Abstract
The antibacterial properties of egg yolk antibodies have been known for many years. Enhanced antibiotic resistance has resulted in increased need for using these antibodies as an alternative. In the present study, generation, capsulation, and inhibition growth properties of IgY directed against Salmonella enterica subsp. enterica serovar Infantis (SI) were evaluated. White Leghorn layer hens were immunized using whole cell of inactivated SI. Salmonella Infantis–specific antibody activities in sera and egg yolk were determined by ELISA. A total of 480 one-day-old male “Cobb 500” chicks were randomly divided into 8 groups, with 6 replications of 10 birds kept for 21 D. All birds from 7 challenged groups were orally inoculated with 1 mL of SI suspension (1 × 107 CFU/mL) at 3 and 4 D of age. Two groups were dietary supplemented with 5 g/kg immune powdered yolk or nonimmune powdered yolk. One group was dietary supplemented with 12.8 g/kg capsulated immune yolk (CIY). Two groups were given 8.3 mL/L of immune water-soluble yolk or nonimmune water-soluble yolk fraction in drinking water. In the antibiotic group, 1 mL/L Enrofloxacin 10% was added to drinking water. All supplements except for the antibiotic (on Day 4 for 10 D) were added on day one and continued during the experiment. Negative and positive control groups received no supplements. During the experiment, among the challenged groups, the minimum SI cecal colonization and the lowest isolation of SI from the liver (P < 0.01) was observed in the antibiotic group. Following antibiotic group, in the group receiving CIY, colonization of bacteria in ceca and liver was significantly reduced during the second and third weeks of the experiment (P < 0.01). According to the results, capsulated specific IgY has a beneficial effect in reducing the colonization of Salmonella under the conditions of this study in comparison with other forms of IgY antibody.
Key words: IgY antibody, capsulation, antibacterial property, Salmonella enterica subsp. enterica serovar Infantis, broiler chicken
Introduction
A new alternative for controlling the infection in humans and animals is use of passive immunity (Gordon et al., 2016). Oral immunotherapy using preformed pathogen-specific egg yolk antibodies has been applied as a method for establishing passive immunity against enteric pathogens (Carlander et al., 2000), such as those caused by Campylobacter jejuni (Tsubokura et al., 1997) and Salmonella enteritidis (Gurtler et al., 2004); the potential in treating and preventing gastrointestinal (GI) infections is thanks to the IgY ability in localizing treatment (Mine and Kovacs-Nolan, 2002).
To produce IgY antibodies, laying hens are immunized with specific microorganisms leading to the production of antibodies (Schade et al., 2005). IgY is deposited in and extracted from the egg yolk, processed, and administered in the feed or water (Chalghoumi et al., 2009a).
However, a significant amount of IgY given orally is considered to be degraded and inactivated under gastric conditions because IgY is not very stable against acid and pepsin digestion (Ebina et al., 1990). The antibodies must survive the GI environment and reach their target areas, while their biological properties remain intact (Bogstedt et al., 1997). For this reason, IgY should be protected against degradation.
Gastroenteritis is regarded as an important disease with high morbidity and mortality among children and elderly (Glass et al., 2001), with Salmonella being one of the most important pathogens causing this condition (Ranjbar et al., 2018). Salmonella enterica subspecies enterica serovar Infantis (SI) spreads from animals to humans mainly through contaminated food (Noda et al., 2010). The European Food Standards Agency (EFSA/ECDC, 2015) stated that Salmonella Infantis has been the fourth most common Salmonella serovar in the EU from 2012 to 2014. Previous studies suggested that the most predominant serogroups isolated from poultry in Iran were of D and B types, and the main serovars were Enteritidis and Typhimurium (Emaddi Chashni et al., 2009, Mirzaie et al., 2010). However, in recent years, a higher prevalence of serogroup C1 and serovar Infantis has been reported (Ghoddusi et al., 2015).
Considering the loss of IgY activity in low pH (i.e., gizzard), the aim of this study was production and capsulation of anti-SI IgY to protect the broiler chickens against SI. It was evaluated whether encapsulation could optimize the efficiency of IgY under in vitro and in vivo conditions of GI tract specially gizzard.
Materials and methods
Experiment 1
Hen Immunization
All procedures were approved by the Animal Care and Use Committee of Tarbiat Modares University. Twenty 32-week-old Salmonella-free White Leghorn hens were divided in 2 groups. Hens in the immune group (I) were hyperimmunized intramuscularly at 2 sites of the breast muscle with SI whole cell antigens (0.5 mL per site). Antigens were obtained by ultrasonication and administrated at a protein concentration of 500 μg/mL (1 × 107 CFU/mL) after centrifugation. The protein concentration of antigenic cells was measured using the Bradford (1976) method. The bacteria were emulsified with an equal volume of Freund's complete adjuvant for the first immunization (Day 0) and Freund's incomplete adjuvant for 4 booster immunizations (Rahimi et al., 2007) at 15-D intervals. Sera and eggs were collected before every injection. The sera and eggs were stored at −20°C and 4°C respectively, until starting the next step.
Egg Yolk Water-Soluble Fraction Preparation
The eggs were broken out, with albumin removed. Then, the yolk membrane was cut, and yolk without vitelline membrane was collected in 50 mL centrifuge tubes to measure yolk volume, and then equal amount of PBS was added to the tube (0.1 M, pH 7.6). This mixture was vortexed, and the resulting mixture was centrifuged at 3,000 × g for 25 min. The superficial lipid layer was removed, and the second phase as the water-soluble fraction of yolk (WSF) was collected and stored at 4°C only for 1 or 2 D as the IgY source (Fulton et al., 2002).
