Abstract
This study investigated the influence of an organic mineral-supplemented broiler diet on the quality of nuggets. The resulting chicken nuggets were enriched with inorganic and organic forms of Zn and Se. The nuggets were processed by incorporating extracts from food industry by-products (rosemary [RH and RL], hydroxytyrosol [HYT], pomegranate [P], grape [GS], and Harpagophytum [H]). The physiochemical, microbiological, and sensory characteristics of the chicken nuggets were evaluated over a 12-month period of frozen storage. The addition of natural extracts did not affect the pH, proximate composition, or color (CIELab) of the nuggets among samples. However, significative differences were found between month of analysis (range from pH 6.16 to 6.63; luminosity from 62.51 to 84.74; redness from 0.16 to 7.14; and yellowness from 10.80 to 33.77). In addition, the combination of phenolic compounds with Zn and Se retarded microbial growth and reduced protein and lipid oxidation, thus maintaining the sensory quality and extending the shelf life of this product. For instance, the combination of RL + GS reduced in 75% the microbiological growth regarding the control sample (C), while samples that incorporated RH + P or HYT + P + H presented 50% less than C. In addition, upon only incorporating organic minerals Zn and Se, microbiological deterioration is reduced in 15%. This mix was significantly effective at reducing the oxidative reactions of lipids and proteins by 40% and 50%, as measured after 9 and 12 mo of frozen storage, respectively. The addition of the natural extracts and Zn and Se did not adversely affect the acceptability of the meat product.
Key words: broiler, nugget, organic mineral-supplemented diet, meat quality, antioxidant activity
Introduction
Chicken meat has positive nutritional characteristics, including a low lipid content, proteins of high biological value, essential amino acids, such as Trp, and a natural source of vitamins B2, B3, and B6 and minerals such as Fe, P, K, Zn, and Se (BEDCA/AESAN, 2019). Based on Spanish Food Consumption Statistics (AESAN/MARM, 2019), a high percentage is consumed in the form of “fast food” or “ready-to-eat” products, such as chicken nuggets, because of the reduced preparation time necessary, their low cost, and long shelf life under frozen storage.
Below −18°C, lipases can produce lipolysis and lipid oxidation due to their hydrolytic action on phospholipids and triglycerides, with the resulting compounds causing a rancid odor and taste. In addition, hemoglobin acts as a catalyst of oxidation reactions, so meat must be properly bled before freezing. On the other hand, frozen storage increases protein oxidation because of the catalytic iron released by alteration of the cell membranes and the possible cryoconcentration of pro-oxidant solutes around the protein molecules in water (Estévez et al., 2012).
To preserve the shelf life under frozen storage and prevent lipid peroxidation, antioxidants are widely used, especially synthetic antioxidants such as butyl-hydroxytoluene, butyl-hydroxyanisole, propyl gallate, and tert-butylhydroquinone (Biswas et al., 2004; Martínez et al., 2014; Nieto et al., 2012; Nieto et al., 2012; Nieto et al., 2013). However, increasing concerns about nutrition and health have resulted in a trend toward “natural products” free of synthetic food additives. This is the origin of “Clean label” meat products that are increasingly demanded by consumers in some European countries and the United States (Naveena et al., 2008).
Another important aspect is that waste management and the reduction of food waste have a high economic cost for the Food Industry (Gustavsson et al., 2011). However, from such wastes, by-products (BPs) rich sources of fiber, minerals, and phytochemicals could be obtained (Pradal et al., 2018). These can be used as functional ingredients and have a great impact on the technological, nutritional, and health-promoting properties of meat products (Mora et al., 2014).
Natural antioxidants obtained in the form of food industrial BP may contribute to avoid the perceived adverse health disorders. Among these natural antioxidants, Rosmarinus officinalis, grape (Vitis vinifera) seed, and Punica granatum are rich in phenolic compounds with known anti-inflammatory, antioxidant, antiaging, antibacterial, and anticancer properties (Nowshehri et al., 2015, Alu'datt et al., 2018, Khwairakpam et al., 2018). For its part, hydroxytyrosol, which is obtained from olive leaves and olive oil production, is one of the most antioxidant compounds known, which has shown many beneficial effects such as immune system protector, anti-inflammatory, anticancer, and antimicrobial (Martínez et al., 2018a, Martínez et al., 2018b). Finally, Harpagophytum procumbens is an herb grown in southern Africa with great anti-inflammatory property because of its high content of iridoid and phenylpropanoid glycosides that can contribute with an added value to the meat products made with it (McWangi et al., 2012).
