Abstract
Histomoniasis is currently a re-emerging disease of major significance for many commercial turkey and broiler breeder production companies because of the unavailability of drugs or vaccines. The protozoa Histomonas meleagridis (HM) requires the presence of enteric microflora to promote the disease. The objectives of this research note were to evaluate the effect of dietary administration of sodium chlorate (SC) and sodium nitrate (SN) in vitro and in vivo for HM prophylaxis in poults. A total of 128 day-of-hatch female poults obtained from a commercial hatchery were wing-tagged and randomly assigned into 1 of 4 experimental groups: negative control (NC), positive control, dietary inclusion of SC (3,200 ppm) and SN (500 ppm). Poults from groups SC and SN started on their respective diets on day 12. All groups, except the NC, were challenged with 2 × 105 HM on day 19. Controls were fed a basal diet, identical to the treatment diets but not supplemented with SC or SN. Body weight gain (BWG) was determined weekly, starting on day 1 until day 28, and postchallenge morbidity and mortality were recorded. On day 28 of age, all surviving poults were lesion scored for hepatic and cecal lesions. Ceca and distal ileum were collected on day 28 for bacterial recovery on selective media for total aerobic, lactic acid bacteria, or gram-negative bacteria. The addition of SC and SN in the in vitro growth of HM greatly reduced the growth of the protozoa after 20 h of incubation when compared with the control nontreated group (P < 0.05). However, dietary supplementation of SC and SN had no effect against HM in vivo, as was demonstrated by BWG, the severity of lesions in the liver and ceca or bacterial recovery of treated poults when compared with the positive control group.
Key words: sodium chlorate, sodium nitrate, Histomonas meleagridis, prophylaxis, Turkey poults
Introduction
Histomoniasis is a re-emerging disease of major significance for many commercial turkey and broiler breeder production companies because of the unavailability of drugs or vaccines (Clark and Kimminau, 2017). The protozoa Histomonas meleagridis (HM) requires the presence of enteric microflora to promote the disease (Doll and Franker, 1963, Franker and Doll, 1964), with evidence for preference of Enterobacteriaceae (Ganas et al., 2012). However, the relationship between HM and bacteria is not completely understood (McDougald, 2005, Hauck et al., 2010). Interestingly, there are 2 factors regarding the immune response towards histomoniasis: (1) a high production of IFN-γ, the main cytokine representing a Th1 response, has been associated with providing resistance to the disease (Kidane et al., 2018) and (2) unlike turkeys, broiler chickens are able to mount an efficient innate immune response restraining the disease (Powell et al., 2009).
The present research note describes a preliminary evaluation of (1) the dependency of the HM protozoa on the cecal microbiota to promote disease and (2) stimulating the host innate immune response to fight the infection. Previous studies have shown that sodium chlorate (SC) has a marked antimicrobial effect against Salmonella in the ceca of chickens and turkeys (McReynolds et al., 2004, Moore et al., 2006). Sodium nitrate (SN) has been reported to have antimicrobial activity and acts to stimulate the innate immune response by increasing the production of nitric oxide (Ascenzi et al., 2003, Tiso and Schechter, 2015). Hence, the objectives of this research note were to evaluate the effect of dietary administration of SC and SN as a HM prophylaxis in poults.
Materials and methods
Antihistomonal Activity In Vitro
Histomonads from a wild-type HM isolated from a field break of histomoniasis in chickens (layer pullets) from the southern United States (previously used by Hauck et al., 2010) were cultured in modified Dwyer's medium and 250 μL of original culture containing 1.5 × 105 histomonads were added to 700 μL of new modified Dwyer's medium enriched with rice. Treatments of sodium chlorate (CAS: 7,775-09-9; SC; Science Company, Lakewood, CO) or sodium nitrate (Science Company, CAS: 7,631-99-4; SN) were reconstituted in water and added to the histomonads at 0.5 mg in 50 μL, whereas controls received 50 μL of the vehicle; final concentration of tested products was 0.5 mg/mL. Each treatment was completed with 3 replicates. After 20 h, the histomonads were enumerated using a hemacytometer, in duplicate.
