Abstract
In the Mekong Delta region of Vietnam, small-scale chicken farming is common. However, high levels of disease or mortality in such flocks impair economic development and challenge the livelihoods of many rural households. We investigated 61 diseased small-scale flocks (122 chickens) for evidence of infection with 5 bacteria, 4 viruses, and helminths. Serological profiles (ELISA) were also determined against 6 of these pathogens. The aims of this study were the following: (1) to investigate the prevalence of different pathogens and to compare the probability of detection of bacterial pathogens using PCR and culture; (2) to investigate the relationship between detection of organisms in birds' tissues and the observed morbidity and mortality, as well as their antibody profile; and (3) to characterize risk factors for infection with specific viral or bacterial pathogens. We used PCR to test for viral (viruses causing infectious bronchitis [IB], highly pathogenic avian influenza [HPAI], Newcastle disease, and infectious bursal disease [IBD]) and bacterial pathogens (Mycoplasma gallisepticum, Pasteurella multocida, Avibacterium paragallinarum, and Ornithobacterium rhinotracheale [ORT]). The latter two were also investigated in respiratory tissues by conventional culture. Colisepticemic Escherichia coli was investigated by liver or spleen culture. In 49 of 61 (80.3%) flocks, at least one bacterial or viral pathogen was detected, and in 29 (47.5%) flocks, more than one pathogen was detected. A. paragallinarum was detected in 62.3% flocks, followed by M. gallisepticum (26.2%), viruses causing IBD (24.6%) and IB (21.3%), septicemic E. coli (14.8%), ORT (13.1%), and HPAI viruses (4.9%). Of all flocks, 67.2% flocks were colonized by helminths. Mortality was highest among flocks infected with HPAI (100%, interquartile range [IQR]: 81.6–100%) and lowest with flocks infected with ORT (5.3%, IQR: 1.1–9.0%). The results indicated slight agreement (kappa ≤ 0.167) between detection by PCR and culture for both A. paragallinarum and ORT, as well as between the presence of cestodes and ORT infection (kappa = 0.317). Control of A. paragallinarum, viruses causing HPAI, IBD, and IB, M. gallisepticum, and gastrointestinal helminths should be a priority in small-scale flocks.
Key words: bacterial pathogen, viral pathogen, helminth, chicken, Vietnam
Introduction
In recent years, the Mekong Delta region of Vietnam (18 million inhabitants in 2017) has experienced considerable development in its chicken production sector, largely driven by the expansion of large-scale (intensive) farms. However, backyard and small-scale chicken farms remain predominant in the region; of a census population of ∼67 million chickens (2017), 42 million (61%) were being raised in backyard and small-scale commercial units (Anon, 2018b). Backyard and small-scale flocks typically consist of long-cycle native breeds that are often housed in conditions of suboptimal biosecurity, resulting in a high incidence of infectious diseases (Bell, 2009).
A recent study reported an average weekly mortality among ∼2.5% chickens in small-scale flocks in the region (Carrique-Mas et al., 2019). Much of the published research on pathogens in poultry in the Mekong Delta region has focused on detecting and characterizing highly pathogenic avian influenza (HPAI). Although overt HPAI outbreaks are now less common than at the beginning of the epidemic in 2003–2004 (Anon, 2018a), HPAI is still transmitted in the region, and outbreaks are still sporadically reported (Phan et al., 2013, Nguyen et al., 2014, Cuong et al., 2016). In addition to HPAI, global viral poultry diseases, such as Newcastle disease (ND) (Choi et al., 2014), infectious bursal disease (IBD), and infectious bronchitis (IB) (de Witt et al., 2010) are suspected to be transmitted in the area (Bui et al., 2001). These pathogens have a considerable economic impact because they can cause disease independently or in association with other bacterial or viral agents (Roussan et al., 2008). A number of bacteria such as Pasteurella multocida (Mariana and Hirst, 2000), Avibacterium paragallinarum (Chukiatsiri et al., 2010), and Mycoplasma gallisepticum (Fujisawa et al., 2019) have all been reported to circulate in Southeast Asia. The low standards of hygiene and biosecurity typical of small-scale chicken farms in the area also facilitate the presence of gastrointestinal helminth burdens in these flocks (Van et al., 2019). However, most studies to date have typically focused on single etiological agents, and the investigation of a wider panel of pathogens is required to prioritize disease control strategies.
There is a lack of information on the prevalence of pathogens circulating in small-scale chicken flocks in the Mekong Delta region of Vietnam. Because small-scale and backyard production systems are so widespread in the area, disease in these flocks may also represent a risk to larger, “intensive” production units that normally consist of birds with reduced genetic resistance.
The aims of this study were as follows: (1) to investigate the prevalence of different pathogens in diseased small-scale chicken flocks and to compare the probability of detection of bacterial pathogens using PCR and bacterial culture; (2) to investigate the potential association between detection of specific pathogens and the observed morbidity and mortality, as well as the birds' ELISA antibody profile; and (3) to investigate risk factors for infection with specific viral or bacterial pathogens. This information is crucial to help prioritize disease control strategies, including diagnostic capacity and vaccination in small-scale poultry flocks in the Mekong Delta and the greater Southeast Asian region.
