Abstract
Reducing dietary CP can reduce N pollution. Much research has been reported in corn-based diets; however, the amino acid (AA) profiles of wheat-based diets differ. Poor performance as a result of reduced protein (RP) has been overcome in corn-based diets with essential AA and glycine (Gly) supplementation. The current study examined RP levels and Gly in wheat-based diets. An industry standard protein (SP) diet plus 3 RP diets with and without Gly supplementation, to match the SP treatment at 0.713 and 0.648% digestible Gly for the grower and finisher periods respectively, were fed to male broilers from day 10 of age. Grower CP included 22.5, 20.6, 18.3, and 17.7% (days 10–21) and finisher CP included 19.7, 17.8, 16.2, and 15.5% (days 21–35). Performance, meat yield, N efficiency, water intake, and apparent ileal digestibility of N and AA were measured. No difference in body weight gain (BWG), feed intake, or feed conversion ratio (FCR) were observed at 20% CP compared to the SP treatment. However, further reducing protein reduced BWG (P < 0.001), feed intake (P < 0.001), and increased FCR (P < 0.001). Supplementation of 0.713% Gly in the grower period increased BWG (P < 0.001) and reduced FCR (P < 0.001). Relative meat yield was not affected by dietary protein, however reducing CP increased relative fat pad weight (P < 0.001). Nitrogen efficiency increased with decreased CP in both grower (R2 = 0.69) and finisher (R2 = 0.80) treatments. Water intake decreased (R2 = 0.83) with decreasing CP intake. Apparent ileal digestibility of AA and N were higher in RP diets (P < 0.05). The benefits of reduced water intake and increased N efficiency and the disadvantages of poor performance and increased body fat in RP corn-based diets have been identified in RP wheat-based diets. Furthermore, at 18.5% CP the supplementation of crystalline AA and Gly can maintain BWG and FCR observed in SP diets.
Key words: meat chickens, amino acids, water intake, reduced protein, digestibility
Introduction
Higher protein levels in broiler diets have been attributed to health and welfare concerns and environmental impacts in the poultry industry. A high protein diet may lead to wet litter that increases the severity and incidence of footpad dermatitis and breast blisters (Harms et al., 1977, Shepherd and Fairchild, 2010). Reducing dietary CP is known to lower water consumption (Marks and Pesti, 1984) and the volume of water excreted (James and Wheeler, 1949) promoting good litter quality and reducing the risk of disease and infection. Additional environmental benefits of reduced protein (RP) diets include lower N waste through improved N efficiency (Belloir et al., 2017, van Emous et al., 2019).
However, when RP diets are fed, broiler performance deteriorates and is attributed to a limited availability of amino acids (AA). The supplementation of essential AA alone has failed to increase performance in RP diets (Dean et al., 2006). Research has identified that the supplementation of nonessential AA, such as glycine (Gly), in RP diets can return performance to that seen in standard protein diets (Ospina-Rojas et al., 2013a). Meat and bone meal is typically removed from RP diets in favor of cost-effective crystalline AA. Meat and bone meal provides a rich source of dietary Gly at approximately 6.6% (NRC, 1994). Glycine is involved in the synthesis of many metabolically important products such as creatine, heme, glutathione, serine (Ser), and uric acid (Baker et al., 1968, Kidd and Kerr, 1996, Stevens, 1996, Shoulders and Raines, 2009). The synthesis of uric acid is believed to be the major contributor to Gly deficiencies in plant-based RP diets (Namroud et al., 2008).
The beneficial effects of Gly supplementation in RP corn-based diets have been well established (Dean et al., 2006); however, poultry nutritionists in some areas rely on wheat-based diets, and limited research has been conducted in RP wheat-based diets. The AA profiles of wheat and corn differ with wheat having a higher CP content, increasing overall AA concentrations. The difference of Gly can be 66% greater in a typical 11.5% CP wheat grain versus a typical 8.5% CP corn grain (NRC, 1994). This supports that AA digestibility of RP diets with differing grain sources will impact birds differently. Heger and Pack (1996) further identified that Gly requirement is influenced by dietary CP. This indicates the research conducted in RP corn-based diets may not be applicable to RP wheat-based diets. A greater understanding of Gly and essential AA supplementation in RP wheat-based diets is required before the implementation of RP diets in wheat-based feeding programs.
The benefits of RP diets identified such as reduced N excretion and water consumption are expected to apply to wheat-based RP diets, but this has not been established using a scientific method. Therefore, the aim of the current study was to investigate the significance of Gly supplementation in and assess possible environmental benefits of RP wheat-based diets.
Materials and methods
All experimental procedures were approved by the Animal Ethics Committee of the University of New England (AEC16-050).
Experimental Design and Diets
A feeding study was conducted to investigate the effects of supplementing essential AA with and without Gly at 3 different RP levels on broiler chicken performance, N efficiency, and water intake in comparison to an industry standard protein diet (SP). This resulted in 7 treatments allocated to a completely randomized design: SP, 20.0% CP (20CP), 20.0% CP with Gly (20CP+Gly), 18.5% CP (18.5CP), 18.5% with Gly (18.5CP+Gly) 17.0% CP (17CP), and 17.0% CP with Gly (17CP+Gly). Serine was not accounted for in Gly supplementation, due to a need to focus on only Gly in meat chicken diets and to establish the necessity of Ser considerations. Dietary CP was reduced changing from grower to finisher treatments to reflect the SP treatment. Glycine was supplemented to match that estimated in the SP diet.
A common starter diet (3,020kcal/kg, 24.0% CP) was fed from day 0 to 10 and contained wheat, sorghum, soybean meal, and meat and bone meal and zinc bacitracin at 0.05%. The treatment diets shown in Tables 1 and 2 were formulated with N corrected metabolizable energy: grower from day 10 to 21 at 3,100 kcal/kg; finisher from day 21 to 35 at 3,200 kcal/kg. Grower and finisher diets were wheat-based with sorghum, soybean meal and canola oil. Meat and bone meal was not included in experimental diets. Crystalline AA offered in the formulation were as follows: D,L methionine (Met), L-lysine (Lys) SO4, L-threonine (Thr), L-valine (Val), L-arginine (Arg), L-isoleucine (Ile), L-phenylalanine (Phe), L-leucine (Leu), L-histidine (His), and L-tryptophan (Trp). Crystalline AA was supplemented when limiting according to AMINOChick 2.0 (Evonik Nutrition and Care GmbH, 2016) software. Glycine was supplemented to 0.71 and 0.65% standardized ileal digestible Gly in the grower and finisher diets, respectively; this level was chosen to reflect the amount of Gly in the grower and finisher SP treatments, respectively. Experimental diets contained 0.5% titanium dioxide as a digestibility marker. Exogenous enzymes xylanase (Econase XT 25) and phytase (Quantum Blue, 5 G) were added to the diets at 1,000 BXU/kg and 500 FTU/kg, respectively, following manufacturer recommendations. The nutrient matrix for the phytase was also considered in the diet formulation. All other nutrient requirements were formulated according to the breed standards (Aviagen, 2014).
Table 1.
Diet compositions for experimental treatments in grower period.
| Ingredients, % | SP | 20CP | 20CP+Gly | 18.5CP | 18.5CP+Gly | 17CP | 17CP+Gly |
|---|---|---|---|---|---|---|---|
| Wheat (10.5% CP) | 43.20 | 50.12 | 50.12 | 57.12 | 57.12 | 59.34 | 59.34 |
| Soybean meal (46.7% CP) | 30.22 | 23.51 | 23.51 | 16.50 | 16.50 | 8.32 | 8.32 |
| Sorghum (10.7% CP) | 20.00 | 20.00 | 20.00 | 20.00 | 20.00 | 20.00 | 20.00 |
| Canola oil | 3.19 | 2.23 | 2.23 | 1.11 | 1.11 | 1.24 | 1.24 |
| Dicalcium phosphate | 0.61 | 0.65 | 0.65 | 0.69 | 0.69 | 0.77 | 0.77 |
| Limestone | 1.06 | 1.07 | 1.07 | 1.09 | 1.09 | 1.10 | 1.10 |
| Sodium chloride | 0.19 | 0.18 | 0.18 | 0.16 | 0.16 | 0.18 | 0.18 |
| Sodium bicarbonate | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 |
| Alpha cellulose/celite | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 3.40 | 3.40 |
| Xylanase1 | 0.005 | 0.005 | 0.005 | 0.005 | 0.005 | 0.005 | 0.005 |
| Phytase2 | 0.010 | 0.010 | 0.010 | 0.010 | 0.010 | 0.010 | 0.010 |
