Abstract
Pioneer colonization of the gastrointestinal tract (GIT) by bacteria is thought to have major influence on neonatal tissue development. Previous studies have shown in ovo inoculation of embryos with saline (S), species of Citrobacter (C, C2), or lactic acid bacteria (L) resulted in an altered microbiome on day of the hatch (DOH). The present study investigated GIT proteomic changes at DOH in relation to different inoculations. Embryos were inoculated in ovo with S or ∼102 cfu of C, C2, or L at 18 embryonic days. On DOH, the GIT was collected, and tissue proteins were extracted for analysis via tandem mass spectrometry. A total of 493 proteins were identified for differential comparison with S at P ≤ 0.10. Different levels were noted in 107, 39, and 78 proteins in C, C2, and L groups, respectively, which were uploaded to Ingenuity Pathway Analysis to determine canonical pathways and biological functions related to these changes. Three members of the cytokine family (interleukin [IL]-1β, IL6, and Oncostatin M) were predicted to be activated in C2, indicated with Z-score ≥ 1.50, which suggested an overall proinflammatory GIT condition. This was consistent with the activation of the acute-phase response signaling pathway seen exclusively in C2 (Z-score = 2.00, P < 0.01). However, activation (Z-score = 2.00) of IL-13, upregulation of peroxiredoxin-1 and superoxide dismutase 1, in addition to activation of nitric oxide signaling in the cardiovascular system of the L treatment may predict a state of increased antioxidant capacity and decreased inflammatory status. The nuclear factor erythroid 2-related factor 2 (NRF2)-mediated oxidative stress response (Z-score = 2.00, P < 0.01) was predicted to be upregulated in C which suggested that chicks were in an inflammatory state and associated oxidative stress, but the impact of these pathways differed from that of C2. These changes in the proteome suggest that pioneer colonizing microbiota may have a strong impact on pathways associated with GIT immune and cellular development.
Key words: inflammation, pioneer colonizers, proteome, gastrointestinal tract
Introduction
The gastrointestinal tract (GIT) acts as a barrier for pathogens (physical and immune) and is where nutrients are absorbed (Klasing, 1998, Furness et al., 1999, Yan and Polk, 2010, Zhang et al., 2015). A recent focus, to optimize growth performance and health in poultry production, has been the modulation of GIT microbiota (Seifert et al., 2011, Kohl, 2012, Ballou et al., 2016, Choi et al., 2018). Establishment of a healthy and diverse GIT microbiota in poultry has been recognized for critical growth performance and flock health (Tellez et al., 2006, Higgins et al., 2010, Latorre et al., 2015). Manipulation of the microflora may, therefore, be an ideal route for optimizing growth performance, and more importantly, exposure to early colonizing bacteria, known as pioneer colonizers, could have beneficial lasting effects on poultry development. Pioneer colonizers may play a critical role in GIT development even before chicks reach the grow-out house, with impacts beginning in hatcher cabinets as they are exposed to bacteria within hatchers and on eggshells.
When chicks hatch, they are first exposed to maternal microbes deposited on the exterior of their eggs' shells, as well as a plethora of endogenous bacteria in commercial hatcheries (Stanley et al., 2013, Smith and Rehberger, 2018). Without intervention, the pioneer colonizers of a chick may be unwanted pathogens (Cox et al., 1990, Byrd et al., 2007, Oliveira et al., 2014). Chicks are especially susceptible to pathogen infections in the GIT because their immune system is naïve, which can lead to a less robust immune response and little clearance (Beal et al., 2004). It has been shown that in ovo application of bacterial candidates as pioneer colonizers may allow colonization of beneficial bacteria before the chick is ever exposed to pathogens within the hatcher cabinet (Teague et al., 2017, Graham et al., 2018). More importantly, appropriate pioneer colonizers can affect important phenotypic attributes in an animal such as body weight. The inclusion of lactic acid bacteria (LAB) probiotic, composed of Lactobacillus salivarius and Pediococcus sp. (Higgins et al., 2007), in ovo can increase the body weight of a chick by 7 D of age (Teague et al., 2017). Wilson (1991) found that every gram increase in body weight at hatch could result in bodyweight increases up to 13 g at market weight. However, bodyweight changes may not be measurable at day of hatch (DOH) and may present instead as developmental changes in the GIT, in relation to immune activation, which may affect the developmental characteristics in the chick.
There are several proteins in the small intestine that are involved in a number of physiological functions, and traditional methods used for detecting differential proteins such as Western blotting, immunohistochemical staining, or ELISA can only identify targeted proteins with a known antibody (Mahmood and Yang, 2012, Bass et al., 2017). On the other hand, analyzing mRNA levels may not coincide with proteins synthesized or identify coexpression of proteins based on their gene functions (Wang et al., 2017). The proteome plays a key role in connecting the genome and the transcriptome to relay potential biological functions. Thus, the advancements of shotgun proteomics enable the identification and quantification of hundreds to thousands of proteins at a given time (Altelaar et al., 2013, Tubaon et al., 2017). Beyond finding the fold changes and expression differences between samples, programs such as Ingenuity Pathway Analysis (IPA) can predict activation or inhibition of upstream regulators along with the relationships of important molecules and pathways associated with the researched data set (Kong et al., 2016). Therefore, the objective of this study was to evaluate the developmental changes of chick GIT at DOH in relation to different pioneer colonizers. By understanding these changes, especially in the context of nondesirable microbiota, potential targets for selection of functional probiotics could be identified.
Materials and methods
Embryo Inoculation, Incubation, and Hatching
A total of 360 Ross 708 fertile broiler eggs were obtained from a local hatchery and placed in a single-stage incubator (Natureform Inc. Jacksonville, FL) until 18 embryonic days (E). Before inoculation, all eggs were confirmed fertile at 18 E by candling. Once confirmed, the air cell end of each egg was sterilized with iodine (Povidone-Iodine 10% topical solution, Drug Mart, Medina, OH). The egg inoculations were performed following the methods previously published by Teague et al., 2017. A small hole was punched into the shell to inoculate each embryo with their treatment in the amnion (Higgins et al., 2007, Prado-Rebolledo et al., 2017). Treatments included 200 uL of 0.9% sterile saline (S) or approximately 102 cells of Citrobacter freundii 97A11 (C), Citrobacter spp. 97A4 (C2), or a mixed inoculum of L. salivarius and Pediococcus ssp. (L). Once all embryos were inoculated, up to 30 eggs were immediately allocated by treatments to separate benchtop hatchers (Hova-Bator model 1602N, Savannah, GA), which had been disinfected with 10% bleach before use. Each treatment was separated into 3 independent hatchers, for a total of 12 across the entire experiment. Incubators were set to standard commercial temperatures and relative humidity (Ven et al., 2013).
Bacterial Inoculum Preparation
The enteric bacteria chosen for this study were of healthy adult poultry cecal origin, formerly identified as C. freundii (C) and Klebsiella oxytoca (C2) using the analytical profile index test strips and 16S sequencing (Bielke et al., 2003). Next-generation sequencing on Miseq Illumina platform from a previous study confirmed that both strains belonged to the genus Citrobacter (Wilson et al., 2019).
Preliminary experimental observations concluded that the inclusion of isolates at approximately 102 cfu did not affect hatchability compared with the S control treatment (data not published). An aliquot of each bacterial isolate was thawed and inoculated in tryptic soy broth (Sigma-Aldrich, St. Louis, MO) at 1% volume and incubated at 37°C for up to 24 h. After incubation, each isolate was washed 3 times in S by centrifugation at 1,800 × g. The approximate concentration was quantified spectrophotometrically (Spectronic 20D+, Spectronic Instruments Thermo Scientific, Madison, WI) at 625 nm. Each isolate was serially diluted in saline, and each egg was inoculated with 200 uL (approximately 2 × 102 cfu). The actual concentration was also determined retrospectively by serial dilution and plating on tryptic soy agar (Sigma-Aldrich, St. Louis, MO). Determined inoculum concentrations were as follows: C, 2.3 × 102 cfu/embryo; C2, 8.0 × 101 cfu/embryo; and L, 6.7 × 101 cfu/embryo.
Sample Collections
Once all chicks were hatched, 9 chicks were randomly chosen from the hatchers within each treatment and immediately euthanized via cervical dislocation. The GIT, spanning from the duodenum to ceca, was removed, and tissues were placed immediately in individual 2-mL tubes and frozen in liquid nitrogen, before being stored at −80°C. All work occurred on stainless steel trays, cleaned with sterile Milli-Q water and Kimwipes (KimTech Low-Lint Wipers, Kimberly-Clark, Irving, TX) between samples, over ice to maintain protein integrity. Equal tissue amounts from every section were cut, and a cumulative total of 0.1 g was placed into 5 mL of buffer (8 mol urea/2 mol thiourea, 2 mmol dithiothreitol, 50 mmol Tris, 5% SDS). The extraction protocol used was a modified version described previously (Iqbal et al., 2004, Kong et al., 2016). In brief, samples were homogenized for 5 s (PRO250 Homogenizer, Pro Scientific, Oxford, CT) and then additionally homogenized using a bead beater (MiniBeadbeater-16, Model 607, BioSpec Products, Bartlesville, OK) using 0.1 g of 0.9- to 2.0-mm diameter stainless steel beads (SSB14 B Next Advance, Averill Park, NY) combined with 1 mL of sample. Samples were homogenized in 30-s intervals for a total of 3 min, with samples placed on ice for 30 s between intervals to prevent overheating. The bead beater was kept in a 4°C cooler to further ensure samples were kept cool. Then, samples were centrifuged at 4°C at 14,000 rpm for 20 min, after which the supernatant was collected, aliquoted, and stored at −80°C for future use.