Materials and methods
Study Area
The study was carried out in Dong Thap province (human population: ∼1.7 million, chicken population: ∼5.1 million in 2017), located in the Mekong Delta of Vietnam. This agroecological region is characterized by intensive production of rice, fruit trees, and the presence of (often mixed) small-scale poultry (ducks, chickens) and pig farms. The area has a tropical monsoon climate, with temperatures ranging from 25°C to 28°C, and a rainy season that generally spans from May to October.
Study Design
Flocks were recruited after running an advertisement campaign on local TV and radio stations requesting farmers raising chickens to notify the veterinary authorities should they observe unusual disease or mortality in their flocks. Flocks experiencing a cumulative morbidity of >4% or at least 5 chickens sick with clinical signs consistent with an infectious etiology were eligible for the study. Data on farm location (global positioning system coordinates), the farm owner's demographic characteristics, the number of chicken flocks, as well as data on flock age and size, morbidity, mortality, and clinical signs were collected using validated questionnaires. From each study flock, 2 representative sick or moribund chickens were selected and transported to the laboratory in Dong Thap province within 1 h. Trained veterinarians affiliated to the Sub-Department of Animal Health and Production of Dong Thap province carried out all farm visits between September 2017 and March 2019. All chickens were subjected to a diagnostic necropsy and were investigated for the presence of 5 bacterial pathogens (P. multocida, A. paragallinarum, Ornithobacterium rhinotracheale [ORT], septicemic Escherichia coli [colibacillosis], and M. gallisepticum) and 4 viral pathogens (viruses causing IB, HPAI, ND, and IBD) as well as for the presence of helminths. In addition, the chickens were investigated for their serological profile (ELISA) for 6 pathogens.
Diagnostic Postmortem Examination
In the laboratory, blood (1 mL) was obtained from chickens by puncture of the metatarsal vein. The blood was left to clot at room temperature, and the serum was collected into a 2-mL Eppendorf tube. The chickens were weighed and euthanized by cervical dislocation following American Veterinary Medical Association guidelines (AVMA, 2016). A diagnostic postmortem examination was conducted. From each bird, the main lesions were systematically described, and the bursa, spleen, and liver samples as well as upper and lower respiratory swabs were collected under aseptic conditions. In addition, the gastrointestinal tract of each bird was investigated for the presence of helminths. The types of tissue or matrix investigated, the diagnostic tests, and the pathogens investigated are shown in Table 1.
Table 1.
Tissues or matrices investigated for poultry pathogens in 61 flocks.
| Diagnostic test | Tissue/matrix | No. of flocks (chickens), no. of samples tested | Pathogen(s) |
|---|---|---|---|
| Conventional PCR | Upper respiratory Lower respiratory |
61 (122), 611 | A. paragallinarum, M. gallisepticum, ORT, viruses causing IB |
| Spleen | 61 (122), 611 | Viruses causing ND and HPAI, P. multocida | |
| Bursa | 61 (122), 611 | Viruses causing IBD | |
| Bacterial culture | Upper respiratory | 61 (122) 611 | A. paragallinarum, ORT |
| Liver/spleen | 61 (122), 611 | E. coli, P. multocida | |
| Detection of antibodies | Serum | 40 (75), 75 | P. multocida, M. gallisepticum, ORT, viruses causing IBD, IB, and ND |
| Full gastrointestinal tract examination | Gastrointestinal tract | 61 (122), 611 | Gastrointestinal helminths |
Abbreviations: HPAI, highly pathogenic avian influenza; IB, infectious bronchitis; IBD, infectious bursal disease; ND, Newcastle disease; ORT, Ornithobacterium rhinotracheale.
Pool of 2 samples (2 chickens) per flock.
Detection of Pathogens by Conventional PCR
About 1 g of spleen and bursa tissue samples was homogenized using a TissueLyser machine (Qiagen, Hilden, Germany) using silica beads. Upper and lower respiratory swab samples were vortexed thoroughly for 2 min. Specimen nucleic acids were extracted using the QIAamp cador Pathogen Mini Kit (Qiagen, Hilden, Germany). Specimen nucleic acids were screened for bacterial pathogens: A. paragallinarum, P. multocida, M. gallisepticum, and ORT by conventional PCR and viral pathogens (viruses causing ND, IBD, IB, and HPAI) by RT-PCR. Highly pathogenic avian influenza–positive samples were investigated for their hemagglutinin and neuraminidase identity (H5, N1, and N6 subtypes) by RT-PCR. The detection primers used in this study are listed in Supplemental Table 1. The PCR products of the expected length were then subjected to electrophoresis in 1% agarose gel.
