Abstract
Variation in egg production exists in commercial turkey hens, with low egg producing hens (LEPH) costing more per egg produced than high egg producing hens (HEPH). Egg production correlates with ovulation frequency, which is governed by the hypothalamic–pituitary–gonadal (HPG) axis. Ovulation is stimulated by a preovulatory surge (PS) of progesterone and luteinizing hormone, triggered by gonadotropin releasing hormone release and inhibited by gonadotropin inhibiting hormone. Differences between LEPH and HEPH were characterized by determining HPG axis plasma hormone profiles and mRNA levels for key genes, both outside and inside of the PS (n = 3 per group). Data were analyzed with a 2-way ANOVA using the mixed models procedure of SAS. In the HPG axis, plasma progesterone levels were not affected by egg production level but were elevated during the PS. In contrast, plasma estradiol levels were higher in HEPH than in LEPH but were not associated with the PS. LEPH exhibited decreased gene expression associated with ovulation stimulation and increased gene expression associated with ovulation inhibition in the hypothalamus and pituitary. In ovarian follicle cells, LEPH displayed decreased gene expression associated with progesterone, androgen, and estradiol production in the F1 follicle granulosa cells, F5 theca interna cells, and small white follicle cells, respectively. Different degrees of stimulation and inhibition within all tissues of the HPG axis were noted between LEPH and HEPH turkey hens, with HEPH showing higher expression of genes related to ovulation and steroidogenesis.
Keywords: egg production, gene expression, hypothalamo–pituitary–gonadal axis, ovulation, steroid hormone
INTRODUCTION
The turkey industry focuses on meat production and has selected heavily for carcass traits over the past 40 yr (Nestor et al., 2008). Selection for carcass traits has negatively affected egg production, causing a reduction in the number of poults per turkey breeding hen (McCartney et al., 1968). Although meat production is the priority of the turkey industry, the number of eggs laid per hen greatly impacts the number of turkey poults that can be reared. In addition to lower overall egg production, there is large variation in egg production within a single commercial flock, creating 2 distinct levels of egg production, low egg producing hens (LEPH) and high egg producing hens (HEPH). LEPH cost more per egg produced than HEPH, which strains the turkey industry.
Egg production begins with follicle ovulation from the ovary, making ovulation frequency and egg production highly correlated. The hypothalamo–pituitary–gonadal (HPG) axis governs the hen's reproductive system and directly regulates ovulation, ultimately regulating egg production. Ovulation is triggered by a preovulatory surge (PS) of progesterone and luteinizing hormone (LH) roughly 8 to 10 h before each ovulation (Yang et al., 1997). The HPG axis can be negatively or positively regulated at the level of the hypothalamus, pituitary, or ovary to impact ovulation timing (Bédécarrats et al., 2016). Within the hypothalamus, gonadotropin releasing hormone (GNRH) and gonadotropin inhibitory hormone (GNIH), both acting through their respective G-protein coupled receptors on pituitary gonadotroph cells, regulate gonadotropin production (Bédécarrats et al., 2009). Within the ovary, steroid hormone feedback loops regulate gene expression locally as well as in the hypothalamus and pituitary (Ottinger and Bakst, 1995; Caicedo Rivas et al., 2016). The largest preovulatory follicle (F1) is responsible for the majority of progesterone production, the fifth largest preovulatory follicle (F5) is responsible for the majority of androgen production, and the small white follicles (SWF) are responsible for the majority of estradiol production (Lee and Bahr, 1994). Avian ovarian steroidogenesis occurs via the 3-cell model of steroidogenesis, where granulosa cells produce progesterone, theca interna cells produce androgens, and theca externa cells produce estradiol (Porter et al., 1989). Progesterone production increases with follicle maturation, whereas androgen and estradiol production decrease with follicle maturation (Porter et al., 1991).
Previous studies examining hens with average egg production rates (roughly 127 eggs/cycle) found that the PS impacts the HPG axis steroid hormone profiles and gene expression (Brady et al., 2019). The PS increased plasma progesterone levels but did not impact plasma estradiol levels. In the hypothalamus and pituitary components of the HPG axis, the PS coincided with a decrease in mRNA levels for genes associated with ovulation stimulation, an increase in expression of genes associated with ovulation inhibition, and an increase in mRNA expression for estradiol receptors. In the follicle cells, increased expression of genes associated with progesterone, androgen, and estradiol production in the F1 granulosa, F5 theca interna, and F5 theca externa, respectively, was seen in response to the PS.
The inner workings of the turkey hen reproductive axis are not consistent within a commercial flock, ultimately resulting in a wide distribution of egg production. Although HPG axis plasma steroid hormone levels and gene expression have been characterized in average egg producing hens, these features remain unknown in hens with poor egg production (LEPH: bottom 15% of flock egg production; <110 eggs/cycle) and with superior egg production (HEPH: top 15% of flock egg production; >145 eggs/cycle). This study sought to characterize the progesterone and estradiol plasma profiles as well as the expression of key HPG axis genes in LEPH and HEPH, both inside and outside of the PS that triggers ovulation. Understanding the perturbations to normal function of the HPG axis that are leading to different egg production levels will be instrumental in improving the egg production capabilities of LEPH.
MATERIALS AND METHODS
Hen Selection and Cell Isolation
A total of 200 females from a commercial line (Hybrid Turkey, Kitchener, Ontario) were housed at the Beltsville Agricultural Research Center (BARC) in individual wire cages. Turkey hens were maintained under standard poultry management practices with artificial lighting (14L:10D with lights on at 6:00 am) and were provided feed ad libitum to NRC standards. Daily egg production records were kept from the onset of lay (around 28 wk of age) until sampling occurred (37 wk of age). Daily egg records were used to calculate each hen's number of eggs per day (EPD) by dividing the total number of eggs produced by the number of days in production. Based on the distribution of flock egg production rates, the bottom and top 15% of egg production were classified as LEPH and HEPH, respectively. Hens were classified as LEPH when EPD <0.6 and as HEPH when EPD >0.8. All animal procedures were approved by the Institutional Animal Care and Use Committee at BARC and at the University of Maryland.
Sampling of turkey hens began at 37 wk of age and was completed for all hens used for the study within 1 wk. All hens were sampled on the second day of the hen's sequence. This allowed for the prediction of the timing of the PS for the third egg of the sequence based on the timing of egg lay for the first egg of the sequence. Hens were also confirmed to have a hard-shell egg in the reproductive tract (second egg of the sequence) prior to sampling to ensure sampling occurred during the clutch period rather than during a pause period. The timing of the PS was predicted based on the oviposition-ovulation cycle pattern as described previously (Brady et al., 2019). A total of 6 LEPH and 6 HEPH, half outside of the PS and half during the PS, were sampled, creating 4 experimental groups (n = 3). The number of experimental replicates was determined through a power analysis (α = 0.05, power = 0.8, |μ1 − μ2| = 0.5, σ2 = 0.2). Sampling for groups during the PS occurred at 8:00 am and sampling for groups outside of the PS occurred at 1:00 pm. To ensure LEPH and HEPH were exposed to the same amount of light prior to sampling, hens laying their first egg of a sequence at 4:00 pm (±30 min) were exclusively used for each experimental group, allowing for hens to be inside of the PS at 8:00 am and outside of the PS at 1:00 pm on the following day. Blood samples taken at the time of sampling were analyzed for progesterone concentrations to confirm the correct timing of sampling.
