Abstract
In poultry production, vaccination is an effective measure to protect chickens from diseases. Vaccination, however, is a stressor that may induce stress responses that interfere with the growth and development of chickens. The interaction between the skeletal and immune systems on bone quality has gained more attention. In the present study, the influence of high frequency vaccinations on the bone development of layer pullets was investigated. Thirty 35-day-old SPF White Leghorn layer pullets were obtained and randomly subjected to the following treatments: vaccinated against Newcastle disease (ND) with LoSota vaccine once at 35-day-old (V1, control); 4 times at 35, 49, 63, and 77 d of age (V4); and 7 times at 35, 42, 49, 56, 63, 70, and 77 d of age (V7). The body weight and organ index of the spleen, thymus, and tibia were recorded. The antibody titer and serum and the tibia calcium and phosphorus concentrations were measured. The transcription levels of the IL-6, IL-17, TNF-α, receptor activator of NF-κB ligand (RANKL), and osteoprotegerin (OPG) genes were determined in spleen, thymus, and the tibia. The results showed that V7 decreased body weight and increased the ND antibody titer, compared to V1-chickens. The expression levels of IL-6, IL-17, and TNF-α were upregulated in spleen, thymus, and the tibia of V7 chickens. In the tibia, RANKL was upregulated, while OPG was downregulated by V7 treatment. The results indicate that high frequency vaccination induces immune stress and impairs bone development. The results suggest that the augmented cytokine expression in immune organs and the tibia is associated with activation of the OPG/RANKL pathway, which, in turn, enhances osteoclastogenesis. The appropriate frequency of vaccination should support optimal bone development and full immunoprotection in layer pullets.
Keywords: vaccination, bone development, OPG/RANKL, immune stress, layer pullets
INTRODUCTION
In cage laying hens, osteoporosis is a condition that involves the progressive loss of structural bone during the laying period, resulting in increased bone fragility and susceptibility to fracture (Whitehead and Fleming, 2000). Cage layer osteoporosis is a serious animal welfare problem (Webster, 2004). The feeding regime during the pullet rearing phase, a period of substantial musculoskeletal growth, offers a proactive approach to reducing osteoporosis by improving bone composition (Casey-Trott et al., 2017).
In human beings, the effect of the skeletal and immune systems on bone quality has gained more attention (Arron and Choi, 2000). Osteoimmunology is an emerging research area that deals with the mutual interactions between bone and the immune system (Rauner et al., 2013). The close relationship between inflammatory disease and bone loss has been established clinically in humans (Redlich and Smolen, 2012; Mbalaviele et al., 2017). In poultry, vaccines are widely applied to prevent and control contagious poultry diseases (Marangon and Busani, 2007). To ensure the health of layers during the laying period, frequent vaccination against antigens is an effective strategy during the pullet rearing period in commercial layer production. Hence, the influence of vaccination on bone development needs to be investigated.
The receptor activator of NF-κB (RANK) ligand (RANKL), which belongs to the TNFR ligand family (Anderson et al., 1997), is a type II transmembrane protein comprising 371 amino acids expressed in osteoblasts and chondrocytes. The binding of RANK and RANKL plays a crucial role in osteoclast survival, differentiation, and activation, and it promotes bone resorption (Malliga et al., 2011; Huang et al., 2013; Xiong et al., 2014). The differentiation of osteoclasts is mainly triggered by RANKL. By binding to preosteoclasts, RANKL stimulates the differentiation of preosteoclasts into osteoclasts (Burgess et al., 1999; Dar et al., 2018). Osteoclast activation results in the resorption of bone matrix that increases serum calcium and phosphate (Mueller et al., 1964). Osteoprotegerin (OPG), a secreted glycoprotein, is a decoy receptor for RANKL. OPG can competitively bind RANKL, hindering the binding between RANKL and RANK, can inhibit osteoclast activity and can impede bone resorption and increase bone density (Simonet et al., 1997; Gori et al., 2000). OPG, RANKL, and RANK interact to form an OPG/RANKL/RANK system that regulates bone metabolic balance (Klejna et al., 2009). The OPG/RANKL pathway plays a dominant role in osteoclastogenesis, which is involved in bone formation and resorption during bone remodelling (Zhang et al., 2009). In chickens, the functions of the avian RANKL and its receptors, RANK and OPG, are evolutionarily conserved (Sutton et al., 2015). Chicken RANKL is an important factor required for inducing osteoclastogenesis similar to its mammalian homologue (Wang et al.,2008). Constructed pcDNA3.1 (+)/chOPG transfected into CEFs expressed bioactive OPG protein that was able to inhibit osteoclast function (Hou et al., 2011). Suppressing the RANKL/OPG pathway may be involved in trace mineral element deficiency and resulted in metaphyseal osteoporosis (Liu et al., 2015). In modern layer production systems, the immune system is frequently challenged by antigens, and frequent vaccinations are required due to the long reproductive cycle. Hence, we hypothesized that the vaccination frequency may have an effect of the bone development of layer pullets.
Moreover, accumulating data indicated RANKL/RANK serves not only as essential player for the development and activation of osteoclasts, but also for immune function. RANK and RANKL have been proved to be important regulators of interactions between T cells and dendritic cells (Anderson et al., 1997). RANKL is involved in the regulation of lymph-node organogenesis and lymphocyte development and the mice deficient in RANKL signaling lack lymph nodes and have severe defects in the spleen microarchitecture (Kong et al., 1999; Weih and Caamano, 2003). The RANKL/RANK signaling axis plays an important role in the orchestration of protective immune responses in the spleen marginal zone, which has important implications for the host response to viral infection and induction of acquired immunity (Habbeddine et al., 2017). RANK/RANKL/OPG axis is involved in the regulation of thymus medullary microenvironments (McCarthy et al., 2015). The correct differentiation of medullary thymic epithelial cells that act as mediators of the central tolerance process by which self-reactive T cells are eliminated while regulatory T cells are generated (Akiyama et al., 2008, 2013; Sobacchi et al., 2019). Hence, the expressions of RANKL and OPG were investigated in the spleen and thymus.
