Abstract
The effect of orally administered hawthorn flavonoid extract (HFE) on growth, electrocardiographic waves, and cardiac parameters of pulmonary hypertensive chickens reared at high altitude (2,100 m above sea level) was examined. A total of 225 one-day-old, mixed broiler chicks (3 treatments with 5 replicates and 15 chicks per each, totally 75 birds/treatment) were assigned to 3 experimental groups: 0, 0.1, and 0.2 ml of HFE per 1 L of drinking water. Birds were administered the drinking water HFE treatments for 42 D. At an age of 28 and 42 D, electrocardiograms were undertaken and cardiac parameters such as the RV:TV, RV:BW, and TV:BW, and indicators of PHS on selected birds were measured. The final BW of chickens receiving the HFE at 0.2 ml/L was greater (2,579 ± 64 g) than that of birds receiving 0.1 ml/L (2,497 ± 62 g) and 0 ml/L (2,323 ± 57 g). Therefore, no supplemented group had a lower final BW than others (P < 0.05). Amplitudes of S and T waves in 0.1- and 0.2-ml/L HFE consumed groups at 28 and 42 D of age decreased compared with that in the control group (P < 0.05). The HFE reduced the heart weight and RV:TV, RV:BW, and TV:BW ratios when supplemented in drinking water at 0.1 and 0.2 mL/L compared with 0 mL/L (P < 0.05). In conclusion, supplementation of HFE in drinking water can reduce the PHS and incidence of cardiac disorders. Owing to the positive effect of HFE on cardiac parameters that mediated through flavonoids bioactive compounds, this product can be used to prevent complications of pulmonary hypertension and disarray of electrocardiographic waves in broiler chickens reared at high altitude.
Key words: chicken, cardiac parameter, electrocardiography, hawthorn, PHS
Introduction
The susceptibility of fast-growing broiler chickens to pulmonary hypertension syndrome (PHS) is augmented when they are raised at high altitudes (generally more than 1,300 m above sea level) where the relative oxygen pressure is declined. Ascites and cardiophysiological disorders are the main health impedance of the modern broiler industry (Hernandez, 1987, Julian, 2000); smaller heart and lung size, due to the increased selection intensity of broilers, has led to further sensitivity of birds to PHS (Wideman et al., 2013). Consequently, advanced pulmonary hypertension and right ventricular (RV) failure are the outcome of hypoxia in birds reared at high altitudes (Izadinia et al., 2010, Ahmadipour et al., 2015). In addition, a number of research studies have shown that a wide range of phenolic compounds in medicinal plants decrease the ratio of right ventricle to total ventricle (RV/TV) in ascetic chickens (Fathi et al., 2015, Shao et al., 2016, Ahmadipour et al., 2017 and 2018). Several studies indicated that the amplitude of the T and S waves increased and that there is a truncation in QRS, QT, and RR intervals from pulmonary hypertension due to high altitude or cold stress (Odom et al., 1992, Hassanpour et al., 2005). Cardiac disorders and heart injuries observed in ascitic birds leads to electrocardiogram (ECG) alterations which can be observed even before clinical signs develop in birds (Wideman and Kirby, 1996, Martinez et al., 1997) Electrocardiography is a noninvasive technique widely used in diagnostic procedures and for the study of cardiac physiopatholgy (Martinez et al., 1997). Among recorded waves, R, S, and T waves are suitable for comparison in birds and poultry (Odom et al., 1991, Odom et al., 1992, Hassanpour et al., 2005, Hassanpour et al., 2008, Hassanpour et al., 2011, Hassanpour and Khadem, 2013).
Hawthorn extract has been used for ameliorating cardiac disorders and pulmonary hypertension. The main chemical constituents of hawthorn flavonoid extract (HFE) include flavonoids (1-2%), oligomeric proanthocyanidins (1-3%), and other bioactive components (e.g., triterpene acids, organic acids, sterols, and cardioactive amines) (Chang et al., 2002, Kao et al., 2005, Long et al., 2006, Kao et al., 2007; Barros et al., 2011, Liu et al., 2011). These compounds are reported to have many pharmacological effects, including neuroprotective, hepatoprotective, cardioprotective, and nephroprotective effects (Chang et al., 2002, Kirakosyan et al., 2003). Furthermore, hawthorn fruits possess tonic effects on the heart and could reduce cardiovascular risk (Long et al., 2006, Salehi et al., 2009). Flavonoids are a natural secondary metabolite in Hawthorn, which is considered to be an antioxidative agent that eliminates oxygen-derived free radicals (Bottje and Wideman, 1995, Chang et al., 2007), improve blood pressure and vascular function (Brixius et al., 2006, Liu et al., 2011), as well as control PHS and cardiac disorders (Horáková, 2011, Surai, 2014). Flavonoids and oligomeric proanthocyanidins are considered to be responsible for the positive health effects of hawthorn extract (Long et al., 2006, Ahmadipour et al., 2017).
Because hawthorn (Crataegus oxyacantha) flavonoids have bioactive antioxidant activity and have potential to modulate the effects of cardiac disorders and prevent PHS and ascites, the objective of the present study is to evaluate the effect of different levels of HFE on electrocardiographic waves and cardiac parameters in broiler chickens that have pulmonary hypertension due to high altitude.
