Abstract
This study was conducted to assess the effect of dietary supplementation of Muramidase 007 to broiler chickens on gastrointestinal functionality, evaluating growth performance, apparent ileal digestibility, intestinal histomorphology, vitamin A in plasma and cecal microbiota. A total of 480 one-day male chicks (Ross 308) were distributed in 16 pens allocated in 2 experimental diets: the control diet (CTR) without feed enzymes, coccidiostat or growth promoters, and the experimental diet (MUR): CTR supplemented with 35,000 units (LSU(F))/kg of the Muramidase 007. Digesta and tissue samples were obtained on days 9 and 36 of the study. A lower feed conversion ratio was observed in the MUR treatment. Apparent ileal digestibility of DM, organic matter and energy were improved by Muramidase 007. It was also observed that MUR improved digestibility of total fatty acids, mono-unsaturated fatty acids and poly-unsaturated fatty acids, and content of vitamin A in plasma at day 9 (P < 0.05). Histomorphological analysis of jejunum samples revealed no differences in the villus height or crypt depth; but a higher number of goblet cells and intraepithelial lymphocytes at day 36 with MUR. No differences were observed in plate counts of enterobacteria or Lactobacillus along the gastrointestinal tract, neither on the cecal short-chain fatty acids. An statistical trend was observed for reduction of cecal clostridia at day 9 for MUR. Analysis of cecal microbiota structure by 16S rRNA gene sequencing revealed relevant changes correlated to age. At day 9, broilers receiving MUR showed decreased alpha diversity compared to CTR that was not detected at day 36. Changes in specific taxonomic groups with an increase in Lactobacillus genus were identified. In conclusion, evaluation of the variables in this study indicates that dietary Muramidase 007 contributes to improve feed conversation ratio and gastrointestinal function in broiler chickens. Effects could have been mediated by slight shifts observed in the intestinal microbiota. More studies are guaranteed to fully understand the mechanisms involved.
Key words: muramidase, broiler, ileal digestibility, 16S rRNA gene, microbiota
INTRODUCTION
A fast growth rate and high feed efficiency are the 2 main targets in modern poultry production. For optimum performance of birds, important factors affecting gastrointestinal health like genetic potential of the birds, quality of the diets, environmental conditions, and disease outbreaks, should be taken into consideration (Yegani and Korver, 2008). Actually, in the modern poultry industry, multi-environment selection is employed by the breeding companies (Neeteson, 2010; Kapell et al., 2012). In addition, an effective functionality of the gastrointestinal tract (GI) is very important in determining animal performance. Optimal GI functionality has been recently defined as a steady state where the microbiome and the intestinal tract (host) exist in symbiotic equilibrium, allowing the maintenance of key physiological functions resulting in improved health and welfare and performance (Celi et al., 2017). Among all components involved in GI functionality and health, the composition and metabolic activity of the GI tract microbiota have a significant impact on the health of the host due to their influence on nutrient absorption, intestinal physiology, immune response, and consequently, resistance to pathogen colonization (Apajalahti et al., 1998; Lu et al., 2003; Santos et al., 2008).
While probiotics, essential oils, organic acids, or prebiotics are used to help stabilizing the gut microbiota, little attention has been paid so far on the massive turnover and dead of the bacterial populations resulting in high amounts of bacterial cell wall fragments, containing peptidoglycans (PGN), that take place in the GI tract (Johnson et al., 2013). PGNs are the mayor structural components of the cell wall, uniquely found in bacteria, and considered as conserved products of bacterial metabolism and activity modulators in the GI tract (Humann and Lenz, 2009).
Muramidase (EC 3.2.1.17), also known as lysozyme or N-acetylmuramidase, belongs to the family of glycosyl hydrolytic enzymes. Muramidase cleaves the β-1,4-glycosidic linkages between N-acetylmuramic acid and N-acetyl glucosamine, the basic elements in the carbohydrate backbone of PGN from bacterial cell walls. The most studied muramidase is the one abundantly found in hen egg white, however, many muramidases are naturally found in a great variety of animal secretions, plants, or micro-organisms. Muramidases are ubiquitous and occur naturally in the GI tract of animals (Sahoo et al., 2012).
Studies with nursery pig using diets supplemented with muramidase (isolated from hen egg white) resulted in ameliorated growth performance probably due to improvements on histomorphology of small intestine (Oliver and Wells, 2013, 2015). Contributing to better gut integrity as well as stronger nonspecific immune response could also explain the performance improvements (Oliver and Wells, 2015; Long et al., 2016). Recently, it was demonstrated that dietary supplementation of a novel microbial muramidase (Muramidase 007) in broiler diets improved the performance variables, increased the number of lactobacilli without disturbing the rest of the functional microbiota in the caecum of broilers, while proven safe for the chickens in a tolerance study (Lichtenberg et al., 2017). Moreover (Goodarzi Boroojeni et al., 2019) supplementing increasing dosis of this muramidase reported improvements in feed conversion ratio (FCR) and apparent ileal digestibility (AID) of nutrients.
The present study aimed to evaluate the impact of dietary supplementation of Muramidase 007 on the GI functionality of broiler chickens by measuring AID of nutrients, intestinal histomorphology, vitamin A content in plasma, microbial ecosystem and metabolism, and animal performance variables.
MATERIALS AND METHODS
The in vivo experimental procedure of this study was performed at the Experimental Unit of the Universitat Autònoma de Barcelona (UAB) and received prior approval from the Animal and Human Experimental Ethical Committee of this Institution. The treatment, management, housing, husbandry and slaughtering conditions strictly conformed to European Union Guidelines (Directive 2010/63/EU).
Muramidase
The novel muramidase used in this study was selected through a discovery program. The gene coding for this muramidase (denominated Muramidase 007) from the fungus Acremonium alcalophilum (strain 114.92) was expressed in an industrial production host, produced in enough amounts and characterized as described by Lichtenberg et al. (2017). This product was produced by Novozymes A/S (Bagsvaerd, Denmark).
Location and Housing
Animals were housed in one single room with 16 floor pens (8 pens (1.5 m × 1 m) at each side of the room). The environmental conditions (lighting program, temperature, relative humidity and ventilation rates) were controlled accordingly to the Ross broiler management guidelines. Animals disposed of nipple drinkers (3 drinkers/pen) and manual pan feeders (1 pan/pen). Litter material was wood shavings.
Animals and Diets
A total of 480 one-day-old male broiler chickens (Ross 308), were obtained from a local hatchery (Pondex SAU, Lleida, Spain), already vaccinated in ovo against Gumboro and Marek diseases. Additionally, chicks were vaccinated against coccidiosis (coarse spray; Hypracox, Amer, Spain) and infectious bronchitis (fine spray) at day 1 after hatching. The birds were individually wing-tagged, weighted and randomly distributed in 16 pens (30 chicks per pen).
Each pen was allocated to one of 2 experimental treatments: A control diet (CTR) or CTR diet supplemented with Muramidase 007 (MUR) at 35,000 muramidase units (LSU(F))/kg. During the experimental period the birds received a feeding program of 2 phases: a starter diet from 0 to 21 d (in crumble form) and a grower diet from 21 to 36 d (in pellet form). Diets were formulated to meet or exceed FEDNA (2008) requirements, as it figures in Table 1. Basal diets did not contain any feed enzymes (other than Muramidase 007 in the experimental diet), coccidiostats, veterinary antibiotics or any other growth promoters. In addition, titanium dioxide (TiO2) was added as indigestible marker at 0.5%.