In the present study, SPF chickens were used to evaluate the effect of vaccination frequency on bone development. The layer pullets were subjected to one of 3 vaccination regimens that involved vaccination either 1, 4, or 7 times from 35 to 77 d of age. Bone development, the expression levels of cytokines in immune organs and the tibia, and the status of the OPG/RANKL pathway were determined.
MATERIALS AND METHODS
All procedures used in this study were approved by the Animal Care Committee of Shandong Agricultural University (P. R. China) and were carried out in accordance with the guidelines for experimental animals of the Ministry of Science and Technology (Beijing, P. R. China).
Experimental Design
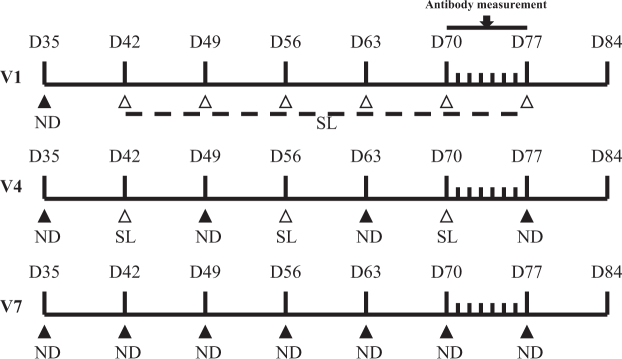
Thirty 35-day-old SPF White Leghorn layer pullets were obtained from Jinan SAIS SPF Poultry Co. Ltd. (Jinan, Shandong). All the chickens were reared in single cages and had free access to feed and water during the whole experimental period. The pullets were divided into three groups and were subjected randomly to the following treatments: vaccinated against Newcastle disease with the LoSota vaccine according to the industry practice (Qilu Animal Health Products Comp. Jinan, P. R. China) by eye drop once at 35 d of age (V1, control); 4 times at 35, 49, 63, and 77 d of age (V4); or 7 times at 35, 42, 49, 56, 63, 70, and 77 d of age (V7). When the experimental chickens were vaccinated against Newcastle vaccine, the other chickens were sham treated with saline and the experimental procedure was schematically graphed in Figure 1. The experiment lasted 8 wk, and the body weights of chickens were recorded before and after the experiment. At 77 d of age, a blood sample was obtained daily for 7 d from each chicken of the three treatments. Serum was separated by centrifugation at 1500 g for 10 min and was stored at −20°C for antibody titer measurement. At 84-day-old, the end of the experiment, all chickens were fasted overnight, and eight chickens were randomly selected from each treatment. After a blood sample was taken from a wing vein, the chicken was euthanized by cervical dislocation. After they were dissected, the thymus, spleen, and right tibia were weighed and sampled. The tissue samples were immediately snap-frozen in liquid nitrogen and were stored at −80°C for further analysis. Serum was separated by centrifugation at 1500 g for 15 min and was stored at −20°C until analysis.
Figure 1.
The schematic graph of experimental protocol. V1, vaccinated one time; V4: vaccinated four times; V7: vaccinated seven times; ND: vaccination against Newcastle Disease with LoSota vaccine by eye drop; SL: sham treated with saline.
Serum HI Antibody Assay
Briefly, 2-fold serial dilutions of serum were made in a 96-well, V-shaped bottom microtiter plates containing 50 μL physiological saline in all wells, and then, 50 μL NDV antigen (4 HA units) was added into all the wells except for the last row, which served as the controls. Serum dilutions ranged from 1:2 to 1:2048. The antigen serum mixture was incubated for 10 min at 37°C. Then, 50 μL 1% rooster erythrocyte suspension was added to each well, and the plate was re-incubated for 30 min. A positive serum, a negative serum, erythrocytes, and antigens were also included as controls. The highest dilution of serum causing complete inhibition was considered the endpoint. The geometric mean titer was expressed as reciprocal log2 values of the highest dilution that displayed HI.
Serum Calcium and Phosphorus
Serum concentrations of Ca and P were measured by using commercial kits (Jiancheng Bioengineering Institute, Nanjing, China). All procedures were carried out according to the manufacturers' instructions.
The Tibia Calcium and Phosphorus Contents
The right tibia sample was treated with a mixture of alcohol and benzene 2:1 for 96 h for degreasing and was then dried at 105°C to constant weight. The degreased bone sample was used for the measurement of calcium and phosphorus content.
Bone Bending Strength
Whole femur mechanical properties (structural strength/stiffness) and material properties (flexural strength/modulus) were quantified using a 3-point bending test (Fleming et al., 1998). The tibia was centred over two supports (4 cm span) with a 1 N preload before loading to failure at a rate of 2 mm/min with the anterior surface in tension. The three-point bending test was carried out by a microcomputer-controlled electronic universal testing machine (Jinan Shi Jin Neng). When the bone fracture occurred, the maximum bending force F was measured according to the formula “aw = (8*F*L)/π/d3,” where L is the spacing of the two support points, and d is the diameter of the bone.
Real-Time PCR Analyses
Total RNA was extracted from the thymus, spleen, and tibia using TransZol Up (TransGen Biotech, China). Then, the concentration of the RNA was measured by spectrophotometry (Eppendorf, Germany), and the RNA purity was verified by calculating the ratio between the absorbance values at 260 and 280 nm (A260/280 ≈ 1.75–2.01). Next, reverse transcription was performed using total RNA (1 μg) for first-strand cDNA synthesis with the Transcriptor First Strand cDNA Synthesis Kit (Roche, Germany). The cDNA was amplified in a 20 μL PCR system containing 0.2 μmol/L each specific primer (Sangon, China) and of the SYBR Green master mix (Roche, Germany) according to the manufacturer's instructions. Real-time PCR was performed at the ABI QuantStudio 5 PCR machine (Applied Biosystems; Thermo, USA); primer sequences are shown in Table 1. The mRNA expression levels of IL-6, IL-17, TNF-α, RANKL, and OPG were measured.
Table 1.