Evaluation of Antihistomonal Activity In Vivo
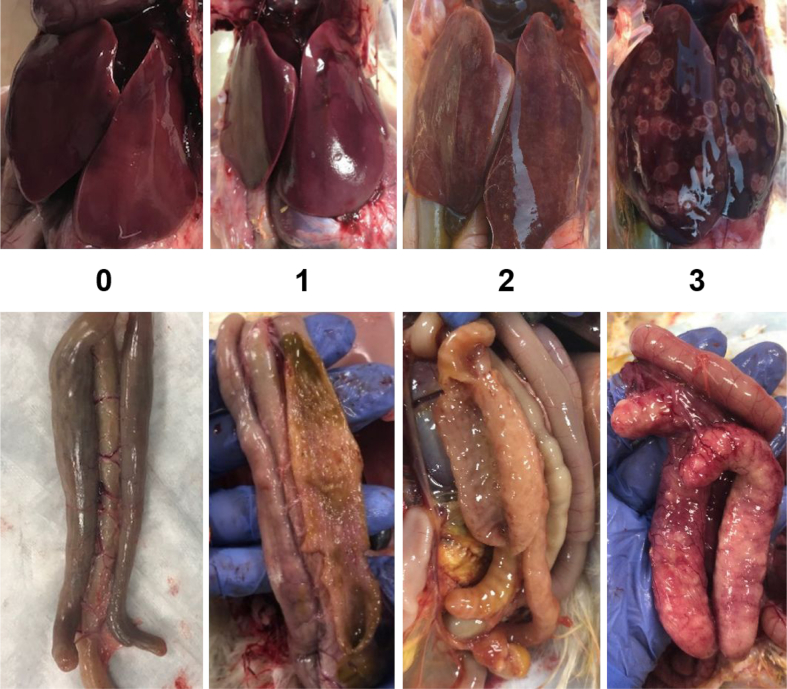
A total of 128 day-of-hatch female poults were obtained from a commercial hatchery (Cargill, Gentry, AR). Poults were wing-tagged and randomly assigned to 1 of 4 experimental groups: negative control (NC), positive control (PC), dietary inclusion of SC (3,200 ppm), SN (500 ppm). Poults were provided ad libitum access to water and a balanced, unmedicated corn and soybean diet meeting the nutritional requirements for turkey poults recommended by the NRC. Controls were fed a basal diet for the duration of the study. Poults from groups SC and SN were fed the basal diet until day 11; after which, they received their respective dietary treatments from day 12 forward. All groups, except the NC, were intracloacally challenged with 2 × 105 histomonads (divided administration, 1 h apart) on day 14 of age with the same wild-type HM described in the in vitro essay. This isolate was deliberately kept at a very low passage in culture to maintain virulence/avoid culture-induced attenuation. Body weight gain (BWG) was determined weekly, starting on day 1 until day 28 of age, and final mortality were recorded. On day 28, all surviving poults were lesion scored for hepatic and cecal lesions on a 0-3 scale as described by Beer et al. (2020) (ceca: 0 = no macroscopic alterations; 1 = detectable thickening of the ceca and small lesions on the mucosa, but normal architecture and cecal content; 2 = meaningful cecal wall thickening with some areas of the mucosa presenting hemorrhages and erosions, abnormal architecture of some portions of the ceca, fluid, and yellowish cecal content; 3 = classic typhlitis with thickened cecal walls, severe inflammation, erosions, and total loss of the normal cecal architecture, presence of caseous cores; liver: 0 = no macroscopic alterations; 1 = few localized necrotic areas; 2 = inflammation and presence of circular necrotic areas in some regions of the liver; 3 = severe inflammation and circular necrotic areas approaching confluency on the surface of the liver consistent with the classic “target-like” lesions characteristic of histomoniasis) (Figure 1). Ceca and distal ileum were collected on day 28 for bacterial recovery on Man Rogosa Sharpe (Rogosa SL Agar, Cat. No. R1148, Sigma, St. Louis, MO, 63,178) and MacConkey (BBL MacConkey Agar, Cat. No. 211387; Becton, Dickinson and Company, Sparks, MD 21152) agar plates. All animal handling procedures were in compliance with the University of Arkansas, Institutional Animal Care and Use Committee (protocol number 19118).
Figure 1.
Pictures of the lesion scores according a 0-3 scale presented by Beer et al. (2020), where in the ceca 0 = no macroscopic alterations; 1 = detectable thickening of the ceca and small lesions on the mucosa, but normal architecture and cecal content; 2 = meaningful cecal wall thickening with some areas of the mucosa presenting hemorrhages and erosions, abnormal architecture of some portions of the ceca, fluid, and yellowish cecal content; 3 = classic typhlitis with thickened cecal walls, severe inflammation, erosions, and total loss of the normal cecal architecture, presence of caseous cores; and in the liver: 0 = no macroscopic alterations; 1 = few localized necrotic areas; 2 = inflammation and presence of circular necrotic areas in some regions of the liver; 3 = severe inflammation and circular necrotic areas approaching confluency on the surface of the liver consistent with the classic “target-like” lesions characteristic of histomoniasis.
