Abstract
To investigate the etiopathogenesis of fatty liver hemorrhagic syndrome (FLHS) and the protective effects of resveratrol (RSV) against FLHS in laying hens, 144 healthy 90-day-old laying hens were randomly divided into 4 groups including control (Con) group, high-energy low-protein (HELP) group, RSV group, and HELP + RSV group, each of which contained 36 hens with 3 replicates. Birds in the 4 groups were fed a basal diet, HELP diet, basal diet supplemented with 400 mg/kg RSV, and HELP diet supplemented with 400 mg/kg RSV. The histopathology of the ovary lesions on day 120, egg production, antioxidative function, and mRNA expression levels of inflammatory cytokines on days 40, 80, and 120 were determined. The lipid accumulation and hemorrhaging were more severe in the HELP group than those in the HELP + RSV group. The laying rate was markedly decreased in the HELP group compared with that in the Con and HELP + RSV groups. Furthermore, the malondialdehyde concentration was significantly increased (P < 0.05), while the levels of superoxide dismutase (SOD), catalase, and glutathione were significantly decreased (P < 0.05) in the HELP group compared with those in the Con and HELP + RSV groups. The mRNA levels of antioxidant genes (Nrf2, SOD-1, and HO-1) were markedly increased (P < 0.05) in the HELP + RSV group compared with those in the HELP group. In addition, the mRNA levels of inflammation-related genes (nuclear factor kappa B, tumor necrosis factor-α, IL-1β, and IL-6) were significantly increased (P < 0.05) in the HELP group compared with those in the Con and HELP + RSV groups. Collectively, these results indicate that oxidative stress and inflammation are involved in the occurrence and development of FLHS in the ovaries of laying hens, but RSV effectively attenuates oxidative stress and inflammation in hens with FLHS. Hence, RSV can be used as an effective feed additive to protect against FLHS.
Key words: resveratrol, fatty liver hemorrhagic syndrome, oxidative stress, inflammation, ovary
Introduction
Fatty liver hemorrhagic syndrome (FLHS) is a metabolic disease mostly observed in female laying hens and can be induced by genetic, environmental epigenetic, and nutritional factors (Butler, 1976, Choi et al., 2012, Trott et al., 2014). Lipid metabolism disorders and oxidative stress are possible mechanisms associated with fatty liver disease (Spurlock and Savage, 1993, Trott et al., 2014). Numerous studies have shown that FLHS is a multisystem disease syndrome that can result in hemorrhage and lipid accumulation in the livers, ovaries, kidneys, and intestines (Yeh et al., 2009, Trott et al., 2014). In addition, the typical characteristics of FLHS are a sudden drop in egg production and a shortened egg production peak, which cause a devastating economic toll on the poultry industry (Walzem et al., 1993). Ovaries are important organs promoting ovulation and egg production, and their dysfunction can lead to a significant reduction in egg number and quality in hens (Liu et al., 2014). Moreover, previous studies reported that FLHS also induces follicular atresia, partial regression of the oviduct, and ovarian hemorrhage (Squires and Leeson, 1988, Walzem et al., 1993, Shini et al., 2019).
Oxidative stress occurs in an environment in which the balance of pro-oxidant species to antioxidant species is altered in favor of the former and is involved in numerous pathophysiological processes of various diseases, including arteriosclerosis, diabetes, and liver steatosis (Kaplowitz, 2000, Bennett et al., 2009, Ghowsi et al., 2018). The balance between hepatic synthesis and secretion of lipids has been shown to be the key point that regulates hepatic and extra hepatic fat deposition in hens (Shini, 2014). Fat accumulation can be increased by many factors, including nutrition, oxidative stress, and inflammatory changes in the liver (Shini, 2014). In addition, oxidative stress can promote the activation of nuclear factor kappa B (NF-κB), which is a redox-sensitive transcription factor that mediates the transcription of a large number of inflammatory genes coding for cytokines and adhesion molecules (Huang et al., 2013, Chen et al., 2016). These cytokines are known as proinflammatory cytokines and include IL-1α, IL-1β, tumor necrosis factor-α (TNF-α), and IL-6 (Asrih and Jornayvaz, 2013); they coordinate the local and systemic inflammatory responses to microbial pathogens (Dantzer et al., 2008).
Resveratrol (RSV, trans-3,5,4-trihydroxystilbene), a natural plant polyphenol found in grapes and red wine, is very popular for its betatrophin and antioxidant activities (Cheng et al., 2015). Thus, it has been used to protect against neurodegeneration, cardiovascular disease, cancer, diabetes, and obesity-related disorders (Ergenoglu et al., 2015). RSV could restrain oxidative stress and alleviate inflammatory responses in a rat model of polycystic ovary syndrome (Sadi et al., 2015). It also plays several roles in antioxidative and anti-inflammatory pathways and ameliorates metabolic syndrome (Javkhedkar et al., 2015). A previous study reported that a diet packed with antioxidants could reduce the morbidity of FLHS (Spurlock and Savage, 1993). RSV also could attenuate hepatic steatosis in high-fat diet–fed mice by decreasing lipogenesis and inflammation (Andrade et al., 2014). However, the effects of RSV on ovarian oxidative stress and inflammation in FLHS have not been fully elucidated. Therefore, this study evaluated the protective effects of RSV on the laying rate, ovarian antioxidant indices, and the mRNA levels of antioxidative and inflammatory genes in ovaries by constructing an FLHS model.
Materials and methods
Animals and Treatments
All experimental procedures were performed as per the guidelines for the care and use of laboratory animals from the Experimental Animal Care and Use Committee of Jiangxi Agricultural University, and the ethics committee of Jiangxi Agricultural University specifically approved this study. A total of 144 clinically healthy 90-day-old Hy-Line brown laying hens were randomly assigned to 4 groups: control (Con) group, high-energy low-protein (HELP) group, RSV group, and HELP + RSV group. Birds in the 4 groups were fed the basal diet, HELP diet, basal diet supplemented with 400 mg/kg RSV, and HELP diet supplemented with 400 mg/kg RSV. The experiment lasted for 120 days, and hens were given free access to standard food and water. The basal diet for hens was prepared according to the standard nutritional requirements of the National Research Council (Dale, 1994), and the HELP diet composition was different in only energy and protein nutritional standards. The basal and HELP diet composition is shown in Table 1.
