Abstract
Curcumin has antioxidant functions, regulates the intestinal microbial composition, and alleviates mycotoxin toxicity. The present study aimed to explore whether curcumin could alleviate ochratoxin A (OTA)-induced liver injury via the intestinal microbiota. A total of 720 mixed-sex 1-day-old White Pekin ducklings were randomly assigned into 4 groups: CON (control group, without OTA), OTA (fed a diet with 2 mg/kg OTA), CUR (ducks fed a diet with 400 mg/kg curcumin), and OTA + CUR (2 mg/kg OTA plus 400 mg/kg curcumin). Each treatment consisted of 6 replicates and 30 ducklings per replicate. Treatment lasted for 21 D. Results were analyzed by a two-tailed Student t test between 2 groups. Our results demonstrated that OTA treatment had the highest serum low-density lipoprotein (LDL) level among 4 groups. Compared with OTA group, OTA + CUR decreased serum LDL level (P < 0.05). OTA decreased liver catalase (CAT) activity in ducks (P < 0.05), while addition of curcumin in OTA group increased liver CAT activity (P < 0.05). 16S ribosomal RNA sequencing suggested that curcumin increased the richness indices (ACE index) and diversity indices (Simpson index) compared with OTA group (P < 0.05) and recovered the OTA-induced alterations in composition of the intestinal microbiota. Curcumin supplementation relieved the decreased abundance of butyric acid producing bacteria, including blautia, butyricicoccus, and butyricimonas, induced by OTA (P < 0.05). OTA also significantly influenced the metabolism of the intestinal microbiota, such as tryptophan metabolism and glyceropholipid metabolism. Curcumin could alleviate the upregulation of oxidative stress pathways induced by OTA. OTA treatment also increased SREBP-1c expression (P < 0.05). The curcumin group had the lowest expression of FAS and PPARG mRNA (P < 0.05) and the highest expression of NRF2 and HMOX1 mRNA. These results indicated that curcumin could alleviate OTA-induced oxidative injury and lipid metabolism disruption by modulating the cecum microbiota.
Key words: curcumin, ochratoxin A, duck, liver oxidative injury, intestinal microbiota
Introduction
Mycotoxins, which are secondary metabolites of fungi, have significant human and animal health, economic, and international trade implications (Malir et al., 2013, Ostry et al., 2013, Luo et al., 2019). Ochratoxin A (OTA) is the most prevalent and relevant fungal toxin and is produced by Aspergillus species and Penicillium species (Liuzzi et al., 2017). OTA is one of the most common contaminants of food such as cereals, coffee, wine, dried fruits and nuts (Fink-Gremmels, 2005), meat products (Altafini et al., 2017), eggs (Juszkiewicz et al., 1982), herbal medicines (Shim et al., 2014), food coloring agents (Solfrizzo et al., 2015), and even in bottled water (Mata et al., 2015). The liver and kidney are key target organs for OTA to exert its toxic effects because of its metabolization and accumulation mainly occurred in the liver and kidney (Vettorazzi et al., 2011). OTA has been shown to be carcinogenic (Polovic et al., 2018), hepatotoxic (Sobral et al., 2018), nephrotoxic (Vettorazzi et al., 2019), and immunotoxic (Hou et al., 2018); however, these roles have now been assigned to reactive oxygen species and oxidative stress induced by OTA (Cui et al., 2013, El-Haleem et al., 2016, Periasamy et al., 2016, Abdel-Wahhab et al., 2017). It has been demonstrated that antioxidants could protect cells against OTA-induced cytotoxicity and genotoxicity (Ramyaa and Padma, 2014, Costa et al., 2016). However, there is a lack of antioxidants to alleviate the toxicity of OTA.
Curcumin is a polyphenolic compound isolated from the rhizome of Curcuma longa Linn, which has a variety of pharmacological activities, including antioxidative, anti-inflammatory, antibacterial, and antifungal properties (Zorofchian Moghadamtousi et al., 2014). Previous studies showed that curcumin could alleviate liver oxidative stress in type 1 diabetic rats by activating the Kelch-like ECH associated protein 1-nuclear factor, erythroid 2 like 2 (NRF2)-antioxidant response element (ARE) signaling pathway (Xie et al., 2018). Gut microbiota dysbiosis was partially caused by increased levels of oxidative stress (Qiao et al., 2013, Borrelli et al., 2018). OTA treatment could increase the relative abundance of Lactobacillaceae while decreasing the relative abundance of Bacteroidaceae in rats (Guo et al., 2014). OTA was given to mice by intragastric application could decrease the relative abundance of Lactobacillus spp. and Bifidotacterium spp. (Oršolić et al., 2017). Curcumin could modify the gut microbial balance. Study has suggested that curcumin could enrich the key phylotype Lactobacillus that was previously associated with alleviated liver weight and improved intestinal integrity (Feng et al., 2017). Curcumin could decrease the relative abundance of Prevotella while increasing the relative abundance of Alistipes (Shen et al., 2017). Curcumin is effective against Aflatoxin B1-induced oxidative stress (El Bahr, 2015, Muhammad et al., 2018) and could ameliorate the toxic effect of OTA and Aflatoxin B1 in broiler chickens (Chavez and Ledoux, 2008, Rangsaz and Ahangaran, 2011, Zhang et al., 2016). Therefore, curcumin treatment could be considered as a potential strategy to modulate the intestinal bacterial composition to ameliorate the toxicity of OTA.
Young animals are always more sensitive with regard to their response to mycotoxin-contaminated feed because of their incomplete organ development (Stoev, 2016, Adetunji et al., 2017). Broiler chicks are relatively sensitive to the toxicity of T-2 toxin, especially in gastrointestinal tissues (Osselaere et al., 2013, Luo et al., 2019). Ducklings are particularly sensitive to OTA delivered via oral gavage (Van der Merwe et al., 1965, Purchase and Theron, 1968, Peckham et al., 1971, Prior et al., 1976). Therefore, the present study aimed to explore the underlying mechanism of liver oxidative injury induced by OTA in ducklings and whether the protective effects of curcumin act by regulating lipid metabolism and intestinal microbiota.
Materials and methods
Preparation of an OTA-Contaminated Diet
The preparation of OTA-contaminated corn performed was according to the study by Ruan et al. (2019). Briefly, corn meal was sterilized at 105°C, inoculated with a suspension of Aspergillus ochraceus conidia, and incubated for 7 D at 29 to 30°C. During the incubation, 3 L of autoclaved Czapek Dox Medium was added twice daily and thoroughly mixed. The OTA level in the moldy corn reached 12.7 mg/kg.
Animals and Treatment
The experimental period lasted for 3 wk. All practices and procedures for this experiment were reviewed and approved by the Animal Care and Use Committee of South China Agricultural University (SCAU-10564).
A total of 720 mixed-sex 1-day-old White Pekin ducklings with an initial body weight of 43.4 ± 0.1 g were randomly assigned into 4 groups: CON (control group, without OTA), OTA (a group fed 2 mg/kg OTA-contaminated diet, birds were fed with 16% moldy corn replacing fresh corn), CUR (ducks fed with 400 mg/kg curcumin in their diet), and OTA + CUR (2 mg/kg OTA plus 400 mg/kg curcumin). Each treatment consisted of 6 replicates, and each replicate contained 30 ducklings. The diet formula is shown in Supplementary Table 1. All diets were formulated to meet or exceed the NRC (1994) for starter ducks. The birds were provided with pelleted diets and ad libitum access to feed and water. The temperature of the room was maintained at 32 to 34°C for the first 3 D and then reduced by 2 to 3°C per wk to a final temperature of 26°C, which was maintained for the remainder of the experiment. Daylight was eliminated, but 18-h/D lighting was provided using incandescent bulbs.
