Abstract
Methylsulfonylmethane (MSM) is an organic, sulfur-containing compound widely used as a dietary supplement to improve joint health and treat arthritic pain. An experiment was conducted to study the effects of feeding 0.05% MSM to broilers exposed to diet-induced oxidative stress on tissue MSM distribution, growth performance, oxidative stress biomarkers, and immune responsivity. A total of 528 birds were allocated to 4 dietary treatments (fresh oil-no MSM, fresh oil-MSM, oxidized oil-no MSM, oxidized oil-MSM) as provided ad libitum to 11 replicate cages of 12 birds per treatment. Blood and tissue samples were collected to analyze MSM concentrations, and oxidative stress biomarkers including concentrations of thiobarbituric acid reactive substances (TBARS), total antioxidant capacity (TAC), total glutathione, and glutathione peroxidase (GPx) and reductase (GR) activities. Additionally, blood samples collected at day 25 were used to quantify T-cell (TC) populations using flow cytometry. Overall, MSM was quantified in all tissues and plasma samples of MSM-treated groups at all time points. Oxidized oil reduced (P = 0.006) feed intake over the 21-d feeding period, but MSM did not affect growth equally across time points. No effects (P > 0.2) of MSM or oil type were observed on TC populations. In the presence of oxidized oil, MSM reduced (P = 0.013) plasma TBARS and increased (P = 0.02) liver GPx at day 21, and increased (P = 0.06) liver GR at day 7. Irrespective of dietary oil type, groups supplemented with MSM showed higher plasma TAC at day 7 (P = 0.023), liver GPx activity at day 21 (P = 0.003), and liver GR activity at day 7 (P = 0.004) compared with groups not receiving MSM. In conclusion, 0.05% dietary MSM supplementation partially protected birds from oxidative stress but did not affect immune cell profiles.
Key words: MSM, oxidative stress, TBARS, broiler
Introduction
Oxidative stress is an important issue influencing health and well-being of broilers in commercial poultry operations (Noguchi et al., 1973, Mézes and Salyi, 1994, Avanzo et al., 2001, Zhang et al., 2011). Oxidative stress is characterized by an accumulation of harmful free radical molecules as the result of disturbance in the redox status. Examples of free radicals are the reactive oxygen species (ROS) which are generated by the mitochondrial electron transport chain during cellular respiration, iron overload, and other physiological reactions, and reactive nitrogen species which act as neurotransmitters (e.g., nitric oxide) and play a role in inflammation (Valko et al., 2007). Studies show that oxidative stress can significantly affect reproductive performance, cellular senescence, and aging (Beckman and Ames, 1998). In poultry, oxidative stress is reported to cause GI disturbances (Dibner et al., 1996), which can lead to poor gut health and subsequent production losses. Hence, oxidative stress related disorders could cause substantial production loss to the poultry industry.
Additionally, genetic selection for fast growth of lean tissues, especially including large breast muscles increases the susceptibility of modern broilers to oxidative stress (Sihvo et al., 2014). Heat stress can cause dysfunction of the mitochondrial respiratory chain to negatively influence ATP production by downregulating avian uncoupling protein, thus leading to increased production of ROS (Mujahid et al., 2006). Despite a subsequent increased activity of antioxidant enzyme systems, heat-stressed animals exhibited many markers of cellular damage characteristic of oxidative stress (Tan et al., 2010). Birds reared in hotter climates are reported to have high mortality (Yahav et al., 1995), greater immune suppression (Young, 1990), and poorer growth (Bottje, and Harrison, 1985), mostly due to subsequent oxidative damage.
Acute heat stress and consumption of oxidized oil in the diet are considered to be major contributors of oxidative stress in poultry (Warnants et al., 1996, Altan et al., 2003). Fats and oils of both plant and animal origin are used in poultry diets to meet energy requirements of fast growing birds. Diet handling, including mixing, pelleting, and storage, can accelerate oxidative damage to dietary lipids, which can increase the production of pro-oxidant compounds in the body when the oxidized lipids are consumed (Warnants et al., 1996). However, it is challenging to quantify the impact of oxidative stress in poultry as it occurs along with other etiologies including heat stress, inflammation, and peroxidized oil intake. Hence, research into novel dietary technologies is needed to reduce the extent of oxidative damage and its influence on poultry health and production.
Methylsulfonylmethane, a naturally occurring, organic, sulfur-containing compound (Pearson et al., 1981), has shown to be an effective prophylactic/therapy against various health ailments in humans. It is widely used as a dietary supplement to improve joint health, and treat arthritic pain (Kim et al., 2006, Debbi et al., 2011, Pagonis et al., 2014). Other major reported health benefits of methylsulfonylmethane (MSM) include moderation of inflammation (Van der Merwe and Bloomer, 2016), reduction of gastric mucosal injury (Amirshahrokhi and Khalili, 2017), and alleviation of exercise-induced oxidative stress (Nakhostin-Roohi et al., 2013). These studies reported that anti-inflammatory and antioxidant property of MSM are largely responsible for most of the associated beneficial health effects. For example, MSM is known to inhibit inducible nitric oxide synthase (iNOS), cyclooxygenase 2 (COX2), prostaglandin E2 (PGE2), IL-6, and TNF-α through downregulation of NFKB signaling (Kim et al., 2009). These mediators can increase free radical generation in cells as a part of the inflammatory response. Although MSM is claimed to be included as a feed additive for better growth performance in animals (Herschler, 1991), there are very few published studies available on the effects of this compound in domestic livestock species. In a previous study, we tested the toxicological effects of MSM in unchallenged broilers, and observed that oral MSM at 2,000 mg/kg BW did not affect growth and health parameters of broilers fed a corn-soybean meal diet. In this study, we hypothesized that dietary MSM supplementation would improve redox and immune status of birds during an oxidative stress challenge. Thus, the current objective was to quantify the effects of dietary MSM ingestion under conditions of oxidative stress (i.e., oxidized oil intake) on redox status and peripheral blood TC profiles, and to quantify tissue distribution of MSM.
Materials and methods
All animal care and experimental procedures were approved by the University of Illinois Institutional Animal Care and Use Committee before initiation of the experiment.
Birds and Husbandry
Day-old Ross 308 male broiler chicks were obtained from Hoover’s Hatchery (Rudd, IA) and raised at the University of Illinois Poultry Research Farm. Broiler chicks were housed in thermostatically controlled Petersime starter batteries with raised-wire flooring in an isolated, environmentally controlled room with continuous lighting. Water and experimental diets were freely available to birds at all times.
Test Material/Experimental Diets
The test material, MSM (Sigma Aldrich, St. Louis, MO), in the form of white crystalline powder, was included in a standard corn-soybean meal based diet at the rate of 0 or 0.05% (50 g/kg of feed or 1 lb/ton of feed). As a secondary dietary factor, a single lot of fresh soybean oil was either preserved intact (fresh oil) or heated to 90°C and oxidized by continuously bubbling air at the rate of 15 L/min for up to 72 h to reach a peroxide value of approximately 244 mEq/kg (Overholt et al., 2018). Briefly, soybean oil was heated in stainless steel heating pots (53 cm circumference, 61 cm high) that were filled only up to two-thirds of the maximum volume to ensure proper oxygen supply. Immersion heaters were used to heat the oil and temperatures were recorded at regular intervals. Multiple batches of oil were prepared and pooled into a container and stored at room temperature in plastic cans until feed manufacture. Feed was mixed in 2 separate batches before the initiation of respective cohorts, and samples of oil were collected and stored at 4°C prior to each feed mixing. All experimental diets contained 5% of oil, either fresh or oxidized, to induce oxidative stress in broilers, and MSM replaced silica sand in the final diet (Table 1). Composition and peroxidation analysis (Barrow-Agee, Memphis, TN) of fresh and oxidized oil is presented in Table 2. Overall, there were 4 dietary treatment groups: (1) fresh oil-no MSM, (2) fresh oil-0.05% MSM, (3) oxidized oil-no MSM, (4) oxidized oil-0.05% MSM.
Table 1.
| Ingredients | Concentration, g/kg |
|---|---|
| Corn | 483.8 |
| Soybean meal | 420.0 |
| Soy oil | 50.0 |
| Salt | 4.0 |
| Limestone | 11.5 |
| Dicalcium phosphate | 19.0 |
| Vitamin premix3 | 2.0 |
| Mineral premix4 | 1.5 |
| L-Lysine HCl | 1.1 |
| DL-Methionine | 3.2 |
| L-Threonine | 0.9 |
| Choline chloride | 2.0 |
| MSM | 0.5 |
| Calculated nutrient content, g/kg as-is basis (unless otherwise noted) | |
| AMEn (kcal/kg) | 3085 |
| CP | 234.7 |
| Ca | 10 |
| nPP | 5 |
| Total P | 7.8 |
| Arg | 15 |
| His | 5.8 |
| Ile | 9.1 |
| Leu | 17.5 |
| Lys | 12.9 |
| Met | 6.4 |
| Met+Cys | 9.5 |
| Phe | 10.5 |
| Phe + Tyr | 16.6 |
| Thr | 8.7 |
| Trp | 2.6 |
| Val | 9.8 |
Abbreviation: MSM, methylsulfonylmethane.