The primers sequences used in the gene expression analyse of via quantitative real-time polymerase chain reaction (qRT-PCR).
| Gene | Primer sequence (5′-3′) |
|---|---|
| β-actin-F | CTGGCACCTAGCACAATGAA |
| β-actin-R | CTGCTTGCTGATCCACATCT |
| IL-6 F | CGCCCAGAAATCCCTCCTC |
| IL-6 R | AGGCACTGAAACTCCTGGTC |
| OPG F | CGCTTGTGCTCTTGGACATT |
| OPG R | GCTGCTTTACGTAGCTCCCA |
| RANKL F | TGTTGGCTCTGATGCTTGTC |
| RANKL R | TCCTGCTTCTGGCTCTCAAT |
| NF-κB-F | CTCTCCCAGCCCATCTATGA |
| NF-κB-R | CCTCAGCCCAGAAACGAAC |
| IL-17-F | CTCCTCTGTTCAGACCACTGC |
| IL-17-R | ATCCAGCATCTGCTTTCTTGA |
| TNF-F | GAGCGTTGACTTGGCTGTC |
| TNF-R | AAGCAACAACCAGCTATGCAC |
Statistical Analysis
The data are expressed as the means ± SEM. The results were analysed using one-way ANOVA via the Statistical Analysis Systems statistical software package (Version 8e; SAS Institute Inc., Cary, NC, USA). Differences between means were evaluated using Duncan's significant difference tests. For variables body weight and ND antibody titre, the Repeated Measurement Analysis was conducted to estimate the main effects of vaccination frequency, with each chicken as replicate. Means were considered significant at P < 0.05.
RESULTS
The increased vaccination frequency had a significant influence (P < 0.05) on body weight from 63-day-old, and the V1-chickens had higher body weights than the V7-chickens (Figure 2). At 70 to 77 d of age, The ND antibody titer was significantly higher in V7-chickens than in V1-chickens at all the measuring time points (P < 0.05, Figure 3 A). Compared to V1, however, the V4 chickens showed higher ND titer only at days 3, 4, 5, and 6 (P < 0.05). In contrast, there was no detectable difference between V4 and V7 treatments (P > 0.05).
Figure 2.
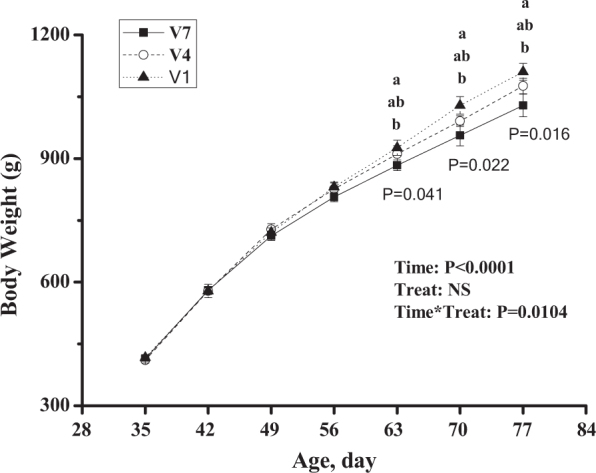
Effect of vaccination frequency (1, 4, and 7 times) on the body weights of layer pullets. V1: vaccinated 2 time; V4: vaccinated 4 times; V7: vaccinated 7 times. a, b: Means sharing different letters in the same column (from top to bottom, V1, V4, V7) are significantly different (P < 0.05). The data are presented as the mean ± SEM (n = 8).
Figure 3.
Effect of vaccination frequency (1, 4, and 7 times) on serum antibody titres against NDV (A) during 70 to 77-day-old, and on serum calcium (B) and serum phosphorus (C) at 84-day-old in layer pullets. V1: vaccinated 1 time; V4: vaccinated 4 times; V7: vaccinated 7 times. The data are presented as the mean ± SEM (n = 7–8); x,y,z: Means labelled with different letters in the same time point differ significantly (P < 0.05).
The serum calcium and phosphorus levels were not changed by vaccination frequency (P > 0.05, Figure 3 B, C). Vaccination frequency had no significant influence (P > 0.05) on the tibia index or on calcium or phosphorus contents (Figure 4 A, B, C). Compared to the bone bending strength of V1-chickens, V7-chickens had lower bone bending strength (P < 0.05, Figure 4 D).
Figure 4.
Effect of vaccination frequency (1, 4, and 7 times) on the tibia index (A), calcium content (B), phosphorus content (C), and bending strength (D) of the tibias of layer pullets. V1: vaccinated 1 time; V4: vaccinated 4 times; V7: vaccinated 7 times. The data are presented as the mean ± SEM (n = 8–10). *, P < 0.05.
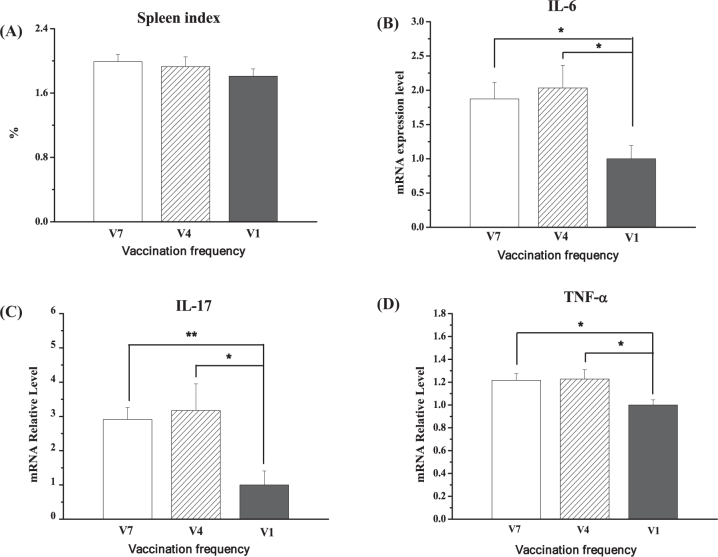
The organ index of the spleen was not changed by different vaccination frequencies (P > 0.05, Figure 5 A). In the spleen, compared to V1-chickens, the mRNA levels of IL-6, IL-17, and TNF-α were significantly increased in V7-chickens (P < 0.01) and in V4-chickens (P < 0.05, Figure 5 B, C, D). In contrast, no detectable difference was observed between V4 and V7 treatments (P > 0.05). Compared to the V1 group, the mRNA level of RANKL was significantly upregulated (P < 0.01) in the V4 and V7 groups, whereas the OPG mRNA level was not different between groups (P > 0.05, Figure 5).
