Abstract
Duck hepatitis A virus type 1 (DHAV) infection causes duck viral hepatitis and results in enormous loss to poultry farming industry. We reported that phosphorylated Codonopsis pilosula polysaccharide (pCPPS) inhibited DHAV genome replication. Here we further explored its underlying antiviral mechanisms. Autophagosomes formation is essential for the genome replication of picornaviruses. In this study, Western blot, confocal microscopy observation, and ELISA methods were performed to analyze polysaccharides' effects on autophagy by the in vitro and in vivo experiments. Results obtained from in vitro and in vivo experiments showed that Codonopsis pilosula polysaccharide did not play a role in regulating autophagy and had no therapeutic effects on infected ducklings. However, pCPPS treatment downregulated LC3-II expression level activated by DHAV and rapamycin, indicating the inhibition of autophagosomes formation. The interdiction of autophagosomes formation resulted in the inhibition of DHAV genome replication. Further study showed that pCPPS treatment reduced the concentration of phosphatidylinositol-3-phosphate (PI3P), an important component of membrane, in cells and serum, and consequently, autophagosomes formation was downregulated. In vivo experiments also verified the therapeutic effect of pCPPS. Phosphorylated Codonopsis pilosula polysaccharide treatment increased the infected ducklings' survival rate and alleviated hepatic injury. Our studies verified the effects of pCPPS against DHAV infection in duck embryo hepatocytes and ducklings and confirmed that phosphorylated modification enhanced the bioactivities of polysaccharides. The results also stated pCPPS's antiviral mechanisms, provided fundamental basis for the development of new anti-DHAV agents.
Key words: duck hepatitis A virus, phosphorylated polysaccharide, antiviral, autophagy
Introduction
Duck hepatitis virus type 1 (DHAV) is an unenveloped small RNA virus in Picornavirus family with positive-stranded RNA genome, resulting in morbidity and high mortality rate in young ducklings and threatening the poultry industry around the world (Thomas, 1969, Tseng et al., 2007, Ming et al., 2019). As for picornaviruses infection, a crucial step for virus proliferation and pathogenicity is genome replication, which is associated with cellular membrane system (Payne, 2017). Also, a drastic rearrangement of intracellular membranes was found in picornavirus-infected cells (Greninger, 2015). However, cells carry out various strategies like innate immune response and autophagy, to protect themselves from being damaged. Meanwhile, to ensure their propagation, viruses have evolved with strong abilities to evade host's weapons.
Autophagic pathway is a highly conserved and well-characterized cellular process leading to the degradation of wasted cell contents (Richards and Jackson, 2013). Cytosolic constituents are packaged in double-membrane vesicles named autophagosomes and subsequently are delivered to lysosomes and thus are digested (Levine and Klionsky, 2004). The digestion product provides substrate for cellular metabolism and scavenges protein accumulation and damaged organelles (Orvedahl et al., 2007). The autophagic pathway has been proven to be of great importance in clearing exogenous pathogens. For instance, viral infection–induced autophagy inhibits the genome replication of transmissible gastroenteritis virus and West Nile virus (Kobayashi et al., 2014, Guo et al., 2016). And as such, autophagic pathway is inhibited or subverted by some viruses. It has been shown that herpes simplex virus type 1 inhibits autophagy by interacting with autophagy-related protein Beclin 1 (Orvedahl et al., 2007). Moreover, autophagic pathway benefits the life cycle of many viruses, positive-strand RNA viruses in particular. Studies demonstrated that the autophagy machinery is necessary to initiate hepatitis C virus and coxsackievirus B3 replication (Wong et al., 2008, Dreux et al., 2009). In the previous study, we found that DHAV recruited class III phosphatidylinositol-3-kinase (PI3KC3), enriched phosphatidylinositol-3-phosphate (PI3P), induced autophagosomes biogenesis, and also interdicted the integrated autophagic pathway, and inhibited the fusion of matured autophagosomes with lysosomes. And as a result, the virus uses autophagosomes' double-membrane structure for their own genome replication (Ming et al., 2019). These research studies established foundation to develop potential antiviral drugs.
To date, there are no effective drugs against DHAV infection in clinic, and injecting egg yolk antibodies is the primary prevention plan. However, intramuscular injection raises the risk of cross infection. Therefore, it is necessary to research easy dosing and effective anti-DHAV drugs. Codonopsis pilosula polysaccharide (CPPS) is one of the main bioactive compounds extracted from traditional Chinese medicine C. pilosula (Ming et al., 2017). Codonopsis pilosula polysaccharide comprises a backbone of (1→3)-linked-β-d-galactopyranosyl, (1→2,3)-linked-β-d-galactopyranosyl, and (1→3)-linked-α-d-rhamnopyranosyl residues, and its molecular weight is determined as 1.1 × 104 Da (Sun and Liu, 2008). Phosphorylated CPPS (pCPPS) is the phosphorylated product of CPPS (Xiong et al., 2014). The infrared spectroscopy analysis shows that 2 specific phosphate absorption peaks are observed in pCPPS, phosphite ester stretching vibration at 1,159.44 cm−1 and pyrophosphate stretching vibration at 941.62 cm−1 (Ming et al., 2017). Codonopsis pilosula polysaccharide possesses good antitumor and immunoenhancement activities (Sun and Liu, 2008, Xin et al., 2012). Meanwhile, it has been confirmed that chemical modification, such as sulfated and phosphorylated modification, enhances or changes the bioactivities of polysaccharides. Song et al. reported the anti-duck enteritis virus activity of sulfated Chuanminshen violaceum polysaccharide (Song et al., 2013). We previously found that phosphorylated modification augments the anti-DHAV activities of CPPS, and pCPPS dramatically inhibits the genome replication and virulence of DHAV while CPPS shows no effect on the life cycle and virulence of DHAV (Ming et al., 2017). However, the molecular mechanism of the antiviral activity is still unclear.
Based on our former research findings, in this study, we explored the effects of CPPS and pCPPS on DHAV-induced autophagy by in vitro and in vivo experiments and further investigated the possible mechanisms. We aim to elaborate the underlying antiviral mechanisms of the phosphorylated polysaccharide and thus provide theory foundation for inventing new anti-DHAV drugs.
Materials and methods
Cell Culture and Virus
Duck embryo hepatocytes (DEHs) were cultured in Dulbecco's modified eagle medium (Gibco, Waltham) containing 10% (v/v) fetal bovine serum, 100 IU/mL penicillin, and 100 IU/mL streptomycin (Chen et al., 2014).
Duck hepatitis A virus type 1 (LQ2 strain), courtesy Shandong Institute of Poultry (Jinan, China), was propagated in DEHs and then was used to infect DEHs and animals.