The concentration of total protein was quantified using the Bradford assay (Bradford reagent, VWR, Suwanee, GA) and a standard bovine serum albumin curve (VWR, Suwanee, GA) on a Synergy HTX Multi-Mode Microplate Reader (BioTek U.S., Winooski, VT). Total protein range for extracted samples was 1.56–1.86 μg/μL. Samples were pooled at equal concentrations into 3 composite samples from each treatment (chicks 1–3; 4–6; 7–9), resulting in 12 total samples of 3 samples per treatment. Pooling of samples was a strategy used to reduce the influence of individual variation which is common in proteomic studies (Zhang et al., 2015, Buzała et al., 2015). To ensure proper extraction, bromophenol blue was added to the sample buffer at 0.0004%, and an SDS-PAGE of each sample was conducted with a 10% acrylamide gel with a 4% stacking gel (RunBlue SDS Gel 10%, Expedeon, San Diego, CA). Gels were run in a vertical electrophoresis box (Vertical Gel Electrophoresis Systems Mini PAGE, VWR, Suwanee, GA) at 100 V for approximately 1.5–2.5 h. Composite samples were sent to the Ohio State University Proteomics Core laboratory for in-solution digestion and mass spectrometry via established methods described in the following section.
In-Solution Digestion
Samples were precipitated with trichloroacetic acid and then resuspended in 50 mmol ammonium bicarbonate. A total of 5 mL of DTT (5 μg/μL in 50 mmol ammonium bicarbonate) was added, and samples were incubated at 56°C for 15 min. After incubation, 5 μL of iodoacetamide (15 mg/mL in 50 mmol ammonium bicarbonate) was added, and the samples were kept in dark at room temperature for 30 min. Sequencing grade–modified trypsin (Promega; Madison, WI) prepared in 50 mmol ammonium bicarbonate was added to each sample with an estimation of 1:20/1:100 enzyme–substrate ratio set at 37°C overnight. The reaction was quenched the following day by adding acetic acid for acidification. Once samples were quenched, the peptide concentration was measured by Nanodrop (Thermo Scientific Nanodrop 2000; Waltham, MA).
Mass Spectrometry
Capillary–liquid chromatography–nanospray tandem mass spectrometry (Capillary-LC/MS/MS) of global protein identification was performed on a Thermo Fisher Fusion mass spectrometer (Thermo Scientific, Waltham, MA). Samples were separated on a Thermo Nano C18 column (UltiMate 3000 HPLC system, Thermo Scientific, Waltham, MA). The MS/MS data sequences were scanned and, based on the preview mode, data-dependent TopSpeed method with collision-induced dissociation and electron-transfer dissociation as fragmentation methods. The raw data were searched on Sequest via Proteome Discoverer (Proteome Discoverer software, Thermo Scientific, Waltham, MA) against the most recent Gallus gallus database for identification of proteins. Only proteins with less than 0.05 false discovery rate were reported. Proteins with a Mascot score of 50 or higher with a minimum of 2 unique peptides from one protein having a –b or –y ion sequence tag of 5 residues or better were accepted. Any modifications or low-score peptide or protein identifications were manually checked for validation.
Quantitation and Statistical Analysis
Label-free quantitation was performed using the spectral count approach, in which the relative protein quantitation was measured by comparing the number of MS/MS spectra identified from the same protein in each of the multiple LC/MS-MS data sets. Scaffold (Scaffold 4.8.4, Proteome Software, Portland, OR) was used for data analysis (Searle, 2010). The Student t test was performed in Scaffold software to evaluate if the fold change for certain proteins were significant (P ≤ 0.05). Volcano plots were also generated to identify the differences between S and C, C2, or L with respect to magnitude of fold difference and a P-value ≤ 0.10 or –log10 (P-value) ≥ 1.0 (Perseus 1.2.0.16; Max Planck Institute of Biochemistry). Fold change of proteins that had a P-value between 0.05 and 0.10 was presented to depict trends in the data (Pearce et al., 2013). The annotations of proteins, relevant upstream regulators, and canonical pathway analysis were analyzed using IPA (Qiagen, Valencia, CA; http://www.ingeunity.com) software, comparing S control with C, C2, or L. To identify changes among microbial challenge treatments, including their predicted upstream regulator changes, fold changes (P ≤ 0.10) among C vs. C2, C vs. L, and C2 vs. L were also analyzed using IPA. If there was a change observed consistent with activation or inhibition of transcriptional regulator in the data, IPA presented a predicted Z-score, a statistical measure of the correlation between relationship direction and gene expression, that predicts activation (Z-score ≥ 2.00) or inhibition (Z-score ≤ -2.00). Qualified predictions were also made for high (Z-score ≥ |1.90| or more) medium (Z-score = |1.70–1.90|), or low (Z-score = |1.50–1.70|) (Kong et al., 2016). The P-value of overlap, which measures any significant statistical overlap between the samples in the data set and the genes that are regulated by the corresponding transcriptional regulator, was also determined and recorded. Fisher exact tests calculated the value at a significance of P < 0.05.
Results
Proteins Isolated
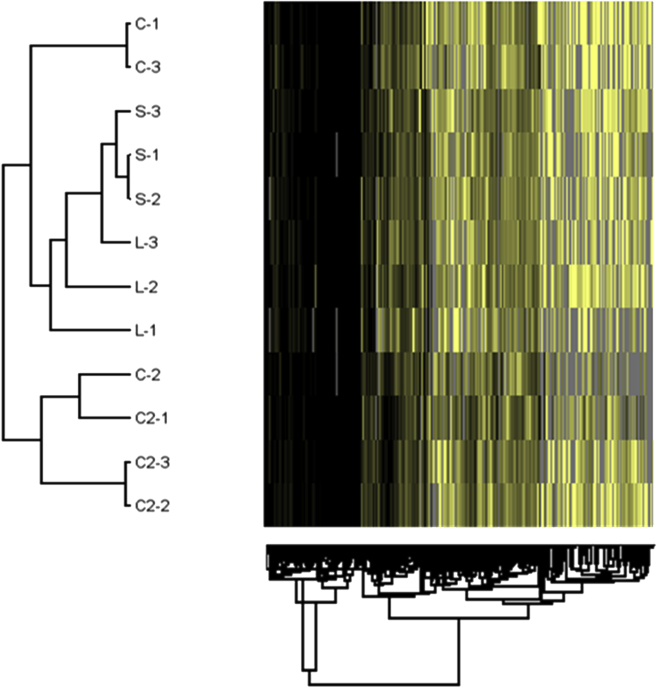
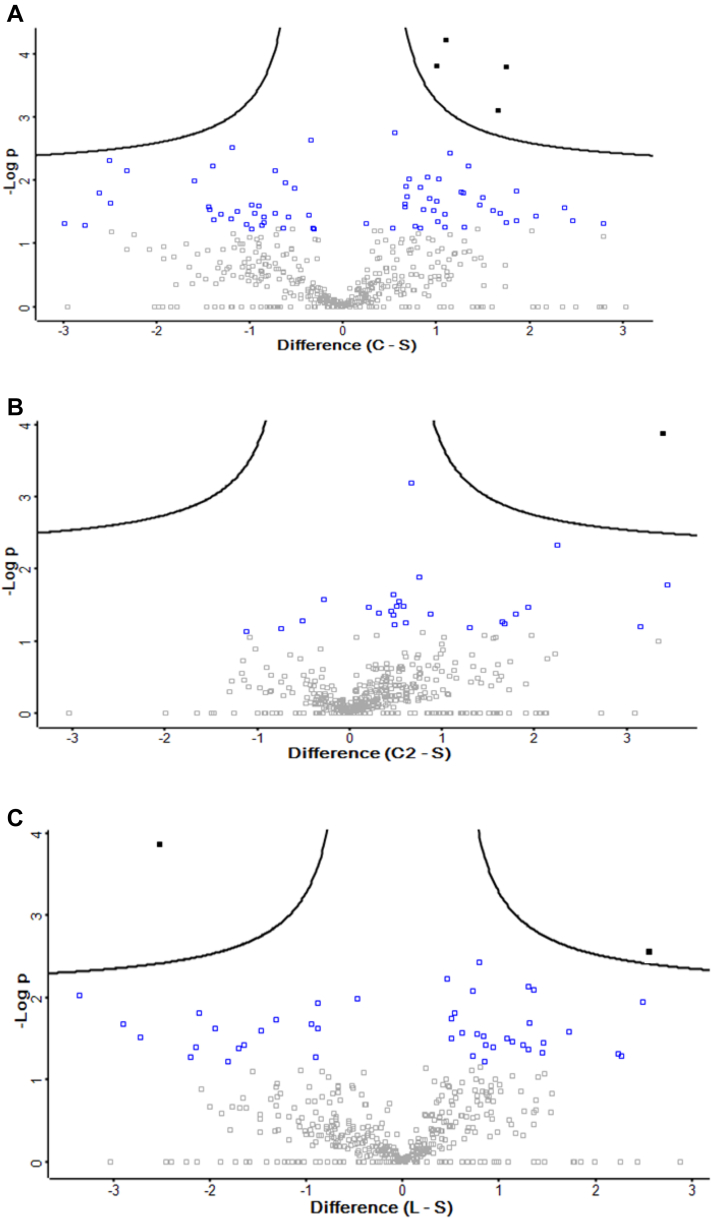
Inoculation of different bacterial isolates in ovo resulted in treatment-distinct microbial communities in accordance with microbiome analysis conducted at the time of collection (Wilson et al., 2019); thus, differences in protein detection here were presumed to be influenced by the altered microbiome. The resulting hierarchical, agglomerative cluster heat map (Figure 1) of the protein profiles for each bioreplicate among each treatment was created to display how similar the samples were from one another. There was an apparent clustering between C, S, and L vs. C2, except for one sample within the C treatment. The volcano plot analysis, presented in Figure 2A to C, showed the distribution of differentially expressed proteins among treatments concerning the magnitude of P-value to fold difference. There were 107, 39, and 78 upregulated or downregulated proteins compared with S control treatment at the level of P-value < 0.10, in C, C2, and L, respectively. Details of selected proteins that were involved in cytoskeletal motility, immune system, and antioxidant systems are listed in Table 1 and are discussed in detail in the following.
