Abstract
The sperm storage tubules located in the mucosal folds of the uterovaginal junction (UVJ) are the primary site of sperm storage in chicken hens after natural mating or artificial insemination (AI). The short-term sperm storage (24 h after mating or AI) in hens was highly associated with immunity and pH-related pathway genes. However, the underlying mechanism of longer duration of sperm storage in female birds remains largely unclear. In the present study, transcriptome analysis was applied to uncover the dynamic gene expression changes in chicken UVJ tissues at two time points (day 3 and day 9) after AI. A total of 574 differentially expressed genes (DEG) were enriched, including 266 upregulated and 308 downregulated DEG. The validation of 5 DEG using quantitative PCR showed a similar expression tendency with RNA sequencing results. The gene ontology terms of DEG were highly enriched in heparin binding (9 genes including COMP, CTGF, and IMPG2), glycosaminoglycan binding (10 genes including PCOLCE, POSTN, and RSPO3), and response to estradiol and ion transport (AREG, RAMP3, SFRP1, and SSTR1). Kyoto encyclopedia of genes and genomes pathway-enrichment analyses of DEG revealed 10 significant pathways (P < 0.05) represented by calcium signaling pathway (7 genes including CACNA1G, PDE1C, PDGFRB, and SLC8A1) and glycosaminoglycan biosynthesis (B3GNT7, CSGALNACT1, GLCE, and ST3GAL1). Protein-protein interaction network of DEG established the connection-regulating epithelial cell or cell-matrix adhesion and migration. The enriched pathways and genes were highly correlated with temporary sperm storage in and possibly sequential sperm release from chicken UVJ overtime after AI. Of these, HIP1, PDE1C, and calcium-related genes were the most interesting candidates associated with sperm storage duration. This report provided a global gene expression profile of the chicken UVJ regarding the capacity of sperm storage overtime after AI. The outcome of this study will contribute to further understanding of the long-term sperm maintenance in avian females and eventually improving the duration of fertile egg performance by selected chicken breeding.
Key words: artificial insemination, gene expression, sperm storage tubule, transcriptome, uterovaginal junction
Introduction
The sperm storage duration is an interesting biological feature that is observed in many female birds. In hens, sperm storage tubules (SST) that are dispersed in the mucosal folds of the uterovaginal junction (UVJ) in oviduct are the primary site for sperm storage (Bobr, et al., 1964). After mating or artificial insemination (AI), the sperms travelled from vagina and entered into SST, where they appeared as packed bundles with their heads directed toward the blind tubular end of SST, and were maintained in the SSTs up to weeks (Gilbert et al., 1968, Bakst et al., 1994). The sperms contain highly compact DNA with inactivated transcription. The regulation of sperm function was largely relying on their extracellular environment (Sullivan and Saez, 2013). Previous studies showed that female birds had potential impacts to regulate the metabolic activity and motility of the residing sperms (Bakst and Bauchan, 2015) and to protect the sperms against the immune threats from the female oviduct (Das, et al., 2010). A series of proteins (enzymes) and signals associated with sperm storage have been identified including carbonic anhydrase (Holm, et al., 1996), aquaporins (Zaniboni and Bakst, 2004), alkaline phosphatase (Bakst and Akuffo, 2007), avidin, avidin-related protein-2, progesterone receptor (Foye-Jackson, et al., 2011), and transforming growth factor-β and its receptors (Das, et al., 2010). The fatty acids (Huang, et al., 2016) and lactic acid (Matsuzaki, et al., 2015) were reported to have a function in sperm storage (Holt and Fazeli, 2016, Matsuzaki and Sasanami, 2017). The detection of high calcium concentration in SST cells and tubular fluid, which was within the range to inhibit the sperm motility, supports the functional role of calcium signaling during the sperm maintenance and storage (Holm, et al., 2000). A handful of immune- and pH-regulatory genes were previously enriched in the transcriptomes of chicken UVJ tissues under the 2 conditions (24 h after mating vs. unmated hens) (Atikuzzaman et al., 2015, Han et al., 2019b). These results addressed that the sperm maintenance, selection, and release are complex processes (Matsuzaki and Sasanami, 2017). Although the short-term sperm storage is largely an immediate immune response that is conserved between hens and pigs (Atikuzzaman, et al., 2017), the mechanisms regulating the lengthy tolerance of spermatozoa or long-term sperm storage in the UVJ remain mostly unclear.
In this study, we hypothesized that as the number of sperms gradually decreases in SSTs with ovulatory cycle, factors beyond the immune response and pH, that are highly correlated with sperm activity and sperm-related genes, would be changing accordingly. This report aimed to identify the gene network regulating the long-term sperm storage in UVJ tissues of chicken hens and provide further understanding of long-term sperm maintenance and release in reservoir beyond the immediate immune response.
Materials and methods
Ethics Statement
All animal experiments were carried out following the protocols (No. 5 proclaim of the Standing Committee of Hubei People's Congress) approved by the Standing Committee of Hubei People's Congress and the ethics committee of Huazhong Agricultural University, China.
Sample Collection and RNA Isolation for Transcriptome Sequencing
White Leghorn roosters and hens at the age of 30 wk were raised individually in stair-step cages under standard management system and fed with isocaloric and isonitrogenous corn-soybean–based diet twice a day with enough drinking water. All chickens experienced 16 h of light and 8 h of dark a day.
A total of 11 hens were artificially inseminated with mixed semen after performing the quality and quantity check as described in previous reports (Atikuzzaman, et al., 2015). Each female bird was inseminated with 30 μL of mixed semen on the same day (day 0) and raised for sample collection on day 3 after AI (5 individuals) and on day 9 after AI (6 individuals). All the selected hens were sacrificed to collect the UVJ tissues containing SST under stereomicroscope. The UVJ tissues were carefully dissected and divided into 2 parts at an angle perpendicular to the ovary-uterus axis. One half of the UVJ tissues were fixed immediately in 4% paraformaldehyde (in phosphate buffered saline) for histological study, while the other half was snap-frozen in liquid nitrogen for RNA isolation.
Histological Study
The histological examination of UVJ tissues was performed following the standard procedures. Briefly, a piece of UVJ tissues or folds containing SST was cut off under the stereomicroscope and submerged in running water for approximately 20 h. Then, samples were sequentially dehydrated, embedded in paraffin, and processed for section at 5-μm thickness using a microtome (Leica Instruments GmbH). The sections were stained by hematoxylin and eosin and photographed by using a microscopy and imaging system (Olympus, Japan).
RNA Isolation and cDNA Preparation
Each frozen UVJ sample was homogenized in 1 mL of TRIzol Reagent to perform RNA isolation and purification (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. A total of 6 RNA samples (3 samples from day 3 after AI and 3 samples from day 9 after AI) were selected for RNA sequencing program by Novogene Biotechnology Company (NOVOGENE, China). In addition, the total RNA (1 μg) of the samples from 2 groups (2 selected time points) were applied for reverse transcription by using TransScript One-Step gDNA Removal and cDNA Synthesis SuperMix kit (TansGen Biotech, Beijing, China) to perform quantitative PCR validation.
