Abstract
The objective of this study was to determine the effect of age at photostimulation on sexual maturity and performance of layer breeders. A total of 192 fourteen-wk-old White Leghorn (WL) breeder hens were randomly allocated to 4 treatments of 48 birds each, with 2 replicates per treatment. The birds were photostimulated at 16 (PS16), 18 (PS18), 20 (PS20), and 22 (PS22) wk of age. Four birds per treatment were randomly selected to evaluate sexual organ development at 1 D before photostimulation and 2, 4, and 6 wk after photostimulation. The ovary weight, large yellow follicles number (LYF), oviduct weight, and oviduct length of PS18 increased sharply after photostimulation. Conversely, the increase in PS16 was not observed until 2 wk after photostimulation. There was no difference in age at sexual maturity between treatments (P > 0.05). The PS16 had the longest interval (28 D) from photostimulation to 5% egg production, while PS22 reached 5% egg production 7 D before photostimulation. The PS22 had lower peak production (P = 0.02) and less egg production (P = 0.02) than other treatments. The PS16 had more broken and abnormal eggs (P = 0.01) and lower hatchability (P = 0.04) than other treatments. In conclusion, photostimulation at 16 and 22 wk of age decreases hatchability and egg production, respectively, and photostimulation at 18 wk is appreciated for the WL breeder hens.
Keywords: photostimulation age, layer breeder, sexual maturity, sexual organ, laying performance
INTRODUCTION
Optimization of chicken reproductive fitness is based on the management of the timing of sexual maturation throughout both the rearing and laying periods. The process of sexual maturation in the breeder hens represents a major shift in its physiological status (Johnson et al., 2009). Chickens are photoperiodic and respond to long photoperiods by activation of the reproductive axis. Once the birds have reached an adequate age, body weight (BW), and frame size, then sexual maturation can be hastened by providing photostimulation. The age at which chicks sexually mature has a direct influence on their laying performance, and genetic stocks have optimal ages at which they reach sexual maturity to produce the maximum possible egg mass (Lewis et al., 2004; Cui et al., 2019; Farghly et al., 2019). Critical age is the point at which the hypothalamo–pituitary–gonadal axis is activated by a photostimulatory cue. The result is the release of gonadotropin-releasing hormone (GnRH) by GnRH neurons. The GnRH stimulates gonadotropin secretion from the anterior pituitary (Sharp, 1993), which supports regulated production of gonadal steroids (Renema et al., 2007a).
The research suggested that there is a minimum age for the ability to respond to photostimulation and sexually mature (Katanbaf et al., 1989). The minimum age after which broiler breeders can be photosensitive is 10 wk (Lewis et al., 2007). Before this age, the onset of lay does not advance when hens are photostimulated and hens respond as if they are maintained on long days from hatch (van der Klein et al., 2018a). There are many researches on broiler breeders showing that photostimulation at 17 or 18 wk of age may result in earlier sexual maturation (Renema et al., 2007a; Pishnamazi et al., 2014). Such manipulation of Hubbard and Ross hens was more likely to produce smaller-sized but a greater number of eggs (Zuidhof et al., 2007). It is known that pullets that are underweight at photostimulation subsequently exhibit lower egg production (van der Klein et al., 2018a). If most of the hens in a flock are physiologically mature at photostimulation, they will commence lay within a narrow range in time and hence go through production as a more uniform group (Robinson et al., 1996). Later, photostimulation ensures that more birds are physically mature enough to respond to a photostimulatory cue, resulting in a more uniform production (Hocking, 1996; Robinson et al., 1996). There are benefits to delaying photostimulation to 22 or 23 wk of age on broiler breeders. A concern with delayed photostimulation is that this may narrow the length of the productive egg-laying period. However, few studies have been performed in layer breeders. Moreover, previous studies focused more on egg production, while how sexual organs development response to photostimulation was missing.
The objective of the current study was to determine the effects of 4 different photostimulation ages (16, 18, 20, and 22 wk) on reproductive performance of White Leghorn (WL) breeder hens including sexual maturity, egg-laying production, eggshell quality, and fertility. This could inform appropriate lighting management of layer breeders.
MATERIALS AND METHODS
Ethics Statement
This study was performed in accordance with local ethical guidelines and met the requirements of the institutional animal care and use committee.
Experimental Design and Birds
A single factor experiment was conducted with 4 treatments representing age at photostimulation. Birds were photostimulated on the first day of 16, 18, 20, and 22 wk of age (PS16, PS18, PS20, and PS22).
