Abstract
Gut inflammation caused by various factors including microbial infection leads to disorder of absorption of dietary nutrients and decrease in egg production in laying hens. We hypothesized that intestinal inflammation may affect egg production in laying hens through its impact on liver function. Dextran sodium sulphate (DSS) is known to induce intestinal inflammation in mammals, but whether it also induces inflammation in laying hens is not known. The goal of this study was to assess whether oral administration of DSS is a useful model of intestinal inflammation in laying hens and to characterize the effects of intestinal inflammation on egg production using this model. White Leghorn hens (350-day old) were administrated with or without 0.9 g of DSS/kg BW in drinking water for 5 D (n = 8, each). All laid eggs were collected, and their whole and eggshell weights were recorded. Blood was collected every day and used for biochemical analysis. Liver and intestinal tissues (duodenum, jejunum, ileum, cecum, cecal-tonsil, and colon) were collected 1 D after the final treatment. These tissue samples were used for histological analysis and PCR analysis. Oral administration of DSS in laying hens caused 1) histological disintegration of the cecal mucosal epithelium and increased monocyte/macrophage infiltration and IL-1β, IL-6, CXCLi2, IL-10, and TGFβ-4 gene expression; 2) decreased egg production; 3) increased leukocyte infiltration and IL-1β, CXCLi2, and IL-10 expression in association with a high frequency of lipopolysaccharide-positive cells in the liver; and 4) decreased expression of genes related to lipid synthesis, lipoprotein uptake, and yolk precursor production. These results suggested that oral administration of DSS is a useful method for inducing intestinal inflammation in laying hens, and intestinal inflammation may reduce egg production by disrupting egg yolk precursor production in association with liver inflammation.
Key words: intestinal inflammation, egg production, liver inflammation, dextran sodium sulphate, egg yolk precursor
Introduction
Intestinal health is important for sustaining normal meat and egg production in chickens. Coccidiosis, a major intestinal infectious disease in poultry caused by Eimeria, leads to decreased feed intake, growth, and egg production (Klasing, 2007, Lensing et al., 2012, Ritzi et al., 2014). Eimeria infection causes histopathological damage, including villous atrophy and higher gene expression of proinflammatory cytokines and chemokines in the intestine (Williams, 2005, Hong et al., 2006). Aging is another factor that leads to decreased egg productivity in hens through reduced egg quality and laying rates (Joyner et al., 1987, Molnar et al., 2016). In mammals, the expression of genes encoding proinflammatory cytokines in the intestinal mucosa increased with aging (Tran and Greenwood-Van Meerveld, 2013, Man et al., 2015). Therefore, these reports indicate that intestinal infection and aging may cause intestinal inflammation along with high cytokine expression. We previously reported that viral antigen stimulation in the oviduct increased interleukin (IL)-1β and IL-6 gene expression in association with eggshell malformation in the uterus of hens (Nii, et al., 2014). We also reported that the proinflammatory cytokines IL-1β and IL-6 directly disrupt the gene and protein expression of eggshell formation–related factors such as calbindin-D28 K (Nii, et al., 2018). Therefore, intestinal inflammation may reduce egg production. However, the relationship between intestinal inflammation and egg production has not been well studied.
Chemokines, such as CXCLi2 (known as IL-8 in mammals), attract monocytes, macrophages, and heterophils into infected areas (Barker et al., 1993, Kaiser et al., 1999, Poh et al., 2008, Kim et al., 2017). Monocytes and macrophages are responsible for inflammation, specifically, they produce proinflammatory cytokines such as IL-1β and IL-6 (Kushner, 1993, Hodge et al., 2005, Netea et al., 2009, Tacke et al., 2011). The intensity of inflammation is regulated not only by proinflammatory cytokines but also by anti-inflammatory cytokines, such as IL-10 and transforming growth factor (TGF) β-2, -3, and -4 (identical to TGFβ-1 in chickens), which play important roles in the suppression of excess inflammation (Babyatsky et al., 1996, Chen and Wahl, 1999).
An egg is formed through a multistep process that includes development of the ovarian follicle, ovulation of the largest follicle, formation of egg white and the eggshell membrane, calcification of the eggshell, and oviposition. Chicken ovaries contain several hierarchical yellow follicles that undergo rapid growth and numerous white follicles before rapid growth (Gilbert, et al., 1983). Egg yolk precursors, such as very low-density lipoprotein (VLDL) and vitellogenin (VTG), that are synthesized in the liver accumulate in the yellow follicles, which reach a maximum size in approximately 7 D (Seol, et al., 2006). Specific VLDLs, with a particle size of 25–44 nm in diameter, that are called VLDLy, accumulate in the developing egg yolk (Yang, et al., 2013). VLDLy consists of 23 units of VLDL-II (also known as ApoVLDL-II) and 1 unit of apolipoprotein (apo) B100 (ApoB), which is a ligand of oocyte triacylglycerol deposition (Bujo et al., 1997, Walzem et al., 1999). The lipid bilayer of VLDLy contains cholesterol and triglyceride (TG), which are supplied from the circulation through low-density lipoprotein (LDL) receptors expressed on hepatocytes and via lipid synthesis in the liver by reactions catalyzed by enzymes such as acetyl-CoA carboxylase (ACC), fatty-acid synthetase (FAS), and stearoyl-CoA desaturase-1 (SCD-1) from glucose (Daval et al., 2000, Alvarenga et al., 2011). VLDLy and VTG synthesis is stimulated by estrogen, which is recognized by estrogenic receptor α (ERα) expressed in the liver (Li, et al., 2014). It has been reported that hepatic inflammation leads to dysfunction in lipid-related metabolism in the liver in rodents (Liang, et al., 2018). In addition, intestinal inflammation leads to hepatic inflammation through macrophage activation caused by an influx of endotoxins, such as lipopolysaccharide (LPS; a component of the gram-negative bacterial cell wall) derived from enteric bacteria, into the liver through the portal vein (Miura, et al., 2016). Accordingly, it is possible that infection-related intestinal inflammation decreases the liver functions involved in lipid metabolism as a result of hepatic inflammation, leading to reduced egg production in hens. However, it is still unclear whether intestinal inflammation leads to reduced egg production through hepatic inflammation.
Dextran sodium sulphate (DSS) causes intestinal inflammation in rodents, and it is used to generate experimental animal models of human colitis, inflammatory bowel disease, and colitis-associated cancer (Saleh and Trinchieri, 2011, Heijmans et al., 2014). DSS is polysaccharide that binds sulfur (1.3 sulphate groups per glucose unit) and has anticoagulant activity similar to heparin (Ricketts, 1952). In mice, administration of DSS in drinking water causes intestinal inflammation, especially in the colon, with body weight loss, bloody diarrhea, ulceration, and granulocyte infiltration into the mucosa (Okayasu et al., 1990, Laroui et al., 2012). It was reported that DSS damages intestinal epithelial cells, impedes tissue repair after the translocation of enteric bacteria into the submucosa, and attracts and activates immune cells, resulting in intestinal inflammation (Saleh and Trinchieri, 2011). Thus, DSS-induced intestinal inflammation likely mimics the inflammation caused by bacterial infection. The DSS treatment model has an excellent advantage in that it can mimic both acute and chronic colitis by altering the treatment schedule and dose of DSS (Wirtz et al., 2007, Bento et al., 2012, Perse and Cerar, 2012, Zheng et al., 2017). Modelling intestinal inflammation by administering DSS is expected to be a useful method for studying enteric inflammation in laying hens. However, in poultry, studies on the inflammation caused by oral administration of DSS are limited to broiler and layer chicks (Kuttappan et al., 2015, Menconi et al., 2015, Kuttappan et al., 2016, Simon et al., 2016). Therefore, it is still unknown whether DSS causes intestinal inflammation in adult laying hens and the effects of colitis on egg production.
The goal of this study was to confirm the usefulness of oral administration of DSS for establishing an intestinal inflammation model in laying hens and to characterize the effects of intestinal inflammation on egg production using this model. We hypothesized that intestinal inflammation may affect egg production through its effects on liver function. Thus, we focused on whether 1) oral administration of DSS caused intestinal inflammation in adult laying hens and 2) DSS-induced intestinal inflammation disrupted egg production. Subsequently, we evaluated whether 3) hepatic inflammation was caused in association with intestinal inflammation and 4) its effects on yolk precursor-related lipid metabolism in the liver.
Materials and methods
Experimental Birds
White Leghorn hens regularly laying 7 or more eggs in a clutch (approximately 350-day old) were housed in individual cages under a 14:10-h light-dark cycle. They were provided feed and water ad libitum. The birds were divided into 2 groups, the control and DSS groups (n = 8, each), which were administered a single oral dose of 4 mL of sterilized water/kg body weight with or without 0.9 g of DSS (molecular weight: 5,000–6,000; Nacalai Tesque, Inc., Kyoto, Japan) (0.9 g DSS/4 mL water/kg BW) daily by cannula for 5 D (DSS and control groups, respectively).
All laid eggs were collected, and their whole and eggshell weights were recorded. Blood was collected every day at 12:00–13:00 (approximately 3 h after oviposition), and these blood samples were inverted with heparin (Mochida Pharmaceutical Co., Ltd., Tokyo, Japan). Plasma samples were separated by centrifugation (1,000 × g, 10 min, room temperature) and stored at −80°C until use for biochemical analysis of TG, total cholesterol (T-CHO), nonesterified fatty acid (NEFA), glucose, alanine aminotransferase (ALT), and aspartate aminotransferase (AST) and for ELISA analysis of estradiol-17β concentration.
