Abstract
Cobb 400, male broilers (n = 4,752) were housed in 12 pens/diet and 33 birds/pen. There were 3 levels of phytate P (0.24, 0.345, or 0.45%) and 4 phytase doses (0, 500, 1,000 or 2,000 phytase units (FTU)/kg) to evaluate the influence of phytate and phytase dose on apparent ileal digestibility (AID) and digestible nutrient intake. Diets were formulated with reduced Ca (0.22%), available P (0.20%), energy (80 to 120 kcal/kg) and amino acids (1 to 5%). On day 21, digesta was collected from 8 birds/pen. Prediction equations determined the linear or non-linear influence of phytate P, log phytase dose, and the interaction. The AID of amino acids, Ca or P and digestible amino acid or Ca intake were influenced by linear or non-linear phytate P × log phytase dose (P < 0.0001). Increasing the dietary phytate P from 0.24 to 0.345 or 0.45% was predicted to reduce the AID of amino acids in a non-linear manner by an average of 6 to 7 percentage points, respectively. This corresponded to a non-linear decrease in digestible amino acid intake of an average of 80 to 90 mg/D. The negative effect of increasing dietary phytate P from 0.24 to 0.45% on AID was greatest for cysteine (−14 percentage points), aspartic acid or glycine (−9 percentage points) and lowest for methionine, tryptophan, serine, or glutamic acid (−5 percentage points). The predicted digestible intake of lysine (−120 mg/D), aspartic acid (−180 mg/D), or glutamic acid (−290 mg/D) were reduced in birds fed diets containing 0.345% vs. 0.24% phytate P. Phytase supplementation was predicted to increase the AID of amino acids, Ca, or P in a non-linear-log or log-linear manner at all levels of phytate P, with the greatest response at higher doses of phytase in diets containing 0.345 or 0.45% phytate P. The effect of phytase on digestible nutrient intake was less clear. Prediction equations can be useful to determine the influence of phytase and phytate P on AID and digestible nutrient intake in broilers.
Key words: apparent ileal digestibility, broiler, digestible intake, phytate, phytase
INTRODUCTION
There is a considerable amount of data in the literature supporting anti-nutritional effects of dietary phytate on apparent ileal amino acid digestibility (AID) and growth performance, particularly as phytate P concentration increases in the diet. However, some of the work evaluating the anti-nutritional effects of phytate was conducted using sodium phytate and synthetic-diets, which has been shown to influence the determined digestibility coefficients from that of standard diets (Gonzalez-Vega et al., 2015; David et al., 2019) and impact feed intake, also reported to have an influence on digestibility coefficients (Kelly et al., 1991). Due to its chemical properties and solubility throughout the intestinal tract, sodium phytate may not fully mimic the anti-nutritional effects of phytate in plant-based feed ingredients (Onyango et al., 2009). However, others have reported increasing concentrations of dietary phytate P from rice bran significantly reduced the AID of amino acids in broilers fed diets with reduced energy, Ca, non-phytate P, and amino acids (Ravindran et al., 2006).
Phytate P in grains is necessary for growth of the seed and can bind minerals, amino acids, and inhibit endogenous enzyme activity in poultry (Liu et al., 2008, 2009). Phytase, is an enzyme capable of breaking down phytate into lower phytate esters and inositol (Sommerfeld et al., 2018; Walk and Olukosi, 2019). This process allows the previously bound P to be available to the animal while also improving Ca, amino acid, and energy digestibility and utilization (Cowieson et al., 2006a). Exogenous phytase increases the availability of nutrients that would otherwise be bound to phytate and therefore the P, Ca, amino acid, and energy content of the diet may be reduced without negatively impacting animal growth performance, while increasing AID (Ravindran et al., 2006) and digestible intake of nutrients to levels comparable to a nutrient adequate control (Walk and Olukosi, 2019). However, as the concentration of phytate increases in the diet, the magnitude of the response or the anti-nutritional effect of phytate on nutrients, such as amino acids, is greater (Cowieson et al., 2006a). To achieve rapid and nearly complete phytate destruction, particularly at higher concentrations of phytate P or in diets severely limited in Ca, P, energy, and amino acids, increasing doses of phytase above 1,000 FTU/kg may be necessary. Therefore, the objective of this experiment was to use prediction equations to determine the impact of increasing phytate P, from rice bran, and increasing doses of phytase, supplemented into diets severely limited in nutrients, on AID and digestible intake of amino acids, Ca, and P. We hypothesized an increase in phytase dose was required to overcome the anti-nutritional properties of the greater phytate P concentration and result in an equivalent AID or digestible intake of nutrients comparable to the lower phytate P diets without and with phytase.
MATERIALS AND METHODS
All experimental procedures complied with Indian ethical standards for use of vertebrate animals in research.
Animals and Husbandry
Cobb 400 male broilers (n = 4,752) were obtained at day of hatch and placed in floor pens on clean rice husk at a stocking density of 14.4 chicks/m2. There were 33 birds/pen and 12 replicate pens/diet. Birds were vaccinated against Newcastle Disease virus and Infectious Bursal Disease virus per label recommendations. For the entire duration of the experiment (42 D), birds were maintained on a lighting program of 23L:1D and allowed ad libitum access to feed and water.
Dietary Treatments
Experimental diets were fed in mash form and based on corn, soybean meal, rice DDGs, and 12% polished rice or rice bran to change the phytate P concentration of the diets (Table 1). Dietary treatments consisted of 3 levels of phytate P (0.24, 0.345, or 0.45%) and 4 concentrations of phytase (0, 500, 1,000, or 2,000 phytase units (FTU)/kg) arranged as a 3 × 4 factorial. The standard (0.24%) and high (0.45%) phytate P diets were mixed as 2 separate basal diets and then splitted 50:50 and mixed to create the moderate (0.345%) phytate diet. Each of the 3 phytate P diets was then split into 4 batches to include the phytase concentrations and 12 treatments total. Due to the influence of phytase and phytate on minerals, amino acids, and energy, all diets were formulated with a reduction of Ca (0.22%), available P (0.20%), energy (80 to 120 kcal/kg), and amino acids (1 to 5%) when compared with the requirements from the VenCobb 400 Broiler Management Guide (Cobb-Vantress Inc., Siloam Spring, AR). Corn was exchanged with phytase where appropriate to equal 100%. The phytase was an enhanced Escherichia coli 6-phytase expressed in Trichoderma reesei with an expected activity of 5,000 FTU/g (Quantum Blue, AB Vista, Marlborough UK). The amount of enzyme required to release 1 µmol of inorganic P/min from sodium phytate at 37°C and pH 5.5 is known as 1 phytase unit. All diets contained a xylanase at 16,000 xylanase units (BXU)/kg (Econase XT, AB Vista, Marlborough UK).
Table 1.
Calculated and analyzed nutrient content of the basal diets.
| Feeding phase |
Starter diets |
Grower diets |
||
|---|---|---|---|---|
| Phytate P | Standard | High | Standard | High |
| Ingredient, % of diet (as-fed basis) | ||||
| Corn | 51.58 | 51.30 | 59.07 | 58.78 |
| Soybean meal, 48% | 27.62 | 25.60 | 20.40 | 18.39 |
| Rice dried distillers grains w/solubles, 47% | 5.00 | 5.00 | 5.00 | 5.00 |
| Polished rice | 12.00 | 12.00 | ||
| De-oiled rice bran | 12.00 | 12.00 | ||
| Soybean oil | 0.52 | 3.31 | 0.99 | 3.78 |
| Salt | 0.42 | 0.43 | 0.37 | 0.37 |
| Limestone | 1.00 | 0.55 | 1.04 | 0.60 |
| Dicalcium phosphate1 | 0.95 | 0.85 | 0.67 | 0.57 |
| Lysine-HCl | 0.20 | 0.23 | 0.14 | 0.18 |
| DL-methionine | 0.19 | 0.20 | 0.12 | 0.13 |
| Threonine | 0.02 | 0.03 | 0.01 | |
| Premix2 | 0.15 | 0.15 | 0.15 | 0.15 |
| Inert (corn/phytase)3 | 0.04 | 0.04 | 0.04 | 0.04 |
| Xylanase4 | 0.01 | 0.01 | 0.01 | 0.01 |
| Chromium | 0.30 | 0.30 | ||
| Nutrient composition, % | ||||
| Crude protein | 21.35 | 21.35 | 18.35 | 18.35 |
| ME, kcal/kg | 2955.00 | 2955.00 | 3060.00 | 3060.00 |
| Dry matter | 87.49 | 87.70 | 87.69 | 87.90 |
| Calcium | 0.72 | 0.72 | 0.66 | 0.66 |
| Total phosphorus | 0.54 | 0.74 | 0.46 | 0.66 |
| Available phosphorus | 0.25 | 0.25 | 0.20 | 0.20 |
| Phytate phosphorus | 0.24 | 0.45 | 0.23 | 0.44 |
| Total methionine + cysteine | 0.93 | 0.94 | 0.78 | 0.79 |
| Total lysine | 1.25 | 1.26 | 1.00 | 1.01 |
| Digestible methionine + cysteine | 0.83 | 0.83 | 0.69 | 0.69 |
| Digestible lysine | 1.13 | 1.13 | 0.90 | 0.90 |
| Sodium | 0.18 | 0.18 | 0.16 | 0.16 |
Dicalcium phosphate supplied 17% P and 21% Ca.
