Abstract
The present study aimed to evaluate the immunopotentiating effect of plant-derived soyasaponin and its immunogenicity in chickens challenged with Newcastle disease virus (NDV). Soyasaponin was extracted from soybean seeds and detected using the phytochemical tests, followed by quantification through the dry-weight method. One-day-old broiler chicks (n = 90) were divided into 3 groups, named as A, B, and C. Group A birds were orally administrated with soyasaponin (5 mg/kg), followed by immunization with inactivated ND vaccine intramuscularly (IM), whereas group B birds were vaccinated with inactivated ND vaccine alone. Group C birds were kept unvaccinated. A booster dose on day 21 was also administered IM to group A and B birds. At day 35, all 3 groups were challenged with NDV. To determine the immunogenicity potential of soyasaponin, antibody titer was measured using the hemagglutination inhibition test before and after the NDV challenge. Histochemical examination was performed to determine the pathological changes associated with NDV infection. Foam formation and hemolytic activity confirmed the presence of saponin in soya bean extract. Group A birds showed a higher antibody response compared with group B and C birds. The disease challenge study showed that soyasaponin-adjuvanted NDV vaccine provided complete protection to group A birds against ND. Moreover, no side effects of soyasaponin were observed on the growth performance of birds during the experiment. Therefore, we can conclude that soyasaponin is a potential immunogenic agent and therefore could be a promising candidate to launch a protective humoral response against ND in chickens.
Key words: Newcastle disease virus, adjuvant, soyasaponin, humoral response, histopathology
Introduction
Newcastle disease (ND) is an economically important contagious disease of wild and domestic birds worldwide and is caused by a negative-sense single-stranded RNA virus known as avian paramyxovirus 1. Its economic importance is due to huge production losses in the form of mortality, decreased egg production, and deterioration of egg quality (Xiao et al. 2009). Current approaches for prevention of ND include biosecurity and vaccination. Both live and killed ND vaccines are available (Abdisa and Tagesu, 2017). Most commercially available ND vaccines are inactivated whole-virus preparations containing oil emulsions as an adjuvant to improve their efficacy. However, the vaccines have been reported to induce poor immune response (Xiao et al. 2009). Therefore, there is a need to improve currently available ND vaccines to effectively protect animals from ND.
Saponins are plant secondary metabolites that contain groups of triterpenoids, glycosides, or sterol glycosides (Burakova, et al., 2018). Among the cultivated crops that mostly contain triterpenoid saponins are legumes such as chickpeas, lucerne, soybean, and peas (Sun et al., 2009). Owing to the immunological and pharmaceutical properties, saponins have aroused a substantial clinical interest. Saponin-based adjuvants have gained a lot of importance in the recent years (Francis et al., 2002). Subsequently, use of saponins in the development of new-generation vaccine adjuvants is at several phases of clinical trials (Leroux-Roels et al., 2016, Burakova et al., 2018). Adjuvants containing saponins increase the antibody (Ab) production and provoke cell-mediated immunity. Saponins either as crude mixture or in the form of purified compounds increase the immune cell proliferation (Cibulski et al., 2018).
Chemically, saponins are hydrophilic and lipophilic in nature having glycosides. The adjuvant action of saponin is related to its branched sugar chains, acyl residues, and aldehyde groups. The aglycone backbone and sugar side chains are responsible for the adjuvant activity of saponins (Oda et al., 2003). Quil-A and many other triterpenoid saponins derived from plants are currently being investigated as vaccine adjuvants as they have ability to mediate cellular and humoral immune responses (T O'Hagan et al., 2017). In contrast to other saponin products, soyasaponins retain their adjuvant activity despite the lack of acyl residues (Rajput et al., 2007). Moreover, soyasaponins are more advantageous than other conventional saponin-based adjuvants owing to their cost effectiveness (de Paula Barbosa, 2014). The present study aimed to determine the immunostimulatory potential of soya bean–derived saponin as a vaccine adjuvant against ND.
