Abstract
The symbiosis of host and intestinal microbiota constitutes a microecosystem and plays an important role in maintaining intestinal homeostasis and regulating the host's immune system. Eimeria tenella, an obligate intracellular apicomplexan parasite, can cause coccidiosis, a serious intestinal disease. In this study, the effects of E. tenella infection on development parameters (villus height, crypt depth, mucosa thickness, muscularis thickness, and serosa thickness) and microbiota in chicken cecum were investigated. Fourteen-day-old male Hy-Line Variety Brown layer chickens were inoculated with sporulated oocysts of E. tenella. Cecal tissues were collected 7 d after inoculation. Relative density of goblet cells and glycoproteins were determined by Alcian blue periodic acid–Schiff staining and periodic acid–Schiff staining, respectively. Intestinal development parameters were also evaluated. Cecal contents were extracted, and the composition of cecal microflora was examined by Illumine sequencing in the V3–V4 region of the 16S rRNA gene. Results indicated that E. tenella infection destroyed the structure of cecal tissue and reduced the relative density of goblet cells and glycoproteins. Sequencing analysis indicated that E. tenella infection altered the diversity and composition of cecal microbiota. The populations of Proteobacteria, Enterococcus, Incertae, and Escherichia–Shigella decreased, and those of Bacteroidales and Rikenella significantly increased in the infected group compared with those in the control group. Hence, the pathological damage caused by E. tenella infection is associated with cecal microbiota dysbiosis, and this finding may be used to develop an alternative measure for alleviating the effect of coccidiosis on the poultry industry.
Key words: Eimeria tenella, cecal microbiota, goblet cells, glycoproteins, homeostasis
Introduction
The intestinal microflora, which exhibits high diversity, is maintained in a relative balance that is critical to the health of host and is susceptible to various diseases (Zhu et al., 2002, Gong et al., 2007, Cui et al., 2017). Coccidiosis, an intestinal disease in poultry, changes the intestinal mucosal structure (Tian et al., 2014). Eimeria tenella infection in chickens has led to production losses and high costs of prevention and treatment, accounting for more than 3.5 billion dollars annually (Blake and Tomley, 2014, Witcombe and Smith, 2014). The life cycle of E. tenella is complex because it involves schizogamy, gametogony, and sporogony developmental stages (Wang et al., 2019). In the process of schizogamy, excessive second-generation merozoites emerge from host cell lysis (Zhou et al., 2010). These merozoites can rapidly and efficiently reinvade the intestinal epithelial cells of chickens, resulting in hemorrhage, malabsorption, and diarrhea (Fernando et al., 1983, Allen, 1997, Chow et al., 2011). E. tenella specifically targets the ceca in chickens, in turn, damaging the intestinal mucosal barrier of the ceca and causing intestinal microecological disturbance (Wu et al., 2014, Macdonald et al., 2017, Okumura and Takeda, 2017).
The chicken intestine has a very diverse microbiota, which interacts with the host and forms a stable and coordinated intestinal system (Stanley et al., 2014). The cecal microbiota affects the health and productivity of chickens by regulating nutrient absorption and metabolism, immune activity and function, and pathogen exclusion (Stanley et al., 2014, Huang et al., 2018). Data have supported that the invasion of pathogenic microorganisms can lead to the absence of normal microbiota in the intestine and the transformation of intestine microbiota from physiological to pathological combinations (Hume et al., 2006). These phenomena result in intestinal microecological disturbance, diarrhea, indigestion, and enteritis (Stanley et al., 2014, Tojo et al., 2014).
In this study, a chicken model of E. tenella infection was established. The developmental parameters (villus height [VH], crypt depth, mucosa thickness, muscularis thickness, and serosa thickness) of the cecum, the relative density of goblet cells, and relative density of glycoproteins were observed under light microscope. The diversity and abundance of the cecal microbiota were detected by 16S rRNA gene sequencing. The findings will clarify the effects of E. tenella infection on the barrier damage and microbiota diversity of chicken cecum and provide further basis to develop an alternative measure for alleviating the effect of coccidiosis on the poultry industry.
Materials and methods
Chemicals and Reagents
E. tenella sporulated oocysts were provided by the Pharmacology Laboratory of Henan University of Science and Technology (Luoyang, China). 2.5% Potassium dichromate (K2Cr2O7) solution, 0.9% physiological saline solution, 4% paraformaldehyde, absolute alcohol, xylene, and paraffin were purchased from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). Periodic acid–Schiff staining, Alcian blue periodic acid–Schiff staining, and agarose were purchased from Servicebio (Wuhan, China).
Preparation of Inoculum
E. tenella oocysts were passaged, purified, allowed to sporulate in accordance with standard procedures, and kept in 2.5% K2Cr2O7 solution (Lin et al., 2015). The sporulated oocysts were washed with physiological saline solution before inoculation and counted using a cytometer.
Experimental Chickens
The experimental scheme conformed strictly to the guidelines of the Institutional Animal Care and Use Committee (No. 201) of Henan University of Science and Technology (Luoyang, Henan, China). One-day-old male Hy-Line Variety Brown layer chickens were purchased from the Hatchery of Huizhong, Luoyang, China and maintained at appropriate temperatures for 14 D in a standard animal house without coccidia.
Experimental Design
A total of 120 14-day-old chickens with similar weight were randomly divided into 2 groups (n = 60), control group (CG) and infected group (IG), with 3 replicates per group (n = 20). Each group were treated as follows: (1) CG: chickens treated without E. tenella sporulated oocysts, with feeding physiological saline (1 ml/chicken) and (2) IG: chickens inoculated by 2 × 104 E. tenella sporulated oocysts through oral gavage. All chickens were housed in disinfected wire-floored metal cages and had ad libitum access to feed and water. Seven day postinfection, in each biological replicates, ceca from 5 chickens selected randomly were aseptically collected and immediately immersed into liquid nitrogen, which was combined into one sequencing sample for microbiota diversity analysis. In each biological replicates, cecal tissue from one chicken selected randomly and immediately fixed with 4% paraformaldehyde for determination of development parameters, goblet cells, and glycoproteins.