Birds were euthanized under anesthesia with sodium pentobarbital (Somnopentyl; Kyoritsu Seiyaku Corporation, Tokyo, Japan), and the liver and intestinal tissues (duodenum, jejunum, ileum, cecum, cecal-tonsil, and colon) were collected. These tissue samples were processed for paraffin sectioning as well as total RNA extraction. Intestinal tissues were also processed to generate frozen sections for immunohistochemical analysis of monocytes and macrophages. All experiments in this study were approved by Hiroshima University Animal Research Committee (approval no.: C17-3). Animal handling was carried out in accordance with its regulations.
Biochemical Assays of Blood Plasma
Blood parameters, namely TG, T-CHO, NEFA, glucose, ALT, and AST, were measured by using a Beckman Coulter AU480 automatic biochemistry analysis system (Beckman Coulter, Inc., Brea, CA) using reagent kits provided by the manufacturer.
Enzyme-Linked Immunosorbent Antibody Assay
Plasma estradiol-17β was analyzed by a competitive enzyme immunoassay as described previously (Isobe and Nakao, 2003). The lipid phase of blood plasma was extracted with dichloromethane. The ether phase was dried in the glass tube, and the residue was diluted with assay buffer (0.31% boric acid, 0.1% bovine serum albumin, and 0.05% potassium dichromate, pH 7.8). Diluted samples were applied to the microplate wells precoated with antirabbit IgG antibody (Sato, et al., 2011) for 4 h, which was followed by the addition of antiestradiol-17β antibody (COSMO BIO, Tokyo, Japan) and horseradish peroxidase (HRP)-conjugated estradiol-17β (FKA 236-E; COSMO BIO) for 4 h at 20°C. The plates were washed 3 times with PBS containing Tween 20, and then 3,3′,5,5′-tetramethylbenzidine substrate solution was added, and the plate was incubated for 30 min. Then, the optical density at 650 nm was read using a microplate reader (Multiskan FC; Thermo Fisher Scientific, Waltham, MA). The estradiol-17β concentration was calculated by the absolute quantification method using a standard curve.
Tissue Preparation and Staining for Histology
Intestinal and liver tissues were fixed with 10% (v/v) formalin in PBS and processed for paraffin sections. Some of the intestinal tissues were also embedded in a medium for frozen tissue specimens (OCT compound; Sakura Co., Tokyo, Japan) and snap-frozen in a mixture of isopentane and solid CO2 to prepare frozen sections. The paraffin sections (4-μm thick) were stained with Hansen's hematoxylin and eosin for histological observation.
Paraffin sections of livers were also used for immunohistochemistry to evaluate LPS-positive cells to confirm the possibility of bacteria or endotoxin influx into the liver. After deparaffinization, the sections were incubated with protease K for 30 min at 37°C. Sections were washed with PBS (3 times for 5 min each), blocked with 10% (v/v) normal horse serum for 30 min, and then incubated with mouse monoclonal antibodies to the common core region of LPS from different gram-negative bacteria (WN1 222-5; Hycult Biotech, Wayne, PA) diluted to 1 μg/mL in PBS overnight at 4°C. After washing the sections with PBS (3 times for 5 min each), the immunoprecipitates were visualized using the Histofine SAB-PO (M) kit (Nichirei Co., Tokyo, Japan). Briefly, the sections were incubated with biotinylated antimouse IgG + IgA + IgM and streptavidin-peroxidase for 1 h and then washed in PBS (3 times for 5 min each) after each step. Immunoprecipitates were visualized by incubating the sections with a reaction mixture containing 0.02% (w/v) 3,3′-diaminobenzidine tetrahydrochloride and 0.005% (v/v) H2O2 in 0.05-mol Tris-HCl buffer (pH 7.6). The sections were counterstained with Hansen's hematoxylin.
Frozen sections (10-μm thick) of intestinal tissues were prepared using a cryostat (Bright Instrument Co., Ltd., Huntingdon, England) and were used to evaluate monocytes/macrophages. They were air-dried on slides, fixed with acetone and methanol on ice for 30 min each, washed with PBS, and then incubated with 10% (v/v) normal horse serum (blocking solution) for 30 min. The primary and secondary antibody incubation and visualization were performed as described for LPS immunostaining of paraffin sections. The mouse monoclonal antibody to chicken monocytes/macrophages (KUL01) (Southern Biotech, Birmingham, AL) was diluted to 1 μg/mL and used as the primary antibodies.
Image Analysis to Determine the Frequencies of Immunopositive Cells
Sections were examined under a light microscope connected to an image analysis software program (NIS-Elements; Nikon, Tokyo, Japan). The number of monocytes/macrophages within the mucosal lamina propria of the duodenum (in an area of 1.6 × 105–2.4 × 105 μm2), jejunum (in an area of 9.9 × 104–2.0 × 105 μm2), ileum (in an area of 1.2 × 105–2.1 × 105 μm2), cecum (in an area of 1.3 × 105–2.5 × 105 μm2), cecal tonsil (in an area of 1.4 × 105–2.4 × 105 μm2), and colon (in an area of 1.9 × 105–2.6 × 105 μm2) and within LPS+ cells in the liver (in an area of 2.7 × 105–2.9 × 105 μm2) were counted. The analysis was performed in quintuplicate in one tissue, and the average was used.
Real Time-PCR Analysis for the Expression of Inflammation and Lipid Metabolism-Related Genes
Total RNA was extracted from the liver using Sepasol RNA I Super (Nacalai Tesque, Inc.). Total RNA was extracted and purified from the intestinal mucosal samples (duodenum, jejunum, ileum, cecum, cecal-tonsil, and colon) using the NucleoSpin RNA kit (Macherey-Nagel GmbH & Co., KG., Duren, Germany), which is a column-type RNA extraction kit, because any DSS remaining in the intestinal tissues would inhibit PCR (Kerr, et al., 2012). The extracted total RNA samples were dissolved in TE buffer (10-mmol Tris-HCl, pH 8.0, with 1-mmol EDTA), and the concentration of total RNA in each sample was measured using a Nano Drop Lite (Thermo Fisher Scientific, Waltham, MA). The RNA samples were reverse-transcribed using ReverTra Ace qPCR RT Master Mix with gDNA Remover (Toyobo Co., Ltd., Osaka, Japan) on a PTC-100 programmable thermal controller (MJ Research, Waltham, MA), programmed according to the manufacturer's instructions. Real-time PCR was performed using the AriaMX real-time PCR system (Agilent Technologies, Santa Clara, CA) with Brilliant III Ultra-Fast SYBR Green QPCR Master Mix (Agilent Technologies). Supplementary Table 1 shows the primers used for PCR. The cycle parameters used for the amplification were as follows: denaturation at 95°C for 5 s and annealing at 58°C (for TGFβ-2, TGFβ-4, ACC, FAS, and ERα), 60°C (for IL-6, CXCLi2, IL-10, TGFβ-3, LDLr, SREBP1, SREBP2, RPS17, GLUT2, GLUT5, and SGLT1), 62°C (for SCD-1, apoVLDL-II, and VTG-II), or 63°C (for IL-1β) for 10 s, and the denaturation and annealing steps were done for 50 cycles. The cycle parameters for the melting step were 95°C for 30 s, 65°C for 30 s, and 95°C for 30 s. RNA expression levels were calculated by the relative quantification method using a standard curve generated with serially diluted PCR products of each target gene. The relative expression level of the target mRNA in each sample was normalized to the RPS17 housekeeping gene and is reported as the mean fold change compared with a standard sample from the control group.
Paired Feeding Experiment
The hens in the DSS group showed decreased feed intake. Thus, we confirmed the effect of anorexia on egg production using a paired feeding (PF) experiment in which the feed intake of the control group was adjusted to the same level as that observed in the DSS group.
White Leghorn laying hens (approximately 350-day old) were used under same conditions as the hens in the DSS experiment. They were divided into 2 groups, the control and PF groups (n = 4, each). Hens in the PF group were served a restricted quantity of feed, which was calculated from mean feed intake in the hens during the 3 D before the start of experiment (percentages were the almost same levels of feed intake in the DSS group (i.e., day 0: 100%, day 1: 83.3%, day 2: 72.2%, day 3: 55.1%, day 4: 68.2%, and day 5: 57.3%). All birds were orally administered a single dose of sterilized water (4 mL/kg body weight).
Egg weight and eggshell weight were recorded (Supplementary Table 2), and blood samples and total RNA from the liver were collected by same method as in the DSS experiment. Blood samples were used for biochemical analysis of TG, T-CHO, NEFA, glucose, and ALT (Supplementary Figure 3). The total RNA from the liver was used for real-time PCR analysis of factors related to lipid synthesis, lipoprotein uptake, and yolk precursor synthesis (Supplementary Figure 4). Handling of birds was carried out in accordance with the regulations of the Hiroshima University Animal Research Committee (no. C17-3).
Statistical Analysis
Values are expressed as the mean ± SEM. The significance of differences in the frequencies of immunopositive cells, namely monocytes/macrophages in intestinal tissues and LPS+ cells in the liver, and the mRNA expression levels between the control and DSS groups in each tissue was evaluated by Student t test for homoscedastic samples and Welch's t test for heteroscedastic samples. Body weight, feed intake, and all blood parameters in the control and DSS groups on days 0, 1, 2, 3, 4, and 5 after first treatment were analyzed by 2-way repeated-measures ANOVA followed by Tukey's post-hoc test to assess the significance of the interaction between time and DSS treatment. Differences were considered significant when the P value was less than 0.05.
