Abstract
Myo-inositol (MI) has gained relevance in physiology research during the last decade. As a constituent of animal cells, MI was proven to be crucial in several metabolic and regulatory processes. Myo-inositol is involved in lipid signaling, osmolarity, glucose, and insulin metabolism. In humans and rodents, dietary MI was assessed to be important for health so that MI supplementation appeared to be a valuable alternative for treatment of several diseases as well as for improvements in metabolic performance. In poultry, there is a lack of evidence not only related to specific species-linked metabolic processes but also about the effects of dietary MI on performance and health. This review intends to provide information about the meaning of dietary MI in animal metabolism as well as to discuss potential implications of dietary MI in poultry health and performance with the aim to identify open questions in poultry research.
Key words: myo-inositol, phytate, metabolism, physiology, poultry nutrition
Introduction
Inositol (cyclohexane-1,2,3,4,5,6-hexol) is a sugar cyclic polyalcohol whose epimerization of its hydroxyl groups generates 9 possible stereoisomeric forms (myo-inositol [MI], scyllo-inositol, muco-inositol, D-chiro-inositol, L-chiro-inositol, neo-inositol, allo-inositol, epi-inositol, and cis-inositol). Among these forms, myo-inositol (cis-1,2,3,5-trans-4,6-cyclohexanehexol) is the predominant form occurring in nature (Turner et al., 2002, Pasta et al., 2015, Thomas et al., 2015). Myo-inositol has been suggested to be a member of the vitamin B group; however, this assumption was refuted because monogastric animals and humans could rely on their cellular biosynthesis (Regidor and Schindler 2016). Requirements for newborn human babies were generally met by body-own synthesis (Brown et al., 2009). However, dietary MI was found to effectively ameliorate certain endocrine diseases such as diabetes and insulin resistance (Croze and Soulange, 2013). Thus, MI may be considered as a semiessential substance, which could be limited at certain physiological and pathophysiological conditions.
It is synthesized de novo from glucose and by catabolism of phosphatidylinositol (PI), phosphoinositides (PIP), and inositol phosphates (InsP). Subsequently, it is utilized by the diacylglycerol pathway to generate new PIP (Di Daniel et al., 2009). Finally, in mammalians, MI is degraded in the kidney (Hankes et al., 1969, Chang et al., 2015a). In addition to the endogenous sources, MI absorbed by the gut epithelium was discussed as an essential source for MI in animal health and performance (Huber, 2016). Myo-inositol has strong physiological importance in a plethora of processes. Among its principal functions, MI participated in cellular signaling as a precursor of relevant biological compounds, including PIP and InsP (Beemster et al., 2002, Indyk et al., 2016). Myo-inositol also acted as an osmolyte in specific tissues, such as brain and kidney medulla, where osmolarity had a crucial biological meaning (Aouameur et al., 2007). It had also been postulated that MI is a modulator of glucose homeostasis and insulin regulation in humans and animal models of insulin resistance (Croze and Soulage, 2013). Several studies showed evidence for MI absorption existing in birds (Isaacks et al., 1982, Isaacks et al., 1989, Kohlmeier, 2003, Lee and Bedford, 2016, Sommerfeld et al., 2018b); however, the relevance of dietary MI in poultry metabolism is unknown. A few studies using MI as a dietary supplement resulted in variations of bone stability and general animal performance (Hegsted et al., 1941, Pearce, 1975, Zyła et al., 2004, Żyła et al., 2012, Żyła et al., 2013a, Cowieson et al., 2013, Pirgozliev et al., 2017, Lee et al., 2017, Farhadi et al., 2017). Scientific interest in the role of MI in poultry metabolism emerged after recent studies showed that the enzyme phytase is able to release MI from myo-inositol-6-phosphate (InsP6) in the gastrointestinal tract of poultry (Cowieson et al., 2015, Sommerfeld et al., 2018a, Pirgozliev et al., 2019). Knowledge about MI intestinal absorption, body-own transport, and metabolism in poultry is scarce, whereas more research has been conducted in other species including man. Therefore, the objective of this review was to summarize the published knowledge about MI metabolism in general and in particular for poultry and to discuss the potential implications of MI in poultry metabolism and nutrition.
Plant and animal sources of myo-inositol
Myo-inositol is contained in fresh fruits, vegetables, grains, meat, fish, eggs, milk, and many other foods (Clements and Darnell, 1980, Croze and Soulage, 2013). Hence, feed ingredients used in grain-based poultry nutrition provide some free MI; however, it had been widely reported that most of the MI in the organism that originated from the diet was obtained from gastrointestinal InsP6 degradation (Holub, 1986, Selle and Ravindran, 2007, Kim et al., 2017). A recent study analyzed the content of the InsP in 7 tree nuts and in 3 grains that are rich in phosphorus and used for animal feeding (Duong et al., 2017). Tree nut species such as Brazil nuts, walnuts, pistachios, hazelnuts, and cashews contained high levels of total InsP (20.08, 6.64, 6.51, 5.15, and 5.02 μmol/g, respectively) in comparison to macadamia and pecan nuts (3.55 and 2.63 μmol/g; Duong et al., 2017). Seeds of different cereal grains contained InsP in different concentrations, but most were present in the form of InsP6, whereas other InsP were present only in traces, except in seeds of barley (Rodehutscord et al., 2016a). For specific grain fractions, InsP levels of wheat aleurone and rice bran (63.85 and 97.36 μmol/g) were significantly higher than of corn germ (9.75 μmol/g). Percentage of InsP1 and InsP2 was higher in corn germ (3.4 and 4.6%, respectively) than in wheat aleurone (0.4 and 0.6%, respectively) and rice bran (0.1%). In these samples, total InsP levels ranged from 66.2 to 96.7% of the organic phosphorus content and from 58.4 to 80.3% of the total phosphorus content (Duong et al., 2017). Besides the sources and their intrinsic properties, factors such as soil type and agronomic details such as fertilization could also affect InsP6 concentrations in plant seeds (Rodehutscord and Rosenfelder, 2016). While these InsP in the feed are an important source of MI for poultry, the dephosphorylation process in the digestive tract is not complete. Some differently phosphorylated InsP were found in the terminal ileum of broilers even when the diet contained high phytase supplements (Sommerfeld et al., 2018a, Sommerfeld et al., 2018b). Hence, the amount of MI absorbed in the intestine cannot be predicted from InsP content of the feed.
Myo-inositol transport and cellular metabolism
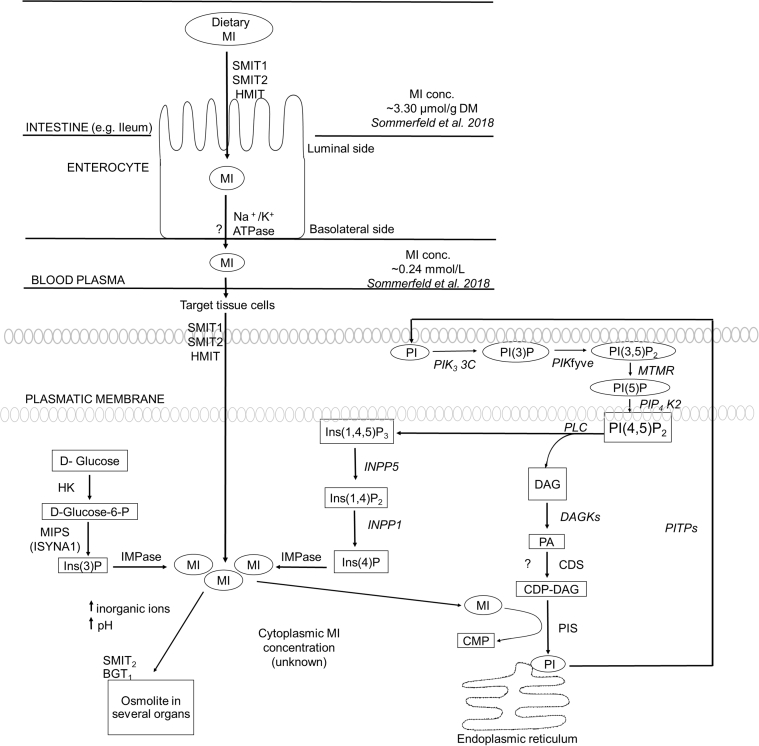
Pathways of MI absorption, transport, and cellular metabolism are summarized in Figure 1. Chicken-specific knowledge about these pathways is also indicated.
Figure 1.
Absorption, transport, and cellular myo-inositol metabolism in KEGG Gallus gallus pathway (Kanehisa et al., 2017, Kanehisa et al., 2019). MI is incorporated into the cell by 3 specific ways: (1) It is transported by SMIT1/2 or HMIT in the enterocyte and subsequently transported to target cells, (2) it is synthesized de novo from glucose metabolism, and (3) it is recycled from InsP phosphorylation. In the cytoplasm, free MI is utilized as osmolyte or transported to endoplasmic reticulum to be converted into PI to synthesize new PI(4,5)P2; however, MI-associated pathways have been not characterized clearly in poultry. Concentrations of free MI in chicken have been reported at intestine and blood plasma; however, cellular values in different tissues remain unknown. Abbreviations: BGT1, betaine cotransporter 1; CDP-DAG, cytidine diphosphate diacylglycerol; CDS, CDP- diacylglycerol synthase; CMP, cytidine monophosphate; DAG, diacylglycerol; glucose-6-P, glucose-6-phosphate; DAGKs, diacylglycerol kinases; HK, hexokinase; HMIT, proton-linked myo-inositol transporter; IMPase, inositol monophosphatase; Ins(3)P, inositol 3-phosphate; Ins(4)P, inositol-4-phosphate; Ins(1,4)P2, inositol (1,4)-bisphosphate; I(1,4,5)P3, inositol-(1,4,5)tris-phosphate; INPP5, inositol-1,4,5-trisphosphate 5-phosphatase; INPP1, the inositol polyphosphate 1-phosphatase; MI, myo-inositol; PA, phosphatidic acid; MIPS (ISYNA1), inositol-3-phosphate synthase 1 isoform X1; Na+/K + -ATPase, sodium-potassium adenosine triphosphatase; pH, potential of hydrogen; MTMR, myotubularin-related protein; PI, phosphatidylinositol; PIP, phosphatidylinositol phosphate; PI(3)P, phosphatidylinositol 4-phosphate; PI(5)P, 1-phosphatidyl-1D-myo-inositol 5-phosphate; PI(3,5)P2, 1-phosphatidyl-1D-myo-inositol 3,5-bisphosphate; PI(4,5)P2, phosphatidylinositol-(4,5)bisphosphate; PIP4K2, phosphatidylinositol 5-phosphate 4-kinase type-2; PIS, phosphatidylinositol synthase; PITPs, phosphatidylinositol transfer proteins; PIK3 3C, phosphatidylinositol 3-kinase catalytic subunit type 3 isoform X2; PIKfyve, 1-phosphatidylinositol 3-phosphate 5-kinase isoform X1; PLC, phospholipase C; SMIT1/2, sodium-linked myo-inositol transporter 1 and 2.
