Abstract
Heat stress is a broiler welfare issue and economic deficit to the broiler industry. Water atomizing with three-dimensional forced ventilation, a holding treatment after summer transport for broiler, has been proved to significantly improve water holding capacity of fresh meat. However, effectiveness of this treatment on water retention after freeze-thaw needs to be conducted. Therefore, the objective of this study was to assess whether water atomizing with forced ventilation could increase freeze-thaw meat quality after birds slaughtering. Arbor Acres broiler (n = 105), undergoing 32°C ambient temperature transport, was randomly categorized into 3 treatments: 1) T group, which underwent 45-min transport without rest; 2) TR group, which underwent 45-min transport with 1-h rest; and the 3) TWFR group, which underwent 45-min transport followed by 15-min water atomizing with three-dimensional forced ventilation and 45-min rest. All birds were hot-deboned within 30-min postmortem. A total of 105 breast fillets were collected and split into halves, which left part fillets were kept in 4°C and for meat analysis, the other part fillets, marked with T-F, TR-F, and TWFR-F, were frozen (−18°C) for 1 mo and then thawed overnight for meat quality analysis. Regardless of fresh or frozen treatment among 3 groups, TWFR has the highest pH which was more than 6.01 (P < 0.05). The L* value, drip loss, and cooking loss of TWFR were significantly lower compared to T and TR groups in both fresh and frozen breast fillets (P < 0.05). Compared with the T group, the TWFR meat shows closely microscopic structure which means less water loss channel. The impedance amplitude of the fresh meat was significantly higher than that of the frozen-thawing meat (P < 0.05). TWFR-treated meat has significantly higher impedance module than T and TR meat at 50 Hz frequency region, for both fresh or thawed meat. Among 6 treatments, TWFR fresh meat has significantly highest Q (modulus change ratio) value (P < 0.05). These results indicate that TWFR treatment for 15 min after transport can improve meat quality, which may be due to the improved welfare of broilers transported in hot summer months.
Key words: water atomizing spray, forced ventilation, frozen-thaw, water retention, impedance properties
Introduction
Freezing is one of the most important and widely used preservation methods to prolong the shelf-life of meat, which can retain meat quality and safety for processors and consumers. However, some detrimentally physical, biochemical, and physicochemical changes, such as freeze-thaw water loss, may happen after thawing of frozen meat. Meat species and freezing rate rank high for the possibility of causing poor water retention of thawed meat (Leygonie et al., 2012). Other research showed that freeze-thaw can be used on breast meat before portioning and vacuum-tumbling marination without negative effect on marinade absorption, marinade retention, or cook loss (Bowker and Zhuang, 2017). However, little is known on the preslaughtering measure to the water retention quality of poultry meat after freeze-thaw, compared to the knowledge on the impact of preslaughter treatments on the fresh meat quality (Jiang et al., 2015).
Among possible preslaughtering factors of decreasing water holding capacity of meat, heat stress is one of the most important environmental stressors that lead to deterioration of meat quality including pH, cook loss, shear value (Holm and Fletcher, 1997, Zhang et al., 2012, Song and King, 2015, Zaboli et al., 2019). Preslaughter chronic heat stress could result in skeletal muscle damage with greater myosin and sarcoplasmic proteins denaturation as well as collagen weakening (Sandercock et al., 2001, Li et al., 2015). Reductions in meat protein functionality lead to reduced yield during further processing procedures such as marination, tumbling, and cooking. Controlling stress response is crucial to animal welfare and meat quality (Jacobs et al., 2017, Zhang et al., 2019). Therefore, we constructed a novel facility (Wang et al., 2016), which is a closed area equipped with forced ventilation and water atomizing sprays, as a strategy to improve broiler welfare during transportation by reducing the effects of heat stress during holding at the processing plant.