Materials and methods
Birds, Experimental Facility, and Treatments
An experiment was performed in the experimental facility of Shahrekord University. The effect of different levels of HFE on growth, electrocardiographic waves, and cardiac parameters of pulmonary hypertensive chickens were evaluated. A total of 225 Ross 308 one-day-old unsexed broiler chicks (3 treatments with 5 replicates and 15 chicks each, totally 75 birds/treatment) were assigned across 15-floor pens randomly. All pens had equal average BW (702 ± 16 g). Birds were reared at high altitude (2,100 m above sea level), and the temperature of the experimental unit was set at 32°C during week 1, then reduced at a rate of 3°C through week 2 to week 4, and finally fixed at 22°C until the end of the trial when the chickens showed an incidence of PHS and ascites. All birds had free access to feed and water, with 23-h light and 1-h dark period throughout the trial. In accordance with the method followed in similar studies (Hassanpour et al., 2005, Hassanpour et al., 2008, Ahmadipour et al., 2015), hypoxia was defined as reduced partial pressure of oxygen that occurs as the altitude increases up to 2,100 m. Therefore, compared with the sea level with partial pressure of oxygen equal to 21%, the partial pressure of oxygen in the mentioned experimental site was calculated to be 15.75%, and it is considered as the hypobaric hypoxia condition which is likely to lead to the PHS and ascites (Julian, 2000, Wideman et al., 2013).
For all experimental groups, a basal diet of corn–soybean meal was formulated for starting (1–3 weeks of age) and growing (3–6 weeks of age) periods according to NRC (1994) recommendations. Experimental treatments were prepared by adding 0.0, 0.1, and 0.2 ml of HFE (HE 00152, Crataegus-Drop 6260) per liter of drinking water (measured pH = 7.05, and total dissolved solids = 2,000 ppm). The HFE contained 0.25 and 0.50 mg/L of total flavonoid compounds. In this way, birds in 0.1-ml and 0.2-ml HFE groups received 0.05 to 0.10 mg of total flavonoid compounds daily. Crataegus oxyacantha (common hawthorn) is an endemic member of the Rosaceae family that grows in Europe, Africa, and Asia, where it is commonly found as a shrub or small tree 5 to 10 m tall (Chang et al., 2002). Hawthorn as a traditional medicinal plant is a plant locally called “Sorkh-e-valik or Zalzalak” that is found in western and central regions of Iran (Salehi et al., 2009).
Oral Crataegus-Drop is a well-known herbal drug therapy which is available at the pharmacy under an approved code and supervision of the Iranian Food and Drug Administration (HE 00152, Crataegus-Drop 6260). The flavonoid extract of this product contains biologically active flavonoid compounds (polyphenols) such as anthocyanidins and proanthocyanidins (also known as bioflavones or procyanidins). Each milliliter of oral Crataegus-Drop 6260 contained 2.5 mg of total flavonoid compounds in the form of hyperoside (21.4% polyphenols and 19.7% procyanidins), produced by Iran Darouk Pharmacy Co, under the production code of 3067-88-02. Determination of total phenolic compounds in Crataegus-Drop 6260 was carried out through the colorimetric method in accordance with the standard extraction procedure of the manufacturer (Ahmadipour et al., 2017).
Electrocardiographic Assessments
At days 28 and 42 of the trial, 15 birds within approximately 5% of the average pen BW from each treatment were randomly selected and weighted, and ECG were recorded using an automatic recorder (Cardimax FX-2111, Fukuda, Co., Ltd, Japan), standardized at 10 mm/mV with a chart speed of 50 mm/s in accordance with the descriptions of (Hassanpour et al., 2011 and Hassanpour and Khadem, 2013). Neither sedation nor anesthesia was used during the ECG recording. All the procedures took place in an isolated room to minimize stress in the birds. The birds were placed in ventral recumbency on a wooden table covered with plastic. The clip electrodes were attached to the propatagium of the left and right wings and to the skin on the left and right stifle joints. Alcohol was used to obtain good clip-to-skin contact. The leads of II, III, augmented vector right (aVR), and augmented vector foot (aVF) were recorded for every chicken, and the amplitude of T, R, and S waves as well as mean electrical axis (MEA) were also measured.
Measurement of heart weight, right ventricle hypertrophy, and the determination of cardiac parameters were performed after electrocardiography on selected noneuthanized birds at an age of 28 and 42 D, in accordance with the method introduced by (Hassanpour et al., 2008, Hassanpour et al., 2011). Briefly, selected birds were euthanized and the heart was dissected from the body. The atria were removed on the same plane of the atrioventricular valves. Then, the total ventricles (TV) were weighted. The RV wall was dissected from the left ventricle and septum. The RV was weighted, and the RV:TV ratio was calculated. The RV:TV is an important index for evaluating pulmonary hypertension. When the RV:TV is greater than 0.25, it is considered as pulmonary hypertension (Ahmadipour et al., 2015). Birds characterized by an accumulation of abdominal fluid were defined as ascitic cases, otherwise they were considered to be nonascitic birds (Wideman et al., 2013).
Statistical Analysis
Results were analyzed using GLM of the SAS (2007; SAS Institute Inc., Cary, NC) software in a completely randomized design. Data were subjected to a nested design when there was sampling effect within pens. The statistical model was Yijk = μ+Ti + eij + ɛijk. In this model, Yij and Yijk are observations; μ is the general mean; Ti is the effect for being in treatment i; eij is random error; and ɛijk is subsampling error. Significant level was fixed as P < 0.05, and means were separated by the Duncan multiple range test.