Figure 5.
Effect of vaccination frequency (1, 4, and 7 times) on the spleen organ index (A), IL-6 mRNA (B), IL-17 mRNA (C), and TNF-α mRNA (D) in the spleens of layer pullets. V1: vaccinated 1 time; V4: vaccinated 4 times; V7: vaccinated 7 times. The data are presented as the mean ± SEM (n = 7–10). *, P < 0.05; **, P < 0.01.
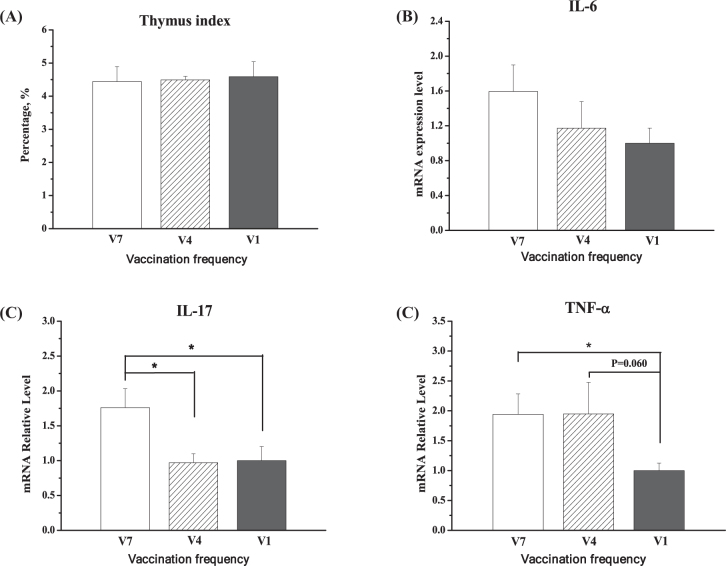
The organ index of thymus was not changed by different vaccination frequencies (P > 0.05, Figure 6 A). In the thymus, V7-chickens had higher expression levels of IL-6 (P = 0.059), IL-17 (P < 0.01), and TNF-α (P < 0.05), compared to V1-chickens (Figure 6 B, C, D). V7-chickens had higher IL-17 than V4-chickens (P < 0.05), whereas V4-chickens tended to have higher TNF-α expression levels (P = 0.06) than V1-chickens.
Figure 6.
Effect of vaccination frequency (1, 4, and 7 times) on the thymus organ index (A), IL-6 mRNA (B), IL-17 mRNA (C), and TNF-α mRNA (D) in the thymus of layer pullets. V1: vaccinated 1 time; V4: vaccinated 4 times; V7: vaccinated 7 times. The data are presented as the mean ± SEM (n = 7–10). *, P < 0.05.
In the tibia, compared to the V1 group, the expression levels of IL-6 (P = 0.098), IL-17 (P < 0.01), and TNF-α (P < 0.01) were increased in V7-chickens (Figure 7 A, B, C). The IL-17 (P < 0.01) and TNF-α mRNA levels (P < 0.05) were higher in the V7 group than that in the V4 group.
Figure 7.
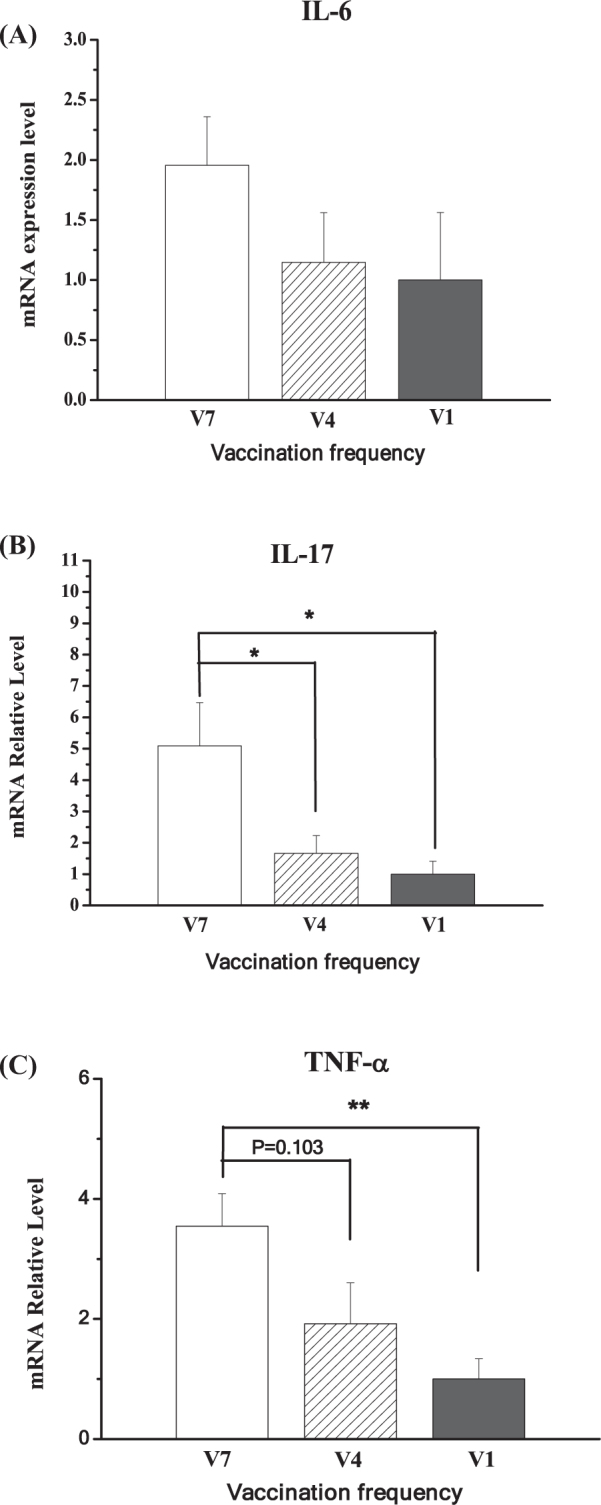
Effect of vaccination frequency (1, 4, and 7 times) on the IL-6 mRNA (A), IL-17 mRNA (B), and TNF-α mRNA (C) in the tibias of layer pullets. V1: vaccinated 1 time; V4: vaccinated 4 times; V7: vaccinated 7 times. The data are presented as the mean ± SEM (n = 6–8). *, P < 0.05; **, P < 0.01.
