Abstract
There is increasing evidence that health and performance of the breeder flock significantly contributes to health and performance of their progeny. Data of broiler performance and health are routinely collected in various stages of the broiler production chain. In the Netherlands, the broiler chain operates at a relatively non-integrated level and the various databases are usually not connected. Connecting databases may however provide important information to improve chain performance. The aim of the present study was to determine systematic effects of broiler breeder production farm or flock on health (mortality and antibiotics use) and performance of their offspring, using data routinely collected at the different stages of the production chain. Broiler flock data collected over 6 yr (daily growth, slaughter weight, carcass weight uniformity, carcass condemnations, first week and total mortality, and antibiotics use) were linked to breeder flocks and farms. In total, 2,174 broiler flock records (at house level) of 74 broiler farms were linked to 88 broiler breeder farms and 209 breeder flocks. A mixed model analysis was applied to simultaneously estimate effects of season, parent flock age, time trend, and the contribution of the different chain phases to broiler performance and health. No systematic effects of breeder farm and only small systematic effects of breeder flock on broiler health and performance were found. The largest breeder flock effect was found for carcass condemnations (estimated contribution to the variance component: 7%). Most variation on broiler health and performance was explained by broiler farm and “day-old chick batch.” The latter refers to the rest variance that could not be explained by other factors, i.e., incidental effects linked to the specific day-old chick batch and the stage between the breeder and broiler farm. Our results suggest that systematic effects of breeder flock and farm could have been overruled by (management in) the hatchery phase and the broiler farm. This indicates room for improvement of management in these production phases.
Key words: broiler, broiler breeder, production chain data, health, performance
INTRODUCTION
Although it is generally known that management and housing on the broiler farm and the conditions during incubation, hatching, and transport of day-old chicks have a large effect on health and production of broiler flocks (e.g., Decuypere et al., 2001; Tona et al., 2003; Yassin et al., 2008, 2009), there is also increasing evidence that housing and management of the parent stock, i.e., the broiler breeders, significantly contribute to welfare, health, and performance of the broiler progeny (Rebel et al., 2004; van der Waaij et al., 2011; Koppenol et al., 2015; Chang et al., 2016; Lesuisse et al., 2018; Li et al. 2018). Hatcheries, nutrition and breeding companies are convinced of the fact that systematic (i.e., repeatable) differences in quality of management between broiler breeder production farms exist that affect broiler production, in addition to effects of breeder age (Peebles et al., 1999) and season (Yassin et al., 2009). An example of systematic breeder farm effects on broiler health and performance are specific breeder farm conditions, such as egg storage conditions on the breeder farm and housing conditions (Decuypere et al., 2001; Tona et al., 2003; Leksrisompong et al., 2014a), whereas breeder flock health and production management (such as age of photostimulation, feeding, and nutrition program) may cause systematic breeder flock effects (Leksrisompong et al., 2014a,b; Urso et al., 2015; van Emous et al., 2018).
Performance and health data (mortality, antibiotics usage) are collected on a routine basis in the broiler production chain, and with developing new (sensor) technologies the amount of data related to performance, health, and welfare of flocks will even further increase (Van Hertem et al., 2017). Slaughter plants register quality, health, welfare, and performance indicators of broiler flocks such as carcass condemnations (Butterworth et al., 2016), carcass uniformity (Vasdal et al., 2019), and footpad dermatitis scores (Butterworth et al., 2016). Broiler farmers register information on growth and mortality of their flocks, hatcheries register data related to the hatching process, such as on hatchability and egg storage time, and breeder production farms register data on performance of their flocks such as egg production, egg weight, growth performance, and mortality. Also national databases are established because of legislation or regulations, such as the database on antibiotics usage in the poultry production chain in the Netherlands (Bos et al., 2013).
However, currently in the Netherlands, in which the broiler production chain operates at a relatively non-integrated level, these data are generally collected and stored solely by the owner and databases are not connected on a routine basis to enable analysis over various production chain phases. These data, however, can be a source of useful information for the production chain and may help chain partners to identify systematic as well as accidental issues affecting broiler and breeder flock health and performance. The information can be used to improve the health and performance of both broiler and breeder flocks, which in turns could improve the economic performance of the whole production chain (Yassin et al., 2008, 2009, 2011).
In contrast to previous studies on transgenerational effects in the broiler production chain that were based on predesigned experimental protocols (e.g., Koppenol et al., 2015; Lesuisse et al., 2018; Rebel et al., 2004), we were interested in whether or not we could find indications of systematic (i.e., repeatable) effects of broiler breeder flock or broiler breeder farm on the performance and health of broiler chickens, using field data routinely collected in the Dutch broiler production chain. In case these systematic effects exist, the results could be a starting point for further research. The aim of the present study therefore was to determine whether or not we could identify systematic effects of broiler breeder flock or farm on the production and health performance of the offspring, based on field data of one conventional, fast-growing broiler breed, registered by chain partners in the broiler production chain over a 6-year period.
MATERIALS AND METHODS
Data Sources
Data were collected by 1 broiler processing plant (with 2 locations), 2 broiler hatcheries, 2 nutrition companies supplying part of the breeder farms that supplied eggs to these hatcheries, 1 breeding company, and by the responsible organization collecting national data on antibiotics usage. Data for the current project were collected from broiler chickens of one conventional fast growing breed and their respective parent flocks. All broiler and breeder farms were located in the Netherlands. Data sources comprised:
-
(1)
Data on broiler flock performance and health stored in the database of the processing plant and collected at depopulation of the flock. This dataset contained records for average daily growth rate, slaughter weight, carcass condemnation percentage (whole carcasses), flock uniformity, and mortality (first week and total mortality). Growth, first week mortality and total mortality were registered by the farmer on the food chain information form that accompanies each flock sent to slaughter. All data were registered per broiler house per farm upon depopulation. In addition, the database contained information on the date of hatch of the broiler flock (which is equal to the date of placement on the broiler farm), an identification of the broiler farm and an identification of the breeder farm;
-
(2)
Data on antibiotic treatments of broiler flocks collected in a national database (Speksnijder et al., 2015); in addition, the database contained information on the broiler house and farm, and the date of hatch of the flock, allowing these data to be linked to the other performance data;
-
(3)
Data on the date of hatch of the breeder flock and the respective breeder farm where the flock was located, and the dates of hatch of the offspring broiler flocks and the broiler farms and houses where these were placed at day old. These data were collected by the hatcheries and breeding company, and used to check the identification of the breeder flock and to check the links between the broiler and breeder farms.