All these natural extracts, except H. procumbens, have been tested in meat products. For example, R. officinalis is authorized by the European Union (Directives 95/2/EC, 2010/67/EU and 2010/69/EU) to be added to meat products with the number E-392. At the same time, Stojanovic-Radic et al. (2018) and Smeti et al. (2018) used it endogenously in chicken and lamb meat, respectively. Lytou et al. (2018) tested pomegranate (P. granatum) extract in marinated chicken meat products also with good results. Nieto et al. (2017) obtained excellent results in sausages made with chicken meat using hydroxytyrosol as a natural antioxidant, while Ragni et al. (2018) and Cosansu and Juneja (2018) endogenously tested grape (Vitis vinífera) extract in lamb meat and exogenously in cooked ground beef.
Moreover, essential minerals such as Zn and Se have been reported as having beneficial effects such as antioxidant compounds and regulators of the immune system and the body function (Oropeza et al., 2015). In chicken, Delles et al. (2014) showed that feeding broilers with Zn and Se combined with vitamin E decreased the lipid and protein oxidation of the meat, a practice that can be considered a good strategy for reducing synthetic additive concentrations. Furthermore, our recent study (Martínez et al., 2018a, Martínez et al., 2018b) showed increases in mineral bioavailability using organic forms of the minerals instead of their inorganic forms.
The aim of the present study was to evaluate the effect of adding natural extracts from food industrial BPs, including rosemary essential oil, which contains rosmarinic acid (RH) and diterpenes (RL), pomegranate extract (P), grape seed (GS) extract, hydroxytyrosol from olive leaf (HYT), H. procumbens (H), and the effects of broiler feed addition of Zn and Se (both organic and inorganic forms) on the nutritional, physiochemical, microbiological, and sensory qualities of frozen chicken nuggets under frozen storage for 12 mo.
Material and Methods
Animal Diet
During the experiments, the corresponding regulations were taken into account: EU regulation (852/2004; 853/2004; 854/2004) and Spanish law RD 32/2007 regarding the care, transport, and slaughter of animals used for experimentation. The procedures used in the study were approved by the Bioethics Committee of the University of Murcia (authorization number: CEEA-92/2014)
Five hundred broilers, since 1 until 40 days old, were randomly placed into 2 different floor pens with 12 replicate pens for each group. Each pen was assigned 2 dietary treatments: (C) basal diet, supplemented with inorganic mineral (0.3 ppm of Na2SeO3 and 80 ppm of ZnO); (SZ) basal diet, supplemented with organic mineral (0.2 ppm and 50 ppm of Se and Zn proteinate). Feed and water were provided at libitum. A starter diet containing 22% soybean meal and 3,025 kcal/kg was provided up to 21 days of age, and a grower diet from 21 to 42 days of age containing 21% soybean meal and 3,105 kcal/kg. Both diets consisted of corn, soybeans, wheat, whole soybeans, sunflower meal (28%), soybean oil, amino acids (methionine, threonine, and lysine), and minerals (CaHPO4, NaHCO3, and CaCO3). After 40 D of feeding, 2 broilers from each pen were slaughtered. Both sides of the thighs were removed and skinned and then kept frozen for 1 wk until chicken nuggets were prepared.
Samples
Eight different chicken nugget batches of samples were prepared, before they were separated into 2 groups: one of them was made with chicken meat enriched endogenously with inorganic Zn and Se (C), and the second group was made with chicken meat enriched endogenously with organic Zn and Se (SZ). Chicken nugget samples were also enriched exogenously by incorporation of natural extracts obtained from plants (rosemary [RH and RL], pomegranate [P], grape seed [GS], hydroxytyrosol [HYT], and Harpagophytum [H]), according to Table 1.
Table 1.