Table 1.
Ingredient and nutrient composition of basal diets.
| Ingredients (%, as-fed basis) | Starter | Grower | ||
|---|---|---|---|---|
| Soybean meal 48 | 34.97 | 27.23 | ||
| Maize/Corn | 23.71 | 31.30 | ||
| Wheat | 20.00 | 20.00 | ||
| Rye | 12.00 | 12.00 | ||
| Soybean oil | 4.86 | − | ||
| Dicalcium phosphate | 1.79 | 1.47 | ||
| Fat 5 FYSFEED1 | − | 5.54 | ||
| Calcium carbonate | 0.92 | 0.80 | ||
| Sodium chloride | 0.40 | 0.36 | ||
| Mineral-vitamin premix2 | 0.30 | 0.30 | ||
| DL-methionine | 0.27 | 0.23 | ||
| L-lysine | 0.17 | 0.19 | ||
| Choline chloride | 0.04 | − | ||
| L-threonine | 0.04 | 0.05 | ||
| Ethoxyquin 66%3 | 0.02 | 0.02 | ||
| TiO2 | 0.50 | 0.50 | ||
| Carophyll Yellow4 | 0.006 | 0.006 | ||
| Calculated nutrients (%, DM basis) | ||||
| Calcium | 0.95 | 0.80 | ||
| Digestible phosphorus | 0.45 | 0.38 | ||
| Digestible lysine | 1.20 | 1.02 | ||
| Digestible methionine + cysteine | 0.87 | 0.76 | ||
| Digestible threonine | 0.76 | 0.66 | ||
| Analyzed nutrients (%, DM basis) | CTR5 | MUR6 | CTR5 | MUR6 |
| Dry Matter | 91.49 | 91.26 | 90.27 | 90.35 |
| Crude protein | 24.10 | 23.48 | 20.07 | 20.23 |
| Crude fat | 6.60 | 6.36 | 7.08 | 7.07 |
| Crude fiber | 3.02 | 3.58 | 2.87 | 2.91 |
| Ash | 6.03 | 6.17 | 5.56 | 5.42 |
| Gross energy (kcal/kg) | 4157 | 4103 | 4096 | 4090 |
| Muramidase activity (LSU(F)/kg) | 1089 | 33,025 | 962 | 28,879 |
Fat 5 FYSFEED (SYSFEED, Granollers, Spain) is a commercial source of animal fat obtained by mechanical pressing after cooking of slaughterhouse by-products coming from animals declared fit from human consumption before and after slaughter.
One kg premix contains vitamin A (all-trans retinol/retynil acetate/retynil palmitate/β-carotene): 3333,334 IU; vitamin D3: 1600,000 IU; vitamin E (dl-α-tocopheryl acetate/dl-α-tocopherol/d-α-tocopherol): 15,000 IU; vitamin K3: 1,000 mg; vitamin B1: 1,000 mg; vitamin B2: 3,000 mg; vitamin B6: 1,500 mg: vitamin B12: 13.3 mg; folic acid: 600 mg; biotin: 50 mg; calcium pantothenate: 5,500 mg; nicotinic acid: 17,000 mg; Mn: 30,000 mg; Zn: 22,000 mg; I: 400 mg; Fe: 18,000 mg; Cu: 4,000 mg; Se: 60 mg; ethoxyquin: 1,333 mg; fumaric acid: 25,0000 mg; and malic acid: 20,000 mg.
Ethoxyquin antioxidant produced by ITPSA (Barcelona, Spain).
Carophyl yellow (10% Apo-ester; DSM Nutritional Products Ltd, Kaiseraugst, Switzerland).
CTR: Control diet.
MUR: Muramidase supplemented diet at 35,000 LSU (F)/kg.
Experimental Procedures
Individual weight of the animals and feed residuals (by pen) were registered at days 0 (arrival), 9 (first sampling), 21 (change of diet), and 36 (final day-second sampling) to monitor individual body weight (BW) as well as the feed intake along the study. From these values the average daily feed intake (ADFI), average daily weight gain (ADG), and the FCR were calculated.
At 9 d of live, 21 birds per cage were randomly selected and euthanized by decapitation, and representative samples of mid-jejunum tissue, ileal digesta and blood, were taken. At 35 d of age, the remaining birds were euthanized, and the same procedure was repeated.
Jejunal tissue was collected from 1 bird per pen for histological analysis. Content from different intestinal segments: crop, ileum and cecum were collected; individually from 2 birds per pen, 1 for classical microbiology and the other for microbiota (1 cecum) and short-chain fatty acids (SCFA) analysis (other ceca). Moreover, representative sample of terminal ileum content of each cage was taken (21 birds at 9 d; 7 birds at 36 d) for AID analyses. Digesta was homogenized, kept frozen at −20°C, freeze-dried, grounded, and kept at 5°C until analyzed.
Pooled sample of blood was sampled via venipuncture (2 or 3 animals on 9 d and individual bird on 36 d from each pen) and was taken in heparinized tubes for vitamin A analyses.
Analytical Methods
AID
AID was calculated using the index marker for a 100% recovery. Analytical determinations of feeds and digesta were performed accordingly to the Association of Official Agricultural Chemists (AOAC) International methods (2005): dry matter (Method 934.01) by desiccating the sample in an air-forced oven at 103°C for 24 h. Organic matter (OM) content was gravimetrically measured by placing samples in a muffle furnace at 550°C for 4 h, (ash, Method 942.05), crude fat (Method 2003.05 for feed and with previous acid hydrolysis, following method 954.02 of the AOAC for excreta) and gross energy content (IKA-Kalorimeter system C4000, Staufen, Germany). The fatty acid (FA) content of the feed and digestive content was determined according to the method of Sukhija and Palmquist (1988). This analytic procedure consists of a direct transesterification (the lipid extraction and FA methylation is achieved in only one step). Samples were incubated at 70°C with methanolic hydrochloric acid (a mixture of methanol and acetyl chloride) for the methylation. After the extraction and methylation potassium carbonate and toluene were added to separate the organic layer. Nonadecanoic acid (C19; Sigma-Aldrich Chemical Co., St. Louis, MO) was used as internal standard. The final extract was injected in a gas chromatograph (HP6890, Agilent Technologies, Waldbronn, Germany) following the method conditions previously described by Cortinas et al. (2004). FAs were identified by matching their retention times with those of their relative standards (Supelco 37 component FAME Mix, Sigma-Aldrich Co., St. Louis, MO) and quantified by internal normalization. Nonadecanoic acid was used for the calibration curves and quantification of FA. Total FAs (TFA) content was calculated as the sum of individual FAs. Nitrogen was analyzed by the DUMAS method. Analysis of Ti of the Titanium dioxide (TiO2) marker was performed by atomic absorption spectroscopy (AAS). The AID of dry matter (DM), OM, nitrogen (N), FA, and energy (E) was calculated as follows:
Where [Ntr]M is the nutrient content in the ileal digesta, [Ntr]D is the nutrient content in the diet, [Ti]M is the concentration of Ti in the ileal digesta, and [Ti]D is the concentration of Ti in the diet.
Vitamin A
Plasma proteins were precipitated with ethanol. Retinol was extracted from the aqueous suspension with n-hexane/butylated hydroxytoluene solution. After centrifugation, an aliquot of the organic phase was dried, re-dissolved in solvent solution and injected into a reversed-phase (C18) HPLC system. The detection was performed with a fluorescence detector.