Table 1.
Composition and nutrients levels of diets (air-dry basis)%.
| Composition of diet % | Basic diet | High-energy low-protein diet |
|---|---|---|
| Corn | 64.00 | 70.00 |
| Wheat bran | 2.00 | 1.20 |
| Soybean meal | 24.00 | 14.58 |
| Fat-soybean oil | 0 | 4.22 |
| Calcium | 8.00 | 8.00 |
| Premix1 | 2.00 | 2.00 |
| Total | 100.00 | 100.00 |
| Nutrient level | ||
| CP | 15.86 | 12.00 |
| AP | 0.51 | 0.46 |
| Arg | 1.03 | 0.74 |
| Met | 0.37 | 0.32 |
| Val | 0.77 | 0.58 |
| Energy (kcal/kg) | 2,678.99 | 3,100.00 |
| Met + Cys | 0.67 | 0.56 |
Abbreviations: AP, Calcium hydrogen phosphate; Arg, arginine; Cys, cysteine; Met, methionine; Val, L-valine.
Per kilogram of additives contained:Cu, 2.50 mg; Fe, 20.00 mg; Zn, 17.50 mg; Mn, 15.00 mg; KI, 4.00 mg; Na2SeO3, 6.00 mg; CoCl2·6H2O, 2.50 mg; C5H11NO2S, 50.00 mg; chromium picolinate, 2.00 mg; vitamins, 15.00 mg; phytase, 10.00 mg; kininase, 7.50 mg; antioxidant, 2.00 mg; betaine, 15.00 mg; choline, 50.00 mg; salt, 200.00 mg; p-ca, 500.00 mg; zeolite powder, 76.00 mg.
Sample Collection
Ovary samples of 12 birds were immediately collected randomly from each group after birds were euthanized with an overdose via intravenous injection of sodium pentobarbital (Nembutal; Abbott Laboratories, Chicago, IL; 100 mg/kg) on days 40, 80, and 120. Each ovary was collected and thoroughly washed with 0.9% normal saline to rinse and remove the blood and separated into 2 parts. One part of the ovary was placed in freezing tubes, lyophilized in liquid nitrogen, and stored at −80°C until used for determining the mRNA expression levels of related genes. The other part of the ovary was stored at −20°C for the detection of antioxidant indices. The remaining ovary sections were obtained on the 120th day to observe the pathological damage.
Histopathological Examination
The ovary tissue specimens were washed with normal saline and then fixed in formalin. After 1 wk, formalin-fixed samples were routinely processed and embedded in paraffin. Then, the tissue was sliced into thin sections (5 μm) and stained with hematoxylin and eosin (H&E). Afterward, pathological sections were observed using an optical microscope, and photographs were taken.
Determination of Laying Rate
The average daily egg production was recorded every day.
Determination of the Antioxidant Indices in Ovary Tissues
The levels of malondialdehyde (MDA), glutathione (GSH), catalase (CAT), and superoxide dismutase (SOD) were assessed by using kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) based on the manufacturer's instructions.
Quantitative Real-Time Polymerase Chain Reaction Analysis
For the measurement of target gene expression, total RNA was isolated using TRIzol reagent (Takara, Shiga, Japan) and then reverse-transcribed into cDNA using a PrimeScript RT reagent kit (Takara, Shiga, Japan) according to the manufacturer's protocol. Primers targeting Nrf2, HO-1, SOD-1, NF-κB, TNF-α, IL-1β, IL-6, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) genes were designed using the Primer Premier Software (PREMIER Biosoft International, Palo Alto, CA) and Net primer (PREMIER Biosoft International, Palo Alto, CA) software. The GenBank accession numbers and primer sequences are shown in Table 2. Quantitative reverse transcription-polymerase chain reaction was performed using a One-Step SYBR PrimeScriptTM RT-PCR Kit II (Takara, Shiga, Japan) on a Real-time PCR Detection System (Thermo Fisher, Beijing, China). Relative changes in mRNA levels of genes were assessed using the 2-△△CT method and normalized to the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
Table 2.
Prime sequences.
| Gene name | Accession number | Primers sequences (5′-3′) |
|---|---|---|
| Nrf2 | NM_205117 | F: ATCACCTCTTCTGCACCGAA |
| R: GCTTTCTCCCGCTCTTTCTG | ||
| HO-1 | NM_205344 | F: CTTCGCACAAGGAGTGTTAAC |
| R: CATCCTGCTTGTCCTCTCAC | ||
| SOD-1 | NM_205064 | F: GGTGCTCACTTTAATCCTG |
| R: CTACTTCTGCCACTCCTCC | ||
| NF-κB | NM_205134 | F: TCAACGCAGGACCTAAAGACAT |
| R: GCAGATAGCCAAGTTCAGGATG | ||
| TNF-α | NM_204267 | F: GCCCTTCCTGTAACCAGATG |
| R: ACACGACAGCCAAGTCAACG | ||
| IL-1β | NM_204524 | F: ACTGGGCATCAAGGGCTA |
| R: GGTAGAAGATGAAGCGGGTC | ||
| IL-6 | NM_204628 | F: AGGACGAGATGTGCAAGAAGT |
| R: TTGGGCAGGTTGAGGTTGTT | ||
| GAPDH | NM_204305 | F: AGAACATCATCCCAGCGTCC |
| R: CGGCAGGTCAGGTCAACAAC |
Abbreviations: F, forward primer; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; NF-κB, nuclear factor kappa B; R, reverse primer; TNF-α, tumor necrosis factor-α.