Purification of Egg Yolk Antibody by Polyethylene Glycol Precipitation Method to Extract IgY
The egg shell was cracked, and the yolk was transferred to a filter paper to remove the remaining egg white, after which the yolk without vitelline membrane was poured into a 50 mL tube. Twice the egg yolk volume of PBS was mixed with the yolk, thereafter 3.5% polyethylene glycol (PEG) 6,000 (in g) of the total volume was added, vortexed, and rolled on a rolling mixer for 10 min. Next, tube was centrifuged at 4°C for 20 min (13,000× g), and the supernatant was poured through a folded filter paper and transferred to a new tube. Then, PEG 6,000 8.5% in gram (calculated according to the new volume) was added to the tube, vortexed, and rolled on a rolling mixer. The supernatant was discarded, and the pellet was dissolved in 1 mL of PBS by means of a glass stick and vortexed. In addition, PBS was added to the final volume of 10 mL. Then, the solution was mixed with 12% PEG 6,000 (w/v, 1.2 g) and vortexed. After centrifugation at 4°C for 20 min (12,000× g), the supernatant was discarded, and the pellet was dissolved in 800 μL of PBS. Further, the IgY extract was subjected to dialysis overnight at 4°C in saline 0.1%, and the saline was replaced by PBS the next morning and again dialyzed for 3 h under agitation. The membrane used had a molecular weight cut-off of 14,000 Da (Sigma-Aldrich, St. Louis, MO). The IgY extract was transferred to 2 mL storage vials, stored at −20°C, and used for further studies (Pauly et al., 2011).
Egg Yolk Powder Preparation
The WSF containing specific IgY and nonspecific IgY as the control was neutralized with 0.1 M NaOH (adjusted to pH 7.0) to ensure that the results would not be confounded by the acidity and then lyophilized by a freeze dryer to obtain IgY powder.
Enzyme Linked Immunosorbent Assay
The specific binding activities of IgY in sera and egg yolk were measured as follows. A flat bottomed polyvinyl chloride ELISA plate was coated overnight at 4°C with 100 μL/well of sonicated SI (5 μg of protein/100 μL) using coating buffer (0.05 M carbonate bicarbonate buffer pH 9.6). The plate was washed 3 times with PBS containing 0.05% between 20 (PBST). After washing, 300 μL per well of 3% skim milk powder in PBS was added to each well and incubated at 37°C for 90 min as the blocking step. The plate was subsequently washed with PBST. Sera and IgY extracts were diluted 1,000×, added to the wells, and incubated at 37°C for 2 h. The control wells had PBST, preimmune sera, and IgY extracts, as well as nonimmune sera and IgY extracts. After incubation, the plate was washed 3 times with PBST, where 100 μL of diluted (1:2,000) goat antichicken IgG conjugated with horseradish peroxidase (Synbiotics Corporation, Kansas City, MO) was added and incubated at 37°C for 90 min. The plate was then washed twice with PBST and once with PBS and incubated at 37°C for an additional 20 min with 100 μL of substrate solution (tetramethyl benzidine). The reaction was stopped by adding 30 μL of 4N H2SO4 with the plate read at 450 nm in an ELISA reader (Anthos 2020, Salzburg, Austria). The agglutination response of ELISA test reflected in form of OD in ELISA reader was considered as the specific antibody activity.
Widal Agglutination Titer Assay
The agglutination method was used to screen sera and IgY extract samples. For each serum and the IgY extraction sample, 11 test tubes were prepared. First, 0.9 and 0.5 mL of physiological serum were transferred into the first and second tubes, respectively. Then, 0.1 mL of the serum or IgY extract was added to the first tube and homogenized. In the next stage, 0.5 mL was taken from the first tube, added to the second tube, and homogenized, where the rest of the tubes were serially diluted to 1/10,240. From the last tube, 0.5 mL of solution was discarded. Next, the 0.5 mL killed whole cell of SI antigen (1 × 108 CFU/mL) was added to all tubes, and after incubating at 37°C for 24 h, the agglutinant formation with considering the dilution series was investigated.
Growth Inhibition Assay
A suspension of SI in brain–heart infusion (BHI) broth was adjusted to an optical density of 0.05 at 600 nm, corresponding to a cell density of approximately 2.7 × 107 CFU/mL. Next, the prepared bacterial culture was mixed with the same volume of BHI and incubated while being shaken at 37°C. The turbidity of the culture (optical density at 600 nm) was measured by a spectrophotometer (JENWAY Genova, UK) at 1-h intervals until reaching the stationary phase. The immune powdered yolks (IPY) and nonimmune powdered yolks (NIPY) were reconstituted to 50, 100, 150, and 200 μg/mL of BHI. The solutions were then centrifuged at 12,000 × g at 4°C for 20 min. The supernatant was sterilized using a 0.22-μm membrane filter (MS CA Syringe Filter). In addition, antibiotic (Enrofloxacin 10%) was reconstituted in BHI at concentrations 10 to 0.0001 μg/mL. Thereafter, 2 mL of IPY, NIPY, and Enrofloxacin solutions was added to the same volume of prepared SI culture and incubated at 37°C while being shaken. The aliquots of samples (10 μL) were taken at 0, 2, 4, and 6 h of incubation and cultured on CHROMagar Salmonella plates in duplicates. Finally, after overnight incubation at 37°C, the plates were investigated in terms of growth or nongrowth of SI.
SDS–PAGE
The purity of IgY extract was monitored by sodium dodecyl sulfate polyacrylamide gel electrophoresis under reducing conditions according to the method suggested by Rosen et al. (2010).
Capsulation
Capsulation of IgY was performed at Darou Pakhsh Pharma. Chem. Co. (Tehran, Iran). Sugar coating method was used for capsulation as follows: sucrose cores (mesh 20–25, IPS, Co. Italy) were coated with WSF of immune yolks using pan instrument at 57°C. In the next stage, cores were coated with methacrylic acid L100 (Evonik, Co. Essen, Germany)—an enteric tablet coater. In this study, 2.56 g capsulated immune yolk (CIY) was calculated to be equivalent to 1 g of IPY.
In Vitro Stability of CIY and IPY to Simulate Gizzard and Intestine Conditions
The GI juice was simulated as described by Martinez-Haro et al. (2009) and Kovacs-Nolan and Mine (2005) with minor modifications. To simulate avian gizzard digestive juice (SGDJ), a solution of NaCl 1 N (Merck, Kenilworth, NJ) was prepared containing 10 g/L of pepsin (Merck), adjusted to pH 2.0 with concentrated HCl (Merck). To simulate intestinal digestive juice (SIDJ), a concentrated solution was prepared containing bile extract (3.5%) and porcine pancreatin (0.35%; Sigma), which were diluted to effective concentrations of 0.35% and 0.035%, respectively.