Materials and methods
Birds
A total of 90 (one-day-old) grade A broiler chicks were purchased from the local hatchery in Faisalabad, Pakistan. All the birds were kept under good management conditions. The birds were provided with feed and water ad libitum with a standard broiler starter and grower diet plan throughout the entire experiment. The present study has been carried out in compliance with the Institutional Bioethics Committee, University of Agriculture, Faisalabad, Pakistan.
Soyasaponin Extraction
Soya saponin was extracted as per the procedure described by Kerem et al., (2005). A total of 10 g of defatted soybean powder was suspended in a 500-mL flask containing 80 mL of 70% aqueous ethanol. The flask was then placed in a microwave oven for 10 min, followed by filtration. The solvent was evaporated, and soyasaponin was procured.
Detection of Soyasaponin
To determine the presence of soyasaponin, 2 screening tests, that is, foam formation and blood hemolysis tests, were performed (Almutairi and Ali, 2015). In brief, 200 mg of soybean extract was mixed thoroughly with 3 mL of distilled water for 10 min. After shaking, the mixture was left undisturbed for 10 min to observe the development of stable foam formation in the test tube. Hemolytic activity of soyasaponin was determined on blood agar medium. About 100 μL of soyasaponin extract was dispensed onto the wells prepared on the solid blood agar medium. A clear zone around the wells was observed 24 h after incubation at 37°C.
Soysaponin Quantification
Saponins were quantified as per the method described by Gestetner et al., (1966). In brief, 150 mL of sulfuric acid was mixed with 50 mL of dioxane. Now, 1.84 g of dried flour of soybean was refluxed with this mixture, followed by filtration and ether extraction of the product. The resultant ether product was washed with water to remove acid and then evaporated. The rest of the residues was dissolved in toluene, and the solution was dispensed onto an alumina column. The column was eluted with methanol solution in toluene. The eluent solution was dried to get solid residual dry mass of soyasaponins.
Newcastle Disease Virus Procurement and Inactivation
Spleen samples from the birds infected with ND were collected, homogenized, and centrifuged as per the standard protocol. The supernatant was inoculated into the allantoic cavity of 9-day-old embryonated eggs, followed by incubation at 37°C. After 48 h of incubation, allantoic fluid from the embryonated eggs was harvested and stored at –20°C until use. The hemagglutination (HA) test was performed to determine ND virus (NDV) titer. Inactivation of NDV was performed by adding formalin. The tubes were then incubated at 38°C for 48 h. Formalin-inactivated NDV was used as the inactivated NDV vaccine.
Experimental Design
One-day-old broiler chicks (n = 90) were divided into 3 groups; A, B, and C, each group comprising 30 birds. At day 5, group A and B birds were immunized with inactivated NDV, intramuscularly (IM). Before immunization, group A birds were fed with soyasaponin in drinking water for 3 consecutive days, whereas no soyasaponin was offered to group B birds. No treatment was given to group C birds (unvaccinated control group). A booster dose of inactivated NDV was administered to both groups (groups A and B) at day 21. Before the booster dose, group A birds were again orally administered with soyasaponin for 3 consecutive days. Blood samples (0.5 mL) were collected from the birds of each group on weekly basis, and the hemagglutination inhibition (HI) test was performed to detect Ab titer on the respective days using the standard procedure (Meijer et al., 2006). At day 35, chickens in the all 3 groups were challenged with NDV (106 EID50/mL). Clinical signs were observed in all experimental groups for 7 D after the challenge on daily basis. The rate of protection was determined until day 7 after the challenge. To determine the adjuvant effect of soyasaponins on the growth rate, all birds were weighted on weekly basis, and mean body weights (BW) were compared.
Histopathological Examination
To determine histopathological changes associated with NDV infection and protective effect of soyasaponin, visceral organs showing gross lesions such as the trachea, spleen, proventriculus, and intestine were collected from birds of all 3 groups during and at the end of the experiment (day 42). The tissue samples were fixed in neutral buffer formalin and processed by paraffin embedding technique (Bancroft et al., 2013). The tissue blocks were cut at 5-μm thickness, and glass slides mounted with the tissue section were stained with hematoxylin and eosin. These stained glass slides were examined at 100× magnification for degenerative cellular changes in these tissue samples.
