Abstract
This research investigated effects of dietary β-sitosterol addition at different levels on serum lipid levels, immune function, oxidative status, and intestinal morphology in broilers. One-day-old broiler chicks were allocated to 5 groups of 6 replicates. Chickens in the 5 groups were fed a basal diet supplemented with 0 (control group), 40, 60, 80, and 100 mg/kg of β-sitosterol for 42 D, respectively. β-Sitosterol linearly decreased (P < 0.05) concentrations of serum total cholesterol, jejunal tumor necrosis factor α (TNF-α), and ileal interleukin 1β (IL-1β) and mRNA relative expressions levels of jejunal TLR4 and ileal MyD88, whereas it linearly increased (P < 0.05) contents of jejunal immunoglobulin G (IgG), ileal secreted IgA and glutathione, jejunal catalase activity and Nrf2 mRNA relative expression level, villus height (VH), and VH-to-crypt depth (CD) ratio (VH:CD) in the jejunum and ileum. Linear and quadratic increases (P < 0.05) in absolute and relative spleen weight were observed by dietary β-sitosterol, whereas malondialdehyde (MDA) concentration in the jejunum and ileum followed the opposite trend (P < 0.05). Compared with the control group, dietary β-sitosterol at higher than or equal to 60 mg/kg level decreased (P < 0.05) contents of serum total cholesterol, ileal MDA, and jejunal TLR4 mRNA relative expression level, whereas it increased (P < 0.05) absolute spleen weight and ileal glutathione content. Higher than or equal to 80 mg/kg level of β-sitosterol enhanced (P < 0.05) jejunal IgG concentration, VH, catalase activity, and Nrf2 relative expression level and ileal secreted IgA content, but reduced (P < 0.05) ileal IL-1β content and MyD88 mRNA relative expression level. β-Sitosterol addition at 60 and 80 mg/kg levels increased (P < 0.05) relative spleen weight, whereas it decreased (P < 0.05) jejunal MDA accumulation. Moreover, 100 mg/kg level of β-sitosterol reduced (P < 0.05) jejunal TNF-α level, but it increased (P < 0.05) VH in the jejunum and VH:CD in the jejunum and ileum. Accordingly, dietary β-sitosterol supplementation could regulate serum cholesterol level, promote immune function, and improve intestinal oxidative status and morphology in broilers.
Key words: β-sitosterol, immune function, intestinal morphology, oxidative status, broiler
Introduction
Environmental stressors (e.g., high temperature) and pathogens (e.g., Clostridium perfringens) can affect health status and production performance of animals (Mujahid et al., 2005, Liu et al., 2018). Fast-growing meat-type broilers are more susceptible to these stressors under large-scale farming condition especially with the ban or restriction of antibiotic usage. These stressful factors induce adverse consequences in broilers, which are related to oxidative stress and immunosuppression (Zhang et al., 2015a, Liu et al., 2018). Nuclear factor (erythroid-derived 2)–like 2 (Nrf2) is a crucial transcription factor of the antioxidant system, and its activation stimulates downstream signals related to antioxidant enzymes. Toll-like receptor 4 (TLR4) is one member of large toll-like family involving immunomodulation, and its stimulation triggers intracellular association of myeloid differentiation factor 88 (MyD88) with its cytosolic domain for the activation of nuclear factor kappa B (NF-κB), resulting in inflammatory response. Therefore, the search for efficient functional feed additives with safety characteristics to improve antioxidant and immune functions in broilers to resist stressors is necessary. Plant-derived extracts, for example, resveratrol (Zhang et al., 2017, Cui et al., 2018), curcumin (Lopresti et al., 2012, Zhang et al., 2015a), Artemisia annua L. (Wan et al., 2016, Song et al., 2017, Song et al., 2018), Ginkgo leaves (Cao et al., 2012, Tan et al., 2018), and plant essential oil (Zeng et al., 2015, Chowdhury et al., 2018), exhibit antioxidant and immunomodulatory effects and therefore have attracted more attention of animal nutritionists by their potential as feed additives.
Phytosterols are plant-derived steroid compounds, and they mainly include β-sitosterol, stigmasterol, campesterol, and brassicasterol. In animal production, phytosterols have been approved as functional feed additives by the Chinese Ministry of Agriculture since 2008. It has been reported that phytosterols improve antioxidant ability, immune function, and intestinal morphology in weaned pigs (Hu et al., 2017a, Hu et al., 2017b). In broiler chickens, available research studies have demonstrated that phytosterols regulate serum lipid levels (Li, et al., 2015) and improve growth performance and meat quality (Naji et al., 2013, Zhao et al., 2019). Owing to the mixed compositions, exploration for action mode and physiological functions of the main component of phytosterols would be beneficial to enhance their application in livestock production. As the most abundant phytosterol, β-sitosterol has similar chemical structure to that of cholesterol and is found in natural products and foods including vegetables, vegetable oils, fruit, berries, and nuts. Extensive in vitro studies have illustrated that β-sitosterol exerts cholesterol-lowering (Hwang, et al., 2008), antioxidant (Vivancos and Moreno, 2005, Wong et al., 2013), anti-inflammatory (Loizou et al., 2010, Shi et al., 2015, Lampronti et al., 2017), and anticancer (Rauf et al., 2016, Rajavel et al., 2018) effects. Moreover, in several medical research studies, it has further been demonstrated that β-sitosterol was efficient in reducing serum lipid levels (Radika, et al., 2013), ameliorating oxidative damage (Gupta, et al., 2011), and attenuating inflammatory response (Kim et al., 2014, Feng et al., 2017) in rats fed high-fat diet or challenged with streptozotocin or dextran sulfate sodium. In addition, improvement on immune response of growing-finishing pigs fed β-sitosterol has been shown by Fraile et al. (2012). Interestingly, our latest findings showed that dietary β-sitosterol could improve growth performance and meat quality in broilers (Cheng, et al., 2019). However, more research studies are necessary to evaluate the effects of β-sitosterol on broilers. We hypothesized that dietary β-sitosterol may exert similar effects to that of phytosterols on animals. The present study was therefore conducted to investigate the effects of dietary β-sitosterol supplementation at different levels on serum lipid levels, immune function, oxidative status, and intestinal morphology in broilers.
Materials and methods
Ethical Statement
The protocol involving animals in the present study was approved by the Animal Care and Use Committee of Nanjing Agricultural University, P. R. China (Certification No.: SYXK (Su) 2011-0036, 11 August 2015).