All experimental diets contained 5% of either fresh or oxidized soy oil, and MSM replaced silica sand in the final diets.
Diets were analyzed to contain no detectable concentration of MSM in fresh oil-no MSM and oxidized oil-no MSM diets, and 57.30 and 43.49 g of MSM/kg of fresh oil-MSM and oxidized oil-MSM diets respectively.
Provided per kg of complete diet Vit A, 4625.5 IU; Vit D3, 1050 IU; Vit E 11.56 IU, Menadione sodium bisulfite complex, 1.27 mg; Vit B12, 0.011 mg; Riboflavin, 4.62 mg; d-pantothenic acid, 10.5 mg; Niacin, 23.12 mg.
Provided per kg of complete diet calcium from CaCO3, 328 mg; Cu from CuSO4, 5.28 mg; I from ethylene diamine dihydroiodide, 0.8 mg; Fe from FeSO4, 80 mg; Mn from MnO, 80 mg; Se from Na2SeO3, 0.11 mg; Zn from ZnO, 80 mg.
Table 2.
| Outcome | Oil type |
|
|---|---|---|
| Fresh | Oxidized | |
| Temperature,°C | 22.5 | 90 |
| Time heated, h | 0 | 72 |
| Air flow, L/min | 0 | 15 |
| Fatty acids, % of total fat | ||
| C14:0, Myristic | 0.06 | 0.07 |
| C16:0, Palmitic | 10.79 | 11.82 |
| C16:1, Palmitoleic | 0.09 | 0.09 |
| C17:0, Margaric | 0.09 | 0.10 |
| C18:0, Stearic | 3.85 | 4.20 |
| C18:1, Oleic | 24.67 | 26.46 |
| C18:2, Linoleic | 52.41 | 49.89 |
| C18:3, Linolenic | 6.71 | 5.62 |
| C20:0, Arachidic | 0.35 | 0.37 |
| C20:1, Gadoleic | 0.21 | 0.21 |
| C22:0, Behenic | 0.35 | 0.39 |
| C24:0, Lignoceric | 0.12 | 0.15 |
| Other FA3 | 0.31 | 0.62 |
| UFA:SFA | 3.60 | 3.31 |
| IV4 | 130 | 124 |
| Free fatty acids, % | 0.02 | 0.28 |
| Moisture, % | 0.02 | 0.02 |
| Insoluble impurities, % | 0.14 | 0.08 |
| Unsaponifiable matter, % | 1.01 | 0.74 |
| Peroxide value, meq/kg | 37.2 | 244 |
| p-Anisidine value | 2.1 | 122 |
| Oxidized FA, % | 1.0 | 2.2 |
| Total tocopherols, mg/kg | 1419 | 87 |
| Alpha | 60 | 87 |
| Beta | < 10 | < 10 |
| Delta | 170 | < 10 |
| Gamma | 1189 | < 10 |
IV, iodine value; UFA:SFA, unsaturated:saturated fatty acid ratio.
There are no units for p-anisidine value, UFA: SFA, and IV.
Other FA detected besides those listed included here.
Iodine values were calculated using the FA profile data following Meadus et al., 2010: VI = (16:1 × 0.95) + (18:1 × 0.86) + (18:2 × 1.732) + (18:3 × 2.616) + (20:1 × 0.795) + (20:2 × 1.57) + (20:3 × 2.38) + (20:4 × 3.19) + (20:5 × 4.01) + (22:4 × 2.93) + (22:5 × 3.68) + (22:6 × 4.64).
Experimental Design
The experiment was conducted in 2 cohorts separated by 1 wk, which was necessary to accommodate facility limitations in terms of replicate pens. As described further in the statistical analysis section, a posthoc analysis that included cohort in the model confirmed that splitting the study into 2 cohorts of birds had minimal effect on study outcomes. Birds were weighed, selected, wing-banded and allocated to 1 of 4 dietary treatment groups. A total of 528 birds (192 and 336 birds in cohorts 1 and 2, respectively) with uniform weights were randomly allocated to brooder battery cages with 4 dietary treatments fed to a total of 4 (cohort 1) and 7 (cohort 2) replicate cages each containing 12 birds. Birds were given ad libitum access to their respective diets and water throughout the study.
Data and Sample Collection
One randomly selected bird in each cage was humanely euthanized with CO2 gas to collect blood by cardiac puncture on study d 7, 14, and 21. The following tissues were also collected immediately after blood collection: liver, spleen, heart, kidney, brain, cecal tonsils, abdominal skin and knee joint (bone and cartilage). Blood (plasma) and tissue samples from each collection day were used to quantify MSM concentrations by gas chromatography as previously described (Rasheed et al., 2019). Individual bird and group feeder weights were recorded weekly throughout the 21-D feeding phase to calculate body weight gain, feed intake, and feed efficiency to assess growth performance. Mortality and culls were monitored daily and used to adjust feed efficiency.
Oxidative Stress Markers
Plasma and liver samples from days 7, 14, and 21 were used to quantify biomarkers of oxidative stress including concentrations of thiobarbituric acid reactive substances (TBARS), total antioxidant capacity (TAC), total glutathione (TGSH), and glutathione peroxidase (GPx) and glutathione reductase (GR) activities using plate-based colorimetric assay kits (Cayman Chemicals, Ann Arbor, MI). Among these parameters, TGSH and GR were measured only in liver samples, as the plasma levels of these markers were too low to be detected. Liver and plasma samples were stored at −80°C until analysis.
Thiobarbituric Acid Reactive Substances
Lipid peroxidation was estimated by measuring malondialdehyde by reacting it with thiobarbituric acid under high temperature and acidic conditions (Janero, 1990). The resultant products were colorimetrically measured at 530 nm using the protocol outlined by the manufacturer. Briefly, 25 mg of liver tissue was homogenized in 250 μL of radioimmunoprecipitation assay buffer (Cayman Chemicals, Ann Arbor, MI) and centrifuged at 1,600× g for 10 min at 4°C. The resultant supernatant was used to conduct the TBARS assay. No prior processing methods were required for plasma samples. Briefly, 100 μL of sample was mixed with 100 μL of 10% trichloroacetic acid and 800 μL of color reagent (20% acetic acid, 0.5% thiobarbituric acid, and 20% 3.5 M sodium hydroxide). The mixture was incubated in a boiling water bath for 1 h, cooled on ice for 10 min, and centrifuged (1,600× g for 10 min at 4°C) prior to the supernatant being used to read the absorbance at 530 nm (BioTek Instruments Inc. Winooski, VT). Sample TBARS values were expressed as μM of malondialdehyde.
Total Antioxidant Capacity
A trolox-equivalent antioxidant assay was performed (Re et., al 1999) on all samples. Briefly, 10% w/v liver tissue was homogenized in a buffer solution containing 5 mM potassium phosphate (pH 7.4) containing 0.9% sodium chloride and 1.5 M potassium chloride. The solution was centrifuged at 1,600× g for 10 min at 4°C. The liver supernatant or blood plasma were initially mixed with metmyoglobin and 2,2-di-3ethylbenzthiazoline sulphonate (ABTS). The reaction was initiated by adding hydrogen peroxide, where metmyoglobin reacts with ABTS to form a blue-green chromophore, which decreases its intensity in the presence of antioxidants with absorbances measured at 750 nm (BioTek Instruments Inc. Winooski, VT). Values were expressed as mmol of trolox-equivalents per gram of tissue or liter of plasma.
Total Glutathione
The TGSH assay is based on the reaction between reduced glutathione (GSH) in the sample and Ellman’s reagent yielding a yellow-colored chromophore, 5-thio-2-nitrobenzoic acid (TNB), which is measured at 405 nm (Brehe and Burch, 1976, Rahman et al., 2006). The concomitant intermediate disulfide product (GS-TNB) formed is reduced to GSH by glutathione reductase in the presence of NADPH to recycle GSH and produce more TNB. The rate of TNB production is directly proportional to this recycling reaction, which is proportional to the amount of GSH present in the sample. In brief, 50 mg of liver tissue was homogenized with cold buffer containing 50 mM 2-(N-morpholino)ethanesulphonic acid, 0.1 M phosphate (pH 6–7), and 1 mM EDTA (MES buffer). The homogenate was centrifuged at 10,000× g for 15 min at 4°C, and the supernatant was deproteinated using 5% (w/v) metaphosphoric acid with the pH adjusted using 4 M triethanolamine. Oxidized glutathione (GSSG) at 25 μM reconstituted using MES buffer was used to prepare the standard curve. Subsequently, 50 μL of standard or sample was mixed with 150 μL of a solution containing MES buffer (11.25 mL), Ellman’s reagent (0.45 mL), glutathione reductase + glucose-6-phophatase mixture (2.1 mL), and NADP+ + glucose-6-phophatase mixture (0.45 mL) and incubated in the dark for 25 min in an orbital shaker (VWR International LLC, Radnor, PA) and absorbance were subsequently read at 405 nm (BioTek Instruments Inc. Winooski, VT).
