Abstract
This study investigated the effects of dietary Paenibacillus xylanexedens ysm1 supplementation on growth performance, intestinal morphology, immune response, and cecal microbiota of broiler chickens challenged with Escherichia coli K88. A total of 320 one-day-old male broiler chicks were randomly allocated to 4 treatments (8 floor pens, 10 birds/pen) including 1) negative control (NC) birds fed a basal diet and not challenged with E. coli K88; 2) positive control (PC) birds fed a basal diet and challenged with of E. coli K88; 3) P. xylanexedens ysm1 treatment (PRO) birds fed a basal diet supplemented with 1 × 109P. xylanexedens ysm1 cfu/kg feed and challenged with E. coli K88; and 4) antibiotic treatment (ANT) birds fed a basal diet supplemented with 20 mg of colistin sulphate/kg of feed and challenged with E. coli K88. The E. coli challenge decreased (P < 0.05) BWG in PC birds compared with the ANT birds on days 21 and 28. The FCR was higher (P < 0.01) in PC birds compared with the NC, PRO, and ANT birds on days 14, 21, and 28. Compared with the NC, PRO, and ANT birds on day 28, PC birds had shorter villi and higher number of goblet cells in both jejunum and ileum (P < 0.001). Irrespective of the dietary treatments, the E. coli challenge reduced the number of PCNA-positive cells in both the jejunum and ileum on day 28. Paenibacillus xylanexedens ysm1 treatment resulted in higher concentration of mucosal sIgA in the jejunum as compared to the other treatment groups on days 14 and 28. The numbers of cecal E. coli were reduced (P = 0.017) in broilers treated with P. xylanexedens ysm1 or antibiotic in comparison with the PC group on day 28. In conclusion, the present study demonstrated that dietary supplementation of this new probiotic bacteria P. xylanexedens ysm1 improved broiler performance by modulating intestinal morphology, enhancing immune response, and reducing the number of E. coli in the cecum.
Key words: broiler, Escherichia coli K88, Paenibacillus xylanexedens ysm1, probiotic, intestinal histomorphology
INTRODUCTION
Avian colibacillosis caused by enterotoxigenic Escherichia coli is an important infection that results in reduced performance, increased mortality, and significant economic losses in poultry production (Cao et al., 2013; Zhang et al., 2014). Antibiotics are typically used to treat or control bacterial diseases in the broiler industry. However, emergence of antibiotic-resistance bacteria and the possibility of antibiotic residues in meat and other animal products have put restrictions on the use of antibiotics (Zhang et al., 2016; Wang et al., 2017). Therefore, there is an increasing demand in the poultry industry for new alternative strategies to improve performance and disease resistance by establishing a favorable intestinal microbiota.
Probiotics influence the host health by maintaining the normal intestinal microbiota, preventing the growth of pathogenic microorganisms, promoting feed intake (FI) and digestion, and enhancing immune function (Kim et al., 2009; Lutful Kabir, 2009). Dietary use of probiotics significantly influenced broiler performance (Mountzouris et al., 2007, 2010; Mookiah et al., 2014), intestinal architecture (Awad et al., 2009; Sen et al., 2012), and the colonization of beneficial microorganisms in the intestines (Mookiah et al., 2014). Moreover, probiotics can decrease pathogen colonization and invasion of the intestinal tract to prevent several enteric infections in chickens (Cao et al., 2013; Wang et al., 2017).
Spore-forming probiotic bacteria, such as Bacillus spp., have been successfully used in animal nutrition and confirmed to promote animal performance and health (Kim et al., 2009; Cao et al., 2018). In general, endospore-forming probiotics have several advantages over Lactobacillus or Bifidobacterium as a probiotic feed additive (Grant et al., 2018). Thanks to their evolutionary advantage of spore-forming ability, these bacteria can withstand harsh environmental conditions, such as feed processing and pelleting, which makes these bacteria a suitable alternative growth promoter to use in the broiler industry (Shivaramaiah et al., 2011; Amerah et al., 2013). Lee et al. (2010) revealed that Bacillus-based direct feed microbials (strains 15AP4 and Bs27) alleviated the Eimeria maxima induced reduction of body weight gain and intestinal lesions. Teo and Tan (2006) reported that Bacillus subtilis PB6 positively influenced performance in broilers challenged with E. coli. Bacillus-based probiotics promote gut health and reduce signs of the enteric diseases via several different mechanisms such as competitive exclusion, antimicrobial peptide production, gut microflora modifications, and immune system stimulation (Grant et al., 2018).
Paenibacillus is a genus of facultative anaerobic, endospore-forming bacteria, which was previously distinguished from the other Bacillus groups by comparative 16S rRNA sequence analysis (Ash et al., 1993). Bacteria belonging to this genus have been isolated or detected in a variety of environments but the majority are found in soils often associated with plant roots (Grady et al., 2016). Plant-associated species of Paenibacillus serve as a plant growth promoter by producing several substances and fixing atmospheric nitrogen (Grady et al., 2016; Weselowski et al., 2016). In addition, they can competitively colonize plant roots and confer biocontrol against a diverse variety of phytopathogens by inducing host defense, producing biochemical substances (Grady et al., 2016), and also synthesizing polysaccharide-hydrolyzing enzymes (Nelson et al., 2009). In contrast to well-known probiotics, there have been limited reports on the effects of Paenibacillus sp. on animal performance and health. Our previous work showed that Paenibacillus xylanexedens ysm1 (P. xylanexedens ysm1) exhibited an adhesion pattern to enterocytes and ability to suppress E. coli proliferation under in vitro conditions (Calik et al., 2017). Moreover, dietary supplementation of P. xylanexedens ysm1 conferred beneficial effects in broilers by improving feed conversion ratio (FCR) and intestinal morphology (Calik et al., 2017). A very recent study showed that dietary use of P. polymyxa 10 improved the intestinal health of broilers by increasing intestinal barrier function, anti-oxidative capacity, and immune response, and by decreasing cell apoptosis (Wu et al., 2019).
Generally, probiotics are recognized as safe for animals and humans. However, due to the increasing number of newly isolated bacteria with probiotic features, assessment of safety of these new microorganisms becomes very important (Kumar et al., 2015). Regulation for novel or newly isolated probiotics may vary from one region to another and be subjected to an extensive safety assessment prior to approval in the markets (Kumar et al., 2015). For safety purposes, biological origin and genome, antibiotic resistance profile, studies on target species, potential toxic effects, and environmental risk are required for the assessment of probiotic feed additives (Anadon et al., 2006; Kumar et al., 2015). Based on previous findings that suggest the benefits of dietary probiotic administrations, and also our in vitro and in vivo study results, the current study hypothesized that dietary supplementation of new isolate P. xylanexedens ysm1 may be an effective method to maintain broiler performance and health by influencing intestinal morphology, gut microflora, and the immune system of broiler chickens challenged with E. coli K88.
MATERIALS AND METHODS
Animal Care and Use
All experimental procedures were approved by the Animal Ethics Committee of Gazi University (G.Ü.ET-15.049).
Probiotic Strain
The P. xylanexedens ysm1 strain was isolated from chyme samples of 8 different cattle between 2 and 4 yr of age and kept in the culture collection of Life Sciences Application and Research Center, Gazi University, Ankara, Turkey. After isolation, resistance to simulated gastric fluids, tolerance to bile salt, sporulation efficiency, spore formation, adhesion, and invasion properties were tested previously (Calik et al., 2017). Additionally, the antimicrobial activity of the isolates against pathogenic bacterial strains (E. coli, Staphylococcus aureus, Klebsiella pneumoniae, Pseudomonas aeruginosa) was evaluated, and the results were reported earlier (Calik et al., 2017).