At 37 wk of age, blood samples were taken from the wing vein immediately before tissue sampling, collected in heparinized tubes, and fractionated by centrifugation (2,000 × g for 10 min at room temperature). Isolated plasma samples were stored at −20°C prior to assessment through radioimmunoassay (RIAs) as described below. Following cervical dislocation, the hypothalamus, pituitary, F1 follicle, F5 follicle, and SWF (a pool of 3 to 5 follicles per hen) were isolated. The entire hypothalamus, pituitary, and SWF were snap-frozen and stored at −80°C for RNA extraction, whereas the F1 and F5 follicles were subjected to isolation of the 3 cell types from the follicle wall. The granulosa, theca interna, and theca externa cells were isolated from the F1 and F5 follicles as previously described (Porter et al., 1989). Briefly, the yolk was drained from each follicle and the follicle was everted to peel off the granulosa layer. The theca interna layer was scraped from the everted follicle and the remaining theca externa layer was minced. All follicle layers were subjected to trypsin dispersion (1 mg/mL) followed by layering onto a Percoll suspension to remove debris and red blood cells. Isolated cells were snap-frozen and stored at −80°C for RNA extraction.
Radioimmunoassays
The RIAs used for progesterone and estradiol were coated tube kits (MP Biomedicals, Solon, OH). For the progesterone and estradiol RIAs, plasma samples were ether extracted prior to the assay. All protocols were performed as directed by the supplier. All samples were assayed in duplicate. The standard curve was assessed for linearity and parallelism using serial plasma dilutions. The intra-assay coefficients of variation determined by pools run every 30 samples were 4.26% for progesterone and 2.48% for estradiol. All samples were measured in a single RIA for each hormone.
RT-qPCR
Total RNA was isolated from the hypothalamus, pituitary, and ovarian SWF and granulosa, theca interna, and theca externa cell from the F1 and F5 follicles with RNeasy Mini kits (Qiagen, Valencia, CA), including on-column deoxyribonuclease digestion. Quantification of RNA, reverse transcription reactions, and RT-qPCR were performed as previously described (Brady et al., 2019). A pool of total RNA was made, and the reaction conducted without reverse transcriptase (No RT) as a control for genomic DNA contamination. Reactions were diluted by tissue type as previously described prior to PCR analysis (Brady et al., 2019). Primers (IDT, Skokie, IL) were designed and used with cycling parameters described previously (Brady et al., 2019). Dissociation curve analysis and gel electrophoresis were conducted to ensure that a single PCR product of appropriate size was amplified in each reaction and was absent from the No RT and water controls. Data were normalized to housekeeping genes and analyzed by the 2−ΔΔ Ct method. For the hypothalamus, GAPDH was used for normalization. For the pituitary, PGK1 was used for normalization. For all of the follicle cell types, GAPDH was used for normalization. All PCR reactions for each gene in a given tissue were analyzed in a single run within a 96-well plate, allowing accurate performance of relative quantification without the need to include a reference control sample in each plate.
Statistics
All data were analyzed using SAS software (SAS Institute, Cary, NC). Normalized RT-qPCR data were log2 transformed before statistical analysis. A 2-way ANOVA using the mixed models procedure (PROC MIXED) was conducted to compare the plasma hormone concentrations and log2-transformed gene expression between LEPH and HEPH, taking the PS into account. The least squares means for each group were compared using the test of least significant difference (PDIFF statement) when this indicated an overall significance level of P < 0.05.
RESULTS
Production differences were noted in egg production rate, clutch length, and pause length between LEPH and HEPH (Figure 1). As expected, HEPH exhibited a higher number of eggs laid per day when compared to LEPH (Figure 1 A) (P < 0.0001). Clutch length, which is the number of eggs laid consecutively, was also higher in HEPH than LEPH (Figure 1 B) (P = 0.0002). Moreover, pause lengths, which is the number of days between clutches, was lower in HEPH in contrast to LEPH (Figure 1 C) (P = 0.0003).
Figure 1.
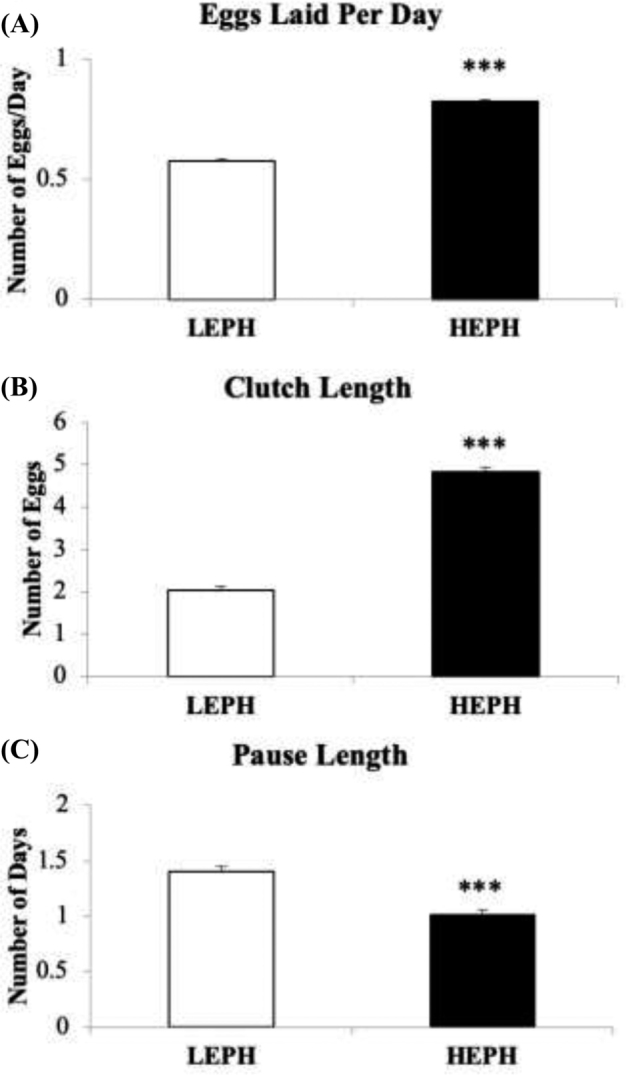
Production calculations showing (A) egg laid per day (EPD), (B) average clutch length, and (C) average pause length in low egg producing hens (LEPH) and high egg producing hens (HEPH). Significance is denoted with an asterisk (*P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001).
Ovarian morphology did not differ between LEPH and HEPH (P ≥ 0.1) (Table 1). The ovaries of LEPH and HEPH did not differ in the number of preovulatory follicles, the weight of the F1 follicle, or the weight of the F5 follicle. Furthermore, the ovary and oviduct weights were not different between LEPH and HEPH.
Table 1.
Ovarian morphology in low egg producing hens (LEPH) and high egg producing hens (HEPH).
| Variable | LEPH (mean ± SEM) | HEPH (mean ± SEM) | P-value |
|---|---|---|---|
| Number of preovulatory follicles | 10.6 ± 1.2 | 11.6 ± 0.6 | 0.3377† |
| F1 follicle weight (g) | 26.5 ± 1.3 | 25.5 ± 1.1 | 0.1423† |
| F5 follicle weights (g) | 19.1 ± 1.5 | 17.5 ± 0.8 | 0.3301† |
| Ovary weight (g) | 197.8 ± 12.4 | 191.4 ± 6.7 | 0.5075† |
| Oviduct weight (g) | 102.6 ± 4.7 | 110.5 ± 3.9 | 0.1137† |
1†P ≥ 0.1.