Figure 1.
Hierarchical clustering analysis based on the differences in protein profile from day of hatch chick intestinal samples treated with either saline control (S), Citrobacter freundii (C), Citrobacter spp. (C2), or lactic acid bacteria (L) into the amnion as pioneer colonizers. Each sample is a composite of 3 chick intestines and 3 bioreplicates for each treatment. Tree connections show the distance from each sample; the shorter distance or closer the 2 samples are together, the more similar their profiles.
Figure 2.
Volcano plot distribution of proteins identified in (A) Citrobacter freundii (C) (B) Citrobacter spp. (C2) and (C) lactic acid bacteria (L) treatment in comparison to saline control (S). The Y-axis shows the–log10 transformation of the P-values for each of the respective proteins. The X-axis represents the log2 transformation of the fold difference between C, C2, or L treatments and S. The red squares indicate proteins that were identified by the volcano plot analysis (shown by the 2 solid black lines separating the points) concerning the magnitude of P-value to fold difference. The blue squares indicate the rest of the proteins which were significantly different (P < 0.05 or [−log10 (P-value)] > 1.3).
Table 1.
Proteins in select biological functional groups that were differentially expressed in the intestinal tract of day of hatch chicks that were administered Citrobacter freundii (C), Citrobacter spp. (C2), or lactic acid bacteria (L) in ovo at 18 embryonic days compared with the saline (S) control treatment. Proteins were identified by LC-MS/MS and quantified with a label-free spectral count approach. The relative protein quantitation was measured by comparing the number of MS/MS spectra identified from the same protein in each of the multiple LC/MS-MS data sets.
|
Biological function |
Symbol ID1 | Fold change relative to S4 |
P-value3 |
||||
|---|---|---|---|---|---|---|---|
| C | C2 | L | C | C2 | L | ||
| Immune system | ALCAM | ---2 | --- | 2.88 | --- | --- | 0.07 |
| CAV1 | --- | --- | −2.41 | --- | --- | <0.01 | |
| H2B-I | −1.99 | --- | --- | 0.02 | --- | --- | |
| H4-I | 2.45 | --- | 1.94 | 0.02 | --- | 0.01 | |
| HMGB2 | --- | --- | −2.06 | --- | --- | 0.05 | |
| HMGB3 | --- | --- | −4.26 | --- | --- | <0.01 | |
| HSP7C | −1.56 | --- | --- | 0.06 | --- | --- | |
| HSP90AB1 | --- | −1.35 | --- | --- | 0.09 | --- | |
| HSPB1 | 1.89 | --- | 1.52 | 0.01 | --- | 0.09 | |
| HSPD1 | −1.46 | --- | --- | 0.06 | --- | --- | |
| LAC | 2.23 | --- | --- | 0.04 | --- | --- | |
| PSMA7 | --- | --- | 2.21 | --- | --- | 0.01 | |
| STAT3 | --- | 2.85 | --- | --- | 0.08 | --- | |
| Antioxidant system | CYC | −2.14 | --- | --- | 0.02 | --- | --- |
| CYB5A | −3.63 | --- | 2.17 | 0.01 | --- | 0.07 | |
| PRDX1 | 3.41 | 1.49 | 2.58 | <0.01 | 0.01 | <0.01 | |
| SOD1 | --- | --- | 2.04 | --- | --- | 0.08 | |
| Cytoskeletal assembly, cell motility, recognition | ACT5 | −1.72 | --- | --- | 0.05 | --- | --- |
| ACTN1 | 1.45 | --- | --- | 0.06 | --- | --- | |
| ACTN2 | 2.14 | --- | --- | <0.01 | --- | --- | |
| ACTN4 | 1.38 | --- | 1.34 | 0.08 | --- | 0.02 | |
| ATP2A2 | −5.74 | --- | --- | 0.03 | --- | --- | |
| COL1A1 | 1.87 | --- | 1.41 | 0.01 | --- | 0.02 | |
| COL6A2 | --- | --- | −1.35 | --- | --- | 0.08 | |
| COL6A3 | --- | 4.42 | --- | --- | 0.08 | --- | |
| CTTN1 | --- | --- | 1.42 | --- | --- | <0.01 | |
| DES | −1.89 | --- | −1.89 | 0.06 | --- | <0.01 | |
| DMD | −1.84 | --- | −1.78 | 0.04 | --- | 0.02 | |
| DPYSL2 | 1.79 | --- | --- | 0.08 | --- | --- | |
| DSTN | −9.00 | 1.66 | --- | <0.01 | 0.02 | --- | |
| DYNC1LI1 | --- | 1.41 | 1.61 | --- | 0.00 | 0.05 | |
| GSN | −1.88 | --- | --- | --- | 0.03 | --- | |
| M7BG31 | −1.92 | --- | −1.73 | 0.05 | --- | 0.08 | |
| MAPRE1 | −1.92 | −1.52 | −1.73 | 0.05 | 0.50 | 0.08 | |
| MARCKS | 1.58 | --- | --- | --- | 0.08 | --- | |
| MLEX | −1.53 | --- | --- | --- | 0.01 | --- | |
| MYH9 | 1.59 | --- | --- | --- | 0.02 | --- | |
| MYLK | −2.04 | −1.82 | −2.61 | 0.08 | 0.04 | 0.08 | |
| MYO1A | 1.45 | --- | --- | --- | --- | 0.06 | |
| PDLIM7 | 1.79 | −1.73 | --- | 0.01 | 0.02 | --- | |
| PLS1 | --- | 3.88 | --- | --- | <0.01 | --- | |
| RDX | −1.65 | --- | --- | 0.08 | --- | --- | |
| SLC9A3R1 | 2.81 | 1.87 | --- | 0.03 | 0.04 | --- | |
| TAGLN | --- | --- | −7.80 | --- | --- | 0.06 | |
| TUBA1A | 1.86 | --- | --- | <0.01 | --- | --- | |
| TBA1 | −2.69 | --- | --- | 0.03 | --- | --- | |
| TBA8 | 1.50 | --- | --- | 0.08 | --- | --- | |
| TPM1 | 1.86 | --- | --- | 0.01 | --- | --- | |
| TBB2 | −1.69 | --- | --- | 0.06 | --- | --- | |
| TNS | --- | 2.61 | −3.10 | --- | 0.05 | 0.09 | |
| TPM1 | 2.55 | --- | --- | 0.01 | --- | --- | |
| TPT1 | --- | --- | −2.07 | --- | --- | 0.05 | |
| VCL | --- | --- | −1.53 | --- | --- | 0.04 | |
Symbol ID, protein identification and function were found on the UniProtKB database in the Gallus gallus reference.
--- = No difference fold change relative to the S control treatment, therefore values were not reported.
P-values were derived with a Student's t-test in relation to the S control treatment. Values recorded were at P ≤ 0.10.
Fold changes with P-values at ≤ 0.05 were bolded.
Ingenuity Pathway Analysis
Proteins listed in Table 1 are associated with several canonical pathways, and IPA indicated that proteins that were differentially expressed (P < 0.10) based on pioneer colonization were mainly associated with immunity and cytoskeletal arrangement/structure (Table 2). Each of the three in ovo treatments elicited different effects on canonical pathways. In the C treatment, pathways included the activation of the nuclear factor erythroid 2-related factor 2 (NRF2)-mediated oxidative stress response (P-value ≤0.01, activation Z-score = 2.00), while in C2, acute-phase response signaling was exclusively activated (P-value <0.01, Z-score = 2.00). In the LAB-probiotic treatment, activation pathways included gluconeogenesis I (Z-score = 2.00, P-value = <0.01) and nitric oxide signaling in the cardiovascular system (Z-score = 2.00, P-value ≤0.01).
Table 2.
Top canonical pathways generated in IPA affected by proteins that were differentially expressed in the gut intestinal tract of day of hatch chicks when compared to the saline (S) control treatment. At 18 embryonic days, embryos were administered 200 μL of S, Citrobacter freundii (C), Citrobacter spp. (C2), or lactic acid bacteria (L) into the amnion.
| Ingenuity canonical pathways1 | Z-score3 |
P-value |
||||
|---|---|---|---|---|---|---|
| C | C2 | L | C | C2 | L | |
| Acute-phase response signaling | --2 | 2.00 | -- | 0.31 | <0.01 | 0.10 |
| Gluconeogenesis I | -- | -- | 2.00 | 0.10 | <0.01 | <0.01 |
| Nitric oxide signaling in the cardiovascular system | -- | -- | 2.00 | 0.09 | <0.01 | <0.01 |
| NRF24-mediated oxidative stress response | 2.00 | -- | -- | <0.01 | 0.01 | <0.01 |
Significane generated in IPA at P ≤ 0.05 from right-tailed Fisher exact test.
-- = Z-score was not provided, so no prediction could not be obtained.
All canonical pathways predicted to be activated (Z-score ≥ 2.00) or inhibited (Z-score ≤ −2.00).
Nuclear factor erythroid 2-related factor 2.
The predicted upstream regulators, which may explain the observed canonical pathway changes in relation to the expression of proteins, resulted in dysregulation of immune-related molecules in C and C2 in relation to S control (Table 3). For the C treatment, this included a strong qualified activation of proinflammatory complexes nuclear factor kappa B (NF-κB) (Z-score = 1.98, P-value overlap = 0.01) in relation to the S control group. In C2, dysregulation was also predicted owing to the upregulation of a variety of anti-inflammatory and proinflammatory cytokines IL-1β (Z-score 1.96, P-value overlap ≤0.01), Oncostatin M (Z-score = 1.880, P-value overlap ≤0.01), IL-6 (Z-score = 2.57, P-value overlap ≤0.01), and protein group vascular endothelial growth factor (Z-score = 2.00, P-value overlap = 0.03) (Table 3). The C2 and L treatments were generally contradictory with respect to proinflammatory cytokines, such as tumor necrosis factor (LPS induced TNF-alpha factor: LITAF in avian species) and NF-κB. Besides, Oncostatin M had opposing qualified predicted activations in C2 relative to L. Compared with C, L showed a predicted upregulation on IL-6 (Z-score = 2.28).