Preparation of P. xylanexedens ysm1 as a Feed Additive
Cultures were grown in 50 mL Difco Sporulation Medium (DSM, USA) at 37°C, 250 rpm for 24 h and centrifuged at 1,280 × g for 5 min. Harvested spores were then inoculated in DSM and incubated at 37°C and 250 rpm for 48 h. After the incubation period, spores were centrifuged at 5,000 × g for 30 min; spore pellets were washed with 20 mL sterile distilled water and resuspended in 5 mL sterile water. Spores of the isolate were then mixed with 10% skim milk for further lyophilization. The lyophilized culture of P. xylanexedens ysm1 was added to the basal diet at 1 × 109 cfu/kg.
Birds, Diets, and Experimental Design
A total of 320 one-day-old male broiler chicks (Ross 308), with average weight of 40.51 ± 1.94 g (mean ± SD), were obtained from a commercial hatchery (Beypiliç, Bolu, Turkey). The birds were randomly allocated to 4 experimental groups each comprising 8 replicate pens with 10 birds per pen. The treatments were as follows: negative control (NC) birds fed a basal diet and not challenged with E. coli K88; positive control (PC) birds fed a basal diet and orally challenged with E. coli K88; P. xylanexedens ysm1 treatment (PRO) birds fed a basal diet supplemented with 1 × 109P. xylanexedens ysm1 cfu/kg feed and orally challenged with E. coli K88; and antibiotic treatment (ANT) birds fed a basal diet supplemented with 20 mg of colistin sulfate/kg of feed and orally challenged with E. coli K88. Birds were housed in a controlled environment for 28 day with the ambient temperature thermostatically controlled and gradually reduced from 34°C on the first day to 22°C at 3 wk, then maintained at 22°C thereafter. To prevent potential cross-contamination, birds within each treatment were placed in individual identical rooms, which had the same conditions throughout the study. The starter and grower diets were based on corn-soybean meal and fed from day 0 to 14 and day 15 to 28, respectively (Table 1). All diets were formulated to meet or exceed the NRC nutrient recommendations (NRC, 1994). Each pen was equipped with a manual plastic feeder and a nipple drinker. Water and the experimental diets (in mash form) were provided ad libitum throughout the study period. All chicks were individually weighed and FI was recorded at weekly intervals. Body weight gain (BWG), FI, and FCR were subsequently calculated based on performance values.
Table 1.
Ingredients and composition of basal diet.
| Basal diet |
||
|---|---|---|
| 0 to 14 D | 15 to 28 D | |
| Ingredient, g/kg | ||
| Corn | 549.40 | 575.00 |
| Soybean meal, CP 48% | 375.00 | 342.40 |
| Vegetable oil | 33.00 | 44.00 |
| Limestone | 5.00 | 3.60 |
| Dicalcium phosphate | 24.50 | 23.40 |
| DL-Methionine (98%) | 3.60 | 3.15 |
| L-Lysine HCI (78%) | 3.00 | 2.35 |
| L-Threonine | 1.50 | 1.10 |
| Salt | 2.50 | 2.50 |
| Vitamin premix1 | 1.00 | 1.00 |
| Mineral premix2 | 1.00 | 1.00 |
| Choline chloride | 0.50 | 0.50 |
| Total | 1,000 | 1,000 |
| Chemical composition (calculated) | ||
| Dry matter, % | 87.93 | 87.93 |
| Crude protein, % | 23.04 | 21.57 |
| AMEn, kcal/kg | 3,006 | 3,105 |
| Lysine, % | 1.44 | 1.30 |
| Methionine + cysteine, % | 1.08 | 0.99 |
| Threonine, % | 1.00 | 0.90 |
| Calcium, % | 0.97 | 0.88 |
| Available phosphorus, % | 0.48 | 0.44 |
Provided per kilogram of complete diet: vitamin A, 15,000 IU; vitamin D3, 5,000 IU; vitamin E, 100 mg; vitamin K3, 3 mg; thiamin, 5 mg; riboflavin, 8 mg; pyridoxine, 5 mg; pantothenic acid, 16 mg; niacin, 60 mg; folic acid, 2 mg; biotin, 200 μg; vitamin B12, 20 μg.
Provided per kilogram of complete diet: Cu, 16 mg; I, 1.5 mg, Co, 500 μg; Se, 350 μg; Fe, 60 mg; Zn, 100 mg; Mn, 120 mg; Mo, 1 mg.
Escherichia coli K88 Preparation and Oral Challenge
Escherichia coli K88, which was previously isolated from an infected broiler flock, was obtained from the culture collection of Ankara University Faculty of Veterinary Medicine, Department of Microbiology. Birds in the PC, PRO, and ANT treatment groups were orally gavaged with 0.1 mL E. coli K88 (2 × 109 cfu/mL) on day 7 and 0.5 mL E. coli K88 (2 × 109 cfu/mL) on days 10, 14, and 21. The NC birds were administrated similarly with the same volume of 0.9% saline solution (Yang et al., 2008).
Sampling Procedures
At 14, 21, and 28 days of age, 1 bird from each replicate pen was selected based on average pen body weight. Birds were euthanized by exsanguination and the intestinal tract was immediately removed. Tissue samples were obtained from the jejunum and ileum for histomorphological analysis on days 14 and 28. A second sample was collected from the jejunum and snap-frozen in liquid nitrogen for sIgA determination on days 14 and 28. On days 14, 21, and 28, both ceca were ligated and aseptically removed from the gastrointestinal tract for cecal microbial analysis. Subsequently, the cecal content was collected in sterile 1.5 mL tubes and stored at −80°C until DNA isolation.
Morphological Measurements of the Jejunum and Ileum
Tissue samples in the formalin solution were dehydrated in graded ethanol solutions, cleared with xylol, and then embedded in paraffin. The intestinal segments were sectioned at a thickness of 5 μ m with a microtome. Cross sections were prepared and stained with Mallory's triple stain, as modified by Crossman, in order to determine the jejunal and ileal morphometry (Culling et al., 1985). Villus height (VH) was measured from the top of the villus to the crypt mouth, and crypt depth (CD) was defined as the depth of the invagination between adjacent crypt mouths. Villus width was measured at the bottom of the villus. Goblet cells were analyzed by staining with combined Alcian Blue (AB) and periodic acid Schiff (PAS) reagent on day 28. Cells were identified as follows: acid mucin was stained by AB (blue), neutral mucin was stained by PAS (pink), and intermediate mucin, which includes both acid and neutral mucins, was stained by AB and PAS (purple) (Geier et al., 2011). All positive cells along the villus were counted, regardless of the mucin type.
A total of 10 well-oriented villi and crypts were randomly selected for histological measurements. Histological sections were examined under a light microscope (Leica DM 2500, Leica Microsystems GmbH, Wetzlar, Germany) and photographed with a digital microscope camera (Leica DFC450, Leica Microsystems GmbH, Wetzlar, Germany). The images were evaluated using the ImageJ software (National Institute of Mental Health, Bethesda, Maryland, USA).
