Abstract
Two independent studies were performed, each with a 3 × 2 factorial arrangement to compare the response in broilers and turkeys to phytase and xylanase supplementation on cecal fermentation and microbial populations. For both studies, 960 Ross 308 and 960 BUT 10 (1-day-old) were allocated to 1 of 6 experimental treatments: (1) control diet, containing the standard dose (100 g/ton) of phytase (STD-Xyl); (2) the control diet with 100 g/ton of xylanase (STD + Xyl); (3) the control diet supplemented on top with 2 fold the standard dose of phytase (200 g/ton), also referred as superdosing (SD-Xyl); (4) the superdosed diet with 100 g/ton of xylanase (SD + Xyl); (5) the control diet supplemented with 5-fold the standard dose of phytase (500 g/ton), also referred as megadosing (MD-Xyl); and (6) the megadosed diet with 100 g/ton of xylanase (MD + Xyl). Each treatment had 8 replicates of 20 animals. Broiler and turkey diets, based on wheat, soybean meal, rapeseed, and barley, and water were available ad libitum. On day 28, the cecal contents from 5 birds per pen were collected. The profile of short-chain fatty acids (SCFA) and microbiome structure (by % guanidine and cytosine [G + C] method) were analyzed. Selected % G + C fractions were used for 16S rDNA sequencing for the identification of bacteria. No treatment effects were noted on SCFA concentrations in either broilers or turkeys. Broilers fed MD diets had greater proportions of unclassified Clostridiales, Mollicutes (RF9) and Faecalibacterium. Xylanase supplementation in broilers resulted in lower proportions of Lactobacillus but increased Mollicutes (RF9), unclassified Ruminococcus, unclassified Clostridiales, and Bifidobacterium. The microbiome in turkeys was unaffected by phytase supplementation, but xylanase supplementation increased the proportions of Lachnospiraceae (Incertae sedis), Lactobacillus, and Bifidobacterium. Supplementation of turkey diets with increasing doses of phytase did not affect the cecal microbiota in contrast to what was observed in broilers. In contrast, xylanase supplementation in both species led to significant changes in the microbial populations, suggesting a positive influence through the provision of oligosaccharides.
Key words: exogenous enzymes, short-chain fatty acids, microbiota, broiler, turkey
Introduction
Exogenous enzymes to date have targeted host animal nutrition by destruction of antinutrients, increasing nutrient digestibility and hence improving animal performance (Choct and Annison, 1990). However, it has been known for more than 20 yr and recently re-emphasized that through their actions, nonstarch polysaccharide degrading enzymes produce nutrients for specific beneficial populations of bacteria, indicating that they are multifactorial in their effects (Bedford and Cowieson, 2012). For example, xylanase ameliorates the antinutritive effects of nonstarch polysaccharides by degrading soluble arabinoxylans, thus reducing digesta viscosity and improving nutrient digestibility (Bedford, 2002), but they have also been shown to release xylo-oligosaccharides (XOS) as a result of xylan degradation (Morgan et al., 2017). The presence of XOS in the distal sections of the gastrointestinal tract (GIT) may have a positive effect on the host as these molecules act as prebiotics or as signaling molecules for specific groups of beneficial bacteria influencing the production of short-chain fatty acids (SCFA) (Samanta et al., 2015, Bedford, 2018). As hypothesized De Maesschalck et al. (2015), the positive outcomes observed by XOS supplementation in broilers may be because of the direct stimulation of lactate producing bacteria, lactate being further fermented to butyrate in the large intestine. Thus, the beneficial effects resulting from inclusion of xylanases in poultry diets could result from the production of XOS and butyrate in addition to their direct activity on the soluble and viscous arabinoxylans.
Phytase can reduce the antinutritional effect of phytate catalyzing the stepwise hydrolysis of inositol phosphate esters (InsPs) and myo-inositol. As a result, phytase improves the digestibility of phosphorous (P), calcium (Ca), amino acids, and energy, as well as reduces excretion of inorganic P into the environment (Humer et al., 2015). Structural and functional changes of the microbiota in the GIT of broilers in response to phytase addition have also been shown (Ptak et al., 2015, Borda-Molina et al., 2016), possibly through alteration of nutrient flow. The combination of higher concentrations of phytase with xylanase in broiler diets has been shown to improve broiler performance (dos Santos et al., 2017), but comparatively little is known about the effects of phytase and xylanase supplementation in turkey rations. In presented the broiler and turkey, comparative response to phytase and xylanase supplementation on performance, nutrient digestibility, and ileal phytate degradation. This article aims to explore the response to phytase and xylanase in the cecal fermentation and the microbiota populations structure in broilers and turkeys from Olukosi et al. (2020).
Materials and methods
Birds and Housing
All the animal experiment procedures in the current studies were approved by Scotland's Rural College's Animal Experiment Committee.
A total of 960 each of Ross 308 male broilers and BUT 10 turkey poults were used in the 2 experiments. Upon arrival, birds were placed immediately in 48 floor pens with white pine shaving in environmentally controlled rooms, with 20 birds per pen on day 0. Each pen for broilers was 2.1 m2 in size, whereas the pens for turkeys were 1.7 m2. All pens were equipped with a hopper feeder and a bell drinker. Test diets and water were provided ad libitum throughout the trials. The rooms were preheated to 36°C 2 D before the commencement of the studies and kept at 36°C for the first 2 D. Then rooms temperature was gradually reduced to 23°C on day 21 (25°C for turkey) and were kept at 22°C (23°C–24°C for turkeys) until the end of the trials. From day 1, the dark hours were decreased daily by 1 h from 24 h light until the light–dark cycle were 18 h light and 6 h dark daily.
Experimental Diets
Wheat, soybean meal, rapeseed, and barley were used as primary ingredients to formulate the experimental diets that met breeder recommendations for Ross 308 broilers and BUT 10 turkeys fed in 1 phase from 0 to 28 D of age. The compositions of the experimental diets and the analyzed chemical composition are shown in Table 1 and Table 2, respectively. For each animal species, 1 basal diet was made, then split equally into 6 subsamples each of which were supplemented with the experimental products: (1) control diet, containing the standard dose (100 g/ton) of phytase (Quantum Blue 5G; AB Vista, Marlborough, UK; 5,000 FTU/g) without xylanase (STD-Xyl); (2) the control diet with 100 g/ton of xylanase (Econase XT 25P; AB Vista, Marlborough, UK; 160,000 BXU/g) (STD + Xyl); (3) the control diet supplemented with 2-fold the standard dose of phytase (200 g/ton), referred to as superdosing (1,500 FTU/kg), without xylanase (SD-Xyl); (4) the superdosed diet with 100 g/ton of xylanase (SD + Xyl); (5) the control diet supplemented with 5-fold the standard dose of phytase (500 g/ton), referred to as megadosing (3,000 FTU/kg), without xylanase (MD-Xyl); and (6) the megadosed diet with 100 g/ton of xylanase (MD + Xyl) resulting in 6 experimental treatments (Table 3). Diets were presented in mash form. Experimental diets did not contain any coccidiostat, antibiotic, or any other growth promoter.
Table 1.