Transcriptome Sequencing and Data Analysis
Sequencing library for mRNA sequencing was carried out following the standard procedure and applied for sequencing using PE-150 at Novogene Biotechnology Company. The sequenced raw data were processed for quality control and mapped to chicken reference genome(ftp://ftp.ensembl.org/pub/release-94/fasta/gallus_gallus/dna/). Index of the reference genome was built, and paired-end clean reads were aligned to the reference genome using Hisat2, version 2.0.5 (Johns Hopkins University Center for Computational Biology, Washington, D.C.). Feature Counts, version 1.5.0-p3 (Bioconductor, Seattle, WA), was used to count the reads mapped to each gene. Then, fragments per kilobase million (FPKM) of each gene was calculated based on the length and reads for estimating gene expression level. Differential expression analysis of 2 groups (3 biological replicates per condition) was performed using the DESeq2 R package (version 1.16.1; Bioconductor). The resulting P values were adjusted using the Benjamini and Hochberg's approach for controlling the false discovery rate. Genes with a P value < 0.05 and | log2 (fold change) | > 0.5 were assigned as threshold for differentially expressed by DESeq2.
Gene Ontology, Kyoto Encyclopedia of Genes and Genomes Enrichment, and Protein-Protein Interaction Analyses of Differentially Expressed Genes.
Gene ontology (GO) enrichment analysis of differentially expressed genes (DEG) was implemented by the clusterProfiler (3.8) R package (Bioconductor), in which gene length bias was corrected. GO terms with corrected P value < 0.05 were considered to be significantly enriched. clusterProfiler (3.8) R package was used to test the statistical enrichment of DEG in Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways. Protein-protein interaction network of DEG in UVJ folds was carried out by the STRING website (https://string-db.org/) at medium confidence (minimum required interaction score: 0.04). Then, data were edited in Cytoscape, version 3.6.1, software (Bethesda, National Institute of General Medical Sciences of the National Institutes of Health).
Analysis of DEGs by Quantitative Real-time PCR
The quantitative real-time PCR primers were designed by the NCBI Primer-BLAST website (https://www.ncbi.nlm.nih.gov/tools/primer-blast/). The primer information was listed in Table S1. Several differentially expressed mRNA were used for quantitative PCR analysis to verify the gene expression tendency in day 3 (n = 5) and day 9 post-AI (n = 6) cDNA samples. The quantitative PCR amplification conditions were as follows: 95°C for 5 min; 39 cycles of 95°C for 15s and 57°C for 15s; 72°C for 15s; 72°C for 5 min. The foldchange was calculated by 2-ΔΔCt method, and the significance testing was conducted by t test.
Results
Histological Observation of Reduced Sperms in SST of UVJ Folds Derived from Mated Hens (day 9 vs. day 3 After AI)
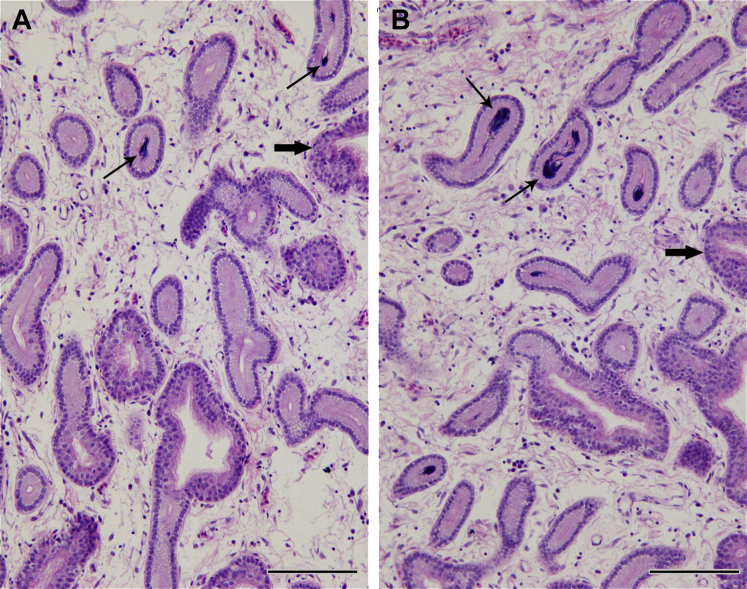
Chicken UVJ is regarded as an important sperm storage site after insemination. To investigate the potential difference relevant to the duration of sperm storage, the UVJ folds containing SSTs of white Leghorn hens were collected on day 3 and day 9 after AI to examine the morphology in the UVJ of the hen oviduct. The mucosa of UVJ folds was characterized as lamina propria and pseudostratified ciliated columnar epithelium that branched into lamina propria to form tubular glands with a single layer of columnar epithelium named SST. The sperms were observed with their heads gathering together as a tightly packed bundle in SST cavity, located closely next to the tubular end and paralleled to SST. The sperm bundles in SST were detected in both sections derived from day 3 and day 9 post-AI samples, whereas the number of SST containing sperm bundles was much less when comparing day 9 samples with day 3 samples (Figure 1). What are the mechanisms governing the dynamic changes remains unclear. Transcriptome sequencing strategy was applied to decipher the genes and networks involved in this progression.
Figure 1.
Histological comparison of uterovaginal junction (UVJ) folds containing sperm storage tubules (SST) stained with hematoxylin and eosin between day 3 and day 9 after artificial insemination (AI) in chicken hens. The epithelium of UVJ folds and SST were pseudostratified ciliated columnar (pointed with bold arrows) and simple columnar, respectively. Sperms were gathered and distributed in the lumen of SST (pointed with narrow arrows) as shown in at day 9 (A) and at day 3 (B) post AI. Scale bar = 100 μm.