Statistical Analysis
Statistical analysis (log-based) was performed using STATA 13.0 (Stata Corp., College Station, TX). One-way analysis of variance was used to compare the difference among all treatment groups. P-values less than or equal to 0.05 (P ≤ 0.05) were considered significant.
Results
Soyasaponin Extraction From Soybean Seeds
Saponin from soybean seeds was extracted by the microwave-assisted method. Defatted soybean powder and aqueous ethanol (extraction solvent) were mixed for extraction of soyasaponins from soybean seeds. Ethanolic solution containing soyasaponin was filtered to separate the solution from the solid residues settled at the bottom of the flask, followed by evaporation of the solvent to obtain soyasaponin.
Quantification of Soyasaponins
Presence of the soyasaponin in the ethanolic extract was confirmed by the foam test and hemolytic assay. The test tube containing soyasaponin showed a positive foam test, whereas no foam was observed in the negative control. Hemolytic ability of soyasaponin on blood agar medium was also determined. Formation of clear zones around the disc containing soyasaponin indicates lysis of red blood cells and confirms the presence of saponin in the ethanolic extract. The results of quantification showed that a total of 500 μg of solid dry mass of soyasaponin was recovered from soybean seeds.
Determination of NDV Titer Isolated From Chicken Embryonated Eggs
After 48 h of NDV inoculation, allantoic fluid from the embryonated eggs was harvested, and HA titer was determined. A high HA titer (1:256) of NDV was observed. Newcastle disease virus was concentrated and inactivated with 0.3% formaldehyde.
Determination of Ab Titer Using the HI Test Before and After the Challenge
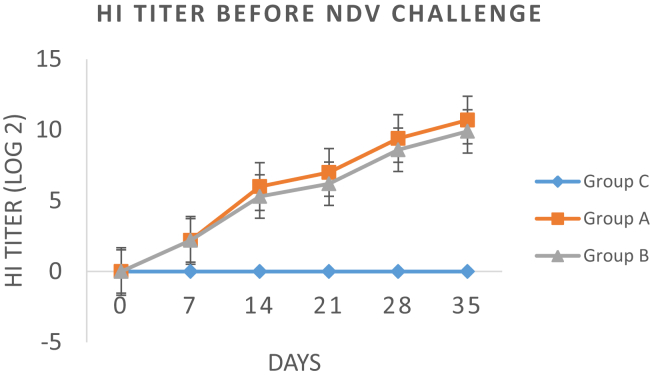
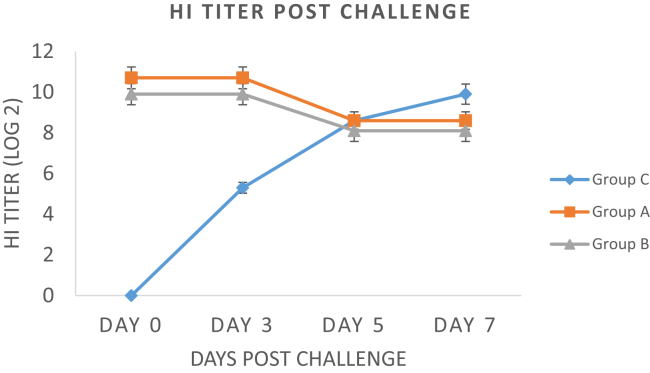
Host Ab response among the three treatment groups was determined by the HI test from the serum samples collected at various days after immunization. Figure. 1 highlights the Ab titer (log2) of all groups at day 0, 7, 14, 21, 28, and 35 after immunization with primary and booster doses. Overall, group A birds orally administered with soyasaponin showed a higher Ab titer than group B birds, whereas group C birds did not show any Ab titer before the NDV challenge. Our results indicated that HI titer of both groups (A and B) start increasing on day 7. At day 14 and 21, group A birds showed a significantly higher log2 HI titer of 6 (1:64) and 7 (1:128), respectively, than group B and C birds on the respective days. A lower log2 HI titer of 5.3 and 6.2 was observed for group B birds during the same time period. After administration of the booster dose at day 21 to both groups (A and B), an enhanced mean HI titer was again observed for group A birds at 28 and 35 D. Maximum Ab titer (log210.7) was observed for group A birds at day 35, whereas group B birds showed a significant lower Ab titer (log2 9.9) titer than group A birds. Figure. 2 describes the mean HI titer of all 3 groups after the NDV challenge. Our results showed that a slight decline in HI titer of both group A and B birds was observed from day 3 to day 5 after the challenge, after which it persisted until day 7 after the NDV challenge. On the other hand, a sudden increase in HI titer of unvaccinated group C birds was observed, which continued to increase until day 7 after the challenge.