Classical Microbiology and Jejunal Histomorphology
Colony forming units (cfu) count of enterobacteria, lactobacilli and clostridia, in the content of crop, ileum and cecum was performed by selective growth medium. Enterobacteria were counted after 24 to 48 h of incubation in MacConkey agar. Lactic acid bacteria were determined in MRS agar and clostridia in SPS Agar used for isolation and enumeration of sulphite‐reducing clostridia. To evaluate possible changes in the microbiota metabolism, the concentration of SCFA was analyzed in cecal samples by gas chromatography following the method of Jouany (1982).
Histological analysis was performed in tissue samples from jejunum fixed by immersion in 10% formalin, paraffin-embedded, and sections stained with hematoxylin/eosin. Measurements analyzed included villus height (μm), crypt depth (μm), villus: crypt ratio, villus width (μm), villus surface (mm2), intraepithelial lymphocytes (IEL) (number cells/100 μm), goblet cells number (GC) (cells/100 μm), and number of mitoses (cells/100 μm).
Molecular Microbiology
DNA from cecal samples was extracted using the QIAamp DNA Stool Mini Kit (Qiagen, Toronto, Canada) according to the manufacturer's instructions. The global structure, dynamics and functionality of the cecal microbial populations was analyzed by high throughput sequencing, targeting V3-V4 region of 16S rRNA, using the kit MiSeq® Reagent Kit v2 (500 cycle) (MiSeq, Illumina, San Diego, CA).
Primers used in the construction of libraries were the following (generating amplicons of putative 460 bp):
F-5′-TCGTCGGCAGCGTCAGATGTGTATAAGA GACAGCCTACGGGNGGCWGCAG
R-5′-GTCTCGTGGGCTCGGAGATGTGTATAAG AGACAGGACTACHVGGGTATCTAATCC
Sequence reads of 16S rRNA gene generated from MiSeq Illumina® system were processed on QIIME v.1.9.1. The quality filter of already demultiplexed sequences was performed at a maximum unacceptable Phred quality score of Q20. Resulting reads were clustered to operational taxonomic unit (OTU) using uclust algorithm with 97% sequence similarity and subsampling pick open reference method (Rideout et al., 2014) at 10% of sequences subsampled. Representative sequences were assigned to taxonomy against bacterial 16S GreenGenes v.13.8 reference database at a 90% confidence threshold and sequence alignment and phylogenetic tree building were obtained through uclust and FastTree. Thereafter, chimeric sequences were removed with ChimeraSlayer with default settings and further quality filtering consisted in removing singletons and OTUs with relative abundance across all samples below 0.005% as recommended by Bokulich et al. (2013).
Statistical Analysis
The results are expressed as means with their standard errors unless otherwise stated. Data were analyzed with ANOVA using the GLM procedure of SAS and the following statistical model:
Where Yij is the dependent variable, µ is the overall mean, Bi is the additive effect, and ɛi is the residual error effect.
When frequencies were analyzed, the Fisher's exact test was used. For the microbiological data, the plate counts values were log-transformed. All statistical analyses were performed using the Statistical Analysis Software SAS version 9.2 (SAS Institute Inc., Cary, NC). The pen was considered as the experimental unit for all the variables analyzed except for classical microbiology and histology. In those analyses, the bird was considered the unit since only 1 bird per pen was sampled. The α level used for the determination of significance for all the analysis was P = 0.05. The statistical trend was also considered for P-values > 0.05 and < 0.10.
Biostatistics of quality filtered sequences of the 16S rRNA gene were performed using an open source software (R v.3.4.0; R Core Team, 2013) after importing the OTU table to R using the phyloseq package. Diversity and ordination analysis (non-multidimensional scaling, NMDS) were performed using the vegan package at OTU level. Richness and alpha diversity were calculated with raw counts while beta diversity and ordination analysis were obtained with relative abundances. Alpha diversity consisted of calculation of three related indexes: Shannon, Simpson and Simpson inverse. For beta diversity, betadiver() function based on Whittaker index was used. To compare any differential effects from treatments, an ANOVA analysis was performed for alpha and beta diversities. Bray-curtis distance matrix was calculated from the dissimilarities between pairs of samples and the relative position of each sample was projected and analyzed in the NMDS plot. Finally, read counts were normalized with the metagenomeSeq package, that uses a cumulative-sum scaling where raw counts are divided by the cumulative sum counts into a particular quantile, by which differential abundance analysis under a zero-inflated Gaussian model could be performed. To achieve that, taxa were aggregated at Phylum and Genus level and expressed as log10 (n + 1).
RESULTS
The trial was successfully carried out and animals showed good health along the whole study. Mortality was very low along the trial being 0.83% and 1.25% for CTR and MUR, respectively with no difference between treatments.
Table 2 shows the evolution of BW, ADG, ADFI, and FCR along the study. There were no differences between treatments in the BW or in the ADG although final BW showed numerical values (P = 0.172) with the addition of MUR. Moreover, animals receiving MUR in the diet showed higher ADFI (P = 0.060) and higher FCR (P = 0.050) when all the experimental period was considered.
Table 2.
Performance of broiler chickens fed a control diet (CTR) or the same diet supplemented with Muramidase 007 (MUR) during the starter and grower periods.
| Treatments1 |
||||
|---|---|---|---|---|
| Variables | CTR2 | MUR3 | SEM | P-value |
| BW (g) | ||||
| 0 d | 42 | 42 | 0.1 | 0.438 |
| 9 d | 259 | 259 | 2.7 | 1.000 |
| 21 d | 1005 | 1030 | 11.1 | 0.134 |
| 36 d | 2787 | 2844 | 27.9 | 0.172 |
| ADG (g/d) | ||||
| 0 to 21 d | 45.7 | 47.1 | 0.61 | 0.137 |
| 21 to 36 d | 118.8 | 119.4 | 1.60 | 0.820 |
| 0 to 36 d | 76.2 | 77.1 | 0.75 | 0.415 |
| ADFI (g/d) | ||||
| 0 to 21 d | 63.9 | 62.1 | 1.09 | 0.277 |
| 21 to 36 d | 172.0 | 165.3 | 3.22 | 0.162 |
| 0 to 36 d | 108.9 | 105.1 | 1.34 | 0.063 |
| FCR4 (g feed:g BW gain) | ||||
| 0 to 21 d | 1.40 | 1.32 | 0.04 | 0.128 |
| 21 to 36 d | 1.45 | 1.39 | 0.03 | 0.207 |
| 0 to 36 d | 1.43 | 1.36 | 0.02 | 0.045 |
Data are means for 8 replicate pens with 30 birds from days 0 to 9 and 9 birds from days 9 to 36.
CTR: Control diet.
MUR: Muramidase supplemented at 35,000 LSU (F)/kg.
Feed Conversion Ratio.
The AID (%) of E, DM, OM, N, TFA, saturated fatty acids (SFA), mono-unsaturated fatty acids, (MUFA), and poly-unsaturated fatty acids (PUFA) estimated using TiO2 as digestibility marker are shown in Table 3. The inclusion of MUR in the diets increased the digestibility of E at day 36 (P < 0.001) and shown numerical higher values at day 9 (P = 0.120). The increased amount of digested energy could be the result of the observed increase in the DM and OM digestibility at day 36 (more than 4 percent; P < 0.001). Digestibility of N showed an increase of more than 2% at day 36, although differences did not reach statistical significance (P = 0.095). Digestibility of TFA was higher with MUR but the effects were only evidenced at day 9 (P = 0.049). This increase in the digestibility with the inclusion of MUR in the diets was seen in all studied fractions: SFA (P = 0.08), MUFA and PUFA.