CPPS and pCPPS
Previous methods were used to extract and purify CPPS (Sun and Liu, 2008). Briefly, herbal medicine C. pilosula was reflux-extracted in 95% ethanol at 75°C to remove lipid, 5 h for each time and 3 times in total. Then the herb was decocted with 10-fold distilled water at 75°C, 3 h for each time and 4 times in total. The liquid was collected and concentrated, and then mixed with 95% ethanol (to 80% of final ethanol concentration), static placed at 4°C for 12 h. The precipitation was collected followed by vacuum drying at 56°C and redissolved in distilled water, Sevag method (Sun et al., 2008) was used to remove superfluous protein in the precipitation, and crude polysaccharide was obtained. The residual protein content was determined as 1.93%. Crude polysaccharide was dissolved in distilled water and submitted to DEAE-Sepharose CL-6B (Pharmacia Biotech) for elution. The eluted solution was purified by using Sephadex G-75 (Pharmacia Biotech) column and salts were cleared by using Sephadex G-25 (Pharmacia Biotech) column. Purified CPPS was obtained followed by freeze drying.
Phosphorylated Codonopsis pilosula polysaccharide was prepared by sodium trimetaphosphate-sodium tripolyphosphate method as described previously (Xiong et al., 2014). The reaction was carried out under 6 h of reaction time, 70°C of reaction temperature, and 8.5 of pH value (Ming et al., 2017). Phenol–sulfuric acid method (Hsieh et al., 2005) was used to analyze the polysaccharide content and molybdenum blue colorimetric method was used to determine the phosphate radical content.
Codonopsis pilosula polysaccharide and pCPPS showed no cytotoxic effects at the concentration of 625 μg/mL and 39 μg/mL, and the most effective antiviral concentration of pCPPS is 19.5 μg/mL.
Autophagy Signaling Pathway In Vitro
The Role of CPPS and pCPPS on DHAV-Induced Autophagy
Duck hepatitis A virus type 1 solution was diluted to 0.01 MOI with growth medium and then was added to 6-well plate covering DEHs and removed after 1.5 h of incubation. Cell control (CC) group was not infected. Codonopsis pilosula polysaccharide and pCPPS were diluted to 625 μg/mL and 19.5 μg/mL with growth medium, respectively, and added to infected cells. Cell control group was treated with equal volume of growth medium. After 24-h incubation, all cells were harvested for analyzing the expression level of LC3-II protein.
The Role of CPPS and pCPPS on Rapamycin-Induced Autophagy
Duck embryo hepatocytes were planted on coverslips or a 6-well cell plate. Cells on coverslips were transfected with pEGFP-LC3 plasmid (Addgene plasmid #21073) by using Effectene Transfection Reagent (Qiagen, Germany). Then the cells on coverslip and cell plate were all pretreated with 100 nmol/L of rapamycin (Amquar, Shanghai, China) (diluted with growth medium) for 4 h (Sun et al., 2014), subsequently replacing the medium with CPPS, pCPPS (at the most effective antiviral concentration), and rapamycin, respectively. Followed by 24-h incubation, coverslips were placed under confocal fluorescence microscopy to observe green fluorescence. Meanwhile, cells on cell plate were harvested for analyzing the expression of LC3-II and p62 proteins.
Effect of CPPS and pCPPS on the Concentration of PI3P
Duck embryo hepatocytes were treated with 100 nmol/L rapamycin or wortmannin (ApexBio, Houston) before the DHAV infection at 500 nmol/L (Sun et al., 2014). After 1.5 h of virus adsorption, virus solution was replaced by CPPS or pCPPS or growth medium. Cells were incubated for another 12 h and then collected. Cell control group avoided viral infection or drugs treatment in the operation.
The product of PI3KC3, PI3P, was measured by duck phosphatidylinositol-3-phosphate ELISA Kit (AngleGene, Nanjing, China) to visualize the function of PI3KC3. According to the manual, DEHs were broken by ultrasonication and supernatants were collected after centrifugation. The collected supernatant was diluted and added it into ELISA plate. After 0.5 h of incubation at 37°C, the ELISA plate was incubated with HRP-conjugated PI3P antibody against for another 0.5 h at 37°C. 3,3′,5,5′-Tetramethylbenzidine was given for chromogenic reaction. Multiskan FC microplate photometer (Thermo Scientific, Waltham) was used to detect the absorbance at 450 nm. The concentration of PI3P was calculated according to the standard curve.
To rule out the influence of virus amounts, non-infected cells were also used to analyze the PI3KC3 activity. Rapamycin treatment and wortmannin treatment were set as positive or negative control, and CPPS and pCPPS treatment were tested groups. After 12-h incubation, DEHs were collected for ELISA analysis.
Animal Experiment
The animal experiment was designed to verify the effects of CPPS and pCPPS in vivo. The animals were housed according to Institutional Animal Care and Use Committee guidelines set by Nanjing Agricultural University (approval number: PTA030). Five-day-old ducklings were randomly divided into 8 groups of 20. Three of the 8 groups were intramuscularly injected DHAV of 0.2 mL per feather and then orally treated with CPPS (3 mg per feather), pCPPS (2.5 mg per feather), or equal volume of clean water. Another 3 groups were intramuscularly injected rapamycin (2 mg/kg of body weight) at 2 h before drugs treatment as well as 2 h after drugs treatment (Sun et al., 2014). Ducklings in one group were intramuscularly injected rapamycin at 2 h before virus injection as well as 2 h after virus injection. Blank control group was segregated from other groups and was only intramuscularly injected sterile normal saline and orally treated with clean water. At 6 h following aforementioned treatment, 3 feathers in each group were randomly captured and executed, and the ducklings' blood, livers, kidneys, brains, and spleens were collected. Tissue homogenates were then subjected to Western blot analysis with LC3B antibody, and blood PI3P concentration was also calculated by ELISA method.
To observe the survival rate, another 180 five-day-old ducklings were randomly divided into 4 groups and then were intramuscularly injected DHAV of 0.2 mL per feather. Ducklings were immediately dosed with CPPS and pCPPS after the injection, once a day for 3 consecutive days. Ducklings in virus control (VC) group were treated with clean water instead of drug solution. For blank control group, sterile normal saline was intramuscularly injected. The survival rate of ducklings in each group was recorded for 60 h, and the clinical symptom was observed daily. The pathological changes of dead ducklings were examined carefully.
Protein Detection by Western Blot
Western blot was performed to observe the LC3-II expression level as mentioned previously. For cell samples, cells were washed with phosphate-buffered saline and then lysed in an RIPA lysis buffer (Biosharp, Hefei, China). As for tissue samples, tissues in equal weight were thoroughly ground in liquid nitrogen and lysed in the RIPA lysis buffer. The lysates were cleared by centrifugation at 12,000 × g for 5 min, and BCA Protein Assay Kit (Beyotime, Shanghai, China) was used to calculate the concentration of proteins. The samples were further denatured in boiled water within the presence of sodium dodecyl sulfate–polyacrylamide gel electrophoresis sample loading buffer (Biosharp, Hefei, China) and then subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis. The separated proteins were transferred onto PVDF membranes (Millipore, Darmstadt, Germany). After 1 h of incubation in 5% non-fat milk at room temperature, the membrane was reacted with the following primary antibodies: LC3B (D11) XP Rabbit mAb (#3868, Cell Signaling Technology, Danvers), SQSTM1/p62 (D5E2) Rabbit mAb (#8025, Cell Signaling Technology, Danvers), β-actin (C4) Antibody (Santa Cruz SC-47778). Goat anti-Rabbit IgG (H + L) Secondary Antibody (Thermo Fisher Scientific, Waltham) was chosen as the secondary antibody.
