Abstract
The aim of the present study was to evaluate the effect of glutamine (Gln) on modulating heat stress–induced oxidative damage in the broiler thigh muscle through nuclear factor erythroid 2–related 2/Kelch-like ECH-associated protein 1 (Nrf2-Keap1) pathway. Three-hundred 22-day-old Arbor Acres broilers were reallocated into 5 groups: a control group (24 °C) fed with basal diet and 4 heat stress (HS) groups (34 °C for 8 h/D) fed with basal diet containing 0, 0.5, 1.0, and 1.5% Gln. This experiment lasted 21 D. Heat stress decreased (P < 0.05) pH, redness, and Gln levels, and increased (P < 0.05) luminance, water loss rate, and cooking loss (CL) values of the thigh meat. Compared with the HS group, supplementation with 1.5% Gln increased (P < 0.05) pH, redness, and Gln levels, but decreased (P < 0.05) luminance and CL values in the thigh meat. There were significant decreases (P < 0.05) in glutathione (GSH), superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GSH-Px), total antioxidant capacity (T-AOC), and Nrf2 levels, but significant increases (P < 0.05) in the malondialdehyde (MDA) and Keap1 levels of the thigh muscle after HS treatment. Compared with the HS group, supplementation with 1.0, and 1.5% Gln decreased (P < 0.05) MDA and Keap1 levels; supplementation with 1.5% Gln increased (P < 0.05) GSH, GSH-Px, T-AOC, CAT, SOD, and Nrf2 levels in the thigh muscle of heat-stressed broilers. Furthermore, HS decreased (P < 0.05) Nrf2, SOD, CAT, and GSH-Px mRNA expression levels, but increased (P < 0.05) Keap1 mRNA level in the thigh muscle of broiler. Dietary supplementation with 1.5% Gln increased (P < 0.05) Nrf2, GSH-Px, CAT, and SOD mRNA expression levels, but decreased (P < 0.05) Keap1 mRNA level in the thigh muscle of heat-stressed broilers. In conclusion, dietary Gln improved the resistance of heat-stressed broiler muscles to oxidative damage possibly through reversing the muscle Gln level and inducing the expression of the Nrf2-Keap1 pathway.
Key words: glutamine, heat stress, meat quality, antioxidant capacity, Nrf2-Keap1 signaling pathway
Introduction
With the development of high-density feeding modes and the deleterious influence of high temperatures during the summer, broilers as heat-sensitive animals are easily susceptible to heat stress in the production process (Lara and Rostagno, 2013). This condition decreases poultry production performance by causing heat exhaustion, which poses a great threat to broiler production (Quinteiro-Filho et al., 2010, Bai et al., 2019). A method commonly used to mitigate the negative effects of heat stress involves regulating dietary additives (Zhang et al. 2015). Because of the growing awareness of the importance of food safety and health, amino acids as constituents of animal proteins are a good choice for regulation based on animal metabolism and nutritional requirements.
Poultry meats are rich in polyunsaturated fatty acids. Therefore, heat stress aggravates muscle oxidation and lipid peroxidation, resulting in pale, soft, exudative meat (Tang et al., 2013, Xing et al., 2015, Zhang et al., 2017). Heat stress also increases the secretion of catecholamines, which increases the content of superoxide free radicals and hydrogen peroxide (Song and King, 2015). The production of excessive reactive oxygen species damages the antioxidant system of the body and induces oxidative stress (Belhadj et al., 2016, Zhang et al., 2018). The nuclear factor erythroid 2–related 2/Kelch-like ECH-associated protein 1 (Nrf2-Keap1) pathway is involved in cellular responses to environmental and oxidative stress (Bryan et al., 2013, Zhang et al., 2018). Activation of the Nrf2-Keap1 pathway increases antioxidant response element–related molecules, including glutathione (GSH), superoxide dismutase (SOD), and catalase (CAT) (Zhang et al., 2018).
Glutamine (Gln), a conditional essential amino acid, is the most abundant amino acid in broiler skeletal muscles. Glutamine, an important nutrient for the conversion of some amino acids and biological macromolecules, is a source of energy for some rapidly dividing cells. Gln is well known to have positive effects on heat-stressed broilers (Shakeri et al., 2014, Wu et al., 2018). Hu et al., 2016a, Jazideh et al., 2014, and Olubodun et al. (2015) suggested that dietary Gln improves the performance, carcass characteristics, serum parameters, intestinal immunity, and meat quality of broilers under heat stress. However, there is little information on the effect and mechanism of action of Gln on oxidative stress in broilers under heat stress. Therefore, the aim of this work was to determine if the effect of heat stress on meat quality and oxidative damage can be alleviated by Gln supplementation. Moreover, regulation of the antioxidant and Nrf2-Keap1 pathway was investigated to elucidate a possible mechanism for Gln-induced protection of meat against oxidation under heat stress.