Supplied per kilogram of diet: iron (ferrous sulfate), 34 mg; manganese (manganese sulfate), 38 mg; zinc (zinc sulfate), 34 mg; copper (basic copper chloride), 6 mg; iodine (calcium iodate), 0.8 mg; selenium (sodium selenite), 113 μg; vitamin A, 9.4 MIU; vitamin D3 2.1 MIU; vitamin E, 22.5 mg; vitamin B12, 11 μg; riboflavin, 3.8 mg; niacin, 25 mg; d-pantothenic acid, 11 mg; vitamin K, 1.5 mg; folic acid, 0.8 mg; vitamin B6, 1.9 mg; thiamine, 1.5 mg; and biotin, 60 μg.
Corn was added in place of phytase in the diets without phytase supplementation. The phytase used was Quantum Blue (AB Vista, Marlborough, UK) with an expected activity of 5,000 FTU/g.
Response Variables
On day 21, 8 birds of average BW/pen were anaesthetized by exposure to CO2 gas for approximately 30 s and euthanized by cervical dislocation for digesta collection. Digesta was obtained from the entire ileum (defined as Meckel's diverticulum to the ileocecal junction), pooled/pen and immediately frozen on dry ice. Digesta was dried at 70°C in a forced air oven for 48 h and ground to pass a 1 mm screen. The use of oven drying was based on availability of equipment and the previously reported use of oven drying at >80°C for determination of amino acid digestibility (Dale et al., 1985; Ravindran et al., 2001; Cowieson et al., 2006b). Dried, ground ileal digesta and the experimental diets were analyzed for amino acids (method 982.30), crude protein (method 984.13 A-D), chromium (method 990.08), Ca (method 975.03 B(b)), and P (method 968.08) according to AOAC (2006) at the University of Missouri Agricultural Experiment Station (Columbia, MO). Phytase activity recovered in the diets was determined according to modified methods of Engelen et al. (2001). Xylanase activity recovered in the diets was determined using birch xylan as a substrate at pH 5.3 and 50°C. The method is based on the end-point determination of reducing sugars using a DNS-based colorimetric system. The color produced is proportional to enzyme activity. Xylanase units are expressed as nanomoles/second of xylose reducing sugar equivalents (BXU/g). Phytate content of the ingredients and the experimental diets was determined using the K-PHYT kit from Megazyme (Bray, Ireland) and phytate P content was calculated as 28.2% of the total phytate.
Calculations and Statistical Analyses
Apparent ileal amino acid digestibility was calculated using chromium ratios in the diets and digesta (Ravindran et al., 1999).
where = the ratio of amino acid to chromium in the diet; and = the ratio of amino acid to chromium in the ileal digesta.
To calculate digestible nutrient intake in g/D, the following equation was used (Walk et al., 2018):
where diet nutrient = the analyzed nutrient concentration of the diet; and AID nutrient = the previously calculated apparent ileal nutrient digestibility.
Data were analyzed as a completely randomized 3 × 4 factorial using the fit model platform of JMP Pro 14.0 (SAS Institute, Cary, NC). Outliers were determined as 3 times the root mean square error plus or minus the mean of response. Plotting the AID or digestible nutrient intake data using a normal quantile plot indicated the means were normally distributed. For all parameters, prediction equations were conducted testing the linear and non-linear effects of phytase log dose, phytate P and the interactions as continuous variables. The full model equation was: y = a + bx + dx2 + ev + fv2 + gxv + hx2v + ixv2 + jx2v2, where y = response variable, a = intercept, b to j = linear and non-linear coefficients, x = calculated phytate P in the experimental diets, and v = calculated log dose of phytase in the experimental diets. The log dose for 0 FTU/kg was estimated as 50 FTU/kg (log dose of 1.699), which is equivalent to the background phytase activity recovered in the diets containing no phytase. Parameter estimates that were not significant in the model and were not included in a significant interaction were removed from the model and the estimates recalculated. Pen served as the experimental unit for all parameters measured. Significance was accepted at P ≤ 0.05.
RESULTS
Phytate P content of the main feed ingredients was determined prior to feed formulation to ensure the expected phytate P levels in the diet were achieved. The phytate P content of the main cereal ingredients was 0.12, 0.21, 0.25, 0.38, and 1.96% for polished rice, corn, rice distillers dried grains with solubles, soybean meal, and de-oiled rice bran, respectively. Phytate P, nutrients analyzed in the diets and enzyme activity recovered in the experimental diets were similar to formulated values (Table 2). Animal performance, bone ash and gizzard phytate, phytate ester, and inositol are presented in a companion paper (Walk and Rama Rao, unpublished).
Table 2.
Analyzed phytate phosphorus content of the main ingredients used to formulate the diets, analyzed nutrient content of the experimental diets, and enzyme activities recovered in the experimental diets.
| Starter basal diets |
Grower basal diets |
|||||
|---|---|---|---|---|---|---|
| Item | Standard phytate P | Moderate phytate P | High phytate P | Standard phytate P | Moderate phytate P | High phytate P |
| Analyzed nutrient composition of the experimental diets, % | ||||||
| Crude protein | 21.54 | 21.67 | 21.84 | 19.30 | 18.73 | 19.52 |
| Total phosphorus | 0.52 | 0.62 | 0.75 | 0.45 | 0.59 | 0.74 |
| Total calcium | 0.67 | 0.63 | 0.56 | 0.65 | 0.53 | 0.55 |
| Total lysine | 1.34 | 1.29 | 1.34 | |||
| Phytate phosphorus | 0.23 | 0.33 | 0.42 | 0.22 | 0.32 | 0.45 |
| Phytase activity recovered in the experimental diets, FTU/kg | ||||||
| 0 | < 50 | < 50 | < 50 | < 50 | < 50 | < 50 |
| 500 | 400 | 329 | 466 | 504 | 543 | 604 |
| 1,000 | 917 | 911 | 1,030 | 847 | 934 | 946 |
| 2,000 | 2,200 | 1,850 | 1,970 | 1,480 | 1,790 | 1,780 |
| Xylanase activity recovered in the experimental diets, BXU/kg | ||||||
| 0 | 14,000 | 15,800 | 15,500 | 18,200 | 18,000 | 18,700 |
| 500 | 14,000 | 18,700 | 16,300 | 18,200 | 18,400 | 18,300 |
| 1,000 | 16,900 | 15,400 | 16,600 | 16,800 | 18,600 | 15,700 |
| 2,000 | 16,600 | 15,400 | 17,200 | 14,200 | 15,600 | 16,700 |