Enrichment of DEGs in UVJ Folds Derived From Mated Hens (day 9 vs. day 3 After AI)
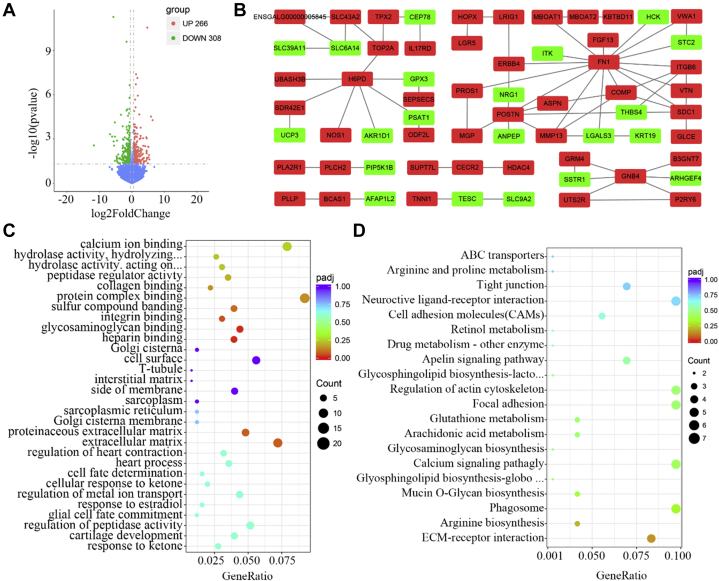
To investigate the molecular mechanisms involved in the decreased sperms in chicken UVJ folds, 2 sets of UVJ folds containing SST that were collected at 2 time points after mating (day 3 and day 9 after AI) with 3 replicates were applied for high throughput RNA sequencing. Illumina sequencing of 6 UVJ tissue samples produced a total of 364,311,924 raw reads and generated 353,509,306 clean reads after quality control (97% of the raw reads). More than 82% of the clean reads were uniquely mapped to the chicken reference genome (Gallus_gallus-5.0) to enrich the DEG between these 2 sets of UVJ samples (day 9 vs. day 3 after AI). A total of 574 genes were identified as differentially expressed genes including 266 upregulated and 308 downregulated transcripts (Figure 2A) and presented in Table S2. Of those, several sperm-related transcripts were enriched and represented as AGO2, ASB9, CECR2, and FGF13, as shown in Table 1.
Figure 2.
(A) The volcano plots of differentially expressed genes (DEG) enriched in chicken uterovaginal junction (UVJ) folds containing sperm storage tubules (SST) (day 9 vs. day 3 after artificial insemination [AI]). The X-axis and Y-axis represented the log2 fold change values and the statistical significance P values (−log10), respectively. The red and green dots represented upregulated and downregulated mRNA expression of DEG, with expression exceeding the threshold value within log2 fold change values between 0.5 and −0.5, P value < 0.05. The blue dots represented the genes with expression level beyond the stated threshold of P value < 0.05. (B) Protein-protein interaction network of DEG in mated chicken UVJ folds (day 9 vs. day 3 after AI). Network nodes and edges represented proteins and protein-protein associations, respectively. The red and green nodes represented downregulated and upregulated genes, respectively. (C) The top 30 enriched biological processes of DEG comparing the UVJ containing SST at day 9 to day 3 after AI. (D) The top 20 enriched Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways of DEG in UVJ comparing between day 9 and day 3 after AI.
Table 1.
The enriched sperm-related genes differentially expressed in the UVJ tissues of white Leghorn hens after insemination (day 9 vs. day 3 after AI).
| Genes | Potential function | Expression pattern | Log2 FC1 | Reference |
|---|---|---|---|---|
| AGO2 | Spermatogenesis-related gene | Seminiferous tubules in rat testis | −0.7723 | PMID:27029769 |
| ASB9 | Spermatogenesis-related gene | Mouse pachytene spermatocytes and spermatids | −0.6590 | PMID: 18776735 |
| CECR2 | Spermatogenesis-related gene | Mice testis | −0.7247 | PMID:22154806 |
| FGF13 | Spermatogenesis-related gene | Mouse testis | −2.6986 | PMID: 23554914 |
| LIPG | Spermatogenesis-related gene | Human testis | −0.8166 | PMID: 19780863 |
| GFRA1 | Related to spermatogenesis (subpopulations of spermatogonia) | Human testis | 1.2107 | PMID: 28827392 |
| HCK | Spermatogenesis-related gene | Bovine spermatozoa and testis | 0.6470 | PMID: 17926339 |
| NRG1 | Spermatogenesis-related gene | Nrg1(Ser−/−) mutant mice | 1.2480 | PMID: 18586089 |
| PIP5K1B | Spermatogenesis-related gene | Pip5k1a-knockout, Pip5k1b-Knockout, and Pip5k1a/Pip5k1b double knockout mice | 0.5860 | PMID: 22321832 |
| SMYD3 | Spermatogenesis-related gene | Human testis and spermatozoa | −1.5791 | PMID: 16081583 |
| MBOAT1 | Related to germline development | Drosophila germ cell | −0.5843 | PMID: 19864461 |
| CFTR | Regulate sperm capacitation | Human sperm | 0.68207 | PMID: 27714810 PMID:19923167 |
| CLIC5 | Regulate sperm function | Bovine epididymal spermatozoa | −1.1024 | PMID: 15147883 |
| DNAJB1 | Chaperone proteins regulating capacitation | 0.84846 | PMID: 17595329 | |
| ERBB4 | Related to sperm motility | Erbb4-knockout mice | −1.2368 | PMID:29453196 PMID:25058600 |
| LGALS3 | Regulate sperm function | Rat, bull testis and epididymis, and human semen prostasomes | 0.5908 | PMID: 19830550 PMID: 30241677 PMID:19830550 |
| PDE1A | Regulate sperm function | Human and mouse sperm | −0.7014 | PMID: 12135876 PMID: 15901640 |
| HES5 | Regulates sperm motility by epididymosomes | Mouse epididymis | −1.1295 | PMID: 26825631 |
| MLPH | sperm DNA methylation patterns | Human spermatozoal DNA | −0.6076 | PMID: 28566207 |
| HIP1 | Related to sperm counts and motility | HIP1 knockout mice | 0.8861 | PMID: 16967501 |
| SFRP1 | Regulates spermatid adhesion in the testis | Rats testis | −0.5969 | PMID: 23073828 |
| SLC22A18 | Responsible for the sperm phenotype | Human sperm | −0.5211 | PMID: 25762640 |
| SLC26A8 | Sperm tail differentiation and motility | Human and mouse male germ cells | 0.5117 | PMID: 17517695 PMID: 23582645 |
| FKBP6 | Related to male fertility | Equine testis and sperm | 1.49906 | PMID: 25495881 or 12764197 |
| GDF9 | Related to sperm quality | Holstein bulls and bovine Sertoli cells | −1.0501 | PMID: 23884762 PMID: 28332739 |
| HDAC4 | Related to male infertility (methylation and gene expression) | Human spermatozoal DNA | −0.5727 | PMID: 30358834 |
| KRT19 | Related to sperm maturation and male fertility | Rat efferent ducts | 0.6199 | PMID: 19906187 |
| RPL28 | Related to fertile and infertile | Human sperm | 0.5329 | PMID: 25973848 |
| CX3CL1 | Related to immune system | Human fallopian tube and ejaculated sperm | −0.7536 | PMID: 14747189 |
| EBP | Immune-related genes | Porcine endometrium | 0.5559 | PMID: 29155178 |
| GPX3 | Sperm antioxidant defences | Epididymal fluid in the goat | 0.6428 | PMID: 27677348 |
| NDRG1 | Sperm RNA elements | Human sperm | −0.5120 | PMID: 29199464 |
| PDGFRB | Associations with semen production traits | Chinese Holstein bulls | −0.7863 | PMID: 28673243 |
| SFMBT1 | Associate semen volume and total sperm number | Holstein-Friesian bulls | −0.6057 | PMID: 25465359 |
Abbreviations: AI, artificial insemination; UVJ, uterovaginal junction.