Results
Body Weight, Feed Consumption, Egg Production, and Blood Parameters
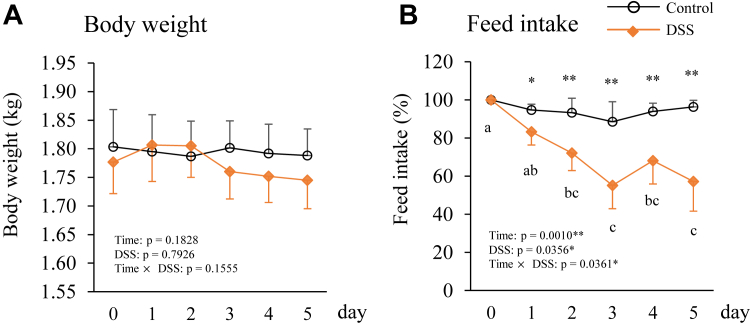
The body weights of the experimental birds did not differ significantly between the control and DSS groups during the experimental period (Figure 1A). However, feed intake in the DSS group was significantly lower on days 1–5 than that on day 0 (Figure 1B). The average feed intake was 51.3 ± 4.29 g/kg body weight in the control group and 52.4 ± 2.49 g/kg body weight in the DSS group on day 0 (which was set to 100%).
Figure 1.
Changes in body weight (A) and feed intake (B) during the experimental period in hens orally administered with dextran sodium sulphate (DSS group) or water (control group). Body weight and feed intake were measured at the same time every day. The open circle plots are the control group, and the orange rhombus plots are the DSS group. The body weights are presented as the measured value (kg) (A), and feed intake is presented as rate of change from the value on day 0 (as a percentage) ± SEM (n = 8) (B). Double asterisks (**) indicate significant differences between the control and DSS groups (P < 0.01). Lowercase superscript letters indicate significant differences among time points (P < 0.05).
Egg production in the DSS group was notably irregular beginning on day 3; only 2 or 3 eggs were laid on days 3, 4, and 5, and 3 hens stopped egg production from day 3 (Table 1). Egg weights and eggshell weights did not change during the experimental period in both the control and DSS groups (Table 1).
Table 1.
Effects of DSS oral administration on the egg production, egg weight, and eggshell weight.
| Group | Day 0 | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | |
|---|---|---|---|---|---|---|---|
| Number of egg laying | Control | 8/8 | 6/8 | 7/8 | 7/8 | 8/8 | 8/8 |
| DSS | 8/8 | 7/8 | 5/8 | 3/8 | 2/8 | 3/8 | |
| Egg weight (%) | Control | 100.0 | 100.0 ± 1.4 | 98.8 ± 2.2 | 98.2 ± 2.0 | 99.2 ± 2.0 | 98.1 ± 2.0 |
| DSS | 100.0 | 100.2 ± 1.5 | 97.9 ± 1.9 | 96.8 ± 4.7 | 88.6 ± 1.7 | 96.1 ± 5.2 | |
| Eggshell weight (%) | Control | 100.0 | 100.7 ± 3.0 | 97.9 ± 2.0 | 98.8 ± 1.0 | 98.8 ± 2.8 | 96.2 ± 2.0 |
| DSS | 100.0 | 101.1 ± 2.4 | 101.5 ± 3.7 | 101.0 ± 6.3 | 87.4 ± 1.2 | 97.5 ± 8.4 |
Number of egg laying means produced egg number/total hen number. Values are given as mean ± SEM of the ratio of egg weight and egg-shell weight against day 0 (%).
Abbreviation: DSS, dextran sodium sulphate.
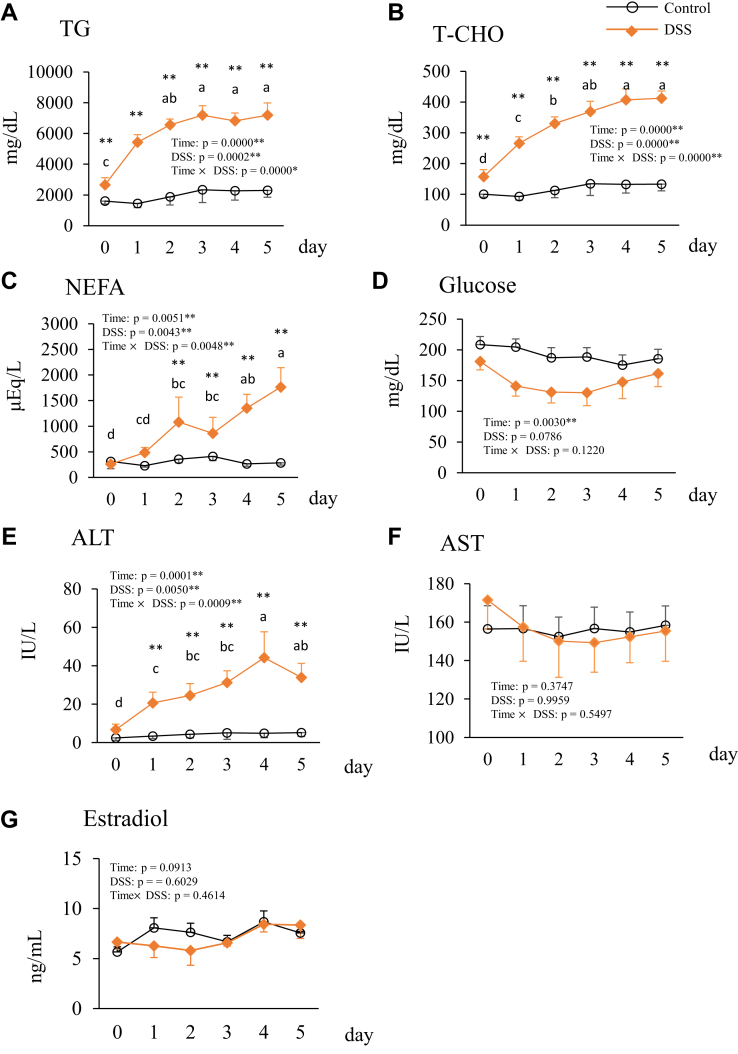
Blood plasma TG and T-CHO in the DSS group increased beginning on day 1 and peaked on day 5 (Figure 2A and B). Both TG and T-CHO were significantly higher in the DSS group than those in the control group at all time points. The plasma NEFA concentration was significantly higher in the DSS group than that in the control group on days 2–5 and was the highest on day 5 (Figure 2C). There was no significant effect of the interaction between time and DSS treatment on glucose levels (Figure 2D). Plasma ALT concentration, an indicator of liver damage, was significantly increased on days 1–5 and peaked on day 4 (Figure 2E). Plasma AST concentration, an indicator of muscle and liver damage, did not change during the experimental period and did not differ significantly between the control and DSS groups (Figure 2F). Plasma estradiol-17β concentration also did not differ significantly between the control and DSS groups and did not change among the different time points in each group (Figure 2G).
Figure 2.
Changes in the blood plasma parameters triglyceride (TG; A), total cholesterol (T-CHO; B), nonesterified fatty acid (NEFA; C), glucose (D), alanine aminotransferase (ALT) (E), aspartate aminotransferase (AST; F), and estradiol-17β (G) during the experimental period in hens administered with dextran sodium sulphate (DSS group) or water (control group). Open circle plots are the control group, and the orange rhombus plots are the DSS group. Values are the mean ± SEM (n = 8). Asterisks (*, **) indicate significant differences between the control and DSS groups at the same time point (P < 0.05 and P < 0.01, respectively). Lower case letters indicate significant differences between time points (P < 0.05).
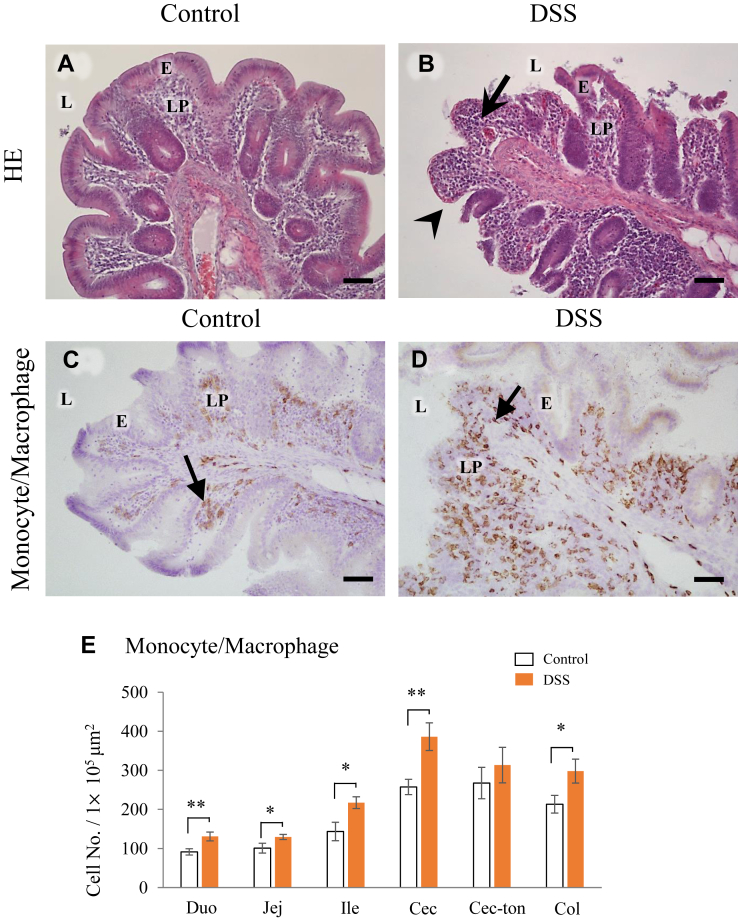
Effects of DSS on Intestinal Inflammation
In this study, the cecum was defined as the cecal-pouch and was identified by the cecal-tonsil. In the control group, the lamina propria of the cecum was lined with luminal epithelium (Figure 3A), whereas in the DSS group, a large area of the luminal epithelium was lost, and many red blood cells and leucocytes had accumulated in the lamina propria (Figure 3B). In contrast, no histological difference was observed between the control and DSS groups in the jejunum (Supplementary Figure 1), ileum (Supplementary Figure 1), and other intestinal segments (data not shown).