Equations to predict the effect of increasing dietary phytate P and log doses of phytase on AID of amino acids, Ca and P are presented in Table 3. There was a phytate P × log dose of phytase interaction (P < 0.05) on the AID of all measured amino acids and Ca and P, with differences in the magnitude and linear or non-linear responses to phytate P or phytase for each amino acid or mineral. In the absence of phytase, the predicted AID of methionine decreased in a non-linear manner by 4.5 to 5.1 percentage points as the phytate P content of the diet increased from 0.24% to 0.345 and 0.45%. Supplementing phytase increased the AID of methionine in a non-linear-log manner in birds fed diets containing 0.24% phytate P, with a maximum AID of methionine achieved at 1,062 FTU/kg (log dose 3.026). However, in birds fed 0.345 or 0.45% phytate P, phytase supplementation increased the AID of methionine in a log-linear manner and the maximum could not be predicted (Table 4; Figure 1 a). In the absence of phytase, the AID of cysteine, serine, or methionine + cysteine decreased in a non-linear manner by 9.5 to 14.1, 5.9 to 5.3, or 6.6 to 8.7 percentage points, respectively, as phytate P content of the diet increased from 0.24 to 0.345 and 0.45%. Supplementing phytase to > 2,000 FTU/kg increased the AID of cysteine (Figure 2 a), serine or methionine + cysteine in a log-linear manner and the maximum could not be predicted (Table 4). In the absence of phytase, the AID of threonine, valine, isoleucine, leucine, phenylalanine, lysine, histidine, arginine, tryptophan, aspartic acid, glutamic acid, proline, glycine, alanine, and tyrosine were reduced in a non-linear manner as the concentration of phytate P increased in the diet from 0.24 to 0.45% (Table 4). The magnitude of the response differed depending on the amino acid, with only a 3.5 to 4.0 percentage point reduction in the AID of arginine as phytate P content of the diet increased from 0.24 to 0.345 and 0.45% to a 7.2 to 8.5 percentage point reduction in the AID of glycine (Figure 3 a) as the phytate P content of the diet increased from 0.24 to 0.345 and 0.45% (Table 4). Supplementing phytase increased the AID of all measured amino acids in a non-linear-log manner and the magnitude of the response to phytase dose was dependent on the phytate P content of the diet. In general, phytase supplementation up to 500 FTU/kg improved the AID amino acids by ∼2 and up to 1,000 FTU/kg improve the AID of amino acids by ∼6 percentage points in birds fed diets containing 0.24 and 0.345% phytate P, respectively. Increasing phytase dose in these diets resulted in no or relatively small (+0.2 to 0.3 percentage point) improvements in the AID amino acids. Whereas, in birds fed diets containing 0.45% phytate P, phytase supplementation of 500 or 1,000 FTU/kg improved the AID of amino acids by ∼2 to 3 percentage points and this was increased to ∼5 percentage points with 2,000 FTU/kg (Table 4). Finally, the AID of Ca or P was predicted to decrease in a non-linear manner as phytate P content in the diet increased from 0.24 to 0.45% (Table 4). Phytase supplementation reduced the AID of Ca in birds fed 0.24% phytate P and increased the AID of Ca in birds fed diets containing 0.345 or 0.45% phytate P, with a greater effect of phytase dose in birds fed 0.45% phytate P (+22 percentage points) compared with birds fed diets containing 0.345% phytate P (+2 percentage points). Phytase was predicted to increase the AID of P by ∼18, 25, and 32 percentage points as phytase dose increased, regardless of the phytate P content of the diet.
Table 3.
Prediction equations of the effect of graded concentrations of phytate P (x) and log doses of phytase (v) on apparent ileal nutrient digestibility of 21-day-old broilers.
| Model |
||||
|---|---|---|---|---|
| Nutrient | Equation | RMSE | Adjusted R2 | P-value |
| Thr | y = 345 − 1803x + 2686x2 − 192v + 33.8v2} + 1298xv − 2000x2v − 228xv2 + 357x2v2 | 1.46 | 0.86 | <0.0001 |
| Val | y = 274 − 1334x + 1983x2 − 131v + 20.8v2 + 893xv − 1385x2v − 142xv2 + 226x2v2 | 1.39 | 0.86 | <0.0001 |
| Met | y = 128 − 290x + 402x2 − 5.4v − 2.1v2 + 76.9xv − 134x2v + 5.1xv2 | 0.60 | 0.93 | <0.0001 |
| Iso | y = 334 − 1673x + 2479x2 − 186v + 32.9v2 + 1253xv − 1920x2v − 221xv2 + 345x2v2 | 1.22 | 0.89 | <0.0001 |
| Leu | y = 284 − 1329x + 1956x2 − 154v + 28.2v2 + 1030xv − 1565x2v - 189xv2 + 291x2v2 | 0.96 | 0.90 | <0.0001 |
| Phe | y = 270 − 1246x + 1832x2 − 140v + 25.6v2 + 948xv − 1448x2v − 173xv2 + 267x2v2 | 0.92 | 0.92 | <0.0001 |
| Lys | y = 277 − 1269x + 1897x2 − 124v + 19.6v2 + 861xv − 1348x2v − 141xv2 + 228x2v2 | 0.86 | 0.88 | <0.0001 |
| His | y = 286 − 1359x + 2033x2 − 160v + 31v2 + 1102xv − 1696x2v − 212xv2 + 329x2v2 | 0.87 | 0.91 | <0.0001 |
| Arg | y = 219 − 839x + 1210{x2 − 91.5v + 15.7v2 + 605xv − 902x2v − 105xv2 + 159x2v2 | 0.62 | 0.88 | <0.0001 |
| Cys | y = 151 − 506x + 599x2 − 23.5v + 166xv − 219x2v | 1.68 | 0.93 | <0.0001 |
| Trp | y = 305 − 1433x + 2086x2 − 166v + 32v2 + 1121xv − 1675x2v − 219xv2 + 331x2v2 | 0.86 | 0.83 | <0.0001 |
| Met + Cys | y = 139 − 391x + 476x2 − 14.5v − 0.65v2 + 121xv − 158x2v | 0.94 | 0.94 | <0.0001 |
| Asp | y = 243 − 1077x + 1564x2 − 109v + 17.8v2 + 736xv − 1128x2v − 120xv2 + 188x2v2 | 1.22 | 0.92 | <0.0001 |
| Ser | y = 163 - 532x + 727x2 - 25.5v + 176v2 - 250x2v | 0.98 | 0.90 | <0.0001 |
| Glu | y = 217 - 869x + 1254x2 - 91.8v + 15.5v2 + 613xv - 922x2v - 103xv2 + 158x2v2 | 0.82 | 0.90 | <0.0001 |
| Pro | y = 242 - 1082x + 1574x2 - 123v + 22.8v2 + 832xv - 1262x2v - 153xv2 + 235x2v2 | 1.15 | 0.91 | <0.0001 |
| Gly | y = 315 - 1613x + 2426x2 - 171v + 30.7v2 + 1176xv - 1842x2v - 211xv2 + 336x2v2 | 1.46 | 0.88 | <0.0001 |
| Ala | y = 264 - 1237x + 1840x2 - 130v + 22.6v2 + 892xv - 1382x2v - 156xv2 + 246x2v2 | 1.06 | 0.89 | <0.0001 |
| Tyr | y = 207 - 826x + 1179x2 - 96.1v + 17.5v2 + 650xv - 980x2v - 118xv2 + 181x2v2 | 0.95 | 0.92 | <0.0001 |
| Ca | y = 727 - 3447x + 4344x2 - 634v + 133v2 + 3337xv - 4404x2v - 717xv2 + 970x2v2 | 2.71 | 0.80 | <0.0001 |
| P | y = 255 - 1499x + 2294x2 - 183v + 40.9v2 + 1257xv - 1970x2v - 256xv2 + 403x2v2 | 1.73 | 0.98 | <0.0001 |
Table 4.