Histomorphological Observation
Cecal tissue samples were fixed with 4% paraformaldehyde for 24 h, dehydrated via gradient alcohol solutions, transferred into xylene, embedded in paraffin, and sliced into a thickness of 5 μm. The sections were deparaffinized, rehydrated, placed in water, and stained with Periodic acid–Schiff staining (G1008) and Alcian blue periodic acid–Schiff staining (G1049) commercial kits (Servicebio) according to the manufacturer's instructions. The development parameters were measured by Pannoramic Viewer (1.15.2 RTM). Glycoprotein and goblet cells were observed, identified, and counted under light microscope (AX70, Olympus, Japan).
DNA Preparation and Sequencing
DNA was prepared from the frozen cecal samples via the PowerSoil DNA isolation kit (12888-50, Anbiosci Tech Ltd.). DNA purity and concentration were detected using 1% agarose gels. The 16S rRNA gene was obtained by amplifying the V3–V4 region with primers 5′-ACTCCTACGGGAGGCAGCA-3′ and 5′-GGACTACHVGGGTWTCTAAT-3′ (Yin et al., 2010, Zhou et al., 2016) and sequenced using an Illumina MiSeq platform in Biomarker (Beijing, China).
Bioinformatics Analysis of Sequencing Data
FLASH (http://ccb.jhu.edu/software/FLASH/) was used to merge the read pairs from the original DNA fragments (Magoč and Salzberg, 2011). Sequences were assigned to samples according to specific barcodes and primers. Trimmomatic software (http://www.usudellab.org/cms/?page=trimmomatic) was used to discard the sequences that were less than 500 bp (Bolger et al., 2014). According to the 97% identity threshold in UCLUST (http://www.drive5.com/uclust/downloads1-2-22q.html), all sequences were clustered into operational taxonomic units (OTUs) (Edgar, 2010). With the OTU definition at a similarity cutoff of 97%, Venn diagrams were analyzed between the 2 groups. The most abundant sequence in each OTU was blasted against Silva databases (Release119, http://www.arb-silva.de) to determine the phylogeny (Quast et al., 2010). In alpha-diversity analyses, Mothur (version v.1.30 http://www.mothur.org/) was used to calculate Chao 1, ACE, Shannon, and Simpson indices (Schloss et al., 2009). R software was used to establish heatmaps by vegan, vegdist, and hclust parameters. The differentially abundant bacterial taxa between the 2 groups were observed by linear discriminant analysis effect size (http://huttenhower.sph.harvard.edu/lefse/). The community structure in each classification level was subjected to taxonomy dendrogram analysis in MEGAN 5 (http://ab.inf.uni-tuebingen.de/software/megan5/) (Huson et al., 2007).
Statistical Analysis
All results were presented as mean ± standard deviation. All data were statistically analyzed by Student t test. P < 0.05 indicated significant differences.
Results
Changes in the Relative Density of Goblet Cells and Glycoproteins in Chickens
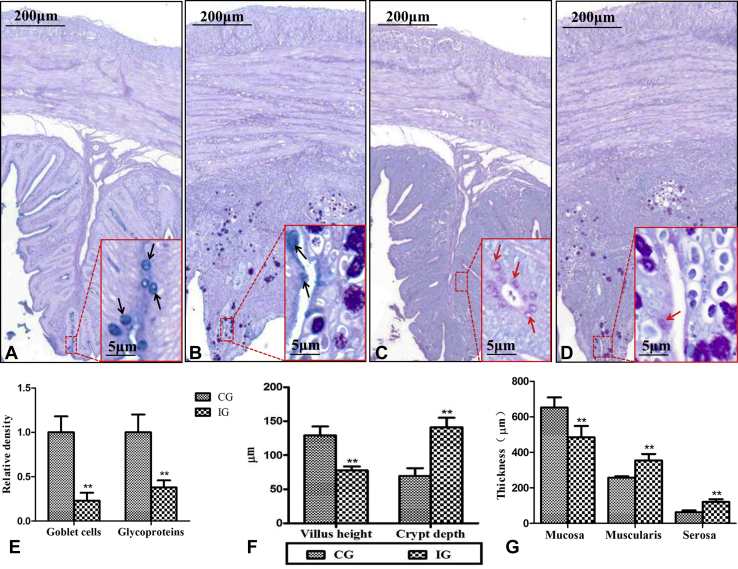
The distribution of goblet cells (Figures 1A and B) and glycoproteins (Figures 1C and D) were determined. Compared with the CG, the relative density of goblet cells and glycoproteins was decreased obviously in IG (Figure 1E, P < 0.01).
Figure 1.
Staining analysis of goblet cells and glycoproteins in cecum. Goblet cells and glycoproteins in cecum were determined through AB-PAS (A and B) and PAS (C and D), respectively. A and C are the sections of the cecum from the control group (CG), and showing the normal intestinal villus and crypt structure; B and D are the sections of the cecum from the infected group (IG), and the mucosa, muscularis, serosa, intestinal villus, and crypt was damaged in E. tenella infection chickens. E presents relative densities of goblet cells and glycoproteins in the cecum. F shows the villus height (VH) and crypt depth (CD) of the cecum. G shows the mucosa thickness, muscularis thickness, and serosa thickness of the cecum. The data are presented with the means ± standard deviation. **P < 0.01, compared with the CG. Black arrows indicate goblet cells. Red arrows indicate glycoproteins. Abbreviations: AB-PAS, Alcian blue periodic acid–Schiff staining; PAS, periodic acid–Schiff staining.