Sample Procedures
Blood samples of approximately 10 mL, selected based on the average weight of each treatment group, were collected at 21 D of age from 6 ducks in each treatment group. Serum was prepared by centrifuging the blood 3,000 rpm for 10 min at 4°C and stored at −30°C for analysis. The sampled ducks were sacrificed, and parts of their livers were snap-frozen in liquid nitrogen for mRNA extraction for quantitation real-time reverse transcription polymerase chain reaction. Cecum digests were collected and stored at −80°C until further analyses.
Serum Lipid Metabolism and Liver Antioxidant Indices
Levels of serum lipid metabolites, including total cholesterol (TC), triglyceride (TG), high-density lipoprotein (HDL), low-density lipoprotein (LDL); as well as liver antioxidant indices including total antioxidation capacity, superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase, and malondialdehyde (MDA) were quantified using commercial diagnostic kits for serum analysis (Jiancheng Bioengineering Institute, Nanjing, China).
Analysis of the Cecal Microbiota Community by 16S rRNA Analysis
DNA was extracted from the cecum digesta and liver using an E.Z.N.A. soil DNA Kit (Omega Bio-tek, Norcross, GA) according to the protocol for isolation of DNA. 16S rRNA sequencing of digesta samples was performed as previously described (Martin et al., 2019). Illumina MiSeq sequencing and general data analyses were performed by a commercial company (Majorbio Bio-Pharm Technology, Shanghai, China).
Metabolite Profiling Analysis
Metabolite concentrations in ducks' cecum digests were quantified using liquid chromatography/mass spectrometry according to a previous study (Rojo et al., 2015). A quality control sample was prepared by mixing aliquots of the all samples as a pooled sample and then analyzed using the same method with the analytic samples. The quality controls were injected at regular intervals throughout the analytical run to provide a set of data from which repeatability could be assessed. Liquid chromatography/mass spectrometry and further data analysis were conducted according to a previously published work (Rojo et al., 2015).
Transcriptional Analysis
Total RNA was isolated from liquid nitrogen frozen liver using a Quick-RNA MiniPrep Plus (Zymo, Irvine, CA) according to the manufacturer's instructions. Synthesis of the first strand (cDNA) was performed using oligo (dT) 20 and Superscript II reverse transcriptase (Takara, Shiga, Japan). The PCR reactions were conducted using the following program: 3 s of denaturation at 95°C; 40 cycles of 5 s at 95°C, 30 s for annealing at 60°C, and 30 s for elongation at 72°C; and store at 4°C. Transcriptional analysis were performed using the following primers: SREBP-1c (sterol regulatory element binding transcription factor 1): 5′- GAGGCCAAGCTCAACAAGTC-3′, 5′- ATCTCCATCACCTCCGCCTT-3′; FAS (fatty acid synthase): 5′-CAATGGATCCTCAGCTTCGC-3′, 5′- AGCTGTTCTGGATCTTGGCT -3′; PPARG (peroxisome proliferator activated receptor gamma): 5′- GGAGCCCAAGTTTGAGTTCG-3′, 5′- GGTCCGTCATTTTCTGGAGC-3′; NRF2 (nuclear factor, erythroid 2 like 2): 5′- GCTGGAGTTAGACGAGGAG-3′, 5′- AGGGCTTGTGATTGTGCT-3′; HMOX1 (Heme oxygennase-1): 5′- ATGCCTACACTCGCTATCTG-3′, 5′- GCAAGGTCCATCTCAAGG-3′; ACTIN (β-actin): 5′- TACGCCAACACGGTGCTG-3′, 5′- GATTCATCATACTCCTGCTTG-3′.
Statistical Analysis
Data are expressed as the means and SEM. Using GraphPad Prism 5.0 software, results were analyzed by a two-tailed Student t test between 2 groups. Significant differences were set at P ≤ 0.05. Orthogonal partial least squares discriminant analysis (OPLS-DA) was undertaken for both positive and negative model construction after log transformation and Pareto scaling.
Results
Serum Liver Function and Lipid Metabolism Indices
The serum liver function and lipid metabolism indices in the different treatment groups are presented in Figure 1. OTA treatment increased serum ALT activity, the marker enzymes for liver function damage, when compared with CON group (P < 0.01). Treatment of ducks with both CUR and OTA + CUR reduced AST activity (P < 0.01). Compared with the control group, ducks fed with the OTA diet had significantly higher LDL levels (P < 0.05). The addition of curcumin to the OTA diet (OTA + CUR) partially ameliorated the increase in LDL. The CUR group had the lowest LDL level (P < 0.05). There were no differences in serum AST activity and levels of TC, TG, and HDL among the 4 dietary treatments (P > 0.05).
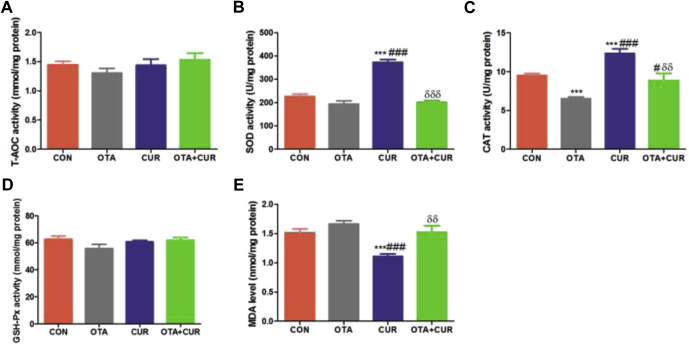
Figure 1.
Effect of ochratoxin A (OTA) and curcumin (CUR) on the serum liver function and lipid metabolism indices of 21 D ducks. (A) Aspartate aminotransferase (AST) activity. (B) Alanine aminotransferase (ALT) activity. (C) Serum total cholesterol (TC) content. (D) Serum triglycerid (TG) content. (E) Serum high-density lipoprotein (HDL) content. (F) Serum low-density lipoprotein (LDL) content. Data are expressed as mean with SEM (n = 6). * vs. CON group; # vs. OTA group; δ vs. CUR group.
Curcumin Improved the Liver Antioxidant Ability in OTA Treated Ducks
Figure 2 shows the effect of OTA and curcumin on the liver antioxidant capacity. Ducks fed with OTA had the lowest SOD and CAT activities and the highest MDA level (Figure 2). Compared with the OTA group, OTA + CUR group significantly increased the CAT activity (P < 0.05). Curcumin treatment had the highest SOD and CAT activities and the lowest MDA level (P < 0.05). No effects on total antioxidation capacity and GPX-Px activities were noted in the ducks fed with OTA and curcumin (P > 0.05).
Figure 2.
Effect of ochratoxin A (OTA) and curcumin (CUR) on liver antioxidative capacity of 21-day ducks. (A) Liver total antioxidation capacity (T-AOC) activity. (B) Liver superoxide dismutase (SOD) activity. (C) Liver catalase (CAT) activity. (D) Liver glutathione peroxidase (GSH-Px) activity. (E) Liver malondialdehyde (MDA) level. Data are expressed as mean with SEM (n = 6). * vs. CON group; # vs. OTA group; δ vs. CUR group.