Proliferating Cell Nuclear Antigen Staining
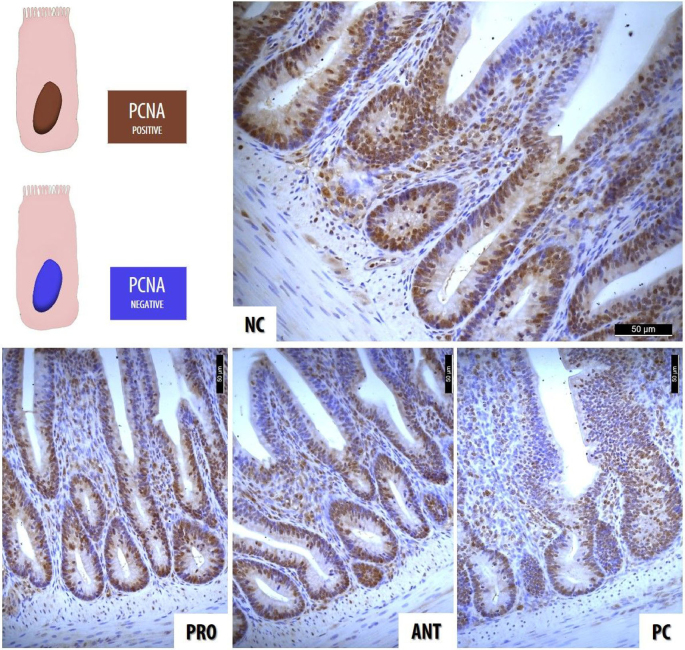
Immunohistochemical staining was performed on the stored 5 µ m thick formalin-fixed paraffin-embedded tissue sections. Tissue sections were placed on poly-L-lysine microscope slides (Thermo Scientific, Braunschweig, Germany), which were incubated at 37°C overnight and de-paraffinized with xylene and rehydrated through graded alcohols. Endogenous peroxidase activity was blocked by quenching with H2 O2 (3% in methanol) for 30 min. The sections were pre-treated by heating for 20 min in 0.01 M citric acid buffer (pH 6) in a microwave oven at 800 W. After cooling for 20 min at room temperature, tissue sections were washed with PBS and incubated with 10% normal goat serum for 30 min for protein blocking to prevent the non-specific binding of antibodies, followed by incubation with the primary antibody to proliferating cell nuclear antigen (PCNA) (MAB424, mouse anti PCNA monoclonal antibody, PC10 clone; EMD Millipore, Darmstadt, Germany) at dilutions of 1:100 overnight at 4°C. After incubation with the primary antibodies, the tissue sections were washed with PBS and incubated with a biotinylated secondary antibody (Goat anti-mouse IgG, Invitrogen, CA, USA) for 30 min at room temperature. Background controls were included by replacing the primary antibodies with PBS. After a PBS wash, tissue sections were incubated using a streptavidin horseradish peroxidase kit (Histostain-Plus IHC Kit, HRP, broad spectrum, Invitrogen, CA, USA) for 30 min at room temperature. A final PBS wash was followed by incubation for color development with 3,3-diaminobenzidine tetrahydrochloride (DAB, Invitrogen, CA, USA) for 3 min at room temperature. Tissue sections were counterstained with Gill's hematoxylin, dehydrated in graded alcohols, applied to a coverslip using Entellan (Merck, Darmstadt, Germany), and examined with a Leica DM2500 light microscope. All images were captured with a digital camera (Leica DFC450, Leica Microsystems GmbH, Wetzlar, Germany) and processed with Image J. PCNA-positive nuclei (Figure 1) of total crypt epithelial cells on 10 different randomly selected intact crypts, regardless of the staining intensity, were counted as described by Bologna-Molina et al. (2011).
Figure 1.
Immunohistochemical distribution of proliferating cell nuclear antigen positive cells on day 28.
Secretory IgA Analysis
Jejunal mucosa was weighed as 0.5 g and diluted into 9 mL of 0.9% physiological saline. After centrifugation at 6,000 × g for 15 min at +4°C, supernatants were transferred into new 1.5 mL sterile microcentrifuge tubes. Secretory IgA (sIgA) ELISA Kit (Elabscience Biotechnology Co., Wuhan, PRC) was used in accordance with the manufacturer's instructions for sIgA concentration on days 14 and 28. Absorbance was read at 450 nm with an automated ELISA reader (BioTek ELx800 Absorbance Microplate Readers, Biotek, VT, USA).
Cecal Microbiota Analysis
DNA Extraction
Genomic DNA from each sample was isolated from 200 mg of cecal content using GeneMatrix Stool DNA Purification Kit (EURx, Gdansk, Poland). All procedures were performed according to the manufacturer's instructions. The quantity of DNA was measured spectrophotometrically at 260 nm using Epoch Microplate Spectrophotometer (Biotek, VT, USA).
Preparation of External Standards and qPCR Analysis
Paenibacillus xylanexedens ysm1 was incubated in nutrient broth, and E. coli (ATCC 2592 strain) was incubated in Triptic soy broth for 24 h at 37°C under aerobic conditions. DNA was extracted from bacterial colonies using the GeneMATRIX Bacterial & Yeast Genomic DNA Purification Kit (EURx, Gdansk, Poland). Standard curves were generated using PCR product of E. coli and P. xylanexedens ysm1. For each bacterial standard, dilutions of 1/10, 1/100, 1/1000, 1/10000, 1/100000 were prepared. The dilutions and each DNA sample from cecal contents were all subjected to the real-time PCR procedure in Qiagen Rotor-Gene Q (Qiagen, MA, USA). Amplicons from E. coli were used for quantification of total bacteria and cecal E. coli, and amplicons from P. xylanexedens ysm1were used for quantification of P. xylanexedens ysm1.
The amplifications were carried out using a final volume of 25 μL containing 12.5 μL GoTaq qPCR master mix (Promega, WI, USA), 100 ng DNA template, 0.5 μL each primer (0.1 μM) [BactF 5′-AGA GTT TGA TCC TGG CTC AG-3′ and BactR 5′-AAG GAG GTG ATC CAG CCG CA-3′ primers (Lane, 1991); E. coli F: 5′-CATGCCGCGTGTATGAAGAA-3′; R: 5′-CGGGTAACGTCAATGAGCAAA-3′ (Huijsdens et al., 2002) and P. xylanexedens ysm1 F: 5′-GTGAGCCATTACCCCACCAA-3′ and R: 5′-GCCCTCAAGTTTGGGACAAC-3′ (this study)], and nuclease free water. The qPCR conditions were 95°C for 2 min, followed by 40 cycles of 95°C for 15 s, 60°C for 60 s.
Statistical Analysis
Data were analyzed using the ANOVA procedure of SPSS version 14.01 (SPSS Inc., Chicago, IL, USA). Significant differences among treatment groups were tested by Tukey multiple range tests. Statistical differences were considered significant at P ≤ 0.05.
RESULTS
Growth Performance
The E. coli K88 challenge decreased BWG in PC birds compared to the ANT birds on day 21 (P = 0.039) and day 28 (P = 0.007) (Table 2). FI was higher (P = 0.028) in PC birds in comparison to NC birds on day 14. No significant differences in FI were observed on days 7, 21, and 28. The FCR was higher in PC birds compared with the NC, PRO, and ANT birds on days 14 (P = 0.004), 21 (P < 0.001), and 28 (P < 0.001). No significant mortality was observed during the entire experimental period.
Table 2.
