Abstract
Impact of feeding n-3 fatty acids (FA) to ISA brown and Shaver white breeders and their progeny on bone development in pullets was investigated. Breeders were fed Control (CON); CON + 1% microalgae (DMA: Aurantiochytrium limacinum) as the source of docosahexaenoic acid; and CON + 2.6% of a co-extruded mixture of full-fat flaxseed (FFF) and pulses mixture as source of α-linolenic acid. Test diets (DMA and FFF) were balanced for total n-3 FA and n-6: n-3 FA ratio. Samples of day-old progeny were euthanized for bone mineral content (BMC) and tibia collagen type II. The remaining pullets were fed posthatch treatments as follows: from breeder CON: CON (CON-CON), DMA (CON-DMA), and FFF (CON-FFF), from breeder DMA: CON (DMA-CON) and DMA (DMA-DMA) and from breeder FFF: CON (FFF-CON) and FFF (FFF-FFF). A total of 60 pullets per posthatch diets were reared in cages (12 pullets/cage, n = 5) with free access to feed and water, bled at 6, 12, and 18 wk of age (WOA) for bone turnover markers and necropsied at 18 WOA for tibia and femur samples. Day-old pullets from breeder fed CON had greater BMC (P < 0.001) relative to those from breeders fed other diets. There was strain and diet interaction (P ≤ 0.024) on tibia breaking strength (TBS) and tibia cortical ash concentration at 18 WOA such that diet responses were only observed in Shaver white pullets. In this context, TBS of DMA-DMA and FFF-FFF was greater than for pullets originating from CON breeder, and the cortical ash weight of DMA-DMA and FFF-FFF pullets was 23.8 and 20.2%, respectively, higher than for CON-CON pullets. In conclusions, the strain effects were strong on tibia attributes on 18-week-old pullets. Breeder feeding of n-3 FA was more effective when concomitant with posthatch feeding of n-3 FA in supporting the skeletal strength and cortical bone development in Shaver white pullets. Further investigations are warranted to establish the impact these strategies on skeletal health during laying cycle.
Key words: breeder feeding, posthatch feeding, pullets, bone development and quality, n-3 fatty acids
Introduction
Osteoporosis, a decrease in the amount of fully mineralized structural bone can cause 20 to 35% of all poultry mortalities in cage housing systems (Whitehead and Fleming, 2000). Approximately 30% of 2 to 3 g Ca required for eggshell formation are derived from skeletal Ca reserves (Gilbert, 1983). This amount of bone remodeling approximate to about 900 g of Ca being drawn out of the body resources in layer hen that is capable of laying more than 300 eggs during the laying cycle (Anderson et al., 2013). A mature bone is a metabolically dynamic organ in which its mass is continuously maintained by replacing old bone with new bone through balanced collaboration of osteoclast-mediated bone resorption and osteoblast-mediated bone formation (Nakanishi and Tsukamoto, 2015). Disruption of the process of bone turnover can lead to bone disorders such as osteoporosis (Beck and Hansen, 2004).
A rise in circulating estrogen levels at the onset of sexual maturity leads to a switch in the bone formation profile from structural bones (e.g., cortical) toward medullary bone (Whitehead and Fleming, 2000). Suboptimal deposition of bone mass of in pullets may lead to permanent damage to the bone structure, as well as increase the risk of osteoporosis (Beck and Hansen, 2004). To prevent osteoporosis, the peak bone mineral density, especially in structural parts, should be achieved and sustained before onset of lay. Proper nutrition is necessary to reach the genetic potential for peak bone mass (Whitehead and Fleming, 2000). Based on in vitro study, fatty acids (FA), especially omega-3 (n-3) polyunsaturated fatty acids (PUFA) are believed to possess various health benefits such as increasing bone mineral content and regulating bone disorders characterized by excessive osteoclast activity (Boeyens et al., 2014). Different FA such as α-linolenic acid (ALA), the precursor of n-3 long-chain PUFA such as docosahexaenoic acid (DHA), can only be synthesized in plants and bacteria and thus have to be supplied through the diet (Boeyens et al., 2014). It is believed that n-3 PUFA may modify bone metabolism mainly through decreasing locally produced prostaglandins (PG, Mazzuco, et al., 2005) and inflammatory cytokines (Kruger et al., 2010). In addition, the type of PUFA consumed during bone remodeling has been reported to be an essential determinant of bone formation (Cohen and Ward, 2005).
Genetic selection for various traits in different strains of poultry has influenced prehatch and posthatch characteristics and attributes such as embryos weight, mortality, residual yolk weight, metabolic hormones (e.g., thyroxine, triiodothyronine, etc.; Everaert et al., 2008). Avian embryos consume nutrients deposited in the eggs by hens. Hence, the breeder-feeding strategy may introduce nutritional stimuli to the embryo (Uni and Ferket, 2004, Uni et al., 2005). Research has reported difference in metabolism and physiological status such as hormones (Everaert et al., 2008), physical, and histological characteristics (e.g., stiffness and osteocytes density, respectively) of bone during the embryonic period (Yair et al., 2017) or at the onset of lay (Khanal et al., 2019) among different strains. These differences suggest the importance of considering the potential effect of genetics (strain) on influencing the development of breed-specific phenotypes.
This study hypothesized that supplementation with n-3 FA via practical and sustainable sources in either the breeder diet, posthatch diets, or both would affect the development of the skeletal system in the progeny. Furthermore, it is hypothesized that these effects may be strain specific. Thus, this study aimed to determine the impact of feeding n-3 FA on skeletal development in layer pullets at 18 wk of age (WOA) by (1) feeding n-3 FA to breeders only, (2) feeding n-3 FA to both breeders and their progeny, and (3) characterizing ISA brown and Shaver white responses.
Materials and methods
The protocol of animal experiment was approved by the University of Guelph Animal Care Committee (#3675), and birds were cared for under the Canadian Council on Animal Care guidelines (Canadian Council on Animal Care, 2009).
Breeders Phase
A total of 240 female and 30 male (per strain) day-old breeders of ISA brown and Shaver white were obtained from Hendrix Genetic Canada (Kitchener, ON, Canada) and kept in floor pens at the Arkell Poultry Research Station (University of Guelph, Guelph, ON, Canada). At 16 WOA, females of each strain were equally divided into separate groups, based on their average body weight (BW). Roosters of each strain were introduced into each flock for a total of 31 birds (27 ♀ and 4 ♂) per flock. During the laying period, birds received 14 h of incandescent light (20 lux, 03:00 to 1,700 h) and 10 h of a dark period. During the laying period, birds were fed commercial feed (Floradale, ON, Canada) with 2,875 kcal/kg AME, 18.0% CP, 4.24% Ca, and 0.68% P until 25 WOA.
At 26 WOA, birds were assigned into 3 dietary treatments: (1) Control (CON), a corn, soybean meal, wheat, and corn gluten diet, (2) CON plus 1% of dried microalgae (Aurantiochytrium limacinum) supplement (DMA) as source of DHA (0.04% ALA; 0.2% eicosapentaenoic acid [EPA]; 17.9% DHA as-is basis with 3.1% moisture [Alltech, Nicholasville, KY]), (3) CON plus 2.6% of a co-extruded blend of full-fat flaxseed extruded and pulses (FFF, 50/50 wt/wt) as a source of ALA (10.5% ALA; 0.0% EPA; 0.0% DHA as-is basis with 5.2% moisture LinPRO, O & T Farms Ltd., Regina, SK, Canada). The inclusion of 1% of DMA was previously shown to double the enrichment of n-3 PUFA in the egg yolk (Ao et al., 2015). The inclusion level of FFF was chosen accordingly to give a similar concentration of total n-3 and n-6 FA. All diets were formulated to supply the nutrients requirement of breeders genetic specification (Parent Stock Management Guide: ISA Brown, 2018; Parent Stock Management Guide: Shaver White, 2018). Dietary treatments were offered for 30 D to allow for peak n-3 PUFA deposition in the egg (Neijat et al., 2016). Then, 10 eggs were collected and submitted for FA analyses to confirm n-3 PUFA deposition.
A total of fertile 3,109 eggs were collected, individually labeled, and stored at 4°C until incubation (within 8 D of the collection). Eggs were incubated at 37.5°C with 55% humidity to day 19 and hatched set at 36.9°C with 66% humidity in a commercial-grade incubator and hatcher (Nature Form, Jacksonville, FL) at the Arkell Poultry Research Station (Guelph, ON, Canada).
Progeny Phase
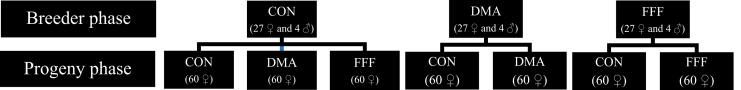
On the day of hatch, day-old pullets were counted and sexed, and all males were euthanized by CO2. Ten females from each experimental diet group were euthanized for the collection of right tibia samples, which were stored at −80°C for future analysis. Ten additional birds were euthanized by CO2, and intact bodies were kept in −20°C for measuring whole-body bone mineral content. The remaining birds were weighed individually, transported to the brooding room, and distributed into posthatch treatments (Figure 1), ensuring BW uniformity among groups. Pullets from breeder CON were divided into 3 posthatch treatments: CON, DMA, and FFF. Pullets from breeder DMA were divided into 2 posthatch treatments: CON and DMA. Pullets from breeder FFF were divided into 2 posthatch treatments: CON and FFF (Figure 1). Posthatch diets were formulated to meet or exceed nutrients requirements from hatch to prelay (Commercial Product Guide- ISA Brown, 2018, Commercial Product Guide- Shaver White, 2018). The diets containing DMA and FFF had the same total amount of total n-3 FA and n3: n-6 FA. The feeding program was as follows (Table 1): starter (0-4 WOA for ISA brown and 0-8 WOA for Shaver White); 2,950 kcal/kg AME, 20.5% CP, 1.1% Ca and 0.48% P), grower (5-10 WOA for ISA brown and 9-12 WOA for Shaver white, 2,850 kcal/kg AME, 19.0% CP, 1.10% Ca, and 0.48% P), developer (11-16 WOA for ISA brown and 13-16 WOA for Shaver white, 2,750 kcal/kg AME, 17.5% CP, 1.20% Ca, and 0.45% P). prelay (16 WOA- 2% egg production, 2,750 kcal/kg AME, 17.0% CP, 2.50% Ca, and 0.45% P). Feed samples from each phase were collected for FA profile analyses.
