Abstract
Guduchi (Tinospora cordifolia) is a well-recognized and widely distributed traditional plant that is used successfully in Indian Ayurveda medicine. T. cordifolia has shown many promising biological activities, such as antioxidative, antimicrobial, antihyperglycemic, anti-inflammatory, osteoprotective, hepatoprotective, antidiarrheal, and antistress effects. Guduchi is a rich source of protein and micronutrients, such as iron, zinc, copper, calcium, phosphorus, and manganese. It also contains many secondary plant metabolites, such as terpenes, alkaloids, flavonoids, steroids, and glycosides. Based on previous studies in poultry, the supplementation levels of Guduchi range from 1 to 5 g/kg of diet (different sources, such as powder, extracts, roots, and leaves, have been used). It was suggested that this variation in supplementation levels depends on different factors, including the extraction method, the supplementation proposed, the method of supplementation (either in feed or drinking water), and the species and physiological status of the birds. Generally, dietary supplementation of poultry broilers with T. cordifolia yielded positive impacts on growth performance, body gains (increased by 4.8%), dressing percentage (increased by 7.1%), meat quality traits, and the shelf life of the meat. In addition, T. cordifolia exerted a palliative effect on the general health status of the birds through reducing live enzymes and plasma uric acids and enhancing the immune response, as indicated by the leukocyte count, hemagglutinin titer, interleukin activity, and mortality levels. Further investigations concluded that T. cordifolia showed strong antimicrobial effects against Escherichia coli and Salmonella enteritidis, with subsequent reductions in mortality. Moreover, T. cordifolia showed an ability to improve humoral and cell-mediated immunity against Newcastle disease, infectious anemia, gout, and aflatoxicosis. The current review discusses many beneficial properties of T. cordifolia, although the lack of pharmacological trials limits the use of this extract in poultry. Further research should be performed regarding the composition of the active compound, the possible mechanisms of action, and the effective doses to fully understand the activities and benefits of T. cordifolia as a growth performance improvement supplement.
Key words: Guduchi, Tinospora cordifolia, clinical pharmacology, feed supplement, poultry
Introduction
In the past, antibiotic growth promoters (ABGPs) have been extensively used to achieve improved animal growth, but since antibiotic resistance has become a significant issue, complete bans or limited use have been implemented in many countries. Therefore, different measures and strategies have been implemented to avoid the use of ABGP-like nutrients by using hygienic antibiotics and applying different microbe-blocking drugs (Baker-Austin et al., 2006, Alagawany et al., 2015, Saeed et al., 2017). Owing to the need to overcome some of the drawbacks of ABGP bans, interest in phytogenic compounds from different plants is growing because there is strong evidence for the beneficial effects of dietary supplementation, such as improved feed conversion ratios (FCRs) and enhanced growth performance (Babazadeh et al., 2011, Waseem Mirza et al., 2016). Medicinal plants and their extracts are also interesting in the context of poultry production as they promote the growth performance, immune response, and general health status of animals (Guilhelmelli et al., 2013). Many different plants have been proven to be safe and inexpensive substitutes for ABGPs, with powerful therapeutic and prophylactic effects against different microorganisms (Dhama et al., 2014, Saeed et al., 2018).
Guduchi (Tinospora cordifolia) is an herbal plant and has promising properties as an auspicious plant growth promoter (Sharma et al., 2013), with potential benefits in poultry. T. cordifolia is accepted as a safe feed supplement because it does not affect DNA integrity, blood lymphocytes, or the bone marrow (Chandrasekaran et al., 2009). For centuries, T. cordifolia has been well established as a successful medication for the treatment of metabolic disorders and diabetes in Indian Ayurvedic medicine (Nadkarni, 1976). T. cordifolia contains various phenolics, steroids, alkaloids, glycosides, diterpenoid lactones, aliphatic compounds, and polysaccharides (Raina et al., 2013). It is also rich in phosphorus and proteins (Khosa and Prasad, 1971). Moreover, T. cordifolia plants and their extracts exert many favorable effects, such as antihepatotoxic (Peer and Sharma 1989), immunomodulatory (Atal et al., 1986), antipyretic (Vedavathy and Rao, 1991), antidiabetic (Wadood et al., 1992), and antiulcer activity (Sarma et al., 1996). Furthermore, T. cordifolia can enhance insulin secretion from pancreatic β-cells in different species (Marles and Farnsworth, 1995), and it contributes to the modulation of lipid- and carbohydrate-related mechanisms in response to excessive doses of fructose. This plant also has positive effects on lipid metabolism, as indicated by high-density lipoprotein, low-density lipoprotein, and very-low-density lipoprotein, in addition to improving hepatic functions and oxidative stress biomarkers and increasing antioxidant enzymes (namely, superoxide dismutase [SOD] and glutathione peroxidase) in different animal models (Shirolkar et al., 2016, Chavan et al., 2017). T. cordifolia could positively affect the lifespan of Drosophila melanogaster in the F1 generation (Pathak et al., 2016). Except for some firm conclusions regarding the positive effects of T. cordifolia against E. coli in vitro, there is a significant gap in our knowledge concerning the beneficial impacts of T. cordifolia in the poultry industry. Therefore, with the current review, we encourage researchers to investigate the effects of T. cordifolia as a natural growth promoter in poultry, improve our pharmacological knowledge, and implement this approach in practice.
Botanical description
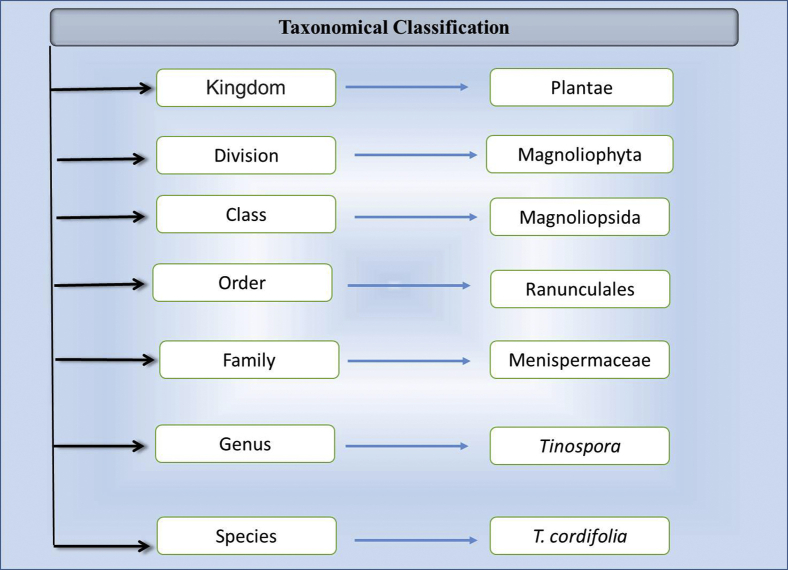
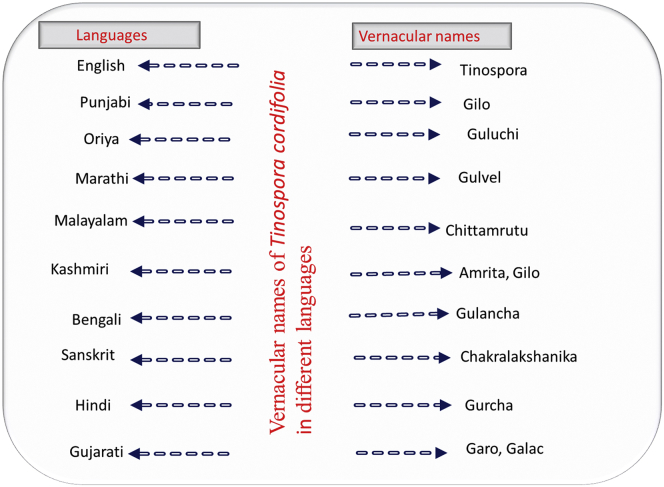
T. cordifolia is a large climbing shrub with many elongated branches. Its leaves are alternate, simple, pulvinate, and roundish, and they have long petioles up to 15-cm long. The lamina is broadly ovate, 7 nerved and deeply cordate at the base, 8- to 15-cm broad, 10- to 20-cm long, and membranous, and there is whitish tomentose with a less prominent reticulum and an upper pubescent region. The taxonomical classification of T. cordifolia is shown in Figure 1. The flowers are small, unisexual, and greenish-yellow on axillary and terminal racemes. Female flowers are usually solitary, but male flowers are clustered. Sepals are in 2 series of 3 each, and the inner sepals are more significant than the outer sepals. The 6 petals are obovate, membranous, and smaller than the sepals. The fruits are in aggregates of 1 to 3 and present as orange and ovoid smooth drupelets on thick stalks with subterminal style scars (Sinha et al., 2004). This plant is widely grown in various parts of the world and is known with different local names (Figure 2).
Figure 1.
Taxonomical classification of Tinospora cordifolia.
Figure 2.
Vernacular names of Guduchi (Tinospora cordifolia) in different languages.