LEPH and HEPH did not differ in plasma progesterone concentrations, either outside or during the PS (Figure 2 A). Both LEPH and HEPH showed an increase in plasma progesterone levels during the PS when compared to basal levels (P = 0.0093 and P = 0.018, respectively). Plasma estradiol levels were different between LEPH and HEPH (Figure 2 B). HEPH exhibited higher plasma estradiol levels both outside and during the PS (P = 0.043 and P = 0.0322, respectively). Neither LEPH nor HEPH displayed a change in plasma estradiol levels due to the PS.
Figure 2.
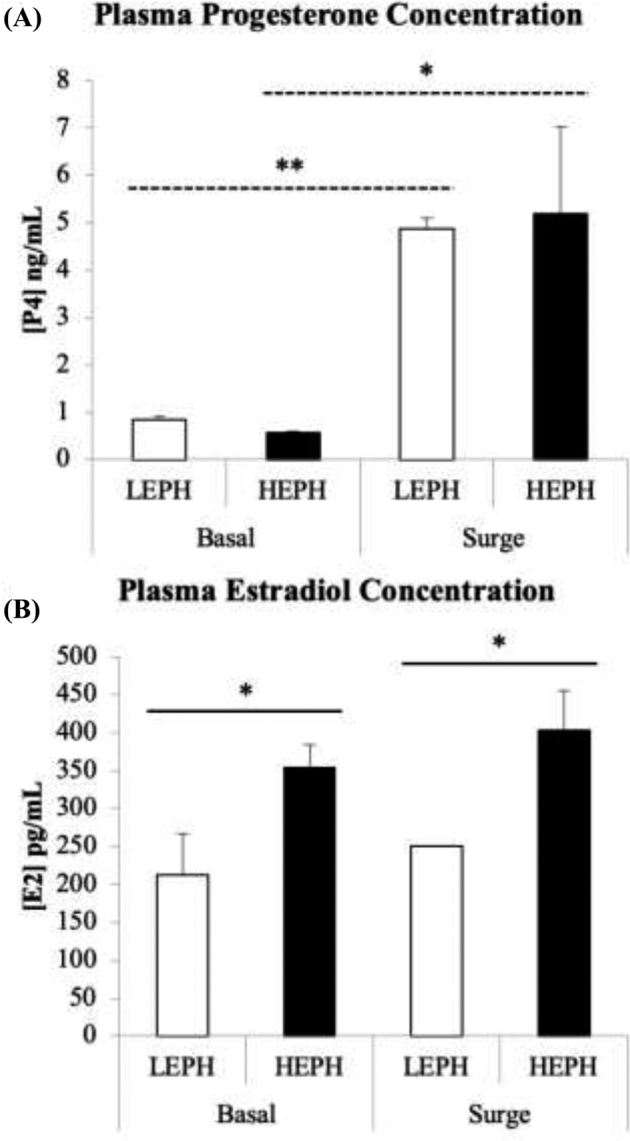
Plasma progesterone and estradiol hormone profiles in low egg producing hens (LEPH) and high egg producing hens (HEPH) sampled outside (basal) and inside (surge) of the preovulatory surge (PS). Significant differences in steroid plasma concentrations are denoted with an asterisk (*P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001). Solid lines indicate a significant difference between LEPH and HEPH for a given condition, whereas dotted lines indicate a significant difference between basal and surge for a given egg production level.
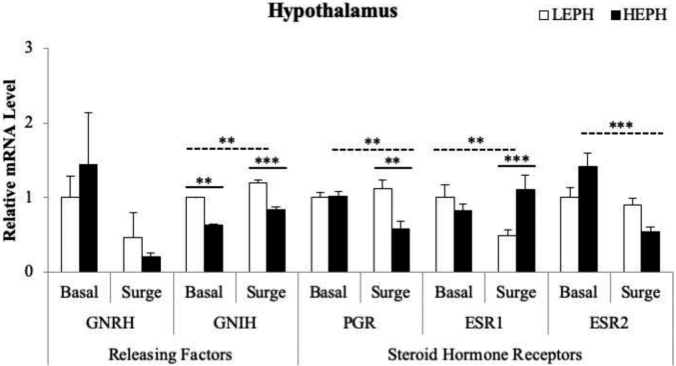
In the hypothalamus, differences between LEPH and HEPH were seen in the mRNA levels of gonadotropin inhibitory hormone (GNIH), the progesterone receptor (PGR), and both estrogen receptors (ESR1 and ESR2) (Figure 3). LEPH exhibited higher mRNA levels for GNIH both outside and inside of the PS (P = 0.0067 and P = 0.0002, respectively). LEPH also showed an increase in GNIH expression in response to the PS (P = 0.0063). Additionally, LEPH showed higher PGR mRNA levels than HEPH during the PS (P = 0.0027). HEPH also displayed downregulation of PGR during the PS compared to levels outside of the PS (P = 0.0057). HEPH displayed increased mRNA levels of ESR1 during the PS when compared to LEPH (P = 0.0063), whereas LEPH displayed downregulation of ESR1 during the PS compared to levels outside of the PS (P = 0.004). Conversely, HEPH showed downregulation of ESR2 during the PS compared to levels outside of the PS (P = 0.0002).
Figure 3.
Hypothalamic gene expression of gonadotropin releasing hormone (GNRH), gonadotropin inhibitory hormone (GNIH), progesterone receptor (PGR), and estrogen receptors 1 and 2 (ESR1 and ESR2) in low egg producing hens (LEPH) and high egg producing hens (HEPH) sampled outside (basal) and inside (surge) of the preovulatory surge (PS). Normalized data are presented relative to LEPH basal expression for each gene. Significant expression differences denoted with an asterisk (*P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001). Solid lines indicate a significant difference between LEPH and HEPH for a given condition, whereas dotted lines indicate a significant difference between basal and surge for a given egg production level.
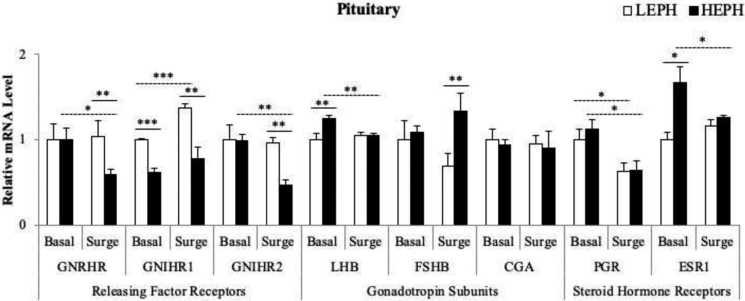
In the pituitary, differences were seen in mRNA levels of LEPH and HEPH for GNRH receptor (GNRHR), both of the GNIH receptors (GNIHR1 and GNIHR2), for the beta subunits of FSH and LH (FSHB and LHB), and for ESR1 (Figure 4). LEPH displayed higher mRNA levels for genes associated with the inhibitory pathways of the HPG axis when compared to HEPH. For example, LEPH showed higher expression of GNIHR1 both inside and outside of the PS and higher expression of GNIHR2 outside of the PS (P = 0.0001,P = 0.0023, and P = 0.0048, respectively). LEPH demonstrated upregulation of GNIHR1 expression during the PS in contrast to expression levels outside of the PS (P = 0.0004), whereas HEPH showed downregulation of GNIHR2 during the PS (P = 0.0039). LEPH also exhibited lower mRNA levels for genes associated with HPG axis stimulation in comparison to HEPH. For example, LEPH showed decreased expression of LHB outside of the PS (P = 0.0045) and decreased expression of FSHB during the PS (P = 0.0036). Interestingly, HEPH showed decreased expression of GNRHR during the PS compared to LEPH (P = 0.0076) and exhibited downregulation of GNRHR expression in response to the PS (P = 0.0227), whereas LEPH did not display expression changes of GNRHR due to the PS. Additionally, only HEPH showed reduced expression of LHB in response to the PS (P = 0.0077). Although both LEPH and HEPH displayed downregulation of PGR during the PS when compared to basal levels (P = 0.039 and P = 0.0245, respectively), only HEPH displayed downregulation of ESR1 during the PS (P = 0.0338). HEPH also exhibited higher ESR1 mRNA levels than LEPH outside of the PS (P = 0.0015).