Ingredient and calculated composition (%) of the experimental basal diets.
| Items | Broiler | Turkey |
|---|---|---|
| Wheat | 62.53 | 50.00 |
| Barley | 5.00 | 5.00 |
| Soybean meal | 17.62 | 26.34 |
| Rapeseed meal | 10.00 | 11.77 |
| Soya oil | 0.50 | 1.03 |
| Salt | 0.22 | 0.18 |
| Limestone | 0.66 | 0.79 |
| Dicalcium phosphate | 1.01 | 2.45 |
| Sodium bicarbonate | 0.10 | 0.10 |
| L-Tryptophan | 0.05 | 0.00 |
| Lysine HCl | 0.55 | 0.65 |
| DL-Methionine | 0.33 | 0.33 |
| L-Threonine | 0.23 | 0.19 |
| L-Valine | 0.19 | 0.12 |
| Trace mineral-vitamin premix1 | 0.50 | 0.50 |
| Quantum Blue 5G2 | 0.01 | 0.01 |
| Titanium oxide | 0.5 | 0.5 |
| Total | 100 | 100 |
| Calculated composition (% as feed) | ||
| AME, kcal/kg | 2,800 | 2,700 |
| Crude protein | 20.52 | 24.00 |
| Ca | 0.90 | 1.34 |
| Available P | 0.31 | 0.60 |
| Fat | 2.13 | 2.65 |
| Crude fiber | 3.33 | 3.58 |
| D Met + Cys | 0.92 | 1.01 |
| D Lys | 1.25 | 1.56 |
| D Trp | 0.26 | 0.25 |
| D Thr | 0.82 | 0.91 |
| D Val | 0.95 | 1.04 |
Vitamin/mineral premix supply per kilogram of diet: vitamin A, 16,000 IU; vitamin D3, 3,000 IU; vitamin E, 25 IU; vitamin B1, 3 mg; vitamin B2, 10 mg; vitamin B6, 3 mg; vitamin B12, 15 μg; nicotinic acid, 60 mg; pantothenic acid, 14.7 mg; folic acid, 1.5 mg; biotin, 125 μg; choline chloride, 25 mg; Fe as iron sulfate, 20 mg; Cu as copper sulfate, 10 mg; Mn as manganese oxide, 100 mg; Co as cobalt oxide, 1.0 mg; Zn as zinc oxide, 82.222 mg; I as potassium iodide, 1 mg; Se as sodium selenite, 0.2 mg; and Mo as molybdenum oxide, 0.5 mg.
Quantum Blue 5G, AB Vista, Marlborough, UK; 5,000 FTU/g.
Table 2.
Analyzed composition (%, as fed) of the experimental diets.
| Treatment | DM | N | Ca | Na | Mg | Cu, ppm | Fe, pp, | Mn, ppm | Zn, ppm | K | P | InsP6, nmol/g |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Broiler | ||||||||||||
| 1 | 89.3 | 3.37 | 0.74 | 0.11 | 0.16 | 16 | 122 | 115 | 98 | 0.86 | 0.54 | 13,733 |
| 2 | 89.2 | 3.20 | 0.74 | 0.11 | 0.16 | 14 | 125 | 123 | 104 | 0.80 | 0.52 | 15,570 |
| 3 | 89.6 | 3.28 | 0.79 | 0.12 | 0.17 | 14 | 149 | 126 | 109 | 0.83 | 0.58 | 13,183 |
| 4 | 89.5 | 3.29 | 0.80 | 0.13 | 0.18 | 16 | 136 | 124 | 102 | 0.89 | 0.58 | 13,639 |
| 5 | 89.0 | 3.24 | 0.70 | 0.11 | 0.16 | 15 | 121 | 119 | 96 | 0.79 | 0.53 | 13,605 |
| 6 | 89.2 | 3.14 | 0.78 | 0.13 | 0.16 | 18 | 134 | 124 | 104 | 0.81 | 0.56 | 10,581 |
| Turkey | ||||||||||||
| 1 | 89.3 | 3.89 | 1.05 | 0.10 | 0.17 | 16 | 130 | 107 | 103 | 0.84 | 0.72 | 8,037 |
| 2 | 89.2 | 3.90 | 1.11 | 0.10 | 0.18 | 15 | 124 | 108 | 104 | 0.96 | 0.77 | 8,098 |
| 3 | 89.6 | 3.79 | 1.02 | 0.08 | 0.17 | 19 | 122 | 100 | 94 | 0.88 | 0.73 | 7,651 |
| 4 | 89.5 | 3.83 | 1.16 | 0.11 | 0.18 | 18 | 125 | 126 | 106 | 0.94 | 0.78 | 8,503 |
| 5 | 89.0 | 3.86 | 1.08 | 0.10 | 0.23 | 17 | 123 | 109 | 101 | 0.90 | 0.75 | 8,396 |
| 6 | 89.2 | 3.87 | 1.07 | 0.10 | 0.17 | 16 | 115 | 102 | 98 | 0.90 | 0.75 | 7,824 |
Table 3.
Analyzed enzyme activities in feed samples.
| Treatments1 |
Broiler |
Turkey |
|||||
|---|---|---|---|---|---|---|---|
| Number | Identification | Phytase (FTU/kg) | Xylanase (BXU/kg) | Phytase2 (FTU/kg) | Xylanase3(BXU/kg) | Phytase (FTU/kg) | Xylanase (BXU/kg) |
| 1 | STD-Xyl | 500 | 0 | 750 | <2,000 | 954 | <2,000 |
| 2 | STD + Xyl | 500 | 16,000 | 865 | 16,100 | 994 | 16,100 |
| 3 | SD-Xyl | 1,500 | 0 | 1,190 | <2,000 | 2,880 | <2,000 |
| 4 | SD + Xyl | 1,500 | 16,000 | 2.250 | 14,300 | 1,840 | 15,000 |
| 5 | MD-Xyl | 3,000 | 0 | 2,720 | <2,000 | 3,790 | <2,000 |
| 6 | MD + Xyl | 3,000 | 16,000 | 3,930 | 17,400 | 2,460 | 13,300 |
Diets consisted in 6 experimental treatments: STD-Xyl (diet containing standard dose of phytase without xylanase), STD + Xyl (diet containing standard dose of phytase with xylanase), SD-Xyl (diet containing superdosing of phytase without xylanase), SD + Xyl (diet containing superdosing with xylanase), MD-Xyl (diet containing megadosing of phytase without xylanase) and MD + Xyl (diet containing megadosing with xylanase).
One FTU is defined as the amount of enzyme required to release 1 μmol of inorganic P per minute from sodium phytate at 37°C and pH 5.5.
One BXU is defined as the amount of enzyme that produces 1 nmol reducing sugars from birchwood xylan in 1 s at 50°C and pH 5.3.
Experimental Procedures
For both experiments, on day 28, 5 birds from each of the 48 floor pens were randomly selected and euthanized by cervical dislocation. The total GIT was removed immediately from the abdominal cavity. The digesta content from the ceca was immediately collected by gently squeezing each section into a tube and pooled per pen, rapidly frozen on dry ice, and stored at −20°C for subsequent analysis of SCFA and the microbial populations structure by the percentage of guanidine and cytosine (G + C) method.