Glutathione Peroxidase
A coupled reaction with NADPH and GR was used to measure the activity of GPx (Maraschiello et al., 1999). In this reaction, GSSG produced due to the reduction of hydroperoxide by GPx was reduced to GSH by GR and NADPH yielding NADP+ that causes a reduction in absorbance at 340 nm. As such, the rate of decrease in sample absorbance is directly proportional to the GPx activity in the sample. Liver samples after homogenization in buffer containing 50 mM Tris-HCl (pH 7.5), 5 mM EDTA, and 1 mM dithiothreitol ( Sigma Aldrich, St. Louis, MO) per gram of tissue was centrifuged at 10,000× g for 15 min at 4°C to obtain the supernatant for performing the assay. The reaction mixture consisted of 0.1 mM NADPH, 1 mM GSH, 1.2 units of GR, 50 mM Tris-HCl, 5 mM EDTA, and 20 μL of sample (liver extract or plasma) in a total volume of 170 μL. The reaction was initiated by adding 20 μL of cumene hydroperoxide. The absorbance was recorded at 340 nm once every minute for 5 min against a blank consisting of all the reagents except the sample, and a positive control containing all reagents + bovine erythrocyte GPx. The activity was expressed as nmol/min/mL for plasma and nmol/min/mg for wet tissue.
Glutathione Reductase
Glutathione reductase activity was measured by determining the rate of NADPH oxidation during the reduction of GSSG to GSH (Bompart et al., 1990). Liver tissue was homogenized in cold buffer containing 50 mM potassium phosphate (pH 7.5) and 1 mM EDTA per gram, centrifuged (10,000× g, 15 min, 4°C), and the resultant supernatant was used for the assay. A total of 20 μL of sample was added to a reaction medium containing 100 μL of cold buffer, and 20 μL of 9.5 mM GSSG. The reaction was initiated by adding 3.6 mM NADPH, and the absorbance was read every minute for 5 min at 340 nm against blank and a positive controls containing GR derived from baker’s yeast. The activity was expressed as nmol/min/mL for plasma and nmol/min/mg for wet tissue.
Immunophenotyping
After completion of 21-D feeding trial, all remaining birds (maximum of 9 birds per cage) were returned to their respective cages with their assigned diets. After 3 additional days on study (i.e., on study day 25), 1 randomly chosen bird per cage was humanely euthanized to permit collection of a whole blood sample. These blood samples were used to isolate peripheral blood mononuclear cells (PBMC) to enrich and quantify the T cell (TC) populations using a flow cytometry technique to understand whether dietary MSM shifted proportions of TC sub-sets in broilers exposed to diet-induced oxidative stress. Briefly, mononuclear cells were isolated by suspending blood cells onto a density gradient (Histopaque; Sigma Aldrich, St. Louis, MO). Following centrifugation (1,800× g for 20 min at 25°C without a brake), mononuclear cells were separated, washed twice, and their concentrations were adjusted to 1 × 106 total cells. Cells were then surface-stained using the following antibody clones and conjugated fluorochromes: anti-CD3-FITC (clone: CT-3; Cat. No.: 8200-02), anti-CD4-PE (clone: CT-4; Cat. No.: 8210-09), and anti-CD8α-APC (clone: CT-8; Cat. No.: 8220-11) (Southern Biotech, Birmingham, AL). After staining, cells were washed and permanently fixed with 2% paraformaldehyde for 10 min at room temperature. This was followed by 3 successive washes, and cells were kept at 4°C overnight until analysis the following day. The relative percentage of different phenotypes of T cells (i.e., single stain for CD4 or CD8 or double-positive T-cells) were determined using a multi-color flow cytometry analyzer (BD Biosciences, San Jose, CA).
Statistical Analyses
The experiment was conducted as a completely randomized design with individual cage as the experimental unit. Data were subjected to analyses of variance using the MIXED procedure of SAS (version 9.4; SAS Institute 2017, Cary, NC). In all cases, the statistical model included a random effect of cohort and replication nested within cohort to account for potential variation resulting from the experimental need to conduct the study using 2 groups of broiler chickens separated by one week. A 2-way ANOVA was performed for growth performance, flow cytometry, and oxidative stress data to determine whether the model was significant and means were separated using a Tukey-Kramer adjustment. Since MSM concentrations of most of the blood and tissue samples in both the control treatments (fresh oil, no MSM and oxidized oil, no MSM) were either not detectable or below quantification limit by gas chromatography, these non-MSM-containing treatment groups were excluded from the statistical analysis. Hence, a 1-way ANOVA was performed over the remaining data to test significance in MSM-containing diets differing only in oil type. All blood and tissue MSM data (i.e., raw concentrations) were log-normal transformed in order to stabilize variance structures for an accurate ANOVA. The data presented in results tables represent the raw least-square means, but model P-values and means separation procedures for MSM data were derived from the transformed datasets. Outliers were identified as having an absolute Studentized residual value of 3 or greater and significance was accepted with a P-value of less than 0.05. For outcomes where there were 1 or more missing values, the highest SEM for any individual was reported as the pooled SEM in results.
Results
Growth Performance
No differences (P > 0.4) in BW or BW gain outcomes were observed between any treatment groups in any of the study periods (data not shown). However, birds receiving oxidized oil-containing diets exhibited decreased feed intake (P < 0.006) and feed:gain (P < 0.006) during days 7 to 14 and days 0 to 21 when compared with birds receiving fresh oil-containing diets.
MSM Tissue Distribution
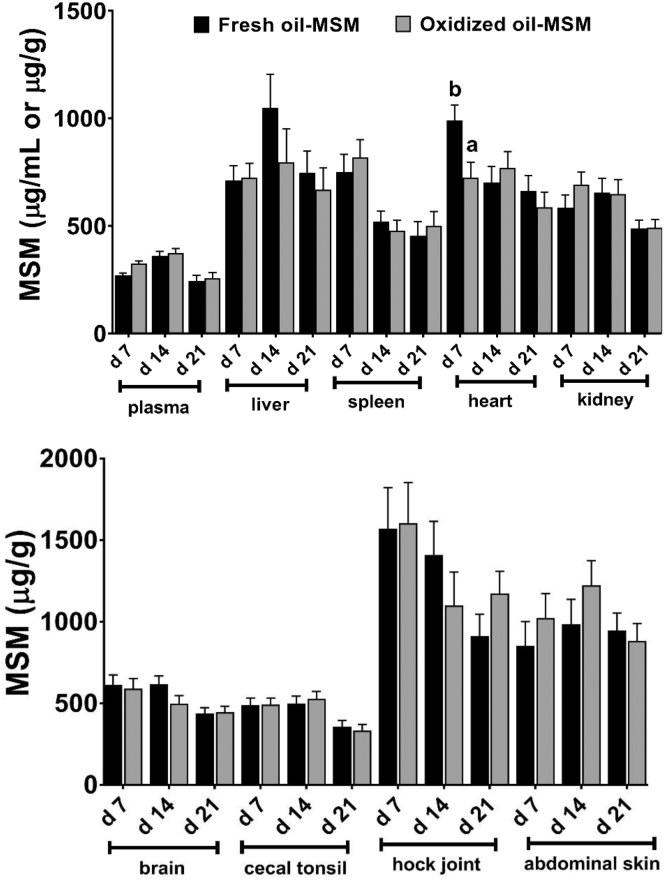
Except in control groups (fresh oil-no MSM and oxidized oil-no MSM), MSM was detected in all tissues and plasma samples at all time points (Figure 1). Overall, MSM was either nondetectable or below quantification limit in treatment groups not supplemented with MSM. As calculated from feed intake data, the estimated amounts of MSM that the birds in MSM groups may have ingested is presented in Table 3. Accordingly, birds may have ingested an estimated 414 (at days 0–7) to 694 (at days 0–14) mg of MSM/kg BW at various times throughout the study. A maximum plasma concentration of 373 μg/mL was observed at day 14 where the birds were estimated to have ingested the maximum dose of 694 mg/kg of MSM during the 0- to 14-D period. With this context, there were no significant differences in MSM concentrations between fresh and oxidized oil groups except in heart at day 7 when MSM was in higher concentration in the fresh oil group (P = 0.01).
Figure 1.
Plasma and tissue concentrations of methylsulfonylmethane (MSM) (μg/mL or μg/g of wet tissue) in birds fed 0.05% MSM with 5% of either fresh (black bars) or oxidized oil (grey bars) in the diet. Values represents least square means derived from 11 replicates total (4 and 7 reps in cohort 1 and 2 respectively; n = 11) initially containing 12 birds per cage. All birds received their assigned diets from study days 0 to 21.
Table 3.
