Abstract
Genetic selection and intensive nutrition for increased growth rate in meat-type ducks has resulted in an imbalance between pectorales increment and sternal mass, which is detrimental to productivity and welfare. Reducing body weight and increasing sternal mass probably reverses these adverse effects. Therefore, 2 experiments (Expt.) were conducted to investigate the effects of 25-hydroxycholecalciferol (25-OH-D3), a vitamin D3 metabolites, on sternal mass. In Expt. 1, 512 1-day-old male ducks were randomly assigned to 4 low-nutrient density diets and received following treatments in a 2 × 2 factorial arrangement: (i) NRC or China Agricultural industry standards (NY/T) vitamin premixes and (ii) 0.069 mg/kg 25-HyD in feed or not. At 49 D of age, regardless of 25-OH-D3, NY/T vitamin regimen inhibited bone turnover and consequently increased sternal trabecular bone volume and mineral deposition compared with NRC vitamin premix. Supplementing 25-OH-D3 to NRC but not NY/T vitamin regimen significantly improved sternal microarchitecture and mineral content, which companied by decreased serum bone resorption markers concentration, as well as downregulation of the gene expressions of osteoclast differentiation and activity. In Expt. 2, 256 1-day-old male ducks were fed a standard nutrient density diet contained NRC vitamin premix with 0 or 0.069 mg/kg of 25-OH-D3. Results also showed that 25-OH-D3 treatment significantly improved sternal mineral accumulation and microarchitecture, along with decreasing osteoblast and osteoclast numbers in bone surface, declining serum bone turnover markers levels, and increasing serum Ca concentration. Collectively, these findings indicated that the dietary administration of 25-OH-D3 increased sternal mass in NRC vitamin diet by suppressing bone resorption in 49-day-old meat duck.
Key words: 25-hydroxycholecalciferol, vitamin, sternal mass, meat duck
Introduction
Increases in growth rate and breast muscle mass through selective breeding and nutrient strategy in meat-type poultry has been accompanied by welfare problems. In addition to an increased incidence of poor gait (Duggan et al., 2015), respiratory problems were also noticed in birds, such as pulmonary diseases (Iyer and Rao, 1971) and ascites (Julian, 1988). In the avian respiratory system, the sternum is considered as primary ventilator (Claessens, 2009), which influences air sac volume, thereby is facilitating a unidirectional flow of air through the lung (Tickle et al., 2007). The rapid musculoskeletal development inadvertently puts stress on sternum and potentially outgrows pulmonary capacity and increases the occurrence rate of these diseases (Julian, 1998, Wideman, 2001). Based on the above phenomenon, slowing weight gain combined with promoting sternal mass might be able to reverse these adverse effects and improve bird's health.
Vitamin D was originally discovered as an antirachitic agent capable of preventing a failure of bone mineralization. Vitamin D3 deficiency results a higher incidence of leg problems in birds (Khan et al., 2010). Supplementing vitamin D3 improved the walking ability and bone quality characteristics and consequently decreased the leg diseases in broilers (Sun et al., 2013, Jiang et al., 2015). A current view is that the beneficial effects of vitamin D3 on bone mineral density and reduction of bone fracture incidence are caused by the suppression of bone resorption (Richy et al., 2005) and/or promoting bone formation (Turner et al., 2014, van der Meijden et al., 2014). Paradoxically, vitamin D3 has been also shown to enhance bone resorption in vitro and in vivo (Sato et al., 2007, Kogawa et al., 2010). Variations in the effects of vitamin D3 on bone homeostasis appear to depend on the physiological context, administration dose, and trial subjects. 25-hydroxycholecalciferol (25-OH-D3) which is an intermediate metabolite of vitamin D3 and is found to significantly promote tibia mineralization of broiler (Wideman et al., 2015, Santiago et al., 2016), and it was approximately twice as active as cholecalciferol in promoting bone strength in broilers (Han et al., 2016). However, Ren et al. (2017) found the positive impacts of maternal canthaxanthin, and 25-OH-D3 supplementation on growth performance and serum phosphorus (P) of ducklings only were observed in a low but not a high vitamin regimen, which has higher levels of all vitamins except nicotinic acid than the low vitamin recommendations, different responses of dietary 25-OH-D3 to distinct vitamin regimen probably because of different doses of vitamin D or interaction among vitamins (Bonjour et al., 2018). There are various recommendations of vitamin premix in the duck industry, and the variations of each vitamin content between each other is wide, especially NRC (NRC, 1994) and China Agricultural Industry Standards (NY/T; NY/T, 2012). Accordingly, this is reasonable to assume that the biological effect of 25-OH-D3 on sternal mass may lie on dietary vitamin regimen.
The rapid body gain in commercial domestic birds seems to be an important factor for the detrimental effects on bone. Accelerating weight gain via increasing dietary nutrient density resulted in a higher incidence of gait abnormality (Brickett et al., 2007), and the intensive nutrition in meat birds has been suggested as main cause for the inadequate bone quality (Williams et al., 2004). Feed withdrawal or reducing the nutrient density of diets played a critical role in alleviation of the gait abnormality of broiler (Brickett et al., 2007). Our previous study also showed that a low nutrient density (LND) diet decreased weight gain and promoted trabecular thickness (Tb.Th) via suppressing bone turnover in meat duck, both relieved the burden acted on the bone and improved the animal welfare (Zhang et al., 2018). Based on these reports, we hypothesized that feeding the LND diet supplemented a rational vitamin premix or 25-OH-D3 might be beneficial to sternal mass, but there is no direct evidence for this so far. Therefore, the aim at this study was to evaluate (1) the effect of dietary 25-OH-D3 on the sternal mass of meat ducks fed a standard nutrient density diet or a LND diet with different vitamin regimens and to determine if 25-OH-D3 could affect osteoblast and/or osteoclast activity to increase sternal quality in meat ducks.
Materials and methods
Care, handling, and sampling procedures were approved by the Animal Care and Use Committee of Sichuan Agricultural University before initiation of the trial. Cherry Valley meat male ducks were reared in cages (2.2 × 1.2 × 0.9 m) in a temperature-controlled and humidity-controlled room and had free access to feed and water throughout the experimental period. Diets were provided in pellet form. All vitamins used in this trial were provided by DSM Ltd. (Shanghai, China).
Experimental Design and Animal Management
There are 2 experiments (Expt.) in this study. In Expt. 1, a total of 512 one-day-old ducks were equally divided into 4 LND diets groups as follows (8 replicate pens; 16 ducks/pen) in a 2 × 2 factorial arrangement: 2 different vitamin regimen from NRC (1994) or NY/T (2012) with 0.069 mg/kg 25-OH-D3 in feed or not. The vitamin composition was displayed in Table 1. Ducklings were all fed a normal nutrient density starter diet until 14 D, and subsequently, they were subjected to an LND diet until 56 D. The starter diet was formulated based on NY/T (2012). The LND diet was designed according to our previous report (Zhang et al., 2018), which is with constant t ratios of CP and essential nutrients, such as limiting amino acids relative to metabolic energy compared with positive control (PC) diet (36–49 D) (Table 2). During the whole rearing period, BW by pen and feed intake (FI) were recorded weekly. Feed conversion was calculated as the feed to gain (F: G) ratio. At 42, 49, and 56 D of age, they were fasted for 12 h, and one bird in each pen was selected based on the average BW of each cage, and whole sternum was removed for fresh weight, morphometry, and mineralization property analysis. In addition, at 49 D of age, another bird in each pen was selected, and the blood was collected via jugular vein, also the serum was obtained. Sternal samples (0.5 × 0.5 cm) that located above the fontanelle and closest to the keel were dissected and put in liquid nitrogen and in phosphate-buffered formaldehyde immediately for gene expression and bone histomorphometry analysis, respectively.
Table 1.
Composition of the vitamin premixes for meat duck1.
| Item | 1–14 D |
15–56 D |
||
|---|---|---|---|---|
| NRC2 | NY/T3 | NRC2 | NY/T3 | |
| A (IU) | 2,500 | 4,000 | 2,500 | 3,000 |
| D3 (IU) | 400 | 2,000 | 400 | 2,000 |
| E (IU) | 10 | 20 | 10 | 20 |
| K3 (mg) | 0.5 | 2 | 0.5 | 2 |
| B1 (mg) | 1.8 | 2 | 1.8 | 1.5 |
| B2 (mg) | 4 | 10 | 4 | 10 |
| B6 (mg) | 2.5 | 4 | 2.5 | 3 |
| B12 (mg) | 0.01 | 0.02 | 0.01 | 0.02 |
| Niacin (mg) | 55 | 50 | 55 | - |
| Pantothenic Acid (mg) | 11 | 20 | 11 | 10 |
| Biotin (mg) | 0.15 | 0.15 | 0.15 | 0.15 |
| Folic acid (mg) | 0.55 | 1 | 0.55 | 1 |
Supplied in per kilogram of diet.
The vitamin levels recommended by the NRC (NRC, 1994).
The vitamin levels recommended by the China Agricultural Industry Standards (NY/T, 2012).
Table 2.