Phytochemistry
Various constituents have been isolated and elucidated from T. cordifolia plants. These components belong to various classes, such as diterpenoid lactones, alkaloids, steroids, glycosides, polysaccharides, aliphatic compounds, phenolics, and sesquiterpenoids. The compounds isolated were reported by several workers (Kidwai et al., 1949, Rao and Rao, 1981, Sharma and Khosla, 1993, Chintalwar et al., 1999, Singh et al., 2003, Jagetia and Rao, 2006) and are listed in Table 1.
Table 1.
Chemical compositions of the Tinospora cordifolia herb.
| Type of chemical | Active principles and their distribution |
|---|---|
| Alkaloids | Tinosporin (L), tinosporic acid (L) (W), berberine (S), palmitine (S) (R), tembatarine (S) (R), mangoflorine (S) (R), choline (S) (R), tinosporin (S) (R), isocolumbin (R), tetrahydropalmatine (R). |
| Glycosides | 18 Nonderodane glycoside (S), furanoid diterpene glycoside (S), tinocordiside (S), tinocordifoliside (S), cordioside (S), cordifolioside A, B, C, D (S), syringin (S), syringinapiosylglycoside (S), palmatosides C and P (S), cordifoliside A, B, C, D, E (S). |
| Diterpenoid lactones | Diterpenoid (S), tinosporon columbin (S), clerodane derivatives (W), tinosporon (W), tinosporisides (W), jateorine (W), columbin (W), tinosporal, tinosporide. |
| Steroids | Sitosterol (S) (O), octacosanol (S), heptacosanol (S), nonacosan-15-one (S), tetrahydrofuran (S), hydroxyecdysone (S) (O), makisterone A (S), giloinsterol (S), ecdysterone (S) |
| Sesquiterpenoids | einocordifolin (S) |
| Miscellaneous compounds | Jatrorrhizine (R), tinosporidin (W), cordifol (W), cordifelone (W), giloin (W), giloinin (W), arabinogalactan (S) |
The letters in brackets indicate the part of the plant from which the chemical constituent has been isolated. S, stem; L, leaf; R, root; W, whole plant; O, other aerial parts.
Promising pharmacological activities of Guduchi (T. cordifolia)
T. cordifolia has many favorable properties in humans, for example, hepatoprotective, antioxidant, antihyperglycemic, antimicrobial, antihyperlipidemic, antipyretic, anti-inflammatory, heart-protective, neuroprotective, osteoprotective, analgesic, antianxiety, antistress, and antidiarrheal activities (Sinha et al., 2004, Dhama et al., 2017), as presented in Table 2 and Figure 3.
Table 2.
Some beneficial applications of the Tinospora cordifolia herb.
| Activities | Modes of action | References |
|---|---|---|
| Cardioprotective activity | Modulates lipid metabolism by inhibiting cholesterol and glucuronides | Kumari et al., 2016 |
| Immunomodulatory activity | Stimulation of a non-specific immune response | Alexander et al., 2010 |
| Hepatoprotective activity | Modulates the activity and synthesis of certain enzymes, including AGPT, AST, and ALP | Kavitha et al., 2011 |
| Neuroprotective activity | Increases the levels of pAkt-1 and CamKII-α | Mishra et al., 2016 |
| Anti-inflammatory activity | Downregulates proinflammatory cytokines | Sannegowda et al., 2015 |
| Antidiabetic activity | Lowers hepatic glucose-6-phosphatase, serum acid phosphatase, alkaline phosphatase, and lactate dehydrogenase activities | Stanely and Menon, 2003 |
| Hypolipidemic activity | Reduces the blood lipid concentration | Stanely et al., 1999 |
| Antitumor | Modulates lipid peroxidation through the release of LDH and a reduction in GST | Jagetia and Rao, 2006 |
Abbreviations: AGPT, agal gel precipitation test; ALP, alkaline phosphatase; AST, aspartate aminotransferase; GST, glutathione S-transferase; LDH, lactic acid dehydrogenase.
Figure 3.
An overview on vital biological effects of Tinospora cordifolia. Abbreviations: TCE, ethanolic extract of Tinospora cordifolia.
Immunomodulatory Activity
Little research has been performed on the immunomodulatory effects of T. cordifolia and its mechanisms of action. Arabinogalactan polysaccharide (G1-4A) is a compound found in T. cordifolia's stem; it has protective effects against lipopolysaccharide-induced endotoxic shock by modulating cytokines and nitric oxide excretion by murine macrophages (Desai et al., 2007). The immunomodulatory effect of T. cordifolia may be linked to different polysaccharides, such as arabinose, glucose, and fructose (Sharma et al., 2012a, Sharma et al., 2012b), and induces a nonspecific immune response (Alexander et al., 2010); however, the mechanism is poorly elucidated. Moreover, supplementation with T. cordifolia in mice leads to splenomegaly and an amplified presence of macrophages, T cells, and B cells, as well as increased expression of antiapoptotic genes in immune cells (Raghu et al., 2009).
Many active compounds, including those in T. cordifolia, such as N-methyl-2-pyrrolidone, N-formylannonain, 11-hydroxymustakone, cordifolioside A, tinocordiside, syringin, and magnoflorine (Sharma et al., 2012a, Sharma et al., 2012b), usually show practical immunomodulatory and cytotoxic effects (Kapil and Sharma, 1997, Tripathi et al., 1997, Subramanian et al., 2002). It was reported that such active components could function through the production of free radicals in human neutrophils and boost the phagocytic property of macrophages (More and Pai, 2012). In addition, these compounds can stimulate the production of nitric oxide from macrophages and splenocytes, which may explain their anticancer effect (Upadhyaya et al., 2011). Moreover, it was concluded that the aqueous extract of Tinospora exerts a favorable impact on the production of cytokines and immunity-enhancer cells (Upadhyaya et al., 2011). In mice, it has been reported that the extract of T. cordifolia can upregulate the cytokine IL-6, with subsequent events that include activation of the inflammatory response and cytotoxic T cells as well as differentiation of B cells (Sudhakaran et al., 2006). However, investigation of T. cordifolia in rats suggested that there is a cytotoxic effect of the active compounds, including compounds in aqueous extracts, such as alkaloids, glycosides di-terpenoid lactones, phenolics, steroids, aliphatic compounds, sesquiterpenoids, and polysaccharides (Jahfar, 2003). Another trial was conducted to investigate the immune-stimulatory effect of T. cordifolia dry crude extract (with a polyclonal B-cell mitogen) in mice; the results demonstrated the immune-stimulatory role of this extract via induction of IL-1 secretion and activation of macrophages (Raghu et al., 2009). In vitro, the (1,4)-alpha-d-glucan derived from T. cordifolia was able to activate human lymphocytes and downregulate the production of inflammatory mediators (Koppada et al., 2009).
Antioxidant Activity
Prince et al. (2004) evaluated the oxidative status of the heart, liver, kidney, and brain after using T. cordifolia extract; such extracts were concluded to be more effective than insulin and glibenclamide. Moreover, T. cordifolia has been used to attenuate ischemic brain damage via preventing ROS production, with consequent amelioration of oxidative stress–mediated cell injuries caused by oxygen/glucose deprivation through direct effects and modulation of gene expression (Jagetia and Baliga, 2004, Rawal et al., 2004). However, Rawal et al. (2004) concluded that the ameliorative effect of T. cordifolia against rat hippocampal slices exposed to low glucose and oxygen levels was due to its strong free radical-scavenging effect. Moreover, oral administration of T. cordifolia methanolic extract to alloxan-induced diabetic rats resulted in the upregulation of lipid peroxide and catalase activity on the erythrocyte membrane and downregulated SOD and GPx activity (Prince and Menon, 2001, Prince and Menon, 2003, Sivakumar and Rajan, 2010). In addition, extracts of T. cordifolia wild (Menispermaceae) exhibited an inhibitory effect against antioxidant agents and aldose reductase (Gacche and Dhole, 2011), with a subsequent reduction in free radical–induced chemotoxicity (Rawal et al., 2004). In addition, extracts of T. cordifolia have been reported to have potent free radical–scavenging activity against hydroxyl radicals (OH), superoxide anion (O2−), peroxynitrite anion (ONOO−), and NO radicals (Rawal et al., 2004). They also reduce the levels of malondialdehyde and ROS and increase glutathione (GSH) and total thiol levels (Shivananjappa and Muralidhara, 2012). On the other hand, T. cordifolia extracts have been demonstrated to play an antioxidant ameliorative role against aflatoxin-induced nephrotoxicity, which may be attributed to their contents of alkaloids, such as tinosporin, choline, palmatine, isocolumbin, magnoflorine, and tetrahydropalmatine (Gupta and Sharma, 2011). In diabetic rats, the tissue content of thiobarbituric acid reactive substance (TBARS) was increased significantly in the brain but decreased in cardiac tissues. In addition, the hepatic production of SOD, GPx, and GSH was enhanced after T. cordifolia treatment (Prince et al., 2004). The arabinogalactan polysaccharide derived from T. cordifolia possessed an acceptable protective property against iron-induced lipid peroxidation in brain homogenates from rats (Subramanian et al., 2002). The hydroalcoholic extract of T. cordifolia aerial roots has been reported to exert potent beneficial effects on the liver contents of antioxidant enzymes, GSH, and lipid peroxidation in Swiss albino mice (Singh et al., 2006). The leaf extract of T. cordifolia has been concluded to have potent antioxidant and ROS-scavenging activities that may be attributed to its content of alpha-glucosidase inhibitor (saponarin) (Sengupta et al., 2009). During aflatoxicosis, it has been reported that treatment with T. cordifolia extract, which is able to scavenge ROS, downregulates TBARS levels and stimulates the activity of SOD, GSH, catalase, glutathione S-transferase, GPx, and glutathione reductase in the kidney (Gupta and Sharma, 2011).