Figure 1.
Dietary treatment layout for the breeder and progeny phases. Day-old female breeder pullets were divided into 3 dietary treatments (CON, DMA, and FFF). Progeny from CON treatment was further divided into 3 posthatch treatments (CON, DMA, and FFF), whereas progeny from the DMA and FFF treatments were divided into 2 posthatch treatments, CON and DMA or CON and FFF. The concentration of total n-3 FA and ratio of n-6: n-3 were identical among DMA and FFF diets in both phases. CON, control; DMA, micro-Algae (Aurantiochytrium limacinum) fermentation product, as a source of docosahexaenoic acid; and FFF, co-extruded full-fat flaxseed and pulses mixture (50/50, wt/wt), as a source of α-linolenic acid.
Table 1.
Calculated composition of experimental diets of the breeders and offspring1.
| Item | AME, kcal/kg | Crude protein, % | Calcium, % | Available phosphorus, % |
|---|---|---|---|---|
| Breeders | ||||
| laying phase | 2,800 | 18.20 | 4.00 | 0.38 |
| Offspring | ||||
| Starter2 | 2,950 | 20.50 | 1.10 | 0.48 |
| Grower3 | 2,850 | 19.00 | 1.10 | 0.48 |
| Developer4 | 2,750 | 17.50 | 1.20 | 0.45 |
| Prelay5 | 2,750 | 17.00 | 2.50 | 0.45 |
Abbreviations: CON, control; DMA, micro-Algae (Aurantiochytrium limacinum) fermentation product, as a source of docosahexaenoic acid; FFF, co-extruded full-fat flaxseed and pulses mixture (50/50, wt/wt), as a source of α-linolenic acid; WOA, weeks of age.
The dietary composition was equal among the treatments (CON, DMA, and FFF) in each period.
Starter diet was offered during 0-4 WOA for ISA brown and 0-8 WOA for Shaver white.
Grower diet was offered during 5-10 WOA for ISA brown and 9-12 WOA for Shaver white.
Developer diet was offered during 11-16 WOA for ISA brown and 13-16 WOA for Shaver white.
Prelay diet was offered during 16 WOA until 2% of egg production for both the strains.
Twelve birds of the same breeder diets were placed in a cage of 20″ × 30″ (Ford Dickson Inc., Mitchell, ON, Canada) to give 5 replications for each posthatch diet and kept until 18 WOA. Pullets were allowed ad-libitum access to feed and water. The temperature was initially set at 34°C on the first day and reduced by 2°C/per week to a constant of 21°C. The lighting program started off on the first day at 40 lux, 02:00 to 18:00 h, and was reduced on the 28th D to 10 Lux, 08:00 to 20:00 h to 16 WOA.
Blood samples were taken from 2 birds from each cage at 6 WOA, and these birds were tagged for repeated blood sampling at 12 WOA and palpation at 18 WOA. All tagged birds were palpated before lighting at 07:00 h and those found with a hard eggshell in the shell gland were selected (Akbari Moghaddam Kakhki et al., 2018a). Seven tagged birds of Shaver white and 2 birds of ISA brown were found without a hard shell in the shell gland and were replaced by untagged birds. The blood samples were taken from the selected birds and centrifuged at 2,000 × g for 30 min at 5°C and serum stored at −20°C until required for analysis. Left and right tibias and femurs were dissected, de-fleshed, and stored at −20°C for further analysis.
Sample Processing and Analyses
Homogenized egg content and ground feed samples were submitted for FA profiling in a commercial lab (Activation Laboratories, Ancaster, ON, Canada) and analyzed according to O'fallon et al. (2007).
Whole-body bone mineral content (BMC) in day-old pullets was analyzed using Prodigy dual-energy X-ray absorptiometry (GE Healthcare, Madison, WI) equipped with enCORE software (version 14.0, GE Healthcare) as described by Akbari Moghaddam Kakhki et al. (2018a). For protein extraction from tibia samples taken at hatch, the whole tibia was crushed in liquid nitrogen using a mortar and pestle. After this process, a 0.1 ± 0.012 g sample was placed in a free-standing microcentrifuge tube (02-682-558, Thermo Fisher, Waltham, MA) followed by addition of T-PER Tissue Protein Extraction Reagent (sample weight × 15; 78,510, Thermo Fisher) supplemented with protease inhibitor (Halt Protease Inhibitor cocktail, CAT# 78,430, Thermo Fisher). Then, 0.2 g ± 0.01 of acid-washed glass beads (2 mm; Z273627-1 EA, Sigma Aldrich, St. Louis, MO) were added into the tubes and homogenized using a bead mill for 2 cycles of 15 s at 5 m/s and one cycle of 150 s at 3 m/s. Samples were then spun at 10,000 × g for 15 min at 4°C. Supernatants were analyzed for protein concentration based on method of Smith et al. (1985) using an assay kit (Pierce BCA protein assay kit, #23225, Thermo Fisher) and kept at −80°C until further analyses.
Left tibia and femur samples taken at 18 WOA were used for measuring the whole bone length as well as dry weight, ash content, and ash percentage in epiphysis, medullary, and cortical subparts. Subparts were separated according to Akbari Moghaddam Kakhki et al. (2018b). The epiphysis, cortical, and medullary sections were dried at 105°C for 24 h and reweighed. Subsequently, dried subparts were ashed at 600°C for 12 h (Akbari Moghaddam Kakhki et al., 2018b) and reweighed for measuring total bone ash content, ash content, and ash percentage in subpart and total bone ash concentration (Akbari Moghaddam Kakhki et al., 2018b). It has been demonstrated that bone attributes are correlated with BW (Erdal et al., 2012). Considering the difference in BW of the strains and possible significant differences among strain originating from the variation in BW among strains, all the bone attributes were normalized to BW, allowing to have a better picture of physiological difference among strains, not the difference in BW. Bone attributes including length, dry weight, and ash content of tibia and femur were normalized by dividing the weight to BW (Akbari Moghaddam Kakhki et al., 2018b).
Tibia is of one the main long-bones in hens where osteoporotic fractures occur (Whitehead and Fleming, 2000, Whitehead, 2004). Therefore, the breaking strength test was done only on the tibia. Right tibia samples taken at 18 WOA were thawed for 48 h at 4°C and used for measuring breaking strength according to Khanal et al. (2019). Breaking strength was measured using a 3-point bending test with an Instron material tester automated with the material test system software BlueHill 3.0 version 3.7.7 (Model: Instron crop, Canton, MA). Briefly, the maximum distance between upper and lower anvil was fixed to 35 mm. Each tibia was underpinned by fulcrum with a span of 4 cm, connecting to the tibia at beginning of the epiphyses (metaphysis region). The crosshead speed was set at 2 mm/s. All bones were kept in the same orientation and maximum load on mid-shaft of tibia to break the bone was considered the value of tibia breaking strength.
The concentration of collagen type II, I in tibia, osteocalcin and tartrate-resistant acid phosphatase (TRAP) in plasma was measured in duplicate using competitive ELISA kits following recommended assay procedures (collagen type II: ECKC0786, collagen type I: CKC0021, osteocalcin: ECKO0008 and TRAP: ECKT0544; ABclonal, Woburn, MA). Antibodies for ELISA kits were rabbit polyclonal and validated using an immunogen of the specific chicken protein. The intraassay CV was 2.19, 8.35, 17.06, 20.31, and 5.08% for collagen type II, I, osteocalcin of 6, 12 WOA, and TRAP, respectively.
Calculations and Statistical Analyses
All data were tested for normality using UNIVARIATE plot normal procedure and submitted for statistical analyses using GLIMMIX procedures (SAS 9.4). Day-old pullet data were subjected to a two-way ANOVA in a 2 (ISA brown and Shaver white) and 3 (CON, DMA, and FFF) factorial arrangement. Posthatch data were subjected to a two-way ANOVA in a 2 (ISA brown and Shaver white) and 7 posthatch diets factorial arraignment. Correlation between bone-turnover markers (osteocalcin at 6 and 12 WOA and TRAP) and bone attributes was performed using CORR procedure. Significance was declared at P < 0.05.
Results
Progeny Egg Production
The age of laying the first egg was day 121 and day 125 for ISA brown and Shaver white, respectively. The age of maturity, the day of 50% egg production, was day 141 and day 137 for ISA brown and Shaver white, respectively. The egg production percentage was 2.0, and 0.86% at the time of sampling of 18 WOA ISA brown and shaver white birds, respectively.