Materials and methods
Animals and Experimental Design
The experimental animal protocol was approved by the Animal Care and Use Committee of Anhui Science and Technology University. Specifically, 400 1-day-old Arbor Acres broilers were obtained from the Anhui Science and Technology University Farm (Chuzhou, People's Republic of China). Broilers (aged 1–21 D) were placed in an environmentally controlled room at 32 to 35°C for the first week and then gradually reduced by 3.5°C per week to a final temperature of 24°C. On day 22, 300 broilers (50% males and females) were randomly divided into the following 5 groups with 6 replicate cages of 10 broilers per cage (50% males and females; there were no differences in initial weight among 5 groups): the control (CON) group was fed basal diet; heat stress (HS) treatment groups, HS-0.5% Gln, HS-1.0% Gln, and HS-1.5% Gln, were circulatory heat-stressed groups and fed the basal diet with 0, 0.5, 1.0, and 1.5% Gln, respectively. Broilers from the CON group were kept under normal-temperature conditions at 24°C (humidity: 45–55%) for 24 h/D, whereas broilers in the heat-stressed groups were kept under hot-temperature conditions at 34°C (humidity: 60–70%) for 8 h/D (9:00–17:00) and at 24°C (humidity: 45–55%) for 16 h/D. All experimental chickens were kept in a stereoscopic cage (120 cm × 70 cm × 40 cm) and enjoyed a 12-h light regimen (10 lux) every day. The plastic feeders (diameter: 25 cm; feed weight: 2 kg) and drinkers (3 L water) were used for free access to water and feed. The growth performance was measured according to the “Technical regulation for performance testing of meat-type chicken (NY/T 828-2004).” The basal diet (Table 1) was corn–soybean meal, and the nutrient level of the formula was designed according to the NRC (1994).
Table 1.
Composition and nutrient levels of the basal diets1.
| Ingredients (g/kg) | |
|---|---|
| Corn | 585 |
| Starch | 10 |
| Soybean meal | 320 |
| Fish meal | 20 |
| Soybean oil | 35 |
| CaHPO4·2H2O | 15 |
| Limestone | 9 |
| Salt | 3 |
| DL-Methionine | 1 |
| Vitamin and trace mineral premix2 | 2 |
| Total | 1000 |
| Nutritional composition | |
| ME (MJ/kg) | 12.73 |
| Crude protein (g/kg) | 203.0 |
| Lysine (g/kg) | 10.8 |
| Methionine + cysteine (g/kg) | 7.6 |
| Ca (g/kg) | 8.9 |
| Available P (g/kg) | 4.2 |
The basal diet was designed according to the NRC (1994) and Bai et al. (2019).
Provided per kilogram of diet: vitamin A: 10,000 IU; cholecalciferol: 2600 IU; vitamin E: 20 IU; vitamin K: 2.0 mg; riboflavin: 6.0 mg; thiamin: 1.6 mg; vitamin B6: 3.0 mg; vitamin B12: 0.014 mg; niacin: 30 mg; choline (as choline chloride): 500 mg; folic acid: 0.8 mg; biotin 0.12 mg; calcium pantothenate: 20 mg; Fe [from Fe2(SO4)3]: 80 mg; Zn (from ZnSO4), 40 mg; Cu (from CuSO4): 8 mg; Se (from Na2SeO3): 0.15 mg; I (from IK): 0.35 mg.
Sample Collection
On day 42, 3 broilers from each replicate were euthanized by dislocation, manual exsanguination, and pulling of feathers. The thigh muscle samples were divided into 2 portions: 1 was stored at 4°C for meat quality analysis, whereas the other was stored at −80°C for biochemical, mRNA, and protein assays.
Meat Quality
Meat quality was determined 45 min after euthanasia. The pH value of the thigh muscle was measured using a pH meter (Mettler Toledo, Zurich, Switzerland) as per the procedure described by Hu et al. (2016a). The luminance (L*), redness (a*), and yellowness (b*) of the thigh muscle were measured using a colorimeter (Minolta, Tokyo, Japan) as described by Hu et al. (2016a).
For determination of drip loss (DL), a thigh muscle sample (approximately 5 g) was weighed (initial weight) and placed in a vacuum bag for 24 h at 4°C. Then, the sample was reweighed (final weight), and the DL (%) was calculated as follows: (initial weight-final weight)/initial weight × 100%.
To assess cooking loss (CL), the muscle samples were weighed (initial weight), placed in a Petri dish, and steamed in an aluminum pot of boiling water for 30 min. Then, the surface juice was gently wiped off using an absorbent paper, followed by cooling and reweighing (final weight). The CL (%) was calculated as follows: (initial weight-final weight)/initial weight × 100%.
The water loss rate (WLR) was measured using a pressure gravimetric method. The muscle sample initial weight was determined. Then, the sample was placed between the 18 layers of the filter paper in a compressor and pressed with a pressure of 2000 psi for 1 min. This meat sample was reweighed immediately (final weight), and the WLR (%) was calculated as follows: (initial weight-final weight)/initial weight × 100%.
Homogenate of the Thigh Muscle
Under cold conditions in an ice water bath, 10% of the tissue homogenate was prepared using physiological saline (0.9%) at a weight (g)-to-volume (mL) ratio of 1:9. The homogenate supernatant was obtained by centrifugation (3500 rpm) for 10 min. The protein concentration of the supernatant was measured using a bicinchoninic acid kit (Beyotime Institute of Biotechnology, Shanghai, China).
Determination of Gln Concentration in the Thigh Muscle
The concentration of Gln was determined in the homogenate supernatant of the thigh muscle using a Gln measurement kit (Jiancheng Bioengineering Research Institute, Nanjing, China).
Determination of Redox State and Antioxidants in the Thigh Muscle
The levels of malondialdehyde (MDA), GSH, glutathione peroxidase (GSH-Px), SOD, CAT, and total antioxidant capacity (T-AOC) were assayed in the homogenate supernatant of the thigh muscle using their respective commercial assay kits purchased from Jiancheng Bioengineering Research Institute (Nanjing, China).
Determination of Nrf2 and Keap1 in the Thigh Muscle
The Nrf2 and Keap1 protein levels in the homogenate supernatant of the thigh muscle were detected using a chicken Nrf2 and Keap1 enzyme-linked immunosorbent assay kit (Jiancheng Bioengineering Research Institute, Nanjing, China). The coefficient of variance of replicates was <10%.