Broilers, Diets, and Experimental Design
A total of 240 broiler chicks (Arbor Acres Plus, aged one day) obtained from commercial hatchery were randomly allocated into 5 treatment groups, and each group consisted of 6 replicates (cages) of 8 broiler chickens each. Chickens in the 5 groups were fed a basal diet, and the basal diet was supplemented with 40, 60, 80, and 100 mg/kg β-sitosterol (the analyzed purity was 88.92%, and kindly gifted by Yichun Dahaigui Life Science Co., Ltd., Yichun, Jiangxi Province, P. R. China) for 42 D. All broiler chickens were raised in wire cages (120 cm × 60 cm × 50 cm; 0.09 m2 per bird) in a three-level battery and housed in a temperature-controlled room. The initial temperature was maintained at 32 to 33°C for the first 3 D, which was decreased gradually by 3°C weekly to a final temperature of 20°C until the end of the experiment. Moreover, a light schedule of 23-h light and 1-h darkness in the housing room was provided during the whole period of the trial. Broiler chickens had access to mash feed and clean water throughout the 42-day study. The basal diet was formulated based on the recommendation by NRC (1994), and the components and nutrients levels of the basal diet are presented in Table 1.
Table 1.
Compositions and nutrient levels of the basal diet (g/kg, as fed basis unless otherwise stated).
| Items | 1–21 D | 22–42 D |
|---|---|---|
| Ingredients | ||
| Corn | 570 | 615.2 |
| Soybean meal | 315.1 | 250 |
| Corn gluten meal | 34 | 46 |
| Soybean oil | 31 | 41 |
| Limestone | 12 | 14 |
| Dicalcium phosphate | 20 | 17 |
| L-Lysine | 3.4 | 3 |
| DL-Methionine | 1.5 | 0.8 |
| Sodium chloride | 3 | 3 |
| Premix1 | 10 | 10 |
| Calculated nutrient levels | ||
| Apparent metabolizable energy (MJ/kg) | 12.55 | 13.05 |
| Crude protein | 233 | 200 |
| Calcium | 10 | 9 |
| Available phosphorus | 4.5 | 3.5 |
| Lysine | 11 | 10 |
| Methionine | 5 | 3.8 |
| Methionine + cystine | 9 | 7.2 |
Premix provided the following per kilogram of diet: vitamin A (transretinyl acetate), 10,000 IU; vitamin D3 (cholecalciferol), 3,000 IU; vitamin E (all-rac-α-tocopherol), 30 IU; menadione, 1.3 mg; thiamin, 2.2 mg; riboflavin, 8 mg; nicotinamide, 40 mg; choline chloride, 400 mg; calcium pantothenate, 10 mg; pyridoxine·HCl, 4 mg; biotin, 0.04 mg; folic acid, 1 mg; vitamin B12 (cobalamin), 0.013 mg; Fe (from ferrous sulfate), 80 mg; Cu (from copper sulfate), 8.0 mg; Mn (from manganese sulfate), 110 mg; Zn (from zinc oxide), 60 mg; I (from calcium iodate), 1.1 mg; Se (from sodium selenite), 0.3 mg.
Sample Collection
At 42 D of age, one broiler chicken (close to the cage average body weight) from each cage was selected and weighed. Blood sample was obtained from jugular vein, and serum was separated and stored at −20°C before analysis after a centrifugation at 3,000 × g for 15 min at 4°C. Then, broiler chickens were euthanized by cervical dislocation and necropsied immediately. After that, the immune organs including the thymus, spleen, and bursa of Fabricius were quickly harvested and weighed for absolute (g) and relative (g/kg live body weight) organ weight calculation. In addition, the whole gastrointestinal tract was also rapidly removed, and the segments of the mid-jejunum and mid-ileum were excised (about 2 cm) and flushed gently with ice-cold phosphate-buffered saline to remove the contents, which were thereafter placed in 10% neutral-buffered saline for morphology measurement. The remaining jejunum and ileum sections were subsequently opened longitudinally, and the contents were flushed with ice-cold phosphate-buffered saline. Mucosa of each sample was collected using a sterile glass microscope slide, rapidly stored in liquid nitrogen, and then frozen at −80°C for further analysis.
Serum Biochemical Parameters Determination
Total cholesterol (TC), triglyceride (TG), total protein (TP), and albumin (ALB) concentrations in the serum were measured using the corresponding commercial kits provided by Shanghai Kehua Bio-Engineering Co., Ltd., (Shanghai, P. R. China) using an Olympus 2700 analyzer (Olympus, Tokyo, Japan).
Intestinal Morphology Examination
The harvested segments of the jejunum and ileum were dehydrated, cleared, and embedded in paraffin after a 24-h fixation in buffered formalin. They were then cut into serial sections at 5-μm depth for subsequent staining with hematoxylin and eosin stain. The villus height (VH) and crypt depth (CD) were determined using a light microscope equipped with a computer-assisted morphometric system (Nikon Corporation, Tokyo, Japan).
Intestinal Immunoglobulin and Cytokine Contents
The aforementioned intestinal mucosa (the jejunum and ileum) supernatant was used to analyze immunoglobulin and cytokine concentrations. Secreted immunoglobulin A (SIgA), immunoglobulin G (IgG), immunoglobulin M (IgM), tumor necrosis factor α (TNF-α), interleukin 1β (IL-1β), and interleukin 2 (IL-2) were determined using chicken-specific SIgA, IgG, IgM, TNF-α, IL-1β, and IL-2 ELISA quantitation kits purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, Jiangsu Province, P. R. China) in accordance with the descriptions by manufacturers. The results were normalized against total protein concentration in each sample for intersample comparison.
Intestinal Oxidative Status
About 0.3 g of each jejunal and ileal mucosa sample was homogenized at a ratio of 1:9 (weight/volume) with ice-cold 154 mmol/L sterile sodium chloride solution using a PRO-PK-02200D homogenizer (Pro Scientific, Inc., Monroe, CT). The homogenate was centrifuged at 3,500 × g for 10 min at 4°C to acquire supernatant, and it was immediately frozen at −20°C for further analysis. Concentrations of malondiadehyde (MDA) and glutathione (GSH), and superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and catalase (CAT) were determined in accordance with the manufacturer's instructions using available commercial kits (Nanjing Jiancheng Institute of Bioengineering, Nanjing, Jiangsu Province, P. R. China). The results were normalized against total protein concentration in each sample for intersample comparison. Total protein content of each mucosal sample was determined using a Coomassie brilliant blue protein assay kit (Nanjing Jiancheng Institute of Bioengineering, Nanjing, Jiangsu Province, P. R. China) using bovine serum albumin as the standard.
mRNA Extraction and Quantitative Real-Time PCR
Total RNA was isolated from jejunal and ileal mucosa using TRIzol reagent in accordance with the instructions of the manufacturer (TaKaRa Biotechnology Co., Ltd., Dalian, Liaoning Province, P. R. China). The concentration and purity of RNA was quantified using a spectrophotometer (NanoDrop 2000c, Thermo Scientific, MA) in accordance with OD260/280 readings. The RNA samples were then diluted with diethyl pyrocarbonate–treated water (Biosharp Biotechnology Co., Ltd., Hefei, P. R. China) to a final concentration of 0.5 μg/μL. After that, RNA was immediately reverse-transcribed into cDNA using the Prime Script RT Master Mix reagent kit in accordance with the manufacturer's protocols (TaKaRa Biotechnology Co., Ltd.). The primer sequences were synthesized by Invitrogen Biotechnology Co., Ltd. (Shanghai, P. R. China) and are listed in Table 2. The cDNA samples were amplified with the SYBR Premix Ex TaqII Tli RNaseH Plus kit based on an ABI7500 Real-time PCR system (Applied Biosystems, Grand Island, NY). Detailed procedures of real-time quantitative PCR were performed following the descriptions by our recent study (Chen, et al., 2018). Each sample was measured in triplicate, and gene expression was calculated relative to β-actin using the 2−ΔΔCT method (Livak and Schmittgen, 2001).