The national registration number of the broiler and breeder flocks, and their dates of hatch were used to connect the datasets and to link each broiler breeder flock to its progeny. After linking the different databases using Microsoft Access software, data were anonymized for further analysis. The data analyzed in the present study were collected over a 6-year period (from 2011 to 2016), to include sufficient subsequent breeder flocks on the same breeder farm in the study.
Definitions
Carcass weight uniformity was registered by slaughterhouse staff according to the quality control standards of the Dutch broiler production chain: the percentage of broilers weighing less than 65% of the arithmetic mean of the slaughter (griller) weight of all broilers supplied (Pluimned, 2017), i.e., a higher percentage meaning a less uniform flock. Use of antibiotics was recorded by veterinarians according to national practice (Speksnijder et al., 2015), and expressed as animals defined daily dosages per year for each broiler flock (addd/y) (addd/y of 1 means that the average animal in the flock was exposed to an antibiotic treatment for 1 D per year; Bos et al., 2013). Carcass condemnation percentages were measured by government veterinarians at the slaughter plant, registered by the plant, and expressed as percentage of whole carcasses not suitable for human consumption (condemnations). This includes condemnations due to disease (e.g., ascites, inflammation, hepatitis, serositis, cachexy), and unacceptable quality (e.g., color, smell). A broiler flock was defined as all broilers from one house at a farm with an identical hatch date. A breeder flock was defined as being all broiler breeders present at one farm at the same time, with an identical hatch date (and could thus comprise more than one breeder house on the same farm).
Number of Records and Data Set
A total of 2,226 records were available in the slaughter plant database for flocks delivered by the 2 hatcheries involved in the study (each record being a broiler flock, i.e., all broilers from 1 house at a farm with an identical hatch date). However, due to missing data, the number of records finally included in the analysis was lower, namely 2,174 records of broiler flocks linked to their parental flocks, 1,985 records containing broiler production and slaughter quality data (daily growth rate, slaughter weight, carcass condemnation percentage, and carcass weight uniformity); 1,978 of these records contained total mortality, and 1,977 of these records also contained first week mortality (Table 1). The 2,174 broiler flock records that could be linked to their parent flock included 74 broiler farms (a farm being defined as having a separate national registration number, i.e., located at one address), 88 breeder farms (a farm being defined as having a separate national registration number, i.e., located at one address), and 209 breeder flocks (a flock being all broiler breeders present at one farm at the same time, with the same date of hatch). Where broiler flocks were separately registered per house at a farm, there was no identification of eggs produced by breeders in a specific house at a farm, so, for the breeders registration was only done at farm level. The database on antibiotics usage of broilers at house level during the same time span from the selected broiler flocks contained 2,156 records (from 70 broiler flocks data were lacking, Table 1). A total of 1,336 broiler flocks (62%) in the antibiotics registration database received no antibiotics, i.e., had an animal defined daily dosage of zero. Separate analyses were performed for flocks with antibiotics usage only (i.e., addd/y >0), and whether or not antibiotics were used (independent of the dosage).
Table 1.
Overview of number of records for the different variables included in the database compared to the total records of broiler flocks, i.e., 2,256 records.
| Variables | Total number of records | Number of missing records |
|---|---|---|
| Broiler flock identification | 2,256 | - |
| Parent flock identification | 2,174 | 52 |
| Antibiotics use of broilers | 2,156 | 70 |
| Daily growth rate, slaughter weight, carcass condemnation percentage and carcass weight uniformity (production variables) | 1,985 | 241 |
| Production variables and total mortality | 1,978 | 278 |
| Production variables, total and first week mortality | 1,977 | 279 |
Statistical Analysis
All analyses were performed using Genstat (version 19, VSN International, Hemel Hempstead, UK). For all variables except the binary variable antibiotics usage yes/no, a mixed model (REML) analysis was performed to simultaneously estimate the fixed effects of season (by Fourier transformation according to Yassin et al. (2009), parent stock age (linear and log-transformed) and year trend (linear, quadratic, and cubic functions), and the effect of the random coefficients of variance (i.e., relative contribution of the broiler breeder farm, broiler breeder flock, broiler farm, house within a broiler farm, combination of broiler breeder/broiler farm, “day-old chick batch,” and house within “day old chick batch,” see below) on the broiler production and health data. The random coefficients of variance include the total rest variance, thus, the variance that could not be explained by effects of season, time and age of the parent stock. This resulted in a quantification of the variance sources, i.e., the broiler breeder farm, broiler breeder flock, broiler farm, house within broiler farm, interaction broiler breeder farm x broiler farm, and rest (“day-old chick batch,” see below for further explanation). Antibiotics usage (addd/y), uniformity percentage, percentage total mortality, and first week mortality were ln + 0.5 transformed. Fixed quadratic and cubic effects of time were removed from the final model when these were not significant.