Predicted effect of graded concentrations of phytate P and log doses of phytase on apparent ileal nutrient digestibility of 21-day-old broilers.1
| 0.24% phytate P |
0.345% phytate P |
0.45% phytate P |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phytate P Phytase dose | 50 | 500 | 1,000 | 2,000 | 50 | 500 | 1,000 | 2,000 | 50 | 500 | 1,000 | 2,000 |
| Thr | 74.5 | 76.7 | 77.1 | 77.5 | 67.1 | 73.8 | 74.8 | 75.4 | 66.8 | 68.5 | 70.3 | 72.7 |
| Val | 73.5 | 75.6 | 76.2 | 76.6 | 67.1 | 73.3 | 74.7 | 75.8 | 66.8 | 68.5 | 70.1 | 72.1 |
| Met | 88.2 | 89.7 | 89.8 | 89.7 | 83.7 | 87.4 | 88.4 | 89.3 | 83.1 | 86.1 | 87.1 | 88.1 |
| Iso | 79.8 | 81.6 | 81.9 | 82.2 | 73.6 | 79.8 | 80.7 | 81.1 | 72.0 | 73.7 | 75.4 | 77.7 |
| Leu | 82.4 | 84.1 | 84.5 | 84.8 | 77.6 | 82.6 | 83.1 | 83.2 | 75.8 | 77.7 | 79.0 | 80.7 |
| Phe | 82.6 | 84.6 | 85.0 | 85.4 | 77.5 | 82.5 | 83.2 | 83.5 | 75.4 | 77.6 | 79.0 | 80.7 |
| Lys | 88.3 | 88.8 | 88.5 | 88.0 | 82.3 | 86.9 | 87.5 | 87.8 | 82.2 | 83.3 | 84.5 | 86.2 |
| His | 85.2 | 87.3 | 87.6 | 87.8 | 81.0 | 85.7 | 86.0 | 85.7 | 79.1 | 80.8 | 82.2 | 84.0 |
| Arg | 89.7 | 90.4 | 90.6 | 90.6 | 86.2 | 89.7 | 90.2 | 90.4 | 85.7 | 87.6 | 88.5 | 89.5 |
| Cys | 70.8 | 74.6 | 75.7 | 76.8 | 61.3 | 69.0 | 71.3 | 73.6 | 56.7 | 63.5 | 65.6 | 67.7 |
| Trp | 89.1 | 89.7 | 89.3 | 88.7 | 84.2 | 87.8 | 87.2 | 85.9 | 83.7 | 85.3 | 86.0 | 86.7 |
| Met + Cys | 80.6 | 83.3 | 83.9 | 84.3 | 74.0 | 79.7 | 81.2 | 82.6 | 71.9 | 77.3 | 78.6 | 79.9 |
| Asp | 79.9 | 82.4 | 83.0 | 83.7 | 73.5 | 79.3 | 80.6 | 81.6 | 71.2 | 73.8 | 75.3 | 77.2 |
| Ser | 80.6 | 82.9 | 83.6 | 84.3 | 74.7 | 80.0 | 81.7 | 83.3 | 75.3 | 78.3 | 79.2 | 80.1 |
| Glu | 84.4 | 86.0 | 86.4 | 86.4 | 80.2 | 84.7 | 85.5 | 86.1 | 79.2 | 81.5 | 82.7 | 84.0 |
| Pro | 79.3 | 81.9 | 82.5 | 83.1 | 74.2 | 79.5 | 80.3 | 80.7 | 71.5 | 74.3 | 75.8 | 77.6 |
| Gly | 75.3 | 78.0 | 78.5 | 78.9 | 68.1 | 74.3 | 75.3 | 75.9 | 66.8 | 68.6 | 70.5 | 73.2 |
| Ala | 79.7 | 81.6 | 81.9 | 82.1 | 74.2 | 79.4 | 80.2 | 80.6 | 73.1 | 75.0 | 76.4 | 78.3 |
| Tyr | 81.5 | 83.4 | 83.8 | 84.2 | 77.2 | 81.6 | 82.3 | 82.7 | 73.7 | 76.6 | 77.9 | 79.3 |
| Ca | 52.9 | 42.7 | 46.5 | 53.3 | 48.4 | 49.3 | 50.2 | 51.5 | 36.4 | 45.4 | 51.0 | 57.9 |
| P | 45.0 | 62.5 | 68.8 | 75.6 | 41.0 | 60.2 | 66.2 | 72.4 | 39.5 | 56.1 | 63.9 | 73.1 |
Means were determined using the equations from Table 3 and the log dose of phytase at 1.7, 2.7, 3.0, and 3.3 for 50, 500, 1,000, and 2,000 FTU/kg, respectively.
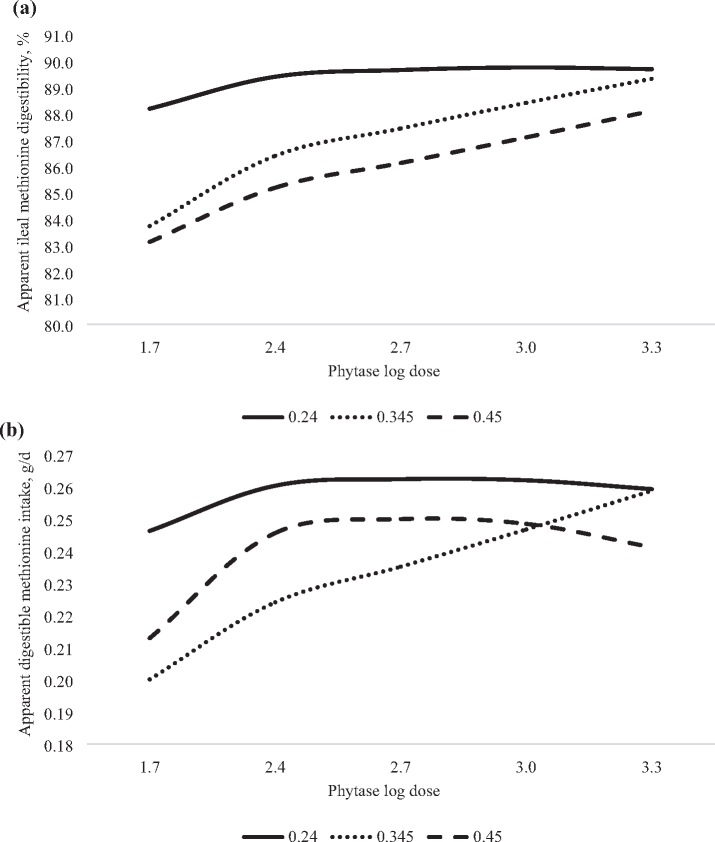
Figure 1.
Predicted effect of graded concentrations of phytate P (x) and log doses of phytase (v) on methionine digestibility in 21-day-old broilers. (a) Apparent ileal methionine digestibility (AID) was predicted with the equation: y = 128 − 290x + 402x2 − 5.4v − 2.11v2 + 76.9xv − 1334x2v + 5.12xv2, adjusted R2 = 0.93, RMSE = 0.60, P < 0.0001. In the absence of phytase, the AID of methionine was predicted to decrease in a non-linear manner from 88.2, 83.7, or 83.1% in birds fed diet containing 0.24, 0.345, or 0.45% phytate P, respectively. However, the inclusion of phytase at 1,062 FTU/kg (log dose 3.026), maximized the AID of methionine in broilers fed diets containing 0.24% phytate P, resulting in a non-linear-log effect of phytase. Whereas phytase supplementation resulted in a log-linear increase in the AID of methionine in birds fed 0.345 or 0.45% phytate P and the maximum was not predicted. (b) Apparent digestible methionine intake was predicted with the equation: y = − 0.38 + 3.82x − 6.62x2 + 0.97v − 0.24v2 − 5.98xv + 9.39x2v + 1.5xv2 − 2.31x2v2, adjusted R2 = 0.83, RMSE = 0.01, P < 0.0001. In the absence of phytase, the digestible intake of methionine was predicted to decrease from 0.25, 0.20, or 0.21 g/D in a non-linear manner as phytate P content of the diet increased from 0.24, 0.345, or 0.45%, respectively. The inclusion of phytase 658 (log dose 2.818) or 598 FTU/kg (log dose 2.777) was predicted to maximize the digestible intake of methionine of birds fed diets containing 0.24 or 0.45% phytate P, respectively, in a non-linear-log manner. Whereas, digestible methionine intake was predicted to increase in a log-linear manner as phytase dose increased to > 2,000 FTU/kg (log dose 3.301) in birds fed diets containing 0.345% phytate P and the maximum digestible methionine intake could not be predicted.
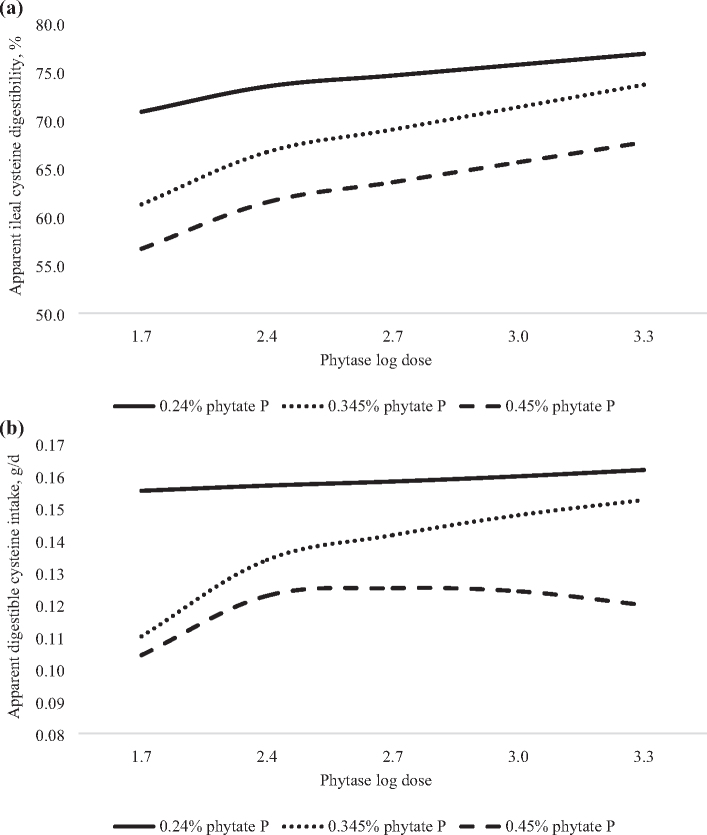
Figure 2.