Figure 4.
Pituitary gene expression of gonadotropin releasing hormone receptor (GNRHR), gonadotropin inhibitory hormone receptors 1 and 2 (GNIHR1 and GNIHR2), luteinizing hormone beta-subunit (LHB), follicle stimulating hormone beta-subunit (FSHB), common alpha-subunit (CGA), progesterone receptor (PGR), and estrogen receptor 1 (ESR1) in low egg producing hens (LEPH) and high egg producing hens (HEPH) sampled outside (basal) and inside (surge) of the preovulatory surge (PS). Normalized data are presented relative to LEPH basal expression for each gene. Significant expression differences denoted with an asterisk (*P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001). Solid lines indicate a significant difference between LEPH and HEPH for a given condition, whereas dotted lines indicate a significant difference between basal and surge for a given egg production level.
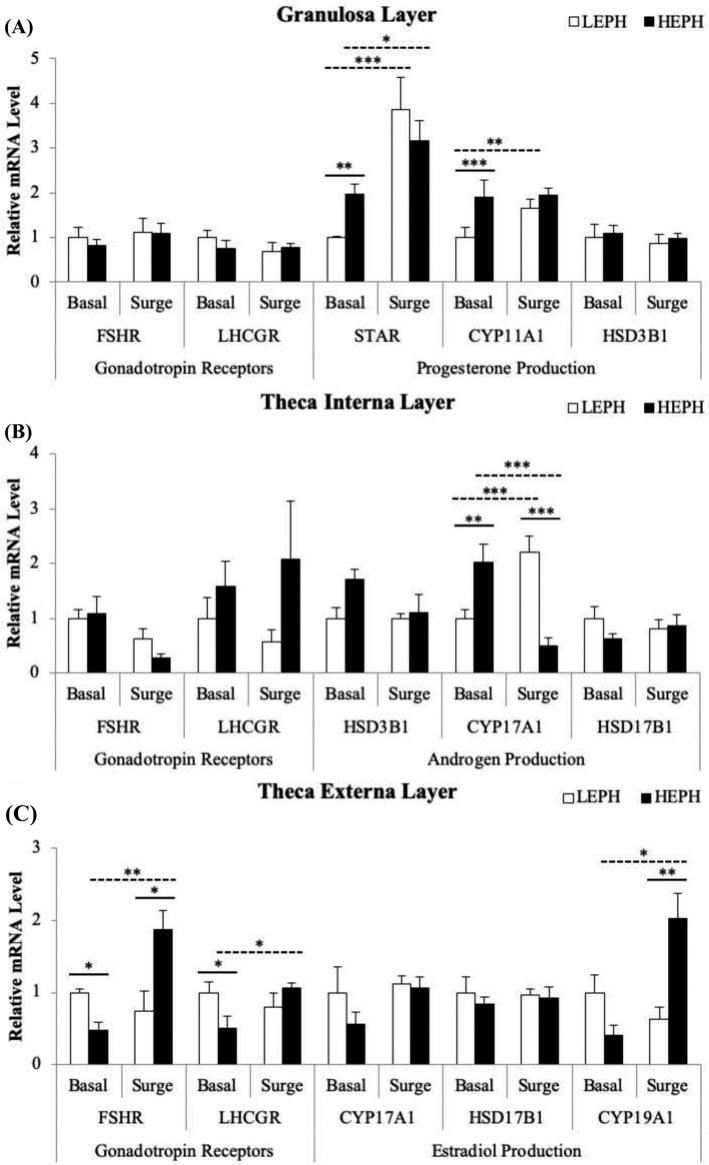
F1 follicle gene expression by cell type is presented in Figure 5. In the F1 granulosa cells, no differences were seen in the expression of FSH or LH receptors (FSHR and LHCGR) but expression differences were seen in 2 of the 3 genes required for progesterone production, namely steroidogenic acute regulatory protein (STAR) and cholesterol side chain cleavage enzyme (CYP11A1) (Figure 5 A). Outside of the PS, LEPH demonstrated decreased mRNA levels when compared to HEPH for the genes encoding STAR (P = 0.0088) and CYP11A1 (P = 0.0009). Both LEPH and HEPH responded to the PS by increasing STAR expression (P = 0.0002 and P = 0.0353, respectively), but only LEPH responded to the PS by increasing CYP11A1 expression (P = 0.0022). In the F1 theca interna cell layer, differences in mRNA levels were observed for 17, 20-lyase (CYP17A1) and for LHCGR (Figure 5 B). CYP17A1 showed higher expression in HEPH under basal conditions (P = 0.0018) but showed higher expression in LEPH during the PS (P < 0.0001). Moreover, LEPH demonstrated upregulation of CYP17A1 in response to the PS (P = 0.008), whereas HEPH demonstrated downregulation in response to the PS (P < 0.0001). In the F1 theca externa cell layer, differences in mRNA levels were observed for FSHR, LHCGR, and aromatase (CYP19A1) (Figure 5 C). FSHR exhibited lower expression in HEPH under basal conditions but showed higher expression in HEPH during the PS (P = 0.0498 and P = 0.0143, respectively). Furthermore, HEPH demonstrated upregulation of FSHR in response to the PS, whereas LEPH did not demonstrate a response to the PS in regard to FSHR expression (P = 0.0037). In addition, HEPH showed decreased expression of LHCGR compared to LEPH outside of the PS (P = 0.0469), with upregulation of LHCGR expression during the PS compared to basal levels (P = 0.0331). CYP19A1 mRNA levels were higher in HEPH during the PS when compared to LEPH (P = 0.0089), and HEPH showed upregulation of CYP19A1 during the PS when compared to basal levels (P = 0.0229).
Figure 5.
F1 follicle gene expression follicle stimulating hormone receptor (FSHR), luteinizing hormone receptor (LHCGR), steroidogenic acute regulatory protein (STAR), cholesterol side chain cleavage enzyme (CYP11A1), 3β-hydroxysteroid dehydrogenase (HSD3B1), 17, 20-lyase (CYP17A1), 17β-hydroxysteroid dehydrogenase (HSD17B1), and aromatase (CYP19A1) in the (A) granulosa, (B) theca interna, and (C) theca externa from low egg producing hens (LEPH) and high egg producing hens (HEPH) sampled outside (basal) and inside (surge) of the preovulatory surge (PS). Normalized data are presented relative to LEPH basal expression for each gene. Significant expression differences denoted with an asterisk (*P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001). Solid lines indicate a significant difference between LEPH and HEPH for a given condition, whereas dotted lines indicate a significant difference between basal and surge for a given egg production level.