Statistical Analysis
All data were analyzed by using SPSS version 17.0 (SPSS Inc., Chicago, IL) and GraphPad Prism 5.01 (GraphPad Inc., La Jolla, CA). Differences between means were assessed by using a one-way ANOVA followed by Dunnett's test for multiple comparisons. Differences were considered significant at levels of P < 0.05.
Results
Histopathology
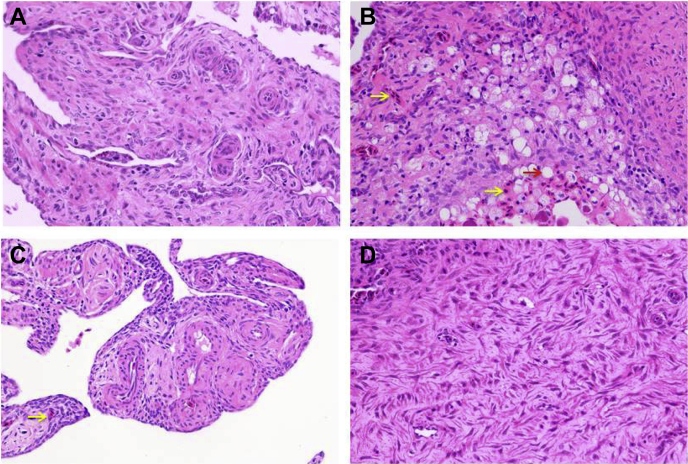
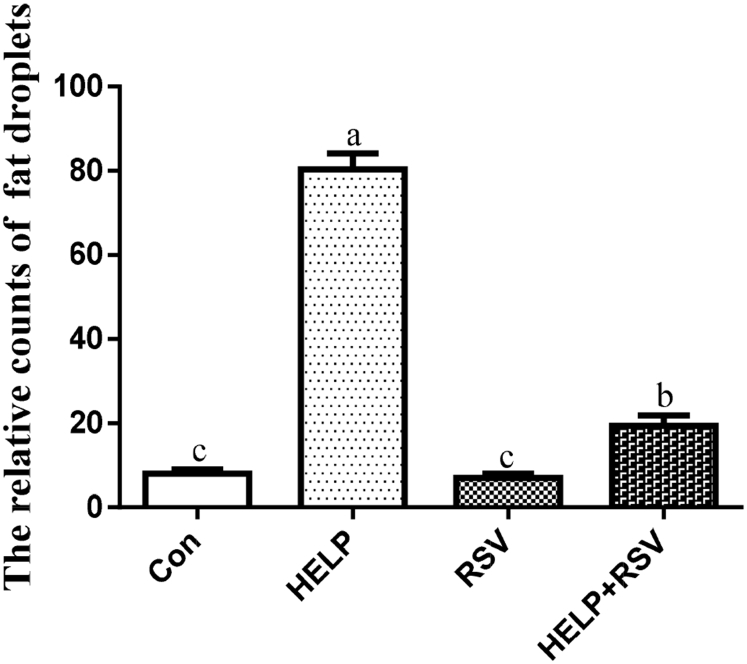
The pathological observation results are shown in Figure 1. The H&E staining showed that the structure of follicular cells was normal in the control group (Figure 1a). However, we found extensive tissue infiltration with fat droplets and erythrocytes in the HELP group (Figure 1b). Compared with those in the HELP group, the fat droplet and erythrocyte numbers were notably reduced in the HELP + RSV group (Figure 1c). Moreover, no follicular cell structural abnormalities were observed in the RSV group (Figure 1d). The relative concentrations of lipid droplets are shown in Figure 2. The relative lipid droplet concentrations in the HELP group were markedly increased (P < 0.05) compared with those in the control group. The relative lipid droplet concentration was significantly decreased (P < 0.05) in the HELP + RSV group throughout the whole experiment compared with that in the HELP group. In addition, the relative lipid droplet concentration in the RSV group was lower than that in the control group (P > 0.05).
Figure 1.
Pathological observation of the ovaries in laying hens. (A) Con group: normal follicular cell structure. (B) HELP group: extensive tissue infiltration by fat droplets and erythrocytes. (C) HELP + RSV group: fewer fat droplets and erythrocytes than the pathological group. (D) RSV group: no obvious lipid vacuoles. The red arrow indicates the lipid vacuolization and the yellow arrow indicates erythrocytes inside the ovary (H&E stain, using 400 × magnification). Abbreviations: H&E, hematoxylin and eosin; HELP, high-energy low-protein; RSV, resveratrol.
Figure 2.
The relative counts of fat droplets in the H&E stained sections. Values are the mean of 3 replicates, with the standard deviation represented by vertical bars. On bars, the same small superscript letters indicate no significant difference (P > 0.05); different small superscript letters indicate significant difference (P < 0.05). The data are presented as means ± SD (n = 3 per group). Abbreviations: Co, control; H&E, hematoxylin and eosin; HELP, high-energy low-protein; RSV, resveratrol; SD, standard deviation.
Laying Rate
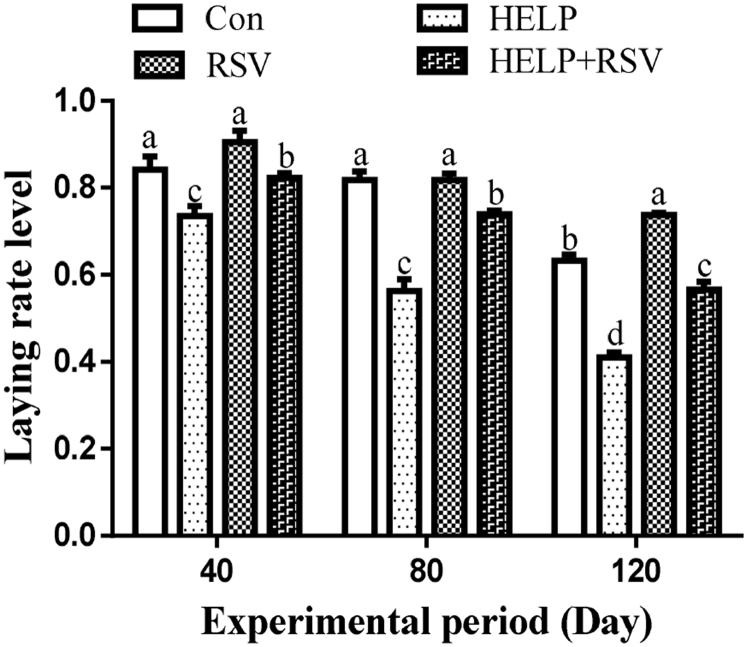
The laying rates on days 40, 80, and 120 are shown in Figure 3. The laying rate in the HELP group on days 40, 80, and 120 was significantly decreased (P < 0.05) compared with that in the Con group. The laying rate was markedly decreased (P < 0.05) in the HELP group on days 40, 80, and 120 compared with that in the HELP + RSV group. Moreover, on days 40 and 80, the laying rate did not differ significantly (P > 0.05) between the Con and RSV groups, whereas it was increased in the RSV group compared with that in the Con group on day 120 (P < 0.05).