Log2 FC = Log2 foldchange of the gene expression changes day 9 vs. day 3 after AI.
GO Terms, KEGG Pathway Enrichment, and Protein-Protein Interaction Analyses of DEG
The 574 DEG were used to perform GO and KEGG analyses. The GO analysis revealed a total of 381 significant (P < 0.05) enriched terms. The top 30 of 381 GO terms were obtained and presented in Table S3 and Figure 2C. Of those, the most prominent enrichment was heparin binding (COMP, CTGF, IMPG2, PCOLCE, POSTN, RSPO3, SFRP1, TGFBR3, and THBS4) and glycosaminoglycan (GAG) binding (CEMIP, COMP, CTGF, IMPG2, PCOLCE, POSTN, RSPO3, SFRP1, TGFBR3, and THBS4)-related terms (q < 0.05) that are highly associated with the characteristics (spermatozoa capacitation) of sperm reservoir (chicken UVJ). In addition, 2 enriched GO terms named as response to estradiol (4 genes enriched) and regulation of metal ion transport (11 genes enriched) were potentially involved in the interaction of oviduct epithelium and sperms to regulate spermatozoa capacitation and sperm release from SST (P < 0.05). There were 2 terms represented as IgE binding (2 genes enriched) and antigen binding (3 genes enriched) that were possibly linked with immune response. The enriched proteinaceous extracellular matrix (12 genes enriched) and regulation of peptidase activity (13 genes enriched) and peptidase regulator activity (8 genes enriched) (P < 0.05)-related GO terms had a potential impact on the hormone regulation of the microenvironment in SST of UVJ. There were additional 10 calcium-related and 11 ion-related (excluding calcium) GO terms represented as calcium ion binding (18 genes enriched), calcium ion transport (10 genes enriched), calcium ion import (7 genes enriched), regulation of calcium ion transport (8 genes enriched), metal ion transport (18 genes enriched), iron ion binding (7 genes enriched), sodium ion transport (7 genes enriched), and regulation of ion transport (15 genes enriched) that potentially mediate the sperm motility as shown in Table 2.
Table 2.
The differentially expressed genes enriched in gene ontology are potentially related to sperm storage duration.
| Description | Count | Differentially expressed genes1 |
|---|---|---|
| Regulation of calcium ion transport | 8 | ATP2C2, CEMIP, LGALS3, NOS1, RAMP3, STAC, STC2, UBASH3B |
| Positive regulation of calcium ion import | 4 | ATP2C2, CEMIP, LGALS3, RAMP3 |
| Calcium ion binding | 18 | ASPN, ASTN2, COMP, ENSGALG00000002553, ENSGALG00000008326, ENSGALG00000012869, ENSGALG00000014854, FAT4, MAN1C1, MCC, MMP13, PCDH19, PLCH2, PROS1, STYL5, TESC, THBS4 |
| Regulation of calcium ion import | 5 | ATP2C2, CEMIP, LGALS3, RAMP3, UBASH3B |
| Calcium ion import | 7 | ATP2C2, CACNA1G, CEMIP, LGALS3, RAMP3, SLC8A1, UBASH3B |
| Positive regulation of calcium ion transport | 4 | ATP2C2, CEMIP, LGALS3, RAMP3 |
| Calcium ion transport | 10 | ATP2C2, CACNA1G, CEMIP, LGALS3, NOS1, RAMP3, SLC8A1, STAC, STC2, UBASH3B |
| Regulation of metal ion transport | 11 | ATP2C2, CEMIP, LGALS3, NOS1, OSR1, RAMP3, STAC, STC2, SNTA1, TESC, UBASH3B |
| Iron ion binding | 7 | ENSGALG00000020876, HBAA, MELTF, NOS1, SCD, TBXAS1, XDH |
| Regulation of ion transport | 15 | ATP2C2, CACNA1G, CEMIP, CFTR, CLIC5, LGALS3, NOS1, OSR1, PLA2R1, RAMP3, SNTA1, STAC, STC2, TESC, UBASH3B |
| Regulation of sodium ion transport | 4 | NOS1, OSR1, SNTA1, TESC |
| Sodium ion transport | 7 | FGF13, NOS1, OSR1, SLC8A1, SLC9A2, SNTA1, TESC |
| Positive regulation of ion transport | 7 | ATP2C2, CEMIP, CFTR, LGALS3, PLA2R1, RAMP3, TESC |
| Sodium ion import | 2 | SLC8A1, SLC9A2 |
| Metal ion transport | 18 | ATP2C2, CACNA1G, CEMIP, FGF13, LGALS3, MELTF, NOS1, OSR1, RAMP3, SLC25A37, SLC39A11, SLC8A1, SLC9A2, SNTA1, STAC, STC2, TESC, UBASH3B |
| Regulation of sodium ion transmembrane transport | 3 | OSR1, SNTA1, TESC, |
| Divalent metal ion transport | 11 | ATP2C2, CACNA1G, CEMIP, LGALS3, NOS1, RAMP3, SLC8A1, SLC39A11, STAC, STC2, UBASH3B |
| Divalent inorganic cation transport | 11 | ATP2C2, CACNA1G, CEMIP, LGALS3, NOS1, RAMP3, SLC8A1, SLC39A11, STAC, STC2, UBASH3B |
| Sodium ion transmembrane transport | 5 | OSR1, SLC8A1, SLC9A2, SNTA1, TESC |
| Heparin binding | 9 | COMP, CTGF, IMPG2, PCOLCE, POSTN, RSPO3, SFRP1, TGFBR3, THBS4 |
| Response to estradiol | 4 | AREG, RAMP3, SFRP1, SSTR1, |
| Glycosaminoglycan binding | 10 | CEMIP, COMP, CTGF, IMPG2, PCOLCE, POSTN, RSPO3, SFRP1, TGFBR3, THBS4 |
The bolded font in the table represents the downregulated differentially expressed genes.
KEGG pathway enrichment revealed 10 significant pathways (P < 0.05) represented by calcium signaling pathway (7 genes enriched) and extracellular matrix (ECM)-receptor interaction (6 genes enriched). The top 20 KEGG terms were presented in Figure 2D. Furthermore, 5 signaling pathways that potentially regulate the sperm storage in UVJ including calcium signaling pathway (7 genes enriched), GAG biosynthesis keratan sulfate (4 genes enriched), ECM-receptor interaction (6 genes enriched), and glutathione metabolism (3 genes enriched) were enriched (Table 3). The most promising candidates displayed the decreasing expression tendency, including PDE1C gene.
Table 3.