Estimated MSM intake of birds throughout the 21-D feeding study.1
| Treatment | Feeding period | Cumulative feed intake, g/bird | Analyzed MSM, μg/g of feed | Ending BW, kg/bird | Calculated MSM intake, mg/kg BW |
|---|---|---|---|---|---|
| Fresh oil-MSM2 | d 0–7 | 136 | 416.8 | 0.137 | 414 |
| d 0–14 | 557 | 416.8 | 0.356 | 652 | |
| d 0–21 | 1124 | 416.8 | 0.751 | 624 | |
| Oxidized oil-MSM3 | d 0–7 | 131 | 478.2 | 0.137 | 457 |
| d 0–14 | 527 | 478.2 | 0.363 | 694 | |
| d 0–21 | 1065 | 478.2 | 0.754 | 676 |
Abbreviation: MSM, methylsulfonylmethane.
Calculated from measured BW and feed intake and dietary MSM analysis data.
Fresh oil diet containing 0.05% MSM.
Oxidized oil containing 0.05% MSM.
Immunophenotyping
T cell populations in blood (i.e., helper T cells, cytotoxic T cells and memory T cells) were not affected (P > 0.2; data not shown) by either dietary oil type or MSM supplementation.
Thiobarbituric Acid Reactive Substances
In the presence of oxidized oil, MSM reduced plasma TBARS at day 21, but did not affect TBARS when supplemented with fresh oil (interaction, P = 0.013; Table 4). In addition, compared with other groups, liver TBARS values were lower in oxidized oil—MSM group at day 14. No significant interactive effects of MSM and oil were observed for plasma or liver TBARS at any other time points. In addition, there were no differences in either plasma or liver TBARS due to main effect of MSM. However, the main effect of oil was observed at days 14 and 21, where plasma TBARS values were higher (P = 0.002) in the oxidized oil group at day 14 and liver TBARS values were higher in the fresh oil group at day 14 (P = 0.017) and day 21 (P = 0.03).
Table 4.
Plasma and liver TBARS of birds after supplementing MSM in feed with either fresh or oxidized oil.1
| Item | Plasma2 |
Liver2 |
||||
|---|---|---|---|---|---|---|
| Day 7 | Day 14 | Day 21 | Day 7 | Day 14 | Day 21 | |
| Interaction | ||||||
| Fresh oil | ||||||
| No MSM | 17.25 | 16.85a | 14.63a,b | 6.90 | 9.07b | 7.11 |
| MSM | 13.59 | 17.55a | 16.07b | 6.88 | 7.43a | 7.04 |
| Oxidized oil | ||||||
| No MSM | 18.11 | 21.83b | 16.43b | 7.30 | 7.07a | 6.76 |
| MSM | 17.81 | 22.12b | 11.74a | 7.53 | 6.95a | 6.27 |
| SEM | 1.776 | 1.116 | 1.196 | 0.692 | 0.533 | 0.274 |
| Main effect | ||||||
| Oil | ||||||
| Fresh | 15.41 | 17.20a | 15.35 | 6.89 | 8.25b | 7.08b |
| Oxidized | 17.96 | 21.98b | 14.09 | 7.41 | 7.01a | 6.51a |
| SEM | 1.268 | 0.808 | 0.846 | 0.458 | 0.358 | 0.181 |
| MSM | ||||||
| No | 17.68 | 19.34 | 15.53 | 7.10 | 8.07 | 6.93 |
| Yes | 15.70 | 19.84 | 13.91 | 7.20 | 7.19 | 6.66 |
| SEM | 1.268 | 0.808 | 0.846 | 0.470 | 0.358 | 0.181 |
| P-values | ||||||
| Cohort | 0.006 | 0.36 | 0.007 | 0.70 | 0.003 | 0.87 |
| Rep (cohort) | 0.08 | 0.42 | 0.32 | 0.35 | 0.18 | 0.66 |
| Oil | 0.15 | 0.002 | 0.28 | 0.38 | 0.017 | 0.030 |
| MSM | 0.26 | 0.65 | 0.17 | 0.86 | 0.08 | 0.27 |
| Oil × MSM | 0.34 | 0.01 | 0.013 | 0.83 | 0.03 | 0.40 |
Abbreviations: MSM, methylsulfonylmethane; TBARS, thiobarbituric acid reactive substances.
a,bMeans lacking a common superscript letter in a column differ (P < 0.05).
Least square means derived from 11 replicates total (4 and 7 reps in cohort 1 and 2 respectively; n = 11) initially containing 12 birds per cage. All birds received their assigned diets from study d 0 to 21. Time points reflects day of study. MSM was fed at 0 or 0.05% with 5% of either fresh or oxidized oil.
TBARS expressed as μmol MDA per gram of wet tissue or liter of plasma.
Total Antioxidant Capacity
No interactive effects (P > 0.4) in TAC were observed in either plasma or liver samples across time (Table 5). However, differences in TAC were observed due to main effects of oil and MSM. Birds supplemented with MSM exhibited a higher (P = 0.023) plasma TAC at d 7 compared with non-MSM-supplemented birds. Additionally, birds fed oxidized oil had higher TAC at days 7 (P = 0.006) and 21 (P = 0.002) in plasma, as well as higher TAC values at day 21 in liver (P < 0.001). However, plasma TAC was reduced (P = 0.002) in the oxidized oil group at day 14.
Table 5.
Total antioxidant capacity (TAC) of birds after supplementing MSM in feed with either fresh or oxidized oil.1
| Item | Plasma2 |
Liver2 |
||||
|---|---|---|---|---|---|---|
| Day 7 | Day 14 | Day 21 | Day 7 | Day 14 | Day 21 | |
| Interaction | ||||||
| Fresh oil | ||||||
| No MSM | 2.54 | 3.24 | 2.97 | 6.74 | 6.69 | 6.81 |
| MSM | 2.89 | 3.22 | 3.61 | 5.90 | 6.72 | 6.87 |
| Oxidized oil | ||||||
| No MSM | 3.14 | 2.26 | 4.03 | 7.50 | 7.07 | 10.42 |
| MSM | 3.60 | 2.42 | 4.17 | 6.04 | 6.62 | 10.19 |
| SEM | 0.182 | 0.211 | 0.316 | 0.695 | 0.867 | 0.600 |
| Main effect | ||||||
| Oil | ||||||
| Fresh | 2.72a | 3.23b | 3.29a | 6.32 | 6.71 | 6.84a |
| Oxidized | 3.67b | 2.34a | 4.10b | 6.77 | 6.84 | 10.31b |
| SEM | 0.126 | 0.151 | 0.205 | 0.496 | 0.585 | 0.428 |
| MSM | ||||||
| No | 2.84a | 2.74 | 3.50 | 7.12 | 6.88 | 8.62 |
| Yes | 3.24b | 2.81 | 3.89 | 5.97 | 6.67 | 8.53 |
| SEM | 0.122 | 0.151 | 0.205 | 0.496 | 0.585 | 0.428 |
| P-values | ||||||
| Cohort | 0.11 | <0.001 | 0.005 | 0.011 | 0.81 | 0.003 |
| Rep (cohort) | 0.005 | 0.47 | 0.71 | 0.72 | 0.84 | 0.049 |
| Oil | 0.006 | 0.002 | 0.002 | 0.51 | 0.85 | <0.001 |
| MSM | 0.023 | 0.73 | 0.16 | 0.10 | 0.78 | 0.88 |
| Oil × MSM | 0.73 | 0.67 | 0.36 | 0.66 | 0.75 | 0.81 |
Abbreviation: MSM, methylsulfonylmethane.
a,bMeans lacking a common superscript letter in a column differ (P < 0.05).
Least square means derived from 11 replicates total (4 and 7 reps in cohort 1 and 2 respectively; n = 11) initially containing 12 birds per cage. All birds received their assigned diets from study days 0 to 21. Time points reflects day of study.MSM was fed at 0 or 0.05% with 5% of either fresh or oxidized oil.
Values expressed as millimole of trolox equivalence per gram of wet tissue or liter of plasma.
Total Glutathione
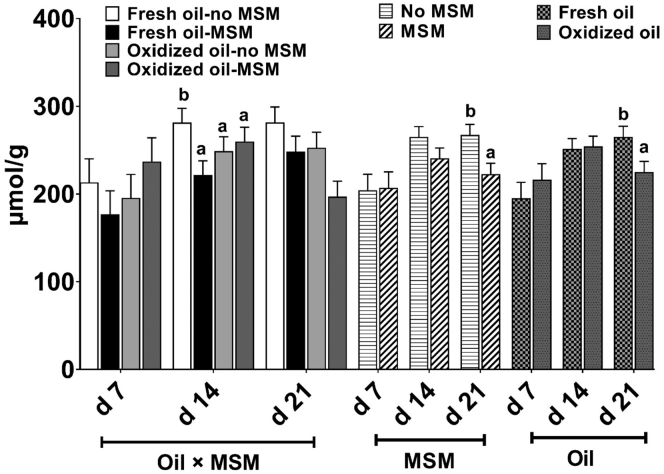
Using the current technique, TGSH was not detectable in plasma samples due to its very low concentration. However, main effects of oil and MSM supplementation, as well as their interaction, were observed for liver TGSH concentrations. Birds assigned to the fresh oil-no MSM treatment had higher (P = 0.03) liver TGSH concentrations at day 14, compared with all other groups (Figure 2). Additionally, birds supplemented with MSM showed lower (P = 0.012) TGSH at day 21 compared with birds not receiving MSM. Compared with birds consuming oxidized oil, TGSH was higher (P = 0.02) in groups that received fresh oil diets at day 21.