Image Quant LAS 4000 (GE Healthcare Life Sciences) was used to observe the protein bands, and BandScan 5.0 software was used to quantify the bands.
Statistical Analysis
Data were shown as means ± standard deviations. GraphPad Prism 7 software was applied for statistical analysis. For comparison between 2 groups, one-way ANOVA was applied. Significant difference was considered to exist when P < 0.05.
Results
pCPPS Inhibits DHAV-Induced Autophagy
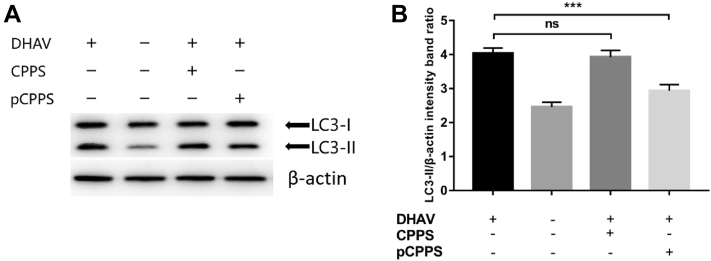
Our research found that pCPPS effectively reduces DHAV gene expression level, while DHAV initiates autophagosomes biogenesis for their genome replication. Thus, we speculated that pCPPS interdicted the formation of autophagosomes and accordingly inhibited viral genome replication. Figure 1 shows the expression level of LC3-II, the autophagy-related protein. The protein conversion from LC3-I to LC3-II was observed in virus-infected cells, suggesting the formation of autophagosomes. Phosphorylated Codonopsis pilosula polysaccharide treatment remarkably reduced the LC3-II expression level; however, CPPS treatment group showed no significant difference compared with the non-treatment group. The result indicates that pCPPS blocked virus-induced autophagosomes formation.
Figure 1.
The polysaccharides' effects on duck hepatitis A virus (DHAV)-induced autophagy. Cells were infected with or without DHAV and then treated with Codonopsis pilosula polysaccharide (CPPS) or phosphorylated C. pilosula polysaccharide (pCPPS). (A) Western blot analysis of LC3-II protein. β-actin was selected as a protein loading control. (B) The ratio of LC3-II/β-actin. The decrease of the ratio represents the inhibition of autophagy. Results are shown as means ± SD (n = 3). Significance was analyzed with ANOVA (***P < 0.001; ns, no significance).
pCPPS Inhibits Rapamycin-Induced Autophagy
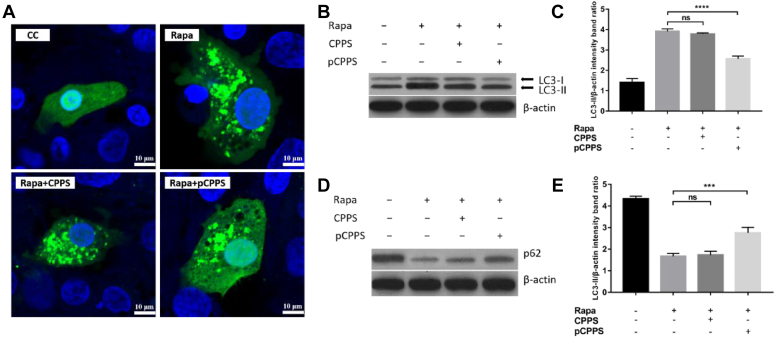
To investigate the direct effects of pCPPS on autophagy and exclude the disturbance of the amount of virus, rapamycin was used to induce autophagy, and 2 methods were adopted to analyze the autophagy level. As shown in Figure 2A, exogenous LC3 protein labeled by GFP was imported into DEHs, and various treatments were also introduced to cells. Rapamycin treatment led to the assembling of punctate green fluorescence, implying that autophagosomes were formed. After the autophagy was initiated, cells were then treated with CPPS or pCPPS. Results showed that pCPPS treatment observably reduced the amount of punctate fluorescence while CPPS did not.
Figure 2.
The polysaccharides' effects on rapamycin-induced autophagy. Cells were treated with rapamycin (Rapa) or polysaccharides (Codonopsis pilosula polysaccharide [CPPS] and phosphorylated Codonopsis pilosula polysaccharide [pCPPS]). (A) The localization of LC3 protein following diverse treatment. (B) Western blot analysis of LC3-II expression. β-actin was selected as a protein loading control. (C) The ratio of LC3-II/β-actin. The increase of the ratio represents autophagy. Results were shown as means ± SD (n = 3). Significance was analyzed with ANOVA (****P < 0.0001; ns, no significance). (D) Western blot analysis of p62 protein. Its decrease represents the fusion of autophagosome with lysosome. β-actin was selected as a protein loading control. (E) The ratio of p62/β-actin. Results are shown as means ± SD (n = 3). Significance was analyzed with ANOVA (***P < 0.001; ns, no significance).
To analyze the autophagy level in the quantitative method, the expression levels of autophagy-related proteins were presented by Western blot. The results were listed in Figure 2B–E. Consistent with the fluorescence images, treating with rapamycin increased the protein conversions from LC3-I to LC3-II and decreased the expression level of p62. This suggested that rapamycin induced a complete autophagy. The expression levels of LC3-II and p62 showed no significant differences between rapamycin treatment group and CPPS treatment group, indicating that the steps of autophagy were not affected by CPPS. However, pCPPS treatment significantly reduced the LC3 protein conversion and increased the p62 protein expression level, indicating that this polysaccharide impaired the autophagy.
pCPPS Diminishes the Production of PI3P
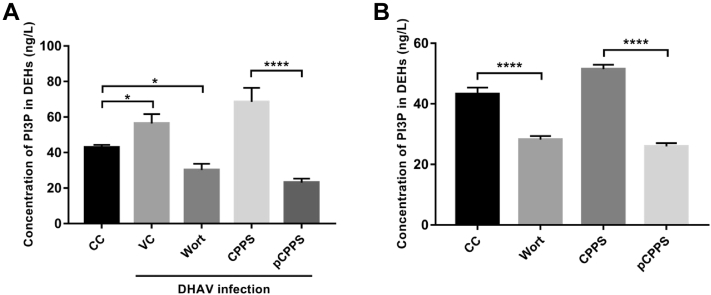
Previous study found that DHAV infection promoted the production of PI3P, which plays a key role in autophagy initiation (Nascimbeni et al., 2017, Ming et al., 2019). Therefore, we explored if pCPPS treatment led to the reduction of PI3P concentration in cells and the results were illustrated in Figure 3. In DHAV-infected cells, the concentration of PI3P (56.39 ng/L) was significantly higher than that in non-infected cells (42.92 ng/L). Pretreating with wortmannin, the specific PI3KC3 inhibitor, reduced the production of this phospholipid (30.19 ng/L). Phosphorylated Codonopsis pilosula polysaccharide treatment effectively diminished the concentration of PI3P (23.09 ng/L) in virus-infected cells. However, CPPS treatment showed facilitation in PI3P concentration (68.56 ng/L) (Figure 3A).
