five-year Plan” Period (no. 2016C02054-16), Team Science and Technology Commissioner Project of Zhejiang Province (no. 22), and the Key Laboratory of Information Traceability for Agricultural Products, Ministry of Agriculture of China.
Footnotes
Supplementary data are available at Poultry Science online.
Contributor Information
L.Z. Lu, Email: 13306813018@163.com.
X.T. Zou, Email: xtzou@zju.edu.cn.
Supplementary data
References
- Aboshi T., Shimizu N., Nakajima Y., Honda Y., Kuwahara Y., Amano H., Mori N. Biosynthesis of linoleic acid in tyrophagus mites (acarina: acaridae) Insect Biochem. Mol. Biol. 2013;43:991–996. doi: 10.1016/j.ibmb.2013.08.002. [DOI] [PubMed] [Google Scholar]
- Apel K., Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004;55:373–399. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- Babior B.M., Takeuchi C., Ruedi J., Gutierrez A., Wentworth P. Investigating antibody-catalyzed ozone generation by human neutrophils. Proc. Natl. Acad. Sci. U. S. A. 2003;100:3031–3034. doi: 10.1073/pnas.0530251100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badinga L., Selberg K., Dinges A., Corner C., Miles R. Dietary conjugated linoleic acid alters hepatic lipid content and fatty acid composition in broiler chickens. Poult. Sci. 2003;82:111–116. doi: 10.1093/ps/82.1.111. [DOI] [PubMed] [Google Scholar]
- Belury M.A. Dietary conjugated linoleic acid in health: physiological effects and mechanisms of action. Annu. Rev. Nutr. 2002;22:505–531. doi: 10.1146/annurev.nutr.22.021302.121842. [DOI] [PubMed] [Google Scholar]
- Bharathi L., Shenoy K.B., Mojamdar M., Hegde S.N. Studies on the growth-stimulatory activity of pigeon milk--comparison and synergistic effects with serum. J. Comp. Physiol. B. 1993;163:332–336. doi: 10.1007/BF00347784. [DOI] [PubMed] [Google Scholar]
- Bogut I., Hasschön E., Čačić M., Milaković Z., Novoselić D., Brkić S. Linolenic acid supplementation in the diet of european catfish (silurus glanis): effect on growth and fatty acid composition. J. Appl. Ichthyol. 2002;18:1–6. [Google Scholar]
- Cheeseman K.H., Slater T.F. An introduction to free radical biochemistry. Br. Med. Bull. 1993;49:481–493. doi: 10.1093/oxfordjournals.bmb.a072625. [DOI] [PubMed] [Google Scholar]
- Davies W.L. The composition of the crop milk of pigeons. Biochem. J. 1939;33:898–901. doi: 10.1042/bj0330898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Veth M.J., Bauman D.E., Koch W., Mann G.E., Pfeiffer A.M., Butler W.R. Efficacy of conjugated linoleic acid for improving reproduction: a multi-study analysis in early-lactation dairy cows. J. Dairy Sci. 2009;92:2662–2669. doi: 10.3168/jds.2008-1845. [DOI] [PubMed] [Google Scholar]
- Djordjević V.B. Free radicals in cell biology. Int. Rev. Cytol. 2004;237:57–89. doi: 10.1016/S0074-7696(04)37002-6. [DOI] [PubMed] [Google Scholar]
- Du M., Ahn D.U. Dietary cla affects lipid metabolism in broiler chicks. Lipids. 2003;38:505–511. doi: 10.1007/s11745-003-1091-z. [DOI] [PubMed] [Google Scholar]
- Du X., Liu Y., Lu L., Wang W., Zeng T., Tian Y., Xu X., Shen J., Niu D., Lu Y. Effects of dietary fats on egg quality and lipid parameters in serum and yolks of Shan partridge duck. Poult. Sci. 2016;96:1184–1190. doi: 10.3382/ps/pew348. [DOI] [PubMed] [Google Scholar]
- Elyassimi A., Hichami A., Besnard P., Khan N.A. Linoleic acid induces calcium signaling, src kinase phosphorylation, and neurotransmitter release in mouse cd36-positive gustatory cells. J. Biol. Chem. 2008;283:12949–12959. doi: 10.1074/jbc.M707478200. [DOI] [PubMed] [Google Scholar]