Composition and nutrient levels in the basal diets (dry matter basis).
| Ingredients and analysis | Starting diet |
PC diet |
LND diet |
|
|---|---|---|---|---|
| 1–14 D | 15–35 D | 36–56 D | 15–56 D | |
| Ingredients, % | ||||
| Maize | 58.05 | 62.1 | 62.71 | 56.61 |
| Soya oil | 3 | 3 | 4.2 | 0 |
| Soybean meal | 34.8 | 23.48 | 17.01 | 0 |
| DDGS | 0 | 3 | 4 | 4 |
| Wheat bran | 0 | 2 | 4 | 26.6 |
| Rapeseed meal | 0 | 2.85 | 4.8 | 8.61 |
| L-Lysine | 0.08 | 0 | 0 | 0.2 |
| DL-Methionine | 0.145 | 0.13 | 0.09 | 0.1 |
| L-Threonine | 0.03 | 0 | 0 | 0.07 |
| L-Tryptophan | 0 | 0 | 0 | 0.12 |
| Choline chloride | 0.2 | 0.2 | 0.2 | 0.2 |
| Sodium chloride | 0.3 | 0.3 | 0.3 | 0.3 |
| Mineral premix1 | 0.2 | 0.2 | 0.2 | 0.2 |
| Vitamin premix2 | 0.03 | 0.03 | 0.03 | 0.03 |
| Limestone | 1.06 | 1.04 | 1.1 | 1 |
| Dicalcium phosphate | 1.75 | 1.6 | 1.25 | 1 |
| Bentonite | 0.355 | 0.07 | 0.11 | 0.96 |
| Total | 100 | 100 | 100 | 100 |
| Calculated nutrient analysis, % | ||||
| AME (MJ kg−1) | 12.14 | 12.14 | 12.35 | 10.25 |
| CP | 20 | 17.5 | 16 | 13.28 |
| ME/CP | 145 | 166 | 184 | 184 |
| Dig Lys, P% | 1.02 | 0.76 | 0.65 | 0.53 |
| Dig Met, P% | 0.42 | 0.38 | 0.32 | 0.27 |
| Calcium | 0.9 | 0.85 | 0.8 | 0.71 |
| Total-phytate P | 0.65 | 0.65 | 0.61 | 0.76 |
| Nonphytic acid P | 0.42 | 0.4 | 0.35 | 0.35 |
Abbreviations: AME, apparent metabolizable energy; CP, crude protein; DDGS, distillers dried grains with soluble; Dig, digestibility; LND, low nutrient density; PC, standard nutrient density positive control.
Provided per kilogram of diet: Cu (CuSO4∙5H2O), 8 mg; Fe (FeSO4∙7H2O), 80 mg; Zn (ZnSO4∙7H2O), 90 mg; Mn (MnSO4∙H2O), 70 mg; Se (NaSeO3), 0.3 mg; I (KI), 0.4 mg.
Provided per kilogram of diet: NRC or NY/T vitamin premixes as shown in Table 1.
Expt. 2 was conducted under a standard nutrient density PC diet to verify the preferential effect of 25-HyD in NRC vitamin premix diet on the sternal mass of meat ducks. 256 one-day-old ducks were equally divided into 2 NRC vitamin diets with or without 0.069 mg/kg of 25-OH-D3 (8 replicate pens; 16 ducks/pen). The ducks were subjected to a 3-period feeding program consisting of starting (0–14 D), growing (15–35 D), and finishing (36–49 D) periods. The diets were formulated to meet all nutrient requirements of meat ducks to NY/T (2012) except vitamin (Table 2). At 49 D of age, BW was recorded per pen basis, then followed by 12 h fasting period, and 2 ducks with similar BW in each pen were selected, and whole sterna were removed from one bird for fresh weight, morphometry, and mineralization property analysis. Serum and sternal samples were also obtained as the same process as the Expt. 1 for bone metabolism analysis.
Sternum Morphometry
For estimating the morphometric change of sternum, 5 parameters including the distance between the 2 coracoids of the sternum, sternum central distance, posterior process distance, sternum length, and sternum depth were obtained from each sternum. Then, the sternum was cut open longitudinally along the keel, and the relative proportion of the sternum cartilage was measured using the method described by Zhang et al. (2019). All measurements were straight-line distances.
Sternum Mineralization Analysis
The fat-free weight and density of sternum were evaluated as previously described (Zhang et al., 2017), subsequently sternum was ashed in a muffle furnace at 550°C for 24 h, and the ash was measured on the basis of the percentage of the fat-free weight. Calcium (Ca) and P contents were determined through ethylene diamine tetraacetic acid titration and ammonium metavanadate colorimetry, respectively, and values were also presented base on the basis of the fat-free weight.
Bone Histological Analysis and Phosphatase Staining
Harvested sternum samples were fixed, embedded, and sliced. For microarchitecture, the sections were stained with toluidine blue, the micrographs of the bone sections were taken using a microscope (Nikon Eclipse TS100; Nikon Corporation, Tokyo, Japan), and an image analyzer (Image Pro-Plus, Rockville, MD) at a magnification of 20×. Bone static histomorphometry parameters were performed by a blinded examiner using Weibel Grid technique, which includes the trabecular bone volume/tissue volume (BV/TV), trabecular number (Tb.N), Tb.Th, and spacing (Tb.Sp). For osteoblasts or osteoclasts detection, the sections were stained with alkaline phosphatase (ALP) or tartrate-resistant acid phosphatase (TRAP) detecting kit (Sigma-Aldrich, St. Louis, MO), respectively. The ALP positive staining represents osteoblast, and TRAP-positive staining represents osteoclasts. Osteoblast and osteoclast number per bone surface (N.Ob/BS and N.Oc/BS) were counted on the external surfaces of the bone using the surgical defect as the field of view. A single operator at 2 separate time points performed the quantification using Image Pro-Plus.
Serum Biochemistry
Serum Ca and P concentrations were determined with Biochemistry Analyzer (Yellow Springs Instrument Co. Inc., Yellow Springs, OH). Serum parathyroid hormone (PTH) and 25-hydroxyvitamin D (25-OH-D) concentration were assayed using commercial ELISA kit (ALPCO Diagnostics, NH) according to the manufacturers' recommendations. For 25-OH-D, samples and pretreatment reagent were combined to release the bound 25(OH)D from vitamin D binding protein. The mixtures are transferred to microplate wells and an anti-25(OH)D antibody is added. After overnight incubation, a peroxidase-conjugated antibody is added to the wells to form a 25(OH)D-peroxidase complex. Subsequently, tetramethylbenzidine, a peroxidase substrate, is then added, initiating a change in the color of the solution. The absorbance was measured at 450 nm on a microplate reader (Bio-Rad Model 680, Hercules, CA).
In addition, Serum procollagen type I N-terminal propeptide (P1NP), ALP (both bone formation markers), C-terminal cross-linked telopeptide of type I collagen (CTx), and TRAP (both bone resorption markers) were measured using commercial assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). All samples were tested in triplicate within each assay.
Gene Expression Assays
mRNA levels of osteocyte-specific, osteoblast-specific, and osteoclast-specific marker genes were determined by quantitative RT-PCR. The frozen sternal samples were subjected to total RNA isolation, and the RNA quality (intact ribosomal RNA 28s/18s) was evaluated by agarose gel electrophoresis. The cDNA was synthesized from 200 ng of total RNA by using the PrimeScript RT Reagent Kit (Takara, Kusatsu, Japan). The RT-PCR analysis was performed on the ABI 7500 detection system (Applied Biosystems, Foster City, CA), and target cDNA was amplified by 40 cycles (1 cycle: 95°C for 5 s, 60°C for 34 s), and the melting curve analysis was performed at the end. The primers were designed using Primer 3 and are shown in Table 3. All reactions were run in duplicate, and a standard curve was generated to estimated reaction efficiency (slope) and genes expression. Glyceraldehyde-3-phosphate dehydrogenase and β-actin were selected as the reference genes, and a normalization factor was obtained by calculating the geometric mean of the values of the selected reference genes, which was subsequently used to normalize the relative amounts of RNAs of interest (Vandesompele et al., 2002).
Table 3.
The primers for quantitative real-time PCR.
| Gene | Gene ID | Primer | Sequence (5′-3′) | Size (bp) |
|---|---|---|---|---|
| Phex | XM_005010837.3 | Reverse | tgccaactatctggtgtgga | 99 |
| Forward | ccgtagatcacccgagaaaa | |||
| Dmp1 | XM_005012780.3 | Reverse | aaccttggtcaccttcatgc | 92 |
| Forward | tcggcaaagtcctgctctat | |||
| Sclerostin | XM_005026106.3 | Reverse | ggaagggtggcaagtgttta | 115 |
| Forward | tgcctggttcattgtgttgt | |||
| Cathepsin K | XM_021277116.1 | Reverse | actgctggtcctgtttgtcc | 98 |
| Forward | gcttgcggtacgttttcttc | |||
| V-ATPase | XM_021267166.1 | Reverse | tccgtgtctggttcatcaaa | 111 |
| Forward | caggacaccagacttcagca | |||
| OPG | XM_005017709.3 | Reverse | gcctaactggctgaacttgc | 106 |
| Forward | gaaggtctgctcttgcgaac | |||
| RANKL | XM_021276016.1 | Reverse | gccttttgcccatctcatta | 100 |
| Forward | taagtttgcctggcctttgt | |||
| β-actin | NM_001310408.1 | Reverse | ccagccatctttcttgggta | 105 |
| Forward | gtgttggcgtacaggtcctt | |||
| GAPDH | XM_005016745.3 | Reverse | tttttaaccgtggctccttg | 94 |
| Forward | actgggcatggaagaacatc |
Abbreviations: Dmp1, dentin matrix protein 1; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; OPG, osteoprotegerin; Phex, phosphate regulating endopeptidase homolog x-linked; RANKL, receptor activator of nuclear factor-κ B ligand; V-ATPase, Vacuolar-type H+-ATPase.