| Titanium dioxide | 0.500 | 0.500 | 0.500 | 0.500 | 0.500 | 0.500 | 0.500 |
| Vitamin premix3 | 0.050 | 0.050 | 0.050 | 0.050 | 0.050 | 0.050 | 0.050 |
| Mineral premix4 | 0.075 | 0.075 | 0.075 | 0.075 | 0.075 | 0.075 | 0.075 |
| L-Lys Sulfate | 0.338 | 0.598 | 0.598 | 0.871 | 0.871 | 1.216 | 1.216 |
| D,L-Met | 0.238 | 0.285 | 0.285 | 0.335 | 0.335 | 0.416 | 0.416 |
| L-Thr | 0.060 | 0.145 | 0.145 | 0.234 | 0.234 | 0.355 | 0.355 |
| L-Val | 0.000 | 0.106 | 0.106 | 0.217 | 0.217 | 0.372 | 0.372 |
| L-Ile | 0.000 | 0.076 | 0.076 | 0.183 | 0.183 | 0.328 | 0.328 |
| L-Arg | 0.000 | 0.117 | 0.117 | 0.305 | 0.305 | 0.552 | 0.552 |
| L-Leu | 0.000 | 0.000 | 0.000 | 0.090 | 0.090 | 0.333 | 0.333 |
| L-Phe | 0.000 | 0.000 | 0.000 | 0.141 | 0.141 | 0.429 | 0.429 |
| L-His | 0.000 | 0.000 | 0.000 | 0.026 | 0.026 | 0.111 | 0.111 |
| L-Trp | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.577 | 0.577 |
| Gly | 0.000 | 0.000 | 0.086 | 0.000 | 0.176 | 0.000 | 0.303 |
| Choline chloride | 0.057 | 0.072 | 0.072 | 0.089 | 0.089 | 0.120 | 0.120 |
| Salinomycin (11.7%) | 0.050 | 0.050 | 0.050 | 0.050 | 0.050 | 0.050 | 0.050 |
| Nutrient composition, % | |||||||
| AMEn, kcal/kg | 3,100 | 3,100 | 3,100 | 3,100 | 3,100 | 3,100 | 3,100 |
| CP | 21.7 | 20.0 | 20.1 | 18.5 | 18.7 | 17.0 | 17.4 |
| SID5 Lys | 1.130 | 1.130 | 1.130 | 1.130 | 1.130 | 1.130 | 1.130 |
| SID Met | 0.510 | 0.529 | 0.529 | 0.550 | 0.550 | 0.587 | 0.587 |
| SID TSAA | 0.830 | 0.830 | 0.830 | 0.830 | 0.830 | 0.830 | 0.830 |
| SID Thr | 0.730 | 0.730 | 0.730 | 0.730 | 0.730 | 0.730 | 0.730 |
| SID Val | 0.900 | 0.900 | 0.900 | 0.900 | 0.900 | 0.900 | 0.900 |
| SID Ile | 0.806 | 0.780 | 0.780 | 0.780 | 0.780 | 0.780 | 0.780 |
| SID Arg | 1.231 | 1.170 | 1.170 | 1.170 | 1.170 | 1.170 | 1.170 |
| SID Leu | 1.462 | 1.296 | 1.296 | 1.210 | 1.210 | 1.210 | 1.210 |
| SID Phe | 0.910 | 0.796 | 0.796 | 0.815 | 0.815 | 0.936 | 0.936 |
| SID Phe + Tyr | 1.576 | 1.379 | 1.379 | 1.310 | 1.310 | 1.310 | 1.310 |
| SID His | 0.463 | 0.405 | 0.405 | 0.370 | 0.370 | 0.370 | 0.370 |
| SID Trp | 0.264 | 0.234 | 0.234 | 0.203 | 0.203 | 0.730 | 0.730 |
| SID Gly | 0.713 | 0.627 | 0.713 | 0.537 | 0.713 | 0.410 | 0.713 |
| SID Ser | 0.885 | 0.780 | 0.780 | 0.670 | 0.670 | 0.514 | 0.514 |
| Calcium | 0.80 | 0.80 | 0.80 | 0.80 | 0.80 | 0.80 | 0.80 |
| Available phosphorus | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 |
| Sodium | 0.18 | 0.18 | 0.18 | 0.17 | 0.17 | 0.18 | 0.18 |
| Potassium. | 0.94 | 0.83 | 0.83 | 0.70 | 0.70 | 0.53 | 0.53 |
| Chloride | 0.17 | 0.17 | 0.17 | 0.16 | 0.16 | 0.18 | 0.18 |
| DEB6 mEq/kg | 271 | 241 | 241 | 210 | 210 | 165 | 165 |
| Choline mg/kg | 1,600 | 1,600 | 1,600 | 1,600 | 1,600 | 1,600 | 1,600 |
| Linoleic acid | 1.74 | 1.50 | 1.50 | 1.22 | 1.22 | 1.20 | 1.20 |
Abbreviations: Lys, lysine; Met, methionine; Thr, threonine; Val, valine; Arg, arginine; Ile, isoleucine; Leu, leucine; Phe, phenylalanine; Tyr, tyrosine; His, histidine; Trp, tryptophan; Gly, glycine; SID, standardized ileal digestible; Ser, serine; DEB, dietary electrolyte balance; SP, standard protein; 20CP, 20% crude protein treatment; 20CP+Gly, 20% crude protein treatment with glycine supplemented; 18.5CP, 18.5% crude protein treatment; 18.5CP+Gly, 18.5% crude protein treatment with glycine supplemented; 17CP, 17% crude protein treatment; 17CP+Gly, 17% crude protein treatment with glycine supplemented.
Econase XT 25 (AB Vista, 1,000 BXU/kg).
Quantum Blue, 5 G (AB Vista, 500 FTU/kg).
Vitamin premix per kg diet: vitamin A, 12 MIU; vitamin D, 5 MIU; vitamin E, 75 mg; vitamin K, 3 mg; nicotinic acid, 55 mg; pantothenic acid, 13 mg; folic acid, 2 mg; riboflavin, 8 mg; cyanocobalamin, 0.016 mg; biotin, 0.25 mg; pyridoxine, 5 mg; thiamine, 3 mg; antioxidant, 50 mg.
Mineral premix per kg diet: Cu, 16 mg as copper sulfate; Mn, 60 mg as manganese sulfate; Mn, 60 mg as manganous oxide; I, 0.125 mg as potassium iodide; Se, 0.3 mg; Fe, 40 mg, as iron sulfate; Zn, 50 mg as zinc oxide; Zn, 50 mg as zinc sulfate.
Standard ileal digestible coefficients for raw ingredients determined using AMINODat 5.0 (Evonik Animal Nutrition).
DEB mEq/kg calculated as 10,000 × (Na+ + K+ −Cl−).
Table 2.
Diet compositions for experimental treatments in finisher period.
| Ingredients, % | SP | 20CP | 20CP+Gly | 18.5CP | 18.5CP+Gly | 17CP | 17CP+Gly |
|---|---|---|---|---|---|---|---|
| Wheat (10.5% CP) | 47.36 | 55.20 | 55.20 | 62.53 | 62.53 | 70.20 | 70.20 |
| Soybean meal (46.7% CP) | 25.36 | 17.70 | 17.70 | 10.28 | 10.28 | 2.39 | 2.39 |
| Sorghum (10.7% CP) | 20.00 | 20.00 | 20.00 | 20.00 | 20.00 | 20.00 | 20.00 |
| Canola oil | 4.09 | 2.99 | 2.99 | 1.77 | 1.77 | 0.44 | 0.44 |
| Dicalcium phosphate | 0.48 | 0.52 | 0.52 | 0.57 | 0.57 | 0.61 | 0.61 |
| Limestone | 1.02 | 1.04 | 1.04 | 1.06 | 1.06 | 1.08 | 1.08 |
| Sodium chloride | 0.19 | 0.18 | 0.18 | 0.16 | 0.16 | 0.15 | 0.15 |
| Sodium bicarbonate | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 |
| Alpha cellulose/celite | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Xylanase1 | 0.005 | 0.005 | 0.005 | 0.005 | 0.005 | 0.005 | 0.005 |
| Phytase2 | 0.010 | 0.010 | 0.010 | 0.010 | 0.010 | 0.010 | 0.010 |
| Titanium dioxide | 0.500 | 0.500 | 0.500 | 0.500 | 0.500 | 0.500 | 0.500 |
| Vitamin premix3 | 0.050 | 0.050 | 0.050 | 0.050 | 0.050 | 0.050 | 0.050 |
| Mineral premix4 | 0.075 | 0.075 | 0.075 | 0.075 | 0.075 | 0.075 | 0.075 |
| L-Lys Sulfate | 0.340 | 0.638 | 0.638 | 0.927 | 0.927 | 1.234 | 1.234 |
| D,L-Met | 0.215 | 0.269 | 0.269 | 0.322 | 0.322 | 0.379 | 0.379 |
| L-Thr | 0.053 | 0.151 | 0.151 | 0.246 | 0.246 | 0.347 | 0.347 |
| L-Val | 0.000 | 0.121 | 0.121 | 0.239 | 0.239 | 0.365 | 0.365 |
| L-Ile | 0.000 | 0.107 | 0.107 | 0.221 | 0.221 | 0.342 | 0.342 |
| L-Arg | 0.000 | 0.176 | 0.176 | 0.376 | 0.376 | 0.588 | 0.588 |
| L-Leu | 0.000 | 0.000 | 0.000 | 0.131 | 0.131 | 0.331 | 0.331 |
| L-Phe | 0.000 | 0.000 | 0.000 | 0.202 | 0.202 | 0.440 | 0.440 |
| L-His | 0.000 | 0.000 | 0.000 | 0.052 | 0.052 | 0.122 | 0.122 |
| L-Trp | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.032 | 0.032 |
| Gly | 0.000 | 0.000 | 0.098 | 0.000 | 0.120 | 0.000 | 0.218 |
| Choline chloride | 0.050 | 0.068 | 0.068 | 0.086 | 0.086 | 0.105 | 0.105 |
| Salinomycin (11.7%) | 0.050 | 0.050 | 0.050 | 0.050 | 0.050 | 0.050 | 0.050 |
| Nutrient composition, % | |||||||
| AMEn, kcal/kg | 3,200 | 3,200 | 3,200 | 3,200 | 3,200 | 3,200 | 3,200 |
| Crude protein | 19.9 | 18.0 | 18.1 | 16.5 | 16.7 | 15.0 | 15.3 |
| SID5 Lys | 1.020 | 1.020 | 1.020 | 1.020 | 1.020 | 1.020 | 1.020 |
| SID Met | 0.466 | 0.488 | 0.488 | 0.510 | 0.510 | 0.533 | 0.533 |
| SID TSAA | 0.770 | 0.770 | 0.770 | 0.770 | 0.770 | 0.770 | 0.770 |
| SID Thr | 0.660 | 0.660 | 0.660 | 0.660 | 0.660 | 0.660 | 0.660 |
| SID Val | 0.820 | 0.820 | 0.820 | 0.820 | 0.820 | 0.820 | 0.820 |
| SID Ile | 0.729 | 0.720 | 0.720 | 0.720 | 0.720 | 0.720 | 0.720 |
| SID Arg | 1.098 | 1.070 | 1.070 | 1.070 | 1.070 | 1.070 | 1.070 |
| SID Leu | 1.335 | 1.146 | 1.146 | 1.090 | 1.090 | 1.090 | 1.090 |
| SID Phe | 0.824 | 0.693 | 0.693 | 0.765 | 0.765 | 0.864 | 0.864 |
| SID Phe + Tyr | 1.427 | 1.201 | 1.201 | 1.180 | 1.180 | 1.180 | 1.180 |
| SID His | 0.419 | 0.353 | 0.353 | 0.340 | 0.340 | 0.340 | 0.340 |
| SID Trp | 0.241 | 0.207 | 0.207 | 0.174 | 0.174 | 0.170 | 0.170 |
| SID Gly | 0.648 | 0.550 | 0.648 | 0.455 | 0.648 | 0.353 | 0.648 |
| SID Ser | 0.805 | 0.685 | 0.685 | 0.568 | 0.568 | 0.442 | 0.442 |
| Calcium | 0.75 | 0.75 | 0.75 | 0.75 | 0.75 | 0.75 | 0.75 |
| Available phosphorus | 0.37 | 0.37 | 0.37 | 0.37 | 0.37 | 0.37 | 0.37 |
| Sodium | 0.18 | 0.18 | 0.18 | 0.17 | 0.17 | 0.17 | 0.17 |
| Potassium | 0.85 | 0.72 | 0.72 | 0.59 | 0.59 | 0.45 | 0.45 |
| Chloride | 0.17 | 0.17 | 0.17 | 0.16 | 0.16 | 0.16 | 0.16 |
| DEB6 mEq/kg | 249 | 215 | 215 | 181 | 181 | 145 | 145 |
| Choline mg/kg | 1,500 | 1,500 | 1,500 | 1,500 | 1,500 | 1,500 | 1,500 |
| Linoleic acid | 1.96 | 1.68 | 1.68 | 1.38 | 1.38 | 1.04 | 1.04 |
Abbreviations: Lys, lysine; Met, methionine; Thr, threonine; Val, valine; Arg, arginine; Ile, isoleucine; Leu, leucine; Phe, phenylalanine; Tyr, tyrosine; His, histidine; Trp, tryptophan; Gly, glycine; SID, standardised ileal digestible; Ser, serine; DEB, dietary electrolyte balance; SP, standard protein; 20CP, 20% crude protein treatment; 20CP+Gly, 20% crude protein treatment with glycine supplemented; 18.5CP, 18.5% crude protein treatment; 18.5CP+Gly, 18.5% crude protein treatment with glycine supplemented; 17CP, 17% crude protein treatment; 17CP+Gly, 17% crude protein treatment with glycine supplemented.