Gene Expression
Total RNA of the thigh muscle was isolated using the RNAprep pure tissue kit (TianGen, Beijing, China). The mRNA expression levels of Nrf2, Keap1, SOD, CAT, GSHPx, and β-actin (reference gene) were measured by quantitative real-time polymerase chain reaction (PCR) technique with the primers shown in Table 2. The quantitative real-time PCR was performed using the TB Green Premix Ex Taq (TaKaRa, Dalian, China). The PCR reaction and program were performed as per the method of Zhang et al. (2018), and the mRNA levels were calculated using the 2− ΔΔCT method (Livak and Schmittgen, 2001).
Table 2.
Specific sequences primers used in the qRT-PCR.
| Gene | Primer (5’→3′) | GenBank number |
|---|---|---|
| β-Actin | F: TGCTGTGTTCCCATCTATCG R: TTGGTGACAATACCGTGTTCA |
NM 205518 |
| SOD | F: GGAGGAGTGGCAGAAGT R: TAAACGAGGTCCAGCAT |
NM 205064 |
| CAT | F: TATCCTTCCTGGTCTTTCTACAT R: CGCCATCTGTTCTACCTCC |
NM 001031215.2 |
| GSH-Px | F: AAGTGCGAGGTGAACGG R: CGGCGACCAGATGATGTAC |
NM 001277853.1 |
| Nrf2 | F: TCGCAGAGCACAGATAC R: AGAAATGAAGACTGGGTC |
NM 205117.1 |
| Keap1 | F: CAACTTCGCCGAGCAGA R: CGTGGAACACCTCCGACT |
KU 321503 179 |
Abbreviations: CAT, catalase; GSH-Px, glutathione peroxidase; Keap1, kelch-like ECH-associated protein 1; Nrf2, nuclear factor erythroid 2–related 2; qRT-PCR, quantitative real-time polymerase chain reaction; SOD, superoxide dismutase.
Statistics Analysis
The data, including those of the CON group, were analyzed by a one-way analysis of variance using SPSS (2008), version 18.0, software (SPSS Inc., Chicago, IL). Duncan's test was used to compare statistical differences among the HS, HS-0.5% Gln, HS-1.0% Gln, and HS-1.5% Gln groups. P < 0.05 was considered significant.
Results
Meat Quality
As shown in Table 3, significant decreases (P < 0.05) in pH and a* values, but significant increases (P < 0.05) in L*, WLR, and CL values of the thigh meat, were observed in the HS group compared with the CON group. Compared with the HS group, supplementation with 1.0% Gln significantly increased (P < 0.05) a* value, but decreased (P < 0.05) L*, WLR, and CL values; supplementation with 1.5% Gln significantly increased (P < 0.05) a* and pH values, but decreased (P < 0.05) L* and CL values in the thigh meat of heat-stressed broilers.
Table 3.
Effect of heat stress and glutamine on meat quality of the thigh muscle in broilers.
| Item | Treatments1 |
SEM | P-value | ||||
|---|---|---|---|---|---|---|---|
| CON | HS | HS-0.5% Gln | HS-1.0% Gln | HS-1.5% Gln | |||
| pH | 6.19a | 5.95b | 6.02b | 6.05b | 6.15a | 0.021 | <0.001 |
| L* | 48.28a | 50.21b | 49.10a,b | 47.95a | 48.38a | 0.240 | 0.013 |
| a* | 8.28a | 6.81b | 7.06b | 8.03a | 7.94a | 0.121 | <0.001 |
| b* | 8.06 | 7.59 | 7.71 | 7.85 | 8.02 | 0.161 | 0.884 |
| DL (%) | 4.60 | 5.10 | 4.88 | 4.73 | 4.66 | 0.073 | 0.204 |
| CL (%) | 37.27a | 41.57d | 41.18c,d | 40.25c | 39.01b | 0.326 | <0.001 |
| WLR (%) | 26.34a | 31.59c | 29.66b,c | 28.34a,b | 29.19b,c | 0.490 | 0.007 |
a–dValues without common superscripts in the same row differ significantly (P < 0.05); Duncan's test was used to compare statistical differences among the CON, HS, HS-0.5% Gln, HS-1.0% Gln, and HS-1.5% Gln groups.
Abbreviations: a*, redness; b*, yellowness; CL, cooking loss; CON, control; DL, drip loss; HS, heat stress; L*, luminance; WLR, water loss rate.
CON = broilers were kept in the normal-temperature environment and fed a basal diet; HS = broilers were kept in the circular heat stress environment and fed a basal diet; HS-0.5% Gln, HS-1.0% Gln, and HS-1.5% Gln = broilers were kept in the circular heat stress environment and fed basal diets supplemented with 0.5, 1.0, and 1.5% Gln.
Gln Concentration in the Thigh Muscle
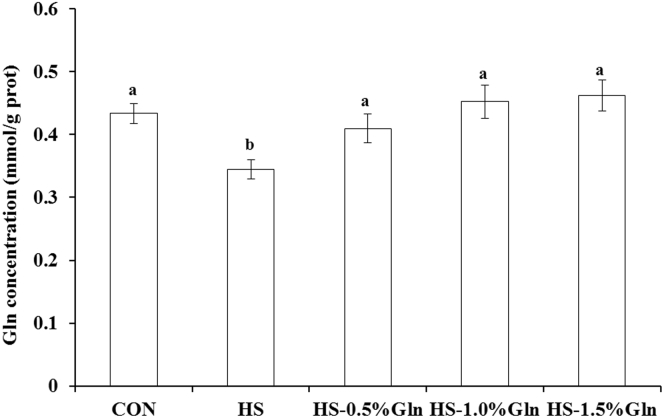
As shown in Figure 1, a significant decrease (P < 0.05) in Gln concentration of the thigh muscle was observed after HS treatment. Compared with the HS group, supplementation with 0.5, 1.0, and 1.5% Gln significantly increased (P < 0.05) the Gln concentration in the thigh muscle of heat-stressed broilers.