Statistical Analysis
All data were expressed as the means and standard deviation (n = 8). Two-way ANOVA followed by Tukey's test (SAS 9.2) were used for analyses of dietary vitamin regimen, 25-HyD level, and their interaction in LND diets. One-way ANOVA was used to compare the effect of 25-OH-D3 on sterna mass in the PC diets with NRC vitamin regimen. Statistical significance was detected at P < 0.05.
Results
Effects on BW and Sternal Characteristics
As shown in Table 4, the addition of 25-OH-D3 increased (P < 0.05) the BW (14 D) and the weight gain (1–14 D). Dietary vitamin regimen significantly increased (P < 0.05) the BW of birds at 35, 42, 49, and 35 D of age, but did not change the gain, FI, and F:G in the whole period. The interaction of vitamin regimen and 25-OH-D3 did not notably affect the performance of ducks, except for F: G from 15 to 42 D of age.
Table 4.
Effects of 25-OH-D3 and vitamin regimen on performance for meat duck1.
| Item | Body weight, g/duck |
Gain, g/duck |
Feed intake, g/duck |
Feed: Gain, g:g |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 14 D | 35 D | 42D | 49 D | 56 D | 1–14 D | 15–42 D | 43–56 D | 1–14 D | 15–42 D | 43–56 D | 1–14 D | 15–42 D | 43–56 D | ||
| Vitamin regimen | 25-OH-D3 | ||||||||||||||
| NRC | − | 628.41b | 2056.86b | 2658.31b | 3096.56 | 3333.99 | 579.93b | 2036.55 | 692.38 | 734.09 | 6025.72b | 4011.51 | 1.27 | 2.97a | 5.82 |
| + | 669.95a | 2111.3a,b | 2703.87a,b | 3155.10 | 3390.64 | 621.41a | 2029.04 | 694.70 | 742.62 | 6019.26b | 3700.29 | 1.19 | 2.97a | 5.36 | |
| NY/T | − | 650.21a,b | 2181.89a | 2827.83a | 3275.51 | 3526.42 | 601.75a,b | 2169.21 | 693.88 | 742.46 | 6554.01a | 3818.48 | 1.23 | 3.02a | 5.56 |
| + | 674.93a | 2213.35a | 2860.70a | 3317.10 | 3567.31 | 626.38a | 2176.16 | 678.86 | 753.08 | 6005.16b | 3751.50 | 1.20 | 2.76b | 5.58 | |
| SEM | 10.2 | 39.01 | 53.71 | 57.67 | 65.14 | 10.44 | 54.12 | 29.15 | 16.66 | 137.82 | 110.49 | 0.02 | 0.06 | 0.19 | |
| Vitamin regimen | |||||||||||||||
| NRC | 649.18 | 2084.08b | 2681.09b | 3125.83b | 3362.32b | 600.67 | 2032.79 | 693.54 | 738.35 | 6022.49 | 3855.90 | 1.23 | 2.97 | 5.59 | |
| NY/T | 662.86 | 2198.02a | 2844.48a | 3296.59a | 3547.14a | 614.36 | 2172.44 | 686.73 | 747.73 | 6288.93 | 3780.78 | 1.22 | 2.89 | 5.58 | |
| SEM | 7.17 | 28.08 | 38.56 | 41.41 | 46.78 | 7.41 | 38.89 | 20.95 | 11.96 | 105.57 | 81.04 | 0.01 | 0.05 | 0.13 | |
| 25-OH-D3 | |||||||||||||||
| − | 639.31b | 2119.37 | 2743.06 | 3168.03 | 3430.21 | 590.84b | 2102.88 | 693.13 | 738.27 | 6289.86a | 3914.99 | 1.25 | 3.00a | 5.69 | |
| + | 672.73a | 2162.72 | 2782.51 | 3236.39 | 3479.25 | 624.19a | 2102.34 | 687.08 | 747.81 | 6021.56b | 3721.68 | 1.20 | 2.87b | 5.46 | |
| SEM | 7.41 | 27.13 | 37.29 | 40.05 | 45.24 | 7.17 | 37.62 | 20.27 | 11.57 | 102.11 | 78.38 | 0.02 | 0.04 | 0.13 | |
| P-value | |||||||||||||||
| Vitamin regimen | 0.208 | 0.008 | 0.006 | 0.007 | 0.010 | 0.207 | 0.071 | 0.811 | 0.583 | 0.078 | 0.534 | 0.598 | 0.194 | 0.907 | |
| 25-OH-D3 | 0.004 | 0.289 | 0.479 | 0.401 | 0.468 | 0.004 | 0.996 | 0.832 | 0.578 | 0.048 | 0.104 | 0.203 | 0.036 | 0.258 | |
| Interaction | 0.425 | 0.774 | 0.908 | 0.886 | 0.906 | 0.442 | 0.897 | 0.772 | 0.951 | 0.064 | 0.287 | 0.329 | 0.038 | 0.219 | |
a-bMean values with unlike letters were significantly different (two-way ANOVA, P < 0.05, Tukey's post hoc test).
Abbreviations: 25-OH-D3, 25-hydroxycholecalciferol; NY/T, China Agricultural industry standards.
Ducklings were all fed a normal nutrient density starter diet until 14 D, subsequently, they were subjected to an LND diet until 56 D.
Except for the sternum depth, the vitamin premix, 25-OH-D3, and their interaction did not affect the sternum dimension (Table 5). Supplementation of NY/T vitamin diet significantly increased the sternum depth of 42-day-old duck compared with NRC vitamin diet. Dietary 25-OH-D3 also increased the sternum depth of 49-day-old duck in this study. The bone weight, Ca, P, and density of sterna were comparable among all groups at 42 D and 56 D (Table 6). Vitamin premix and the interaction of vitamin regimen and 25-OH-D3 did not significantly change the sternal weight, ash, mineral content, and density at 49 D. Administration of 25-OH-D3 remarkably increased the sternal bone weight, mineral content, and density of 49-day-old ducks in NY/T but not NY/T vitamin regimens (Table 6).
Table 5.
Effects of 25-OH-D3 and vitamin regimen on sternal dimension for meat duck.
| Item | Coracoid distance, mm |
Sternum central distance, mm |
Posterior process distance, mm |
Sternum length, mm |
Sternum depth, mm |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 42 D | 49 D | 56 D | 42 D | 49 D | 56 D | 42 D | 49 D | 56 D | 42 D | 49 D | 56 D | 42 D | 49 D | 56 D | ||
| Vitamin regimen | 25-OH-D3 | |||||||||||||||
| NRC | − | 50.53 | 54.12 | 55.31 | 52.58 | 55.50 | 57.66 | 67.42 | 75.72 | 78.64 | 116.94 | 129.60 | 137.63 | 23.12b | 29.90b | 35.44 |
| + | 51.35 | 54.20 | 56.50 | 53.34 | 55.48 | 58.38 | 68.30 | 75.25 | 74.63 | 116.42 | 131.27 | 142.22 | 23.45b | 30.67a,b | 35.74 | |
| NY/T | − | 49.99 | 54.58 | 55.77 | 51.96 | 55.50 | 58.08 | 67.50 | 76.09 | 77.74 | 112.79 | 132.05 | 141.23 | 24.80a,b | 29.95a,b | 35.37 |
| + | 51.67 | 55.53 | 56.09 | 52.77 | 55.51 | 61.05 | 66.89 | 74.94 | 78.87 | 117.40 | 135.63 | 141.56 | 25.16a | 32.63a | 36.67 | |
| SEM | 0.51 | 0.79 | 0.99 | 0.57 | 0.85 | 0.89 | 1.36 | 1.01 | 1.55 | 1.49 | 2.35 | 1.57 | 0.59 | 0.60 | 0.76 | |
| Vitamin regimen | ||||||||||||||||
| NRC | 50.94 | 54.16 | 55.90 | 52.96 | 55.49 | 58.02 | 67.86 | 75.49 | 76.64 | 116.68 | 130.44 | 139.92 | 23.28b | 30.03 | 35.59 | |
| NY/T | 50.83 | 55.05 | 55.93 | 52.37 | 55.50 | 59.56 | 67.20 | 75.51 | 78.31 | 115.10 | 133.84 | 141.39 | 24.98a | 30.79 | 36.02 | |
| SEM | 0.36 | 0.56 | 0.70 | 0.40 | 0.60 | 0.64 | 0.96 | 0.71 | 1.09 | 1.05 | 1.66 | 1.11 | 0.41 | 0.42 | 0.54 | |
| 25-OH-D3 | ||||||||||||||||
| − | 50.26 | 54.35 | 55.54 | 52.27 | 55.50 | 57.87 | 67.46 | 75.90 | 78.19 | 114.86 | 130.82 | 139.43 | 23.95 | 29.92b | 35.40 | |
| + | 52.51 | 54.86 | 56.29 | 53.05 | 55.49 | 59.71 | 67.60 | 75.09 | 76.75 | 116.91 | 133.45 | 141.89 | 24.30 | 32.71a | 36.21 | |
| SEM | 0.36 | 0.56 | 0.70 | 0.40 | 0.60 | 0.64 | 0.96 | 0.71 | 1.09 | 1.05 | 1.67 | 1.11 | 0.41 | 0.42 | 0.54 | |
| P-value | ||||||||||||||||
| Vitamin regimen | 0.832 | 0.268 | 0.977 | 0.308 | 0.991 | 0.097 | 0.628 | 0.979 | 0.289 | 0.297 | 0.159 | 0.357 | 0.006 | 0.217 | 0.557 | |
| 25-OH-D3 | 0.203 | 0.518 | 0.455 | 0.181 | 0.989 | 0.501 | 0.922 | 0.430 | 0.361 | 0.181 | 0.273 | 0.129 | 0.557 | 0.044 | 0.297 | |
| Interaction | 0.402 | 0.587 | 0.667 | 0.968 | 0.994 | 0.221 | 0.588 | 0.738 | 0.109 | 0.096 | 0.687 | 0.187 | 0.974 | 0.251 | 0.516 | |
a-bMean values with unlike letters were significantly different (two-way ANOVA, P < 0.05, Tukey's post hoc test).