Econase XT, 25 (AB Vista, 1,000 BXU/kg).
Quantum Blue, 5 G (AB Vista, 500 FTU/kg).
Vitamin premix per kg diet: vitamin A, 12 MIU; vitamin D, 5 MIU; vitamin E, 75 mg; vitamin K, 3 mg; nicotinic acid, 55 mg; pantothenic acid, 13 mg; folic acid, 2 mg; riboflavin, 8 mg; cyanocobalamin, 0.016 mg; biotin, 0.25 mg; pyridoxine, 5 mg; thiamine, 3 mg; antioxidant, 50 mg.
Mineral premix per kg diet: Cu, 16 mg as copper sulfate; Mn, 60 mg as manganese sulfate; Mn, 60 mg as manganous oxide; I, 0.125 mg as potassium iodide; Se, 0.3 mg; Fe, 40 mg, as iron sulfate; Zn, 50 mg as zinc oxide; Zn, 50 mg as zinc sulfate.
Standard ileal digestible coefficients for raw ingredients determined using AMINODat 5.0 (Evonik Animal Nutrition).
DEB mEq/kg calculated as 10,000 × (Na+ + K+ − Cl−).
Animal Husbandry
Ross 308 male broilers (n = 546) from Baiada hatchery in Tamworth, Australia, were delivered to the Centre of Animal Research and Teaching at the University of New England, Armidale, Australia. On day 0, chicks were randomly assigned to 42 floor pens of equal size (120 × 75 cm) across 2 rooms with wood shavings as bedding material, with 13 individuals per replicate and 6 replicates per treatment. Rooms were temperature-controlled and well-ventilated with feed and water provided ad libitum throughout the experiment. On day 7, all birds were weighed and re-assigned to pens of approximate equal weight within 5% of experiment mean for body weight.
Data Measurement
Chickens and feed were weighed on days 10, 14, 21, 28 and 35. Mortality was recorded and feed conversion ratio (FCR) was calculated with correction for mortalities. On days 21 and 35, 2 birds per pen were sampled by electrical stunning and cervical dislocation and their ileal digesta collected. The ileum was defined as between Meckel’s diverticulum to 1 cm distal to the ileocecal junction. To avoid contaminating the digesta with intestinal secretions, the ileum was longitudinally cut to collect the contents. Birds sampled on day 35 were weighed and breasts, fat-pad, and thigh and drumsticks were removed and weighed for meat yield. Only the left breast and the left thigh and drumstick were weighed and multiplied by 2. Water intake was recorded during the final week. Water consumed per pen was calculated as water added to the container of each pen during the period minus that remaining at the end of the period. Covers were used on water containers to minimize evaporation.
Amino Acid Analysis
Samples of digesta were stored at −20°C until preparation by freeze drying with the freeze dryer Christ Alpha 1-4 LDplus (Osterode am Harz, Germany) and grinding. Feed and digesta samples were ground and sent to AMINOLab, Singapore (Evonik SEA) for CP and AA profile analysis. The CP was estimated using Dumas method, and AA analysis was done by standard procedures (AOAC, 1994) using an AA analyzer (Biochrom 30+, Cambridge, UK). Tryptophan was determined in ground digesta and feed samples by high-performance liquid chromatography following preparation by hydrolysis. Titanium dioxide was measured in the diets and digesta in duplicate by colorimetric method (Short et al., 1996).
Calculations and Statistical Analysis
The FCR was calculated for each pen by dividing the total pen feed intake by the gain in pen weight for each period. Both live and dead bird weights were included for each period. Average feed intake was then calculated by multiplying the pen FCR by the average pen body weight gain (BWG) for each period.
Apparent ileal digestibility (AID) of CP and AA were calculated (Gracia et al., 2007) (1).
| (1) |
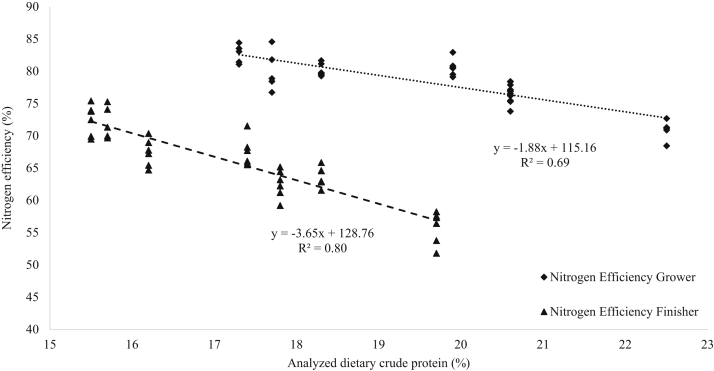
The N efficiency (4) calculation followed those defined by Belloir et al. (2017) which divided N retention (g/bird) (3) by N intake (g/bird) (2). For each pen, the N intake was determined by multiplying the average feed intake by the CP of the diet divided by 6.25. Nitrogen retention was then calculated by multiplying the constant 29 g/kg described by (ITAVI, 2013) for whole body N by the average BWG divided by 1000 for unit correction. This enables the determination of N efficiency by dividing N retention by N intake. Nitrogen efficiency was then plotted against analyzed treatment CP (Figure 1), and the equation and R2 value were determined using linear regression equations in Microsoft Excel 2016 (Microsoft Corp., Redmond, WA).
| (2) |
| (3) |
| (4) |
Figure 1.
Nitrogen efficiency (%) scatter plot against analyzed dietary crude protein (%). Three replicate outliers identified in the Nitrogen Efficiency Grower and 1 replicate outlier from Nitrogen Efficiency were removed. One outlier identified in the Nitrogen Efficiency Finisher was removed.
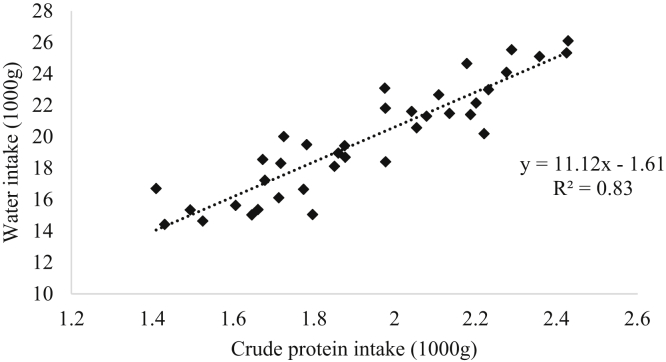
The water intake was calculated by measuring total pen water intake from days 28 to 35 and then plotted against total pen analyzed CP intake (Figure 2), and the equation and R2 value were determined using linear regression equations in Microsoft Excel 2016 (Microsoft Corp., Redmond, WA). The water intake to feed intake ratio was calculated by dividing the total pen water intake by the total pen feed intake.
Figure 2.
Water intake (g) scatter plot against analyzed dietary CP intake (%) from day 28 to 35. Two replicates from standard protein treatment, 1 replicate from 20CP treatment, and 3 replicates from 17CP+Gly were removed from analysis due to compromised water intake data and outliers.
To determine statistical significance of P < 0.05 between all treatments, the IBM SPSS statistical package (v. 24.0.0.0) was employed using a one-way ANOVA. Due to the non-normal distribution of livability values, those data were subject to Kruskal-Wallis nonparametric test for significance. Extreme outliers were defined as values more than 3 interquartile ranges from the upper or lower quartiles and were removed from analysis. Data were also examined using a 3 × 2 factorial arrangement of treatments to test for interactions between CP level (20CP, 18.5CP, and 17CP) and Gly supplementation (–, +) using a general-linear model with Minitab statistical package (v. 17.1.0) to analyze all results. Treatment and main effect means were separated by Tukey’s test at P < 0.05.
Results
The results from the diet analysis are presented in Tables 3 and 4 for the grower and finisher treatments respectively.
Table 3.