Sample Analyses
Feed samples were analyzed for dry matter, nitrogen, and minerals. The analyses were performed according to (AOAC, 2006) Official Methods. Dry matter was determined by drying the samples in a drying oven (Uniterm, Russel-Lindsey Engineering Ltd., Birmingham, England, UK) at 100°C for 24 h (AOAC Method 934.01). Total nitrogen content was determined by the Dumas combustion method (Method 968.06). Mineral content was determined using inductively coupled plasma–optical emission spectroscopy following digestion, in turn, in concentrated HNO3 and HCl.
The SCFA were analyzed as free acids by gas chromatography, using pivalic acid as an internal standard (Apajalahti et al., 2019). Briefly, 1 mL of H2O was mixed with 1 g of ceca content, and then 1 mL of 20 mmol/L pivalic acid solution was added as an internal standard. After mixing, 1 mL of perchloric acid was added, and SCFA were extracted by shaking the mixture for 5 min. After centrifugation, perchloric acid in the supernatant was precipitated by adding 50 μL of 4 mol KOH in 500 μL of supernatant. After 5 min, saturated oxalic acid was added, and the mixture incubated at 4°C for 60 min and then centrifuged at 18,000 × g for 10 min. Samples were analyzed by gas chromatography using a glass column packed with 80/120 Carbopack B-DA/4% Carbowax 20 mol stationary phase (Supelco, Bellefonte, PA), using helium as the carrier gas and a flame ionization detector. The acids measured were acetic, propionic, butyric, iso-butyric, 2-methyl-butyric, iso-valeric, and lactic acid.
Cecal digesta samples pooled from 5 birds per pen were collected, and bacterial DNA was extracted as previously described (Apajalahti et al., 1998). The DNA samples were then fractionated by 72 h CsCl equilibrium density gradient ultracentrifugation (100,000 × g), which separates chromosomes with different G + C content (Apajalahti et al., 1998). This separation is based on differential density imposed by the adenine–thymine–dependent DNA-binding dye bisbenzimidazole. Following the ultracentrifugation, the formed gradients were pumped through a flow-through UV absorbance detector set to 280 nm, and % G + C fractions were collected at 5 to 7% intervals. Finally, the % G + C content represented by each gradient fraction was determined by linear regression analysis (r2 > 0.99).
Fifteen pooled % G + C fractions were used for 16S rDNA sequencing. Altogether 15 DNA pools were subjected to desalting with PD-10 columns (GE Healthcare, UK) for subsequent 16S rRNA gene PCR amplification with the universal broad-range primer pair. The PCR products were sequenced with the Illumina MiSeq (Illumina, San Diego, CA) next-generation sequencing platform. Raw sequence data were subjected to standard next-generation sequencing data preprocessing and data analysis: demultiplexing of all libraries for each sequence lane using Illumina bcl2fastq 2.17.1.14 software (Illumina), sorting of reads by amplicon inline barcodes, clipping of the adapters, primer-based sorting, sequence alignment, combining of the forward and reverse reads using BBMerge 34.48, generating consensus sequences, and grouping. 16S rRNA gene data were processed, and operational taxonomic units (OTU) were picked from amplicons with Mothur 1.35.1 program: alignment was done against 16S rRNA SILVA SEED r119 reference alignment; short alignments (truncated or unspecific PCR products) and chimeras were filtered; sequences were taxonomically classified against the SILVA databases and sequences from other domains of life were removed; OTU were picked by clustering at the 97% identity level; and OTU consensuses were taxonomically classified to genus level.
Operational taxonomic units diversity analysis was performed with QIIME 1.9.0 (Caporaso et al., 2010): within-sample diversity was analyzed at minimum and median sample sequence count rarefaction levels (“alpha diversity”), including creation of plots and tables with taxonomical sample composition; between-sample diversity was analyzed at minimum and median sample sequence count rarefaction levels (“beta diversity”).
Statistical Analysis
The effect of treatments for SCFA for each study were subjected to 2-way analysis of variance using JMP 14 Pro (SAS). Additionally, the effect of bird species for SCFA were subjected to one-way analysis of variance. The effect of treatments for % G + C profile were subjected to t-test analysis. Pen was the experimental unit. Means were separated only when the treatment P-value was significant and then by using the least significant difference test. Statements of significance were based on P-value of equal to or less than 0.05.
Results
All growth performance, ileal digestibility, and digesta inositol phosphate concentration results from the experiments are presented in (Olukosi et al., 2020). The nutrient profile in the diets (Table 2) and the xylanase activities (Table 3) were met, but the analyzed phytase in the diets were greater than expected (Table 3).
Cecal Fermentation
The effects of experimental treatments on the concentration of SCFA in the cecum of broilers and turkeys at 28 D of age are presented in Tables 4 and 5, respectively. There were no treatment effects observed for any of the SCFA measured in broilers or turkeys.
Table 4.
| Phytase, FTU/kg | Xylanase, BXU/kg | Acetic | Propionic | Butyric | Iso-butyric | 2-Me-butyric | Iso-valeric | Lactic | BCFAs4 | VFAs5 | Total SCFAs6 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Main effects means for phytase | |||||||||||
| 500 (STD) | 90.69 | 4.39 | 22.61 | 1.00 | 0.21 | 0.31 | 9.19 | 1.52 | 119.19 | 128.63 | |
| 1,500 (SD) | 93.09 | 4.04 | 21.93 | 0.92 | 0.11 | 0.23 | 10.69 | 1.25 | 120.25 | 131.13 | |
| 3,000 (MD) | 94.10 | 4.01 | 23.40 | 0.91 | 0.08 | 0.21 | 9.94 | 1.19 | 122.75 | 132.63 | |
| Least significant difference | 8.56 | 0.78 | 4.26 | 0.12 | 0.26 | 0.22 | 4.91 | 0.48 | 11.22 | 13.26 | |
| Main effect means for xylanase | |||||||||||
| 0 (−Xyl) | 91.89 | 4.08 | 22.28 | 0.97 | 0.15 | 0.29 | 10.04 | 1.41 | 119.63 | 129.88 | |
| 16,000 (+Xyl) | 93.37 | 4.22 | 23.01 | 0.91 | 0.10 | 0.20 | 9.83 | 1.23 | 121.83 | 131.71 | |
| Least significant difference | 6.99 | 0.63 | 3.48 | 0.10 | 0.21 | 0.18 | 4.01 | 0.39 | 9.16 | 10.82 | |
| Standard deviation | 11.99 | 1.09 | 5.97 | 0.16 | 0.36 | 0.31 | 6.88 | 0.68 | 15.73 | 18.58 | |
| P-value for main effects and interaction3 | |||||||||||
| Phytase | 0.719 | 0.552 | 0.778 | 0.217 | 0.549 | 0.613 | 0.825 | 0.332 | 0.808 | 0.832 | |
| Xylanase | 0.676 | 0.652 | 0.668 | 0.219 | 0.625 | 0.345 | 0.916 | 0.332 | 0.631 | 0.737 | |
| Phytase × Xylanase | 0.205 | 0.483 | 0.913 | 0.598 | 0.788 | 0.809 | 0.532 | 0.859 | 0.301 | 0.242 | |
Data are means of 5 birds per pen with 8 pens per treatment.
Diets consisted in 6 experimental treatments: STD-Xyl (diet containing standard dose of phytase without xylanase), STD + Xyl (diet containing standard dose of phytase with xylanase), SD-Xyl (diet containing superdosing of phytase without xylanase), SD + Xyl (diet containing superdosing with xylanase), MD-Xyl (diet containing megadosing of phytase without xylanase) and MD + Xyl (diet containing megadosing with xylanase).