Figure 3.
Polysaccharides' effects on the concentration of PI3P in duck embryo hepatocyte (DEHs). Infected or non-infected cells were treated with polysaccharides (Codonopsis pilosula polysaccharide [CPPS] and phosphorylated Codonopsis pilosula polysaccharide [pCPPS]) or wortmannin (Wort). (A) The concentration of PI3P in infected DEHs. Results were shown as means ± SD (n = 3). Significance was analyzed with ANOVA (*P < 0.05; ****P < 0.0001). (B) The concentration of PI3P in non-infected DEHs. Results are shown as means ± SD (n = 3). Significance was analyzed with ANOVA (****P < 0.0001).
To eliminate the influence of virus amount on phospholipid concentration, we further explored the polysaccharides' effect by making them directly act on DEHs and obtained similar result. As illustrated in Figure 3B, compared with CC group, wortmannin inhibited the activity of PI3KC3 and consequently interdicted the synthesis of PI3P, and pCPPS also remarkably inhibited the production of PI3P. The concentration of PI3P in the CPPS treatment group was higher than that in the CC group.
pCPPS Inhibits DHAV-Induced Autophagy in Vivo
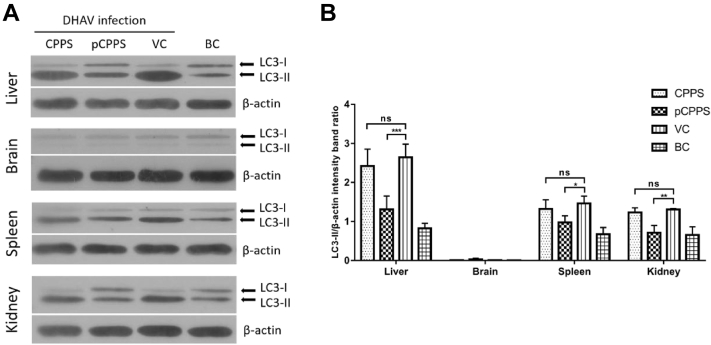
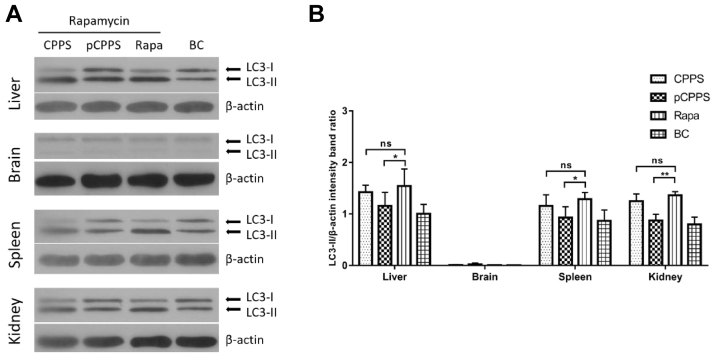
Animal experiment confirmed that DHAV infection triggered autophagosomes formation in various tissues. As shown in Figure 4, after the ducklings being infected, the protein conversion from LC3-I to LC3-II happened in the liver, spleen, and kidney. Among these 3 tissues, liver showed the most apparent conversion, suggesting that liver is the main target organ in DHAV infection. Animal experiment obtained similar results with in vitro experiment. In all these 3 tissues, the LC3-II protein expression level in the pCPPS treatment group was significantly lower than that in the VC group, and there was no significant difference between the CPPS treatment group and VC group. However, there was no detectable protein conversion from LC3-I to LC3-II found in the brain. This result indicated that DHAV-induced autophagy was tissue dependent.
Figure 4.
The LC3-II expression levels in tissues of ducklings infected with duck hepatitis A virus type 1 (DHAV). (A) Western blot analysis of LC3-II expression. β-actin was selected as a protein loading control. (B) The ratio of LC3-II/β-actin. The increase of the ratio represents autophagy. Results are shown as means ± SD (n = 3). Significance was analyzed with ANOVA (*P < 0.05; **P < 0.01; ***P < 0.0001; ns, no significance).
pCPPS Inhibits Rapamycin-Induced Autophagy in Vivo
Ducklings of another group were also intramuscularly injected rapamycin to induce autophagy, and the polysaccharides' effects in vivo were observed. As shown in Figure 5, the upregulation of LC3-II protein was observed in the liver, spleen, and kidney, suggesting that rapamycin successfully triggered autophagy in these 3 tissues. No detectable LC3-II expression was found in the brain in rapamycin treatment ducklings. The result also illustrated that pCPPS treatment significantly reduces the expression level of LC3-II in the liver, spleen, and kidney. CPPS treatment showed no significant change in comparison with the group without polysaccharide treatment.
Figure 5.
The LC3-II expression levels in tissues of ducklings treated with rapamycin. (A) Western blot analysis of LC3-II expression. β-actin was selected as a protein loading control. (B) The ratio of LC3-II/β-actin. The increase of the ratio represents autophagy. Results are shown as means ± SD (n = 3). Significance was analyzed with ANOVA (*P < 0.05; **P < 0.01; ns, no significance).
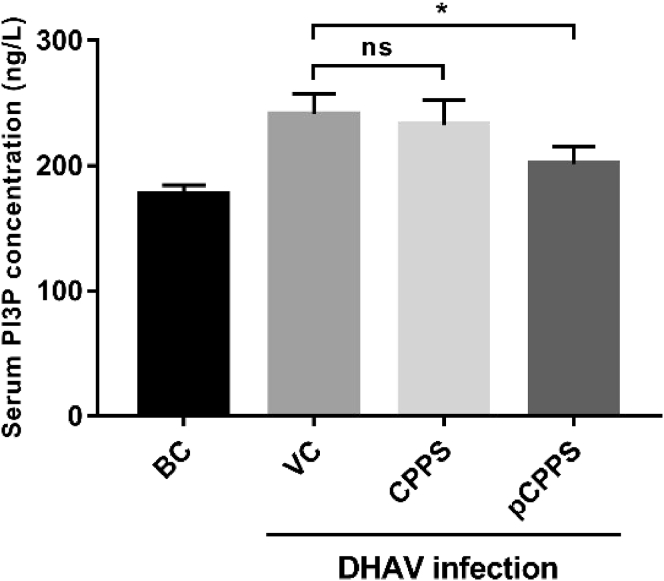
pCPPS Treatment Downregulates the Serum PI3P Concentration
The serum concentration of PI3P of infected ducklings was measured by ELISA method and the result was illustrated in Figure 6. Similar with in vitro experiment, serum PI3P concentration increased after virus infection and significantly decreased after pCPPS treatment. The PI3P concentration in CPPS treatment showed no significant change in comparison with the VC group.