Ethical Considerations
This study was performed in accordance with the recommendations in the Guide for the Care and Use Committee of Shahrekord University in accordance with international standards for care and use of animals. At all stages of study, breeding operations were ethically approved by the Ethical Review Committee of College of Public Health and Medical Sciences of Shahrekord University, Shahrekord, Iran. Owing to negative effects of disturbance and environmental stress on heart waves, electrocardiography was performed in a quiet place by reducing stress in the environment.
Results
Our results indicated unchanged R wave amplitude in lead II, III, and aVF (Table 1). But inversely, an increased R wave amplitude in lead aVR of birds consumed 0.1 or 0.2 ml of HFE was observed, which was significant when compared with the control group (P < 0.05). The S wave amplitude in leads II and III was decreased for birds at 28 days of age that consumed HFE at levels of 0.1 and 0.2 mL (P < 0.05). An increased S wave amplitude in lead aVR and aVF was also observed (P < 0.05). Decreased amplitude for leads II and III were recorded in case of T wave significantly (P < 0.05), but unchanged T wave amplitude in leads aVR and aVF were observed.
Table 1.
Effect of HFE on electrocardiogram waves and MEA of birds at 28 days of agea.
| Drinking levels of hawthorn flavonoid extract (mL) | |||||
|---|---|---|---|---|---|
| Leads | Control (0) | 0.1 | 0.2 | SEM | P-value |
| R (mV) | |||||
| II | 0.22 | 0.17 | 0.18 | 0.03 | 0.623 |
| III | 0.18 | 0.16 | 0.14 | 0.03 | 0.548 |
| aVR | 0.09b | 0.13a | 0.12a | 0.02 | 0.014 |
| aVF | 0.19 | 0.17 | 0.18 | 0.04 | 0.704 |
| S (mV) | |||||
| II | 0.24a | 0.19a,b | 0.11b | 0.04 | 0.001 |
| III | 0.25a | 0.11b | 0.12b | 0.05 | 0.001 |
| aVR | 0.20a | 0.15b | 0.16b | 0.02 | ≤0.021 |
| aVF | 0.18a | 0.09b | 0.11b | 0.02 | 0.001 |
| T (mV) | |||||
| II | 0.18a | 0.13b | 0.11b | 0.02 | ≤0.011 |
| III | 0.19a | 0.16b | 0.12b | 0.01 | 0.000 |
| aVR | 0.08 | 0.08 | 0.09 | 0.04 | 0.539 |
| aVF | 0.09 | 0.11 | 0.12 | 0.03 | 0.851 |
| MEA (mV) | 183.33 | 177.65 | 166.75 | 32.4 | - |
a,bSuperscripts in the same row with different letters are significantly different (P < 0.05). Each mean represents values from 15 observations.
Abbreviations: aVF, augmented vector foot; aVR, augmented vector right; HFE, hawthorn flavonoid extract; MEA, mean electrical axis; mV, milli-Volt; R, heart wave type R; S, heart wave type S; T, heart wave type T.
A reduced R wave amplitude in leads II and III was recorded for birds that consumed 0.1 or 0.2 mL of HFE which was significant only in lead III when compared with the control group (P < 0.05; Table 2). The R wave amplitude in lead aVR or aVF did not change when compared with the control group. The S wave amplitude in leads II, III, and aVR decreased for birds at 42 days of age that consumed 0.1 or 0.2 ml of HFE compared with the control group (P < 0.05; Table 2). An increased S wave amplitude in lead aVF was observed for birds that consumed 0.1 or 0.2 mL of HFE (P < 0.05). Decreased amplitude of T waves in leads of II and III were observed (P < 0.05), but the changes of T wave amplitude in aVR and aVF were not significant.
Table 2.
Effect of HFE on electrocardiogram waves and MEA of birds at 42 days of agea.
| Drinking levels of hawthorn flavonoid extract (mL) | |||||
|---|---|---|---|---|---|
| Leads | Control (0) | 0.1 | 0.2 | SEM | P-value |
| R (mV) | |||||
| II | 0.22 | 0.23 | 0.21 | 0.03 | 0.711 |
| III | 0.21a | 0.15b | 0.16b | 0.04 | ≤0.023 |
| aVR | 0.09 | 0.12 | 0.13 | 0.02 | 0.552 |
| aVF | 0.19 | 0.17 | 0.18 | 0.04 | 0.811 |
| S (mV) | |||||
| II | 0.34a | 0.21b | 0.19b | 0.06 | ≤0.039 |
| III | 0.25a | 0.11b | 0.12b | 0.05 | ≤0.041 |
| aVR | 0.20a | 0.15b | 0.16b | 0.02 | 0.001 |
| aVF | 0.21a | 0.19a | 0.14b | 0.02 | 0.001 |
| T (mV) | |||||
| II | 0.18a | 0.13b | 0.11b | 0.02 | ≤0.012 |
| III | 0.19a | 0.16b | 0.12b | 0.01 | ≤0.010 |
| aVR | 0.08 | 0.12 | 0.10 | 0.04 | 0.643 |
| aVF | 0.12 | 0.09 | 0.09 | 0.03 | 0.511 |
| MEA (mV) | 225.65 | 221.45 | 194.50 | 45.12 | - |
a,bSuperscripts in the same row with different letters are significantly different (P < 0.05). Each mean represents values from 15 observations.