The statistical model was as follows:
With:
β0: Intercept
β1.1, β1.2, β1.3: linear term, quadratic and cubic term for fixed time (year) trends between 2011 and 2016
t: Time (D) (from first date of hatch in the data set)
β2, β3: Parameters for effect of season (after Fourier transformation of calendar day number, see below)
X1, X2: Sinus , cosinus , d being day number in a year (based on date of hatch of the broilers, thus ranging from 1 to 365). The seasonal sinus wave represents the spring-autumn variation (1st of March vs. 1st of September); the seasonal cosinus wave represents the summer–winter variation (1st of January vs. 1st of June)
β4, β5: Parameters for non-linear effect of parent stock age
A: Estimated parent stock age, 3 wk before the date of hatch of broilers (in weeks minus 18 wk (which is the estimated rearing period duration for broiler breeders)
εi, εij: Random effect of breeder farm i, and breeder flock j (within i)
εk,εkm: Random coefficients of broiler farm k, and broiler house m (within k)
εik: Random coefficients of a specific combination ik of (breeder farm—broiler farm)
εijkl,εijklm: Random coefficients of the day-old chick delivery batch (date of hatch l within the combination breeder production flock—broiler farm), and the specific combination of chick delivery batch and broiler house (record level)
The residual variance (Var {y}) could be split up into the following sources of variance:
Thus, after correction for season, parent stock age, and time trend we estimated:
Var{breeder farm}: estimates the effects of all breeder farms
Var{breeder flock}: estimates the effects of all breeder flocks of the same farm (a breeder flock being an age group of parent birds, all with the same date of hatch, regardless of whether these were kept in one or more houses at the broiler breeder farm)
Var{broiler farm}: estimates the effects of all broiler farms
Var{broiler house}: estimates the effects of the different houses on one broiler farm
Var{interaction (breeder farm; broiler farm)}: estimates the effects for specific combinations of broiler farms and breeder farms
Var{“day-old chick batch”}: estimates incidental effects linked to a specific batch of day-old chicks, presumably influenced by sources of variation in the stage between breeder farm and placement of the day-old chicks on the broiler farm
Var{interaction (“day-old chick batch”; broiler house)}: estimates the effects for a specific batch of day-old chicks which is divided over two broiler houses on the same farm
It should be noted that Var{“day-old chick batch”} and Var {interaction (“day-old chick batch”; broiler house)} estimate the variance in performance of day-old chicks that cannot be explained by the effects of season, time trend, parent stock age, broiler breeder farm, broiler farm, broiler breeder flock, house within broiler farm, and the specific combination of breeder and broiler farm, and thus actually represent the contribution of a specific batch of day-old chicks in the stage between the breeder farm and placement of day-old chicks on the broiler farm.
The ratio between Var{“day-old chick batch”} /Var{“day-old chick batch”}+{interaction(day-old chick batch; broiler house)} provides the estimated correlation (Fisher's Intra Class Correlation) between houses on the same broiler farm where chicks of the same batch of eggs (same breeder farm, same date of hatch) were placed at day-old.
For the variable “antibiotics usage yes/no,” data were first translated into a binomial variable (yes or no antibiotics used), and subsequently analyzed with a General Linear Mixed Model with binomial distribution and logit link, using the same model as defined above.
RESULTS
Time Trends and Effects of Season and Parent Stock Age
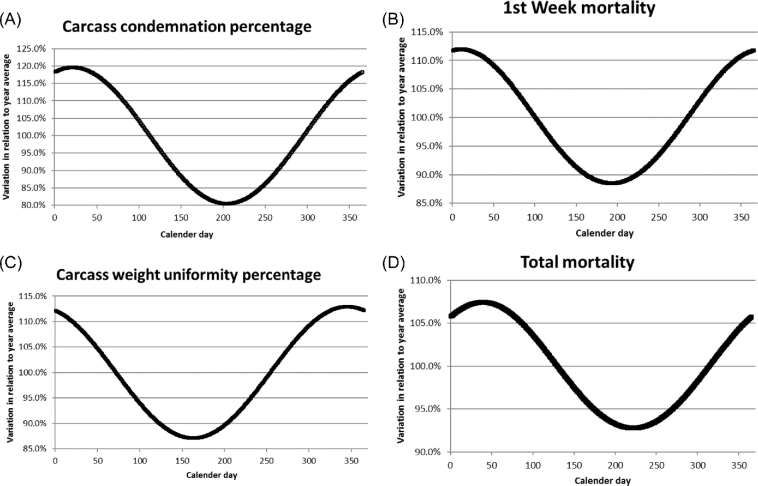
The average effects of season, parent stock age, and time trend (between 2011 and 2016) were estimated for the different health and production parameters on broiler flock level. Table 2 shows the estimations of these fixed effects in the model and whether or not these effects were significant. Significant effects of time trend were found for all health and production parameters on broiler level except for first week mortality, for which a tendency for a time effect was found. Average daily growth showed a quadratic increase over time (Table 2). Slaughter weight and carcass weight uniformity first decreased and then increased over time; carcass condemnation percentage increased over time (Table 2). For carcass weight uniformity, this means that it first improved and later on became worse. The significant cubic component represents a correction on the increasing or decreasing time trend (because a relatively large time interval was taken into account). Total mortality and first week mortality showed a significant increase over time, whereas use of antibiotics significantly decreased over time (Table 2). Significant effects of season were found for carcass condemnation percentage, carcass weight uniformity, and first week and total mortality. A tendency for a seasonal effect was found for slaughter weight and average daily growth. Figure 1 graphically presents seasonal effects in case these were significant. Significant effects of parent stock age were found for average daily growth and slaughter weight, and first week mortality. Figure 2 shows significant parent stock age effects.
Table 2.
Estimates of the fixed effects in the statistical model, i.e., of time trend, season and parent stock age, per parameter on broiler flock level. † significant effect (P < 0.05 at least); # trend (P < 0.10); where no superscript is provided no significant effect or trend was found. β1–5 Refers to the respective terms in the statistical model, see materials and methods section and footnotes under the table.
| β1.1 Time trend (Linear) | β1.2 Time trend (Quadratic) | β1.3 Time trend (Cubic)1 | β2 Season (Sinus)2 | β3 Season (Cosinus)3 | β4 Parent stock age (Linear) | β5 Parent stock age (Loglineair) | |
|---|---|---|---|---|---|---|---|
| Average daily growth | −5.68* 10−4† | 1.81* 10−6† | 3 | 0.03 | 0.20# | −0.04 | 2.25† |
| Slaughter weight | −2.23* 10−4† | 4.41* 10−7† | −1.35* 10−10† | 5.27* 10−3 | 0.01# | −7.13* 10−4 | 6.46† |
| Carcass condemnation % | 5.42* 10−6† | 3 | 3 | 1.81* 10−3† | 4.61* 10−3† | 6.21* 10−5 | −4.82* 10−4 |
| Carcass weight uniformity %4 | −1.86* 10−5,† | 3.67* 10−8 | −1.51* 10−11† | 7.07* 10−4 | 1.46* 10−3† | −1.21* 10−4 | 1.73* 10−3 |
| First week mortality % | 6.84* 10−5,# | 3 | 3 | 0.01 | 0.07† | 8.83* 10−3† | −0.233† |
| Total mortality % | 1.04* 10−4† | 3 | 3 | 0.04† | 0.05† | 5.02* 10−3 | −0.10 |
| Antibiotics usage (addd/y)5 | −4.61* 10−4† | 3 | 3 | 0.02 | −0.01 | −3.56* 10−3 | −0.02 |
The seasonal sinus wave represents the spring-autumn variation (1st of March vs. 1st of September).