Table 3.
Apparent ileal digestibility (%) of energy and nutrients in broiler chickens fed a control diet (CTR) or the same diet supplemented with Muramidase 007 (MUR) during the starter and grower periods.
| Treatments2 |
|||||
|---|---|---|---|---|---|
| Apparent ileal digest. (%)1 | Day | CTR3 | MUR4 | SEM | P-value |
| Energy | 9 | 71.2 | 72.4 | 0.52 | 0.123 |
| 36 | 71.8 | 75.6 | 0.50 | <0.001 | |
| Dry Matter | 9 | 68.4 | 69.6 | 0.45 | 0.085 |
| 36 | 68.1 | 72.3 | 0.49 | <0.001 | |
| Organic Matter | 9 | 69.7 | 70.9 | 0.44 | 0.077 |
| 36 | 69.8 | 74.0 | 0.47 | <0.001 | |
| Nitrogen | 9 | 82.2 | 82.9 | 0.40 | 0.264 |
| 36 | 75.2 | 77.5 | 0.91 | 0.095 | |
| Total fatty acids (TFA) | 9 | 86.5 | 89.1 | 0.85 | 0.049 |
| 36 | 88.9 | 90.3 | 0.98 | 0.324 | |
| Saturated fatty acids (SFA) | 9 | 61.5 | 67.6 | 2.29 | 0.080 |
| 36 | 83.9 | 85.1 | 1.37 | 0.530 | |
| Mono-unsaturated fatty acids (MUFA) | 9 | 82.4 | 86.3 | 1.29 | 0.048 |
| 36 | 91.3 | 92.5 | 0.96 | 0.407 | |
| Poly-unsaturated fatty acids (PUFA) | 9 | 95.6 | 96.7 | 0.26 | 0.012 |
| 36 | 90.6 | 92.2 | 0.85 | 0.208 | |
Apparent ileal digestibility was estimated using TiO2 as digestibility marker.
Data are means for 8 replicate pens with 19 pooled birds from days 9 and 7 pooled birds from day 36.
CTR: Control diet.
MUR: Muramidase supplemented at 35,000 LSU (F)/kg.
Regarding plasma vitamin A concentration, it was found an increase in total retinol at day 9 with the supplementation of MUR (MUR: 615.6 ng/mL vs. CTR: 521.4 ng/mL; P = 0.040). At day 36, both diets showed higher values, without differences (CTR: 775.5 ng/mL and MUR: 740.9 ng/mL; P = 0.556).
Histomorphological observations in jejunum are shown in Table 4. None of the experimental diets modified the villus height or crypt depth, neither the villus/crypt ratio. Increases (P < 0.05) were detected with the MUR treatment at day 36 in IEL and GC, regardless if they were expressed in absolute or relative terms.
Table 4.
Histomorphometry of jejunum in broiler chickens fed a control diet (CTR) or the same diet supplemented with Muramidase 007 (MUR) during the starter and grower periods.
| Treatment1 |
|||||
|---|---|---|---|---|---|
| Variables | Day | CTR2 | MUR3 | SEM | P-value |
| Villus height (μm) | 9 | 442.3 | 444.1 | 15.60 | 0.936 |
| 36 | 1027.8 | 996.3 | 38.50 | 0.572 | |
| Crypt depth (μm) | 9 | 147.5 | 157.6 | 6.67 | 0.303 |
| 36 | 185.3 | 170.5 | 6.08 | 0.108 | |
| Villus:crypt ratio (μm: μm) | 9 | 3.1 | 2.9 | 0.13 | 0.288 |
| 36 | 5.7 | 6.1 | 0.23 | 0.258 | |
| IEL4 (cells) | 9 | 15.4 | 18.0 | 2.59 | 0.495 |
| 36 | 46.0 | 59.6 | 2.49 | 0.002 | |
| GC5 (cells) | 9 | 22.1 | 23.6 | 1.61 | 0.500 |
| 36 | 57.6 | 72.7 | 2.96 | 0.003 | |
n = 8 animals.
CTR: Control diet.
MUR: Muramidase supplemented at 35,000 LSU (F)/kg.
IEL: intraepithelial lymphocytes.
GC: goblet cells regardless they were expressed in absolute or relative terms.
Regarding possible changes induced by the experimental diets on cecal profile and concentration of total SCFA, no statistically significant differences among treatments on the profile, concentration of SCFA or their molar percentage at any of the sampling days were detected (data not shown).
Regarding possible effects on microbiota, neither lactobacilli nor enterobacteria (log cfu/g fresh matter) were modified by the experimental treatments in any gastrointestinal section and any sampling day (Table 5). For clostridia, as plate counts were in some animals below detection level of the method, a frequency analysis was performed (Table 6). By this mean, it could be seen that MUR tended to decrease the number of animals with detectable clostridia in cecum at day 9 (P = 0.060).
Table 5.
Plate counts (log cfu/g fresh matter) for different microbial groups in crop, ileum, and cecum digesta in broiler chickens fed a control diet (CTR) or the same diet supplemented with Muramidase 007 (MUR) during the starter and grower periods.
| Treatment1 |
||||||
|---|---|---|---|---|---|---|
| Section | Day | Microbial group | CTR2 | MUR3 | SEM | P-value |
| Crop | 9 | Lactobacillus | 8.1 | 7.9 | 0.75 | 0.799 |
| Enterobacteria | 6.5 | 6.9 | 1.08 | 0.810 | ||
| 36 | Lactobacillus | 7.5 | 7.6 | 0.35 | 0.805 | |
| Enterobacteria | 4.5 | 5.3 | 0.37 | 0.176 | ||
| Ileum | 9 | Lactobacillus | 8.1 | 8.3 | 0.60 | 0.885 |
| Enterobacteria | 5.3 | 5.8 | 0.75 | 0.645 | ||
| 36 | Lactobacillus | 9.5 | 8.8 | 0.40 | 0.201 | |
| Enterobacteria | 7.1 | 6.4 | 0.49 | 0.296 | ||
| Cecum | 9 | Lactobacillus | 11.0 | 9.9 | 0.62 | 0.219 |
| Enterobacteria | 11.3 | 11.4 | 0.37 | 0.815 | ||
| 36 | Lactobacillus | 10.5 | 11.3 | 0.42 | 0.224 | |
| Enterobacteria | 11.3 | 11.1 | 0.38 | 0.821 | ||
n = 8 animals.
CTR: Control diet.
MUR: Muramidase supplemented at 35,000 LSU (F)/kg.
Table 6.
Number of animals with detectable number of clostridia along the gastrointestinal tract (minimum level of detection 10 cfu/g). Broiler chickens were fed a control diet (CTR) or the same diet supplemented with Muramidase 007 (MUR) during the starter and grower periods.
| Treatment1 |
||||
|---|---|---|---|---|
| Clostridium (+) | Day | CTR2 | MUR3 | P-value |
| Crop | 9 | 4/8 (4.4) | 5/8 (5.3) | 0.343 |
| 36 | 4/8 (4.4) | 6/8 (6.2) | 0.245 | |
| Ileum | 9 | 7/8 (7.1) | 3/8 (3.5) | 0.056 |
| 36 | 6/8 (6.2) | 6/8 (6.2) | 0.431 | |
| Cecum | 9 | 7/8 (7.1) | 5/8 (5.3) | 0.246 |
| 36 | 5/8 (5.3) | 4/8 (4.4) | 0.343 | |
n = 8 animals.