Oral administration of T. cordifolia root extracts can restore antioxidant markers, such as GPx, SOD, and GSH (Patel and Mishra, 2011). T. cordifolia extracts have been found to regulate the oxidative stress induced in the maternal liver in diabetic animals via decreased levels of malondialdehyde and ROS and increased GSH levels (Shivananjappa and Muralidhara, 2012).
Anti-Inflammatory and Antipyretic Activity
Fever, inflammation, and pain are traditionally treated with T. cordifolia preparations (Ashok et al., 2011, Hussain et al., 2015). These preparations can reduce carrageenan-induced edema, indicating their anti-inflammatory potential (Biswajyoti et al., 2014). Anti-inflammatory effects of T. cordifolia were also detected in autoimmune arthritis mediated by decreased synthesis of proinflammatory cytokines, for example, IL-1β, IL-17, tumor necrosis factor-α, and IL-17 (Sannegowda et al., 2015). Some data also suggest that T. cordifolia can mediate the peripheral and central nervous system mechanisms and exert analgesic effects (Goel et al., 2014). In addition, T. cordifolia has antipyretic effects against fever (Pushpangadan et al., 2006).
The anticancer drug cyclophosphamide has been concluded to downregulate the cytokines IL-2 and interferon-γ and to upregulate the proinflammatory cytokine TNF-α; such effects could be successfully reversed via treatment with T. cordifolia (Hamsa and Kuttan, 2012).
Hepatoprotective Activity
T. cordifolia was reported to be an efficient hepatoprotective agent because of its ability to scavenge free ROS, which enhances its hepatic regeneration effect (Kumar et al., 2013). Currently, several polyherbal preparations have been marketed for the treatment of different liver diseases, many of which contain T. cordifolia. In addition, T. cordifolia root extracts have shown hepatoprotective properties against rifampicin- and pyrazinamide-induced liver injuries (Adhvaryu et al., 2007). T. cordifolia also showed ameliorative effects against carbon tetrachloride (CCL4)-induced liver damage via reducing expression of the liver enzymes aspartate aminotransferase (AST), alkaline phosphatase (ALP), and alanine aminotransferase (ALT), as well as total bilirubin (Kavitha et al., 2011), preventing fibrous proliferation and activating tissue regeneration (Rege et al., 1984). Similarly, Sharma and Pandey (2010b) reported the ameliorative effect of T. cordifolia against lead-induced liver damage. In another study, morphological, biochemical, and functional hepatic changes in albino rats intoxicated with CCL4 were investigated with concurrent treatment with chloroform extract from T. cordifolia at a dose of 200 mg/kg. Treatment resulted in a decrease in liver weight; however, it failed to affect the elevated levels of serum ALT, AST, ALP, and serum bilirubin (Reddy et al., 1993). Moreover, HPN-12, an Ayurvedic preparation containing T. cordifolia, was found to have ameliorative effects against CCL4-induced liver injury in male Sprague Dawley albino rats (Latha et al., 1999).
Leaf and stem aqueous extracts of T. cordifolia have significant hepatoprotective effects against lead nitrate intoxication in male Swiss albino mice (Sharma and Pandey, 2010a, Sharma and Pandey, 2010b). The results revealed that lead nitrate toxicity caused the downregulation of SOD and catalase levels and the upregulation of ALT, AST, and ALP levels (Sharma and Pandey, 2010b). However, the concurrent administration of stem and leaf aqueous extract of T. cordifolia improved such parameters.
Neuroprotective and Antistress Activity
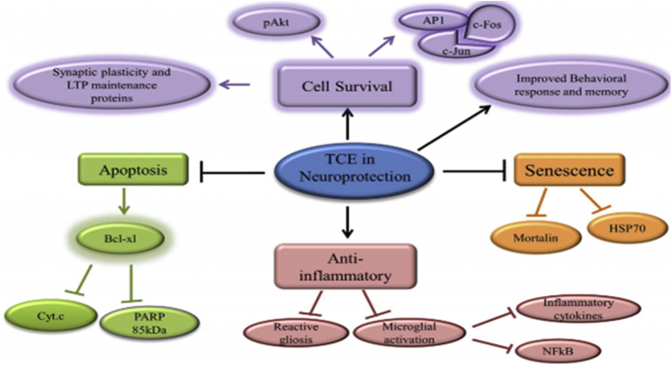
Little is known about the ability of T. cordifolia to improve mental skills and memory in subjects with mental deficits and behavioral disorders (Phukan et al., 2015). Moreover, T. cordifolia has beneficial properties in patients with depression (Walia, 2015), senile and presenile dementia, and impaired memory (Pushpangadan et al., 2010). Mishra et al. (2016) observed that T. cordifolia ethanolic extract (TCE) increased the levels of CamKII-α and pAkt-1 in sleep-deprived animals, proving its neuroprotective effects, possibly due to the inhibition of changes in 2 discrete brain regions. Thus, the TCE seems to inhibit stress-induced changes in 2 discrete brain regions and inhibit the activation of apoptotic pathways by inducing cell survival (the schematic diagram in Figure 4 presents the possible molecular pathways associated with the TCE in sleep-deprived animals). Moreover, the antistress properties of T. cordifolia ethanol extract (100 mg/kg) were reported to be comparable to those of diazepam (2.5 mg/kg) (Sarma et al., 1996).
Figure 4.
Schematic figure depicting a possible molecular pathway in the neuroprotective activity of ethanolic extract of Tinospora cordifolia (Mishra et al., 2016).
Cardioprotective and Renoprotective Activity
Berberine is an alkaloid found in T. cordifolia. It can decrease endothelial inflammation, with subsequent cardioprotective effects (Cicero and Baggioni, 2016). T. cordifolia supplementation also modulates lipid metabolism via inhibiting glucuronide and cholesterol (Kumari et al., 2016) and protecting against infarction due to its antioxidant properties (Rao et al., 2005), such as peroxide levels in heart tissue and serum (Mary et al., 2003, Rao et al., 2005). In addition, the water extract of T. cordifolia produced a marked transient decrease in blood pressure, along with diuresis in rats and stronger ventricular contraction in dogs (Singh et al., 1975). Moreover, it was concluded that the water extract of T. cordifolia was able to dissolve urinary calculi (Rai and Gupta, 1967) and downregulate the levels of urea in the blood of uremic dogs and patients (Singh et al., 1975).
Antiulcerative, Digestive, and Hypolipidemic Activity
Compared with diazepam, the TCE exhibited significant antiulcerative effects under stress conditions (Sarma et al., 1995). Moreover, the antiamoebic impact of T. cordifolia against Entamoeba histolytica was investigated; the results revealed various degrees of inhibition of different digestion-related enzymes, such as DNase, RNase, ALP, aldolase, α-amylase, acid phosphatase, and protease activities (Sohni et al., 1995). In addition, the hypolipidemic effect of T. cordifolia aqueous extract (2.5 and 5.0 g/kg BW) was evaluated in alloxan diabetic rats after administration for 6 wk, and the results revealed significant decreases in serum and tissue cholesterol, free fatty acids, and phospholipids. Compared with glibenclamide, the most potent hypolipidemic effect of the aqueous extract is achieved at a dose of 5.0 g/kg body weight (Prince et al., 1999).
Effects of T. cordifolia in poultry farming
Recently, many researchers reported the favorable impacts of T. cordifolia on poultry health and production parameters. The results of those studies showed that T. cordifolia has a positive effect on poultry growth performance (Bhardwaj et al., 2011, Singh et al., 2014) via improving the FCR, antioxidant activity, and immunomodulatory properties of T. cordifolia. Oral administration of alcoholic T. cordifolia (100 mg/kg body weight) induced a significant elevation in TBARS in the liver (0.78 ± 0.03) and kidney (0.42 ± 0.06) compared with normal rates (0.78 ± 0.06 and 0.41 ± 0.08, respectively). There was also a significant decrease in liver GSH (50.8 ± 2.4) compared with normal levels (51.4 ± 3.9) (Prince et al., 2004); in addition, the mean body weight gain of treated birds was increased significantly (P = 0.05) after 6 wk of age compared with that of control birds (1,165 g vs. 1,148 g, respectively), and the mean FCR of treated birds was significantly (P = 0.05) lower than that of control birds (1.45 vs. 1.54, respectively) (Singh et al., 2009).