Abbreviations: aVF, augmented vector foot; aVR, augmented vector right; HFE, hawthorn flavonoid extract; MEA, mean electrical axis; mV, milli-Volt; R, heart wave type R; S, heart wave type S; T, heart wave type T.
The effects of HFE on the MEA of broilers at 28 and 42 days of age are shown in Tables 1 and 2. No differences were observed between the experimental groups at age of 28 or 42 D, but the level of MEA in the control group was higher than that of HFE-supplemented groups.
The effects of different levels of HFE at 42 days of age on heart weight, ascites index (RV:TV ratio), and relevant PHS indices are shown in Table 3. Birds that consumed drinking water containing 0.1 and 0.2 mg/L of HFE had a lower RV:TV ratio than the birds of the control group (0 mg/L) (P < 0.05; Table 3). Observations of pulmonary hypertensive birds were greater in the control group (P < 0.05; Table 3). In addition, inclusion of HFE in broiler drinking water at both 0.1 and 0.2 mL/L reduced (P < 0.05) bird deaths associated with PHS compared with the control treatment with 0 mg of HFE/L.
Table 3.
Effect of HFE on RV:TV ratio and PHS indices of birds at 42 days of agea.
| Drinking levels of hawthorn extract (mL) | |||||
|---|---|---|---|---|---|
| Item | Control (0) | 0.1 | 0.2 | SEM | P-value |
| Heart (gram) | 16.95 | 15.23 | 13.67 | 0.82 | 0.688 |
| Heart (%) | 0.73 | 0.61 | 0.53 | 0.05 | 0.596 |
| RV:TV (ratio)1 | 0.32a | 0.251 | 0.221 | 0.02 | ≤0.030 |
| Affected birds with Pulmonary hypertension (%) | 57.34a | 44.661 | 36.84c | 3.85 | 0.001 |
| PHS mortality (%) | 28.72a | 22.321 | 18.411 | 2.88 | 0.001 |
aSuperscripts in the same row with different letters are significantly different (P < 0.05).
Abbreviations: HFE, hawthorn flavonoid extract; PHS, pulmonary hypertension syndrome, RV:TV, right ventricle to total ventricle weight ratio.
Mean represents values from 15 observations.
The effects of HFE on BW, right ventricle weight, and TV weight and also on the ratio of RV:BW and TV:BW are shown in Table 4. Broilers that consumed drinking water containing 0.1 or 0.2 mg of HFE/L had a greater BW but had lower RV and TV weights or RV:BW and TV:BW ratio than the birds of the control group (P < 0.05).
Table 4.
Effects of HFE on BW and cardiac parameters of birds at 42 days of agea.
| Drinking levels of hawthorn extract (mL) | |||||
|---|---|---|---|---|---|
| Item (gram) | Control (0) | 0.1 | 0.2 | SEM | P-value |
| BWb at 28 day | 1,160.3b | 1,212.2a,b | 1,249.7a | 33.84 | ≤0.011 |
| BW at 42 day | 2,323.3b | 2,497.2a | 2,579.4a | 58.42 | 0.001 |
| RVc | 3.19a | 2.21b | 1.91b | 0.23 | 0.001 |
| TV1 | 9.98a | 8.99b | 8.77b | 0.35 | 0.001 |
| RV/BW (%) | 0.14a | 0.09b | 0.07b | 0.01 | 0.000 |
| TV/BW (%) | 0.43a | 0.36b | 0.34b | 0.02 | 0.000 |
a,b,cSuperscripts in the same row with different letters are significantly different (P < 0.05).
Abbreviations: RV, right ventricle; TV, total ventricle.
Mean represents values from 15 observations.
Discussion
Modern genotype broiler chickens are highly susceptible to pulmonary hypertension syndrome due to high demands on oxygen for metabolism to support growth and performance efficiency (Izadinia et al., 2010, Ahmadipour et al., 2015). Hypobaric hypoxia due to high altitudes reduces the availability of atmospheric oxygen for red blood cells passing through the lung vessels and induces an increased load on the cardiac system leading to increased arterial pressure on the pulmonary vessels. Inordinate pulmonary arterial pressure causes hypertrophy and subsequent dilation of the right ventricle. Ultimately, imbalance between cardiac output and capacity of the pulmonary vessels to facilitate of blood flow result in an improper elevated electrocardiogram owing to increased degree of constriction (Julian, 2000, Wideman et al., 2013). Hypoxia is the main factor that increases the pulmonary vascular resistance and accelerates the pathophysiological progression leading to PHS and ascites (Hernandez, 1987).
Flavonoids and oligomeric proanthocyanidins of Crataegus oxyacantha are biologically active polyphenols such as anthocyanidins and proanthocyanidins (Chang et al., 2002, Kirakosyan et al., 2003, Kao et al., 2005, Kao et al., 2007, Barros et al., 2011). Extracts of hawthorn fruits, leaves, or flowers are considered potent antioxidant and free radical scavengers, owing to the impact of epicatechin, hyperoside, and chlorogenic acid compounds (Chang et al., 2002, Barros et al., 2011). These compounds are reported to have many pharmacological effects, including neuroprotective, hepatoprotective, cardioprotective, and nephroprotective effects (Chang et al., 2002, Kirakosyan et al., 2003).