Initially, 12 mL of SGDJ was added to 24, 50 mL polypropylene centrifuge tubes. All tubes were divided into 2 groups of A and B, with each group including 6 tubes containing 5 g of IPY, and 6 tubes containing 12.8 g of CIY. The tubes were incubated at 42°C with shaking. At intervals of 0.5, 1, 2, and 3 h, sampling was done from A replicate, and the samples were neutralized with sodium carbonate 0.1 M buffer, at pH 9.6. The remaining IgY activity of SGDJ samples was assessed by ELISA.
To simulate intestine conditions, after 3 h of incubation in SGDJ, B replicate was centrifuged at 20,000 × g for 10 min. Specifically, 1.5 mL of the supernatant (in a 1.5 mL microtube) was adjusted to pH 6.2 using a saturated solution of NaHCO3 (Merck). After vortex mixing, 150 μL was discarded, while 150 μL of SIDJ was added, and solutions were incubated at 42°C with shaking at a slow speed. The sampling was performed at 0.5, 1, 2, and 3 h of incubation with the samples placed on ice. The remaining IgY activity in SIDJ samples was determined by ELISA.
In Vivo Gastrointestinal Stability of CIY and IPY
According to Kovacs-Nolan and Mine (2005) with minor modifications, twelve 21-day-old male “Cobb 500” chicks were randomly assigned into 2 groups of 6 birds. The chickens were given feed and water ad libitum during the 24 h experiment to maintain normal digestive functions. Control chicks were given 5 g/kg IPY, and the test chicks given 12.8 g/kg of CIY granules on top of their diets. After 24 h, the chickens were euthanized, and necropsy was immediately carried out to collect contents from proventriculus, gizzard, proximal intestine (duodenum to Meckel's diverticulum), and distal intestine (Meckel's diverticulum to ceca). The contents of proventriculus, proximal, and distal intestine were added to 3 and gizzard contents added to 4 volumes (to neutralize gizzard acid) of PBS containing complete protease inhibitor cocktail tablets (Merck) and kept on ice. The samples were homogenized and centrifuged at 5,500 × g for 10 min at 4°C. The supernatants were then concentrated to 1/5 of their original volume by ultrafiltration using a 50 kDa molecular weight cut-off (MWCO) cellulose membrane. The remaining IgY activity of samples was determined by ELISA.
Experiment 2
Bacterial Strain and Growth Condition
The standard strain of Salmonella Infantis was first obtained from the collection of Department of Microbiology and Immunology, Faculty of Veterinary Medicine, University of Tehran. For the preparation of the inoculate, bacteria were grown in nutrient broth at 37°C for 24 h. The cultures were centrifuged for 20 min at 4,000 ×g and resuspended in fresh broth to produce a highly concentrated culture. Ten-fold dilution series for inoculation of chickens were made in sterile saline, and the viable cell concentration of the inoculum was determined by counting the CFU on CHROMagar Salmonella plates, following a pour plate procedure (Bjerrum et al., 2003). The dose for the experiment was chosen based on pre-experiments. The goal was to achieve a life-lasting infection of the chicks with counts of Salmonella remaining as stable as possible. The birds received 1 mL of bacterial suspension containing 1 × 107 CFU/mL of SI.
Experimental Animal and Design
A total of 480 male “Cobb 500” day-old broiler chicks were obtained from a Salmonella-free parent flock and randomly assigned into 8 groups and 6 replications of 10 birds. Chicken had ad libitum access to feed and water during the 21 D of the experimental trial. The feed was conventional for broilers without antibacterial and coccidiostat and was analyzed for Salmonella content before the experiment trial, following an enrichment procedure (Barrow, 1991).
All birds except for negative control group (NC) were orally inoculated with 1 mL of bacterial suspension containing 1 × 107 CFU/mL SI at 3 and 4 D of age. Negative control group was kept in a separated room from challenged groups and received no supplements. Salmonella Immune powdered yolk (SIPY) and Salmonella non immune powdered yolk (SNIPY) groups were dietary supplemented with 5 g/kg of diet IPY or NIPY. Salmonella capsulated immune yolk (SCIY) group was dietary supplemented with 12.8 g/kg of CIY. The supplements were added on top to diets. Salmonella, immune water-soluble yolk fraction and Salmonella, nonimmune water-soluble yolk fraction groups were given 8.3 mL/L of drinking water, immune WSF of yolk or nonimmune WSF of yolk. In Salmonella antibiotic (SA) group, 1 mL/L of Enrofloxacin 10% antibiotic (A) was added to drinking water. All supplements except for the antibiotic (on day 4 for 10 D) were added on day one and continued during the experiment.
Bacterial Culture
On Days 7, 14, and 21, one chicken was euthanized from each replicate. To screen Salmonella, the liver was homogenized, and 1 g of the homogenized tissue from each bird was serially (1:10) diluted to 103 using sterile saline. In case of cecal samples, whole cecal contents of each bird were pooled, approximately 1 g weighed, diluted serially by 102, 104, and 106 times in sterile saline. 10 μL of each dilution of liver and cecal samples were cultured on CHROMagar Salmonella plates, and after 24 h of incubation, the CFU number of SI was counted.
Statistical Analysis
A completely randomized design was employed for all data analyses. All assays except for the sera and egg yolks ELISA test (n = 10) were performed with 6 replications. The model was as follows: Yij = μ + Ai + eij, where Yij = observed value for a particular character; μ = overall mean; Ai = effect of the ith treatment; and eij = random error associated with the ijth recording. Significant differences (P < 0.05) between means were separated using the Duncan's multiple range test (SAS Institute, 2004). All figures were made in GraphPad prism 8.
Results
SDS PAGE
IgY extract purity was confirmed through SDS PAGE experiment according to Figure 1. The molecular weight of the purified IgY was confirmed as 180 kDa; under reducing conditions, it exhibited 2 high and low protein bands (about 65 kDa and 35 kDa, respectively).
Figure 1.