Figure 3.
Micrographs showing the histology of the cecum in hens orally administered with dextran sodium sulphate (DSS group) or water (control group), and the effects of DSS on the localization of monocytes/macrophages in intestinal tissues. The cecum of hens in the control (A) and DSS (B) groups. Hematoxylin-Eosin (HE) staining. Leukocytes are distributed in the lamina propria in the cecum of hens in the DSS group (arrows). The luminal epithelial cells of the cecum in the DSS group had disintegrated or were lost (arrow head). Frozen sections of the cecum of hens in the control (C) and DSS (D) groups stained with an antimonocyte/macrophage antibody. Monocytes/macrophages are in the lamina propria (triangle arrows) in all sections (data not shown). E = mucosal epithelium; L = lumen; LP = lamina propria. Scale bars = 50 μm. (E) Frequencies of monocytes/macrophages in the lamina propria of all intestinal segments. Open bars are the control group, and orange filled bars are the DSS group. Values are the number of monocytes/macrophages in an area of 1 × 105 μm2 (n = 8). Asterisks (*, **) indicate significant differences between the control and DSS groups (P < 0.05 and P < 0.01, respectively).
Monocytes/macrophages were mainly localized to the lamina propria of the cecum (Figure 3C and D) and all other segments of intestine (data not shown). Some of these cells were also located in the submucosal tissues. The frequencies of monocytes/macrophages were significantly higher in the DSS group than those in the control group in all segments of intestine, except for the cecal-tonsil (Figure 3E).
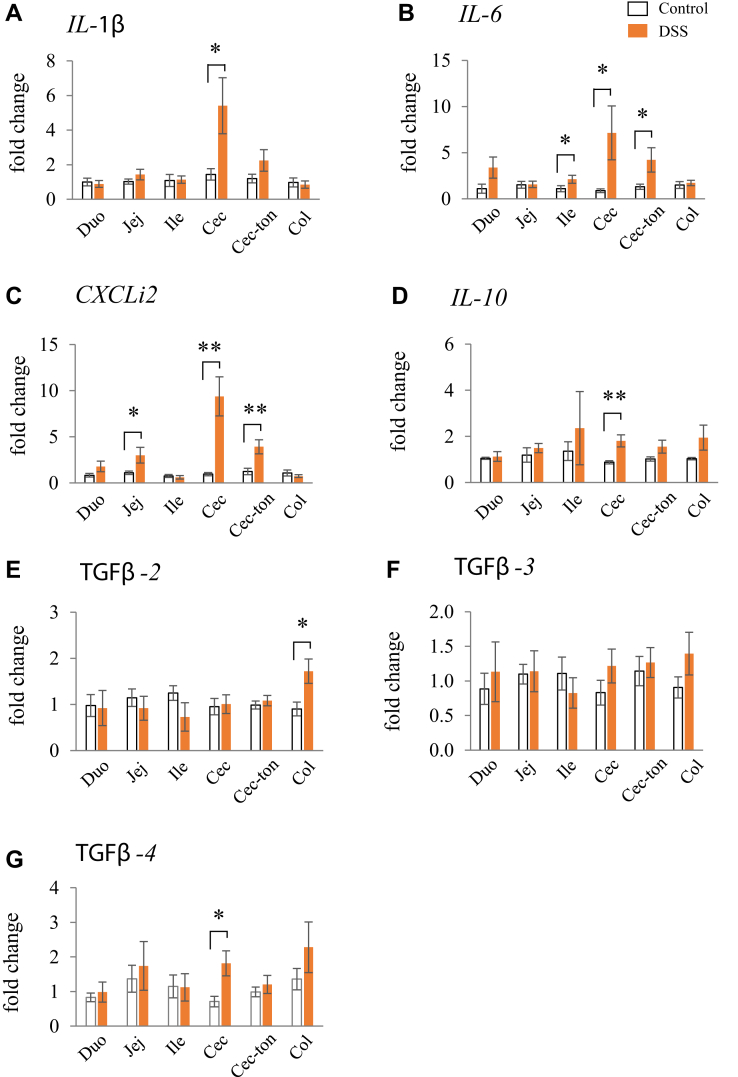
The gene expression levels of IL-1β in the cecum; IL-6 in the ileum, cecum, and cecal-tonsil; and CXCLi2 in the jejunum, cecum, and cecal-tonsil were significantly higher in the DSS group than those in the control group (Figure 4A–C). However, the expression levels of these genes in the other segments did not differ significantly between the DSS and control groups. The gene expression levels of IL-10 and TGF-β2, 3, and 4 were assessed in the mucosal tissues of all intestinal segments (Figure 4D–G). IL-10 and TGFβ-4 in the cecum and TGFβ-2 in the colon were significantly higher in the DSS group than those in the control group (Figure 4D, E, and G). However, no significant differences in expression were observed in the other segments, and TGFβ-3 gene expression levels in all segments were not significantly different between the control and DSS groups (Figure 4F).
Figure 4.
Effects of dextran sodium sulphate (DSS) on the mRNA expression of proinflammatory (A, B and C) and anti-inflammatory (D, E, F and G) cytokines in the mucosal tissues of the duodenum, jejunum, ileum, cecum, cecal-tonsil, and colon. Open bars are the control group, and orange filled bars are the DSS group. Values are the fold change in target gene expression compared with a standard duodenum sample from the control group (n = 8). Target gene expression was normalized to the house-keeping gene RPS17. Asterisks (*, **) indicate significant differences between the water (control) and DSS-treated groups (P < 0.05 and P < 0.01, respectively).
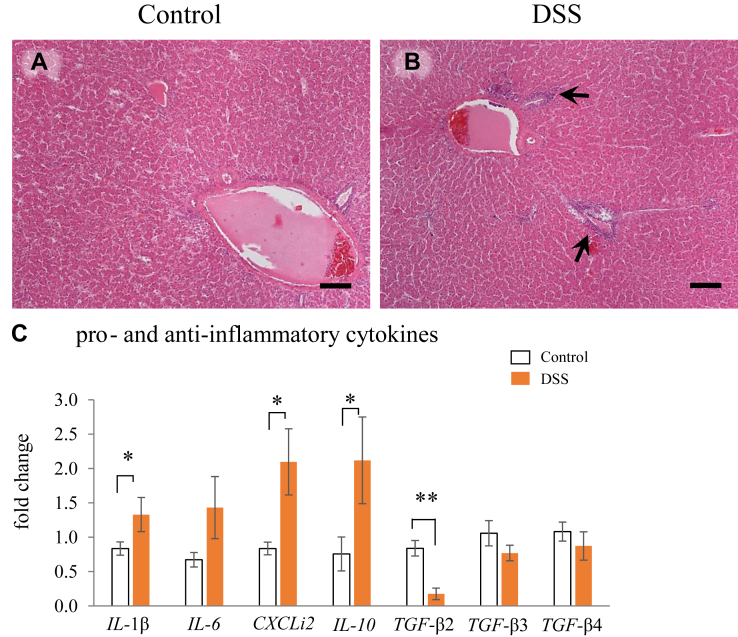
Effects of DSS on Hepatic Inflammation
The liver tissue was filled with hepatic cells and many sinusoidal capillaries, and the interlobular vein, arteria, and bile duct were observed (Figure 5A and B). Leucocytes were mainly localized in the connective tissue surrounding the vein, arteria, and sinusoidal capillary in both groups. Higher frequencies of lymphocyte infiltration, specifically around the blood vessels, were observed in the liver of the DSS group than in the control group. The gene expression level of IL-1β and CXCLi2 in the liver was significantly higher in the DSS group than that in the control group; however, IL-6 gene expression levels did not differ between the groups (Figure 5C). In contrast, the expression of IL-10 was significantly increased and TGFβ-2 was significantly lower in the DSS group than that in the control group.
Figure 5.
Effects of oral administration of dextran sodium sulphate (DSS) on hepatic inflammation in hens. (A and B) Micrographs showing the histology of the livers of hens treated with water (A; control group) and DSS (B; DSS group). Leukocytes were mainly localized around the veins and arteria in the liver in the DSS group (arrows). Hematoxylin-Eosin (HE) staining. Scale bars = 50 μm. Effects of oral administration of DSS on the mRNA expression of proinflammatory and anti-inflammatory cytokines (C). Open bars are the control group, and orange filled bars are the DSS group. Values are the fold change in the target gene expression compared to that in a standard sample from the control group (n = 8). Target gene expression is normalized to the house-keeping gene RPS17. Asterisks (*, **) indicate significant difference between the control and DSS groups (P < 0.05 and P < 0.01, respectively).