- Fantel A.G. Reactive oxygen species in developmental toxicity: review and hypothesis. Birth Defects Res. Part. A. 1996;53:196–217. doi: 10.1002/(SICI)1096-9926(199603)53:3<196::AID-TERA7>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Gupta S., Kihara Y., Maurya M.R., Norris P.C., Dennis E.A., Subramaniam S. Computational modeling of competitive metabolism between ω3- and ω6-polyunsaturated fatty acids in inflammatory macrophages. J. Phys. Chem. B. acs.Jpcb. 2016;120:8346–8353. doi: 10.1021/acs.jpcb.6b02036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hullar I., Meleg I., Fekets S., Romvari R. Studies on the energy content of pigeon feeds I. determination of digestibility and metabolizable energy content. Poult. Sci. 1999;78:1757–1762. doi: 10.1093/ps/78.12.1757. [DOI] [PubMed] [Google Scholar]
- Honda K., Kamisoyama H., Motoori T., Saneyasu T., Hasegawa S. Effect of dietary coenzyme q10 on cholesterol metabolism in growing chickens. J. Poul. Sci. 2010;47:41–47. [Google Scholar]
- Inoguchi T., Li P., Umeda F., Yu H.Y., Kakimoto M., Imamura M., Aoki T., Etoh T., Hashimoto T., Naruse M., Sano H., Utsumi H., Nawata H. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase c--dependent activation of nad(p)h oxidase in cultured vascular cells. Diabetes. 2000;49:1939–1945. doi: 10.2337/diabetes.49.11.1939. [DOI] [PubMed] [Google Scholar]
- Jaeschke H. Mechanisms of oxidant stress-induced acute tissue injury. Exp. Biol. Med. 1995;209:104–111. doi: 10.3181/00379727-209-43885b. [DOI] [PubMed] [Google Scholar]
- Jogl G., Hsiao Y.S., Tong L. Structure and function of carnitine acyltransferases. Ann. N. Y. Acad. Sci. 2010;1033:17–29. doi: 10.1196/annals.1320.002. [DOI] [PubMed] [Google Scholar]
- Joseph S.B., Laffitte B.A., Patel P.H., Watson M.A., Matsukuma K.E., Walczak R., Collins J., Osborne T., Tontonoz P. Direct and indirect mechanisms for regulation of fatty acid synthase gene expression by liver x receptors. J. Biol. Chem. 2002;277:11019–11025. doi: 10.1074/jbc.M111041200. [DOI] [PubMed] [Google Scholar]
- Kalmijn S., Feskens E.J.M., Launer L.J., Kromhout D. Polyunsaturated fatty acids, antioxidants, and cognitive function in very old men. Am. J. Epidemiol. 1997;145:33–41. doi: 10.1093/oxfordjournals.aje.a009029. [DOI] [PubMed] [Google Scholar]
- Kersten S. Physiological regulation of lipoprotein lipase. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2014;1841:919–933. doi: 10.1016/j.bbalip.2014.03.013. [DOI] [PubMed] [Google Scholar]
- Khang N.T.K., Jennen D.G.J., Tholen E., Tesfaye D., Mennicken L., Hoelker M., Schellander K., Ponsuksili S., Murani E., Wimmers K. Association of the fads2 gene with ω-6 and ω-3 pufa concentration in the egg yolk of Japanese quail. Anim. Biotechnol. 2007;18:189–201. doi: 10.1080/10495390701201390. [DOI] [PubMed] [Google Scholar]
- Kim H.K., Kim S.R., Ahn J.Y., Cho I.J., Yoon C.S., Ha T.Y. Dietary conjugated linoleic acid reduces lipid peroxidation by increasing oxidative stability in rats. J. Nutr. Sci. Vitaminol. 2005;51:8–15. doi: 10.3177/jnsv.51.8. [DOI] [PubMed] [Google Scholar]
- Li R.G. MS Thes. Univ. Shandong; China: 2011. Effects of Dietary Linoleic Acid Levels on Growth Performance, Fatty Acid Composition of Tissue and Lipid Metabolism of Meat Rabbits. [Google Scholar]
- Liu W.M., Zhang J., Lu L.Z., Shi F.X., Niu D., Wang D.L., Tian Y. Effects of perilla extract on productive performance, serum values and hepatic expression of lipid-related genes in shaoxing ducks. Bri. Poult. Sci. 2011;52:381–387. doi: 10.1080/00071668.2011.577053. [DOI] [PubMed] [Google Scholar]