Abbreviations: 25-OH-D3, 25-hydroxycholecalciferol; NY/T, China Agricultural industry standards.
Table 6.
Effects of 25-OH-D3 and vitamin regimen on sternal weight and minerals content for meat duck.
| Item | Fat-free weight, g |
Relative fresh weight (g/100 g) |
Density (g/cm3) |
Ash, % fat-free weight |
Ca, % fat-free weight |
P, % fat-free weight |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 42 D | 49 D | 56 D | 42 D | 49 D | 56 D | 42 D | 49 D | 56 D | 42 D | 49 D | 56 D | 42 D | 49 D | 56 D | 42 D | 49 D | 56 D | ||
| Vitamin level | 25-OH-D3 | ||||||||||||||||||
| NRC | − | 4.13 | 8.92 | 13.07 | 0.72 | 1.12b | 1.10 | 0.26 | 0.32b | 0.44 | 14.27b | 38.23b | 44.53 | 5.77 | 21.08b | 25.63 | 3.13 | 9.86b | 11.38 |
| + | 4.38 | 9.53 | 13.96 | 0.76 | 1.22a | 1.11 | 0.27 | 0.37a | 0.45 | 15.14b | 45.27a | 45.26 | 5.90 | 24.35a | 26.88 | 3.37 | 11.32a,b | 11.50 | |
| NY/T | − | 4.45 | 9.53 | 13.84 | 0.77 | 1.18a,b | 1.08 | 0.27 | 0.35a,b | 0.47 | 16.42a,b | 42.94a,b | 45.85 | 5.92 | 23.66a,b | 25.80 | 3.37 | 10.35b | 11.90 |
| + | 4.62 | 9.67 | 14.36 | 0.79 | 1.26a | 1.12 | 0.27 | 0.37a | 0.47 | 18.30a | 46.49a | 45.61 | 6.12 | 25.52a | 26.83 | 3.69 | 12.20a | 12.11 | |
| SEM | 0.41 | 0.41 | 0.63 | 0.03 | 0.03 | 0.05 | 0.01 | 0.01 | 0.03 | 0.80 | 1.65 | 1.02 | 0.30 | 1.06 | 0.76 | 0.21 | 0.61 | 0.43 | |
| Vitamin regimen | |||||||||||||||||||
| NRC | 2.25 | 9.23 | 13.51 | 0.74 | 1.17 | 1.11 | 0.27 | 0.34 | 0.45 | 14.71 | 41.75 | 44.89 | 5.83 | 22.71 | 26.25 | 3.25 | 10.59 | 11.44 | |
| NY/T | 4.53 | 9.60 | 14.10 | 0.78 | 1.21 | 1.10 | 0.27 | 0.36 | 0.47 | 17.36 | 44.71 | 45.73 | 6.02 | 24.59 | 26.32 | 3.53 | 11.27 | 12.01 | |
| SEM | 0.29 | 0.29 | 0.45 | 0.02 | 0.02 | 0.03 | 0.01 | 0.01 | 0.02 | 0.57 | 1.17 | 0.72 | 0.21 | 0.75 | 0.53 | 0.15 | 0.43 | 0.30 | |
| 25-OH-D3 | |||||||||||||||||||
| − | 4.29 | 9.22 | 13.48 | 0.75 | 1.14b | 1.09 | 0.27 | 0.33a | 0.45 | 15.34 | 40.58a | 45.19 | 5.84 | 22.36a | 25.71 | 3.25 | 10.11a | 11.64 | |
| + | 4.50 | 9.59 | 14.16 | 0.77 | 1.23a | 1.11 | 0.27 | 0.37b | 0.46 | 16.72 | 45.88b | 45.43 | 6.01 | 24.94b | 26.85 | 3.52 | 11.76b | 11.80 | |
| SEM | 0.29 | 0.29 | 0.45 | 0.02 | 0.02 | 0.03 | 0.01 | 0.01 | 0.02 | 0.57 | 1.17 | 0.72 | 0.21 | 0.75 | 0.53 | 0.15 | 0.43 | 0.30 | |
| P-value | |||||||||||||||||||
| Vitamin regimen | 0.497 | 0.370 | 0.364 | 0.260 | 0.070 | 0.921 | 0.996 | 0.114 | 0.371 | 0.004 | 0.088 | 0.425 | 0.549 | 0.091 | 0.936 | 0.195 | 0.279 | 0.199 | |
| 25-OH-D3 | 0.611 | 0.369 | 0.281 | 0.392 | 0.002 | 0.591 | 0.973 | 0.048 | 0.884 | 0.102 | 0.004 | 0.813 | 0.587 | 0.025 | 0.146 | 0.206 | 0.014 | 0.707 | |
| Interaction | 0.923 | 0.559 | 0.775 | 0.839 | 0.612 | 0.726 | 0.691 | 0.531 | 0.984 | 0.538 | 0.305 | 0.639 | 0.915 | 0.512 | 0.887 | 0.848 | 0.754 | 0.928 | |
a-bMean values with unlike letters were significantly different (two-way ANOVA, P < 0.05, Tukey's post hoc test).
Abbreviations: 25-OH-D3, 25-hydroxycholecalciferol; Ca, calcium; NY/T, China Agricultural industry standards; P, phosphorus.
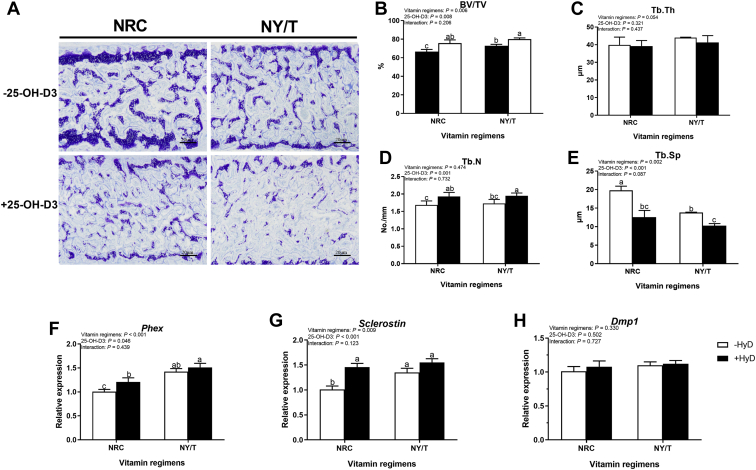
Considered with the above sternal characterization results, therefore, more attention was given to the effect of 25-OH-D3 on bone phenotype between 42 and 49 D of age. Toluidine blue stain has shown that marrow area was intuitionally decreased, and the BV was obviously increased by 25-OH-D3 treatment in all vitamin regimen diets (Figure 1A). Outcomes of morphometric analysis confirmed that the diet contained NY/T vitamin, markedly increased the BV/TV and reduced the Tb.Sp compared with the NRC vitamin diet under the no 25-OH-D3-treated condition (Figures 1B, 1E). Dietary 25-OH-D3 manipulation significantly increased the BV/TV and the Tb.N and decreased the Tb.Sp among all groups (Figures 1B, 1D, 1E). Osteocyte markers genes, such as Phe, Sclerostin, and Dmp1, were quantified using RT-PCR and shown that the expression of Phex and Sclerostin mRNA were pronouncedly upregulated by 25-OH-D3 treatment in NRC but not in NY/T vitamin regimen (Figures 1F, 1G). The interaction of vitamin regimen and 25-OH-D3 did not significantly affect the bone phenotypes of 49-day-old duck. These results suggest that the LND diet with NY/T vitamin premix produced positive role on sterna mass of 49-day-old duck compared with NRC vitamin diet and supplementation 25-OH-D3 to NRC, but not NY/T vitamin regimens could significantly promote the mineral deposition of sternum, even though sternal microarchitecture was improved in NY/T vitamin diet.
Figure 1.
Bone phenotypes responses to 25-OH-D3 and vitamin regimen in LND diet for meat duck at 49 D. (A) Toluidine blue stained and the morphometric analysis for (B) trabecular bone volume/tissue volume (BV/TV), (C) trabecular thickness (Tb.Th), (D) number (Tb.N), and (E) spacing (Tb.Sp). Real-time RT-PCR analysis of osteocyte markers gene including (F) Phosphate regulating endopeptidase homolog x-linked (Phex), (G) Sclerostin, and (H) Dentin matrix protein1 (Dmp1) in the sternum. Values are means and standard deviation represented by vertical bars (n = 8). a–cMean values with different letters are significantly different (two-way ANOVA, P < 0.05, Tukey's post hoc test). Abbreviations: 25-OH-D, 25-hydroxycholecalciferol; NY/T, China Agricultural industry standards; LND, low nutrient density.