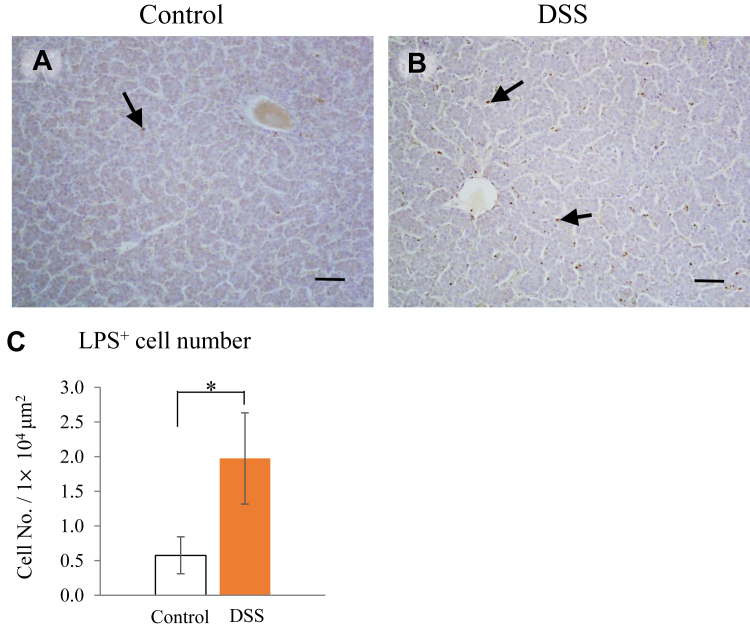
The localization pattern of LPS + cells was like that of leucocytes, that is, they were in the connective tissue surrounding the vessels in the liver (Figure 6A and B). The frequencies of LPS + cells in the liver tissue, as determined by microscope image analysis, were significantly higher in the DSS group than those in the control group (Figure 6C).
Figure 6.
Effects of dextran sodium sulphate (DSS) administration on the localization of lipopolysaccharide (LPS)+ cells in the liver. (A and B) Paraffin sections of the livers from hens in the control (A) and DSS (B) groups stained with an anti-LPS antibody. LPS+ cells are in the connective tissue surrounding the vessels (arrows). Scale bars = 50 μm. (C) Frequencies of LPS+ cells in the liver. Open bars are the control group, and orange filled bars are the DSS group. Values are the number of LPS+ cells in a 1 × 104 μm2 area (n = 8). An asterisk (*) indicates a significant difference between the water (control) and DSS groups (P < 0.05).
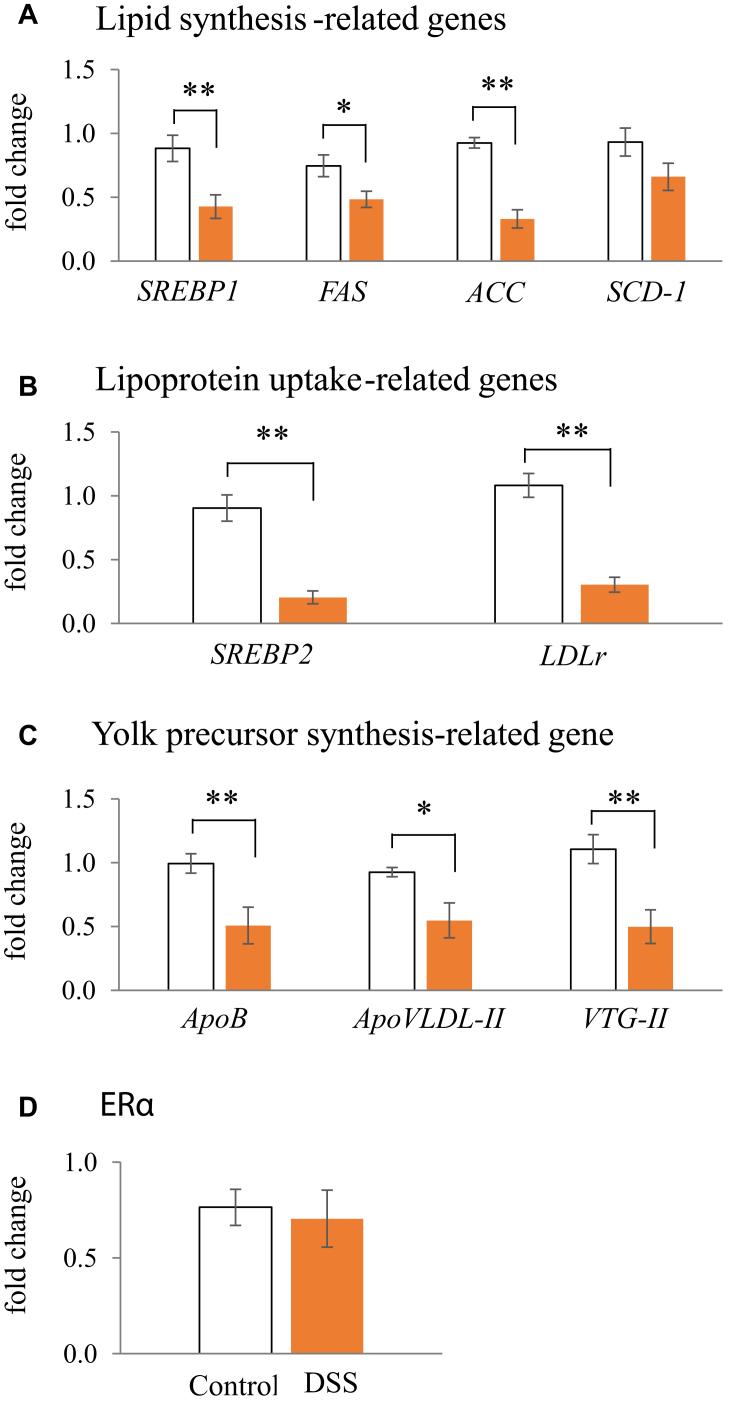
Effects of DSS on Yolk Precursor-Related Lipid Metabolism in the Liver
Figure 7 shows the mRNA expression levels of several lipid synthesis–related genes (SREBP1, FAS, ACC, and SCD-1; Figure 7A), lipoprotein uptake–related genes (SREBP2 and LDLr; Figure 7B), yolk precursor synthesis–related genes (ApoB, ApoVLDL-II, and VTG-II; Figure 7C), and oestrogen receptor (ERα: Figure 7D) in the hen liver. The expression levels of these 10 genes, excluding SCD-1 and ERα, in the liver were significantly lower in the DSS group than those in the control group.
Figure 7.
Effects of dextran sodium sulphate (DSS) on the mRNA expression of lipid synthesis–related genes (A), lipoprotein uptake–related genes (B), yolk precursor–related genes (C), and estrogen receptor (D) in the liver of hens. Open bars are the control group, and orange filled bars are the DSS group. Values are the fold change in the target gene expression as compared to that in a standard sample from the control group (n = 8). Target gene expression is normalized to the house-keeping gene RPS17. Asterisks (*, **) indicate significant differences between the water (control) and DSS groups (P < 0.05 and P < 0.01, respectively).
Effects of DSS on Glucose Transporter Expression
GLUT5 and SGLT1 expressions in the ileum were significantly lower in the DSS group than those in the control group (Supplementary Figure 2A). However, GLUT2 in both segments and GLUT5 and SGLT1 in the jejunum did not differ significantly between the control and DSS groups (Supplementary Figure 2).
Effects of PF on Egg Production, Plasma Parameters, and Lipid Metabolism in the Liver
Egg production was not altered by PF (Supplementary Table 2). However, the weights of the eggs and eggshells were significantly lower in the PF group than those in the control group on days 4 and 5.
The pattern of changes in plasma parameters differed greatly between the DSS experiment and the PF experiment (Supplementary Figure 3). The plasma T-CHO concentration in the PF group decreased significantly in a time-dependent manner and reached a minimum on day 3 (Supplementary Figure 3B). TG in the PF group did not differ significantly nor was there an interaction between time and feed regulation. However, the pattern was like that for T-CHO in the PF group (Supplementary Figure 3). The plasma NEFA level was significantly higher in the PF group than that in the control group (Supplementary Figure 3). However, the glucose and ALT concentrations did not differ between the PF and control groups (Supplementary Figure 3).
SREBP-1 gene expression was higher, and FAS and ApoVLDL-II expression were lower in the liver in the PF group than those in the control group. However, there were no differences in the other lipid metabolism–related and yolk precursor synthesis–related genes (Supplementary Figure 4).
Discussion
This is the first report showing the effects of orally administered DSS on egg laying in laying hens. We observed that oral administration of DSS caused intestinal inflammation, hepatic inflammation, and impaired lipid metabolism in the liver in association with a detriment in egg production. The significant findings in this study were that oral administration of DSS caused 1) histological disintegration of the mucosal epithelial tissue in the cecum, an increased frequency of monocytes/macrophages, and increased expression of IL-1β, IL-6, CXCLi2, IL-10 and TGFβ-4; 2) decreased egg production; (3) increased levels of ALT, leukocyte infiltration, and IL-1β, CXCLi2, and IL-10 expression as well as decreased TGFβ-2 expression in association with high frequencies of LPS + cells in the liver; and 4) decreased expression of genes related to lipid synthesis (SREBP-1, FAS, and ACC), lipoprotein uptake (SREBP-2 and LDLr), and yolk precursor production (ApoB, ApoVLDL-II, and VTG-II).
Oral administration of DSS caused clear damage to the cecum mucosa, including detachment of a portion of the luminal epithelium (Figure 3B) and increased macrophage/monocyte infiltration in all segments of the intestine (Figure 3E). Previous reports showed similar observations in the intestinal mucosa of DSS-treated rodents and chicks (Wirtz et al., 2007, Kim et al., 2010, Dharmani et al., 2011, Banerjee et al., 2015, Simon et al., 2016). DSS treatment also showed direct toxicity in Caco-2 cells, a strain of human intestinal epithelial cells, namely, decreased cell viability associated with increased IL-6 and IL-8 production in an in vitro study (Araki, et al., 2006). DSS directly affects epithelial cells, causing a loss of these cells, which is followed by mucosal inflammation as a result of the invasion of enteric microbes (Saleh and Trinchieri, 2011). M1 macrophage is a well-known inflammation-mediating macrophage that secretes IL-1β and IL-6 (Kushner, 1993, Hodge et al., 2005, Netea et al., 2009, Tacke et al., 2011). The increase in the number of monocytes/macrophages and the levels of proinflammatory cytokines (IL-1β, IL-6, and CXCLi2) together with the increase in anti-inflammatory cytokines (IL-10 and TGFβ-4) in the DSS group indicate local inflammation in the mucosal layer of the cecum (Figures 3E and 4). The mucosa of the cecal-tonsil also showed features of inflammation, including increased levels of proinflammatory and anti-inflammatory cytokines (Figure 4B, C, and E). This evidence suggested that DSS damaged the cecal epithelial cells, and microbes invading the lamina propria caused mucosal inflammation through the secretion of proinflammatory cytokines produced by infiltrating macrophages induced by chemokines.