Transepithelial Myo-Inositol Absorption in the Intestine and Transport Into Tissues
Dietary InsP were partially degraded by brush border membrane-associated endogenous phytases, phosphatases, pancreatic phospholipases, and microbial phytases, resulting in lower InsP and free MI in the digestive tract (Holub, 1986, Huber, 2016). Free MI was easily absorbed from the small intestine (Kohlmeier, 2003). It has been demonstrated in healthy humans that 99.8% of MI was absorbed in the gastrointestinal tract (Clements and Reynerston, 1977).
In rats, MI was transported by sodium and proton-dependent processes across the apical membrane of the enterocytes as the key step of MI absorption (Aouameur et al., 2007) as it was observed also in epithelial cells of rabbit kidney (Lahjouji et al. 2007). In chickens, mRNA of sodium/MI co-transporter 1 (SMIT1), sodium/MI co-transporter 2 (SMIT2), and H+/MI co-transporter (HMIT) has been found in jejunum and ileum, which suggested that all 3 transporters could be involved in intestinal MI absorption (Walk et al., 2018). Most likely, the majority of MI was transferred into the bloodstream (Lewin et al., 1976) by an unknown secondary active transport system across the basolateral side of enterocytes driven by the sodium/potassium (Na+/K+)-ATPase (Holub, 1986).
After intestinal absorption, MI can reach body tissues via the bloodstream (Lewin et al. 1976); hypothetically, a portion of absorbed MI could be catabolized in the liver (Lee and Bedford, 2016). Basal MI concentrations in the blood of chicken ranged from 0.19 to 0.28 mmol/l (Oshima et al., 1964, Cowieson et al., 2015, Schmeisser et al., 2017, Sommerfeld et al., 2018b). All animal cells appeared to contain MI; however, its concentrations varied in different tissues (Battaglia et al., 1961). It has been reported that broilers supplemented with 3% MI (total diet) increased total weight of liver (Pirgozliev et al., 2019); however, until now, no data about MI concentrations in poultry tissues were reported. Myo-inositol concentrations in mouse liver and kidney were about 0.5 and 3.5 μmol/g wet weight, respectively (Croze et al., 2015). Intraperitoneal injection of radioactively labeled MI in male rats revealed that organs such as thyroid, brain, liver, spleen, kidney, reproductive tract, pituitary, and prostate gland actively accumulated MI (Eisenberg and Bolden, 1963, Lewin et al., 1976). In contrast, adipose tissue and muscle accumulate less because of their limited de novo synthesis capacity (Lewin et al., 1976, Croze and Soulage, 2013), which will be explained in a later chapter. It is unknown the extent chicken tissues rely on MI biosynthesis. Entrance of MI into cells is transporter-dependent; SMIT1 and SMIT2 activity appeared to be induced by extracellular hyperosmolarity in cultured kidney cells (Bissonnette et al., 2008). Both transporters have been identified in many body tissues but predominantly in brain, intestine and kidney (Aouameur et al., 2007). The SMIT2, but not SMIT1, has been mainly located in kidney cortex where it potentially participated in MI reabsorption from the glomerular filtrate (Aouameur et al., 2007). The HMIT function (also known as GLUT 13) was restricted to brain inositol metabolism; however, mRNA (SLC2A13) has also been identified in rodent adipose tissue and kidney (Uldry et al. 2001) and in chicken intestine, kidney, and liver (Walk et al., 2018).
Cellular Synthesis of Myo-Inositol
As shown in Figure 1 and according to the KEGG's MI pathway for Gallus gallus (Kanehisa et al., 2017, Kanehisa et al., 2019), chicken MI is derived from de novo synthesis from glucose and generated from PIP and InsP (Di Daniel et al., 2009, Kanehisa et al., 2019). Regardless of the source, the ability of a cell to maintain sufficient levels of MI was crucial for the re-synthesis of PIPs and the maintenance and efficiency of signal transduction (Di Daniel et al., 2009). De novo synthesis of MI has been observed in rat liver, testis, kidney, and brain (Eisenberg and Bolden, 1963, Hauser and Finelli, 1963). As shown in Figure 1, MI was synthesized from D-glucose through 3 biochemical reactions. First, D-glucose was phosphorylated by hexokinase to glucose-6-phosphate; second, glucose-6-phosphate was isomerized by the NADH-dependent, cytosolic inositol-3-phosphate synthase to inositol 3-phosphate; and finally, inositol 3-phosphate was dephosphorylated to free MI by inositol-monophosphatase (IMPase) for further use in the PI synthesis pathway (Dinicola et al., 2017). Clements and Diethelm (1979) demonstrated this process was of quantitative importance; for instance, in human kidneys, around 4 g MI/d were synthesized.
Dephosphorylation of InsP was the other important route of cellular MI generation (Figure 1). The critical intermediates were Ins(1,4,5)P3 and Ins(1,3,4)P3, both isoforms of InsP3. Briefly, each isoform of InsP3 was dephosphorylated by the inositol-1,4,5-trisphosphate 5-phosphatase into Ins(1,4) P2. Ins(1,4)P2 was converted to Ins(4)P1 by the action of the inositol polyphosphate 1-phosphatase. InsP1 was then converted into free MI by IMPase (Downes and Macphee, 1990, Abel et al., 2001, Di Daniel et al., 2009). Free MI fueled the PI synthesis in the endoplasmic reticulum by PI synthase using cytidine diphosphate diacylglycerol (CDP-DAG) as educt (Downes and Macphee, 1990, Vance, 2015). Then, PI was transported to the plasmatic membrane by PI transfer proteins (Selitrennik and Lev, 2016, Muallem et al., 2017). In the plasmatic membrane, PI was phosphorylated to PIns(3)P, which in turn was converted into PIns(5)P and then into PIns(4,5)P2 (Kanehisa et al., 2019). PIns(4,5)P2 has been reported to be hydrolyzed to diacylglycerol (DAG) by phospholipase C. DAG phosphorylation by the DAG kinase to phosphatidic acid (Muallem et al., 2017) was a crucial step in generating new liponucleotide CDP-DAG via CDP-DAG synthase (phosphatidate cytidylyltransferase). All pathways are deposited as chicken-specific at https://www.genome.jp/kegg-bin/show_pathway?gga00562 (Kanehisa et al., 2017, Kanehisa et al., 2019).
Linkages Between Myo-Inositol and Glucose Metabolism
Myo-inositol and glucose metabolism are interlinked. There is a competition for sodium availability of the transport processes of MI (SMIT) and glucose (sodium-linked glucose transporter 1) as it was observed that elevated glucose concentrations in medium reduced MI uptake in rabbit peripheral nerve tissue (Greene and Lattimer, 1982). Furthermore, in cultured rat glomerular mesangial cells, Haneda et al. (1990) reported that an increase of glucose concentrations in the medium from 0 to 55 mmol/L decreased intracellular MI from about 12 nmol/mg protein to about 5 nmol/mg protein linearly. Increasing concentrations of MI in medium also decreased jejunal sodium-dependent glucose absorption in an ex vivo everted sac model (Chukwuma et al., 2016).
Myo-inositol could act as insulin mimetics and could play a relevant role in insulin-related diseases (Kim et al., 2014). Dietary MI has been demonstrated to reduce postprandial glucose levels and increase peripheral insulin sensitivity proved by indices in humans (Ortmeyer, 1996, Corrado et al., 2011). Some studies indicated that also inositol phosphoglycans (phospholipid-derived putative second messengers of insulin) might have an insulin-mimicking effect. In regard to mammalian species, detailed information about effects of MI on insulin metabolism can be found elsewhere (Larner et al., 2010, Croze and Soulage, 2013, Unfer et al., 2017, Bevilacqua and Bizzarri, 2018).
In chickens, a study conducted by Cowieson et al. (2013) showed MI added to a diet low in Ca and P increased blood glucose, insulin, and glucagon concentrations in comparison to a control diet. These findings may be explained by the aforementioned competition between MI and glucose for sodium to enable a secondary active transport (Greene and Lattimer, 1982). Dietary MI was shown to increase both the expression of protein kinase B (PKB/Akt) and the translocation of GLUT4 in presence of insulin in skeletal muscle of mice (Dang et al., 2010, Croze et al., 2012).Together with the up-regulation of PInsP3 through activation of the insulin receptor substrate proteins (Croze et al., 2012), it indicates that high concentrations of circulating MI could lead to increases in levels of insulin to activate the PI pathway.
Renal Myo-Inositol Catabolism and Handling
Catabolism of MI is essential for regulation of inositol homeostasis, and it occurs mainly in the kidney (Howard and Anderson, 1967, Hankes et al., 1969, Chang et al., 2015b) via myo-inositol oxygenase (MIOX; Thorsell et al., 2008). Myo-inositol oxygenase is a nonheme iron enzyme, which converts MI to D-glucuronic acid (Arner et al., 2001, González-Álvarez et al., 2017). The subsequent steps involve the conversion of D-glucuronic acid to D-xylulose-5-phosphate, which subsequently goes into the pentose phosphate pathway (Arner et al., 2004). End products are used for oxidative energy production (Hankes et al., 1969, Lewin et al., 1976). Renal myo-inositol handling included excretion of MI into the primary urine and reabsorption into the blood of about 98% of excreted MI (Sarashina et al., 2004).
Therefore, the kidney appears to be the most important organ in regulation of plasma inositol concentration in animals and humans (Holub, 1986). Higher glucose concentrations appeared to upregulate MIOX enzyme activity (Nayak et al., 2005). This regulation could be based (1) on polymorphisms in the promoter regions of MIOX gene (Yang et al., 2010), (2) on activation of several transcription factors by different forms of stress induced by hyperglycemia (Nayak et al., 2011), and (3) on posttranslational modification of MIOX. The latter is based on an increased phosphorylation of MIOX by kinases such as protein kinase A and C and the 3-phosphoinositide-dependent protein kinase 1 (Nayak et al., 2011). A significant concomitant effect caused by glucose-induced MIOX activity in human and porcine kidney cell cultures was the impairment of mitochondrial integrity which was based on increases in reactive oxygen species, on a higher apoptosis rate and on the reduction in autophagy processes (Zhan et al., 2015). It has not been reported whether these pathways are relevant in birds as well, and further research is necessary to clarify MI breakdown in poultry.