A total of 192 WL breeder hens of 14 wk of similar BW (average ± 10%) were acquired from the Institute of Animal Science of the Chinese Academy of Agriculture Sciences and randomly allocated to 1 of the 4 treatments. There were 2 rooms (replicates) per treatment, and each room was included 8 cages and 3 birds per cage. Three birds were kept per cage. Feed and water were provided according to chicken standard feeding (Ministry of Agriculture, 2004). From 14 wk to age at 5% egg production, birds were fed commercial corn- and soybean-based diet with 16.00% crude protein (CP), 11.70 MJ/kg metabolizable energy (ME), and 1.00% calcium. From age at 5% egg production to 51 wk, birds were fed 16.50% CP, 11.50 MJ/kg ME, and 3.50% calcium.
The chickens were housed in light-controlled facility. In the first week of photostimulation, the light intensity was increased from 5–10 lx to 80 lx. From the second to the fourth week, the lighting regimen was changed from 8L:16D to 14L:10D by increasing day length during the second week by 4 h and then by 1 h during each of the third and fourth weeks. Light intensity was measured at the birds' eye level with the photoreceptor sensor of a light meter (model: DT-1301; Shenzhen Everbest Machinery Industry Co. Ltd., China). LED lamps were used with bulbs suspended 2 m above the ground, and the replicate was light-tight. Rooms had independent temperature controls, and all rooms were held at 18 to 24°C for the duration of the study.
Estradiol Hormone
Eight birds from each replicate were randomly selected and bled via the wing vein to collect 1 mL blood samples at the 4 time points: 1 D before photostimulation, and 2, 4, and 6 wk after photostimulation. All blood samples were immediately centrifuged at 3,000 × g for 2 min at 4 to 8°C to collect the serum. The serum samples were stored at −20°C. The estradiol (E2) concentration was assayed straight with a commercial kit (label: B05PZB, Beijing North Institute of Biological Technology Co. Ltd., China).
Sexual Organ Development
Two birds from 2 random cages of each replicate were selected to sacrifice using direct cervical dislocation, and the development of their sexual organs was characterized at 4 time points mentioned above. The oviduct (emptied of contents) and ovary were weighed and photographed to measure oviduct length by Digimizer 5.3.4 MedCalc software (Ostend, Belgium). Ovarian follicles greater than 10 mm in diameter were classified as large yellow follicles (LYF), and the number was recorded (Robinson and Etches, 1986). The weights of the oviduct and ovary were calculated as a percentage of BW.
Sexual Maturity
Sexual maturity was defined as age at 5% egg production, and at this age the birds' BW was recorded for each replicate. Age at 5 and 50% egg production was recorded for each replicate, and interval time between photostimulation to 5% egg production, and between 5 and 50% egg production, was calculated accordingly.
Sexual Organ at Peak Laying
Two birds from 2 random cages of each replicate were sacrificed at 28 wk of age (peak laying) to measure ovary and oviduct weight as a percentage of BW, and to determine the number of LYF and oviduct length.
Egg Production Performance
The numbers of eggs in each replicate were recorded daily until the end of 51 wk of age, including number of defective eggs. Mean egg production per replicate was expressed on a surviving-bird basis. An egg production curve model (Yang et al., 1989) was used to fit the egg production curve. This model is described as:
The biological interpretations of the relevant variables are as follows: yt = laying rate (%), t = age (wk),a = a scale variable, b = rate of decrease in laying ability, c = the reciprocal indicator of the variation in sexual maturity, and d = mean age of sexual maturity.
Defective eggs, calculated as percentages of total egg number, were classified as broken, soft-shelled, and other abnormal eggs.
Fertility and Hatchability
Hens were inseminated once with 20 μL of pooled semen from WL males at 50 wk of age. All eggs were collected for 7 D after insemination for incubation to calculate fertility, hatchability of setting eggs, and hatchability of fertile eggs. The setting egg number was around 30 for each replicate.
Eggshell Quality
On the third day after reaching sexual maturity, peak laying period, and 43 wk of age, eggs were collected for each replicate, marked, and placed in cool storage for 3 to 5 D at 15 to 18°C. Egg and eggshell weight were recorded; eggshell strength was measured by a detector (model: EFR-01; ORKA. Israel); and eggshell thickness was measured using an echo-meter (model: ESTG-1; ORKA. Israel).
Statistical Analysis
Sexual maturity and reproductive performance data were analyzed using GLM procedure of SAS (SAS 9.1, SAS Institute Inc., Cary, NC). The main effect of the model is photostimulation age, and the percentage was arcsine transformed before analysis. Significance was designated as P < 0.05. Means were compared by Student–Newman–Keuls multiple-range tests when a significant difference was detected.