Changes in Development Parameters in the Cecum of E. tenella-Infected Chickens
As shown in Figures 1F, the cecum VH was lower (P < 0.01) and crypt depth significantly increased in IG (P < 0.01) than CG. In Figure 1G, the cecum mucosa thickness was markedly decreased (P < 0.01), and muscularis thickness and serosa thickness were notably increased in IG (P < 0.01) compared with that in CG.
Changes in Richness and Diversity of Cecal Microbiota in Chickens
In Table 1, cecal microbiota richness was evaluated by OTU counts. The number of OTUs was increased in IG compared with that in CG. The microbiota richness and diversity between IG and CG were also assessed by the abundance (Ace and Chao 1) and diversity (Shannon and Simpson) indices. With E. tenella infection, the microbiota richness and overall diversity increased in IG than the CG.
Table 1.
Number of OTUs and estimators of sequence diversity and richness.
| Sample_ID | OTUs | ACE | Chao1 | Simpson | Shannon |
|---|---|---|---|---|---|
| CG | 230 ± 16.04 | 248.5 ± 15.47 | 248.59 ± 14.74 | 0.12 ± 0.01 | 2.79 ± 0.08 |
| IG | 253 ± 15.31 | 263.68 ± 10.31 | 266.58 ± 19.03 | 0.13 ± 0.05 | 2.79 ± 0.42 |
Abbreviations: CG, control group; IG, infected group; OTU, operational taxonomic unit.
Cecal Microbiota Changes at Various Levels
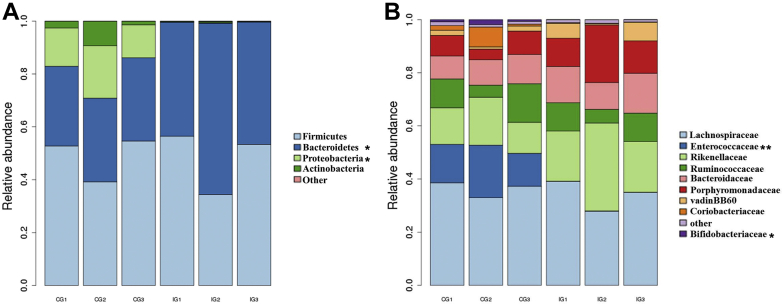
At the phylum level, the overall microbiota composition of each group is shown in Figure 2A. The microbiota in CG mainly included Firmicutes (48.86%), Bacteroidetes (31.09%), Proteobacteria (15.58%), and Actinobacteria (4.42%). E. tenella infection increased the population of Bacteroidetes (51.42%) but decreased those of Proteobacteria (0.08%) and Actinobacteria (0.31%).
Figure 2.
Relative contributions of the dominant phyla (A) and family (B) in the cecal microbiota through V3–V4 amplicon sequencing. *P < 0.05, **P < 0.01.
At the family level (Figure 2B), some bacteria were uniformly affected by E. tenella infection. Lachnospiraceae (32.86%) and Enterobacteriaceae (14.32%) were the most abundant families in CG, followed by Rikenellaceae (13.46%), Ruminococcaceae (11.98%), and Bifidobacteriaceae (2.36%). The population of Rikenellaceae (21.43%) was significantly enriched and those of Bifidobacteriaceae (0.26%) and Ruminococcaceae (8.95%) decreased in IG.
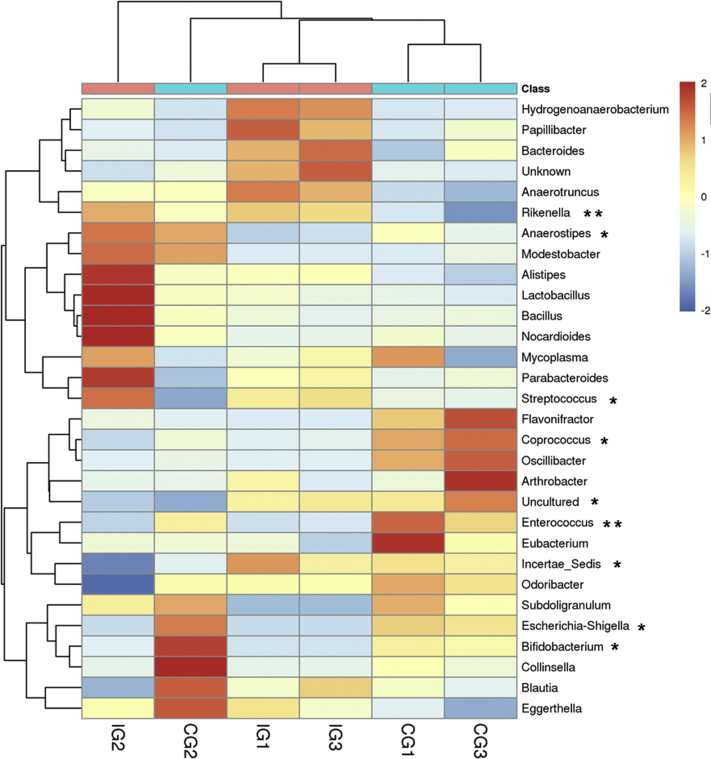
At the genus level (Figure 3), the identified genera in CG were mainly Incertae (34.94%), Escherichia–Shigella (15.55%), and Bifidobacterium (1.07%). E. tenella infection enriched Rikenella (53%) and Streptococcus (17.8%) and reduced Escherichia–Shigella (0.09%), Enterococcus (0.04%), and Incertae (32.38%).