Figure 6.
Concentration of PI3P in serum. Results are shown as means ± SD (n = 3). Significance was analyzed with ANOVA (*P < 0.05; ns, no significance).
The Therapeutic Effect of Polysaccharides
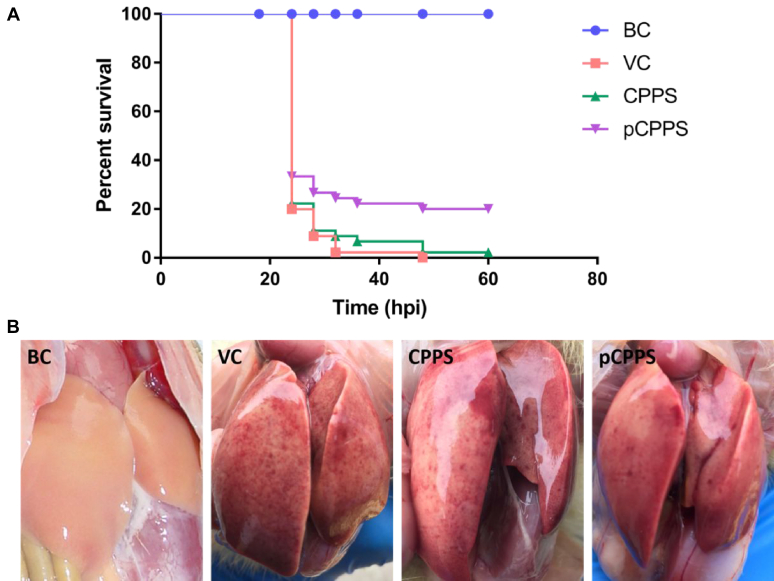
The duckling survival rate was counted and the hepatic injury was observed. The results in Figure 7A showed that the survival rate of ducklings increased by pCPPS treatment. All ducklings in the VC group died at 48 h after infection, and only one duckling survived in the CPPS treatment group. This difference was not statistically significant.
Figure 7.
The evaluation of polysaccharides' therapeutic effects. (A) Survival rates of ducklings that were treated with Codonopsis pilosula polysaccharide (CPPS), phosphorylated Codonopsis pilosula polysaccharide (pCPPS), or normal saline after being infected with duck hepatitis A virus type 1 (DHAV) (n = 10). (B) Visual pathological changes of livers in different groups.
Liver visual pathological changes were shown in Figure 7B. Serious liver lesions were observed in the VC group and CPPS treatment group; the lesions mainly included a mass of hemorrhagic spots and gores. However, the lesions were visibly alleviated after pCPPS treatment. These results suggested that pCPPS treatment performed good therapeutic effects on DHAV infection.
Discussion
In Picornavirus family, DHAV is a unique one because of the viral gene sequence. It now has been classified into Avihepatovirus, a new genus in Picornavirus family (Tseng et al., 2007). Duck hepatitis virus type 1 is characterized by rapid transmission, and its infection-induced duck virus hepatitis is fatal for ducklings within 3-week-olds. Injecting egg yolk antibody is still the primary method to prevent duck viral hepatitis because of the deficiency of effective anti-DHAV drugs. However, once immunization fails, it may cause great economic loss. Therefore, developing new effective anti-DHAV drugs is necessary.
Traditional herbal medicines have been used for thousands of years around the world. The bioactive ingredients extracted from these herbal medicines possess great therapeutic effect against infection and tumor. For example, the active ingredient in Curcuma longa, Curcumin, has a stupendously extensive bioactivities, including antioxidant, anti-inflammatory, and chemotherapeutic activity (Hatcher et al., 2008). In plenty of herbal medicines, polysaccharides are primary ingredient and bioactive substance (Dwek, 1996). Many studies found the biological activities of polysaccharides, such as antiviral activity, antitumor activity, anti-diabetes activity, anticoagulant activity, antioxidant activity, and so on (Yu et al., 2018). Thus, natural extraction could be considered as promising candidates in drugs development.
Virus replication is a key step in virus life cycle, and in many cases, drugs play antiviral efficacy by impairing virus genome replication (Fu et al., 2016, Kato et al., 2016). We also previously confirmed that pCPPS treatment distinctly inhibited DHAV gene replication while CPPS did not (Ming et al., 2017). However, the potential mechanisms are still unclear. In recent years, growing evidences point out that virus replication is related to autophagy of host cells (Alexander et al., 2007, Dreux et al., 2009, Zhang et al., 2011), and also, we have verified that DHAV recruits autophagosomes for their gene replication (Ming et al., 2019). Meanwhile, research studies showed that some polysaccharides affect autophagy of host cells. For instance, Tremella polysaccharide inhibits autophagy induced by Pseudomonas aeruginosa lipopolysaccharide (Shi et al., 2018). Liu et al. also found that selenizing Astragalus polysaccharide showed inhibitory effects on autophagy (Liu et al., 2018). Based on these studies, we speculated about if CPPS and pCPPS made impact on autophagy level.
In the present study, DEHs were infected by 0.01 MOI DHAV, and consequently, autophagosomes formation was observed. The conversion from LC3-I to LC3-II decreased greatly when the infected cells were treated with pCPPS (Figure 1), indicating that the autophagosomes biogenesis was strongly inhibited. This result suggested that pCPPS had an effect on DHAV-triggered autophagosomes formation. Codonopsis pilosula polysaccharide did not affect the autophagy level of DHAV-infected host cells. However, one thing should be mentioned is that polysaccharides have broad effects on virus life cycle. Bush sophora root polysaccharide inhibits DHAV proliferation by interdicting viral protein translation and leads to the reduction of viral amounts (Chen et al., 2018). Thus, to accurately analyze the polysaccharides' effect on autophagy and rule out the possible impact caused by viral amounts, rapamycin was used to trigger autophagy, and the polysaccharides' activity was observed.
Both confocal images and Western blot results confirmed that autophagy was successfully initiated in DEHs by rapamycin treatment, and pCPPS reduced the rapamycin-induced autophagy level (Figure 2). These results implied that pCPPS affected autophagy by acting on cellular process instead of decreasing virus amounts. Rapamycin stimulates cellular autophagy by inhibiting target of rapamycin (TOR) kinase complex in a wide variety of eukaryotes (Alvers et al., 2009, Kim et al., 2011). Akt/mTOR pathway plays indispensable role in initiating autophagy. Sun et al. found that foot-and-mouth disease virus dramatically downregulated the phosphorylation of Akt S473 and consequently downregulated the phosphorylation of mTOR (Sun et al., 2018). The downregulation of the phosphorylation of Akt was also observed in DHAV infection (Ming et al., 2019); therefore, it is sufficient to demonstrate that DHAV induces autophagy partly in the same way the rapamycin does. Phosphorylated Codonopsis pilosula polysaccharide inhibited rapamycin-induced autophagy initiation, suggesting that this polysaccharide might have effects on Akt/mTOR pathway, but this should be further explored. Moreover, given the role of autophagy in DHAV replication and pCPPS's effect on autophagosomes formation, there is reason to believe that pCPPS inhibited DHAV replication by inhibiting autophagosomes formation.