Figure 2.
Total liver glutathione concentration of birds fed 0 (control) or 0.05% methylsulfonylmethane (MSM) with 5% of either fresh or oxidized oil in the diet. Values represents least square means derived from 11 replicates total (4 and 7 reps in cohort 1 and 2 respectively; n = 11) initially containing 12 birds per cage. All birds received their assigned diets from study days 0 to 21.
Glutathione Peroxidase
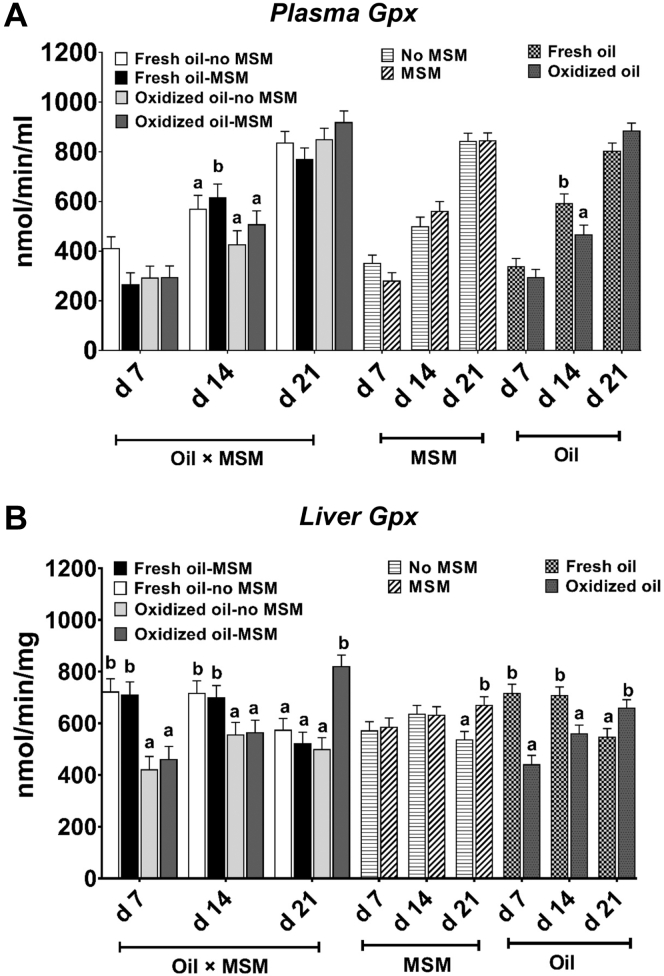
There were no interactive effects (P > 0.1) of MSM and oil on plasma GPx activity at any time points (Figure 3A). Irrespective of oil type, there were also no main effects (P > 0.09) of MSM on plasma GPx activity at any time point. However, plasma GPx activity was higher (P = 0.01) in birds receiving fresh oil, as compared with oxidized oil, at day 14. An interactive effect of oil and MSM supplementation was observed for liver GPx activity (Figure 3B), where increased (P = 0.001) activity was observed in oxidized oil-MSM-fed birds compared with all other treatment groups at day 21. Additionally, MSM supplemented groups had higher (P = 0.003) liver GPx activity at day 21 compared with nonsupplemented groups. Compared with groups receiving fresh oil, oxidized oil groups had lower (P < 0.001) liver GPx at days 7 and 14, and higher (P = 0.01) liver GPx at d 21.
Figure 3.
(A) Plasma and (B) liver glutathione peroxidase activity of birds fed 0 (control) or 0.05% methylsulfonylmethane (MSM) with 5% of either fresh or oxidized oil in the diet. Values represents least square means derived from 11 replicates total (4 and 7 reps in cohort 1 and 2 respectively; n = 11) initially containing 12 birds per cage. All birds received their assigned diets from study days 0 to 21.
Glutathione Reductase
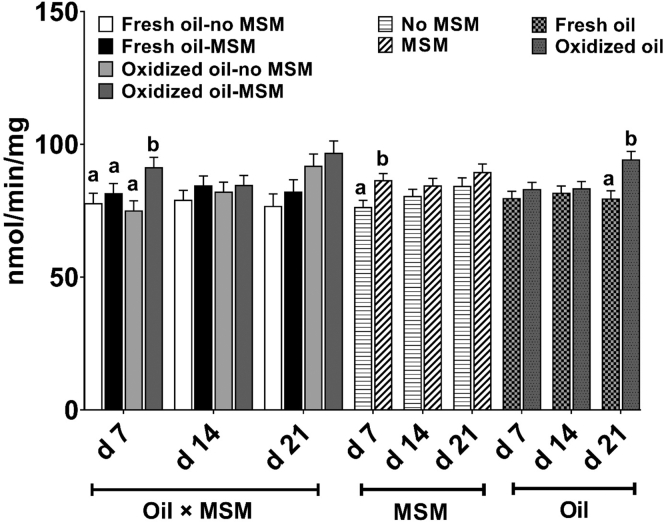
Due to very low concentration, GR activity was not detectable in plasma at any time points. There were no interactive effects (P > 0.06) of oil type and MSM supplementation on liver GR activity at any time points (Figure 4). Groups of birds supplemented with MSM showed higher (P = 0.004) liver GR activity at d 7 compared with groups not receiving MSM. Additionally, liver GR activity was higher (P = 0.001) in groups fed oxidized oil, compared with those receiving fresh oil, at d 21.
Figure 4.
Glutathione reductase activity of birds fed 0 (control) or 0.05% methylsulfonylmethane (MSM) with 5% of either fresh or oxidized oil in the diet. Values represents least square means derived from 11 replicates total (4 and 7 reps in cohort 1 and 2 respectively; n = 11) initially containing 12 birds per cage. All birds received their assigned diets from study days 0 to 21.
Discussion
Oxidative stress is defined as the imbalance between pro-oxidants and antioxidant systems (Seis, 1985) or the disruption of redox signaling and its control (Jones, 2006) in the body. Many studies reported deleterious effects of feeding excess oil or oxidized oil in poultry diets; reduced growth performance and feed utilization (Inoue et al., 1984, Calabotta and Shermer, 1985), gastrointestinal tract disturbances, and increased hepatic cell proliferation (Dibner et al., 1996) are commonly reported effects. In the current experiment, we tested the antioxidant property of MSM supplementation in broilers experiencing mild oxidative stress due to ingestion of oxidized oil in the diet. Because rancid oil/fat in the feed is one of the main causes of oxidative stress in poultry (Engberg et al., 1996), we used thermally peroxidized soybean oil delivered via feed to induce oxidative stress. The temperature selected for lipid peroxidation in the current study has close resemblance to the temperature used in rendering of animal fat. This would simulate field conditions as poultry producers use rendered fat to meet the high energy-requirements of their birds.
Growth performance parameters of birds in the current study were not affected by supplementing 0.05% MSM in the diet. We obtained similar results in a previous study involving oral gavage of MSM at 1500 mg/kg daily for 21 D (Rasheed et al., 2019). Most of the previous studies on MSM (Hui-fang and An-guo, 2008, Hwang et al., 2017) also reported no effects on growth performance, except Jiao et al. (2017), where they observed a growth performance improvement in broilers fed 0.2% MSM. We expected to observe decreased growth of broilers fed oxidized oil as it may affect nutrient absorption and utilization (Engberg et al., 1996). Interestingly, growth parameters of broilers in the current study were not affected by oxidized oil, leading us to conclude we had induced mild oxidative stress. This observation was a contradiction to most of the previous studies involving oxidized oil intake by broilers, where inclusion of 0.055% sunflower oil (PV = 400 mEq/kg; Lin et al., 1989), 11% rapeseed plus soy oil (PV = 156 mEq/kg; Engberg et al., 1996), or 4% poultry fat (PV = 175 mEq/kg; Cabel et al., 1988) caused a clear reduction in growth performance of broilers. The authors attributed this growth depression to the lower biological value of oxidized oil/fat in these diets. However, there are also reports that dietary oxidation status had no effect on growth performance of broilers (Carpenter and L'estrange, 1966, Açıkgöz et al., 2011). Similar effects were observed in rats (Lea et al., 1966), turkeys (L'Estrange et al., 1966), and calves (Jenkins and Emmons, 1984). According to Cabel et al (1988), adverse effects of dietary lipid-peroxidation on growth performance is not consistent, and conflicting opinion exists on the threshold level of rancidity required to induce a negative effect on broiler growth. In support of this premise, a recent experiment in pigs fed peroxidized soy oil with 3 levels of peroxidation (PV = 17.4, 123.6, and 194.0 mEq/kg) caused a reduction in ADG at a PV 123.6 mEq/kg, but not when included at 17.4 mEq/kg (Overholt et al., 2018). Interestingly, the authors did not observe any growth adversities due to inclusion of oil at the highest peroxidation level (PV = 194.0 mEq/kg). Hence, it can be speculated that the threshold value of peroxidation in dietary lipid to induce a negative effect in poultry growth performance may be higher than the current level of peroxidation (PV = 244 mEq/kg), or that pigs and chickens simply handle/metabolize dietary oxidized oils differently. In addition, DeRouchey et al. (2004) observed that ADG of pigs fed 6% rancid choice white grease (PV = 105 mEq/kg) was decreased due to reduction in feed intake and not due to the nutritional inadequacies of the oxidized diet, as they observed no effect of oxidized oil on the digestibility of fatty acids. We obtained similar results that the birds fed oxidized oil had decreased feed intake but BW gain was never affected, thereby leading to an unexpected improvement in FCR of birds fed oxidized oil throughout the study. This suggests that fat digestibility might not have been affected, which is why no effects of peroxidation on growth performance were observed.