The seasonal cosinus wave represents the summer–winter variation (1st of January vs. 1st of June).
Non-significant quadratic and cubic time effects have not been fitted in the final model.
Uniformity: % carcasses with a griller weight lower than 65% of the average griller weight of a broiler flock, i.e., a higher percentage meaning a less uniform flock.
addd/y: animals defined daily dosages per year (Bos et al., 2013).
Figure 1.
Estimated seasonal effects for percentages of (A) carcass condemnation, (B) carcass weight uniformity, (C) first week mortality, and (D) total mortality of the broiler flocks. The X-axis represents the day of the year when day-old chickens are placed in the broiler house, starting at the 1st of January (Day 1). The Y-axis shows the variation in relation to the year average, which is set at 100%. Note: a higher uniformity percentage means a less uniform flock.
Figure 2.
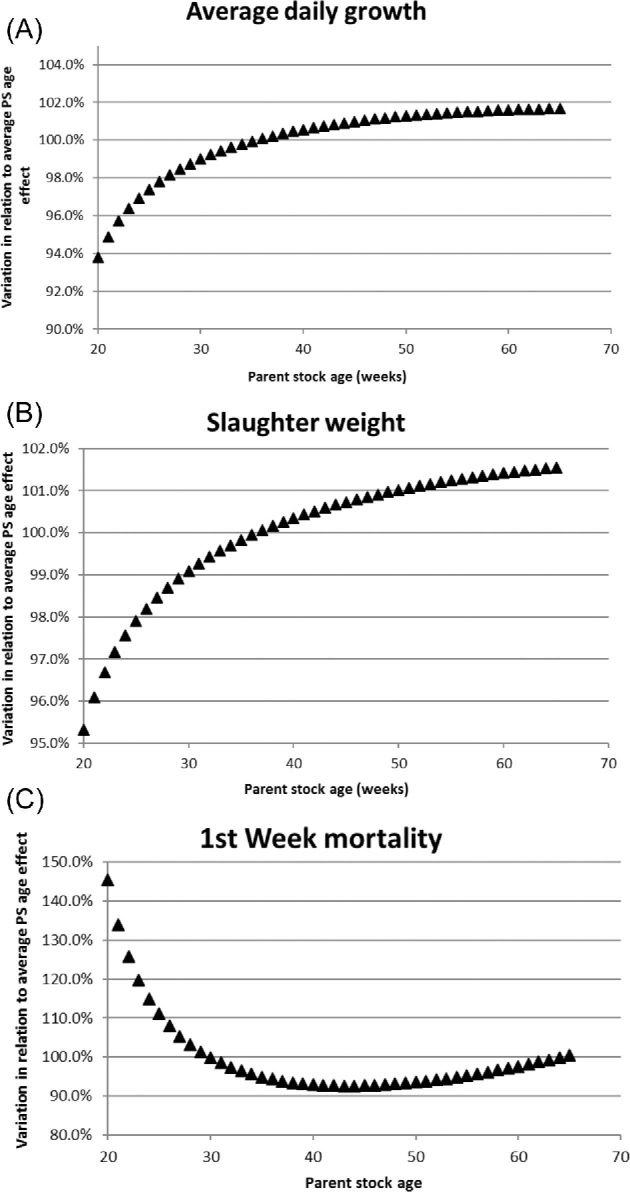
Estimated parent stock age effects for daily growth rate (A), slaughter weight (B) and first week mortality percentage (C). The X-axis represents the parent stock age in weeks, the Y-axis shows the variation in relation to the average parent stock age effect, which is set at 100%. PS: Parent stock.
Relative Contribution of Chain Phases to Broiler Flock Health and Performance
Table 3 presents the estimated contribution of the different phases in the production chain to the variance in health and production indicators on broiler flock level, which is determined by the variance components of the random variables. The variance components are presented in Table S1 in Supplementary Material. Taking into consideration the significant effects of time, season, and parent flock age, no systematic contribution of the broiler breeder farm and only small contributions of broiler breeder flock on the health and performance indicators of broiler flocks was found. The largest systematic effects of the broiler breeder flock were found for carcass condemnation percentage (7%) and carcass weight uniformity (5%) of the offspring, and smaller effects for first week and total mortality (4%) (Table 3).
Table 3.
Estimations of the relative size of the variance components for the different broiler chain phases. BB Farm: broiler breeder farm; BB Flock: broiler breeder flock (all birds of same age group); B Farm: broiler farm; CB: “day-old chick batch”; see also table footnotes and materials and methods section for further explanation.
| BB farm (%) | BB flock (%) | B farm (%) | House within B farm (%) | BB/B farm combination (%) | CB (%)1 | House within CB (%)1 | Fisher's ICC2 | |
|---|---|---|---|---|---|---|---|---|
| Average daily growth (g) | 0 | 2 | 40 | 2 | 2 | 33 | 20 | 0.63 |
| Slaughter weight (kg) | 0 | 2 | 32 | 0 | 0 | 37 | 29 | 0.56 |
| Carcass condemnation % | 0 | 7 | 15 | 1 | 0 | 27 | 50 | 0.35 |
| Carcass weight uniformity %3 | 0 | 5 | 24 | 5 | 0 | 38 | 29 | 0.57 |
| First week mortality % | 0 | 4 | 18 | 0 | 2 | 42 | 33 | 0.55 |
| Total mortality % | 0 | 4 | 32 | 1 | 0 | 40 | 23 | 0.63 |
| Antibiotics usage, (ddda/year)4 | 0 | 2 | 14 | 1 | 0 | 52 | 31 | 0.63 |
| Antibiotics use (yes/no)5 | 0 | 3 | 35 | 0 | 0 | 27 | 34 | 0.45 |
| Level of antibiotics use >0 (addd/year)4,6 | 0 | 0 | 4 | 2 | 0 | 42 | 51 | 0.45 |
Columns indicated with CB (“day-old chick batch”) and House within CB (House within “day-old chick batch”): “day-old chick batch” indicates the relative variance in broiler flock health and performance that cannot be explained by average seasonal, time trend and parent stock age effects (see Table 2), average effects of BB flock and farm, average effects of B farm and house within broiler farm, and average effect of the specific combination of BB flock and B farm. These columns CB and House within CB thus include the rest variance, i.e., incidental effects linked to the specific day-old chick batch and the stage between breeder farm and placement of day-old chicks on the broiler farm (see Materials and Methods).