Figure 3.
Heatmap of the genus-level changes in the control group (CG) and infected group (IG). The double hierarchical dendrogram shows the bacterial distribution. The heatmap plot depicts the relative percentage of each bacterial genus within each sample. The relative values of the bacterial families are indicated by color intensity with the legend next to the heatmap. *P < 0.05, **P < 0.01.
Microbiota Taxon Populations Associated With E. tenella Infection
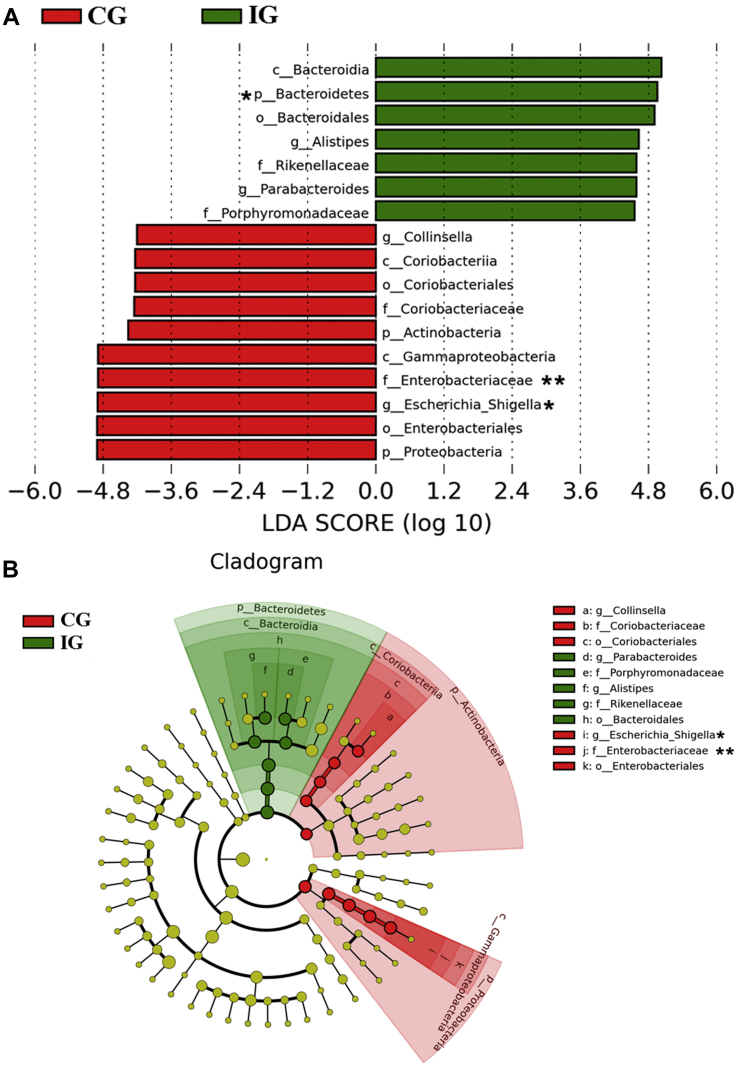
The microbiota was compared between CG and IG by using the linear discriminant analysis effect size algorithm to identify taxonomic differences associated with E. tenella infection (Figure 4A). Important taxonomic differences were identified using a logarithmic linear discriminant analysis score cutoff of 4.0 (α = 0.01) (Figure 4B). A total of 17 bacterial groups were differentially expressed between the CG and IG. Bacteroidetes were overrepresented, while Actinobacteria, Collinsella, Escherichia–Shigella, and Proteobacteria were underrepresented in chickens with E. tenella infection.
Figure 4.
Different abundances of bacterial communities between control group (CG) and infected group (IG). (A) Histogram of the LDA scores computed for differentially abundant bacterial taxa between CG and IG. (B) Taxonomic distribution of bacterial between CG and IG. A total of 17 differentially abundant bacterial taxa were detected (α = 0.01, LDA score = 4.0). Of those, 7 bacterial taxa were overrepresented in samples from IG (green), and 10 bacterial taxa were overrepresented in samples from CG (red).*P < 0.05, **P < 0.01. Abbreviations: LDA, linear discriminant analysis.