Autophagosomes formation is substantially the rearrangement or synthesis of lipid, which is the main component of cellular membrane. Phosphatidylinositol-3-phosphate (PI3P) synthesis is one of the hallmarks of autophagosomes initiation (Nascimbeni et al., 2017). Once the autophagy-related proteins were activated and targeted to the autophagosomes initiation site, autophagosome-specific pool of PI3P was produced (Lamb et al., 2013). Positive-strand RNA viruses recruit PI3KC3 and enrich PI3P to facilitate their replication (Feng et al., 2019). Our previous research also found that DHAV infection increased the concentration of PI3P in DEHs, which implied the initiation of autophagy. Phosphorylated Codonopsis pilosula polysaccharide treatment significantly reduced the PI3P concentration in DHAV-infected cells and normal cells (Figure 3). The result suggested that pCPPS directly acted on the pathway that produces PI3P, such as PI3KC3 signaling pathway. This mechanism partly accounted for the inhibition of autophagosomes formation. Also, this result provided another evidence and made it clear that pCPPS inhibited DHAV gene replication by impairing autophagosome formation pathway. Recent studies also showed the existence of PI3KC3-independent autophagosome formation. For example, foot-and-mouth disease virus triggered autophagy via a PI3KC3-independent pathway (Berryman et al., 2012). However, the role of PI3P in the initiation of autophagosomes should not be neglected. PI3KC3 is not the only one that account for the production of PI3P, and class II PI3K also produces PI3P (Nascimbeni et al., 2017). Thus, it is worthwhile to carry out research studies on the interaction between phosphorylated polysaccharide and phosphatidylinositol-3-kinase.
In all in vitro experiments, CPPS treatment showed no effects on autophagosomes formation induced by DHAV or rapamycin, suggesting that CPPS did not take part in and affect the autophagy-mediated antiviral pathway. In traditional Chinese medicine, C. pilosula is usually used as a tonic agent (Lin et al., 2013) and used to relieve fatigue and increase appetite (Wang et al., 1997). As the primary bioactive ingredient of C. pilosula, CPPS has effects on immunomodulatory, anticancer, and attenuating cognitive impairments (Zhang et al., 2018, Sun et al., 2019, Zhang et al., 2019) but no effect on antivirus. However, pCPPS showed good anti-DHAV activities in our research, which suggested that new bioactivity appeared after phosphorylated modification. This also confirmed the successful chemical modification in our experiment. It is worth noting that CPPS treatment increased the concentration of PI3P in DEHs (Figure 3), which implied that CPPS possesses other bioactivities.
Numerous studies have demonstrated the critical role of autophagy initiation in virus proliferation. However, only few studies have been conducted in animal model. Huang et al. found that Enterovirus 71 (EV71) infection triggered autophagosomes formation both in vitro and in vivo, and autophagosomes benefit the EV71 viral replication (Huang et al., 2009). Our data showed that in the DHAV infection group, the autophagy level significantly increased in various tissues, including the liver, spleen, and kidney (Figure 4). The primary DHAV target is liver (Yugo et al., 2016), in which the viral particles process their gene replication. Thus, the early autophagy level in the liver is higher than that in the spleen and kidney. Phosphorylated Codonopsis pilosula polysaccharide treatment effectively reduced the expression level of LC3-II in these 3 tissues, which confirmed the effect of pCPPS on autophagy. Also, the result suggested that polysaccharide treatment plays systemic effect instead of organ targeting effect. Similar results were observed in rapamycin treatment group (Figure 5), suggested that pCPPS inhibited rapamycin-induced autophagy in the in vivo experiment. Interestingly, no LC3-II expression was detected in the brain in both DHAV treatment and rapamycin treatment groups. For DHAV infection ducklings, the autophagy was undetectable possibly because virus had not invaded their brains at 6 h after infection, whereas for rapamycin treatment group, autophagy cannot be induced possibly because of the blood–brain barrier. We also determined the concentration of PI3P in blood and obtained similar result with the in vitro experiment, implying that pCPPS treatment impaired the production of PI3P and affected the related signaling pathways. However, CPPS treatment did not change the PI3P concentration in the in vivo model (Figure 6). The survival rate of infected ducklings was calculated to evaluate the therapeutic effects of the polysaccharides. Phosphorylated Codonopsis pilosula polysaccharide remarkably increased the survival rate of infected ducklings, which confirmed the pCPPS's antiviral effect in our previous in vitro experiment (Ming et al., 2017). In addition, pCPPS treatment effectively alleviated the hepatic injury caused by DHAV infection (Figure 7).
In the present study, we first explored the effects of C. pilosula polysaccharide and its phosphorylated modification on autophagy. We found that pCPPS significantly inhibited the formation of autophagosomes and reduced the concentration of PI3P. Besides DHAV-induced autophagy, pCPPS also impaired the rapamycin-induced autophagy, suggesting that the decrease of the autophagy level has nothing to do with the virus amounts. Phosphorylated Codonopsis pilosula polysaccharide treatment reduced the production of PI3P, which is the component of autophagosomes. Codonopsis pilosula polysaccharide showed no effects on autophagy but increased the concentration of PI3P in DEHs, suggesting that this polysaccharide maybe plays a role in other bioactivities. This also further confirmed that phosphorylated modification gave new bioactivity to CPPS. The in vivo experiment verified that pCPPS inhibited autophagy in animals. As a complex cellular process, autophagy is not only a catabolic process but also affects other signaling pathways such as innate immunity (Faure and Lafont, 2013). Autophagy could be a modulator of innate immune, and autophagy-related proteins participate in the innate immune response (Saitoh and Akira, 2010). Previous research reported that autophagy-related proteins Atg5-Atg12 conjugate negatively, regulating the production of IFN-I (Jounai et al., 2007). These research studies remind us that the effects of polysaccharides on innate immune system could be further studied in our future works.
Conclusion
In conclusion, we proved that pCPPS inhibited the formation of autophagosomes by impaired production of PI3P and consequently inhibited the replication of DHAV genome. Animal experiments confirmed the good therapeutic effect of pCPPS. This study further demonstrated that phosphorylated modification changes the bioactivities of polysaccharide and provided fundamental basis for the development of new antiviral agents.
Acknowledgments
The project was funded by National Natural Science Foundation of China (grant no. 31572557, 31772784) and the Special Fund for Agro-scientific Research in the Public Interest (201403051). The project was also supported by the Priority Academic Program Development (PAPD) of Jiangsu Higher Education Institutions. The authors thank all other staff in the Institute of Traditional Chinese Veterinary Medicine of Nanjing Agricultural University for their assistances in their work.