Dietary protein and total amino acid content of the grower experimental diets.1
| Ingredients, % | SP | 20CP | 20CP+Gly | 18.5CP | 18.5CP+Gly | 17CP | 17CP+Gly |
|---|---|---|---|---|---|---|---|
| CP | 21.7 (22.5) | 20.0 (20.6) | 20.1 (20.6) | 18.5 (18.3) | 18.7 (19.9) | 17.0 (17.7) | 17.4 (17.3) |
| Total Lys | 1.247 (1.260) | 1.229 (1.147) | 1.229 (1.140) | 1.210 (1.125) | 1.210 (1.265) | 1.186 (1.197) | 1.186 (1.086) |
| Total Met | 0.540 (0.574) | 0.556 (0.509) | 0.556 (0.510) | 0.573 (0.545) | 0.573 (0.648) | 0.605 (0.629) | 0.605 (0.600) |
| Total Cys | 0.379 (0.352) | 0.352 (0.324) | 0.352 (0.337) | 0.323 (0.297) | 0.323 (0.312) | 0.275 (0.259) | 0.275 (0.259) |
| Total Thr | 0.840 (0.857) | 0.824 (0.800) | 0.824 (0.792) | 0.807 (0.785) | 0.807 (0.843) | 0.786 (0.792) | 0.786 (0.744) |
| Total Val | 1.006 (1.017) | 0.993 (0.970) | 0.993 (1.002) | 0.978 (0.958) | 0.978 (1.024) | 0.959 (0.993) | 0.959 (0.952) |
| Total Ile | 0.907 (0.912) | 0.864 (0.840) | 0.864 (0.872) | 0.846 (0.812) | 0.846 (0.893) | 0.824 (0.846) | 0.824 (0.801) |
| Total Arg | 1.356 (1.392) | 1.282 (1.261) | 1.282 (1.263) | 1.268 (1.237) | 1.268 (1.336) | 1.247 (1.282) | 1.247 (1.219) |
| Total Leu | 1.651 (1.671) | 1.461 (1.512) | 1.461 (1.614) | 1.349 (1.324) | 1.349 (1.454) | 1.316 (1.379) | 1.316 (1.359) |
| Total Phe | 1.019 (1.082) | 0.889 (0.945) | 0.889 (0.980) | 0.891 (0.928) | 0.891 (1.004) | 0.993 (1.097) | 0.993 (1.051) |
| Total His | 0.517 (0.538) | 0.453 (0.463) | 0.453 (0.467) | 0.411 (0.399) | 0.411 (0.440) | 0.402 (0.423) | 0.402 (0.395) |
| Total Trp | 0.297 (0.273) | 0.264 (0.239) | 0.264 (0.246) | 0.229 (0.200) | 0.229 (0.215) | 0.751 (0.683) | 0.751 (0.636) |
| Total Gly | 0.906 (0.897) | 0.804 (0.771) | 0.906 (0.835) | 0.696 (0.658) | 0.906 (0.884) | 0.544 (0.541) | 0.906 (0.743) |
| Total Ser | 1.010 (1.029) | 0.886 (0.902) | 0.886 (0.916) | 0.754 (0.740) | 0.754 (0.809) | 0.573 (0.602) | 0.573 (0.596) |
| Total Ala | 0.972 (0.994) | 0.862 (0.904) | 0.862 (0.979) | 0.746 (0.738) | 0.746 (0.822) | 0.588 (0.644) | 0.588 (0.645) |
| Total Pro | 1.286 (1.403) | 1.197 (1.324) | 1.197 (1.360) | 1.103 (1.219) | 1.103 (1.245) | 0.935 (1.045) | 0.935 (1.070) |
Abbreviations: Lys, lysine; Met, methionine; Cys, cysteine; Thr, threonine; Val, valine; Arg, arginine; Ile, isoleucine; Leu, leucine; Phe, phenylalanine; Tyr, tyrosine; His, histidine; Trp, tryptophan; Gly—, glycine; Ser, serine; Pro, proline; Ala, alanine; Asp, aspartic acid; Glu, Glutamic acid; SP, standard protein; 20CP, 20% crude protein treatment; 20CP+Gly, 20% crude protein treatment with glycine supplemented; 18.5CP, 18.5% crude protein treatment; 18.5CP+Gly, 18.5% crude protein treatment with glycine supplemented; 17CP, 17% crude protein treatment; 17CP+Gly, 17% crude protein treatment with glycine supplemented.
Measured as-is values in parentheses.
Table 4.
Dietary protein and total amino acid content of the finisher experimental diets.1
| Ingredients, % | SP | 20CP | 20CP+Gly | 18.5CP | 18.5CP+Gly | 17CP | 17CP+Gly |
|---|---|---|---|---|---|---|---|
| CP | 19.9 (19.7) | 18.0 (17.8) | 18.1 (18.3) | 16.5 (16.2) | 16.7 (17.4) | 15.0 (15.5) | 15.3 (15.7) |
| Total Lys | 1.124 (1.059) | 1.103 (1.034) | 1.103 (1.055) | 1.083 (0.938) | 1.083 (1.150) | 1.062 (1.007) | 1.062 (0.992) |
| Total Met | 0.493 (0.463) | 0.511 (0.503) | 0.511 (0.495) | 0.530 (0.468) | 0.530 (0.601) | 0.549 (0.566) | 0.549 (0.564) |
| Total Cys | 0.357 (0.331) | 0.326 (0.306) | 0.326 (0.305) | 0.296 (0.295) | 0.296 (0.277) | 0.263 (0.249) | 0.263 (0.251) |
| Total Thr | 0.758 (0.719) | 0.740 (0.702) | 0.740 (0.706) | 0.722 (0.653) | 0.722 (0.732) | 0.704 (0.661) | 0.704 (0.668) |
| Total Val | 0.916 (0.905) | 0.900 (0.887) | 0.900 (0.895) | 0.885 (0.851) | 0.885 (0.935) | 0.869 (0.884) | 0.869 (0.895) |
| Total Ile | 0.818 (0.807) | 0.789 (0.779) | 0.789 (0.789) | 0.770 (0.733) | 0.770 (0.818) | 0.750 (0.749) | 0.750 (0.756) |
| Total Arg | 1.212 (1.142) | 1.170 (1.141) | 1.170 (1.153) | 1.155 (1.082) | 1.155 (1.224) | 1.139 (1.108) | 1.139 (1.123) |
| Total Leu | 1.506 (1.587) | 1.289 (1.333) | 1.289 (1.389) | 1.206 (1.267) | 1.206 (1.271) | 1.177 (1.244) | 1.177 (1.265) |
| Total Phe | 0.921 (0.958) | 0.773 (0.803) | 0.773 (0.820) | 0.829 (0.849) | 0.829 (0.917) | 0.910 (0.973) | 0.910 (0.977) |
| Total His | 0.469 (0.459) | 0.395 (0.391) | 0.395 (0.399) | 0.375 (0.374) | 0.375 (0.407) | 0.368 (0.375) | 0.368 (0.379) |
| Total Trp | 0.272 (0.250) | 0.234 (0.214) | 0.234 (0.218) | 0.196 (0.179) | 0.196 (0.181) | 0.189 (0.174) | 0.189 (0.174) |
| Total Gly | 0.828 (0.772) | 0.711 (0.674) | 0.828 (0.727) | 0.597 (0.565) | 0.828 (0.722) | 0.475 (0.469) | 0.828 (0.642) |
| Total Ser | 0.916 (0.902) | 0.773 (0.768) | 0.773 (0.783) | 0.634 (0.641) | 0.634 (0.655) | 0.485 (0.511) | 0.485 (0.518) |
| Total Ala | 0.889 (0.957) | 0.763 (0.791) | 0.763 (0.828) | 0.640 (0.700) | 0.640 (0.667) | 0.509 (0.564) | 0.509 (0.583) |
| Total Pro | 1.213 (1.298) | 1.112 (1.169) | 1.112 (1.219) | 1.011 (1.124) | 1.011 (1.093) | 0.902 (1.047) | 0.902 (1.035) |
Abbreviations: Lys, lysine; Met, methionine; Cys, cysteine; Thr, threonine; Val, valine; Arg, arginine; Ile, isoleucine; Leu, leucine; Phe, phenylalanine; Tyr, tyrosine; His, histidine; Trp, tryptophan; Gly—, glycine. Ser, serine; Pro, proline; Ala, alanine; Asp, aspartic acid; Glu, glutamic acid; SP, standard protein; 20CP, 20% crude protein treatment; 20CP+Gly, 20% crude protein treatment with glycine supplemented; 18.5CP, 18.5% crude protein treatment; 18.5CP+Gly, 18.5% crude protein treatment with glycine supplemented; 17CP, 17% crude protein treatment; 17CP+Gly, 17% crude protein treatment with glycine supplemented.
Measured as-is values in parentheses.
Growth Performance
As shown in Table 5, reducing protein had a significant effect on grower BWG, feed intake, and FCR (P < 0.001). Birds fed 17CP treatments had 18% lower BWG, 12% lower feed intake, and 11 points higher FCR as compared to those fed the SP diet. No significant differences were observed in BWG, feed intake, and FCR between 20CP and 18.5CP. Supplementing Gly increased BWG by 8% and reduced FCR by nearly 7 points compared to nonsupplemented RP diets (P < 0.001). Furthermore, comparing all grower treatments, birds fed the SP diet showed no difference in BWG, feed intake, and FCR as compared to those fed 20CP, 20CP+Gly, and 18.5+Gly treatments. During the finisher period, reducing CP to that seen in 17CP treatments reduced feed intake by up to 12% (P < 0.001), compared to 20CP and 18.5CP treatments. Supplementing Gly in finisher RP diets increased feed intake by 8%, and decreased FCR by 6 points (P ≤ 0.001). An interaction between protein and Gly was observed in finisher BWG (P < 0.05), with Gly supplementation increasing BWG in 20CP and 18.5CP treatments by up to 21%; however, no difference was observed at 17CP. Additionally, birds fed the SP diet in the finisher period had no significantly higher BWG, feed intake, or lower FCR than other treatments.
Table 5.
Average body weight gain, feed intake, and feed conversion ratio in grower (days 10–21) and finisher (days 21–35) period.
| Treatment | Days 10–21 BWG (g) | Days 10–21 Feed intake (g) | Days 10–21 FCR (g/g) | Days 21–35 BWG (g) | Days 21–35 Feed intake1 (g) | Days 21–35 FCR1 (g/g) | |
|---|---|---|---|---|---|---|---|
| SP | 784a | 995a | 1.273c | 1,268c | 2,088b–d | 1.650a,b | |
| 20CP | 723a,b | 974a | 1.347b,c | 1,319b,c | 2,145a–c | 1.629a,b | |
| 20CP+Gly | 776a | 991a | 1.276c | 1,489a | 2,315a | 1.555a | |
| 18.5CP | 677b,c | 945a,b | 1.396a,b | 1,198c | 1,989c,d | 1.661b | |
| 18.5CP+Gly | 755a | 976a | 1.293c | 1,452a,b | 2,253a,b | 1.553a | |
| 17CP | 602d | 864c | 1.439a | 1,195c | 1,926d | 1.614a,b | |
| 17CP+Gly | 621c,d | 877b,c | 1.413a | 1,222c | 1,984c,d | 1.604a,b | |
| SEM | 12.05 | 9.70 | 0.012 | 21.69 | 26.20 | 0.010 | |
| Main effect | |||||||
| Protein | 20CP | 750 | 982 | 1.312b | 1,404 | 2,230a | 1.592 |
| 18.5CP | 716 | 961 | 1.345b | 1,325 | 2,121a | 1.607 | |
| 17CP | 612 | 871 | 1.426a | 1,209 | 1,955b | 1.609 | |
| Gly | − | 667 | 928 | 1.394a | 1,237 | 2,020b | 1.635a |
| + | 718 | 948 | 1.327b | 1,388 | 2,184a | 1.571b | |
| P-value | ANOVA | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.006 |
| Protein | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.654 | |
| Gly | <0.001 | 0.151 | <0.001 | <0.001 | <0.001 | 0.001 | |
| Protein × Gly | 0.184 | 0.855 | 0.066 | 0.017 | 0.094 | 0.075 | |
a–eDiffering superscripts indicate significant differences between means (P < 0.05).