Figure 3.
Laying rate on days 40, 80, and 120. On bars, the same small superscript letters indicate no significant difference (P > 0.05); different small superscript letters indicate significant difference (P < 0.05). Abbreviations: Con, control; HELP, high-energy low-protein; RSV, resveratrol.
The Levels of MDA, CAT, SOD, and GSH in Ovaries
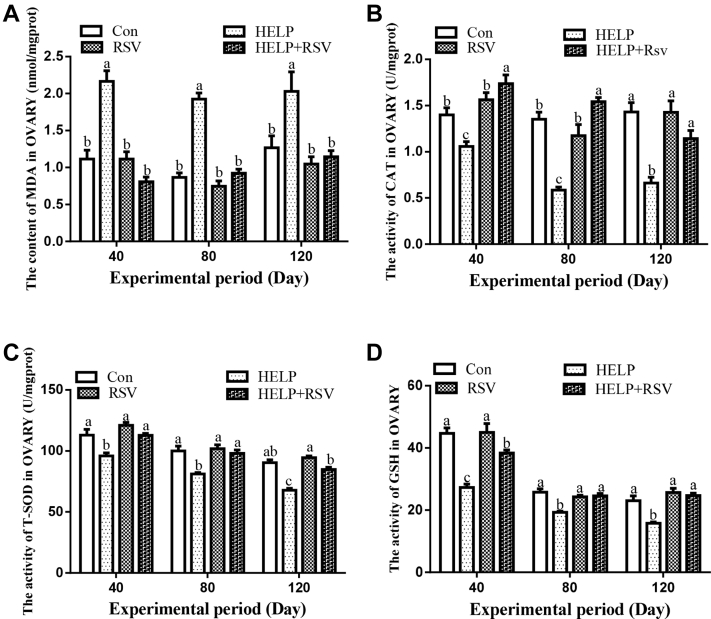
The levels of MDA, CAT, SOD, and GSH in ovaries on days 40, 80, and 120 are shown in Figure 4. MDA concentration on days 40, 80, and 120 was significantly increased (P < 0.05) in the HELP group compared with that in the Con group. Nevertheless, in the HELP + RSV group, the concentration of MDA was significantly decreased (P < 0.05) compared with that in the HELP group on days 40, 80, and 120 (Figure 4a). The activity of CAT, SOD, and GSH was obviously increased (P < 0.05) in the Con group on days 40, 80, and 120 compared with that in the HELP group (Figure 4b-d). In addition, the activity of CAT, SOD, and GSH was significantly increased (P < 0.05) in the HELP + RSV group compared with that in the HELP group on days 40, 80, and 120. The activity of CAT, SOD, and GSH was obviously decreased (P < 0.05) in the HELP group on days 40, 80, and 120 compared with that in the RSV group.
Figure 4.
Determination of (A) MDA, (B) CAT, (C) SOD, and (D) GSH activity in hen ovaries on days 40, 80, and 120. On bars, the same small superscript letters indicate no significant difference (P > 0.05); different small superscript letters indicate significant difference (P < 0.05). Abbreviations: CAT, catalase; Con, control; GSH, glutathione; HELP, high-energy low-protein; MDA, malondialdehyde; RSV, resveratrol; SOD, superoxide dismutase.
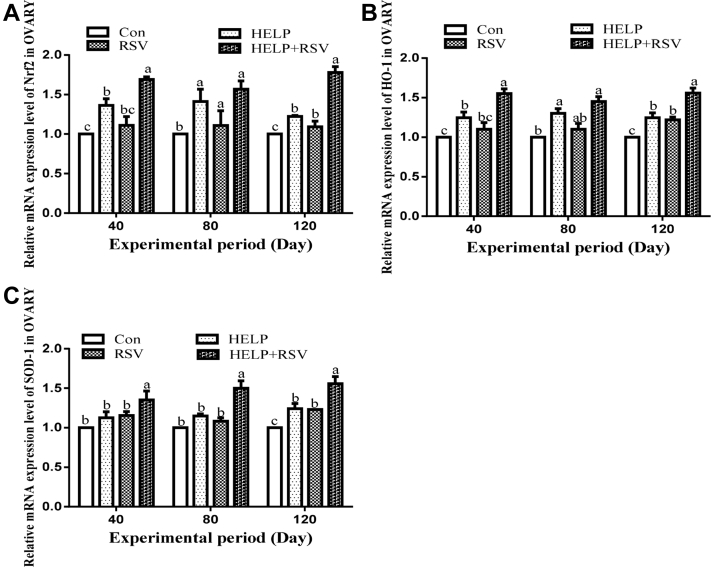
The mRNA Expression Levels of Nrf2, HO-1, and SOD-1 in Ovaries
The alterations in the mRNA expression levels of Nrf2, HO-1, and SOD-1 in the ovaries of hens are described in Figure 5. The mRNA expression levels of Nrf2, HO-1, and SOD-1 were decreased in the Con group at all experimental time points compared with those in the HELP group (Figure 5a–c). Nevertheless, the mRNA expression levels in the HELP + RSV group were higher than those in the HELP group on days 40, 80, and 120. In addition, the mRNA expression level of Nrf2 was notably increased (P < 0.05) in the RSV group compared with that in the Con group on days 80 and 120 (Figure 5a). The HO-1 mRNA expression level in the Con group was significantly increased (P < 0.05) on day 120 compared with that in the RSV group (Figure 5b).