This research is the further application of our device in poultry meat freeze-thaw quality assurance. Freeze-thaw processes are known to have negative impacts on the water holding capacity of meat through tissue damage by the formation of extracellular ice crystals. This structural change of biological tissues can be reflected by changes in impedance characteristics (Chen et al., 2016, Chen et al., 2017). Electrical impedance detection is fast and nondestructive to solid, liquid, semi-liquid food samples. In recent years, more and more studies have taken impedance properties as an index to detect the quality of meat (Yu et al., 2004, Chen et al., 2016, Chen et al., 2017). The study of impedance characteristics of pork shows that freezing damage to biological tissues can cause significant changes in their impedance characteristics (Yu et al., 2004).
With the aim to assess the effects of forced ventilation cooling system during holding of broilers transported under heat stress on breast meat quality, we conducted this study using water atomizing and 3-dimensional forced ventilation facilities after summer transportation with the detection methods of electrical impedance and microscopic structure.
Materials and methods
Birds Management and Sample Preparation
In this experiment, total number of 105 (5 replicates with 7 birds × 3 treatments) 45-D old mixed-sex Arbor Acres broilers were used. The broilers were transported in a truck for 45 min from the farm to the processing plant under 32°C ambient temperature. Upon arrival at the slaughterhouse, 5 fully loaded containers trucks (5 replicates, all on the same day but from different farms) were treated with 3 approaches: the broilers of T group was slaughtered without rest, and the TR group was rested for 1 h with temperature of 29°C and a relative humidity of 60% under quiet area, while TWFR group was treated with 15 min water atomizing sprays with forced ventilation (0.05 mm diameter water droplet and wind speed of 2.5 m/s) and 45-min rest. The water-misting spray with forced ventilation treatment was conducted in a closed shed. Three-dimensional forced ventilation is characterized by the coordination of 2 processes: 1) supplying air from 6 fans placed on the right and left sides of the shed wall and 2) providing updraught air from a fan placed on the roof. Water atomizing sprays were completed with 6 sprinkle nozzles that permit a 0.05 mm size of water-misting sprays at a pressure of 0.6 MPa. The ambient temperature of the water-misting sprays with forced ventilation shed was 26°C with a relative humidity of 70%. All procedures were approved by the Animal Care and Use Committee of the Food Science College of Nanjing Agricultural University. All birds were stunned electrically (15 V: alternating current, 750 Hz for 10 s for each one) and slaughtered immediately. Within 30-min postmortem, both pectoralis major muscles were removed at 4°C and then were brought to the laboratory for the detection of meat quality at 24-h postmortem. Whole-breast fillets were split into right and left parts; all right-part fillets were tagged as fresh group with 3 different transportation groups (T, TR, TWFR), and all left-part fillets were frozen under −18°C for 1 mo, followed by thawing at +4°C for 16 h, marked with T-F, TR-F, TWFR-F, respectively.
pH
The pH of breast meat was determined at 24-h postmortem for fresh meat or thoroughly thawed meat. Approximately 5 g of minced breast meat was homogenized in 45 mL of ice-cold buffer containing sodium iodoacetate (5 mmol/L) and potassium chloride (150 mmol/L) with pH 7.0 using an Ultra Turrax T25 (IKA, Germany) at 6,000 rpm for 30 s. The pH of the homogenate was detected using a pH meter (FE-20, Mettler-Toledo Instruments Co., Ltd., Zurich, Switzerland).
Color
The meat color of cranial, medial surface (bone side) was assessed at 24-h postmortem or the time of complete thaw using Minolta CR-400 (illuminate D and 65° standard observer) device (Minolta Camera Co., Osaka, Japan). The color values L* (lightness), a* (redness), and b* (yellowness) were expressed as average of these 3 measurements.
Drip Loss
Each fresh meat sample was trimmed into a specific shape (1 cm × 1 cm × 3 cm) and then suspended on a hook from the lid of an air-tight container for 24 h at 4°C. Drip loss was expressed as a percentage of the weight loss in 24 h compared with the initial sample weight.