Predicted effect of graded concentrations of phytate P (x) and log doses of phytase (v) on cysteine digestibility in 21-day-old broilers. (a) Apparent ileal cysteine digestibility (AID) was predicted with the equation: y = 151 − 506x + 599x2 − 23.5v + 166xv − 219x2v, adjusted R2 = 0.93, RMSE = 1.68, P < 0.0001. In the absence of phytase, the AID of cysteine was predicted to decrease in a non-linear manner from 70.8, 61.3, or 56.7% in birds fed diet containing 0.24, 0.345, or 0.45% phytate P, respectively. However, the inclusion of phytase was predicted to increase the AID of cysteine in a log-linear manner and the maximum AID of cysteine could not be calculated at phytase dose > 2,000 FTU/kg (log dose 3.301), regardless of the phytate P content of the diet. (b) Apparent digestible cysteine intake (g/D) was predicted with the equation: y = 0.88 − 4.15x + 4.8x2 − 0.32v + 0.025v2 + 1.74xv − 1.78x2v − 0.097xv2, adjusted R2 = 0.93, RMSE = 0.01, P < 0.0001. In the absence of phytase, the digestible intake of cysteine was predicted to decrease in a non-linear manner from 0.16, 0.11, or 0.10 g/D in birds fed diets containing 0.24, 0.345, or 0.45% phytate P, respectively. However, in the presence of phytase, digestible cysteine intake was predicted to increase in a log-linear manner in birds fed diets containing 0.24 or 0.345% phytate P, and the maximum cysteine intake could not be determined at phytase dose > 2,000 FTU/kg (log dose 3.301). Whereas, in birds fed 0.45% phytate, the phytase dose predicted to maximize digestible cysteine intake was 579 FTU/kg (log dose 2.763), resulting in a non-linear-log influence of phytase dose on digestible cysteine intake.
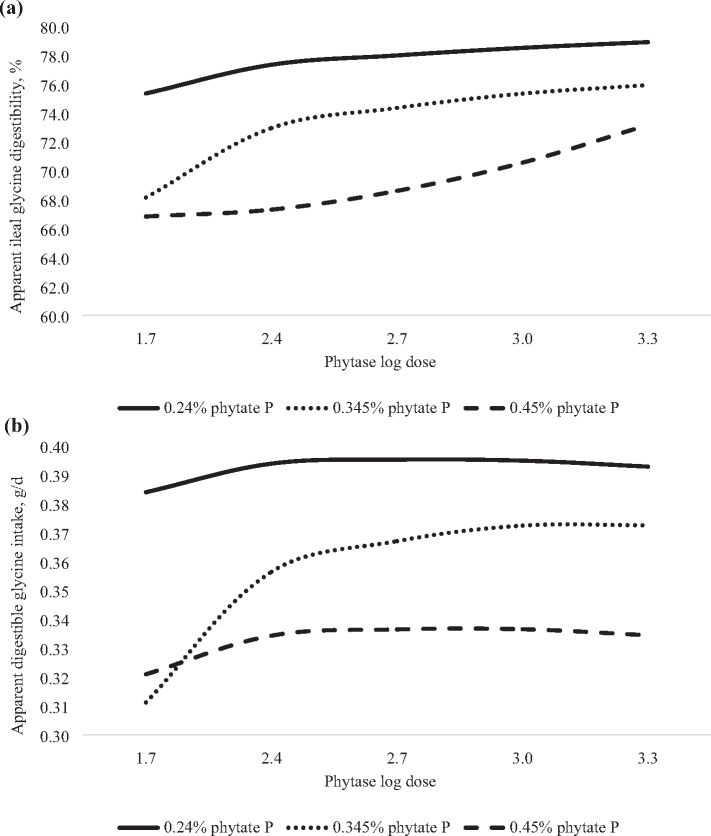
Figure 3.
Predicted effect of graded concentrations of phytate P (x) and log doses of phytase (v) on glycine digestibility in 21-day-old broilers. (a) Apparent ileal glycine digestibility (AID) was predicted with the equation: y = 315 − 1613x + 2426x2 − 171v + 30.7v2 + 1176xv − 1842x2v − 211xv2 + 336x2v2, adjusted R2 = 0.88, RMSE = 1.46, P < 0.0001. In the absence of phytase, the AID of glycine was predicted to decrease in a non-linear manner from 75.3, 68.1, or 66.8% in birds fed diet containing 0.24, 0.345, or 0.45% phytate P, respectively. However, the inclusion of phytase at > 2,000 FTU/kg (log dose 3.301) was predicted to increase the AID of glycine in a non-linear-log manner, regardless of the phytate P content of the diet, and the maximum could not be predicted. (b) Apparent digestible glycine intake (g/D) was predicted with the question: y = 2.35 − 12.8x + 17.9x2 − 1.17v + 0.17v2 + 7.8xv − 11.2x2v − 1.17xv2 + 1.67x2v2, adjusted R2 = 0.84, RMSE = 0.01, P < 0.0001. In the absence of phytase, the digestible intake of glycine was predicted to decrease in a non-linear manner from 0.38, 0.31, or 0.32 g/D in birds fed diets containing 0.24, 0.345, or 0.45% phytate P, respectively. The inclusion of phytase was predicted to increase the digestible intake of glycine in a non-linear-log manner, with 618 (log dose 2.791), 1,416 (log dose 3.151), or 724 FTU/kg (log dose 2.86) of phytase predicted to maximize the digestible intake of glycine in birds fed diets containing 0.24, 0.345, or 0.45% phytate P, respectively.
Equations to predict the effect of increasing dietary phytate P and log doses of phytase on the digestible intake of amino acids, Ca, and P are presented in Table 5. There was a phytate P × log dose of phytase interaction (P < 0.05) on the digestible intake all measured amino acids and Ca and P, with differences in the magnitude and linear or non-linear responses to phytate P or phytase for each amino acid or mineral. In the absence of phytase, the predicted digestible intake of methionine, tryptophan, glycine, or methionine + cysteine decreased in a non-linear manner by 10 to 90 mg/D as the phytate P content of the diet increased from 0.24% to 0.345 or 0.45%. Supplementing phytase influenced the digestible intake of methionine (Figure 1b), tryptophan, glycine (Figure 3b), or methionine + cysteine in a non-linear-log manner, with no or minimal effects of phytase dose >500 FTU/kg (log dose 2.699) on digestible amino acid intake in birds fed diets containing 0.24 or 0.45% phytate P and a log-linear increase in digestible intake of amino acids in birds fed diets containing 0.345% phytate P (Table 6). In the absence of phytase, the predicted digestible intake of cysteine (Figure 2b) or serine decreased in a non-linear manner, with a greater decrease in digestible amino acid intake between birds fed 0.24 or 0.345% phytate P when compared with those fed 0.24 or 0.45%. However, in the presence of phytase, digestible cysteine intake was predicted to increase in a log-linear manner in birds fed diets containing 0.24 or 0.345% phytate P, and the maximum cysteine intake could not be determined at phytase dose >2,000 FTU/kg (log dose 3.301). Whereas, in birds fed 0.45% phytate, the phytase dose predicted to maximize digestible cysteine intake was 579 FTU/kg (log dose 2.763), resulting in a non-linear-log influence of phytase dose on digestible cysteine intake. In the birds fed diets containing 0.345 or 0.45% phytate P, digestible serine intake was predicted to increase as phytase dose increased to 1,660 (log dose 3.22) or 296 (log dose 2.472) FTU/kg, respectively, in a non-linear-log manner; whereas, there was no effect of phytase on serine intake in birds fed 0.24% phytate P. In general, the digestible intake of threonine, valine, isoleucine, leucine, phenylalanine, lysine, histidine, arginine, aspartic acid, glutamic acid, proline, alanine, or tyrosine were decreased in a non-linear manner as phytate P content in the diet increased from 0.24 to 0.45%. Phytase supplementation was predicted to increase the digestible intake of all amino acids in a log-linear manner in birds fed diets containing 0.345% phytate P, but had no effect on the digestible intake of amino acids in birds fed diets containing 0.24 or 0.45% phytate P. Digestible Ca intake was predicted to decrease in a non-linear manner as phytate P content in the diet increased from 0.24 to 0.45%, with the greatest decrease in digestible Ca intake in birds fed diets containing 0.45% phytate P compared with those fed 0.24 or 0.345% phytate P. Phytase supplementation was predicted to influence digestible Ca intake in a non-linear-log manner, with a decrease or no effect of increasing phytase dose on digestible Ca intake in birds fed diets containing 0.24 or 0.345% phytate P and an increase in digestible Ca intake in birds fed diets containing 0.45% phytate P (Table 6). Finally, Digestible P intake was predicted to increase in a log-linear manner as both phytate P content and phytase dose increased in the diet, with the greatest digestible P intake predicted in birds fed diets containing 0.45% phytate P and 2,000 FTU/kg (Table 6).