Effects on the Formation and Bone Resorption
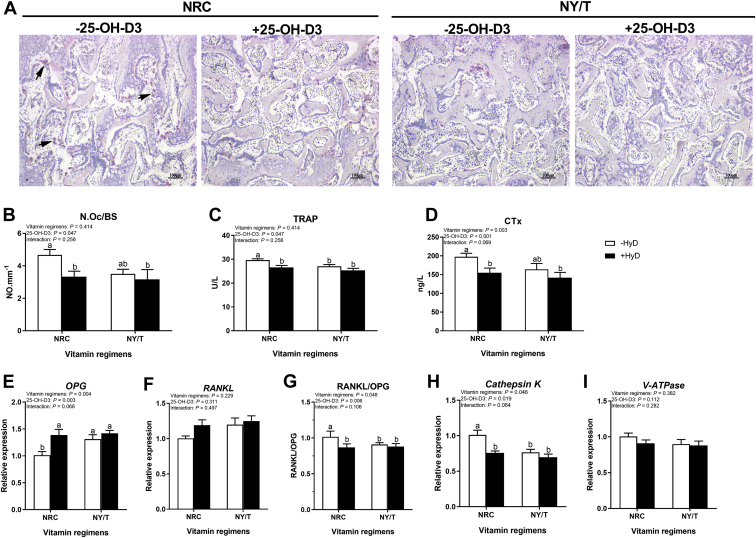
Effects of 25-OH-D3 on bone resorption under 2 different vitamin regimens was assessed histologically and biochemically. TRAP-positive cells are intuitionally higher in NRC vitamin regimens in the absence of 25-OH-D3 (Figure 2A). In NRC but not NY/T vitamin diet, supplementation of 25-OH-D3 markedly reduced the N.Oc/BS (Figure 2B), and circulating TRAP and CTx, both were resorption markers (Figures 2C, 2D). The osteoclast-related factors including OPG, RANKL, cathepsin K, and H+-ATPase were examined using RT-PCR and found that 25-OH-D3 treatment failed to affect the expression of RANKL but increased the expression of OPG mRNA, thereby decreasing the RANKL/OPG ratio in NRC, but not in NY/T vitamin groups (Figures 2E–2G). Furthermore, dietary 25-OH-D3 administration significantly depressed the cathepsin K mRNA abundance in NRC vitamin group (Figure 2H), but it has not notably changed the mRNA expression of H+-ATPase among all groups (Figure 2I). Taken together, these results suggest that 25-OH-D3 treatment increases sternal mass by suppressing bone resorption, especially in NRC vitamin groups.
Figure 2.
Bone absorption responses to 25-OH-D3 and vitamin regimen in LND diet for meat duck at 49 D. (A) Tartrate-resistant acid phosphatase (TRAP) staining of sternal sections, and (B) osteoclast number per bone surface (N.Oc/BS) was determined by histomorphometry. Serum (C) TRAP activity and (D) C-terminal cross-linked telopeptide of type I collagen (CTx) concentrations. Real-time RT-PCR analysis for mRNA expression of osteoclastogenesis-related factors including (E) Osteoprotegerin (OPG), (F) Receptor activator of nuclear Factor-κ B ligand (RANKL), (G) The ratio of RANKL/OPG, (H) Cathepsin K, and (I) Vacuolar-type H+-ATPase (V-ATPase) in the sternum. Values are means and standard deviation represented by vertical bars (n = 8). a, bMean values with different letters are significantly different (two-way ANOVA, P < 0.05, Tukey's post hoc test). Abbreviations: 25-OH-D3, 25-hydroxycholecalciferol; NY/T, China Agricultural industry standards; LND, low nutrient density.
Effects of 25-OH-D3 on bone formation under 2 different vitamin regimens was also assessed biochemically. The serum indicators for bone formation, ALP and P1NP, were reduced significantly by the 25-OH-D3 treatment in NRC but not in NY/T vitamin regimen (Table 7). Responded to 25-OH-D3 supplementation, serum 25-OH-D and Ca concentrations were notably higher in 25-OH-D3-treated duck than that in untreated ducks (Table 7), but serum P and PTH concentrations were not altered by dietary 25-OH-D3 in any of the groups (Table 7). Thus, the administration of 25-OH-D3 promoted the sternal mass not through enhancing bone formation, perhaps as the result of indirect increase in Ca absorption from tissues.
Table 7.
Effects of 25-OH-D3 and vitamin regimen on serum biochemistry for meat duck at 49 D of age.
| Item | Ca, mmol/L | P, mmol/L | 25-OH-D, ng/L | PTH, ng/L | ALP, UI/L | P1NP, μg/L | |
|---|---|---|---|---|---|---|---|
| Vitamin level | 25-OH-D3 | ||||||
| NRC | − | 2.33c | 3.61 | 435.78c | 39.84 | 773.50a | 14.49a |
| + | 2.44a,b | 3.72 | 475.62a,b | 37.79 | 741.83b | 12.35b | |
| NY/T | − | 2.37b,c | 3.66 | 453.65b | 38.40 | 755.83a,b | 13.40a,b |
| + | 2.46a | 3.76 | 488.78a | 37.96 | 7,340b | 11.89b | |
| SEM | 0.03 | 0.04 | 8.36 | 1.64 | 20.53 | 0.42 | |
| Vitamin regimen | |||||||
| NRC | 2.39 | 3.66 | 455.70b | 38.81 | 757.67 | 13.42 | |
| NY/T | 2.41 | 3.71 | 471.21a | 38.18 | 744.92 | 12.64 | |
| SEM | 0.02 | 0.03 | 5.91 | 1.16 | 14.52 | 0.310 | |
| 25-OH-D3 | |||||||
| − | 2.34b | 3.63 | 444.72b | 39.12 | 764.67a | 13.94a | |
| + | 2.45a | 3.74 | 482.19a | 37.87 | 737.92b | 12.11b | |
| SEM | 0.02 | 0.03 | 5.91 | 1.16 | 14.52 | 0.31 | |
| P-value | |||||||
| Vitamin regimen | 0.433 | 0.259 | 0.041 | 0.705 | 0.398 | 0.083 | |
| 25-OH-D3 | 0.004 | 0.058 | 0.003 | 0.456 | 0.032 | 0.004 | |
| Interaction | 0.833 | 0.929 | 0.755 | 0.630 | 0.068 | 0.471 | |
a–c Mean values with unlike letters were significantly different (two-way ANOVA, P < 0.05, Tukey's post hoc test).
Abbreviations: 25-OH-D, 25-hydroxycholecalciferol; ALP, alkaline phosphatase; Ca, calcium; NY/T, China Agricultural industry standards; P, phosphorus; P1NP, procollagen type I N-terminal propeptide; PTH, parathyroid hormone.
Effect on Sternal Characteristics and Bone Metabolism in PC Diet
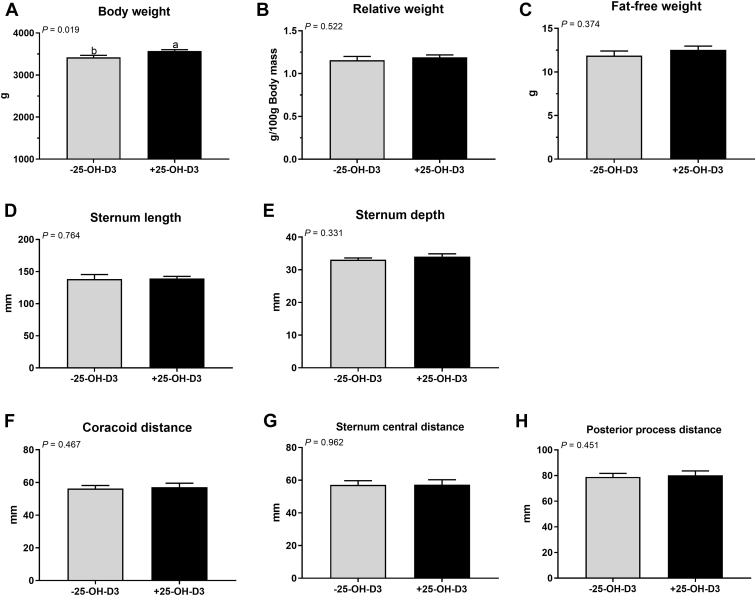
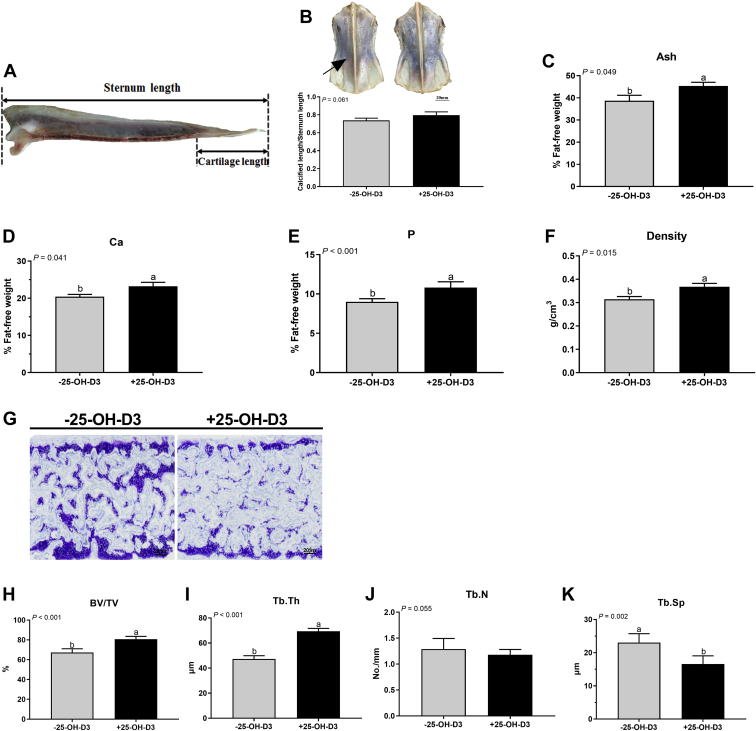
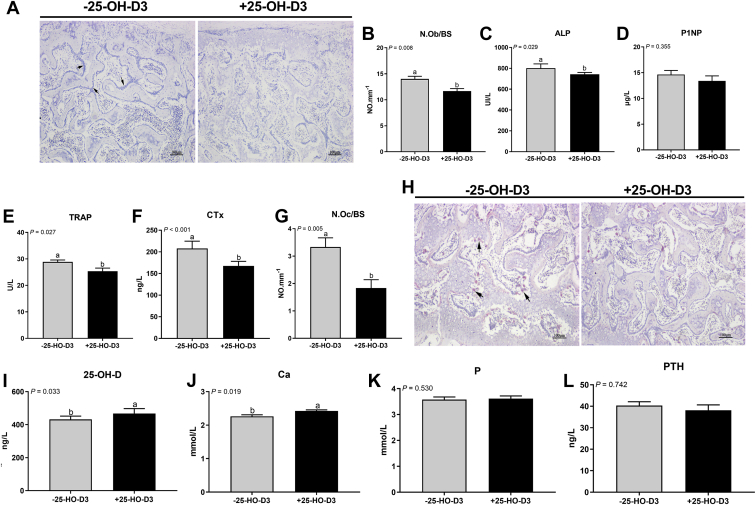
To verify the preferential effect of 25-OH-D3 in NRC vitamin premix diet on the sternal mass of meat ducks, another animal trial was conducted in a standard nutrient density diet contained NRC vitamin premix with 0 or 0.069 mg/kg of 25-OH-D3, and the results showed that 25-OH-D3 administered to birds for 49 D significantly increased the final BW but has no apparent change the sternal relative weight and dimension (Figure 3). Dietary 25-OH-D3 addition increased the mineralization area (darker colors) and sternal minerals deposition in 49-day-old ducks (Figures 4B–4F). Toluidine blue stained and morphometric analysis revealed that parameters of the trabecular bone structure, such as BV/TV and Tb.Th were significantly increased, and Tb.Sp was significantly decreased by 25-OH-D3 treatment (Figures 4G–4K). The influence of 25-OH-D3 on bone turnover also was determined histologically and biochemically and showed that both osteoblast and osteoclast number were remarkably decreased by 25-OH-D3 (Figures 5B, 5G). Serum levels of CTx, TRAP, and ALP activity were significantly suppressed by dietary 25-OH-D3 supplementation (Figures 5C, 5E, 5F). Meanwhile, 25-OH-D3 treatment markedly increased serum 25-OH-D and Ca concentrations (Figures 5I, 5J) but not significantly altered serum P and PTH level in 49-day-old ducks (Figures 5K, 5L). Overall, supplementation 25-OH-D3 to PC diet with NRC vitamin regimen improved the sternal mass through decreasing bone turnover and inducing Ca resorption from intestine and/or kidney.