Detection of Pathogens by Bacterial Culture
Swabs from the upper respiratory tract of chickens were inoculated directly into nonselective media, including blood agar and chocolate agar (Oxoid, Cheshire, England). The agar plates were then incubated for 18–30 h at 35°C ± 2°C in 5% CO2. Liver and spleen samples were cultured onto MacConkey agar and blood agar (Oxoid) and incubated at 37°C for 20–24 h. Identification of bacterial colonies was performed by matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS) (Bruker, Germany). Because the use of MALDI-TOF MS resulted in difficulties in assigning species of the Avibacterium genus, all isolates identified as belonging to this genus were further investigated by PCR using specific A. paragallinarum primers.
Serological Tests
Chicken serum samples were tested for the presence of IgY antibodies against 6 pathogens, including viruses causing IB, ND, and IBD, M. gallisepticum, ORT, and P. multocida. Antibodies against P. multocida were investigated using IDEXX Antibody Test kits (IDEXX, Westbrook, Maine), whereas IgY antibodies against other pathogens were investigated using BioChek Elisa kits (BioChek, Reeuwijk, Netherland). Positive and negative controls were included in the commercial kits. The manufacturers' guidelines were carefully followed for the calculation of serological titers. Based on the cutoff values provided by the manufacturers, the results were converted into positive or negative.
Investigation of Gastrointestinal Helminths
During the postmortem examination, the gastrointestinal tract of each bird was extracted and systematically separated into 3 parts: (1) gizzard and proventriculus; (2) small intestine (duodenum, jejunum, and ileum); and (3) ceca. The gizzard and proventriculus were dissected and examined using a binocular microscope. The small intestine and ceca were dissected in a Petri dish containing saline solution to facilitate detachment of worms from the intestinal mucosa and digestive content. All worms were isolated under a binocular microscope and were then transferred onto 70% ethyl alcohol–containing tubes. Recovered helminths were identified based on their morphological characteristics under the microscope (Soulsby, 1982, Gomes et al., 2004).
Data Analyses
Because daily flock mortality was not recorded by most farmers, the cumulative mortality since the onset of disease was calculated by dividing the total number of chickens dying by the number of chickens present at the date of onset of clinical signs. The morbidity was calculated by dividing the number of sick chickens observed by the total number of chickens in the flock at the onset of disease. Diagnostic results using different tests were compared using the Kappa statistic, which takes into account the possibility of the agreement occurring by chance. The positive predictive values (PPV) of the ELISA test results in relation to pathogen detection were calculated. The probability of positivity between the rainy (November to April) and the dry (May to October) season was compared for all diagnostic results. Linear regression models were built to investigate the potential association between age (in weeks) and serological titer (log-transformed). Logistic regression models were built to investigate the association between the detection of a particular pathogen (either by culture, PCR, or RT-PCR) and the following variables: (1) age of chicken (weeks); (2) number of chickens in the flock (log) (for normal distribution); (3) presence of more than one chicken flock in the farm; (4) experience in chicken farming of the farm owner (years); (5) ratio of chicken weight to “normal” weight; (6) presence of helminths; and (7) rainy season (May to October). The normal weight by age had been previously estimated from a study using weekly weight measurements of 10 native birds from 10 flocks in the same area that did not experience disease (Cuong et al., 2019).
Results
Clinical Signs
On average, investigated flocks had a median of 200 chickens (interquartile range [IQR]: 100–300 chickens), and chickens had a median age of 7 wk (IQR: 4–10 wk old) and weighed a median of 435 g (IQR: 200–690 g). The median number of days from the onset of clinical signs to investigation was 4 D (IQR: 2–7 D). Flocks had experienced a median cumulative mortality of 6.1% (IQR: 2.1–17.7%) and a median morbidity of 27.0% (IQR: 7.8–99.4%) since the onset of clinical signs. The most commonly reported clinical signs were as follows (in decreasing order): diarrhea (68.9% flocks), respiratory system (57.4% flocks), joint or foot (11.5%), and central nervous system (11.5%). The description of the main disease features (morbidity, mortality, and clinical signs) in 61 flocks enrolled is shown in Supplemental Table 2.
Detection of Pathogens by PCR
At least one pathogen was detected in 48 (78.7%) flocks. The prevalence of detected pathogens was as follows (in decreasing order): A. paragallinarum (62.3% flocks), followed by M. gallisepticum (26.2%), viruses causing IBD (24.6%) and IB (21.3%), ORT, and HPAI viruses (4.9%). Neither ND nor P. multocida were detected. All three flocks with HPAI were infected with H5N1. The prevalence of respiratory pathogens in the 2 different sections of the respiratory tract (upper and lower) is shown in Table 2. For A. paragallinarum, there was a higher probability of detection in samples of the upper respiratory tract than in those of the lower respiratory tract (P = 0.015).
Table 2.