Table 2.
Sequences for real-time PCR primers.
| Gene1 | GenBank ID | Primer sequence, sense/antisense | Length |
|---|---|---|---|
| Keap1 | XM_015274015 | GCATCACAGCAGCGTGGAGAG | 117 |
| GCGTACAGCAGTCGGTTCAGC | |||
| Nrf2 | NM_205117.1 | GATGTCACCCTGCCCTTAG | 215 |
| CTGCCACCATGTTATTCC | |||
| TLR4 | NM_001030693.1 | AGGCACCTGAGCTTTTCCTC | 96 |
| TACCAACGTGAGGTTGAGCC | |||
| MyD88 | NM_001030962.1 | ATCCGGACACTAGAGGGAGG | 115 |
| GGCAGAGCTCAGTGTCCATT | |||
| β-actin | NM_205518.1 | TTGGTTTGTCAAGCAAGCGG | 100 |
| CCCCCACATACTGGCACTTT |
Keap1, Kelch-like ECH-associated protein 1; Nrf2, nuclear factor (erythroid-derived-2)–like 2; TLR4, toll-like receptor 4; MyD88, myeloid differentiation factor 88.
Statistical Analysis
All data were analyzed by one-way analysis of variance using SPSS, version 19.0, for windows (2010, SPSS Inc., Chicago, IL). Replicate was defined as an experimental unit for the trial. Polynomial contrasts were used to test the linear and quadratic response to the increasing levels of β-sitosterol in diets. Multiple comparisons were conducted using the Tukey test to evaluate the difference among groups. Statistical significance was considered if P < 0.05 in all analyses. Results are presented as means and standard error of means (tables) or standard errors (figures).
Results
Serum Biochemical Parameters
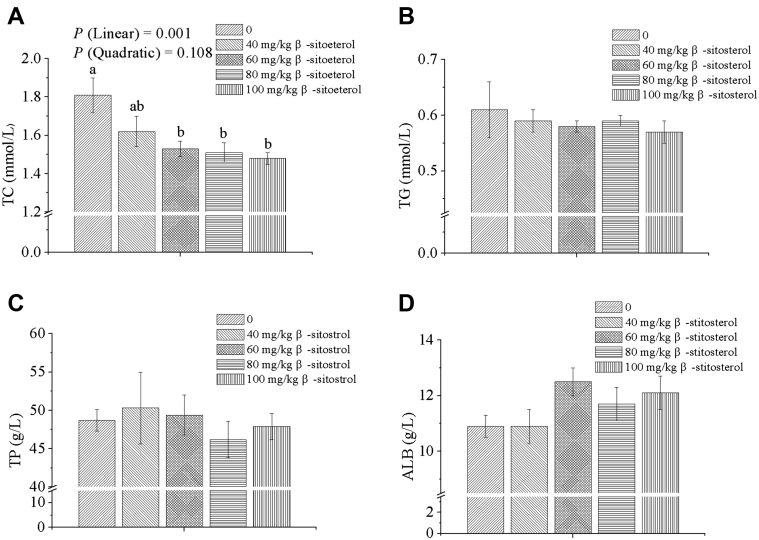
Compared with the control group (Figure 1), the supplementation of dietary β-sitosterol linearly reduced serum TC concentration (P = 0.001). Higher than or equal to 60 mg/kg level of dietary β-sitosterol decreased TC content than in the control group (P < 0.05). However, serum TG, TP, and ALB levels were not significantly different among treatments (P > 0.05).
Figure 1.
Effects of dietary β-sitosterol inclusion at various levels on serum biochemical indicators in broilers (A–D). Data are shown as means and standard errors (n = 6). TC, total cholesterol; TG, triglyceride; TP, total protein; ALB, albumin. a, bDifferent letters indicate significant differences among the treatments (P < 0.05).
Immune Organ Weight
Both absolute and relative weights of the spleen in broiler chickens were linearly and quadratically enhanced by increasing dietary β-sitosterol levels (Table 3, P < 0.05). Compared with the control group, higher than or equal to 60 mg/kg level of β-sitosterol increased relative (except 100 mg/kg) and absolute weights of the spleen (P < 0.05). Treatments, however, did not affect the weights of the thymus and bursa of Fabricius (P > 0.05).
Table 3.
Effect of dietary β-sitosterol inclusion at various levels on immune organ weight in broilers.
| Items | β-Sitosterol level (mg/kg) |
SEM1 |
P Value |
|||||
|---|---|---|---|---|---|---|---|---|
| 0 | 40 | 60 | 80 | 100 | Linear | Quadratic | ||
| Absolute weight (g) | ||||||||
| Thymus | 7.51 | 8.00 | 8.15 | 8.10 | 8.48 | 0.30 | 0.354 | 0.870 |
| Spleen | 2.65b | 3.32a,b | 4.16a | 3.86a | 3.91a | 0.15 | 0.001 | 0.022 |
| Bursa of Fabricius | 4.01 | 5.12 | 4.82 | 4.51 | 4.74 | 0.20 | 0.547 | 0.286 |
| Relative weight (g/kg) | ||||||||
| Thymus | 2.98 | 2.90 | 2.99 | 3.15 | 3.02 | 0.10 | 0.678 | 0.992 |
| Spleen | 1.02b | 1.23a,b | 1.56a | 1.51a | 1.39a,b | 0.06 | 0.007 | 0.016 |
| Bursa of Fabricius | 1.56 | 1.89 | 1.82 | 1.78 | 1.69 | 0.07 | 0.758 | 0.187 |
a, bMeans within a row with different superscripts are different at P < 0.05.
SEM, total standard error of means (n = 6).
Intestinal Immunoglobulin and Cytokine Concentrations
Broilers given the basal diet supplemented with β-sitosterol exhibited linear increases in concentrations of IgG in the jejunum (P < 0.001) and SIgA in the ileum (P = 0.001), whereas they exhibited linear decreases (P < 0.001) in contents of TNF-α in the jejunum and IL-1β in the ileum (Table 4). Compared with the control group, higher than or equal to 60 mg/kg level of dietary β-sitosterol increased jejunal SIgA and ileal IgG concentrations (P < 0.05), whereas it decreased ileal IL-1β content (P < 0.05). In contrast, 100 mg/kg of β-sitosterol decreased jejunal TNF-α concentration (P < 0.05). However, treatment did not alter intestinal mucosal IL-2 and IgM levels (P > 0.05).
Table 4.