Figure 1.
Effect of heat stress and glutamine on glutamine concentration of thigh muscle in broilers. a,bGroups without common letters differ significantly (P < 0.05); Duncan's test was used to compare statistical differences among the CON, HS, HS-0.5% Gln, HS-1.0% Gln, and HS-1.5% Gln groups. CON = broilers were kept in the normal-temperature environment and fed a basal diet; HS = broilers were kept in the circular heat stress environment and fed a basal diet; HS-0.5% Gln, HS-1.0% Gln, and HS-1.5% Gln = broilers were kept in the circular heat stress environment and fed basal diets supplemented with 0.5, 1.0, and 1.5 Gln. CON, control; Gln, glutamine; HS, heat stress.
Redox State and Antioxidants in the Thigh Muscle
Table 4 shows the redox state and antioxidants in the thigh muscle. There were significant decreases (P < 0.05) in the GSH, SOD, GSH-Px, CAT, and T-AOC levels but significant increases (P < 0.05) in the MDA level of the thigh muscle after HS treatment. Compared with the HS group, supplementation with 0.5, 1.0, and 1.5% Gln significantly decreased (P < 0.05) MDA concentration; supplementation with 1.0 and 1.5% Gln significantly increased (P < 0.05) GSH, GSH-Px, and T-AOC levels; supplementation with 1.5% Gln significantly increased (P < 0.05) CAT and SOD levels in the thigh muscle of heat-stressed broilers.
Table 4.
Effect of heat stress and glutamine on redox state and antioxidants of the thigh muscle in broilers.
| Item | Treatments1 |
SEM | P-value | ||||
|---|---|---|---|---|---|---|---|
| CON | HS | HS-0.5% Gln | HS-1.0% Gln | HS-1.5% Gln | |||
| MDA (nmol/mg protein) | 0.40a | 0.66d | 0.58c | 0.52b,c | 0.46a,b | 0.020 | <0.001 |
| GSH (mg/g protein) | 5.92a | 3.33b | 4.89a,b | 5.87a | 5.86a | 0.312 | 0.023 |
| SOD (U/mg protein) | 109.97a | 85.53c | 89.33b,c | 96.53a,b,c | 106.57a,b | 2.930 | 0.021 |
| GSH-Px (U/mg protein) | 25.88a | 19.36b | 23.08a,b | 25.17a | 26.91a | 0.734 | 0.003 |
| CAT (U/mg protein) | 3.80a | 2.29b | 2.79a,b | 3.17a,b | 3.85a | 0.179 | 0.015 |
| T-AOC (U/mg protein) | 2.84a | 1.98b | 2.74a,b | 3.56a | 3.40a | 0.144 | 0.004 |
a–cValues without common superscripts in the same row differ significantly (P < 0.05); Duncan's test was used to compare statistical differences among the CON, HS, HS-0.5% Gln, HS-1.0% Gln, and HS-1.5% Gln groups.
Abbreviations: CAT, catalase; CON, control; GSH, glutathione; GSH-Px, glutathione peroxidase; HS, heat stress; MDA, malondialdehyde; SOD, superoxide dismutase; T-AOC, total antioxidant capacity.
CON = broilers were kept in the normal-temperature environment and fed a basal diet; HS = broilers were kept in the circular heat stress environment and fed a basal diet; HS-0.5% Gln, HS-1.0% Gln, and HS-1.5% Gln = broilers were kept in the circular heat stress environment and fed basal diets supplemented with 0.5, 1.0, and 1.5% Gln.
The mRNA Expression Levels of Antioxidant Enzymes
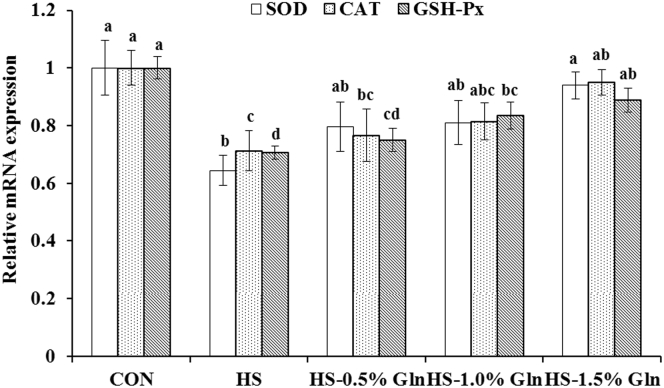
There were significant decreases (P < 0.05) in the SOD, GSH-Px, and CAT mRNA expression levels of the thigh muscle after HS treatment (Figure 2). Compared with the HS group, supplementation with 1.0 and 1.5% Gln significantly increased (P < 0.05) the GSH-Px mRNA expression level; supplementation with 1.5% Gln significantly increased (P < 0.05) CAT and SOD mRNA expression levels in the thigh muscle of heat-stressed broilers (Figure 2).
Figure 2.