Effects of Paenibacillus xylanexedens ysm1 on growth performance in broilers.1
| Dietary treatment2 |
||||||
|---|---|---|---|---|---|---|
| Item3 | NC | PC | PRO | ANT | SEM | P-value |
| 0 to 7 D | ||||||
| BWG (g) | 128.3 | 128.6 | 128.2 | 129.0 | 0.18 | 0.392 |
| FI (g) | 141.9 | 144.4 | 143.4 | 144.3 | 0.88 | 0.754 |
| FCR | 1.106 | 1.122 | 1.119 | 1.118 | 0.01 | 0.881 |
| 0 to 14 D | ||||||
| BWG (g) | 358.7 | 354.6 | 362.8 | 368.3 | 1.92 | 0.063 |
| FI (g) | 425.1b | 446.8a | 434.7a,b | 439.7a,b | 2.72 | 0.028 |
| FCR | 1.185b | 1.261a | 1.199b | 1.194b | 0.01 | 0.004 |
| 0 to 21 D | ||||||
| BWG (g) | 797.2a,b | 751.7b | 784.4a,b | 803.4a | 7.09 | 0.039 |
| FI (g) | 1,013 | 1,009 | 996.0 | 1,016 | 6.41 | 0.711 |
| FCR | 1.271b | 1.343a | 1.270b | 1.267b | 0.01 | <0.001 |
| 0 to 28 D | ||||||
| BWG (g) | 1,382a,b | 1,332b | 1,407a,b | 1,454a | 13.48 | 0.007 |
| FI (g) | 1,823 | 1,870 | 1,845 | 1,890 | 14.69 | 0.417 |
| FCR | 1.320b | 1.404a | 1.311b | 1.300b | 0.01 | <0.001 |
Means with different superscripts in the same row are significantly different (P < 0.05).
Data represent mean values of 8 replicates per treatment.
NC: birds fed a basal diet and not challenged with Escherichia coli K88; PC: birds fed a basal diet and orally challenged with of E. coli K88; PRO: birds fed a diet supplemented with 1 × 109P. xylanexedens ysm1 cfu/kg feed and orally challenged with E. coli K88; ANT: birds fed a diet supplemented with 20 mg of colistin sulfate/kg of feed and orally challenged with E. coli K88.
BWG: body weight gain; FI: feed intake; FCR: feed conversion ratio.
Morphological Measurements of the Jejunum and Ileum
Day 14
Morphological measurements of jejunal and ileal tissues are shown in Table 3. No significant differences were observed among the dietary treatment groups in terms of VH, CD, and VH:CD ratio in the jejunum on day 14. However, the E. coli K88 challenge reduced (P = 0.004) ileum VH in the PC and PRO birds when compared with non-challenged birds. Dietary supplementation of P. xylanexedens ysm1 and colistin sulfate significantly increased CD (P = 0.006) and reduced VH:CD ratio (P < 0.001) in the ileum.
Table 3.
Effects of Paenibacillus xylanexedens ysm1 on intestinal morphology of the jejunum and ileum on days 14 and 28.1
| Dietary treatment2 |
||||||
|---|---|---|---|---|---|---|
| Item | NC | PC | PRO | ANT | SEM | P-value |
| Day 14 | ||||||
| Jejunum | ||||||
| Villus height (μm) | 496.8 | 455.6 | 485.1 | 487.9 | 6.78 | 0.154 |
| Crypt depth (μm) | 86.38 | 79.38 | 82.00 | 80.75 | 1.43 | 0.351 |
| VH:CD3 | 5.77 | 5.75 | 6.01 | 6.05 | 0.10 | 0.653 |
| Ileum | ||||||
| Villus height (μm) | 380.1a | 357.9b,c | 351.4c | 374.6a,b | 3.44 | 0.004 |
| Crypt depth (μm) | 68.64a,b | 67.94b | 73.20a | 72.93a | 0.73 | 0.006 |
| VH:CD | 5.54a | 5.28a,b | 4.80c | 5.14b,c | 0.06 | <0.001 |
| Day 28 | ||||||
| Jejunum | ||||||
| Villus height (μm) | 1,067a | 910.4c | 985.1b | 1016a,b | 13.58 | <0.001 |
| Crypt depth (μm) | 108.0a | 90.21d | 96.10c | 101.7b | 1.33 | <0.001 |
| VH:CD | 9.89 | 10.09 | 10.25 | 9.99 | 0.08 | 0.414 |
| Ileum | ||||||
| Villus height (μm) | 532.8a | 413.9b | 511.8a | 521.3a | 9.07 | <0.001 |
| Crypt depth (μm) | 95.43a | 80.05c | 91.06b | 95.11a | 1.16 | <0.001 |
| VH:CD | 5.58a | 5.17b | 5.62a | 5.48a,b | 0.05 | 0.003 |
Means with different superscripts in the same row are significantly different (P < 0.05).
Data represent mean values of 8 replicates per treatment.
NC: birds fed a basal diet and not challenged with Escherichia coli K88; PC: birds fed a basal diet and orally challenged with of E. coli K88; PRO: birds fed a diet supplemented with 1 × 109P. xylanexedens ysm1 cfu/kg feed and orally challenged with E. coli K88; ANT: birds fed a diet supplemented with 20 mg of colistin sulfate/kg of feed and orally challenged with E. coli K88.
Villus height to crypt depth ratio.
Day 28
Morphological measurements of intestinal tissues are shown in Table 3. In both jejunum and ileum, VH was decreased in the PC birds compared with the NC, PRO, and ANT birds (P < 0.001). Similarly, the lowest CD (P < 0.001) was observed in both jejunum and ileum of PC birds on day 28.
Goblet Cells and PCNA-Positive Cells
Goblet cells and PCNA-positive cell counts of the jejunum and ileum are shown in Figure 2 and Figure 3, respectively. The number of goblet cells was significantly higher (P < 0.001) in both jejunum and ileum of the PC group when compared with the other treatment groups on day 28. Irrespective of the dietary treatments, inoculation of E. coli K88 reduced (P < 0.001) the number of PCNA-positive cells in both jejunum and ileum on day 28.
Figure 2.
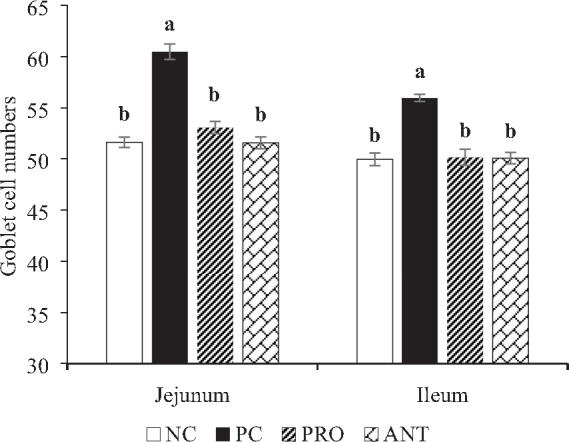
Effects of Paenibacillus xylanexedens ysm1 on jejunum and ileum goblet cell numbers on day 28. Each bar represents mean ± SE values of 8 replicates per treatment. Bars with different letters (a,b) differ significantly (P < 0.001).
Figure 3.
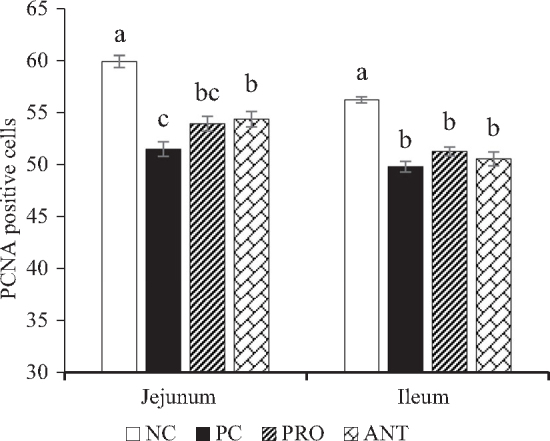
Effects of Paenibacillus xylanexedens ysm1 on proliferating cell nuclear antigen (PCNA) cell counts in jejunum and ileum on day 28. Each bar represents mean ± SE values of 8 replicates per treatment. Bars with different letters (a-c) differ significantly (P < 0.001).