Effect of dietary β-sitosterol inclusion at various levels on intestinal immunoglobulin and cytokine concentrations in broilers.
| Items1 | β-Sitosterol level (mg/kg) |
SEM2 |
P Value |
|||||
|---|---|---|---|---|---|---|---|---|
| 0 | 40 | 60 | 80 | 100 | Linear | Quadratic | ||
| Jejunum | ||||||||
| SIgA (μg/mg protein) | 13.3 | 12.4 | 13.4 | 14.0 | 14.2 | 0.4 | 0.248 | 0.589 |
| IgG (μg/mg protein) | 22.2c | 22.1c | 23.6b,c | 31.1a | 29.6a,b | 1.0 | <0.001 | 0.612 |
| IgM (μg/mg protein) | 6.99 | 6.05 | 6.54 | 8.49 | 7.01 | 0.5 | 0.458 | 0.928 |
| TNF-α (pg/mg protein) | 5.83a | 5.45a,b | 4.94a,b | 4.56a,b | 4.20b | 0.18 | 0.001 | 0.905 |
| IL-1β (pg/mg protein) | 3.52 | 3.07 | 3.21 | 3.26 | 3.45 | 0.09 | 0.914 | 0.108 |
| IL-2 (pg/mg protein) | 0.31 | 0.32 | 0.30 | 0.30 | 0.37 | 0.01 | 0.519 | 0.283 |
| Ileum | ||||||||
| SIgA (μg/mg protein) | 7.87b | 9.99a,b | 10.5ab | 12.4a | 13.2a | 0.49 | <0.001 | 0.718 |
| IgG (μg/mg protein) | 32.3 | 30.8 | 32.9 | 34.5 | 37.1 | 1.2 | 0.130 | 0.452 |
| IgM (μg/mg protein) | 10.5 | 10.3 | 10.4 | 11.8 | 11.2 | 0.4 | 0.349 | 0.898 |
| TNF-α (pg/mg protein) | 5.89 | 5.17 | 5.73 | 5.51 | 6.05 | 0.20 | 0.654 | 0.318 |
| IL-1β (pg/mg protein) | 2.89a | 2.68a,b | 2.65a,b | 2.25b,c | 2.32c | 0.06 | <0.001 | 0.585 |
| IL-2 (pg/mg protein) | 0.30 | 0.33 | 0.29 | 0.30 | 0.29 | 0.01 | 0.634 | 0.914 |
a–cMeans within a row with different superscripts are different at P < 0.05.
SIgA, secreted immunoglobulin A; IgG, immunoglobulin G; IgM, immunoglobulin G; TNF-α, tumor necrosis factor α; IL-1β; interleukin 1β; IL-2; interleukin 2.
SEM, total standard error of means (n = 6).
Intestinal Morphology
The VH and VH:CD values in the both jejunal and ileal mucosa of broilers were linearly improved by the supplementation of dietary β-sitosterol (Table 5, P < 0.05). Compared with the control group, higher than or equal to 80 mg/kg level of β-sitosterol increased jejunal VH (P < 0.05). In contrast, 100 mg/kg of dietary β-sitosterol enhanced (P < 0.05) ileal VH and VH:CD in the both jejunal and ileal mucosa. However, chickens fed the different levels of β-sitosterol exhibited no difference on intestinal CD value compared with those fed the basal diet (P > 0.05).
Table 5.
Effect of dietary β-sitosterol inclusion at various levels on intestinal morphology in broilers.
| Items1 | β-Sitosterol level (mg/kg) |
SEM2 |
P value |
|||||
|---|---|---|---|---|---|---|---|---|
| 0 | 40 | 60 | 80 | 100 | Linear | Quadratic | ||
| Jejunum | ||||||||
| VH (μm) | 1,437c | 1,423c | 1,481b,c | 1,538a,b | 1,573a | 13 | <0.001 | 0.173 |
| CD (μm) | 196 | 191 | 200 | 201 | 198 | 2 | 0.246 | 0.806 |
| VH:CD (μm: μm) | 7.34b | 7.47a,b | 7.42a,b | 7.64a,b | 7.97a | 0.07 | 0.003 | 0.216 |
| Ileum | ||||||||
| VH (μm) | 1,234b | 1,251a,b | 1,253a,b | 1,306a,b | 1,329a | 10 | <0.001 | 0.378 |
| CD (μm) | 189 | 192 | 195 | 191 | 185 | 2 | 0.507 | 0.870 |
| VH:CD (μm: μm) | 6.53b | 6.55a,b | 6.78a,b | 6.86a,b | 7.18a | 0.04 | 0.001 | 0.361 |
a-cMeans within a row with different superscripts are different at P < 0.05.
VH, villus height; CD, crypt depth; VH:CD, villus height-to-crypt depth ratio.
SEM, total standard error of means (n = 6).
Oxidative Status in the Intestinal Mucosa
Dietary β-sitosterol supplementation linearly increased (P < 0.001) CAT activity in the jejunal mucosa and GSH content in the ileal mucosa (Table 6). Meanwhile, jejunal and ileal mucosal MDA concentration in chickens was linearly and quadratically decreased by increasing β-sitosterol inclusion (P < 0.05). Compared with the control group, broilers fed β-sitosterol at higher than or equal to level of 60 mg/kg exhibited increase in ileal GSH content (P < 0.05), whereas they exhibited decrease in the MDA accumulation (P < 0.05). Moreover, 80 and 100 mg/kg of dietary β-sitosterol increased jejunal CAT activity (P < 0.05), and 60 and 80 mg/kg of dietary β-sitosterol reduced jejunal MDA content (P < 0.05). However, Treatments did not affect intestinal SOD and GSH-Px activities (P > 0.05).
Table 6.
Effect of dietary β-sitosterol inclusion at various levels on intestinal antioxidant capacity in broilers.
| Items1 | β-Sitosterol level (mg/kg) |
SEM2 |
P Value |
|||||
|---|---|---|---|---|---|---|---|---|
| 0 | 40 | 60 | 80 | 100 | Linear | Quadratic | ||
| Jejunum | ||||||||
| MDA (nmol/mg protein) | 0.38a | 0.32a,b | 0.20b | 0.22b | 0.25a,b | 0.02 | 0.005 | 0.025 |
| GSH (mg/g protein) | 1.83 | 1.87 | 1.85 | 1.88 | 1.87 | 0.07 | 0.863 | 0.953 |
| SOD (U/mg protein) | 186 | 200 | 196 | 192 | 206 | 3 | 0.205 | 0.991 |
| GSH-Px (U/mg protein) | 2.79 | 2.82 | 2.73 | 3.04 | 2.89 | 0.08 | 0.475 | 0.978 |
| CAT (U/mg protein) | 1.20b | 1.83a,b | 1.72a,b | 2.53a | 2.53a | 0.14 | <0.001 | 0.742 |
| Ileum | ||||||||
| MDA (nmol/mg protein) | 0.39a | 0.29a,b | 0.25b | 0.28b | 0.27b | 0.01 | 0.002 | 0.012 |
| GSH (mg/g protein) | 1.63c | 1.66b,c | 2.03a,b | 2.13a | 2.09a | 0.05 | <0.001 | 0.263 |
| SOD (U/mg protein) | 155 | 153 | 147 | 153 | 161 | 3 | 0.600 | 0.271 |
| GSH-Px (U/mg protein) | 2.20 | 2.48 | 2.14 | 2.21 | 2.25 | 0.09 | 0.796 | 0.930 |
| CAT (U/mg protein) | 3.52 | 3.61 | 3.83 | 4.19 | 3.73 | 0.10 | 0.174 | 0.260 |
a–cMeans within a row with different superscripts are different at P < 0.05.