Values in the same column with different letters are significantly different (P < 0.05).
BCFA: branched-chain fatty acids (iso-butyric + 2-me-butyric + iso-valeric).
VFA: volatile fatty acids (acetic acid + propionic acid + butyric acid + BCFA).
Total SCFA: total short-chain fatty acids (VFA + lactic acid).
Table 5.
| Phytase, FTU/kg | Xylanase, BXU/kg | Acetic | Propionic | Butyric | Iso-butyric | 2-Me-butyric | Iso-valeric | Lactic | BCFAs4 | VFAs5 | Total SCFAs6 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Main effect means for phytase | |||||||||||
| 500 (STD) | 62.11 | 1.01 | 11.33 | 0.04 | 0.05 | 0.00 | 25.69 | 0.09 | 74.69 | 100.25 | |
| 1,500 (SD) | 63.78 | 1.28 | 11.83 | 0.12 | 0.03 | 0.03 | 23.70 | 0.17 | 77.04 | 100.88 | |
| 3,000 (MD) | 67.31 | 1.51 | 11.88 | 0.09 | 0.00 | 0.02 | 29.56 | 0.11 | 80.69 | 110.31 | |
| Least significant difference | 8.42 | 0.60 | 3.61 | 0.11 | 0.07 | 0.05 | 10.02 | 0.14 | 11.85 | 9.30 | |
| Main effect means for xylanase | |||||||||||
| 0 (−Xyl) | 65.37 | 1.40 | 12.05 | 0.09 | 0.00 | 0.01 | 25.13 | 0.10 | 78.92 | 104.13 | |
| 16,000 (+Xyl) | 63.43 | 1.14 | 11.31 | 0.08 | 0.05 | 0.02 | 27.51 | 0.14 | 76.02 | 103.50 | |
| Least significant difference | 6.87 | 0.49 | 2.95 | 0.09 | 0.06 | 0.04 | 8.18 | 0.11 | 9.68 | 7.59 | |
| Standard deviation | 11.79 | 0.84 | 5.06 | 0.15 | 0.10 | 0.07 | 14.03 | 0.20 | 16.60 | 13.02 | |
| P-value for main effects and interactions3 | |||||||||||
| Phytase | 0.452 | 0.254 | 0.943 | 0.353 | 0.344 | 0.541 | 0.500 | 0.505 | 0.592 | 0.062 | |
| Xylanase | 0.577 | 0.310 | 0.623 | 0.733 | 0.069 | 0.759 | 0.564 | 0.496 | 0.554 | 0.870 | |
| Phytase × Xylanase | 0.862 | 0.139 | 0.922 | 0.465 | 0.344 | 0.203 | 0.093 | 0.428 | 0.838 | 0.166 | |
Data are means of 5 birds per pen with 8 pens per treatment.
Diets consisted in 6 experimental treatments: STD-Xyl (diet containing standard dose of phytase without xylanase), STD + Xyl (diet containing standard dose of phytase with xylanase), SD-Xyl (diet containing superdosing of phytase without xylanase), SD + Xyl (diet containing superdosing with xylanase), MD-Xyl (diet containing megadosing of phytase without xylanase) and MD + Xyl (diet containing megadosing with xylanase).
Values in the same column with different letters are significantly different (P < 0.05).
BCFA: branched-chain fatty acids (iso-butyric + 2-me-butyric + iso-valeric).
VFA: volatile fatty acids (acetic acid + propionic acid + butyric acid + BCFA).
Total SCFA: total short-chain fatty acids (VFA + lactic acid).
Microbial Population Structure
The effects of experimental treatments on the percentage of G + C in the cecum of broilers and turkeys at 28 D of age are presented in Figure 1A and B, respectively. The regions between 28 and 29% G + C fraction (P < 0.05) and 73 to 75% G + C fraction (P < 0.05) in broiler chickens were significantly lesser in abundance in birds fed the standard dose of phytase compared with those fed the megadosed diet (Figure 1A). In turkeys, no differences were observed when all treatments were considered in the statistical analysis (Figure 1B).
Figure 1.
Effect of phytase and xylanase on cecal microbiota of broiler chickens (A) and turkeys (B) at 28 D of age. Diets consisted in 6 experimental treatments: STD-Xyl (diet containing standard dose of phytase without xylanase), STD + Xyl (diet containing standard dose of phytase with xylanase), SD-Xyl (diet containing superdosing of phytase without xylanase), SD + Xyl (diet containing superdosing with xylanase), MD-Xyl (diet containing megadosing of phytase without xylanase) and MD + Xyl (diet containing megadosing with xylanase). Data are means of 5 birds per pen with 8 pens per treatment. The colored regions indicates the % G + C profile which are statistically different (P ≤ 0.05) between treatment groups by Student t-test.
Turkeys differed significantly from broilers with regards to density of population of microbes between 21 to 29, 50 to 61, 64 to 69, and 75% G + C fractions (P < 0.05) (data not shown). The identification of the microbiota profile behind these regions and the comparison between both bird species will be covered in the discussion section.
The effect of phytase on broiler microbiota was explored isolating only STD and MD treatments without xylanase (Figure 2A). MD phytase increased species between 21 and 29% G + C fraction, (P < 0.05) and the 60 to 63% G + C (P ≤ 0.10). In contrast, the highest dose of phytase tended to decrease the numbers of bacteria found in the regions 39 to 41 and 50 to 53% G + C (P ≤ 0.10). Bacteria behind these shifts were identified through 16S rDNA sequencing of the selected % G + C fractions. In % G + C fraction from 24 to 29%, unclassified Ruminococcaceae, Clostridiales, Mollicutes (RF9 group), unclassified Bacteria, and Lactobacillus were the most abundant bacterial genera in both treatments (Figure 2B). High doses of phytase increased the proportion unclassified Clostridiales by approximately 10% and the proportion of Mollicutes (RF9 group) by 4%. In % G + C fraction from 44 to 49%, Lachnospiraceae (Incertae sedis), unclassified Lachnospiraceae, and Lactobacillus were the most abundant bacteria (Figure 2C); however, proportions of the different bacteria did not differ with high doses of phytase when compared with the standard dose. In % G + C fraction from 64 to 69%, Bifidobacterium made up more than half of the total bacterial sequences in both treatments (Figure 2D). Megadosing reduced the proportion of Bifidobacterium by 5% units and increased the proportion Faecalibacterium by approximately 16% units.
Figure 2.
(A) Effect of high doses of phytase (MD) on cecal microbiota of broiler chickens compared to standard doses of phytase (STD) without xylanase at 28 D of age. Data are means of 5 birds per pen with 8 pens per treatment. The tildes (∼) indicate the % G + C profile which are P ≤ 0.10 > 0.05 and asterisks (*) indicate the % G + C profile which are statistically different (P ≤ 0.05) by Student t-test. (B) Distribution of the bacterial genera identified in the low % G + C fraction (24–29%) in broilers fed standard doses of phytase (STD) and high doses of phytase (MD) without xylanase. (C) Distribution of bacterial general identified in the mid % G + C fraction (44–49%) in broilers fed standard doses of phytase (STD) and high doses of phytase (MD) without xylanase. (D) Distribution of bacterial general identified in the high % G + C fraction (64–69%) in broilers fed standard doses of phytase (STD) and high doses of phytase (MD) without xylanase.