Figure 1.
Comparison of mean antibody titer (log2) of all groups at various days before the challenge. HI, hemagglutination inhibition; NDV, Newcastle disease virus.
Figure 2.
Comparison of mean antibody titer (log2) of all groups at various days after the NDV challenge. NDV, Newcastle disease virus; HI, hemagglutination inhibition.
Results of the Challenge Protection Study
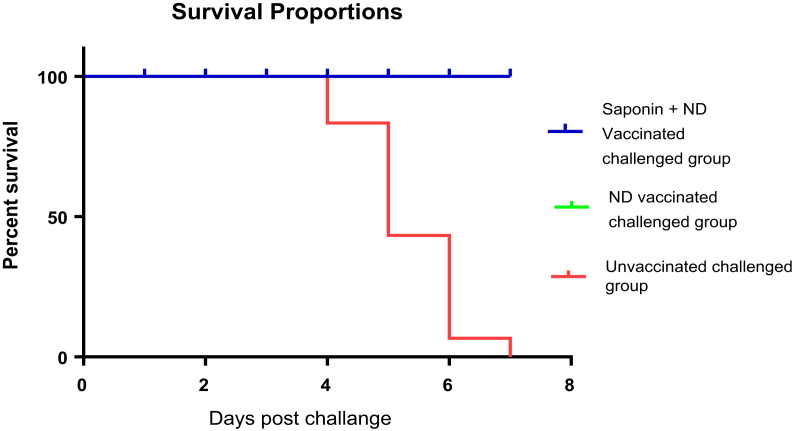
All birds of group A administered with soyasaponin-adjuvanted NDV vaccine survived NDV infection and did not develop any disease symptoms. Group B birds administered with the vaccine also showed complete protection to disease after NDV infection. Conversely, the unvaccinated group C chickens developed disease symptoms 2 D after infection, and all birds died on day 7 after infection. Our results suggest that the rate of protection in saponin-administered chicken was 100% (Figure. 3).
Figure 3.
Percentage survival curve of A, B, and C groups challenged with NDV. Rate of protection in group A and B birds was 100%, whereas group C birds showed mortality at various days after the challenge. NDV, Newcastle disease virus.