Effect of heat stress and glutamine on the mRNA expression of antioxidant enzymes of thigh muscle in broilers. a,b,c,dGroups without common letters differ significantly (P < 0.05); Duncan's test was used to compare statistical differences among the CON, HS, HS-0.5% Gln, HS-1.0% Gln, and HS-1.5% Gln groups. CON = broilers were kept in the normal-temperature environment and fed a basal diet; HS = broilers were kept in the circular heat stress environment and fed a basal diet; HS-0.5% Gln, HS-1.0% Gln, and HS-1.5% Gln = broilers were kept in the circular heat stress environment and fed basal diets supplemented with 0.5, 1.0, and 1.5 Gln. SOD, superoxide dismutase; GSH-Px, glutathione peroxidase; CAT, catalase. The mRNA expression of each gene of the CON was set to be 1. CON, control; Gln, glutamine; HS, heat stress.
Expression of Nrf2-Keap1 Signaling Pathway–Related Proteins
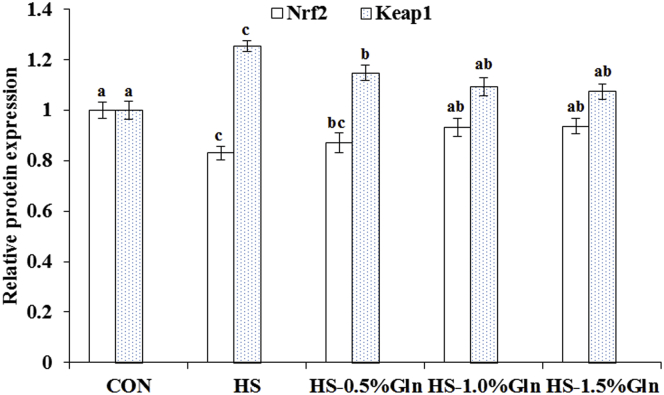
As shown in Figure 3, the Nrf2 protein level significantly decreased (P < 0.05), whereas that of Keap1 significantly increased (P < 0.05) in the thigh muscle after HS treatment. Compared with the HS group, supplementation with 1.0 and 1.5% Gln significantly increased (P < 0.05) the protein level of Nrf2, whereas supplementation with 0.5, 1.0, and 1.5% Gln significantly decreased (P < 0.05) the protein level of Keap1 in the thigh muscle of heat-stressed broilers (Figure 3).
Figure 3.
Effect of heat stress and Gln on the protein expression of Nrf2 and Keap1 of the thigh muscle in broilers. a,b,c Groups without common letters differ significantly (P < 0.05); Duncan's test was used to compare statistical differences among the CON, HS, HS-0.5% Gln, HS-1.0% Gln, and HS-1.5% Gln groups. CON = broilers were kept in the normal-temperature environment and fed a basal diet; HS = broilers were kept in the circular heat stress environment and fed a basal diet; HS-0.5% Gln, HS-1.0% Gln, and HS-1.5% Gln = broilers were kept in the circular heat stress environment and fed basal diets supplemented with 0.5, 1.0, and 1.5 Gln. Nrf2, nuclear factor erythroid 2–related 2; Keap1, kelch-like ECH-associated protein 1. The protein expression of each protein of the CON was set to be 1. CON, control; Gln, glutamine; HS, heat stress.
Expression of Nrf2-Keap1 Signaling Pathway–Related Genes
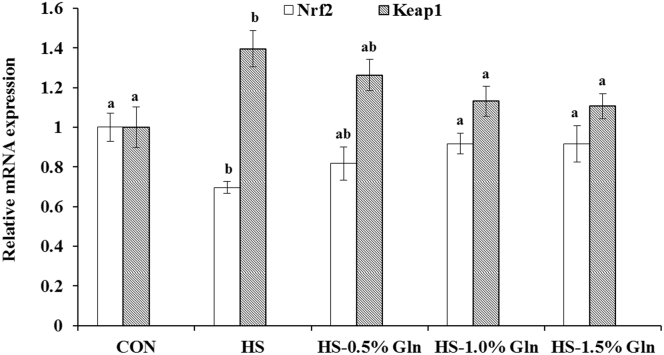
As shown in Figure 4, there was a significant decrease (P < 0.05) in the expression level of the Nrf2 gene, whereas that of Keap1 significantly increased (P < 0.05) in the thigh muscle after HS treatment. Compared with the HS group, supplementation with 1.0 and 1.5% Gln significantly increased (P < 0.05) the Nrf2 mRNA expression level but decreased (P < 0.05) that of Keap1 in the thigh muscle of heat-stressed broilers (Figure 4).
Figure 4.
Effect of heat stress and Gln on the mRNA expression of Nrf2 and Keap1 of the thigh muscle in broilers. a,b Groups without common letters differ significantly (P < 0.05); Duncan's test was used to compare statistical differences among the CON, HS, HS-0.5% Gln, HS-1.0% Gln, and HS-1.5% Gln groups. CON = broilers were kept in the normal-temperature environment and fed a basal diet; HS = broilers were kept in the circular heat stress environment and fed a basal diet; HS-0.5% Gln, HS-1.0% Gln, and HS-1.5% Gln = broilers were kept in the circular heat stress environment and fed basal diets supplemented with 0.5%, 1.0%, and 1.5% Gln. Nrf2, nuclear factor erythroid 2–related 2; Keap1, kelch-like ECH-associated protein 1. The mRNA expression of each gene of the CON was set to be 1. CON, control; Gln, glutamine; HS, heat stress.
Discussion
Meat quality of broilers is a complex characteristic that is generally determined by meat color, water-holding capacity, and pH. It has been reported that a hot environment could be detrimental to meat quality (Gregory, 2010, Lara and Rostagno, 2013, Lu et al., 2017). Heat stress can depress postmortem energy metabolism and accelerate glycolysis and lactic acid formation, which leads to rapid pH decline (Lu et al., 2017). A pH that is too low can easily lead to the appearance of pale, soft, exudative meat, which is characterized by paleness and decreased WHC of the meat (Petracci et al., 2015, Hu et al., 2016a). In this experiment, the decreased (P < 0.05) pH, a*, WLR, and CL and increased (P < 0.05) L* values observed in the thigh meat after the high-temperature treatment were prevented by Gln supplementation. Similar results were also found by Hu et al. (2016a), who reported that dietary Gln increased the pH and WHC values, but decreased CL values of broiler thigh meat after acute heat stress. Preslaughter intervention by adding Gln to the diet may provide an attractive strategy for improving meat quality of broilers exposed to a hot environment.