Abbreviations: SP, standard protein; 20CP, 20% crude protein treatment; 20CP+Gly, 20% crude protein treatment with glycine supplemented; 18.5CP, 18.5% crude protein treatment; 18.5CP+Gly, 18.5% crude protein treatment with glycine supplemented; 17CP, 17% crude protein treatment; 17CP+Gly, 17% crude protein treatment with glycine supplemented; Gly, glycine; BWG, body weight gain; FCR, feed conversion ratio.
One replicate from 17CP+Gly removed from analysis.
When the birds were placed into treatment groups on day 7, no significant differences in body weight were detected (data not included); however, at day 10 a significant difference (P < 0.01) of 12 g was identified between SP and 18.5+Gly treatments (Table 6). Lowering dietary CP reduced overall feed intake by up to 11% (P < 0.001) and the supplementation of Gly increased feed intake by 6% (P < 0.001). An interaction between protein and Gly was observed in overall BWG and FCR (P < 0.01). No difference was observed in 17CP treatments, however, in 20CP and 18.5CP treatments supplementing Gly increased overall BWG by up to 18% and decreased FCR by up to 10 points. Additionally, an interaction was observed in final body weight as Gly supplementation increased final body weight in 20CP and 18.5CP treatments by up to 15% (P < 0.01). Comparing all treatments, birds fed 20CP+Gly had 10 and 8% greater BWG and feed intake respectively. However, birds fed 18.5CP, 17CP, and 17CP+Gly had reduced BWG and feed intake by up to 12 and 9%, respectively (P < 0.001). Additionally, FCR was higher in 18.5CP, 17CP, and 17CP+Gly treatments by up to 7 points (P < 0.001). No significant differences were seen in livability between all treatments.
Table 6.
Average starting weight, body weight gain, feed intake, feed conversion ratio, final body weight, and livability in the entire experimental period (days 10–35).
| Treatment | Day 10 weight (g) | Days 10–35 BWG (g) | Days 10–35 Feed intake1 (g) | Days 10–35 FCR1 (g/g) | Final body weight (g) | Livability (%) | |
|---|---|---|---|---|---|---|---|
| SP | 306a | 2,052b,c | 3,026b–d | 1.475b,c | 2358b | 99 | |
| 20CP | 305a,b | 2,042b,c | 3,080a–c | 1.509a,b | 2347b,c | 99 | |
| 20CP+Gly | 296a,b | 2,266a | 3,257a | 1.438c | 2561a | 96 | |
| 18.5CP | 301a,b | 1,875c,d | 2,901c,d,e | 1.547a | 2176c,d | 96 | |
| 18.5CP+Gly | 294b | 2,207a,b | 3,191a,b | 1.446c | 2501a,b | 99 | |
| 17CP | 302a,b | 1,797d | 2,768e | 1.542a | 2099d | 99 | |
| 17CP+Gly | 295a,b | 1,844d | 2,840d,e | 1.523a | 2139d | 97 | |
| SEM | 1.28 | 29.78 | 31.52 | 0.008 | 29.68 | 0.5 | |
| Main effect | |||||||
| Protein | 20CP | 300 | 2,154 | 3,168a | 1.473 | 2,454 | 97 |
| 18.5CP | 298 | 2,041 | 3,046a | 1.497 | 2,339 | 97 | |
| 17CP | 299 | 1,821 | 2,804b | 1.532 | 2,119 | 98 | |
| Gly | − | 303a | 1,905 | 2,916b | 1.532 | 2,208 | 98 |
| + | 295b | 2,106 | 3,096a | 1.469 | 2,400 | 97 | |
| P-value | ANOVA | 0.009 | <0.001 | <0.001 | <0.001 | <0.001 | 0.632 |
| Protein | 0.602 | <0.001 | <0.001 | <0.001 | <0.001 | 0.880 | |
| Gly | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.721 | |
| Protein × Gly | 0.924 | 0.007 | 0.139 | 0.003 | 0.007 | – | |
a–eDiffering superscripts indicate significant differences between means (P < 0.05).
Abbreviations: SP, standard protein; 20CP, 20% crude protein treatment; 20CP+Gly, 20% crude protein treatment with glycine supplemented; 18.5CP, 18.5% crude protein treatment; 18.5CP+Gly, 18.5% crude protein treatment with glycine supplemented; 17CP, 17% crude protein treatment; 17CP+Gly, 17% crude protein treatment with glycine supplemented; Gly, glycine. BWG, body weight gain; FCR, feed conversion ratio.
One replicate from 17CP+Gly removed from analysis.
Breast Meat, Fat-pad, and Thigh and Drumstick Yield
In Table 7, an interaction was observed between protein and Gly for breast meat yield, with birds fed 17CP+Gly having 0.9% points less breast meat yield than 17CP treatment (P < 0.01). No significant differences were observed in thigh and drumstick yield between treatments. However, reducing CP increased relative fat-pad weight by up to 31% (P < 0.001). Additionally, comparing all treatments to SP, birds fed 17CP+Gly had lower breast meat yield by 0.9% points and birds fed 17CP and 17CP+Gly had up to 58% greater relative fat-pad (P < 0.01).
Table 7.
Breast, thigh and drumstick, fat-pad yield (% of body weight, day (d) 35), nitrogen efficiency in grower (days 10–21) and finisher (days 21–35) period, and water to feed intake ration (day 28–35).
| Treatment | Breast (%) | Thigh and drumstick (%) | Fat-pad (%) | Days 10 to 21 N efficiency1 (%) | Days 21 to 35 N efficiency1 (%) | WI:FI2 (g/g) | |
|---|---|---|---|---|---|---|---|
| SP | 10.29a | 9.14 | 0.66b | 70.89d | 55.84d | 2.08a | |
| 20CP | 10.05a,b | 9.34 | 0.77b | 75.59c | 62.57c | 1.86a,b | |
| 20CP+Gly | 10.33a | 9.09 | 0.67b | 77.62b,c | 63.73b,c | 2.00a | |
| 18.5CP | 9.99a,b | 9.44 | 0.85a,b | 80.16a,b | 67.41b | 1.64c,d | |
| 18.5CP+Gly | 10.48a | 9.32 | 0.86a,b | 80.57a | 67.47b | 1.71b,c | |
| 17CP | 10.24a | 9.31 | 1.00a | 80.08a,b | 72.06a | 1.49d | |
| 17CP+Gly | 9.35b | 9.47 | 1.04a | 82.71a | 74.50a | 1.59c,d | |
| SEM | 0.09 | 0.06 | 0.03 | 0.62 | 0.91 | 0.04 | |
| Main effect | |||||||
| Protein | 20CP | 10.19 | 9.21 | 0.72c | 76.60 | 63.15c | 1.930a |
| 18.5CP | 10.23 | 9.38 | 0.86b | 80.37 | 67.44b | 1.676b | |
| 17CP | 9.80 | 9.39 | 1.02a | 81.39 | 72.28a | 1.540c | |
| Gly | − | 10.10 | 9.36 | 0.87 | 78.61 | 67.49 | 1.666b |
| + | 10.05 | 9.29 | 0.86 | 80.30 | 72.28 | 1.765a | |
| P-value | ANOVA | 0.006 | 0.489 | <0.001 | <0.001 | <0.001 | <0.001 |
| Protein | 0.060 | 0.405 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Gly | 0.796 | 0.571 | 0.708 | 0.004 | 0.725 | 0.026 | |
| Protein × Gly | 0.003 | 0.392 | 0.351 | 0.235 | 0.669 | 0.744 | |
a–dDiffering superscripts indicate significant differences between means (P < 0.05).
Abbreviations: SP, standard protein; 20CP, 20% crude protein treatment; 20CP+Gly, 20% crude protein treatment with glycine supplemented; 18.5CP, 18.5% crude protein treatment; 18.5CP+Gly, 18.5% crude protein treatment with glycine supplemented; 17CP, 17% crude protein treatment; 17CP+Gly, 17% crude protein treatment with glycine supplemented; Gly, glycine; N, nitrogen. WI: FI, water intake to feed intake ratio.
Three replicates identified in the Nitrogen Efficiency Grower from treatments SP, 17CP, and 17CP+Gly and removed. One outlier identified in the Nitrogen efficiency finisher from treatment 17CP+Gly and removed.
Two replicates from standard protein treatment, one replicate from 20CP treatment, and 3 replicates from 17CP +Gly were removed from analysis due to compromised water intake data and outliers.
Nitrogen Efficiency and Water Consumption
During the grower period, N efficiency (Table 7) increased with decreasing CP by 4.8% points and increased by 1.7% points with Gly supplementation (P < 0.001). Compared to the SP treatment, all other treatments had increased N efficiency by up to 11.8% points (P < 0.001). During the finisher period only reducing CP increased N efficiency, with 9.1% points greater N efficiency in 17CP. Similar to the results observed in the grower period, all other treatments had increased N efficiency by up to 18.7% points compared to the SP treatment (P < 0.001). Reducing CP increased N efficiency in the grower (R2 = 0.69) and finisher (R2 = 0.80) periods (Figure 1). Reducing CP decreased water intake relative to feed intake by up to 20% (P < 0.001); however, Gly supplementation increased water intake relative to feed intake by 6% (P < 0.05). Compared to the SP treatment, 18.5CP, 18.5CP+Gly, 17CP, and 17CP+Gly all had up to 28% lower water intake relative to feed intake. Additionally, decreased CP intake resulted in decreased water intake in the final week of the experiment as shown in Figure 2 (R2 = 0.83).