Intracellular Myo-Inositol Depletion
Intracellular MI depletion depends on intestinal MI absorption, cellular MI synthesis, efflux from organ cells, and increases of renal MI excretion (Croze and Soulage, 2013, Dinicola et al., 2017). Causes of cellular MI depletion are mainly associated with the reduction of IMPase, myo-inositol-3-phosphate synthase (MIPS), and glycogen synthase kinase 3 activity (Llewelyn, 2003, Harwood, 2005). Consequences of cellular MI depletion are the reduction of PIP and DAG concentrations, the decreases of Na+/K+ -ATPase activity (Azab et al., 2007, Deranieh and Greenberg, 2009), and also the impairment of cell development, transformation, and differentiation (Oishi et al., 1990, Steele et al., 1993, Hamada et al., 1996).
Intracellular MI depletion was observed to be associated with intracellular osmotic stress. An increase in intracellular osmolarity provoked MI release, and under chronic conditions, this led to intracellular MI depletion (Croze and Soulage, 2013). Furthermore, inhibitory substances like lithium and valproic acid most likely caused intracellular MI depletion. Lithium is a mood stabilizer that increased serotonin accumulation in the central nervous system but also blocked MI synthesis by inhibition of phosphatases inositol-1,4 bisphosphate 1-phosphatase (IPP) and IMPase (Harwood, 2005). Valproic acid (a branched, short-chain fatty acid) has been related to the inhibition of MIPS, blocking the conversion of glucose-6-phosphate to MI (Deranieh et al., 2015). Interestingly, it has been hypothesized that valproic acid and the concomitant depletion of MI led to an increase in the level of the phospholipid cardiolipin (Ju and Greenberg, 2003). Cardiolipin has been shown to be essential for the osmotic stability of the mitochondrial membrane, the maintenance of the mitochondrial membrane potential, and the oxidative phosphorylation efficiency (Jiang et al., 2000, Deranieh and Greenberg, 2009).
Physiological meaning of myo-inositol
Besides its insulin-mimetics properties, dietary MI appeared to be of biological importance for several metabolic functions. A deficiency of dietary MI was related to enhanced intestinal mucosa inflammation and apoptosis and to diminished cell proliferation, antioxidant capacity, and intestinal bacterial activity in grass carps (Li et al., 2017, Li et al., 2018). Dietary MI has also been considered crucial for lipid metabolism, bone formation, skeletal muscle metabolism, reproduction, and nervous system development in humans and animals. Furthermore, an artificial knock-out mouse model with total SMIT1 depletion (SMIT−/−) was used to determine the impact of MI for biological functions. The SMIT−/− mice died soon after birth due to respiratory failure because of a lack of functional surfactant (Hallman et al., 1985) but also because of malfunction of respiratory nerves (Chau et al., 2005); however, neonatal mortality was prevented by prenatal maternal MI supplementation (Chau et al., 2005). The SMIT−/− mice also expressed deficiencies of MI in brain, kidney, skeletal muscle, liver, and sciatic nerve causing detrimental impact on nerve conduction velocity (Chau et al., 2005), prenatal skeletal development, and postnatal bone remodeling (Dai et al., 2011).
Effects of Dietary Myo-Inositol on Lipid Metabolism
Although relationships between dietary MI and lipid metabolism have not been fully understood yet, it was hypothesized in recent years that MI could be relevant for adipocyte differentiation and for fatty acid metabolism. Dietary MI deficiency was associated with increases of liver triglycerides (TG) concentration in male rats (Hayashi et al., 1974).
Murine preadipocyte 3T3-L1 cells incubated with 10, 50, and 200 μmol/L MI increased differentiation in a dose-dependent manner but also enhanced capacity for lipid storage, glucose uptake, and decreased lipolysis rate (Kim et al., 2014). This was confirmed earlier by Croze et al. (2012), detecting that dietary MI decreased nonesterifed fatty acids in plasma of mice. Furthermore, it was shown that dietary MI increased plasma adiponectin concentrations which correlated negatively with TG content in liver of rats (Okazaki et al., 2018). Higher lipid storage was associated with activation of transcription factors (CCAAT/enhancer-binding protein α, peroxisome proliferator-activated receptor γ, and sterol regulatory element-binding protein 1c, higher extent of tyrosine phosphorylation and increased insulin receptor substrate 1, fatty acid synthase, and GLUT4 expression) (Kim et al., 2014). These results suggested that MI is an insulin mimetic also promoting adipose tissue lipid storage capacity and preventing ectopic fat deposition (Plows et al., 2017). In poultry, controversial effects of MI on lipid metabolism were reported. Whereas, Farhadi et al. (2017) showed MI supplementation (0.15% total diet) to a diet low in Ca and P did not change concentrations of total cholesterol and TG in 21-day-old broilers, Cowieson et al. (2013) showed that MI (0.15% total diet) added to a diet low in Ca and P decreased both total cholesterol and TG in comparison to a standard diet in 42 D old broilers. Differences in these results could be likely attributed to a plethora of reasons, one of them may be the duration of MI supplementation, which would suggests the importance of tracking dietary MI over lifespan as a key molecule for lipid metabolism in poultry; this from the fact basal total cholesterol and TG concentrations have shown to increase along broiler's life span (Prasad et al., 2009). Broilers supplemented with MI showed decreases in 5 phosphatidylcholines (PCaaC34:1, PCaaC36:1, PCaaC40:3, PCaaC36:1, PCaaC36:3) and 2 lysophosphatidylcholines (lysoPC C16:1 and lysoPC C18:1), both important components of cell membrane and plasma phospholipids (Gonzalez-Uarquin et al., 2019, accepted). It could be explained by the fact that more MI availability could increase the use of phosphatidic acid for PI synthesis at the expense of phosphatidylcholine. In hens, despite lack of conclusive evidence, dietary MI (0.1% total diet) added to a corn-based diet showed to decrease polyunsaturated fatty acids of 20 carbon atoms (C-20) and total lipids content in comparison to hens supplemented a diet low in nonphytate phosphorus (Żyła et al., 2012).
Effects of Dietary Myo-Inositol on Bone Formation
Myo-inositol could be associated with mineral absorption and bone mineralization. For example, in rats, supplementation of inositol alone by orogastric intubation (20 mg/100 g of BW) caused a 48% increase of 45Ca accumulation in bone within 24 h (Angeloff et al., 1977). Moreover, supplementations with arginine silicate inositol complex (Arg: 49.47%; silicone: 8.2%; inositol: 25%) significantly improved bone mineral density as well as Ca, P, and Mg concentrations in tibia ash in control and heat-stressed quails (Sahin et al., 2006). However, potential mechanisms of MI effects are unknown. In transgenic SMIT1−/− mice, SMIT1 deficiency caused reductions in postnatal bone mass and changes in bone morphometry generated by an impairment in embryonic bone development and remodeling. Furthermore, postweaning dietary MI supplementation partially rescued bone defects in adult SMIT1−/− mice (Dai et al., 2011). Myo-inositol supplementation by maternal milk in SMIT1−/− mice restored skeletal malformation developed during their embryonic phase. Even in healthy subjects, dietary MI improved bone structure and increased BW (Dai et al., 2011).
In poultry, significant increases in blood alkaline phosphatase, an enzyme related to osteoblastic activity, were reported after 3% MI (total diet) supplementation (Pirgozliev et al., 2019), suggesting that MI could reform new InsP at the intestinal lumen provoking new synthesis of alkaline phosphatase (Farhadi et al., 2017). Lee et al. (2017) detected that dietary MI tended to decrease bone strength, while Żyła et al., 2013a, Farhadi et al., 2017, and Sommerfeld et al. (2018b) found no changes in tibia ash content and thereby, most likely no changes in bone strength. Perhaps, the inconsistency of data from poultry studies was caused by different basal MI concentrations in the diet or by different concentrations of phosphorus and calcium in the diet. In this regard, a supplementation of monocalcium phosphate increased egg production and egg mass in comparison to hens fed MI (Żyła et al., 2012). Moreover, Żyła et al. (2004) found that supplementation of 0.1% MI (total diet) resulted in significant decreases of P retention. Interestingly, when MI was supplemented together with a high Ca/P ratio diet, toe ash concentration increased significantly.
Effects of Dietary Myo-Inositol on Skeletal Muscle Metabolism
Myo-inositol could affect glucose transporter and therefore glucose uptake in muscle cells. For example, oral application of MI to mice 30 min before an oral glucose tolerance test resulted not only in an increase of GLUT4 transporter translocation into skeletal muscle cell membranes compared with control but also in a lower glucose and insulin concentration in plasma (Dang et al., 2010). In SMIT1−/− mice, individuals supplemented with MI from birth until 10 weeks of age had higher levels of MI in skeletal muscle than individuals supplemented with MI until weaning only (Chau et al., 2005). Chickens fed a moderately phosphorus-deficient diet plus supplementation of microbial 6-phytase (1000 FTU/kg) increased blood MI concentrations in comparison to chickens fed a nonsupplemented diet. Furthermore, a higher breast meat weight and an increased expression of genes associated with muscle development (calmodulin/calcineurin and insulin-like growth factor) were observed. However, this could not be solely related to MI because lower InsP and free phosphate (Pi) might also influence muscle metabolism (Schmeisser et al., 2017).
Effects of Dietary Myo-Inositol on Reproduction
Free MI concentrations were significantly higher in testes than in plasma (Setchell et al., 1968). An importance of MI for male reproduction was considered based on the following points: (1) high levels of MIPS and IMPase exist within the testis of patient diagnosed with asthenozoospermia (Chauvin and Griswold, 2004), (2) MI functions in the regulation of osmolarity in Sertoli cells and seminal fluid, and (3) spermatozoa concentrations increase in patients with oligoasthenoteratozoospermia by MI supplementation (Condorelli et al., 2017). Sperms incubated with MI for 2 h had a significantly higher motility and a higher mitochondrial membrane potential, probably by increasing mitochondrial Ca2+ concentrations (Condorelli et al., 2011, Condorelli et al., 2012, Gulino et al., 2016). In human female patients with polycystic ovary syndrome, an oral dose of 2 g MI/twice a day increased the number of mature oocytes of top quality embryos and of successful pregnancies (Unfer et al., 2011). Data from laying hen studies are rare, and more research is needed in this field; however, dietary MI appeared to decrease egg production in comparison to a standard diet (Żyla, et al., 2012).
Effects of Dietary Myo-Inositol on Peripheral Nerve Function
Peripheral neuropathy, a damage of peripheral nerves, is common in diabetic patients and is accompanied by sensory loss and decreased nerve conduction velocity (Jolivalt et al., 2016). Effects of dietary MI on peripheral neuropathy remain unknown; however, it has been demonstrated that dietary MI supplementation maintained neuronal MI concentrations in diabetic rats and also improved sciatic motor nerve conduction velocity regardless of persistent hyperglycemia and elevated nerve sorbitol and fructose concentrations in diabetic rats (Greene et al., 1975). Furthermore, it has been observed that SMIT1−/− mice expressed dysfunctions in peripheral nerves such as brachial plexus, sciatic, facial, vagus, intercostal, and phrenic nerves, indicating that MI metabolism could be crucial in neuronal signaling, hypothetically through regulation of Ca2+ release (Takei et al., 1998, Chau et al., 2005).