Fatty Acids Concentration in the Diets and Eggs
Supplementation with DMA increased concentration of DHA and EPA in total fat across breeders to prelay diets by an average of 3.46 ± 0.22 and 0.06 ± 0.02%, respectively (Table 2). Compared with CON, supplementation with FFF increased the concentration of ALA and DHA in breeders to prelay diets by an average of 3.94 ± 1.19 and 0.19 ± 0.06%, respectively. The FA analyses in egg yolk showed that feeding DMA increased the concentration of DHA in ISA brown and Shaver white layer breeders compared with CON. Feeding FFF to ISA brown and Shaver white breeders increased the concentration of ALA compared with CON (Akbari Moghaddam Kakhki et al., 2019).
Table 2.
Analyzed fatty acid profile in experimental diets1, % of total fat.
| Item | 18:2n-62 | 18:3n-33 | 20:4n-64 | 20:5n-35 | 22:6n-36 | ∑n3 | ∑n6 | ∑n6: ∑n3 | Total fat7 |
|---|---|---|---|---|---|---|---|---|---|
| Breeders | |||||||||
| CON | 47.63 | 6.30 | 0.00 | 0.00 | 0.00 | 6.30 | 47.63 | 7.56 | 4.57 |
| DMA | 45.68 | 5.52 | 0.05 | 0.08 | 3.80 | 9.48 | 45.89 | 4.84 | 4.56 |
| FFF | 45.36 | 9.26 | 0.06 | 0.05 | 0.25 | 9.58 | 45.60 | 4.76 | 4.49 |
| Starter | |||||||||
| CON | 55.50 | 4.18 | 0.00 | 0.00 | 0.00 | 4.18 | 55.50 | 13.28 | 2.95 |
| DMA | 50.16 | 5.46 | 0.00 | 0.05 | 3.45 | 9.07 | 50.16 | 5.53 | 3.08 |
| FFF | 50.53 | 9.50 | 0.00 | 0.02 | 0.15 | 9.67 | 50.62 | 5.23 | 3.01 |
| Grower | |||||||||
| CON | 55.27 | 6.32 | 0.00 | 0.00 | 0.00 | 6.32 | 55.47 | 8.78 | 3.53 |
| DMA | 51.42 | 5.86 | 0.00 | 0.06 | 3.24 | 9.20 | 51.53 | 5.60 | 3.62 |
| FFF | 51.16 | 9.01 | 0.00 | 0.00 | 0.11 | 9.12 | 51.35 | 5.63 | 3.56 |
| Developer | |||||||||
| CON | 57.16 | 5.44 | 0.00 | 0.00 | 0.00 | 5.48 | 57.39 | 10.48 | 4.17 |
| DMA | 50.09 | 5.58 | 0.02 | 0.07 | 3.28 | 9.00 | 50.19 | 5.58 | 4.31 |
| FFF | 50.15 | 9.15 | 0.01 | 0.06 | 0.19 | 9.42 | 50.42 | 5.36 | 4.25 |
| Prelay | |||||||||
| CON | 58.06 | 4.14 | 0.00 | 0.00 | 0.00 | 4.14 | 58.06 | 14.03 | 3.64 |
| DMA | 52.05 | 5.25 | 0.00 | 0.04 | 3.52 | 8.87 | 52.05 | 5.87 | 3.89 |
| FFF | 52.50 | 9.18 | 0.00 | 0.00 | 0.23 | 9.41 | 52.50 | 5.58 | 3.71 |
CON, control; DMA, micro-Algae (Aurantiochytrium limacinum) fermentation product, as a source of docosahexaenoic acid; and FFF, co-extruded full-fat flaxseed and pulses mixture (50/50, wt/wt), as a source of α-linolenic acid.
Linoleic.
α-linolenic acid.
Arachidonic acid.
Eicosapentaenoic acid.
Docosahexaenoic acid.
Expressed as a gram per 100 g of feed, as fed basis.
Whole Bone Mineral Content and Tibia Collagen in Day-Old Pullets
Day-old pullet BW was affected by the interaction between strain and diet (P = 0.006) with Shaver white pullets from breeders fed CON being 4.89 g heavier than Shaver white pullets from breeders fed FFF (Table 3). The interaction between strain and diet did not affect BMC and collagen type II (P > 0.05). Shaver white pullets had more BMC than ISA brown (P < 0.05). The BW and collagen type II content in tibia were not affected by the main effect of strain (P > 0.05). Pullets from breeders fed the CON diet had greater BMC (g) compared with breeders fed either DMA or FFF (P < 0.001, Table 3). Pullets from breeders fed CON had more BMC (%) compared with pullets from breeders fed the FFF diet (P < 0.001). Tibia collagen type II was increased (P < 0.05) in pullets from breeders fed FFF compared with those from breeders fed CON, whereas pullets from breeders fed DMA were numerically intermediate between CON and FFF (P > 0.05). The collagen type I was neither affected by the interaction of strain and diet nor the main effects of strain and diet (P < 0.05).
Table 3.
Effects of feeding sources of docosahexaenoic and α-linolenic acids to ISA brown and Shaver White breeders and/progeny on body weight, bone mineral content and collagen type II in tibia of day-old pullets1.
| Items | BW, g | BMC2 |
Tibia collagen II |
Tibia collagen I |
||||
|---|---|---|---|---|---|---|---|---|
| g | % | ng/mg | ng/μg protein | ng/mg | ng/μg protein | |||
| Strain | Diet3 | |||||||
| ISA brown | CON | 33.22a,b | 0.38 | 1.08 | 3,487 | 4.712 | 96.41 | 0.130 |
| ISA brown | DMA | 33.18a,b | 0.32 | 0.98 | 3,493 | 4.713 | 97.48 | 0.131 |
| ISA brown | FFF | 35.44a,b | 0.28 | 0.78 | 3,498 | 4.723 | 97.62 | 0.132 |
| Shaver white | CON | 37.16a | 0.55 | 1.27 | 3,490 | 4.693 | 95.40 | 0.128 |
| Shaver white | DMA | 34.43a,b | 0.41 | 1.03 | 3,495 | 4.714 | 96.88 | 0.131 |
| Shaver white | FFF | 32.27b | 0.36 | 0.94 | 3,505 | 4.736 | 96.39 | 0.130 |
| SEM | 1.050 | 0.033 | 0.059 | 3.581 | 0.006 | 1.022 | 0.001 | |
| Main effect | ||||||||
| Strain | ||||||||
| ISA brown | 33.95 | 0.33b | 0.82b | 3,492 | 4.716 | 97.17 | 0.13 | |
| Shaver white | 34.62 | 0.44a | 1.09a | 3,497 | 4.715 | 96.22 | 0.13 | |
| SEM | 0.631 | 0.013 | 0.032 | 2.067 | 0.004 | 0.590 | 0.0007 | |
| Diet | ||||||||
| CON | 35.19 | 0.46a | 1.17a | 3,488b | 4.702b | 95.90 | 0.129 | |
| DMA | 33.81 | 0.37b | 1.01a,b | 3,494a,b | 4.714a,b | 97.18 | 0.131 | |
| FFF | 33.85 | 0.32b | 0.86b | 3,502a | 4.729a | 97.01 | 0.131 | |
| SEM | 0.788 | 0.023 | 0.053 | 2.532 | 0.005 | 0.722 | 0.001 | |
| Probabilities (P-value) | ||||||||
| Strain | 0.447 | <0.001 | 0.005 | 0.142 | 0.806 | 0.263 | 0.211 | |
| Diet | 0.362 | <0.001 | <0.001 | 0.002 | <0.001 | 0.407 | 0.401 | |
| Strain × Diet | 0.006 | 0.111 | 0.397 | 0.792 | 0.051 | 0.952 | 0.915 | |
a,bValues with uncommon superscripts within each column are significantly different (P < 0.05).
n = 10.
Bone mineral content; the minimum detectable amount was 0.1 g.
CON, control; DMA, micro-Algae (Aurantiochytrium limacinum) fermentation product, as a source of docosahexaenoic acid; and FFF, co-extruded full-fat flaxseed and pulses mixture (50/50, wt/wt), as a source of α-linolenic acid.
Body Weight of Pullets
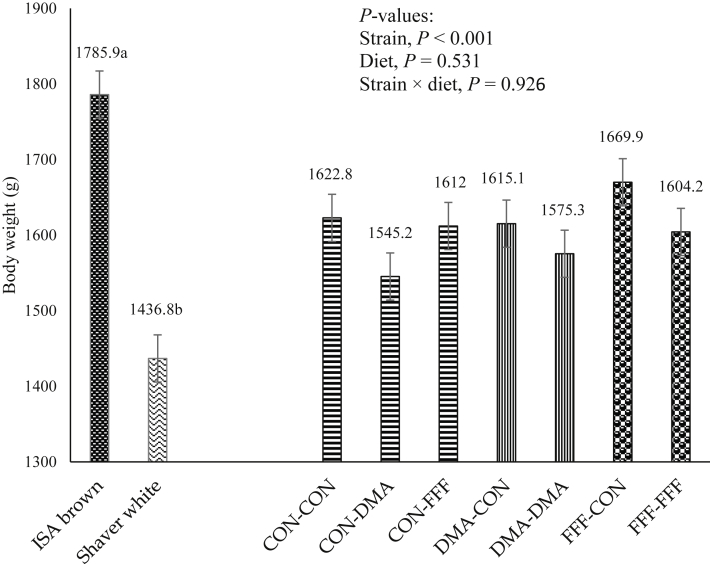
There was no interaction between strains and diet (P = 0.926) or the main effect of diets (P = 0.531) on the BW. ISA brown pullets were 349.1 g heavier than Shaver white pullets at 18 WOA (P < 0.001, Figure 2).
Figure 2.