References
- Alexander D.E., Ward S.L., Mizushima N., Levine B., Leib D.A. Analysis of the role of autophagy in replication of herpes simplex virus in cell culture. J. Virol. 2007;81:12128–12134. doi: 10.1128/JVI.01356-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvers A.L., Wood M.S., Hu D., Kaywell A.C., William J., Dunn A., Aris J.P. Autophagy is required for extension of yeast chronological life span by rapamycin. Autophagy. 2009;5:847–849. doi: 10.4161/auto.8824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berryman S., Brooks E., Burman A., Hawes P., Roberts R., Netherton C., Monaghan P., Whelband M., Cottam E., Elazar Z., Jackson T., Wileman T. Foot-and-Mouth disease virus induces autophagosomes during cell entry via a class III phosphatidylinositol 3-kinase-independent pathway. J. Virol. 2012;86:12940–12953. doi: 10.1128/JVI.00846-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Xiong W., Zeng L., Wang D., Liu J., Wu Y., Hu Y. Comparison of Bush Sophora Root polysaccharide and its sulfate’s anti-duck hepatitis A virus activity and mechanism. Carbohydr. Polym. 2014;102:333–340. doi: 10.1016/j.carbpol.2013.11.065. [DOI] [PubMed] [Google Scholar]
- Chen Y., Yang Y., Yuan W., Wang Z., Ming K., Zeng L., Liu J. Effects of Bush Sophora Root polysaccharide and its sulfate on DHAV-1 replication. Carbohydr. Polym. 2018;197:508–514. doi: 10.1016/j.carbpol.2018.06.039. [DOI] [PubMed] [Google Scholar]
- Dreux M., Gastaminza P., Wieland S.F., V Chisari F. The autophagy machinery is required to initiate hepatitis C virus replication. Proc. Natl. Acad. Sci. U. S. A. 2009;106:14046–14051. doi: 10.1073/pnas.0907344106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwek R.A. Glycobiology: toward understanding the function of Sugars. Chem. Rev. 1996;96:683–720. doi: 10.1021/cr940283b. [DOI] [PubMed] [Google Scholar]
- Faure M., Lafont F. Pathogen-induced autophagy signaling in innate immunity. J. Innate Immun. 2013;5:456–470. doi: 10.1159/000350918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Z., Xu K., Kovalev N., Nagy P.D. Recruitment of Vps34 PI3K and enrichment of PI3P phosphoinositide in the viral replication compartment is crucial for replication of a positive-strand RNA virus. PLoS Pathog. 2019;15:e1007530. doi: 10.1371/journal.ppat.1007530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y., Gaelings L., Söderholm S., Belanov S., Nandania J., Nyman T.A., Matikainen S., Anders S., Velagapudi V., Kainov D.E. JNJ872 inhibits influenza A virus replication without altering cellular antiviral responses. Antiviral Res. 2016;133:23–31. doi: 10.1016/j.antiviral.2016.07.008. [DOI] [PubMed] [Google Scholar]
- Greninger A.L. Picornavirus-host interactions to construct viral secretory membranes. Pages 189–212 in molecular basis of viral infection. In: Klasse P.J., editor. Progress in Molecular Biology and Translational Science. Elsevier Academic Press Inc.; San Diego, CA: 2015. [DOI] [PubMed] [Google Scholar]
- Guo L., Yu H., Gu W., Luo X., Li R., Zhang J., Xu Y. Autophagy negatively regulates transmissible gastroenteritis virus replication. Sci. Rep. 2016;6:23864. doi: 10.1038/srep23864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatcher H., Planalp R., Cho J., Torti F.M., V Torti S. Curcumin: from ancient medicine to current clinical trials. Cell. Mol. Life Sci. 2008;65:1631–1652. doi: 10.1007/s00018-008-7452-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh C., Hsu T.-H., Yang F.-C. Production of polysaccharides of Ganoderma lucidum (CCRC36021) by reusing thin stillage. Process. Biochem. 2005;40:909–916. [Google Scholar]
- Huang S.-C., Chang C.-L., Wang P.-S., Tsai Y., Liu H.-S. Enterovirus 71-induced autophagy detected in vitro and in vivo promotes viral replication. J. Med. Virol. 2009;81:1241–1252. doi: 10.1002/jmv.21502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jounai N., Takeshita F., Kobiyama K., Sawano A., Miyawaki A., Xin K., Ishii K.J., Kawai T., Akira S., Suzuki K., Okuda K. The Atg5 – Atg12 conjugate associates with innate antiviral immune responses. Proc. Natl. Acad. Sci. U. S. A. 2007;104:14050–14055. doi: 10.1073/pnas.0704014104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato F., Ishida Y., Oishi S., Fujii N., Watanabe S., Vasudevan S.G., Tajima S., Takasaki T., Suzuki Y., Ichiyama K., Yamamoto N., Yoshii K., Takashima I., Kobayashi T., Miura T., Igarashi T., Hishiki T. Novel antiviral activity of bromocriptine against dengue virus replication. Antiviral Res. 2016;131:141–147. doi: 10.1016/j.antiviral.2016.04.014. [DOI] [PubMed] [Google Scholar]
- Kim J., Kundu M., Viollet B., Guan K.-L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 2011;13:132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S., Orba Y., Yamaguchi H., Takahashi K., Sasaki M., Hasebe R., Kimura T., Sawa H. Autophagy inhibits viral genome replication and gene expression stages in West Nile virus infection. Virus Res. 2014;191:83–91. doi: 10.1016/j.virusres.2014.07.016. [DOI] [PubMed] [Google Scholar]
- Lamb C.A., Yoshimori T., Tooze S.A. The autophagosome: origins unknown, biogenesis complex. Nat. Rev. Mol. Cell Biol. 2013;14:759–774. doi: 10.1038/nrm3696. [DOI] [PubMed] [Google Scholar]
- Levine B., Klionsky D.J. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev. Cell. 2004;6:463–477. doi: 10.1016/s1534-5807(04)00099-1. [DOI] [PubMed] [Google Scholar]
- Lin L.-C., Tsai T.-H., Kuo C.-L. Chemical constituents comparison of Codonopsis tangshenCodonopsis pilosula var. modesta and Codonopsis pilosula. Nat. Prod. Res. 2013;27:1812–1815. doi: 10.1080/14786419.2013.778849. [DOI] [PubMed] [Google Scholar]
- Liu D., Xu J., Qian G., Hamid M., Gan F., Chen X., Huang K. Selenizing astragalus polysaccharide attenuates PCV2 replication promotion caused by oxidative stress through autophagy inhibition via PI3K/AKT activation. Int. J. Biol. Macromol. 2018;108:350–359. doi: 10.1016/j.ijbiomac.2017.12.010. [DOI] [PubMed] [Google Scholar]
- Ming K., Chen Y., Yao F., Shi J., Yang J., Du H., Wang X., Wang Y., Liu J., Ph D. Phosphorylated Codonopsis pilosula polysaccharide could inhibit the virulence of duck hepatitis A virus compared with Codonopsis pilosula polysaccharide. Int. J. Biol. Macromol. 2017;94:28–35. doi: 10.1016/j.ijbiomac.2016.10.002. [DOI] [PubMed] [Google Scholar]