Apparent Ileal Digestibility of Nitrogen and Amino Acids
Tables 8 and 9 depict the N and AA AID values in the grower and finisher period treatments, respectively. In the grower period, an interaction was observed between protein and Gly in N and all AA AID values (P < 0.05). Comparing the AID of AA and N between 18.5CP+Gly and 18.5CP during the grower phase, birds fed 18.5CP+Gly had higher AID in total N, His, Gly, alanine (Ala), and aspartic acid (Asp) (P < 0.01). The poorest apparent ileal N and AA digestibility observed in the grower treatments were in birds fed 20CP+Gly, except for Gly, Ala, and Asp as 17CP had the lowest digestibility of these AA (P < 0.05). Additionally, grower N and AA AID values in the 18.5CP+Gly treatment were higher than or equal to birds fed the SP diet (P < 0.01). During the finisher period reducing CP improved N and all AA AID (P < 0.001), with further improvements in Gly digestibility with Gly supplementation (P < 0.001). Treatments with 18.5CP, 18.5CP+Gly, 17CP, and 17CP+Gly increased (P < 0.001) cysteine (Cys) and glutamic acid (Glu) digestibility compared to the SP treatment regardless of Gly supplementation. However, 20CP and 20CP+Gly treatments reduced N, His, Leu, Gly, Ser, Ala, Pro, Asp, and Glu (P < 0.01) digestibility compared to the SP treatment.
Table 8.
Nitrogen and amino acid digestibility at the grower (days 7–21) period (%)
| Amino acid | Treatment |
SEM | Main effects |
P-value |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein |
Gly |
||||||||||||||||
| SP | 20CP | 20CP +Gly | 18.5CP | 18.5CP +Gly | 17CP1 | 17CP +Gly2 | 20CP | 18.5CP | 17CP | – | + | ANOVA | Protein | Gly | Protein × Gly | ||
| N | 84.3a,b | 83.8b | 81.6b | 84.2bb | 88.2a | 83.3b | 84.9a,b | 0.43 | 82.7 | 86.2 | 84.1 | 83.8 | 84.9 | 0.001 | 0.001 | 0.100 | 0.002 |
| Lys | 88.8b,c | 88.8b,c | 86.6c | 89.8a–c | 92.6a | 90.2a,b | 90.1a,b | 0.39 | 87.7 | 91.2 | 90.2 | 89.6 | 89.8 | <0.001 | <0.001 | 0.777 | 0.007 |
| Met | 92.9b,c | 92.7b,c | 91.5c | 93.4a,b,c | 95.7a | 94.1a,b | 94.5a,b | 0.28 | 92.1 | 94.6 | 94.3 | 93.4 | 93.9 | <0.001 | <0.001 | 0.256 | 0.006 |
| Cys | 69.2b,c | 72.4a–c | 67.7c | 77.6a,bb | 80.3a | 72.1a-c | 77.9a,b | 1.06 | 70.0 | 78.9 | 75.0 | 74.0 | 75.3 | 0.001 | <0.001 | 0.433 | 0.030 |
| Thr | 80.5b,c | 80.7b,c | 77.8c | 82.5a,b | 87.0a | 82.0b,c | 82.7a,b | 0.57 | 79.2 | 84.8 | 82.4 | 81.7 | 82.5 | <0.001 | <0.001 | 0.364 | 0.002 |
| Val | 83.4b,c | 84.5b,c | 82.0c | 85.8a–c | 89.3a | 86.3a,b | 87.3a,b | 0.50 | 83.3 | 87.5 | 86.8 | 85.5 | 86.2 | <0.001 | <0.001 | 0.367 | 0.005 |
| Ile | 84.7b,c | 85.7b,c | 83.2c | 86.9a–c | 90.2a | 87.9a,b | 88.7a,b | 0.49 | 84.5 | 88.6 | 88.3 | 86.8 | 87.4 | <0.001 | <0.001 | 0.444 | 0.005 |
| Arg | 90.9a,b | 90.9a,b | 88.5b | 92.0a | 93.5a | 92.1a | 92.7a | 0.33 | 89.7 | 92.7 | 92.4 | 91.6 | 91.5 | <0.001 | <0.001 | 0.829 | 0.004 |
| Phe | 85.5b,c | 85.7b,c | 82.9c | 87.2a,b | 90.0a | 89.5a,b | 90.4a | 0.53 | 84.3 | 88.6 | 90.0 | 87.5 | 87.8 | <0.001 | <0.001 | 0.658 | 0.009 |
| His | 85.9a,b | 85.0a,b | 82.4b | 84.4b | 88.3a | 84.4b | 85.3a,b | 0.41 | 83.7 | 86.3 | 84.9 | 84.6 | 85.4 | 0.007 | <0.010 | 0.256 | 0.002 |
| Leu | 83.6a,b | 84.5a,b | 82.2b | 83.5a,b | 87.9a | 85.1a,b | 87.2a | 0.48 | 83.3 | 85.7 | 86.1 | 84.4 | 85.7 | 0.004 | 0.018 | 0.099 | 0.005 |
| Gly | 81.4b | 79.5b | 78.2b | 78.7b | 87.1a | 72.9c | 82.2a,b | 0.73 | 78.8 | 82.9 | 77.6 | 77.0 | 82.5 | <0.001 | <0.001 | <0.001 | <0.001 |
| Ser | 82.4a,b | 81.7a,b | 78.9b | 80.4a,b | 85.2a | 75.2a,b | 78.1a | 0.59 | 80.3 | 82.8 | 76.7 | 79.1 | 80.7 | <0.001 | <0.001 | 0.062 | 0.002 |
| Ala | 80.1a,b | 80.2a,b | 78.5b,c | 76.7b | 83.9a | 74.2c | 77.7b,c | 0.64 | 79.4 | 80.3 | 76.0 | 77.1 | 80.0 | 0.001 | 0.010 | 0.010 | 0.006 |
| Pro | 86.0a,b | 86.4a,b | 84.6b | 86.7a,b | 89.4a | 83.8b | 86.3a,b | 0.38 | 85.5 | 88.0 | 85.0 | 85.6 | 86.8 | 0.001 | <0.001 | 0.063 | 0.005 |
| Asp | 82.4a,b | 80.8a,b,c | 77.7b,c,d | 79.4b–d | 84.6a | 74.5d | 76.0c,d | 0.64 | 79.3 | 82.0 | 75.3 | 78.3 | 79.4 | <0.001 | <0.001 | 0.200 | 0.002 |
| Glu | 89.1a,b | 89.3a,b | 87.3b | 89.3a,b | 91.6a | 88.1b | 89.7a,b | 0.33 | 88.3 | 91.4 | 88.9 | 88.9 | 89.5 | 0.009 | 0.012 | 0.291 | 0.008 |
a–dDiffering superscripts indicate significant differences between means (P < 0.05).
Abbreviations: N, nitrogen; Lys, lysine; Met, methionine; Cys, cysteine; Thr, threonine; Val, valine; Arg, arginine; Phe, phenylalanine; His, histidine; Leu, leucine; Gly, glycine; Ser, serine; Ala, alanine; Pro, proline; Asp, aspartic acid; Glu, glutamic acid; SP, standard protein; 20CP, 20% crude protein treatment; 20CP+Gly, 20% crude protein treatment with glycine supplemented; 18.5CP, 18.5% crude protein treatment; 18.5CP+Gly, 18.5% crude protein treatment with glycine supplemented; 17CP, 17% crude protein treatment; 17CP+Gly, 17% crude protein treatment with glycine supplemented.
Two replicates removed due to insufficient digesta samples for amino acid analysis.
One replicate removed due to insufficient digesta samples for amino acid analysis.
Table 9.
Nitrogen and amino acid digestibility at the finisher (days 21–35) period (%).
| Amino acid | Treatment |
SEM | Main effects |
P-value |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein |
Gly |
||||||||||||||||
| SP | 20CP | 20CP +Gly | 18.5CP | 18.5CP +Gly | 17CP1 | 17CP +Gly2 | 20CP | 18.5CP | 17CP | – | + | ANOVA | Protein | Gly | Protein × Gly | ||
| N | 82.9a | 77.0b | 76.7b | 83.7a | 87.0a | 84.3a | 84.3a | 0.68 | 76.8b | 85.4a | 84.3a | 81.7 | 82.7 | 0.001 | <0.001 | 0.269 | 0.186 |
| Lys | 86.6b,c | 83.4c | 83.2c | 88.3a,b | 91.4a | 90.0a,b | 89.6a,b | 0.58 | 83.3b | 89.9a | 89.8a | 87.2 | 88.1 | <0.001 | <0.001 | 0.293 | 0.139 |
| Met | 91.3b,c | 89.2c | 88.4c | 92.7a,b | 94.8a | 94.0a,b | 93.8a,b | 0.43 | 88.8b | 93.8a | 93.9a | 92.0 | 92.3 | <0.001 | <0.001 | 0.506 | 0.088 |
| Cys | 71.6b | 68.0b | 69.1b | 81.0a,a | 83.1a | 80.2a | 79.7a | 1.09 | 68.4b | 82.0a | 80.0a | 76.3 | 77.3 | 0.001 | <0.001 | 0.455 | 0.719 |
| Thr | 78.0b,c | 73.1c | 73.4c | 82.0a,b | 86.3a | 83.7a | 83.6a | 0.87 | 73.3b | 84.2a | 83.6a | 79.6 | 81.1 | <0.001 | <0.001 | 0.127 | 0.134 |
| Val | 82.1b,c | 77.9c,d | 76.8d | 85.5a,b | 88.6a | 87.5a | 87.2a | 0.80 | 77.4b | 87.1a | 87.3a | 83.7 | 84.2 | <0.001 | <0.001 | 0.542 | 0.143 |
| Ile | 84.0b,c | 80.2c,d | 78.7d | 87.0a,b | 89.9a | 89.0a | 88.7a | 0.76 | 79.4b | 88.4a | 88.8a | 85.4 | 85.7 | <0.001 | <0.001 | 0.697 | 0.144 |
| Arg | 88.7b,c | 87.2c | 86.3c | 91.3a,b | 93.0a | 92.5a | 92.0a | 0.46 | 86.8b | 92.1a | 92.3a | 90.3 | 90.4 | <0.001 | <0.001 | 0.904 | 0.122 |
| Phe | 85.7b | 80.1c | 78.1c | 88.1a,b | 90.3a | 91.0a | 90.5a | 0.86 | 79.1b | 89.2a | 90.8a | 86.4 | 86.3 | <0.001 | <0.001 | 0.869 | 0.194 |
| His | 84.1a | 77.7b | 77.0b | 84.9a | 88.1a | 86.2a | 85.7a | 0.73 | 77.3b | 86.5a | 85.9a | 82.9 | 83.6 | 0.007 | <0.001 | 0.449 | 0.136 |
| Leu | 84.2a | 76.7b | 75.1b | 84.4a | 86.8a | 86.2a | 85.8a | 0.86 | 75.9b | 85.6a | 86.0a | 82.4 | 82.6 | 0.004 | <0.001 | 0.919 | 0.373 |
| Gly | 79.1b,c | 72.3e | 74.1d,e | 78.7b-d | 85.7a | 76.4c-e | 82.0a,b | 0.78 | 73.2c | 82.2a | 79.2b | 75.8b | 80.6a | <0.001 | <0.001 | <0.001 | 0.059 |
| Ser | 81.9a,b | 75.1c | 74.5c | 81.0a,b | 84.1a | 78.6a-c | 78.3b,c | 0.68 | 74.8c | 82.6a | 78.5b | 78.3 | 79.0 | <0.001 | <0.001 | 0.505 | 0.299 |
| Ala | 81.0a | 69.8b,c | 69.2c | 77.6a,b | 80.6a | 74.5a-c | 75.1a-c | 0.93 | 69.5b | 79.4a | 74.8a | 74.0 | 75.0 | 0.001 | <0.001 | 0.517 | 0.610 |
| Pro | 85.7a | 80.5b | 80.3b | 86.9a | 89.0a | 87.1a | 86.5a | 0.59 | 80.4b | 87.9a | 86.8a | 84.8 | 85.2 | 0.001 | <0.001 | 0.613 | 0.292 |
| Asp | 79.9a | 72.6b | 71.7b | 78.1a,b | 82.0a | 72.4b | 71.8b | 0.83 | 72.1b | 80.1a | 72.1b | 74.4 | 75.2 | <0.001 | <0.001 | 0.535 | 0.247 |
| Glu | 88.3a,b | 84.4b,c | 82.8c | 89.0a | 90.8a | 92.1a | 91.7a | 0.62 | 83.6b | 89.9a | 91.9a | 88.5 | 88.4 | 0.009 | <0.001 | 0.928 | 0.202 |
a–eDiffering superscripts indicate significant differences between means (P < 0.05).