Preventing colibacillosis caused by different serotypes of E. coli is a challenging task because these organisms are resistant to different antimicrobials (Rahman et al., 2004, Li et al., 2007). Therefore, much effort has been made to investigate natural antimicrobials. In addition, T. cordifolia extract exerted antibacterial activity against E. coli in vitro studies; the maximum activity of the extract was observed at a 1:32 dilution, and the minimum inhibitory concentration was reported at a 1:64 dilution. All the dilutions showed significantly fewer colony-forming units than the positive control. However, the lowest count was observed at a 1:32 dilution of 15% extract, with a maximum percent inhibition of bacterial growth of 53.59% compared with all other dilutions (1:2 to 1:128) (Mam and Jakhar, 2016). Furthermore, Baishya et al. (2008) observed a significant reduction in the mortality rate of broilers infected with Salmonella enteritidis when they were supplemented with a polyherbal extract containing T. cordifolia at 5 wk of age; the mean bodyweight of the prophylactically treated birds was significantly higher (1,350 ± 1.78 g) than that of the infected birds (1,000 ± 2.04 g), while that of noninfected, nonmedicated birds was 1,470 ± 1.51 g. High mortality of up to 32% was observed in the infected untreated group; however, this value was 4 and 10% in groups I and III, respectively. The effect of T. cordifolia might be due to its antimicrobial, hepatoprotective, and immunomodulatory effects (Kolte et al., 2007). T. cordifolia methanolic extract increased avian T- and B-cell proliferation, showing a potential immunomodulatory effect in poultry (Sonu et al., 2013). Rajkumar et al. (2009) showed that supplementation of the broiler diet (1%) with T. cordifolia resulted in increased humoral and cell-mediated immunity against Newcastle disease. Furthermore, Chaudhari et al. (2009) concluded that an aqueous extract of T. cordifolia showed better efficacy against gout in broilers as an alcoholic extract, suggesting the water solubility of active substances in T. cordifolia. Similarly, Alexander et al. (2010) reported the immune-stimulatory properties of the water-soluble fraction of the T. cordifolia leaf fraction in fish.
T. cordifolia is known to enhance general immunity, prevent oxidative stress and different diseases, increase leukocyte counts, and decrease neutropenia in rats (Bishayi et al., 2002). Lakra (2008) showed that dietary supplementation with T. cordifolia significantly improved humoral and cellular immunity in broilers, and the maximum antibody titer was observed in groups fed with T. cordifolia. The increase in antibody titer in the treated group might be due to the effect of IL-1, which stimulates B-cell proliferation and immunoglobulin secretion. Similarly, Dhote et al. (2005) reported improved leukocyte counts and IL-1 and IL-2 levels in animals fed with an herbal preparation containing T. cordifolia. Latheef et al. (2013) conducted a study to assess the immunomodulatory potential of T. cordifolia (1% pure extracts) against infectious anemia in chicks. The chickens showed improved humoral and cellular immune responses and reduced viral loads. Furthermore, Maryamma et al. (1990) conducted a study on ducks with aflatoxicosis, indicating a higher packed cell volume, hemoglobin concentration, and body weight gain in T. cordifolia–supplemented (100 g/L of drinking water) birds. Many studies have investigated the effects of T. cordifolia on broiler performance. Among those studies, Kulkarni et al. (2011) showed that dried T. cordifolia stem (0.1–0.3%) improved body weight and the FCR and reduced feed intake and drumstick and breast yields. On the other hand, T. cordifolia supplementation did not affect hemoglobin levels, the relative weights of immune organs, the cell-mediated immune response, or oxidative stress but positively affected the humoral immune response.
Bhardwaj et al. (2012) evaluated the impacts of aqueous extracts of T. cordifolia dried stem (1 g/kg feed) on the serum biochemical and immune responses of broiler chickens. Experimental results indicated that the hemagglutination antibody titers displayed an overall increasing trend in the treated groups with the growing age of the birds. According to later authors, T. cordifolia increased total lymphocyte (26.166 ± 0.307) and erythrocyte counts (261.830 ± 7.162) and hemoglobin levels (8.61 ± 0.113) vs. the control (23.667 ± 0.421, 219.333 ± 7.723, and 8.12 ± 0.108, respectively). As studied by Sharma et al. (2008), the positive effects of dietary T. cordifolia on weight gain led to a maximum final bodyweight of 1,614 g, which was higher than that of the untreated control group (1,492 g), after 6 wk, a lower FCR (1.86) than that of the control (2.03), and a lower mortality rate in treated groups (4.2%) than that in the untreated control (8.8%). Joshi et al. (2015) studied the influences of supplementation with T. cordifolia stem powder on broiler growth performance. In a completely randomized design with 3 treatments, 135 chicken broilers were fed a 0, 1, or 2 g/kg basal diet for 6 wk. The final body weight was improved (4.8%) by T. cordifolia supplementation (1 g/kg feed) in comparison to the control, and a lower mortality rate was recorded after dietary administration (2.2 vs. 6.7 in the treated and control groups, respectively). The improvement in body weight and the reduction of the mortality rate may be due to the presence of some constituents in T. cordifolia that alleviate the physiological consequences of environmental stressors (Joshi et al., 2015).
Latheef et al. (2017) estimated the immunomodulatory effects of pure extracts of T. cordifolia (with active tinosporins at 1.5% as a powder form) in immunosuppressed chickens with chicken infectious anemia virus. T. cordifolia–supplemented birds showed reduced viral loads and increased CD4+ T-cell counts (1,296 ± 8.7) in comparison to those of the infected control (984.3 ± 11.3). Similarly, Nety et al. (2017) found that supplementation with a dried stem powder of T. cordifolia in the amount of 0.4 g/L of drinking water significantly (P < 0.05) increased the levels of hemagglutinin titer values (6.03) compared with those of the control, BMD antibiotic, and dried leaf powder from Azadirachta indica groups (3.33, 3.87, and 4.12, respectively). In addition, the cell-mediated immune responses of the T. cordifolia group were 3.35 vs. 2.12 and 3.82 vs. 2.63 in comparison to those of the control group at 24 and 48 h, respectively. The supplementation of T. cordifolia may enhance immune function in the birds by increasing the thickness of the abdominal skin at both 24 and 48 h after challenge (Nety et al., 2017).
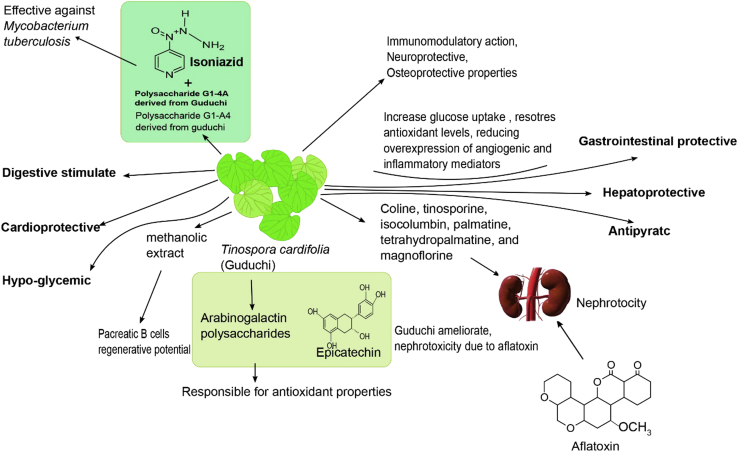
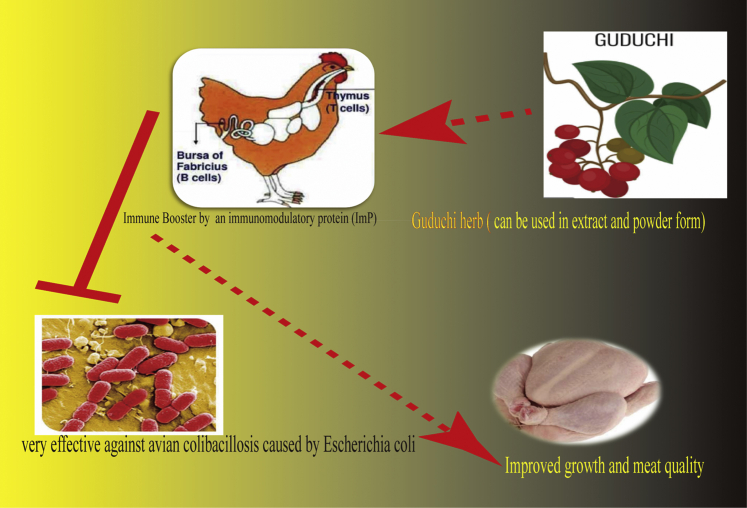
Sharma et al. (2008) reported that broilers fed with herbal liver stimulants (250 g/ton) containing T. cordifolia for 6 wk exhibited a significantly (P < 0.05) improved dressing percentage (7.1%) and eviscerated weight percentage (5.56%), and meat color scores (5.56 vs. 5.63) and meat pH (5.7 vs. 5.84) were significantly reduced compared with those of the control. This result proved that T. cordifolia significantly prevented color loss in the meat, thus improving meat quality, and suggested that some of the constituent herbs successfully inhibited lipid peroxidation of the meat, leading to better quality and enhancing the shelf life of the meat. Dietary T. cordifolia was reported to strengthen the activity of the heart and liver and protect the cells against inflammation, enlargement, and injury. The effects of the herbal immunomodulatory liquid and powder form on sensory qualities of Giriraja chickens were compared with those of antibiotics (levamisole powder) for 8 wk. Dharmaraj et al. (2017a) found that T. cordifolia can affect sensory qualities, including flavor (4.65), taste (4.10), and juiciness (3.95), in free-range Giriraja chickens vs. the control (3.25, 3.70, and 3.65, respectively). However, the dressing percentage, breast, and thigh muscle ratio, and relative visceral organs were not significantly improved (Dharmaraj et al., 2017b). Recently, Sharma et al. (2018) investigated the influences of T. cordifolia (0.5% leaf meal powder) to identify suitable alternatives to antibiotics in diet turkeys for 8 wk. Increased body weight gain was observed from 5 to 8 wk of age, and no differences were found in the feed conversion rate between groups. In addition, plasma uric acid and ALP in the turkeys were significantly reduced (P < 0.05) by 36.6% and 3.5% for the T. cordifolia supplementation and control groups, respectively. The proposed effects of this herb in avian production are shown in Figure 5.