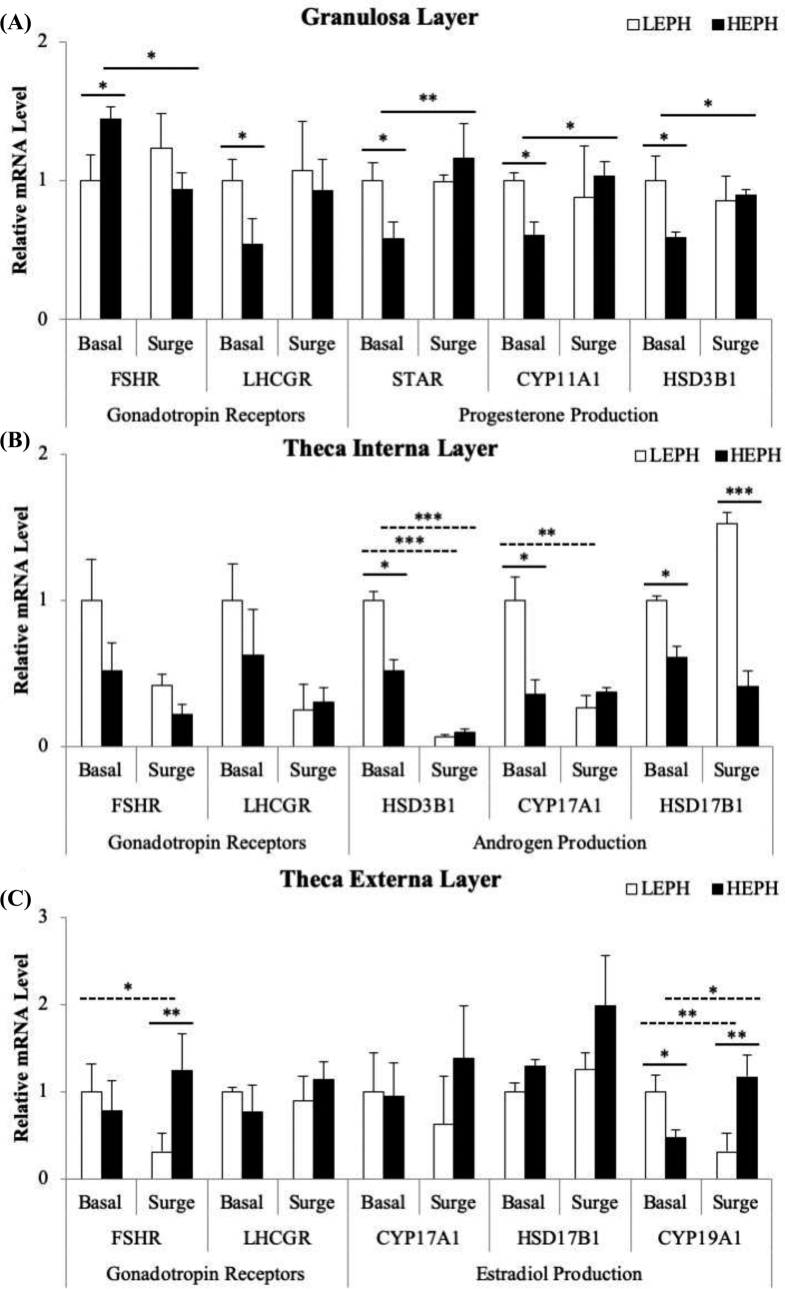
F5 follicle gene expression by cell type is presented in Figure 6. In the F5 granulosa cells, differences were seen in the expression of FSHR and LHCGR. Additionally, expression differences were found in all 3 of the enzymes required for progesterone production, STAR, CYP11A1, and 3β-hydroxysteroid dehydrogenase (HSD3B1) (Figure 6 A). HEPH demonstrated increased mRNA levels for FSHR but decreased mRNA levels for LHCGR outside of the PS when compared to LEPH (P = 0.0271 and P = 0.012, respectively). Additionally, HEPH responded to the PS by reducing FSHR expression (P = 0.0141) and increasing LHCGR expression (P = 0.0462), where LEPH did not change expression of either receptor during the PS. In regard to progesterone production, LEPH showed increased mRNA levels for the genes encoding STAR, CYP11A1, and HSD3B1 outside of the PS (P = 0.0247, P = 0.0258, and P = 0.017, respectively). Furthermore, HEPH displayed increased expression of STAR, CYP11A1, and HSD3B1 in response to the PS, whereas LEPH did not respond to the PS (P = 0.0085, P = 0.0202, andP = 0.0265, respectively). In the F5 theca interna cell layer, differences in mRNA levels were observed for HSD3B1, CYP17A1, and 17β-hydroxysteroid dehydrogenase (HSD17B1) (Figure 6 B). LEPH exhibited higher mRNA levels for HSD3B1, CYP17A1, and HSD17B1 under basal conditions when compared to HEPH (P = 0.0255, P = 0.0137, and P = 0.0372, respectively). Additionally, LEPH displayed higher mRNA levels than LEPH for HSD17B1 during the PS (P = 0.0002). Downregulation of HSD3B1 and CYP17A1 was seen in LEPH during the PS (P < 0.0001 and P = 0.0038, respectively), whereas only downregulation of HSD3B1 was seen in HEPH during the PS (P = 0.0002). In the F5 theca externa cell layer, differences in mRNA levels were observed for FSHR as well as for CYP19A1 (Figure 6 C). HEPH exhibited higher mRNA levels for FSHR during the PS compared to LEPH (P = 0.0056), whereas LEPH showed downregulation of FSHR expression during the PS compared to basal levels (P = 0.0115). LEPH exhibited downregulation of CYP19A1 during the PS compared to basal levels (P = 0.0035). On the other hand, HEPH showed lower CYP19A1 levels when compared to LEPH during basal conditions (P = 0.0387) but showed upregulation of CYP19A1 during the PS (P = 0.0182), resulting in higher mRNA levels than LEPH during the PS (P = 0.0019).
Figure 6.
F5 follicle gene expression of follicle stimulating hormone receptor (FSHR), luteinizing hormone receptor (LHCGR), steroidogenic acute regulatory protein (STAR), cholesterol side chain cleavage enzyme (CYP11A1), 3β-hydroxysteroid dehydrogenase (HSD3B1), 17, 20-lyase (CYP17A1), 17β-hydroxysteroid dehydrogenase (HSD17B1), and aromatase (CYP19A1) in the (A) granulosa, (B) theca interna, and (C) theca externa from low egg producing hens (LEPH) and high egg producing hens (HEPH) sampled outside (basal) and inside (surge) of the preovulatory surge (PS). Normalized data are presented relative to LEPH basal expression for each gene. Significant expression differences denoted with an asterisk (*P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001). Solid lines indicate a significant difference between LEPH and HEPH for a given condition, whereas dotted lines indicate a significant difference between basal and surge for a given egg production level.