Abbreviations: N, nitrogen; Lys, lysine; Met, methionine; Cys, cysteine; Thr, threonine; Val, valine; Arg, arginine; Phe, phenylalanine; His, histidine; Leu, leucine; Gly, glycine; Ser, serine; Ala, alanine; Pro, proline; Asp, aspartic acid; Glu, glutamic acid; SP, standard protein; 20CP, 20% crude protein treatment; 20CP+Gly, 20% crude protein treatment with glycine supplemented; 18.5CP, 18.5% crude protein treatment; 18.5CP+Gly, 18.5% crude protein treatment with glycine supplemented; 17CP, 17% crude protein treatment; 17CP+Gly, 17% crude protein treatment with glycine supplemented.
One replicate removed due to insufficient digesta samples for amino acid analysis.
Discussion
The impacts of reducing CP on performance have been well documented (Dean et al., 2006, Ospina-Rojas et al., 2014, Awad et al., 2015). The findings from this study suggest that reducing CP below 19% in the grower period reduces BWG and increases FCR. During the grower period chicks are more responsive to dietary changes in CP than the finisher period (Kriseldi et al., 2017). Protein plays an important role in younger birds due to early gastrointestinal tract development (Lilburn and Loeffler, 2015) and the relative higher AA requirement compared to birds in older phases (NRC, 1994). The results from this study suggest that protein plays an important role in early development up to 21 D. Many studies consider Gly and Ser as Gly equivalents (Glyequiv) as the same nutrient following the equation Gly + (0.7143 × Ser) (Dean et al., 2006), due to their interchangeability in vivo (Sugahara and Kandatsu, 1976). Heger and Pack (1996) identified that different levels of CP changes the requirement of Glyequiv to achieve maximum growth and efficiency. Corzo et al. (2004) recommended 1.537% Glyequiv at 18.0% CP for birds aged 7 to 21 days old. However, the findings from this study demonstrated increasing Glyequiv from 1.187 to 1.462% can increase BWG and reduced the FCR to that seen in birds fed the SP diet. This Glyequiv level is above that recommended by the NRC (1994) but below the Glyequiv recommendations given by Schutte et al. (1997). However, other dietary differences must be acknowledged such as increases in other AA. In the current study, increasing Glyequiv from 0.971 to 1.169% did not increase BWG or decrease FCR, suggesting limiting Glyequiv may have reduced performance. Alternate causes of poor performance in birds fed the 17CP diets, are proline (Pro), and dietary electrolyte balance. Proline is reduced to 1.045 and 1.047% in the grower and finisher 17CP treatments, respectively, which is above literature recommendations of 0.5% (Sugahara and Ariyoshi, 1967); however, these recommendations are given for a purified AA diet. The dietary electrolyte balance in the 17CP grower treatment was below 250 mEq/kg, recommended by Murakami et al. (2003), for this age range, from 271 to 165 mEq/kg. Nutrients associated with reducing dietary CP must be considered as potential reasons for reduced performance in RP diets. This study has demonstrated that with the supplementation of six crystalline AA and Gly, protein can be lowered to 19.9% with 1.462% Glyequiv and 17.4% with 1.190% Glyequiv in the grower and finisher phases respectively and still produce performance exceeding that of birds fed the SP diet.
Diet analysis results are consistent with formulated values; however, some discrepancies exist in AA density comparing Gly supplemented and nonsupplemented treatments at each protein level, particularly the 18.5CP treatments. Increasing AA density has been shown to impact weight gain, breast meat yield, and FCR (Vieira and Angel, 2012). This will be acknowledged in all analysis regarding Gly supplementation. A formulation oversight also appears in 17CP grower treatments with excess Trp at 0.6%. Feeding Trp at this level, particularly with reference to supplementing highly digestible crystalline L-Trp, has not been investigated. However, Blair et al. (1993) and Corzo et al. (2005) found no significant effects on performance at 0.4% Trp. Nevertheless, the inconsistencies identified in the diets should be considered in the interpretation of the results. It must also be acknowledged that significant differences were observed in starting live body weights at day 10 between SP and 18.5CP+Gly treatments; however, as no difference in BWG between days 10 to 21, days 21 to 35, and days 10 to 35 was observed between these treatments, the early effect is negligible to the overall experiment.
Relative fat-pad weight in the current study was similar to those in Fancher and Jensen (1989), with increased relative fat-pad weight observed as CP was reduced. Increasing fat-pad in response to reducing dietary CP may be a result of maintaining energy levels at 3,100 and 3,200 kcal/kg in the grower and finisher treatments, respectively. Additionally, lowering CP unavoidably increases the starch content of the diet and this may also affect intestinal glucose transport and subsequent fat deposition (Barekatain et al., 2018). Mabray and Waldroup (1981) found that increasing energy increased relative fat-pad weight and this effect was reversed with increased AA density, suggesting an interaction between energy and AA with fat-pad deposition. This study supports such an interaction by reducing CP and maintains energy levels.
In the present study significant differences in feeding RP diets were observed for apparent ileal N and AA digestibility values. In contrast, Hernández et al. (2012) found no significant differences in male broilers of similar age in CP digestibility. The increased AID values in the grower treatments reflect the increased inclusion rates of highly digestible crystalline AA as the respective AA supplemented had higher AID digestibility. Additionally, the supplementation of Gly has been known to increase nutrient utilization (Ospina-Rojas et al., 2013b). One possible mechanism can be due to increased mucin excretion as Gly supplementation spares Thr and Ser, AA that make up a significant portion of mucin (Bansil and Turner, 2006). Additionally, other proteins, where synthesis was limited by Gly availability, could increase in production enabling better digestibility of those AA. This was demonstrated in Gly-supplemented treatments having greater AID of AA. Further support for this can be seen in the increased BWG response with Gly supplementation. Therefore, increasing AA and Gly supplementation can increase AA digestibility and increase nutrient utilization, maintaining an efficient production system.
Nitrogen efficiency provides an indication into N waste, a key area when dealing with environmental impacts of feeding higher protein diets to livestock. The increased N efficiency as a result of decreasing CP observed in this study is consistent with findings of Bregendahl et al. (2002) and Belloir et al. (2017). However, using the constant of 29 g/kg for body N must be reviewed as it has been demonstrated in this study and others that body fat is increasing, therefore altering total body N (Bregendahl et al., 2002). As a result, this would increase the accuracy of the N efficiency findings of this study and others. Further studies should be performed to examine N utilization efficiency with measured N excretion to confirm the results of this study. Reducing CP decreased the water intake to feed intake ratio. This trend supports findings of Alleman and Leclercq (1997) and Wheeler and James (1950), who observed a similar correlation between reducing CP and reducing water consumption and excretion. It is known that excretion of excess catabolized AA causes osmotic diuresis. The observed correlation is therefore expected as in birds fed RP diet, less excretion of surplus nutrients in particular catabolized urea would require less water compared with a higher level of protein (Pfeiffer et al., 1995). Reducing water consumption can further contribute to improving industry sustainability but can also reduce the occurrence of wet litter and thus the health and welfare concerns associated with this issue. Together, these findings provide further evidence that RP diets reduce N excretion and can improve sustainability of the poultry industry, particularly in regard to N pollution during the finisher phases.
In conclusion, reducing dietary CP reduces growth performance, decreases water intake, and increases N efficiency. Glycine plays an important role in younger birds and this study found that at 19.9% CP, a Glyequiv level of 1.462% is sufficient to support growth similar to birds fed the SP diet; however, other increases in AA may also explain this result. The benefits of Gly supplementation during the grower period are limited as other protein related nutrients become limiting at 17.7% CP. Additionally, increasing highly digestible crystalline AA in an RP diet can also help increase BWG and decrease FCR, performance parameters hindered by reduced CP. Further research into the roles of both essential and nonessential AA in RP diets is required. The findings of this research show that reducing CP in wheat-based diets has a similar effect on performance to that seen in literature investigating RP corn-based diets, and that the supplementation of essential AA and Gly can increase performance.
Acknowledgments
The authors thank the Poultry Research and Teaching Unit at the University of New England for their help throughout the experiment. The authors further acknowledge Evonik (South East Asia) Pte. Ltd. (Singapore) for funding the project and expert AA analysis of feed and digesta samples. The authors also acknowledge AgriFutures Australia, Chicken Meat for the scholarship awarded to postgraduate student Matthew Hilliar.