The mechanism of inflammation caused by oral administration of DSS is similar to that of the inflammation caused by enteric bacterial infections. In aged animals, the tight junctions between the intestinal epithelial cells are weakened, and enteric bacteria enter the lamina propria to cause intestinal inflammation (Tran and Greenwood-Van Meerveld, 2013, Man et al., 2015). Therefore, the mechanisms of the enteric inflammation caused by oral administration of DSS are similar to one caused by infection and aging in laying hens. However, the clear sign of inflammation was observed in only cecum in this DSS experiment, while an enteric infectious disease, coccidiosis, causes clear mucosal inflammation and damage in not only cecum but also small intestine (Williams, 2005, Hong et al., 2006, Belote et al., 2018). In addition, aging causes mucosal inflammation in both small and large intestines in mammals (Tran and Greenwood-Van Meerveld, 2013, Man et al., 2015). Thus, the DSS method in this experiment can be used as an intestinal inflammation model, but not as the direct model of intestinal inflammation caused by coccidiosis or aging. Meanwhile, some reports that used different methods of DSS treatment indicated clear mucosal damage in both small and large intestines in chicks (Kuttappan et al., 2015, Menconi et al., 2015). Accordingly, DSS methods may be expected to application for various direct experiment models of intestinal inflammation such as infection and aging, by modulation of DSS treatment condition.
In contrast, only a portion of the intestinal inflammation, that is, the number of macrophages/monocytes infiltrating the lamina propria, was observed in the other segments of the intestine (the duodenum, jejunum, ileum, and colon) (Figure 3E), while the heavy mucosal damage was observed in the cecum in the DSS group. The retention time of the cecum contents is 24–48 h, which is much longer than the retention times for the other segments of the intestinal tract (McNab, 1973, Svihus et al., 2013). The reason why clear damage and signs of inflammation were specifically observed in the cecum is not unknown; however, we assume that the longer retention time of the luminal contents including DSS in the cecum compared with the other segments may be related to this observation.
Many leukocytes were observed in the liver tissues of hens in the DSS group along with higher expression levels of IL-1β, CXCLi2, and IL-10 and lower expression of TGFβ-2 when compared with the levels in the control group (Figure 5). In addition, ALT, a known indicator of functional disorder in the liver, was markedly increased in the DSS group compared with the levels in the control group (Figure 2E). These results indicate that liver damage occurred with hepatic inflammation after oral administration of DSS. Destruction of the intestinal mucosal barrier led to the invasion of enteric bacteria into the lamina propria followed by an influx of bacteria and/or endotoxin to other organs, such as the liver, though the blood stream (Jahnel et al., 2009, Qin et al., 2018). In this study, we detected higher frequencies of LPS + cells in the liver in the DSS group than in the control group (Figure 6C). LPS is recognized by toll-like receptor 4, and its recognition leads to an inflammatory reaction (Zhang, et al., 2013). These results suggest that the intestinal inflammation caused by DSS may lead to an influx of enteric gram-negative bacteria or LPS from the cecum to the liver, causing hepatic inflammation. Brachyspira pilosicoli infection in the cecum and colon of chickens led to tissue damage in the liver, including hemorrhage with leukocytes (Mappley, et al., 2013). Infection by Eimeria and Clostridium perfringens caused heavier damage to the small and large intestine and the liver in association with inflammation and higher expression levels of proinflammatory cytokines in broiler chicks (Belote et al., 2018, Fasina and Lillehoj, 2019). The results of these reports are similar to our findings.
Our results showed high levels of plasma TG, T-CHO, NEFA, and ALT in the DSS group (Figure 2A, B and E), indicating that lipid metabolism in the liver was affected by hepatic inflammation (Liang, et al., 2018). Therefore, we assumed that the liver lipid supply is abnormally high, namely there was an increase in the expression of lipid synthesis–related genes and lipoprotein uptake–related genes caused by hepatic inflammation. However, contrary to our expectations, the gene expression of lipid synthesis–related genes (SREBP-1, FAS, and ACC) and lipoprotein uptake–related genes (SREBP-2 and LDLr) was significantly lower in the DSS group than that in the control group (Figure 7A and B). Thus, it is likely that lipid synthesis and lipid uptake were suppressed in association with the high TG level. Meanwhile, we showed that the yolk precursor synthesis–related genes ApoB, ApoVLDL-II, and VTG-II in the liver were significantly lower in the DSS group than those in the control group (Figure 7C). This suggested that the decrease in yolk precursor synthesis in the liver directly caused a malfunction of normal follicle growth in the ovary. In addition, TG is a principal constitution of VLDLy, which is the egg yolk precursor (Seol, et al., 2006). Thus, we assumed that the high blood TG level may reflect a decrease in VLDLy synthesis and decreased TG utilization. However, we still do not know why yolk precursor synthesis was suppressed despite the TG-rich conditions under intestinal inflammation in laying hens. Further studies are necessary to answer this question.
Plasma estradiol-17β levels and ERα gene expression in the liver were not changed by DSS treatment (Figures 2G and 7D). Oestrogen controls lipid metabolism and yolk precursor synthesis in the liver (Li, et al., 2014). Therefore, the downregulation of lipid synthesis and yolk precursor synthesis in the liver was caused by the effects of oral administration of DSS, including intestinal and hepatic inflammation, but not by estrogen.
Egg production gradually stopped in the DSS group (Table 1). Oral administration of DSS also caused anorexia, intestinal inflammation, and liver malfunction. Thus, we hypothesized that these 3 factors may affect egg production. The hens in the DSS group showed decreased feed intake (Figure 1B). Many researchers have reported that oral administration of DSS induced anorexia not only in mammals but also in broiler and layer chicks (Kim et al., 2010, Hakansson et al., 2015, Simon et al., 2016). First, we confirmed the effect of anorexia on egg production using a PF experiment in which the feed intake of the control group was adjusted to same level as that observed in the DSS group. Thus, we prepared a PF group in which the feed intake level was adjusted to the same level as that in the DSS group. Then, we confirmed the effects of anorexia on egg production. In the PF group, egg production did not differ from that in the DSS group (Supplementary Table 2). This result suggested that anorexia caused by DSS treatment may not be the main reason for the reduction in egg production. Second, we considered the effects of nutrient absorption through histological observations and gene expression analysis of glucose transporters (Supplementary Figure 1 and 2). Histological analysis showed that the mucosal structure of the duodenum (data not shown), jejunum, and ileum (Supplementary Figure 1) did not differ between the control and DSS groups. However, the gene expression levels of GLUT5 and SGLT1, but not GLUT2 in the ileum, were significantly lower in the DSS group than those in the control group (Supplementary Figure 2). These results suggested that glucose absorption in the ileum may be impaired without the histological damage caused by DSS treatment. However, the effect may not strong because the blood glucose concentration was not decreased during DSS treatment (Figure 2D). Thus, we assumed that the decrease in nutrient absorption in the ileum caused by DSS treatment is one factor affecting egg production. Third, we considered the effects of liver malfunction on egg production. In this study, yolk precursor synthesis was suppressed in association with hepatic inflammation as mentioned previously. In addition, the obvious high blood levels of TG and T-CHO and decreased expression of lipid metabolism–related genes were not detected in the PF group (Supplementary Figures 3A, B and 4). This suggested that the decrease in egg yolk precursor synthesis caused by intestinal inflammation directly affected normal follicle growth and subsequent egg production. In addition, we also considered the effects of DSS on the oviduct and ovary. The oviduct did not show clear difference between the control and DSS groups, but several ovarian follicles showed atresia in the DSS group (data not shown). The result suggests that DSS treatment may affect the ovarian function. Further studies are necessary to clear the effect of DSS on ovarian function. Therefore, we assumed that the disfunction of yolk precursor synthesis in the liver as well as decreased nutrient absorption are 2 factors leading to reduced egg production in laying hens under intestinal inflammation.
In conclusion, we confirmed that DSS treatment is a useful method for inducing intestinal inflammation in laying hens, and we assessed the effect of induced intestinal inflammation on egg production using this method. Intestinal inflammation caused by DSS led to hepatic inflammation through endotoxin influx into the liver. Hepatic inflammation is likely to suppress yolk precursor synthesis followed by a disruption of normal follicle growth and reduced egg production in association with high blood TG and T-CHO levels. This is the first report to show the effects of intestinal inflammation on egg production in laying hens, and it also shows that oral administration of DSS is an effective technique for studying the pathogenesis of infectious intestinal inflammation in adult laying hens.
Acknowledgments
The authors thank Ms. Haruna Kakuya, undergraduate student, Hiroshima University, for support of dairy rearing management of hens and oral administration during experiment. The authors would like to thank Editage (www.editage.jp) for English language editing.
This work was supported by a Grant-in-Aid for Research Activity Start-up (No. 17H06892) and Grant-in-Aid for Early-Career Scientists (No. 18K14569) from the Japan Society for the Promotion of Science (JSPS) to T. N.