CTR: Control diet.
MUR: Muramidase supplemented at 35,000 LSU (F)/kg.
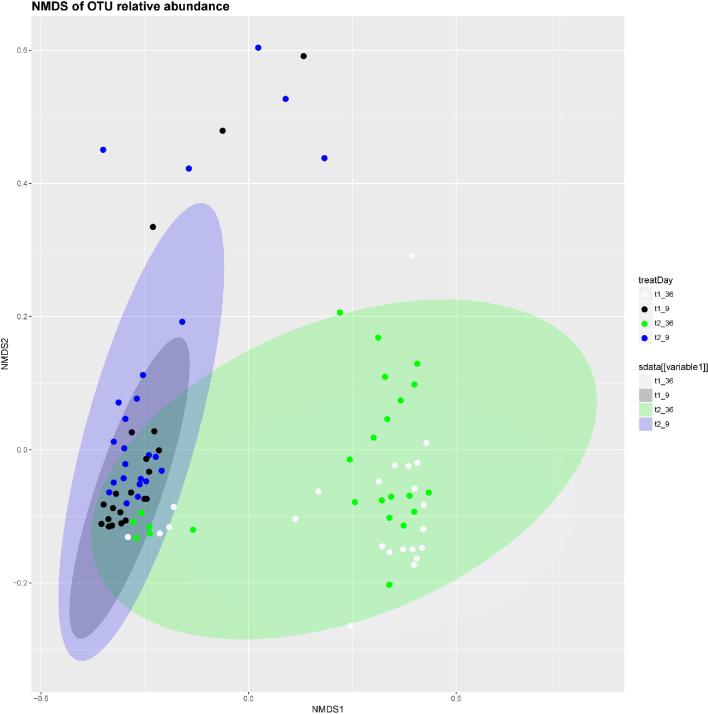
The analysis of the cecal microbiota profile by sequencing of the V3-V4 region of the 16S rRNA gene revealed relevant structural changes related to the development of the cecal microbiota with age. Figure 1 shows the community structure based on Bray-Curtis distance matrix with OTUs. A clear impact of the age was observed on cecal microbiota structure being clustered closer microbial structures of day 9 than those of day 36, coincident with numerical higher beta diversity value for the later (data not shown). Interestingly, at day 36 a group of animals from both treatments clustered separately to the rest (4 from CTR treatment and 6 from MUR treatment) with a profile closer to those animals of day 9, suggesting that these animals had not evolved to a mature ecosystem. Nonetheless, animals of day 9 presented higher richness range (600 to 1000 OTU) than those from day 36 (250 to 800 OTU), despite similar number of reads between both ages. Phyla distribution was similar between ages, being Firmicutes (60 and 55% for 9 and 36 d, respectively) and Bacteroidetes (26 and 35% for 9 and 36 d, respectively) the main phyla registered. When analyzing differences in relative abundance of genera (Tables 7 and 8), it was registered a relevant increase in the percentage of Bacteroides with age (from 7 to 29%). Regarding to changes promoted by experimental diets, animals receiving MUR showed a decrease in alpha diversity (Simpson Index (P = 0.052); Shannon index (P = 0.091); Simpson inverse index (P = 0.001)) but no differences were detected in beta diversity. At day 36 no changes were detected in alpha or beta diversity. When differences in abundance of taxa promoted by diets were analyzed, no changes were seen in phyla at day 9, nor at day 36. Only minor changes were found in genera at day 36 (Table 8) with an increase in Lactobacillus (3.28 ± 2.319% vs. 4.90 ± 1.087%) and cc-115 (0.01 ± 0.003% vs 0.15 ± 0.046%) genera when analyzed as normalized counts (P < 0.05).
Figure 1.
Community structure based on Bray-Curtis distance matrix. Non-metric multidimensional scaling (NMDS) of operational taxonomic unit (OTU) relative abundances in broiler chickens fed a control diet (CTR) or the same diet supplemented with Muramidase 007 (MUR) during the starter and grower periods. Data are clustered by the age of the animal (sampling days at 9 and 36 d of life) and the experimental diets (CTR diet and MUR diet). Black: CTR day 9; blue: MUR day 9; white; CTR day 36; and green: MUR day 36.
Table 7.
Microbiota composition at the genus level for the 15 most abundant genera in cecal digesta of broilers of 9 d of live receiving control diet (CTR) or the same diet supplemented with Muramidase (MUR). Results are expressed as relative abundance (% ± SE) by diet. No statistical differences were found between experimental diets.
| Treatment1 |
||
|---|---|---|
| Genus | CTR2 | MUR3 |
| Bacteroides | 7.45 ± 0.601 | 5.89 ± 0.572 |
| Ruminococcus | 3.98 ± 0.465 | 7.48 ± 2.114 |
| Oscillospira | 4.13 ± 0.420 | 4.82 ± 0.491 |
| Prevotella | 4.34 ± 0.350 | 3.35 ± 0.340 |
| Lactobacillus | 3.28 ± 1.267 | 2.88 ± 0.887 |
| Parabacteroides | 3.23 ± 0.266 | 2.58 ± 0.259 |
| Akkermansia | 1.99 ± 0.974 | 1.07 ± 0.197 |
| Ruminococcus | 1.54 ± 0.207 | 1.19 ± 0.151 |
| Coprococcus | 1.44 ± 0.172 | 1.94 ± 0.608 |
| Anaerotruncus | 0.58 ± 0.206 | 0.60 ± 0.236 |
| Enhydrobacter | 0.72 ± 0.071 | 0.59 ± 0.075 |
| Clostridium | 0.40 ± 0.173 | 0.30 ± 0.063 |
| Bifidobacterium | 0.72 ± 0.067 | 0.51 ± 0.061 |
| Dorea | 0.45 ± 0.101 | 0.41 ± 0.094 |
| Actinobacillus | 0.57 ± 0.058 | 0.45 ± 0.057 |
n = 21 for CTR, and n = 24 for MUR.
CTR: Control diet.
MUR: Muramidase supplemented at 35,000 LSU (F)/kg.
Table 8.
Microbiota composition at the genus level for the 15 most abundant genera in cecal digesta of broilers of 36 d of live receiving control diet (CTR) or the same diet supplemented with Muramidase 007 (MUR). Results are expressed as relative abundance (% ± SE) by diet.
| Treatment1 |
||
|---|---|---|
| Genus | CTR2 | MUR3 |
| Bacteroides | 32.67 ± 2.253 | 24.41 ± 1.801 |
| Oscillospira | 4.21 ± 0.409 | 4.90 ± 0.316 |
| Lactobacillus | 3.28b ± 2.319 | 4.90a ± 1.087 |
| Akkermansia | 3.83 ± 0.714 | 2.08 ± 0.370 |
| Sutterella | 2.22 ± 0.290 | 1.96 ± 0.323 |
| Ruminococcus | 1.46 ± 0.210 | 2.64 ± 0.396 |
| Megamonas | 0.92 ± 0.574 | 0.69 ± 0.261 |
| Ruminococcus | 1.28 ± 0.126 | 1.26 ± 0.096 |
| Prevotella | 0.89 ± 0.332 | 1.47 ± 0.430 |
| Coprococcus | 0.64 ± 0.113 | 1.67 ± 0.408 |
| Parabacteroides | 0.65 ± 0.242 | 0.99 ± 0.275 |
| Anaerotruncus | 0.73 ± 0.261 | 0.41 ± 0.116 |
| Blautia | 0.18 ± 0.026 | 0.44 ± 0.162 |
| Bifidobacterium | 0.15 ± 0.060 | 0.21 ± 0.061 |
| Dorea | 0.20 ± 0.035 | 0.16 ± 0.018 |
n = 24 for CTR and n = 25 for MUR.