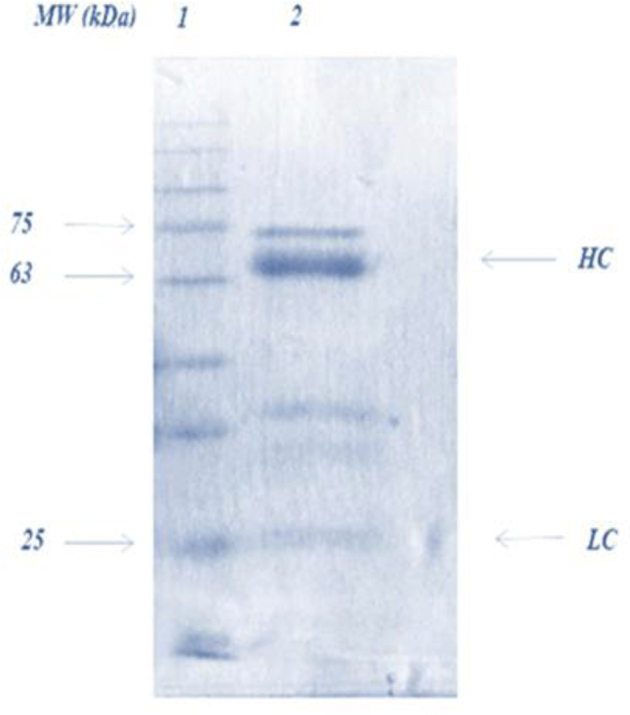
SDS PAGE of IgY extract under the reducing conditions, IgY extract was obtained from egg yolk by PEG method. Lane 1 shows the molecular weight marker (Sinaclone, Tehran, Iran) Lane 2 is IgY extraction sample. HC, heavy chains; LC, light chains; PEG, polyethylene glycol.
Measurement of Salmonella-Specific Antibody Activity
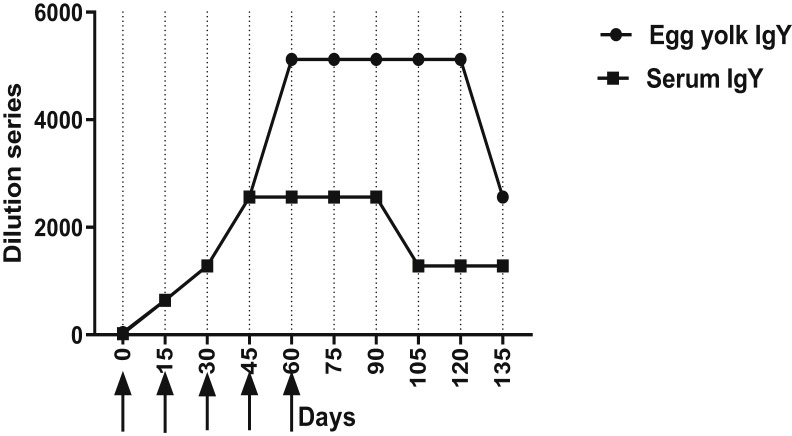
Anti-SI antibody activities in serum and egg yolk were determined by ELISA as demonstrated in Figure 2. The specific antibody activity in sera and egg yolk was low before the first immunization, whereas the egg yolk had a higher antibody activity (0.62 ± 0.05) as compared with the sera (0.46 ± 0.04) (P < 0.01). After the first immunization, the specific antibody activity in sera increased (0.82 ± 0.27), which had a difference with the antibody activity before the injection (P < 0.01). A rapid rise occurred after the second injection (1.77 ± 0.31), and serum IgY reached the maximum level (2.75 ± 0.17) 60 D after the initial immunization. Specific antibody activity slightly began to decline (2.73 ± 0.18) on day 75. With a 2-wk lag, egg yolk–specific antibody activity reached its maximum level (3.3 ± 0.29) on day 75 and then began to decline (3.28 ± 0.37) on Day 90, although the maximum specific antibody activity was higher than that of the serum (data not shown).
Figure 2.
Time-dependent changes of specific antibody activity in serum and egg yolk during the experiment period. The arrows indicate the immunization time. Antibody activities in 1/1,000 diluted sera and IgY extraction from chickens were measured by ELISA and shown as OD 450 nm. IgY extracts were purified by PEG method from egg yolks. Error bars indicate the standard deviation (n = 10). The arrows indicate the immunization times. PEG, polyethylene glycol.
Widal Agglutination Titer Assay
The results of ELISA test were confirmed by the Widal test as displayed in Figure 3, where elevation and reduction of agglutination (increase and decrease in amount of antibody) were observed; however, note that Widal test is a qualitative test and is not as sensitive as ELISA test.
Figure 3.
Time-dependent qualitative (dilution series) changes of antibody agglutination in serum and egg yolk (IgY extract) during the experiment period. The arrows indicate the immunization time. Each sample is a pool of 10 replicates. Serum and IgY extract have been serially diluted to 1/10,240.
Growth Inhibition
As presented in Table 1, growth inhibition assay confirmed the specific activity of anti-SI IgY against SI. Bacterial growth was observed at all levels of control groups during all hours of sampling. However, among different levels of IPY, bacterial growth was observed only at 50 μg/mL level at all hours of sampling. In case of Enrofloxacin, the growth of bacteria was observed at 0.001 μg/mL at 4 and 6 h as well as at 0.001 μg/mL across all hours of sampling.
Table 1.
The effect of IPY, NIPY, and Enrofloxacin (Enro) on bacterial growth.
| Concentration (mg/mL) | Time (h) |
|||
|---|---|---|---|---|
| 0 | 2 | 4 | 6 | |
| IPY | ||||
| 50 | + | + | + | + |
| 100 | − | − | − | − |
| 150 | − | − | − | − |
| 200 | − | − | − | − |
| NIPY | ||||
| 50 | + | + | + | + |
| 100 | + | + | + | + |
| 150 | + | + | + | + |
| 200 | + | + | + | + |
| Enro | ||||
| 0.0001 | + | + | + | + |
| 0.001 | − | − | + | + |
| 0.01 | − | − | − | − |
| 0.1 | − | − | − | − |
| 1 | − | − | − | − |
| 10 | − | − | − | - |
Abbreviations: IPY, immune powdered yolk; NIPY, Nonimmune powdered yolk; Enro, Enrofloxacin.