Curcumin Recovered the Altered Cecum α-Diversity and Composition Induced by OTA
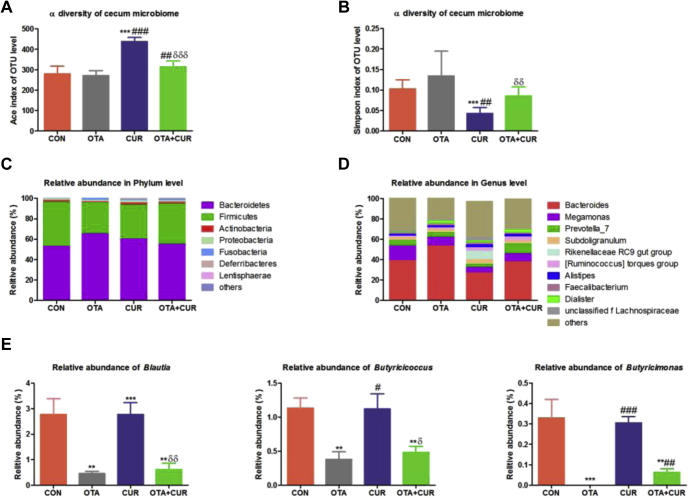
The effects of OTA and curcumin on the cecum α-diversity and composition are shown in Figure 3. Compared with the CON group, OTA treatment had no significant effect on the cecum microbiota richness and diversity (Figures 3A and 3B; P > 0.05). Adding curcumin to the diet increased the ACE and Simpson indexes compared with those in the OTA group (P < 0.05). Overall, the microbiomes of the individual ducks were dominated by the phyla Bacteroidetes and Firmicutes. The other phyla present in low abundance (less than 2.0%) were Actinobacteria, Proteobacteria, Fusobacteria, and Tenericutes (Figure 3C). At the genus level, ducks fed with OTA increased the relative abundance of Bacteroides (Figure 3D). The cecum microbiota composition in the OTA + CUR group was similar to that of the CON group. We further compared the relative abundance of butyric acid–producing bacteria among the 4 groups (Figure 3E). The result showed that the relative abundance of butyric acid–producing bacteria was very different in the duck fed with the different diets; however, the change in blautia was the same as those of the butyricicoccus and butyricimonas in each group; however, their levels were significantly higher in the CON and CUR groups than in the groups fed with diets containing OTA (P < 0.05). Compared with the OTA group, OTA + CUR increased the relative abundance of butyricimonas (P < 0.05). These results indicated that curcumin could recovery the micriobiota composition of ducks fed with OTA.
Figure 3.
The α-diversity and composition of the cecum microbiota in different groups. (A) ACE index. (B) Simpson index. (C) Relative abundance of bacteria at the phylum level. Operational taxonomic units (OTUs) with an occurrence lower than 1% are not represented (n = 6). (D) Relative abundance of top 10 genera in each group (n = 6). (E) Relative abundance of butyric acid producing bacteria at the genus level (n = 6). Abbreviations: CUR, curcumin; OTA, ochratoxin A. Data are expressed as mean with SEM (n = 6). * vs. CON group; # vs. OTA group; δ vs. CUR group.
OTA and Curcumin Altered the Metabolism of Intestinal Microbiota in Ducks
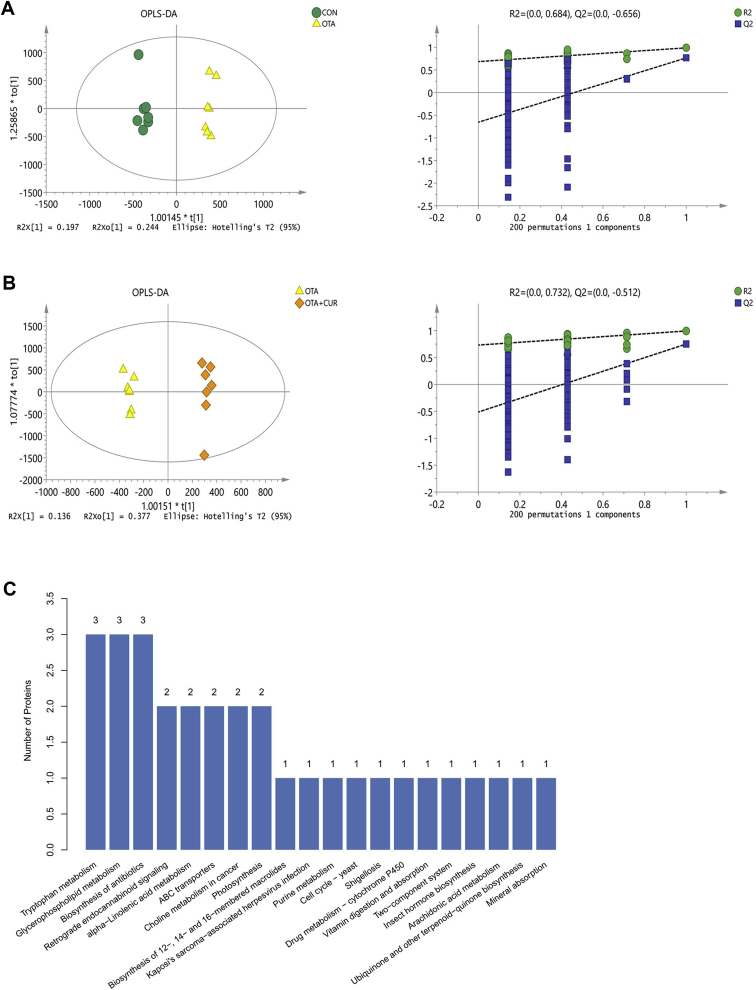
To further explore the influence of OTA supplementation on intestinal microbiota, we analyzed the contents of metabolites in the fecal samples in the 4 groups (Figure 4). An OPLS-DA was applied to better understand the different metabolic patterns. In the OPLS-DA score plot, the OTA-treated group and CON ducks were separated from each other (Figure 4A). Similarly, the OTA treated group and OTA + CUR ducks were also separated from each other (Figure 4B). The qualities of the resulting discriminant model are shown in Figures 4C and 4D, suggesting that the model was robust and had good fitness and prediction. Kyoto Encyclopedia of Genes and Genomes analysis showed that OTA highly altered microbial metabolism, including tryptophan metabolism, glycerophospholipid metabolism, biosynthesis of antibiotics, retrograde endocannabinoid signaling, alpha-Linolenic acid metabolism, ABC transporters, and choline metabolism in cancer and photosynthesis (Figure 4E). We further collected the Kyoto Encyclopedia of Genes and Genomes pathways associated with oxidative stress (Table 1), including the calcium signaling pathway, the nuclear factor kappa b signaling pathway, Th17 cell differentiation, the mitogen activated protein kinase signaling pathway, the Ras signaling pathway, the chemokine signaling pathway, the T cell receptor signaling pathway, and hepatocellular carcinoma. The result showed that OTA could upregulate oxidative stress–related pathways, and the level of the metabolite of these pathways diacylglycerol was 9.6 times higher than that in the CON group. We also compared OTA + CUR and CON (OTA + CUR vs. CON) groups for these pathways and found that they were not upregulated. Collectively, curcumin supplementation significantly alters the metabolism of intestinal microbiota in ducks fed with OTA.
Figure 4.