- Ming K., Yuan W., Chen Y., Du H., He M., Hu Y., Wang D. PI3KC3-dependent autophagosomes formation pathway is of crucial importance to anti-DHAV activity of Chrysanthemum indicum polysaccharide. Carbohydr. Polym. 2019;208:22–31. doi: 10.1016/j.carbpol.2018.12.035. [DOI] [PubMed] [Google Scholar]
- Nascimbeni A.C., Codogno P., Morel E. Phosphatidylinositol-3-phosphate in the regulation of autophagy membrane dynamics. FEBS J. 2017;284:1267–1278. doi: 10.1111/febs.13987. [DOI] [PubMed] [Google Scholar]
- Orvedahl A., Alexander D., Tallóczy Z., Sun Q., Wei Y., Zhang W., Burns D., Leib D.A., Levine B. HSV-1 ICP34.5 confers neurovirulence by targeting the Beclin 1 autophagy protein. Cell Host Microbe. 2007;1:23–35. doi: 10.1016/j.chom.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Payne S. Chapter 11 - family Picornaviridae. In: Payne S.B.T.-V., editor. Viruses from Understanding to Investigation. Academic Press; Cambridge, MA: 2017. pp. 107–114. [Google Scholar]
- Richards A.L., Jackson W.T. How positive-strand RNA viruses benefit from autophagosome maturation. J. Virol. 2013;87:9966–9972. doi: 10.1128/JVI.00460-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh T., Akira S. Regulation of innate immune responses by autophagy-related proteins. J. Cell Biol. 2010;189:925–935. doi: 10.1083/jcb.201002021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X., Wei W., Wang N. Tremella polysaccharides inhibit cellular apoptosis and autophagy induced by Pseudomonas aeruginosa lipopolysaccharide in A549 cells through sirtuin 1 activation. Oncol. Lett. 2018;15:9609–9616. doi: 10.3892/ol.2018.8554. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Song X., Yin Z., Li L., Cheng A., Jia R., Xu J., Wang Y., Yao X., Lv C., Zhao X. Antiviral activity of sulfated Chuanminshen violaceum polysaccharide against duck enteritis virus in vitro. Antiviral Res. 2013;98:344–351. doi: 10.1016/j.antiviral.2013.03.012. [DOI] [PubMed] [Google Scholar]
- Sun Q.-L., Li Y.-X., Cui Y.-S., Jiang S.-L., Dong C.-X., Du J. Structural characterization of three polysaccharides from the roots of Codonopsis pilosula and their immunomodulatory effects on RAW264.7 macrophages. Int. J. Biol. Macromol. 2019;130:556–563. doi: 10.1016/j.ijbiomac.2019.02.165. [DOI] [PubMed] [Google Scholar]
- Sun Y., Liu J. Structural characterization of a water-soluble polysaccharide from the roots of Codonopsis pilosula and its immunity activity. Int. J. Biol. Macromol. 2008;43:279–282. doi: 10.1016/j.ijbiomac.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Sun Y., Wang S., Li T., Li X., Jiao L., Zhang L. Purification, structure and immunobiological activity of a new water-soluble polysaccharide from the mycelium of Polyporus albicans (Imaz.) Teng. Bioresour. Technol. 2008;99:900–904. doi: 10.1016/j.biortech.2007.01.029. [DOI] [PubMed] [Google Scholar]
- Sun Y., Yu S., Ding N., Meng C., Meng S., Zhang S., Zhan Y., Qiu X., Tan L., Chen H., Song C., Ding C. Autophagy benefits the replication of Newcastle disease virus in chicken cells and tissues. J. Virol. 2014;88:525–537. doi: 10.1128/JVI.01849-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun P., Zhang S., Qin X., Chang X., Cui X., Li H., Zhang S., Gao H., Wang P., Zhang Z., Luo J., Li Z. Foot-and-mouth disease virus capsid protein VP2 activates the cellular EIF2S1-ATF4 pathway and induces autophagy via HSPB1. Autophagy. 2018;14:336–346. doi: 10.1080/15548627.2017.1405187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T.E. Studies of an agent causing mortality among ducklings immune to duck virus hepatitis. Avian Dis. 1969;13:834–846. [PubMed] [Google Scholar]
- Tseng C.-H., Knowles N., Tsai H.-J. Molecular analysis of duck hepatitis virus type 1 indicates that it should be assigned to a new genus. Virus Res. 2007;123:190–203. doi: 10.1016/j.virusres.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Wang Z.-T., Du Q., Xu G.-J., Wang R.-J., Fu D.-Z., Ng T.-B. Investigations on the protective action of Condonopsis pilosula (Dangshen) extract on experimentally-induced gastric ulcer in rats. Gen. Pharmacol. 1997;28:469–473. doi: 10.1016/s0306-3623(96)00047-x. [DOI] [PubMed] [Google Scholar]
- Wong J., Zhang J., Si X., Gao G., Mao I., McManus B.M., Luo H. Autophagosome supports coxsackievirus B3 replication in host cells. J. Virol. 2008;82:9143–9153. doi: 10.1128/JVI.00641-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin T., Zhang F., Jiang Q., Chen C., Huang D., Li Y., Shen W., Jin Y., Sui G. The inhibitory effect of a polysaccharide from Codonopsis pilosula on tumor growth and metastasis in vitro. Int. J. Biol. Macromol. 2012;51:788–793. doi: 10.1016/j.ijbiomac.2012.07.019. [DOI] [PubMed] [Google Scholar]
- Xiong W., Chen Y., Wang Y., Liu J. Roles of the antioxidant properties of icariin and its phosphorylated derivative in the protection against duck virus hepatitis. BMC Vet. Res. 2014;10:226. doi: 10.1186/s12917-014-0226-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y., Shen M., Song Q., Xie J. Biological activities and pharmaceutical applications of polysaccharide from natural resources: a review. Carbohydr. Polym. 2018;183:91–101. doi: 10.1016/j.carbpol.2017.12.009. [DOI] [PubMed] [Google Scholar]
- Yugo D.M., Hauck R., Shivaprasad H.L., Meng X.-J. Hepatitis virus infections in poultry. Avian Dis. 2016;60:576–588. doi: 10.1637/11229-070515-Review.1. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Li Z., Ge X., Guo X., Yang H. Autophagy promotes the replication of encephalomyocarditis virus in host cells. Autophagy. 2011;7:613–628. doi: 10.4161/auto.7.6.15267. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Xia Y., Luo H., Huang S., Wang Y., Shentu Y., Mahaman Y.A.R., Huang F., Ke D., Wang Q., Liu R., Wang J.-Z., Zhang B., Wang X. Codonopsis pilosula polysaccharide attenuates tau Hyperphosphorylation and cognitive impairments in htau infected mice. Front. Mol. Neurosci. 2018;11:437. doi: 10.3389/fnmol.2018.00437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Zhang Y., Xu H. Effect of Codonopsis pilosula polysaccharides on the growth and Motility of Hepatocellular Carcinoma HepG2 cells by regulating -Catenin/TCF4 pathway. Int. J. Polym. Sci. 2019;2019:1–7. [Google Scholar]