T. N. conceived, designed, performed, and analyzed the experiments. T. B. and Y. Y. contributed to critical discussion and reviewing of the manuscript. N. I. contributed to writing and reviewing of the manuscript.
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.psj.2019.11.028.
Supplementary data
References
- Alvarenga R.R., Zangeronimo M.G., Pereira L.J., Rodrigues P.B., Gomide E.M. Lipoprotein metabolism in poultry. World Poult. Sci. J. 2011;67:431–440. [Google Scholar]
- Araki Y., Sugihara H., Hattori T. In vitro effects of dextran sulfate sodium on a Caco-2 cell line and plausible mechanisms for dextran sulfate sodium-induced colitis. Oncol. Rep. 2006;16:1357–1362. [PubMed] [Google Scholar]
- Babyatsky M.W., Rossiter G., Podolsky D.K. Expression of transforming growth factors alpha and beta in colonic mucosa in inflammatory bowel disease. Gastroenterology. 1996;110:975–984. doi: 10.1053/gast.1996.v110.pm8613031. [DOI] [PubMed] [Google Scholar]
- Banerjee A., Bizzaro D., Burra P., Di Liddo R., Pathak S., Arcidiacono D., Cappon A., Bo P., Conconi M.T., Crescenzi M., Pinna C.M., Parnigotto P.P., Alison M.R., Sturniolo G.C., D'Inca R., Russo F.P. Umbilical cord mesenchymal stem cells modulate dextran sulfate sodium induced acute colitis in immunodeficient mice. Stem Cell Res. Ther. 2015;6:79. doi: 10.1186/s13287-015-0073-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker K.A., Hampe A., Stoeckle M.Y., Hanafusa H. Transformation-associated cytokine 9E3/CEF4 is chemotactic for chicken peripheral blood mononuclear cells. J. Virol. 1993;67:3528–3533. doi: 10.1128/jvi.67.6.3528-3533.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belote B.L., Tujimoto-Silva A., Hummelgen P.H., Sanches A.W.D., Wammes J.C.S., Hayashi R.M., Santin E. Histological parameters to evaluate intestinal health on broilers challenged with Eimeria and Clostridium perfringens with or without enramycin as growth promoter. Poult. Sci. 2018;97:2287–2294. doi: 10.3382/ps/pey064. [DOI] [PubMed] [Google Scholar]
- Bento A.F., Leite D.F., Marcon R., Claudino R.F., Dutra R.C., Cola M., Martini A.C., Calixto J.B. Evaluation of chemical mediators and cellular response during acute and chronic gut inflammatory response induced by dextran sodium sulfate in mice. Biochem. Pharmacol. 2012;84:1459–1469. doi: 10.1016/j.bcp.2012.09.007. [DOI] [PubMed] [Google Scholar]
- Bujo H., Hermann M., Lindstedt K.A., Nimpf J., Schneider W.J. Low density lipoprotein receptor gene family members mediate yolk deposition. J. Nutr. 1997;127:801s–804s. doi: 10.1093/jn/127.5.801S. [DOI] [PubMed] [Google Scholar]
- Chen W., Wahl S.M. Manipulation of TGF-beta to control autoimmune and chronic inflammatory diseases. Microbes Infect. 1999;1:1367–1380. doi: 10.1016/s1286-4579(99)00249-x. [DOI] [PubMed] [Google Scholar]
- Daval S., Lagarrigue S., Douaire M. Messenger RNA levels and transcription rates of hepatic lipogenesis genes in genetically lean and fat chickens. Genet. Sel. Evol. 2000;32:521–531. doi: 10.1186/1297-9686-32-5-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmani P., Leung P., Chadee K. Tumor necrosis factor-alpha and Muc2 mucin play major roles in disease onset and progression in dextran sodium sulphate-induced colitis. PLoS One. 2011;6:e25058. doi: 10.1371/journal.pone.0025058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasina Y.O., Lillehoj H.S. Characterization of intestinal immune response to Clostridium perfringens infection in broiler chickens. Poult. Sci. 2019;98:188–198. doi: 10.3382/ps/pey390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert A.B., Perry M.M., Waddington D., Hardie M.A. Role of atresia in establishing the follicular hierarchy in the ovary of the domestic hen (Gallus domesticus) J. Reprod. Fertil. 1983;69:221–227. doi: 10.1530/jrf.0.0690221. [DOI] [PubMed] [Google Scholar]
- Hakansson A., Tormo-Badia N., Baridi A., Xu J., Molin G., Hagslatt M.L., Karlsson C., Jeppsson B., Cilio C.M., Ahrne S. Immunological alteration and changes of gut microbiota after dextran sulfate sodium (DSS) administration in mice. Clin. Exp. Med. 2015;15:107–120. doi: 10.1007/s10238-013-0270-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heijmans J., Wielenga M.C., Rosekrans S.L., van Lidth de Jeude J.F., Roelofs J., Groothuis P., Ederveen A., de Jonge-Muller E.S., Biemond I., Hardwick J.C., D'Haens G., Hommes D.W., Muncan V., van den Brink G.R. Oestrogens promote tumorigenesis in a mouse model for colitis-associated cancer. Gut. 2014;63:310–316. doi: 10.1136/gutjnl-2012-304216. [DOI] [PubMed] [Google Scholar]
- Hodge D.R., Hurt E.M., Farrar W.L. The role of IL-6 and STAT3 in inflammation and cancer. Eur. J. Cancer. 2005;41:2502–2512. doi: 10.1016/j.ejca.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Hong Y.H., Lillehoj H.S., Lee S.H., Dalloul R.A., Lillehoj E.P. Analysis of chicken cytokine and chemokine gene expression following Eimeria acervulina and Eimeria tenella infections. Vet. Immunol. Immunopathol. 2006;114:209–223. doi: 10.1016/j.vetimm.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Isobe N., Nakao T. Direct enzyme immunoassay of progesterone in bovine plasma. Anim. Sci. J. 2003;74:369–373. [Google Scholar]
- Jahnel J., Fickert P., Langner C., Hogenauer C., Silbert D., Gumhold J., Fuchsbichler A., Trauner M. Impact of experimental colitis on hepatobiliary transporter expression and bile duct injury in mice. Liver Int. 2009;29:1316–1325. doi: 10.1111/j.1478-3231.2009.02044.x. [DOI] [PubMed] [Google Scholar]
- Joyner C.J., Peddie M.J., Taylor T.G. The effect of age on egg production in the domestic hen. Gen. Comp. Endocrinol. 1987;65:331–336. doi: 10.1016/0016-6480(87)90117-1. [DOI] [PubMed] [Google Scholar]
- Kaiser P., Hughes S., Bumstead N. The chicken 9E3/CEF4 CXC chemokine is the avian orthologue of IL8 and maps to chicken chromosome 4 syntenic with genes flanking the mammalian chemokine cluster. Immunogenetics. 1999;49:673–684. doi: 10.1007/s002510050664. [DOI] [PubMed] [Google Scholar]
- Kerr T.A., Ciorba M.A., Matsumoto H., Davis V.R., Luo J., Kennedy S., Xie Y., Shaker A., Dieckgraefe B.K., Davidson N.O. Dextran sodium sulfate inhibition of real-time polymerase chain reaction amplification: a poly-A purification solution. Inflamm. Bowel Dis. 2012;18:344–348. doi: 10.1002/ibd.21763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C.J., Kovacs-Nolan J.A., Yang C., Archbold T., Fan M.Z., Mine Y. l-Tryptophan exhibits therapeutic function in a porcine model of dextran sodium sulfate (DSS)-induced colitis. J. Nutr. Biochem. 2010;21:468–475. doi: 10.1016/j.jnutbio.2009.01.019. [DOI] [PubMed] [Google Scholar]
- Kim W.H., Lillehoj H.S., Lim Y., Min W., Sullivan Y.B., Kakach L., LaBresh J.W. Development and characterization of mouse monoclonal antibodies reactive with chicken CXCLi2. Dev. Comp. Immunol. 2017;72:30–36. doi: 10.1016/j.dci.2017.02.008. [DOI] [PubMed] [Google Scholar]
- Klasing K.C. Nutrition and the immune system. Br. Poult. Sci. 2007;48:525–537. doi: 10.1080/00071660701671336. [DOI] [PubMed] [Google Scholar]
- Kushner I. Regulation of the acute phase response by cytokines. Perspect. Biol. Med. 1993;36:611–622. doi: 10.1353/pbm.1993.0004. [DOI] [PubMed] [Google Scholar]
- Kuttappan V.A., Berghman L.R., Vicuna E.A., Latorre J.D., Menconi A., Wolchok J.D., Wolfenden A.D., Faulkner O.B., Tellez G.I., Hargis B.M., Bielke L.R. Poultry enteric inflammation model with dextran sodium sulfate mediated chemical induction and feed restriction in broilers. Poult. Sci. 2015;94:1220–1226. doi: 10.3382/ps/pev114. [DOI] [PubMed] [Google Scholar]
- Kuttappan V.A., Vicuna E.A., Faulkner O.B., Huff G.R., Freeman K.A., Latorre J.D., Menconi A., Tellez G.I., Hargis B.M., Bielke L.R. Evaluation of changes in serum chemistry in association with feed withdrawal or high dose oral gavage with dextran sodium sulfate- (DSS-) induced gut leakage in broiler chickens. Poult. Sci. 2016;95:2565–2569. doi: 10.3382/ps/pew171. [DOI] [PubMed] [Google Scholar]