The metabolism of intestinal microbiota in ducks of different groups. (A) Orthogonal partial least squares discriminant analysis (OPLS-DA) and score plot derived from LC-MS analysis in the digesta after ochratoxin A (OTA) treatment (n = 6). (B) OPLS-DA and score plot derived from LC-MS analysis in the cecum digesta after OTA and curcumin (CUR) treatment (OTA, n = 6; OTA + CUR, n = 7). (C) Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis of different metabolites identified from LC-MS analysis in the cecum digesta after curcumin supplementation (n = 6).
Table 1.
KEGG pathways associated with oxidative stress (OTA vs. CON).
| Metabolites | Regulate | Fold change | Pathway | Pathway name |
|---|---|---|---|---|
| DG (18:0/16:0/0:0) | Upregulated | 9.563 | ko04020 | Calcium signaling pathway |
| DG (18:0/16:0/0:0) | Upregulated | 9.563 | ko04064 | NF-kappa B signaling pathway |
| DG (18:0/16:0/0:0) | Upregulated | 9.563 | ko04659 | Th17 cell differentiation |
| DG (18:0/16:0/0:0) | Upregulated | 9.563 | ko04010 | MAPK signaling pathway |
| DG (18:0/16:0/0:0) | Upregulated | 9.563 | ko04014 | Ras signaling pathway |
| DG (18:0/16:0/0:0) | Upregulated | 9.563 | ko04062 | Chemokine signaling pathway |
| DG (18:0/16:0/0:0) | Upregulated | 9.563 | ko04660 | T cell receptor signaling pathway |
| DG (18:0/16:0/0:0) | Upregulated | 9.563 | ko05225 | Hepatocellular carcinoma |
Abbreviations: DG, diacylglycerol; OTA, ochratoxin A.
Effect of OTA and Curcumin on Liver mRNA Expression Related to Liver Lipid Metabolism and Antioxidant Capacity
Compared with the CON group, OTA treatment upregulated the relative mRNA expression of SREBP-1c (Figure 5A; P < 0.05) but had no significant effect on the expression of FAS and PPARG mRNA (Figures 5B and 5C; P > 0.05). Compared with the OTA group, OTA + CUR treatment decreased the expression of FAS mRNA (Figure 5B; P < 0.05). The curcumin group had the lowest expression level of FAS and PPARG mRNA (Figures 5B and 5C; P < 0.05). Figures 5D and 5E shows the effect of OTA on liver antioxidant gene expression. OTA presented the lowest expression of NRF2 and HMOX1 mRNA but showed no significant decrease compared with that in the CON group (P > 0.05). By contrast, curcumin treatment had the highest expression of NRF2 and HMOX1 mRNA, but the difference was not significant (P > 0.05).
Figure 5.
Relative mRNA expressions of lipid metabolism and antioxidant capacity related genes in liver of ducks. (A) FAS, (B) SREBP-1c, (C) PPARG, (D) NRF2, (E) HMOX1. Abbreviations: CUR, curcumin; OTA, ochratoxin A. Data are expressed as mean with SEM (n = 6). * vs. CON group; # vs. OTA group; δ vs. CUR group.
Discussion
Numerous studies reported that OTA could induce oxidative stress and lead to liver injury. Curcumin has antioxidant functions, regulates the intestinal microbial composition, and alleviates mycotoxin toxicity (Saint-Cyr et al., 2013, Muhammad et al., 2018). In the present study, our data showed that dietary supplementation of curcumin reversed serum biochemical changes, ameliorated liver oxidative injury, and recovered composition and metabolism of intestinal microbiota induced by OTA.
Activities of serum AST and ALT would be the reflection of hepatic functionality. In this experiment, OTA could increase the serum ALT activity. Our previous study has shown that ducks fed with the OTA diet had lower 21-day BW, ADFI, and ADG and higher absolute or relative liver weight. Addition of curcumin to the OTA diet (CUR + OTA) improved growth performance of the OTA group while partially ameliorated the increase of relative liver weight (Ruan et al., 2019). This indicated that curcumin could ameliorate the liver damage induced by OTA. Serum TC, TG, LDL, and HDL concentrations are regarded as diagnostic markers in lipid metabolism. Most fatty acids are synthesized in the liver and are transported via LDL or chylomicrons for storage in adipose tissue as TC. In contrast, HDL promotes the uptake of cholesterol from peripheral tissues and facilitates the transport of cholesterol to the liver for catabolism (Hermier, 1997). In the present study, OTA treatment increased the serum LDL-C level. This indicated that body lipid metabolism of ducks was disturbed by OTA. Curcumin supplementation decreased the serum LDL level increased by OTA. Consistent with our results, Yuan (2016) showed that serum LDL level decreased after treatment with curcumin in hyperlipidemic rats. The susceptibility to stress of ducks is a major problem in the modern intensive poultry industry. Antioxidant ability is crucial for the health and growth performance of ducks. Our results showed that OTA largely decreased liver CAT activity, and the addition of curcumin in OTA + CUR group was significantly increased the CAT level in liver. SOD was numerically decreased, and MDA was increased, but not significantly, in the OTA group. The antioxidant enzymes CAT and SOD are considered the first line of cellular defense against oxidative damage (Ferreccio et al., 1998). It is reported that curcumin could increase the CAT mRNA expression in rat via activation of Keap1-Nrf2-ARE signaling pathway (Xie et al., 2018). MDA is one of several low-molecular-weight end products formed via the decomposition of certain primary and secondary lipid peroxidation products (Janero, 1990). MDA production alters membrane fluidity and increases membrane fragility (Chen and Yu, 1994). In our experiment, addition of curcumin was slightly decreased the liver MDA in the OTA + CUR group, even though there were no significant differences among control, OTA, and OTA + CUR groups. Curcumin could alleviate the reduction of antioxidant capacity induced by OTA. This is consistent with previous studies that reported that curcumin could alleviate the Aflatoxin B1 and OTA toxin by increasing the antioxidant capacity (El-Haleem et al., 2016, Muhammad et al., 2018). Research has demonstrated that abnormalities in lipid metabolism are associated with oxidative stress and inflammation (Rizvi et al., 2003, Chaudhari et al., 2012). It is reported that the serum LDL level was positively correlated with liver MDA levels and negatively correlated with liver glutathione peroxidase and CAT (Zheng, 2018). The results of the present study confirmed this result. These findings showed that curcumin could alleviate the oxidative injury induced by OTA.