Effects of feeding sources of docosahexaenoic and α-linolenic acids to ISA brown and Shaver White breeders and/progeny on body weight of 18-weeks-old pullets. The day-old female pullets from breeders fed CON, DMA, and FFF were divided into 3 (CON, DMA, and FFF), 2 (CON and DMA), and 2 (CON, FFF) posthatch treatments, respectively. The concentration of total n-3 fatty acid and ratio of n-6: n-3 were identical among DMA and FFF diets in both breeder and progeny phases. CON, control; DMA, micro-Algae (Aurantiochytrium limacinum) fermentation product, as a source of docosahexaenoic acid; FFF, co-extruded full-fat flaxseed and pulses mixture (50/50, wt/wt), as a source of α-linolenic acid.
Plasma Bone Turnover Markers During Rearing
The effects of strain and diet on plasma bone turnover markers are shown in Table 4. The interactive effect of strain and diet affected the concentration of osteocalcin at 6 WOA such that ISA brown pullets of FFF-FFF had more concentration compared with pullets of CON-CON CON-FFF and those originating from breeders fed DMA (P < 0.001). There was no interaction between strain and diet on plasma osteocalcin at 12 WOA (P = 0.107). Shaver white pullets had 43.5% greater concentration of plasma osteocalcin compared with ISA brown pullets (P < 0.001). Diets influenced the plasma concentration of osteocalcin such that pullets from the FFF-FFF treatment and pullets from CON-DMA had a greater concentration of osteocalcin compared with rest of the treatments (P < 0.001). The concentration of plasma TRAP was not affected by the interaction between strain and diet (P = 0.682) or the main effect of diet (P = 0.175). ISA brown pullets had 84.9% greater TRAP concentration compared with Shaver white pullets (P = 0.002).
Table 4.
Effects of feeding sources of docosahexaenoic and α-linolenic acids to ISA brown and Shaver White breeders and/progeny on serum bone formation and resorption markers in pullets (ng/mL)1.
| Items | Osteocalcin |
Tartrate resistant acid phosphatase |
||
|---|---|---|---|---|
| 6-wk | 12-wk | 18-wk | ||
| Strain | Diet2 | |||
| ISA brown | CON-CON | 15.46b | 8.06 | 11.46 |
| ISA brown | CON-DMA | 25.45a,b | 11.11 | 9.04 |
| ISA brown | CON-FFF | 12.76b | 6.71 | 6.32 |
| ISA brown | DMA-CON | 19.62b | 4.55 | 7.90 |
| ISA brown | DMA-DMA | 14.98b | 6.88 | 4.28 |
| ISA brown | FFF-CON | 22.71a,b | 7.22 | 12.96 |
| ISA brown | FFF-FFF | 38.04a | 12.85 | 10.80 |
| Shaver white | CON-CON | 16.66b | 9.02 | 7.57 |
| Shaver white | CON-DMA | 28.45a,b | 15.20 | 6.43 |
| Shaver white | CON-FFF | 17.66b | 10.02 | 2.94 |
| Shaver white | DMA-CON | 28.00a,b | 12.04 | 5.53 |
| Shaver white | DMA-DMA | 18.40b | 8.55 | 3.35 |
| Shaver white | FFF-CON | 25.92a,b | 11.76 | 4.63 |
| Shaver white | FFF-FFF | 12.34b | 15.78 | 3.53 |
| SEM | 3.409 | 1.168 | 2.350 | |
| Main effect | ||||
| Strain | ||||
| ISA brown | 21.29 | 8.20b | 8.97a | |
| Shaver white | 21.06 | 11.77a | 4.85b | |
| SEM | 1.288 | 0.409 | 0.880 | |
| Diet | ||||
| CON-CON | 16.06b | 8.54b | 9.52 | |
| CON-DMA | 26.95a | 13.16a | 7.74 | |
| CON-FFF | 15.21b | 8.36b | 4.63 | |
| DMA-CON | 23.81a,b | 8.30b | 6.71 | |
| DMA-DMA | 16.69a,b | 7.71b | 3.82 | |
| FFF-CON | 24.32a,b | 9.49b | 8.79 | |
| FFF-FFF | 25.19a,b | 14.32a | 7.16 | |
| SEM | 2.410 | 0.791 | 1.661 | |
| Probabilities (P-value) | ||||
| Strain | 0.903 | <0.001 | 0.002 | |
| Diet | 0.001 | <0.001 | 0.175 | |
| Strain × Diet | <0.001 | 0.107 | 0.682 | |
a,bValues with uncommon superscripts within each column are significantly different (P < 0.05).
n = 10.
The day-old female pullets from breeders fed CON, DMA, and FFF were divided into 3 (CON, DMA, and FFF), 2 (CON and DMA), and 2 (CON, FFF) posthatch treatments, respectively. CON, control; DMA, micro-Algae (Aurantiochytrium limacinum) fermentation product, as a source of docosahexaenoic acid; and FFF, co-extruded full-fat flaxseed and pulses mixture (50/50, wt/wt), as a source of α-linolenic acid.
Tibia and Femur Attributes in 18 Wk of Age Pullets
The effect of strain and diet on the whole tibia and femur attributes in 18 WOA pullets are shown in Table 5. The interaction between strain and diet improved tibia breaking strength in Shaver white pullets from the DMA-DMA and FFF-FFF groups compared with pullets from all 3 posthatch treatments originating from this breeder CON (P = 0.024). Tibia length, weight, ash weight, and ash concentration were neither affected by the interaction between the strain and diet nor the main effect of diet (P > 0.05). Shaver white pullets had longer (P < 0.001) and lighter (P < 0.001) tibia with greater ash concentration (P < 0.001) compared with tibia from ISA brown pullets. There was an interaction between strain and diet for femur ash weight (P = 0.017), where it was higher for Shaver white pullets from DMA-DMA group compared with pullets of CON-FFF. Femur length, weight, and ash concentration were not influenced by the interaction between strains and diet as well as the main effect of diet. Shaver white pullets had longer (P < 0.001) and lighter (P < 0.001) femurs with higher ash concentration compared with ISA brown pullets.
Table 5.
Effects of feeding sources of docosahexaenoic and α-linolenic acids to ISA brown and Shaver White breeders and/progeny on attributes of whole tibia and femur in 18-wk old pullets1.
| Items | Tibia |
Femur |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BS, N2 | Length3 | Wt 4 | Ash 4 | Ash, % | Length3 | Wt 4 | Ash4 | Ash, % | ||
| Strain | Diet5 | |||||||||
| ISA brown | CON-CON | 225.2a,b,c | 0.067 | 4.88 | 1.73 | 35.35 | 0.047 | 3.65 | 1.37a,b,c | 37.20 |
| ISA brown | CON-DMA | 229.6a,b,c | 0.072 | 4.99 | 1.79 | 36.34 | 0.049 | 3.74 | 1.42a,b,c | 38.64 |
| ISA brown | CON-FFF | 246.0a,b,c | 0.067 | 4.81 | 1.71 | 35.55 | 0.046 | 3.54 | 1.35a,b,c | 38.18 |
| ISA brown | DMA-CON | 216.5b,c | 0.069 | 4.83 | 1.65 | 34.31 | 0.047 | 3.58 | 1.27b,c | 35.71 |
| ISA brown | DMA-DMA | 211.8b,c | 0.068 | 4.56 | 1.68 | 36.92 | 0.047 | 3.43 | 1.34a,b,c | 39.35 |
| ISA brown | FFF-CON | 191.2c | 0.065 | 4.78 | 1.73 | 35.74 | 0.045 | 3.29 | 1.16c | 35.76 |
| ISA brown | FFF-FFF | 193.4c | 0.070 | 5.20 | 1.64 | 31.64 | 0.050 | 3.66 | 1.23a,b,c | 33.76 |
| Shaver white | CON-CON | 190.8c | 0.079 | 4.03 | 1.59 | 39.49 | 0.053 | 2.98 | 1.31a,b,c | 44.13 |
| Shaver white | CON-DMA | 206.7c | 0.082 | 4.31 | 1.66 | 38.62 | 0.056 | 3.28 | 1.37a,b,c | 42.03 |
| Shaver white | CON-FFF | 199.5c | 0.083 | 4.49 | 1.65 | 36.94 | 0.058 | 3.19 | 1.25b,c | 39.31 |
| Shaver white | DMA-CON | 237.6a,b,c | 0.080 | 4.14 | 1.65 | 40.22 | 0.056 | 3.44 | 1.63a,b | 47.66 |
| Shaver white | DMA-DMA | 281.3a | 0.081 | 4.23 | 1.76 | 41.83 | 0.057 | 3.55 | 1.73a | 48.98 |
| Shaver white | FFF-CON | 216.1b,c | 0.078 | 4.36 | 1.68 | 38.70 | 0.055 | 3.30 | 1.37a,b,c | 41.34 |
| Shaver white | FFF-FFF | 259.7a,b | 0.083 | 4.48 | 1.76 | 39.35 | 0.057 | 3.38 | 1.41a,b,c | 41.77 |
| SEM | 25.41 | 0.002 | 0.183 | 0.089 | 1.572 | 0.002 | 0.135 | 0.085 | 2.593 | |
| Main effect | ||||||||||
| Strain | ||||||||||
| ISA brown | 216.2 | 0.068b | 4.86a | 1.70 | 35.12b | 0.047b | 3.55a | 1.30b | 36.95b | |
| Shaver white | 227.4 | 0.081a | 4.29b | 1.68 | 39.31a | 0.056a | 3.30b | 1.44a | 43.60a | |
| SEM | 7.718 | 0.0009 | 0.069 | 0.034 | 0.594 | 0.0007 | 0.051 | 0.032 | 0.980 | |
| Diet | ||||||||||
| CON-CON | 208.0 | 0.073 | 4.45 | 1.66 | 37.42 | 0.050 | 3.31 | 1.34a,b | 40.67 | |
| CON-DMA | 218.2 | 0.077 | 4.65 | 1.73 | 37.48 | 0.052 | 3.51 | 1.40a,b | 40.34 | |
| CON-FFF | 222.7 | 0.075 | 4.65 | 1.68 | 36.24 | 0.052 | 3.37 | 1.30a,b | 38.75 | |
| DMA-CON | 227.1 | 0.074 | 4.48 | 1.65 | 37.27 | 0.052 | 3.51 | 1.45a,b | 41.68 | |
| DMA-DMA | 246.5 | 0.075 | 4.39 | 1.72 | 39.37 | 0.052 | 3.49 | 1.54a | 44.16 | |
| FFF-CON | 203.7 | 0.072 | 4.57 | 1.71 | 37.22 | 0.050 | 3.29 | 1.26b | 38.55 | |
| FFF-FFF | 226.6 | 0.076 | 4.84 | 1.70 | 35.49 | 0.053 | 3.52 | 1.32a,b | 37.78 | |
| SEM | 14.438 | 0.002 | 0.130 | 0.06 | 1.111 | 0.001 | 0.095 | 0.060 | 1.833 | |
| Probabilities (P-value) | ||||||||||
| Strain | 0.312 | <0.001 | <0.001 | 0.609 | <0.001 | <0.001 | <0.001 | 0.005 | <0.001 | |
| Diet | 0.463 | 0.358 | 0.248 | 0.968 | 0.334 | 0.534 | 0.373 | 0.035 | 0.220 | |
| Strain × diet | 0.024 | 0.944 | 0.679 | 0.701 | 0.452 | 0.737 | 0.068 | 0.017 | 0.432 | |
a-cValues with uncommon superscripts within each column are significantly different (P < 0.05).