Bacterial growth indicated by +.
In Vitro Stability of CIY and IPY Under Simulated Gizzard and Intestine Conditions
According to this assay as reported in Table 2, uncapsulated IgY could not tolerate the simulated gizzard conditions even for 0.5 h. In comparison, CIY presented a greater resistance to incubation under simulated gizzard conditions; after 1 and 2 h of incubation, the capsule began to disintegrate where the remaining antibody activity of CIY was 1.35 ± 0.67 and 1.78 ± 0.36, respectively, which were higher (P < 0.01) than those of IPY at all hours of sampling. Part of the CIY granules that have been able to tolerate the simulated gizzard conditions for 3 h, after 2 and 3 h incubation in simulated intestine conditions were opened and showed the remaining antibody activity of 1.36 ± 0.26 and 1.13 ± 0.11, respectively.
Table 2.
In vitro stability of CIY and IPY to simulated gizzard and intestine conditions as assessed by ELISA.
| Treatments | Time (h) |
|||
|---|---|---|---|---|
| 0.5 | 1 | 2 | 3 | |
| Gizzard | ||||
| IPY | 0.57 ± 0.06a | 0.51 ± 0.13a | 0.47 ± 0.10b | 0.45 ± 0.07a |
| CIY | 0.46 ± 0.10a | 1.35 ± 0.7a | 1.8 ± 0.36a | 0.43 ± 0.05a |
| P-value | 0.87 | 0.047 | 0.0002 | 0.67 |
| Intestine | ||||
| IPY | 0.42 ± 0.09a | 0.41 ± 0.07a | 0.41 ± 0.11b | 0.44 ± 0.11b |
| CIY | 0.45 ± 0.06a | 0.50 ± 0.12a | 1.36 ± 0.26a | 1.13 ± 0.11a |
| P-value | 0.83 | 0.86 | 0.0001 | 0.0001 |
a-bMeans within a column lacking a common superscripts differ (P < 0.01).
Abbreviations: CIY, capsulated immune yolk; IPY, immune powdered yolk; SGDJ, simulate avian gizzard digestive juice; SIDJ, simulate intestinal digestive juice.
Anti-SI IgY activity remaining over 3 h incubation in SGDJ and SIDJ was measured by ELISA.
Values represent the mean ± SD, (n = 6).
In Vivo Gastrointestinal Stability of CIY and IPY
As outlined in Table 3, among the contents of GI sections, the maximum remaining antibody activities of CIY were observed in proximal and distal intestine (2.13 ± 0.61 and 1.53 ± 0.65, respectively), which had a difference (P < 0.01) with the remaining antibody activity of IPY in all GI parts. Further, CIY showed a higher remaining antibody activity in proventriculus and gizzard, through it was not significant (1.06 ± 0.76 and 0.98 ± 0.70, respectively).
Table 3.
In vivo gastrointestinal stability of IPY and CIY as assessed by ELISA.
| Treatments | Proventriculus | Gizzard | Proximal intestine | Distal intestine |
|---|---|---|---|---|
| IPY | 0.38 ± 0.16a | 0.55 ± 0.20a | 0.42 ± 0.09b | 0.49 ± 0.11b |
| CIY | 1.06 ± 0.76a | 0.98 ± 0.69a | 2.13 ± 0.61a | 1.53 ± 0.65a |
| P-value | 0.13 | 0.26 | 0.001 | 0.008 |
a-bMeans within a column lacking a common superscripts differ (P < 0.01).
Abbreviations: CIY, capsulated immune yolk; IPY, immune powdered yolk.
Anti-SI IgY activity in each region of the gastrointestinal tract was assessed by ELISA.
Values represent the mean ± SD, (n = 6).
S. enterica subsp. Enterica Serovar Infantis Counts of the Cecal Content Samples and Liver Tissue
The effect of anti-SI IgY in reducing the colonization of SI was evaluated by cecal content samples and liver tissue on Days 7, 14, and 21, as summarized in Table 4. The cecal content samples and liver tissue from the NC chicks were free of Salmonella throughout the experiment period. The minimum colony counts of the cecal contents and liver tissue among the challenged groups belonged to SA treatment during the 21 D of experiment (P < 0.01). There was a difference (P < 0.01) between SCIY group, which showed the lowest cecal content colony count when compared with other treatments on Days 14 and 21. In case of liver tissue after SA, SCIY treatment revealed the minimum colony counts during the 21 D of the experimental trial, which was also significant (P < 0.01).
Table 4.
The effect of treatments on Salmonella enterica subsp. enterica serovar Infantis population (Log10 cfu*/g) of cecal content and liver tissue.
| Treatments | Age (D) |
||
|---|---|---|---|
| 7 | 14 | 21 | |
| Cecum | |||
| S | 9.24 ± 0.20a | 6.82 ± 0.35a | 4.36 ± 0.15a |
| SA | 7.73 ± 0.89c | 3.66 ± 0.37c | 0.48 ± 0.24e |
| SIPY | 8.89 ± 0.57a,b | 6.39 ± 0.32a | 3.83 ± 0.22b,c |
| SCIY | 8.51 ± 0.83a,b | 4.82 ± 0.28b | 1.60 ± 0.27d |
| SIWY | 8.89 ± 0.50a,b | 6.61 ± 0.45a | 3.74 ± 0.45c |
| SNIPY | 9.10 ± 0.56a,b | 6.86 ± 0.33a | 4.23 ± 0.39a,b |
| SNIWY | 9.20 ± 0.37a,b | 6.87 ± 0.41a | 4.25 ± 0.28a,b |
| C | 0.0 ± 0.0d | 0.0 ± 0.0d | 0.0 ± 0.0f |
| P-value | 0.0001 | 0.0001 | 0.0001 |
| Treatments | Liver | ||
| S | 5.39 ± 0.27a | 3.85 ± 0.64a | 1.74 ± 0.56a |
| SA | 2.47 ± 0.19c | 0.08 ± 0.11c | 0.0 ± 0.0b |
| SIPY | 5.07 ± 0.51a | 3.43 ± 0.72a | 1.6 ± 0.45a |
| SCIY | 3.46 ± 0.79b | 1.47 ± 0.38b | 0.0 ± 0.0b |
| SIWY | 5.21 ± 0.26a | 3.68 ± 1.24a | 1.54 ± 0.43a |
| SNIPY | 5.49 ± 0.27a | 3.97 ± 0.71a | 1.69 ± 0.48a |
| SNIWY | 5.13 ± 0.29a | 3.78 ± 0.99a | 1.71 ± 0.32a |
| C | 0.0 ± 0.0d | 0.0 ± 0.0c | 0.0 ± 0.0b |
| P-value | 0.0001 | 0.0001 | 0.0001 |
a-bMeans within a column lacking a common superscripts differ (P < 0.01).