After supplementing 0.05% MSM via the feed, MSM was quantified in plasma and all tissues at all time points throughout the study. Although the birds ingested varying amounts of MSM at various periods of their growth (see Table 3), this exogenous MSM was clearly quantified in tissues throughout the body. In our previous study with broilers, MSM was observed to have been well absorbed and distributed throughout various tissues after daily oral gavage of MSM in water at 1,500 mg/kg (Rasheed et al., 2019). Similar kinetics were observed in the current study, which shows that a continuous supply of MSM at 0.05% of the diet appears to be rapidly absorbed and distributed throughout the body. Moreover, a maximal plasma concentration of 373 μg/mL was observed at day 14, at which point those birds would have ingested the estimated maximum dose of 694 mg/kg BW of MSM during the 0- to 14-D period. There were no differences in MSM concentrations quantified in plasma and tissues of fresh and oxidized oil groups, except in abdominal skin. This suggests that dietary oxidation status may not affect MSM absorption and distribution, which matches with evidence from rat studies where MSM was shown to be well absorbed and distributed throughout the body (Otsuki et al., 2002, Magnuson et al., 2007) with a carrier independent unsaturable absorption from the small intestine (Wong et al., 2017). In agreement with our previous study regarding MSM distribution after oral gavage in broilers, the current data suggest that MSM supplemented via feed also has similar tissue distribution patterns.
Reduction in cytokine synthesis during inflammation is thought to be a major mechanism by which MSM reduces inflammation (Kim et al., 2009). However, to the best of our knowledge, no studies have attempted to explore the direct effect of MSM on B and T lymphocytes. Current results show that MSM supplemented at 0.05% of the diet did not affect T cell subpopulations in broilers, regardless of the oil type they ingested. This could be because of the low inclusion rate of MSM in the feed or the compound itself may not have any effect on T cell population. However, dietary lipids are reported to affect immune cell functions. Grimble (1998) and Miles and Calder (1998) explain the mechanisms involving altered immune cell functions in response to dietary lipid composition as influencing fluidity of cell membranes, signaling molecules, binding affinity, and the overall inflammatory response. In addition, comparative studies have investigated dietary fatty acids effects on inhibition of lymphocyte proliferation in cell cultures (Mertin et al., 1974, Mihas et al., 1975, Buttke, 1984). Many unsaturated fatty acids (e.g., linoleic, α-linolenic, γ-linolenic, dihomo-γ-linolenic, arachidonic, eicosapentaenoic, and docosahexaenoic acids) have the ability to reduce T cell proliferation. This ability of polyunsaturated fatty acids is used to prevent atherosclerosis and other chronic inflammatory processes (Calder, 1998). However, compared to oleic acid, most of the monounsaturated fatty acids have reduced ability to modulate lymphocyte proliferation. In the current study, the fresh and peroxidized oils were analyzed to contain numerically different concentrations of oleic and linoleic acids, even though the peroxidized oil was produced using the exact same fresh oil as a starting material. As supported by previous literature cited above, we believe fatty acid composition may be one of the reasons why we were unable to detect an effect of oil peroxidation on T cell profiles.
To assess antioxidant potential of MSM in broilers, we measured redox status of birds by quantifying various oxidant-antioxidant defense biomarkers. Inconsistent results have been observed on the various oxidative stress biomarkers due to MSM, oxidized oil, or their interaction. The TBARS assay, which measures a carbonyl compound derived due to lipid peroxidation in the body, is one of the most widely used measures of oxidative stress. Previous studies testing antioxidant properties of MSM showed contradictory results, as MSM reduced TBARS when supplemented at 8 mg/kg BW for 7 D in horses (Marañón et al., 2008), 50 mg/kg BW for 10 D in humans (Nakhostin-Roohi et al., 2011), and 400 mg/kg BW for 5 D (Kamel and El Morsy, 2013) or 10 D in rats (Mohammadi et al., 2012). In contrast to these data, Withee et al. (2017) reported no effects of MSM supplementation at 3 g/D for 21 D on TBARS in men or women after an exercise-induced oxidative stress. Birds in the current study did not show a main effect of MSM on TBARS either in plasma or liver samples. However, in the presence of oxidized oil, MSM reduced TBARS in plasma (day 21) and liver (day 14) but did not have any effects in fresh oil treatments. This suggests that MSM may help lower TBARS during diet-induced oxidative stress as oxidized oil is expected to increase TBARS in the body. However, TBARS were either unaffected at most of the time points or actually lower in some cases (e.g., in liver at day 21). Total antioxidant capacity, another good indicator of redox potential, was also not influenced by either MSM supplementation or oil type. While Barmaki et al (2012) and Nakhostin-Roohi et al. (2011) reported MSM increased TAC during exercise-induced muscle damage, Kalman et al (2013) did not observe any effect of MSM on TAC. In agreement, there was no evidence from the current study to support that MSM improved TAC of broilers. When under oxidative stress conditions, one would expect to experience decreased overall antioxidant capacity. However, when compared with the fresh oil groups, TAC in birds ingesting oxidized oil was either increased (at days 7 and 14 in plasma, and day 21 in liver) or remained unaffected (at all other time points). It is possible that the production of antioxidants have increased in response to the oxidative stress in oxidized oil group, and then remained at a peak level. However, further investigation to study the temporal changes in TAC in response to an oxidative stress will be beneficial.
Previous reports suggest that MSM may be helpful in counteracting oxidative stress in broilers through regulation of the glutathione system (TGSH, GPx, and GR). Glutathione represents a primary endogenous antioxidant. In the current study, TGSH concentration was not improved in MSM-fed groups, which suggests that MSM may not help increase the concentration of glutathione in the body or the level of MSM used in the study might not have been enough to see any positive response. As expected, TGSH concentrations were lower in birds receiving oxidized oil at day 21, which may be due to glutathione being used to counteract oxidative stress. Overall, MSM-supplemented birds exhibited overall increased activity of liver GPx at day 21 and GR at day 7. In support of this observation, previous studies have reported beneficial effects of MSM on glutathione enzymes in rats and humans (DiSilvestro et al., 2008, Mohammadi et al., 2012, Kamel and El Morsy, 2013). However, MSM did not affect GPx and GR at any other time points. Induction of oxidative stress is expected to have enhanced glutathione enzyme activity, as these enzymes help in eliminating excess free radicals; nevertheless, we observed a lower plasma GPx (at day 14) and liver GPx (days 7 and 14) activity in oxidized oil group compared to fresh oil group.
There are no published data regarding the effect of MSM on oxidative stress biomarkers during mild diet-induced oxidative stress in broilers. Our study provides preliminary evidence that MSM may partially improve redox potential of birds during mild oxidative stress, but TC populations do not appear to be altered under these conditions. Overall, the following conclusions can be drawn from our study: (1) MSM included at 0.05% of the diet is well absorbed and distributed throughout various tissues, and ingestion of oxidized oil does not appear to influence MSM distribution kinetics, (2) neither ingestion of 0.05% MSM or 5% oxidized oil to induce mild oxidative stress influence BW gain of broilers, (3) 0.05% dietary MSM supplementation for 21 d may partially protect birds from mild oxidative stress by reducing TBARS and improving glutathione-related outcomes, and (4) at the levels used, neither MSM nor oxidized oil appear to affect subsets of TC population in broiler chickens. Compared to other MSM studies on oxidative stress and immune function, dose of MSM used in the present study was low (estimated 414–694 mg/kg BW when supplemented at the rate of 0.05% of the diet). Therefore, this level of inclusion might not have been sufficient to observe a significant change in the outcomes measured. Perhaps effects are also easier to observe in an oxidative stress model severe enough to show physiological effects on growth performance. Additional studies are warranted to establish whether a higher inclusion rate of MSM may be helpful in promoting antioxidant potential and immune status of broilers undergoing mild oxidative stress.