n = 10.
Breaking strength.
mm/kg BW.
g/kg BW.
The day-old female pullets from breeders fed CON, DMA and FFF were divided into 3 (CON, DMA, and FFF), 2 (CON and DMA), and 2 (CON, FFF) posthatch treatments, respectively. CON, control; DMA, micro-Algae (Aurantiochytrium limacinum) fermentation product, as a source of docosahexaenoic acid; and FFF, co-extruded full-fat flaxseed and pulses mixture (50/50, wt/wt), as a source of α-linolenic acid.
The tibia epiphysis and medullary weight, ash content, and ash concentration, as well as the cortical weight and ash concentration, were not affected by the interaction between strain and diet and the main effect of diet (P > 0.05, Table 6). The tibia cortical ash weight was improved (P = 0.007) in Shaver white pullets from DMA-DMA and FFF-FFF compared with CON-CON. The main effect of strain was such that tibia epiphysis and medullary region of Shaver white pullets had lower dry weight (P < 0.001), as well as greater concentration of ash in the medullary (P = 0.001) and cortical (P < 0.001) regions compared with ISA brown pullets.
Table 6.
Effects of feeding sources of docosahexaenoic and α-linolenic acids to ISA brown and Shaver White breeders and/progeny on attributes of tibia subparts in 18-wk old pullets1.
| Items | Epiphysis |
Medullary |
Cortical |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Wt2 | Ash2 | Ash, % | Wt2 | Ash2 | Ash, % | Wt2 | Ash2 | Ash, % | ||
| Strain | Diet3 | |||||||||
| ISA brown | CON-CON | 2.74 | 0.83 | 30.21 | 0.52 | 0.004 | 0.79 | 1.61 | 0.89a,b,c | 37.20 |
| ISA brown | CON-DMA | 2.70 | 0.84 | 31.33 | 0.67 | 0.005 | 0.98 | 1.62 | 0.95a,b,c | 38.64 |
| ISA brown | CON-FFF | 2.63 | 0.79 | 31.13 | 0.61 | 0.003 | 0.55 | 1.58 | 0.92a,b,c | 38.18 |
| ISA brown | DMA-CON | 2.57 | 0.77 | 29.81 | 0.69 | 0.003 | 0.44 | 1.57 | 0.88a,b,c | 35.71 |
| ISA brown | DMA-DMA | 2.52 | 0.80 | 31.84 | 0.55 | 0.005 | 0.96 | 1.49 | 0.87a,b,c | 39.35 |
| ISA brown | FFF-CON | 2.63 | 0.93 | 33.71 | 0.69 | 0.004 | 0.72 | 1.46 | 0.80c | 35.76 |
| ISA brown | FFF-FFF | 2.81 | 0.76 | 27.06 | 0.78 | 0.003 | 0.42 | 1.60 | 0.88a,b,c | 33.78 |
| Shaver white | CON-CON | 2.29 | 0.74 | 32.08 | 0.35 | 0.005 | 1.48 | 1.38 | 0.84b,c | 44.13 |
| Shaver white | CON-DMA | 2.35 | 0.75 | 32.08 | 0.46 | 0.005 | 1.47 | 1.50 | 0.90a,b,c | 42.03 |
| Shaver white | CON-FFF | 2.48 | 0.74 | 29.82 | 0.48 | 0.004 | 0.73 | 1.52 | 0.91a,b,c | 39.31 |
| Shaver white | DMA-CON | 2.17 | 0.70 | 32.08 | 0.46 | 0.003 | 0.74 | 1.51 | 0.95a,b,c | 47.66 |
| Shaver white | DMA-DMA | 2.24 | 0.72 | 32.08 | 0.37 | 0.006 | 1.74 | 1.62 | 1.04a | 48.98 |
| Shaver white | FFF-CON | 2.43 | 0.75 | 31.12 | 0.46 | 0.005 | 1.23 | 1.47 | 0.93a,b,c | 41.34 |
| Shaver white | FFF-FFF | 2.39 | 0.74 | 31.12 | 0.47 | 0.004 | 1.07 | 1.61 | 1.01a | 41.77 |
| SEM | 0.114 | 0.070 | 1.628 | 0.108 | 0.0009 | 0.278 | 0.086 | 0.035 | 2.593 | |
| Main effect | ||||||||||
| Strain | ||||||||||
| ISA brown | 2.66a | 0.82a | 30.59 | 0.64a | 0.004 | 0.69b | 1.56 | 0.94a | 36.95b | |
| Shaver white | 2.34b | 0.73b | 31.48 | 0.44b | 0.005 | 1.21a | 1.52 | 0.88b | 43.60a | |
| SEM | 0.043 | 0.026 | 0.615 | 0.041 | 0.0004 | 0.105 | 0.032 | 0.013 | 0.980 | |
| Diet | ||||||||||
| CON-CON | 2.52 | 0.78 | 31.15 | 0.44 | 0.005 | 1.13 | 1.49 | 0.87 | 40.67 | |
| CON-DMA | 2.52 | 0.80 | 31.71 | 0.56 | 0.005 | 1.22 | 1.56 | 0.93 | 40.34 | |
| CON-FFF | 2.56 | 0.76 | 29.98 | 0.54 | 0.003 | 0.64 | 1.55 | 0.91 | 38.75 | |
| DMA-CON | 2.37 | 0.73 | 30.95 | 0.57 | 0.003 | 0.59 | 1.54 | 0.92 | 41.68 | |
| DMA-DMA | 2.37 | 0.76 | 31.96 | 0.49 | 0.005 | 1.35 | 1.56 | 0.96 | 44.16 | |
| FFF-CON | 2.53 | 0.84 | 32.41 | 0.58 | 0.005 | 0.97 | 1.47 | 0.86 | 38.55 | |
| FFF-FFF | 2.60 | 0.75 | 29.09 | 0.63 | 0.004 | 0.74 | 1.61 | 0.94 | 37.78 | |
| SEM | 0.080 | 0.049 | 1.151 | 0.076 | 0.0007 | 0.197 | 0.061 | 0.025 | 1.833 | |
| Probabilities (P-value) | ||||||||||
| Strain | <0.001 | 0.031 | 0.306 | <0.001 | 0.199 | 0.001 | 0.306 | 0.004 | <0.001 | |
| Diet | 0.328 | 0.784 | 0.420 | 0.578 | 0.131 | 0.076 | 0.727 | 0.076 | 0.220 | |
| Strain × diet | 0.797 | 0.960 | 0.541 | 0.987 | 0.998 | 0.935 | 0.521 | 0.007 | 0.432 | |
a-cValues with uncommon superscripts within each column are significantly different (P < 0.05).
n = 10.
g/kg BW.
The day-old female pullets from breeders fed CON, DMA, and FFF were divided into 3 (CON, DMA, and FFF), 2 (CON and DMA), and 2 (CON, FFF) posthatch treatments, respectively. CON, control; DMA, micro-Algae (Aurantiochytrium limacinum) fermentation product, as a source of docosahexaenoic acid; and FFF, co-extruded full-fat flaxseed and pulses mixture (50/50, wt/wt), as a source of α-linolenic acid. of α-linolenic acid.
The interaction between strain and diet did not affect femur epiphysis, medullary, and cortical dry weight, ash weight, and ash concentration (P > 0.05, Table 7). The femurs of ISA brown pullets had greater epiphysis, cortical weight, and ash weight but had lower ash concentration and heavier medullary compared with Shaver white (P < 0.05). The main effect of diets only affected the cortical dry weight (P < 0.001) and ash weight (P < 0.001), in which dry weight was improved in pullets from DMA-DMA compared with pullets from CON-CON, CON-FFF, FFF-CON, and FFF-FFF. The ash weight in pullets from DMA-CON and DMA-DMA was greater than the rest of the dietary treatments (P < 0.05).