The differentially expressed genes enriched in different signaling pathways are potentially related with sperm storage duration.
| Pathway | Count | Differentially expressed genes1 |
|---|---|---|
| Calcium signaling pathway | 7 | CACNA1G, ERBB4, HTR7, NOS1, PDE1C, PDGFRB, SLC8A1 |
| Glycosaminoglycan biosynthesis | 4 | B3GNT7, CSGALNACT1, GLCE, ST3GAL1 |
| ECM-receptor interaction | 6 | SDC1, FN1, ITGB6, VTN, COMP, THBS4 |
Abbreviation: ECM, extracellular matrix.
The bolded font in the table represents the downregulated differentially expressed gene.
Protein-protein interaction network established a broad interconnection regulating epithelial cell or cell-matrix adhesion and migration centered by FN1, POSTN, and SDC1. The other 2 subnetworks potentially contributing to ion binding or transport including NOS1 and SLC39A11, and the protein releasing or inflammatory response mediated by G-protein coupled receptors GRM4, SSTR1, and P2RY6, were also set up as shown in Figure 2B.
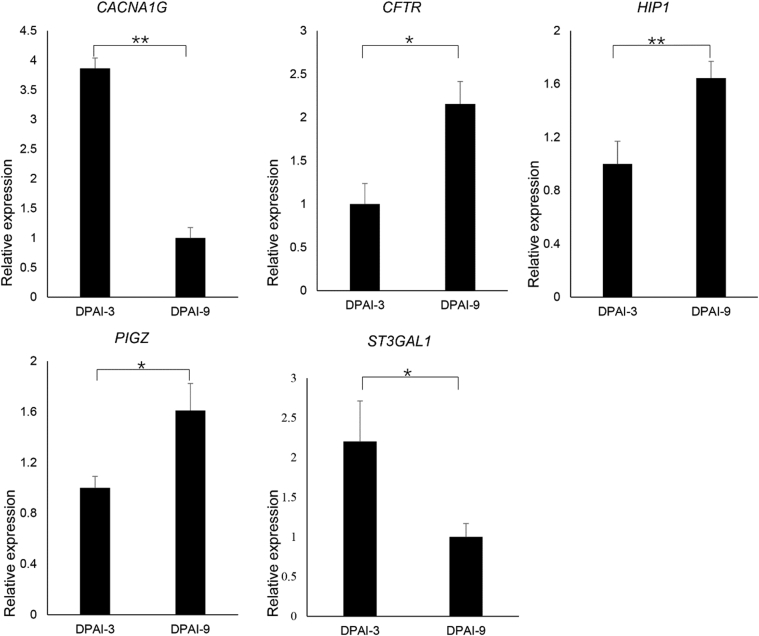
Quantitative Real-time PCR Validation of Candidate Genes Regulating the Sperm Storage Capacity of Chicken UVJ
To gain the information of dynamic gene expression changes occurring in the chicken UVJ folds containing SST after AI, we performed quantitative PCR to evaluate the reliability of our RNA-seq result, and a total of 5 genes (CACNA1G, CFTR, HIP1, PIGZ, and ST3GAL1) were randomly selected for detailed investigation of gene expression patterns by using the quantitative PCR technique. The downregulation of CACNA1G and ST3GAL1 and the upregulation of CFTR, PIGZ, and HIP1 in UVJ of mated chicken hens (day 9 vs. day3) were consistent with the tendency of RNA sequencing results (Figure 3).
Figure 3.
The quantitative real-time PCR validation of randomly selected differentially expressed genes (CACNA1G, CFTR, HIP1, ST3GAL1, and PIGZ) in mated chicken uterovaginal junction folds (day 9 vs. day 3 after artificial insemination [AI]). Day post-AI (DPAI)-3 represented day 3 post-AI group (n = 5), whereas DPAI-9 represented day 9 post-AI group (n = 6). Error bars represented SEM. “*” and “**” represented the levels of significant differences at P < 0.05 and P < 0.01, respectively.
Discussion
Sperm reservoir is widely accepted as the temporary location for sperm maintenance in female reproductive tracts in many animals, such as cows, pigs, reptiles, insects, and birds (Matsuzaki and Sasanami, 2017). In mated chicken hens, a large amount of spermatozoa were supposed to experience sequential processes including escaping the immune attack at the site of vagina, travelling to the UVJ for short-term or long-term stay, engineering progressive release from the UVJ to the uterus, and migrating upward to infundibulum to meet the oocyte and reach the final destination for successful fertilization (Gilbert et al., 1968, Bakst et al., 1994). The detailed movements of sperms during these processes involved arriving at the UVJ, swimming into SSTs and possibly staying inactive in the SST fluid, and eventually capacitating to release from SST. During the later step, the sperms are possibly transferred from inactive to active phase to travel out of the SST tubular epithelium. The mechanisms underlying the intake, maintenance, and release of spermatozoa in SST of UVJ are poorly understood. Many questions are yet to be answered: 1) Why the duration or length of sperm storage is different among the individuals? 2) What are the immediate and long-term molecular responses to the spermatozoa? And 3) What signals trigger or regulate the progressive release of spermatozoa from the SST of UVJ? Most of the previous studies focused on interpreting the gene network regulating the immediate response of UVJ to the sperm intake by comparing mated hens (24 h after mating) with unmated controls using RNA sequencing strategy (Atikuzzaman et al., 2015, Han et al., 2019a). It is not surprising that most of the enriched differentially expressed transcripts (lncRNAs, mRNAs, and microRNAs) were highly related with immune- and pH-regulatory genes in UVJ of chicken hens. This mechanism was proved to be conserved among different species because the comparison of UVJ between chicken hens and the counterparts in sows exhibited similar gene regulatory network (Atikuzzaman, et al., 2017). While these studies observed the early impact of spermatozoa on the sperm reservoir, the followed-up dynamic balance between the maintenance and progressive release of sperms from the UVJ remains largely unclear. Hence, it would be interesting to reveal the mystery of long-term sperm storage in chicken UVJ and to further understand the diversity of sperm reservoir among chicken and other animals. The present study focused on deciphering the molecular network governing the short (3 D) and long length (9 D) of sperm storage in chicken UVJ (day 9 vs. day 3) after mating. The histological analysis of the UVJ revealed sperm bundles in the SST of UVJ collected at day 9 and day 3 time points, whereas the number of bundles greatly reduced at day 9 samples. The morphological changes support the notion that the spermatozoa experienced progressive release from UVJ over time (Ito, et al., 2011). The observation of spermatozoa in the SST of mated white Leghorn hens (day 9) in our study directly supports the good performance of egg fertility of this egg layer.
During the processes of sperm storage in SST, the potential sperm activation or capacitation requires the complex network including ion (calcium) and pH fluctuation (Holm et al., 1996, Froman and Feltmann, 2005). The RNA-sequencing was performed to find out what gene network governed this phenomenon. By comparing UVJ tissues (day 9 vs. day 3 after AI) derived from white Leghorn chickens, the enriched 4 GO terms (heparin binding, GAG binding, response to estradiol, and regulation of metal ion transport) were predicted to be highly involved with the sperm storage and release.