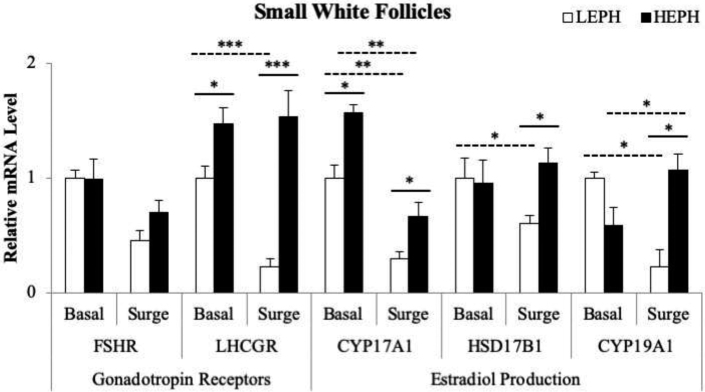
SWF gene expression is presented in Figure 7. Gene expression differences between LEPH and HEPH were seen in LHCGR and in all 3 of the genes involved in estradiol production. LHCGR mRNA levels were higher in HEPH than in LEPH, both outside and during the PS (P = 0.0428 and P < 0.0001, respectively). LEPH exhibited downregulation of LHCGR in response to the PS, whereas expression in HEPH did not change (P = 0.0003). Additionally, HEPH displayed higher gene expression of CYP17A1 than in LEPH, both outside and inside of the PS (P = 0.04 and P = 0.036, respectively). However, both LEPH and HEPH showed decreased mRNA levels for CYP17A1 during the PS when compared to basal levels (P = 0.006 and P = 0.004, respectively). HSD17B1 and CYP19A1 expression only differed between LEPH and HEPH during the PS, with both HSD17B1 and CYP19A1 mRNA levels higher in HEPH than LEPH (P = 0.0228 and P = 0.0126, respectively). HSD17B1 expression did not change in HEPH during the PS. In contrast, HSD17B1 expression decreased in LEPH during the PS (P = 0.0474). CYP19A1 expression, however, was downregulated in LEPH during the PS (P = 0.0141), whereas expression was upregulated in HEPH during the PS (P = 0.0488).
Figure 7.
Small white follicle gene expression of follicle stimulating hormone receptor (FSHR), luteinizing hormone receptor (LHCGR), 17, 20-lyase (CYP17A1), 17β-hydroxysteroid dehydrogenase (HSD17B1), and aromatase (CYP19A1) in low egg producing hens (LEPH) and high egg producing hens (HEPH) sampled outside (basal) and inside (surge) of the preovulatory surge (PS). Normalized data are presented relative to LEPH basal expression for each gene. Significant expression differences denoted with an asterisk (*P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001). Solid lines indicate a significant difference between LEPH and HEPH for a given condition, whereas dotted lines indicate a significant difference between basal and surge for a given egg production level.
DISCUSSION
This is the first study to compare production characteristic, ovarian morphology, steroid hormone profiles, and HPG axis mRNA levels in LEPH and HEPH of the same breed, strain, and age. Previous studies have examined the impact of genetic selection on HPG axis function through examination of production characteristics of chicken and turkey lines divergently selected for meat production and egg production (Velleman et al., 2007). Additionally, there have been studies that examined gene expression changes in chicken strains with low and high egg production (Chen et al., 2007, Yang et al., 2008). Understanding HPG axis function at the macroscopic and molecular levels, as well as how this axis is perturbed in hens with differential egg production, is imperative to improving the egg production rates in birds selected for meat production.
Though differences were seen in egg production rates, clutch length, and pause length in LEPH and HEPH, these differences were not explained by the morphological structure of the reproductive tract. LEPH did not show reduced follicle numbers, signs of follicle atresia, or an abnormal follicular hierarchy. LEPH also did not appear to have issues attaining the hormone levels for the PS to occur or issues with ovulation. Lastly, the development of the reproductive tract, in terms of individual follicle weight, ovary weight, and oviduct weight, did not appear to be impeded in LEPH when compared to HEPH. In broiler breeders, which are also selected predominantly for meat production causing lowered reproductive success, follicular hierarchy issues are common and lead to decreased egg production (Decuypere et al., 2010). Broiler breeders have increased numbers of internal ovulations associated with ad libitum feeding, which was not seen in turkey hens with lower egg production (Hocking, 1993). Ovarian morphology issues common to birds heavily selected for meat production purposes were not seen in turkey hens with lowered egg production, indicating that selection for meat production has impacted turkey and broiler hens to different degrees and possibly through different mechanisms.
In the present study, both groups of hens displayed basal and peak progesterone levels similar to previously reported levels (Yang et al., 1997). Additionally, both groups of hens exhibited a roughly 6-fold increase in plasma progesterone levels, with no apparent differences between LEPH and HEPH in plasma progesterone concentration during the ovulatory cycle. Plasma estradiol concentrations were not affected by the PS in either LEPH or HEPH, but HEPH did display higher plasma estradiol levels both outside and during the PS when compared to LEPH. The role of estradiol in the regulation of egg production is not fully understood; however, estradiol has been shown to bind in the hypothalamus, pituitary, ovary, and oviduct. In laying hens, estradiol binding affinity changes in the neurohypophysis during the ovulatory cycle and induces progesterone receptor expression in gonadotrophs of the pituitary, implicating a role in ovulation regulation (Gasc and Baulieu, 1988; Takahashi and Kawashima, 2009). Additionally, estradiol injection in laying hens resulted in increased binding affinity of progesterone in the oviduct, indicating that estradiol regulates the action of other sex steroid hormones (Kawashima et al., 1996).
In the hypothalamus and pituitary, LEPH showed higher mRNA levels for GNIH, GNIHR1, and GNIHR2, as well as lower mRNA levels for FSHB and LHB, which at the transcript level, is consistent with increased ovulation inhibition and decreased follicular development in these hens. Though differences in the follicular hierarchy structure were not seen between LEPH and HEPH, decreased FSHB compared to HEPH may slow the movement of follicles through the follicular hierarchy in LEPH. Studies examining goat breeds with low and high fertility found that FSHB and LHB expression was also upregulated in breeds with high fertility compared to breeds with low fertility (Zi et al., 2013). LEPH also showed upregulation of the progesterone receptor in the hypothalamus during the PS relative to HEPH, whereas HEPH showed upregulation of estradiol receptor, ESR1, in the hypothalamus and pituitary. Higher plasma estradiol levels coupled with increased gene expression of estradiol receptors in the hypothalamus and pituitary of HEPH suggest that estradiol feedback mechanisms may differ in LEPH and HEPH. Downregulation of PGR in the hypothalamus and GNRHR in the pituitary during the PS was previously seen (Brady et al., 2019); however, only HEPH exhibited downregulation of both receptors during the PS, whereas LEPH showed no expression changes of the receptors during the PS. PGR and GNRHR stimulate the HPG axis and have been shown to decrease receptor binding and gene expression, respectively, during the PS in chickens (Kawashima et al., 1994; Lovell et al., 2005). Downregulation of these receptors may serve to prime the HPG axis for the next ovulation to occur.