MDA, malondialdehyde; GSH, glutathione; SOD, superoxide dismutase; GSH-Px, glutathione peroxidase; CAT, catalase.
SEM, total standard error of means (n = 6).
Intestinal Mucosal Gene Expression Levels
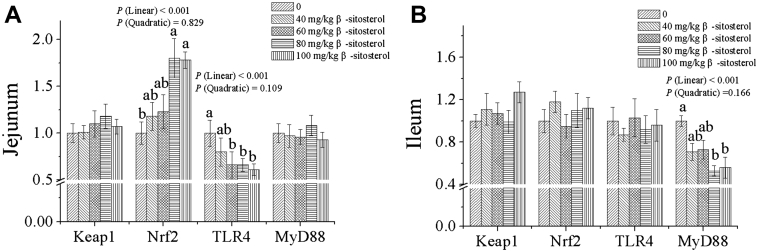
Dietary β-sitosterol supplementation linearly upregulated (Figure 2, P < 0.001) jejunal Nrf2 mRNA relative expression level, whereas it linearly downregulated (P < 0.001) mRNA relative expression levels of jejunal TLR4 and ileal MyD88. Compared with the control group, β-sitosterol at higher than or equal to 80 mg/kg level increased jejunal Nrf2 gene relative expression level, whereas it decreased ileal MyD88 mRNA relative expression level (P < 0.05), and its dosage higher than or equal to 60 mg/kg level reduced jejunal TLR4 mRNA expression level (P < 0.05). Keap1 gene expression level in the jejunal and ileal mucosa, however, was not altered by increasing dietary β-sitosterol inclusion (P > 0.05).
Figure 2.
Effect of dietary β-sitosterol inclusion at various levels on intestinal mucosal mRNA relative expression levels in broilers (A and B). Data are shown as means and standard errors (n = 6). Keap1, Kelch-like ECH-associated protein 1; Nrf2, nuclear factor (erythroid-derived-2)–like 2; TLR4, toll-like receptor 4; MyD88, myeloid differentiation factor 88. a, bDifferent letters indicate significant differences among the treatments (P < 0.05).
Discussion
Phytosterols were initially investigated because of their cholesterol-lowering activity; in vitro pertinent study showed this result (Hwang, et al., 2008). In addition, such effect was observed in several clinical trials (Demonty et al., 2009, Ras et al., 2016, Li et al., 2018). In livestock production, phytosterols, as functional feed additive, could reduce serum TC concentration in laying hens (Raeini-Sarjaz, et al., 2006) and weaned pigs (Hu, et al., 2017a). In the present research, we observed that dietary β-sitosterol supplementation at higher than or equal to 40 mg/kg level decreased serum TC level, indicating that dietary β-sitosterol could regulate serum lipid levels in broilers. This finding was consistent with the results of in vitro and in vivo studies in which β-sitosterol exerted cholesterol-reducing effect (Hwang et al., 2008, Ponnulakshmi et al., 2018). On the one hand, β-sitosterol has similar structure to cholesterol, and cholesterol absorption is eventually decreased as a result of a competitive absorption by β-sitosterol. In addition, it has been illustrated that phytosterols could downregulate fatty acid synthetase mRNA abundance and inactivate its activity, whereas they could activate hormone-sensitive lipase activity (Li, et al., 2015), which could increase cholesterol catabolism but reduce its synthesis in broilers. Those consequences would account for the reduced serum TC level in broilers by β-sitosterol inclusion in the present work.
The thymus and bursa of Fabricius are central lymphoid organs. The spleen is the biggest peripheral immune organ, which involves in cellular and humoral immunity. Therefore, the development of the immune organ can partly reflect immune function (Liu, et al., 2016). In this study, we observed that higher than or equal to 60 mg/kg level of β-sitosterol enhanced absolute spleen weight, and 60 and 80 mg/kg level of β-sitosterol increased its relative weight. Those results were in agreement with findings of previous studies in which plant-derived extracts improved immune organ weight in animals, as evidenced by increasing weight of thymus, spleen, and/or bursa of Fabricius (Bin-Hafeez et al., 2003, Dong et al., 2007, Khan et al., 2012). In the domestic chicken, 3 classes of immunoglobulins have been identified as the homologs of mammalian IgM, IgA, and IgG. They bind antigens specifically and remove them through precipitation and phagocytosis. In the present work, dietary β-sitosterol at higher than or equal to 60 mg/kg level increased jejunal IgG content and its dosage higher than or equal to 80 mg/kg level improved ileal SIgA content. Furthermore, our research showed that dietary β-sitosterol at higher than or equal to 80 mg/kg level decreased ileal MyD88 gene expression level and IL-1β content, its levels higher than or equal to 60 mg/kg level downregulated jejunal TLR4 mRNA relative expression level, and its dosage at 100 mg/kg reduced jejunal TNF-α concentration. These findings were in the same line with results of available research studies in which β-sitosterol could downregulate mRNA relative expression level of TLR4 and inactivate phosphorylation of NF-κB p65 and therefore decreased proinflammatory cytokine concentrations both in cells and animals challenged with various adverse factors (Gupta et al., 2010, Loizou et al., 2010, Valerio et al., 2013, Kim et al., 2014). The increased immune organ weight and intestinal immunoglobulins contents, coupled with the inactivation of TLR4/MyD88 signal and decreased proinflammatory cytokine level in the intestine by β-sitosterol inclusion in this study, suggested that dietary β-sitosterol addition can improve immune function in broilers.
Maintenance of normal microarchitecture in the small intestine is essential for resistance to exogenous toxins and harmful substrates, nutrient absorption, as well as body health, and thus plays an important role in an individual's proper growth and development. The VH and CD are used as criteria that can reflect gross intestinal morphology. To the best of our knowledge, there is no information regarding the effect of β-sitosterol on intestinal morphology in broilers. In the present study, higher than or equal to 80 mg/kg level of β-sitosterol addition increased jejunal VH, and 100 mg/kg of β-sitosterol enhanced ileal VH and VH:CD in the both jejunal and ileal mucosa. Those findings were consistent with results of a recently published article, in which dietary phytosterol inclusion could improve VH:CD in the duodenal and jejunal mucosa of weaned piglets (Hu, et al., 2017a). Our findings suggested that dietary β-sitosterol inclusion is an efficient way to enhance intestinal morphology in broilers. Rosero et al. (2015) have demonstrated that lipid peroxidation could impair villus morphology in the intestine of pigs. Therefore, simultaneously decreased MDA concentration in the jejunal and ileal mucosa by β-sitosterol supplementation would partly account for the improved intestinal villus morphology in the present work. In addition, our research also showed that dietary β-sitosterol supplementation improved intestinal immune function, which could contribute to the better intestinal health status, and this beneficial consequence may also explain the improved intestinal morphology of broilers fed β-sitosterol. However, more comprehensive investigations are needed in terms of β-sitosterol on broilers concerning the relationships of morphology, immune function, and oxidative status in the intestine.