The effect of xylanase in broiler microbiota was studied only in treatments having standard doses of phytase without and with xylanase (Figure 3A). Xylanase supplementation tended to increase the abundance of bacteria at % G + C 63 to 64 and to reduce the abundance of 32 to 37%, the latter being mainly attributed to Lactobacillus, Mollicutes (RF9 group), unclassified Ruminococcus, and unclassified Clostridiales (Figure 3B). Broilers receiving xylanase had 9% less Lactobacillus with a concomitant increase in the proportion of Mollicutes (RF9 group), unclassified Ruminococcus, and unclassified Clostridiales. Figure 3C illustrates the distribution of bacteria identified in the high % G + C fractions (64 to 69%) of the STD treatment with and without xylanase. Bifidobacterium was the most abundant genus. Broilers receiving xylanase-supplemented diet had greater proportion of bifidobacterial sequences by 15%.
Figure 3.
(A) Effect of xylanase supplementation in diets with standard dose of phytase (STD) on cecal microbiota of broiler chickens at 28 D of age. Data are means of 5 birds per pen with 8 pens per treatment. The cross marks (†) indicate the % G + C profile which are P ≤ 0.17 > 0.10, and the tildes (∼) indicate the % G + C profile which are P ≤ 0.10 > 0.05 between the treatments by Student t-test. (B) Distribution of the bacterial genera identified in the % G + C fraction 32 to 37%. (C) Distribution of bacterial general identified in the % G + C fraction 64 to 69%.
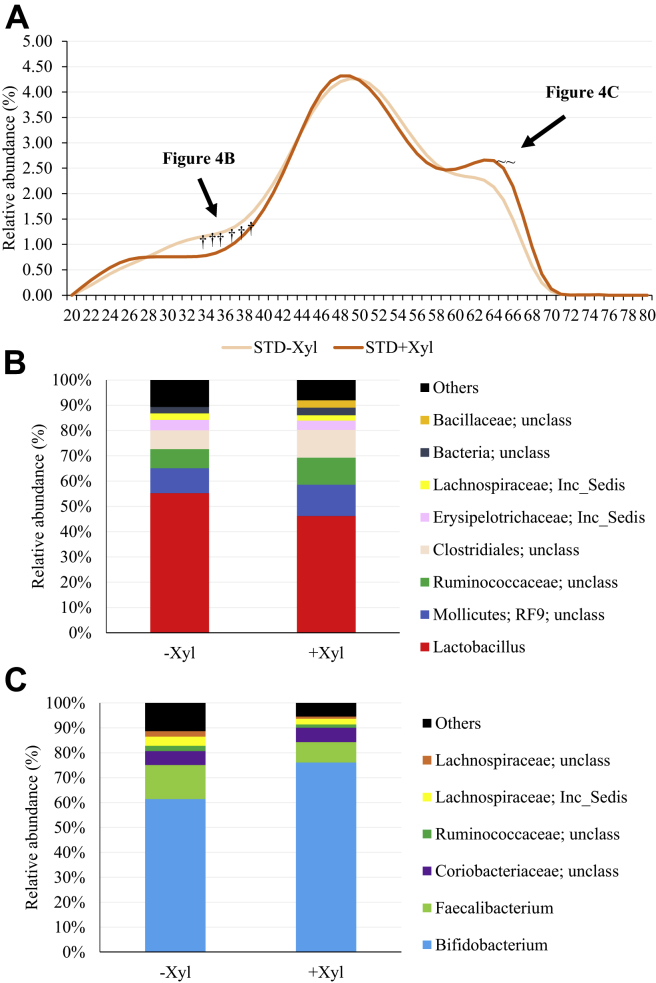
The increasing doses of phytase did not result in any significant change in turkey's microbiota using % G + C profiling (data not shown). However, more interesting results were observed when xylanase was included in the diet regardless the dose of phytase. Figure 4A shows the % G + C shifts of the cecal microbiota of turkeys without and with xylanase, when all phytase doses were combined. Xylanase reduced the abundance of bacteria representing the % G + C region 36 to 48 (P < 0.05) but increased the proportion of bacteria in the % G + C region from 64 to 69% (P < 0.05). Xylanase addition increased bacterial diversity in the % G + C from 44 to 49% (Figure 4B). Lachnospiraceae (I. sedis), unclassified Lachnospiraceae, Anaerofilum, Faecalibacterium, Lactobacillus, Subdoligranulum, and Erysipelotruchaceae (I. sedis) were the most dominant bacterial genera in both treatments. Xylanase supplementation increased proportion of Lachnospiraceae (I. sedis) by 8% and Lactobacillus by 2.5%, but the proportion of Anaerofilum was reduced by about 3% units. Finally, sequence identification of the % G + C region from 64 to 69% is presented in Figure 4C. Sequences of Bifidobacterium dominated this region. Supplementation with xylanase increased the proportion of Bifidobacterium by 13%.
Figure 4.
(A) Effect of xylanase supplementation combining all doses of phytase on cecal microbiota of turkeys at 28 D of age. Data are means of 5 birds per pen with 8 pens per treatment. The tildes (∼) indicate the % G + C profile which are P ≤ 0.10 > 0.05, and asterisks (*) indicate the % G + C profile which are statistically different (P ≤ 0.05) between the treatments by Student t-test. (B) Distribution of bacterial general identified in the % G + C fraction (44 to 49%) in turkeys fed without xylanase (−Xyl) or with xylanase (+Xyl) combining all doses of phytase. (C) Distribution of bacterial general identified in the % G + C fraction (64–69%) in turkeys fed without xylanase (−Xyl) or with xylanase (+Xyl) combining all doses of phytase.
Discussion
The aim of the current experiment was to explore the effects of phytase and xylanase in broilers and turkeys on the concentration of SCFA and the microbiome in the ceca. Diets were formulated to meet or exceed nutrient recommendations fed in 1 single phase from day 0 to day 28. As a result, the diets for both species were different because of their significantly different requirements. This introduces a source of variation which is likely significant to interpretation of the results for comparison purposes. Protein, Ca, and P were considerably greater in turkey compared with broiler diets. High Ca/P diets may induce greater buffering capacity which increases digesta pH and may result in undigested protein ultimately being fermented in the ceca. However, the concentration of BCFA (considered an indication of protein fermentation) in the ceca of turkeys was lower when compared with broilers suggesting no such problem was apparent in this study. The concentration of all SCFA in the ceca of both poultry species indicate differences in nutrient flow, microbial colonization, and gut maturation (Adebiyi and Olukosi, 2015). At day 28, the proportion of lactic acid to the total SCFA in turkeys was 3-fold greater than for broilers. As the animal ages, flow of rapidly fermentable carbohydrates is reduced and the microbiome biodiversity in the hindgut increases, reducing the production of lactic acid at the expense of other SCFA such as acetic acid, propionic acid, or butyric acid (Oakley et al., 2014, Lee et al., 2017, Wilkinson et al., 2017, Gonzalez-Ortiz et al., 2019). Although all the differences regarding animal species and dietary composition, this may indicate that the microbiome in turkeys at 28 D of age is not as mature compared with broilers.