Figure 5.
Determination of the mRNA expression levels of (A) Nrf2, (B) HO-1, and (C) SOD-1 in hen ovaries on days 40, 80, and 120. On bars, the same small superscript letters indicate no significant difference (P > 0.05); different small superscript letters indicate significant difference (P < 0.05). Abbreviations: Con, control; HELP, high-energy low-protein; RSV, resveratrol; SOD, superoxide dismutase.
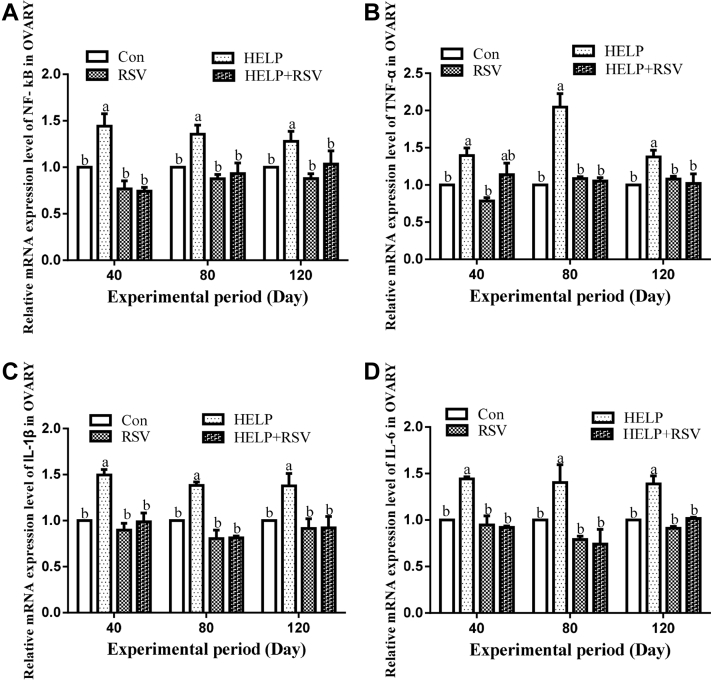
The mRNA Expression Levels of NF-κB, TNF-α, IL-1β, and IL-6 in Ovaries
The mRNA expression levels of NF-κB, TNF-α, IL-1β, and IL-6 in the ovaries of hens are shown in Figure 6. The mRNA expression levels of NF-κB, TNF-α, IL-1β, and IL-6 were significantly elevated (P < 0.05) in the HELP group compared with those in the Con group at all experimental time points (Figure 6a–d). In addition, on days 40, 80, and 120, the mRNA levels of NF-κB, TNF-α, IL-1β, and IL-6 were decreased in the Con group compared with those in the RSV group, but the differences were not significant (P > 0.05). Nevertheless, the mRNA expression levels of NF-κB, IL-1β, and IL-6 in the HELP + RSV group were obviously decreased (P < 0.05) compared with those in the HELP group at all the experimental time points (Figure 6a, c, d). In addition, the TNF-α expression level in the HELP + RSV group was significantly decreased (P < 0.05) compared with that in the HELP group on days 80 and 120 (Figure 6b). Moreover, the mRNA level of TNF-α in the RSV group was markedly reduced (P < 0.05) compared with that in the Con group on day 120 (Figure 6b).
Figure 6.
Determination of the mRNA expression levels of (A) NF-κB, (B) TNF-α, (C) IL-1β, and (D) IL-6 in hen ovaries on days 40, 80, and 120. On bars, the same small superscript letters indicate no significant difference (P > 0.05); different small superscript letters indicate significant difference (P < 0.05). Abbreviations: Con, control; HELP, high-energy low-protein; NF-κB, nuclear factor kappa B; RSV, resveratrol; TNF-α, tumor necrosis factor-α.
Discussion
FLHS is a metabolic disorder disease presumably related to oxidative stress and is characterized by a sudden drop in egg production and an increase in mortality among adult layers (Yang et al., 2017, Shini et al., 2019). Previous reports have shown that administering exogenous oestradiol or HELP diets dramatically increase the incidence of FLHS, which stops egg production and causes rapid regression of the oviduct and ovaries (Haghighi-Rad and Polin, 1981, Rozenboim et al., 2016). In this study, we used a HELP diet combined with caged feeding technology and high temperature in summer to construct an FLHS model, which is the same approach as that used by Yang (Yang et al., 2017). The results indicated that the laying rate was significantly decreased in the HELP group compared with that in the Con group, which was consistent with previous FLHS reports (Trott et al., 2014, Shini et al., 2019). Moreover, it was evident that ovary in the pathology group had fatty degeneration and hemorrhaging. Hence, animal models of FLHS were established successfully.