The F1 follicle is responsible for progesterone production, which occurs in the granulosa cells. In the granulosa layer, HEPH showed higher basal mRNA levels of STAR and CYP11A1, indicating a greater capacity for progesterone production. Movement of cholesterol from the outer mitochondrial membrane to the inner membrane by STAR is the rate-limiting step of steroidogenesis (Stocco, 2001). Higher expression of STAR in HEPH may allow for increased initiation of steroidogenesis. Increased expression of STAR and CYP11A1 in preovulatory follicles was also seen in goat breeds with high fertility when compared to breeds with low fertility (Zi et al., 2018). Both LEPH and HEPH upregulated STAR during the PS, which is consistent with previous studies (Johnson et al., 2002); however, HEPH, but not LEPH, downregulated CYP11A1 during the PS, which was seen in previous studies (Brady et al., 2019). In the theca interna and theca externa layers of the F1 follicle, HEPH also showed upregulation of genes involved in androgen and estradiol production, such as HSD3B1, CYP17A1, and CYP19A1 when compared to LEPH. Despite the main function of the F1 follicle being progesterone production, theca interna and externa layers of the F1 follicle in HEPH may be contributing to total androgen and estradiol concentrations. Increased mRNA levels of STAR, CYP11A1, CYP17A1, and CYP19A1 in HEPH may indicate that the F1 follicle of HEPH is more capable of steroidogenesis compared to the F1 follicle of LEPH.
The F5 follicle is responsible for androgen production, which occurs in the theca interna cells. In the theca interna layer, LEPH showed upregulation of HSD3B1, CYP17A1, and HSD17B1, indicating a greater capacity for androgen production. Androgens are necessary for normal reproductive function and have been shown to have positive and negative action on the HPG axis (Rangel and Gutierrez, 2014). Testosterone injections increased the number of internal ovulations in broiler breeders, ultimately decreasing egg production (Navara et al., 2015). On the other hand, testosterone treatment increased progesterone production and related gene expression in chicken granulosa cells (Rangel et al., 2009). Interestingly, LEPH also showed upregulation of all 3 genes involved in progesterone production in the F5 granulosa layer when compared to HEPH. In the F5 theca externa layer, LEPH exhibited higher expression of CYP19A1, the key enzyme involved in estradiol production, outside of the PS but opposite gene expression trends were seen during the PS. Increased mRNA levels of STAR, CYP11A1, HSD3B1, CYP17A1, HSD17B1, and CYP19A1 in LEPH may indicate an increased steroidogenic capacity.
The SWF are responsible for most of the hen's total estradiol production. In the SWF cells, HEPH showed higher mRNA levels of CYP17A1, HSD17B1, and CYP19A1, indicating a greater capacity for estradiol production in HEPH than in LEPH. In addition to upregulation of estradiol production genes at the follicle level, plasma estradiol levels and estradiol receptor gene expression in the hypothalamus and pituitary were also increased in HEPH compared to LEPH. Decreased levels of CYP17A1 and CYP19A1 expression in the SWF along with decreased plasma estradiol levels have been associated with incubation behavior and follicle atresia in the turkey hen (Tabibzadeh et al., 1994). Although visual signs of follicle atresia were not seen in the present study (for example, decreased follicle numbers or atretic follicles), decreased egg production may exhibit molecular mechanisms similar to follicle atresia, such as increased granulosa cell apoptosis in LEPH. LH receptor expression was also upregulated in HEPH, both outside and during the PS, compared to levels in LEPH. Studies comparing sheep breeds with low and high fertility also found upregulation of LHCGR in follicles in early development (1 to 3 mm in size) from high fertility breeds (Abdennebi et al., 1999). Similar to the F1 follicle, increased mRNA levels of CYP17A1, HSD17B1, and CYP19A1 in HEPH may indicate an increased ability for steroid production in the SWF of HEPH compared to LEPH.
In the current study, LEPH and HEPH exhibited clutch and pause length differences but did not exhibit differences in ovarian or oviduct morphology. Differences were not seen in plasma progesterone levels but plasma estradiol levels were higher in HEPH compared to LEPH. Gene expression differences were established in each tissue of the HPG axis, with LEPH and HEPH displaying different degrees of stimulation and inhibition in all of the tissues of the HPG axis at the mRNA level. Increased egg production was associated with mRNA levels consistent with increased ovulation stimulation, decreased ovulation inhibition, increased progesterone synthesis in the F1 follicle granulosa layer, decreased androgen synthesis in the F5 follicle theca interna layer, and increased estradiol synthesis in the SWF. The gene expression differences reported in this study may indicate that HPG axis function and regulation differ in LEPH and HEPH, with HEPH more adept at gonadotropin stimulation and steroidogenesis and LEPH more proficient at gonadotropin inhibition, which could ultimately lead to the observed differences in egg production rates. This study has provided novel insights into the inner workings of the HPG axis in turkey hens. The influence of egg production levels on HPG axis function has been defined through production, morphology, and gene expression measures and lays the foundation for future research to improve the reproductive efficiency of breeding hens in a meat focused industry.
ACKNOWLEDGMENTS
This project was supported by Agriculture and Food Research Initiative Competitive Grant # 2019-67015-29472 from the USDA National Institute of Food and Agriculture to T.E.P.
REFERENCES
- Abdennebi L., Monget P., Pisselet C., Remy J.J., Salesse R., Monniaux D. Comparative expression of luteinizing hormone and follicle-stimulating hormone receptors in ovarian follicles from high and low prolific sheep breeds. Biol. Reprod. 1999;60:845–854. doi: 10.1095/biolreprod60.4.845. [DOI] [PubMed] [Google Scholar]
- Brady K., Porter T.E., Liu H.C., Long J.A. Characterization of gene expression in the hypothalamo-pituitary-gonadal axis during the preovulatory surge in the turkey hen. Poult. Sci. 2019 doi: 10.3382/ps/pez437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bédécarrats G.Y., Baxter M., Sparling B. An updated model to describe the neuroendocrine control of reproduction in chickens. Gen. Comp. Endocrinol. 2016;227:58–63. doi: 10.1016/j.ygcen.2015.09.023. [DOI] [PubMed] [Google Scholar]
- Bédécarrats G.Y., McFarlane H., Maddineni S.R., Ramachandran R. Gonadotropin-inhibitory hormone receptor signaling and its impact on reproduction in chickens. Gen. Comp. Endocrinol. 2009;163:7–11. doi: 10.1016/j.ygcen.2009.03.010. [DOI] [PubMed] [Google Scholar]