Results of Histopathological Examination
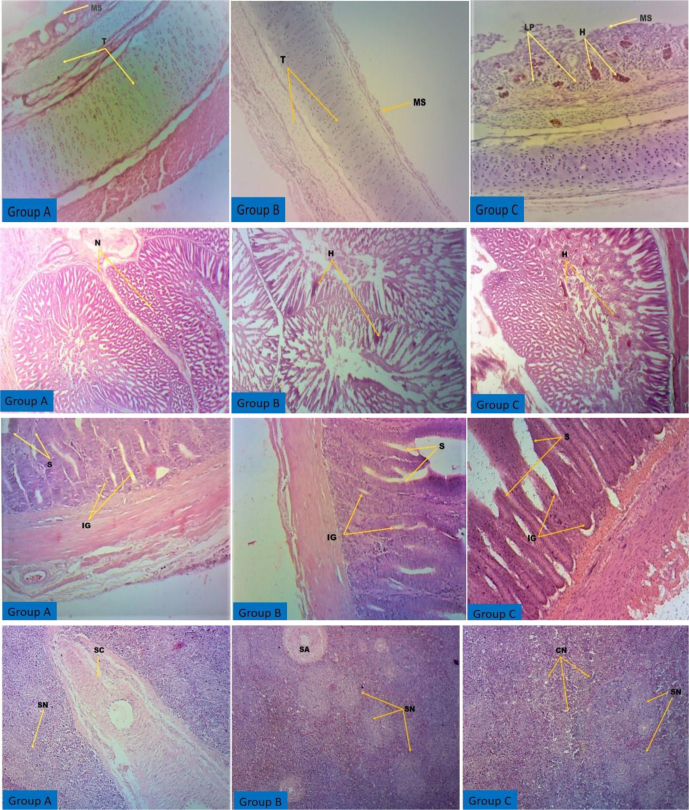
Histopathologically, the challenged group A chickens showed no lesions in the trachea, spleen, proventriculus, and intestine. Although challenged group B chickens survived the disease, their organs showed slight degenerative changes (less severe lesions). The challenged unvaccinated group C chickens showed significant pathological changes in the trachea, proventriculus, spleen, and intestine (Figure. 4). Histopathological lesions observed in challenged unvaccinated chickens include severe hemorrhages in the tracheal lumen and lymphocyte infiltration, hemorrhages in the proventriculus, disrupted intestinal glands and sloughing of the intestinal epithelium, and severe coagulative necrosis in the splenic parenchyma. Histopathology of group B birds showed mild sloughing of the tracheal cartilage as well as minor hemorrhages in the proventriculus and intestinal glands. No such lesions were observed in tissues of the organs collected from the soyasaponin-administered group.
Figure 4.
Histological micrograph of different organs of chickens collected from different groups (H&E, 100×). Group A: soyasaponin + ND-challenged vaccinated group; group B: ND-challenged vaccinated group; group C: unvaccinated challenged group. Yellow arrows indicate normal and pathological changes in tissues. First row: the trachea of group A birds showing the normal mucosal surface (MS) with cilia and the normal structure of the submucosa and tracheal cartilage (T). Group B birds exhibited slight sloughing of mucosal cells (MS), whereas group C birds had severe hemorrhages (H) in the tracheal lumen with sloughed mucosal cells (MS) and infiltration of lymphocytes (LP). Second row: the proventriculus of group A birds showed the normal structure of glands (N). In group B, minor hemorrhages (H) in the glands were observed, but these hemorrhages (H) were found to be severe in group C birds. Third row: the normal intestinal structure such as the surface epithelium (S) and intestinal glands (IG) was present in group A birds. Sloughing of the epithelium surface (S) and disrupted intestinal glands (IG) were observed in group B and C birds. Fourth row: normal splenic structure having the splenic nodule (SN) and splenic cord (SC) in group A birds. In group B and C birds, coagulative necrosis (CN) was seen in the splenic parenchyma. H&E, hematoxylin and eosin.
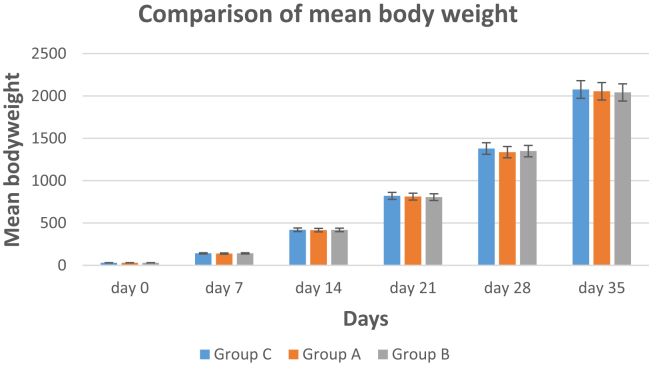
Effect of Soyasaponin on Growth Performance
Figure. 5 describes the comparison among the mean BW of 3 groups at day 0, 7, 14, 21, 28, and 35. No significant differences were observed in BW of all 3 groups, suggesting that soyasaponin did not have any effect on the growth rate of birds.
Figure 5.