Ingredients (g) of samples of frozen chicken nuggets.
| Ingredients (g) | Chicken nuggets |
|||||||
|---|---|---|---|---|---|---|---|---|
| Enriched with inorganic forms of Zn and Se |
Enriched with organic forms of Zn and Se |
|||||||
| C | CRH+P | CRL+GS | CHYT+P+H | SZ | SZRH+P | SZRL+GS | SZHYT+P+H | |
| Chicken meat (g) | 675 | 672.5 | 672.5 | 672.25 | 675 | 672.5 | 672.5 | 672.25 |
| Plant extract (ppm) | ||||||||
| RH | 1000 | 1000 | ||||||
| P | 1500 | 1500 | 1500 | 1500 | ||||
| RL | 1000 | 1000 | ||||||
| GS | 1500 | 1500 | ||||||
| HYT | 750 | 750 | ||||||
| H | 500 | 500 | ||||||
| Water (ml) | 150 | 150 | 150 | 150 | 150 | 150 | 150 | 150 |
| Ice (g) | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Commercial mix® (g/kg) | 75 | 75 | 75 | 75 | 75 | 75 | 75 | 75 |
| Total | 1000 | 1000 | 1000 | 1000 | 1000 | 1000 | 1000 | 1000 |
Abbreviations: C, control; CRH+P, 1000 ppm rosemary extract + 1500 ppm pomegranate extract; CRL+GS, 1000 ppm Nutrox OS + 1500 ppm grape seed extract; CHYT+P+H, 1500 ppm pomegranate extract + 750 ppm hydroxytyrosol + 500 ppm Harpagophytum; GS, grape seed; H, Harpagophytum; HYT, hydroxytyrosol; P, pomegranate; RH, rosemary extract; RL, rosemary extract (Nutrox OS); SZ, control fortified with Zn and Se meat; SZRH+P, 1000 ppm rosemary extract + 1500 ppm pomegranate extract; SZRL+GS, 1000 ppm Nutrox OS + 1500 ppm grape seed extract; SZHYT+P+H, 1500 ppm pomegranate extract + 750 ppm hydroxytyrosol + 500 ppm Harpagophytum.
Commercial mix: mix of spices for the preparation of chicken nuggets, without preservatives or synthetic colors, supplied by Pimursa S.L. (Murcia, Spain).
After mixing all the ingredients, including natural extracts, trimmed chicken meat was placed in a cutter and homogenized for 1 min or until reaching a temperature of 15°C in a room at 4°C (knife and bowl speeds 3,000 and 10 rpm, respectively). Chicken nuggets were prepared in characteristic shapes of 5 × 3 × 1 cm, each weighing 20 g, and frozen at −18°C. Subsequently, all the nugget batches were prefried using a household fryer (Taurus S.L., Lérida, Spain) for 30 s at 165°C in sunflower oil (Koipesol Semillas S.A., Sevilla, Spain). Fifty prefried nuggets per batch (10 per day of analysis) were packaged in polyethylene bags (one per sample and day of analysis) and stored at −18°C until analysis at month 0, 3, 6, 9, and 12. All analyses were carried out at least in triplicate.
Plant Extracts
The following plant extracts were used: rosemary extract (RH, rich in rosmarinic acid, and RL, rich in diterpenes), P. granatum (P), grape (V. vinifera) seed (GS), hydroxytyrosol (HYT), from olive leaf, and H. procumbens (H). All were supplied by the Nutrafur-Frutarom group (Alcantarilla, Murcia, Spain).
Characterization of Plant Extracts
For the quantification of plant extracts, each one was diluted and filtered through a 0.45-μm nylon membrane. The high-performance liquid chromatography equipment used was a Hewlett-Packard HP 1100 equipped with a diode array detector. The stationary phase was a C18 LiChrospher 100 analytical column (250 × 4 mm i.d.) with a particle size of 5 μm (Merck, Darmstadt, Germany). The injection volume was 20 μl of each extract. The quantification of phenolic compounds was carried out by comparing the chromatographic areas of the corresponding standards. Table 2 summarizes the conditions applied for each analysis.
Table 2.