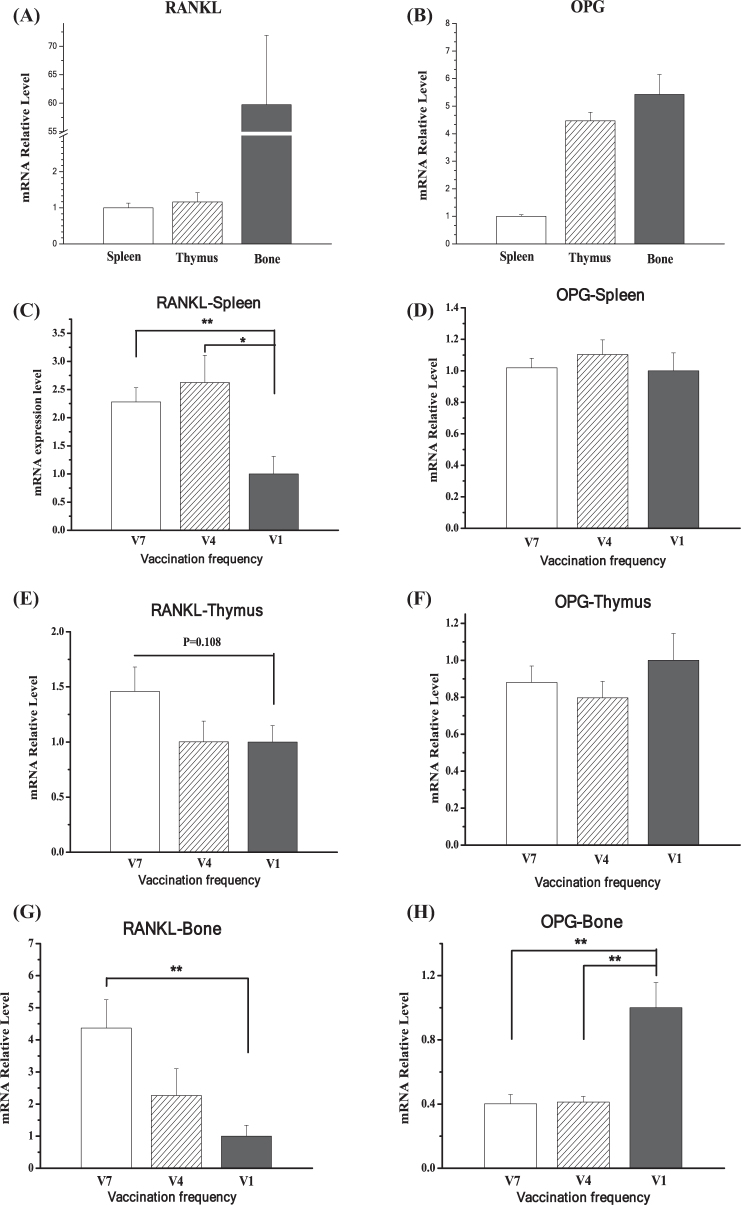
Compared to the tibia, the expression level of RANKL was relatively lower in the spleen and thymus (P < 0.001, Figure 8 A). In contrast, OPG expressed at a comparable level in the thymus compared to the tibia, while the spleen had lower expression levels of OPG (P < 0.001, Figure 8 B). In the spleen, compared to the level in V1 chickens, the mRNA level of RANKL was increased in V7 (P < 0.01) and V4 chickens (P < 0.05, Figure 8 C). In contrast, RANKL was not altered in the thymus (P > 0.05, Figure 8 E). The gene expression of OPG was not changed by vaccination frequency in the spleen or in the thymus (P > 0.05, Figure 8 D, F). Compared to RANKL expression in the V1 group, the mRNA level of RANKL was increased in the V7 group (P < 0.01) but not in the V4 group (P > 0.05, Figure 8 G). Conversely, the OPG expression level was significantly decreased in the V4 and V7 groups compared to the level in the V1 group (P < 0.01, Figure 8 H).
Figure 8.
The relative expression profile of receptor activator of nuclear factor κB ligand (RANKL) and osteoprotegerin (OPG) in thymus, spleen, and tibia (A, B) and the effect of vaccination frequency (1, 4, and 7 times) on the expression in thymus (C, D), spleen (E, F), and tibia (G, H) of layer pullets. V1: vaccinated 1 time; V4: vaccinated 4 times; V7: vaccinated 7 times. The data are presented as the mean ± SEM (n = 6–8). *, P < 0.05; **, P < 0.01.
DISCUSSION
In the present study, the influence of vaccination frequency on the bone development of layer pullets was investigated. The results indicate that high frequency vaccination induces immune stress and impairs the tibia development. This result suggests that augmented cytokine expression in immune organs and the tibia is associated with activation of the OPG/RANKL pathway, which, in turn, enhances osteoclastogenesis.