Statistical Analysis
Data from BWG were subjected to multiway analysis of variance for the randomized design using the General Linear Models procedure in SAS (version 9.1, SAS Institute Inc., Cary, NC). Means were separated with Tukey's multiple-range test and considered significant at P < 0.05. Data were reported as mean ± SE. A PROC MIXED, ANOVA program was used to test statistical significance for lesion scores. For BWG, each of the replicate pens was considered as the experimental unit (n = 4/treatment); for lesion scores, each bird was the experimental unit (n = 32/treatment); for bacterial recovery, 3 birds were randomly selected from all replicates of each group (n = 12/group). Mortality was compared with all possible combinations using the chi-square test of independence to determine significance (P < 0.05)
Results and discussion
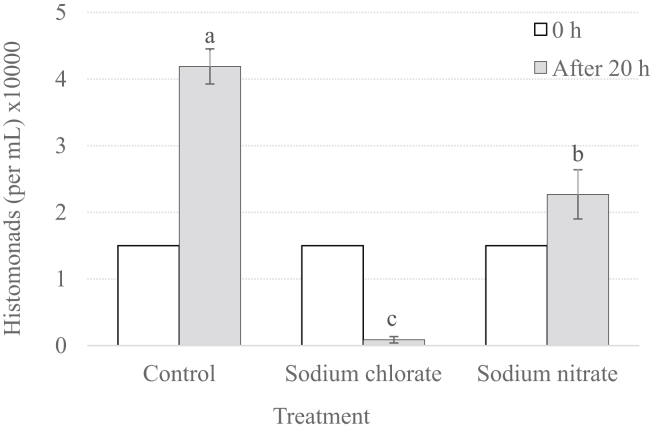
The addition of SC and SN to in vitro culture of HM significantly (P < 0.05) reduced the growth of the protozoa after 20 h of incubation when compared with the nontreated control group (Figure 2). However, dietary supplementation of SC and SN had no effect against HM in the host (Table 1). Turkeys fed SC had a lower BW compared with the other groups (Table 2), suggesting that higher levels of SC would not be practical; in addition, the mortality for the SC group reached 30% on day 25, and the remaining birds were euthanized on the same day because of Institutional Animal Care and Use Committee protocol requirements. The selected dose of SC was based on experiments where SC was administered in the drinking water, considering the approximate proportion of feed to water consumption (3x the water concentration). Feed consumption was not measured in the present experiment, but it is possible that the birds rejected the feed, leading to weaker birds more susceptible to the disease.
Figure 2.
Histomonas meleagridis response to in vitro treatment with sodium chlorate or sodium nitrate. Histomonads (150,000 cells seeding density) were treated with 0.5 mg sodium chlorate or sodium nitrate (0.5 mg/mL); sterile water treatment served as a negative control. Histomonads proliferation/density enumerated after 20 h of treatment. Statistical significance (P > 0.05) indicated with nonmatching letters (a–c).
Table 1.
Lesion scores (liver and cecal) and total aerobic, presumptive gram-negative, and lactic acid bacteria (cecal and lower ileum) of turkeys receiving dietary sodium chlorate (SC) or sodium nitrate (SN) challenged with Histomonas meleagridis.
| Treatment | NC | PC | SC | SN | P-value |
|---|---|---|---|---|---|
| Lesion score liver (0-3) | 0.00 ± 0.00b | 2.00 ± 0.23a | 2.38 ± 0.19a | 2.31 ± 0.21a | <0.0001 |
| Lesion score ceca (0-3) | 0 ± 0.00b | 2.13 ± 0.22a | 2.31 ± 0.18a | 2.38 ± 0.21a | <0.0001 |
| Recoverable gram-negative bacteria (Log10 CFU/g) 1 | 6.34 ± 0.20 | 6.81 ± 0.19 | 6.79 ± 0.16 | 6.97 ± 0.17 | 0.0989 |
| Recoverable lactic-acid bacteria (Log10 CFU/g) | 7.16 ± 0.18 | 6.42 ± 0.17 | 6.65 ± 0.25 | 6.78 ± 0.20 | 0.0793 |
| Mortality (%) | 0b | 18.8a | 31.3a,1 | 18.8a | <0.05 |
a,bValues within rows with different superscripts differ significantly (P < 0.05).