Comparison between the probability of detection of pathogens in upper and lower respiratory tract samples by PCR.
| Pathogen | U (+) L (+) | U (+) L (−) | U (−) L (+) | U (−) L (−) | Total U (+)/Total (+) | Total L (+)/Total (+) | χ2 (P-value) |
|---|---|---|---|---|---|---|---|
| A. paragallinarum | 25 | 11 | 2 | 23 | 36/38 | 27/38 | 5.94 (P = 0.015) |
| M. gallisepticum | 10 | 3 | 3 | 47 | 13/16 | 13/16 | 0.0 (P = 1.0) |
| Viruses causing IB | 5 | 6 | 2 | 48 | 11/13 | 7/13 | 1.63 (P = 0.202) |
| ORT | 1 | 2 | 0 | 60 | 3/3 | 1/3 | 0.75 (P = 0.387) |
| Any pathogen | 29 | 12 | 2 | 18 | 41/43 | 31/43 | 6.91 (P = 0.008) |
Abbreviations: IB, infectious bronchitis; L, lower; ND, Newcastle disease; ORT, Ornithobacterium rhinotracheale; U, upper.
Detection of Pathogens by Bacterial Culture
A total of 6 of 7 (85.7%) isolates identified as Avibacterium spp. by MALDI-TOF MS were confirmed as A. paragallinarum by PCR. Overall, the most common bacterial pathogens detected by bacterial culture were E. coli (9/61, 14.8% flocks), followed by A. paragallinarum (9.8%) and ORT (9.8%). P. multocida was not isolated from any flock. The comparison between the probability of detection by bacterial culture and PCR of upper respiratory samples is shown in Table 3. For A. paragallinarum and ORT, there was limited (albeit significant) agreement between PCR and culture methods. For ORT, the time period from the onset of disease to the test was significantly shorter for flocks with positive culture and negative PCR results (median: 4 D) than for those with positive PCR results but negative culture (median: 10 D) (Kruskal–Wallis, χ2 = 3.81, P = 0.051).
Table 3.
Comparison of the detection of bacterial pathogens by bacterial culture by PCR in 61 upper respiratory samples.
| Test results | A. paragallinarum | ORT |
|---|---|---|
| Total positive | 36 | 8 |
| C(+) P(+) | 6 | 1 |
| C(+) P(−) | 0 | 5 |
| C(−) P(+) | 30 | 2 |
| C(−) P(−) | 25 | 53 |
| Total C(+) | 6 | 6 |
| Total P(+) | 36 | 3 |
| Kappa (P-value) | 0.141 (P = 0.016) | 0.167 (P = 0.081) |
| Level of agreement | Slight | Slight |
Abbreviations: C, culture; ORT, Ornithobacterium rhinotracheale; P, PCR.
Summary of Pathogens by Frequency
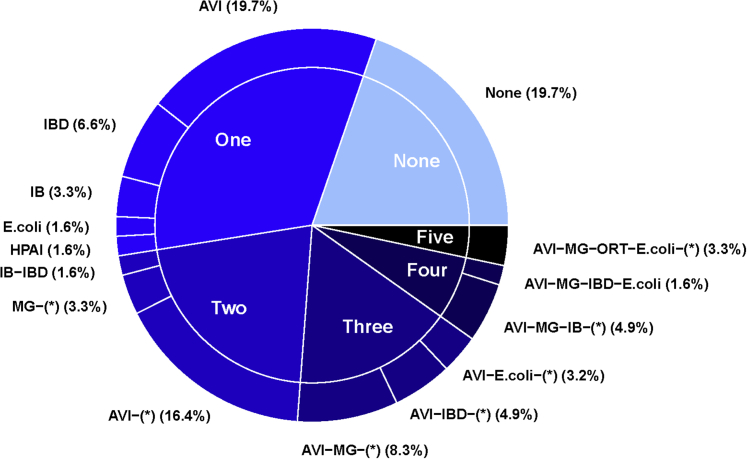
In 49 of 61 (80.3%) flocks, at least one pathogen was detected. The number of flocks with 0, 1, 2, 3, 4, and 5 pathogens detected were 12 of 61 (19.7%), 20 of 61 (32.8%), 13 of 61 (21.3%), 10 of 61 (16.4%), 4 of 61 (6.6%), and 2 of 61 (3.3%), respectively (Figure 1). The most common pathogens detected were as follows (in decreasing order): A. paragallinarum (62.3% flocks), followed by M. gallisepticum (26.2%), viruses causing IBD (24.6%) and IB (21.3%), E. coli (14.8%), ORT (13.1%), and HPAI H5N1 viruses (4.9%). The most prevalent pathogen combination was A. paragallinarum and M. gallisepticum (detected in 23.0% flocks). Among three flocks confirmed with HPAI H5N1 viruses, chickens were aged 9, 14, and 20 wk. Only one of these flocks (14-wk-old flock) had received one shot of vaccine against HPAI.
Figure 1.
Percentage of flocks detected with 0 to 5 pathogens (N = 61). AVI, A. paragallinarum; HPAI, highly pathogenic avian influenza; IBD, infectious bursal disease; IB, infectious bronchitis; MG, M. gallinarum; ORT, O. rhinotracheale; X-(*): combination of X with other pathogen(s).