Table 3.
Predicted upstream regulators in select molecule types generated in IPA based on observed proteins that were differentially expressed and their corresponding genes in the data set in relation to the saline (S) control treatment. Proteins were derived from the intestinal tract of day of hatch chicks that were inoculated at 18 embryonic days with either Citrobacter freundii (C) Citrobacter spp. (C2), or lactic acid bacteria (L) in ovo.
|
Molecule type |
Upstream regulator | Z-score1,2 |
P-value of overlap3 |
||||
|---|---|---|---|---|---|---|---|
| C | C2 | L | C | C2 | L | ||
| Complex | TCR | --4 (--)2 | -- (--) | 1.91 (--) | -- | -- | <0.01 |
| NF-kβ | 1.98 (1.98) | -- (--) | -- (-1.98) | 0.01 | -- | -- | |
| Membrane receptor | PDGF BB | 1.49 (--) | -- (--) | -- (--) | <0.01 | -- | -- |
| CD3 | – | 1.63 (--) | -- (--) | -- | <0.01 | -- | |
| Cytokines | TNFα | -- (2.08) | -- (--) | -- (--) | -- | -- | -- |
| IL1β | -- (--) | 1.96 (1.53) | -- (--) | -- | <0.01 | -- | |
| OSM | -- | 1.88 (--) | -- (-1.55) | -- | 0.002 | -- | |
| IL6 | -- (--) | 2.57 (2.28) | -- (--) | -- | <0.01 | -- | |
| IL13 | -- (--) | -- (--) | 2.00 (--) | -- | -- | 0.001 | |
| Group | Vegf | -- (--) | 2.00 (2.23) | -- (--) | -- | 0.03 | -- |
| Pkc(s) | -- (--) | -- (2.00) | -- (--) | -- | -- | -- | |
| ERK | 1.98 (--) | -- (--) | -- (--) | <0.01 | -- | -- | |
| Growth factor | FGF2 | -- (--) | -- (--) | 1.92 (--) | -- | -- | 0.01 |
| Ligand nuclear receptor | PPARα | -- (--) | −1.94 (--) | -- (--) | -- | <0.01 | -- |
| Kinase | ERBB2 | 1.82 (--) | -- (1.97) | -- (--) | <0.01 | -- | -- |
| MKNK1 | −2.00 (--) | – | -- (--) | <0.01 | -- | -- | |
| Transcription regulators | XBP1 | -- (--) | -- (−1.55) | 2.40 (--) | <0.01 | -- | -- |
| FOS | -- (--) | -- (--) | 1.73 (--) | -- | -- | <0.01 | |
| GATA6 | −2.16 (−1.63) | -- (1.63) | −2.00 (--) | <0.01 | -- | <0.01 | |
| STAT3 | -- (−2.42) | 1.50 (2.42) | -- (−1.62) | -- | <0.01 | -- | |
| PRDM1 | -- (--) | 1.98 (--) | -- (--) | -- | <0.01 | -- | |
| RB1 | −1.38 (--) | -- (−1.96) | -- (1.96) | 0.01 | -- | -- | |
| NFE2L2 | 1.73 (--) | -- (−1.95) | 2.91 (1.95) | <0.01 | -- | <0.01 | |
| MYCN | -- (--) | -- (--) | 1.50 (1.81) | -- | -- | <0.01 | |
| MYC | −1.86 (−2.73) | -- (−1.69) | 1.82 (2.73) | <0.01 | -- | <0.01 | |
| SMAD3 | -- (--) | -- (1.96) | -- (−1.96) | -- | -- | -- | |
| SMAD7 | -- (--) | -- (−1.96) | -- (1.96) | -- | -- | -- | |
| HNF1α | −2.00 (--) | 1.92 (--) | -- (--) | <0.01 | 0.027 | -- | |
| HNF4α | −1.96 (−1.96) | -- (--) | 1.94 (--) | <0.01 | -- | <0.01 | |
| MKL1 | -- (--) | -- (--) | −1.95 (--) | -- | -- | <0.01 | |
The top Z-score value represents the up-regulator prediction in relation to S. The scores that were activated (Z-score ≥ 2.00) or inhibited (Z-score ≤ −2.00). Qualified predictions were ranked as (Z-score ≥ |1.90| or more) medium (Z-score = |1.70 – 1.90|) or low (Z-score = |1.50 – 1.70|).
The Z-score in ( ) represents the prediction of the corresponding up-regulator among the C, C2 and L treatments, all P-value of overlap ≤ 0.05.
P-value of overlap generated in IPA observed the significant statistical overlap between the samples in the data set and the genes that are regulated by the corresponding transcriptional regulator. The values represent treatment difference in relation to S. All values presented had a Fisher's exact test significant value of P ≤ 0.05.
-- = Z-score or P-value of overlap was not provided so no prediction could not be obtained.
Canonical pathways and predicted upstream regulators helped to identify the downstream effects and predict biological functions that are likely to be affected by the upregulated or downregulated proteins (Table 4). In all microbial inoculated treatments, there was an increased activity of up to 34 molecules associated with cellular movement, and migration of cells (P-value overlap ≤0.01). The C treatment had 19 molecules exclusively involved in the activation of protein synthesis, 14 molecules related to activation of cell signaling, and 21 molecules related to inflammation (Z-score ≥ 1.45, P-value overlap ≤0.01). However, the C2 treatment showed predicted activation of cellular movement and migration of cells, while LAB-probiotic–treated chicks had synthesis and production of reactive oxygen species (Z-score ≤ −1.46, P-value overlap ≤0.01).
Table 4.
Predicted activation state in the gut intestinal tract of day of hatch chicks. The table depicts top biological and disease functions with a predicted activation or inhibition relative to the saline (S) control group.
|
Categories |
Diseases or functions annotation |
P-value1 |
Z-score3 |
||||
|---|---|---|---|---|---|---|---|
| C | C2 | L | C | C2 | L | ||
| Carbohydrate metabolism | Synthesis of carbohydrate | -- | -- | <0.01 | -- | --2 | 1.91 |
| Cellular movement, Hematological system development and function, immune cell trafficking | Migration of cells | <0.01 | <0.01 | <0.01 | 2.10 | 2.24 | 1.57 |
| Cell movement of endothelial cells | <0.01 | -- | <0.01 | 2.00 | -- | -- | |
| Cell movement | <0.01 | <0.01 | -- | 2.24 | 2.31 | -- | |
| Invasion of cells | <0.01 | <0.01 | 2.30 | -- | -- | ||
| Protein synthesis | Metabolism of protein | <0.01 | -- | <0.01 | 1.71 | -- | -- |
| Synthesis of protein | <0.01 | -- | <0.01 | 1.45 | -- | -- | |
| Translation of protein | <0.01 | -- | <0.01 | 1.12 | -- | -- | |
| Free radical scavenging | Synthesis of reactive oxygen species | -- | -- | <0.01 | -- | -- | −1.88 |
| Production of reactive oxygen species | -- | -- | <0.01 | -- | -- | −1.46 | |
| Inflammatory response | Inflammation of organ | <0.01 | -- | -- | 2.21 | -- | -- |
| Cell-to-cell signaling | Aggregation of cells | <0.01 | -- | -- | 1.74 | -- | -- |
| Cell-cell contact | <0.01 | -- | -- | 2.59 | -- | -- | |
Significane generated in IPA was set at P ≤ 0.05 from Fisher's exact test.
-- = Z-score or P-value of overlap was not provided so no prediction could not be obtained.
All biological and disease functions that were predicted to be activated (Z-score ≥ 2.00) or inhibited (Z-score ≤ −2.00). Qualified predictions were categorized as high (Z-score ≥ |1.90| or more) medium (Z-score = |1.70 – 1.90|) or low (Z-score = |1.50 – 1.70|).
Discussion
This study aimed to explore the effects of introducing pioneer colonizers in ovo on the intestinal (mucosal and serosal layers) proteome of DOH chicks. The microbiota plays a pivotal role in animal health and mucosal barrier integrity of the GIT because the initial bacterial exposure of perinatal animals sets a foundation for the mucosal and systemic immune system (Jiang et al., 2013). This study demonstrated that exposure to 2 different strains of Citrobacter or LAB-probiotic isolates induced different developmental and immunological effects in the GIT at DOH. The perinatal and neonatal GIT is more sensitive to bacteria, as these intestinal cells have increased plasticity to reprogram the transcriptional and proteomic profile, which may predispose the host to disease or improved performance into adult life (Cortese et al., 2016). Knowledge about the role of commensal Enterobacteriaceae found in chicks is generally limited, beyond their role as pathogens (Crhanova et al., 2011, Awad et al., 2016, Zeng et al., 2018). The in ovo technique applied here may allow for the control of pioneer colonization and to influence the GIT development based on the pioneering microbiota in the host. There were clear proteomic profile differences between C, S, and L vs. C2 (Figure 1). The minor differences in clustering (Figure 1) may allude to the complexity of the intestinal protein network and pioneer colonization impact on the host. Investigating the magnitude of differential changes seen in volcano plots (Figure 2), and the differential expression changes are important, even though only biological relevance was confirmed using spectral count t tests.