Figure 3.
Body weight and sternal characters of 49-day-old meat duck responses to 25-OH-D3 in PC diet with NRC vitamin regimen. (A) Body weight, (B) Sternum relative weight (per 100 g body weight), (C) fat-free weight, (D) sternum length, (E) depth, (F) coracoid distance, (G) sternum central distance, and (H) posterior process distance. Values are means and standard deviation represented by vertical bars (n = 8). a, bMean values with different letters are significantly different (one-way ANOVA, P < 0.05, Tukey's post hoc test). Abbreviations: 25-OH-D3, 25-hydroxycholecalciferol; PC, positive control.
Figure 4.
Sternal mineralization and microarchitecture of 49-day-old meat duck responses to 25-OH-D3 in PC diet with NRC vitamin regimen. (A) Picture showing the measure location of calcified ration, (B) the visually the mineralization area (darker colors) and calcified ration. (C) ash, (D) calcium (Ca), (E) phosphorus (P), and (F) density. (G) Toluidine blue stained and the morphometric analysis for (H) trabecular bone volume/tissue volume (BV/TV), (I) trabecular thickness (Tb.Th), (J) number (Tb.N), and (K) spacing (Tb.Sp). Values are means and standard deviation represented by vertical bars (n = 8). a, bMean values with different letters are significantly different (one-way ANOVA, P < 0.05, Tukey's post hoc test). Abbreviations: 25-OH-D3, 25-hydroxycholecalciferol; PC, positive control.
Figure 5.
Bone metabolism of 49-day-old meat duck responses to 25-OH-D3 in PC diet with NRC vitamin regimen. To reflect bone formation, sternum was subjected to a (A) alkaline phosphatase (ALP) staining for (B) osteoblast number (N.Ob/BS) calculation, and serum was obtain for (C) ALP and (D) procollagen type I N-terminal propeptide (P1NP) analyses. Serum (E) tartrate-resistant acid phosphatase (TRAP) activity and (F) C-terminal cross-linked telopeptide of type I collagen (CTx) were measured, and (G) the osteoclast number (N.Oc/BS) was determined via (H) TRAP staining for bone resorption analyses. Serum levels of (I) 25-hydroxyvitamin D (25-OH-D), (J) calcium (Ca), (K) phosphorus (P), and (L) parathyroid hormone (PTH) were also further measured. Values are means and standard deviation represented by vertical bars (n = 8). a, bMean values with different letters are significantly different (one-way ANOVA, P < 0.05, Tukey's post hoc test). Abbreviations: 25-OH-D3, 25-hydroxycholecalciferol; PC, positive control.
Discussion
In the current study, we examined the effect on sternal mass and bone metabolism of the daily administration of 25-OH-D3 in LND diet with different vitamin regimen and PC diet. NY/T vitamin regimen increased sternal mass compared with NRC vitamin premix in LND diet. Supplementing 25-OH-D3 to NRC vitamin regimen improved the bone mass of the sterna with the suppression of both bone resorption and bone formation in LND and PC diet. It was clear that the increase in sternal mass was mainly because of the suppression of bone resorption. The decrease in bone formation caused by 25-OH-D3 may be related to the coupling reaction induced by the suppression of bone resorption.
Recent studies have indicated that diets supplemented with 2,000 IU/kg vitamin D3 improved broiler's BW in comparison with birds fed according to NRC (1994) recommendations (Gomezverduzco et al., 2013). The positive effect of supplement 25-OH-D3 was also reflected in an increased BW gain and feed conversion ratio (Santiago et al., 2016). Coincidentally, the present study revealed 25-OH-D3 administered significantly increased the final BW of 49-day-old ducks in PC diet, as well as increased BW (14 D), gain (1–14 D) and decreased FI (15–42 D) and F: G in LND diet with any given vitamin premix. A positive impact of NY/T vitamin diet on BW also noticed in the present study. The birds fed NY/T vitamin diet had a significantly higher BW at 35, 42, 49, and 35 D of age, which was consistent with our previous observation that feeding high-vitamin level diet increases the BW of meat duck than low-vitamin level diet (Ren et al., 2017, Zhang et al., 2019), and it is partly because NY/T had higher levels of all vitamins except nicotinic acid than NRC recommendations (Table 1).
Vitamin D3 also exerts a variety of actions on maintaining a healthy mineralized skeleton (Goltzman, 2018). In an excellent review published by Świątkiewicz et al. (2017), it was shown that the supplementation about 3,000 IU/kg vitamin D3, which much higher than NRC (1994) recommendations, is optimal for mineral digestibility and bone quality of broilers. Meat-type duck is no exception, and the present study confirmed that supplementing 25-OH-D3 (the equivalent of 2,760 IU/kg vitamin D3) to NRC vitamin regimen significantly increased the mineral deposition and density of sternum in LND diet in the 49-day-old duck. These findings were in accordance with previous studies conducted in broilers (Wideman et al., 2015, Santiago et al., 2016). However, an apparent increment of sternal mass induced by 25-OH-D3 was not observed in NY/T vitamin diets, suggesting the effect of 25-OH-D3 on sternum quality of meat duck probably depended on dietary vitamin regimen (Ren et al., 2017). Also, it was noticed that these positive effects of 25-OH-D3 on sternal mass were not prominently stood out in 42-day-old and 56-day-old duck in the LND diet. It is likely related to the sternum mineralization kinetic. Our laboratory has recently proposed the rapid mineralization of sternum occurs in between 42 and 49 D of age and achieve the plateau phase after 49 D for meat duck (Zhang et al., 2017). Thus, more attention was given to the impact of 25-OH-D3 on sternal mass at 49-day-old duck. As previously reported (Nakamichi et al., 2017), morphometric analysis has further demonstrated that dietary 25-OH-D3 administration significantly promoted sternal mineralization at 49 D, evidenced by the increase of BV/TV and Tb.N, and the decrease of Tb.Sp among all diets in a similar fashion. Overall, LND diet with NY/T vitamin premix is beneficial to sterna mass of 49-day-old duck compared with NRC vitamin diet and supplementation 25-OH-D3 to NRC but not NY/T vitamin regimens could significantly promote mineral accumulation of sternum in spite of perfect sternal microarchitecture in NY/T vitamin diet.
It is well established that the main function of vitamin D3 is the maintenance of Ca homeostasis, although it is also involved in multiple other biological effects (Dittmer and Thompson, 2011). During hypocalcemia, vitamin D3 will activate the Ca absorption in intestines and kidney and mobilize the Ca from bone to maintain normal Ca concentration in blood, which also depends on the PTH concentration (Lips and van Schoor, 2011). Hence, the effect of vitamin D3 on the bone (stimulation or inhibition of bone resorption and mineralization) also depends on the PTH level in blood (Sun et al., 2016). In the present study, serum Ca concentration was increased because of the dietary 25-OH-D3 supplementation LND diets at 49 D, but the PTH concentration was did not significantly change by the dietary 25-OH-D3, which indicates that the blood Ca level was still within the normal range, and the Ca content in LND diets is sufficient to meet the requirements of meat duck. Alternatively, the increased serum Ca concentration might suggest that the positive effect of 25-OH-D3 on sternal mass in vivo at least partly is through inducing the Ca absorption in intestine or kidney.