Mortality and Morbidity by Pathogen
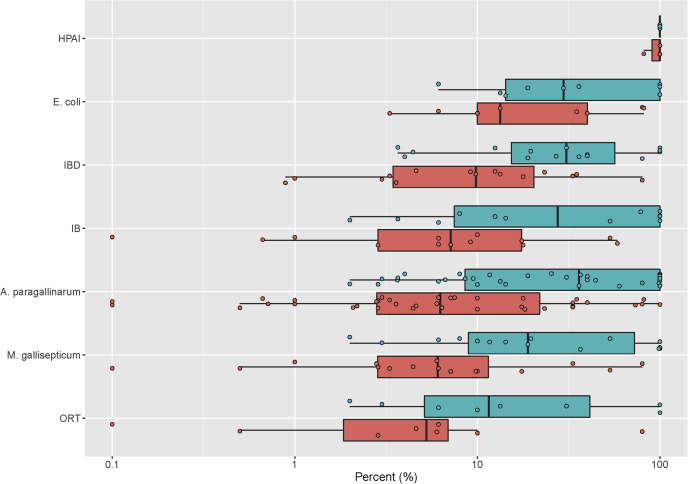
The median cumulative mortality of flocks by pathogen was as follows (in decreasing order): HPAI H5N1 viruses (100%, IQR: 81.6–100%), E. coli (13.3%, IQR: 8.1–60.0%), viruses causing IBD (9.8%, IQR: 3.3–23.3%) and IB (7.1%, IQR: 1.9–17.7%), A. paragallinarum (6.3%, IQR: 2.6–25.8%), M. gallisepticum (6.1%, IQR: 2.8–15.6%), and ORT (5.3%, IQR: 1.1–9.0%) (Figure 2).
Figure 2.
Median cumulative mortality (%) (red) and morbidity (%) (blue) in flocks by pathogen detected. Each dot represents one flock. The boxes represent the median and IQR; the whiskers indicate extreme values. HPAI, highly pathogenic avian influenza; IBD, infectious bursal disease; IB, infectious bronchitis; ORT, O. rhinotracheale.
Seasonality
There was no significant difference between the prevalence of detection by season for all pathogens or tests except for A. paragallinarum and seropositivity for M. gallisepticum (both higher probability of detection during the dry season) (Supplemental Table 3).
Serological Test Results
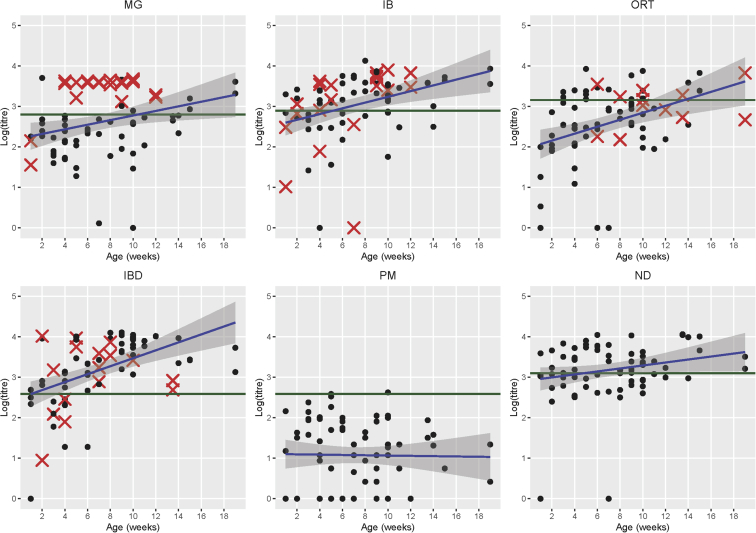
The relationship between age (weeks) and titer (log-transformed) is displayed in Figure 3. The overall seroprevalence of pathogens in decreasing order was as follows: viruses causing IBD (80.0%), ND (65.3%), and IB (64.0%), M. gallisepticum (37.3%), ORT (32.0%), and P. multocida (2.7%). Titers significantly increased with age for IBD (linear model coefficient = 2.4, P < 0.001), IB (coefficient = 1.9, P = 0.001), O. rhinotracheale (coefficient = 1.8, P < 0.001), and M. gallisepticum (coefficient = 1.5, P = 0.012). The serological test result of M. gallisepticum had the highest PPV for the detection of the organism (71.4%; confidence interval [CI]: 56.6–82.7%), followed by IB (27.1%; CI: 20.3–35.2%), ORT (25.0%; CI: 14.4–39.9%), and IBD (20.0%; CI: 15.5–25.4%) (Supplemental Table 4).
Figure 3.
Association between pathogen titer (log10-transformed) and chicken age (weeks). Red mark indicates positive detection by PCR and/or bacterial culture. The green horizontal line is the positive cutoff value as indicated by the manufacturer. IBD, infectious bursal disease; MG, M. gallinarum; ND, Newcastle disease PM, P. multocida.