Total sIgA Concentration in the Jejunum Mucosa
Dietary supplementation with P. xylanexedens ysm1 resulted in significantly higher concentrations of the jejunum mucosal sIgA (Figure 4A) as compared with the other treatment groups on both days 14 and 28 (P < 0.001).
Figure 4.
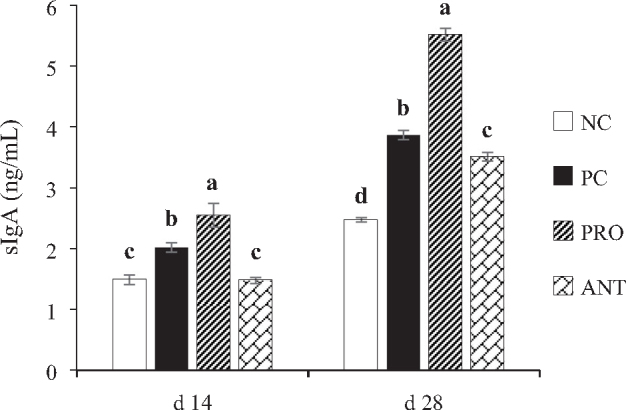
Effects of Paenibacillus xylanexedens ysm1 on concentration of sIgA in the jejunum of broilers. Each bar represents mean ± SE values of 8 replicates per treatment. Within the same day, bars with different letters (a-d) differ significantly (P < 0.001)
Cecal Microbial Analysis
On days 21 and 28, the ratio of cecal E. coli was higher in the PC group compared to the NC group. As in the PRO group, there was no difference between E. coli cecal content on days 14 and 21 compared to the PC group. When comparing the cecal content of PC, PRO, and ANT groups on day 28, the amount of E. coli was lower (P = 0.017) in the PRO and ANT groups (Figure 5). The P. xylanexedens ysm1 was found only in cecal contents of the PRO group (data not shown).
Figure 5.
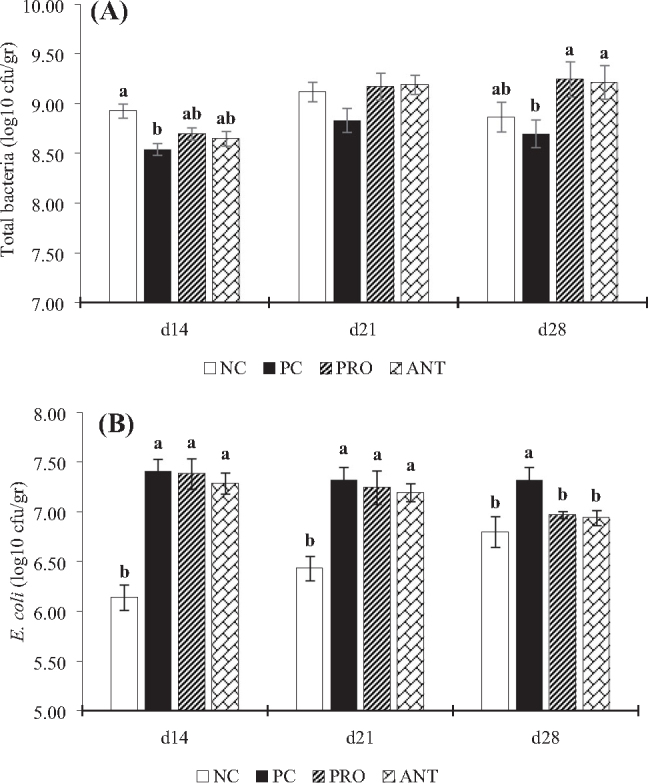
Effects of Paenibacillus xylanexedens ysm1 on cecal total bacteria (A) and Escherichia coli (B) populations in broilers. Each bar represents mean ± SE values of 8 replicates per treatment. Within the same day, bars with different letters (a-b) differ significantly (P < 0.05).
DISCUSSION
Among the strategies to reduce the use of antibiotics in the broiler industry, the use of dietary probiotics is becoming an accepted alternative because of their beneficial effects on health and performance without having the risk of drug residues or antibacterial resistance. In this context, the present study investigated the effect of a new isolate P. xylanexedens ysm1 on broiler performance, morphology, gut microbiota, and immune response of broiler chickens challenged with E. coli K88.
As an expected outcome, the E. coli challenge retarded the growth of broilers in the PC group. However, dietary supplementation of P. xylanexedens ysm1 alleviated the growth suppression effect of the E. coli challenge but no significant differences were observed between the probiotic and antibiotic treated birds in terms of their BWG, FI, and FCR during the entire experimental period (day 0 to 28). Our results are in agreement with those of Calik et al. (2017) who reported that dietary supplementation of P. xylanexedens ysm1 (1 × 109 cfu/feed) resulted in improved performance from day 0 to 21. Other studies also revealed that dietary supplementation of several probiotics, such as Enterococcus faecium (Cao et al., 2013), Clostridium butyricum (Zhang et al., 2014), Lactobacillus plantarum B1 (Wang et al., 2017), and B. subtilis (Manafi et al., 2017), significantly improved broiler performance during an E. coli challenge by improving intestinal integrity and immune status of the broiler chickens. The exact mechanisms underlying the growth promoting effects of P. xylanexedens ysm1 remain unclear, as there has been no available information of its effect on broilers. However, P. xylanexedens ysm1 might affect the growth performance through several mechanism(s), such as competitive colonization, improved intestinal integrity, and selectively stimulating intestinal microbiota (Daudelin et al., 2011).
Morphological changes in the small intestine, such as increased villus height and villus height to crypt depth ratio, are important parameters that affect broiler performance by improving nutrient digestion and absorption (Calik and Ergun, 2015). Previous studies showed that a diverse variety of probiotics might be used to maintain intestinal integrity (Awad et al., 2009; Zhang et al., 2016). Based on the present findings, there was no improvement in villus height and VH:CD in jejunum and ileum on day 14, which was likely due to the acute phase of the infection or consecutive oral gavaging with E. coli on days 7 and 10. However, as hypothesized in this study, dietary supplementation of P. xylanexedens ysm1 had a pronounced effect on villus height and VH:CD in both jejunum and ileum on day 28, which was accompanied by enhanced growth performance. Similar results were reported by Calik et al. (2017) where dietary application of P. xylanexedens ysm1 improved intestinal histomorphology in non-challenged broiler chickens. However, in contrast to well-established probiotics, there is limited reporting on the effects of P. xylanexedens ysm1 on broiler intestinal morphology during a pathogenic challenge. In agreement with our results, Cao et al. (2013) reported that dietary E. faecium supplementation increased jejunal villus height after an E. coli K88 challenge in broilers. More recently, Wang et al. (2017) concluded that L. plantarum B1 could alleviate the negative effects of colibacillosis on the intestinal epithelium. Such enhancements in intestinal integrity might be related to the antibacterial activity of the P. xylanexedens ysm1 against E. coli K88 and modification of the intestinal microbiota and increased abundance of bacterial metabolites such as butyrate, which induces enterocyte differentiation and proliferation.