Glutamine is not only an important amino acid in proteins and peptides but also the biosynthetic precursor of pyrimidine and purine nucleotides, nucleic acids, and amino sugars in vivo. Under pathological conditions such as trauma, long-term stress, and infection, the consumption of Gln increases in the broiler muscle. However, the endogenous Gln levels in the muscle cannot meet the requirements, leading to the need to exogenously supplement Gln in the diet (Jazideh et al., 2014, Dai et al., 2018, Hu et al., 2016b). Barekatain and Toghyani (2019) suggested that there is need for exogenous addition of Gln in broiler diet under long-term stress rather than under normal conditions. In the present study, heat stress markedly decreased (P < 0.05) the Gln levels in the broiler thigh muscle, whereas exogenous Gln supplementation reversed this effect. This observation suggests that Gln deficiency in the body could be improved by exogenous supplementation to relieve the damage of heat stress.
Malondialdehyde is the stable end product of the lipid oxidation reaction, and its content reflects the degree of damage induced by free radicals in body fat (Wang et al., 2009, Ayala et al., 2014). Generally, the MDA content is a direct quantitative index of meat lipid peroxidation, which is related to its quality (Luna et al., 2010, Zhang et al., 2018). Many studies have shown that heat stress results in oxidative damage to the organism with a concomitant increase in the MDA level (Pamok et al., 2009, Yang et al., 2010). The MDA content in the thigh muscle of the high-temperature stress group was significantly higher (P < 0.05) than that of the CON group in the present study. This observation indicated that fat oxidation of the thigh muscle was damaged by heat stress.
It has been convincingly demonstrated that nutritional regulation (such as Se, vitamin C, curcumin, mannan oligosaccharide, and so on) alleviates the deleterious consequences including the excessive lipid peroxidation in heat-stressed broilers (Zhang et al., 2018, Cheng et al., 2018). Dai et al. (2018) showed that Gln significantly depressed lipid peroxidation and alleviated oxidative stress in the thigh muscle of broilers subjected to acute heat-stressed condition. Similarly, the present results showed that the addition of Gln effectively reduced (P < 0.05) MDA levels in the thigh muscle of broilers. We hypothesized that this may be caused by the antioxidant and protective function of Gln under heat stress conditions.
When poultry is subjected to heat stress, the production of free radicals increases, whereas the activities of antioxidant enzymes and free radical scavenging ability decreases, inducing a state of oxidative stress in broilers (Lara and Rostagno, 2013; Akbarian et al., 2016). Antioxidants in the body include GSH, GSH-Px, CAT, and SOD. Zhang et al. (2015) showed that the GSH, GSH-Px, and CAT levels of the HS group were lower than those of the normal-temperature group. Long-term stress results in oxidative damage. In the present study, dietary Gln effectively increased (P < 0.05) the levels of GSH, GSH-Px, CAT, and T-AOC in the HS group, which was consistent with the results of Dai et al. (2018). The antioxidant effect of Gln is achieved by its participation in the synthesis of GSH, which prevents damaging oxidative effects of oxygen free radicals on biofilm (Cruzat, 2019). Thus, Gln plays a key role in maintaining homeostasis of the internal environment.
The Nrf2-Keap1 signaling pathway, which mediates the transcription of antioxidant enzyme genes, is considered the most important cellular antioxidant mechanism (Nguyen et al., 2009, Kobayashi et al., 2009, Kaspar et al., 2009). Under normal conditions, Nrf2 is located in the cytoplasm bound to Keap1; however, under oxidative stress, the Nrf2-Keap1 complex disassociates to release Nrf2, which subsequently translocates to the nucleus. The Nrf2 induces the expression of the antioxidant response element, thereby enhancing cellular antioxidant capacity (Kobayashi and Yamamoto, 2005). Wang et al. (2015) and Hartmann et al. (2017) observed that Gln markedly increased the expression of Nrf2 and decreased that of Keap1 in oxidative stressed rats induced by ischemia-reperfusion injury. Our findings that Gln upregulated (P < 0.05) the expression of Nrf2 and downregulated (P < 0.05) that of Keap1 in the thigh muscle of heat-stressed broilers were consistent with these results. Furthermore, Gln pretreatment also significantly increased (P < 0.05) the expression of SOD, CAT, and GSH-Px in the thigh muscle of heat-stressed broilers, suggesting that it restored the antioxidant system by reducing oxidative stress through modulation of the Nrf2-Keap1 pathway.
In conclusion, our study suggested that heat stress induced Gln deficiency, oxidative damage, and meat quality reduction in the broiler thigh muscle. Supplementation with exogenous Gln improved the resistance of heat-stressed broiler muscles to oxidative damage by increasing muscle Gln levels and activating the antioxidant defense mechanism. These Gln-induced effects may have been achieved by the enhancement of antioxidant activity through the Nrf2-Keap1 signaling pathway. These results suggested that Gln could serve as an antistress additive in broiler production to improve the muscle redox status and meat quality under hot environmental conditions.
Acknowledgments
This study was supported by National Natural Science Foundation of China (No. 31601983), and the key discipline fund of Animal Science in Anhui Science and Technology University [AKZDXK2015B03].