Summary of conditions applied for characterization of studied food industry by-products by HPLC.
| Extracts |
RL |
RH |
GS |
P |
HYT |
H |
|---|---|---|---|---|---|---|
| Solvent | MeOH | Water | MeOH | DMSO | DMSO | DMSO |
| Dilution (mg/ml) | 0.2–4 | 5 | 3 | 4 | 5 | 5 |
| Temperature (ºC) | 30 | 30 | 30 | 30 | 30 | 30 |
| Flow rate (ml/min) | 0.75 | 1 | 1 | 1 | 1 | 1 |
| Mobile phase | Acetonitrile (65%), water (35%), and phosphoric acid (0.2%) | Acetonitrile (65%), water (35%), and phosphoric acid (0.2%) | (A) Acetic acid/water (1:99) and (B) acetonitrile | (A) Acetic acid/water (0.1:99.5) and (B) methanol | (A) Acetic acid/water (2.5:97.5) and (B) acetonitrile | (A) Methanol and (B) water (50:50 v/v) |
| Linear gradient | - | - | From 96% (A) and 4% (B) to 90% (A) in 25 min; changing to 87% (A) in 5 min; changing to 50% (A), before re-equilibrating in 10 min | 15 min with 100% (A) was followed by a linear gradient from 100% (A) to 90% (A) and 10% (B) in 15 min; 10 min maintained before equilibration in 10 min to the initial composition | From 95% (A) and 5% (B) to 75% (A) and 25% (B) in 20 min; changing to 50% (A) and (B) in 20 min; changing to 20% (A) and 80% (B), and re-equilibrated in 10 min | - |
| Total time (min) | 25 | 25 | 45 | 50 | 60 | 25 |
| Wavelength (nm) | 230 | 340 | 280 | 280 | 280 | 280 |
Abbreviations: DMSO, dimethyl sulfoxide; GS, grape seed; H, Harpagophytum; HPLC, high-performance liquid chromatography; HYT, hydroxytyrosol; MeOH, methanol; P, pomegranate; RH, rosemary extract; RL, rosemary extract (Nutrox OS).
Chicken Nuggets
Proximate Composition
Macronutrients
Chicken nuggets were analyzed for their moisture, ash, lipid, and total protein contents following to Association of Official Analytical Chemists methods (AOAC, 2012).
Minerals
The mineral concentrations of chicken nuggets were measured by plasma spectroscopy (inductively coupled plasma, optical emission spectrometer) using an ICAP THERMO DUO 6500 computer. Inductively coupled plasma is an ionized gas, electrically neutral and confined in a discharge tube, which, together with an optical emission spectrometer, form the inductively coupled plasma, optical emission spectrometer equipment. The Zn and Se concentrations are expressed in mg/100 g.
Measurement of pH, Color (CIELab), Lipid, and Protein Oxidation
The pH of nugget samples was measured in triplicate using Crison GLP21 equipment (Crison Instruments S.A., Barcelona, Spain) (ISO 2917, 1999). For that, 5 g of sample was mixed with 20 ml of MilliQ water and homogenized. The pH was assessed at 0, 3, 6, 9, and 12 mo in triplicate.
Color was measured using a Konika Minolta CR 400 chromameter standardized using a white calibration plate (Minolta Camera Co., Osaka, Japan) (8-mm-diameter aperture, d/0 illumination system, D65 illuminant, and a 2-standard observer angle) in sextuplicate. Lightness (L*), green-red chromaticity (a*), and blue-yellow chromaticity (b*) were measured according to the CIELab system. For that, samples were cut into pieces of 5 × 3 × 0.5 cm. The color coordinates were analyzed at 0, 3, 6, 9, and 12 mo in triplicate.
Thiobarbituric acid reactive substances (TBARs) were measured according to the method described by Wang and Xiong (2005). The analysis was repeated in triplicate at 0, 3, 6, 9, and 12 mo to measure lipid oxidation.
Protein oxidation was related to the thiol concentration. The concentration of the thiol groups was determined spectrophotometrically after derivatization by Ellman's reagent, 5,5'-Dithiobis (2-nitrobenzoic acid), following the method described by Nieto et al. (2017). The analysis was carried out at 0, 3, 6, 9, and 12 mo.