Previous studies have indicated that abnormal antioxidant (MDA, SOD, and GSH) and inflammatory cytokine (TNF-α, IL-6 IL-1, SAAL1, and iNOS2) production plays a role in the pathogenesis of FLHS (Guo XQ GLH, 2010, Shini, 2014). MDA, a product of lipid peroxidation, is a commonly known marker of oxidative stress and antioxidant status (Shen et al., 2016). The results of the present study revealed that the MDA concentration was significantly increased in the HELP group compared with that in the Con group. Squires and Wu also reported that hens with severe liver hemorrhagic scores had increased MDA level in the liver (Squires and Wu, 1992). This finding may be due to an increase in free radicals, causing the overproduction of MDA. In addition, our results showed that the activity of SOD, CAT, and GSH was decreased in ovaries in the HELP group compared with that in the Con group. SOD, CAT, and GSH, 3 important endogenous free radical scavengers, play a crucial role in maintaining the oxidative and antioxidative balance of the body, as they scavenge free radicals and protect cells from oxidative damage (Shen et al., 2016). The reduced of SOD, CAT, and GSH activity shows that the antioxidant system was damaged by free radicals. As a pivotal regulator of the antioxidant response element, Nrf2 has attracted great attention for its role in preventing the development of oxidative stress by regulating Nrf2-related antioxidant activity (Liu et al., 2015). Nrf2 is a significant transcription factor that plays a role in the transcription of enzymes (HO-1 and SOD-1) by binding to DNA sequences called antioxidant-response elements (Zhang et al., 2013). HO-1 is an important Nrf2-regulated antioxidant enzyme that regulates intracellular ROS levels in response to diverse stimuli (Bao et al., 2018). The results of the present study revealed that the mRNA transcription levels of Nrf2, HO-1, and SOD-1 in ovaries were significantly increased in the HELP group compared with those in the Con group. The results from the present study revealed increased Nrf2, HO-1, and SOD-1 mRNA expression levels in FLHS models, which indicated that the oxidative stress might be involved in triggering ovary injuries in FLHS hens. A previous study showed that the activation of NF-κB signaling is mediated by the upstream kinase inhibitor of kappaB kinase which is triggered by hypoxia, ROS, and several inflammatory mediators (Lan et al., 2016). NF-κB is an important transcription factor that plays a critical role in the regulation of a variety of important genes in cellular responses (Liu et al., 2008). Whenever it is activated, NF-κB regulates the expression of a wide array of inflammatory mediators, such as IL-1β, cyclooxygenase-2, TNF-α, IL-6, inducible nitric oxide synthase, and matrix metalloproteinase-9, all of which play crucial roles in fatty liver damage (Shini, 2012, Li et al., 2018). Herein, the results showed that the mRNA transcription levels of NF-κB, TNF-α, IL-1β, and IL-6 in ovaries were significantly increased in the HELP group compared with those in the Con group which is in consistent with a previous inflammation and nonalcoholic fatty liver disease study (Asrih and Jornayvaz, 2013).
Growing evidence suggests that RSV plays a role in the prevention of pathologies such as inflammation, oxidative stress, carcinogenesis, and cardiovascular disease (Fremont et al., 1999, Zhang et al., 2016). In addition, researchers have indicated that RSV reduces or inhibits the generation of ROS, inhibits lipid peroxidation, and regulates the activity of antioxidant enzyme (Breinholt et al., 2003). In this study, there were fewer lipid vacuoles and erythrocytes in the HELP + RSV group than those in the HELP group, assessed by H&E staining. Furthermore, the laying rate was increased in the HELP + RSV group compared with that in the HELP group. In the HELP + RSV group, the level of MDA was significantly decreased. In contrast, the activity of SOD, CAT, and GSH was significantly increased in the HELP + RSV group compared with that in the HELP group. Ergenoglu et al. also reported the effects of RSV supplementation on alleviating oxidative damage in ovaries with age-associated infertility disorder in mice (Ergenoglu et al., 2015). Taken together, these findings suggest that RSV pretreatment attenuates oxidative damage in ovaries. Moreover, we found that the mRNA expression levels of Nrf2, SOD, and HO-1 were significantly increased in the HELP + RSV group compared with those in the HELP group. The anti-inflammatory capability of RSV is associated with its capacity to reduce the activation of NF-κB and TNF-α (Elisabetta Ferrero and Ferrero, 2005). Andrade reported that RSV supplementation attenuated hepatic steatosis in high-fat fed mice by decreasing lipogenesis and inflammation (Andrade et al., 2014). In the present study, the mRNA levels of NF-κB, TNF-α, IL-1β, and IL-6 in ovaries were found to be significantly decreased in the HELP + RSV group compared with those in HELP group. Considering that RSV plays an important role in antioxidative stress and anti-inflammatory processes, we conclude that the FLHS-induced damage in hen ovaries may be alleviated by RSV.
Conclusion
Taken together, our findings show that FLHS induces oxidative stress and inflammation in the ovaries of hens, but this induction is compromised by RSV supplementation.
Acknowledgments
This project was supported by grants from the National Important Research Plan (2018YFD0501302, 2016YFD0501205-2), the National Natural Science Foundation of China (No.31560715), and Academic and Technical Leaders of Major Disciplines in Jiangxi Province (611227202153). The authors declare that there are no conflicts of interest.
Contributor Information
Huabin Cao, Email: chbin20020804@163.com.
Guoliang Hu, Email: hgljx3818@163.com.