Figure 5.
The figure showing the promising effects of Guduchi in poultry.
Toxicological effects
Until now, no study has reported the toxicological effects of T. cordifolia, even being tested in a very high dose (900 mg/D) in allergic (Badar et al., 2005) and HIV individuals (Kalikar et al., 2008). Similarly, in animal models, no adverse effects were recorded in the renal system (Nayampalli et al., 1988), gastrointestinal system (Sheth et al., 2001), and central nervous system (Agarwal et al., 2002) when Guduchi is used for 2–4 wk. Further studies should be warranted using specially designed projects to explore the long-term supplemental effects of T. cordifolia in various animal models, including chicken.
Conclusion
Guduchi (T. cordifolia) is a well-known plant, especially in traditional medicine, and is one of the most commercially exploited plants in the pharmaceutical industry. It has many beneficial effects, such as antioxidant, hepatoprotective, antimicrobial, antihyperglycemic, antipyretic, antihyperlipidemic, cardiovascular-protective, anti-inflammatory, osteoprotective, neuroprotective, antianxiety, analgesic, antidiarrheal, and antistress effects. In poultry, T. cordifolia can be used as a potent immunomodulator and an active antimicrobial agent against colibacillosis or S. enteritidis. It could be used as 1% extract or dried powder for inclusion in broilers' diet. In conclusion, there is a severe gap in our knowledge concerning the use of T. cordifolia as a successful supplement, particularly concerning its mechanisms of action and sufficient concentrations, of which almost no investigations have been performed. For practical use of T. cordifolia in poultry rearing, further research is strongly suggested, especially with extracted substances to confirm the effectiveness of pure compounds.
Acknowledgments
This work was supported by the National Nature Science Foundation of China (# 31572365) and the Key Sci-Tech Innovation Team of Shaanxi Province (# 2017KCT-24) P.R. China. The authors also thanks Department of Poultry Science, Cholistan University of Veterinary and Animal Sciences, Bahawalpur 63100, Pakistan.
References
- Adhvaryu M.R., Reddy N., Parabia M.H. Effects of four Indian medicinal herbs on isoniazid-, rifampicin-and pyrazinamide-induced hepatic injury and immunosuppression in Guinea pigs. World J. Gastroenterol. 2007;13:3199–3205. doi: 10.3748/wjg.v13.i23.3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal A.S., Bairy M.K.L., Rao M.S. Effect of Tinospora cardifolia on learning and memory in normal and memory deficit rats. Indian J. Pharmacol. 2002;34:339–349. [Google Scholar]
- Alagawany M.M., Farag M.R., Dhama K. Nutritional and biological effects of turmeric (Curcuma longa) supplementation on performance, serum biochemical parameters and oxidative status of broiler chicks exposed to endosulfan in the diets. AJAVA. 2015;10:86–96. [Google Scholar]
- Alexander C.P., Kirubakaran C.J.W., Michael R.D. Water soluble fraction of TC leaves enhanced the non-specific immune mechanisms and disease resistance in Oreochromis mossambicus. Fish Shellfish Immunol. 2010;29:765–772. doi: 10.1016/j.fsi.2010.07.003. [DOI] [PubMed] [Google Scholar]
- Ashok B.K., Ravishankar B., Prajapati P.K., Bhat S.D. Antipyretic activity of Guduchi Ghrita formulations in albino rats. Ayu. 2011;31:331–367. doi: 10.4103/0974-8520.77162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atal C.K., Sharma M.L., Kaul A., Khajuria A. Immunomodulating agents of plant origin. I: preliminary screening. J. Ethnopharmacol. 1986;18:133–141. doi: 10.1016/0378-8741(86)90025-5. [DOI] [PubMed] [Google Scholar]
- Babazadeh D., Vahdatpour T., Nikpiran H., Jafargholipour M.A., Vahdatpour S. Effects of probiotic, prebiotic and synbiotic intake on blood enzymes and performance of Japanese quails (Coturnix japonica) Indian J. Anim. Sci. 2011;81:870–874. [Google Scholar]
- Badar V.A., Thawani V.R., Wakode P.T., Shrivastava M.P., Gharpure K.J., Hingorani L.L., Khiyani R.M. Efficacy of Tinospora cordifolia in allergic rhinitis. J. Ethnopharmacol. 2005;96:445–449. doi: 10.1016/j.jep.2004.09.034. [DOI] [PubMed] [Google Scholar]
- Baishya K.K., Maini S., Ravikanth K. A polyherbal formulation to control bacterial enteritis in poultry: a case report in Salmonella enteritidis induced experimental model. Internet J. Vet. Med. 2008;5 [Google Scholar]
- Baker-Austin C., Wright M.S., Stepanauskas R., Mcarthur J. Co-selection of antibiotic and metal resistance. Trends Microbiol. 2006;14:176–182. doi: 10.1016/j.tim.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Bhardwaj U., Tiwary B., Prasad A., Ganguly S. Use of Tinospora cordifolia as poultry feed supplement. DHR. 2011;1:18–22. [Google Scholar]
- Bhardwaj U., Tiwary B., Prasad A., Ganguly S. Effect of Tinospora cordifolia extract on immune response and serum biochemical profile in broilers. Indian J. Anim. Sci. 2012;82:379–381. [Google Scholar]
- Bishayi B., Roychowdhury S., Ghosh S., Sengupta M. Hepatoprotective and immunomodulatory properties of Tinospora cordifolia in CCL4 intoxicated mature albino rats. J. Toxicol. Sci. 2002;27:139–146. doi: 10.2131/jts.27.139. [DOI] [PubMed] [Google Scholar]
- Biswajyoti P., Umretia B.L., Vaishnav P.U., Kumar P.P., Shukla V.J., Ravishankar B. Anti-inflammatory activity ofguduchi ghana (aqueous extract of tinospora cordifoliamiers) Ayu. 2014;35:108–110. doi: 10.4103/0974-8520.141958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekaran C., Mathuram L., Daivasigamani P., Bhatnagar U. Tinospora cordifolia, a safety evaluation. Toxicol. Vitro. 2009;23:1220–1226. doi: 10.1016/j.tiv.2009.07.030. [DOI] [PubMed] [Google Scholar]
- Chaudhari G.H., Shitut M.R., Deshmukh P.V., Gatne M.M. Evaluation of anti-gout activity of acqueous and alcoholic extract of Tinospora cordifolia in diclofenac induced gout in chicken. J. Bombay Vet. Coll. 2009;17:39–42. [Google Scholar]
- Chavan T., Ghadge A., Karandikar M., Pandit V., Ranjekar P., Kulkarni O., Kuvalekar A., Mantri N. Hepatoprotective activity of satwa, an ayurvedic formulation, against alcohol-induced liver injury in rats. Altern. Ther. Health Med. 2017;23:34–40. [PubMed] [Google Scholar]
- Chintalwar G., Jain A., Sipahimalani A., Banerji A., Sumariwalla P., Ramakrishnan R., Sainis K. An immunologically active arabinogalactan from Tinospora cordifolia. Phytochemistry. 1999;52:1089–1093. doi: 10.1016/s0031-9422(99)00386-6. [DOI] [PubMed] [Google Scholar]
- Cicero A.F., Baggioni A. Berberine and its role in chronic disease. Adv. Exp. Med. Biol. 2016;928:27–45. doi: 10.1007/978-3-319-41334-1_2. [DOI] [PubMed] [Google Scholar]
- Desai V.R., Ramkrishnan R., Chintalwar G.J., Sainis K.B. G1-4a, an immunomodulatory polysaccharide from tinospora cordifolia, modulates macrophage responses and protects mice against lipopolysaccharide induced endotoxic shock. Int. Immunopharmacol. 2007;7:1375–1386. doi: 10.1016/j.intimp.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Dhama K., Sachan S., Khandia R., Munjal A., Hmn I., Latheef S.K., Karthik K., Samad H.A., Tiwari R., Dadar M. Medicinal and beneficial health applications of Tinospora cordifolia (guduchi): a miraculous herb countering various diseases/disorders and its immunomodulatory effects. Recent Pat. Endocr. Metab. Immune Drug Discov. 2017;10:96. doi: 10.2174/1872214811666170301105101. [DOI] [PubMed] [Google Scholar]