Microbiological Analysis
The microbiological parameters of chicken nuggets, mesophiles, Escherichia coli, Staphylococcus aureus, Lysteria monocytogenes, and Salmonella were determined following the methods described by ICMSF (2000), pursuant RD 474/2014 of Spanish law. All the samples were analyzed in triplicate, and the counts were expressed as colony forming units per gram (cfu/g). Samples were prepared in a horizontal laminar flow cabinet (Telstar, BIO-II-A, Spain) sterilized by UV irradiation. Ten grams of sample were aseptically weighed into a stomacher bag with filter, and 90 ml of peptone water was added and homogenized. Dilutions were obtained from this mix. Plates for total plate counts (Plate Count Agar) were incubated at 37 ± 1°C for 48 h; for E. coli (Rapid E. coli), at 45 ± 1°C for 24 h; and for S. aureus (Bair-Parker), at 37 ± 1°C for 48 h. In case of Salmonella, 25 ± 0.1 g of sample was aseptically weighed, and 225 ml of peptone water was added. This mix was incubated at 37° ± 1°C for 18 h. After this time, 1 ml of this mix was added to 10 ml of Rappaport Vassiliadis Soya and incubated at 41 ± 1°C for 24 h. Then, samples were plated in Rapid Salmonella and XLD Agar. For L. monocytogenes analysis, 25 ± 0.1 g of sample was weighed, and 225 ml of Fraser Broth was added and incubated at 37°C for 22 h. Then, the bacteria were seeded on Rapid Listeria plates, which were incubated at 45°C for 24 h. The media used and peptone water were autoclaved (Steam sterilizer Raypa) at 121°C for 20 min.
Sensory Analysis
The tasting room for sensory evaluation was air-conditioned and free of disturbing factors. The prefried nugget samples were fried in a household deep fat fryer (Taurus S.L., Lerida, Spain) at 165°C for 3 min, until reaching an internal temperature of 72°C, as measured by a portable T200 thermometer (Digitron Instrumentation LD., Hertford, United Kingdom). Rectangular pieces of 2 × 1.5 cm were obtained and presented to the panelists. Sensory analysis was carried out at 0 and 12 mo after manufacture.
Ten panelists were trained according to ISO (2012. Samples were coded with a random three-digit number and were presented individually to the panelists. Mineral water and bread were provided for mouth rinsing between samples. The attributes measured for the color, odor, and taste characteristics were “Own Colour”, “Brown Colour”, “Extract Colour”, “Own Odour”, “Rancid Odour”, “Extract Odour”, “Own Flavour”, “Rancid Flavour”, “Extract Flavour”, and “Acceptability”. For that, an intensity scale, from 1 to 5, was used to measure the intensity of the several attributes, where 1 = no attribute perception and 5 = very intense attribute perception. Otherwise, “Acceptability” was measured with a hedonic scale, where 1 = “I do not like the product” and 5 = “I like it a lot. I will buy the product”.
Statistical Analysis
Data were analyzed with the statistical package SPSS 23.0 (Statistical Package for the Social Science for Window [IBM, Armonk, NY]). The effect of the different types of extract on chicken nugget quality was analyzed using ANOVA and Scheffe test. A value of P < 0.05 was considered statistically significant. Pearson's correlation was applied to test differences between groups.
Results and discussion
Characterization of Natural Extracts From Food Industry BPs
The concentrations of bioactive compounds in the natural extracts obtained as food industry BPs are represented in Table 3. The antioxidant and antimicrobial capacities of these extracts depend on the concentrations of the phenolic compounds contained in them. The extracts obtained from R. officinalis L. had 8.10% of rosmarinic acid (RH) and 5.76% of diterpenes (RL), such as, carnosol, isorosmanol, rosmadial, rosmaridiphenol, picrosalvin, and rosmariquinone. The H. procumbens extract (H) had 3.05% of harpagoside, as the bioactive compound. Grape (Vitis vinífera) seed extract (GS) contained 95.6% of oligomeric proanthocyanidins, 2.2% catechin, and also 2.2% epicatechin. Pomegranate (P) had 41.38% punicalagin as the principal bioactive compound. Finally, the hydroxytyrosol extract (HYT) obtained from olive leaf during the manufacture of olive oil contained 7.26% of hydroxytyrosol.
Table 3.