Cook Loss
All breast fillets were placed into a thin-walled plastic bag and then cooked in an 80°C water bath until the internal temperature reached 75°C. After cooking, the package was transferred from the water bath to tap water for 20 min. The muscles were then obtained from the bags, followed by drying with filter paper, and then weighed. Cook loss was expressed as the percentage change of weight before and after cooking and was calculated from the average of 3 measurements.
Thawing Loss
After 1-mo freezing, all frozen samples were thawed at 4°C for 16 h. Thawing loss was calculated as a percentage of weight loss before and after thawing.
Impedance Measurements
Impedance of fresh or frozen-thawed samples were measured using an LCR electric bridge (AT2827-1, Tonghui Electricity Technology Co., Ltd., Changzhou, China) as described by Chen et al. (2016). Briefly, the impedance at 15 frequencies from 50 to 200 kHz (0.05, 0.06, 0.08, 0.1, 0.12, 0.15, 0.2, 0.25, 0.3, 0.4, 1, 1.2, 50, 100, 200 kHz) was measured, followed by calculating the magnitude and phase angle. Then, the modulus change ratio (P value) was defined as Q = (ZL−ZH) × 100/ZH, where ZL and ZH are the modulus of the samples at 50 Hz and 200 kHz, respectively.
Light Microscopy
Light microscopy of fresh or frozen-thawed sample measurements were performed with the method described in the following. Muscle samples at 24-h postmortem or the time of completely thawed were prefixed in 10% formalin for 10 h, dehydrated in 75, 85, 95, and 100% ethanol solution followed by xylene. After embedding in paraffin, samples were cut into 4- to 6-μm-thick sections and then were stained with hematoxylin and eosin. Observation of microscopic image was achieved by using a light microscope with the magnification of 200× (Carl Zeiss, Axio Scope.A1, Germany).
Statistical Analysis
Using Proc MEANS and Proc ANOVA in SAS (version 9.2; SAS Institute Inc., Cary, NC), descriptive statistics and one-way analysis of variance with a significant level of P < 0.05 were performed with the mean value of each broiler as a replicate. Two effects (holding treatment and frozen treatment) were evaluated using multiple comparisons by Duncan's least significant difference, without regard to interaction.
Results and discussion
Water Holding Capacity
Although 7 birds/rep is a small sample size that is not directly relevant on a commercial scale, our aim is to discover the relationship between preslaughter procedure and meat quality from the aspect of scientific research. TWFR treatment shows relieving effect for both fresh and frozen-thawed meat (Table 1). This water holding capacity increase should be attributed to the preslaughter welfare improvement of thermal microenvironment on holding stage (Sandercock et al., 2001, Jiang et al., 2015). Summer transportation, especially on afternoon, plays an important role on the chicken heat stress that can make 1.04% and 1.21% higher water loss for drip loss and cooking loss, respectively (Zhu et al., 2011, Zhang et al., 2012, Song and King, 2015). In the summer, the high-temperature transportation before slaughter will affect the metabolism of poultry and eventually lead to poultry meat inferior in texture, water holding capacity, color, pH, and other basic quality (Sandercock et al., 2001, Zaboli et al., 2019). Water holding capacity is an important index to evaluate meat quality, which is reflected by drip loss, cooking loss, and thawing loss. Among 3 treatments, TWFR exhibited highest water holding capacity value for both fresh and thawed meat (P < 0.05). TR treatments decreased drip loss and cook loss of fresh meat compared with T treatments (P < 0.05). However, there was no significant thawing loss and cooking loss difference between T and TR treatments for frozen-thawed meat (P > 0.05). In our study, T-treated meat lost 4.81% and 5.66% more moisture than TWFR-treated meat during cooking for fresh and frozen-thawed condition, respectively. Therefore, it can be found that the water holding capacity of the meat can be improved by TWFR treatment after summer transportation.
Table 1.