- Dhama K., Tiwari R., Chakraborty S., Saminathan M., Kumar A., Karthik K., Wani M.Y., Singh S.V.A., Rahal A. Evidence based antibacterial potentials of medicinal plants and herbs countering bacterial pathogens especially in the era of emerging drug resistance: an integrated update. Int. J. Pharmacol. 2014;10:1–43. [Google Scholar]
- Dharmaraj G.Y., Indresh H.C.J., Prabhu T.M., Munegowda T. Effect of herbal immunomodulator on sensory evaluation in Giriraja birds. J. Entomol. Zool. Stud. 2017;5:1603–1605. [Google Scholar]
- Dharmaraj G.Y., Indresh H.C.J., Munegowda T. Effect of herbal immunomodulator on dressing percentage and carcass characteristics of Giriraja birds. Int. J. Cur. Microbiol. App. Sci. 2017;6:1436–1441. [Google Scholar]
- Dhote B.S., Singh G.K., Chauhan R.S. Effect of immuplus (a herbal immunomodulator) on paraspecific immune responses in chicks. ISAH. 2005;2:60–65. [Google Scholar]
- Gacche R.N., Dhole N.A. Profile of aldose reductase inhibition, anti-cataract and free radical scavenging activity of selected medicinal plants: an attempt to standardize the botanicals for amelioration of diabetes complications. Food Chem. Toxicol. 2011;49:1806–1813. doi: 10.1016/j.fct.2011.04.032. [DOI] [PubMed] [Google Scholar]
- Goel B., Pathak N., Nim D.K., Singh S.K., Dixit R.K., Chaurasia R. Clinical evaluation of analgesic activity of Guduchi (Tinospora cordifolia) using animal model. J. Clin. Diag. Res. 2014;8:01–04. doi: 10.7860/JCDR/2014/9207.4671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilhelmelli F., Vilela N., Albuquerque P., Derengowski L. da S., Silva-pereirai I., Kyaw C.M. Antibiotic development challenges: the various mechanisms of action of antimicrobial peptides and of bacterial resistance. Front. Microbiol. 2013;4:353. doi: 10.3389/fmicb.2013.00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R., Sharma V. Ameliorative effects of Tinospora cordifolia root extract on histopathological and biochemical changes induced by aflatoxin-b (1) in mice kidney. Toxicol. Int. 2011;18:94–98. doi: 10.4103/0971-6580.84259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamsa T.P., Kuttan G. Tinospora cordifolia ameliorates urotoxic effect of cyclophosphamide by modulating GSH and cytokine levels. Exp. Toxicol. Pathol. 2012;64:307–314. doi: 10.1016/j.etp.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Hussain L., Akash M.S., Ain N.U., Rehman K., Ibrahim M. The analgesic, anti-inflammatory and anti-pyretic activities of tinospora cordifolia. Adv. Clin. Exp. Med. 2015;24:957–964. doi: 10.17219/acem/27909. [DOI] [PubMed] [Google Scholar]
- Jagetia G.C., Baliga M.S. The evaluation of nitric oxide scavenging activity of certain indian medicinal plants in vitro: a preliminary study. J. Medic. Food. 2004;7:343–348. doi: 10.1089/jmf.2004.7.343. [DOI] [PubMed] [Google Scholar]
- Jagetia G.C., Rao S.K. Evaluation of cytotoxic effects of dichloromethane extract of Guduchi (Tinospora cordifolia Miers ex hook F & THOMS) on cultured HeLa cells. Evid. Based Complement. Alternat. Med. 2006;3:267–272. doi: 10.1093/ecam/nel011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahfar M. Glycosyl composition of polysaccharide from Tinospora cordifolia. Acta Pharm. 2003;53:65–69. [PubMed] [Google Scholar]
- Joshi S., Ingle P., Bhagwat S., Pawar M., Prajapati K., Kulkarni R. Effect of dietary addition of ashwagandha (Withania somnifera) and guduchi (Tinospora cordifolia) powder on broiler performance. Indian J. Anim. Sci. 2015;85:1358–1361. [Google Scholar]
- Kalikar M.V., Thawani V.R., Varadpande U.K., Sontakke S.D., Singh R.P., Khiyani R.K. Immunomodulatory effect of Tinospora cordifolia extract in human immuno-deficiency virus positive patients. Indian J. Pharmacol. 2008;40:107–110. doi: 10.4103/0253-7613.42302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapil A., Sharma S. Immunopotentiating compounds from Tinospora cordifolia. J. Ethnopharmacol. 1997;58:89–95. doi: 10.1016/s0378-8741(97)00086-x. [DOI] [PubMed] [Google Scholar]
- Kavitha B., Shruthi S., Rai S.P., Ramachandra Y. Phytochemical analysis and hepatoprotective properties of Tinospora cordifolia against carbon tetrachloride-induced hepatic damage in rats. J. Basic Clin. Pharm. 2011;2:139. [PMC free article] [PubMed] [Google Scholar]
- Khosa R.L., Prasad S. Pharmacognostical studies on guduchi (Tinospora cordifolia Miers) Indian J. Med. Res. 1971;6:261–269. [Google Scholar]
- Kidwai A., Salooja K., Sharma V., Siddiqui S. Chemical examination of Tinospora cordifolia. J. Sci. Ind. Res. 1949;8:115–118. [Google Scholar]
- Kolte A., Siddiqui M., Mode S. Immunomodulating effect of Withania somnifera and Tinospora cordifolia in broiler birds. Indian J. Vet. Med. 2007;27:33. [Google Scholar]
- Koppada R., Norozian F.M., Torbati D., Kalomiris S., Ramachandran C., Totapally B.R. Physiological effects of a novel immune stimulator drug, (1,4)-α-D-glucan, in rats. Basic Clin. Pharmacol. Toxicol. 2009;105:217–221. doi: 10.1111/j.1742-7843.2009.00383.x. [DOI] [PubMed] [Google Scholar]
- Kulkarni R., Mandal A., Munj C., Dan A., Saxena A., Tyagi P.K. Response of coloured broilers to dietary addition of geloi (Tinospora cordifolia) during extreme summer. Indian J. Poult. Sci. 2011;46:70–74. [Google Scholar]
- Kumar V., Modi P.K., Saxena K. Exploration of hepatoprotective activity of aqueous extract of Tinospora cordifolia-an experimental study. Studies. 2013;1:2. [Google Scholar]
- Kumari S., Mittal A., Dabur R. Moderate alcohol consumption in chronic form enhances the synthesis of cholesterol and c-21 steroid hormones, while treatment with Tinospora cordifolia modulate these events in men. Steroids. 2016;114:68–77. doi: 10.1016/j.steroids.2016.03.016. [DOI] [PubMed] [Google Scholar]
- Lakra M. Indira Gandhi Krishi Vishwavidayalaya; Raipur: 2008. Evaluation of Immune Response Against Hydropericardium Syndrome Virus in Broilers. Doctoral dissertation. [Google Scholar]
- Latha U., Rajesh M.G., Latha M.S. Hepatoprotective effect of an ayurvedic medicine. Indian Drugs. 1999;36:470. [Google Scholar]
- Latheef S.K., Dhama K., Wani M.Y., Samad H.A., Barathidasan R., Tiwari R., Singh S.D., Rai R.B. Ameliorative effects of four herbs (Withania somnifera, Azadirachta indica, Tinospora cordifolia and E Care Se Herbal) on the pathogenesis of chicken infectious anaemia virus. Int. J. Curr. Res. 2013;5:2327–2331. [Google Scholar]
- Latheef S.K., Dhama K., Samad H.A., Wani M.Y., Kumar M.A., Palanivelu M., Malik Y.S., Singh S., Singh R. Immunomodulatory and prophylactic efficacy of herbal extracts against experimentally induced chicken infectious anaemia in chicks: assessing the viral load and cell mediated immunity. Virus Dis. 2017;28:115–120. doi: 10.1007/s13337-016-0355-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.S., Wang G.Q., Du X.D., Cui B.A., Zhang S.M., Shen J.Z. Antimicrobial susceptibility and molecular detection of chloramphenicol and florfenicol resistance among Escherichia coli isolates from diseased chickens. J. Vet. Sci. 2007;8:243–247. doi: 10.4142/jvs.2007.8.3.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mam T.A., Jakhar K.K. Studies on in vitro antibacterial activity of Tinospora cordifolia stem extract on Escherichia coli. Vet. Res. Int. 2016;4:74–77. [Google Scholar]