- Laroui H., Ingersoll S.A., Liu H.C., Baker M.T., Ayyadurai S., Charania M.A., Laroui F., Yan Y., Sitaraman S.V., Merlin D. Dextran sodium sulfate (DSS) induces colitis in mice by forming nano-lipocomplexes with medium-chain-length fatty acids in the colon. PLoS One. 2012;7:e32084. doi: 10.1371/journal.pone.0032084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lensing M., van der Klis J.D., Yoon I., Moore D.T. Efficacy of Saccharomyces cerevisiae fermentation product on intestinal health and productivity of coccidian-challenged laying hens. Poult. Sci. 2012;91:1590–1597. doi: 10.3382/ps.2011-01508. [DOI] [PubMed] [Google Scholar]
- Li J., Leghari I.H., He B., Zeng W., Mi Y., Zhang C. Estrogen stimulates expression of chicken hepatic vitellogenin II and very low-density apolipoprotein II through ER-alpha. Theriogenology. 2014;82:517–524. doi: 10.1016/j.theriogenology.2014.05.003. [DOI] [PubMed] [Google Scholar]
- Liang J., Chen S., Hu Y., Yang Y., Yuan J., Wu Y., Li S., Lin J., He L., Hou S., Zhou L., Huang S. Protective roles and mechanisms of Dendrobium officinal polysaccharides on secondary liver injury in acute colitis. Int. J. Biol. Macromol. 2018;107:2201–2210. doi: 10.1016/j.ijbiomac.2017.10.085. [DOI] [PubMed] [Google Scholar]
- Man A.L., Bertelli E., Rentini S., Regoli M., Briars G., Marini M., Watson A.J., Nicoletti C. Age-associated modifications of intestinal permeability and innate immunity in human small intestine. Clin. Sci. (Lond) 2015;129:515–527. doi: 10.1042/CS20150046. [DOI] [PubMed] [Google Scholar]
- Mappley L.J., Tchorzewska M.A., Nunez A., Woodward M.J., Bramley P.M., La Ragione R.M. Oral treatment of chickens with Lactobacillus reuteri LM1 reduces Brachyspira pilosicoli-induced pathology. J. Med. Microbiol. 2013;62:287–296. doi: 10.1099/jmm.0.051862-0. [DOI] [PubMed] [Google Scholar]
- McNab J. The avian caeca: a review. World Poult. Sci. J. 1973;29:251–263. [Google Scholar]
- Menconi A., Hernandez-Velasco X., Vicuna E.A., Kuttappan V.A., Faulkner O.B., Tellez G., Hargis B.M., Bielke L.R. Histopathological and morphometric changes induced by a dextran sodium sulfate (DSS) model in broilers. Poult. Sci. 2015;94:906–911. doi: 10.3382/ps/pev054. [DOI] [PubMed] [Google Scholar]
- Miura K., Ishioka M., Minami S., Horie Y., Ohshima S., Goto T., Ohnishi H. Toll-like receptor 4 on macrophage Promotes the development of Steatohepatitis-related Hepatocellular Carcinoma in mice. J. Biol. Chem. 2016;291:11504–11517. doi: 10.1074/jbc.M115.709048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar A., Maertens L., Ampe B., Buyse J., Kempen I., Zoons J., Delezie E. Changes in egg quality traits during the last phase of production: is there potential for an extended laying cycle? Br. Poult. Sci. 2016;57:842–847. doi: 10.1080/00071668.2016.1209738. [DOI] [PubMed] [Google Scholar]
- Netea M.G., Nold-Petry C.A., Nold M.F., Joosten L.A., Opitz B., van der Meer J.H., van de Veerdonk F.L., Ferwerda G., Heinhuis B., Devesa I., Funk C.J., Mason R.J., Kullberg B.J., Rubartelli A., van der Meer J.W., Dinarello C.A. Differential requirement for the activation of the inflammasome for processing and release of IL-1beta in monocytes and macrophages. Blood. 2009;113:2324–2335. doi: 10.1182/blood-2008-03-146720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nii T., Isobe N., Yoshimura Y. Effects of avian infectious bronchitis virus antigen on eggshell formation and immunoreaction in hen oviduct. Theriogenology. 2014;81:1129–1138. doi: 10.1016/j.theriogenology.2014.02.002. [DOI] [PubMed] [Google Scholar]
- Nii T., Isobe N., Yoshimura Y. Effects of interleukin-1β and -6 on the expression of ion transporters for mineralization of eggshell in cultured uterine mucosal tissue of hen. J. Poult. Sci. 2018;55:142–149. doi: 10.2141/jpsa.0170138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayasu I., Hatakeyama S., Yamada M., Ohkusa T., Inagaki Y., Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694–702. doi: 10.1016/0016-5085(90)90290-h. [DOI] [PubMed] [Google Scholar]
- Perse M., Cerar A. Dextran sodium sulphate colitis mouse model: traps and tricks. J. Biomed. Biotechnol. 2012;2012:718617. doi: 10.1155/2012/718617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poh T.Y., Pease J., Young J.R., Bumstead N., Kaiser P. Re-evaluation of chicken CXCR1 determines the true gene structure: CXCLi1 (K60) and CXCLi2 (CAF/interleukin-8) are ligands for this receptor. J. Biol. Chem. 2008;283:16408–16415. doi: 10.1074/jbc.M800998200. [DOI] [PubMed] [Google Scholar]
- Qin C., Zhang H., Zhao L., Zeng M., Huang W., Fu G., Zhou W., Wang H., Yan H. Microbiota transplantation reveals beneficial impact of berberine on hepatotoxicity by improving gut homeostasis. Sci. China Life Sci. 2018;61:1537–1544. doi: 10.1007/s11427-017-9202-0. [DOI] [PubMed] [Google Scholar]
- Ricketts C.R. Dextran sulphate–a synthetic analogue of heparin. Biochem. J. 1952;51:5. doi: 10.1042/bj0510129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritzi M.M., Abdelrahman W., Mohnl M., Dalloul R.A. Effects of probiotics and application methods on performance and response of broiler chickens to an Eimeria challenge. Poult. Sci. 2014;93:2772–2778. doi: 10.3382/ps.2014-04207. [DOI] [PubMed] [Google Scholar]
- Saleh M., Trinchieri G. Innate immune mechanisms of colitis and colitis-associated colorectal cancer. Nat. Rev. Immunol. 2011;11:9–20. doi: 10.1038/nri2891. [DOI] [PubMed] [Google Scholar]
- Sato M., Sugino T., Yoshimura Y., Isobe N. Follicular persistence induced by adrenocorticotropic hormone administration in goats. J. Reprod. Dev. 2011;57:212–216. doi: 10.1262/jrd.10-091t. [DOI] [PubMed] [Google Scholar]
- Seol H.S., Sato K., Murakami H., Toyomizu M., Akiba Y. Changes in gene expression involved in energy utilization during chicken follicle development. Anim. Reprod. Sci. 2006;95:283–294. doi: 10.1016/j.anireprosci.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Simon K., Arts J.A., de Vries Reilingh G., Kemp B., Lammers A. Effects of early life dextran sulfate sodium administration on pathology and immune response in broilers and layers. Poult. Sci. 2016;95:1529–1542. doi: 10.3382/ps/pew074. [DOI] [PubMed] [Google Scholar]
- Svihus B., Choct M., Classen H. Function and nutritional roles of the avian caeca: a review. World Poult. Sci. J. 2013;69:249–264. [Google Scholar]
- Tacke R.S., Tosello-Trampont A., Nguyen V., Mullins D.W., Hahn Y.S. Extracellular hepatitis C virus core protein activates STAT3 in human monocytes/macrophages/dendritic cells via an IL-6 autocrine pathway. J. Biol. Chem. 2011;286:10847–10855. doi: 10.1074/jbc.M110.217653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran L., Greenwood-Van Meerveld B. Age-associated remodeling of the intestinal epithelial barrier. J. Gerontol. A. Biol. Sci. Med. Sci. 2013;68:1045–1056. doi: 10.1093/gerona/glt106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walzem R.L., Hansen R.J., Williams D.L., Hamilton R.L. Estrogen induction of VLDLy assembly in egg-laying hens. J. Nutr. 1999;129:467s–472s. doi: 10.1093/jn/129.2.467S. [DOI] [PubMed] [Google Scholar]
- Williams R.B. Intercurrent coccidiosis and necrotic enteritis of chickens: rational, integrated disease management by maintenance of gut integrity. Avian Pathol. 2005;34:159–180. doi: 10.1080/03079450500112195. [DOI] [PubMed] [Google Scholar]
- Wirtz S., Neufert C., Weigmann B., Neurath M.F. Chemically induced mouse models of intestinal inflammation. Nat. Protoc. 2007;2:541–546. doi: 10.1038/nprot.2007.41. [DOI] [PubMed] [Google Scholar]
- Yang S., Suh Y., Choi Y.M., Shin S., Han J.Y., Lee K. Loss of fat with increased adipose triglyceride lipase-mediated lipolysis in adipose tissue during laying stages in quail. Lipids. 2013;48:13–21. doi: 10.1007/s11745-012-3742-6. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Guo F., Ni Y., Zhao R. LPS-induced inflammation in the chicken is associated with CCAAT/enhancer binding protein beta-mediated fat mass and obesity associated gene down-regulation in the liver but not hypothalamus. BMC Vet. Res. 2013;9:257. doi: 10.1186/1746-6148-9-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B., Morgan M.E., van de Kant H.J.G., Garssen J., Folkerts G., Kraneveld A.D. Transcriptional modulation of pattern recognition receptors in chronic colitis in mice is accompanied with Th1 and Th17 response. Biochem. Biophys. Rep. 2017;12:29–39. doi: 10.1016/j.bbrep.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.