In poultry production, vaccination is an effective measure to protect chickens from diseases (Marangon and Busani, 2007). Vaccination, however, is a stressor that may induce a stress response. For example, a combined Newcastle disease and infectious bronchitis vaccine administration to SPF chicks via the intraocular route results in an acute phase response and an increased heterophil/lymphocyte ratio (Kaab et al., 2018). Hence, we speculated that high frequency vaccination may have a disadvantageous influence on the development of layer pullets. In the present study, the relatively lower body weight in the V7 treatment group indicated that high frequency vaccination has an undesirable effect on the development of layer pullets. Augmented immune stress induced by vaccination is speculated to be associated with the undesirable influence of high frequency vaccination. This speculation was supported by the previous work by (Wang et al., 2015), who reported that ND vaccine challenge reduces growth performance of broilers during an early period after the first immunization and that relatively lower doses of ND vaccine inoculation are beneficial for feed efficiency of broiler chickens. Indeed, it has been reported that the physiological and metabolic levels of chickens will change significantly after LPS challenge (Webel et al., 1998; Xie et al., 2000; Koutsos and Klasing, 2001). The bacteriological challenges by Clostridium spp., E. coli, or Salmonella spp. suppress the feed intake and body weight gain of broilers, and a meta-analysis indicated that all of these challenges increased maintenance requirements (Remus et al., 2014). Hence, the result suggests that high-frequency vaccination-induced stress could be responsible for the suppressed body weight gain. Moreover, the difference of body weight among V1, V4, and V7 chickens showed a trend of decreasing body weight with increasing time in a vaccination-frequency-dependent manner, suggesting that the higher the vaccination frequency is, the greater the undesirable impact on the growth and development of chickens.
In broilers, bone homeostasis appears to be severely disturbed during an inflammatory response (Mireles et al., 2005). In line with a previous study, the present results showed that high frequency vaccination reduced bending strength of the tibia, indicating that high frequency vaccination may impair the bone development of layer pullets. The bone bending strength is considered a sensitive indicator of acute phase response during immune challenge (Mireles et al., 2005). Calcium is important for maintaining the normal shape and function of bone (Onyango et al., 2003; Garcia and Dale, 2006). In this study, the unchanged calcium and phosphorus concentrations in serum and the tibia indicated that circulating and tibial calcium and phosphorus levels may not effectively reflect the influence of immune challenge. In LPS-challenged broilers, the plasma calcium concentration is changed as the catabolism of bone is induced during the acute phase response to inflammation (Mireles et al., 2005). Although the calcium and phosphorus contents in the tibia were not significantly influenced by treatment, there was a similar trend in calcium and phosphorus levels to decrease with vaccination frequency. The relatively poor density and mineral content of bone is likely to reduce effective bending strength of the tibiotarsus of broilers (Williams et al., 2000). This result may imply that bone calcium and phosphorus is associated with reduced bone quality in pullets subjected to high frequency vaccination.
In this study, the expression of RANKL and OPG were investigated in the tibia. The significantly upregulated RANKL and downregulated OPG in V7-chickens compared to V1-chickens indicated that osteoclastogenesis was activated. Decreased OPG/RANKL mRNA expression is suggested to be involved in the metaphyseal osteoporosis in tibia of broilers (Liu et al., 2015). Hence, the results of this study suggest that high frequency vaccination has a detrimental influence on bone development via the OPG/RANKL pathway in layer pullets.
The immune system is closely related to bone metabolism (Okamoto, et al., 2017; Takayanagi, 2007). The ND antibody titer was highest in the V7-chickens, demonstrating that repeated immunizations stimulated the immune system. In accordance with the result, the expression levels of IL-6, IL-17, and TNF-α were increased by V7 treatments in the spleen, thymus, and tibia, indicating enhanced cytokine expression in immune organs and in the tibia. In humans, the immune system is involved in the development of bone metabolic diseases such as rheumatoid arthritis, osteoporosis and osteolysis (Schett, 2009). Inflammatory factors, such as IL-6, IL-1, TNF, IL-7, and IL-17, primarily promote bone resorption and tend to have a negative balance of bone metabolism. In the present study, the expression levels of IL-6, IL-17, and TNF-α were upregulated in V7-chickens in the immune organs and in the tibia, indicating that high frequency vaccination activates the immune system, leading to increased expression of inflammatory cytokines not only in immune organs but also in the tibia. Cytokines such as IL-6 and TNF-α are important for bone resorption, and they can promote bone resorption by increasing RANKL expression. IL-17 can upregulate the expression of RANKL, thereby destroying the balance of RANKL/OPG and causing bone destruction. Taken together, the present results suggest that high frequency vaccination-induced immune challenge evokes the OPG/RANKL pathway and impairs bone development.
In conclusion, high frequency vaccination may induce immune stress and impair bone development. The augmented cytokine expression in both immune organs and in the tibia is associated with the activation of the OPG/RANKL pathway, which, in turn, enhances osteoclast activity. The optimal frequency of vaccination should allow for optimal bone development and for full immunoprotection in layer pullets.
ACKNOWLEDGMENT
This work was supported by the National Key Research Program of China (grant number 2016YFD0500510), the Modern Agro-industry Technology Research System (CARS-40-K09), and the Taishan Scholars Program (201511023).
Conflict of interest statement
The authors have declared no conflicts of interest.
Footnotes
The Author(s) 2019. Published by Oxford University Press on behalf of Poultry Science Association. This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/), which permits non-commercial re-use, distribution, and reproduction in any medium, provided the original work is properly cited. For commercial re-use, please contact journals.permissions@oup.com.