Table 7.
Effects of feeding sources of docosahexaenoic and α-linolenic acids to ISA brown and Shaver White breeders and/progeny on attributes of femur subparts in 18 wk-old-pullets1.
| Items | Epiphysis |
Medullary |
Cortical |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Wt2 | Ash2 | Ash, % | Wt2 | Ash2 | Ash, % | Wt2 | Ash2 | Ash, % | |
| Main effect | |||||||||
| Strain | |||||||||
| ISA brown | 2.01a | 0.67 | 33.87b | 0.40a | 0.003 | 0.87 | 1.14b | 0.63b | 55.25b |
| Shaver white | 1.80b | 0.70 | 38.71a | 0.27b | 0.004 | 1.67 | 1.23a | 0.74a | 59.60a |
| SEM | 0.034 | 0.020 | 0.994 | 0.023 | 0.0002 | 0.146 | 0.027 | 0.015 | 0.954 |
| Diet3 | |||||||||
| CON-CON | 1.93 | 0.72 | 37.11 | 0.28 | 0.003 | 1.33 | 1.11b,c | 0.62b | 56.63 |
| CON-DMA | 1.99 | 0.73 | 37.33 | 0.33 | 0.003 | 1.23 | 1.20a,b,c | 0.66b | 55.80 |
| CON-FFF | 1.92 | 0.67 | 35.11 | 0.34 | 0.004 | 1.37 | 1.11b,c | 0.62b | 56.72 |
| DMA-CON | 1.83 | 0.66 | 36.72 | 0.36 | 0.003 | 1.00 | 1.32a,b | 0.79a | 59.16 |
| DMA-DMA | 1.82 | 0.69 | 38.41 | 0.28 | 0.004 | 1.63 | 1.39a | 0.84a | 59.85 |
| FFF-CON | 1.88 | 0.66 | 35.33 | 0.32 | 0.003 | 1.23 | 1.10c | 0.60b | 54.78 |
| FFF-FFF | 1.98 | 0.67 | 34.02 | 0.43 | 0.004 | 1.11 | 1.10b,c | 0.65b | 59.06 |
| SEM | 0.064 | 0.039 | 1.859 | 0.044 | 0.0005 | 0.274 | 0.052 | 0.029 | 1.785 |
| Probabilities (P-value) | |||||||||
| Strain | <0.001 | 0.550 | 0.001 | <0.001 | 0.064 | <0.001 | 0.023 | <0.001 | 0.002 |
| Diet | 0.364 | 0.799 | 0.675 | 0.224 | 0.236 | 0.773 | <0.001 | <0.001 | 0.336 |
| Strain × diet | 0.322 | 0.646 | 0.891 | 0.503 | 0.917 | 0.908 | 0.201 | 0.111 | 0.250 |
a-cValues with uncommon superscripts within each column are significantly different (P < 0.05).
n = 10.
g/kg BW.
The day-old female pullets from breeders fed CON, DMA, and FFF were divided into 3 (CON, DMA, and FFF), 2 (CON and DMA), and 2 (CON, FFF) posthatch treatments, respectively. CON, control; DMA, micro-Algae (Aurantiochytrium limacinum) fermentation product, as a source of docosahexaenoic acid; and FFF, co-extruded full-fat flaxseed and pulses mixture (50/50, wt/wt), as a source of α-linolenic acid.
Correlation Among Parameters
There was a correlation between osteocalcin concentration measured at 6 WOA and osteocalcin measured at 12 WOA (correlation coefficient = 0.409), tibia ash weight (correlation coefficient = 0.441), and ash concentration (correlation coefficient = 0.421) in ISA brown pullets (P < 0.05, Table 8). The TRAP concentration had no correlation (P > 0.05) with any bone attributes in ISA brown pullets. The osteocalcin measured at 6 WOA correlated with osteocalcin measured at 12 WOA (correlation coefficient = 0.563, P < 0.001) in Shaver white pullets. The concentration of TRAP correlated with ash concentration in whole tibia (correlation coefficient = −0.305), whole femur (correlation coefficient = −0.387), and femur cortical (correlation coefficient = −0.305) in Shaver white pullets.
Table 8.
Coefficient of correlation analysis between serum bone-turnover markers and bone attributes in ISA brown and Shaver white pullets1.
| Item | OST2 - 6 week | OST3 - 12 week | TRAP4-18 week |
|---|---|---|---|
| ISA brown | |||
| OST-12 wk | 0.409* | ||
| TRAP-18 wk | 0.102 | −0.031 | |
| Tibia ash content (g: g BW) | 0.441** | 0.237 | 0.044 |
| Tibia ash percentage (%) | 0.421* | 0.008 | −0.057 |
| Femur ash content (g: g BW) | 0.006 | −0.145 | 0.040 |
| Femur ash percentage (%) | 0.077 | −0.148 | −0.074 |
| Tibia cortical ash content (g: g BW) | 0.047 | 0.005 | 0.101 |
| Tibia cortical ash percentage (%) | 0.010 | 0.007 | −0.123 |
| Femur cortical ash content (g: g BW) | 0.011 | −0.077 | 0.188 |
| Femur cortical ash percentage (%) | −0.021 | 0.143 | −0.198 |
| Shaver white | |||
| OST-12 wk | 0.563** | ||
| TRAP-18 wk | 0.072 | −0.045 | |
| Tibia ash content (g: g BW) | 0.112 | 0.087 | 0.074 |
| Tibia ash percentage (%) | −0.038 | −0.054 | −0.305* |
| Femur ash content (g: g BW) | −0.028 | −0.089 | −0.206 |
| Femur ash percentage (%) | −0.071 | −0.155 | −0.387* |
| Tibia cortical ash content (g: g BW) | 0.167 | 0.177 | −0.089 |
| Tibia cortical ash percentage (%) | 0.113 | −0.207 | −0.037 |
| Femur cortical ash content (g: g BW) | −0.088 | −0.093 | −0.305* |
| Femur cortical ash percentage (%) | −0.123 | −0.374 | −0.024 |
* Indicates <0.05 probability and ** indicates <0.001 probability.
Osteocalcin measured at 6 wk.
Osteocalcin measured at 12 wk.
Tartrate resistant acid phosphatase measured at 18 wk.
Discussion
The FA analyses in the diets and eggs confirmed that the DMA and FFF mainly increased DHA and ALA, respectively. Composition of hatching eggs may modify the skeletal morphological features of developing embryo and hatchling (Yair et al., 2012). Bone development during the embryonic period has been shown to peak after day 14 of incubation (Yair et al., 2012). The reduction in BMC in day-old pullets from n-3 FA fed breeders might be associated with the impact of the increased concentration of n-3 FA in the eggs, resulting in greater DHA uptake but lower uptake of other FA from the residual yolk (Akbari Moghaddam Kakhki et al., 2019). This connection highlights the vital role of yolk FA content in modifying nutrient utilization by the embryo (Yalcin et al., 2008). Another possible factor involved in the change in BMC may be the difference in egg component percentages, as egg yolk and eggshell are the main sources of nutrients and mineral for bone development (Yalcin et al., 2008, Torres and Korver, 2018). However, the absolute value and percentage of yolk, albumen, and eggshell were not different among the dietary treatments (Akbari Moghaddam Kakhki et al. 2019).
Endochondral ossification ensures the process of longitudinal growth occurs, which is initiated by the secretion of the extracellular matrix via chondrocytes (Whitehead, 2004). The extracellular matrix has been shown to contain a high content of collagen type II (Whitehead, 2004). As chondrocytes becomes enlarged in hypertrophic zone, the extracellular matrix is surrounded by collagen type X (Whitehead, 2004). After chondrocytes undergo apoptosis and are resorbed, osteoblasts secrete collagen type I and form a scaffold for hydroxyapatite crystals (Rath et al., 2000). The greater concentration of collagen type II in embryos of the FFF compared with CON and equal concentration of collagen type I among treatments could explain why the reduced tibia mineralization in embryos of DMA and FFF was not associated with a delay in collagen synthesis/development. On the other hand, it can be deduced that embryos of FFF had more collagen type II, which might provide more area for osteoblasts to secrete collagen type I, meaning more scaffold to host hydroxyapatite crystals and more potential for greater bone mineral content in later stages of life. These observations emphasize the alteration in functions of osteoblasts by the change in the availability of PUFA (Kushwaha et al., 2018).
Disruption of the process of bone resorption and formation, such as osteoporosis, is the result of reduced number and activity of osteoblast cells derived from mesenchymal stem cells and increased number and activity of osteoclast cells derived from hematopoietic stem cells (Nakanishi and Tsukamoto, 2015). In the current study, the concentration of osteocalcin in plasma at 12 WOA showed that only posthatch feeding of DMA and combination of the breeder and posthatch feeding of FFF resulted in greater osteocalcin. However, the concentration of osteocalcin at 12 WOA was not correlated with bone characteristics. One reason for this discrepancy might be the high variability of the observations as the intra-assay coefficient variation was 20.31%. The differences in the concentration of biomarker for activity of osteoblast (osteocalcin at 6 and 12 WOA) and osteoclast (TRAP at 18 WOA) demonstrated differences in bone turnover rate in the 2 strains. R. Pottgueter (2015) reported there is a peak of bone formation in pullet starting from 6 until 12 WOA, explaining the importance of measuring biomarker of bone formation at these ages. After sexual maturity, the role of osteoblast in structural bones is important as structural bones start to loss their mineral content. Khanal et al. (2019) observed differences in femur physical attributes between Lohmann Brown and Lohmann LSL-Lite at different time points in the lay cycle. Lohmann LSL-Lite had greater femur mineral density and content compared with Lohmann Brown (Khanal et al., 2019). Similarly, in the current study, 18 WOA Shaver white pullets had greater tibia and femur ash concentration compared with ISA brown pullets.