RESULTS AND DISCUSSION
Estradiol Hormone
Serum E2 is an important hormone produced in response to photostimulation, and preferentially directs nutrients to the ovary (Renema et al., 1999). Increase of serum E2 of PS16, PS18, and PS20 was detected in immediate response to photostimulation, and PS20 and PS22 decreased at 26 and 28 wk of age (Figure 1). The result was similar to Bacon et al. (2002), who found that E2 increased from 4 to 6 D after photostimulation and then did not change much during the egg production period in turkey hens. In our previous study using Beijing-You chicken breeders, serum E2 of PS18, PS20, and PS22 also increased sharply from 0 to 6 wk after photostimulation (Shi et al., 2019). Therefore, photostimulation maybe an important cue for E2 secretion. However, the study in layer hens suggested that E2 levels of Lohmann LSL-Lite breed peaked at 16 wk of age, 2 wk before photostimulation (Baxter and Bédécarrats, 2019), and the study in Beijing-You breed suggested that increase of serum E2 in PS16 was relatively slow (Shi et al., 2019). Therefore, both age and photostimulation are important for the secretion of E2. Age may determine the E2 profile, while the photostimulation at a right age may also accelerate the increase of E2.
Figure 1.

Change in serum estradiol concentration of White Leghorn breeder hens photostimulated at different ages. Note: “PS16,” “PS18,” “PS20,” “PS22,” and “None” indicated that the hens were photostimulated at 16, 18, 20, 22 wk of age, and before photostimulation of each treatment, respectively.
Sexual Organ Development
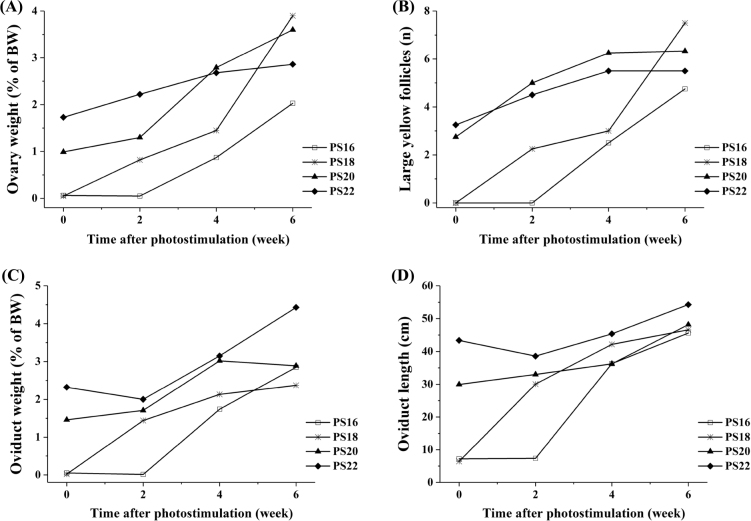
The ovary weight and LYF number of PS18 increased sharply from 0 to 6 wk after photostimulation. Conversely, the increase in PS16 was not observed until 2 wk after photostimulation. Before photostimulation, the ovary weight in PS22 was 28-fold higher than that in PS16 and PS18 (Figure 2 A). The LYF number of PS18 increased sharply after photostimulation, while that of PS20 and PS22 hens increased before photostimulation. The LYF number was seen in the ovary of PS16 hens at 2 wk after photostimulation. The LYF number in PS20 and PS22 increased before photostimulation (Figure 2 B). The 17 wk of age may be a critical age for WL that sexual organs and LYF number spontaneously increased regardless of photostimulation, and the peak of ovary weight and LYF number decreased with delaying of photostimulation. This result was substantially different from native chicken breeders (Shi et al., 2019) and broiler breeders that delaying photostimulation to 20 or 23 wk of age is the benefit for production (Robinson et al., 1996; Renema et al., 2001; Pishnamazi et al., 2014; Shi et al., 2017). Broiler breeders are kept on a strict level of feed restriction to manage their BW and frame size, and also restrict the growth of sexual gonad during rearing period (Renema et al., 2007b). Hens with a high BW started laying 34 D earlier than hens on the standard BW for Ross 708 broiler breeders (van der Klein et al., 2018b). In marked contrast, layer breeders, which have lighter BW and reach sexual maturity at a younger age, are reared on rearing period and facilitate to synchronize sexual maturation and physical maturation. As a direct correlation between age at first egg and BW was identified, metabolic cues most likely served as a primary trigger to initiate sexual maturation prior to photostimulation in Lohmann LSL-Lite hens (Baxter and Bédécarrats, 2019). In the current study, the sexual organs of WL increased initiatively at 17 wk of age. The results are different from native chicken breeders that the sexual organs grow spontaneous at 21 wk of age (Shi et al., 2019). The peak of ovary weight and LYF number decreased with delaying of photostimulation (Figure 2 A and B). The LYF number found in high-producing breeder hens at sexual maturity ranged from 7 to 8 (Renema and Robinson, 2004). During sexual maturation, birds show high sensitivity to estrogen, and surplus nutrients are guided under the influence of estrogen to the liver and ultimately to the ovarian hierarchy (Renema et al., 2007a). In the current study, the LYF number of PS18 increased steadily after photostimulaiton, and reached 7.5 at 6 wk after photostimulation. The LYF numbers in PS20 and PS22 were 6.3 and 5.5 at sexual maturation, respectively. Typically, hens that have LYF of less than 7.0 are more prone to problems with persistency than hens with more LYF and hens that have more than 8.0 LYF typically exhibit problems associated with erratic oviposition and defective egg syndrome (Renema et al., 2007a). The data indicated that PS18 had normal LYF number, and may present a better egg production.