Effects of Dietary Myo-Inositol on Brain
How dietary MI is associated with brain functions appears to be multivariate and remains unclear. Several mechanisms might be involved, such as the role of MI in osmotic balance (Macri et al., 2006, Dai et al., 2016), the role of PI, PIP, and InsP as important signaling molecules in cell regulation, and the role of molecules such as DAG, InsP3, and InsP4 as second messengers, among others. For mental health in humans, there was no conclusive evidence that dietary MI affected depression, anxiety, and obsessive-compulsive behavioral problems (Mukai et al., 2014). However, MI has been associated with amelioration of premenstrual dysphoric disorder in women (Gianfranco et al., 2011). Dietary MI was also associated with the functionality of the dopaminergic system, because dietary MI applied 12 wk increased significantly dopamine receptor (D2) density in guinea pigs (Harvey et al., 2001). Furthermore, dietary MI applied 21 D was associated with increased plasma serotonin concentrations in broiler chickens (Gonzalez-Uarquin et al. 2019, accepted paper). Serotonin and dopamine are modulators of brain functions and behavioral patterns in animals and humans. In chicken, an increase in serotonin concentration was associated with a decrease in feather pecking and aggressive behavior (de Haas and van der Eijk, 2018). Even though a direct relationship between central and peripheral serotonin has not been proved yet, significant correlations between cerebrospinal fluid and plasma have been observed in humans and rats (Audhya et al., 2012), indicating that dietary MI in poultry may be a promising candidate for functional feeding to improve animal health and welfare.
Myo-inositol supplementation in poultry nutrition
Relevance of MI in poultry nutrition and physiology has received attention in recent years. Myo-inositol may be supplemented pure or through complete dephosphorylation of dietary InsP6. Phytase supplementation has shown to increase MI concentrations in intestinal chymus and blood (Walk et al., 2014; Gautier et al., 2017, Farhadi et al., 2017, Beeson et al., 2017, Schmeisser et al., 2017, Walk et al., 2018, Sommerfeld et al., 2018b, Babatunde et al., 2019). Phytase supplementation also has been related with increases in the expression of the MI transporters-associated genes SLC5A11 (SMIT2) and SLC2A13 (HMIT) in jejunum and ileum, respectively (Walk et al., 2018). Studies on the effect of dietary MI on poultry health and performance are summarized in Table 1. Generally, the response to MI is inconsistent, but clearly, a better understanding is needed on the physiological effects of MI.
Table 1.
Published studies on dietary myo-inositol in poultry.
| Reference | Poultry type | MI % (total diet) | Lifespan | Main diet components | Measured variable | MI effects |
|---|---|---|---|---|---|---|
| Hegsted et al., 1941 | Broiler chickens | 0.1 | Day 1 to 28 | Dextrin/casein/cartilage. | BW gain. | ↑BW. |
| Dam, 1944 | White leghorn chicks | 0.15 | From day 1 | Sucrose/casein/yeast or Lard/starch/casein. | A staircase curve for exudate and encephalomalacia. | ↓Encephalomalacia and exudates. |
| Pearce, 1975 | Ross broiler chickens | 0.25 | Week 1 to 8 | Corn (conventional). Wheat + choline. Wheat + inositol. |
BW gain. Mortality by FLKS. Liver weight, lipid content, DM, and lipogenic enzyme activities. |
↓BW but did not affect the incidence of FLKS nor liver lipid metabolism. |
| Żyła et al., 2004 | Ross broiler chickens | 0.1 | Day 1 to 21 | Corn–soybean + 0.27% NPP/0.65% Ca or 0.47 NPP/0.80% Ca. | Performance (BW gain and feed intake). Bone mineralization (toe ash). Phosphorus and Ca retention. |
↑BW gain. ↓P retention (In birds fed just with the diet with 0.27% NPP and 0.65% Ca diet). |
| Żyła et al., 2012 | Bovans Brown laying hens | 0.1 | Week 50 to 62 | Corn and wheat/soybean. | Layer performance, eggshell quality, yolk cholesterol, and fatty acid deposition. | Added to corn–soybean diets: ↑ egg palmitoleic acid ↓egg total lipid content ↓ egg arachidonic, eicosatrienoic, and gadoleic fatty acids (In comparison to hens fed the negative control diet −3.65% Ca and 0.08& NPP). |
| Żyła et al., 2013a | Ross broiler chickens | 0.1 | First experimental period: Day 1 to 21 Second experimental period: Day 22 to 42 |
In the first period animals were fed with corn or wheat/soybean. For the second period, experiment proceeded only with broilers fed the low NPP corn-based, grower type diets. |
Performance (body weight gain, feed intake, feed conversion ratio, and mortality). Bone mineralization (toe ash). Concentration of triglycerides and HDL cholesterol. 42-day-old broilers fed the corn-based starter and grower diets. |
↑feed intake, BW gain and feed convertion ratio (In comparison to broilers fed the negative control diet). ↑the growth more efficiently in the starter than in the grower period. |
| Żyła et al., 2013b | Bovans Brown laying hens | 0.1 | Week 50 to 62 | Corn/soybean. | Hematological indices (Hemoglobin, hematocrit, erythrocytes, leucocytes, lymphocytes, monocytes, heterophils, eosinophils, basophils, H/L3 as well as AGP levels. | ↓basophils percent in the white blood cells in comparison to hens fed the NC diet. |
| Cowieson et al., 2013 | Ross broiler chickens | 0.15 | Different measurements within day 1 to 42 | Wheat/corn/soybean. Randomized positive Control - PC (adequated in P and Ca) Negative Control - NC (P and Ca reduced in 0.12 and 0.14%, respectively), Both of them supplemented with MI |
Experiment 1: Performance (BW gain, feed intake and feed conversion ratio) Experiment 2: Performance (BW gain, feed intake and feed conversion ratio) Blood biochemistry (glucagon, insulin, glucose, total cholesterol, HDL cholesterol, and triglycerides) |
Significant variations in feed intake and feed conversion ratio according to the age. Significant variations in BW and feed conversion ratio according to age. ↑insulin concentrations in birds fed the PC diet at the 42 d old. ↑glucagon and glucose concentration increased significantly in birds fed the NC diet at the 42 d old. |
| Lee et al., 2017 | Cobb Broiler chickens | 0.3 | First phase: Day 0 to 21 Second phase: Day 22 to 42 |
Corn/soybean | Performance (feed intake, BW gain, and feed conversion ratio). Bone mineralization (Ash, Ca and Phosphorus) |
MI supplementation had no significant effect on general performance respect to the other treatments. ↓significantly reduction in bone strength. |
| Pirgozliev et al., 2017 | Ross Broiler chickens | 2.5 5.0 7.0 |
Day 7 to 17 | Corn/soybean 2.5 g/kg NPP |
Performance (feed intake, daily weight gain, feed conversion efficiency). Dietary AMEn, AMEn intake, and AMEn: GE. DM digestibility, N, fat, P digestibility Excreta sialic acid concentration and sialic acid secretion. |
↑feed intake, daily weight gain, AMEn intake and DM digestibility in quadratic (higher in animals supplemented with 0.25% MI). ↓concentration and secretion of sialic acid in excreta (the higher the level of MI, the lower the sialic acid concentration). |
| Farhadi et al., 2017 | Ross Broiler chickens | 0.15 | First phase: Day 0 to 21 Second phase: Day 22 to 42 |
Corn/soybean + 0.45, 0.42 and 0.39% of nPP during starter, grower and finisher period, respectively (PC). Corn-soybean + 0.30, 0.27 and 0.24% of nPP during starter, grower and finisher, respectively (NC). Mi only was added to the NC diet. |
Growth performance, nutrient digestibility, serum metabolites, tibia mineralization. Litter moisture content. Foot problems |
↑significantly Ca, ALP, total protein, and gait score in comparison to NC, PC or both. ↓P content, crude protein digestibility, and tibia bone mineralization in comparison to NC, PC or both. ↓litter moisture % in comparison to NC diet but ↑compared with PC. |
| Sommerfeld et al., 2018b | Unsexed Ross Broiler chickens | 0.38 0.35 |
Started phase: Day 0 to 11 Grower phase: Day 11 to 22 |
Wheat/Corn-soybean 1.5 g aP, 1.65 g Ca, + 0.3 g Na/kg feed. |
Performance (Average daily gain, average daily feed intake and gain: feed ratio). Inositol phosphate degradation. Concentrations of MI in the digestive tract and blood. Bone mineralization, Prececal digestibility of amino acids (AA). |
↑gain-feed ratio ↑MI concentration in crop, ileum, and blood plasma during the grower phase. ↑concentrations of InsP3 and InsP4 in crop digesta and InsP5 in ileum digesta of 22-day-old broilers. ↓digestibility of the amino acids arginine, isoleucine, leucine, phenylananine, asparagine, glutamic acid, proline and tyrosine. |
| Pirgozliev et al., 2019 | Male Ross broiler chickens | 0.3 3.0 |
Day 7 to 21 | Corn/soybean + 4.8 g/kg NPP (PC). Corn/soybean + 2.5 g/kg NPP (NC). |
Performance (BW, average daily feed intake, average daily gain and gain: feed ratio). AME, DM retention, nitrogen retention and fat digestibility. Nutrient retention, liver N, fat, and vitamin E contents, MI and ALP concentrations in blood plasma. |
MI inclusion at 3.0% significantly ↑ blood plasma MI and ALP concentrations. ↑liver weight and hepatic N retention in a dose dependent manner. |
| Farhadi et al., 2019 | Ross broiler chickens | 0.15 | Day 1 to 23 | Corn/soybean +4.8 g/kg NPP (PC). Started phase: (NPP: 0.45%). Grower phase: (NPP: 0.42%). Corn/soybean + 2.5 g/kg NPP (NC). Started phase: (NPP: 0.30%). Grower phase: (NPP: 0.27%). |
pH of digesta and diets in gizzard and jejunum. Ca, P, Zn, and Fe solubility of digesta and diets in gizzard and jejunum. |
↓ P gizzard solubility in comparison to animals fed the PC. |
| Gonzalez -Uarquin et al., 2019. (unpublished data) | Ross Broiler chickens | 0.4 | Day 1 to 21 | Wheat/corn/soybean. | Blood plasma MI quantification. Targeted metabolomics approach in blood plasma (acylcarnitines, amino acids, biogenic amines, glycerophospholipids1, sphingolipids and hexoses). |
↑ blood plasma MI. ↑ serotonin and dopamine concentrations. MI supplementation also associated with decreases of acylcarnitines and glycerophospholipids. |
This table shows information strictly related to dietary myo-inositol (MI) supplementation. For more details (e.g., diets, phytase supplementation and interactions between MI, phytase, and minerals), please see the original sources. Search was done with Google Scholar and PubMed using the following key words: “myo-inositol supplementation in poultry, avian, broilers, laying hens, layers, chicks, birds, and chickens,” “dietary myo-inositol in poultry, avian, broilers, laying hens, layers, chicks, birds, and chicken”.