Table 5.
Prediction equations of the effect of graded concentrations of phytate P (x) and log doses of phytase (v) on apparent digestible nutrient intake of 21-day-old broilers.
| Model |
||||
|---|---|---|---|---|
| Nutrient | Equation | RMSE | Adjusted R2 | P-value |
| Thr | y = 1.31 - 6.64x + 9.20x2 - 0.26v - 0.018v2 + 2.25xv - 3.23x2v | 0.01 | 0.85 | <0.0001 |
| Val | y = 1.58 - 8.16x + 11.4x2 - 0.35v - 0.025v2 + 3.09xv - 4.50x2v | 0.01 | 0.88 | <0.0001 |
| Met | y = - 0.38 + 3.82x - 6.62x2 + 0.97v - 0.24v2 - 5.98xv + 9.39x2v + 1.5xv2 - 2.31x2v2 | 0.01 | 0.83 | <0.0001 |
| Iso | y = 1.42 - 7.29x + 10.1x2 - 0.303v - 0.027v2 + 2.85xv - 4.14x2v | 0.01 | 0.90 | <0.0001 |
| Leu | y = 2.25 - 11.3x + 15.8x2 - 0.35v - 0.063v2 + 4.34xv - 6.35x2v | 0.02 | 0.86 | <0.0001 |
| Phe | y = 1.6 - 8.2x + 11.4x2 - 0.29v - 0.036v2 + 3.02xv - 4.41x2v | 0.01 | 0.88 | <0.0001 |
| Lys | y = 2.61 - 12.7x + 17.3x2 - 0.59v - 0.028v2 + 4.37xv - 6.09x2v | 0.02 | 0.72 | <0.0001 |
| His | y = 0.72 - 3.55x + 4.95x2 - 0.11v - 0.018v2 + 1.31xv - 1.92x2v | 0.01 | 0.84 | <0.0001 |
| Arg | y = 2.28 - 11.3x + 15.7x2 - 0.41v - 0.046v2 + 4.02xv - 5.79x2v | 0.02 | 0.77 | <0.0001 |
| Cys | y = 0.88 - 4.15x + 4.8x2 - 0.32v + 0.025v2 + 1.74xv - 1.78x2v - 0.097xv2 | 0.01 | 0.93 | <0.0001 |
| Trp | y = 0.037 + 0.63x - 1.52x2 + 0.25v - 0.07v2 - 1.59xv + 2.72x2v + 0.42xv2 - 0.68x2v2 | 0.00 | 0.76 | <0.0001 |
| Met + Cys | y = 0.58 - 0.81x - 1.11x2 + 0.58v - 0.20v2 - 3.8xv + 6.97x2v + 1.31xv2 - 2.17x2v2 | 0.01 | 0.90 | <0.0001 |
| Asp | y = 3.3 - 17.1x + 23.7x2 - 0.61v - 0.065v2 + 6.12xv - 8.96x2v | 0.03 | 0.91 | <0.0001 |
| Ser | y = 2.36 - 11.5x + 14.4x2 - 0.71v + 0.038v2 + 4.29xv - 4.71x2v - 0.21xv2 | 0.01 | 0.88 | <0.0001 |
| Glu | y = 5.46 - 27.8x + 38.6x2 - 0.88v - 0.135v2 + 10.1xv - 14.7x2v | 0.05 | 0.88 | <0.0001 |
| Pro | y = 1.50 - 7.14x + 9.68x2 - 0.26v - 0.031v2 + 2.69xv - 3.87x2v | 0.02 | 0.87 | <0.0001 |
| Gly | y = 2.35 - 12.8x + 17.9x2 - 1.17v + 0.17v2 + 7.8xv - 11.2x2v - 1.17xv2 + 1.67x2v2 | 0.01 | 0.84 | <0.0001 |
| Ala | y = 1.36 - 6.78x + 9.49x2 - 0.21v - 0.033v2 + 2.43xv - 3.52x2v | 0.01 | 0.79 | <0.0001 |
| Tyr | y = 0.93 - 4.3x + 5.76x2 - 0.13v - 0.022v2 + 1.56xv - 2.25x2v | 0.01 | 0.87 | <0.0001 |
| Ca | y = 2.71 - 12.2x + 14.9x2 - 2.42v + 0.51v2 + 12.3xv - 15.9x2v - 2.67xv2 + 3.54x2v2 | 0.02 | 0.60 | <0.0001 |
| P | y = 0.054 - 0.092x + 0.026v + 0.123xv | 0.01 | 0.96 | <0.0001 |
Table 6.
Predicted effect of graded concentrations of phytate P and log doses of phytase on apparent digestible nutrient intake (g/D) of 21-day-old broilers.1
| 0.24% phytate P |
0.345% phytate P |
0.45% phytate P |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phytate P Phytase dose | 50 | 500 | 1,000 | 2,000 | 50 | 500 | 1,000 | 2,000 | 50 | 500 | 1,000 | 2,000 |
| Thr | 0.35 | 0.36 | 0.36 | 0.35 | 0.28 | 0.33 | 0.34 | 0.34 | 0.29 | 0.31 | 0.31 | 0.30 |
| Val | 0.43 | 0.45 | 0.45 | 0.44 | 0.35 | 0.42 | 0.43 | 0.44 | 0.36 | 0.38 | 0.38 | 0.37 |
| Met | 0.25 | 0.26 | 0.26 | 0.26 | 0.20 | 0.24 | 0.25 | 0.26 | 0.21 | 0.25 | 0.25 | 0.24 |
| Iso | 0.41 | 0.43 | 0.43 | 0.42 | 0.34 | 0.41 | 0.42 | 0.42 | 0.34 | 0.36 | 0.35 | 0.34 |
| Leu | 0.81 | 0.85 | 0.84 | 0.82 | 0.70 | 0.81 | 0.82 | 0.82 | 0.70 | 0.74 | 0.72 | 0.70 |
| Phe | 0.50 | 0.52 | 0.51 | 0.50 | 0.42 | 0.49 | 0.49 | 0.49 | 0.42 | 0.44 | 0.43 | 0.42 |
| Lys | 0.67 | 0.66 | 0.64 | 0.62 | 0.55 | 0.62 | 0.63 | 0.63 | 0.58 | 0.60 | 0.59 | 0.58 |
| His | 0.26 | 0.27 | 0.27 | 0.26 | 0.22 | 0.26 | 0.26 | 0.26 | 0.23 | 0.24 | 0.23 | 0.23 |
| Arg | 0.72 | 0.73 | 0.72 | 0.70 | 0.61 | 0.69 | 0.70 | 0.70 | 0.63 | 0.65 | 0.64 | 0.62 |
| Cys | 0.16 | 0.16 | 0.16 | 0.16 | 0.11 | 0.14 | 0.15 | 0.15 | 0.10 | 0.13 | 0.12 | 0.12 |
| Trp | 0.12 | 0.12 | 0.12 | 0.12 | 0.10 | 0.11 | 0.11 | 0.11 | 0.11 | 0.12 | 0.11 | 0.11 |
| Met + Cys | 0.40 | 0.42 | 0.42 | 0.42 | 0.31 | 0.38 | 0.39 | 0.41 | 0.32 | 0.37 | 0.37 | 0.36 |
| Asp | 0.95 | 1.00 | 0.99 | 0.97 | 0.77 | 0.91 | 0.93 | 0.94 | 0.77 | 0.81 | 0.80 | 0.77 |
| Ser | 0.48 | 0.48 | 0.48 | 0.47 | 0.37 | 0.44 | 0.44 | 0.44 | 0.39 | 0.43 | 0.41 | 0.39 |
| Glu | 1.79 | 1.88 | 1.85 | 1.80 | 1.50 | 1.75 | 1.77 | 1.77 | 1.52 | 1.59 | 1.57 | 1.51 |
| Pro | 0.53 | 0.55 | 0.54 | 0.53 | 0.45 | 0.52 | 0.52 | 0.53 | 0.44 | 0.46 | 0.46 | 0.45 |
| Gly | 0.38 | 0.40 | 0.39 | 0.39 | 0.31 | 0.37 | 0.37 | 0.37 | 0.32 | 0.34 | 0.34 | 0.33 |
| Ala | 0.47 | 0.50 | 0.49 | 0.48 | 0.41 | 0.47 | 0.48 | 0.49 | 0.42 | 0.45 | 0.44 | 0.43 |
| Tyr | 0.35 | 0.37 | 0.37 | 0.36 | 0.30 | 0.34 | 0.34 | 0.34 | 0.29 | 0.30 | 0.30 | 0.29 |
| Ca | 0.19 | 0.15 | 0.17 | 0.20 | 0.17 | 0.17 | 0.17 | 0.18 | 0.11 | 0.13 | 0.15 | 0.17 |
| P | 0.12 | 0.18 | 0.20 | 0.21 | 0.14 | 0.20 | 0.23 | 0.25 | 0.15 | 0.23 | 0.25 | 0.28 |
Means were determined using the equations from Table 5 and the log dose of phytase at 1.7, 2.7, 3.0, and 3.3 for 50, 500, 1,000, and 2,000 FTU/kg, respectively.