Changes of the Cecal Microbiota Communities in Chickens
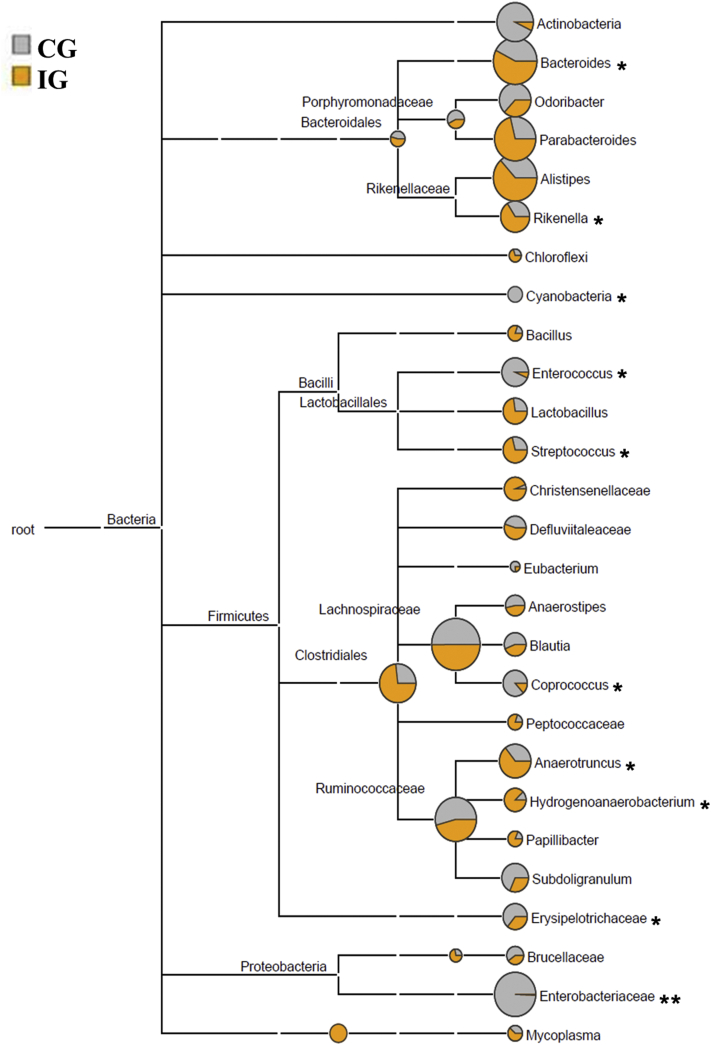
Bacterial communities are presented in Figure 5. Actinobacteria, Bacteroides, Odoribacter, Parabacteriodes, Alistipes, Rikenella, Lachnospiracoeae, Clostridiales, Anaerrotruncus, Ruminococcaceae, and Enterobacteriaceae were the dominant bacteria in the cecal microbiota of chickens. E. tenella infection increased the abundance of Bacteroides, Parabacteriodes, Alistipes, Rikenella, Clostridiales, and Anaerrotruncus and decreased those of Actinobacteria, Odoribacter, Ruminococcaceae, and Enterobacteriaceae in IG compared with CG.
Figure 5.
MEGAN 5 cladogram representing the bacterial community composition of control group (CG, gray dots) and infected group (IG, yellow dots) from cecal content of chickens. The area of pie chart represents the relative abundance of bacteria at this level. The fan-shaped area represents the size of the corresponding flora abundance. *P < 0.05, **P < 0.01.
Discussion
In birds, the intestine plays an important role in fermentation, transport, and absorption of water and nutrients (Stanley et al., 2014). E. tenella infection seriously damaged the intestinal structure of chickens (Zhou et al., 2010), reducing feed intake (Morris et al., 2007) and increasing risk of secondary infection and mortality (Wang et al., 2018). Recently reports mentioned that E. tenella infection lead to the cecum microbiota disturbance (Macdonald et al., 2017) and intestinal dysfunction (Bortoluzzi et al., 2019). The present study further explored the underlying mechanism of E. tenella infection on cecum microbiota from the perspective of intestinal barrier damage and homeostasis.
The morphology and structure of cecal tissues can reflect physiological functions in terms of exercise, digestion, and absorption (Rubin and Levin, 2016, Greig and Cowles, 2017, Wang et al., 2019). In this study, E. tenella invasion damaged the cecum villus, sloughed off intestinal epithelial cells, and significantly increased the thickness of the muscularis and serosa. Concomitantly, the intestinal villus ruptured and VH and mucosal thickness decreased significantly, which caused the intestinal crypt to compensate for the loss of intestinal villus. Goblet cells, which are distributed in intestinal epithelial cells, are derived from pluripotent stem cells inhabiting the intestinal crypt and involved in promoting the development and absorption of intestinal epithelial cells (van der Flier and Clevers, 2009, McCauley and Guasch, 2015). Glycoproteins secreted by goblet cells can flush foreign pathogenic factors and prevent harmful substances from contacting the intestinal epithelial cells (Hansson and Johansson, 2010, Round et al., 2012). In the present study, E. tenella propagation damaged the morphology of the cecum, resulting in extensive loss of goblet cells and reduced glycoproteins secretion. These phenomena consequently affect the intestinal mucosal immune response (Laurent et al., 2001). Changes in the cecum environment may alter the composition of endogenous microbiota.
16S rRNA sequencing technology was used to enhance our understanding of the pathogenic mechanism of E. tenella on cecal microbiota composition and abundance. Based on the preliminary analysis of richness and diversity indices, E. tenella infection increased the cecal microbial diversity. Actinobacteria, Bacteroidetes, Firmicutes, and Proteobacteria are the dominant phylum in chicken cecum, consistent with previous reports (Stanley et al., 2014, Waite and Taylor, 2014, Jandhyala et al., 2015). Bacteroidetes and Firmicutes are the most important microbiota in the intestine (Hidalgo-Cantabrana et al., 2017). Firmicutes can promote the effective absorption of nutrients and calories and maintain health (Ley et al., 2005, de Clercq et al., 2016). With the infection of E. tenella, the population of Firmicutes in the cecum became less than that of Bacteroidetes, which may be 1 of the reasons for the chickens weight loss, malnutrition, and anemia of the chickens (Pastor-Fernández et al., 2019). Previous studies reported that the loss of intestinal probiotics can stimulate exfoliation in the mucosa layer, shrinking of the intestinal villus, and reduction in the number of epithelial cells (Handelsman, 2004, Biggs and Parsons, 2008, Jandhyala et al., 2015, Gallo et al., 2016, Liu et al., 2019). In the present study, the abundance of members of Ruminococcaceae, Coprococcus, Ruminococcus, Lachnospira, Bifidobacterium, Incertae, and Actinobacteria remarkably decreased, thereby affecting the absorption capacity, immune function, and self-repair function of infected chickens (Duncan et al., 2002, Frank et al., 2007, Lee et al., 2013). Proteobacteria contain a wide variety of remarkable conditional pathogens, such as Escherichia–Shigella (Ma et al., 2017), which significantly decreased from 15.55% in CG for 0.09% in IG (P < 0.05). E. tenella infection is speculated to influence the colonization of Escherichia–Shigella in the cecum (Cui et al., 2017). E. tenella infection probably interferes with the growth and colonization of resident bacteria, resulting in changes in the richness and diversity of the cecal microbiota, increased susceptibility to other pathogens, and exacerbated cecal injury.