Mean body weight of three groups at various days before and after immunization. No significant differences were observed among all groups until day 35.
Discussion
In the present study, soyasaponin was used as a vaccine adjuvant to analyze its potential to mediate humoral immune response in broiler chicks against ND, followed by the challenge protection study. First, soyasaponin was extracted from soybean seeds by the microwave-assisted solvent extraction method. Successful extraction of soyasaponin by this method was also reported by Deore et al., (2015). Waziiroh and Kamilia (2018) also suggested this solvent extraction as a reliable method for soyasaponin extraction from plant sources.
In the second step, soyasaponins were administered in chickens via the oral route to determine the enhancement in humoral responses. Previously, adjuvant activity of saponins from different sources have been also reported by Burakova et al., 2018, Cibulski et al., 2018, Yendo et al., 2016; and Yu et al., 2015. Both the parental and oral routes for administration of saponins have been reported previously (Song and Hu, 2009, Zhai et al., 2011a, Zhai et al., 2011b). The present study demonstrated that oral administration of soyasaponin-adjuvanted inactivated NDV vaccine significantly increased serum Ab titer compared with administration of inactivated ND vaccine alone in broiler chicks at each weak followed by immunization (primary and booster doses). A significantly increase in log2 HI titer was observed among group A birds at 14, 21, 28, 35, and 42 D. On the other hand, a significant lower Ab titer was observed for group B birds in the same time period. This difference in HI titer continued even after administration of booster doses to both groups. Zhai et al. (2011a) reported a time-dependent increase in HI titer after oral administration of ginseng stem leaf saponins (GSLS), followed by immunization with live NDV vaccine through the intranasal route, in chickens. Before vaccination, oral administration of GSLS via drinking water significantly enhanced the serum Ab titer and mucosal immunity in birds compared with those of the control group immunized with the NDV vaccine only (Zhai et al., 2011a). Our results were also in line with those of the study by Zhai et al., (2011b), who reported an increase in HI titer in serum of chickens vaccinated with the inactivated ND vaccine IM after oral administration with GSLS. An enhanced protection was also reported when chickens were vaccinated with Avian influenza and Infectious bursal disease vaccines after oral treatment with GSLS (Zhai et al., 2011b, Zhai et al., 2014). Xiao et al., (2009) measured an elevated level of both humoral and cellular immune responses in chickens by indirect ELISA, when immunized with the extract of Momordica cochinchinensis along with the ND vaccine. Similar findings were also reported from the study of Ibrahim et al., (2016), and a high serum Ab level was found to be directly related to the protective effect against NDV. Enhancement in Ab titer due to combined adjuvant effects of plant-derived saponin and Se in response to a live vaccine in chickens was also demonstrated by Ma et al., 2019. Our data showed that soyasaponin provided 100% protection to all the birds in group A against NDV infection compared with the unvaccinated birds. A complete protection was also observed in the vaccinated group B birds. But group A birds maintained a higher Ab titer before and after the NDV challenge than group B birds. Our results regarding the challenge protection test were also in line with the results of the studies conducted by El-Dabae et al., 2018, in which saponin-adjuvanted ND vaccine provided complete protection to chickens against the velogenic NDV strain. Histopathological examination of the soyasaponin-administered group revealed a lack of lesions in various organs, indicating that the immune response induced by soyasaponin is highly protective compared with the immune response observed in groups B and C, in which mild and severe degenerative changes were observed, respectively. No significant differences were observed in mean BW of all 3 groups before the challenge, suggesting that soyasaponin did not affect the growth performance. The results of the present study showed that soyasaponin was also able to produce an appreciable amount of immune response as soyasaponin gave a more consistent and elevated immunostimulatory effect than inactivated ND vaccine alone. Naturally extracted soyasaponins are highly effective in provoking humoral immune responses and therefore may be considered as potential and cost-effective candidates for vaccine adjuvants in vaccination compared with other synthetic and conventional adjuvants.
Acknowledgments
The authors would like to acknowledge all the coauthors for their cooperation.