Abbreviations: AGP, α-1 acid glycoprotein; ALP, alkaline phosphatase; AMEn, nitrogen-corrected apparent metabolizable energy; day-old, posthatching-day; AMEn:GE: metabolizability of gross energy; dP, digestible phosphorus; FLKS, Fatty liver and kidney syndrome; HDL, high-density lipoproteins; H/L3, heterophils to lymphocytes ratio; MI, myo-inositol; NPP, nonphytate phosphorus.
The glycerophospholipids mainly consists of phosphatidylcholines and lysophosphatidylcholines.
Further experimental data demonstrated that dietary MI was associated with increases of MI concentrations in intestinal chymus and in blood plasma (Sommerfeld et al., 2018b). However, conclusive evidence about its effects on health is insufficient because body MI concentrations depended on several factors such as production system, bird's age, diet type, and MI doses (Pirgozliev et al., 2017). Some studies have demonstrated that pure dietary MI supplementation did not affect P and Ca digestibility (Sommerfeld et al., 2018b, Pirgozliev et al., 2019). Combined supplementation of MI and phytase influenced metabolic conditions such as glucose and mineral homeostasis. For example, Cowieson et al. (2013) found that Ross 308 male broilers fed a diet with low available P plus 500 FTU/kg phytase and 0.15% MI supplementation significantly lower blood glucose concentrations (11.8 vs. 12.8 mmol/L) in comparison to the same feed without phytase. Furthermore, a dietary level of MI of 2.0, 5.0, and 7.5 g/kg of feed increased BW gain and nitrogen-corrected apparent metabolizable energy intake, whereas decreased total sialic acid excretion. When MI was supplemented in the presence of 500 FTU/kg phytase, a supplementation of 7.5 g/kg MI led to a reduction in P digestibility (Pirgozliev et al., 2017). Increases in Ca and P concentration of the diet may be associated with reductions in the efficacy of endogenous microbial or epithelial phosphatases, what could explain the decrease found in MI concentration in the intestinal digesta. Interestingly, these effects were counteracted by the addition of 1500 FTU phytase/kg feed (Sommerfeld et al., 2018a). Evidence of P × Ca × phytase interactions on InsP degradation is provided by several studies (Zyła et al., 2004, Tamim et al., 2004, Amerah et al., 2014, Dersjant-Li et al., 2015, Zeller et al., 2015, Beeson et al., 2017; and Sommerfeld et al., 2018a). However, insufficient work was done to allow an understanding of MI, Ca, and P relationships.
Many factors appear to influence the ability of MI to mitigate animal performance. It appeared that in vivo MI efficiency depended on (1) animal-related factors (e.g., species, age of animals, genetic background and endogenous, and microbial phosphatases), (2) dietary-related factors (e.g., MI content, type of substrates, intrinsic phytases or phosphatases, total Ca, P levels, and Ca:P ratio), and (3) MI-related factors (e.g., MI doses, and source). Exact knowledge about its functions and potential advantage in poultry production is lacking. Myo-inositol plays key roles in a number of different metabolic pathways, and a clearer understanding makes MI an important topic for future research in poultry.
Acknowledgements
This project was financially supported by the German Research Foundation (DFG HU838/16-1)
References
- Abel K., Anderson R.A., Shears S.B. Phosphatidylinositol and inositol phosphate metabolism. J.Cell Sci. 2001;114:2207–2208. doi: 10.1242/jcs.114.12.2207. [DOI] [PubMed] [Google Scholar]
- Amerah A.M., Plumstead P.W., Barnard L.P., Kumar A. Effect of calcium level and phytase addition on ileal phytate degradation and amino acid digestibility of broilers fed corn-based diets. Poult. Sci. 2014;93:906–915. doi: 10.3382/ps.2013-03465. [DOI] [PubMed] [Google Scholar]
- Angeloff L.G., Skoryna S.C., Henderson I.W. Effects of the hexahydroxyhexane myoinositol on bone uptake of radiocalcium in rats: effect of inositol and vitamin D2 on bone uptake of 45Ca in rats. Acta Pharmacol. Toxicol. (Copenh). 1977;40:209–215. doi: 10.1111/j.1600-0773.1977.tb02070.x. [DOI] [PubMed] [Google Scholar]
- Aouameur R., Da C.S., Bissonnette P., Coady M.J., Lapointe J.Y. SMIT2 mediates all myo-inositol uptake in apical membranes of rat small intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;293:1300–1307. doi: 10.1152/ajpgi.00422.2007. [DOI] [PubMed] [Google Scholar]
- Arner R.J., Prabhu K.S., Thompson J.T., Hildenbrandt G.R., Liken A.D., Reddy C.C. Myo-Inositol oxygenase: molecular cloning and expression of a unique enzyme that oxidizes myo-inositol and D-chiro-inositol. Biochem. J. 2001;360:313–320. doi: 10.1042/0264-6021:3600313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arner R.J., Prabhu S., Reddy C.C. Molecular cloning, expression, and characterization of myo-inositol oxygenase from mouse, rat, and human kidney. Biochem. Biophys. Res. Commun. 2004;324:1386–1392. doi: 10.1016/j.bbrc.2004.09.209. [DOI] [PubMed] [Google Scholar]
- Audhya T., Adams J.B., Johansen L. Correlation of serotonin levels in CSF, platelets, plasma, and urine. Biochim. Biophys. Acta. 2012;1820:1496–1501. doi: 10.1016/j.bbagen.2012.05.012. [DOI] [PubMed] [Google Scholar]
- Azab A.N., He Q., Ju S., Li G., Greenberg M.L. Glycogen synthase kinase-3 is required for optimal de novo synthesis of inositol. Mol. Microbiol. 2007;63:1248–1258. doi: 10.1111/j.1365-2958.2007.05591.x. [DOI] [PubMed] [Google Scholar]
- Babatunde O.O., Cowieson A.J., Wilson J.W., Adeola O. Influence of age and duration of feeding low-phosphorus diet on phytase efficacy in broiler chickens during the starter phase. Poult. Sci. 2019;98:2588–2597. doi: 10.3382/ps/pez014. [DOI] [PubMed] [Google Scholar]
- Battaglia F.C., Meschia G., Blechner J.N., Barron D.H. The free myo-inositol concentration of adult and fetal tissues of several species. J. Exp. Physiol. Cogn. Med. Sci. 1961;46:188–193. doi: 10.1113/expphysiol.1961.sp001532. [DOI] [PubMed] [Google Scholar]
- Beemster P., Groenen P., Steegers-Theunissen R. Involvement of inositol in reproduction. Nutr. Rev. 2002;60:80–87. doi: 10.1301/00296640260042748. [DOI] [PubMed] [Google Scholar]
- Beeson L.A., Walk C.L., Bedford M.R., Olukosi O.A. Hydrolysis of phytate to its lower esters can influence the growth performance and nutrient utilization of broilers with regular or super doses of phytase. Poult. Sci. 2017;96:2243–2253. doi: 10.3382/ps/pex012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua A., Bizzarri M. Inositols in insulin signaling and glucose metabolism. Int. J. Endocrinol. 2018;2018:1968450. doi: 10.1155/2018/1968450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissonnette P., Lahjouji K., Coady M.J., Lapointe J.Y. Effects of hyperosmolarity on the Na+ -myo-inositol cotransporter SMIT2 stably transfected in the Madin-Darby canine kidney cell line. Am. J. Physiol. Cell. Physiol. 2008;295:C791–C799. doi: 10.1152/ajpcell.00390.2007. [DOI] [PubMed] [Google Scholar]
- Brown L.D., Cheung A., Harwood J.E., Battaglia F.C. Inositol and mannose utilization rates in term and late-preterm infants exceed nutritional intakes. J. Nutr. 2009;139:1648–1652. doi: 10.3945/jn.109.109108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H.H., Choong B., Phillips A.R., Loomes K.M. The diabetic rat kidney mediates inosituria and selective urinary partitioning of D-chiroinositol. Exp. Biol. Med. 2015;240:8–14. doi: 10.1177/1535370214543064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H.H., Chao H.N., Walker C.S., Choong S.Y., Phillips A., Loomes K.M. Renal depletion of myo-inositol is associated with its increased degradation in animal models of metabolic disease. Am. J. Physiol. Renal Physiol. 2015;309:755–763. doi: 10.1152/ajprenal.00164.2015. [DOI] [PubMed] [Google Scholar]
- Chau J.F., Lee M.K., Law J.W., Chung S.K., Chung S.S. Sodium/myo-inositol cotransporter-1 is essential for the development and function of the peripheral nerves. FASEB J. 2005;19:1887–1889. doi: 10.1096/fj.05-4192fje. [DOI] [PubMed] [Google Scholar]
- Chauvin T.R., Griswold M.D. Characterization of the expression and regulation of genes necessary for myo-inositol biosynthesis and transport in the seminiferous epithelium. Biol. Reprod. 2004;70:744–751. doi: 10.1095/biolreprod.103.022731. [DOI] [PubMed] [Google Scholar]
- Chukwuma C.I., Ibrahim M.A., Islam M.S. Myo-inositol inhibits intestinal glucose absorption and promotes muscle glucose uptake: a dual approach study. J. Physiol. Biochem. 2016;72:791–801. doi: 10.1007/s13105-016-0517-1. [DOI] [PubMed] [Google Scholar]
- Clements R.S., Reynerston R. Myo-inositol metabolism in diabetes mellitus: effect of insulin treatment. Diabetes. 1977;26:215–221. doi: 10.2337/diab.26.3.215. [DOI] [PubMed] [Google Scholar]
- Clements R.S., Jr., Diethelm A.G. The metabolism of myo-inositol by the human kidney. J. Lab. Clin. Med. 1979;93:210–219. [PubMed] [Google Scholar]
- Clements R.S., Jr., Darnell B. Myo-inositol content of common foods: development of a high-myo-inositol diet. Am. J. Clin. Nutr. 1980;33:1954–1967. doi: 10.1093/ajcn/33.9.1954. [DOI] [PubMed] [Google Scholar]
- Condorelli R.A., La Vignera S., Di Bari F., Unfer V., Calogero A.E. Effects of myoinositol on sperm mitochondrial function in-vitro. Eur. Rev. Med. Pharmacol. Sci. 2011;15:129–134. [PubMed] [Google Scholar]