CTR: Control diet.
MUR: Muramidase supplemented at 35,000 LSU (F)/kg.
Means within a row lacking a common superscript differ (P < 0.05).
DISCUSSION
Effect on Growth Performance and AID
In the present study, the inclusion of the Muramidase 007 in broiler chicken diets, throughout the whole growing period, had a relevant impact on their FCR at the end of the trial. FCR improved from 1.43 to 1.36 g feed: g BW gain (P = 0.045) at day 36. These data agree with those obtained by Lichtenberg et al. (2017) and Goodarzi Boroojeni et al. (2019), who reported a significant improvement in BW gain and FCR when Muramidase 007 was included at different dose levels. Other studies have also reported similar effects on growth performance when other muramidases, from different origins, were included on broiler diets. Humphrey et al. (2002) found that chickens fed a conventional diet containing 10% modified rice expressing lysozyme had an improved feed efficiency (P = 0.01). Liu et al. (2010) reported that supplemental hen egg white lysozyme (HEWL) significantly improved FCR and tended to increase weight gain (P = 0.082). Abdel-Latif et al. (2017) also reported that BW gain and FCR were significantly improved when HEWL was included in poultry diets (P < 0.05). However, Gong et al. (2016) reported no effect found on growth variables by the inclusion of HEWL on broiler diets (P > 0.05).
This improvement observed in FCR is consistent with the improvement reported in ileal apparent digestibility of energy, DM, and OM that also was reported by Goodarzi Boroojeni et al. (2019) for this muramidase. It can be hypothesized that an improved digestibility of nutrients could be due to a better intestinal absorption (Noy and Skylan, 1995). However, attending to the histomorphological effect of the diets, we were not able to show changes in the height of jejunal villi or crypt depth despite Goodarzi Boroojeni et al. (2019) were able to find an increase in ileal villus length to crypt depth ratio with this same muramidase. However, at the end of the study, we observed an increment on the number of GC in the jejunum of chickens fed the MUR diet. Mucus constitutes a protective barrier against microorganisms, physical and chemical attacks, and has the role of lubrication of the digestive tract (Deplancke and Gaskins, 2001) it is considered as a major component of innate immunity. We could therefore hypothesize that the increase observed in GC, and presumably in the mucus layer density, could have facilitated a better nutrient digestion and absorption, explaining the improved efficiency of feed utilization. However it is truth that the role of intestinal mucus is complex and still unknown, and that a higher numbers of goblet cells not necessarily means a higher amount of mucus. Mucus layer is known to provide a niche for bacterial colonization offering attachment site and carbon source for resident bacteria. Both, bacteria and the host can influence the composition for the mucus layer, and mucus can turn in a way to the host to modulate its indigenous microbiota although mucus-derived components could also offer cues for adaptation and pathogenesis of some bacteria (Sicard et al., 2017). Some authors also have described stimulation of mucin productions by molecular patterns such as lipopolysaccharide (Petersson et al., 2011) relating mucin production and inflammation. Taking all this in consideration is therefore simplistic to draw conclusions just from an observed increase in GC.
Our results show that inclusion of the Muramidase 007 increased ileal apparent digestibility of fat (TFA, SFA (P = 0.08), MUFA and PUFA), particularly at day 9 suggesting an early development of intestinal capacity to digest fat nutrient. Accordingly, plasma levels of vitamin A also were increased at day 9 with Muramidase 007, being another indication of a better digestion and absorption of fats. In poultry, the jejunum is the major site of digestion and absorption of fat, with the digestion continuing in the upper ileum (Ravindran et al., 2016). It has been proposed that he immature digestive system of young chickens could lead to the misuse of nutrients (Kaczmarek et al., 2014) and particularly at first ages when chickens show an impaired digestion of fat. According to Kussaibati et al. (1982), the intestinal microbiota would be responsible for the decreased apparent fecal digestibility of vegetable lipids in chickens younger than 3 wk. This reduction in fat digestibility would be the result of the deconjugation of the bile salts by certain bacterial species, in particular lactobacilli. Since the conjugated bile salts serve for the formation of micelles, their low concentration would reduce lipid solubilization and thus their absorption, in particular those containing long chain saturated fatty acids (Gabriel et al., 2005). In this regard, the analysis of the cecal structure by the 16S rRNA gene sequencing revealed important structural changes in the microbiota between days 9 and 36 with significant increases with age in the members of Bacteroides genus.
Effects on Intestinal Architecture and Immune Response
As we have seen before, improvements in AID of nutrients were not related to changes in intestinal histomorphometry in terms of villus: crypt ratio. However, significant changes in the number of GC and IEL were found with supplementation of Muramidase 007 at day 36 that could reflect an intestinal response conditioned to differential luminal stimuli. Changes in the microbiota, or an increase in PGNs from a higher bacterial turnover, or other metabolites, could be behind these effects. Lichtenberg et al. (2017) showed higher number of Lactobacillus in the ceca of the broilers supplemented with Muramidase 007 compared to the un-supplemented broilers. In the present study, we were unable to demonstrate quantitative changes in lactobacilli or enterobacteria by plate counts among treatments. However, analysis by 16S rRNA gene sequencing showed a decrease in alpha diversity at day 9 and also an increase in the relative abundance of sequences from the Lactobacillus genus supplementing Muramidase 007. Therefore, it cannot be discarded that some changes in minor microbial groups or in microbiota structure could have induced those changes in the local immune response.
Changes promoted in IEL numbers were quantitatively low, being within the physiological range and could reflect an activation of the intestinal mucosal immune response of the animals without inducing inflammation. IEL are considered part of the gut-associated lymphoid tissue and provide a first line of defense against intestinal microbial invasion. These frontline lymphocytes residing within the epithelial layer are incredibly heterogeneous regarding their function and phenotype. IEL subsets can provide immune surveillance at the epithelial barrier to prevent or impair infection in the intestine either through innate-like mechanisms or as antigen-specific effectors or memory CD8αβ+ T cells. However, we are only recently starting to unravel the complex and sophisticated network of mucosal immune responses and function and regulation of these cells (Sheridan and Lefrançois, 2011).
Similarly, goblet cells contribute to the protection of the intestinal epithelium by the production and maintenance of the protective mucus blanket by synthesizing and secreting high-molecular-weight glycoproteins known as mucins. Changes in GC functions and in the chemical composition of intestinal mucus have been described in response to a broad range of luminal insults, including alterations of the normal microbiota (Montagne et al., 2004; Sicard et al., 2017). Available data indicate that intestinal microbes may affect GC dynamics and the mucus layer directly via the local release of bioactive factors or indirectly via activation of host immune cells (Deplancke and Gaskins, 2001). Changes promoted by Muramidase 007, directly or indirectly, in intestinal ecosystem or in the release of bioactive factors could therefore be behind the observed effects.