Effects of preslaughtering TWFR treatment after transport during summer on water holding capacity of broiler muscle.
| Treatments | Drip loss (%)m | Thawing loss (%) | Cook loss (%) |
|---|---|---|---|
| T | 7.67 ± 0.54a | - | 17.47 ± 2.40c |
| TR | 5.12 ± 0.28b | - | 15.25 ± 2.20d |
| TWFR | 3.65 ± 0.35c | - | 12.66 ± 1.54e |
| T-F | - | 11.67 ± 1.51a | 23.79 ± 2.21a |
| TR-F | - | 10.49 ± 1.32a,b | 22.24 ± 1.65a,b |
| TWFR-F | - | 4.27 ± 0.66c | 18.13 ± 2.02c |
a,b,c,d,eMeans (n = 9) in the same column with no common superscript differ significantly (P < 0.05). Error bars represent the standard deviation of 3 determinations.
Abbreviations: T-group, 45-min transport group; TR-group, 45-min transport with 1-h rest; TWER-group, 45-min transport with 15-min water atomizing sprays with forced ventilation and 45-min rest. T-F, TR-F, and TWFR-F are the above groups frozen under −18°C for 1 mo.
pH and Color
Means of pH and color parameters from breast meat of T-, TR-, and TWFR-treated broiler chickens are presented in Table 2. The T treatment meat showed the lowest pH (5.64) and highest L* value (57.18) and the value of above 2 index for T treatment meat has significant difference compared with the value of TWFR meat (P < 0.05). TWFR meat had significantly higher pH and L* value compared with T and TWFR meat (P < 0.05). According to the criteria values for normal (46 < L∗< 53, 5.7 < pH24 h < 6.1) and PSE-like (L*> 53, pH24 h < 5.7) meat, T-treated meat are consistent with the general characteristic for PSE-like meat in poultry (Zhang and Barbut, 2005). pH is a very important factor in determining meat quality. Lactic acid is excessively produced and accumulated in the muscle under heat stress, resulting in rapidly declining pH (Zaboli et al., 2019). Heat stress causes a decrease in meat pH due to enzymatic changes in physiological metabolic processes. For example, increased activity of creatine kinase is an indicator of muscle damage (myopathy) and the malfunction of sarcolemma in muscle cells, which could be associated with the disturbance of intracellular Ca2+ homeostasis in the muscle (Zaboli et al., 2019). Higher pH of TWFR treatment meat of our study suggested that this treatment can reduce the initial rate of glycolysis after slaughter and can reduce the rate of pH decline. Researchers found that the higher pH of muscle, the more molecular water that can be combined with protein, and then the less water loss (De la Fuente et al., 2010).
Table 2.
Effects of preslaughtering TWFR treatment after transport during summer on pH and color of broiler muscle.
| Treatments | pH | L* | a* | b* |
|---|---|---|---|---|
| T | 5.64 ± 0.18b,c | 57.18 ± 2.78a | 3.57 ± 0.36c,d | 10.54 ± 1.27b |
| TR | 5.73 ± 0.14b | 56.47 ± 2.47a | 4.34 ± 0.56b,c | 10.75 ± 1.33b |
| TWFR | 6.04 ± 0.12a | 49.75 ± 2.13b | 4.73 ± 0.21b | 8.66 ± 1.12c |
| T-F | 5.56 ± 0.19c | 56.85 ± 1.54a | 3.24 ± 0.22d | 13.26 ± 1.35a |
| TR-F | 5.70 ± 0.15b | 56.26 ± 1.75a | 3.66 ± 0.31c,d | 11.23 ± 0.97b |
| TWFR-F | 6.01 ± 0.28a | 49.30 ± 1.30b | 5.92 ± 0.27a | 9.97 ± 1.40b |
a,b,c,dMeans (n = 9) in the same column with no common superscript differ significantly (P < 0.05). Error bars represent the standard deviation of 3 determinations.
Abbreviations: T-group, 45-min transport group; TR-group, 45-min transport with 1-h rest; TWER-group, 45-min transport with 15-min water atomizing sprays with forced ventilation and 45-min rest. T-F, TR-F, and TWFR-F are the above groups frozen under −18°C for 1 mo.