Figure 2.
Change in ovary characteristics of White Leghorn breeder hens photostimulated at different ages. Note: “PS16,” “PS18,” “PS20,” and “PS22” indicated that the hens were photostimulated at 16, 18, 20, and 22 wk of age, respectively. “0,” “2,” “4,” and “6” on the x axis means 1 D before photostimulation, 2, 4, and 6 wk after photostimulation, respectively.
The oviduct weight and oviduct length of PS18 increased upon photostimulation, but that in PS16 did not increase until 2 wk after. The oviduct weight and oviduct length in PS22 were 46-fold and 6-fold higher than in PS16, before photostimulation (Figure 2 C and D). The results suggest that sexual organs can develop without photostimulation. However, the development could be accelerated by the photostimulation at an optimal age. The ovary and oviduct weight at laying peak were not different between groups (Table 2). This suggests that the effect of photostimulation on gonad development can be substantial during sexual maturity, but disappears with aging. The results were similar to our previous study using Beijing-You chicken breeders (Shi et al., 2019).
Table 2.
Effect of age at photostimulation on ovary and oviduct characteristics of White Leghorn breeders at 28 wk of age.
| Item | Age at photostimulation (wk) |
SEM | P-value | |||
|---|---|---|---|---|---|---|
| 16 | 18 | 20 | 22 | |||
| Ovary weight, % | 3.03 | 2.92 | 2.98 | 2.86 | 0.05 | 0.96 |
| Large yellow follicles, n | 5.25 | 6.00 | 5.25 | 5.50 | 0.21 | 0.66 |
| Oviduct weight, % | 4.10 | 3.45 | 3.64 | 4.43 | 0.22 | 0.58 |
| Oviduct length, cm | 49.46 | 49.81 | 44.22 | 53.82 | 1.53 | 0.07 |
Data are the mean of 2 replicates with 2 birds each.
Sexual Maturity
Table 1 presents the effect of age at photostimulation on the sexual maturity of WL breeders. There was no difference in age at sexual maturity between treatments (P > 0.05). In contrast, results in the previous research studies showed that delaying photostimulation from 16 to 22 wk of age delayed sexual maturity in native chicken breeders (Shi et al., 2019) and broiler breeders (Renema et al., 2007a; Pishnamazi et al., 2014; van der Klein et al., 2018a). The PS20 and PS22 had the shortest interval from photostimulation to 5% egg production, while PS22 reached 5% egg production 7 D before photostimulation. This is in agreement with the findings of Pishnamazi et al. (2014) that delaying photostimulation narrowed the interval, but different from the study in broiler breeders showing that the interval time of PS22 was short (Renema et al., 2007a). The age at peak egg production, the intervals from 5% to 50% egg production, and from 5% egg production to peak laying were not affected by age at photostimulation (P > 0.05), but PS22 had lower rate of peak production than other treatments (P = 0.02). However, PS20 and PS22 had the shortest interval from photostimulation to 5% egg production.
Table 1.
Effect of age at photostimulation on sexual maturity variables of White Leghorn breeder hens.
| Item | Age at photostimulation (wk) |
SEM | P-value | |||
|---|---|---|---|---|---|---|
| 16 | 18 | 20 | 22 | |||
| BW at sexual maturity, g | 1372.39 | 1381.40 | 1438.77 | 1454.57 | 16.15 | 0.18 |
| Age at 5% egg production, D | 134.00 | 132.50 | 141.00 | 141.00 | 1.85 | 0.21 |
| Age at 50% egg production, D | 149.50 | 148.50 | 154.50 | 154.00 | 1.32 | 0.28 |
| Photostimulation to 5% egg production interval, D | 28.00a | 12.50b | 7.00b | −7.00c | 4.86 | 0.01 |
| 5% to 50% egg production interval, D | 15.50 | 16.00 | 13.50 | 13.00 | 1.07 | 0.79 |
| Age at peak of laying, D | 176.00 | 179.50 | 186.50 | 179.50 | 4.17 | 0.90 |
| Peak of laying, % | 91.96a | 93.37a | 88.91a | 78.81b | 2.26 | 0.02 |
Data are the mean of 2 replicates.
1Age at sexual maturity was defined as age at 5% egg production.
Values within a row with different superscripts differ significantly at P < 0.05.