Abbreviations: S, Salmonella challenged groups; SA, Salmonella antibiotic; SIPY, Salmonella immune powdered yolk; SCIY, Salmonella capsulated immune yolk; SIWY, Salmonella immune water-soluble yolk fraction; SNIWY, Salmonella nonimmune water-soluble yolk fraction; NC, negative control.
*Log CFU SI per gram of cecal content and mixed liver tissue (n = 6).
Values represent the mean ± SD.
Discussion
The intestinal colonization of Salmonella serovars plays a significant role in carcass contamination, where reducing the intestinal colonization during the growth period is highly effective in improvement of carcass quality (Tellez et al., 2001). With concerns about antibiotic resistance, oral administration of hen egg yolk antibody (IgY) is an emerging and promising nutritional strategy to control infections in broiler chicken industry (Chalghoumi et al., 2009b). However, to maintain the efficacy of IgY for prevention and treatment of Salmonella infection in chickens, IgY stability should be preserved under GI conditions.
Following the second immunization, a rapid growth occurred in the specific antibody activity of sera in response to the second and subsequent exposure of the same antigen. The fifth injection failed to have any improvement in the antibody activity, as Chalghoumi et al. (2008) reported a slower antibody activity-elevating rate after the third injection. The specific antibody activity in egg yolk was higher (P < 0.01) than that of the serum after the third injection until the end of experiment because the secretion of IgY into the hen circulatory system was selectively accumulated in egg follicle (Rose and Orlans, 1981, Chalghoumi et al., 2008). In addition, the delay is associated to the fact that it takes time to reflect the events occurring in plasma in the yolk (Li et al., 1998).
The specific activity of antibody against SI without indicating any bacterial growth occurred at the concentration of 100 μg/mL IPY; in case of Enrofloxacine, nongrowth of bacteria was observed at a concentration of 0.01 μg/mL Enrofloxacin 10%, after 6 h of incubation. Note that IgY antibody and antibiotics have a different mode of action to inhibit the growth of bacteria. In Salmonella serovars., the A subunit of DNA gyrase is a target of quinolones such as Enrofloxacin (Delsol et al., 2004), whereas IgY antibody does not possess any bactericidal or bacteriostatic effect (Tsubokura et al., 1997). Instead, IgY binding to the specific components on the bacterial surface is considered as the most important mechanism leading to the impairment of the biological functions, which plays a significant role in the bacterial growth (Sim et al., 2000, Diraviyam et al., 2011). On the other hand, the ability of IgY in agglutination of bacteria should also be considered.
Cecal contents and ceca tonsils of chickens were found to be the superior sites for Salmonella isolation (Brownell et al., 1969). In this study, having investigated the cecal contents and liver tissue, it was observed that during 21 D of the experiment, the antibiotic was notably the best supplement in reducing the colonization of SI in the ceca and liver of the challenged chickens, followed by CIY as compared with other supplements. Although there are studies such as Mahdavi et al. (2010) that with inoculating a maximum level of 4 g/kg of diet IgY powder confirmed the successful performance of noncapsulated IgY in reducing the intestinal colonization of bacteria, the results of the present study showed greater success of capsulated IgY as compared with noncapsulated IgY. Methacrylic acid which was used for the coating is a common pharmaceutical coater which is insoluble in acid medium but will begin to dissolve around pH above 5.5 (Lehmann et al., 1999). Through coating via this enteric polymer, we expected to produce antibodies which are resistant to gizzard conditions and will dissolve in the small intestine, which is the primary reservoir of this zoonotic bacterium (Cosby et al., 2015). Therefore, we assumed that CIY granules after reaching target area were disintegrated, and IgY inhibited bacterial growth and blocked bacteria to adhere to the intestinal wall by sticking to them (Ma et al., 1990, Chalghoumi et al., 2009a). In agreement with our claim, in vivo experiment showed the highest antibody activity of CIY in proximal intestine (P < 0.01) followed by the distal intestine, proventriculus, and gizzard, respectively. All these suggest that IgY was more available to recognize and bind to SI while passing through the small intestine. However, in vitro stability of CIY was not completely in agreement with our expectation. Over time, the acidic conditions of simulated gizzard finally managed to disintegrate the polymer coater as the highest antibody activity was observed after 2 and 1 h of incubation, respectively, where the activity of the released antibodies diminished. Hatta et al. (1993) reported that digestion of IgY with pepsin at pH 2 resulted in complete hydrolysis of the antibody molecule, leaving only small peptides. The unaffected CIY granules after tolerating the gizzard conditions, in simulated intestine conditions, were opened and showed the acceptable antibody activity after 2 and 3 h incubation. Kovacs-Nolan and Mine (2005) also used methacrylic acid polymer to coat IgY. They observed that after more than 2 h incubation in simulated pig gastric conditions, polymer began gradually to disintegrate. In in vivo experiment, they observed the highest activity of IgY in pig stomach. Likewise, in agreement with our in vitro experiment, the noncapsulated IgY was extremely sensitive to simulated gastric conditions and was rapidly inactivated.
At the end, it should be mentioned that sugar coating method used for capsulation has some limitations such as relatively high cost and long coating time, and high bulk have led to the use of other coating materials (Hussan et al., 2012). In addition, diet supplementation with yolk products probably increases the concentration of protein received in chickens, while the performance of birds and preparing the equal amount of protein in this study was not considered as a major factor.