DISCUSSION
Prediction equations were used in the current experiment to determine the relationship between increasing dietary phytate P concentrations and varying doses of phytase on the AID and digestible intake of amino acids, Ca, and P. It was hypothesized that increasing doses of exogenous phytase are required as dietary phytate P content increases to result in nearly complete phytate degradation and improvements in nutrient utilization. Ravindran et al. (2006) reported increasing dietary phytate P, from rice bran, significantly reduced AID of amino acids. This in agreement with the current results, with the greatest impact of increasing dietary phytate P on the digestibility of amino acids such as cysteine (minus 10 to 14 percentage units), glycine (minus 7 to 9 percentage points), and threonine (minus 7 to 8 percentage points). Threonine, glycine, and cysteine make up a large proportion of endogenous amino acid losses associated with dietary phytate (Cowieson et al., 2008; Onyango et al., 2009), thereby explaining the large negative influence of increasing dietary phytate on these amino acids. However, previous authors have reported no effect of dietary phytate on cysteine or methionine (Onyango et al., 2009). This is contrary to the current results and may be indicative of the amino acid deficiencies formulated into the experimental diets, the animals' amino acid requirements, type of phytate and basal diet, and the inhibitory impact of dietary phytate on pepsin and trypsin activity (Liu et al., 2009; Yu et al., 2012); thereby predicting a large negative impact on the AID of essential amino acids not always reported to be influenced by dietary phytate.
Phytase supplementation significantly improved the AID of all amino acids and this has been previously reported in corn−soy−rice bran-based diets (Ravindran et al., 2006). The dose of phytase employed to improve the AID of amino acids and the magnitude of the response to phytase, was dependent on the phytate P content of the diet and the amino acid evaluated. In diets containing 0.24% phytate P, the average uplift of phytase supplementation on the AID of amino acids was +2 percentage points, achieved at 500 FTU/kg with a +3 percentage points uplift as phytase dose increased to 2,000 FTU/kg. However, in diets containing 0.345 or 0.45% phytate P, the initial 500 FTU/kg of phytase increased the average AID of amino acids by +5 or +3 percentage points, respectively, and this was further increased to +6 or +7 percentage points as phytase dose increased to 2,000 FTU/kg. Greater responses were observed for specific amino acids, such as +4 to 12 or +2 to 8 percentage points for cysteine or glycine. Large and significant beneficial effects of phytase supplementation on the AID of cysteine (Walk and Rama Rao, 2018) and glycine (Cowieson et al., 2017) have been previously reported. The current results confirm greater doses of phytase continue to result in increases in the AID of amino acids as the dietary phytate P content increases. However, the current results are contradictory to those of Ravindran et al. (2006) who reported 750 to 1,000 FTU/kg was required to improve the AID of amino acids in diets containing 0.28% phytate P and no further benefits on the AID of amino acids were reported by increasing phytase above 500 FTU/kg diets containing 0.33 or 0.38% phytate P. The authors suggested increasing the concentration of dietary phytate promoted phytate−protein complex formation and reduced phytase activity. A challenge that may now be overcome in high phytate diets with novel commercially available phytases, reported to rapidly degrade phytate at low pH where phytate is soluble (Menezes-Blackburn et al., 2015).
Other factors to consider when comparing the results from Ravindran et al. (2006) to the current results are factors that can influence the digestibility response, such as the length of time the phytase was fed (1-week vs. 3-weeks in the current trial; Babatunde et al., 2019), oven drying of the digesta samples vs. freeze drying (Lagos and Stein, 2018), total ileal digesta collection vs. the distal half, and genetic changes in broilers over the past 13 yr (Ten Doeschate et al., 1993), particularly regarding voluntary feed intake. To try and account for the influence of feed intake on the AID of amino acids, the digestible amino acid intake was calculated. Previous authors have reported a better relationship between digestible amino acid intake and BWG when compared with that of the AID of amino acids and BWG (Walk and Olukosi, 2019). In the current trial (and similar to the AID of amino acids), increasing dietary phytate P from 0.24 to 0.345 and 0.45% reduced the digestible intake of amino acids in a non-linear manner by an average of −91 to −84 mg/D, respectively. The greatest impact of dietary phytate P concentration on digestible amino acid intake was noted in birds fed diets containing 0.345% phytate P. This maybe an artifact of similar feed intake to that of birds fed 0.24% phytate P and a significant reduction in the AID of amino acids, whereas birds fed 0.45% phytate P had a significant reduction in intake and similar AID of amino acids to that of birds fed 0.345% phytate P.
Phytase supplementation increased digestible amino acid intake, particularly in birds fed diets containing 0.345% phytate P, with improvements of an average of +86, 95, or 98 mg/D at 500, 1,000 or 2,000 FTU/kg, respectively. However, phytase supplementation in birds fed diets containing 0.45% phytate P could not overcome the reduction of feed intake, even with the improvement in AID of amino acids, and increases in digestible amino acid intake were only apparent up to 500 FTU/kg. Until more information becomes available for the use of digestible amino acid intake as a response variable, comparisons with previous data and conclusions on the current results are difficult to make. However, pairwise correlations between BWG from hatch to day 21 and the AID of a few amino acids, such as lysine (r = 0.53, < 0.0001), methionine + cysteine (r = 0.60, P < 0.0001), or glycine (r = 0.57, P < 0.0001) or the digestible intake of lysine (r = 0.66, P < 0.0001), methionine + cysteine (r = 0.75, P < 0.0001), or glycine (r = 0.75, P < 0.0001) support a better relationship between digestible intake and BWG and this has been previously reported (Walk and Olukosi, 2019). Growth performance data from the current trial is presented in a companion paper (Walk and Rama Rao, unpublished).
Finally, due to the use of oven drying, the AID of amino acids reported in the current experiment may be greater than that if the samples were freeze dried (Lagos and Stein, 2018). However, in the current experiment, the digesta samples were all exposed to the same drying conditions and any denaturation of amino acids, particularly lysine, arginine, or alanine would have occurred equally between the experimental diets. Therefore, the effect of phytase or phytate P on the AID of amino acids can still be described as reported because the magnitude of the response to phytase or phytate P would be the same regardless of the drying conditions. The absolute amino acid digestibility coefficients obtained from any experiment are an estimate of the average amino acid digestibility over the duration of the trial and obtained as a point in time measurement. The most important factor in the current trial is the relative effect of phytase or phytate P on the AID of amino acids and Ca and P, information that is further supported by growth performance, phytate and phytate ester degradation, and bone ash (Walk and Rama Rao, unpublished).