References
- Andrade J.M., Paraiso A.F., de Oliveira M.V., Martins A.M., Neto J.F., Guimaraes A.L., de Paula A.M., Qureshi M., Santos S.H. Resveratrol attenuates hepatic steatosis in high-fat fed mice by decreasing lipogenesis and inflammation. Nutrition. 2014;30:915–919. doi: 10.1016/j.nut.2013.11.016. [DOI] [PubMed] [Google Scholar]
- Asrih M., Jornayvaz F.R. Inflammation as a potential link between nonalcoholic fatty liver disease and insulin resistance. J. Endocrinol. 2013;218:R25–R36. doi: 10.1530/JOE-13-0201. [DOI] [PubMed] [Google Scholar]
- Bao L., Li J., Zha D., Zhang L., Gao P., Yao T., Wu X. Chlorogenic acid prevents diabetic nephropathy by inhibiting oxidative stress and inflammation through modulation of the Nrf2/HO-1 and NF-kB pathways. Int. Immunopharmacol. 2018;54:245–253. doi: 10.1016/j.intimp.2017.11.021. [DOI] [PubMed] [Google Scholar]
- Bennett S., Grant M.M., Aldred S. Oxidative stress in vascular dementia and Alzheimer's disease: a common pathology. J. Alzheimers Dis. 2009;17:245–257. doi: 10.3233/JAD-2009-1041. [DOI] [PubMed] [Google Scholar]
- Breinholt V.M., Molck A.M., Svendsen G.W., Daneshvar B., Vinggaard A.M., Poulsen M., Dragsted L.O. Effects of dietary antioxidants and 2-amino-3-methylimidazo[4,5-f]-quinoline (IQ) on preneoplastic lesions and on oxidative damage, hormonal status, and detoxification capacity in the rat. Food Chem. Toxicol. 2003;41:1315–1323. doi: 10.1016/s0278-6915(03)00122-4. [DOI] [PubMed] [Google Scholar]
- Butler E.J. Fatty liver diseases in the domestic fowl--a review. Avian Pathol. 1976;5:1–14. doi: 10.1080/03079457608418164. [DOI] [PubMed] [Google Scholar]
- Chen X., Wei S.Y., Li J.S., Zhang Q.F., Wang Y.X., Zhao S.L., Yu J., Wang C., Qin Y., Wei Q.J., Lv G.X., Li B. Overexpression of heme oxygenase-1 prevents renal interstitial inflammation and fibrosis induced by Unilateral Ureter obstruction. Plos One. 2016;11:147–184. doi: 10.1371/journal.pone.0147084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L., Jin Z., Zhao R., Ren K., Deng C., Yu S. Resveratrol attenuates inflammation and oxidative stress induced by myocardial ischemia-reperfusion injury: role of Nrf2/ARE pathway. Int. J. Clin. Exp. Med. 2015;8:10420–10428. [PMC free article] [PubMed] [Google Scholar]
- Choi Y.I., Ahn H.J., Lee B.K., Oh S.T., An B.K., Kang C.W. Nutritional and hormonal induction of fatty liver syndrome and effects of dietary Lipotropic factors in egg-type Male Chicks. Asian-australas J. Anim. Sci. 2012;25:1145–1152. doi: 10.5713/ajas.2011.11418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale N. National Research Council Nutrient Requirements of Poultry - Ninth Revised Edition (1994) J. Appl. Poultry. Res. 1994;3(1):101. [Google Scholar]
- Dantzer R., O'Connor J.C., Freund G.G., Johnson R.W., Kelley K.W. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elisabetta Ferrero A.F., Ferrero A.M.E. Anti-inflammatory and anti-Neoplastic Actions of resveratrol. Curr. Nutr. Food Sci. 2005;1:189–199. [Google Scholar]
- Ergenoglu M., Yildirim N., Yildirim A.G., Yeniel O., Erbas O., Yavasoglu A., Taskiran D., Karadadas N. Effects of resveratrol on ovarian Morphology, plasma anti-Mullerian hormone, IGF-1 levels, and oxidative stress parameters in a rat model of polycystic ovary syndrome. Reprod. Sci. 2015;22:942–947. doi: 10.1177/1933719115570900. [DOI] [PubMed] [Google Scholar]
- Fremont L., Belguendouz L., Delpal S. Antioxidant activity of resveratrol and alcohol-free wine polyphenols related to LDL oxidation and polyunsaturated fatty acids. Life Sci. 1999;64:2511–2521. doi: 10.1016/s0024-3205(99)00209-x. [DOI] [PubMed] [Google Scholar]
- Ghowsi M., Khazali H., Sisakhtnezhad S. The effect of resveratrol on oxidative stress in the liver and serum of a rat model of polycystic ovary syndrome: an experimental study. Int. J. Reprod. Biomed. (yazd). 2018;16:149–158. [PMC free article] [PubMed] [Google Scholar]
- Guo XQ GLH H.B.C.C. Ntioxidative functions and liver injury in laying hens with fatty liver hemorrhagic syndrome induced by high energy-low protein diet. Chin. Journay Vet. Sci. 2010;30:829–839. [Google Scholar]
- Haghighi-Rad F., Polin D. The relationship of plasma estradiol and progesterone levels to the fatty liver hemorrhagic syndrome in laying hens. Poult. Sci. 1981;60:2278–2283. doi: 10.3382/ps.0602278. [DOI] [PubMed] [Google Scholar]
- Huang W., Xu L., Zhou X., Gao C., Yang M., Chen G., Zhu J., Jiang L., Gan H., Gou F., Feng H., Peng J., Xu Y. High glucose induces activation of NF-kappaB inflammatory signaling through IkappaBalpha sumoylation in rat mesangial cells. Biochem. Biophys. Res. Commun. 2013;438:568–574. doi: 10.1016/j.bbrc.2013.07.065. [DOI] [PubMed] [Google Scholar]
- Javkhedkar A.A., Quiroz Y., Rodriguez-Iturbe B., Vaziri N.D., Lokhandwala M.F., Banday A.A. Resveratrol restored Nrf2 function, reduced renal inflammation, and mitigated hypertension in spontaneously hypertensive rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015;308:840–R846. doi: 10.1152/ajpregu.00308.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplowitz N. Mechanisms of liver cell injury. J. Hepatol. 2000;32:39–47. doi: 10.1016/s0168-8278(00)80414-6. [DOI] [PubMed] [Google Scholar]