The concentration of 6 different ions (sodion, phosphonium, sulfion, chloridion, and potassium) was shown to be different between SST tubular fluid and SST epithelial cells of chicken, quail, and turkey hens (Holm, et al., 2000). The sperm motility was regulated by the mitochondrial calcium cycling driven by extracellular sodium (Froman and Feltmann, 2005). The fluctuation of ions in the SST fluid may be one of the drivers for sperm maintenance in or release from SST. Our analyses confirmed this observation from molecular level by enriching a series of solute carrier family genes represented by solute carrier family genes (SLC8A1, SLC16A5, SLC22A18, SLC26A8, and SLC43A2) that may function in transporting lactic acid, hydrogen, and calcium ions (Hediger et al., 2004, Perland and Fredriksson, 2017). SLC43A2 is an amino acid transporter mainly mediating the exchange of amino acids across the placental membrane to the fetus. Solute carrier family 26 member 8 (SLC26A8) is one of 11 members of the SLC26 family (solute carrier family) that interacts with CFTR to transport anions required for sperm motility and capacitation (Fong, 2012, Dirami et al., 2013). CFTR is involved in regulating Cl− and HCO3− transport and secretion in epithelial lining tissues including reproductive tracts. Activation of CFTR is responsible for the sustained uptake of HCO3− into sperms to trigger the sperm capacitation when exposed to high concentration of HCO3− in seminal plasma or reproductive tract (Riordan et al., 1989, Wang et al., 2003, Muchekehu and Quinton, 2010). The lower expression of CFTR in chicken UVJ tissues at day 3 after AI was supposed to reduce the sperm capacitation in SST, whereas the acidic fluid in SST also contributed to the sperm inactivation.
The response to estradiol GO terms (RAMP3) and calcium signaling pathway (CACNA1G, ERBB4, HTR7, NOS1, PDE1C, PDGFRB, and SLC8A1) were enriched in our data. Estradiol, particularly E2 (17β-estradiol), was shown to have a negative impact on the binding of bovine oviduct epithelial cells to spermatozoa in cell culture systems (Lamy et al., 2017, Yoshimoto et al., 2017). The administration of estradiol triggered adrenomedullin to induce uterine artery relaxation by increasing RAMP3 expression (Ross, et al., 2010). RAMP3 acts as chaperone to assist the activation of adrenomedullin which increases the concentration of intercellular calcium ions and maintains the ion microenvironment in rat oviduct. RAMP3 was shown to express both in the ciliated and secretory cells of the ampullary tissue of bovine oviduct and regulate the oviductal fluid flow, particularly to release calcium from intracellular storage (Yoshimoto, et al., 2017). SLC8A1, a calcium extrusion regulatory molecule encoding the NCX1 antiporter member protein, was shown to regulate the calcium homeostasis. The dysregulation of NCX1 was highly associated with heart failure and Kawasaki disease caused by impaired calcium flux (Shimizu, et al., 2016). The downregulation of SLC8A1 implied that the sperm release from the reservoir was highly correlated with calcium extrusion. STAC was identified as a new regulatory family functioned in voltage-gated calcium channel trafficking (Campiglio, et al., 2018). The downregulation of RAMP3, SLC8A1, and STAC indicated the potential roles involved in high calcium concentration in SST to maintain the sperm to be inactive. PDE1C is calcium/calmodulin-dependent phosphodiesterase that was highly related to male mating behavior and infertility in Drosophila melanogaster (Morton, et al., 2010). Although PDE1C male mutants with decreased expression displayed normal spermatogenesis and maturation, they were presumably not favored by the females in courtship behavior. The normal females that successfully mated with PDE1C male mutants showed normal sperm maintenance in uterus 24 h after mating and few sperms in seminal receptacle (sperm reservoir) 72 h after mating comparing with those of control group with abundant sperm distribution. The greatly decreased sperm storage in seminal receptacle implied that PDE1C was functionally important for female sperm storage in sperm reservoir with unknown mechanisms. The downregulation of PDE1C gene with nearly 1.5 folds (UVJ day 9 vs. day 3 after AI) in our sequencing data highly implied that PDE1C expression pattern was positively correlated with the duration of spermatozoa in UVJ of hens. HIP1 is an endocytic membrane adaptor protein that is involved in clathrin-mediated endocytosis. The HIP1 mutant mice displayed obvious defects in testis with 50% reduction of sperm production comparing with those of controls. The most interesting observation was the increase of static sperm by 143% and decrease of sperm velocity by 25% in HIP1 mutant mice (Rao et al., 2001, Khatchadourian et al., 2007). These data highly indicate that HIP1 was positively correlated with the sperm activity. The upregulation of HIP1 gene expression in UVJ tissue (day 9 vs. day 3 after AI) suggested that the low expression of HIP1 helps the spermatozoa to keep quiescence for short-term maintenance in SST of UVJ in day 3 samples.
The GAG unit has been identified in different parts of the chicken oviduct including SST to potentially function in regulating sperm storage process (Trainis, 1967, Bakst and Bauchan, 2016). GAG were reported to have crucial roles in stimulating acrosome reactions in bovine, porcine, and rabbit sperms in vitro. The GAG heparin worked as the most potent inducer of capacitation in bovine and ovine female reproductive tracts (Handrow et al., 1982, Handrow et al., 1984, Therien et al., 2005). The ST3GAL1 gene was an important enzyme in GAG biosynthesis. The ST3GAL1 gene was differentially expressed in UVJ tissues (day 9 vs. day 3 after AI) and enriched in GAG binding and heparin in GO analysis, indicating its potential roles in sperm functional regulation, such as metabolic activity, motility, and acrosomal enzyme activity.
In conclusion, the sperms stored in SST of chicken UVJ were decreasing overtime (day 9 vs. day 3) after insemination. The enrichment of ion-related genes and cell migratory–related genes implied their important roles in maintaining and releasing the sperms in and from the SST of UVJ. A series of genes such as RAMP3, PDE1C, HIP1, and ST3GAL1 may be candidates to further study the short and long length of sperm storage in sperm reservoir of chicken hens. This is the first report addressing the global gene expression correlated with relatively long-term sperm storage in chicken hens. The molecular network identified in this report will contribute to further explore the differential capacity of sperm storage among individuals and eventually understand the diversity of the sperm storage displayed in different breeds and species of female animals in nature.
Acknowledgements
This work was supported by the grants from the National Key R&D Program of China (2018YFD0501301) and National Natural Science Foundation of China (31772585).
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.psj.2019.10.021.
Contributor Information
Shijun Li, Email: lishijun@mail.hzau.edu.cn.
Chunyan Mou, Email: chunyanmou@mail.hzau.edu.cn.