Colonization With Gastrointestinal Helminths
A total of 41 of 61 flocks (67.2%) and 65 of 122 (53.3%) birds were colonized with gastrointestinal helminths. Nematodes were the most common type of helminth (67.2% flocks colonized), followed by cestodes (16.4%), and trematodes (1.6%). Two species of nematodes were identified: Heterakis gallinarum (59.0% flocks, 44.3% birds) and Ascaridia galli (49.2% flocks, 38.5% birds). There was no overall agreement between colonization with helminths and overall detection of bacterial or viral pathogens (kappa = 0.005; P = 0.482). However, there was statistical (P < 0.05) agreement between colonization with ORT and cestodes and with A. galli and H. gallinarum, as well as between Mycoplasma gallinarum and A. galli (Table 4 and Supplemental Table 5).
Table 4.
Comparison between the presence of helminths and different types of pathogens.
| Comparison | (1) (+) (2) (+) | (1) (+) (2) (−) | (1) (−) (2) (+) | (1) (−) (2) (−) | Kappa value | P-value | Level of agreement |
|---|---|---|---|---|---|---|---|
| (1) ORT; (2) CE | 4 | 4 | 7 | 46 | 0.317 | 0.006 | Fair |
| (1) ORT; (2) AG | 7 | 1 | 23 | 30 | 0.203 | 0.010 | Slight |
| (1) ORT; (2) HG | 8 | 0 | 28 | 25 | 0.190 | 0.006 | Slight |
| (1) MG; (2) AG | 11 | 5 | 19 | 26 | 0.207 | 0.034 | Slight |
Only associations with P < 0.05 are shown.
Abbreviations: AG, Ascaridia galli; CE, cestode; HG, Heterakis gallinarum; MG, M. gallisepticum; ORT, O. rhinotracheale.
Risk Factor Analysis
Helminth colonization was significantly associated with detection of IBD in the final model (protective) (P = 0.045). For A. paragallinarum, rainy season (protective) (P = 0.010) and age (borderline significant, P = 0.070) remained in the final model. For HPAI, only age of chickens remained (borderline significant, P = 0.068) (Supplemental Table 6).
Discussion
Using a range of molecular and bacteriological or parasitological approaches, we investigated circulating poultry pathogens in small-scale chicken flocks in the Mekong Delta region of Vietnam. The most commonly detected bacterial or viral pathogens were, in decreasing order, A. paragallinarum (62.3% flocks), M. gallisepticum (26.2%), viruses causing IBD (24.6%) and IB (21.3%), E. coli (14.8%), ORT (13.1%), and HPAI viruses (4.9%). We found mixed infections in a high percentage of flocks (∼50%). Detection of HPAI was associated with the highest mortality (100%), whereas ORT was associated with the lowest mortality (5.3%).
The most widespread bacterial infection was A. paragallinarum (62.3% flocks). This organism is thought to be common in chicken flocks in India, Thailand, and Korea (Chukiatsiri et al., 2010, Muhammad and Sreedevi, 2015, Han et al., 2016, Patil et al., 2017). We found a significant association between age and the probability of detection of this organism, suggesting increased susceptibility of older birds or the detection of chronic, long-term infections (Blackall, 1999). However, detection of A. paragallinarum and ORT needs to be interpreted cautiously because both organisms are often carried by healthy birds in the study area (authors' observation). Furthermore, in our study, 21% cases (8/38) of A. paragallinarum infections were from birds that did not show any respiratory signs (a typical sign of coryza). Further work is needed to understand the significance and impact of these infections in the field.
Most of the A. paragallinarum infections were detected by PCR and not by culture. It has been suggested that PCR should be the test of choice for A. paragallinarum because it is a relatively slow-growing organism, being easily overgrown by commensal bacteria of the nasal and upper respiratory passages. In contrast, culture was more sensitive than PCR in detecting ORT infection. We hypothesize that this may reflect differences in antimicrobial susceptibility profiles between the 2 organisms and the antimicrobials used by farmers.
We confirmed severe HPAI H5N1 infection in 3 flocks experiencing high (>80%) mortality. According to the farmers' records, one of these flocks (aged 14 wk) had previously been vaccinated (at 5 wk of age) with an injectable inactivated H5N1-based vaccine. This confirms the challenge of controlling HPAI infection using vaccination alone.
There are some limitations in our study. First, our disease panel was limited to some of the most known “global” poultry diseases. Owing to logistic and economic constraints, we were obliged to restrict our study to a panel of 9 bacterial or viral pathogens in addition to gastrointestinal helminths. We included diseases that had been previously detected in other research studies performed in the area (i.e., Gumboro disease, IB, ND, M. gallisepticum infection, P. multocida infection, HPAI) or were suspected to be transmitted in the area (i.e., A. paragallinarum infection, ORT infection). The serological panel chosen was limited by the availability of distributors available in Vietnam. We also had to work within the technical limitations of a small provincial veterinary laboratory. We acknowledge that in doing so, we may have left out a number of important pathogens (i.e., coccidial protozoa) that may potentially be relevant in the area. Second, because of high levels of antimicrobial usage (AMU) in flocks in the area (Cuong et al., 2019), the results may be biased toward detection of viral infections and resistant bacterial strains. Unfortunately, we had no reliable information on the use of antimicrobials and vaccines before disease investigation, but it is highly likely that farmers had given antimicrobials to their flocks immediately after the disease onset in most cases. The choice of antimicrobials during the disease episode may have affected the subsequent outcome. It has been shown that treatment efficacy will be depending on its match with the putative etiological agent (Choisy et al., 2019). Preliminary analyses of our samples indicate that phenotypic antimicrobial resistance is more common in ORT than in Avibacterium spp. (data not shown). This may have conferred a higher probability of detection of the latter.