Egg Production
The effect of age at photostimulation on egg production performance is presented in Table 3. The PS22 had less egg production than other treatments (P = 0.02). The PS16 had higher number of broken eggs than other groups (P = 0.01). There was a tendency for PS16 birds to have more abnormal egg than other groups (P = 0.08). The broken eggs and abnormal eggs of PS16 occurred mostly at sexual maturity. The result was similar to Zuidhof et al. (2007), who found that the early production advantage of the early photostimulation treatment was lost when settable eggs were considered. E2 is implicated in shell formation indirectly by acting on organs involved in calcium metabolism and an injection of E2 increases the circulating levels of calcium in plasma (Bar et al., 1996). The poor E2 profile of PS16 at sexual maturity may be a reason of high incidence of broken eggs and abnormal eggs by affecting the shell formation. Age at photostimulation had no effect on the number of soft-shelled eggs (P > 0.05).
Table 3.
Effect of age at photostimulation on egg production of White Leghorn breeder hens throughout laying period until the end of 51 wk of age.
| Item | Age at photostimulation (wk) |
SEM | P-value | |||
|---|---|---|---|---|---|---|
| 16 | 18 | 20 | 22 | |||
| Egg number1, n | 161.18a | 162.64a | 145.53a | 133.54b | 4.80 | 0.02 |
| Broken eggs2, % | 1.84a | 0.96b | 1.16b | 1.31b | 0.13 | 0.01 |
| Soft-shelled eggs, % | 0.37 | 0.28 | 0.12 | 0.20 | 0.06 | 0.55 |
| Other abnormal eggs, % | 2.36 | 1.16 | 1.30 | 1.38 | 0.20 | 0.08 |
Data are the mean of 2 replicates.
Values within a row with different superscripts differ significantly at P < 0.05.
Mean eggs per bird per day × days between age at first egg and 51 wk of age.
Broken eggs include broken-shelled eggs, cracked eggs, holed eggs, and entirely broken eggs.
Fitting of egg-laying rate curves is shown in Figure 3. The fitting of egg-laying rate curves of 4 treatments is as follows:
Figure 3.
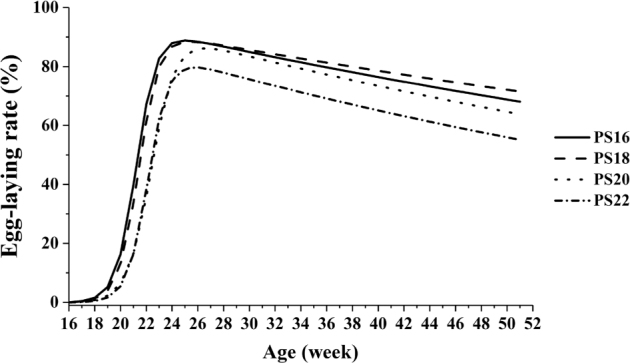
Fitting of egg-laying rate curves of White Leghorn breeder hens photostimulated at different ages. Note: “PS16,” “PS18,” “PS20,” and “PS22” indicated that the hens were photostimulated at 16, 18, 20, and 22 wk of age, respectively.
In the current study, the laying rate of PS18 decreased slowly after peak laying, and PS22 had lower rate of peak production and egg production than other treatments. This situation may be related with physiological characteristics of layer breeders that sexual organs increased initiatively at 17 wk of age. Photostimulation at 22 wk of age was extreme late for layer breeder. This is different from the observations in broiler breeders of Starbro (Robinson et al., 1996), Ross (Pishnamazi et al., 2014), and Hubbard (Zuidhof et al., 2007). If most of the hens in a flock are physiologically mature at photostimulation, they will commence lay within a narrow range in time and hence go through production as a more uniform group (Robinson et al., 1996). Based on the above, the light-sensitive period of layer chickens may be narrower and earlier than native chicken and broiler breeders.
Fertility, Hatchability, and Eggshell Quality
As shown in Table 4, the fertility and hatchability were not affected by age at photostimulation (P > 0.05), but PS16 had lower hatchability than other groups (P = 0.04).
Table 4.
Effect of age at photostimulation on fertility and hatching efficiency of White Leghorn breeder hens at 50 wk of age.
| Item | Age at photostimulation (wk) |
SEM | P-value | |||
|---|---|---|---|---|---|---|
| 16 | 18 | 20 | 22 | |||
| Fertility, % | 97.83 | 98.75 | 97.62 | 96.00 | 1.07 | 0.90 |
| Hatchability of fertile eggs, % | 71.33 | 88.94 | 84.66 | 90.68 | 3.39 | 0.14 |
| Hatchability, % | 69.65b | 87.85a | 82.51a | 86.86a | 2.98 | 0.04 |
Data are the mean of 2 replicates.
Values within a row with different superscripts differ significantly at P < 0.05.