References
- Açıkgöz Z., Bayraktar H., Altan Ö, Akhisaroglu S.T., Kırkpınar F., Altun Z. The effects of moderately oxidised dietary oil with or without vitamin E supplementation on performance, nutrient digestibility, some blood traits, lipid peroxidation and antioxidant defense of male broilers. J. Sci. Food Agric. 2011;91:1277–1282. doi: 10.1002/jsfa.4311. [DOI] [PubMed] [Google Scholar]
- Altan Ö, Pabuçcuoğlu A., Altan A., Konyalioğlu S., Bayraktar H. Effect of heat stress on oxidative stress, lipid peroxidation and some stress parameters in broilers. Brit. Poult. Sci. 2003;44:545–550. doi: 10.1080/00071660310001618334. [DOI] [PubMed] [Google Scholar]
- Amirshahrokhi K., Khalili A.R. Methylsulfonylmethane is effective against gastric mucosal injury. Eur. J. Pharmacol. 2017;811:240–248. doi: 10.1016/j.ejphar.2017.06.034. [DOI] [PubMed] [Google Scholar]
- Avanzo J.L., de Mendonça C.X., Jr., Pugine S.M.P., de Cerqueira Cesar M. Effect of vitamin E and selenium on resistance to oxidative stress in chicken superficial pectoralis muscle. Pharmacol. Toxicol. 2001;129:163–173. doi: 10.1016/s1532-0456(01)00197-1. [DOI] [PubMed] [Google Scholar]
- Barmaki S., Bohlooli S., Khoshkhahesh F., Nakhostin-Roohi B. Effect of methylsulfonylmethane supplementation on exercise—induced muscle damage and total antioxidant capacity. J. Sports Med. Phys. Fitness. 2012;52:170. [PubMed] [Google Scholar]
- Beckman K.B., Ames B.N. The free radical theory of aging matures. Physiol. Rev. 1998;78:547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- Bottje W.G., Harrison P.C. Effect of carbonated water on growth performance of cockerels subjected to constant and cyclic heat stress temperatures. Poult. Sci. 1985;64:1285–1292. doi: 10.3382/ps.0641285. [DOI] [PubMed] [Google Scholar]
- Bompart G.J., Prévot D.S., Bascands J.L. Rapid automated analysis of glutathione reductase, peroxidase, and S-transferase activity: application to cisplatin-induced toxicity. Clin. Biochem. 1990;23:501–504. doi: 10.1016/0009-9120(90)80039-l. [DOI] [PubMed] [Google Scholar]
- Brehe J.E., Burch H.B. Enzymatic assay for glutathione. Anal. Biochem. 1976;74:189–197. doi: 10.1016/0003-2697(76)90323-7. [DOI] [PubMed] [Google Scholar]
- Buttke T.M. Inhibition of lymphocyte proliferation by free fatty acids. I. Differential effects on mouse B and T lymphocytes. Immunol. 1984;53:235. [PMC free article] [PubMed] [Google Scholar]
- Cabel M.C., Waldroup P.W., Shermer W.D., Calabotta D.F. Effects of ethoxyquin feed preservative and peroxide level on broiler performance. Poult. Sci. 1988;67:1725–1730. doi: 10.3382/ps.0671725. [DOI] [PubMed] [Google Scholar]
- Calder P.C. Fat chance of immunomodulation. Immunol. Today. 1998;19:244–247. doi: 10.1016/s0167-5699(98)01264-x. [DOI] [PubMed] [Google Scholar]
- Calabotta D.F., Shermer W.D. Controlling feed oxidation can be rewarding. Feedstuffs. 1985;25:24. [Google Scholar]
- Carpenter K.J., L'estrange J.L. Effects of moderate levels of oxidized fat in animal diets under controlled conditions. Proc. Nutr. Soc. 1966;25:25–31. doi: 10.1079/pns19660007. [DOI] [PubMed] [Google Scholar]
- Debbi E.M., Agar G., Fichman G., Ziv Y.B., Kardosh R., Halperin N., Debi R. Efficacy of methylsulfonylmethane supplementation on osteoarthritis of the knee: a randomized controlled study. BMC Complement Altern. Med. 2011;11:50. doi: 10.1186/1472-6882-11-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRouchey J.M., Hancock J.D., Hines R.H., Maloney C.A., Lee D.J., Park J.S. Effects of rancidity and free fatty acids in choice white grease on growth performance and nutrient digestibility in weanling pigs. J. Anim. Sci. 2004;82:2937–2944. doi: 10.2527/2004.82102937x. [DOI] [PubMed] [Google Scholar]
- Dibner J.J., Atwell C.A., Kitchel M.L., Shermer W.D., Ivey F.J. Feeding of oxidized fats to broilers and swine: effects on enterocyte turnover, hepatocyte proliferation and the gut associated lymphoid tissue. Anim. Feed Sci. Technol. 1996;62:1–13. [Google Scholar]
- DiSilvestro R.A., DiSilvestro D.J., DiSilvestro D.J. Methylsulfonylmethane (MSM) intake in mice produces elevated liver glutathione and partially protects against carbon tetrachloride-induced liver injury. FASEB J. 2008;22:445–448. [Google Scholar]
- Engberg R.M., Lauridsen C., Jensen S.K., Jakobsen K. Inclusion of oxidized vegetable oil in broiler diets. Its influence on nutrient balance and on the antioxidative status of broilers. Poult. Sci. 1996;75:1003–1011. doi: 10.3382/ps.0751003. [DOI] [PubMed] [Google Scholar]
- Grimble R.F. Dietary lipids and the inflammatory response. Proc. Nutr. Soc. 1998;57:535–542. doi: 10.1079/pns19980078. [DOI] [PubMed] [Google Scholar]
- Herschler R.J., Herschler R.J. U.S. Patent 5071878; 1991. Use of methylsulfonylmethane to enhance diet of an animal. [Google Scholar]
- Hui-fang L.U.I., An-guo Z.H.O. Effects of plant extracts, cysteamine and methylsulfonylmethane on productive performance and slaughter characteristics in meat ducks. Nat. Prod. Res. 2008;20:302–306. [Google Scholar]
- Hwang J.W., Cheong S.H., Kim Y.S., Lee J.W., You B.I., Moon S.H., Park P.J. Effects of dietary supplementation of oriental herbal medicine residue and methyl sulfonyl methane on the growth performance and meat quality of ducks. Anim. Prod. Sci. 2017;57:948–957. [Google Scholar]
- Inoue T., Kurashige A., Minetoma T., Shigyo F. XVII World’s Poultry Congress; Helsinki, Finland: 1984. Nutritional effect of oxidized soybean oil in broiler diets; pp. 368–369. [Google Scholar]
- Jenkins K.J., Emmons D.B. Tolerance of calves to fat peroxides in milk replacer. J. Dairy Sci. 1984;67:592–597. [Google Scholar]
- Jiao Y., Park J.H., Kim Y.M., Kim I.H. Effects of dietary methyl sulfonyl methane (MSM) supplementation on growth performance, nutrient digestibility, meat quality, excreta microbiota, excreta gas emission, and blood profiles in broilers. Poult. Sci. 2017;96:2168–2175. doi: 10.3382/ps/pew480. [DOI] [PubMed] [Google Scholar]
- Janero D.R. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Rad. Biol. Med. 1990;9:515–540. doi: 10.1016/0891-5849(90)90131-2. [DOI] [PubMed] [Google Scholar]
- Jones D.P. Redefining oxidative stress. Antiox. Redox Signal. 2006;8:1865–1879. doi: 10.1089/ars.2006.8.1865. [DOI] [PubMed] [Google Scholar]
- Kalman D., Feldman S., Samson A., Krieger D. A randomized double blind placebo controlled evaluation of MSM for exercise induced discomfort/pain. FASEB J. 2013;27:1076–1077. [Google Scholar]
- Kamel R., El Morsy E.M. Hepatoprotective effect of methylsulfonylmethane against carbon tetrachloride-induced acute liver injury in rats. Arch. Pharm. Res. 2013;36:1140–1148. doi: 10.1007/s12272-013-0110-x. [DOI] [PubMed] [Google Scholar]
- Kim L.S., Axelrod L.J., Howard P., Buratovich N., Waters R.F. Efficacy of methylsulfonylmethane (MSM) in osteoarthritis pain of the knee: a pilot clinical trial. Osteoarthritis Cartilage. 2006;14:286–294. doi: 10.1016/j.joca.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Kim Y.H., Kim D.H., Lim H., Baek D.Y., Shin H.K., Kim J.K. The anti-inflammatory effects of methylsulfonylmethane on lipopolysaccharide-induced inflammatory responses in murine macrophages. Biol. Pharm. Bull. 2009;32:651–656. doi: 10.1248/bpb.32.651. [DOI] [PubMed] [Google Scholar]
- Lea C.H., Parr L.J., L'Estrange J.L., Carpenter K.J. Nutritional effects of autoxidized fats in animal diets. 3. The growth of turkeys on diets containing oxidized fish oil. Br. J. Nutr. 1966;20:123–133. doi: 10.1079/bjn19660014. [DOI] [PubMed] [Google Scholar]
- L'estrange J.L., Carpenter K.J., Lea C.H., Parr L.J. Nutritional effects of autoxidized fats in animal diets: Beef fat in the diet of broiler chicks. Br. J. Nutr. 1966;20:113–122. doi: 10.1079/bjn19660013. [DOI] [PubMed] [Google Scholar]