Supplementary data
Differentially expressed genes enriched in chicken uterovaginal junction tissues at two time points (day 9 vs. day 3) after artificial insemination.
All terms enriched in gene ontology analysis.
References
- Atikuzzaman M., Alvarez-Rodriguez M., Vicente-Carrillo A., Johnsson M., Wright D., Rodriguez-Martinez H. Conserved gene expression in sperm reservoirs between birds and mammals in response to mating. BMC Genomics. 2017;18:98. doi: 10.1186/s12864-017-3488-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atikuzzaman M., Mehta Bhai R., Fogelholm J., Wright D., Rodriguez-Martinez H. Mating induces the expression of immune- and pH-regulatory genes in the utero-vaginal junction containing mucosal sperm-storage tubuli of hens. Reproduction. 2015;150:473–483. doi: 10.1530/REP-15-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakst M.R., Akuffo V. Alkaline phosphatase reactivity in the vagina and uterovaginal junction sperm-storage tubules of turkeys in egg production: implications for sperm storage. Br. Poult. Sci. 2007;48:515–518. doi: 10.1080/00071660701381761. [DOI] [PubMed] [Google Scholar]
- Bakst M.R., Bauchan G. Apical blebs on sperm storage tubule epithelial cell microvilli: their release and interaction with resident sperm in the Turkey hen oviduct. Theriogenology. 2015;83:1438–1444. doi: 10.1016/j.theriogenology.2015.01.016. [DOI] [PubMed] [Google Scholar]
- Bakst M.R., Bauchan G. Lectin staining of the uterovaginal junction and sperm-storage tubule epithelia in broiler hens. Poult. Sci. 2016;95:948–955. doi: 10.3382/ps/pev440. [DOI] [PubMed] [Google Scholar]
- Bakst M.R., Wishart G.J., Brillard J.P. Oviducal sperm selection, transport, and storage in poultry. Poult.sci.rev. 1994;5:117–143. [Google Scholar]
- Bobr L.W., Lorenz F.W., Ogasawara F.X. Distribution of spermatozoa in the oviduct and fertility in domestic birds. I. Residence sites of spermatozoa in Fowl oviducts. J. Reprod. Fertil. 1964;8:39–47. doi: 10.1530/jrf.0.0080039. [DOI] [PubMed] [Google Scholar]
- Campiglio M., Coste de Bagneaux P., Ortner N.J., Tuluc P., Van Petegem F., Flucher B.E. STAC proteins associate to the IQ domain of CaV1.2 and inhibit calcium-dependent inactivation. Proc. Natl. Acad. Sci. U S A. 2018;115:1376–1381. doi: 10.1073/pnas.1715997115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S.C., Isobe N., Yoshimura Y. Analysis of changes in the expression of transforming growth factor-βs in the utero-vaginal junction of hen oviduct in response to sperm Concerning their significance in sperm Survivability. J. Poult. Sci. 2010;47:326–332. [Google Scholar]
- Dirami T., Rode B., Jollivet M., Da Silva N., Escalier D., Gaitch N., Norez C., Tuffery P., Wolf J.P., Becq F., Ray P.F., Dulioust E., Gacon G., Bienvenu T., Toure A. Missense mutations in SLC26A8, encoding a sperm-specific activator of CFTR, are associated with human asthenozoospermia. Am. J. Hum. Genet. 2013;92:760–766. doi: 10.1016/j.ajhg.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong P. CFTR-SLC26 transporter interactions in epithelia. Biophys. Rev. 2012;4:107–116. doi: 10.1007/s12551-012-0068-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foye-Jackson O.T., Long J.A., Bakst M.R., Blomberg L.A., Akuffo V.G., Silva M.V.B., Guthrie H.D., Mcmurtry J.P. Oviductal expression of avidin, avidin-related protein-2, and progesterone receptor in Turkey hens in relation to sperm storage: effects of oviduct tissue type, sperm presence, and Turkey line. Poult. Sci. 2011;90:1539–1547. doi: 10.3382/ps.2010-01159. [DOI] [PubMed] [Google Scholar]
- Froman D.P., Feltmann A.J. Fowl (Gallus domesticus) sperm motility depends upon mitochondrial calcium cycling driven by extracellular sodium. Biol. Reprod. 2005;72:97–101. doi: 10.1095/biolreprod.104.033209. [DOI] [PubMed] [Google Scholar]
- Gilbert A.B., Reynolds M.E., Lorenz F.W. Distribution of spermatozoa in the oviduct and fertility in domestic birds. V. Histochemistry of the uterovaginal sperm-host glands of the domestic hen. J. Reprod. Fertil. 1968;16:433–444. doi: 10.1530/jrf.0.0160433. [DOI] [PubMed] [Google Scholar]
- Han J., Ahmad H.I., Jiang X., Liu G. Role of genome-wide mRNA-seq profiling in understanding the long-term sperm maintenance in the storage tubules of laying hens. Trop. Anim. Health Prod. 2019 doi: 10.1007/s11250-019-01821-5. [DOI] [PubMed] [Google Scholar]
- Han J., Ahmad H.I., Jiang X., Liu G. Role of genome-wide mRNA-seq profiling in understanding the long-term sperm maintenance in the storage tubules of laying hens. Trop. Anim. Health Prod. 2019;51:1441–1447. doi: 10.1007/s11250-019-01821-5. [DOI] [PubMed] [Google Scholar]
- Handrow R.R., Boehm S.K., Lenz R.W., Robinson J.A., Ax R.L. Specific binding of the glycosaminoglycan 3H-heparin to bull, monkey, and rabbit spermatozoa in vitro. J. Androl. 1984;5:51–63. doi: 10.1002/j.1939-4640.1984.tb00776.x. [DOI] [PubMed] [Google Scholar]
- Handrow R.R., Lenz R.W., Ax R.L. Structural comparisons among glycosaminoglycans to promote an acrosome reaction in bovine spermatozoa. Biochem. Biophys. Res. Commun. 1982;107:1326–1332. doi: 10.1016/s0006-291x(82)80143-5. [DOI] [PubMed] [Google Scholar]
- Hediger M.A., Romero M.F., Peng J.B., Rolfs A., Takanaga H., Bruford E.A. The ABCs of solute carriers: physiological, pathological and therapeutic implications of human membrane transport proteinsIntroduction. Pflugers Arch. 2004;447:465–468. doi: 10.1007/s00424-003-1192-y. [DOI] [PubMed] [Google Scholar]
- Holm L., Ekwall H., Wishart G.J., Ridderstrale Y. Localization of calcium and zinc in the sperm storage tubules of chicken, quail and Turkey using X-ray microanalysis. J. Reprod. Fertil. 2000;118:331–336. [PubMed] [Google Scholar]