Phytase concentration or xylanase did not influence the concentrations of any of the SCFA in the cecum of broilers or turkeys. Greater butyric acid concentrations in the digesta of broiler chickens have been reported when xylanase has been supplemented (Masey-O'Neill et al., 2014, González-Ortiz et al., 2016, Lee et al., 2017), but this is not always the case, either for broilers (Gonzalez-Ortiz et al., 2019) or turkeys (González-Ortiz et al., 2017). However, when the ceca microbiome of control and xylanase fed birds were used as inoculum in an ex vivo fermentation study, the inoculum from xylanase supplemented birds produced significantly more butyric acid than the control birds (Bedford and Apajalahti, 2018), suggesting that the exposure of a broiler to a xylanase augments the capacity of the microbiome to utilize xylan-containing fiber sources, which has implications for intestinal function. Butyric acid is the preferred energy source for colonocytes and influences the integrity of the gut epithelium (Lopetuso et al., 2013).
The effect of phytase on SCFA production is less straight-forward. On the one hand, there is a minimal P requirement for the fermentation of carbohydrates as observed with rumen bacteria (Komisarczuk et al., 1987), thus differences in the concentration of P arriving at the hindgut due to phytase use could be thought to be unimportant on cecal fermentation. However, Ptak et al., (2015) observed that reducing the Ca/P content in the diet was related to a reduction in total SCFA, DL-lactate, and acetic acid in the ileum and that phytase addition increased concentrations of these acids only in birds fed the mineral reduced diets suggesting that phosphate supply may well have been a limiting factor for fermentation in the ileum. Phytase supplementation increased acetic acid and butyric acid concentrations in the cecal digesta of 29-day-old chickens (Smulikowska et al., 2010), whereas phytase supplementation did not affect ileal concentrations of SCFA, but reduced butyric acid concentrations in the excreta of pigs (Metzler-Zebeli et al., 2010). Such variable results suggest the mechanisms by which these effects are brought about are poorly comprehended.
It is worth noting that the concentration of an acid in the ceca does not necessarily reflect the rate of its production by bacteria. Approximately 95 to 99% of SCFA produced in the hindgut are absorbed (von Engelhardt et al., 1998), and the rate of absorption from the intestine into the blood is extremely fast (Pouteau et al., 2008). Moreover, digesta flow is dynamic, and reverse peristalsis may result in variability in local concentrations of the different SCFA (Godwin and Russell, 1997). Thus the poor repeatability and reproducibility of SCFA data is not surprising, and in fact, their value in interpretation of treatment effects is questionable.
Differences in the cecal microbiome between broilers and turkeys were observed. In the current study, the biggest differences were observed in the mid region of the G + C profile (44–49%), where broilers had a lower diversity in bacterial composition compared with turkeys. Lachnospiraceae (Incertae Sedis), unclassified Lachnospiraceae, Lactobacillus, Faecalibacterium, and Ruminoccocus (Incertae Sedis) were common to both species, but turkeys had at least 10 other genera vs. the 4 additional genera sequenced in broilers. Bacteria sequenced from the other regions of the G + C profile in broilers and turkeys were similar in identity but not in proportions. Unclassified Ruminococcaceae, unclassified Clostridiales, Mollicutes (RF9 group), and Bifidobacterium were the other dominant groups present in both broilers and turkeys. In spite of the variety of techniques and procedures established in different labs, the composition of the microbiome obtained in this study are in agreement with others previously reported. According to Borda-Molina et al., (2018), the most abundant families within the cecum of broilers are Clostrodicaceae, Bacteroidaceae, Lactobacillaceae, and butyrate producers such as Lachnospiraceae. Wilkinson et al., (2017) identified Lactobacillus, Streptococcus, and Clostridium XI as the dominant bacteria in the caecum of turkeys.
Xylanase supplementation increased the proportion of Bifidobacterium in the ceca of both broilers and turkeys. These results are in agreement with Lee et al., (2017) who also observed an increase of Bifidobacterium counts of broiler chickens supplemented with xylanase for 42 D. Bifidobacteria produce acetate and lactate as end products of sugar fermentation. One of the reasons for such effects may be because of the release of XOS as a result of xylan degradation in the distal sections of the GIT in the presence of xylanase (Bedford, 2018). The beneficial effects of XOS on broiler performance can be explained by the direct stimulation of lactate producing bacteria, such as Bifidobacterium. Butyrate producing bacteria can utilize lactate to further ferment to butyrate in the large intestine as hypothesized by De Maesschalck et al., (2015). Although in the current study, the increase in Bifidobacterium proportions were not linked to higher concentrations of SCFA measured, other studies have demonstrated that XOS selectively increases the abundance of Bifidobacterium and the production of butyrate and acetate (Van Craeyveld et al., 2008). In any case, in this study, xylanase supplementation improved feed conversion ratio in broilers and turkeys (Olukosi et al., 2020), suggesting a possible link between this bacterial group and performance.
In the current study, the highest concentration of phytase coincided with some changes in the microbiota populations in the ceca of broilers. Faecalibacterium, unclassified Clostridiales, and unclassified Mollicutes (RF9 group) were increased with megadosing, whereas the proportion of Bifidobacterium decreased. Faecalibacterium is one of the most prominent genus in the hindgut and includes butyrate producers. As noted earlier, butyrate provides energy to the colonic mucosa and is known to regulate gene expression, inflammation, differentiation, and apoptosis in host cells (Luo et al., 2013). Regarding Clostridiales group, Borda-Molina et al., (2016) identified a genus of Clostridium associated with the production of a cysteine phytase. In addition, other bacterial species belonging to Megasphaera elsdenii and Mitsuokella spp., common members of the rumen microbiota which have the ability to produce phytases (Yanke et al., 1998), were also detected in the ileum and ceca samples from birds receiving diets supplemented with Ca, P, or P with phytase (Borda-Molina et al., 2016), and although these were not identified in the current study, we cannot discard their participation. P, Ca, and phytase influence the microbiome (Witzig et al., 2015, Borda-Molina et al., 2016), and it thus can be speculated that microbiome associated phytase activity complemented phytate degradation by phytase (Palacios et al., 2008). No influence of phytase supplementation on the microbiome in turkeys were observed in the current study, and to the best of our knowledge, no such studies have been reported previously. Broilers possess a greater capacity for InsP6 degradation and hydrolysis for lower inositol phosphates compared with turkeys (Ingelmann et al., 2019, Olukosi et al., 2020), and this difference can be maximized with phytase supplementation. The differences observed between turkeys and broilers in their capacity for InsP6 hydrolysis and P digestibility may be the result of differences in small intestine maturity (Adebiyi and Olukosi, 2015), endogenous P loss, pH along the GIT, passage rate (Rodehutscord and Dieckmann, 2005, Adebiyi and Olukosi, 2015), and differences in their microbiome (Pan and Yu, 2014). Furthermore, when comparing the effects of phytase and xylanase on broilers and turkeys, it is important to recall the differences in diet composition, especially the differences in Ca and P content which has been shown to influence ileal and ceca microbial diversity in broilers (Borda-Molina et al., 2016). It is known that higher doses of Ca in the diets can lead to an increase in pH (Ptak et al., 2015) and lower prececal P digestibility (Adeola and Walk, 2013, Hamdi et al., 2015), which could possibly influence the presence or absence of some bacteria as observed in turkeys by the different G + C profile obtained.