Footnotes
The authors have declared that no competing interests exist.
References
- Ayala A., Muñoz M.F., Argüelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014;2014:360438. doi: 10.1155/2014/360438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbarian A., Michiels J., Degroote J., Majdeddin M., Golian A., De Smet S. Association between heat stress and oxidative stress in poultry; mitochondrial dysfunction and dietary interventions with phytochemicals. J. Anim. Sci. Biotechno. 2016;7:37. doi: 10.1186/s40104-016-0097-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai X., Dai S., Li J., Xiao S., Wen A., Hu H. Glutamine improves the growth performance, serum biochemical profile and antioxidant status in broilers under medium-term chronic heat stress. J. Appl. Poult. Res. 2019;28:1248–1254. [Google Scholar]
- Barekatain R., Toghyani M. High dietary zinc and glutamine do not improve the performance or reduce excreta moisture of broiler chickens fed diets with and without magnesium supplementation. Poult. Sci. 2019;9:4066–4072. doi: 10.3382/ps/pez098. [DOI] [PubMed] [Google Scholar]
- Belhadj S.I., Najar T., Ghram A., Abdrrabba M. Heat stress effects on livestock: molecular, cellular and metabolic aspects, a review. J. Anim. Physiol. N. 2016;100:401–412. doi: 10.1111/jpn.12379. [DOI] [PubMed] [Google Scholar]
- Bryan H.K., Olayanju A., Goldring C.E., Park B.K. The Nrf2 cell defence pathway: Keap1-dependent and-independent mechanisms of regulation. Biochem. Pharmacol. 2013;85:705–717. doi: 10.1016/j.bcp.2012.11.016. [DOI] [PubMed] [Google Scholar]
- Cheng Y., Du M., Xu Q., Chen Y., Wen C., Zhou Y. Dietary mannan oligosaccharide improves growth performance, muscle oxidative status, and meat quality in broilers under cyclic heat stress. J. Therm. Biol. 2018;75:106–111. doi: 10.1016/j.jtherbio.2018.06.002. [DOI] [PubMed] [Google Scholar]
- Cruzat V.F. Nutrition and Skeletal Muscle. Humana Press; New York, NY: 2019. Glutamine and Skeletal Muscle. Pages 299-313. [Google Scholar]
- Dai S.F., Bai X., Zhang D., Hu H., Wu X., Wen A., He S., Zhao L. Dietary glutamine improves meat quality, skeletal muscle antioxidant capacity and glutamine metabolism in broilers under acute heat stress. J. Appl. Anim. Res. 2018;46:1412–1417. [Google Scholar]
- Gregory N.G. How climatic changes could affect meat quality. Food Res. Int. 2010;43:1866–1873. [Google Scholar]
- Hu H., Bai X., Wen A., Shah A.A., Dai S., Ren Q., Wang S., He S., Wang L. Assessment of interactions between glutamine and glucose on meat quality, AMPK, and glutamine concentrations in pectoralis major meat of broilers under acute heat stress. J. Appl. Poult. Res. 2016;25:370–378. [Google Scholar]
- Hu H., Bai X., Shah A.A., Dai S., Wang L., Hua J., Che C., He S., Wen A., Jiang J. Interactive effects of glutamine and gamma-aminobutyric acid on growth performance and skeletal muscle amino acid metabolism of 22–42-day-old broilers exposed to hot environment. Int. J. Biometeorol. 2016;60:907–915. doi: 10.1007/s00484-015-1084-9. [DOI] [PubMed] [Google Scholar]
- Hartmann R.M., Licks F., Schemitt E.G., Colares J.R., do Couto Soares M., Zabot G.P., Fillmann H.S., Marroni N.P. Protective effect of glutamine on the main and adjacent organs damaged by ischemia-reperfusion in rats. Protoplasma. 2017;254:2155–2168. doi: 10.1007/s00709-017-1102-3. [DOI] [PubMed] [Google Scholar]
- Jazideh F., Farhoomand P., Daneshyar M., Najafi G. The effects of dietary glutamine supplementation on growth performance and intestinal morphology of broiler chickens reared under hot conditions. Turk. J. Vet. Anim. Sci. 2014;38:264–270. [Google Scholar]
- Kobayashi M., Li L., Iwamoto N., Nakajima-Takagi Y., Kaneko H., Nakayama Y., Eguchi M., Wada Y., Kumagai Y., Yamamoto M. The antioxidant defense system Keap1-Nrf2 comprises a multiple sensing mechanism for responding to a wide range of chemical compounds. Mol. Cell. Biol. 2009;29:493–502. doi: 10.1128/MCB.01080-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaspar J.W., Niture S.K., aiswal J.A.K. Nrf2: INrf2 (Keap1) signaling in oxidative stress. Free Radic. Bio. Med. 2009;47:1304–1309. doi: 10.1016/j.freeradbiomed.2009.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M., Yamamoto M. Molecular mechanisms activating the Nrf2-Keap1 pathway of antioxidant gene regulation. Antioxid. Redox Sign. 2005;7:385–394. doi: 10.1089/ars.2005.7.385. [DOI] [PubMed] [Google Scholar]
- Lara L., Rostagno M. Impact of heat stress on poultry production. Animals. 2013;3:356–369. doi: 10.3390/ani3020356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lu Z., He X., Ma B., Zhang L., Li J., Jiang Y., Zhou G., Gao F. Chronic heat stress impairs the quality of breast-muscle meat in broilers by affecting redox status and energy-substance metabolism. J. Agr. Food Chem. 2017;65:11251–11258. doi: 10.1021/acs.jafc.7b04428. [DOI] [PubMed] [Google Scholar]