Effects on Gastrointestinal Microbial groups and Cecal Fermentation
Firmicutes and Bacteroidetes were the two main bacterial phyla in the cecum of broilers representing more than 80% of total sequencing. These values are in accordance to values described by other authors using 16S rRNA gene sequencing methodology (Kers et al., 2018). Changes observed with the age of the animals are, however, contradictory to some previous results. Whereas variation between individuals has been considered to decrease with the age of the animals (Crhanova et al., 2011), in the present study we found an increase of beta diversity with the age of animals accordingly to what has been described by other authors (Ballou et al.,2016). Nevertheless, dynamics and structure of intestinal microbiota is not only affected by age but also by other factors like diet, housing, litter, or climate (Kers et al., 2018).
Including exogenous muramidase into chicken diets, depending on dose and specificity, could have an impact on the intestinal microbiota considering that in the gut, natural endogenous muramidase is part of the primary protective factors in epithelial secretions (Harder et al., 2007; Dave et al., 2016). HEWL is more effective against gram positive bacteria, but also has been demonstrated to kill gram negative bacteria in vitro (Masschalck and Michiels, 2003). In vitro experiments with transgenic human lysozyme have shown activity against several gastrointestinal pathogens, including Clostridium perfringens (Brundige et al., 2008). Liu et al. (2010) showed that the administration of HEWL in broiler diets reduced C. perfringens, Escherichia coli and lactobacilli populations in ileal digesta of birds with and/or without and C. perfringens challenge. In the current study, the plate count results for Lactobacillus and enterobacteria did not show a significant difference with the inclusion of the microbial muramidase, in any, crop, ileum or cecum samples. However, a statistical trend for a decrease was observed for Clostridium in the ileum (P = 0.06) at day 9, suggesting a possible impact of the additive in the microbiota structure at early age, reducing the growth of this particular microbial group. Moreover, the 16 rRNA gene sequencing also was able to show that at day 9 animals receiving Muramidase 007 showed lower alpha diversity although we were not able to detect changes in particular taxonomic groups. Nevertheless, such variations were small and could be the indirect consequence of the modification of the microenvironment in the GI tract by the hydrolysis products of the muramidase activity or by indirect effects associated to the increased digestibility of nutrients.
Differences between results of these studies could be explained by the different types (origin) of the muramidases evaluated: HEWL, microbial, etc., with different properties in vitro and in vivo.
In summary, results of this study demonstrate the positive effects of the inclusion of Muramidase 007 in the diet of broiler chickens resulting in increased feed efficiency and improved FCR. This improvement can be explained by a significant improvement in the ileal digestibility of energy and nutrients. Effects on cecal microbiota and their metabolisms seems to be minimal, despite this some changes could be detected in particular microbial genera, like an increase in Lactobacillus. Additional studies might bring a better understanding of the mechanisms underlying these effects.
ACKNOWLEDGEMENTS
The Mediterranean Agronomic Institute of Zaragoza (IAMZ) of Zaragoza (Spain) is acknowledged for funding scholarships for Mounira Sais.
REFERENCES
- Abdel-Latif M.A., Ali H., Elbestawy A.R., Ghanem R., Mousa S.A., El-Hamid H.S.A. Exogenous dietary lysozyme improves the growth performance and gut microbiota in broiler chickens targeting the antioxidant and non-specific immunity mRNA expression. PLoS One. 2017;12 doi: 10.1371/journal.pone.0185153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apajalahti J.H.A., Rkilahti L.K.S.A., Maki B.R.E., Heikkinen P.J., Nurminen P.H., Holben W.E. Effective recovery of bacterial DNA and percent-guanine-plus-cytosine-based analysis of community structure in the gastrointestinal tract of broiler chickens. Appl. Environ. Microbiol. 1998;64:4084–4088. doi: 10.1128/aem.64.10.4084-4088.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballou A.L., Ali R.A., Mendoza M.A., Ellis J.C., Hassan H.M., Croom W.J., Koci M.D. Development of the chick microbiome: how early exposure influences future microbial diversity. Front. Vet. Sci. 2016;3:2. doi: 10.3389/fvets.2016.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokulich N.A., Subramanian S., Faith J.J., Gevers D., Gordon J.I., Knight R., Mills D.A., Caporaso J.G. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat. Methods. 2013;10:57–59. doi: 10.1038/nmeth.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brundige D.R., Maga E.A., Klasing K.C., Murray J.D. Lysozyme transgenic goats' milk influences gastrointestinal morphology in young pigs. J. Nutr. 2008;138:921–926. doi: 10.1093/jn/138.5.921. [DOI] [PubMed] [Google Scholar]
- Celi P., Cowieson A.J., Fru-Nji F., Steinert R.E., Kluenter A.M., Verlhac V. Gastrointestinal functionality in animal nutrition and health: New opportunities for sustainable animal production. Anim. Feed Sci. Technol. 2017;234:88–100. [Google Scholar]
- Cortinas L., Villaverde C., Galobart J., Baucells M.D., Codony R., Barroeta A.C. Fatty acid content in chicken thigh and breast as affected by dietary polyunsaturation level. Poult. Sci. 2004;83:1155–1164. doi: 10.1093/ps/83.7.1155. [DOI] [PubMed] [Google Scholar]
- Crhanova M., Hradecka H., Faldynova M., Matulova M., Havlickova H., Sisak F. Immune response of chicken gut to natural colonization by gut microflora and to Salmonella enterica serovar Enteritidis infection. Infect. Immun. 2011;79:2755–2763. doi: 10.1128/IAI.01375-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dave L.A., Hayes M., Mora L., Montoya C.A., Moughan P.J., Rutherfurd S.M. Gastrointestinal endogenous protein-derived bioactive peptides: an in vitro study of their gut modulatory potential. Int. J. Mol. Sci. 2016;17:482. doi: 10.3390/ijms17040482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deplancke B., Gaskins H.R. Microbial modulation of innate defense: goblet cells and the intestinal mucus layer. J. Clin. Nutr. 2001;73:1131S–1141S. doi: 10.1093/ajcn/73.6.1131S. [DOI] [PubMed] [Google Scholar]
- Fundación Española para el Desarrollo de la Nutrición Animal (FEDNA) FEDNA; Madrid, Spain: 2008. Necesidades nutricionales para avicultura: pollos de carne y aves de puesta. [Google Scholar]
- Gabriel I., Mallet S., Sibille P. La microflore digestive des volailles: facteurs de variation et conséquences pour l'animal. INRA Prod. Anim. 2005;18:309–322. [Google Scholar]
- Gong M., Anderson D., Rathgeber B., Isaac J.M. The effect of dietary lysozyme with EDTA on growth performance and intestinal microbiota of broiler chickens in each period of the growth cycle. J. Appl. Poult. 2016;26:1–8. [Google Scholar]