- Lan Zhanga B.C., Xiangjian Z.B.C.N., Cong Z.B.C., Xue B., Jian Zhanga B.C., Xumeng Z.B.C., Linyu C.B.C., Lina W.B.C., Chunhua Zhua B.C., Lili C.B.C., Rong C.C., Ting Z., Yuan Z.B.C. Nobiletin promotes antioxidant and anti-inflammatory responses and elicits protection against ischemic stroke in vivo. Brain Res. 2016;1636:130–141. doi: 10.1016/j.brainres.2016.02.013. [DOI] [PubMed] [Google Scholar]
- Li J., Li L., Wang S., Zhang C., Zheng L., Jia Y., Xu M., Zhu T., Zhang Y., Rong R. Resveratrol alleviates inflammatory responses and oxidative stress in rat kidney ischemia-reperfusion injury and H2O2-induced NRK-52E cells via the Nrf2/TLR4/NF-kappaB pathway. Cell. Physiol. Biochem. 2018;45:1677–1689. doi: 10.1159/000487735. [DOI] [PubMed] [Google Scholar]
- Liu G.H., Qu J., Shen X. NF-kappaB/p65 antagonizes Nrf2-ARE pathway by depriving CBP from Nrf2 and facilitating recruitment of HDAC3 to MafK. Biochim. Biophys. Acta. 2008;1783:713–727. doi: 10.1016/j.bbamcr.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Liu Y., Zhang L., Liang J. Activation of the Nrf2 defense pathway contributes to neuroprotective effects of phloretin on oxidative stress injury after cerebral ischemia/reperfusion in rats. J. Neurol. Sci. 2015;351:88–92. doi: 10.1016/j.jns.2015.02.045. [DOI] [PubMed] [Google Scholar]
- Liu Z.C., Xie Y.L., Chang C.J., Su C.M., Chen Y.H., Huang S.Y., Walzem R.L., Chen S.E. Feed intake alters immune cell functions and ovarian infiltration in broiler hens: implications for reproductive performance. Biol. Reprod. 2014;90:134. doi: 10.1095/biolreprod.113.115824. [DOI] [PubMed] [Google Scholar]
- Rozenboim I., Mahato J., Cohen N.A., Tirosh O. Low protein and high-energy diet: a possible natural cause of fatty liver hemorrhagic syndrome in caged White Leghorn laying hens. Poult. Sci. 2016;95:612–621. doi: 10.3382/ps/pev367. [DOI] [PubMed] [Google Scholar]
- Sadi G., Pektas M.B., Koca H.B., Tosun M., Koca T. Resveratrol improves hepatic insulin signaling and reduces the inflammatory response in streptozotocin-induced diabetes. Gene. 2015;570:213–220. doi: 10.1016/j.gene.2015.06.019. [DOI] [PubMed] [Google Scholar]
- Shen C., Cheng W., Yu P., Wang L., Zhou L., Zeng L., Yang Q. Resveratrol pretreatment attenuates injury and promotes proliferation of neural stem cells following oxygen-glucose deprivation/reoxygenation by upregulating the expression of Nrf2, HO-1 and NQO1 in vitro. Mol. Med. Rep. 2016;14:3646–3654. doi: 10.3892/mmr.2016.5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shini A.S.S.F. 2014. Fatty Liver Haemorrhagic Syndrome in Laying Hens: Field and Experimental Investigations.http://espace.library.uq.edu.au/view/UQ:346438/s4185012_phd_submission.pdf [Google Scholar]
- Shini A.S.S.L. 2012. Role of Inflammation in the Pathogenesis of Fatty Liver Haemorrhagic Syndrome in Laying Hens. 23rd Annual Australian Poultry Science Symposium. Sydney, Australia. [Google Scholar]
- Shini A., Shini S., Bryden W.L. Fatty liver haemorrhagic syndrome occurrence in laying hens: impact of production system. Avian Pathol. 2019;48:25–34. doi: 10.1080/03079457.2018.1538550. [DOI] [PubMed] [Google Scholar]
- Spurlock M.E., Savage J.E. Effect of dietary protein and selected antioxidants on fatty liver hemorrhagic syndrome induced in Japanese quail. Poult. Sci. 1993;72:2095–2105. doi: 10.3382/ps.0722095. [DOI] [PubMed] [Google Scholar]
- Squires E.J., Wu J. Enhanced induction of hepatic lipid peroxidation by ferric nitrilotriacetate in chickens susceptible to fatty liver rupture. Br. Poult. Sci. 1992;33:329–337. doi: 10.1080/00071669208417471. [DOI] [PubMed] [Google Scholar]
- Squires E.J., Leeson S. Aetiology of fatty liver syndrome in laying hens. Br. Vet. J. 1988;144:602–609. doi: 10.1016/0007-1935(88)90031-0. [DOI] [PubMed] [Google Scholar]
- Trott K.A., Giannitti F., Rimoldi G., Hill A., Woods L., Barr B., Anderson M., Mete A. Fatty liver hemorrhagic syndrome in the backyard chicken: a retrospective histopathologic case series. Vet. Pathol. 2014;51:787–795. doi: 10.1177/0300985813503569. [DOI] [PubMed] [Google Scholar]
- Walzem R.L., Simon C., Morishita T., Lowenstine L., Hansen R.J. Fatty liver hemorrhagic syndrome in hens overfed a purified diet. Selected enzyme activities and liver histology in relation to liver hemorrhage and reproductive performance. Poult. Sci. 1993;72:1479–1491. doi: 10.3382/ps.0721479. [DOI] [PubMed] [Google Scholar]
- Yang F., Ruan J., Wang T., Luo J., Cao H., Song Y., Huang J., Hu G. Improving effect of dietary soybean phospholipids supplement on hepatic and serum indexes relevant to fatty liver hemorrhagic syndrome in laying hens. Anim. Sci. J. 2017;88:1860–1869. doi: 10.1111/asj.12832. [DOI] [PubMed] [Google Scholar]
- Yeh E., Wood R.D., Leeson S., Squires E.J. Effect of dietary omega-3 and omega-6 fatty acids on clotting activities of Factor V, VII and X in fatty liver haemorrhagic syndrome-susceptible laying hens. Br. Poult. Sci. 2009;50:382–392. doi: 10.1080/00071660902942767. [DOI] [PubMed] [Google Scholar]
- Zhang N., Li Z., Xu K., Wang Y., Wang Z. Resveratrol protects against high-fat diet induced renal pathological damage and cell Senescence by activating SIRT1. Biol. Pharm. Bull. 2016;39:1448–1454. doi: 10.1248/bpb.b16-00085. [DOI] [PubMed] [Google Scholar]
- Zhang W., Guo C., Gao R., Ge M., Zhu Y., Zhang Z. 2013. The protective role of resveratrol against Arsenic Trioxide-induced Cardiotoxicity. Evid. Based Complement Alternat. Med. 2013:407839. doi: 10.1155/2013/407839. [DOI] [PMC free article] [PubMed] [Google Scholar]