Meat color is an important indicator to distinguish abnormal, PSE (pale, soft, exudative) or DFD (dark, firm, dry) meat. In this study, the breast L* value of T treatment for fresh and frozen-thawed group reached 57.18 and 56.85, respectively. According to the provisions (Qiao et al., 2001), if 24-h slaughter meat L* greater than 53, the sample can be determined as PSE category. The L* values of TWFR group, for fresh and frozen-thawed group, are 49.75 and 49.30, respectively, which means that, TWFR treatment is effective to improve the meat color of birds suffered from high temperature transportation in summer. The results of our study are similar to former researcher that found the reduced brightness of chicken breasts and the decreased incidence of PSE if shower was available (Guarnieri et al., 2004).
Impedance
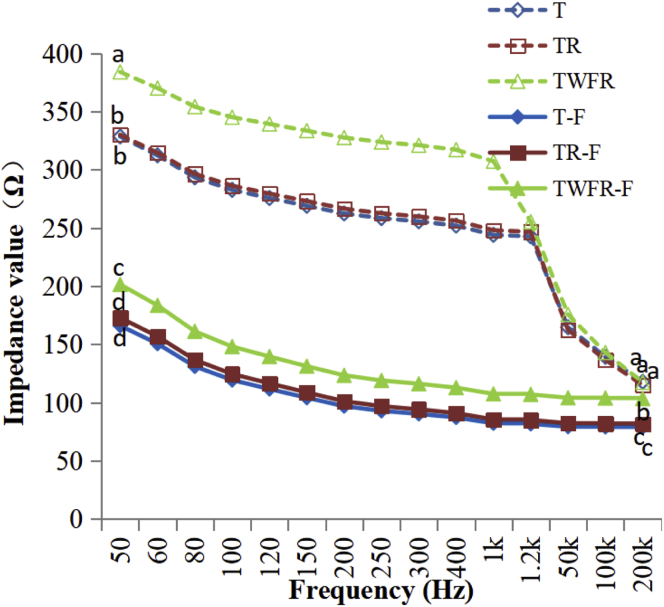
As shown in Figure 1, the impedance module of fresh and frozen-thawed chicken breast meat samples decreased with the increasing frequency from 50 Hz to 200 kHz. Compared to frozen-thawed meat, fresh meat shows sharper downward trend with the increasing frequency. Statistical analysis revealed that fresh meat had significantly higher impedance module than that of frozen-thawed meat at frequencies of 50 Hz and 200 kHz (P < 0.05). Previous studies on fish muscle also found this impedance decrease phenomenon due to frozen storage (Fuentes et al., 2013). Low-frequency region, between 400 Hz and 2 kHz, has been used to establish quality assessment relevance for process of meat (Pliquett, 2010). In our research, TWFR-treated fresh or frozen-thawed meat has significantly higher impedance module than T and TR meat during low-frequency region. However, impedance module of 200 kHz cannot effectively distinguish 3 fresh samples. The Q value of the fresh and frozen-thawed samples is shown in Table 3. The Q value of fresh meat was significantly higher than that of frozen-thawed samples. Among 6 treatments, TWFR fresh meat has significantly highest Q value (P < 0.05) that reaches 228.04. This may be due to integrity of chicken tissue structure brought by TWFR. By contrast, heat stress or frozen-thawed will lead to the destruction of the tissue structure and then relative change in impedance values. Research on the impedance characteristics of frozen Tibetan chicken shows that the impedance amplitude of the same frequency decreases with the extension of frozen storage time (Chen et al., 2017). Similar results were obtained, and the impedance of frozen-thawed chicken was significantly lower than that of fresh meat. Freezing destroys the structure of biological tissue, damages the structure of biological cell membrane (Hansen et al., 2004), increases the number of free electrons in the tissue fluid, and decreases the resistance of the cell membrane. In the long process of freezing, ice crystal growth and recrystallization may further damage the structure of biological tissue, lipid oxidation, and protein denaturation and dielectric environment of the organizations causes further changes, resulting in muscle tissue impedance decrease after thawing.