The intestinal mucus layer can provide a habitat and energy source for intestinal microbiota (Schroeder, 2019). The intestinal microbiota produces short-chain fatty acids, which can act as energy sources for intestinal epithelial cells (Kuru et al., 2004). The intestinal mucus layer interacts with the intestinal microbiota to maintain intestinal homeostasis (Okumura and Takeda, 2018). In the present study, the intestinal barrier damaged by E. tenella infection led to changes in cecal composition and homeostasis, which might be related to the abundance and diversity of the cecum microbiota. Knowledge between coccidian and cecum microbiota will provide a foundation for developing prevention and treatment strategies against the ubiquitous parasite in the poultry industry.
Acknowledgments
This work is sponsored by the National Natural Science Foundation of China (grant nos. 31472238 and 31101855), Henan province science and technology planning project (grant no. 182102110214) and Young Backbone Teachers Training Project of Colleges and Universities in Henan Province, China (grant no. 2016GGJS-061).
Contributor Information
Bian-hua Zhou, Email: zhoubh@haust.edu.cn.
Hong-wei Wang, Email: wanghw@haust.edu.cn.
References
- Allen P. Nitric oxide production during Eimeria tenella infections in chickens. Poult. Sci. 1997;76:810–813. doi: 10.1093/ps/76.6.810. [DOI] [PubMed] [Google Scholar]
- Biggs P., Parsons C.M. The effects of several organic acids on growth performance, nutrient digestibilities, and cecal microbial populations in young chicks. Poult. Sci. 2008;87:2581–2589. doi: 10.3382/ps.2008-00080. [DOI] [PubMed] [Google Scholar]
- Blake D.P., Tomley F.M. Securing poultry production from the ever-present Eimeria challenge. Trends Parasitol. 2014;30:12–19. doi: 10.1016/j.pt.2013.10.003. [DOI] [PubMed] [Google Scholar]
- Bolger A.M., Lohse M., Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortoluzzi C., Vieira B.S., Hofacre C., Applegate T.J. Effect of different challenge models to induce necrotic enteritis on the growth performance and intestinal microbiota of broiler chickens. Poult. Sci. 2019;98(7):2800–2812. doi: 10.3382/ps/pez084. [DOI] [PubMed] [Google Scholar]
- Chow Y.P., Wan K.L., Blake D.P., Tomley F., Nathan S. Immunogenic Eimeria tenella glycosylphosphatidylinositol-anchored surface antigens (SAGs) induce inflammatory responses in avian macrophages. PLoS One. 2011;6(9):e25233. doi: 10.1371/journal.pone.0025233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui N., Wang X., Wang Q., Li H., Wang F., Zhao X. Effect of dual infection with Eimeria tenella and subgroup J avian leukosis virus on the cecal microbiome in specific-pathogen-free Chicks. Front. Vet. Sci. 2017;4:177. doi: 10.3389/fvets.2017.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Clercq N.C., Groen A.K., Romijn J.A., Nieuwdorp M. Gut microbiota in obesity and undernutrition. Adv. Nutr. 2016;7(6):1080–1089. doi: 10.3945/an.116.012914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan S.H., Barcenilla A., Stewart C.S., Pryde S.E., Flint H.J. Acetate utilization and butyryl coenzyme A (CoA): acetate-CoA transferase in butyrate-producing bacteria from the human large intestine. Appl. Environ. Microbiol. 2002;68:5186–5190. doi: 10.1128/AEM.68.10.5186-5190.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26:2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- Fernando M.A., Lawn A.M., Rose M.E., Al-Attar M.A. Invasion of chicken caecal and intestinal lamina propria by crypt epithelial cells infected with coccidian. Parasitol. 1983;86:391–398. doi: 10.1017/s0031182000050587. [DOI] [PubMed] [Google Scholar]
- Frank D.N., Amand A.L.S., Feldman R.A., Boedeker E.C., Harpaz N., Pace N.R. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc. Natl. Acad. Sci. U S A. 2007;104:13780–13785. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo A., Passaro G., Gasbarrini A., Landolfi R., Montalto M. Modulation of microbiota as treatment for intestinal inflammatory disorders: an uptodate. World J. Gastroenterol. 2016;22(32):7186–7202. doi: 10.3748/wjg.v22.i32.7186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong J., Si W., Forster R.J., Huang R., Yu H., Yin Y., Yang C., Han Y. 16S rRNA gene-based analysis of mucosa-associated bacterial community and phylogeny in the chicken gastrointestinal tracts: from crops to ceca. FEMS Microbiol. Ecol. 2007;59(1):147–157. doi: 10.1111/j.1574-6941.2006.00193.x. [DOI] [PubMed] [Google Scholar]
- Greig C.J., Cowles R.A. Muscarinic acetylcholine receptors participate in small intestinal mucosal homeostasis. J. Pediatr. Surg. 2017;52(6):1031–1034. doi: 10.1016/j.jpedsurg.2017.03.037. [DOI] [PubMed] [Google Scholar]