Fisher's ICC (Intra Class Correlation) provides the correlation between two houses on the same farm, for chickens from the same BB flock and the same date of hatch, for the different indicators of health and performance at B flock level.
Carcass uniformity: % chickens with a griller weight lower than 65% of the average griller weight of a broiler flock, i.e., a higher percentage meaning a less uniform flock.
addd/y: animals defined daily dosages per year (Bos et al., 2013).
In a substantial amount of B flocks (houses) no antibiotics were applied. This analysis shows the variance components for the binomial (yes/no) variable “antibiotics usage in a broiler flock.”
This analysis provides the relative variance components for only the flocks in which antibiotics were applied (addd/y >0) (see also Materials and Methods).
Broiler farm, “day-old chick batch,” and house within “day-old chick batch” represented the largest contribution to the variance in health and performance on broiler level (Table 3). “Day-old chick batch” represents the incidental effects that were linked to a specific batch of day-old chickens and the stage between breeder farm and broiler flock. The largest systematic effects of “day-old chick batch” were found for first week and total mortality (42 resp. 40%), and use of antibiotics in broiler flocks (52% for whether or not antibiotics were used; 42% for the level of antibiotics use in case addd/y>0) (Table 3). The largest systematic broiler farm effects were found for average daily growth (40%) and whether or not antibiotics were applied in the broiler flocks (binary variable, 35%). Systematic effects of house on the broiler farm within a specific “day-old chick batch” were relatively large (Table 3). By contrast, there were only very small systematic effects of broiler house (when not within the same “day-old chick batch”). Only very small effects were also found for specific combinations of breeder farm and broiler farm (Column ɛik in Table 3; but note that the number of repeated breeder-broiler farm combinations was relatively small). Fishers ICC (Table 3) shows that there is a moderate correlation between performance of chickens from the same origin (same broiler breeder flock and date of hatch, i.e., “day-old chick batch”) placed in different houses on the same farm. The lowest correlation was found for carcass condemnation percentage (0.35; Table 3), the highest correlations were found for average daily growth, total mortality and antibiotics usage (all 0.63).
DISCUSSION
The present analysis of field data collected over a 6-year period by commercial partners in the Dutch broiler production chain showed that systematic effects of breeder production farm and broiler breeder flock on broiler health (mortality, antibiotics usage) and performance were absent or relatively small. Broiler farm, “day-old chick batch” (incidental effects linked to the specific batch of day-old chicks and the stage between breeder farm and broiler farm), and house on the broiler farm within “day-old chick batch” had the largest effects on health and production of broiler flocks. These findings do not support our hypothesis that systematic effects of broiler breeder farm and broiler breeder flock on broiler production and health indicators may be substantial and could be found in routinely collected production chain data. However, the results do not exclude the possibility that more substantial systematic effects of breeder production farm or flock on broiler health and performance exist. The current results indicate that the effects of other phases in the production chain (hatchery, broiler farm) have been larger and seem to overrule systematic effects of the preceding broiler breeder phase. It should further be pointed out that effects of accidental issues, such as a disease in a breeder flock, would probably only affect part of the performance of the offspring of the breeders and might therefore not be found in the present analysis that focused on systematic effects of breeder farm and flock. Such temporary effects were appropriately included in “day-old chick batch” effects. Finally, we included performance parameters of broiler flocks that were recorded by the plant, but it cannot be excluded that larger breeder farm or flock effects could have been found on other performance indicators such as cumulative feed conversion in the broiler flocks.
Although systematic breeder farm effects were absent, we found small systematic breeder flock effects on carcass condemnation and carcass weight uniformity in broiler flocks at the slaughter plant, and on broiler first week and total mortality (and very small effects on growth, slaughter weight, and antibiotics use). Management practices on the breeder farm, such as pre-peak feeding and photostimulation programs, may affect egg quality and as a result day-old chicken quality, (Robinson et al., 1996; Hudson et al., 2001; Chang et al., 2016), and as a result carcass condemnations, carcass weight uniformity, and mortality levels of the offspring. Yassin et al. (2009) found a significant effect of feed company of the breeder farm on first week mortality, confirming the importance of broiler breeder nutrition in relation to day-old chick quality. In predesigned experimental setups, nutrition of the breeder flocks was shown to be related to mortality, immune status (Enting et al., 2007), and growth performance (Enting et al., 2007; Van Emous et al., 2015; Lesuisse et al., 2018) of the offspring, but contrasts between treatments were larger as compared to commercial practice. These management practices may interact with broiler breeder flock aspects, such as body weight uniformity at the start of the production period which is found to affect egg weight uniformity and as a result body weight uniformity of the progeny (e.g., Petitte et al., 1982; Hudson et al., 2001). Despite the small effects, our findings could be a starting point for a more in-depth analysis of the relationship between broiler breeder flock characteristics and production management, and broiler performance, and how to adjust management both on breeder and broiler level to improve health and performance. The fact that we did not find any systematic breeder farm effects but small systematic breeder flock effects at least suggests that specific breeder flock characteristics and their interaction with farm management seem to be more important in relation to broiler health and performance than specific breeder farm characteristics more generally.
The relatively large systematic effects of “day-old chick batch” on carcass weight uniformity, carcass condemnation percentage, first week and total mortality, suggest a relatively large effect of the conditions during egg storage, incubation, hatching, handling, and transport on broiler performance and health. In addition to the management on the broiler farm, day-old chick quality is an important factor for the final performance of a broiler flock (Bergoug et al., 2013). It is well known that even when all conditions for good quality hatching eggs are fulfilled, egg storage, incubation, and hatching conditions and post-hatch handling are crucial to produce good quality day-old chicks (Decuypere et al., 2001; Willemsen et al., 2010; Bergoug et al., 2013; de Jong et al., 2017) and affect post-hatch performance of broilers (Decuypere and Bruggeman, 2005; Tona et al., 2005). With respect to first week mortality, Yassin et al. (2009) found a large effect of the specific hatchery and egg storage length in their analysis of field data, which is in line with the results of the present study. Our results thus support the importance of factors between egg-laying and placement on the broiler farm, and suggest that broiler performance can be largely improved by further optimizing these conditions in practice. It is therefore advised to include hatchery records in future production chain data analysis.