- Lin C.F., Asghar A., Gray J.I., Buckley D.J., Booren A.M., Crackel R.L., Flegal C.J. Effects of oxidised dietary oil and antioxidant supplementation on broiler growth and meat stability. Brit. Poult. Sci. 1989;30:855–864. doi: 10.1080/00071668908417212. [DOI] [PubMed] [Google Scholar]
- Magnuson B.A., Appleton J., Ryan B., Matulka R.A. Oral developmental toxicity study of methylsulfonylmethane in rats. Food Chem. Toxicol. 2007;45:977–984. doi: 10.1016/j.fct.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Marañón G., Muñoz-Escassi B., Manley W., García C., Cayado P., De la Muela M.S., Begoña O., Rosa L., Vara E. The effect of methyl sulphonyl methane supplementation on biomarkers of oxidative stress in sport horses following jumping exercise. Acta Vet. Scand. 2008;50:45. doi: 10.1186/1751-0147-50-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maraschiello C., Sárraga C., Garcia Regueiro J.A. Glutathione peroxidase activity, TBARS, and α-tocopherol in meat from chickens fed different diets. J. Agric. Food Chem. 1999;47:867–872. doi: 10.1021/jf980824o. [DOI] [PubMed] [Google Scholar]
- Meadus W.J., Duff P., Uttaro B., Aalhus J.L., Rolland D.C., Gibson L.L., Dugan M.E.R. Production of docosahexaenoic acid (DHA) enriched bacon. J. Agric. Food Chem. 2010;58:465–472. doi: 10.1021/jf9028078. [DOI] [PubMed] [Google Scholar]
- Mertin J., Hughes D., Stewart-Wynne E. PHA transformation in MS: inhibition by linoleic acid. The Lancet. 1974;303:1005–1006. doi: 10.1016/s0140-6736(74)91334-8. [DOI] [PubMed] [Google Scholar]
- Mézes M., Salyi G. Effect of acute selenium toxicosis on the lipid peroxide status and the glutathione system of broiler chickens. Acta Vet. Hung. 1994;42:459–463. [PubMed] [Google Scholar]
- Mihas A.A., Gibson R.G., Hirschowitz B.I. Suppression of lymphocyte transformation by 16,(16) dimethyl prostaglandin E2 and unsaturated fatty acids. Exp. Biol. Med. 1975;149:1026–1028. doi: 10.3181/00379727-149-38949. [DOI] [PubMed] [Google Scholar]
- Miles E.A., Calder P.C. Modulation of immune function by dietary fatty acids. Proc. Nutr. Soc. 1998;57:277–292. doi: 10.1079/pns19980042. [DOI] [PubMed] [Google Scholar]
- Mohammadi S., Najafi M., Hamzeiy H., Maleki-Dizaji N., Pezeshkian M., Sadeghi-Bazargani H., Garjani A. Protective effects of methylsulfonylmethane on hemodynamics and oxidative stress in monocrotaline-induced pulmonary hypertensive rats. Adv. Pharmacol. Sci. 2012;2012:507278. doi: 10.1155/2012/507278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mujahid A., Sato K., Akiba Y., Toyomizu M. Acute heat stress stimulates mitochondrial superoxide production in broiler skeletal muscle, possibly via downregulation of uncoupling protein content. Poult. Sci. 2006;85:1259–1265. doi: 10.1093/ps/85.7.1259. [DOI] [PubMed] [Google Scholar]
- Nakhostin-Roohi B., Barmaki S., Khoshkhahesh F., Bohlooli S. Effect of chronic supplementation with methylsulfonylmethane on oxidative stress following acute exercise in untrained healthy men. J. Pharm. Pharmacol. 2011;63:1290–1294. doi: 10.1111/j.2042-7158.2011.01314.x. [DOI] [PubMed] [Google Scholar]
- Nakhostin-Roohi B., Niknam Z., Vaezi N., Mohammadi S., Bohlooli S. Effect of single dose administration of methylsulfonylmethane on oxidative stress following acute exhaustive exercise. Iran J. Pharm. Res. 2013;12:845. [PMC free article] [PubMed] [Google Scholar]
- Noguchi T., Cantor A.H., Scott M.L. Mode of action of selenium and vitamin E in prevention of exudative diathesis in chicks. J. Nutr. 1973;103:1502–1511. doi: 10.1093/jn/103.10.1502. [DOI] [PubMed] [Google Scholar]
- Otsuki S., Qian W., Ishihara A., Kabe T. Elucidation of dimethylsulfone metabolism in rat using a 35S radioisotope tracer method. Nutr. Res. 2002;22:313–322. [Google Scholar]
- Overholt M.F., Dilger A.C., Boler D.D., Kerr B.J. Influence of feeding thermally peroxidized soybean oil on growth performance, digestibility, and gut integrity in finishing pigs. J. Anim. Sci. 2018;96:2789–2803. doi: 10.1093/jas/sky091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagonis T.A., Givissis P.A., Kritis A.C., Christodoulou A.C. The effect of methylsulfonylmethane on osteoarthritic large joints and mobility. Int. J. Orthopaed. 2014;1:19–24. [Google Scholar]
- Pearson T.W., Dawson H.J., Lackey H.B. Naturally occurring levels of dimethyl sulfoxide in selected fruits, vegetables, grains, and beverages. J. Agric. Food Chem. 1981;29:1089–1091. doi: 10.1021/jf00107a049. [DOI] [PubMed] [Google Scholar]
- Rahman I., Kode A., Biswas S.K. Assay for quantitative determination of glutathione and glutathione dislfide levels using enzymatic recycling method. Nat. Protoc. 2006;1:3159. doi: 10.1038/nprot.2006.378. [DOI] [PubMed] [Google Scholar]
- Rasheed A., Oelschlager M.L., Smith B.N., Bauer L.L., Whelan R.A., Dilger R.N. Toxicity and tissue distribution of methylsulfonylmethane following oral gavage in broilers. Poult. Sci. 2019;98:4972–4981. doi: 10.3382/ps/pez265. [DOI] [PubMed] [Google Scholar]
- Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999;26:1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- SAS Institute. SAS Institute; Cary, NC.: 2017. Base SAS 9.4 procedures guide: Statistical procedures. [Google Scholar]
- Seis H. Oxidative stress: introductory remarks. In: Seis H., editor. Oxidative Stress. Acad. Press; New York, NY: 1985. pp. 1–8. [Google Scholar]
- Sihvo H.K., Immonen K., Puolanne E. Myodegeneration with fibrosis and regeneration in the pectoralis major muscle of broilers. Vet. Pathol. 2014;51:619–623. doi: 10.1177/0300985813497488. [DOI] [PubMed] [Google Scholar]
- Tan G.Y., Yang L., Fu Y.Q., Feng J.H., Zhang M.H. Effects of different acute high ambient temperatures on function of hepatic mitochondrial respiration, antioxidative enzymes, and oxidative injury in broiler chickens. Poult. Sci. 2010;89:115–122. doi: 10.3382/ps.2009-00318. [DOI] [PubMed] [Google Scholar]
- Valko M., Leibfritz D., Moncol J., Cronin M.T., Mazur M., Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Van der Merwe M., Bloomer R.J. The influence of methylsulfonylmethane on inflammation-associated cytokine release before and following strenuous exercise. J. Sports Med. 2016;2016:7498359. doi: 10.1155/2016/7498359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnants N., Van Oeckel M.J., Boucqué C.V. Incorporation of dietary polyunsaturated fatty acids in pork tissues and its implications for the quality of the end products. Meat Sci. 1996;44:125–144. doi: 10.1016/s0309-1740(96)00029-0. [DOI] [PubMed] [Google Scholar]
- Withee E.D., Tippens K.M., Dehen R., Tibbitts D., Hanes D., Zwickey H. Effects of Methylsulfonylmethane (MSM) on exercise-induced oxidative stress, muscle damage, and pain following a half-marathon: a double-blind, randomized, placebo-controlled trial. J. Int. Soc. Sports Nutr. 2017;14:24. doi: 10.1186/s12970-017-0181-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong T., Bloomer R.J., Benjamin R.L., Buddington R.K. Small intestinal absorption of methylsulfonylmethane (msm) and accumulation of the sulfur moiety in selected tissues of mice. Nutrients. 2017;10:19. doi: 10.3390/nu10010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahav S., Goldfeld S., Plavnik I., Hurwitz S. Physiological responses of chickens and turkeys to relative humidity during exposure to high ambient temperature. J. Therm. Biol. 1995;20:245–253. [Google Scholar]
- Young R.A. Stress proteins and immunology. Annu. Rev. Immunol. 1990;8:401–420. doi: 10.1146/annurev.iy.08.040190.002153. [DOI] [PubMed] [Google Scholar]
- Zhang W., Xiao S., Lee E.J., Ahn D.U. Consumption of oxidized oil increases oxidative stress in broilers and affects the quality of breast meat. J. Agric. Food Chem. 2011;59:969. doi: 10.1021/jf102918z. [DOI] [PubMed] [Google Scholar]