REFERENCES
- Akiyama T., Shimo Y., Yanai H., Qin J., Ohshima D., Maruyama Y., Asaumi Y., Kitazawa J., Takayanagi H., Penninger J.M., Matsumoto M., Nitta T., Takahama Y., Inoue J. The tumor necrosis factor family receptors RANK and CD40 cooperatively establish the thymic medullary microenvironment and self-tolerance. Immunity. 2008;29:423–437. doi: 10.1016/j.immuni.2008.06.015. [DOI] [PubMed] [Google Scholar]
- Akiyama T., Shinzawa M., Qin J., Akiyama N. Regulations of gene expression in medullary thymic epithelial cells required for preventing the onset of autoimmune diseases. Front. Immunol. 2013;4:249. doi: 10.3389/fimmu.2013.00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D.M., Maraskovsky E., Billingsley W.L., Dougall W.C., Tometsko M.E., Roux E.R., Teepe M.C., Dubose R.F., Cosman D., Galibert L. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature. 1997;390:175–179. doi: 10.1038/36593. [DOI] [PubMed] [Google Scholar]
- Arron J.R., Choi Y. Bone versus immune system. Nature. 2000;408:535–536. doi: 10.1038/35046196. [DOI] [PubMed] [Google Scholar]
- Burgess T.L., Qian Y., Kaufman S., Ring B.D., Van G., Capparelli C., Kelley M., Hsu H., Boyle W.J., Dunstan C.R., Hu S., Lacey D.L. The ligand for osteoprotegerin (OPGL) directly activates mature osteoclasts. J. Cell Biol. 1999;145:527–538. doi: 10.1083/jcb.145.3.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey-Trott T.M., Korver D.R., Guerin M.T., Sandilands V., Torrey S., Widowski T.M. Opportunities for exercise during pullet rearing, Part I: Effect on the musculoskeletal characteristics of pullets. Poult. Sci. 2017;96:2509–2517. doi: 10.3382/ps/pex059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dar H.Y., Azam Z., Anupam R., Mondal R.K., Srivastava R.K. Osteoimmunology: the Nexus between bone and immune system. Front Biosci (Landmark Ed) 2018;23:464–492. doi: 10.2741/4600. [DOI] [PubMed] [Google Scholar]
- Fleming R.H., McCormack H.A., McTeir L., Whitehead C.C. Medullary bone and humeral breaking strength in laying hens. Res. Vet. Sci. 1998;64:63–67. doi: 10.1016/s0034-5288(98)90117-5. [DOI] [PubMed] [Google Scholar]
- Garcia A.R., Dale N.M. Foot ash as a means of quantifying bone mineralization in chicks. J. Appl. Poult. Res. 2006;15:103–109. [Google Scholar]
- Gori F., Hofbauer L.C., Dunstan C.R., Spelsberg T.C., Khosla S., Riggs B.L. The expression of osteoprotegerin and RANK ligand and the support of osteoclast formation by stromal-osteoblast lineage cells is developmentally regulated. Endocrinology. 2000;141:4768–4776. doi: 10.1210/endo.141.12.7840. [DOI] [PubMed] [Google Scholar]
- Habbeddine M., Verthuy C., Rastoin O., Chasson L., Bebien M., Bajenoff M., Adriouch S., den Haan J.M.M., Penninger J.M., Lawrence T. Receptor activator of NF-kappaB orchestrates activation of antiviral memory CD8 T cells in the spleen marginal zone. Cell Rep. 2017;21:2515–2527. doi: 10.1016/j.celrep.2017.10.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou L., Hou J., Yao J., Zhou Z. Effects of osteoprotegerin from transfection of pcDNA3.1(+)/chOPG on bioactivity of chicken osteoclasts. Acta Vet. Scand. 2011;53:21. doi: 10.1186/1751-0147-53-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.L., Liu Y.W., Huang Y.J., Chiou W.F. A special ingredient (VtR) containing oligostilbenes isolated from vitis thunbergii prevents bone loss in ovariectomized mice: in vitro and in vivo study. Evid. Based Complement Alternat. Med. 2013;2013 doi: 10.1155/2013/409421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaab H., Bain M.M., Eckersall P.D. Acute phase proteins and stress markers in the immediate response to a combined vaccination against Newcastle disease and infectious bronchitis viruses in specific pathogen free (SPF) layer chicks. Poult. Sci. 2018;97:463–469. doi: 10.3382/ps/pex340. [DOI] [PubMed] [Google Scholar]
- Klejna K., Naumnik B., Gasowska K., Mysliwiec M. OPG/RANK/RANKL signaling system and its significance in nephrology. Folia. Histochem. Cytobiol. 2009;47:199–206. doi: 10.2478/v10042-009-0035-x. [DOI] [PubMed] [Google Scholar]
- Kong Y.Y., Yoshida H., Sarosi I., Tan H.L., Timms E., Capparelli C., Morony S., Oliveira-dos-Santos A.J., Van G., Itie A., Khoo W., Wakeham A., Dunstan C.R., Lacey D.L., Mak T.W., Boyle W.J., Penninger J.M. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397:315–323. doi: 10.1038/16852. [DOI] [PubMed] [Google Scholar]
- Koutsos E.A., Klasing K.C. The acute phase response in Japanese quail (Coturnix coturnix japonica) Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2001;128:255–263. doi: 10.1016/s1532-0456(00)00199-x. [DOI] [PubMed] [Google Scholar]
- Liu R., Jin C., Wang Z., Wang Z., Wang J., Wang L. Effects of manganese deficiency on the microstructure of proximal tibia and OPG/RANKL gene expression in chicks. Vet. Res. Commun. 2015;39:31–37. doi: 10.1007/s11259-015-9626-5. [DOI] [PubMed] [Google Scholar]
- Malliga D.E., Wagner D., Fahrleitner-Pammer A. The role of osteoprotegerin (OPG) receptor activator for nuclear factor kappaB ligand (RANKL) in cardiovascular pathology – a review. Wien. Med. Wochenschr. 2011;161:565–570. doi: 10.1007/s10354-011-0022-7. [DOI] [PubMed] [Google Scholar]
- Marangon S., Busani L. The use of vaccination in poultry production. Rev. Sci. Tech. 2007;26:265–274. [PubMed] [Google Scholar]