In the present study, curcumin alleviated the decreased cecum microbiota richness and diversity induced by OTA. Its inferior systemic bioavailability (Ji and Shen, 2014) means that curcumin has a high concentration in gastrointestinal tract after oral administration; therefore, it could interact with gut microbiota to increase the bacterial diversity and richness (Zhang et al., 2017). OTA treatment increased the relative abundance of Bacteroidetes significantly, which was similar to the results of a previous study in which rats fed with deoxynivalenol had a significant increase of 0.5 log10 was observed for the Bacteroides/Prevotella group during the first 3 wk of administration (Saint-Cyr et al., 2013). Bacteroidetes, normal commensals of the gastrointestinal tract, are thought to be generally beneficial to human and animal health via their production of polysaccharides, volatile fatty acids, and other nutrients; however, when they escape this environment, they can cause substantial inflammatory pathology with significant morbidity and mortality (Lukiw, 2016). At the genus level, the relative abundance of Bacteroides in the OTA group was higher than that in other groups. A certain number of Bacteroides in the gut is benefit for the host, providing nutrients for intestinal bacterial by fermenting carbohydrates (Hooper et al., 2002), and has a role in preventing infection with Clostridium difficile (Hopkins and Macfarlane, 2002). However, Bacteroides also have negative effects sometimes if they release higher than normal concentrations of LPS, which has demonstrable toxicity (Delahooke et al., 1995). The cecum microbiota composition in the OTA + CUR group was similar to that of the CON and CUR groups, which indicated that curcumin could partially recovery the cecal microbiota disturbances caused by OTA. OTA treatment decreased the relative abundance of butyric acid–producing bacteria. Butyric acid could minimize oxidative stress–induced diabetes in rat (Kumar et al., 2010). Butyrate is known to decrease the gut mucosal pH, thus creating an acidic environment for the growth of normal commensals (Moquet et al., 2016). Studies have shown that a diet with sodium butyrate could alleviate the decreased liver antioxidant capacity induced by LPS in broilers (Ju et al., 2015). This may partially explain the decreased liver antioxidant capacity and richness of cecum microbiota induced by OTA. These results suggested that curcumin might alleviate oxidative damage by regulating the intestinal microbiota.
In addition to affecting the composition of the intestinal microbiota, OTA and curcumin also altered the metabolism of the intestinal microbiota. The intestinal microbiota interacts with numerous physiological functions and the pathogenesis of various diseases in the host through its metabolic products (Lee and Hase, 2014, Tang et al., 2017). In this study, we found that OTA altered the contents of intestinal metabolites and upregulated signaling pathways associated with oxidative stress and liver injury, including the calcium signaling pathway, nuclear factor kappa b signaling pathway, Th17 cell differentiation, the mitogen-activated protein kinase signaling pathway, the Ras signaling pathway, the chemokine signaling pathway, the T cell receptor signaling pathway, and hepatocellular carcinoma. This confirmed that OTA treatment could lead to oxidative stress, and curcumin treatment could alleviate this oxidative stress. Studies have shown intestinal microbiota are closely related to oxidative stress in host (Hollister et al., 2015, Shin et al., 2017). Collectively, curcumin might decrease oxidative injury by regulating the metabolism of the intestinal microbiota.
Sterol regulatory element–binding proteins (SREBPs) are key lipogenic transcription factors in cellular lipid metabolism and homeostasis. Among the isoforms of SREBPs, SREBP-1c is involved in fatty acid synthesis and energy storage and is reported that the mutual interaction between SREBP-1c activation and endoplasmic reticulum stress and/or inflammation is bidirectional (Shimano and Sato, 2017). Therefore, SREBP-1c activation could increase the production of reactive oxygen species and lead to oxidative stress. Studies have shown that SREBP-1c activation is usually companied by an increase in LDL-C (Sharawy et al., 2015, Zhang et al., 2018). In our results, addition of curcumin decreased the SREBP-1c mRNA levels by 20% compared with the OTA group. Many studies have suggested that curcumin could decrease SREBP-1c mRNA levels to protect the liver from lipid metabolism disruption (Liu et al., 2017, Lu et al., 2018). These studies remind us that curcumin could decrease serum LDL-C by decrease the mRNA expression of SREBP-1c. FAS is known to be regulated by SREBP-1. In this study, our data indicated that curcumin suppressed FAS mRNA expression, and this is consistent with the previous study by Um et al. (2013). Studies have shown that the liver lipid metabolism was influenced when rats were given 210 μg/kg body weight OTA (Qi et al., 2014) and decreased the expression of PPARG in human embryonic kidney (HEK) 293 cells treated with OTA (Yang et al., 2017). There was no difference of PPARG expression between CON and OTA groups, which may be due to the animal species and the OTA dosage. Research has demonstrated that OTA downregulated NRF2 expression, thus decreased the antioxidant capacity (Periasamy et al., 2014). Curcumin could alleviate the Aflatoxin B1-induced hepatotoxicity involving the NRF2/HMOX1 signaling pathway in chicken (Muhammad, 2018). In the present study, OTA and curcumin had no effect on the liver NRF2 and HMOX1 expression, which may be due to the OTA dosage or the treatment period was insufficient to activate these 2 genes expression. Further investigation would be focused on the hepatic lipid metabolism signal pathway OTA and curcumin involved.
Conclusions
Collectively, the results of the present study showed that dietary OTA negatively affected body lipid metabolism, reduced the activities of liver anti-oxidative enzymes, disrupted the function of metabolites of the cecum microbiota, increased liver SREBP-1c expression, while dietary curcumin significantly reversed these OTA-induced alterations in serum lipid metabolism parameters and enhanced liver antioxidant enzyme activities. Moreover, curcumin restored the cecum microbiota composition that had been altered by OTA. Therefore, we concluded that curcumin could alleviate liver oxidative injury by modulating the disruption to the cecum microbiota and lipid metabolism induced by OTA. Curcumin is recommended as a prophylactic measure to prevent OTA-induced hepatic oxidative injury.
Acknowledgments
This study was sponsored by the National Key Research Program (2016YFD0500509-07), National Youth Fund Project of China (31501959), China Agriculture Research System (CARS-42-15), the Special Fund for Agro-scientific Research in the Public Interest (201303143), and Senior Cooperation Research Program of China Scholarship Council with the North America and Australia.
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.psj.2019.10.041.
Contributor Information
W.C. Wang, Email: wangwence@scau.edu.cn.