In accordance with the results of this study, the proportion of heart weight and RV:TV ratio as well as S and T wave amplitudes of electrocardiogram were reduced as the supplementation level of HFE increased in drinking water from 0 to 0.2 mL per liter. Decreased development of pulmonary hypertension observed in experimental groups of this study is as a result of reduction in the number of factors including RV:TV, RV:BW, and TV:BW ratios.
Xiang et al. (2002) reported that some of cardiac parameters such as RV:TV decreased during the supplementation of vitamin C as an antioxidant in pulmonary hypertensive chickens, but Teshfam et al., 2006 showed that cardiac indices increased in situations in which cold temperature induced pulmonary hypertension. Hassanpour et al., 2009 reported that RV:TV and RV:BW decreased when citric acid was supplemented at levels of 0.5, 1.0, and 1.5 g per liter of drinking water.
Because of low oxygen concentration at high altitudes, the heart pumping activity increases, which in turn reflects on increased relative weight of the heart for greater oxygenation (Izadinia et al., 2010). An subsequent increase in the RV:TV ratio and heart wave amplitudes reflects the hypertrophy of the RV wall (S wave) which can be directly linked to increased pulmonary arterial pressure (Hassanpour et al., 2009, Hassanpour and Khadem, 2013, Wideman et al., 2013). Increased pulmonary arterial pressure theoretically can be related to increase in cardiac output and/or be accompanied by a pulmonary vascular resistance. The RV:TV ratio can be as an important index to express the severity of pulmonary hypertension in broiler chickens (Hassanpour et al., 2005, Izadinia et al., 2010). Therefore, in accordance with this index, the results of the present study suggested that HFE in drinking water of broilers could reduce pulmonary hypertension, in agreement with the findings by Ahmadipour et al., 2015 (Figure 1, Figure 2, Figure 3).
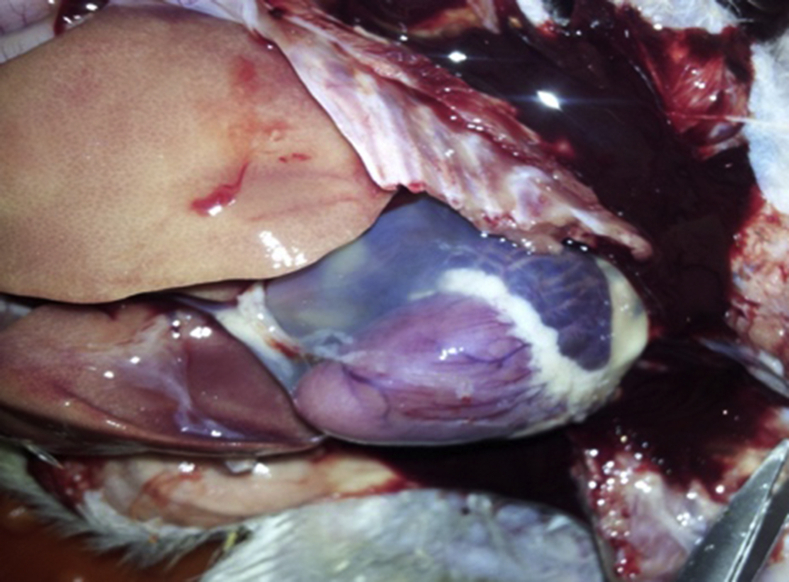
Figure 1.
Overall view of an ascetic chicken.
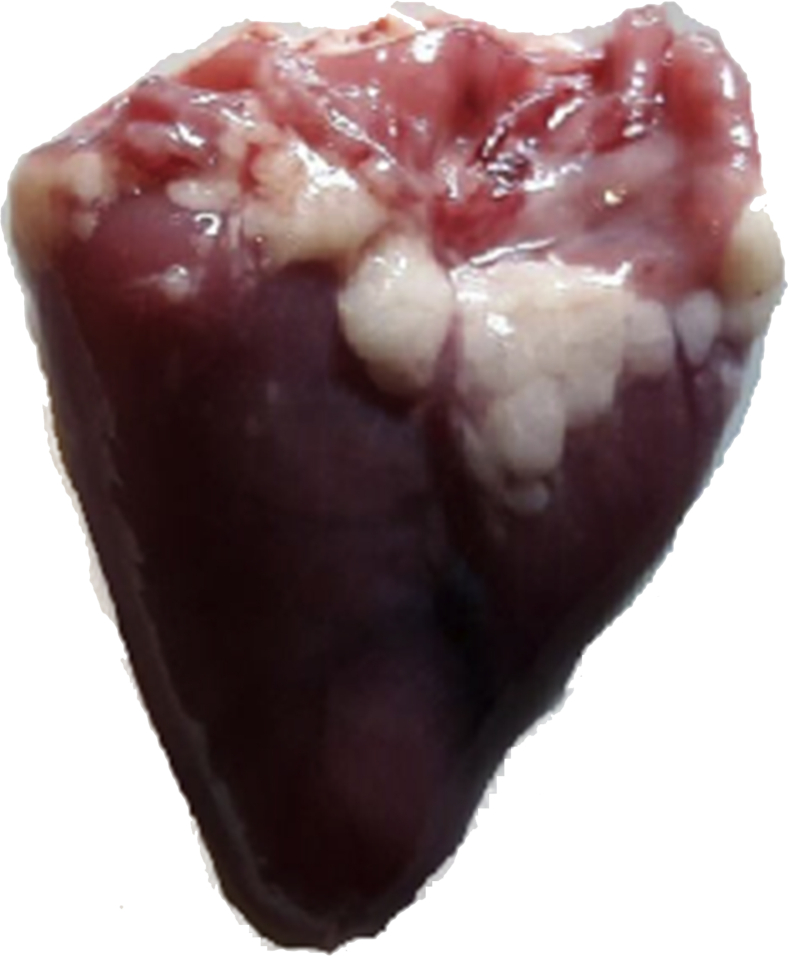
Figure 2.