A major function of the intestine is to maintain a barrier between the lumen and internal tissues with a single layer of epithelial cells (Marchiando et al., 2011). Actin cytoskeletal proteins are involved in maturation, migration, adhesion, and renewal of epithelial cells along the crypt and villus (Roffers-Agarwal et al., 2005, Di Garbo et al., 2010, Li and Schroeder, 2012, Zhang et al., 2015). Although it has been suggested that cytoskeletal remodeling may be an important event during early infections in the intestine (Li and Schroeder, 2012), this may also be essential for bacteria introduced as pioneer colonizers. Bacteria-induced actin cytoskeletal remodeling is crucial for bacterial attachment and entry into the host cells, which inadvertently results in host immune response (Carabeo et al., 2002). Typically colonization of bacteria, including commensal bacteria, usually leads to mild inflammation as part of the development of innate immunity (Crhanova et al., 2011). However, administering bacteria in ovo may stimulate the early onset of the immune system. During the late embryonic stage, cell-mediated immunity components may be developing, altering predicted upregulators, resulting in the manipulated phenotype of the chick GIT (Table 1, Table 2, Table 3, Table 4).
Oxidative and Lipopolysaccharide Responses
Lipopolysaccharides are a significant component of any gram-negative bacteria's outer membrane and can alter cytoskeletal assembly, such as changing intestinal actin filaments to appear to have irregular spikes vs. straight fibers in cells (Chakravortty and Nanda Kumar, 2000). This disassembly may induce potent proinflammatory cytokines, including IL-6, and affect the intestinal barrier function (Goldblum et al., 1993, Chakravortty and Nanda Kumar, 2000). These may be due to the activation of acute-phase response signaling, which is a rapid inflammatory response typically triggered by tissue injury or immunological dysregulation associated with tissue macrophages (Kushner, 1993, Baumann and Gauldie, 1994). In the C treatment, however, NRF-2–mediated oxidative response was upregulated, likely involved in the enhancing antioxidant capacity during oxidative stress to maintain cellular homeostasis (Nguyen et al., 2009, Schneider and Chan, 2013, Bryan et al., 2013). Oxidative stress is associated with an imbalance between the production of reactive oxygen and the detoxification of reactive intermediates, which can help trigger apoptosis (Liu et al., 2010). The predicted activation of protein synthesis seen exclusively in C (Table 4, P-value overlap <0.01, Z-score ≥ 1.12) may further elude to the continual growth of the GIT as well as previous damage and repair. However, bacterial densities in the GIT are dramatically changing both in terms of density and species diversity in the community (Rinttilä and Apajalahti, 2013, Awad et al., 2016, Ballou et al., 2016). This coevolution of C introduction produced specific host–microbe interactions that affected intestinal growth and development), although protein synthesis in L was the opposite (Table 4). The predicted biological function was influenced by differences among proteins involved in the initiation, rate, and efficiency of translational processes, regulation of assembly/disassembly of actin filaments (GSN) and epithelial–matrix interaction (FN1) (Anderson et al., 2007, Kolachala et al., 2007). The alterations may go beyond the structural component of the intestine and involve immunological tissue proliferation as with ribosomal protein L22 (upregulated in L, downregulated in C). Ribosomal protein L22 is directly associated with T-cell lymphocyte proliferation (Kolachala et al., 2007, Rao et al., 2012), which correlates with the predicted T-cell receptor activation as an upstream regulator (Table 3, TCR P-value overlap <0.01, Z-score = 1.91). This study demonstrated microbiota manipulation during late embryonic development after in ovo inoculation. Along with the ability of probiotics to increase T-regulatory cells, this method could provide the means to reduce or prevent intestinal stress (Vinderola et al., 2005, Zhao et al., 2013).
The mediation of oxidative stress can be modulated by antioxidants, and the results showed that the inclusion of bacteria in ovo did promote the upregulation of different antioxidants (Table 1). A highly influenced protein seen in the C, C2, and L treatments was peroxiredoxin-1 was peroxiredoxin-1 (PRDX1) (Table 1). Peroxiredoxins metabolize to reduce their oxidative capability and protect proteins from oxidative damage induced by reactive oxygen species (ROS) (Rhee, 2016). Increased PRDX1 may act as an antistress response protein that protects cells from damage by ROS and hydrogen peroxide. Probiotic candidates, including Enterococcus faecium, have been reported to upregulate PRDX1 in the adult chicken intestine (Luo et al., 2013). In addition to PRDX1, superoxide dismutase 1 (SOD1) was upregulated exclusively in the L treatment (Table 1; fold change = 2.048, P-value = 0.084). Superoxide dismutase serves as an enzymatic scavenger to convert superoxide into hydrogen peroxide and participates in numerous catalases that convert hydrogen peroxide into water (Jones et al., 2012, Rhee, 2016). During embryonic development, the prenatal chick has an elevated portion of polyunsaturated fatty acids. Therefore, chicks must have an antioxidant defense including SOD1 to maintain embryonic health during the late stages of development (Speake et al., 1998, Surai et al., 2016). Other Lactobacillus-based probiotics have been shown to increase SOD1 (Wang et al., 2012, Hou et al., 2014), helping to ameliorate intestinal oxidative stress. This mechanism possibly occurs because Lactobacillus cells have high viability in hydrogen peroxide, the precursor to water-mediated by SOD, as it is an antimicrobial compound produced by Lactobacillus (Reis et al., 2012). Therefore, antioxidant and immune activation may be modulated during embryogenesis, and SOD1 proliferation may be elevated in the intestines, particularly the L treatment by DOH. If intestinal oxidative stress continues, tight and adheren junctions can be disrupted by the production of ROS.
Adheren and Tight Junctions
Several proteins are involved in the formation and function of tight junctions beyond zona occludins and claudins (Ulluwishewa et al., 2011, Su et al., 2013, Takeichi, 2014, Hatte et al., 2018). Proteins involved in cell-to-cell contact, a foundation for the cytoskeleton and their transcription factors, are necessary to have the formation of tight and adheren junctions complexes (Anderson et al., 2010). Together, adherens and tight junctions regulate the intestinal permeability, which is crucial for the integrity of the intestinal epithelial barrier (Farquhar and Palade, 1963, Ulluwishewa et al., 2011). As adheren junctions are located below the tight junction, they are closely connected to actin cytoskeletal proteins. Although all microbial-treated groups showed had fold change differences relative to the S control treatment in proteins related to the structural constitutes of cytoskeletons (Table 1), the C treatment had the most differentially expressed proteins related to cytoskeleton assembly, cell motility, and recognition (25 vs. 8 in C2 and 13 in L). The top 6 canonical pathways modulated included adheren junction signaling and remodeling, actin cytoskeletal signaling, integrin-linked kinase signaling, calcium signaling, and tight junction signaling (P-values = <0.01). A Z-score for predicted activity was not provided and was, therefore, not included in Table 2 (see Supplementary material). The proteins involved in the modulation of these pathways included actins, myosins, tubulins, and microtubules (Table 1), which are involved in intestinal regulation development, angiogenesis, and T-cell migration (Assi et al., 2011).
The C treatment alone had decreased levels of destrin (DSTN, Table 1, fold change -9.00, P-value <0.01), an actin-depolymerizing factor that typically enhances actin filament turnover (Hotulainen et al., 2005). When downregulated, DSTN has previously shown to attenuate adheren and tight junction turnover if cells were disrupted (Wang et al., 2016). While the embryogenesis remains to be elucidated, the data suggest that C-treated chicks at DOH were in a state of attenuated cytoskeletal development. The C. freundii isolate, used in the C treatment, may be able to reorganize the endothelial cell junctions including fPDZ-LIM domain 7 (PDZLIM7) which is known to function as an anchor to cytoskeletal proteins actinin alpha 1 (ACTN1) and alpha 4 (ACTN4) along with and tight junction proteins such as zona occludins (Youssoufian et al., 1990, Honda et al., 1998, Vallenius et al., 2000, Groschwitz and Hogan, 2009, Suzuki, 2013). This also coincides with the predicted increase in cellular movement observed across multiple tissue types in C (Table 4). Although all microbial challenged groups had cellular movement, different proteins were involved and differentially expressed (Table 4). With the upregulation of ACTN1, ACTN4, and PDZLIM7 (Table 1; 1.45, 1.38, and 1.79, respectively P ≤ 0.05) in C and downregulation of PDZLIM7 (Table 1; -1.73, P-value <0.01) in C2 and upregulation of ACTN4 (Table 1; 1.34, P-value = 0.026) in L, these proteins may be perturbed before the chick is hatched. This suggests that these bacteria may play a role in the disruption and/or maturation of the intestinal barrier integrity. It is important to note that maturation of intestinal flora is positively correlated with epithelial cell turnover, which may suggest here that in ovo inoculation may enhance GIT maturation by upregulating proteins involved in the synthesis. Furthermore, key proteins involved in structural components may need to be further researched.
Gluconeogenesis
The increase of gluconeogenesis through the upregulation of aldolase, fructose-bisphosphate B, enolase 1, glyceraldehyde-3-phosphate, malate dehydrogenase 1 (see Supplementary material) could be hypothesized to support the high energy demand of intestinal epithelial cells (Berger et al., 2017). Importantly, glycogen concentrations are most concentrated within the yolk of an embryo just before hatch to provide immediate energy (Yadgary and Uni, 2012). The results showed that the canonical pathway associated with gluconeogenesis was enhanced only in L treatment. Further research is necessary to investigate whether exposure of LAB-based probiotics might affect the energy availability at DOH.
The IPA software system contains hundreds of thousands of published literature citations of expected effects between transcriptional regulators and their target genes (Kong et al., 2016). Therefore, the upstream regulator analysis results by IPA facilitate the discovery of new aspects of biological systems under study, while the confirmation of previously reported results strengthens the validity and relevance of the overall data set. Although different canonical responses may be due to bacterial cell wall differences among gram-positive and gram-negative bacteria (Derrien et al., 2011), the clear differences between C and C2 indicate that early intestinal modifications can occur among different gram-negative bacteria. However, we must note that less than 25% proteins were differentially expressed, which may lead in difficulty to identify predictive activation or inhibition of pathways, upstream regulators thus downstream biological functions (Li et al., 2011). Although not directly tested in this study, it is clear that species and strain selection may need to be taken into consideration as choosing the appropriate candidates for in ovo inoculation, is similar to identifying role on probiotics on intestinal integrity and maintenance dependent on experimental conditions (Mujagic et al., 2017). A majority of the intestinal proteomic research has been conducted in mice, humans, and porcine (Arce et al., 2014), while avian GIT proteomic studies are limited.