Figure 1.
Effects of preslaughtering TWFR treatment after transport during summer on impedance value of broilers breast meat. a,b,cMeans (n = 9) at the same frequency with no common superscript differ significantly (P < 0.05). T-group, 45-min transport group; TR-group, 45-min transport with 1-h rest; TWER-group, 45-min transport with 15-min water atomizing sprays with forced ventilation and 45-min rest. T-F, TR-F, and TWFR-F are the above groups frozen under −18°C for 1 mo.
Table 3.
Effects of preslaughtering TWFR treatment after transport during summer on Q value of broiler muscle impedance.
| Treatments | T | TR | TWFR | T-F | TR-F | TWFR-F |
|---|---|---|---|---|---|---|
| Q value | 179.23 ± 13.97b | 187.83 ± 18.62b | 228.04 ± 23.86a | 110.79 ± 13.55c | 112.21 ± 15.58c | 95.80 ± 12.77d |
a,b,c,dMeans (n = 9) in the same line with no common superscript differ significantly (P < 0.05). Error bars represent the standard deviation of 3 determinations.
Abbreviations: T-group, 45-min transport group; TR-group, 45-min transport with 1-h rest; TWER-group, 45-min transport with 15-min water atomizing sprays with forced ventilation and 45-min rest. T-F, TR-F, and TWFR-F are the above groups frozen under −18°C for 1 mo.
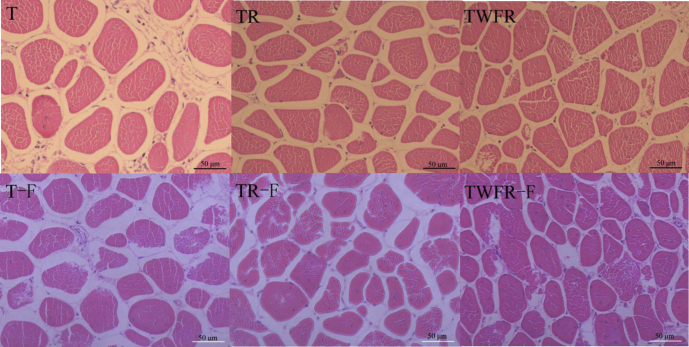
Microstructure
Figure 2 shows the effects of TWFR after the summer transportation on the microstructure of the muscle after 24-h postmortem or a month frozen storage. Loose structure of fresh meat muscle of T group can be clearly observed. High contraction of the samples may form a water channel, which allows water easily flow from the myofibrillar compartment to the extracellular region. When the discharged liquid is enough, a drop is formed. By contrast, TR and TWFR can significantly reduce muscle contraction evidenced by more closely arranged microscopic structure. Freeze-thaw created structure changes to meat sample. After thawing, the fractured myofibrils morphological became pronounced in the T group and the interstices between the muscle fibers became larger compared with nonfreezing T sample. Although the tissue damage during the thawing process is inevitable, we can still find that the tissue damage to the TWFR group (frozen-thaw treatment) is not obvious. This may be probably due to the avoidance of serious heat stress, which can cause more shrinking and depolymerization of myofilaments and Z-lines disorganization and leads to increased collapse of the sarcomere structure compared with normal meat (Wilhelm et al., 2010). This abnormal structure indicates a great variation in the environment of the water proton population of the meat system and drive the migration of water to form water loss.
Figure 2.
Effects of preslaughtering TWFR treatment after transport during summer on light microscopy of broilers breast meat. T-group, 45-min transport group; TR-group, 45-min transport with 1-h rest; TWER-group, 45-min transport with 15-min water atomizing sprays with forced ventilation and 45-min rest. T-F, TR-F, and TWFR-F are the above groups frozen under −18°C for 1 mo.