- Caicedo Rivas R.E., Paz-Calderón Nieto M., Kamiyoshi M. Effects of steroid hormone in avian follicles. Asian-Australas J. Anim. Sci. 2016;29:487–499. doi: 10.5713/ajas.15.0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L.R., Chao C.H., Chen C.F., Lee Y.P., Chen Y.L., Shiue Y.L. Expression of 25 high egg production related transcripts that identified from hypothalamus and pituitary gland in red-feather Taiwan country chickens. Anim. Reprod. Sci. 2007;100:172–185. doi: 10.1016/j.anireprosci.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Decuypere E., Bruggeman V., Everaert N., Li Y., Boonen R., De Tavernier J., Janssens S., Buys N. The broiler breeder paradox: ethical, genetic and physiological perspectives, and suggestions for solutions. Br. Poult. Sci. 2010;51:569–579. doi: 10.1080/00071668.2010.519121. [DOI] [PubMed] [Google Scholar]
- Gasc J.M., Baulieu E.E. Regulation by estradiol of the progesterone receptor in the hypothalamus and pituitary: an immunohistochemical study in the chicken. Endocrinology. 1988;122:1357–1365. doi: 10.1210/endo-122-4-1357. [DOI] [PubMed] [Google Scholar]
- Hocking P.M. Effects of body weight at sexual maturity and the degree and age of restriction during rearing on the ovarian follicular hierarchy of broiler breeder females. Br. Poult. Sci. 1993;34:793–801. doi: 10.1080/00071669308417638. [DOI] [PubMed] [Google Scholar]
- Johnson A.L., Solovieva E.V., Bridgham J.T. Relationship between steroidogenic acute regulatory protein expression and progesterone production in hen granulosa cells during follicle development. Biol. Reprod. 2002;67:1313–1320. doi: 10.1095/biolreprod67.4.1313. [DOI] [PubMed] [Google Scholar]
- Kawashima M., Kamiyoshi M., Tanaka K. Changes in progesterone receptor binding of preoptic hypothalamus during an ovulatory cycle of the hen. Poult. Sci. 1994;73:855–863. doi: 10.3382/ps.0730855. [DOI] [PubMed] [Google Scholar]
- Kawashima M., Takahashi T., Kamiyoshi M., Tanaka K. Effects of progesterone, estrogen, and androgen on progesterone receptor binding in hen oviduct uterus (shell gland) Poult. Sci. 1996;75:257–260. doi: 10.3382/ps.0750257. [DOI] [PubMed] [Google Scholar]
- Lee K., Bahr J. Utilization of substrates for testosterone and estradiol-17β production by small follicles of the chicken ovary. Endocrinology. 1994;11:307–314. doi: 10.1016/0739-7240(94)90022-1. [DOI] [PubMed] [Google Scholar]
- Lovell T.M., Knight P.G., Gladwell R.T. Variation in pituitary expression of mRNAs encoding the putative inhibin co-receptor (betaglycan) and type-I and type-II activin receptors during the chicken ovulatory cycle. J. Endocrinol. 2005;186:447–455. doi: 10.1677/joe.1.06303. [DOI] [PubMed] [Google Scholar]
- McCartney M.G., Nestor K.E., Harvey W.R. Genetics of growth and reproduction in the Turkey: 2. Selection for increased body weight and egg production. Poult. Sci. 1968;47:981–990. doi: 10.3382/ps.0470981. [DOI] [PubMed] [Google Scholar]
- Navara K.J., Pinson S.E., Chary P., Taube P.C. Higher rates of internal ovulations occur in broiler breeder hens treated with testosterone. Poult. Sci. 2015;94:1346–1352. doi: 10.3382/ps/pev103. [DOI] [PubMed] [Google Scholar]
- Nestor K.E., Anderson J.W., Patterson R.A., Velleman S.G. Genetics of growth and reproduction in the Turkey. 17. Changes in genetic parameters over forty generations of selection for increased sixteen-week body weight. Poult. Sci. 2008;87:1971–1979. doi: 10.3382/ps.2008-00137. [DOI] [PubMed] [Google Scholar]
- Ottinger M.A., Bakst M.R. Endocrinology of the avian reproductive system. J. Avian. Med. Surg. 1995;9:242–250. [Google Scholar]
- Porter T.E., Hargis B.M., Silsby J.L., El Halawani M.E. Differential steroid production between theca interna and theca externa cells: a three-cell model for follicular steroidogenesis in avian species. Endocrinology. 1989;125:109–116. doi: 10.1210/endo-125-1-109. [DOI] [PubMed] [Google Scholar]
- Porter T.E., Hargis B.M., Silsby J.L., El Halawani M.E. Characterization of dissimilar steroid productions by granulosa, theca interna and theca externa cells during follicular maturation in the turkey (Meleagris gallopavo) Gen. Comp. Endocrinol. 1991;84:1–8. doi: 10.1016/0016-6480(91)90058-e. [DOI] [PubMed] [Google Scholar]
- Rangel P.L., Gutierrez C.G. Reproduction in hens: Is testosterone necessary for the ovulatory process? Gen. Comp. Endocrinol. 2014;203:250–261. doi: 10.1016/j.ygcen.2014.03.040. [DOI] [PubMed] [Google Scholar]
- Rangel P.L., Rodríguez A., Rojas S., Sharp P.J., Gutierrez C.G. Testosterone stimulates progesterone production and STAR, P450 cholesterol side-chain cleavage and LH receptor mRNAs expression in hen (Gallus domesticus) granulosa cells. Reproduction. 2009;138:961–969. doi: 10.1530/REP-09-0071. [DOI] [PubMed] [Google Scholar]
- Stocco D.M. StAR Protein and the regulation of steroid hormone biosynthesis. Annu. Rev. Physiol. 2001;63:193–213. doi: 10.1146/annurev.physiol.63.1.193. [DOI] [PubMed] [Google Scholar]
- Tabibzadeh C., Silsby J.L., Rozenboim I., Pitts G.R., Foster D.N., El Halawani M.E. Theca cell cytochrome-P450 17-hydroxylase and aromatase messenger-ribonucleic-acid abundance and serum steroid-levels during follicular atresia associated with incubation behavior in the domestic turkey hen. Biol. Reprod. 1994;51:731–738. doi: 10.1095/biolreprod51.4.731. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Kawashima M. Properties of estrogen binding components in the plasma membrane of neurohypophysis in hens and changes in its binding before and after oviposition. Poult. Sci. 2009;88:2206–2211. doi: 10.3382/ps.2009-00114. [DOI] [PubMed] [Google Scholar]
- Velleman S.G., Coy C.S., Anderson J.W., Nestor K.E. The effect of genetic increases in egg production and age and sex on breast muscle development of turkeys. Poult. Sci. 2007;86:2134–2138. doi: 10.1093/ps/86.10.2134. [DOI] [PubMed] [Google Scholar]
- Yang K.T., Lin C.Y., Huang H.L., Liou J.S., Chien C.Y., Wu C.P., Huang C.W., Ou B.R., Chen C.F., Lee Y.P., Lin E.C., Tang P.C., Lee W.C., Ding S.T., Cheng W.T., Huang M.C. Expressed transcripts associated with high rates of egg production in chicken ovarian follicles. Mol. Cell. Probes. 2008;22:47–54. doi: 10.1016/j.mcp.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Yang J., Long D.W., Bacon W.L. Changes in plasma concentrations of luteinizing hormone, progesterone, and testosterone in turkey hens during the ovulatory cycle. Gen. Comp. Endocrinol. 1997;106:281–292. doi: 10.1006/gcen.1997.6884. [DOI] [PubMed] [Google Scholar]
- Zi X.D., Huang L., Wang Y., Lu J.Y. Comparative messenger RNA expression of FSHβ, LHβ, FSHR, LHR, and ERβ in high and low prolific goat breeds. Anim. Biotechnol. 2013;24:307–311. doi: 10.1080/10495398.2013.790824. [DOI] [PubMed] [Google Scholar]
- Zi X.D., Lu J.Y., Zhou H., Ma L., Xia W., Xiong X.R., Lan D.L., Wu X.H. Comparative analysis of ovarian transcriptomes between prolific and non-prolific goat breeds via high-throughput sequencing. Reprod. Domest. Anim. 2018;53:344–351. doi: 10.1111/rda.13111. [DOI] [PubMed] [Google Scholar]