Free radical overproduction can be quenched by nonenzymatic and enzymatic antioxidant systems. MDA is an end product of lipid peroxidation in which carbon–carbon double bond of lipid is attacked by a free radical, which serves as an indicator to reflect lipid peroxidation. In vitro studies have illustrated that β-sitosterol can improve antioxidant enzymes activities, such as SOD, GSH-Px, and/or CAT in the cells under both normal and adverse conditions (Moreno, 2003, Vivancos and Moreno, 2005). Furthermore, Baskar et al. (2012) have reported that β-sitosterol could reduce MDA concentration, whereas it could increase activities of SOD, GSH-Px, and CAT, as well as GSH content, in the livers of rats induced by 1,2-dimethylhydrazine. In livestock production, it has been illustrated that dietary phytosterol supplementation could increase GSH content and/or activities of SOD and total antioxidant capacity, whereas it could decrease MDA content of muscle (or serum) in pigs and broilers (Naji et al., 2013, Hu et al., 2017a, Zhao et al., 2019). Consistently, in this work, dietary β-sitosterol at higher than or equal to 60 mg/kg increased ileal GSH content, whereas it decreased its MDA accumulation; 80 and 100 mg/kg of dietary β-sitosterol increased jejunal CAT activity; and 60 and 80 mg/kg of dietary β-sitosterol reduced jejunal MDA content in broilers. In addition, we further observed that dietary β-sitosterol at higher than or equal to 60 mg/kg increased jejunal Nrf2 mRNA relative expression level, and this consequence could account for the simultaneous increase in CAT activity, whereas it could decrease MDA accumulation in the intestine. These findings were consistent with results of available literature in which β-sitosterol (or phytosterol) could upregulate Nrf2 mRNA relative expression level and consequently alleviate oxidative damage both in vitro and in vivo studies (Almazari et al., 2012, Zhang et al., 2015b, Sharmila et al., 2016).
Conclusion
In conclusion, higher than or equal to 60 mg/kg of β-sitosterol supplementation reduced serum TC and intestinal mucosal MDA levels, whereas it promoted immune organ development; higher than or equal to 80 of β-sitosterol improved intestinal immune function, oxidative status, and jejunal villus development; 100 mg/kg of which enhanced ileal villus development. Herein, a level of 80 mg/kg of β-sitosterol was recommended in broiler diet.
Acknowledgments
This work was financially supported by the Jiangsu Natural Science Foundation (BK20190537). The technical assistance of colleagues in our laboratories is gratefully acknowledged.
References
- Almazari I., Park J.M., Park S.A., Suh J.Y., Na H.K., Cha Y.N., Surh Y.J. Guggulsterone induces heme oxygenase-1 expression through activation of Nrf2 in human mammary epithelial cells: PTEN as a putative target. Carcinogenesis. 2012;33:368–376. doi: 10.1093/carcin/bgr259. [DOI] [PubMed] [Google Scholar]
- Baskar A.A., Al Numair K.S., Gabriel Paulraj M., Alsaif M.A., Muamar M.A., Ignacimuthu S. β-sitosterol prevents lipid peroxidation and improves antioxidant status and histoarchitecture in rats with 1,2-dimethylhydrazine-induced colon cancer. J. Med. Food. 2012;15:335–343. doi: 10.1089/jmf.2011.1780. [DOI] [PubMed] [Google Scholar]
- Bin-Hafeez B., Haque R., Parvez S., Pandey S., Sayeed I., Raisuddin S. Immunomodulatory effects of fenugreek (Trigonella foenum graecum L.) extract in mice. Int. Immunopharmacol. 2003;3:257–265. doi: 10.1016/S1567-5769(02)00292-8. [DOI] [PubMed] [Google Scholar]
- Cao F.L., Zhang X.H., Yu W.W., Zhao L.G., Wang T. Effect of feeding fermented Ginkgo biloba leaves on growth performance, meat quality, and lipid metabolism in broilers. Poult. Sci. 2012;91:1210–1221. doi: 10.3382/ps.2011-01886. [DOI] [PubMed] [Google Scholar]
- Chen Y., Zhang H., Cheng Y., Li Y., Wen C., Zhou Y. Dietary l-threonine supplementation attenuates lipopolysaccharide-induced inflammatory responses and intestinal barrier damage of broiler chickens at an early age. Br. J. Nutr. 2018;119:1254–1262. doi: 10.1017/S0007114518000740. [DOI] [PubMed] [Google Scholar]
- Cheng Y.F., Chen Y.P., Li J., Qu H.M., Zhao Y.R., Wen C., Zhou Y. Dietary β-sitosterol improves growth performance, meat quality, antioxidant status, and mitochondrial biogenesis of breast muscle in broilers. Animals. 2019;9:71. doi: 10.3390/ani9030071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury S., Mandal G.P., Patra A.K., Kumar P., Samanta I., Pradhan S., Samanta A.K. Different essential oils in diets of broiler chickens: 2. Gut microbes and morphology, immune response, and some blood profile and antioxidant enzymes. Anim. Feed Sci. Technol. 2018;236:39–47. [Google Scholar]
- Cui Q., Fu Q., Zhao X., Song X., Yu J., Yang Y., Sun K., Bai L., Tian Y., Chen S., Jia R., Zou Y., Li L., Liang X., He C., Yin L., Ye G., Lv C., Yue G., Yin Z. Protective effects and immunomodulation on piglets infected with rotavirus following resveratrol supplementation. PLoS One. 2018;13:e0192692. doi: 10.1371/journal.pone.0192692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demonty I., Ras R.T., van der Knaap H.C.M., Duchateau G.S.M.J.E., Meijer L., Zock P.L., Geleijnse J.M., Trautwein E.A. Continuous dose-response relationship of the LDL-cholesterol-lowering effect of phytosterol intake. J. Nutr. 2009;139:271–284. doi: 10.3945/jn.108.095125. [DOI] [PubMed] [Google Scholar]
- Dong X.F., Gao W.W., Tong J.M., Jia H.Q., Sa R.N., Zhang Q. Effect of polysavone (Alfalfa Extract) on abdominal fat deposition and immunity in broiler chickens. Poult. Sci. 2007;86:1955–1959. doi: 10.1093/ps/86.9.1955. [DOI] [PubMed] [Google Scholar]