We found considerable systematic effects of broiler farm and house (on the same farm) within “day-old chick batch” on growth rate, slaughter weight, mortality and use of antibiotics. Without doubt farm management plays an important role in relation to final performance; this comprises factors such as feed composition and feeding regimes, temperature and relative humidity profiles, light schedules, but also detecting health problems and decisions whether or not to apply antibiotics or other health treatments in a flock (Averos and Estevez, 2018). Effects of a specific house on the performance of a “day-old chick batch” may refer to differences in climate (control), pathogen load, heating, ventilation, age of the building, and other aspects that may differ between broiler houses on the same farm and under similar management, that are generally known by farmers. The differences between houses on the same farm are also reflected by the fact that correlations (Fishers ICC) were only moderate between houses on the same farm when chickens from the same batch are distributed over different houses. In contrast, house effects are almost absent for chickens from different origin, indicating that in other factors than house effect (such as farm and day-old chick batch effects) play a more important role in explaining the variation in health and performance. Although the effects of farm management on broiler health and production are well known, the relatively large effects of broiler farm on several production and health indicators suggest that there is still room for improvement on individual farms, both in management and housing aspects.
Significant time trends between 2011 and 2016 were found for nearly all broiler production and health indicators (only a tendency was found for first week mortality). The start of the registration in a national database in with the purpose of benchmarking and defining targets for veterinary consumption of antibiotics by the government played an important role in the amounts of antibiotics used on farms (Bos et al., 2013). Use of antibiotics in the broiler production chain showed a sharp decrease over the years, both on broiler farms and broiler breeder farms (Stichting Autoriteit Diergeneesmiddelen, 2017). This also explains the relatively large proportion of farms with a defined daily dose of zero in the database. The significant linear decrease in use of antibiotics over time may explain the increase in total mortality, first week mortality and carcass condemnations at the slaughter plant. Farmers may wait longer to apply antibiotics, or apply narrow spectrum antibiotics instead of broad spectrum antibiotics when their flock shows a health problem, as they aim to stay under the defined targets set by the government. Moreover, preventive application of antibiotic treatments was banned. Finally, the significant quadratic increase over time in daily growth rate may represent the genetic progress over the years (Zuidhof et al., 2014) whereas the variation in slaughter weight may represent market trends.
A significant effect of parent stock age was found on average daily growth rate and slaughter weight, showing an increase with increasing parent stock age which is in line with earlier studies (Jacobs et al., 2017). Older breeders generally produce larger eggs, and as a result heavier day-old chicks (Wilson, 1991; Nangsuay et al., 2011), which in turn results in a heavier daily weight gain and slaughter weight (Jacobs et al., 2017). The effect of parent stock age on first week mortality confirmed previous research using field data collected in the Netherlands (Yassin et al., 2009). Increased first week mortality was found in young breeders. It was explained by the relatively low egg weights in young breeders, leading to less available nutrients, as well as a reduced mobilization of energy from the yolk sac (Wilson, 1991). Increased first week mortality in older breeders, on the other hand, could be explained by a generally worse chick quality (bad navel quality and navel-yolk sac infections) and the fact that they hatch earlier, increasing the risk of dehydration and a longer period until first feeding, which in turn increases the risk for mortality (Bergoug et al., 2013; de Jong et al., 2017).
Carcass condemnations at the plant, carcass weight uniformity, first week and total mortality all showed a significant seasonal variation, with the best performance in flocks placed in the broiler house around the summer season (i.e., just before the summer for carcass weight uniformity, during or just after the summer for condemnations, first week and total mortality). Yassin et al. (2009) related the lower first week mortality during the summer season to an improved climate, and this may also hold true for flock body weight and thus carcass weight uniformity (Gous, 2018). A relationship between flock health (i.e., low mortality), carcass weight uniformity and carcass condemnation percentages has been suggested (Vasdal et al., 2019). Healthy flocks usually show a more uniform growth and lower condemnation percentage at slaughter, whereas during the more cold and wet seasons, usually more health problems occur, for instance due to suboptimal environmental conditions in the broiler house.
A possible shortcoming of the present analysis is that we only used data collected at depopulation of the broiler flocks, and we did not take into account a possible effect of thinning frequency and the actual proportion of birds being thinned. Although not registered, it is likely that the majority of flocks have been thinned at least once as this is more or less standard practice on Dutch broiler farms rearing conventional, fast growing breeds. It can, however, not be excluded that the proportion of birds being thinned and the frequency of thinning were a possible additional source of variation in the data, that were not taken into account. We also did not have records of the specific veterinarian assessing carcass condemnations, being a possible source of variation which was not taken into account in the present analysis.
The present analysis was performed using data of a relatively non-integrated broiler production chain. The major focus of chain partners, therefore, is probably to optimize their own results instead of aiming at optimal performance of the whole chain. Moreover, communication between chain partners may be suboptimal, leading to management practices not adjusted to the actual status of the breeder flock, egg quality, and day-old chick quality. As a consequence, the present results may not be fully translatable to more integrated production chains, and it would be valuable to perform a similar study in a fully integrated production chain.
In conclusion, our analysis of field data in a relatively non-integrated broiler production chain in the Netherlands showed large systematic effects on indicators of broiler health and performance of broiler farm and “day-old chick batch” (incidental effects linked to the specific batch of day-old chickens and the stage between breeder farm and broiler farm). These results indicate there is (still) room for improvement in these stages of the production chain. Although there is increasing evidence that broiler breeder management may important for broiler performance as well, we found no systematic effects of breeder farm and only a small systematic effect of breeder flock on broiler performance. The possible systematic effects of breeder flock or farm seem to have been overruled by (management at) the hatchery phase and the broiler farm.
ACKNOWLEDGEMENTS
The present study was financed by the Public-private partnership project “Healthy broiler chain,”TKI-AF-14216 (BO-23.05-001-013). Marc Bracke, Annemarie Rebel and Rick van Emous (Wageningen Livestock Research) are acknowledged for reviewing the draft version of the paper.