- Luna A., Labaque M.C., Zygadlo J.A., Marin R.H. Effects of thymol and carvacrol feed supplementation on lipid oxidation in broiler meat. Poult. Sci. 2010;89:366–370. doi: 10.3382/ps.2009-00130. [DOI] [PubMed] [Google Scholar]
- National Research Council . 9th rev. Natl. Acad. Press; Washington, DC: 1994. Nutrient Requirements of Poultry. [Google Scholar]
- Nguyen T., Nioi P., Pickett C.B. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J. Biol. Chem. 2009;284:13291–13295. doi: 10.1074/jbc.R900010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olubodun J.O., Zulkifli I., Farjam A.S., Hair-Bejo M., Kasim A. Glutamine and glutamic acid supplementation enhances performance of broiler chickens under the hot and humid tropical condition. Ital. J. Anim. Sci. 2015;14:3263. [Google Scholar]
- Petracci M., Mudalal S., Soglia F., Cavani C. Meat quality in fast-growing broiler chickens. World's Poult. Sci. J. 2015;71:363–374. [Google Scholar]
- Pamok S., Aengwanich W., Komutrin T. Adaptation to oxidative stress and impact of chronic oxidative stress on immunity in heat-stressed broilers. J. Therm. Biol. 2009;34:353–357. [Google Scholar]
- Quinteiro-Filho W.M., Ribeiro A., Ferraz-de-Paula V., Pinheiro M.L., Sakai M., Sá L.R.M., Ferreira A.J.P., Palermo-Neto J. Heat stress impairs performance parameters, induces intestinal injury, and decreases macrophage activity in broiler chickens. Poult. Sci. 2010;89:1905–1914. doi: 10.3382/ps.2010-00812. [DOI] [PubMed] [Google Scholar]
- Song D.J., King A.J. Effects of heat stress on broiler meat quality. World's Poult. Sci. J. 2015;71:701–709. [Google Scholar]
- SPSS . SPSS Inc; Chicago: 2008. Statistical Package for Social Sciences (Release 18.0.) for Windows. [Google Scholar]
- Shakeri M., Zulkifli I., Soleimani A.F., O’Reilly E.L., Eckersall P.D., Anna A.A., Kumari S., Abdullah F.F.J. Response to dietary supplementation of L-glutamine and L-glutamate in broiler chickens reared at different stocking densities under hot, humid tropical conditions. Poult. Sci. 2014;93:2700–2708. doi: 10.3382/ps.2014-03910. [DOI] [PubMed] [Google Scholar]
- Tang S., Yu J., Zhang M., Bao E. Effects of different heat stress periods on various blood and meat quality parameters in young Arbor Acer broiler chickens. Can. J. Anim. Sci. 2013;93:453–460. [Google Scholar]
- Wu Q.J., Liu N., Wu X.H., Wang G.Y., Lin L. Glutamine alleviates heat stress-induced impairment of intestinal morphology, intestinal inflammatory response, and barrier integrity in broilers. Poult. Sci. 2018;97:2675–2683. doi: 10.3382/ps/pey123. [DOI] [PubMed] [Google Scholar]
- Wang R.R., Pan X.J., Peng Z.Q. Effects of heat exposure on muscle oxidation and protein functionalities of pectoralis majors in broilers. Poult. Sci. 2009;88:1078–1084. doi: 10.3382/ps.2008-00094. [DOI] [PubMed] [Google Scholar]
- Wang A.L., Niu Q., Shi N., Wang J., Jia X.F., Lian H.F., Liu Z., Liu C.X. Glutamine ameliorates intestinal ischemia-reperfusion Injury in rats by activating the Nrf2/Are signaling pathway. Int. J. Clin. Exp. Patho. 2015;8:7896. [PMC free article] [PubMed] [Google Scholar]
- Xing T., Xu X.L., Zhou G.H., Wang P., Jiang N.N. The effect of transportation of broilers during summer on the expression of heat shock protein 70, postmortem metabolism and meat quality. J. Anim. Sci. 2015;93:62–70. doi: 10.2527/jas.2014-7831. [DOI] [PubMed] [Google Scholar]
- Yang L., Tan G.Y., Fu Y.Q., Feng J.H., Zhang M.H. Effects of acute heat stress and subsequent stress removal on function of hepatic mitochondrial respiration, ROS production and lipid peroxidation in broiler chickens. Comp. Biochem. Phys. C. 2010;151:204–208. doi: 10.1016/j.cbpc.2009.10.010. [DOI] [PubMed] [Google Scholar]
- Zhang J., Hu Z., Lu C., Bai K., Zhang L., Wang T. Effect of various levels of dietary curcumin on meat quality and antioxidant profile of breast muscle in broilers. J. Agr. Food Chem. 2015;63:3880–3886. doi: 10.1021/jf505889b. [DOI] [PubMed] [Google Scholar]
- Zhang C., Zhao X., Wang L., Yang L., Chen X., Geng Z. Resveratrol beneficially affects meat quality of heat-stressed broilers which is associated with changes in muscle antioxidant status. Anim. Sci. J. 2017;88:1569–1574. doi: 10.1111/asj.12812. [DOI] [PubMed] [Google Scholar]
- Zhang J.F., Bai K.W., Su W.P., Wang A.A., Zhang L.L., Huang K.H., Wang T. Curcumin attenuates heat-stress-induced oxidant damage by simultaneous activation of GSH-related antioxidant enzymes and Nrf2-mediated phase II detoxifying enzyme systems in broiler chickens. Poult. Sci. 2018;97:1209–1219. doi: 10.3382/ps/pex408. [DOI] [PubMed] [Google Scholar]