Conclusion
In conclusion, water-misting spray and three-dimensional ventilation can significantly improve the water retention of broiler by reducing drip loss,cooking loss, and thawing loss of broiler. Further impedance and microstructure study have found that the improvement of water retention of meat is achieved mainly by more intact structure even after frozen-thawed treatment, and so fluidity of water in meat decreased.
Acknowledgments
This work was supported by Fundamental Research Funds for the Central Universities (KYDS201812), the National Natural Science Foundation (31571769), and China Agriculture Research System (CARS-41). The authors certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
References
- Bowker B., Zhuang H. Freezing-thawing and sub-sampling influence the marination performance of chicken breast meat. Poult. Sci. 2017;96:3482–3488. doi: 10.3382/ps/pex117. [DOI] [PubMed] [Google Scholar]
- Chen T.H., Zhu Y.P., Wang P., Han M.Y., Wei R., Xu X.L., Zhou G.H. The use of the impedance measurements to distinguish between fresh and frozen-thawed chicken breast muscle. Meat Sci. 2016;116:151–157. doi: 10.1016/j.meatsci.2016.02.003. [DOI] [PubMed] [Google Scholar]
- Chen T.H., Zhu Y.P., Han M.Y., Wang P., Wei R., Xu X.L., Zhou G.H. Classification of chicken muscle with different freeze-thaw cycles using impedance and physicochemical properties. J. Food Eng. 2017;196:94–100. [Google Scholar]
- De la Fuente J., Sanchez M., Perez C., Lauzurica S., Vieira C., De Chavarri E.G., Diaz M.T. Physiological response and carcass and meat quality of suckling lambs in relation to transport time and stocking density during transport by road. Animal. 2010;4:250–258. doi: 10.1017/S1751731109991108. [DOI] [PubMed] [Google Scholar]
- Fuentes A., Masot R., Fernandez-Segovia I., Ruiz-Rico M., Alcaniz M., Barat J.M. Differentiation between fresh and frozen-thawed sea bream (Sparus aurata) using impedance spectroscopy techniques. Innov. Food Sci. Emerg. 2013;19:210–217. [Google Scholar]
- Guarnieri P.D., Soares A.L., Olivo R., Schneider J.P., Macedo R.M., Ida E.I., Shimokomaki M. Preslaughter handling with water shower spray inhibits PSE (pale, soft, exudative) broiler breast meat in a commercial plant. biochemical and ultrastructural observations. J. Food Biochem. 2004;28:269–277. [Google Scholar]
- Hansen E., Lauridsen L., Skibsted L.H., Moawad R.K., Andersen M.L. Oxidative stability of frozen pork patties: effect of fluctuating temperature on lipid oxidation. Meat Sci. 2004;68:185–191. doi: 10.1016/j.meatsci.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Holm C.G.P., Fletcher D.L. Antemortem holding temperatures and broiler breast meat quality. J. Appl. Poult. Res. 1997;6:180–184. [Google Scholar]
- Jacobs L., Delezie E., Duchateau L., Goethals K., Tuyttens F.A.M. Impact of the separate pre-slaughter stages on broiler chicken welfare. Poult. Sci. 2017;96:266–273. doi: 10.3382/ps/pew361. [DOI] [PubMed] [Google Scholar]
- Jiang N.N., Xing T., Wang P., Xie C., Xu X.L. Effects of water-misting sprays with forced ventilation after transport during summer on meat quality, stress parameters, glycolytic potential and microstructures of muscle in broilers. Asian-Australas. J. Anim. Sci. 2015;28:1767–1773. doi: 10.5713/ajas.15.0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leygonie C., Britz T.J., Hoffman L.C. Impact of freezing and thawing on the quality of meat: review. Meat Sci. 2012;91:93–98. doi: 10.1016/j.meatsci.2012.01.013. [DOI] [PubMed] [Google Scholar]