The intestinal mucous layers, synthesized and secreted by goblet cells, cover the gut epithelium with compact viscous, permeable, and gel-forming mucin to provide a frontline defense against microbes, pathogenic microorganisms, environmental toxins, and other dietary components (Kim and Ho, 2010; Calik et al., 2017). Although mucin is continuously secreted at a basal level, the composition and thickness of this mucous layer can change rapidly in response to pathogenic microorganisms or microbial products and are modulated by the underlying innate and adaptive immune responses (Kim and Ho, 2010; Ashida et al., 2011; McGuckin et al., 2011). In addition to its adverse effect on intestinal morphology, our results revealed that the E. coli challenge also had a pronounced effect on goblet cells by increasing their numbers in the jejunum and ileum. In agreement with our results, previous studies reported an increase in the intestinal goblet cell numbers during E. coli (Almeida et al., 2013; Manafi et al., 2017) and Salmonella enterica serovar Typhimurium (Almeida et al., 2014) challenges. As an important finding of this study, dietary supplementation of P. xylanexedens ysm1 significantly reduced the number of goblet cells as efficiently as the antibiotic treatment, most likely due to the reduced population of E. coli, modification of the intestinal bacteria, or intestinal immune responses. Increase in villus height and decrease in the goblet cell numbers in the probiotic group might help to explain the improvements in growth performance, because thicker mucus may limit the diffusion of nutrients (Bontempo et al., 2006), and increased demand for mucosal secretion inevitably leads to increased endogenous loss, since it contains important nutrients such as serine, threonine, proline, and cysteine (Cowieson et al., 2004).
Intestinal epithelial cells have a short lifespan and need to be replenished rapidly and continuously via the replication of undifferentiated cells. Precursor cells or proliferative cells, which are being continuously generated in the crypt regions, terminally differentiate into secretory cells (goblet, enteroendocrine, and Paneth) or absorptive enterocytes (Fre et al., 2005; van der Flier and Clevers, 2009). These proliferating cells can be observed by immunohistochemical staining of an endogenous protein called PCNA, also known as cyclin or DNA-polymerase delta auxiliary protein (Foley et al., 1993; Uni et al., 1998; Gulbahar et al., 2005). In the current study, while challenged birds had similar numbers of PCNA-positive cells, birds in the probiotic group had higher intestinal villus height and lower goblet cell numbers compared to positive control birds. We could speculate that dietary supplementation of P. xylanexedens ysm1 might have influenced the cell fate decision, directly or indirectly, during the differentiation steps towards absorptive enterocytes rather than mucin-filled secretory goblet cells. The exact mechanisms of how the microbiota modulates the proliferation and differentiation of cell lineages to absorptive or secretary cells are still unclear. However, previous studies reported relationships between intestinal microbiota and cell fate decision pathways (Broderick et al., 2014; Troll et al., 2018). Further investigations on host–microbiome interaction, using RNA-seg methods, could help to reveal differentiation and proliferation dynamics of the intestinal epithelial cells.
The intestinal mucosal surface has a variety of defense mechanisms which protect the host against pathogens and toxins to maintain local homeostasis. Secretory IgA acts as a first line defense mechanism that promotes immune exclusion by entrapping harmful microorganisms and keeping the commensal bacteria in balance to ensure their controlled survival (Cerutti and Rescigno, 2008; Corthesy, 2013). The concentration of sIgA in the intestine is an important parameter to assess mucosal immunity (Wang et al., 2017). We observed a significantly higher level of sIgA concentration in the jejunum by dietary addition of the new probiotic P. xylanexedens ysm1. Previous studies revealed that dietary probiotic supplementation stimulated the intestinal sIgA production compared to control birds under challenge (Wang et al., 2017) and non-challenge (Amerah et al., 2013; Peng et al., 2016) conditions. Furthermore, increased levels of sIgA might help to increase nutrient absorption (Peng et al., 2016). Along with the improvements in intestinal morphology, this finding suggests that dietary addition of P. xylanexedens ysm1 appears to improve intestinal mucosa health, maintain barrier functions, and alleviate inflammatory response under E. coli K88 challenge.
Along with the modulatory effects on intestinal epithelial barrier and immune function, one of the well-known modes of action of probiotics is competitive exclusion or inhibition of pathogens in the gastrointestinal tract (Lebeer et al., 2010). On day 28 of the study, despite the recurrence of E. coli challenge, probiotic and antibiotic administration had equivalent effects against E. coli K88 infection, considering the decrease in E. coli rates in PRO and ANT groups compared to the PC group. This can be correlated with our previous in vitro study results where P. xylanexedens ysm1 exhibited an antimicrobial activity against E. coli. Moreover, our findings are consistent with previous reports whereby administration of probiotics (such as B. subtilis, E. faecium, Lactobacillus sp., etc.) reduced colonization of E. coli (Cao et al., 2013), Salmonella Enteritidis (Mountzouris et al., 2009), and Clostridium perfringens (Jayaraman et al., 2013).
In conclusion, the present study demonstrated that dietary supplementation of a new P. xylanexedens-based probiotic improved broiler performance by modulating intestinal morphology, enhancing immune response, and reducing the number of E. coli in the cecum. Due to its spore-forming ability and potential beneficial effects on broiler performance and health, it might be used in the broiler industry as a promising alternative to antibiotic growth promoters.
ACKNOWLEDGEMENTS
This study was funded by the Scientific and Technological Research Council of Turkey, Ankara, Turkey [Grant Number: 215O716]. The authors thank Rami A. Dalloul (Virginia Tech, USA) for proofreading and Mallory White (Virginia Tech, USA) for technical editing of the manuscript.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
REFERENCES
- Almeida J.A., Liu Y., Song M., Lee J.J., Gaskins H.R., Maddox C.W., Osuna O., Pettigrew J.E. Escherichia coli challenge and one type of smectite alter intestinal barrier of pigs. J. Anim. Sci. Biotechnol. 2013;4:52. doi: 10.1186/2049-1891-4-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida J.A., Ponnuraj N.P., Lee J.J., Utterback P., Gaskins H.R., Dilger R.N., Pettigrew J.E. Effects of dietary clays on performance and intestinal mucus barrier of broiler chicks challenged with Salmonella enterica serovar Typhimurium and on goblet cell function in vitro. Poult. Sci. 2014;93:839–847. doi: 10.3382/ps.2013-03587. [DOI] [PubMed] [Google Scholar]