Table 5 shows that there was no difference on most eggshell quality parameters at sexual maturity, peak of laying, or 43 wk of age (P > 0.05). The eggshell percentage in PS16 was lower than other groups at sexual maturity (P = 0.01). The birds of PS16 had higher rate of broken eggs and abnormal eggs than other groups, which was consistent with the results of Morris and Perry (2002) and Tyler and Gous (2012), who found that advanced sexual maturity would reduce egg mass throughout the laying period because of reduction in the rate of recruitment or yellow-yolky follicles as well as an increased incidence of follicular atresia, internal ovulation, and the production of membranous of soft-shelled eggs.
Table 5.
Effects of age at photostimulation on eggshell quality at sexual maturity, peak of laying, and 43 wk of age of White Leghorn breeder hens.
| Period | Item | Age at photostimulation (wk) |
SEM | P-value | |||
|---|---|---|---|---|---|---|---|
| 16 | 18 | 20 | 22 | ||||
| Sexual maturity1 | Egg weight, g | 37.04 | 37.76 | 37.37 | 40.03 | 0.70 | 0.52 |
| Eggshell weight, g | 3.63 | 4.00 | 4.14 | 4.24 | 0.11 | 0.19 | |
| Eggshell, % of egg weight | 9.82b | 10.48a | 11.14a | 10.57a | 0.19 | 0.01 | |
| Eggshell thickness, mm | 0.34 | 0.37 | 0.39 | 0.37 | 0.01 | 0.13 | |
| Eggshell strength, kg • cm−2 | 2.61 | 3.06 | 3.22 | 3.25 | 0.11 | 0.10 | |
| Peak of laying2 | Egg weight, g | 48.22 | 50.40 | 49.09 | 50.68 | 0.50 | 0.28 |
| Eggshell weight, g | 5.09 | 5.41 | 5.20 | 5.40 | 0.07 | 0.26 | |
| Eggshell, % of egg weight | 10.58 | 10.75 | 10.62 | 10.63 | 0.08 | 0.93 | |
| Eggshell thickness, mm | 0.39 | 0.40 | 0.39 | 0.40 | <0.01 | 0.72 | |
| Eggshell strength, kg • cm−2 | 3.77 | 4.04 | 3.99 | 3.94 | 0.06 | 0.53 | |
| 43 wk of age | Egg weight, g | 57.21 | 59.16 | 56.68 | 58.44 | 0.48 | 0.27 |
| Eggshell weight, g | 5.75 | 6.21 | 5.91 | 5.98 | 0.08 | 0.26 | |
| Eggshell, % of egg weight | 10.30 | 10.49 | 10.48 | 10.23 | 0.07 | 0.50 | |
| Eggshell thickness, mm | 0.39 | 0.42 | 0.41 | 0.40 | <0.01 | 0.20 | |
| Eggshell strength, kg • cm−2 | 3.59 | 3.91 | 3.70 | 3.73 | 0.08 | 0.62 | |
Data are the mean of 2 replicates.
Values within a row with different superscripts differ significantly at P < 0.05.
Age at sexual maturity was defined as age at 5% egg production.
Peak of laying was defined as 32 wk of age.
The eggshell is a complex and highly structured calcific structure. Approximately 94% of eggshell mineral is calcium carbonate, with other inorganic minerals such as magnesium carbonate, calcium phosphate, and magnesium phosphate (Rodriguez-Navarro et al., 2015). The eggshell plays an important role in the resistance of eggs to physical and microbial invasion. Moreover, the eggshell must permit the exchange of gas and water and serve as a source of calcium for the growing embryo (Qi et al., 2016). The strength of the eggshell is directly related to its thickness and the structure of the external surface as well as the shell membrane. Increased resistance of eggshells is a desirable feature that has economic importance in a commercial laying sector (Fathi et al., 2007; Iqbal et al., 2017; Lopez et al., 2018). In general, PS16 had pool eggshell weight during laying period. This is in agreement with the finding of Silversides et al. (2006) that later photostimulation resulted in higher shell weight. This situation may be attributed to the complete development of the reproductive system prior to the onset of laying. The low eggshell quality of PS16 could be a causing factor of its lower hatchability. The WL layer breeder may different from the indigenous and broiler breeder hens in the responses to age at photostimulation. The recommended photostimulation age of layer breeders used in this study is 18 wk. Photostimulation at 16 wk of age may decrease hatchability, and photostimulation at 22 wk of age may decrease egg production.
ACKNOWLEDGMENTS
Financial support of this study was provided by the National Key Research and Development Program of China (No. 2016YFD0500502), the Chinese Agricultural Research System (No. CARS-40), and the Agricultural Science and Technology Innovation Program (No. ASTIPIAS04).