- Feng S., Dai Z., Liu A., Wang H., Chen J., Luo Z., Yang C.S. β-sitosterol and stigmasterol ameliorate dextran sulfate sodium-induced colitis in mice fed a high fat western-style diet. Food Funct. 2017;8:4179–4186. doi: 10.1039/c7fo00375g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraile L., Crisci E., Córdoba L., Navarro M.A., Osada J., Montoya M. Immunomodulatory properties of beta-sitosterol in pig immune responses. Int. Immunopharmacol. 2012;13:316–321. doi: 10.1016/j.intimp.2012.04.017. [DOI] [PubMed] [Google Scholar]
- Gupta P., Balwani S., Kumar S., Aggarwal N., Rossi M., Paumier S., Caruso F., Bovicelli P., Saso L., DePass A.L., Prasad A.K., Parmar V.S., Ghosh B. β-sitosterol among other secondary metabolites of piper galeatum shows inhibition of TNFα-induced cell adhesion molecule expression on human endothelial cells. Biochimie. 2010;92:1213–1221. doi: 10.1016/j.biochi.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Gupta R., Sharma A.K., Dobhal M.P., Sharma M.C., Gupta R.S. Antidiabetic and antioxidant potential of β-sitosterol in streptozotocin-induced experimental hyperglycemia. J. Diabetes. 2011;3:29–37. doi: 10.1111/j.1753-0407.2010.00107.x. [DOI] [PubMed] [Google Scholar]
- Hu Q., Li S., Zhang Y., Zhuo Z., Feng J. Phytosterols on growth performance, antioxidant enzymes and intestinal morphology in weaned piglets. J. Sci. Food Agric. 2017;97:4629–4634. doi: 10.1002/jsfa.8333. [DOI] [PubMed] [Google Scholar]
- Hu Q., Zhuo Z., Fang S., Zhang Y., Feng J. Phytosterols improve immunity and exert anti-inflammatory activity in weaned piglets. J. Sci. Food Agric. 2017;97:4103–4109. doi: 10.1002/jsfa.8277. [DOI] [PubMed] [Google Scholar]
- Hwang S.L., Kim H.N., Jung H.H., Kim J.E., Choi D.K., Hur J.M., Lee J.Y., Song H., Song K.S., Huh T.L. Beneficial effects of β-sitosterol on glucose and lipid metabolism in L6 myotube cells are mediated by AMP-activated protein kinase. Biochem. Biophys. Res. Commun. 2008;377:1253–1258. doi: 10.1016/j.bbrc.2008.10.136. [DOI] [PubMed] [Google Scholar]
- Khan S.H., Ansari J., Haq A.U., Abbas G. Black cumin seeds as phytogenic product in broiler diets and its effects on performance, blood constituents, immunity and caecal microbial population. Ital. J. Anim. Sci. 2012;11:e77. doi: 10.3382/ps.2011-01393. [DOI] [PubMed] [Google Scholar]
- Kim K.A., Lee I.A., Gu W., Hyam S.R., Kim D.H. β-sitosterol attenuates high-fat diet-induced intestinal inflammation in mice by inhibiting the binding of lipopolysaccharide to toll-like receptor 4 in the NF-κB pathway. Mol. Nutr. Food Res. 2014;58:963–972. doi: 10.1002/mnfr.201300433. [DOI] [PubMed] [Google Scholar]
- Lampronti I., Dechecchi M.C., Rimessi A., Bezzerri V., Nicolis E., Guerrini A., Tacchini M., Tamanini A., Munari S., D’Aversa E., Santangelo A., Lippi G., Sacchetti G., Pinton P., Gambari R., Agostini M., Cabrini G. β-sitosterol reduces the expression of chemotactic cytokine genes in cystic fibrosis bronchial epithelial cells. Front. Pharmacol. 2017;8:236. doi: 10.3389/fphar.2017.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B., Hussain A., Wang Y.X., Jun J., Wang T. Effects of phytosterols on growth performance and fat metabolism in broilers. Pak. J. Zool. 2015;47:111–118. [Google Scholar]
- Li Y.C., Li C.L., Li R., Chen Y., Zhang M., Guo P.P., Shi D., Ji X.N., Feng R.N., Sun C.H. Associations of dietary phytosterols with blood lipid profiles and prevalence of obesity in Chinese adults, a cross-sectional study. Lipids Health Dis. 2018;17:54. doi: 10.1186/s12944-018-0703-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Fu C., Yan M., Xie H., Li S., Yu Q., He S., He J. Resveratrol modulates intestinal morphology and HSP70/90, NF-κB and EGF expression in the jejunal mucosa of black-boned chickens on exposure to circular heat stress. Food Funct. 2016;7:1329–1338. doi: 10.1039/c5fo01338k. [DOI] [PubMed] [Google Scholar]
- Liu N., Lin L., Wang J., Zhang F., Wang J.P. Dietary cysteamine hydrochloride protects against oxidation, inflammation, and mucosal barrier disruption of broiler chickens challenged with Clostridium perfringens. J. Anim. Sci. 2018;96:4339–4347. doi: 10.1093/jas/sky292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Loizou S., Lekakis I., Chrousos G.P., Moutsatsou P. β-Sitosterol exhibits anti-inflammatory activity in human aortic endothelial cells. Mol. Nutr. Food Res. 2010;54:551–558. doi: 10.1002/mnfr.200900012. [DOI] [PubMed] [Google Scholar]
- Lopresti A.L., Hood S.D., Drummond P.D. Multiple antidepressant potential modes of action of curcumin: a review of its anti-inflammatory, monoaminergic, antioxidant, immune-modulating and neuroprotective effects. J. Psychopharmacol. 2012;26:1512–1524. doi: 10.1177/0269881112458732. [DOI] [PubMed] [Google Scholar]
- Moreno J.J. Effect of olive oil minor components on oxidative stress and arachidonic acid mobilization and metabolism by macrophages RAW 264.7. Free Radic. Biol. Med. 2003;35:1073–1081. doi: 10.1016/s0891-5849(03)00465-9. [DOI] [PubMed] [Google Scholar]
- Mujahid A., Yoshiki Y., Akiba Y., Toyomizu M. Superoxide radical production in chicken skeletal muscle induced by acute heat stress. Poult. Sci. 2005;84:307–314. doi: 10.1093/ps/84.2.307. [DOI] [PubMed] [Google Scholar]
- Naji T.A., Amadou I., Abbas S., Zhao R.Y., Shi Y.H., Le G.W. Phytosterol supplementation improves antioxidant enzymes status and broiler meat quality. Pak. J. Food Sci. 2013;23:163–171. [Google Scholar]
- NRC. 9th ed. Natl. Acad. Press; Washington, DC, USA: 1994. Nutrient Requirements of Poultry. [Google Scholar]
- Ponnulakshmi R., Shyamaladevi B., Vijayalakshmi P., Selvaraj J. In silico and in vivo analysis to identify the antidiabetic activity of beta sitosterol in adipose tissue of high fat diet and sucrose induced type-2 diabetic experimental rats. Toxicol. Mech. Methods. 2018;29:276–290. doi: 10.1080/15376516.2018.1545815. [DOI] [PubMed] [Google Scholar]