A heart with an enlarged ventricles.
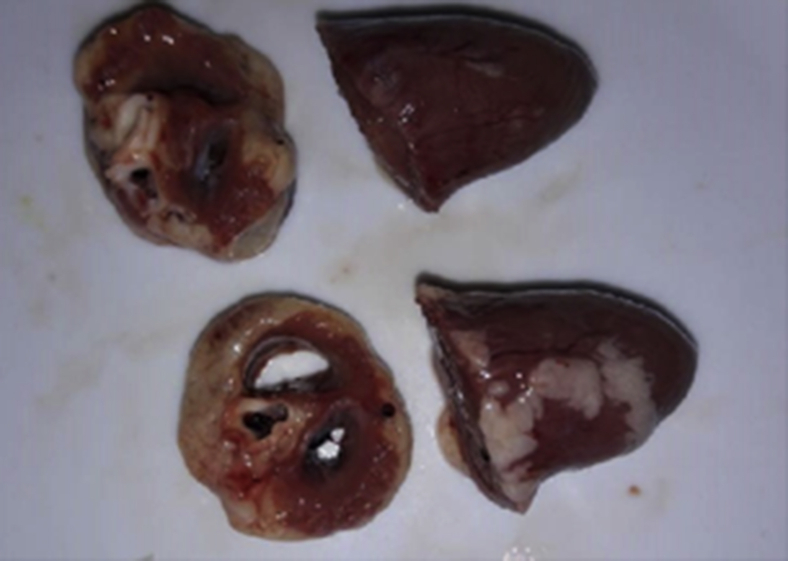
Figure 3.
Comparison between a normal heart (top) with an ascetic heart (bottom).
An elevation of T and S waves occurs in cold-induced pulmonary hypertensive broilers (Owen et al., 1995, Martinez et al., 1997, Hassanpour et al., 2005). Dilation and hypertrophy of the ventricles are the primary cause of the increased S wave amplitude (prolonged ventricular depolarization) (Hassanpour et al., 2005, Hassanpour et al., 2011) It was shown in the present study that the amplitude of the S and T waves decreased during consumption of HFE at both 28 and 42 days of age. Therefore, it is suggested that broilers orally receiving HFE supplementation have a lower rate of ventricular hypertrophy and dilation.
Flavonoids are precursor of the most antioxidant agents that act as the key pulmonary vasoprotectives against oxidative stress and modulator of vasodestruction (Chang et al., 2007, Procházková et al., 2011) Flavonoids improve endothelial function (Brixius et al., 2006), decrease blood pressure, and improve vasoconstriction (Procházková et al., 2011). In accordance with recent research findings, an important mechanism by which flavonoids may exert health benefits is modulation of calcium homeostasis and cell signaling via Ca2+-ATPase (Horáková, 2011). Flavonoids have been suggested to exert health benefits through different antioxidant mechanisms and metal chelators and also have been recognized to possess anti-inflammatory and anticarcinogenic activities (Chang et al., 2007, Liu et al., 2011, Surai, 2014).
Adding HFE to broiler drinking water protects the vascular system and facilitated normal pulmonary vasodilation in response to large increases in blood flow and modulated the pulmonary hypertension and restricted the increase in the pulmonary arterial pressure including cardiac load–associated hypertrophy specified by an elevated RV:TV ratio and accelerated rates of blood flow through the lungs (Hassanpour et al., 2005, Wideman et al., 2013). In the present study, using HFE in drinking water of broilers considerably decreased the proportion of the heart weight which be the reason for the reduced RV:TV ratio and consequently reduced pulmonary hypertension as well as heart wave amplitudes.
Conclusion
In conclusion, supplementation of HFE particularly at the level of 0.2 ml/L in drinking water can help prevent cardiac disorders including arrhythmia, increased pulmonary arterial pressure, and cardiac hypertrophy as a result of PHS and ascites in hypertensive broiler chickens. Positive effects of HFE on electrocardiographic waves and cardiac parameters are attributed to cardiotropic, vasodilatory, and antioxidant actions of HFE that mediated through flavonoid bioactive compounds. Therefore, in accordance with the positive effects on physiological responses, reduced mortality, and better performance, using HFE at the level of 0.2 ml/L of drinking water is an effective prophylaxis of pulmonary hypertension and cardiac disorders in broiler chickens reared at high altitude.
Acknowledgments
The authors are grateful for the financial support of Shahrekord University, Shahrekord, Iran. The authors also thank for technical supports of the Animal Science Department of Agricultural Research Center of Qom (QARC), Qom, Iran. All the authors acknowledge and thank their respective institutes and universities. The authors of the manuscript declare no conflict of interests for financial and personal relationships with other people or organizations that might inappropriately influence or bias this work.