Contributor Information
Sajjad ur Rahman, Email: sajjadur@gmail.com.
Faisal Rasheed Anjum, Email: drfaissaltarar@gmail.com.
References
- Abdisa T., Tagesu T. Review on Newcastle disease of poultry and its public Health importance. J. Vet. Sci. Technol. 2017;8:441–442. [Google Scholar]
- Almutairi M.S., Ali M. Direct detection of saponins in crude extracts of soapnuts by FTIR. Nat. Prod. Res. 2015;29:1271–1275. doi: 10.1080/14786419.2014.992345. [DOI] [PubMed] [Google Scholar]
- Bancroft J.D., Layton C., Suvarna S.K. Churchill Livingstone Elsevier; 2013. Bancroft's theory and practice of histological techniques; p. 151. [Google Scholar]
- Burakova Y., Madera R., Wang L., Buist S., Lleellish K., Schlup J.R., Shi J. Food- Grade saponin extract as an Emulsifier and Immunostimulant in Emulsion-based Subunit vaccine for Pigs. J. Immunol. Res. 2018;2018:1–8. doi: 10.1155/2018/8979838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cibulski S.P., Rivera-Patron M., Mourglia-Ettlin G., Casaravilla C., Yendo A.C.A., Fett-Neto A.G., Chabalgoity J.A., Moreno M., Roehe P.M., Silveira F. Quillaja brasiliensis saponin-based nanoparticulate adjuvants are capable of triggering early immune responses. Sci. Rep. 2018;8:13582. doi: 10.1038/s41598-018-31995-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Paula Barbosa A. Saponins as immunoadjuvant agent: a review. Afr. J. Pharm. Pharmacol. 2014;8:1049–1057. [Google Scholar]
- Deore S.L., Baviskar B.A., Rangari A.S. Rapid and high yield Extraction method for Saponins from Safed musli. J. Pharmacogn. 2015;7:210–214. [Google Scholar]
- El-Dabae W.H., Hussein H.A., Rohaim M.A., El-Safty M.M., Ata N.S., Reda I.M. Saponin-adjuvanted vaccine protects chickens against velogenic Newcastle disease virus. Arch. Virol. 2018;163:2423–2432. doi: 10.1007/s00705-018-3917-4. [DOI] [PubMed] [Google Scholar]
- Francis G., Kerem Z., Makkar H.P., Becker K. The biological action of saponins in animal systems: a review. Br. J. Nutr. 2002;88:587–605. doi: 10.1079/BJN2002725. [DOI] [PubMed] [Google Scholar]
- Gestetner B., Birk Y., Bondi A., Tencer Y. Soya bean saponins—VII: a method for the determination of sapogenin and saponin contents in soya beans. Phytochemistry. 1966;5:803–806. [Google Scholar]
- Ibrahim I.U., Lawal J.R., El-Yuguda A.D. Efficacy of feed Coated Newcastle disease I2 vaccine in Village chickens in Gombe state, Nigeria. J. Vet. Sci. Technol. 2016;7:349. [Google Scholar]
- Kerem Z., German-Shashoua H., Yarden O. Microwave-assisted extraction of bioactive saponins from chickpea (Cicer arietinum L) J. Sci. Food Agric. 2005;85:406–412. [Google Scholar]
- Leroux-Roels G., Marchant A., Levy J., Van Damme P., Schwarz T.F., Horsmans Y., Jilg W., Kremsner P.G., Haelterman E., Clément F., Gabor J.J. Impact of adjuvants on CD4+ T cell and B cell responses to a protein antigen vaccine: results from a phase II, randomized, multicenter trial. Clin. Immunol. 2016;169:16–27. doi: 10.1016/j.clim.2016.05.007. [DOI] [PubMed] [Google Scholar]