In summary, this study demonstrated that exposure to 2 different strains of Citrobacter or LAB-probiotic isolates induced different developmental and immunological effects in the GIT at DOH. In L treatment, the early GIT microbiota–mediated proteomic changes associated with a state of increased antioxidant capacity and gluconeogenesis, while the neonatal colonization by Citrobacter spp may have triggered a cellular oxidative stress status and an inflammatory stimulus in the GIT of newly hatched chicks. These findings may reflect some to-be-determined physiological behaviors during and soon after the initial settlement of the GIT. Exploration of the intestinal proteome from a model relatable to the commercial chick may provide a better understanding of the functional relationship, identifying proteins that can act as key downstream molecular regulators that mediate the effects of initial external stressors or benefactors of intestinal development.
Acknowledgments
The authors would like to thank the farm personnel at the OARDC Poultry Research Farm, including Keith Patterson and Jarrod Snell for providing the eggs. Kendal Searer and Claudia Telleman are greatly acknowledged for their technical assistance. The authors would like to acknowledge Liwen Zhang and thank her for her technical assistance and expertise in mass spectrometry. Finally, the authors would like to acknowledge and thank the OARDC Research Enhancement Committee Grants Program (SEEDS) and the Arkansas Biosciences Institute for providing the funding of this research.
References
- Altelaar A.F.M., Munoz J., Heck A.J.R. Next-generation proteomics: towards an integrative view of proteome dynamics. Nat. Rev. Genet. 2013;14:35–48. doi: 10.1038/nrg3356. [DOI] [PubMed] [Google Scholar]
- Anderson R.C., Cookson A.L., McNabb W.C., Park Z., McCann M.J., Kelly W.J., Roy N.C. Lactobacillus plantarum MB452 enhances the function of the intestinal barrier by increasing the expression levels of genes involved in tight junction formation. BMC Microbiol. 2010;10:316. doi: 10.1186/1471-2180-10-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S.J., Lauritsen J.P.H., Hartman M.G., Foushee A.M.D., Lefebvre J.M., Shinton S.A., Gerhardt B., Hardy R.R., Oravecz T., Wiest D.L. Ablation of ribosomal protein L22 Selectively Impairs αβ T cell development by activation of a p53-dependent checkpoint. Immunity. 2007;26:759–772. doi: 10.1016/j.immuni.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Arce C., Lucena C., Moreno A., Garrido J.J. Proteomic analysis of intestinal mucosa responses to Salmonella enterica serovar typhimurium in naturally infected pig. Comp. Immunol. Microbiol. Infect. Dis. 2014;37:59–67. doi: 10.1016/j.cimid.2013.10.008. [DOI] [PubMed] [Google Scholar]
- Assi K., Patterson S., Dedhar S., Owen D., Levings M., Salh B. Role of epithelial integrin-linked kinase in promoting intestinal inflammation: effects on CCL2, fibronectin and the T cell repertoire. BMC Immunol. 2011;12:42. doi: 10.1186/1471-2172-12-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awad W.A., Mann E., Dzieciol M., Hess C., Schmitz-Esser S., Wagner M., Hess M. Age-related differences in the luminal and mucosa-associated gut microbiome of broiler chickens and Shifts associated with Campylobacter jejuni infection. Front. Cell. Infect. Microbiol. 2016;6:1–17. doi: 10.3389/fcimb.2016.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballou A.L., Ali R.A., Mendoza M.A., Ellis J.C., Hassan H.M., Croom W.J., Koci M.D. Development of the chick microbiome: how early exposure influences future microbial diversity. Front. Vet. Sci. 2016;3:1–12. doi: 10.3389/fvets.2016.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass J.J., Wilkinson D.J., Rankin D., Phillips B.E., Szewczyk N.J., Smith K., Atherton P.J. An overview of technical considerations for Western blotting applications to physiological research. Scand. J. Med. Sci. Sports. 2017;27:4–25. doi: 10.1111/sms.12702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann H., Gauldie J. The acute phase response. Immunol. Today. 1994;15:74–80. doi: 10.1016/0167-5699(94)90137-6. [DOI] [PubMed] [Google Scholar]
- Beal R.K., Wigley P., Powers C., Hulme S.D., Barrow P.A., Smith A.L. Age at primary infection with Salmonella enterica serovar Typhimurium in the chicken influences persistence of infection and subsequent immunity to re-challenge. Vet. Immunol. Immunopathol. 2004;100:151–164. doi: 10.1016/j.vetimm.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Berger C.N., Crepin V.F., Roumeliotis T.I., Wright J.C., Carson D., Pevsner-Fischer M., Furniss R.C.D., Dougan G., Dori-Bachash M., Yu L., Clements A., Collins J.W., Elinav E., Larrouy-Maumus G.J., Choudhary J.S., Frankel G. Citrobacter rodentium Subverts ATP Flux and Cholesterol homeostasis in intestinal epithelial cells in vivo. Cell Metab. 2017;26:738–752.e6. doi: 10.1016/j.cmet.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielke L.R., Elwood A.L., Donoghue D.J., Donoghue A.M., Newberry L.A., Neighbor N.K., Hargis B.M. Approach for selection of individual enteric bacteria for competitive exclusion in Turkey poults. Poult. Sci. 2003;82:1378–1382. doi: 10.1093/ps/82.9.1378. [DOI] [PubMed] [Google Scholar]
- Bryan H.K., Olayanju A., Goldring C.E., Park B.K. The Nrf2 cell defence pathway: Keap1-dependent and -independent mechanisms of regulation. Biochem. Pharmacol. 2013;85:705–717. doi: 10.1016/j.bcp.2012.11.016. [DOI] [PubMed] [Google Scholar]
- Buzała M., Janicki B., Czarnecki R. Consequences of different growth rates in broiler breeder and layer hens on embryogenesis, metabolism and metabolic rate: a review. Poult. Sci. 2015;94:728–733. doi: 10.3382/ps/pev015. [DOI] [PubMed] [Google Scholar]
- Byrd J., Bailey R.H., Wills R., Nisbet D. Recovery of Campylobacter from commercial broiler hatchery Trayliners. Poult. Sci. 2007;86:26–29. doi: 10.1093/ps/86.1.26. [DOI] [PubMed] [Google Scholar]
- Carabeo R.A., Grieshaber S.S., Fischer E., Hackstadt T. Chlamydia trachomatis induces remodeling of the actin cytoskeleton during attachment and entry into HeLa cells. Infect. Immun. 2002;70:3793–3803. doi: 10.1128/IAI.70.7.3793-3803.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravortty D., Nanda Kumar K.S. Bacterial lipopolysaccharide induces cytoskeletal rearrangement in small intestinal lamina propria fibroblasts: actin assembly is essential for lipopolysaccharide signaling. Biochim. Biophys. Acta BBA - Mol. Basis Dis. 2000;1500:125–136. doi: 10.1016/s0925-4439(99)00098-8. [DOI] [PubMed] [Google Scholar]
- Choi J.-H., Lee K., Kim D.-W., Kil D.Y., Kim G.-B., Cha C.-J. Influence of dietary avilamycin on ileal and cecal microbiota in broiler chickens. Poult. Sci. 2018;97:970–979. doi: 10.3382/ps/pex360. [DOI] [PubMed] [Google Scholar]
- Cortese R., Lu L., Yu Y., Ruden D., Claud E.C. Epigenome-Microbiome crosstalk: a potential new paradigm influencing neonatal susceptibility to disease. Epigenetics. 2016;11:205–215. doi: 10.1080/15592294.2016.1155011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox N.A., Bailey J.S., Mauldin J.M., Blankenship L.C. Research note: presence and impact of Salmonella Contamination in commercial broiler hatcheries. Poult. Sci. 1990;69:1606–1609. doi: 10.3382/ps.0691606. [DOI] [PubMed] [Google Scholar]
- Crhanova M., Hradecka H., Faldynova M., Matulova M., Havlickova H., Sisak F., Rychlik I. Immune response of chicken gut to natural colonization by gut microflora and to Salmonella enterica serovar enteritidis infection. Infect. Immun. 2011;79:2755–2763. doi: 10.1128/IAI.01375-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrien M., van Baarlen P., Hooiveld G., Norin E., Muller M., de Vos W. Modulation of mucosal immune response, Tolerance, and proliferation in mice colonized by the Mucin-Degrader Akkermansia muciniphila. Front. Microbiol. 2011;2 doi: 10.3389/fmicb.2011.00166. https://www.frontiersin.org/articles/10.3389/fmicb.2011.00166/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Garbo A., Johnston M.D., Chapman S.J., Maini P.K. Variable renewal rate and growth properties of cell populations in colon crypts. Phys. Rev. E. 2010;81:061909. doi: 10.1103/PhysRevE.81.061909. [DOI] [PubMed] [Google Scholar]
- Farquhar M.G., Palade G.E. Junctional complexes in various Epithelia. J. Cell Biol. 1963;17:375–412. doi: 10.1083/jcb.17.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness J.B., Kunze W.A.A., Clerc N. II. The intestine as a sensory organ: neural, endocrine, and immune responses. Am. J. Physiol.-Gastrointest. Liver Physiol. 1999;277:G922–G928. doi: 10.1152/ajpgi.1999.277.5.G922. [DOI] [PubMed] [Google Scholar]