In addition to the well-known vitamin D3 stimulation of the intestinal absorption of Ca, this positive effect on sternum was also identified to directly regulate the several bone cell types including osteoblasts, osteocytes, and osteoclasts (Goltzman, 2018). Vitamin D3 was reported to act on osteoclasts by stimulating or inhibiting bone resorption in vivo (Sato et al., 2007, Harada et al., 2012), although vitamin D3 has been used as therapeutic agents for osteoporosis (Richy et al., 2005). The discrepancies could be partly explained by the dose used in these studies (Zarei et al., 2016). In terms of circulating bone resorption markers (TRAP and CTx) which were examined in this study, both TRAP activity and CTx concentration were significantly decreased by the 25-OH-D3 treatment in the LND diet with NRC but not with NY/T vitamin premix, which indicates that the supplementation of 25-OH-D3 to NRC vitamin premix could suppress bone resorption of sterna in 49-day-old meat ducks. This inhibitory effect of 25-OH-D3 probably was because of the decreasing osteoclast differentiation in this study, which was evidence in the treatment of 25-OH-D3, in which RANKL/OPG ratio, an important regulator of osteoclastogenesis markedly declined, and consequently the osteoclast number decreased in NRC vitamin regimen. In agreement with our results, previous findings also showed administration of vitamin D3 decreased the ratio of RANKL to OPG in vivo and in vitro (Tang and Meng, 2009, Nakamichi et al., 2017). In addition, some important enzymes, such as vacuolar H+-ATPases (V-ATPases) and cathepsin K, identified in numerous studies could also associated with the function of osteoclast through dissolving the organic and inorganic components of bone in the process of bone resorption (Fujisaki et al., 2007, Riihonen et al., 2007). Downregulated expression of cathepsin K because of 25-OH-D3-treated in NRC but not NY/T vitamin diets implied the suppression of osteoclast activity induced by 25-OH-D3, and this may be another a contributor to the decreased bone resorption. Taken together, these results may indicate that the addition of 25-OH-D3 to the LND diet with NRC vitamin regimen acted on osteoclast to inhibit bone resorption of sterna in 49-day-old ducks.
Vitamin D3 was reported to enhance osteoblast differentiation and mineralization (Turner et al., 2014, van der Meijden et al., 2014). In contrast, consistent with the previous findings on eldecalcitol, an active vitamin D analog (Harada et al., 2012, Nakamichi et al., 2017), 25-OH-D3 supplementation to NRC vitamin regimen significantly decreased the serum ALP activity and P1NP levels, which suggests that 25-HyD is unlikely to be a positive regulator for bone formation. This suppressive effect on bone formation appears to be an indirect action of 25-OH-D3 and is a consequence of coupling of bone resorption to bone formation (Nakamura et al., 2003). Sclerostin was recently proposed to be a key mediator for the coupling process (Masuki et al., 2010). In the current study, 25-OH-D3 treatment upregulated osteocyte-specific genes transcription including sclerostin, suggesting that excessive bone mass induces a higher expression of sclerostin and subsequently sclerostin in turn regulated bone formation to return to the normal level. Further studies would be essential to exclude this possibility.
Under a standard nutrient density diet, we further showed that dietary 25-OH-D3 treatment remarkably decreased osteoblast and osteoclast number, as well as serum bone turnover markers concentration. Meanwhile, dietary 25-OH-D3 also significantly increased the serum total vitamin D3 and Ca concentrations. As a result, supplementation 25-OH-D3 to the PC diet with NRC vitamin regimen has a positive role in promoting sternal microstructure and bone mass in 49-day-old meat duck.
NY/T vitamin regimen increased the minerals accumulation of sternum and declined these indictors that reflect both the bone formation and resorption compared with NRC vitamin premix in LND diet. More importantly, the outcome of histomorphometry showed that dietary 25-OH-D3 administration significantly improved the trabecular microstructure of sternum, but it did not significantly alter the mineral content of the whole sternum and bone metabolism of sternum in NY/T vitamin diet. It is possible that the vitamin composition and proportion of NY/T vitamin regimen are adequate to sternal mass. Additionally, the higher bone mass in the NY/T diet compared with the NRC diet could be the result of a lower bone turnover ratio (Kolp et al., 2017). Further experiments would be essential for understanding the overall mechanisms of the NY/T vitamin regimen actions on bone metabolism.
Taken together, the present findings demonstrated that the LND diet with NY/T vitamin regimen had a favorable effect on the sternal mass of duck through decreasing bone turnover, and the 25-OH-D3 administration notably induced an increase in bone mass in NRC vitamin regimen, which is mediated by suppressing bone resorption in 49-day-old meat duck.
Acknowledgments
This work was supported by Modern Agri-industry Technology Research System (CARS-42-10), China, and the 111 Project, China. The authors thank Jeyanathan Jeyamalar for the revision of English.
References
- Bonjour J.P., Dontot-Payen F., Rouy E., Walrand S., Rousseau B. Evolution of serum 25OHD in response to vitamin D3-fortified yogurts consumed by healthy menopausal women: a 6-month randomized controlled trial assessing the interactions between doses, baseline vitamin D status, and seasonality. J. Am. Coll. Nutr. 2018;37:34–43. doi: 10.1080/07315724.2017.1355761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickett K.E., Dahiya J.P., Classen H.L., Annett C.B., Gomis S. The impact of nutrient density, feed form, and photoperiod on the walking ability and skeletal quality of broiler chickens. Poult. Sci. 2007;86:2117–2125. doi: 10.1093/ps/86.10.2117. [DOI] [PubMed] [Google Scholar]
- Claessens L.P. The skeletal kinematics of lung ventilation in three basal bird taxa (emu, tinamou, and Guinea fowl) J. Exp. Zool. A. Ecol. Genet. Physiol. 2009;311:586–599. doi: 10.1002/jez.501. [DOI] [PubMed] [Google Scholar]
- Dittmer K.E., Thompson K.G. Vitamin D metabolism and rickets in domestic animals: a review. Vet. Pathol. 2011;48:389–407. doi: 10.1177/0300985810375240. [DOI] [PubMed] [Google Scholar]
- Duggan B.M., Hocking P.M., Schwarz T., Clements D.N. Differences in hindlimb morphology of ducks and chickens: effects of domestication and selection. Genet. Sel. Evol. 2015;47:88. doi: 10.1186/s12711-015-0166-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisaki K., Tanabe N., Suzuki N., Kawato T., Takeichi O., Tsuzukibashi O., Makimura M., Ito K., Maeno M. Receptor activator of NF-kappaB ligand induces the expression of carbonic anhydrase II, cathepsin K, and matrix metalloproteinase-9 in osteoclast precursor RAW264.7 cells. Life Sci. 2007;80:1311–1318. doi: 10.1016/j.lfs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- Goltzman D. Functions of vitamin D in bone. Histochem. Cell Biol. 2018;149:305–312. doi: 10.1007/s00418-018-1648-y. [DOI] [PubMed] [Google Scholar]
- Gomezverduzco G., Moraleslopez R., Avilagozalez E. Use of 25-hydroxycholecalciferol in diets of broiler chickens effects on growth performance, immunity and bone calcification. J. Poult. Sci. 2013;50:60–64. [Google Scholar]
- Han J.C., Chen G.H., Wang J.G., Zhang J.L., Qu H.X., Zhang C.M., Yan Y.F., Cheng Y.H. Evaluation of relative bioavailability of 25-hydroxycholecalciferol to cholecalciferol for broiler chickens. Asian-Australas. J. Anim. Sci. 2016;29:1145–1151. doi: 10.5713/ajas.15.0553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada S., Mizoguchi T., Kobayashi Y., Nakamichi Y., Takeda S., Sakai S., Takahashi F., Saito H., Yasuda H., Udagawa N., Suda T., Takahashi N. Daily administration of eldecalcitol (ED-71), an active vitamin D analog, increases bone mineral density by suppressing RANKL expression in mouse trabecular bone. J. Bone Miner. Res. 2012;27:461–473. doi: 10.1002/jbmr.555. [DOI] [PubMed] [Google Scholar]
- Iyer P.K., Rao P.P. Suspected pulmonary nocardiosis in a duck. Sabouraudia. 1971;9:79–80. doi: 10.1080/00362177185190201. [DOI] [PubMed] [Google Scholar]