For IBD, IB, ORT, and M. gallisepticum infection, we found statistically significant higher titers in older chickens. This is likely to reflect field circulation of pathogens rather than vaccination. In addition to ND and HPAI, vaccination of flocks against IBD and IB was frequently practiced in the area (normally in the first 3–4 wk of life). We found a high agreement (PPV) between ELISA and detection of M. gallisepticum. However, the ongoing challenge with these pathogens is likely to limit the use of ELISA as a diagnostic tool.
The present investigation revealed a high prevalence (67.2%) of colonization with gastrointestinal helminths, the most common species being the nematodes H. gallinarum and A. galli. A recent study in the same area reported a higher burden of A. galli in diseased chickens than in healthy ones (Van et al., 2019).
Our results show that control of HPAI, IBD, IB, and M. gallisepticum infection should be a priority in small-scale chicken flocks. Effective control of these diseases requires stepping up on-farm biosecurity practices (including cleaning and disinfection) while improving the quality of day-old chicks. Because vaccines against these organisms are widely available, we also recommend the optimization of vaccination regimes to include these pathogens.
Acknowledgments
The authors are grateful to all participating farmers, as well as to staff affiliated to SDAHP-DT and the Microbiology section at Oxford University Clinical Research Unit (Ho Chi Minh City). This work has been carried out under the umbrella of the Vietnamese Platform of Antimicrobial Reductions in Chicken Production (ViParc project) (www.viparc.org), funded by the Wellcome Trust through an Intermediate Clinical Fellowship awarded to J.C.-M. (grant no. 110085/Z/15/Z).
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.psj.2019.10.033.
Supplementary data
References
- Anon . 2018. Situation Report for Highly Pathogenic Avian Influenza. World Organization of Animal Health. Accessed September 1, 2019. https://www.oie.int/fileadmin/Home/eng/Animal_Health_in_the_World/docs/pdf/OIE_AI_situation_report/OIE_SituationReport_AI_9_11July2017.pdf. [Google Scholar]
- Anon . 2018. Statistics on livestock in Vietnam (in Vietnamese) Accessed September 1, 2019. http://channuoivietnam.com/ [Google Scholar]
- AVMA . 2016. Guideline for the Humane Slaughter of Animal: 2016 Edition. American Veterinary Medical Association. Accessed September 1, 2019. https://www.avma.org/KB/Resources/Reference/AnimalWelfare/Documents/Humane-Slaughter-Guidelines.pdf. [Google Scholar]
- Bell J.G. Factors limiting production efficiency and profitability from smallholder poultry production. World's Poult. Sci. J. 2009;65:207–210. [Google Scholar]
- Blackall P.J. Infectious coryza: overview of the disease and new diagnostic options. Clin. Microbiol. Rev. 1999;12:627–632. doi: 10.1128/cmr.12.4.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui T.A.D., Tripodi A., Carles M., Bodin G. Newcastle disease, Gumboro disease and avian infectious bronchitis in Viet Nam. Medical and economic interest of the one vaccinal program applied in Ho Chi Minh City. Rev. Med. Vet. 2001;152:239–246. [Google Scholar]
- Carrique-Mas J., Van N.T.B., Cuong N.V., Truong B.D., Kiet B.T., Thanh P.T.H., Lon N.N., Giao V.T.Q., Hien V.B., Padungtod P., Choisy M., Setyawan E., Rushton J., Thwaites G. Mortality, disease and associated antimicrobial use in commercial small-scale chicken flocks in the Mekong Delta of Vietnam. Prev. Vet. Med. 2019;165:15–22. doi: 10.1016/j.prevetmed.2019.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K.S., Kye S.J., Kim J.Y., To T.L., Nguyen D.T., Lee Y.J., Choi J.G., Kang H.M., Kim K.I., Song B.M., Lee H.S. Molecular epidemiology of Newcastle disease viruses in Vietnam. Trop. Anim. Health Prod. 2014;46:271–277. doi: 10.1007/s11250-013-0475-3. [DOI] [PubMed] [Google Scholar]
- Choisy M., Cuong N.V., Bao T.D., Kiet B.T., Hien B.V., Thu H.V., Chansiripornchai N., Setyawan E., Thwaites G., Rushton J., Carrique-Mas J. Assessing antimicrobial misuse in small-scale chicken farms in Vietnam from an observational study. BMC Vet. Res. 2019;15:206. doi: 10.1186/s12917-019-1947-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chukiatsiri K., Chotinun S., Chansiripornchai N. An outbreak of Avibacterium paragallinarum serovar B in a Thai layer farm. Thai J. Vet. Med. 2010;40:441–444. [Google Scholar]