References
- Ahmadipour B., Hassanpour H., Asadi E., Khajali F., Rafiei F., Khajali F. Kelussia odoratissima Mozzaf–A promising medicinal herb to prevent pulmonary hypertension in broiler chickens reared at high altitude. J. Ethnopharmacol. 2015;159:49–54. doi: 10.1016/j.jep.2014.10.043. [DOI] [PubMed] [Google Scholar]
- Ahmadipour B., Kalantar M., Hosseini S.M., Yang L.G., Kalantar M.H., Raza S.H.A., Schreurs N.M. Hawthorn (Crataegus oxyacantha) extract in the drinking water of broilers on growth and incidence of pulmonary hypertension syndrome (PHS) Rev. Bras. Cienc. Avic. 2017;19:639–644. [Google Scholar]
- Ahmadipour B. Securigera securidaca seed medicinal herb supplementation to diets improves pulmonary hypertensive response in broiler chickens reared at high altitude. J. Anim. Physiol. Anim. Nutr. 2018;102:1601–1607. doi: 10.1111/jpn.12981. [DOI] [PubMed] [Google Scholar]
- Barros L., Carvalho A.M., Ferreira I.C.F.R. Comparing the composition and bioactivity of Crataegus monogyna flowers and fruits used in folk medicine. Phytochem. Anal. 2011;22:181–188. doi: 10.1002/pca.1267. [DOI] [PubMed] [Google Scholar]
- Bottje W.G., Wideman R.F. Potential role of free radicals in the pathogenesis of pulmonary hypertension syndrome. Poult. Avian Biol. Rev. 1995;6:211–231. [Google Scholar]
- Brixius K., Willms S., Napp A., Tossios P., Ladage D., Bloch W., Mehlhorn U., Schwinger R.H.G. Crataegus special extract WS® 1442 induces an endothelium-dependent, NO-mediated vasorelaxation via eNOS-phosphorylation at serine 1177. Cardiovasc. Drugs Ther. 2006;20:177–184. doi: 10.1007/s10557-006-8723-7. [DOI] [PubMed] [Google Scholar]
- Chang W.-T., Shao Z.-H., Yin J.-J., Mehendale S., Wang C.-Z., Qin Y., Li J., Chen W.-J., Chien C.-T., Becker L.B. Comparative effects of flavonoids on oxidant scavenging and ischemia-reperfusion injury in cardiomyocytes. Eur. J. Pharmacol. 2007;566:58–66. doi: 10.1016/j.ejphar.2007.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Q., Zuo Z., Harrison F., Chow M.S.S. Hawthorn. J. Clin. Pharmacol. 2002;42:605–612. doi: 10.1177/00970002042006003. [DOI] [PubMed] [Google Scholar]
- Fathi M., Haydari M., Tanha T. Effects of enalapril on growth performance, ascites mortality, antioxidant Status and blood parameters in broiler chickens under cold-induced ascites. Poult. Sci. 2015. 2015;3:121–127. [Google Scholar]
- Hassanpour H., Khadem P. Normal electrocardiogram patterns and values in Muscovy ducks (Cairina moschata) J. Avian Med. Surg. 2013;27:280–284. doi: 10.1647/2012-045. [DOI] [PubMed] [Google Scholar]
- Hassanpour H., Moghadam A.K., Teshfam M., Zarei H. Effect of ascorbic acid o the electrocardiogram of broiler chickens raised at high altitude. Niger. Vet. J. 2008;29:8–14. [Google Scholar]
- Hassanpour H., Teshfam M., Modirsanei M., Emadi L. Comparative studies of the electrocardiographic parameters, mean electrical axis (MEA) and cardiac index (RV/TV) in normal and experimentally ascites (using cold) groups of broilers. BEL Stud. Sp. Z Oo. 2005:17–20. [Google Scholar]
- Hassanpour H., Yazdani A., Khabir Soreshjani K., Asgharzadeh S. Evaluation of endothelial and inducible nitric oxide synthase genes expression in the heart of broiler chickens with experimental pulmonary hypertension. Br. Poult. Sci. 2009;50:725–732. doi: 10.1080/00071660903141005. [DOI] [PubMed] [Google Scholar]
- Hassanpour H., Zarei H., Hojjati P. Analysis of electrocardiographic parameters in helmeted Guinea fowl (Numida meleagris) J. Avian Med. Surg. 2011;25:8–14. doi: 10.1647/2009-048.1. [DOI] [PubMed] [Google Scholar]
- Hernandez A. Hypoxic ascites in broilers: a review of several studies done in Colombia. Avian Dis. 1987;31:658–661. [PubMed] [Google Scholar]
- Horáková Ľ. Flavonoids in prevention of diseases with respect to modulation of Ca-pump function. Interdiscip. Toxicol. 2011;4:114–124. doi: 10.2478/v10102-011-0019-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izadinia M., Nobakht M., Khajali F., Faraji M., Zamani F., Qujeq D., Karimi I. Pulmonary hypertension and ascites as affected by dietary protein source in broiler chickens reared in cool temperature at high altitudes. Anim. Feed Sci. Technol. 2010;155:194–200. [Google Scholar]