- Goldblum S.E., Ding X., Brann T.W., Campbell-Washington J. Bacterial lipopolysaccharide induces actin reorganization, intercellular gap formation, and endothelial barrier dysfunction in pulmonary vascular endothelial cells: concurrent F-actin depolymerization and new actin synthesis. J. Cell. Physiol. 1993;157:13–23. doi: 10.1002/jcp.1041570103. [DOI] [PubMed] [Google Scholar]
- Graham L.E., Teague K.D., Latorre J.D., Yang Y., Baxter M.F.A., Mahaffey B.D., Hernandez-Velasco X., Bielke L.R., Hargis B.M., Tellez G. Use of probiotics as an alternative to formaldehyde fumigation in commercial broiler chicken hatch cabinets. J. Appl. Poult. Res. 2018;27:371–379. [Google Scholar]
- Groschwitz K.R., Hogan S.P. Intestinal barrier function: molecular regulation and disease pathogenesis. J. Allergy Clin. Immunol. 2009;124:3–22. doi: 10.1016/j.jaci.2009.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatte G., Prigent C., Tassan J.-P. Tight junctions negatively regulate mechanical forces applied to adherens junctions in vertebrate epithelial tissue. J. Cell Sci. 2018;131:jcs208736. doi: 10.1242/jcs.208736. [DOI] [PubMed] [Google Scholar]
- Higgins S.E., Erf G.F., Higgins J.P., Henderson S.N., Wolfenden A.D., Gaona-Ramirez G., Hargis B.M. Effect of probiotic treatment in broiler chicks on intestinal macrophage numbers and phagocytosis of Salmonella enteritidis by abdominal exudate cells. Poult. Sci. 2007;86:2315–2321. doi: 10.3382/ps.2007-00123. [DOI] [PubMed] [Google Scholar]
- Higgins J.P., Higgins S.E., Wolfenden A.D., Henderson S.N., Torres-Rodriguez A., Vicente J.L., Hargis B.M., Tellez G. Effect of lactic acid bacteria probiotic culture treatment timing on Salmonella Enteritidis in neonatal broilers. Poult. Sci. 2010;89:243–247. doi: 10.3382/ps.2009-00436. [DOI] [PubMed] [Google Scholar]
- Honda K., Yamada T., Endo R., Ino Y., Gotoh M., Tsuda H., Yamada Y., Chiba H., Hirohashi S. Actinin-4, a novel actin-bundling protein associated with cell motility and Cancer Invasion. J. Cell Biol. 1998;140:1383–1393. doi: 10.1083/jcb.140.6.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotulainen P., Paunola E., Vartiainen M.K., Lappalainen P. Actin-depolymerizing factor and Cofilin-1 play overlapping roles in promoting rapid F-actin depolymerization in Mammalian nonmuscle cells. Mol. Biol. Cell. 2005;16:649–664. doi: 10.1091/mbc.E04-07-0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou C.L., Zhang J., Liu X.T., Liu H., Zeng X.F., Qiao S.Y. Superoxide dismutase recombinant Lactobacillus fermentum ameliorates intestinal oxidative stress through inhibiting NF-κB activation in a trinitrobenzene sulphonic acid-induced colitis mouse model. J. Appl. Microbiol. 2014;116:1621–1631. doi: 10.1111/jam.12461. [DOI] [PubMed] [Google Scholar]
- Iqbal M., Pumford N.R., Tang Z.X., Lassiter K., Wing T., Cooper M., Bottje W. Low feed efficient broilers within a single genetic line exhibit higher oxidative stress and protein expression in breast muscle with lower mitochondrial complex activity. Poult. Sci. 2004;83:474–484. doi: 10.1093/ps/83.3.474. [DOI] [PubMed] [Google Scholar]
- Jiang P., Wan J.M.-F., Cilieborg M.S., Sit W.-H., Sangild P.T. Premature Delivery reduces intestinal cytoskeleton, metabolism, and stress response proteins in newborn Formula-fed Pigs. J. Pediatr. Gastroenterol. Nutr. 2013;56:615. doi: 10.1097/MPG.0b013e318288cf71. [DOI] [PubMed] [Google Scholar]
- Jones R.M., Mercante J.W., Neish A.S. Reactive oxygen production induced by the gut microbiota: pharmacotherapeutic Implications. Curr. Med. Chem. 2012;19:1519–1529. doi: 10.2174/092986712799828283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klasing K.C. Nutritional modulation of resistance to infectious diseases. Poult. Sci. 1998;77:1119–1125. doi: 10.1093/ps/77.8.1119. [DOI] [PubMed] [Google Scholar]
- Kohl K.D. Diversity and function of the avian gut microbiota. J. Comp. Physiol. B. 2012;182:591–602. doi: 10.1007/s00360-012-0645-z. [DOI] [PubMed] [Google Scholar]
- Kolachala V.L., Bajaj R., Wang L., Yan Y., Ritzenthaler J.D., Gewirtz A.T., Roman J., Merlin D., Sitaraman S.V. Epithelial-derived fibronectin expression, signaling, and function in intestinal inflammation. J. Biol. Chem. 2007;282:32965–32973. doi: 10.1074/jbc.M704388200. [DOI] [PubMed] [Google Scholar]
- Kong B.-W., Lassiter K., Piekarski-Welsher A., Dridi S., Reverter-Gomez A., Hudson N.J., Bottje W.G. Proteomics of breast muscle tissue associated with the phenotypic expression of feed efficiency within a Pedigree Male broiler line: I. Highlight on Mitochondria. PLoS One. 2016;11:e0155679. doi: 10.1371/journal.pone.0155679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner I. Regulation of the acute phase response by cytokines. Perspect. Biol. Med. 1993;36:611–622. doi: 10.1353/pbm.1993.0004. [DOI] [PubMed] [Google Scholar]
- Latorre J.D., Hernandez-Velasco X., Kuttappan V.A., Wolfenden R.E., Vicente J.L., Wolfenden A.D., Bielke L.R., Prado-Rebolledo O.F., Morales E., Hargis B.M., Tellez G. Selection of Bacillus spp. for cellulase and xylanase production as direct-fed microbials to reduce digesta viscosity and clostridium perfringens proliferation using an in vitro digestive model in different poultry diets. Front. Vet. Sci. 2015;2 doi: 10.3389/fvets.2015.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F., Glinskii O.V., Zhou J., Wilson L.S., Barnes S., Anthony D.C., Glinsky V.V. Identification and analysis of signaling networks potentially involved in breast Carcinoma Metastasis to the Brain. PLoS One. 2011;6 doi: 10.1371/journal.pone.0021977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R.W., Schroeder S.G. Cytoskeleton remodeling and alterations in smooth muscle contractility in the bovine jejunum during nematode infection. Funct. Integr. Genomics. 2012;12:35–44. doi: 10.1007/s10142-011-0259-7. [DOI] [PubMed] [Google Scholar]
- Liu X., Lu R., Xia Y., Sun J. Global analysis of the eukaryotic pathways and networks regulated by Salmonella typhimurium in mouse intestinal infection in vivo. BMC Genomics. 2010;11:722. doi: 10.1186/1471-2164-11-722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J., Zheng A., Meng K., Chang W., Bai Y., Li K., Cai H., Liu G., Yao B. Proteome changes in the intestinal mucosa of broiler (Gallus gallus) activated by probiotic Enterococcus faecium. J. Proteomics. 2013;91:226–241. doi: 10.1016/j.jprot.2013.07.017. [DOI] [PubMed] [Google Scholar]
- Mahmood T., Yang P.-C. Western blot: technique, theory, and Trouble Shooting. North Am. J. Med. Sci. 2012;4:429–434. doi: 10.4103/1947-2714.100998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchiando A.M., Shen L., Graham W.V., Edelblum K.L., Duckworth C.A., Guan Y., Montrose M.H., Turner J.R., Watson A.J.M. The epithelial barrier is maintained by in vivo tight junction Expansion during pathologic intestinal epithelial Shedding. Gastroenterology. 2011;140:1208–1218.e2. doi: 10.1053/j.gastro.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mujagic Z., de Vos P., Boekschoten M.V., Govers C., Pieters H.-J.H.M., de Wit N.J.W., Bron P.A., Masclee A.A.M., Troost F.J. The effects of Lactobacillus plantarum on small intestinal barrier function and mucosal gene transcription; a randomized double-blind placebo controlled trial. Sci. Rep. 2017;7:40128. doi: 10.1038/srep40128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T., Nioi P., Pickett C.B. The nrf2-antioxidant response Element signaling pathway and its activation by oxidative stress. J. Biol. Chem. 2009;284:13291–13295. doi: 10.1074/jbc.R900010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira J. E. de, van der Hoeven-Hangoor E., van de Linde I.B., Montijn R.C., van der Vossen J.M.B.M. In ovo inoculation of chicken embryos with probiotic bacteria and its effect on posthatch Salmonella susceptibility. Poult. Sci. 2014;93:818–829. doi: 10.3382/ps.2013-03409. [DOI] [PubMed] [Google Scholar]
- Pearce S.C., Mani V., Boddicker R.L., Johnson J.S., Weber T.E., Ross J.W., Rhoads R.P., Baumgard L.H., Gabler N.K. Heat stress reduces intestinal barrier integrity and Favors intestinal Glucose Transport in growing Pigs. PLoS One. 2013;8:e70215. doi: 10.1371/journal.pone.0070215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado-Rebolledo O.F., Delgado-Machuca J. de J., Macedo-Barragan R.J., Garcia-Márquez L.J., Morales-Barrera J.E., Latorre J.D., Hernandez-Velasco X., Tellez G. Evaluation of a selected lactic acid bacteria-based probiotic on Salmonella enterica serovar Enteritidis colonization and intestinal permeability in broiler chickens. Avian Pathol. 2017;46:90–94. doi: 10.1080/03079457.2016.1222808. [DOI] [PubMed] [Google Scholar]