References
- Alleman F., Leclercq B. Effect of dietary protein and environmental temperature on growth performance and water consumption of male broiler chickens. Brit. Poult. Sci. 1997;38:607–610. doi: 10.1080/00071669708418044. [DOI] [PubMed] [Google Scholar]
- AOAC . Association of Official Analytical Chemists; Washington, D.C: 1994. Official Methods of Analysis. [Google Scholar]
- Aviagen . Aviagen; Newbridge, Midlothian, Scotland, UK: 2014. Ross 308 Broiler: Nutrition Specifications. [Google Scholar]
- Awad E.A., Zulkifli I., Soleimani A.F., Loh T.C. Individual non-essential amino acids fortification of a low-protein diet for broilers under the hot and humid tropical climate. Poult. Sci. 2015;94:2772–2777. doi: 10.3382/ps/pev258. [DOI] [PubMed] [Google Scholar]
- Baker D.H., Sugahara M., Scott H.M. The glycine-serine interrelationship in chick nutrition. Poult. Sci. 1968;47:1376–1377. doi: 10.3382/ps.0471376. [DOI] [PubMed] [Google Scholar]
- Bansil R., Turner B.S. Mucin structure, aggregation, physiological functions and biomedical applications. Curr. Opin. Colloid. In. 2006;11:164–170. [Google Scholar]
- Barekatain R., Nattrass G., Tilbrook A.J., Chousalkar K., Gilani S. Reduced protein diet and amino acid concentration alter intestinal barrier function and performance of broiler chickens with or without synthetic glucocorticoid. Poult. Sci. 2018;98:3662–3675. doi: 10.3382/ps/pey563. [DOI] [PubMed] [Google Scholar]
- Belloir P., Meda B., Lambert W., Corrent E., Juin H., Lessire M., Tesseraud S. Reducing the CP content in broiler feeds: impact on animal performance, meat quality and nitrogen utilization. Animal. 2017;11:1881–1889. doi: 10.1017/S1751731117000660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair R., Newberry R.C., Gardiner E.E. Effects of lighting pattern and dietary tryptophan supplementation on growth and mortality in broiler. Poult. Sci. 1993;72:495–502. doi: 10.3382/ps.0720495. [DOI] [PubMed] [Google Scholar]
- Bregendahl K., Sell J.L., Zimmerman D.R. Effect of low protein diet on performance and body composition of broiler chicks. Poult. Sci. 2002;81:1156–1167. doi: 10.1093/ps/81.8.1156. [DOI] [PubMed] [Google Scholar]
- Corzo A., Kidd M.T., Burnham D.J., Kerr B.J. Dietary glycine needs of broiler chicks. Poult. Sci. 2004;83:1382–1384. doi: 10.1093/ps/83.8.1382. [DOI] [PubMed] [Google Scholar]
- Corzo A., Kidd M.T., Thaxton J.P., Kerr B.J. Dietary tryptophan effects on growth and stress responses of male broiler chicks. Brit. Poult. Sci. 2005;46:475–484. doi: 10.1080/00071660500157974. [DOI] [PubMed] [Google Scholar]
- Dean D., Bidner T.D., Southern L.L. Glycine supplementation to low crude protein, amino acid supplemented diets supports optimal performance of broiler chicks. Poult. Sci. 2006;85:288–296. doi: 10.1093/ps/85.2.288. [DOI] [PubMed] [Google Scholar]
- Evonik Nutrition and Care GmbH . 2016. AMINOChick® 2.0. 2010. [Google Scholar]
- Fancher B.I., Jensen L.S. Dietary protein level and essential amino acid content: influence upon female broiler performance during the grower period. Poult. Sci. 1989;68:897–908. doi: 10.3382/ps.0680897. [DOI] [PubMed] [Google Scholar]
- Gracia A.R., Batal A.B., Dale N.M. A comparison of methods to determine amino acid digestibility. Poult. Sci. 2007;86:94–101. doi: 10.1093/ps/86.1.94. [DOI] [PubMed] [Google Scholar]
- Harms R.H., Damron B.L., Simpson C.F. Effect of wet litter and supplemental biotin and/or whey on the production of foot pad dermatitis in broilers. Poult. Sci. 1977;56:291–296. doi: 10.3382/ps.0560291. [DOI] [PubMed] [Google Scholar]
- Heger J., Pack M. Effects of glycine+serine on starting broiler performance as influenced by dietary crude protein concentrations. Agribiol. Res. 1996;49:257–265. [Google Scholar]
- Hernández F., Lopez M., Martinez S., Megias M.D., Catala P., Madrid J. Effect of low-protein diets and single sex on production performance, plasma metabolites, digestibility, and nitrogen excretion in 1- to 48-day-old broilers. Poult. Sci. 2012;91:683–692. doi: 10.3382/ps.2011-01735. [DOI] [PubMed] [Google Scholar]
- ITAVI. Estimation des rejets d’azote, phosphore, potassium, calcium, cuivre, zinc par les élevages avicoles. I. T. d. l. A. (ITAVI); Paris, France: 2013. p. 63. [Google Scholar]
- James E.C., Wheeler R.S. Relation of dietary protein content to water intake, water elimination and amount of cloacal excreta produced by growing chickens. Poult. Sci. 1949;28:465–467. [Google Scholar]
- Kidd M.T., Kerr B.J. L-Threonine for poultry: a review. J. Appl. Poult. Res. 1996;5:358–367. [Google Scholar]
- Kriseldi R., Tillman P.B., Jiang Z., Dozier W.A., III Effects of glycine and glutamine supplementation to reduced crude protein diets on growth performance and carcass characteristics of male broilers during a 41-day production period. J. Appl. Poult. Res. 2017;26:558–572. [Google Scholar]
- Lilburn M.S., Loeffler S. Early intestinal growth and development in poultry. Poult. Sci. 2015;94:1569–1576. doi: 10.3382/ps/pev104. [DOI] [PubMed] [Google Scholar]
- Mabray C.J., Waldroup P.W. The influence of dietary energy and amino acid levels on abdominal fat pad development of the broiler chicken. Poult. Sci. 1981;60:151–159. [Google Scholar]
- Marks H.L., Pesti G.M. The roles of protein level and diet form in water consumption and abdominal fat pad deposition of broilers. Poult. Sci. 1984;63:1617–1625. doi: 10.3382/ps.0631617. [DOI] [PubMed] [Google Scholar]
- Murakami A.E., Franco J.R.G., Martins E.N., Oviedo Rondon E.O., Sakamoto M.I., Pereira M.S. Effect of electrolyte balance in low-protein diets on broiler performance and tibial dyschondroplasia incidence. J. Appl. Poult. Res. 2003;12:207–216. [Google Scholar]
- Namroud N.F., Shivazad M., Zaghari M. Effects of fortifying low crude protein diet with crystalline amino acids on performance, blood ammonia level, and excreta characteristics of broiler chicks. Poult. Sci. 2008;87:2250–2258. doi: 10.3382/ps.2007-00499. [DOI] [PubMed] [Google Scholar]
- NRC . National Academies Press; Washington, DC: 1994. Nutrient Requirements for Poultry. [Google Scholar]
- Ospina-Rojas I.C., Murakami A.E., Duarte C.R., Eyng C., Oliveira C.A., Janeiro V. Valine, isoleucine, arginine and glycine supplementation of low-protein diets for broiler chickens during the starter and grower phases. Brit. Poult. Sci. 2014;55:766–773. doi: 10.1080/00071668.2014.970125. [DOI] [PubMed] [Google Scholar]
- Ospina-Rojas I.C., Murakami A.E., Moreira I., Picoli K.P., Rodrigueiro R.J., Furlan A.C. Dietary glycine+serine responses of male broilers given low-protein diets with different concentrations of threonine. Brit. Poult. Sci. 2013;54:486–493. doi: 10.1080/00071668.2013.794257. [DOI] [PubMed] [Google Scholar]
- Ospina-Rojas I.C., Murakami A.E., Oliveira C.A., Guerra A.F.Q.G. Supplemental glycine and threonine effects on performance, intestinal mucosa development, and nutrient utilization of growing broiler chickens. Poult. Sci. 2013;92:2724–2731. doi: 10.3382/ps.2013-03171. [DOI] [PubMed] [Google Scholar]
- Pfeiffer A., Henkel H., Verstegen M.W.A., Philipczyk I. The influence of protein intake on water balance, flow rate and apparent digestibilty of nutrients at the distal ileum in growing pigs. Livest. Prod. Sci. 1995;44:179–187. [Google Scholar]
- Schutte J.B., Smink W., Pack M. Requirement of young broiler chicks for glycine + serine. Arch. Geflügelk. 1997;61:43–47. [Google Scholar]
- Shepherd E.M., Fairchild B.D. Footpad dermatitis in poultry. Poult. Sci. 2010;89:2043–2051. doi: 10.3382/ps.2010-00770. [DOI] [PubMed] [Google Scholar]
- Short F.J., Gorton P., Wiseman J., Boorman K.N. Determination of titanium dioxide added as an inert marker in chicken digestibility studies. Anim. Feed Sci. Technol. 1996;59:215–221. [Google Scholar]
- Shoulders M., Raines R. Collagen structure and stability. Annu. Rev. Biochem. 2009;78:929–958. doi: 10.1146/annurev.biochem.77.032207.120833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens L. Avian Biochemistry and Molecular Biology. Cambridge University Press; Cambridge, UK: 1996. Avian nutrition; pp. 9–28. [Google Scholar]
- Sugahara M., Ariyoshi S. The nonessentiality of glycine and the essentiality of L-proline in the chick nutrition. Agric. Biol. Chem. 1967;31:106–110. [Google Scholar]
- Sugahara M., Kandatsu M. Glycine serine interconversion in the rooster. Agric. Biol. Chem. 1976;40:833–837. [Google Scholar]
- van Emous R.A., Winkel A., Aarnink A.J.A. Effects of dietary crude protein levels on ammonia emission, litter and manure composition, N losses, and water intake in broiler breeders. Poult. Sci. 2019;98:6618–6625. doi: 10.3382/ps/pez508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira S.L., Angel C.R. Optimizing broiler performance using different amino acid density diets: What are the limits? J. Appl. Poult. Res. 2012;21:149–155. [Google Scholar]
- Wheeler R.S., James E.C. The problem of wet poultry house litter: Influence of total dietary protein and soybean meal content on water intake and urinary and fecal water elimination in growing chickens. Poult. Sci. 1950;29:496–500. [Google Scholar]