- Ma X., Bi S., Wang Y., Chi X., Hu S. Combined adjuvant effect of ginseng stem-leaf saponins and selenium on immune responses to a live bivalent vaccine of Newcastle disease virus and infectious bronchitis virus in chickens. Poult. Sci. 2019;98:3548–3556. doi: 10.3382/ps/pez207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer A., Bosman A., van de Kamp E.E., Wilbrink B., van Beest Holle M.D.R., Koopmans M. Measurement of antibodies to avian influenza virus A (H7N7) in humans by hemagglutination inhibition test. J.Virol. Methods. 2006;132:113–120. doi: 10.1016/j.jviromet.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Oda K., Matsuda H., Murakami T., Katayama S., Ohgitani T., Yoshikawa M. Relationship between adjuvant activity and amphipathic structure of soyasaponins. Vaccine. 2003;21:2145–2151. doi: 10.1016/s0264-410x(02)00739-9. [DOI] [PubMed] [Google Scholar]
- Rajput Z.I., Hu S.H., Xiao C.W., Arijo A.G. Adjuvant effects of saponins on animal immune responses. J. Zhej. Uni. Sci. B. 2007;3:153–161. doi: 10.1631/jzus.2007.B0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X., Hu S. Adjuvant activities of saponins from traditional Chinese medicinal herbs. Vaccine. 2009;27:4883–4890. doi: 10.1016/j.vaccine.2009.06.033. [DOI] [PubMed] [Google Scholar]
- Sun H.X., Xie Y., Ye Y.P. Advances in saponin-based adjuvants. Vaccine. 2009;27:1787–1796. doi: 10.1016/j.vaccine.2009.01.091. [DOI] [PubMed] [Google Scholar]
- T O’Hagan D., Friedland L.R., Hanon E., M Didierlaurent A. Towards an evidence based approach for the development of adjuvanted vaccines. Curr. Opin. Immunol. 2017;47:93–102. doi: 10.1016/j.coi.2017.07.010. [DOI] [PubMed] [Google Scholar]
- Waziiroh E., Kamilia K. Microwave-assisted extraction (MAE) of bioactive saponin from mahogany seed (Swietenia mahogany Jacq) IOP Conf. Ser. Earth Environ. Sci. 2018;1:012006. [Google Scholar]
- Xiao C., Bao G., Hu S. Enhancement of immune responses to Newcastle disease vaccine by a supplement of extract of Momordica cochinchinensis (Lour.) Spreng. Seeds. Poult. Sci. 2009;88:2293–2297. doi: 10.3382/ps.2009-00059. [DOI] [PubMed] [Google Scholar]
- Yendo A.C., de Costa F., Cibulski S.P. A rabies vaccine adjuvanted with saponins from leaves of the soap tree (Quillaja brasiliensis) induces specific immune responses and protection against lethal challenge. Vaccine. 2016;34:2305–2311. doi: 10.1016/j.vaccine.2016.03.070. [DOI] [PubMed] [Google Scholar]
- Yu J., Shi F.S., Hu S. Improved immune responses to a bivalent vaccine of Newcastle disease and avian influenza in chickens by ginseng stem-leaf saponins. Vet. Immunol. Immunopathol. 2015;15:147–155. doi: 10.1016/j.vetimm.2015.07.017. [DOI] [PubMed] [Google Scholar]
- Zhai L., Li Y., Wang W., Hu S. Enhancement of humoral immune responses to inactivated Newcastle disease and avian influenza vaccines by oral administration of ginseng stem-and-leaf saponins in chickens. Poult. Sci. 2011;90:1955–1959. doi: 10.3382/ps.2011-01433. [DOI] [PubMed] [Google Scholar]
- Zhai L., Li Y., Wang W., Wang Y., Hu S. Effect of oral administration of ginseng stem-and-leaf saponins (GSLS) on the immune responses to Newcastle disease vaccine in chickens. Vaccine. 2011;29:5007–5014. doi: 10.1016/j.vaccine.2011.04.097. [DOI] [PubMed] [Google Scholar]
- Zhai L., Wang Y., Yu J., Hu S. Enhanced immune responses of chickens to oral vaccination against infectious bursal disease by ginseng stem-leaf saponins. Poult. Sci. 2014;93:2473–2481. doi: 10.3382/ps.2014-04056. [DOI] [PubMed] [Google Scholar]