L. Yang, Email: ylin@scau.edu.cn.
Supplementary data
References
- Abdel-Wahhab M.A., Aljawish A., El-Nekeety A.A., Abdel-Aziem S.H., Hassan N.S. Chitosan nanoparticles plus quercetin suppress the oxidative stress, modulate DNA fragmentation and gene expression in the kidney of rats fed ochratoxin A-contaminated diet. Food Chem. Toxicol. 2017;99:209–221. doi: 10.1016/j.fct.2016.12.002. [DOI] [PubMed] [Google Scholar]
- Adetunji M.C., Atanda O.O., Ezekiel C.N. Risk assessment of mycotoxins in stored maize grains consumed by infants and young children in Nigeria. Children. 2017;4:58. doi: 10.3390/children4070058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altafini A., Armorini S., Zaghini A., Sardi L., Roncada P. Tissue distribution of ochratoxin a in pigs after administration of two-levels contaminated diets. World Mycotoxin J. 2017;10:263–272. [Google Scholar]
- Borrelli A., Bonelli P., Tuccillo F.M., Goldfine I.D., Evans J.L., Buonaquro F.M., Mancini A. Role of gut microbiota and oxidative stress in the progression of non-alcoholic fatty liver disease to hepatocarcinoma: current and innovative therapeutic approaches. Redox Biol. 2018;15:467–479. doi: 10.1016/j.redox.2018.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhari H.S., Bhandari U., Khanna G. Preventive effect of embelin from embelia ribes on lipid metabolism and oxidative stress in high-fat diet-induced obesity in rats. Planta Med. 2012;78:651–657. doi: 10.1055/s-0031-1298379. [DOI] [PubMed] [Google Scholar]
- Chavez C., Ledoux D.R. 2008 Undergraduate Research and Creative Achievements Forum (MU) University of Missouri–Columbia. Office of Undergraduate Research; 2008. “Efficacy of curcumin in ameliorating the toxic effects of ochratoxin a and aflatoxin in young broilers”. [Google Scholar]
- Chen J.J., Yu B.P. Alterations in mitochondrial membrane fluidity by lipid peroxidation products. Free Radic. Biol. Med. 1994;17:411–418. doi: 10.1016/0891-5849(94)90167-8. [DOI] [PubMed] [Google Scholar]
- Costa J.G., Saraiva N., Guerreiro P.S., Louro H., Silva M.J., Castro M., Batinic-Haberle I., Fernandes A.S., Oliveira N.G. Ochratoxin A-induced cytotoxicity, genotoxicity and reactive oxygen species in kidney cells: an integrative approach of complementary endpoints. Food Chem. Toxicol. 2016;87:65–76. doi: 10.1016/j.fct.2015.11.018. [DOI] [PubMed] [Google Scholar]
- Cui J., Liu J., Wu S., Wang Y., Shen H., Xing L., Wang J., Yan X., Zhang X. Oxidative DNA damage is involved in ochratoxin A-induced G 2 arrest through ataxia telangiectasia-mutated (ATM) pathways in human gastric epithelium GES-1 cells in vitro. Arch. Toxicol. 2013;87:1829–1840. doi: 10.1007/s00204-013-1043-3. [DOI] [PubMed] [Google Scholar]
- Delahooke D.M., Barclay G.R., Poxton I.R. A re-appraisal of the biological activity of bacteroides LPS. J. Med. Microbiol. 1995;42:102–112. doi: 10.1099/00222615-42-2-102. [DOI] [PubMed] [Google Scholar]
- El Bahr S.M. Effect of curcumin on hepatic antioxidant enzymes activities and gene expressions in rats intoxicated with aflatoxin B1. Phytother. Res. 2015;29:134–140. doi: 10.1002/ptr.5239. [DOI] [PubMed] [Google Scholar]
- El-Haleem M.R., Kattaia A.A., El-Baset S.A., Mostafa S.H. Alleviative effect of myricetin on ochratoxin A-induced oxidative stress in rat renal cortex: histological and biochemical study. Histolo. Histopathol. 2016;31:441–451. doi: 10.14670/HH-11-689. [DOI] [PubMed] [Google Scholar]
- Feng W., Wang H., Zhang P., Gao C., Tao J., Ge Z., Zhu D., Bi Y. Modulation of gut microbiota contributes to curcumin-mediated attenuation of hepatic steatosis in rats. Biochim. Biophys. Acta Gen. Subj. 2017;1861:1801–1812. doi: 10.1016/j.bbagen.2017.03.017. [DOI] [PubMed] [Google Scholar]
- Ferreccio C., Gonzã L.P.C., Milosavjlevic S.V., Marshall G.G., Sancha A.M. Lung cancer and arsenic exposure in drinking water: a case-control study in northern Chile. Cad Saude Publica. 1998;14:193–198. doi: 10.1590/s0102-311x1998000700021. [DOI] [PubMed] [Google Scholar]
- Fink-Gremmels J. Proceedings of the workshop ochratoxin a in food: recent developments and significance. Baden, Austria, 29 June-1 July 2005. Food Addit. Contam. 2005;22:1–5. [PubMed] [Google Scholar]
- Guo M., Huang K., Chen S., Qi X., He X., Cheng W.H., Luo Y., Xia K., Xu W. Combination of metagenomics and culture-based methods to study the interaction between ochratoxin a and gut microbiota. Toxicol. Sci. 2014;141:314–323. doi: 10.1093/toxsci/kfu128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermier D. Lipoprotein metabolism and fattening in poultry. J. Nutr. 1997;127:805S. doi: 10.1093/jn/127.5.805S. [DOI] [PubMed] [Google Scholar]
- Hollister E.B., Riehle K., Luna R.A., Weidler E.M., Rubio-Gonzales M., Mistretta T.A., Raza S., Doddapaneni H.V., Metcalf G.A., Muzny D.M., Gibbs R.A., Petrosino J.F., Shulman R.J., Versalovic J. Structure and function of the healthy pre-adolescent pediatric gut microbiome. Microbiome. 2015;3:36. doi: 10.1186/s40168-015-0101-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper L.V., Midtvedt T., Gordon J.I. How host-microbial interactions shape the nutrient environment of the mammalian intestine. Annu. Rev. Nutr. 2002;22:283–307. doi: 10.1146/annurev.nutr.22.011602.092259. [DOI] [PubMed] [Google Scholar]
- Hopkins M.J., Macfarlane G.T. Changes in predominant bacterial populations in human faeces with age and with Clostridium difficile infection. J. Med. Microbiol. 2002;51:448–454. doi: 10.1099/0022-1317-51-5-448. [DOI] [PubMed] [Google Scholar]
- Hou L., Gan F., Zhou X., Zhou Y., Qian G., Liu Z., Huang K. Immunotoxicity of ochratoxin a and aflatoxin B1 in combination is associated with the nuclear factor kappa B signaling pathway in 3D4/21 cells. Chemosphere. 2018;199:718–727. doi: 10.1016/j.chemosphere.2018.02.009. [DOI] [PubMed] [Google Scholar]
- Janero D.R. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic. Biol. Med. 1990;9:515–540. doi: 10.1016/0891-5849(90)90131-2. [DOI] [PubMed] [Google Scholar]
- Ji H.F., Shen L. Can improving bioavailability improve the bioactivity of curcumin? Trends Pharmacol. Sci. 2014;35:265–266. doi: 10.1016/j.tips.2014.04.001. [DOI] [PubMed] [Google Scholar]
- Ju T.T., Guo X.Y., Sui J.J., Xiao X., Zhan X.A. Effect of different type of sodium butyrate on serum biochemical indies, antioxidant and anti-inflammatory function of broilers challenged with lipopolysaccharide. Chinese Anim. Nutr. 2015;27:3146–3154. [Google Scholar]
- Juszkiewicz T., Piskorska-Pliszczynska J., Wisniewska H. Pages 122–125 in Mycotoxins and Phycotoxins, 5th International IUPAC Symposium on Mycotoxins and Phytotoxins. Technical University; Vienna, Austria: 1982. Ochratoxin A in laying hens: tissue deposition and passage into eggs. [Google Scholar]