REFERENCES
- Bacon W.L., Vizcarra J.A., Morgan J.L.M., Jing Y., Han-Ken L., Long D.W., Kirby J.D. Changes in plasma concentrations of luteinizing hormone, progesterone, and estradiol-17beta in peripubertal turkey hens under constant or diurnal lighting. Biol. Reprod. 2002;67:591–598. doi: 10.1095/biolreprod67.2.591. [DOI] [PubMed] [Google Scholar]
- Bar A., Vax E., Hunziker W., Halevy O., Striem S. The role of gonadal hormones in gene expression of calbindin (Mr 28,000) in the laying hen. Gen. Comp. Endocrinol. 1996;103:115–122. doi: 10.1006/gcen.1996.0100. [DOI] [PubMed] [Google Scholar]
- Baxter M., Bédécarrats G.Y. Evaluation of the impact of light source on reproductive parameters in laying hens housed in individual cages. J. Poult. Sci. 2019;56:148–158. doi: 10.2141/jpsa.0180054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y.M., Wang J., Zhang H.J., Feng J., Wu S.G., Qi G.H. Effect of photoperiod on ovarian morphology, reproductive hormone secretion, and hormone receptor mRNA expression in layer ducks during the pullet phase. Poult. Sci. 2019;98:2439–2447. doi: 10.3382/ps/pey601. [DOI] [PubMed] [Google Scholar]
- Farghly M.F.A., Mahrose K.M., Rehman Z.U. Intermittent lighting regime as a tool to enhance egg production and eggshell thickness in Rhode Island Red laying hens. Poult. Sci. 2019;98:2459–2465. doi: 10.3382/ps/pez021. [DOI] [PubMed] [Google Scholar]
- Fathi M.M., El-Dein A.Z., El-Safty S.A., Radwan L.M. Using scanning electron microscopy to detect the ultrastructural variations in eggshell quality of Fayoumi and Dandarawi chicken breeds. Int. J. Poult. Sci. 2007;6:236–241. [Google Scholar]
- Hocking P.M. Role of body weight and food intake after photostimulation on ovarian function at first egg in broiler breeder females. Br. Poult. Sci. 1996;37:841–851. doi: 10.1080/00071669608417913. [DOI] [PubMed] [Google Scholar]
- Iqbal J., Mukhtar N., Rehman Z.U., Khan S.H., Ahmad T., Anjum M.S., Pasha R.H., Umar S. Effects of egg weight on the egg quality, chick quality, and broiler performance at the later stages of production (week 60) in broiler breeders. Appl. Sci. Res. 2017;26:183–191. [Google Scholar]
- Johnson E.L., Cunningham T.W., Marriner S.M., Kovacs J.L., Hunt B.G., Bhakta D.B., Goodisman M.A.D. Resource allocation in a social wasp: effects of breeding system and life cycle on reproductive decisions. Mol. Ecol. 2009;18:2908–2920. doi: 10.1111/j.1365-294X.2009.04240.x. [DOI] [PubMed] [Google Scholar]
- Katanbaf M.N., Dunnington E.A., Siegel P.B. Restricted feeding in early and late-feathering chickens. 2. Reproductive responses. Poult. Sci. 1989;68:352–358. doi: 10.3382/ps.0680352. [DOI] [PubMed] [Google Scholar]
- Lewis P.D., Ciacciariello M., Backhouse D., Gous R.M. Effect of age and body weight at photostimulation on the sexual maturation of broiler breeder pullets transferred from 8L:16D to 16L:8D. Br. Poult. Sci. 2007;48:601–608. doi: 10.1080/00071660701573052. [DOI] [PubMed] [Google Scholar]
- Lewis P.D., Sharp P.J., Wilson P.W., Leeson S. Changes in light intensity can influence age at sexual maturity in domestic pullets. Br. Poult. Sci. 2004;45:123–132. doi: 10.1080/00071660410001668950. [DOI] [PubMed] [Google Scholar]
- Lopez J.C., Kitto L., Hulet R.M. Effect of eggshell temperature on survival rate, development at hatch, and 7-day growth. J. Appl. Poult. Res. 2018;27:249–252. [Google Scholar]
- Ministry of Agriculture . China Agriculture Press; Beijing, China: 2004. Feeding Standard of Chicken (NY/T33-2004) [Google Scholar]
- Morris T.R., Perry G.C. A model for predicting the age at sexual maturity for growing pullets of layer strains given a single change in photoperiod. J. Agric. Sci. 2002;138:441–458. [Google Scholar]
- Pishnamazi A., Renema R.A., Zuidhof M.J., Robinson F. Effect of age at photostimulation on sexual maturation in broiler breeder pullets. Poult. Sci. 2014;93:1274–1281. doi: 10.3382/ps.2012-02834. [DOI] [PubMed] [Google Scholar]