Data are expressed as the mean ± SEM.
Abbreviations: NC, negative control; PC, positive control; SC, dietary sodium chlorate (3,200 ppm); SN, dietary sodium nitrate (500 ppm); IACUC, Institutional Animal Care and Use Committee. Poults from groups NC and PC were fed a basal diet; poults from groups SC and SN started to be fed with the respective compounds on day 12.
Poults from groups PC, SC, and SN were intracloacally challenged with 2 × 105 histomonads on day 14.
Birds from group SC were euthanized on day 25 because mortality reached 30% as required in IACUC protocol.
Table 2.
Body weight (BW) and body weight gain (BWG) of turkeys receiving sodium chlorate (SC) and sodium nitrate (SN) in feed.
| Treatment | NC | PC | SC | SN | P-value |
|---|---|---|---|---|---|
| BW, g/poult | |||||
| Day 0 | 57.88 ± 1.33 | 58.16 ± 1.15 | 58.81 ± 0.37 | 59.53 ± 0.76 | 0.6451 |
| Day 7 | 161.78 ± 3.53 | 166.06 ± 2.27 | 161.78 ± 2.83 | 164.94 ± 2.97 | 0.6519 |
| Day 14 | 363.47 ± 16.29 | 381.84 ± 5.94 | 356.88 ± 6.33 | 385.47 ± 6.23 | 0.1626 |
| Day 21 | 560.63 ± 39.35 | 569.19 ± 13.31 | 494.41 ± 3.73 | 582.25 ± 14.68 | 0.0653 |
| Day 28 | 816.44 ± 62.87a | 704.62 ± 44.35a,b | 578.26 ± 21.68b | 641.32 ± 36.24a,b | 0.0142 |
| BWG, g/poult | |||||
| Day 0–14 | 305.59 ± 17.14 | 323.69 ± 6.51 | 298.06 ± 6.33 | 325.94 ± 5.91 | 0.1984 |
| Day 14–28 | 452.97 ± 47.54a | 318.99 ± 48.25a,b | 227.87 ± 25.27b | 257.67 ± 40.60b | 0.0109 |
| Day 0–28 | 758.56 ± 63.89a | 646.40 ± 44.26a,b | 518.74 ± 21.61b | 581.97 ± 36.01a,b | 0.0141 |
a,bValues within rows with different superscripts differ significantly (P < 0.05).
Data are expressed as the mean ± SEM.
Poults from groups PC, SC, and SN were intracloacally challenged with 2 × 105 histomonads on day 14.
Abbreviations: NC, negative control; PC, positive control; SC, dietary sodium chlorate (3,200 ppm); SN, dietary sodium nitrate (500 ppm). Poults from groups NC and PC were fed a basal diet; poults from groups SC and SN started to be fed with the respective compounds on day 12.
The conversion of nitrate to nitric oxide involves complex pathways requiring the participation of different bacteria and enzymes (Tiso and Schechter, 2015). Previous researchers reported that turkeys are more sensitive to nitrate toxicity than chickens (Adams et al., 1966). Although in the present study, until the turkeys started showing clinical signs related to histomoniasis, the poults receiving SN did not exhibit suppression in body weight (Table 2), higher concentrations could be detrimental to the bird (Marrett and Sunde, 1968).
Bacterial recovery was not significantly different between groups (Table 1), although a tendency of higher gram-negative and lower lactic-acid bacteria recovery was observed in the birds inoculated with HM, which can be explained by a dysbacteriosis caused by the disease.
No mortalities or lesions were observed in the NC group and mortalities ranged from 18.8 to 31.3% (P > 0.05) in all challenged groups. Similarly, lesion scores were not markedly different between treatments for either cecal or hepatic lesions (Table 1).
Previous researchers also showed a discrepancy between in vitro and in vivo results (Thøfner et al., 2012). The bioavailability of the compounds was not evaluated; it is possible that the compounds did not reach the ceca, not impacting the protozoa.
These data are not encouraging for these candidate approaches for controlling HM as no beneficial effects of these selected treatments and time frames were observed. Higher dietary concentrations of SC and SN are not likely candidates for evaluation because of known negative effects (SC and SN) in turkeys.