Different strains possess different traits and characteristics such as feed intake, body growth and development, sexual maturity, egg production efficiency, and skeletal health (Singh et al., 2009, Khanal et al., 2019). However, the phenotypic differences among strains start during the embryonic period (Ho et al., 2011). The differences in bone metabolism among strains may explain why bone attributes of ISA brown pullets were not affected by feeding n-3 FA; this difference highlighted the importance of the impact of genetics on bone turnover and phenotypic differences. Feeding either source of n-3 FA to Shaver white breeders and their progeny increased the tibia breaking strength. The increase in strength suggested that feeding n-3 FA to layer breeders and their progeny was more effective than feeding n-3 FA to layer breeders only. The tibia ash weight of pullets from layer breeders fed either source of n-3 FA was greater compared with those from CON-CON. This showed the effectiveness of feeding n-3 FA to both breeders and progeny compared with feeding n-3 FA to progeny in improving bone quality. However, the response of femur cortical to feeding FFF was different from feeding DMA. Feeding DMA to both breeders and progeny increased the femur weight and feeding DMA to either only progeny or both breeders and progeny increased femur ash weight compared with all the dietary treatment. Feeding FFF to either breeders, progeny, or both did not influence femur cortical ash weight.
One mechanism by which n-3 PUFA work to influence bone metabolism is through modifying the production of eicosanoids, in particular, PG. The PG family is made up of fast-acting hormones originating from PUFA (Kruger et al., 2010). The dominant PG in bone, PGE2 (Kruger et al., 2010), is derived from arachidonic acid while PGE3, equipotent to PGE2 in bone resorption, is obtained from EPA (Kajarabille et al., 2013). Both precursors undergo the same enzymatic reaction when being converted (Kruger al., 2010); however, EPA undergoes a less efficient enzymatic reaction than arachidonic acid, subsequently favoring PGE2 production over PGE3 (Kruger et al., 2010). In addition, PGE1 derived from gamma-linolenic acid, possesses anti-inflammatory properties, and reduces PGE2 levels (Kruger et al., 2010). Thus, dietary EPA and gamma-linolenic acid can regulate PGE2 production and subsequently impact bone resorption (Kruger et al., 2010). A study by Liu and Denbow (2001) reported that ex-vivo biosynthesis of PGE2 was higher in the femur of newly hatched quail layers fed diets supplemented with 50 g/kg soybean oil and poultry fat (P < 0.01) compared with those supplemented by menhaden fish oil, which was correlated with arachidonic acid content in the femur. However, these researchers did not measure bone characteristics (Liu and Denbow, 2001). In the current study, the concentration of EPA was only detectable (at 0.04%) in the eggs of ISA brown breeders fed DMA. In addition, the concentration of gamma-linolenic acid remained constant in eggs of ISA brown layer breeders across all the treatments. While Shaver white breeders fed CON and DMA maintained a constant concentration of gamma-linolenic acid, those fed by FFF did not deposit detectable amount of gamma-linolenic acid in their eggs. These data suggest another mechanism of action other than the change in the profile of PG, which might be involved in the response of long-bones to dietary n-3 FA.
Different factors are required for osteoclastogenesis and osteoclast differentiation such as receptors for activation of nuclear factor-KB ligand (RANKL; Nakanishi and Tsukamoto, 2015). The expression of RANKL by osteoblast is increased by proinflammatory cytokines such as TNF-α, IL1β, and IL6 (Boeyens et al., 2014). The RANKL eventually binds to its receptor, RANK, on the surface of osteoclast and a cascade of downstream pathways is initiated, which results in an increase in osteoclastogenesis, activation of osteoclasts, and bone resorption (Boeyens et al., 2014). Polyunsaturated FA are able to modify inflammatory mediators and therefore have the potential to affect bone development and growth (Kruger et al., 2010). Dietary n-6 PUFA have the potential to increase inflammation, whereas n-3 PUFA have been shown to decrease mediators of inflammation (Kajarabille et al., 2013). In addition, the microalgae, supplemental source of DMA diets, contain several groups of bioactive components of pigments with anticarcinogenic, antioxidative, and antihypertensive properties. These bioactive components can elicit immunomodulating responses (Raja and Hemaiswarya, 2010). Nakanishi et al. (2013) observed an improvement in the tibia and femur BMC along with the reduction in TNF-α and IL-6 in ovariectomized rats fed a diet supplemented with 45 g/kg of menhaden oil. These authors also observed lower expression (P < 0.05) of RANKL, and RANK in ovariectomized rats fed diets supplemented by menhaden oil compared with corn oil (Nakanishi et al., 2013). Kruger and Schollum (2005) reported an increase in femur BMC and Ca content in mice fed diets supplemented with tuna oil at 4% (26.3% DHA and 6.79% EPA of total fat) for 6 wk compared with those fed diets supplemented with corn oil. However, test diet containing 4% evening primrose oil (11.67% DHA and 17.86% EPA of total fat) did not affect BMC and Ca content in the femur. These findings demonstrated that the effect of n-3 PUFA on skeletal metabolism is also dependent on the type of n-3 FA (Kruger and Schollum, 2005). On the other hand, Anez-Bustillos et al. (2018) did not observe any differences in tibia microstructural and blood-based biomarkers of bone metabolism in 3-wk old mice of both sexes fed diets supplemented with soybean oil (DHA, 8.4 g/kg) and DHA with arachidonic acid at a 20:1 ratio (DHA: arachidonic acid). It has been suggested that the potential effect of dietary n-3 PUFA may be more evident in the presence of disease (Anez-Bustillos et al., 2018). The discrepancies in the results might be related to the various factors including type, dose, and duration of the intervention (Rajaram et al., 2017) and to the target population, which included the presence of disease or provoked immune response (Anez-Bustillos et al., 2018). However, whether the protective effects of n-3 PUFA can translate to bone metabolism remains unknown and must be further explored.
In this study feeding a source of n-3 FA led to a decrease in BMC and delay in prenatal bone mineralization in the tibia of day-old pullets, which was not associated with collagen synthesis/development. Impact of supplementing n-3 FA in the breeder or posthatch diet on bone development in pullets was strain dependent. Feeding n-3 FA as ALA or DHA to both breeders and progeny was an effective feeding strategy in supporting structural bones (cortical) in Shaver White pullets at 18 WOA. The implications of these feeding strategies on maintenance of skeletal health in laying hens warrant further investigations.
Acknowledgments
The research was funded by Ontario Agri-Food Innovation Alliance, National Sciences and Engineering Research Council of Canada-CRD Program, Egg Farmers of Canada, Alltech Canada, and O & T Farms. Authors appreciate the assistance of members of Monogastric laboratory (University of Guelph, ON, CA) in performing the animal trial and Dr. Tina Widowski for her scientific and technical review of the manuscript. R. Akbari Moghaddam Kakhki is a recipient of the Ontario Trillium Doctoral and Mary Edmunds Williams Scholarships. The donation of the parent flocks by Hendrix Genetics is greatly appreciated.