- Condorelli R.A., La Vignera S., Bellanca S., Vicari E., Calogero A.E. Myoinositol: does it improve sperm mitochondrial function and sperm motility? Urology. 2012;79:1290–1295. doi: 10.1016/j.urology.2012.03.005. [DOI] [PubMed] [Google Scholar]
- Condorelli R.A., La Vignera S., Mongioì L.M., Vitale S.G., Laganà A.S., Cimino L., Calogero A.E. Myo-inositol as a male fertility molecule: speed them up! Eur. Rev. Med. Pharmacol. Sci. 2017;21(2 Suppl):30–35. [PubMed] [Google Scholar]
- Corrado F., D'Anna R., Di Vieste G., Giordano D., Pintaudi B., Santamaria A., Di Benedetto A. The effect of myoinositol supplementation on insulin resistance in patients with gestational diabetes. Diabet. Med. 2011;28:972–975. doi: 10.1111/j.1464-5491.2011.03284.x. [DOI] [PubMed] [Google Scholar]
- Cowieson A.J., Ptak A., Maćkowiak P., Sassek M., Pruszynska-Oszmałek E., Żyła K., Świątkiewicz S., Kaczmarek S., Jozefiak D. The effect of microbial phytase and myo-inositol on performance and blood biochemistry of broiler chickens fed wheat/corn-based diets. Poult. Sci. 2013;92:2124–2134. doi: 10.3382/ps.2013-03140. [DOI] [PubMed] [Google Scholar]
- Cowieson A.J., Aureli R., Guggenbuhl P., Fru-Nji F. Possible involvement of myo inositol in the physiological response of broilers to high doses of microbial phytase. Anim. Prod. Sci. 2015;55:710–719. [Google Scholar]
- Croze M.L., Vella R.E., Pillon N.J., Soula H.A., Hadji L., Guichardant M., Soulage C.O. Chronic treatment with myo-inositol reduces white adipose tissue accretion and improves insulin sensitivity in female mice. J. Nutr. Biochem. 2012;24:457–466. doi: 10.1016/j.jnutbio.2012.01.008. [DOI] [PubMed] [Google Scholar]
- Croze M.L., Soulage C.O. Potential role and therapeutic interests of myo-inositol in metabolic diseases. Biochimie. 2013;95:1811–1827. doi: 10.1016/j.biochi.2013.05.011. [DOI] [PubMed] [Google Scholar]
- Croze M.L., Geloen A., Soulage C.O. Abnormalities in myo-inositol metabolism associated with type 2 diabetes in mice fed a high-fat diet: benefits of a dietary myo-inositol supplementation. Br. J. Nutr. 2015;113:1862–1875. doi: 10.1017/S000711451500121X. [DOI] [PubMed] [Google Scholar]
- Dai Z., Chung S.K., Miao D., Lau K.S., Chan A.W., Kung A.W. Sodium/myo-inositol cotransporter 1 and myo-inositol are essential for osteogenesis and bone formation. J. Bone. Miner. Res. 2011;26:582–590. doi: 10.1002/jbmr.240. [DOI] [PubMed] [Google Scholar]
- Dai G., Yu H., Kruse M., Traynor-Kaplan A., Hille B. Osmoregulatory inositol transporter SMIT1 modulates electrical activity by adjusting PI (4,5)P2 levels. Proc. Natl. Acad. Sci. 2016;113:E3290–E3299. doi: 10.1073/pnas.1606348113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dam H. Studies on vitamin E deficiency in chicks. J. Nutr. 1944;27:193–211. [Google Scholar]
- Dang N.T., Mukai R., Yoshida K., Ashida H. D-pinitol and myo-inositol stimulate translocation of glucose transporter 4 in skeletal muscle of C57BL/6 mice. Biosci. Biotechnol. Biochem. 2010;74:1062–1067. doi: 10.1271/bbb.90963. [DOI] [PubMed] [Google Scholar]
- de Haas E.N., van der Eijk J.A.J. Where in the serotonergic system does it go wrong? Unravelling the route by which the serotonergic system affects feather pecking in chickens. Neurosci. Biobehav. Rev. 2018;95:170–188. doi: 10.1016/j.neubiorev.2018.07.007. [DOI] [PubMed] [Google Scholar]
- Deranieh R.M., Greenberg M.L. Cellular consequences of inositol depletion. Biochem. Soc. Trans. 2009;37:1099–1103. doi: 10.1042/BST0371099. [DOI] [PubMed] [Google Scholar]
- Deranieh R.M., Shi Y., Tarsio M., Chen Y., McCaffery J.M., Kane P.M., Greenberg M.L. Perturbation of the vacuolar ATPase: a novel consequence of inositol depletion. J. Biol. Chem. 2015;290:27460–27472. doi: 10.1074/jbc.M115.683706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dersjant-Li Y., Awati A., Schulze H., Partridge G. Phytase in non-ruminant animal nutrition: a critical review on phytase activities in the gastrointestinal tract and influencing factors. J. Sci. Food Agric. 2015;95:878–896. doi: 10.1002/jsfa.6998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Daniel E., Kew J.N., Maycox P.R. Investigation of the H (+)-myo-inositol transporter (HMIT) as a neuronal regulator of phosphoinositide signalling. Biochem. Soc. Trans. 2009;37:1139–1143. doi: 10.1042/BST0371139. [DOI] [PubMed] [Google Scholar]
- Dinicola S., Minini M., Unfer V., Verna R., Cucina A., Bizzarri M. Nutritional and acquired deficiencies in inositol bioavailability. Correlations with metabolic disorders. Int. J. Mol. Sci. 2017;18:E2187. doi: 10.3390/ijms18102187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes C.P., Macphee C.H. Myo-inositol metabolites as cellular signals. Eur. J. Biochem. 1990;193:1–18. doi: 10.1111/j.1432-1033.1990.tb19297.x. [DOI] [PubMed] [Google Scholar]
- Duong Q.H., Clark K.D., Lapsley K.G., Pegg R.B. Determination of myo-inositol phosphates in tree nuts and grain fractions by HPLC–ESI–MS. J. Food Compost. Anal. 2017;59:74–82. [Google Scholar]
- Eisenberg F., Jr., Bolden A.H. Biosynthesis of inositol in rat testis homogenate. Biochem. Biophys. Res. Commun. 1963;12:72–77. [Google Scholar]
- Farhadi D., Karimi A., Sadeghi G.H., Rostamzadeh J., Bedford M.R. Effects of a high dose of microbial phytase and myo-inositol supplementation on growth performance, tibia mineralization, nutrient digestibility, litter moisture content, and foot problems in broiler chickens fed phosphorus-deficient diets. Poult. Sci. 2017;96:3664–3675. doi: 10.3382/ps/pex186. [DOI] [PubMed] [Google Scholar]
- Farhadi D., Karimi A., A Sadeghi A., Rostamzadeh J., Bedford M.R. Effect of a high dose of exogenous phytase and supplementary myo-inositol on mineral solubility of broiler digesta and diets subjected to in vitro digestion assay. Poult. Sci. 2019;0:1–14. doi: 10.3382/ps/pez104. [DOI] [PubMed] [Google Scholar]
- Gautier A.E., Walk C.L., Dilger R.N. Effects of a high level of phytase on broiler performance, bone ash, phosphorus utilization, and phytate dephosphorylation to inositol. Poult. Sci. 2017;97:211–218. doi: 10.3382/ps/pex291. [DOI] [PubMed] [Google Scholar]
- Gianfranco C., Vittorio U., Silvia B., Francesco D. Myo-inositol in the treatment of premenstrual dysphoric disorder. Hum. Psychopharmacol. 2011;26:526–530. doi: 10.1002/hup.1241. [DOI] [PubMed] [Google Scholar]
- González-Álvarez R., Pérez-Ibave D.C., Garza-Rodríguez M.L., Lugo-Trampe Á, Delgado-Enciso I., Tejero-Barrera M.E., Rodríguez-Sánchez I.P. Molecular cloning of the myo-inositol oxygenase gene from the kidney of baboons. Biomed. Rep. 2017;7:301–305. doi: 10.3892/br.2017.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Uarquin F., Kenez A., Rodehutscord M., Huber K. Dietary phytase and myo-inositol supplementation are associated with distinct plasma metabolite profile in broiler chickens. Animal. 2019:1–11. doi: 10.1017/S1751731119002337. [DOI] [PubMed] [Google Scholar]
- Greene D.A., De Jesus P.V., Winegrad A.I. Effects of insulin and dietary myoinositol on impaired peripheral motor nerve conduction velocity in acute streptozotocin diabetes. J. Clin. Investig. 1975;55:1326–1336. doi: 10.1172/JCI108052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene D.A., Lattimer S.A. Sodium- and energy-dependent uptake of myo-inositol by rabbit peripheral nerve. Competitive inhibition by glucose and lack of an insulin effect. J. Clin. Investig. 1982;70:1009–1018. doi: 10.1172/JCI110688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulino F.A., Leonardi E., Marilli I., Musmeci G., Vitale S.G., Leanza V., Palumbo M.A. Effect of treatment with myo-inositol on semen parameters of patients undergoing an IVF cycle: in vivo study. Gynecol. Endocrinol. 2016;32:65–68. doi: 10.3109/09513590.2015.1080680. [DOI] [PubMed] [Google Scholar]
- Hallman M., Saugstad O.D., Porreco R.P., Epstein B.L., Gluck L. Role of myoinositol in regulation of surfactant phospholipids in the newborn. Early Hum. Dev. 1985;10:245–254. doi: 10.1016/0378-3782(85)90055-6. [DOI] [PubMed] [Google Scholar]
- Hamada Y., Araki N., Koh N., Nakamura J., Horiuchi S., Hotta N. Rapid formation of advanced glycation end products by intermediate metabolites of glycolytic pathway and polyol pathway. Biochem. Biophys. Res. Commun. 1996;228:539–543. doi: 10.1006/bbrc.1996.1695. [DOI] [PubMed] [Google Scholar]
- Haneda M., Kikkawa R., Arimura T., Ebata K., Togawa M., Maeda S., Sawada T., Horide N., Shigeta Y. Glucose inhibits myo-inositol uptake and reduces myo-inositol content in cultured rat glomerular mesangial cells. Metabolism. 1990;39:40–45. doi: 10.1016/0026-0495(90)90145-3. [DOI] [PubMed] [Google Scholar]
- Hankes L.V., Politzer W.M., Touster O., Anderson L. Myo-Inositol catabolism in human pentosurics: the predominant role of the glucuronate-xylulose-pentose phosphate pathway. Ann. NY Acad. Sci. 1969;165:564–576. [PubMed] [Google Scholar]
- Harvey B.H., Scheepers A., Brand L., Stein D.J. Chronic inositol increases striatal D (2) receptors but does not modify dexamphetamine-induced motor behavior. Relevance to obsessive-compulsive disorder. Pharmacol. Biochem. Behav. 2001;68:245–253. doi: 10.1016/s0091-3057(00)00459-7. [DOI] [PubMed] [Google Scholar]
- Harwood A.J. Lithium and bipolar mood disorder: the inositol-depletion hypothesis revisited. Mol. Psychiatry. 2005;10:117–126. doi: 10.1038/sj.mp.4001618. [DOI] [PubMed] [Google Scholar]