- Rao S., Lee S.-Y., Gutierrez A., Perrigoue J., Thapa R.J., Tu Z., Jeffers J.R., Rhodes M., Anderson S., Oravecz T., Hunger S.P., Timakhov R.A., Zhang R., Balachandran S., Zambetti G.P., Testa J.R., Look A.T., Wiest D.L. Inactivation of ribosomal protein L22 promotes transformation by induction of the stemness factor, Lin28B. Blood. 2012;120:3764–3773. doi: 10.1182/blood-2012-03-415349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis J.A., Paula A.T., Casarotti S.N., Penna A.L.B. Lactic acid bacteria antimicrobial compounds: characteristics and applications. Food Eng. Rev. 2012;4:124–140. [Google Scholar]
- Rhee S.G. Overview on peroxiredoxin. Moleucles Cells. 2016;39:1–5. doi: 10.14348/molcells.2016.2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinttilä T., Apajalahti J. Intestinal microbiota and metabolites—Implications for broiler chicken health and performance1. J. Appl. Poult. Res. 2013;22:647–658. [Google Scholar]
- Roffers-Agarwal J., Xanthos J.B., Miller J.R. Regulation of actin cytoskeleton architecture by Eps8 and Abi1. BMC Cell Biol. 2005;6:36. doi: 10.1186/1471-2121-6-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider K.S., Chan J.Y. Emerging role of Nrf2 in Adipocytes and Adipose Biology. Adv. Nutr. 2013;4:62–66. doi: 10.3945/an.112.003103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searle B.C. Scaffold: a bioinformatic tool for validating MS/MS-based proteomic studies. PROTEOMICS. 2010;10:1265–1269. doi: 10.1002/pmic.200900437. [DOI] [PubMed] [Google Scholar]
- Seifert S., Fritz C., Carlini N., Barth S.W., Franz C.M.a.P., Watzl B. Modulation of innate and adaptive immunity by the probiotic Bifidobacterium longum PCB133 in turkeys. Poult. Sci. 2011;90:2275–2280. doi: 10.3382/ps.2011-01560. [DOI] [PubMed] [Google Scholar]
- Smith A.H., Rehberger T.G. Bacteria and fungi in day-old turkeys vary among companies, collection periods, and breeder flocks. Poult. Sci. 2018;0:1–11. doi: 10.3382/ps/pex429. [DOI] [PubMed] [Google Scholar]
- Speake B.K., Murray A.M.B., Noble R.C. Transport and transformations of yolk lipids during development of the avian embryo. Prog. Lipid Res. 1998;37:1–32. doi: 10.1016/s0163-7827(97)00012-x. [DOI] [PubMed] [Google Scholar]
- Stanley D., Geier M.S., Hughes R.J., Denman S.E., Moore R.J. Highly variable microbiota development in the chicken gastrointestinal tract. PLoS One. 2013;8:e84290. doi: 10.1371/journal.pone.0084290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su L., Nalle S.C., Shen L., Turner E.S., Singh G., Breskin L.A., Khramtsova E.A., Khramtsova G., Tsai P.-Y., Fu Y.-X., Abraham C., Turner J.R. TNFR2 activates MLCK-dependent tight junction dysregulation to cause apoptosis-mediated barrier loss and experimental colitis. Gastroenterology. 2013;145:407–415. doi: 10.1053/j.gastro.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surai P.F., Fisinin V.I., Karadas F. Antioxidant systems in chick embryo development. Part 1. Vitamin E, carotenoids and selenium. Anim. Nutr. 2016;2:1–11. doi: 10.1016/j.aninu.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T. Regulation of intestinal epithelial permeability by tight junctions. Cell. Mol. Life Sci. CMLS. 2013;70:631–659. doi: 10.1007/s00018-012-1070-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeichi M. Dynamic contacts: rearranging adherens junctions to drive epithelial remodelling. Nat. Rev. Mol. Cell Biol. 2014;15:397–410. doi: 10.1038/nrm3802. [DOI] [PubMed] [Google Scholar]
- Teague K.D., Graham L.E., Dunn J.R., Cheng H.H., Anthony N., Latorre J.D., Menconi A., Wolfenden R.E., Wolfenden A.D., Mahaffey B.D., Baxter M., Hernandez-Velasco X., Merino-Guzman R., Bielke L.R., Hargis B.M., Tellez G. In ovo evaluation of FloraMax®-B11 on Marek’s disease HVT vaccine protective efficacy, hatchability, microbiota composition, morphometric analysis, and Salmonella enteritidis infection in broiler chickens. Poult. Sci. 2017;96:2074–2082. doi: 10.3382/ps/pew494. [DOI] [PubMed] [Google Scholar]
- Tellez G., Higgins S.E., Donoghue A.M., Hargis B.M. Digestive physiology and the role of Microorganisms. J. Appl. Poult. Res. 2006;15:136–144. [Google Scholar]
- Tubaon R.M., Haddad P.R., Quirino J.P. Sample Clean-up Strategies for ESI mass spectrometry applications in Bottom-up proteomics: trends from 2012 to 2016. Proteomics. 2017;17:1700011. doi: 10.1002/pmic.201700011. [DOI] [PubMed] [Google Scholar]
- Ulluwishewa D., Anderson R.C., McNabb W.C., Moughan P.J., Wells J.M., Roy N.C. Regulation of tight junction permeability by intestinal bacteria and dietary components. J. Nutr. 2011;141:769–776. doi: 10.3945/jn.110.135657. [DOI] [PubMed] [Google Scholar]
- Vallenius T., Luukko K., Mäkelä T.P. CLP-36 PDZ-LIM protein associates with nonmuscle alpha-actinin-1 and alpha-actinin-4. J. Biol. Chem. 2000;275:11100–11105. doi: 10.1074/jbc.275.15.11100. [DOI] [PubMed] [Google Scholar]
- Ven V. D. L.J., V. Wagenberg A.V., Decuypere E., Kemp B., van den Brand H. Perinatal broiler physiology between hatching and chick collection in 2 hatching systems. Poult. Sci. 2013;92:1050–1061. doi: 10.3382/ps.2012-02534. [DOI] [PubMed] [Google Scholar]
- Vinderola G., Matar C., Perdigon G. Role of intestinal epithelial cells in immune effects mediated by gram-positive probiotic bacteria: Involvement of Toll-like receptors. Clin. Diagn. Lab. Immunol. 2005;12:1075–1084. doi: 10.1128/CDLI.12.9.1075-1084.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Ji H.F., Wang S.X., Zhang D.Y., Liu H., Shan D.C., Wang Y.M. Lactobacillus plantarum ZLP001: in vitro Assessment of antioxidant capacity and effect on growth performance and antioxidant status in Weaning Piglets. Asian-australas. J. Anim. Sci. 2012;25:1153–1158. doi: 10.5713/ajas.2012.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Ma Z., Carr S.A., Mertins P., Zhang H., Zhang Z., Chan D.W., Ellis M.J.C., Townsend R.R., Smith R.D., McDermott J.E., Chen X., Paulovich A.G., Boja E.S., Mesri M., Kinsinger C.R., Rodriguez H., Rodland K.D., Liebler D.C., Zhang B. Proteome profiling outperforms transcriptome profiling for coexpression based gene function prediction. Mol. Cell. Proteomics. 2017;16:121–134. doi: 10.1074/mcp.M116.060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Naydenov N.G., Feygin A., Baranwal S., Kuemmerle J.F., Ivanov A.I. Actin-depolymerizing factor and Cofilin-1 have unique and overlapping functions in regulating intestinal epithelial junctions and mucosal inflammation. Am. J. Pathol. 2016;186:844–858. doi: 10.1016/j.ajpath.2015.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J.H. Bone strength of caged layers as affected by dietary calcium and phosphorus concentrations, reconditioning, and ash content. Br. Poult. Sci. 1991;32:501–508. doi: 10.1080/00071669108417374. [DOI] [PubMed] [Google Scholar]
- Wilson K.M., Rodrigues D.R., Briggs W.N., Duff A.F., Chasser K.M., Bielke L.R. Evaluation of the impact of in ovo administered bacteria on microbiome of chicks through 10 days of age. Poult. Sci. 2019;98:5949–5960. doi: 10.3382/ps/pez388. [DOI] [PubMed] [Google Scholar]
- Yadgary L., Uni Z. Yolk sac carbohydrate levels and gene expression of key gluconeogenic and glycogenic enzymes during chick embryonic development. Poult. Sci. 2012;91:444–453. doi: 10.3382/ps.2011-01669. [DOI] [PubMed] [Google Scholar]
- Yan F., Polk D.B. Probiotics: progress toward novel therapies for intestinal diseases. Curr. Opin. Gastroenterol. 2010;26:95–101. doi: 10.1097/MOG.0b013e328335239a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youssoufian H., McAfee M., Kwiatkowski D.J. Cloning and chromosomal localization of the human cytoskeletal alpha-actinin gene reveals linkage to the beta-spectrin gene. Am. J. Hum. Genet. 1990;47:62–71. [PMC free article] [PubMed] [Google Scholar]
- Zeng Y., Zeng D., Zhang Y., Ni X.Q., Wang J., Jian P., Zhou Y., Li Y., Yin Z.Q., Pan K.C., Jing B. Lactobacillus plantarum BS22 promotes gut microbial homeostasis in broiler chickens exposed to aflatoxin B1. J. Anim. Physiol. Anim. Nutr. 2018;102:e449–e459. doi: 10.1111/jpn.12766. [DOI] [PubMed] [Google Scholar]
- Zhang J., Li C., Tang X., Lu Q., Sa R., Zhang H. Proteome changes in the small intestinal mucosa of broilers (Gallus gallus) induced by high concentrations of atmospheric ammonia. Proteome Sci. 2015;13 doi: 10.1186/s12953-015-0067-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H.-M., Huang X.-Y., Zuo Z.-Q., Pan Q.-H., Ao M.-Y., Zhou F., Liu H.-N., Liu Z.-Y., Liu D.-Y. Probiotics increase T regulatory cells and reduce severity of experimental colitis in mice. World J. Gastroenterol. WJG. 2013;19:742–749. doi: 10.3748/wjg.v19.i5.742. [DOI] [PMC free article] [PubMed] [Google Scholar]