The results of this study confirmed the importance of capsuling in maximizing the efficiency of specific antibodies in reducing the colonization of bacteria. Nevertheless, further studies are required to examine other methods for coating and using other types of polymer coaters, which could be completely stable under poultry's gizzard conditions.
References
- Barrow M. Factor influencing the intestinal infection of chickens with Salmonella typhimurium. Avian Dis. 1991;13:804–816. [PubMed] [Google Scholar]
- Bjerrum L., Engberg R.M., Pedersen K. Infection models for Salmonella typhimurium DT110 in day-old and 14-day-old broiler chickens, kept in isolators. Avian Dis. 2003;47:1474–1480. doi: 10.1637/7051. [DOI] [PubMed] [Google Scholar]
- Bogstedt A.K., Hammarstrfm L., Robertson A.K. Survival of immunoglobulins from different species through the gastrointestinal tract in healthy adult volunteers: implications for human therapy. Antimicrob. Agents Chemother. 1997;41:2320. doi: 10.1128/aac.41.10.2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M.M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brownell J.R., Sadier W.W., Fanelli M.J. Factors influencing the intestinal infection of chickens with Salmonella typhimurium. Avian Dis. 1969;13:804–816. [PubMed] [Google Scholar]
- Carlander D., Kollberg H., Wejaker P.E., Larsson A. Peroral immunotherapy with yolk antibodies for the prevention and treatment of enteric infections. Immunol. Res. 2000;21:16. doi: 10.1385/IR:21:1:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalghoumi R., Thewis A., Portetelle D., Beckers Y. Production of hen egg yolk immunoglobulins simultaneously directed against Salmonella Enteritidis and Salmonella Typhimurium in the same egg yolk. Poult. Sci. 2008;87:32–40. doi: 10.3382/ps.2007-00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalghoumi R., Beckers Y., Portetelle D., Thewis A. Hen egg yolk antibodies (IgY) production and use for passive immunization against bacterial enteric infections in chicken: a review. Biotechnol. Agron. Societ. Environ. 2009;13:295–308. [Google Scholar]
- Chalghoumi R., Marcq C., Thewis A., Portetelle D., Beckers Y. Effects of feed supplementation with specific hen egg yolk antibody (immunoglobin Y) on Salmonella species cecal colonization and growth performances of challenged broiler chickens. Int. J. Poult. Sci. 2009;88:2081–2092. doi: 10.3382/ps.2009-00173. [DOI] [PubMed] [Google Scholar]
- Cosby D.E., Cox N.A., Harrison M.A., Wilson J.L., Buhr R.J., Fedorka Cray P.J. Salmonella and antimicrobial resistance in broilers: a review. J. Appl. Poult. Res. 2015;24:408–426. [Google Scholar]
- Delsol A.A., Woodward M.J., Roe1 M.J. Effect of a 5 day enrofloxacin treatment on Salmonella enterica serotype Typhimurium DT104 in the pig. J. Antimicrob. Agents Chemother. 2004;53:396–398. doi: 10.1093/jac/dkh038. [DOI] [PubMed] [Google Scholar]
- Diraviyam T., Jeevitha T., Saravanan P., Michael A., Meenatchisundaram S. Preparation of chicken (IgY) antibodies consortium for the prevention of enteric infections in poultry. J. Microbiol. Biotech. Res. 2011;1:95–103. [Google Scholar]
- Ebina T., Tsukada K., Umezu K., Nose M., Tsuda K., Hatta H., Kim M., Yamamoto T. Gastroenteritis in suckling mice caused by human rotavirus can be prevented with egg yolk immunoglobulin (IgY) and treated with a protein bound polysaccharide preparation (PSK) Microbiol. Immunol. 1990;34:617–629. doi: 10.1111/j.1348-0421.1990.tb01037.x. [DOI] [PubMed] [Google Scholar]
- EFSA/ECDC European food Safety Authority, European Centre for disease prevention and control. The European union summary report on trends and sources of zoonosis, zoonotic agents and food-borne Outbreaks in 2014. EFSA. J. 2015;13:4329. [Google Scholar]
- Emaddi Chashni S., Hassanzadeh M., Bozorgmehri Fard M.H., Mirzaie S. Characterization of the Salmonella isolates from backyard chickens in north of Iran, by serotyping, multiplex PCR and antibiotic resistance analysis. Arch. Razi. Inst. 2009;64:77–83. [Google Scholar]
- Fulton R.M., Nersessian B.N., Reed W.M. Prevention of Salmonella enteritidis infection in commercial ducklings by oral chicken egg-derived antibody alone or in combination with probiotics. Poult. Sci. 2002;81:34–40. doi: 10.1093/ps/81.1.34. [DOI] [PubMed] [Google Scholar]
- Ghoddusi A., Nayeri Fasaei B., Karimi V., Ashrafi Tamai I., Moulana Z., Zahraei Salehi T. Molecular identification of Salmonella Infantis isolated from backyard chickens and detection of their resistance genes by PCR. Iranian. J. Vet. Res. 2015;163:293–297. [PMC free article] [PubMed] [Google Scholar]
- Glass R.I., Bresee J., Jiang B., Gentsch J., Ando T., Fankhauser R., Noel J., Parashar U., Rosen B., Monroe S.S. Gastroenteritis viruses: an overview. Novartis Found. Symp. 2001;238:5–19. doi: 10.1002/0470846534.ch2. [DOI] [PubMed] [Google Scholar]
- Gordon G.,G., Morán L, Ayala., Seqqat R., Fernández R., Torres M. Generation and characterization of IgY antibodies from Lohmann Brown hens immunized with Salmonella spp. for their subsequent application in Nano therapy. Biol. Med. 2016;8:284–300. [Google Scholar]