- Liu W.M., Lai S.J., Lu L.Z., Shi F.X., Zhang J., Liu Y., Yu B., Tao Z.R., Shen J.D., Li G.Q., Wang D.Q., Li J.J., Tian Y. Effect of dietary fatty acids on serum parameters, fatty acid compositions, and liver histology in Shaoxing laying ducks. J. Zhejiang Univ. Sci. B. 2011;12:736–743. doi: 10.1631/jzus.B1000329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z., Zhang J., Ma H., Dai B., Zheng L., Miao J., Zhang Y. The influence of dietary taurine and reduced housing density on hepatic functions in laying hens. Poult. Sci. 2014;93:1724–1736. doi: 10.3382/ps.2013-03654. [DOI] [PubMed] [Google Scholar]
- Malcicka M., Visser B., Ellers J. An evolutionary perspective on linoleic acid synthesis in animals. Evol. Biol. 2018;45:15–26. doi: 10.1007/s11692-017-9436-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marnett L.J. Lipid peroxidation-DNA damage by malondialdehyde. Mutat. Res. Fundam. Mol. Mech. Mutagen. 1999;424:83–95. doi: 10.1016/s0027-5107(99)00010-x. [DOI] [PubMed] [Google Scholar]
- Mensink R.P., Katan M.B. Effect of dietary fatty acids on serum lipids and lipoproteins. a meta-analysis of 27 trials. Arterioscler. Thromb. 1992;12:911–919. doi: 10.1161/01.atv.12.8.911. [DOI] [PubMed] [Google Scholar]
- Miller G., Suzuki N., Ciftci-Yilmaz S., Mittler R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2010;33:453–467. doi: 10.1111/j.1365-3040.2009.02041.x. [DOI] [PubMed] [Google Scholar]
- Niu Z.Y., Liu F.Z., Min Y.N., Li W.C. Effects of dietary dihydropyridine supplementation on growth performance and lipid metabolism of broiler chickens. Czech J. Anim. Sci. 2010;55:116–122. [Google Scholar]
- Oshodi A.A., Ipinmoroti K.O., Adeyeye E.I., Hall G.M. Amino and fatty acids composition of African yam bean (Sphenostylis stenocarpa) flour. Food Chem. 1995;53:1–6. [Google Scholar]
- Patsouris D., Mandard S., Voshol P.J., Escher P., Tan N.S., Havekes L.M., Koenig W., März W., Tafuri S., Wahli W., Müller M., Kersten S. Pparα governs glycerol metabolism. J. Clin. Invest. 2004;114:94–103. doi: 10.1172/JCI20468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng M., Li L., Yu L., Ge C., Ma H. Effects of (−)-hydroxycitric acid on lipid droplet accumulation in chicken embryos. Anim. Sci. J. 2018;89:237–249. doi: 10.1111/asj.12887. [DOI] [PubMed] [Google Scholar]
- Perret B., Mabile L., Martinez L., Tercé F., Barbaras R., Collet X. Hepatic lipase: structure/function relationship, synthesis, and regulation. J. Lipid Res. 2002;43:1163–1169. [PubMed] [Google Scholar]
- Qi X., Wu S., Zhang H., Yue H., Xu S., Ji F., Qi G. Effects of dietary conjugated linoleic acids on lipid metabolism and antioxidant capacity in laying hens. Arch. Anim. Nutr. 2011;65:354–365. doi: 10.1080/1745039x.2011.617546. [DOI] [PubMed] [Google Scholar]
- Renobales M.D., Ryan R.O., Heisler C.R., Mclean D.L., Blomquist G.J. Linoleic acid biosynthesis in the pea aphid, acyrthosiphon pisum (harris) Arch. Insect Biochem. Physiol. 2010;3:193–203. [Google Scholar]
- Sales J., Janssens G.P.J. Nutrition of the domestic pigeon (Columba livia domestica) World's Poult. Sci. J. 2003;59:221–232. doi: 10.1093/ps/82.9.1457. [DOI] [PubMed] [Google Scholar]
- Schmittgen T.D. Real-time quantitative PCR. Methods. 2001;25:383–385. doi: 10.1006/meth.2001.1260. [DOI] [PubMed] [Google Scholar]
- Shetty S., Hegde S.N. Changes in lipids of pigeon “milk” in the first week of its secretion. Lipids. 1991;26:930–933. [Google Scholar]
- Shimizu N., Naito M., Mori N., Kuwahara Y. De novo biosynthesis of linoleic acid and its conversion to the hydrocarbon (z,z)-6,9-heptadecadiene in the astigmatid mite, carpoglyphus lactis: incorporation experiments with 13c-labeled glucose. Insect Biochem. Mol. Biol. 2014;45:51–57. doi: 10.1016/j.ibmb.2013.11.006. [DOI] [PubMed] [Google Scholar]