In conclusion, prediction equations can be a method to determine the influence of- and interactions-between increasing dietary phytate P and varying phytases doses on AID and digestible nutrient intake of amino acids, Ca, and P. Dietary phytate P, particularly at concentrations greater than 0.24%, significantly reduced AID and digestible intake of amino acids. Phytase supplementation improved the AID of amino acids and greater concentrations of phytase continued to improve AID of amino acids in birds fed diets containing higher levels of phytate. Digestible amino acid intake is strongly correlated with BWG and may be a good response variable to include in future trials evaluating the influence of enzymes on broiler performance and nutrient digestibility.
REFERENCES
- AOAC. 2006. Official Methods of Analysis of AOAC International. 18th ed., Arlington, VA.
- Babatunde O.O., Cowieson A.J., Wilson J.W., Adeola O. Influence of age and duration of feeding low-phosphorus diet on phytase efficacy in broiler chickens during the starter phase. Poult. Sci. 2019;98:2588–2597. doi: 10.3382/ps/pez014. [DOI] [PubMed] [Google Scholar]
- Cowieson A.J., Ruckebusch J.-P., Sorbara J.O.B., Wilson J.W., Guggenbuhl P., Roos F.F. A systematic view on the effect of phytase on ileal amino acid digestibility in broilers. Anim. Feed Sci. Technol. 2017;225:182–194. [Google Scholar]
- Cowieson A.J., Ravindran V., Selle P.H. Influence of dietary phytic acid and source of microbial phytase on ileal endogenous amino acid flows in broiler chickens. Poult. Sci. 2008;87:2287–2299. doi: 10.3382/ps.2008-00096. [DOI] [PubMed] [Google Scholar]
- Cowieson A.J., Acamovic T., Bedford M.R. Phytic acid and phytase: implications for protein utilization by poultry. Poult. Sci. 2006;85:878–885. doi: 10.1093/ps/85.5.878. [DOI] [PubMed] [Google Scholar]
- Cowieson A.J., Acamovic T., Bedford M.R. Supplementation of corn-soy-based diets with an Eschericia coli-derived phytase: Effects on broiler chick performance and the digestibility of amino acids and metabolizability of minerals and energy. Poult. Sci. 2006;85:1389–1397. doi: 10.1093/ps/85.8.1389. [DOI] [PubMed] [Google Scholar]
- Dale N., Fuller H.L., Pesti G.M., Dixon Phillips R. Freeze drying versus oven drying of excreta in true metabolizable energy, nitrogen-corrected true metabolizable energy, and true amino acid availability bioassays. Poult. Sci. 1985;64:362–365. [Google Scholar]
- David L.S., Abdollahi M.R., Walk C.L., Ravindran V. Calcium digestibility of limestone in broiler chickens as influenced by age and dietary crude protein content. Poult. Sci. 2019;98(E-Suppl. 1):209. doi: 10.3382/ps/pez314. abstr. 547P. [DOI] [PubMed] [Google Scholar]
- Engelen A.J., van der Heeft F.C., Randsdorp P.H.G., Somers W.A.C. Determination of phytase activity in feed by colorimetric enzymatic method: collaborative interlaboratory study. J. AOAC. Int. 2001;84:629–633. [PubMed] [Google Scholar]
- Gonzalez-Vega J.C., Walk C.L., Stein H.H. Effect of phytate, microbial phytase, fiber, and soybean oil on calculated values for apparent and standardized total tract digestibility of calcium and apparent total tract digestibility of phosphorus in fish meal fed to growing pigs. J. Anim. Sci. 2015;93:4808–4818. doi: 10.2527/jas.2015-8992. [DOI] [PubMed] [Google Scholar]
- Kelly D., Smyth J.A., McCracken K.J. Digestive development of the early-weaned pig. 2. Effect of level of food intake on digestive enzyme activity during the immediate post-weaning period. Br. J. Nutr. 1991;65:181–188. doi: 10.1079/bjn19910079. [DOI] [PubMed] [Google Scholar]
- Lagos L.V., Stein H.H. Effect of drying method of ileal digesta on the digestibility of crude protein and amino acids by pigs. J. Anim. Sci. 2018;96:181. [Google Scholar]
- Liu N., Ru Y.J., Li F.D., Wang J.-P., Lei X.-Q. Effect of dietary phytate and phytase on proteolytic digestion and growth regulation of broilers. Arch. Anim. Nutr. 2009;63:292–303. doi: 10.1080/17450390903020422. [DOI] [PubMed] [Google Scholar]
- Liu N., Ru Y.J., Li F.D., Cowieson A.J. Effect of diet containing phytate and phytase on the activity and messenger ribonucleic acid expression of carbohydrase and transporter in chickens. J. Anim. Sci. 2008;86:3432–3439. doi: 10.2527/jas.2008-1234. [DOI] [PubMed] [Google Scholar]
- Menezes-Blackburn D., Gabler S., Greiner R. Performance of seven commercial phytases in an in vitro simulation of poultry digestive tract. J. Agri. Food Chem. 2015;63:6142–6149. doi: 10.1021/acs.jafc.5b01996. [DOI] [PubMed] [Google Scholar]
- Onyango E.M., Asem E.K., Adeola O. Phytic acid increases mucin and endogenous losses from the gastrointestinal tract of chickens. Br. J. Nutr. 2009;101:836–842. doi: 10.1017/S0007114508047740. [DOI] [PubMed] [Google Scholar]
- Ravindran V., Morel P.C.H., Partridge G.G., Hruby M., Sands J.S. Influence of an Escherichia coli-derived phytase on nutrient utilization in broiler starters fed diets containing varying concentrations of phytic acid. Poult. Sci. 2006;85:82–89. doi: 10.1093/ps/85.1.82. [DOI] [PubMed] [Google Scholar]
- Ravindran V., Selle P.H., Ravindran G., Morel PCH, Kies A.K., Bryden W.L. Microbial phytase improves performance, apparent metabolizable energy, and ileal amino acid digestibility of broilers fed a lysine-deficient diet. Poult. Sci. 2001;80:338–344. doi: 10.1093/ps/80.3.338. [DOI] [PubMed] [Google Scholar]
- Ravindran V., Cabahug S., Ravindran G., Bryden W.L. Influence of microbial phytase on apparent ileal amino acid digestibility of feedstuffs for broilers. Poult. Sci. 1999;78:699–706. doi: 10.1093/ps/78.5.699. [DOI] [PubMed] [Google Scholar]
- Sommerfeld V., Kunzel S., Schollenberger M., Kuhn I., Rodehutscord M. Influence of phytase or myo-inositol supplements on performance and phytate degradation products in the crop, ileum, and blood of broiler chickens. Poult. Sci. 2018;97:920–929. doi: 10.3382/ps/pex390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ten Doeschate R.A.H.M., Scheele C.W., Schreurs V.V.A.M., Van Der Klis J.D. Digestibility studies in broiler chickens: influence of genotype, age, sex, and method of determination. Br. Poult. Sci. 1993;34:131–146. [Google Scholar]
- Walk C.L., Olukosi O.A. Influence of graded concentrations of phytase in high-phytate diets on growth performance, apparent ileal amino acid digestibility, and phytate concentration in broilers from hatch to 28 D post-hatch. Poult. Sci. 2019 doi: 10.3382/ps/pez106. [DOI] [PubMed] [Google Scholar]
- Walk C.L., Pirgozliev V., Juntunen K., Paloheimo M., Ledoux D.R. Evaluation of novel protease enzymes on growth performance and nutrient digestibility of poultry: enzyme screening. Poult. Sci. 2018;97:2123–2138. doi: 10.3382/ps/pey080. [DOI] [PubMed] [Google Scholar]
- Walk C.L., Rama Rao S.V. High doses of phytase on growth performance and apparent ileal amino acid digestibility of broilers fed diets with graded concentrations of digestible sulfur amino acids. Poult. Sci. 2018;97:3610–3621. doi: 10.3382/ps/pey218. [DOI] [PubMed] [Google Scholar]
- Yu S., Cowieson A., Gilbert C., Plumstead P., Dalsgaard S. Interactions of phytate and myo-inositol phosphate esters (IP1-5) including IP5 isomers with dietary protein and iron and inhibition of pepsin. J. Anim. Sci. 2012;90:1824–1832. doi: 10.2527/jas.2011-3866. [DOI] [PubMed] [Google Scholar]