- Amerah A.M., Quiles A., Medel P., Sánchez J., Lehtinen M.J., Gracia M.I. Effect of pelleting temperature and probiotic supplementation on growth performance and immune function of broilers fed maize/soy-based diets. Anim. Feed Sci. Tech. 2013;180:55–63. [Google Scholar]
- Anadon A., Martinez-Larranaga M.R., Aranzazu Martinez M. Probiotics for animal nutrition in the European Union. Regulation and Safety Assessment. Regul. Toxicol. Pharmacol. 2006;45:91–95. doi: 10.1016/j.yrtph.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Ash C., Priest F.G., Collins M.D. Molecular identification of rRNA group 3 bacilli (Ash, Farrow, Wallbanks and Collins) using a PCR probe test. Proposal for the creation of a new genus Paenibacillus. Antonie Van Leeuwenhoek. 1993;64:253–260. doi: 10.1007/BF00873085. [DOI] [PubMed] [Google Scholar]
- Ashida H., Ogawa M., Kim M., Mimuro H., Sasakawa C. Bacteria and host interactions in the gut epithelial barrier. Nat. Chem. Biol. 2011;8:36–45. doi: 10.1038/nchembio.741. [DOI] [PubMed] [Google Scholar]
- Awad W.A., Ghareeb K., Abdel-Raheem S., Bohm J. Effects of dietary inclusion of probiotic and synbiotic on growth performance, organ weights, and intestinal histomorphology of broiler chickens. Poult. Sci. 2009;88:49–56. doi: 10.3382/ps.2008-00244. [DOI] [PubMed] [Google Scholar]
- Bologna-Molina R., Damián-Matsumura P., Molina-Frechero N. An easy cell counting method for immunohistochemistry that does not use an image analysis program. Histopathology. 2011;59:801–803. doi: 10.1111/j.1365-2559.2011.03954.x. [DOI] [PubMed] [Google Scholar]
- Bontempo V., Di Giancamillo A., Savoini G., Dell'Orto V., Domeneghini C. Live yeast dietary supplementation acts upon intestinal morpho-functional aspects and growth in weanling piglets. Anim. Feed Sci. Tech. 2006;129:224–236. [Google Scholar]
- Broderick N.A., Buchon N., Lemaitre B. Microbiota-induced changes in drosophila melanogaster host gene expression and gut morphology. MBio. 2014;5:e01117–01114. doi: 10.1128/mBio.01117-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calik A., Ekim B., Bayraktaroğlu A.G., Ergün A., Saçaklı P. Effects of dietary probiotic and synbiotic supplementation on broiler growth performance and intestinal histomorphology. Ankara Üniv. Vet. Fak. 2017;64:183–189. [Google Scholar]
- Calik A., Ergun A. Effect of lactulose supplementation on growth performance, intestinal histomorphology, cecal microbial population, and short-chain fatty acid composition of broiler chickens. Poult. Sci. 2015;94:2173–2182. doi: 10.3382/ps/pev182. [DOI] [PubMed] [Google Scholar]
- Cao G., Zhan X., Zhang L., Zeng X., Chen A., Yang C. Modulation of broilers' caecal microflora and metabolites in response to a potential probiotic Bacillus amyloliquefaciens. J. Anim. Physiol. Anim. Nutr. (Berl) 2018;102:e909–e917. doi: 10.1111/jpn.12856. [DOI] [PubMed] [Google Scholar]
- Cao G.T., Zeng X.F., Chen A.G., Zhou L., Zhang L., Xiao Y.P., Yang C.M. Effects of a probiotic, Enterococcus faecium, on growth performance, intestinal morphology, immune response, and cecal microflora in broiler chickens challenged with Escherichia coli K88. Poult. Sci. 2013;92:2949–2955. doi: 10.3382/ps.2013-03366. [DOI] [PubMed] [Google Scholar]
- Cerutti A., Rescigno M. The biology of intestinal immunoglobulin A responses. Immunity. 2008;28:740–750. doi: 10.1016/j.immuni.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corthesy B. Role of secretory IgA in infection and maintenance of homeostasis. Autoimmun. Rev. 2013;12:661–665. doi: 10.1016/j.autrev.2012.10.012. [DOI] [PubMed] [Google Scholar]
- Cowieson A.J., Acamovic T., Bedford M.R. The effects of phytase and phytic acid on the loss of endogenous amino acids and minerals from broiler chickens. Br. Poult. Sci. 2004;45:101–108. doi: 10.1080/00071660410001668923. [DOI] [PubMed] [Google Scholar]
- Culling C.F.A., Allison R., Barr W. 4th ed. Butterworth and Co. Ltd; London: 1985. Cellular Pathology Technique. [Google Scholar]
- Daudelin J.F., Lessard M., Beaudoin F., Nadeau E., Bissonnette N., Boutin Y., Brousseau J.P., Lauzon K., Fairbrother J.M. Administration of probiotics influences F4 (K88)-positive enterotoxigenic Escherichia coli attachment and intestinal cytokine expression in weaned pigs. Vet. Res. 2011;42:69. doi: 10.1186/1297-9716-42-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley J., Ton T., Maronpot R., Butterworth B., Goldsworthy T.L. Comparison of proliferating cell nuclear antigen to tritiated thymidine as a marker of proliferating hepatocytes in rats. Environ. Health Perspect. 1993;101:199–205. doi: 10.1289/ehp.93101s5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fre S., Huyghe M., Mourikis P., Robine S., Louvard D., Artavanis-Tsakonas S. Notch signals control the fate of immature progenitor cells in the intestine. Nature. 2005;435:964–968. doi: 10.1038/nature03589. [DOI] [PubMed] [Google Scholar]
- Geier M.S., Torok V.A., Guo P., Allison G.E., Boulianne M., Janardhana V., Bean A.G., Hughes R.J. The effects of lactoferrin on the intestinal environment of broiler chickens. Br. Poult. Sci. 2011;52:564–572. doi: 10.1080/00071668.2011.607429. [DOI] [PubMed] [Google Scholar]
- Grady E.N., MacDonald J., Liu L., Richman A., Yuan Z.-C. Current knowledge and perspectives of Paenibacillus: a review. Biomed. Central. 2016;15:203. doi: 10.1186/s12934-016-0603-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant A., Gay C.G., Lillehoj H.S. Bacillus spp. as direct-fed microbial antibiotic alternatives to enhance growth, immunity, and gut health in poultry. Avian Pathol. 2018;47:339–351. doi: 10.1080/03079457.2018.1464117. [DOI] [PubMed] [Google Scholar]
- Gulbahar M.Y., Yuksel H., Guvenc T., Okut H. Assessment of proliferative activity by AgNOR and PCNA in prostatic tissues of ram lambs implanted with zeranol. Reprod. Domest. Anim. 2005;40:468–474. doi: 10.1111/j.1439-0531.2005.00604.x. [DOI] [PubMed] [Google Scholar]
- Huijsdens X.W., Linskens R.K., Mak M., Meuwissen S.G.M., Vandenbroucke-Grauls C.M.J.E., Savelkoul P.H.M. Quantification of bacteria adherent to gastrointestinal mucosa by real-time PCR. J. Clin. Microbiol. 2002;40:4423–4427. doi: 10.1128/JCM.40.12.4423-4427.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraman S., Thangavel G., Kurian H., Mani R., Mukkalil R., Chirakkal H. Bacillus subtilis PB6 improves intestinal health of broiler chickens challenged with Clostridium perfringens-induced necrotic enteritis. Poult. Sci. 2013;92:370–374. doi: 10.3382/ps.2012-02528. [DOI] [PubMed] [Google Scholar]
- Kim K.M., Kim M.J., Kim D.H., Park Y.S., Kang J.S. Characterization of Bacillus polyfermenticus KJS-2 as a probiotic. J. Microbiol. Biotechnol. 2009;19:1013–1018. doi: 10.4014/jmb.0903.113. [DOI] [PubMed] [Google Scholar]
- Kim Y.S., Ho S.B. Intestinal goblet cells and mucins in health and disease: recent insights and progress. Curr. Gastroenterol. Rep. 2010;12:319–330. doi: 10.1007/s11894-010-0131-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar H., Salminen S., Verhagen H., Rowland I., Heimbach J., Banares S., Young T., Nomoto K., Lalonde M. Novel probiotics and prebiotics: road to the market. Curr. Opin. Biotechnol. 2015;32:99–103. doi: 10.1016/j.copbio.2014.11.021. [DOI] [PubMed] [Google Scholar]