- Qi X.F., Tan D., Wu C.Q., Tang C., Li T., Han X.Y., Wang J., Liu C.H., Li R.Q., Wang J.Y. Deterioration of eggshell quality in laying hens experimentally infected with H9N2 avian influenza virus. Vet. Res. 2016;47:35. doi: 10.1186/s13567-016-0322-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renema R.A., Robinson F.E. Defining normal: comparison of feed restriction and full feeding of female broiler breeders. Worlds Poult. Sci. J. 2004;60:508–522. [Google Scholar]
- Renema R.A., Robinson F.E., Feddes J.J.R., Fasenko G.M., Zuidhoft M.J. Effects of light intensity from photostimulation in four strains of commercial egg layers: 2. Egg production parameters. Poult. Sci. 2001;80:1121–1131. doi: 10.1093/ps/80.8.1121. [DOI] [PubMed] [Google Scholar]
- Renema R., Robinson F., Proudman J., Newcombe M., McKay R. Effects of body weight and feed allocation during sexual maturation in broiler breeder hens. 2. Ovarian morphology and plasma hormone profiles. Poult. Sci. 1999;78:629–639. doi: 10.1093/ps/78.5.629. [DOI] [PubMed] [Google Scholar]
- Renema R.A., Robinson F.E., Zuidhof M.J. Reproductive efficiency and metabolism of female broiler breeders as affected by genotype, feed allocation, and age at photostimulation. 2. Sexual maturation. Poult. Sci. 2007;86:2267–2277. doi: 10.1093/ps/86.10.2267. [DOI] [PubMed] [Google Scholar]
- Renema R.A., Rustad M.E., Robinson F.E. Implications of changes to commercial broiler and broiler breeder body weight targets over the past 30 years. Worlds Poult. Sci. J. 2007;63:457–472. [Google Scholar]
- Robinson F.E., Etches R.J. Ovarian steroidogenesis during follicular maturation in the hen (Gallus domesticus) Biol. Reprod. 1986;35:1096–1105. doi: 10.1095/biolreprod35.5.1096. [DOI] [PubMed] [Google Scholar]
- Robinson F.E., Wautier T.A., Hardin R.T., Robinson N.A., Wilson J.L., Newcombe M., McKay R.I. Effects of age at photostimulation on reproductive efficiency and carcass characteristics. 1. Broiler breeder hens. Can. J. Anim. Sci. 1996;76:275–282. [Google Scholar]
- Rodriguez-Navarro A.B., Marie P., Nys Y., Hincke M.T., Gautron J. Amorphous calcium carbonate controls avian eggshell mineralization: a new paradigm for understanding rapid eggshell calcification. J. Struct. Biol. 2015;190:291–303. doi: 10.1016/j.jsb.2015.04.014. [DOI] [PubMed] [Google Scholar]
- Sharp P.J. Photoperiodic control of reproduction in the domestic hen. Poult. Sci. 1993;72:897–905. doi: 10.3382/ps.0720897. [DOI] [PubMed] [Google Scholar]
- Shi L., Sun Y.Y., Xu H., Liu Y.F., Li Y.L., Huang Z.Y., Ni A.X., Chen C., Wang P.L., Ye J.H., Ma H., Li D.L., Chen J.L. Effect of age at photostimulation on reproductive performance of Beijing-You Chicken breeders. Poult. Sci. 2019 doi: 10.3382/ps/pez267. [DOI] [PubMed] [Google Scholar]
- Shi L., Sun Y.Y., Xu H., Liu Y.F., Xu S.S., Li Y.L., Ye J.H., Chen C., Li D.L., Chen Y., Guo Y.L., Chen J.L. Effect of age at photostimulation on sexual maturation in broiler breeders. Acta. Vet. Et Zootech. Sin. 2017;48:2107–2114. [Google Scholar]
- Silversides F.G., Korver D.R., Budgell K.L. Effect of strain of layer and age at photostimulation on egg production, egg quality, and bone strength. Poult. Sci. 2006;85:1136–1144. doi: 10.1093/ps/85.7.1136. [DOI] [PubMed] [Google Scholar]
- Tyler N.C., Gous R.M. Photorefractoriness in avian species – could this be eliminated in broiler breeders? Worlds Poult. Sci. J. 2012;68:645–650. [Google Scholar]
- van der Klein S.A.S., Bédécarrats G.Y., Robinson F.E., Zuidhof M.J. Early photostimulation at the recommended body weight reduced broiler breeder performance. Poult. Sci. 2018;97:3736–3745. doi: 10.3382/ps/pey215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Klein S.A.S., Bédécarrats G.Y., Zuidhof M.J. The effect of rearing photoperiod on broiler breeder reproductive performance depended on body weight. Poult. Sci. 2018;97:3286–3294. doi: 10.3382/ps/pey199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang N., Wu C., Mcmillan I. New mathematical model of poultry egg production. Poult. Sci. 1989;68:476–481. [Google Scholar]
- Zuidhof M.J., Renema R.A., Robinson F.E. Reproductive efficiency and metabolism of female broiler breeders as affected by genotype, feed allocation, and age at photostimulation. 3. Reproductive efficiency. Poult. Sci. 2007;86:2278–2286. doi: 10.1093/ps/86.10.2278. [DOI] [PubMed] [Google Scholar]