Supplementation of broiler and turkey diets with xylanase resulted in differences in the microbiome in the ceca, which may be due to the release of fermentable XOS, although no effects of xylanase were noted on SCFA in the current study. Supplementation of turkey diets with increasing doses of phytase did not affect the cecal microbiome, contrary to that observed in broilers. There are several factors influencing such differences between bird species; however, the differences in gut maturation, microbiome colonization, feed formulation, passage rate, and pH profile in the different sections of the GIT may have conditioned the microbiome in the cecal of turkeys to the same extent as broilers. For a better understanding of exogenous enzymes effect on broiler and turkey gut function and the microbial community, digesta samples should be studied to determine the rate of oligosaccharide production and InsP6 degradation at different ages until slaughter.
References
- Adebiyi A.O., Olukosi O.A. Metabolizable energy content of wheat distillers' dried grains with solubles supplemented with or without a mixture of carbohydrases and protease for broilers and turkeys. Poult. Sci. 2015;94:1270–1276. doi: 10.3382/ps/pev089. [DOI] [PubMed] [Google Scholar]
- Adeola O., Walk C.L. Linking ileal digestible phosphorus and bone mineralization in broiler chickens fed diets supplemented with phytase and highly soluble calcium. Poult. Sci. 2013;92:2109–2117. doi: 10.3382/ps.2013-03068. [DOI] [PubMed] [Google Scholar]
- AOAC . 18th ed. 2006. Official Methods of Analysis of AOAC International Official Methods of Analysis of AOAC International. AOAC International, Gaithersburg, MD. [Google Scholar]
- Apajalahti J.H.A., Särkilahti L.K., Mäki B.R.E., Pekka Heikkinen J., Nurminen P.H., Holben W.E. Effective recovery of bacterial DNA and percent-guanine-plus-cytosine- based analysis of community structure in the gastrointestinal tract of broiler chickens. Appl. Environ. Microbiol. 1998;64:4084–4088. doi: 10.1128/aem.64.10.4084-4088.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apajalahti J.H.A., Vienola K., Raatikainen K., Holder V., Moran C.A. Conversion of branched-chain amino acids to corresponding isoacids - an in vitro tool for estimating ruminal protein degradability. Front. Vet. Sci. 2019;6:311. doi: 10.3389/fvets.2019.00311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedford M.R. The role of carbohydrases in feedstuff digestion. In: McNab J.M., Boorman K.N., editors. Poultry Feedstuffs: Supply, Composition and Nutritive Value. CABI; Roslin, Midlothian, UK: 2002. pp. 319–336. [Google Scholar]
- Bedford M.R. The evolution and application of enzymes in the animal feed industry: the role of data interpretation. Br. Poult. Sci. 2018;59:486–493. doi: 10.1080/00071668.2018.1484074. [DOI] [PubMed] [Google Scholar]
- Bedford M.R., Apajalahti J. 2018. Exposure of a Broiler to a Xylanase for 35d Increases the Capacity of Cecal Microbiome to Ferment Soluble Xylan. Proc. Poultry Science Association 107th Annual Meeting, San Antonio, Texas, US. E-Supplement 1; pp. 98–99. [Google Scholar]
- Bedford M.R., Cowieson A.J. Exogenous enzymes and their effects on intestinal microbiology. Anim. Feed Sci. Tech. 2012;173:76–85. [Google Scholar]
- Borda-Molina D., Vital M., Sommerfeld V., Rodehutscord M., Camarinha-Silva A. Insights into broilers' gut microbiota fed with phosphorus, calcium, and phytase supplemented diets. Front. Microbiol. 2016;7:2033. doi: 10.3389/fmicb.2016.02033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borda-Molina D., Seifert J., Camarinha-Silva A. Current perspectives of the chicken gastrointestinal tract and its microbiome. Comput. Struct. Biotechnol. J. 2018;16:131–139. doi: 10.1016/j.csbj.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso J.G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F.D., Costello E.K., Fierer N., Peña A.G., Goodrich J.K., Gordon J.I., Huttley G.A., Kelley S.T., Knights D., Koenig J.E., Ley R.E., Lozupone C.A., McDonald D., Muegge B.D., Pirrung M., Reeder J., Sevinsky J.R., Turnbaugh P.J., Walters W.A., Widmann J., Yatsunenko T., Zaneveld J., Knight R. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choct M., Annison G. Anti-nutritive activity of wheat pentosans in broiler diets. Br. Poult. Sci. 1990;31:811–821. doi: 10.1080/00071669008417312. [DOI] [PubMed] [Google Scholar]
- De Maesschalck C., Eeckhaut V., Maertens L., De Lange L., Marchal L., Nezer C., De Baere S., Croubels S., Daube G., Dewulf J., Haesebrouck F., Ducatelle R., Taminau B., Van Immerseel F. Effects of xylo-oligosaccharides on broiler chicken performance and microbiota. Appl. Environ. Microbiol. 2015;81:5880–5888. doi: 10.1128/AEM.01616-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- dos Santos T.T., Masey O'Neill H.V., González-Ortiz G., Camacho-Fernández D., López-Coello C. Xylanase, protease and superdosing phytase interactions in broiler performance, carcass yield and digesta transit time. Anim. Nutr. 2017;3:121–126. doi: 10.1016/j.aninu.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godwin I.R., Russell W.J. Reverse peristalsis in the chicken digestive tract. In: Corbett J.L., Choct M., Nolan J.V., Rowe J.B., editors. Proceedings of the Recent Advances in Animal Nutrition in Australia. University of New England; Armidale, Australia: 1997. p. 229. [Google Scholar]
- González-Ortiz G., Vienola K., Apajalahti J., Bedford M.R. 2016. Xylanase Supplementation Influences Performance and Intestinal Fermentation in Broiler Chickens. Proc. Gut Microbiology. 10th Symposium INRA-Rowett 2016, Clermont-Ferrand, France; p. 128. [Google Scholar]
- González-Ortiz G., Kozłowski K., Drażbo A., Bedford M.R. Response of turkeys fed wheat-barley-rye based diets to xylanase supplementation. Anim. Feed Sci. Tech. 2017;229:117–123. [Google Scholar]
- Gonzalez-Ortiz G., Dos Santos T.T., Vienola K., Vartiainen S., Apajalahti J., Bedford M.R. Response of broiler chickens to xylanase and butyrate supplementation. Poult. Sci. 2019;98:3914–3925. doi: 10.3382/ps/pez113. [DOI] [PubMed] [Google Scholar]
- Hamdi M., Lopez-Verge S., Manzanilla E.G., Barroeta A.C., Perez J.F. Effect of different levels of calcium and phosphorus and their interaction on the performance of young broilers. Poult. Sci. 2015;94:2144–2151. doi: 10.3382/ps/pev177. [DOI] [PubMed] [Google Scholar]
- Humer E., Schwarz C., Schedle K. Phytate in pig and poultry nutrition. J. Anim. Physiol. Anim. Nutr. (Berl) 2015;99:605–625. doi: 10.1111/jpn.12258. [DOI] [PubMed] [Google Scholar]