- Jiang S., Jiang Z., Yang K., Chen F., Zheng C., Wang L. Dietary vitamin D3 requirement of Chinese yellow-feathered broilers. Poult. Sci. 2015;94:2210–2220. doi: 10.3382/ps/pev163. [DOI] [PubMed] [Google Scholar]
- Julian R.J. Ascites in meat-type ducklings. Avian Pathol. 1988;17:11–21. doi: 10.1080/03079458808436424. [DOI] [PubMed] [Google Scholar]
- Julian R.J. Rapid growth problems: ascites and skeletal deformities in broilers. Poult. Sci. 1998;77:1773–1780. doi: 10.1093/ps/77.12.1773. [DOI] [PubMed] [Google Scholar]
- Khan S.H., Shahid R., Mian A.A., Sardar R., Anjum M.A. Effect of the level of cholecalciferol supplementation of broiler diets on the performance and tibial dyschondroplasia. J. Anim. Physiol. Anim. Nutr. (Berl) 2010;94:584–593. doi: 10.1111/j.1439-0396.2009.00943.x. [DOI] [PubMed] [Google Scholar]
- Kogawa M., Findlay D.M., Anderson P.H., Ormsby R., Vincent C., Morris H.A., Atkins G.J. Osteoclastic metabolism of 25(OH)-vitamin D3: a potential mechanism for optimization of bone resorption. Endocrinology. 2010;151:4613–4625. doi: 10.1210/en.2010-0334. [DOI] [PubMed] [Google Scholar]
- Kolp E., Wilkens M.R., Pendl W., Eichenberger B., Liesegang A. Vitamin D metabolism in growing pigs: influence of UVB irradiation and dietary vitamin D supply on calcium homeostasis, its regulation and bone metabolism. J. Anim. Physiol. Anim. Nutr. (Berl) 2017;101 Suppl 1:79–94. doi: 10.1111/jpn.12707. [DOI] [PubMed] [Google Scholar]
- Lips P., van Schoor N.M. The effect of vitamin D on bone and osteoporosis. Best Pract. Res. Clin. Endocrinol. Metab. 2011;25:585–591. doi: 10.1016/j.beem.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Masuki H., Li M., Hasegawa T., Suzuki R., Ying G., Zhusheng L., Oda K., Yamamoto T., Kawanami M., Amizuka N. Immunolocalization of DMP1 and sclerostin in the epiphyseal trabecule and diaphyseal cortical bone of osteoprotegerin deficient mice. Biomed. Res. 2010;31:307–318. doi: 10.2220/biomedres.31.307. [DOI] [PubMed] [Google Scholar]
- Nakamichi Y., Udagawa N., Horibe K., Mizoguchi T., Yamamoto Y., Nakamura T., Hosoya A., Kato S., Suda T., Takahashi N. VDR in osteoblast-lineage cells primarily mediates vitamin D treatment-induced increase in bone mass by suppressing bone resorption. J. Bone Miner. Res. 2017;32:1297–1308. doi: 10.1002/jbmr.3096. [DOI] [PubMed] [Google Scholar]
- Nakamura M., Udagawa N., Matsuura S., Mogi M., Nakamura H., Horiuchi H., Saito N., Hiraoka B.Y., Kobayashi Y., Takaoka K., Ozawa H., Miyazawa H., Takahashi N. Osteoprotegerin regulates bone formation through a coupling mechanism with bone resorption. Endocrinology. 2003;144:5441–5449. doi: 10.1210/en.2003-0717. [DOI] [PubMed] [Google Scholar]
- NRC . National Academies Press; Washington, DC: 1994. Nutrient Requirements of Poultry, 9th rev. ed. [Google Scholar]
- NY/T . China Agricultural Industry Standards; Beijing: 2012. Nutrient Requirement of Meat-Type Ducks (NY/T 2122-2012) [Google Scholar]
- Ren Z.Z., Jiang S.Z., Zeng Q.F., Ding X.M., Bai S.P., Wang J.P., Luo Y.H., Su Z.W., Xuan Y., Zhang K.Y. Effect of maternal canthaxanthin and 25-hydroxycholecalciferol supplementation on the performance of ducklings under two different vitamin regimens. J. Anim. Physiol. Anim. Nutr. (Berl) 2017;101:359–368. doi: 10.1111/jpn.12453. [DOI] [PubMed] [Google Scholar]
- Richy F., Schacht E., Bruyere O., Ethgen O., Gourlay M., Reginster J.Y. Vitamin D analogs versus native vitamin D in preventing bone loss and osteoporosis-related fractures: a comparative meta-analysis. Calcif. Tissue Int. 2005;76:176–186. doi: 10.1007/s00223-004-0005-4. [DOI] [PubMed] [Google Scholar]
- Riihonen R., Supuran C.T., Parkkila S., Pastorekova S., Vaananen H.K., Laitala-Leinonen T. Membrane-bound carbonic anhydrases in osteoclasts. Bone. 2007;40:1021–1031. doi: 10.1016/j.bone.2006.11.028. [DOI] [PubMed] [Google Scholar]
- Santiago M., David S., Alexandra N., Eduardo A., Jimmy Q. Effect of 25-hydroxycholecalciferol (25-OH-D3) on productive performance and bone mineralization in broiler. Open J. Anim. Sci. 2016;6:180–184. [Google Scholar]
- Sato M., Nakamichi Y., Nakamura M., Sato N., Ninomiya T., Muto A., Nakamura H., Ozawa H., Iwasaki Y., Kobayashi E., Shimizu M., DeLuca H.F., Takahashi N., Udagawa N. New 19-nor-(20S)-1alpha,25-dihydroxyvitamin D3 analogs strongly stimulate osteoclast formation both in vivo and in vitro. Bone. 2007;40:293–304. doi: 10.1016/j.bone.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Sun Z.W., Yan L., G Y.Y., Zhao J.P., Lin H., Guo Y.M. Increasing dietary vitamin D3 improves the walking ability and welfare status of broiler chickens reared at high stocking densities. Poult. Sci. 2013;92:3071–3079. doi: 10.3382/ps.2013-03278. [DOI] [PubMed] [Google Scholar]
- Sun J., Sun B., Wang W., Han X., Liu H., Du J., Feng W., Liu B., Amizuka N., Li M. Histochemical examination of the effects of high-dose 1,25(OH)2D3 on bone remodeling in young growing rats. J. Mol. Histol. 2016;47:389–399. doi: 10.1007/s10735-016-9681-4. [DOI] [PubMed] [Google Scholar]
- Świątkiewicz S., Arczewskawłosek A., Bederskalojewska D., Jozefiak D. Efficacy of dietary vitamin D and its metabolites in poultry - review and implications of the recent studies. Worlds Poult. Sci. J. 2017;73:57–68. [Google Scholar]
- Tang X., Meng H. Osteogenic induction and 1,25-dihydroxyvitamin D3 oppositely regulate the proliferation and expression of RANKL and the vitamin D receptor of human periodontal ligament cells. Arch. Oral Biol. 2009;54:625–633. doi: 10.1016/j.archoralbio.2009.04.009. [DOI] [PubMed] [Google Scholar]
- Tickle P.G., Ennos A.R., Lennox L.E., Perry S.F., Codd J.R. Functional significance of the uncinate processes in birds. J. Exp. Biol. 2007;210:3955–3961. doi: 10.1242/jeb.008953. [DOI] [PubMed] [Google Scholar]
- Turner A.G., Hanrath M.A., Morris H.A., Atkins G.J., Anderson P.H. The local production of 1,25(OH)2D3 promotes osteoblast and osteocyte maturation. J. Steroid Biochem. Mol. Biol. 2014;144(Pt A):114–118. doi: 10.1016/j.jsbmb.2013.10.003. [DOI] [PubMed] [Google Scholar]
- van der Meijden K., Lips P., van Driel M., Heijboer A.C., Schulten E.A., den Heijer M., Bravenboer N. Primary human osteoblasts in response to 25-hydroxyvitamin D3, 1,25-dihydroxyvitamin D3 and 24R,25-dihydroxyvitamin D3. PLoS One. 2014;9:e110283. doi: 10.1371/journal.pone.0110283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandesompele J., De Preter K., Pattyn F., Poppe B., Van Roy N., De Paepe A., Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-7-research0034. Research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wideman R.F. Pathophysiology of heart/lung disorders: pulmonary hypertension syndrome in broiler chickens. Worlds Poult. Sci. J. 2001;57:289–307. [Google Scholar]
- Wideman R.F., Jr., Blankenship J., Pevzner I.Y., Turner B.J. Efficacy of 25-OH vitamin D3 prophylactic administration for reducing lameness in broilers grown on wire flooring. Poult. Sci. 2015;94:1821–1827. doi: 10.3382/ps/pev160. [DOI] [PubMed] [Google Scholar]
- Williams B., Waddington D., Murray D.H., Farquharson C. Bone strength during growth: influence of growth rate on cortical porosity and mineralization. Calcif. Tissue Int. 2004;74:236–245. doi: 10.1007/s00223-002-2124-0. [DOI] [PubMed] [Google Scholar]
- Zarei A., Morovat A., Javaid K., Brown C.P. Vitamin D receptor expression in human bone tissue and dose-dependent activation in resorbing osteoclasts. Bone Res. 2016;4:16030. doi: 10.1038/boneres.2016.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H.Y., Liao H., Zeng Q.F., Wang J.P., Ding X.M., Bai S.P., Zhang K.Y. A study on the sternum growth and mineralization kinetic of meat duck from 35 to 63 days of age. Poult. Sci. 2017;96:4103–4115. doi: 10.3382/ps/pex223. [DOI] [PubMed] [Google Scholar]
- Zhang H., Zeng Q., Bai S., Wang J., Ding X., Xuan Y., Su Z., Zhang K. Effect of graded calcium supplementation in low-nutrient density feed on tibia composition and bone turnover in meat ducks. Br. J. Nutr. 2018;120:1217–1229. doi: 10.1017/S0007114518002556. [DOI] [PubMed] [Google Scholar]
- Zhang H., Liao H., Zeng Q., Wang J., Ding X., Bai S., Zhang K. Effects of commercial premix vitamin level on sternum growth, calcification and carcass traits in meat duck. J. Anim. Physiol. Anim. Nutr. (Berl) 2019;103:53–63. doi: 10.1111/jpn.13001. [DOI] [PubMed] [Google Scholar]