- Gurtler M., Methner U., Koblike H., Fehlhaber K. Effect of orally administered egg yolk antibodies on Salmonella enteritidis contamination of hen’s eggs. J. Vet. Med. B. Infec. Dis.Vet Public Health. 2004;51:129–134. doi: 10.1111/j.1439-0450.2004.00739.x. [DOI] [PubMed] [Google Scholar]
- Hatta H., Tsuda K., Akachi S., Kim M., Yamamoto T. Productivity and some properties of egg yolk antibody (IgY) against human rotavirus compared with rabbit IgG. Biosci. Biotechnol. Biochem. 1993;57:450–454. doi: 10.1271/bbb.57.450. [DOI] [PubMed] [Google Scholar]
- Hussan S.D., Santanu R., Verma P., Bhandari V. A review on recent advances of enteric coating. IOSR. J. Pharma. 2012;2:5–11. [Google Scholar]
- Kovacs-Nolan J., Mine Y. Microencapsulation for the gastric passage and controlled intestinal release of immunoglobulin Y. J. Immunol. Methods. 2005;296:199–209. doi: 10.1016/j.jim.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Lehmann K., Agmus M., Bössler H., Dreher D., Liddiard C., Petereit H.U., Rothgang G., Weisbrod W., Beckert T. Röhm, Pharma Polymers; Darmstadt, Germany: 1999. Practical Course in Film Coating of Pharmaceutical Dosage Forms with EUDRAGITR®. [Google Scholar]
- Li X., Nakano T., Sunwoo H.H., Paek B.H., Chae H.S., Sim J.S. Effects of egg and yolk weights on yolk antibody (IgY) production in laying chickens. Poult. Sci. 1998;77:266–270. doi: 10.1093/ps/77.2.266. [DOI] [PubMed] [Google Scholar]
- Ma J.K., Hunjan M., Smith R., Lehner T. An investigation in to the mechanism of protection by local passive immunization with monoclonal antibodies against Streptococcus mutants. Infect. Immun. 1990;58:3407–3414. doi: 10.1128/iai.58.10.3407-3414.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahdavi A.H., Rahmani H.R., Nili N., Samie A.H., Soleimanian-Zad S., jahanian R. Effects of dietary egg yolk antibody powder on growth performance, intestinal Escherichia coli colonization, and immunocompetence of challenged broiler chicks. Poult. Sci. 2010;89:484–494. doi: 10.3382/ps.2009-00541. [DOI] [PubMed] [Google Scholar]
- Martinez-Haro M., Taggart M.A., Green A.J., Mateo R. Avian digestive tract simulation to study the effect of grit geochemistry and food on Pb Shot Bio accessibility. Environ. Sci. Technol. 2009;43:9480–9486. doi: 10.1021/es901960e. [DOI] [PubMed] [Google Scholar]
- Mine Y., Kovacs-Nolan J. Chicken egg yolk antibodies as therapeutics in enteric infectious disease: a Review. J. Med. Food. 2002;5:159–169. doi: 10.1089/10966200260398198. [DOI] [PubMed] [Google Scholar]
- Mirzaie S., Hassanzadeh M., Ashrafi I. Identification and characterization of Salmonella isolates from captured house sparrows. Turk. J. Vet. Med. 2010;34:181–186. [Google Scholar]
- Noda T., Koichi M., Yasuhisa I., Tetsuo A. Chicken meat is an infection source of Salmonella serovar Infantis for humans in Japan. Foodborne Pathog. Dis. 2010;7:727–735. doi: 10.1089/fpd.2009.0438. [DOI] [PubMed] [Google Scholar]
- Pauly D., Chacana P.A., Calzado J.E.G., Brembs B., Schade R. IgY technology: extraction of chicken antibodies from egg yolk by polyethylene glycol (PEG) precipitation. J. Vis. Exp. 2011;51(e3084):1–5. doi: 10.3791/3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimi S., Moghadam Shiraz Z., Zahraei Salehi T., Karimi Torshizi M.A., Grimes J.L. Prevention of Salmonella infection in poultry by specific egg-derived antibody. Int. J. Poult. Sci. 2007;6:230–235. [Google Scholar]
- Ranjbar R., Rahmati H., Shokoohizadeh L. Detection of common clones of Salmonella enterica serotype Infantis from human sources in Tehran hospitals. Gastroenterol. Hepatol. Bed. Bench. 2018;11:54–59. [PMC free article] [PubMed] [Google Scholar]
- Rose M.E., Orlans E. Immunoglobulins in the egg, embryo, and young chick. Dev. Comp. Immunol. 1981;5:15–20. doi: 10.1016/s0145-305x(81)80003-1. [DOI] [PubMed] [Google Scholar]
- Rosen R.F., Tomidokoro Y., Ghiso J.A., Walker L.C. SDS-PAGE/immunoblot detection of Aβ multimers in human cortical tissue homogenates using antigen-epitope retrieval. J. Vis. Exp. 2010;38:1916. doi: 10.3791/1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAS Institute . SAs Institute, Inc; Cary, NC: 2004. SAS Institute for Windows: Version 9.1.2. [Google Scholar]
- Schade R., Calzado E.G., Sarmiento R., Chacana P.A., Porankiewicz-Asplund J., Terzolo H.R. Chicken egg yolk antibodies (IgY-technology): a review of progress in production and use in research and human and veterinary medicine. Altern. Lab. Anim. 2005;33:129–154. doi: 10.1177/026119290503300208. [DOI] [PubMed] [Google Scholar]
- Sim J.S., Sunwoo H.H., Lee E.N. Ovoglobulin Y. In: Naidu A.S., editor. Natural Food Antimicrobial Systems. CRC Press; New York: 2000. pp. 227–252. [Google Scholar]
- Tellez G., Petrone V.M., Escorcia M., Morishita T.Y., Cobb C.W., Villasenor L. Evaluation of avian specific probiotic and Salmonella enteritidis, Salmonella typhimurium and Samonella heidelberg-specific antibodies on cecal colonization and organ invasion of Salmonella enteritidis in broiler. J. Food Prot. 2001;64:287–291. doi: 10.4315/0362-028x-64.3.287. [DOI] [PubMed] [Google Scholar]
- Tsubokura K., Berndtson E., Bogstedt A., Kaijser B., Kim M. Oral administration of antibodies as prophylaxis and therapy in Campylobacter jejuni infected chickens. Clin. Immunol. 1997;108:451–455. doi: 10.1046/j.1365-2249.1997.3901288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]