References
- Adams A., Emerick R., Carlson C. Effects of nitrate and nitrite in the drinking water on chicks, poults and laying hens. Poult. Sci. 1966;45:1215–1222. [Google Scholar]
- Ascenzi P., Bocedi A., Gradoni L. The anti-parasitic effects of nitric oxide. IUBMB Life. 2003;55:573–578. doi: 10.1080/15216540310001639265. [DOI] [PubMed] [Google Scholar]
- Beer L.C., Vuong C.N., Barros T.L., Latorre J.D., Tellez G., Fuller A.L., Hargis B.M. Research note: evaluation of boric acid as a chemoprophylaxis candidate to prevent histomoniasis. Poult. Sci. 2020;99:1978–1982. doi: 10.1016/j.psj.2019.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark S., Kimminau E. Critical review: future control of blackhead disease (histomoniasis) in poultry. Avian Diseases. 2017;61:281–288. doi: 10.1637/11593-012517-ReviewR. [DOI] [PubMed] [Google Scholar]
- Doll J.P., Franker C.K. Experimental histomoniasis in gnotobiotic turkeys. I. Infection and histopathology of the bacteria-free host. J. Parasitol. 1963:411–414. [PubMed] [Google Scholar]
- Franker C.K., Doll J.P. Experimental histomoniasis in gnotobiotic turkeys. II. Effects of some cecal bacteria on pathogenesis. J. Parasitol. 1964:636–640. [PubMed] [Google Scholar]
- Ganas P., Liebhart D., Glӧsmann M., Hess C., Hess M. Escherichia coli strongly supports the growth of Histomonas meleagridis, in a monoxenic culture, without influence on its pathogenicity. Int. Journal Parasitol. 2012;42:893–901. doi: 10.1016/j.ijpara.2012.07.007. [DOI] [PubMed] [Google Scholar]
- Hauck R., Armstrong P., McDougald L. Histomonas meleagridis (Protozoa: Trichomonadidae): analysis of growth requirements in vitro. J. Parasitol. 2010;96:1–8. doi: 10.1645/GE-1985.1. [DOI] [PubMed] [Google Scholar]
- Kidane F.A., Mitra T., Wernsdorf P., Hess M., Liebhart D. Allocation of interferon gamma mrna Positive cells in caecum hallmarks a Protective Trait against histomonosis. Front. Immunol. 2018;9 doi: 10.3389/fimmu.2018.01164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrett L., Sunde M. The use of Turkey poults and chickens as test animals for nitrate and nitrite toxicity. Poult. Sci. 1968;47:511–519. [Google Scholar]
- McDougald L. Blackhead disease (histomoniasis) in poultry: a critical review. Avian Diseases. 2005;49:462–476. doi: 10.1637/7420-081005R.1. [DOI] [PubMed] [Google Scholar]
- McReynolds J., Byrd J., Moore R., Anderson R., Poole T., Edrington T., Kubena L., Nisbet D. Utilization of the nitrate reductase enzymatic pathway to reduce enteric pathogens in chickens. Poult. Science. 2004;83:1857–1860. doi: 10.1093/ps/83.11.1857. [DOI] [PubMed] [Google Scholar]
- Moore R.W., Byrd J.A., Knape K.D., Anderson R.C., Callaway T.R., Edrington T., Kubena L.F., Nisbet D.J. The effect of an experimental chlorate product on Salmonella recovery of turkeys when administered prior to feed and water withdrawal. Poult. Sci. 2006;85:2101–2105. doi: 10.1093/ps/85.12.2101. [DOI] [PubMed] [Google Scholar]
- Powell F., Rothwell L., Clarkson M., Kaiser P. The Turkey, compared to the chicken, fails to mount an effective early immune response to Histomonas meleagridis in the gut. Parasite Immunol. 2009;31:312–327. doi: 10.1111/j.1365-3024.2009.01113.x. [DOI] [PubMed] [Google Scholar]
- Thøfner I.C.N., Liebhart D., Hess M., Schou T.W., Hess C., Ivarsen E., Fretté X., Christensen L.P., Grevsen K., Engberg R.M., others Antihistomonal effects of artemisinin and Artemisia annua extracts in vitro could not be confirmed by in vivo experiments in turkeys and chickens. Avian Pathol. 2012;41:487–496. doi: 10.1080/03079457.2012.714459. [DOI] [PubMed] [Google Scholar]
- Tiso M., Schechter A.N. Correction: nitrate Reduction to nitrite, nitric oxide and Ammonia by gut bacteria under Physiological Conditions. PLoS One. 2015;10:e0127490. doi: 10.1371/journal.pone.0119712. [DOI] [PMC free article] [PubMed] [Google Scholar]