- Kumar A.P., Chougala M., Nandini C.D., Salimath P.V. Effect of butyric acid supplementation on serum and renal antioxidant enzyme activities in streptozotocin-induced diabetic rats. J. Food Biochem. 2010;34:15–30. [Google Scholar]
- Lee W.J., Hase K. Gut microbiota-generated metabolites in animal health and disease. Nat. Chem. Biol. 2014;10:416–424. doi: 10.1038/nchembio.1535. [DOI] [PubMed] [Google Scholar]
- Liu Z., Cui C., Xu P., Dang R., Cai H., Liao D., Yang M., Feng Q., Yan X., Jiang P. Curcumin activates AMPK pathway and regulates lipid metabolism in rats following prolonged clozapine exposure. Front. Neurosci. 2017;11:558–565. doi: 10.3389/fnins.2017.00558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liuzzi V.C., Fanelli F., Tristezza M., Haidukowski M., Picardi E., Manzari C., Lionetti C., Grieco F., Logrieco A.F., Thon M.R., Pesole G., Mulè G. Transcriptional analysis of Acinetobacter sp. Neg1 capable of degrading ochratoxin A. Front. Microbiol. 2017;7:2162. doi: 10.3389/fmicb.2016.02162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu N., Li X., Yu J., Li Y., Zhong X. Curcumin sttenuates lipopolysaccharide-induced hepatic lipid metabolism disorder bymodification of m 6 a RNA methylation in piglets. Lipids. 2018;53:53–63. doi: 10.1002/lipd.12023. [DOI] [PubMed] [Google Scholar]
- Lukiw W.J. Bacteroides fragilis lipopolysaccharide and inflammatory signaling in Alzheimer’s disease. Front. Microbiol. 2016;7:1544. doi: 10.3389/fmicb.2016.01544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J.J., Zhang Y., Sun H., Wei J.T., Khalil M.M., Wang Y.W., Dai J.F., Zhang N.Y., Qi D.S., Sun L.H. The Response of Glandular Gastric Transcriptome to T-2 Toxin in Chicks. Food Chem. Toxicol. 2019;132:110658. doi: 10.1016/j.fct.2019.110658. [DOI] [PubMed] [Google Scholar]
- Malir F., Ostry V., Novotna E. Toxicity of the mycotoxin ochratoxin a in the light of recent data. Toxin Rev. 2013;32:19–33. [Google Scholar]
- Martin O.C.B., Olier M., Ellero-Simatos S., Naud N., Dupuy J., Huc L., Taché S., Graillot V., Levêque M. Haem iron reshapes colonic luminal environment: impact on mucosal homeostasis and microbiome through aldehyde formation. Microbiome. 2019;7:72–89. doi: 10.1186/s40168-019-0685-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata A.T., Ferreira J.P., Oliveira B.R., Batoréu M.C., Crespo M.B., Pereira V.J., Bronze M.R. Bottled water: analysis of mycotoxins by LC–MS/MS. Food Chem. 2015;176:455–464. doi: 10.1016/j.foodchem.2014.12.088. [DOI] [PubMed] [Google Scholar]
- Moquet P., Onrust L., Van Immerseel F., Ducatelle R., Hendriks W.H., Kwakkel R. Importance of release location on the mode of action of butyrate derivatives in the avian gastrointestinal tract. Worlds Poul. Sci. J. 2016;72:61–80. [Google Scholar]
- Muhammad I., Wang H., Sun X., Wang X., Han M., Lu Z., Cheng P., Hussain M.A., Zhang X. Dual role of dietary curcumin through attenuating AFB1-induced oxidative stress and liver injury via modulating liver phase-I and phase-II enzymes involved in AFB1 bioactivation and detoxification. Front. Pharmacol. 2018;9:554. doi: 10.3389/fphar.2018.00554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhammad I.W.X.L. Curcumin confers hepatoprotection against AFB 1-induced toxicity via activating autophagy and ameliorating inflammation involving Nrf2/HO-1 signaling pathway. Mol. Biol. Rep. 2018;45:1775–1785. doi: 10.1007/s11033-018-4323-4. [DOI] [PubMed] [Google Scholar]
- Oršolić N., Jazvinšćak Jembrek M., Terzić S. Honey and quercetin reduce ochratoxin A-induced DNA damage in the liver and the kidney through the modulation of intestinal microflora. Food Agric. Immunol. 2017;28:812–833. [Google Scholar]
- Osselaere A., Li S.J., De Bock L., Devreese M., Goossens J., Vandenbroucke V., Van Bocxlaer J., Boussery K., Pasmans F., Martel A., De Backer P., Croubels S. Toxic effects of dietary exposure to T-2 toxin on intestinal and hepatic biotransformation enzymes and drug transporter systems in broiler chickens. Food Chem. Toxicol. 2013;55:150–155. doi: 10.1016/j.fct.2012.12.055. [DOI] [PubMed] [Google Scholar]
- Ostry V., Malir F., Ruprich J. Producers and important dietary sources of ochratoxin a and citrinin. Toxins. 2013;5:1574–1586. doi: 10.3390/toxins5091574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peckham J.C., Doupnik B., Jones O.H. Acute toxicity of ochratoxins a and B in chicks. Appl. Environ. Microbiol. 1971;21:492–494. doi: 10.1128/am.21.3.492-494.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Periasamy R., Kalal I.G., Krishnaswamy R., Viswanadha V. Quercetin protects human peripheral blood mononuclear cells from OTA-induced oxidative stress, genotoxicity, and inflammation. Environ. Toxicol. 2016;31:855–865. doi: 10.1002/tox.22096. [DOI] [PubMed] [Google Scholar]
- Periasamy R., Rajashree K., Viswanadha Vijaya P. Quercetin modulates OTA-induced oxidative stress and redox signalling in HepG2 cells - up regulation of Nrf2 expression and down regulation of NF-κB and COX-2. Biochim. Biophys. Acta. 2014;1840:681–692. doi: 10.1016/j.bbagen.2013.10.024. [DOI] [PubMed] [Google Scholar]
- Polovic M., Dittmar S., Hennemeier I., Humpf H., Seliger B., Fornara P., Theil G., Azinovic P., Nolze A., Köhn M., Schwerdt G., Gekle M. Identification of a novel lncRNA induced by the nephrotoxin ochratoxin a and expressed in human renal tumor tissue. Cell. Mol. Life Sci. 2018;75:2241–2256. doi: 10.1007/s00018-017-2731-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prior M.G., Sisodia C.S., Neil J.B. Acute oral ochratoxicosis in day-old white leghorns, turkeys and Japanese quail. Poul. Sci. 1976;55:786–790. doi: 10.3382/ps.0550786. [DOI] [PubMed] [Google Scholar]
- Purchase I., Theron J.J. The acute toxicity of ochratoxin a to rats. Food Cosmet. Toxicol. 1968;6:479–483. doi: 10.1016/0015-6264(68)90138-7. [DOI] [PubMed] [Google Scholar]
- Qiao Y., Sun J., Ding Y., Le G., Shi Y. Alterations of the gut microbiota in high-fat diet mice is strongly linked to oxidative stress. Appl. Microbiol. Biotechnol. 2013;97:1689–1697. doi: 10.1007/s00253-012-4323-6. [DOI] [PubMed] [Google Scholar]
- Qi X., Yang X., Chen S., He X., Dweep H., Guo M., Cheng W., Xu W., Luo Y., Gretz N., Dai Q., Huang K. Ochratoxin A induced early hepatotoxicity: new mechanistic insights from microRNA, mRNA and proteomic profiling studies. Sci. Rep. 2014;4:5163. [Google Scholar]
- Ramyaa P., Padma V.V. Quercetin modulates OTA-induced oxidative stress and redox signalling in HepG2 cells—up regulation of Nrf2 expression and down regulation of NF-κB and COX-2. Biochim. Biophys. Acta Gen. Subj. 2014;1840:681–692. doi: 10.1016/j.bbagen.2013.10.024. [DOI] [PubMed] [Google Scholar]
- Rangsaz N., Ahangaran M.G. Evaluation of turmeric extract on performance indices impressed by induced aflatoxicosis in broiler chickens. Toxicol. Ind. Health. 2011;27:956–960. doi: 10.1177/0748233711401262. [DOI] [PubMed] [Google Scholar]