- Marles R.J., Farnsworth N.R. Antidiabetic plants and their active constituents. Phytomedicine. 1995;2:137–189. doi: 10.1016/S0944-7113(11)80059-0. [DOI] [PubMed] [Google Scholar]
- Mary N.K., Babu B.H., Padikkala J. Antiatherogenic effect of caps ht2, a herbal ayurvedic medicine formulation. Phytomedicine. 2003;10:474–482. doi: 10.1078/094471103322331412. [DOI] [PubMed] [Google Scholar]
- Maryamma K., Ismail P., Manomohan C., Rajan A. Ameliorating effect of amruthu (Tinospora cordifolia) in aflatoxicosis of ducks. J. Vet. Anim. Sci. 1990;21:93–96. [Google Scholar]
- Mishra R., Manchanda S., Gupta M., Kaur T., Saini V., Sharma A., Kaur G. Tinospora cordifolia ameliorates anxiety-like behavior and improves cognitive functions in acute sleep deprived rats. Sci. Rep. 2016;6:25564. doi: 10.1038/srep25564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- More P., Pai K. In vitro NADH-oxidase, NADPH-oxidase and myeloperoxidase activity of macrophages after Tinospora cordifolia (guduchi) treatment. Immunopharmacol. Immunotoxicol. 2012;34:368–372. doi: 10.3109/08923973.2011.606324. [DOI] [PubMed] [Google Scholar]
- Nadkarni A.K. (3rd ed.) Vol I. Popular Prakasan Pvt. Ltd.; Mumbai: 1976. (Indian Medicinal Plants.). [Google Scholar]
- Nayampalli S.S., Ainapure S.S., Samant B.D., Kudtarkar R.G., Desai N.K., Gupta K.C. A comparative study of diuretic effects of Tinospora cordifolia and hydrochlorothiazide in rats and a preliminary phase I study in human volunteers. J. Postgrad. Med. 1988;34:233. [PubMed] [Google Scholar]
- Nety S., Koley K.M., Choudhary M., Chourasia D., Kumar V. Comparative study of immunomodulatory effect of Tinospora cordifolia stem and azadirachta indica leaf extract in broiler chicks. Vet. Pract. 2017;18:286–288. [Google Scholar]
- Patel M.B., Mishra S. Hypoglycemic activity of alkaloidal fraction of Tinospora cordifolia. Phytomedicine. 2011;18:1045–1052. doi: 10.1016/j.phymed.2011.05.006. [DOI] [PubMed] [Google Scholar]
- Pathak P., Vyas M., Vyas H., Naria M. Rasayana effect of Guduchi Churnaon the life span of Drosophila melanogaster. Ayu. 2016;37:67–70. doi: 10.4103/ayu.AYU_11_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peer F., Sharma M. Therapeutic evaluation of Tinospora cordifolia in ccl4 induced hepatopathy in goats. Indian J. Vet. Med. 1989;9:154–156. [Google Scholar]
- Phukan P., Bawari M., Sengupta M. Promising neuroprotective plants from north-east India. Int. J. Pharm. Pharmaceut. Sci. 2015;7:28–39. [Google Scholar]
- Prince P., Menon V.P., Gunasekaran G., Stanely M. Hypolipidaemic action of Tinospora cordifolia roots in alloxan diabetic rats. J. Ethnopharmacol. 1999;64:53. doi: 10.1016/s0378-8741(98)00106-8. [DOI] [PubMed] [Google Scholar]
- Prince P.S., Kamalakkannan N., Menon V.P. Restoration of antioxidants by ethanolic Tinospora cordifolia in alloxan-induced diabetic Wistar rats. Acta Pol. Pharm. 2004;61:283–287. [PubMed] [Google Scholar]
- Prince S.M.P., Menon V.P. Antioxidant action of Tinospora cordifolia root extract in alloxan diabetic rats. Phytother. Res. 2001;15:213–218. doi: 10.1002/ptr.707. [DOI] [PubMed] [Google Scholar]
- Prince S.M.P., Menon V.P. Hypoglycaemic and hypolipidaemic action of alcohol extract of Tinospora cordifolia roots in chemical induced diabetes in rats. Phytother Res. 2003;17:410–413. doi: 10.1002/ptr.1130. [DOI] [PubMed] [Google Scholar]
- Pushpangadan P., Rao C.V., Kishore K., Kartik R., Gupta Y.K., Govindarajan R. 2006. Herbal formulation as memory enhacher in Alzheimer condition. Council of Scientific & Industrial Research, New Delhi (IN), assignee. PAT - WO2006067796. [Google Scholar]
- Pushpangadan P., Rawat A.K.S., Rao C.V., Srivastava S.K., Khatton S. 2010. Synergistic antipyretic formulation. Council of Scientific & Industrial Research, New Delhi (IN), assignee. US Pat. No. 7,658,954. [Google Scholar]
- Raghu R., Sharma D., Ramakrishnan R., Khanam S., Chintalwar G.J., Sainis K.B. Molecular events in the activation of b cells and macrophages by a non-microbial tlr4 agonist, g1-4a from tinospora cordifolia. Immunol. Lett. 2009;123:60–71. doi: 10.1016/j.imlet.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Rahman M.A., Samad M.A., Rahman M.B., Kabir S. In vitro antibiotic sensitivity and therapeutic efficacy of experimental salmonellosis, colibacillosis and pasteurellosis in broiler chickens. Bangladesh J. Vet. Med. 2004;2:99–102. [Google Scholar]
- Rai M., Gupta S.S. Experimental evaluation of Tinospora cordifolia (Guduchi) for dissolution of urinary calculi. J. Res. Ind. Med. 1967;2:115. [Google Scholar]
- Raina M.T.N., Arora M., Madan S. Standardization and evaluation of formulation parameters of Tinospora cordifolia tablet. J. Adv. Pharm. Edu. Res. 2013;3 [Google Scholar]
- Rajkumar R., Yadav A., Kirupasankar M., Saxena V., Sangeeta S. Effect of Tinospora cordifolia supplementation on immunity of broiler chicks. Indian Vet. J. 2009;86:1244–1245. [Google Scholar]
- Rao E., Rao M. Studies on the polysaccharide preparation (Guduchi satwa) derived from Tinospora cordifolia. Indian J. Pharmaceut. Sci. 1981;43:103–106. [Google Scholar]
- Rao P.R., Kumar V.K., Viswanath R.K., Subbaraju G.V. Cardioprotective activity of alcoholic extract of Tinospora cordifolia in ischemia-reperfusion induced myocardial infarction in rats. Biol. Pharm. Bull. 2005;28:2319–2322. doi: 10.1248/bpb.28.2319. [DOI] [PubMed] [Google Scholar]
- Rawal A., Muddeshwar M., Biswas S. Effect of Rubia cordifolia, Fagonia cretica linn, and Tinospora cordifolia on free radical generation and lipid peroxidation during oxygen-glucose deprivation in rat hippocampal slices. Biochem. Biophys. Res. Commun. 2004;324:588–596. doi: 10.1016/j.bbrc.2004.09.094. [DOI] [PubMed] [Google Scholar]
- Reddy B.P., Murthy V.N., Venkateshwarlu V., Kokate C.K., Rambhau D. Antihepatotoxic activity of Phyllanthus niruri, Tinospora cordifolia and Ricinus communis. Indian Drugs. 1993;30:338. [Google Scholar]
- Rege N., Dahanukar S., Karandikar S.M. Hepatotoxic effects of Tinospora cordifolia against carbon tetrachloride induced liver damage. Indian Drugs. 1984;21:544. [Google Scholar]
- Saeed M., Abd Elhack M.E., Arif M., Elhindawy M.M., Attia A.I., Mahrose K.M., Bashir I., Siyal F.A., Arain M.A., Fazlani S.A. Impacts of distiller's dried grains with solubles as replacement of soybean meal plus vitamin e supplementation on production, egg quality and blood chemistry of laying hens. Ann. Anim. Sci. 2017;17 [Google Scholar]
- Saeed M., Yatao X., Hassan F.U., Arain M.A., Abd El-Hack M.E., Noreldin A.E., Sun C. Influence of graded levels of l-theanine dietary supplementation on growth performance, carcass traits, meat quality, organs histomorphometry, blood chemistry and immune response of broiler chickens. Int. J. Mol. Sci. 2018;19:462. doi: 10.3390/ijms19020462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sannegowda K., Venkatesha S., Moudgil K. Tinospora cordifolia inhibits autoimmune arthritis by regulating key immune mediators of inflammation and bone damage. Int. J. Immunopathol. Pharmacol. 2015;28:521–531. doi: 10.1177/0394632015608248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarma D.N.K., Khosa R.L., Chaurasia J.P.N., Sahai M. Antiulcer activity of Tinospora cordifolia Meirs and Centella asiatica linn extracts. Phytother. Res. 1995;9:589–590. [Google Scholar]