- Goodarzi Boroojeni F., Männer K., Rieger J., Pérez Calvo E., Zentek J. Evaluation of a microbial muramidase supplementation on growth performance, apparent ileal digestibility, and intestinal histology of broiler chickens. Poult. Sci. 2019;98:2080–2086. doi: 10.3382/ps/pey556. [DOI] [PubMed] [Google Scholar]
- Harder J., Gläser R., Schröder J.M. The role and potential therapeutical applications of antimicrobial proteins in infectious and inflammatory diseases. Endocr. Metab. Immune. Disord. Drug Targets. 2007;7:75–82. doi: 10.2174/187153007780832091. [DOI] [PubMed] [Google Scholar]
- Humann J., Lenz L.L. Bacterial peptidoglycan-degrading enzymes and their impact on host muropeptide detection. J. Innate Immun. 2009;1:88–97. doi: 10.1159/000181181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey B.D., Huang N., Klasing K.C. Rice expressing lactoferrin and lysozyme has antibiotic-like properties when fed to chicks. J. Nutr. 2002;132:1214–1218. doi: 10.1093/jn/132.6.1214. [DOI] [PubMed] [Google Scholar]
- Johnson J.W., Fisher J.F., Mobashery S. Bacterial cell-wall recycling. Ann. N. Y. Acad. Sci. 2013;1277:54–75. doi: 10.1111/j.1749-6632.2012.06813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapell D.N., Hill W.G., Neeteson A.M., McAdam J., Koerhuis A.N., Avendaño S. Genetic parameters of foot-pad dermatitis and body weight in purebred broiler lines in 2 contrasting environments. Poult. Sci. 2012;91:565–574. doi: 10.3382/ps.2011-01934. [DOI] [PubMed] [Google Scholar]
- Kaczmarek S.A., Rogiewicz A., Mogielnicka M., Rutkowski A., Jones R.O., Slominski B.A. The effect of protease, amylase, and nonstarch polysaccharide-degrading enzyme supplementation on nutrient utilization and growth performance of broiler chickens fed corn-soybean meal-based diets. Poult. Sci. 2014;93:1745–1753. doi: 10.3382/ps.2013-03739. [DOI] [PubMed] [Google Scholar]
- Kers J.G., Velkers F.C., Fischer E.A.J., Hermes G.D.A., Stegeman J. A, Smidt H. Host and environmental factors affecting the intestinal microbiota in chickens. Front. Microbiol. 2018;16:235. doi: 10.3389/fmicb.2018.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kussaibati R., Guillaume J., Leclercq B. The effect of gut microflora on the digestibility of starch and proteins in young chicks. Ann. Zootech. 1982;31:483–488. [Google Scholar]
- Lichtenberg J., Pérez Calvo E., Madsen K., Østergaard Lund T., Kramer Birkved F., van Cauwenberghe S., Mourier M., Wulf-Andersen L., Jansman A.J.M., Lopez-Ulibarri R. Safety evaluation of a novel muramidase for feed application. Regul. Toxicol. Pharmacol. 2017;89:57–69. doi: 10.1016/j.yrtph.2017.07.014. [DOI] [PubMed] [Google Scholar]
- Liu D., Guo Y., Wang Z., Yuan J. Exogenous lysozyme influences Clostridium perfringens colonization and intestinal barrier function in broiler chickens. Avian Pathol. 2010;39:17–24. doi: 10.1080/03079450903447404. [DOI] [PubMed] [Google Scholar]
- Long Y., Lin S., Zhu J., Pang X., Fang Z., Lin Y., Che L., Xu S., Li J., Huang Y., Su X., Wu D. Effects of dietary lysozyme levels on growth performance, intestinal morphology, non-specific immunity and mRNA expression in weanling piglets. Anim. Sci. J. 2016;87:411–418. doi: 10.1111/asj.12444. [DOI] [PubMed] [Google Scholar]
- Lu J., Idris U., Harmon B., Hofacre C., Maurer J.J., Lee M.D. Diversity and succession of the intestinal bacterial community of the maturing broiler chicken. Appl. Environ. Microbiol. 2003;69:6816–6824. doi: 10.1128/AEM.69.11.6816-6824.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masschalck B., Michiels C.W. Antimicrobial properties of lysozyme in relation to foodborne vegetative bacteria. Microbiol. 2003;29:191–214. doi: 10.1080/713610448. [DOI] [PubMed] [Google Scholar]
- Montagne L., Piel C., Lallès J.P. Effect of diet on mucin kinetics and composition: nutrition and health implications. Nutr. Rev. 2004;62:105–114. doi: 10.1111/j.1753-4887.2004.tb00031.x. [DOI] [PubMed] [Google Scholar]
- Neeteson A.M. Striking the balance. J. Anim. Breed. Genet. 2010;127:85–86. doi: 10.1111/j.1439-0388.2010.00866.x. [DOI] [PubMed] [Google Scholar]
- Noy Y., Sklan D.E. Digestion and absorption in the young chick. Poult. Sci. 1995;74:366–373. doi: 10.3382/ps.0740366. [DOI] [PubMed] [Google Scholar]
- Oliver W.T., Wells J.E. Lysozyme as an alternative to antibiotics improves growth performance and small intestinal morphology in nursery pigs. J. Anim. Sci. 2013;91:3129–3136. doi: 10.2527/jas.2012-5782. [DOI] [PubMed] [Google Scholar]
- Oliver W.T., Wells J.E. Lysozyme as an alternative to growth promoting antibiotics in swine production. J. Anim. Sci. Biotechnol. 2015;6:7. doi: 10.1186/s40104-015-0034-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersson J., Schreiber O., Hansson G.C., Gendler S.J., Velcich A., Lundberg J.O., Roos S., Holm L., Phillipson M. Importance and regulation of the colonic mucus barrier in a mouse model of colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2011;300:G327–G333. doi: 10.1152/ajpgi.00422.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2013. R: A language and environment for statistical computing. [Google Scholar]
- Ravindrana V., Tancharoenrata P., Zaefariana F., Ravindranb G. Fats in poultry nutrition: Digestive physiology and factors influencing their utilization. Anim. Feed Sci. Technol. 2016;213:1–21. [Google Scholar]
- Rideout J.R., He Y., Navas-Molina J.A., Walters W.A., Ursell L.K., Gibbons S.M., Chase J., McDonald D., Gonzalez A., Robbins-Pianka A., Clemente J.C., Gilbert J.A., Huse S.M., Zhou H.W., Knight R., Caporaso J.G. Subsampled open-reference clustering creates consistent, comprehensive OTU definitions and scales to billions of sequences. Peer. J. 2014;2:e545. doi: 10.7717/peerj.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahoo N.R., Kumar P., Bhusan B., Bhattacharya T.K., Dayal S., Sahoo M. Lysozyme in livestock: A guide to selection for disease resistance. J. Anim. Sci. Adv. 2012;2:347–360. [Google Scholar]
- Santos J.A.A., Ferket P.R., Santos F.B.O., Nakamura N., Collier C. Change in the ileal bacterial population of turkeys fed different diets and after infection with Salmonella as determined with denaturing gradient gel electrophoresis of amplified 16S ribosomal DNA. Poult. Sci. 2008;87:1415–1427. doi: 10.3382/ps.2006-00462. [DOI] [PubMed] [Google Scholar]
- Sheridan B.S., Lefrançois L. Regional and mucosal memory T cells. Nat. Immunol. 2011;12:485–491. doi: 10.1038/ni.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicard J.F., Le Bihan G., Vogeleer P., Jacques M., Harel J. Interactions of intestinal bacteria with components of the intestinal mucus. Front. Cell Infect. Microbiol. 2017;5:387. doi: 10.3389/fcimb.2017.00387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukhija P.S., Palmquist D.L. Rapid method for determination of total fatty acid content and composition of feedstuffs and feces. J. Agric. Food Chem. 1988;36:1202–1206. [Google Scholar]
- Yegani M., Korver D.R. Factors affecting intestinal health in poultry. Poult. Sci. 2008;87:2052–2063. doi: 10.3382/ps.2008-00091. [DOI] [PMC free article] [PubMed] [Google Scholar]