- Lane D. 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M, editors. Nucleic Acid Techniques in Bacterial Systematics. John Wiley and Sons; Chichester, UK: 1991. pp. 115–175. [Google Scholar]
- Lebeer S., Vanderleyden J., De Keersmaecker S.C. Host interactions of probiotic bacterial surface molecules: comparison with commensals and pathogens. Nat. Rev. Microbiol. 2010;8:171–184. doi: 10.1038/nrmicro2297. [DOI] [PubMed] [Google Scholar]
- Lee K.-W., Lillehoj H.S., Jang S.I., Li G., Lee S.-H., Lillehoj E.P., Siragusa G.R. Effect of Bacillus-based direct-fed microbials on Eimeria maxima infection in broiler chickens. Comp. Immunol. Microbiol. Infect. Dis. 2010;33:e105–e110. doi: 10.1016/j.cimid.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Lutful Kabir S.M. The role of probiotics in the poultry industry. Int. J. Mol. Sci. 2009;10:3531–3546. doi: 10.3390/ijms10083531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manafi M., Khalaji S., Hedayati M., Pirany N. Efficacy of Bacillus subtilis and bacitracin methylene disalicylate on growth performance, digestibility, blood metabolites, immunity, and intestinal microbiota after intramuscular inoculation with Escherichia coli in broilers. Poult. Sci. 2017;96:1174–1183. doi: 10.3382/ps/pew347. [DOI] [PubMed] [Google Scholar]
- McGuckin M.A., Linden S.K., Sutton P., Florin T.H. Mucin dynamics and enteric pathogens. Nat. Rev. Microbiol. 2011;9:265–278. doi: 10.1038/nrmicro2538. [DOI] [PubMed] [Google Scholar]
- Mookiah S., Sieo C.C., Ramasamy K., Abdullah N., Ho Y.W. Effects of dietary prebiotics, probiotic and synbiotics on performance, caecal bacterial populations and caecal fermentation concentrations of broiler chickens. J. Sci. Food Agric. 2014;94:341–348. doi: 10.1002/jsfa.6365. [DOI] [PubMed] [Google Scholar]
- Mountzouris K.C., Balaskas C., Xanthakos I., Tzivinikou A., Fegeros K. Effects of a multi-species probiotic on biomarkers of competitive exclusion efficacy in broilers challenged with Salmonella enteritidis. Br. Poult. Sci. 2009;50:467–478. doi: 10.1080/00071660903110935. [DOI] [PubMed] [Google Scholar]
- Mountzouris K.C., Tsirtsikos P., Kalamara E., Nitsch S., Schatzmayr G., Fegeros K. Evaluation of the efficacy of a probiotic containing Lactobacillus, Bifidobacterium, Enterococcus, and Pediococcus strains in promoting broiler performance and modulating cecal microflora composition and metabolic activities. Poult. Sci. 2007;86:309–317. doi: 10.1093/ps/86.2.309. [DOI] [PubMed] [Google Scholar]
- Mountzouris K.C., Tsitrsikos P., Palamidi I., Arvaniti A., Mohnl M., Schatzmayr G., Fegeros K. Effects of probiotic inclusion levels in broiler nutrition on growth performance, nutrient digestibility, plasma immunoglobulins, and cecal microflora composition. Poult. Sci. 2010;89:58–67. doi: 10.3382/ps.2009-00308. [DOI] [PubMed] [Google Scholar]
- Nelson D.M., Glawe A.J., Labeda D.P., Cann I.K., Mackie R.I. Paenibacillus tundrae sp. nov. and Paenibacillus xylanexedens sp. nov., psychrotolerant, xylan-degrading bacteria from Alaskan tundra. Int. J. Syst. Evol. Microbiol. 2009;59:1708–1714. doi: 10.1099/ijs.0.004572-0. [DOI] [PubMed] [Google Scholar]
- NRC . 9th ed. National Academy Press; Washington, DC: 1994. Nutrient Requirements of Poultry. [Google Scholar]
- Peng Q., Zeng X.F., Zhu J.L., Wang S., Liu X.T., Hou C.L., Thacker P.A., Qiao S.Y. Effects of dietary Lactobacillus plantarum B1 on growth performance, intestinal microbiota, and short chain fatty acid profiles in broiler chickens. Poult. Sci. 2016;95:893–900. doi: 10.3382/ps/pev435. [DOI] [PubMed] [Google Scholar]
- Sen S., Ingale S.L., Kim Y.W., Kim J.S., Kim K.H., Lohakare J.D., Kim E.K., Kim H.S., Ryu M.H., Kwon I.K., Chae B.J. Effect of supplementation of Bacillus subtilis LS 1–2 to broiler diets on growth performance, nutrient retention, caecal microbiology and small intestinal morphology. Res. Vet. Sci. 2012;93:264–268. doi: 10.1016/j.rvsc.2011.05.021. [DOI] [PubMed] [Google Scholar]
- Shivaramaiah S., Pumford N.R., Morgan M.J., Wolfenden R.E., Wolfenden A.D., Torres-Rodriguez A., Hargis B.M., Tellez G. Evaluation of Bacillus species as potential candidates for direct-fed microbials in commercial poultry. Poult. Sci. 2011;90:1574–1580. doi: 10.3382/ps.2010-00745. [DOI] [PubMed] [Google Scholar]
- Teo A.-L., Tan H.-M. Effect of Bacillus subtilis PB6 (CloSTAT) on broilers infected with a pathogenic strain of Escherichia coli. J. Appl. Poult. Res. 2006;15:229–235. [Google Scholar]
- Troll J.V., Hamilton M.K., Abel M.L., Ganz J., Bates J.M., Stephens W.Z., Melancon E., van der Vaart M., Meijer A.H., Distel M., Eisen J.S., Guillemin K. Microbiota promote secretory cell determination in the intestinal epithelium by modulating host Notch signaling. Development. 2018;145 doi: 10.1242/dev.155317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uni Z., Platin R., Sklan D. Cell proliferation in chicken intestinal epithelium occurs both in the crypt and along the villus. J. Comp. Physiol. B. Biochem. Syst. Environ. Physiol. 1998;168:241–247. doi: 10.1007/s003600050142. [DOI] [PubMed] [Google Scholar]
- van der Flier L.G., Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu. Rev. Physiol. 2009;71:241–260. doi: 10.1146/annurev.physiol.010908.163145. [DOI] [PubMed] [Google Scholar]
- Wang S., Peng Q., Jia H.M., Zeng X.F., Zhu J.L., Hou C.L., Liu X.T., Yang F.J., Qiao S.Y. Prevention of Escherichia coli infection in broiler chickens with Lactobacillus plantarum B1. Poult. Sci. 2017;96:2576–2586. doi: 10.3382/ps/pex061. [DOI] [PubMed] [Google Scholar]
- Weselowski B., Nathoo N., Eastman A.W., MacDonald J., Yuan Z.-C. Isolation, identification and characterization of Paenibacillus polymyxa CR1 with potentials for biopesticide, biofertilization, biomass degradation and biofuel production. BMC Microbiol. 2016;16:244. doi: 10.1186/s12866-016-0860-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Wang B., Zeng Z., Liu R., Tang L., Gong L., Li W. Effects of probiotics Lactobacillus plantarum 16 and Paenibacillus polymyxa 10 on intestinal barrier function, antioxidative capacity, apoptosis, immune response, and biochemical parameters in broilers. Poult. Sci. 2019 doi: 10.3382/ps/pez226. 10.3382/ps/pez226. [DOI] [PubMed] [Google Scholar]
- Yang Y., Iji P.A., Kocher A., Mikkelsen L.L., Choct M. Effects of mannanoligosaccharide and fructooligosaccharide on the response of broilers to pathogenic Escherichia coli challenge. Br. Poult. Sci. 2008;49:550–559. doi: 10.1080/00071660802290408. [DOI] [PubMed] [Google Scholar]
- Zhang L., Cao G.T., Zeng X.F., Zhou L., Ferket P.R., Xiao Y.P., Chen A.G., Yang C.M. Effects of Clostridium butyricum on growth performance, immune function, and cecal microflora in broiler chickens challenged with Escherichia coli K88. Poult. Sci. 2014;93:46–53. doi: 10.3382/ps.2013-03412. [DOI] [PubMed] [Google Scholar]
- Zhang L., Zhang L., Zhan X., Zeng X., Zhou L., Cao G., Chen A., Yang C. Effects of dietary supplementation of probiotic, Clostridium butyricum, on growth performance, immune response, intestinal barrier function, and digestive enzyme activity in broiler chickens challenged with Escherichia coli K88. J. Anim. Sci. Biotechnol. 2016;7:3. doi: 10.1186/s40104-016-0061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]