- Skoie I.M., Dalen I., Omdal R., Jonsson G. Malondialdehyde and advanced oxidation protein products are not increased in psoriasis: a controlled study. Arch. Dermatol. Res. 2019;311:299–308. doi: 10.1007/s00403-019-01903-2. [DOI] [PubMed] [Google Scholar]
- Tan X.Y., Zhi L., Ping X., Liu X.J. Effect of dietary linolenic acid/linoleic acid ratio on growth performance, hepatic fatty acid profiles and intermediary metabolism of juvenile yellow catfish pelteobagrus fulvidraco. Aquaculture. 2009;296:96–101. [Google Scholar]
- Uwayjan M.G., Azar E.J., Daghir N.J. Sunflower seed in laying hen rations. Poult. Sci. 1983;62:1247–1253. [Google Scholar]
- Vandeputte-Poma J. Feeding, growth and metabolism of the pigeon, columba livia domestica: duration and role of crop milk feeding. J. Comp. Physiol. 1980;135:97–99. [Google Scholar]
- Wang S., Chen W., Dong R., Wang S., Lin Y., Zheng C. Effects of dietary linoleic acid level on laying performance,egg quality and lipids metabolism of ducks during the early laying period. Chin. J. Anim. Nutr. 2015;27:731–739. [Google Scholar]
- Wnuk A., Mroczeksosnowska N.M., Łukasiewicz M., Batorska M., Niemiec J. Influence of the system of rearing on cholesterol level and its fraction in blood serum of slow-growing chickens. Ann. Warsaw Univ. Life Sci. - SGGW, Anim. Sci. 2013;52:219–225. [Google Scholar]
- Wood D.A., Butler S., Macintyre C., Riemersma R.A., Thomson M., Elton R.A., Oliver M.F. Linoleic and eicosapentaenoic acids in adipose tissue and platelets and risk of coronary heart disease. Lancet. 1987;329:177–183. doi: 10.1016/s0140-6736(87)90001-8. [DOI] [PubMed] [Google Scholar]
- Xie P., Wang Y., Wang C., Yuan C., Zou X. Effect of different fat sources in parental diets on growth performance, villus morphology, digestive enzymes and colorectal microbiota in pigeon squabs. Arch. Anim. Nutr. 2013;67:147–160. doi: 10.1080/1745039X.2013.776329. [DOI] [PubMed] [Google Scholar]
- Xu H., Min J., Zhu T., Li C., Lu Y., Yuan Y., Xiong J., Zhou Q. Moderate levels of dietary arachidonic acid reduced lipid accumulation and tended to inhibit cell cycle progression in the liver of Japanese seabass lateolabrax japonicus. Sci. Rep-uk. 2018;8:10682. doi: 10.1038/s41598-018-28867-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q.Q., Zhang X.Y., Zou X.T., Dong X.Y. Effects of in ovo injection of L-histidine on hatch performance and post-hatch development in domestic pigeons (Columba livia) Poult. Sci. 2019;98:3194–3203. doi: 10.3382/ps/pez046. [DOI] [PubMed] [Google Scholar]
- Zhang X.T. MS Thes. Univ. Henan; China: 2018. Application Effect of Different Proportion of Linoleic Acid/linolenic Acid in Diet on Sows during Late Gestation and Lactation. [Google Scholar]
- Zhang X.Y., Wan X.P., Miao L.P., Zou X.T., Dong X.Y. Effects of in ovo injection of l-arginine on hatchability, hatching time, early posthatch development, and carcass traits in domestic pigeons (Columba livia) J. Anim. Sci. 2017;95:4462–4471. doi: 10.2527/jas2017.1776. [DOI] [PubMed] [Google Scholar]
- Zhang, X. Y X.Y., Dong X. C. Bu, Zou X.T. Bioactive Constituents in pigeon milk during 2~10 Days secretion. Chin. J. Anim. Nutr. 2016;52:39–42. [Google Scholar]
- Zhang Y.Y., Wang B.W., Ge W.H., Zhang M.A., Yue B., Zhang H.W. Linoleic acid on growth performance, slaughter performance, meat quality and nutrient availabilities of meat geese aged from 5 to 16 weeks. Chin. J. Anim. Nutr. 2016;28:3473–3482. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.