- Hauser G., Finelli V.N. The biosynthesis of free and phosphatide myo-inositol from glucose by mammalian tissue slices. J. Biol. Chem. 1963;238:3224–3228. [PubMed] [Google Scholar]
- Hayashi E., Maeda T., Tomita T. The effect of myo-inositol deficiency on lipid metabolism in rats. II. The mechanism of triacylglycerol accumulation in the liver of myo-inositol deficient rats. Biochim. Biophys. Acta. 1974;360:146–155. doi: 10.1016/0005-2760(74)90164-7. [DOI] [PubMed] [Google Scholar]
- Hegsted D.M., Briggs G.M., Mills R.C., Elvehjem C.A., Hart E.B. Inositol in chick nutrition. Exp. Biol. Med. 1941;47:376e7. [Google Scholar]
- Holub B.J. Metabolism and function of myo-inositol and inositol phospholipids. Annu. Rev. Nutr. 1986;6:563–597. doi: 10.1146/annurev.nu.06.070186.003023. [DOI] [PubMed] [Google Scholar]
- Howard C.F., Anderson L. Metabolism of myo-inositol in animals. II. Complete catabolism of myo-inositol-14C by rat kidney slices. Arch. Biochem. Biophys. 1967;118:332–339. doi: 10.1016/0003-9861(67)90357-8. [DOI] [PubMed] [Google Scholar]
- Huber K. Cellular myo-inositol metabolism Pages 53–60. In: Walk C.L., Kühn I., Stein H.H., Kidd M.T., Rodehutscord M., editors. Phytate Destruction – Consequences for Precision Animal Nutrition. Wageningen Academic Publishers; The Netherlands: 2016. ISBN: 978-90-8686-290-0. [Google Scholar]
- Indyk H.E., Saldo S.C., White P.M., Dole M.N., Gill B.D., Woollard D.C. The free and total myo-inositol contents of early lactation and seasonal bovine milk. Int. Dairy J. 2016;56:33–37. [Google Scholar]
- Isaacks R.E., Kim C.Y., Johnson A.E., Jr., Goldman P.H., Harkness D.R. Studies on avian erythrocyte metabolism. XII. The syn- thesis and degradation of inositol pentakis (dihy- drogen phosphate) Poult. Sci. 1982;61:2271–2281. doi: 10.3382/ps.0612271. [DOI] [PubMed] [Google Scholar]
- Isaacks R.E., Lai L.L., Kim C.Y., Goldman P.H., Kim H.D. Studies on avian erythrocyte metabolism. XVII. Kinetics and transport properties of myo-inositol in chicken reticulocytes. Arch. Biochem. Biophys. 1989;274:564–573. doi: 10.1016/0003-9861(89)90471-2. [DOI] [PubMed] [Google Scholar]
- Jiang F., Ryan M.T., Schlame M., Zhao M., Gu Z., Klingenberg M., Pfanner N., Greenberg M.L. Absence of cardiolipin in the crd1 null mutant results in decreased mitochondrial membrane potential and reduced mitochondrial function. J. Biol. Chem. 2000;275:22387–22394. doi: 10.1074/jbc.M909868199. [DOI] [PubMed] [Google Scholar]
- Jolivalt C.G., Frizzi K.E., Guernsey L., Marquez A., Ochoa J., Rodriguez M., Calcutt N.A. Peripheral neuropathy in mouse models of diabetes. Curr. Protoc. Mouse Biol. 2016;6:223–255. doi: 10.1002/cpmo.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju S., Greenberg M.L. Valproate disrupts regulation of inositol responsive genes and alters regulation of phospholipid biosynthesis. Mol. Microbiol. 2003;49:1595–1603. doi: 10.1046/j.1365-2958.2003.03641.x. [DOI] [PubMed] [Google Scholar]
- Kanehisa M., Furumichi M., Tanabe M., Sato Y., Morishima K. KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017;45:D353–D361. doi: 10.1093/nar/gkw1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanehisa M., Sato Y., Furumichi M., Morishima K., Tanabe M. New approach for understanding genome variations in KEGG. Nucleic Acids Res. 2019;47:D590–D595. doi: 10.1093/nar/gky962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.N., Han S.N., Kim H.K. Phytic acid and myo-inositol support adipocyte differentiation and improve insulin sensitivity in 3 T3-L1 cells. Nutr. Res. 2014;34:723–731. doi: 10.1016/j.nutres.2014.07.015. [DOI] [PubMed] [Google Scholar]
- Kim J.H., Pitargue F.M., Jung H., Han G.P., Choi H.S., Kil D.Y. Effect of superdosing phytase on productive performance and egg quality in laying hens. Asian-Australas. J. Anim. Sci. 2017;30:994–998. doi: 10.5713/ajas.17.0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlmeier M. Inositol. Pages 634-642. In: Kohlmeier M., editor. Nutrient Metabolism. Food Science and Technology; 2003. [DOI] [Google Scholar]
- Lahjouji K., Aouameur R., Bissonnette P., Coady M.J., Bichet D.G., Lapointe J.Y. Expression and functionality of the Na+/myo-inositol cotransporter SMIT2 in rabbit kidney. Biochim. Biophys. Acta. 2007;1768:1154–1159. doi: 10.1016/j.bbamem.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Larner J., Brautigan D.L., Thorner M.O. D-Chiro-Inositol glycans in insulin signaling and insulin resistance. Mol. Med. 2010;16:543–552. doi: 10.2119/molmed.2010.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Bedford M. Inositol-An effective growth promotor? World's Poult. Sci. J. 2016;72:743–760. [Google Scholar]
- Lee S.A., Nagalakshmi D., Raju M.V.L.N., Rama Rao S.V., Bedford M.R. Effect of phytase superdosing, myo-inositol and available phosphorus concentrations on performance and bone mineralisation in broilers. Anim. Nutr. 2017;3:247–251. doi: 10.1016/j.aninu.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin L.M., Yannai Y., Sulimovici S., Kraicer P.F. Studies on the metabolic role of myo-inositol distribution of radioactive myo-inositol in the male rat. Biochem. J. 1976;156:375–380. doi: 10.1042/bj1560375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S.A., Jiang W.D., Feng L., Liu Y., Wu P., Jiang J., Kuang S.Y., Tang L., Tang W.N., Zhang Y.A., Tang X., Shi H.Q., Zhou X.Q. Dietary myo-inositol deficiency decreased the growth performances and impaired intestinal physical barrier function partly relating to nrf2, jnk, e2f4 and mlck signaling in young grass carp (Ctenopharyngodon idella) Fish Shellfish Immunol. 2017;67:475–492. doi: 10.1016/j.fsi.2017.06.032. [DOI] [PubMed] [Google Scholar]
- Li S.A., Jiang W.D., Feng L., Liu Y., Wu P., Jiang J., Kuang S.Y., Tang L., Tang W.N., Zhang Y.A., Yang J., Tang X., Shi H.Q., Zhou X.Q. Dietary myo-inositol deficiency decreased intestinal immune function related to NF-κB and TOR signaling in the intestine of young grass carp (Ctenopharyngodon idella) Fish Shellfish Immunol. 2018;76:333–346. doi: 10.1016/j.fsi.2018.03.017. [DOI] [PubMed] [Google Scholar]
- Llewelyn J. The diabetic neuropathies: types, diagnosis and management. J. Neurol. Neurosurg. Psychiatr. 2003;74:15–19. doi: 10.1136/jnnp.74.suppl_2.ii15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macri M.A., D'Alessandro N., Di Giulio C., Di Iorio P., Di Luzio S., Giuliani P., Bianchi G., Esposito E. Regional changes in the metabolite profile after long-term hypoxia-ischemia in brains of young and aged rats: a quantitative proton MRS study. Neurobiol. Aging. 2006;27:98–104. doi: 10.1016/j.neurobiolaging.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Muallem S., Chung W.Y., Jha A., Ahuja M. Lipids at membrane contact sites: cell signaling and ion transport. EMBO Rep. 2017;18:1893–1904. doi: 10.15252/embr.201744331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai T., Kishi T., Matsuda Y., Iwata N. A meta-analysis of inositol for depression and anxiety disorders. Hum. Psychopharmacol. 2014;29:55–63. doi: 10.1002/hup.2369. [DOI] [PubMed] [Google Scholar]
- Nayak B., Xie P., Akagi S., Yang Q., Sun L., Wada J., Thakur A., Danesh F.R., Chugh S.S., Kanwar Y.S. Modulation of renal-specific oxidoreductase/myo-inositol oxygenase by high-glucose ambience. Proc. Natl. Acad. Sci. U S A. 2005;102:17952–17957. doi: 10.1073/pnas.0509089102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak B., Kondeti V.K., Xie P., Lin S., Viswakarma N., Raparia K., Kanwar Y.S. Transcriptional and post-translational modulation of myo-inositol oxygenase by high glucose and related pathobiological stresses. J. Biol. Chem. 2011;286:27594–27611. doi: 10.1074/jbc.M110.217141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi K., Zheng B., Kuo J.F. Inhibition of Na, K-ATPase and sodium pump by protein kinase C regulators sphingosine, lysophosphatidylcholine, and oleic acid. J. Biol. Chem. 1990;265:70–75. [PubMed] [Google Scholar]
- Okazaki Y., Sekita A., Katayama T T. Intake of phytic acid and myo-inositol lowers hepatic lipogenic gene expression and modulates gut microbiota in rats fed a high-sucrose diet. Biomed. Rep. 2018;8:466–474. doi: 10.3892/br.2018.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortmeyer H.K. Dietary myoinositol results in lower urine glucose and in lower postprandial plasma glucose in obese insulin resistant rhesus monkeys. Obes. Res. 1996;4:569–575. doi: 10.1002/j.1550-8528.1996.tb00271.x. [DOI] [PubMed] [Google Scholar]
- Oshima M., Taylor T.G., Williams A. Variations in the concentration of phytic acid in the blood of the domestic fowl. Biochem. J. 1964;92:42–46. doi: 10.1042/bj0920042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasta V., Gullo G., Giuliani A., Harrath A.H., Alwasel S.H., Tartaglia F., Cucina A., Bizzarri M. An association of boswellia, betaine and myo-inositol in the treatment of mammographic breast density: a randomized, double-blind study. Eur. Rev. Med. Pharmacol. Sci. 2015;19:4419–4426. [PubMed] [Google Scholar]
- Pearce J. The effects of choline and inositol on hepatic lipid metabolism and the incidence of the fatty liver and kidney syndrome in broilers. Br. Poult. Sci. 1975;16:565–570. doi: 10.1080/00071667508416230. [DOI] [PubMed] [Google Scholar]