- Mbalaviele G., Novack D.V., Schett G., Teitelbaum S.L. Inflammatory osteolysis: a conspiracy against bone. J. Clin. Invest. 2017;127:2030–2039. doi: 10.1172/JCI93356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy NI, Cowan JE, Nakamura K, Bacon A, Baik S, White AJ, Parnell SM, Jenkinson EJ, Jenkinson WE, Anderson G. Osteoprotegerin-mediated homeostasis of rank+ thymic epithelial cells does not limit Foxp3+ regulatory T cell development. J. Immunol. 2015;195:2675–2682. doi: 10.4049/jimmunol.1501226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mireles A.J., Kim S.M., Klasing K.C. An acute inflammatory response alters bone homeostasis, body composition, and the humoral immune response of broiler chickens. Poult. Sci. 2005;84:553–560. doi: 10.1093/ps/84.4.553. [DOI] [PubMed] [Google Scholar]
- Mueller W.J., Schraer R., Schraer H. Calcium metabolism and skeletal dynamics of laying pullets. J. Nutr. 1964;84:20–26. doi: 10.1093/jn/84.1.20. [DOI] [PubMed] [Google Scholar]
- Okamoto K., Nakashima T., Shinohara M., Negishi-Koga T., Komatsu N., Terashima A., Sawa S., Nitta T., Takayanagi H. Osteoimmunology: the conceptual framework unifying the immune and skeletal systems. Physiol. Rev. 2017;97:1295–1349. doi: 10.1152/physrev.00036.2016. [DOI] [PubMed] [Google Scholar]
- Onyango E.M., Hester P.Y., Stroshine R., Adeola O. Bone densitometry as an indicator of percentage tibia ash in broiler chicks fed varying dietary calcium and phosphorus levels. Poult. Sci. 2003;82:1787–1791. doi: 10.1093/ps/82.11.1787. [DOI] [PubMed] [Google Scholar]
- Rauner M., Sipos W., Thiele S., Pietschmann P. Advances in osteoimmunology: pathophysiologic concepts and treatment opportunities. Int. Arch. Allergy Immunol. 2013;160:114–125. doi: 10.1159/000342426. [DOI] [PubMed] [Google Scholar]
- Redlich K., Smolen J.S. Inflammatory bone loss: pathogenesis and therapeutic intervention. Nat. Rev. Drug Discov. 2012;11:234–250. doi: 10.1038/nrd3669. [DOI] [PubMed] [Google Scholar]
- Remus A., Hauschild L., Andretta I., Kipper M., Lehnen C.R., Sakomura N.K. A meta-analysis of the feed intake and growth performance of broiler chickens challenged by bacteria. Poult. Sci. 2014;93:1149–1158. doi: 10.3382/ps.2013-03540. [DOI] [PubMed] [Google Scholar]
- Schett G. Osteoimmunology in rheumatic diseases. Arthritis Res. Ther. 2009;11:210. doi: 10.1186/ar2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonet W.S., Lacey D.L., Dunstan C.R., Kelley M., Chang M.S., Luthy R., Nguyen H.Q., Wooden S., Bennett L., Boone T., Shimamoto G., DeRose M., Elliott R., Colombero A., Tan H.L., Trail G., Sullivan J., Davy E., Bucay N., Renshaw-Gegg L., Hughes T.M., Hill D., Pattison W., Campbell P., Sander S., Van G., Tarpley J., Derby P., Lee R., Boyle W.J. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–319. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- Sobacchi C, Menale C, Villa A. The RANKL-RANK Axis: a Bone to thymus round trip. Front Immunol. 2019;10:629. doi: 10.3389/fimmu.2019.00629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton K.M., Hu T., Wu Z., Siklodi B., Vervelde L., Kaiser P. The functions of the avian receptor activator of NF-kappaB ligand (RANKL) and its receptors, RANK and osteoprotegerin, are evolutionarily conserved. Dev. Comp. Immunol. 2015;51:170–184. doi: 10.1016/j.dci.2015.03.006. [DOI] [PubMed] [Google Scholar]
- Takayanagi H. Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat. Rev. Immunol. 2007;7:292–304. doi: 10.1038/nri2062. [DOI] [PubMed] [Google Scholar]
- Wang F.Y., Liu J.M., Luo H.H., Liu A.H., Jiang Y. Potential protective effects of Clostridium butyricum on experimental gastric ulcers in mice. World J. Gastroenterol. 2015;21:8340–8351. doi: 10.3748/wjg.v21.i27.8340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Hou J.F., Zhou Z.L. Chicken receptor activator of nuclear factor-kappaB ligand induces formation of chicken osteoclasts from bone marrow cells and also directly activates mature osteoclasts. Poult. Sci. 2008;87:2344–2349. doi: 10.3382/ps.2008-00142. [DOI] [PubMed] [Google Scholar]
- Webel D.M., Johnson R.W., Baker D.H. Lipopolysaccharide-induced reductions in body weight gain and feed intake do not reduce the efficiency of arginine utilization for whole-body protein accretion in the chick. Poult. Sci. 1998;77:1893–1898. doi: 10.1093/ps/77.12.1893. [DOI] [PubMed] [Google Scholar]
- Webster A.B. Welfare implications of avian osteoporosis. Poult. Sci. 2004;83:184–192. doi: 10.1093/ps/83.2.184. [DOI] [PubMed] [Google Scholar]
- Weih F., Caamano J. Regulation of secondary lymphoid organ development by the nuclear factor-kappaB signal transduction pathway. Immunol. Rev. 2003;195:91–105. doi: 10.1034/j.1600-065x.2003.00064.x. [DOI] [PubMed] [Google Scholar]
- Whitehead C.C., Fleming R.H. Osteoporosis in cage layers. Poult. Sci. 2000;79:1033–1041. doi: 10.1093/ps/79.7.1033. [DOI] [PubMed] [Google Scholar]
- Williams B., Solomon S., Waddington D., Thorp B., Farquharson C. Skeletal development in the meat-type chicken. Br. Poult. Sci. 2000;41:141–149. doi: 10.1080/713654918. [DOI] [PubMed] [Google Scholar]
- Xie H., Rath N.C., Huff G.R., Huff W.E., Balog J.M. Effects of Salmonella typhimurium lipopolysaccharide on broiler chickens. Poult. Sci. 2000;79:33. doi: 10.1093/ps/79.1.33. [DOI] [PubMed] [Google Scholar]
- Xiong J., Piemontese M., Thostenson J.D., Weinstein R.S., Manolagas S.C., O'Brien C.A. Osteocyte-derived RANKL is a critical mediator of the increased bone resorption caused by dietary calcium deficiency. Bone. 2014;66:146–154. doi: 10.1016/j.bone.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Liu C., Huang P., Zhou S., Ren J., Kitamura Y., Tang P., Bi Z., Gao B. The affinity of human RANK binding to its ligand RANKL. Arch. Biochem. Biophys. 2009;487:49–53. doi: 10.1016/j.abb.2009.04.008. [DOI] [PubMed] [Google Scholar]