- Li K., Zhao Y.Y., Kang Z.L., Wang P., Han M.Y., Xu X.L., Zhou G.H. Reduced functionality of PSE-like chicken breast meat batter resulting from alterations in protein conformation. Poult. Sci. 2015;94:111–122. doi: 10.3382/ps/peu040. [DOI] [PubMed] [Google Scholar]
- Pliquett U. Bioimpedance: a review for food processing. Food Eng. Rev. 2010;2:74–94. [Google Scholar]
- Qiao M., Fletcher D.L., Smith D.P., Northcutt J.K. The effect of broiler breast meat color on pH, moisture, water-holding capacity, and emulsification capacity. Poult. Sci. 2001;80:676–680. doi: 10.1093/ps/80.5.676. [DOI] [PubMed] [Google Scholar]
- Sandercock D.A., Hunter R.R., Nute G.R., Mitchell M.A., Hocking P.M. Acute heat stress-induced alterations in blood acid-base status and skeletal muscle membrane integrity in broiler chickens at two ages: implications for meat quality. Poult. Sci. 2001;80:418–425. doi: 10.1093/ps/80.4.418. [DOI] [PubMed] [Google Scholar]
- Song D.J., King A.J. Effects of heat stress on broiler meat quality. Worlds Poult. Sci. J. 2015;71:701–709. [Google Scholar]
- Wang P., Zhao Y., Jiang N., Li K., Xing T., Chen L., Wang X., Tang Y., Xu X. Effects of water-misting spray combined with forced ventilation on heat induced meat gelation in broiler after summer transport. Poult. Sci. 2016;95:2441–2448. doi: 10.3382/ps/pew203. [DOI] [PubMed] [Google Scholar]
- Wilhelm A.E., Maganhini M.B., Hernández-Blazquez F.J., Ida E.I., Shimokomaki M. Protease activity and the ultrastructure of broiler chicken PSE (pale, soft, exudative) meat. Food Chem. 2010;119:1201–1204. [Google Scholar]
- Yu T.H., Liu J., Zhou Y.X. Using electrical impedance detection to evaluate the viability of biomaterials subject to freezing or thermal injury. Anal. Bioanal. Chem. 2004;378:1793–1800. doi: 10.1007/s00216-004-2508-2. [DOI] [PubMed] [Google Scholar]
- Zaboli G., Huang X., Feng X., Ahn D.U. How can heat stress affect chicken meat quality? -a review. Poult. Sci. 2019;98:1551–1556. doi: 10.3382/ps/pey399. [DOI] [PubMed] [Google Scholar]
- Zhang L., Barbut S. Rheological characteristics of fresh and frozen PSE, normal and DFD chicken breast meat. Br. Poult. Sci. 2005;46:687–693. doi: 10.1080/00071660500391516. [DOI] [PubMed] [Google Scholar]
- Zhang Z.Y., Jia G.Q., Zuo J.J., Zhang Y., Lei J., Ren L., Feng D.Y. Effects of constant and cyclic heat stress on muscle metabolism and meat quality of broiler breast fillet and thigh meat. Poult. Sci. 2012;91:2931–2937. doi: 10.3382/ps.2012-02255. [DOI] [PubMed] [Google Scholar]
- Zhang L., Li J.L., Wang X.F., Zhu X.D., Gao F., Zhou G.H. Attenuating effects of guanidinoacetic acid on preslaughter transport-induced muscle energy expenditure and rapid glycolysis of broilers. Poult. Sci. 2019;98:3223–3232. doi: 10.3382/ps/pez052. [DOI] [PubMed] [Google Scholar]
- Zhu X., Ruusunen M., Gusella M., Zhou G., Puolanne E. High post-mortem temperature combined with rapid glycolysis induces phosphorylase denaturation and produces pale and exudative characteristics in broiler Pectoralis major muscles. Meat Sci. 2011;89:181–188. doi: 10.1016/j.meatsci.2011.04.015. [DOI] [PubMed] [Google Scholar]