- Handelsman J. Metagenomics: application of genomics to uncultured microorganisms. Microbiol. Mol. Biol. Rev. 2004;68:669–685. doi: 10.1128/MMBR.68.4.669-685.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson G.C., Johansson M.E. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Gut Microbes. 2010;1(1):51–54. doi: 10.4161/gmic.1.1.10470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo-Cantabrana C., Delgado S., Ruiz L., Ruas-Madiedo P., Sánchez B., Margolles A. Bifidobacteria and their health-promoting effects. Microbiol. Spectr. 2017;5(3) doi: 10.1128/microbiolspec.BAD-0010-2016. [DOI] [PubMed] [Google Scholar]
- Huang G., Tang X., Bi F., Hao Z., Han Z., Suo J., Zhang S., Wang S., Duan C., Yu Z., Yu F., Yu Y., Lv Y., Suo X., Liu X. Eimeria tenella infection perturbs the chicken gut microbiota from the onset of oocyst shedding. Vet. Parasitol. 2018;258:30–37. doi: 10.1016/j.vetpar.2018.06.005. [DOI] [PubMed] [Google Scholar]
- Hume M.E., Clemente-Hernández S., Oviedo-Rondón E.O. Effects of feed additives and mixed eimeria species infection on intestinal microbial ecology of broilers. Poult. Sci. 2006;85(12):2106–2111. doi: 10.1093/ps/85.12.2106. [DOI] [PubMed] [Google Scholar]
- Huson D.H., Auch A.F., Qi J., Schuster S.C. MEGAN analysis of metagenomic data. Genome Res. 2007;17(3):377–386. doi: 10.1101/gr.5969107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jandhyala S.M., Talukdar R., Subramanyam C., Vuyyuru H., Sasikala M., Nageshwar Reddy D. Role of the normal gut microbiota. World J. Gastroenterol. 2015;21:8787–8803. doi: 10.3748/wjg.v21.i29.8787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuru B., Dinc S., Altinok G., Aksoz T., Camlibel M., Gulcelik M.A., Alagol H. Effect of different enteral nutrients on bacterial translocation in experimental obstructive jaundice. Eur. Surg. Res. 2004;36(1):45–52. doi: 10.1159/000075074. [DOI] [PubMed] [Google Scholar]
- Laurent F., Mancassola R., Lacroix S., Menezes R., Naciri M. Analysis of chicken mucosal immune response to Eimeria tenella and Eimeria maxima infection by quantitative reverse transcription-PCR. Infect. Immun. 2001;69(4):2527–2534. doi: 10.1128/IAI.69.4.2527-2534.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.A., Kim S.H., Kim E.K., Ha E.M., You H., Kim B., Kim M.J., Kwon Y., Ryu J.H., Lee W.J. Bacterial-derived uracil as a modulator of mucosal immunity and gut-microbe homeostasis in Drosophila. Cell. 2013;153:797–811. doi: 10.1016/j.cell.2013.04.009. [DOI] [PubMed] [Google Scholar]
- Ley R.E., Bäckhed F., Turnbaugh P., Lozupone C.A., Knight R.D., Gordon J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. U. S. A. 2005;102(31):11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z., Shi Y., Deng B., Mao X., Yu D., Li W. Protective immunity against Eimeria tenella infection in chickens following oral immunization with Bacillus subtilis expressing Eimeria tenella 3-1E protein. Parasitol. Res. 2015;114:3229–3236. doi: 10.1007/s00436-015-4539-3. [DOI] [PubMed] [Google Scholar]
- Liu J., Wang H.W., Lin L., Miao C.Y., Zhang Y., Zhou B.H. Intestinal barrier damage involved in intestinal microflora changes in fluoride-induced mice. Chemosphere. 2019;234:409–418. doi: 10.1016/j.chemosphere.2019.06.080. [DOI] [PubMed] [Google Scholar]
- Ma X., Wang Q., Li H., Xu C., Cui N., Zhao X. 16S rRNA genes Illumina sequencing revealed differential cecal microbiome in specific pathogen free chickens infected with different subgroup of avian leukosis viruses. Vet. Microbiol. 2017;207:195–204. doi: 10.1016/j.vetmic.2017.05.016. [DOI] [PubMed] [Google Scholar]
- Macdonald S.E., Nolan M.J., Harman K., Boulton K., Hume D.A., Tomley F.M., Stabler R.A., Blake D.P. Effects of Eimeria tenella infection on chicken caecal microbiome diversity, exploring variation associated with severity of pathology. PLoS One. 2017;12(9):e0184890. doi: 10.1371/journal.pone.0184890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magoč T., Salzberg S.L. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics. 2011;27:2957–2963. doi: 10.1093/bioinformatics/btr507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCauley H.A., Guasch G. Three cheers for the goblet cell: maintaining homeostasis in mucosal epithelia. Trends Mol. Med. 2015;21(8):492–503. doi: 10.1016/j.molmed.2015.06.003. [DOI] [PubMed] [Google Scholar]
- Morris G.M., Woods W.G., Richards D.G., Gasser R.B. Investigating a persistent coccidiosis problem on a commercial broilerebreeder farm utilizing PCR-coupled capillary electrophoresis. Parasitol. Res. 2007;101:583–589. doi: 10.1007/s00436-007-0516-9. [DOI] [PubMed] [Google Scholar]
- Okumura R., Takeda K. Roles of intestinal epithelial cells in the maintenance of gut homeostasis. Exp. Mol. Med. 2017;49(5):e338. doi: 10.1038/emm.2017.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumura R., Takeda K. Maintenance of intestinal homeostasis by mucosal barriers. Inflamm. Regen. 2018;38:5. doi: 10.1186/s41232-018-0063-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor-Fernández I., Pegg E., Macdonald S.E., Tomley F.M., Blake D.P., Marugán-Hernández V. Laboratory growth and genetic manipulation of Eimeria tenella. Curr. Protoc. Microbiol. 2019;53(1):e81. doi: 10.1002/cpmc.81. [DOI] [PubMed] [Google Scholar]