- Rizvi F., Iftikhar M., George J.P. Beneficial effects of fish liver preparations of sea bass (Lates calcarifer) versus gemfibrozil in high fat diet-induced lipid-intolerant rats. J. Med. Food. 2003;6:123–128. doi: 10.1089/109662003322233521. [DOI] [PubMed] [Google Scholar]
- Rojo D., Gosalbes M.J., Ferrari R., Pérez-Cobas A.E., Hernández E., Oltra R., Buesa J., Latorre A., Barbas C., Ferrer M., Moya A. Clostridium difficile heterogeneously impacts intestinal community architecture but drives stable metabolome responses. ISME J. 2015;9:2206–2220. doi: 10.1038/ismej.2015.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan D., Wang W.C., Lin C.X., Fouad A.M., Chen W., Xia W.G., Wang S.Q., Luo X., Zhang W.H., Yan S.J., Zheng C.T., Yang L. Effects of curcumin on performance, antioxidation, intestinal barrier and mitochondrial function in ducks fed corn contaminated with ochratoxin A. Animal. 2019;13:42–52. doi: 10.1017/S1751731118000678. [DOI] [PubMed] [Google Scholar]
- Saint-Cyr M.J., Perrin-Guyomard A., Houée P., Rolland J., Laurentie M. Evaluation of an oral subchronic exposure of deoxynivalenol on the composition of human gut microbiota in a model of human microbiota-associated rats. PLoS One. 2013;8:e80578. doi: 10.1371/journal.pone.0080578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharawy M.H., Awady M.S.E., Megahed N., Gameil N.M. The ergogenic supplement [beta]-hydroxy-[beta]-methylbutyrate (HMB) attenuates insulin resistance through suppressing GLUT-2 in rat liver. Can. J. Physiol. Pharmacol. 2015;94:1. doi: 10.1139/cjpp-2015-0385. [DOI] [PubMed] [Google Scholar]
- Shen L., Liu L., Ji H. Regulative effects of curcumin spice administration on gut microbiota and its pharmacological implications. Food Nutr. Res. 2017;61:1361780. doi: 10.1080/16546628.2017.1361780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim W., Ha K., Kim M., Kim J., Chung D. Evaluation of the transfer rate of ochratoxin a to decoctions of herbal medicines. Food Sci. Biotechnol. 2014;23:2103–2108. [Google Scholar]
- Shimano H., Sato R. SREBP-regulated lipid metabolism: convergent physiology [mdash] divergent pathophysiology. Nat. Rev. Endocrinol. 2017;13:710–730. doi: 10.1038/nrendo.2017.91. [DOI] [PubMed] [Google Scholar]
- Shin N.R., Bose S., Wang J.H., Ansari A., Lim S.K., Chin Y.W., Choi H.S., Kim H. Flos lonicera combined with metformin ameliorates hepatosteatosis and glucose intolerance in association with gut microbiota modulation. Front. Microbiol. 2017;8:2271. doi: 10.3389/fmicb.2017.02271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobral M.M.C., Faria M.A., Cunha S.C., Ferreira I.M. Toxicological interactions between mycotoxins from ubiquitous fungi: impact on hepatic and intestinal human epithelial cells. Chemosphere. 2018;202:538–548. doi: 10.1016/j.chemosphere.2018.03.122. [DOI] [PubMed] [Google Scholar]
- Solfrizzo M., Piemontese L., Gambacorta L., Zivoli R., Longobardi F. Food coloring agents and plant food supplements derived from Vitis vinifera: a new source of human exposure to ochratoxin A. J. Agric. Food Chem. 2015;63:3609–3614. doi: 10.1021/acs.jafc.5b00326. [DOI] [PubMed] [Google Scholar]
- Stoev S.D. Food security and foodborne mycotoxicoses, risk assessment, preventive measures, and underestimated hazard of masked mycotoxins or joint mycotoxin interaction. Food Toxicol. 2016;9:169–199. doi: 10.1016/j.etap.2015.01.022. [DOI] [PubMed] [Google Scholar]
- Tang W.H., Kitai T., Hazen S.L. Gut microbiota in cardiovascular health and disease. Circ. Res. 2017;120:1183–1196. doi: 10.1161/CIRCRESAHA.117.309715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Um M.Y., Hwang K.H., Ahn J., Ha T.Y. Curcumin attenuates diet-induced hepatic steatosis by activating AMP-activated protein kinase. Basic Clin. Pharmacol. Toxicol. 2013;113:152–157. doi: 10.1111/bcpt.12076. [DOI] [PubMed] [Google Scholar]
- Van der Merwe K.J., Steyn P.S., Fourie L., Scott D.B., Theron J.J. Ochratoxin A, a toxic metabolite produced by Aspergillus ochraceus Wilh. Nature. 1965;205:1112. doi: 10.1038/2051112a0. [DOI] [PubMed] [Google Scholar]
- Vettorazzi A., de Trocóniz I.F., González-Peñas E., Arbillaga L., Corcuera L., Gil A.G., de Cerain A.L. Kidney and liver distribution of ochratoxin A in male and female F344 rats. Food Chem. Toxicol. 2011;49:1935–1942. doi: 10.1016/j.fct.2011.04.021. [DOI] [PubMed] [Google Scholar]
- Vettorazzi A., Pastor L., Guruceaga E., de Cerain A.L. Sex-dependent gene expression after ochratoxin a insult in F344 rat kidney. Food Chem. Toxicol. 2019;123:337–348. doi: 10.1016/j.fct.2018.10.057. [DOI] [PubMed] [Google Scholar]
- Xie Z., Wu B., Shen G., Li X., Wu Q. Curcumin alleviates liver oxidative stress in type 1 diabetic rats. Mol. Med. Rep. 2018;17:103–108. doi: 10.3892/mmr.2017.7911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Wang H., Huang H.,C., He X., Xu W., Luo Y., Huang K. Zinc enhances the cellular energy supply to improve cell motility and restore impaired energetic metabolism in a toxic environment induced by OTA. Sci. Rep. 2017;7:14669. doi: 10.1038/s41598-017-14868-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y.Y. Hebei Medical University; Shijiazhuang: 2016. The Effect of Curcumin Compounds on the Lipid Metabolism and its Possible Mechanism on Acute Gyperlipidemia Rats. Master Diss. [Google Scholar]
- Zhang N.Y., Qi M., Zhao L., Zhu M., Guo J., Liu J., Gu C.Q., Rajput S.A., Krumm C.S., Qi D.S., Sun L.H. Curcumin prevents aflatoxin B1 hepatoxicity by inhibition of cytochrome P450 isozymes in chick liver. Toxins. 2016;8:327. doi: 10.3390/toxins8110327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Qian Z.Y., Zhou P.H., Zhou X.L., Zhang D.L., He N., Zhang J., Liu Y.H., Gu Q. Effects of oral selenium and magnesium co-supplementation on lipid metabolism, antioxidative status, histopathological lesions, and related gene expression in rats fed a high-fat diet. Lipids Health Dis. 2018;17:165–176. doi: 10.1186/s12944-018-0815-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Chen Y., Xiang L., Wang Z., Xiao G.G., Hu J. Effect of curcumin on the diversity of gut microbiota in ovariectomized rats. Nutrients. 2017;9:1146. doi: 10.3390/nu9101146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L.C. Shenyang Medical College; Shengyang: 2018. Effects of diethylhexyl phthalate and bisphenol a on lipid metabolism and the relationship with oxidative stress of liver in rat. Master Diss. [Google Scholar]
- Zorofchian Moghadamtousi S., Abdul Kadir H., Hassandarvish P., Tajik H., Abubakar S., Zandi K. A review on antibacterial, antiviral, and antifungal activity of curcumin. Biomed. Res. Int. 2014;2014:186864. doi: 10.1155/2014/186864. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.