- Julian R.J. Physiological, management and environmental triggers of the ascites syndrome: a review. Avian Pathol. 2000;29:519–527. doi: 10.1080/03079450020016751. [DOI] [PubMed] [Google Scholar]
- Kao E.-S., Wang C.-J., Lin W.-L., Chu C.-Y., Tseng T.-H. Effects of polyphenols derived from fruit of Crataegus pinnatifida on cell transformation, dermal edema and skin tumor formation by phorbol ester application. Food Chem. Toxicol. 2007;45:1795–1804. doi: 10.1016/j.fct.2007.03.016. [DOI] [PubMed] [Google Scholar]
- Kao E.-S., Wang C.-J., Lin W.-L., Yin Y.-F., Wang C.-P., Tseng T.-H. Anti-inflammatory potential of flavonoid contents from dried fruit of Crataegus pinnatifida in vitro and in vivo. J. Agric. Food Chem. 2005;53:430–436. doi: 10.1021/jf040231f. [DOI] [PubMed] [Google Scholar]
- Kirakosyan A., Seymour E., Kaufman P.B., Warber S., Bolling S., Chang S.C. Antioxidant capacity of polyphenolic extracts from leaves of Crataegus laevigata and Crataegus monogyna (Hawthorn) subjected to drought and cold stress. J. Agric. Food Chem. 2003;51:3973–3976. doi: 10.1021/jf030096r. [DOI] [PubMed] [Google Scholar]
- Liu P., Kallio H., Yang B. Phenolic compounds in hawthorn (Crataegus grayana) fruits and leaves and changes during fruit ripening. J. Agric. Food Chem. 2011;59:11141–11149. doi: 10.1021/jf202465u. [DOI] [PubMed] [Google Scholar]
- Long S.R., Carey R.A., Crofoot K.M., Proteau P.J., Filtz T.M. Effect of hawthorn (Crataegus oxycantha) crude extract and chromatographic fractions on multiple activities in a cultured cardiomyocyte assay. Phytomedicine. 2006;13:643–650. doi: 10.1016/j.phymed.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Martinez L.A., Jeffrey J.S., Odom T.W. Electrocardiographic diagnosis of cardiomyopathies in aves. Poult. Avian Biol. Rev. 1997;8:9–20. [Google Scholar]
- Odom T.W., Hargis B.M., Lopez C.C., Arce M.J., Ono Y., Avila G.E. Use of electrocardiographic analysis for investigation of ascites syndrome in broiler chickens. Avian Dis. 1991;35:738–744. [PubMed] [Google Scholar]
- Odom T.W., Rosenbaum L.M., Hargis B.M. Evaluation of vectorelectrocardiographic analysis of young broiler chickens as a predictive index for susceptibility to ascites syndrome. Avian Dis. 1992;36:78–83. [PubMed] [Google Scholar]
- Owen R.L., Wideman R.F., Jr., Leach R.M., Cowen B.S., Dunn P.A., Ford B.C. Physiologic and electrocardiographic changes occurring in broilers reared at simulated high altitude. Avian Dis. 1995;39:108–115. [PubMed] [Google Scholar]
- Procházková D., Boušová I., Wilhelmová N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia. 2011;82:513–523. doi: 10.1016/j.fitote.2011.01.018. [DOI] [PubMed] [Google Scholar]
- Salehi S., Long S.R., Proteau P.J., Filtz T.M. Hawthorn (Crataegus monogyna Jacq.) extract exhibits atropine-sensitive activity in a cultured cardiomyocyte assay. J. Nat. Med. 2009;63:1–8. doi: 10.1007/s11418-008-0278-4. [DOI] [PubMed] [Google Scholar]
- Shao J., Wang P., Liu. A., Du. X., Bai J.*, Chen M. Punicalagin prevents hypoxic pulmonary hypertension via anti-oxidant effects in rats. Am. J. Chin. Med. 2016;44:1–17. doi: 10.1142/S0192415X16500439. [DOI] [PubMed] [Google Scholar]
- Surai P.F. Polyphenol compounds in the chicken/animal diet: from the past to the future. J. Anim. Physiol. Anim. Nutr. (Berl). 2014;98:19–31. doi: 10.1111/jpn.12070. [DOI] [PubMed] [Google Scholar]
- Teshfam M., Brujeni G.N., Hassanpour H. Evaluation of endothelial and inducible nitric oxide synthase mRNA expression in the lung of broiler chickens with developmental pulmonary hypertension due to cold stress. Br. Poult. Sci. 2006;47:223–229. doi: 10.1080/00071660600611169. [DOI] [PubMed] [Google Scholar]
- Wideman R.F., Kirby Y.K. Electrocardiographic evaluation of broilers during the onset of pulmonary hypertension initiated by unilateral pulmonary artery occlusion. Poult. Sci. 1996;75:407–416. doi: 10.3382/ps.0750407. [DOI] [PubMed] [Google Scholar]
- Wideman R.F., Rhoads D.D., Erf G.F., Anthony N.B. Pulmonary arterial hypertension (ascites syndrome) in broilers: a review. Poult. Sci. 2013;92:64–83. doi: 10.3382/ps.2012-02745. [DOI] [PubMed] [Google Scholar]
- Xiang R.P., Sun W.D., Wang J.Y., Wang X.L. Effect of vitamin C on pulmonary hypertension and muscularisation of pulmonary arterioles in broilers. Br. Poult. Sci. 2002;43:705–712. doi: 10.1080/0007166021000025064. [DOI] [PubMed] [Google Scholar]