- Ingelmann C.J., Witzig M., Mohring J., Schollenberger M., Kuhn I., Rodehutscord M. Phytate degradation and phosphorus digestibility in broilers and turkeys fed different corn sources with or without added phytase. Poult. Sci. 2019;98:912–922. doi: 10.3382/ps/pey438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komisarczuk S., Durand M., Beaumatin P., Hannequart G. Effects of phosphorous deficiency on rumen microbial activity associated with the solid and liquid phase of a fermenter. Reprod. Nutr. Dev. 1987;27:907–919. doi: 10.1051/rnd:19870703. [DOI] [PubMed] [Google Scholar]
- Lee S.A., Apajalahti J., Vienola K., González-Ortiz G., Fontes C.M.G.A., Bedford M.R. Age and dietary xylanase supplementation affects ileal sugar residues and short chain fatty acid concentration in the ileum and caecum of broiler chickens. Anim. Feed Sci. Tech. 2017;234:29–42. [Google Scholar]
- Lopetuso L.R., Scaldaferri F., Petito V., Gasbarrini A. Commensal Clostridia: leading players in the maintenance of gut homeostasis. Gut Pathog. 2013;5:23. doi: 10.1186/1757-4749-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y.H., Peng H.W., Wright A.D.G., Bai S.P., Ding X.M., Zeng Q.F., Li H., Zheng P., Su Z.W., Cui R.Y., Zhang K.Y. Broilers fed dietary vitamins harbor higher diversity of cecal bacteria and higher ratio of Clostridium, Faecalibacterium, and Lactobacillusthan broilers with no dietary vitamins revealed by 16S rRNA gene clone libraries. Poult. Sci. 2013;92 doi: 10.3382/ps.2012-02935. [DOI] [PubMed] [Google Scholar]
- Masey-O'Neill H.V., Singh M., Cowieson A.J. Effects of exogenous xylanase on performance, nutrient digestibility, volatile fatty acid production and digestive tract thermal profiles of broilers fed on wheat- or maize-based diet. Br. Poult. Sci. 2014;55:351–359. doi: 10.1080/00071668.2014.898836. [DOI] [PubMed] [Google Scholar]
- Metzler-Zebeli B.U., Vahjen W., Baumgartel T., Rodehutscord M., Mosenthin R. Ileal microbiota of growing pigs fed different dietary calcium phosphate levels and phytase content and subjected to ileal pectin infusion. J. Anim. Sci. 2010;88:147–158. doi: 10.2527/jas.2008-1560. [DOI] [PubMed] [Google Scholar]
- Morgan N.K., Wallace A., Bedford M.R., Choct M. Efficiency of xylanases from families 10 and 11 in production of xylo-oligosaccharides from wheat arabinoxylans. Carbohydr. Polym. 2017;167:290–296. doi: 10.1016/j.carbpol.2017.03.063. [DOI] [PubMed] [Google Scholar]
- Oakley B.B., Buhr R.J., Ritz C.W., Kiepper B.H., Berrang M.E., Seal B.S., Cox N.A. Successional changes in the chicken cecal microbiome during 42 days of growth are independent of organic acid feed additives. BMC Vet. Res. 2014;10:282. doi: 10.1186/s12917-014-0282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olukosi O.A., González-Ortiz G., Whitfield H., Bedford M.R. Comparative aspects of phytase and xylanase effects on performance, mineral digestibility, and ileal phytate degradation in broilers and turkeys. Poult. Sci. 2020;99:1528–1539. doi: 10.1016/j.psj.2019.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios M.C., Haros M., Sanz Y., Rosell C.M. Selection of lactic acid bacteria with high phytate degrading activity for application in whole wheat breadmaking. LWT-Food Sci. Technol. 2008;41:82–92. [Google Scholar]
- Pan D., Yu Z. Intestinal microbiome of poultry and its interaction with host and diet. Gut Microb. 2014;5:108–119. doi: 10.4161/gmic.26945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouteau E., Rochat F., Jann A., Meirim I., Sanchez-Garcia J.L., Ornstein K., German B., Ballevre O. Chicory increases acetate turnover, but not propionate and butyrate peripheral turnovers in rats. Br. J. Nutr. 2008;99:287–296. doi: 10.1017/S0007114507815790. [DOI] [PubMed] [Google Scholar]
- Ptak A., Bedford M.R., Swiatkiewicz S., Zyla K., Jozefiak D. Phytase modulates ileal microbiota and enhances growth performance of the broiler chickens. PLoS One. 2015;10:e0119770. doi: 10.1371/journal.pone.0119770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodehutscord M., Dieckmann A. Comparative studies with three-week-old chickens, turkeys, ducks, and quails on the response in phosphorus utilization to a supplementation of monobasic calcium phosphate. Poult. Sci. 2005;84:1252–1260. doi: 10.1093/ps/84.8.1252. [DOI] [PubMed] [Google Scholar]
- Samanta A.K., Jayapal N., Jayaram C., Roy S., Kolte A.P., Senani S., Sridhar M. Xylooligosaccharides as prebiotics from agricultural by-products: production and applications. Bioact. Carbohydr. Diet. Fibre. 2015;5:62–71. [Google Scholar]
- Smulikowska S., Czerwiński J., Mieczkowska A. Effect of an organic acid blend and phytase added to a rapeseed cake-containing diet on performance, intestinal morphology, caecal microflora activity and thyroid status of broiler chickens. J. Anim. Physiol. Anim. Nutr. (Berl) 2010;94:15–23. doi: 10.1111/j.1439-0396.2008.00876.x. [DOI] [PubMed] [Google Scholar]
- Van Craeyveld V., Swennen K., Dornez E., Van de Wiele T., Marzorati M., Verstraete W., Delaedt Y., Onagbesan O., Decuypere E., Buyse J., De Ketelaere B., Broekaert W.F., Delcour J.A., Courtin C.M. Structurally different wheat-derived arabinoxylooligosaccharides have different prebiotic and fermentation properties in rats. J. Nutr. 2008;138:2348–2355. doi: 10.3945/jn.108.094367. [DOI] [PubMed] [Google Scholar]
- von Engelhardt W., Bartels J., Kirschberger S., zu Düttingdorf H.D.M., Busche R. Role of short-chain fatty acids in the hind gut. Vet. Q. 1998;20:52–59. [PubMed] [Google Scholar]
- Wilkinson T.J., Cowan A.A., Vallin H.E., Onime L.A., Oyama L.B., Cameron S.J., Gonot C., Moorby J.M., Waddams K., Theobald V.J., Leemans D., Bowra S., Nixey C., Huws S.A. Characterization of the microbiome along the gastrointestinal tract of growing turkeys. Front. Microbiol. 2017;8 doi: 10.3389/fmicb.2017.01089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witzig M., Camarinha da Silva A., Green-Engert R., Hoelzle K., Zeller E., Seifert J., Hoelzle L.E., Rodehutscord M. Spatial variation of the gut microbiota in broiler chickens as affected by dietary available phosphorus and assessed by T-RFLP analysis and 454 pyrosequencing. PLoS One. 2015;10:e0143442. doi: 10.1371/journal.pone.0143442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanke L.J., Bae H.D., Selinger L.B., Cheng K.J. Phytase activity of anaerobic ruminal bacteria. Microbiology. 1998;144(Pt 6):1565–1573. doi: 10.1099/00221287-144-6-1565. [DOI] [PubMed] [Google Scholar]