- Cuong N.V., Truc V.N., Nhung N.T., Thanh T.T., Chieu T.T., Hieu T.Q., Men N.T., Mai H.H., Chi H.T., Boni M.F., van Doorn H.R., Thwaites G.E., Carrique-Mas J.J., Hoa N.T. Highly pathogenic avian influenza virus A/H5N1 infection in vaccinated meat duck flocks in the Mekong Delta of Vietnam. Transbound. Emerg. Dis. 2016;63:127–135. doi: 10.1111/tbed.12470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuong N.V., Phu D.H., Van N.T.B., Dinh Truong B., Kiet B.T., Hien B.V., Thu H.T.V., Choisy M., Padungtod P., Thwaites G., Carrique-Mas J. High-resolution monitoring of antimicrobial consumption in Vietnamese small-scale chicken farms highlights discrepancies between study metrics. Front. Vet. Sci. 2019;6:174. doi: 10.3389/fvets.2019.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Witt J.J., Cook J.K.A., Van D. Infectious bronchitis virus in Asia, Africa, Australia and Latin America - history, current situation and control measures. Braz. J. Poult. Sci. 2010;12:97–106. [Google Scholar]
- Fujisawa S., Murata S., Takehara M., Katakura K., Hmoon M.M., Win S.Y., Ohashi K. Molecular detection and genetic characterization of Mycoplasma gallisepticum, Mycoplama synoviae, and infectious bronchitis virus in poultry in Myanmar. BMC Vet. Res. 2019;15:261. doi: 10.1186/s12917-019-2018-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes D.C., Menezes R.C., Vicente J.J., Lanfredi R.M., Pinto R.M. New morphological data on Cheilospirura hamulosa (Nematoda, Acuarioidea) by means of bright-field and scanning electron microscopy. Parasitol. Res. 2004;92:225–231. doi: 10.1007/s00436-003-1047-7. [DOI] [PubMed] [Google Scholar]
- Han M.S., Kim J.N., Jeon E.O., Lee H.R., Koo B.S., Min K.C., Lee S.B., Bae Y.J., Mo J.S., Cho S.H., Jang H.S., Mo I.P. The current epidemiological status of infectious coryza and efficacy of PoulShot Coryza in specific pathogen-free chickens. J. Vet. Sci. 2016;17:323–330. doi: 10.4142/jvs.2016.17.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariana S., Hirst R. The immunogenicity and pathogenicity of Pasteurella multocida isolated from poultry in Indonesia. Vet. Microbiol. 2000;72:27–36. doi: 10.1016/s0378-1135(99)00184-4. [DOI] [PubMed] [Google Scholar]
- Muhammad T.M., Sreedevi B. Detection of Avibacterium paragallinarum by Polymerase chain reaction from outbreaks of Infectious coryza of poultry in Andhra Pradesh. Vet. World. 2015;8:103–108. doi: 10.14202/vetworld.2015.103-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen L.V., Stevenson M., Schauer B., Nguyen D.T., Tran Q.D., Tien T.N., Tran P.T., Jones G., Prattley D., Morris R. Descriptive results of a prospective cohort study of avian influenza in the Mekong River Delta of Viet Nam. Transbound. Emerg. Dis. 2014;61:511–525. doi: 10.1111/tbed.12055. [DOI] [PubMed] [Google Scholar]
- Patil V.V., Mishra D., Mane D.V. 16S ribosomal RNA sequencing and molecular serotyping of Avibacterium paragallinarum isolated from Indian field conditions. Vet. World. 2017;10:1004–1007. doi: 10.14202/vetworld.2017.1004-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan M.Q., Henry W., Bui C.B., Do D.H., Hoang N.V., Thu N.T., Nguyen T.T., Le T.D., Diep T.Q., Inui K., Weaver J., Carrique-Mas J. Detection of HPAI H5N1 viruses in ducks sampled from live bird markets in Vietnam. Epidemiol. Infect. 2013;141:601–611. doi: 10.1017/S0950268812001112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussan D.A., Haddad R., Khawaldeh G. Molecular survey of avian respiratory pathogens in commercial broiler chicken flocks with respiratory diseases in Jordan. Poult. Sci. 2008;87:444–448. doi: 10.3382/ps.2007-00415. [DOI] [PubMed] [Google Scholar]
- Soulsby E.J. Bailliere Tindall; London: 1982. Helminths, Arthropods and Protozoa of Domesticated Animals. [Google Scholar]
- Van N.T.B., Cuong N.V., Yen N.T.P., Nhi N.T.H., Kiet B.T., Hoang N.V., Hien V.B., Thwaites G., Carrique-Mas J.J., Ribas A. Characterisation of gastrointestinal helminths and their impact in commercial small-scale chicken flocks in the Mekong Delta of Vietnam. Trop. Anim. Health Prod. 2019:1–10. doi: 10.1007/s11250-019-01982-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.