- Quast C., Pruesse E., Yilmaz P., Gerken J., Schweer T., Yarza P., Peplies J., Glöckner F.O. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2010;41:D590–D596. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Round A.N., Rigby N.M., Garcia de la Torre A., Macierzanka A., Mills E.N., Mackie A.R. Lamellar structures of MUC2-rich mucin: a potential role in governing the barrier and lubricating functions of intestinal mucus. Biomacromolecules. 2012;13(10):3253–3261. doi: 10.1021/bm301024x. [DOI] [PubMed] [Google Scholar]
- Rubin D.C., Levin M.S. Mechanisms of intestinal adaptation. Best Pract. Res. Clin. Gastroenterol. 2016;30(2):237–248. doi: 10.1016/j.bpg.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloss P.D., Westcott S.L., Ryabin T., Hall J.R., Hartmann M., Hollister E.B., Lesniewski R.A., Oakley B.B., Parks D.H., Robinson C.J., Sahl J.W., Stres B., Thallinger G.G., Van Horn D.J., Weber C.F. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009;75:537–7541. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder B.O. Fight them or feed them: how the intestinal mucus layer manages the gut microbiota. Gastroenterol. Rep. (Oxf). 2019;7(1):3–12. doi: 10.1093/gastro/goy052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley D., Hughes R.J., Moore R.J. Microbiota of the chicken gastrointestinal tract: influence on health, productivity and disease. Appl. Microbiol. Biotechnol. 2014;98:4301–4310. doi: 10.1007/s00253-014-5646-2. [DOI] [PubMed] [Google Scholar]
- Tian E.J., Zhou B.H., Wang X.Y., Zhao J., Deng W., Wang H.W. Effect of diclazuril on intestinal morphology and SIgA expression in chicken infected with Eimeria tenella. Parasitol. Res. 2014;113(11):4057–4064. doi: 10.1007/s00436-014-4074-7. [DOI] [PubMed] [Google Scholar]
- Tojo R., Suárez A., Clemente M.G., de los Reyes-Gavilán C.G., Margolles A., Gueimonde M., Ruas-Madiedo P. Intestinal microbiota in health and disease: role of bifidobacteria in gut homeostasis. World J. Gastroenterol. 2014;20(41):15163–15176. doi: 10.3748/wjg.v20.i41.15163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Flier L.G., Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu. Rev. Physiol. 2009;71:241–260. doi: 10.1146/annurev.physiol.010908.163145. [DOI] [PubMed] [Google Scholar]
- Waite D.W., Taylor M.W. Characterizing the avian gut microbiota: membership, driving influences, and potential function. Front. Microbiol. 2014;5:223. doi: 10.3389/fmicb.2014.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Zhou L., Li W., Zhou H., Hou G. Anticoccidial effects of areca nut (Areca catechu L.) extract on broiler chicks experimentally infected with Eimeria tenella. Exp. Parasitol. 2018;184:16–21. doi: 10.1016/j.exppara.2017.11.002. [DOI] [PubMed] [Google Scholar]
- Wang L., Zhu S., Zhao Q., Huang B., Lv L., Liu G., Li Z., Zhao H., Han H., Dong H. Effects of host fatty acid-binding protein 4 on Eimeria tenella sporozoites invasion of cells. Parasitol. Res. 2019;118(6):1919–1926. doi: 10.1007/s00436-019-06321-x. [DOI] [PubMed] [Google Scholar]
- Wang H.W., Liu J., Zhao W.P., Zhang Z.H., Li S.Q., Li S.H., Zhu S.Q., Zhou B.H. Effect of fluoride on small intestine morphology and serum cytokine contents in rats. Biol. Trace. Elem. Res. 2019;189(2):511–518. doi: 10.1007/s12011-018-1503-y. [DOI] [PubMed] [Google Scholar]
- Witcombe D.M., Smith N.C. Strategies for anti-coccidial prophylaxis. Parasitology. 2014;141:1379–1389. doi: 10.1017/S0031182014000195. [DOI] [PubMed] [Google Scholar]
- Wu S.B., Stanley D., Rodgers N., Swick R.A., Moore R.J. Two necrotic enteritis predisposing factors, dietary fishmeal and Eimeria infection, induce large changes in the caecal microbiota of broiler chickens. Vet. Microbiol. 2014;169(3-4):188–197. doi: 10.1016/j.vetmic.2014.01.007. [DOI] [PubMed] [Google Scholar]
- Yin Y., Lei F., Zhu L., Li S., Wu Z., Zhang R., Gao G.F., Zhu B., Wang X. Exposure of different bacterial inocula to newborn chicken affects gut microbiota development and ileum gene expression. ISME J. 2010;4:367–376. doi: 10.1038/ismej.2009.128. [DOI] [PubMed] [Google Scholar]
- Zhou B.H., Wang H.W., Wang X.Y., Zhang L.F., Zhang K.Y., Xue F.Q. Eimeria tenella: effects of diclazuril treatment on microneme genes expression in second-generation merozoites and pathological changes of caeca in parasitized chickens. Exp. Parasitol. 2010;125(3):264–270. doi: 10.1016/j.exppara.2010.01.022. [DOI] [PubMed] [Google Scholar]
- Zhou X., Jiang X., Yang C., Ma B., Lei C., Xu C., Zhang A., Yang X., Xiong Q., Zhang P., Men S., Xiang R., Wang H. Cecal microbiota of Tibetan Chickens from five geographic regions were determined by 16S rRNA sequencing. Microbiologyopen. 2016;5:753–762. doi: 10.1002/mbo3.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X.Y., Zhong T., Pandya Y., Joerger R.D. 16S rRNA-based analysis of microbiota from the cecum of broiler chickens. Appl. Environ. Microbiol. 2002;68:124–137. doi: 10.1128/AEM.68.1.124-137.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]