- Sarma D.N.K., Khosa R.L., Chaurasia J.P.N., Sahai M. Antistress activity of Tinospora cordifolia and Centella asiatica extracts. Phytother. Res. 1996;10:181. [Google Scholar]
- Sengupta S., Mukherjee A., Goswami R., Basu S. Hypoglycemic activity of the antioxidant saponarin, characterized as alpha-glucosidase inhibitor present in Tinospora cordifolia. J. Enzyme Inhib. Med. Chem. 2009;24:684–690. doi: 10.1080/14756360802333075. [DOI] [PubMed] [Google Scholar]
- Sharma D., Khosla R. Chemistry and pharmacology of Tinospora cordifolia Miers. Indian Drugs. 1993;30:549–554. [Google Scholar]
- Sharma V., Pandey D. Beneficial effects of Tinospora cordifolia on blood profiles in male mice exposed to lead. Toxicol. Int. 2010;17:8–11. doi: 10.4103/0971-6580.68341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma V., Pandey D. Protective role of Tinospora cordifolia against lead-induced hepatotoxicity. Toxicol. Int. 2010;17:12–17. doi: 10.4103/0971-6580.68343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R.K., Maini S., Ravikanth K. Beneficial effects of superliv ds and xlivpro on growth promotion and carcass quality traits in broilers. Vet. World. 2008;1:363–365. [Google Scholar]
- Sharma U., Bala M., Kumar N., Singh B., Munshi R.K., Bhalerao S. Immunomodulatory active compounds from Tinospora cordifolia. J. Ethnopharmacol. 2012;141:918–926. doi: 10.1016/j.jep.2012.03.027. [DOI] [PubMed] [Google Scholar]
- Sharma U., Bala M., Saini R., Verma P.K., Kumar N., Singh B., Munshi R.K., Bhalerao S. Polysaccharide enriched immunomodulatory fractions from Tinospora cordifolia (willd) miers ax hook. F. & Thoms. Indian J. Exp. Biol. 2012;50:612–617. [PubMed] [Google Scholar]
- Sharma P., Velu V., Indrani D., Singh R.P. Effect of dried guduchi (Tinospora cordifolia) leaf powder on rheological, organoleptic and nutritional characteristics of cookies. Food Res. Int. 2013;50:704–709. [Google Scholar]
- Sharma A., Shukla P.K., Bhattacharyya A., Kumar U., Roy D., Yadav B., Prakash A. Effect of dietary supplementation of sea buckthorn and giloe leaf meal on the body weight gain, feed conversion ratio, biochemical attributes and meat composition of Turkey poults. Vet. World. 2018;11:93–98. doi: 10.14202/vetworld.2018.93-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheth M.D., Rege N.N., Dahanukar S.A. Effect of Tinospora cordifolia on gastrointestinal dysmotility induced by chronic, unpredictable wrap-restraint. Indian J. Pharmacol. 2001;33:135. [Google Scholar]
- Shirolkar A., Sharma B., Lata S., Dabur R. Guduchi sawras (Tinospora cordifolia): an ayurvedic drug treatment modulates the impaired lipid metabolism in alcoholics through dopaminergic neurotransmission and anti-oxidant defense system. Biomed. Pharmacother. 2016;83:1265–1277. doi: 10.1016/j.biopha.2016.08.051. [DOI] [PubMed] [Google Scholar]
- Shivananjappa M.M., Muralidhara Abrogation of maternal and fetal oxidative stress in the streptozotocin-induced diabetic rat by dietary supplements of Tinospora cordifolia. Nutrition. 2012;28:581–587. doi: 10.1016/j.nut.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Singh K.P., Gupta A.S., Pendse V.K., Mahatma O.P., Bhandari D.S., Mahawar M.M. Experimental and clinical studies on Tinospora cordifolia. J. Res. Ind. Med. 1975;10:9. [Google Scholar]
- Singh S., Pandey S., Srivastava S., Gupta V., Patro B., Ghosh A. Chemistry and medicinal properties of Tinospora cordifolia (guduchi) Indian J. Pharmacol. 2003;35:83–91. [Google Scholar]
- Singh R.P., Banerjee S., Kumar P.V., Raveesha K.A., Rao A.R. Tinospora cordifolia induces enzymes of carcinogen/drug metabolism and antioxidant system and inhibits lipid peroxidation in mice. Phytomedicine. 2006;13:74–84. doi: 10.1016/j.phymed.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Singh V.K., Chauhan S.S., Ravikanth K., Maini S., Rekhe D.S. Effect of dietary supplementation of polyherbal liver stimulant on growth performance and nutrient utilization in broiler chicken. Vet. World. 2009;2:350–352. [Google Scholar]
- Singh A., Kaushik P., Yadav P., Yadav P. Effect of bael (Aegle marmelos) and giloy (Tinospora cordifolia) alone and in combination on growth and feed conversion of broiler chicks. Glob. J. Res. Anal. 2014;3:96–99. [Google Scholar]
- Sinha K., Mishra N.P., Singh J., Khanuja S.P.S. Tinospora cordifolia (guduchi): a reservoir plant for therapeutic applications: a review. Indian J. Tradit. Know. 2004;3:257–270. [Google Scholar]
- Sivakumar V., Rajan M.S. Antioxidant effect of Tinospora cordifolia extract in alloxan-induced diabetic rats. Indian J. Pharm. Sci. 2010;72:795–798. doi: 10.4103/0250-474X.84600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohni Y.R., Kaimal P., Bhatt R.M. The antiamoebic effect of a crude drug formulation of herbal extracts against Entamoeba histolytica in vitro and in vivo. J. Ethnopharmacol. 1995;45:43. doi: 10.1016/0378-8741(94)01194-5. [DOI] [PubMed] [Google Scholar]
- Sonu A., Sonam S., Jyoti V., Osman M.S., Uma M. In vitro immunopotentiating effects of Tinospora cordifolia in chicken lymphocytes culture system. J. Immunol. Immunopathol. 2013;15:113–114. [Google Scholar]
- Stanely M.P.P., Menon V.P. Hypoglycaemic and hypolipidaemic action of alcohol extract of Tinospora cordifolia roots in chemical induced diabetes in rats. Phytother. Res. 2003;17:410–413. doi: 10.1002/ptr.1130. [DOI] [PubMed] [Google Scholar]
- Stanely M.P.P., Menon V.P., Gunasekaran G. Hypolipidaemic action of Tinospora cordifolia roots in alloxan diabetic rats. J. Ethnopharmacol. 1999;64:53–57. doi: 10.1016/s0378-8741(98)00106-8. [DOI] [PubMed] [Google Scholar]
- Subramanian M., Chintalwar G.J., Chattopadhyay S. Antioxidant properties of a Tinospora cordifolia polysaccharide against iron-mediated lipid damage and gamma-ray induced protein damage. Redox Rep. 2002;7:137–143. doi: 10.1179/135100002125000370. [DOI] [PubMed] [Google Scholar]
- Sudhakaran D.S., Srirekha P., Devasree L.D., Premsingh S., Michael R.D. Immunostimulatory effect of Tinospora cordifolia Miers leaf extract in Oreochromis mossambicus. Indian J. Exp. Biol. 2006;44:726–733. [PubMed] [Google Scholar]
- Tripathi Y.B., Sharma M., Manickam M. Rubia 5 din, a new antioxidant from Rubia cordifolia. Indian J. Biochem. Biophys. 1997;34:302–306. [PubMed] [Google Scholar]
- Upadhyaya R., Pandey R.P., Sharma V., Anita K.V. Assessment of the multifaceted immunomodulatory potential of the aqueous extract of Tinospora cordifolia. Res. J. Chem. Sci. 2011;1:71–79. [Google Scholar]
- Vedavathy S., Rao K.N. Antipyretic activity of six indigenous medicinal plants of Tirumala hills, Andhra Pradesh, India. J. Ethnopharmacol. 1991;33:193–196. doi: 10.1016/0378-8741(91)90178-g. [DOI] [PubMed] [Google Scholar]
- Wadood N., Wadood A., Shah S.A.W. Effect of Tinospora cordifolia on blood glucose and total lipid levels of normal and alloxan-diabetic rabbits. Planta Med. 1992;58:131–136. doi: 10.1055/s-2006-961414. [DOI] [PubMed] [Google Scholar]
- Walia V. Review: Tinospora cordifolia in the treatment of depression. Pharma Tutor. 2015;3:32–34. [Google Scholar]
- Waseem Mirza M., Rehman Z., Mukhtar N. Use of organic acids as potential feed additives in poultry production. J. World's Poult. Res. 2016;6:105–116. [Google Scholar]