- Holm L., Ridderstrale Y., Knutsson P.G. Localisation of carbonic anhydrase in the sperm storing regions of the domestic hen oviduct. Acta Anat. (basel) 1996;156:253–260. doi: 10.1159/000147853. [DOI] [PubMed] [Google Scholar]
- Holt W.V., Fazeli A. Sperm storage in the female reproductive tract. Annu. Rev. Anim. Biosci. 2016;4:291–310. doi: 10.1146/annurev-animal-021815-111350. [DOI] [PubMed] [Google Scholar]
- Huang A., Isobe N., Obitsu T., Yoshimura Y. Expression of lipases and lipid receptors in sperm storage tubules and possible role of fatty acids in sperm survival in the hen oviduct. Theriogenology. 2016;85:1334–1342. doi: 10.1016/j.theriogenology.2015.12.020. [DOI] [PubMed] [Google Scholar]
- Ito T., Yoshizaki N., Tokumoto T., Ono H., Yoshimura T., Tsukada A., Kansaku N., Sasanami T. Progesterone is a sperm-releasing factor from the sperm-storage tubules in birds. Endocrinology. 2011;152:3952–3962. doi: 10.1210/en.2011-0237. [DOI] [PubMed] [Google Scholar]
- Khatchadourian K., Smith C.E., Metzler M., Gregory M., Hayden M.R., Cyr D.G., Hermo L. Structural abnormalities in spermatids together with reduced sperm counts and motility underlie the reproductive defect in HIP1-/- mice. Mol. Reprod. Dev. 2007;74:341–359. doi: 10.1002/mrd.20564. [DOI] [PubMed] [Google Scholar]
- Lamy J., Corbin E., Blache M.C., Garanina A.S., Uzbekov R., Mermillod P., Saint-Dizier M. Steroid hormones regulate sperm-oviduct interactions in the bovine. Reproduction. 2017;154:497–508. doi: 10.1530/REP-17-0328. [DOI] [PubMed] [Google Scholar]
- Matsuzaki M., Mizushima S., Hiyama G., Hirohashi N., Shiba K., Inaba K., Suzuki T., Dohra H., Ohnishi T., Sato Y., Kohsaka T., Ichikawa Y., Atsumi Y., Yoshimura T., Sasanami T. Lactic acid is a sperm motility inactivation factor in the sperm storage tubules. Sci. Rep. 2015;5:17643. doi: 10.1038/srep17643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki M., Sasanami T. Sperm storage in the female reproductive tract: a conserved reproductive strategy for Better fertilization success. Adv. Exp. Med. Biol. 2017;1001:173–186. doi: 10.1007/978-981-10-3975-1_11. [DOI] [PubMed] [Google Scholar]
- Morton D.B., Clemens-Grisham R., Hazelett D.J., Vermehren-Schmaedick A. Infertility and male mating behavior deficits associated with Pde1c in Drosophila melanogaster. Genetics. 2010;186:159–165. doi: 10.1534/genetics.110.118018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchekehu R.W., Quinton P.M. A new role for bicarbonate secretion in cervico-uterine mucus release. J. Physiol. 2010;588:2329–2342. doi: 10.1113/jphysiol.2010.187237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perland E., Fredriksson R. Classification systems of Secondary active transporters. Trends Pharmacol. Sci. 2017;38:305–315. doi: 10.1016/j.tips.2016.11.008. [DOI] [PubMed] [Google Scholar]
- Rao D.S., Chang J.C., Kumar P.D., Mizukami I., Smithson G.M., Bradley S.V., Parlow A.F., Ross T.S. Huntingtin interacting protein 1 Is a clathrin coat binding protein required for differentiation of late spermatogenic progenitors. Mol. Cell Biol. 2001;21:7796–7806. doi: 10.1128/MCB.21.22.7796-7806.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riordan J.R., Rommens J.M., Kerem B., Alon N., Rozmahel R., Grzelczak Z., Zielenski J., Lok S., Plavsic N., Chou J.L. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- Ross G.R., Yallampalli U., Gangula P.R., Reed L., Sathishkumar K., Gao H., Chauhan M., Yallampalli C. Adrenomedullin relaxes rat uterine artery: mechanisms and influence of pregnancy and estradiol. Endocrinology. 2010;151:4485–4493. doi: 10.1210/en.2010-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu C., Eleftherohorinou H., Wright V.J., Kim J., Alphonse M.P., Perry J.C., Cimaz R., Burgner D., Dahdah N., Hoang L.T., Khor C.C., Salgado A., Tremoulet A.H., Davila S., Kuijpers T.W., Hibberd M.L., Johnson T.A., Takahashi A., Tsunoda T., Kubo M., Tanaka T., Onouchi Y., Yeung R.S., Coin L.J., Levin M., Burns J.C., International Kawasaki Disease Genetics Genetic Variation in the SLC8A1 calcium signaling pathway is associated with Susceptibility to Kawasaki disease and Coronary artery abnormalities. Circ. Cardiovasc. Genet. 2016;9:559–568. doi: 10.1161/CIRCGENETICS.116.001533. [DOI] [PubMed] [Google Scholar]
- Sullivan R., Saez F. Epididymosomes, prostasomes, and liposomes: their roles in mammalian male reproductive physiology. Reproduction. 2013;146:R21–R35. doi: 10.1530/REP-13-0058. [DOI] [PubMed] [Google Scholar]
- Therien I., Bergeron A., Bousquet D., Manjunath P. Isolation and characterization of glycosaminoglycans from bovine follicular fluid and their effect on sperm capacitation. Mol. Reprod. Dev. 2005;71:97–106. doi: 10.1002/mrd.20287. [DOI] [PubMed] [Google Scholar]
- Trainis K.V. [Mucopolysaccharides of the oviduct wall in the laying hen] Arkh Anat. Gistol Embriol. 1967;53:102–107. [PubMed] [Google Scholar]
- Wang X.F., Zhou C.X., Shi Q.X., Yuan Y.Y., Yu M.K., Ajonuma L.C., Ho L.S., Lo P.S., Tsang L.L., Liu Y., Lam S.Y., Chan L.N., Zhao W.C., Chung Y.W., Chan H.C. Involvement of CFTR in uterine bicarbonate secretion and the fertilizing capacity of sperm. Nat. Cell Biol. 2003;5:902–906. doi: 10.1038/ncb1047. [DOI] [PubMed] [Google Scholar]
- Yoshimoto Y., Nishie T., Ito S., Kobayashi Y., Yamamoto Y., Okuda K., Kimura K. Adrenomedullin regulates the speed of oviductal fluid flow in cattle. Mol. Reprod. Dev. 2017;84:712–718. doi: 10.1002/mrd.22852. [DOI] [PubMed] [Google Scholar]
- Zaniboni L., Bakst M.R. Localization of aquaporins in the sperm storage tubules in the Turkey oviduct. Poult. Sci. 2004;83:1209–1212. doi: 10.1093/ps/83.7.1209. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Differentially expressed genes enriched in chicken uterovaginal junction tissues at two time points (day 9 vs. day 3) after artificial insemination.
All terms enriched in gene ontology analysis.