Footnotes
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.3382/ps/pez562.
Table S1. Variance components ± standard error for the different broiler chain phases as provided by the statistical models. BB Farm: broiler breeder farm; BB Flock: broiler breeder flock (all birds of same age group); B Farm: broiler farm; CB: “day-old chick batch”; see also table footnotes and materials and methods section for further explanation.
Supplementary Material
REFERENCES
- Averos X., Estevez I. Meta-analysis of the effects of intensive rearing environments on the performance and welfare of broiler chickens. Poult. Sci. 2018;97:3767–3785. doi: 10.3382/ps/pey243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergoug H., Burel C., Guinebretiere M., Tong Q., Roulston N., Romanini C.E.B., Exadaktylos V., McGonnell I.M., Demmers T.G.M., Verhelst R., Bahr C., Berckmans D., Eterradossi N. Effect of pre-incubation and incubation conditions on hatchability, hatch time and hatch window, and effect of post-hatch handling on chick quality at placement. Worlds Poult. Sci. J. 2013;69:313–334. [Google Scholar]
- Bos M.E.H., Taverne F.J., van Geijlswijk I.M., Mouton J.W., Mevius D.J., Heederik D.J.J. Consumption of antimicrobials in pigs, veal calves, and broilers in The Netherlands: quantitative results of nationwide collection of data in 2011. Plos ONE. 2013;8 doi: 10.1371/journal.pone.0077525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth A., de Jong I.C., Keppler C., Knierim U., Stadig L., Lambton S. What is being measured, and by whom? Facilitation of communication on technical measures amongst competent authorities in the implementation of the European Union Broiler Directive (2007/43/EC) Animal. 2016;10:302–308. doi: 10.1017/S1751731115001615. [DOI] [PubMed] [Google Scholar]
- Chang A., Halley J., Silva M. Can feeding the broiler breeder improve chick quality and offspring performance? Anim. Prod. Sci. 2016;56:1254–1262. [Google Scholar]
- de Jong I.C., van Riel J., Bracke M.B.M., van den Brand H. A ‘meta-analysis’ of effects of post-hatch food and water deprivation on development, performance and welfare of chickens. PLoS ONE. 2017;12 doi: 10.1371/journal.pone.0189350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decuypere E., Bruggeman V. Endocrine aspects of development: new challenges for the control of incubation process. Worlds Poult. Sci. J. 2005;61:278–283. [Google Scholar]
- Decuypere E., Tona K., Bruggeman V., Bamelis E. The day-old chick: a crucial hinge between breeders and broilers. Worlds Poult. Sci. J. 2001;57:127–138. [Google Scholar]
- Enting H., Boersma W.J.A., Cornelissen J., van Winden S.C.L., Verstegen M.W.A., van der Aar P.J. The effect of low-density broiler breeder diets on performance and immune status of their offspring. Poult. Sci. 2007;86:282–290. doi: 10.1093/ps/86.2.282. [DOI] [PubMed] [Google Scholar]
- Gous R.M. Nutritional and environmental effects on broiler uniformity. Worlds Poult. Sci. J. 2018;74:21–33. [Google Scholar]
- Hudson B.P., Lien R.J., Hess J.B. Effects of body weight uniformity and pre-peak feeding programs on broiler breeder hen performance. J. Appl. Poult. Res. 2001;10:24–32. [Google Scholar]
- Jacobs L., Delezie E., Duchateau L., Goethals K., Ampe B., Buyse J., Tuyttens F.A.M. Impact of transportation duration on stress responses in day-old chicks from young and old breeders. Res. Vet. Sci. 2017;112:172–176. doi: 10.1016/j.rvsc.2017.04.015. [DOI] [PubMed] [Google Scholar]
- Koppenol A., Delezie E., Wang Y., Franssens L., Willems E., Ampe B., Buyse J., Everaert N. Effects of maternal dietary EPA and DHA supplementation and breeder age on embryonic and post-hatch performance of broiler offspring. J. Anim. Physiol. Anim. Nutr. 2015;99:36–47. doi: 10.1111/jpn.12308. [DOI] [PubMed] [Google Scholar]
- Leksrisompong N., Romero-Sanchez H., Oviedo-Rondon E.O., Brake J. Effect of feeder space during the growing and laying periods and the rate of feed increase at the onset of lay on broiler breeder female reproductive function. Poult. Sci. 2014;93:1599–1607. doi: 10.3382/ps.2013-03277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leksrisompong N., Romero-Sanchez H., Oviedo-Rondon E.O., Brake J. Effects of feeder space allocations during rearing, female strain, and feed increase rate from photostimulation to peak egg production on broiler breeder female performance. Poult. Sci. 2014;93:1045–1052. doi: 10.3382/ps.2013-03219. [DOI] [PubMed] [Google Scholar]
- Lesuisse J., Schallie S., Li C., Bautil A., Li B., Leblois J., Buyse J., Everaert N. Multigenerational effects of a reduced balanced protein diet during the rearing and laying period of broiler breeders. 2. Zootechnical performance of the F1 broiler offspring. Poult. Sci. 2018;97:1666–1676. doi: 10.3382/ps/pey014. [DOI] [PubMed] [Google Scholar]
- Li C., Lesuisse J., Schallier S., Climaco W., Wang Y., Bautil A., Everaert N., Buyse J. The effects of a reduced balanced protein diet on litter moisture, pododermatitis and feather condition of female broiler breeders over three generations. Animal. 2018;12:1493–1500. doi: 10.1017/S1751731117002786. [DOI] [PubMed] [Google Scholar]
- Nangsuay A., Ruangpanit Y., Meijerhof R., Attamangkune S. Yolk absorption and embryo development of small and large eggs originating from young and old breeder hens. Poult. Sci. 2011;90:2648–2655. doi: 10.3382/ps.2011-01415. [DOI] [PubMed] [Google Scholar]