References
- Akbari Moghaddam Kakhki R., Heuthorst T., Wornath-Vanhumbeck A., Neijat M., Kiarie E. Interactive effects of calcium and top-dressed 25-hydroxy vitamin D3 on egg production, egg shell quality, and bones attributes in aged Lohmann LSL-lite layers. Poult. Sci. 2018;98:1254–1262. doi: 10.3382/ps/pey446. [DOI] [PubMed] [Google Scholar]
- Akbari Moghaddam Kakhki R., Heuthorst T., Wornath-Vanhumbeck A., Neijat M., Kiarie E. Medullary bone attributes in aged Lohmann LSL-lite layers fed different levels of calcium and top-dressed 25-hydroxy vitamin D3. Can. J. Anim. Sci. 2018;99:138–149. [Google Scholar]
- Akbari Moghaddam Kakhki R., Ma D.W.L., Price K.R., Moats J., Karrow N.A., Kiarie E.G. Enriching Isa brown and Shaver white breeder diets with sources of n-3 polyunsaturated fatty acids increased embryonic utilization of docosahexaenoic acid. Poult. Sci. 2020 doi: 10.1016/j.psj.2019.09.002. In-Press Accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson K., Havenstein G., Jenkins P., Osborne J. Changes in commercial laying stock performance, 1958-2011: thirty-seven flocks of the North Carolina random sample and subsequent layer performance and management tests. World. Poult. Sci. J. 2013;69:489–514. [Google Scholar]
- Anez-Bustillos L., Cowan E., Cubria M.B., Villa-Camacho J.C., Mohamadi A., Dao D.T., Pan A., Fell G.L., Baker M.A., Nandivada P. Effects of dietary omega-3 fatty acids on bones of healthy mice. Clin. Nutr. 2018;5614:32430–33250. doi: 10.1016/j.clnu.2018.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ao T., Macalintal L., Paul M., Pescatore A., Cantor A., Ford M., Timmons B., Dawson K.A. Effects of supplementing microalgae in laying hen diets on productive performance, fatty-acid profile, and oxidative stability of eggs. J. Appl. Poult. Res. 2015;24:394–400. [Google Scholar]
- Beck M.M., Hansen K.K. Role of estrogen in avian osteoporosis. Poult. Sci. 2014;83:200–206. doi: 10.1093/ps/83.2.200. [DOI] [PubMed] [Google Scholar]
- Boeyens J., Deepak V., Chua W.-H., Kruger M., Joubert A., Coetzee M. Effects of ω3-and ω6-polyunsaturated fatty acids on RANKL-induced osteoclast differentiation of RAW264. 7 cells: a comparative in vitro study. Nutrients. 2014;6:2584–2601. doi: 10.3390/nu6072584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canadian Council on Animal Care. Canadian Council on Animal Care in Science. 2009. The care and use of farm animals in research, teaching and testing. Ottawa, Canada. [Google Scholar]
- Cohen S.L., Ward W.E. Flaxseed oil and bone development in growing male and female mice. J. Toxicol. Env. Heal. A. 2005;68:1861–1870. doi: 10.1080/15287390500226516. [DOI] [PubMed] [Google Scholar]
- Commercial Product Guide- ISA Brown . Hendrix Genetic; Boxmeer, The Netherlands.: 2018. North American Version.https://www.isa-poultry.com/documents/302/ISA_Brown_cs_product_guide_North_America_L8110-2-NA.pdf Accessed Sept. 2018. [Google Scholar]
- Commercial Product Guide- Shaver White . Hendrix Genetic; Boxmee, The Netherlands.: 2018. North American Version.https://www.shaver-poultry.com/documents/304/Shaver_White_cs_product_guide_NA.pdf Accessed Sept. 2018. [Google Scholar]
- Erdal R., Richardson I., Ljøkjel K., Haug A. Sensorial quality and bone strength of female and male broiler chickens are influenced by weight and growth rate. Br. Poult. Sci. 2012;53:616–622. doi: 10.1080/00071668.2012.736611. [DOI] [PubMed] [Google Scholar]
- Everaert N., Willemsen H., De Smit L., Witters A., De Baerdemaeker J., Decuypere E., Bruggeman V. Comparison of a modern broiler and layer strain during embryonic development and the hatching process. Br. Poult. Sci. 2008;49:574–582. doi: 10.1080/00071660802357025. [DOI] [PubMed] [Google Scholar]
- Gilbert A. Calcium and reproductive function in the hen. P. Nutr. Soc. 1983;42:195–212. doi: 10.1079/pns19830024. [DOI] [PubMed] [Google Scholar]
- Ho D.H., Reed W.L., Burggren W.W. Egg yolk environment differentially influences physiological and morphological development of broiler and layer chicken embryos. J. Exp. Biol. 2011;214:619–628. doi: 10.1242/jeb.046714. [DOI] [PubMed] [Google Scholar]
- Kajarabille N., Díaz-Castro J., Hijano S., López-Frías M., López-Aliaga I., Ochoa J.J. A new insight to bone turnover: role of-3 polyunsaturated fatty acids. Sci. World J. 2013;2013:589641. doi: 10.1155/2013/589641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanal T., Widowski T., Bédécarrats G., Kiarie E. Effects of pre-lay dietary calcium (2.5 vs. 4.0%) and pullet strain (Lohmann Brown vs. Selected Leghorn LSL-Lite) on calcium utilization and femur quality at 1st through to the 50th egg. Poult. Sci. 2019;98:4919–4928. doi: 10.3382/ps/pez245. [DOI] [PubMed] [Google Scholar]
- Kruger M.C., Coetzee M., Haag M., Weiler H. Long-chain polyunsaturated fatty acids: selected mechanisms of action on bone. Prog. Lipid. Res. 2010;49:438–449. doi: 10.1016/j.plipres.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Kruger M.C., Schollum L.M. Is docosahexaenoic acid more effective than eicosapentaenoic acid for increasing calcium bioavailability? Prostag. Leukotr. Ess. 2005;73:327–334. doi: 10.1016/j.plefa.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Kushwaha P., Wolfgang M.J., Riddle R.C. Fatty acid metabolism by the osteoblast. Bone. 2018;115:8–14. doi: 10.1016/j.bone.2017.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D., Denbow D. Maternal dietary lipids modify composition of bone lipids and ex vivo prostaglandin E2 production in early postnatal Japanese quail. Poult. Sci. 2001;80:1344–1352. doi: 10.1093/ps/80.9.1344. [DOI] [PubMed] [Google Scholar]
- Mazzuco H., McMurtry J., Kuo A., Hester P. The effect of pre-and postmolt diets high in n-3 fatty acids and molt programs on skeletal integrity and insulin-like growth factor-I of White Leghorns. Poult. Sci. 2005;84:1735–1749. doi: 10.1093/ps/84.11.1735. [DOI] [PubMed] [Google Scholar]
- Nakanishi A., Iitsuka N., Tsukamoto I. Fish oil suppresses bone resorption by inhibiting osteoclastogenesis through decreased expression of M-CSF, PU. 1, MITF and RANK in ovariectomized rats. Mol. Med. Rep. 2013;7:1896–1903. doi: 10.3892/mmr.2013.1446. [DOI] [PubMed] [Google Scholar]
- Nakanishi A., Tsukamoto I. n-3 polyunsaturated fatty acids stimulate osteoclastogenesis through PPARγ-mediated enhancement of c-Fos expression, and suppress osteoclastogenesis through PPARγ-dependent inhibition of NFKB activation. J. Nutr. Biochem. 2015;26:1317–1327. doi: 10.1016/j.jnutbio.2015.06.007. [DOI] [PubMed] [Google Scholar]
- Neijat M., Ojekudo O., House J. Effect of flaxseed oil and microalgae DHA on the production performance, fatty acids and total lipids of egg yolk and plasma in laying hens. Prostag. Leukotr. Ess. 2016;115:77–88. doi: 10.1016/j.plefa.2016.10.010. [DOI] [PubMed] [Google Scholar]
- O'fallon J., Busboom J., Nelson M., Gaskins C. A direct method for fatty acid methyl ester synthesis: application to wet meat tissues, oils, and feedstuffs. J. Anim. Sci. 2007;85:1511–1521. doi: 10.2527/jas.2006-491. [DOI] [PubMed] [Google Scholar]
- Parent Stock Management Guide: ISA Brown. Edition L7140. Hendrix Genetic; Boxmeer, The Netherlands: 2018. [Google Scholar]
- Parent Stock Management Guide: Shaver White. Edition L7140. Hendrix Genetic; Boxmeer, The Netherlands: 2018. [Google Scholar]
- Pottgueter R. World Poultry Science Association; Beekbergen, The Netherlands: 2015. 20th European Symposium on Poultry Nutrition, 24–27 August 2015. Prague, Czech Republic. [Google Scholar]
- Raja R., Hemaiswarya S. Dietary Components and Immune Function. Springer. Berlin, Germany; 2010. Microalgae and immune potential; pp. 515–527. [Google Scholar]
- Rajaram S., Yip E., Reghunathan R., Mohan S., Sabaté J. Effect of altering dietary n-6: n-3 polyunsaturated fatty acid ratio with plant and Marine-based supplement on biomarkers of bone turnover in healthy Adults. Nutrients. 2017;9:1162–1171. doi: 10.3390/nu9101162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rath N., Huff G., Huff W., Balog J. Factors regulating bone maturity and strength in poultry. Poult. Sci. 2000;79:1024–1032. doi: 10.1093/ps/79.7.1024. [DOI] [PubMed] [Google Scholar]
- Singh R., Cheng K., Silversides F. Production performance and egg quality of four strains of laying hens kept in conventional cages and floor pens. Poult. Sci. 2009;88:256–264. doi: 10.3382/ps.2008-00237. [DOI] [PubMed] [Google Scholar]
- Smith P.e., Krohn R.I., Hermanson G., Mallia A., Gartner F., Provenzano M., Fujimoto E., Goeke N., Olson B., Klenk D. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Torres C., Korver D. Influences of trace mineral nutrition and maternal flock age on broiler embryo bone development. Poult. Sci. 2018;97:2996–3003. doi: 10.3382/ps/pey136. [DOI] [PubMed] [Google Scholar]
- Uni Z., Ferket P., Tako E., Kedar O. In ovo feeding improves energy status of late-term chicken embryos. Poult. Sci. 2005;84:764–770. doi: 10.1093/ps/84.5.764. [DOI] [PubMed] [Google Scholar]
- Uni Z., Ferket R. Methods for early nutrition and their potential. World Poult. Sci. J. 2004;60:101–111. [Google Scholar]
- Whitehead C. Overview of bone biology in the egg-laying hen. Poult. Sci. 2004;83:193–199. doi: 10.1093/ps/83.2.193. [DOI] [PubMed] [Google Scholar]
- Whitehead C., Fleming R. Osteoporosis in cage layers. Poult. Sci. 2000;79:1033–1041. doi: 10.1093/ps/79.7.1033. [DOI] [PubMed] [Google Scholar]
- Yair R., Cahaner A., Uni Z., Shahar R. Maternal and genetic effects on broiler bone properties during incubation period. Poult. Sci. 2017;96:2301–2311. doi: 10.3382/ps/pex021. [DOI] [PubMed] [Google Scholar]
- Yair R., Uni Z., Shahar R. Bone characteristics of late-term embryonic and hatchling broilers: bone development under extreme growth rate. Poult. Sci. 2012;91:2614–2620. doi: 10.3382/ps.2012-02244. [DOI] [PubMed] [Google Scholar]
- Yalcin S., Bağdatlioğlu N., Bruggeman V., Babacanoğlu E., Uysal İ, Buyse J., Decuypere E., Siegel P. Acclimation to heat during incubation. 2. Embryo composition and residual egg yolk sac fatty acid profiles in chicks. Poult. Sci. 2008;87:1229–1236. doi: 10.3382/ps.2007-00436. [DOI] [PubMed] [Google Scholar]