- Pirgozliev V.R., Bedford M.R., Rose S.P., Whiting I.M., Oluwatosin O.O., Oso A.O., Oke F.O., Ivanova S.G., Staykova G.P. Phosphorus utilisation and growth performance of broiler chicken fed diets containing graded levels of supplementary myo-inositol with and without exogenous phytase. J. World Poult. Res. 2017;7:1–7. [Google Scholar]
- Pirgozliev V., Brearley C.A., Rose S.P., Mansbridge S.C. Manipulation of plasma myo-inositol in broiler chickens: effect on growth performance, dietary energy, nutrient availability, and hepatic function. Poult. Sci. 2019;98:260–268. doi: 10.3382/ps/pey341. [DOI] [PubMed] [Google Scholar]
- Plows J.F., Budin F., Andersson R.A., Mills V.J., Mace K., Davidge S.T., Vickers M.H., Baker P.N., Silva-Zolezzi I., Stanley J.L. The Effects of myo-Inositol and B and D vitamin supplementation in the db/+ mouse model of gestational Diabetes Mellitus. Nutrients. 2017;9:141. doi: 10.3390/nu9020141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad R., Rose M.K., Virmani M., Garg S.L., Puri J.P. Lipid profile of chicken (Gallus domesticus) in response to dietary supplementation of garlic (Allium sativum) Int. J. Poult. Sci. 2009;8 270276. [Google Scholar]
- Regidor P.A., Schindler A.E. Myoinositol as a safe and alternative approach in the treatment of infertile PCOS women: a German observational study. Int. J. Endocrinol. 2016;2016 doi: 10.1155/2016/9537632. 9537632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodehutscord M., Rückert C., Maurer H.P., Schenkel H., Schipprack W., Bach Knudsen K.E., Schollenberger M., Laux M., Eklund M., Siegert W., Mosenthin R. Variation in chemical composition and physical characteristics of cereal grains from different genotypes. Arch. Anim. Nutr. 2016;70:87–107. doi: 10.1080/1745039X.2015.1133111. [DOI] [PubMed] [Google Scholar]
- Rodehutscord M., Rosenfelder P. Update on phytate degradation pattern in the gastrointestinal tract of pigs and broiler chickens. Pages 53–60. In: Walk C.L., Kühn I., Stein H.H., Kidd M.T., Rodehutscord M., editors. Phytate Destruction – Consequences for Precision Animal Nutrition. Wageningen Academic Publishers; The Netherlands: 2016. ISBN: 978-90-8686-290-0. [Google Scholar]
- Sahin K., Onderci M., Sahin N., Balci T.A., Gursu M.F., Juturu V., Kucuk O. Dietary arginine silicate inositol complex improves bone mineralization in quail. Poult. Sci. 2006;85:486–492. doi: 10.1093/ps/85.3.486. [DOI] [PubMed] [Google Scholar]
- Sarashina G., Yamakoshi M., Noritake M., Takahashi M., Kure M., Katsura Y., Shiomi H., Tsuboi I., Kawazu S., Yamagata F., Tominaga M., Matsuoka T. A study of urinary myo-inositol as a sensitive marker of glucose intolerance. Clin. Chim. Acta. 2004;344:181–188. doi: 10.1016/j.cccn.2004.02.026. [DOI] [PubMed] [Google Scholar]
- Schmeisser J., Seon A.A., Aureli R., Friedel A., Guggenbuhl V., Duval S., Cowieson A.J., Fru-Nji F. Exploratory transcriptomic analysis in muscle tissue of broilers fed a phytase-supplemented diet. J. Anim. Physiol. Anim. Nutr. (Berl). 2017;101:563–575. doi: 10.1111/jpn.12482. [DOI] [PubMed] [Google Scholar]
- Selitrennik M., Lev S. The role of phosphatidylinositol-transfer proteins at membrane contact sites. Biochem. Soc. Trans. 2016;44:419–424. doi: 10.1042/BST20150182. [DOI] [PubMed] [Google Scholar]
- Selle P.H., Ravindran V. Microbial phytase in poultry nutrition. Anim. Feed Sci. Technol. 2007;135:1–41. [Google Scholar]
- Setchell B.P., Dawson R.M., White R.W. The high concentration of free myo-inositol in rete testis fluid from rams. J. Reprod. Fertil. 1968;17:219–220. doi: 10.1530/jrf.0.0170219. [DOI] [PubMed] [Google Scholar]
- Sommerfeld V., Schollenberger M., Kühn I., Rodehutscord M. Interactive effects of phosphorus, calcium, and phytase supplements on products of phytate degradation in the digestive tract of broiler chickens. Poult. Sci. 2018;97:1177–1188. doi: 10.3382/ps/pex404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommerfeld V., Künzel S., Schollenberger M., Kühn I., Rodehutscord M. Influence of phytase or myo-inositol supplements on performance and phytate degradation products in the crop, ileum, and blood of broiler chickens. Poult. Sci. 2018;97:920–929. doi: 10.3382/ps/pex390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele J.W., Faulds D., Goa K.L. Epalrestat. A review of its pharmacology, and therapeutic potential in late-onset complications of diabetes mellitus. Drugs Aging. 1993;3:532–555. doi: 10.2165/00002512-199303060-00007. [DOI] [PubMed] [Google Scholar]
- Takei K., Shin R.M., Inoue T., Kato K., Mikoshiba K. Regulation of nerve growth mediated by inositol 1, 4, 5-trisphosphate receptors in growth cones. Science. 1998;282:1705–1708. doi: 10.1126/science.282.5394.1705. [DOI] [PubMed] [Google Scholar]
- Tamim N.M., Angel R., Christman M. Influence of dietary calcium and phytase on phytate phosphorus hydrolysis in broiler chickens. Poult. Sci. 2004;83:1358–1367. doi: 10.1093/ps/83.8.1358. [DOI] [PubMed] [Google Scholar]
- Thomas M.P., Mills S.J., Potter B.V. The “Other” inositols and their phosphates: synthesis, biology, and medicine (with recent advances in myo-inositol chemistry) Angew. Chem. Int. Ed. Engl. 2015;54:2–39. doi: 10.1002/anie.201502227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorsell A.G., Persson C., Voevodskaya N., Busam R.D., Hammarström M., Gräslund S., Gräslund A., Hallberg B.M. Structural and biophysical characterization of human myo-inositol oxygenase. J. Biol. Chem. 2008;283:15209–15216. doi: 10.1074/jbc.M800348200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner B.L., Papházy M.J., Haygarth P.M., McKelvie I.D. Inositol phosphates in the environment. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2002;357:449–469. doi: 10.1098/rstb.2001.0837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uldry M., Ibberson M., Horisberger J.D., Chatton J.Y., Riederer B.M., Thorens B. Identification of a mammalian H(+)-myo-inositol symporter expressed predominantly in the brain. EMBO J. 2001;20:4467–4477. doi: 10.1093/emboj/20.16.4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unfer V., Carlomagno G., Rizzo P., Raffone E., Roseff S. Myo-inositol rather than D-chiro-inositol is able to improve oocyte quality in intracytoplasmic sperm injection cycles. A prospective, controlled, randomized trial. Eur. Rev. Med. Pharmacol. Sci. 2011;15:452–457. [PubMed] [Google Scholar]
- Unfer V., Facchinetti F., Orrù B., Giordani B., Nestler J. Myo-inositol effects in women with PCOS: a meta-analysis of randomized controlled trials. Endocr. Connect. 2017;6:647–658. doi: 10.1530/EC-17-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance J.E. Phospholipid synthesis and transport in mammalian cells. Traffic. 2015;16:1–18. doi: 10.1111/tra.12230. [DOI] [PubMed] [Google Scholar]
- Walk C.L., Santos T.T., Bedford M.R. Influence of superdoses of a novel microbial phytase on growth performance, tibia ash, and gizzard phytate and inositol in young broilers. Poult. Sci. 2014;93:1172–1177. doi: 10.3382/ps.2013-03571. [DOI] [PubMed] [Google Scholar]
- Walk C.L., Bedford M.R., Olukosi O.A. Effect of phytase on growth performance, phytate degradation and gene expression of myo-inositol transporters in the small intestine, liver and kidney of 21 D old broilers. Poult. Sci. 2018;97:1155–1162. doi: 10.3382/ps/pex392. [DOI] [PubMed] [Google Scholar]
- Yang B., Hodgkinson A., Millward B.A., Demaine A.G. Polymorphisms of myo-inositol oxygenase gene are associated with Type 1 diabetes mellitus. J. Diabetes Complicat. 2010;24:404–408. doi: 10.1016/j.jdiacomp.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Zeller E., Schollenberger M., Witzig M., Shastak Y., Kühn I., Hoelzle L.E., Rodehutscord M. Interactions between supplemented mineral phosphorus and phytase on phytate hydrolysis and inositol phosphates in the small intestine of broilers. Poult. Sci. 2015;94:1018–1029. doi: 10.3382/ps/pev087. [DOI] [PubMed] [Google Scholar]
- Zhan M., Usman I.M., Sun L., Kanwar Y.S. Disruption of renal tubular mitochondrial quality control by myo-inositol oxygenase in diabetic kidney disease. J. Am. Soc. Nephrol. 2015;26:1304–1321. doi: 10.1681/ASN.2014050457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zyła K., Mika M., Stodolak B., Wikiera A., Koreleski J., Swiatkiewicz S. Towards complete dephosphorylation and total conversion of phytates in poultry feeds. Poult. Sci. 2004;83:1175–1186. doi: 10.1093/ps/83.7.1175. [DOI] [PubMed] [Google Scholar]
- Żyła K., Mika M., Duliński R., Świątkiewicz S., Koreleski J., Pustkowiak H., Piironen J. Effects of inositol, inositol-generating phytase B applied alone, and in combination with 6-phytase A to phosphorus-deficient diets on laying performance, eggshell quality, yolk cholesterol, and fatty acid deposition in laying hens. Poult. Sci. 2012;91:1915–1927. doi: 10.3382/ps.2012-02198. [DOI] [PubMed] [Google Scholar]
- Żyła K., Duliński R., Pierzchalska M., Grabacka M., Józefiak D., Świątkiewicz S. Phytases and myo-inositol modulate performance, bone mineralization and alter lipid fractions in serum of broilers. J. Anim. Feed Sci. 2013;22:56–62. [Google Scholar]
- Żyla K., Grabacka M., Pierzchalska M., Duliński R., Starzyńska A. Effect of inositol and Phytase on hematological indices and α-1 acid glycoprotein levels in laying hens fed phosphorus-deficient cornsoybean meal-based diets. Poult. Sci. 2013;92:199–204. doi: 10.3382/ps.2012-02651. [DOI] [PubMed] [Google Scholar]