- Peebles E.D., Doyle S.M., Pansky T., Gerard P.D., Latour M.A., Boyle C.R., Smith T.W. Effects of breeder age and dietary fat an subsequent broiler performance. 1. Growth, mortality, and feed conversion. Poult. Sci. 1999;78:505–511. doi: 10.1093/ps/78.4.505. [DOI] [PubMed] [Google Scholar]
- Petitte J.N., Hawes R.O., Gerry R.W. The influence of flock uniformity on the reproductive performance of broiler breeder hens housed in cages and floor pens. Poult. Sci. 1982;61:2166–2171. doi: 10.3382/ps.0612166. [DOI] [PubMed] [Google Scholar]
- Pluimned IKB Kip version 4. 2017. http://pluimned.avined.nl/sites/pluimned.avined.nl/files/8-broiler_evaluation_system_ikb_kip_version_4.pdf
- Rebel J.M.J., van Dam J.T.P., Zekarias B., Balk F.R.M., Post J., Minambres A.F., ter Huurne A. Vitamin and trace mineral content in feed of breeders and their progeny: effects of growth, feed conversion and severity of malabsorption syndrome of broilers. Br. Poult. Sci. 2004;45:201–209. doi: 10.1080/00071660410001715803. [DOI] [PubMed] [Google Scholar]
- Robinson F.E., Wautier T.A., Hardin R.T., Robinson N.A., Wilson J.L., Newcombe M., McKay R.I. Effects of age at photostimulation on reproductive efficiency and carcass characteristics. 1. Broiler breeder hens. Can. J. Anim. Sci. 1996;76:275–282. [Google Scholar]
- Speksnijder D.C., Mevius D.J., Bruschke C.J.M., Wagenaar J.A. Reduction of veterinary antimicrobial use in the Netherlands. The Dutch success model. Zoonoses Public Health. 2015;62:79–87. doi: 10.1111/zph.12167. [DOI] [PubMed] [Google Scholar]
- Stichting Autoriteit Diergeneesmiddelen Het gebruik van antibiotica bij landbouwhuisdieren in 2017, SDa/1152/2018, Utrecht, The Netherlands. 2017. https://cdn.i-pulse.nl/autoriteitdiergeneesmiddelen/userfiles/sda%20jaarrapporten%20ab-gebruik/sda-rapport-2017.pdf
- Tona K., Bamelis F., De Ketelaere B., Bruggeman V., Moraes V.M.B., Buyse J., Onagbesan O., Decuypere E. Effects of egg storage time on spread of hatch, chick quality, and chick juvenile growth. Poult. Sci. 2003;82:736–741. doi: 10.1093/ps/82.5.736. [DOI] [PubMed] [Google Scholar]
- Tona K., Bruggeman V., Onagbesan O., Bamelis F., Gbeassor M., Mertens K., Decuypere E. Day-old chick quality: Relationship to hatching egg quality, adequate incubation practice and prediction of broiler performance. Avian Poult. Biol. Rev. 2005;16:109–119. [Google Scholar]
- Urso U.R.A., Dahlke F., Maiorka A., Bueno I.J.M., Schneider A.F., Surek D., Rocha C. Vitamin E and selenium in broiler breeder diets: effect on live performance, hatching process, and chick quality. Poult. Sci. 2015;94:976–983. doi: 10.3382/ps/pev042. [DOI] [PubMed] [Google Scholar]
- van der Waaij E.H., van den Brand H., van Arendonk J.A.M., Kemp B. Effect of match or mismatch of maternal-offspring nutritional environment on the development of offspring in broiler chickens. Animal. 2011;5:741–748. doi: 10.1017/S1751731110002387. [DOI] [PubMed] [Google Scholar]
- van Emous R.A., de la Cruz C.E., Naranjo V.D. Effects of dietary protein level and age at photo stimulation on reproduction traits of broiler breeders and progeny performance. Poult. Sci. 2018;97:1968–1979. doi: 10.3382/ps/pey053. [DOI] [PubMed] [Google Scholar]
- van Emous R.A., Kwakkel R.P., van Krimpen M.M., van den Brand H., Hendriks W.H. Effects of growth patterns and dietary protein levels during rearing of broiler breeders on fertility, hatchability, embryonic mortality, and offspring performance. Poult. Sci. 2015;94:681–691. doi: 10.3382/ps/pev024. [DOI] [PubMed] [Google Scholar]
- Van Hertem T., Rooijakkers L., Berckmans D., Fernandez P.A., Norton T., Berckmans D., Vranken E. Appropriate data visualisation is key to Precision Livestock Farming acceptance. Comput. Electron. Agric. 2017;138:1–10. [Google Scholar]
- Vasdal G., Granquist E.G., Skjerve E., de Jong Ingrid C., Berg C., Michel V., Moe R.O. Associations between carcass weight uniformity and production measures on farm and at slaughter in commercial broiler flocks. Poult. Sci. 2019;98:4261–4268. doi: 10.3382/ps/pez252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willemsen H., Debonne M., Swennen Q., Everaert N., Careghi C., Han H., Bruggeman V., Tona K., Decuypere E. Delay in feed access and spread of hatch: importance of early nutrition. Worlds Poult. Sci. J. 2010;66:177–188. [Google Scholar]
- Wilson H.R. Interrelationships of egg size, chick size, posthatching growth and hatchability. Worlds Poult. Sci. J. 1991;47:5–20. [Google Scholar]
- Yassin H., Velthuis A.G.J., Boerjan M., Lourens A., Lansink A. Standardized data in the broiler value chain. Poult. Sci. 2011;90:498–506. doi: 10.3382/ps.2010-00820. [DOI] [PubMed] [Google Scholar]
- Yassin H., Velthuis A.G.J., Boerjan M., van Riel J. Field study on broilers' first-week mortality. Poult. Sci. 2009;88:798–804. doi: 10.3382/ps.2008-00292. [DOI] [PubMed] [Google Scholar]
- Yassin H., Velthuis A.G.J., Boerjan M., van Riel J., Huirne R.B.M. Field study on broiler eggs hatchability. Poult. Sci. 2008;87:2408–2417. doi: 10.3382/ps.2007-00515. [DOI] [PubMed] [Google Scholar]
- Zuidhof M.J., Schneider B.L., Carney V.L., Korver D.R., Robinson F.E. Growth, efficiency, and yield of commercial broilers from 1957, 1978, and 2005. Poult. Sci. 2014;93:2970–2982. doi: 10.3382/ps.2014-04291. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.