- Radika M.K., Viswanathan P., Anuradha C.V. Nitric oxide mediates the insulin sensitizing effects of β-sitosterol in high fat diet-fed rats. Nitric Oxide. 2013;32:43–53. doi: 10.1016/j.niox.2013.04.007. [DOI] [PubMed] [Google Scholar]
- Raeini-Sarjaz M., Vasoukolaei M.N., Sayahzadeh H., Ansari Z., J Jones P.H. EPC 2006–12th European Poultry Conference. 2006. The effects of plant sterols on serum lipid profiles of laying hens. Verona, Italy. Eur. Poult. Sci. [Google Scholar]
- Rajavel T., Packiyaraj P., Suryanarayanan V., Singh S.K., Ruckmani K., Pandima Devi K. β-Sitosterol targets Trx/Trx1 reductase to induce apoptosis in A549 cells via ROS mediated mitochondrial dysregulation and p53 activation. Sci. Rep. 2018;8:2071. doi: 10.1038/s41598-018-20311-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ras R.T., Koppenol W.P., Garczarek U., Otten-Hofman A., Fuchs D., Wagner F., Trautwein E.A. Increases in plasma plant sterols stabilize within four weeks of plant sterol intake and are independent of cholesterol metabolism. Nutr. Metab. Cardiovasc. Dis. 2016;26:302–309. doi: 10.1016/j.numecd.2015.11.007. [DOI] [PubMed] [Google Scholar]
- Rauf A., Uddin G., Khan H., Raza M., Zafar M., Tokuda H. Anti-tumour-promoting and thermal-induced protein denaturation inhibitory activities of β-sitosterol and lupeol isolated from Diospyros lotus L. Nat. Prod. Res. 2016;30:1205–1207. doi: 10.1080/14786419.2015.1046381. [DOI] [PubMed] [Google Scholar]
- Rosero D.S., Odle J., Moeser A.J., Boyd R.D., van Heugten E. Peroxidised dietary lipids impair intestinal function and morphology of the small intestine villi of nursery pigs in a dose-dependent manner. Br. J. Nutr. 2015;114:1985–1992. doi: 10.1017/S000711451500392X. [DOI] [PubMed] [Google Scholar]
- Sharmila R., Sindhu G., Arockianathan Pushpam M. Nephroprotective effect of β-sitosterol on N-diethylnitrosamine initiated and ferric nitrilotriacetate promoted acute nephrotoxicity in Wistar rats. J. Basic Clin. Physiol. Pharmacol. 2016;27:473–482. doi: 10.1515/jbcpp-2015-0085. [DOI] [PubMed] [Google Scholar]
- Shi C., Luo X., Wang J., Long D. Incorporation of β-sitosterol into the membrane prevents tumor necrosis factor-α-induced nuclear factor-κB activation and gonadotropin-releasing hormone decline. Steroids. 2015;96:1–6. doi: 10.1016/j.steroids.2014.12.014. [DOI] [PubMed] [Google Scholar]
- Song Z., Cheng K., Zhang L., Wang T. Dietary supplementation of enzymatically treated Artemisia annua could alleviate the intestinal inflammatory response in heat-stressed broilers. J. Therm. Biol. 2017;69:184–190. doi: 10.1016/j.jtherbio.2017.07.015. [DOI] [PubMed] [Google Scholar]
- Song Z.H., Cheng K., Zheng X.C., Ahmad H., Zhang L.L., Wang T. Effects of dietary supplementation with enzymatically treated Artemisia annua on growth performance, intestinal morphology, digestive enzyme activities, immunity, and antioxidant capacity of heat-stressed broilers. Poult. Sci. 2018;97:430–437. doi: 10.3382/ps/pex312. [DOI] [PubMed] [Google Scholar]
- Tan X., Sun Z., Liu Q., Ye H., Zou C., Ye C., Wang A., Lin H. Effects of dietary ginkgo biloba leaf extract on growth performance, plasma biochemical parameters, fish composition, immune responses, liver histology, and immune and apoptosis-related genes expression of hybrid grouper (Epinephelus lanceolatus♂ × Epinephelus fuscoguttatus♀) fed high lipid diets. Fish Shellfish Immunol. 2018;72:399–409. doi: 10.1016/j.fsi.2017.10.022. [DOI] [PubMed] [Google Scholar]
- Valerio M.S., Minderman H., Mace T., Awad A.B. β-sitosterol modulates TLR4 receptor expression and intracellular MyD88-dependent pathway activation in J774A.1 murine macrophages. Cell. Immunol. 2013;285:76–83. doi: 10.1016/j.cellimm.2013.08.007. [DOI] [PubMed] [Google Scholar]
- Vivancos M., Moreno J.J. β-sitosterol modulates antioxidant enzyme response in RAW 264.7 macrophages. Free Radic. Biol. Med. 2005;39:91–97. doi: 10.1016/j.freeradbiomed.2005.02.025. [DOI] [PubMed] [Google Scholar]
- Wan X.L., Niu Y., Zheng X.C., Huang Q., Su W.P., Zhang J.F., Zhang L.L., Wang T. Antioxidant capacities of Artemisia annua L. leaves and enzymatically treated Artemisia annua L. in vitro and in broilers. Anim. Feed Sci. Technol. 2016;221:27–34. [Google Scholar]
- Wong H.S., Chen N., Leong P.K., Ko K.M. β-sitosterol enhances cellular glutathione redox cycling by reactive oxygen species generated from mitochondrial respiration: protection against oxidant injury in H9c2 cells and rat hearts. Phytother. Res. 2013;28:999–1006. doi: 10.1002/ptr.5087. [DOI] [PubMed] [Google Scholar]
- Zeng Z., Zhang S., Wang H., Piao X. Essential oil and aromatic plants as feed additives in non-ruminant nutrition: a review. J. Anim. Sci. Biotechnol. 2015;6:7. doi: 10.1186/s40104-015-0004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Yang L., Zhao X., Chen X., Wang L., Geng Z. Effect of dietary resveratrol supplementation on meat quality, muscle antioxidative capacity and mitochondrial biogenesis of broilers. J. Sci. Food Agric. 2017;98:1216–1221. doi: 10.1002/jsfa.8576. [DOI] [PubMed] [Google Scholar]
- Zhang J.F., Hu Z.P., Lu C.H., Yang M.X., Zhang L.L., Wang T. Dietary curcumin supplementation protects against heat-stress-impaired growth performance of broilers possibly through a mitochondrial pathway. J. Anim. Sci. 2015;93:1656–1665. doi: 10.2527/jas.2014-8244. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Song M., Rui X., Pu S., Li Y., Li C. Supplemental dietary phytosterin protects against 4-nitrophenol-induced oxidative stress and apoptosis in rat testes. Toxicol. Rep. 2015;2:664–676. doi: 10.1016/j.toxrep.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y.R., Chen Y.P., Cheng Y.F., Qu H.M., Li J., Wen C., Zhou Y.M. Effects of dietary phytosterols on growth performance, antioxidant status, and meat quality in Partridge Shank chickens. Poult. Sci. 2019;98:3715–3721. doi: 10.3382/ps/pez059. [DOI] [PubMed] [Google Scholar]