Characterization (%) of natural extracts obtained as food industry by-products measured by HPLC.
| Extracts | Rosmarinic acid | Diterpenes | Catechin | Epicatechin | OPCs | Punicalagin | Hydroxytyrosol | Harpagoside |
|---|---|---|---|---|---|---|---|---|
| RH | 8.10 | |||||||
| RL | 5.76 | |||||||
| GS | 2.2 | 2.2 | 95.6 | |||||
| P | 41.38 | |||||||
| HYT | 7.16 | |||||||
| H | 3.05 |
Abbreviations: GS, grape seed; H, Harpagophytum procumbens; HPLC, high-performance liquid chromatography; HYT, hydroxytyrosol from olive leaf; OPCs, oligomers of proanthocyanidins; P, pomegranate; RH, rosmarinic extract; RL, Nutrox OS.
Proximate Composition
The proximate composition of the frozen chicken nuggets enriched with natural extracts from fruits, seeds, and herbs is shown in Table 4. The moisture, ash, protein, and lipid contents (%) represented in this table point to no significant differences between samples. The moisture percentage ranged from 63%, in CRH+P to 67% in CRL+GS, while the ash content ranged from 1.17% in SZRL+GS to 1.61% in SZRH+P. Similar results were obtained for the protein and lipid contents. The protein content ranged from 10.65% in SZHYT+P+H to 12.24% in CHYT+P+H, while the lipid percentage ranged from 3.90% in sample C to 5.36% in sample CRH+P. Thomas et al. (2014 and 2016) showed comparable results in pork nuggets enriched with kordoi (Averrhoa carambola) fruit and bamboo (Bambusa polymorpha) shoot, the moisture content being 60-70%, and ash representing 1% of the proximate composition. Recently, Carvalho et al. (2018) published similar results in chicken nuggets enriched with omega-3 and fiber by chia (Salvia hispanica L.) flour, although they obtained higher values for the lipid (25-28 g/100 g) and ash (4 g/100 g) content because of the incorporation of flour and omega-3 in the formula.
Table 4.
Results of nutritional analysis and mineral content in frozen chicken nuggets enriched with inorganic and organic Zn and Se.
| Treatment | Samples | Proximate composition (M ± SD) |
|||||
|---|---|---|---|---|---|---|---|
| Moisture (%) | Ash (%) | Protein (%) | Lipid (%) | Se (mg/100g) | Zn (mg/100g) | ||
| Enriched with inorganic forms of Zn and Se | C | 66.56 ± 0.12 | 1.44 ± 0.04 | 10.84 ± 0.01 | 3.90 ± 0.05 | 3.10 × 10−3c | 0.44b |
| CRH+P | 63.00 ± 0.25 | 1.36 ± 0.02 | 11.26 ± 0.02 | 5.36 ± 0.03 | 4.20 × 10−3b,c | 0.53b | |
| CRL+GS | 66.97 ± 0.97 | 1.33 ± 0.11 | 10.95 ± 0.04 | 4.83 ± 0.02 | 4.00 × 10−3b,c | 0.51b | |
| CHYT+P+H | 66.82 ± 0.18 | 1.18 ± 0.05 | 12.24 ± 0.05 | 5.08 ± 0.01 | 3.50 × 10−3c | 0.49b | |
| Enriched with organic forms of Zn and Se | SZ | 64.43 ± 0.49 | 1.57 ± 0.07 | 11.26 ± 0.02 | 4.46 ± 0.04 | 6.70 × 10−3a | 0.58a,b |
| SZRH+P | 64.39 ± 0.21 | 1.61 ± 0.10 | 11.23 ± 0.06 | 5.35 ± 0.02 | 5.70 × 10−3a,b | 0.74a | |
| SZRL+GS | 64.83 ± 0.88 | 1.17 ± 0.08 | 11.19 ± 0.03 | 4.57 ± 0.03 | 4.20 × 10−3b,c | 0.77a | |
| SZHYT+P+H | 65.61 ± 0.67 | 1.39 ± 0.06 | 10.67 ± 0.02 | 5.09 ± 0.02 | 4.60 × 10−3b | 0.72a | |
Different letters among data in the same column indicate significant differences between samples (P < 0.05).
Abbreviations: C, control; CRH+P, 1000 ppm rosemary extract + 1500 ppm pomegranate extract; CRL+GS, 1000 ppm Nutrox OS + 1500 ppm grape seed extract; CHYT+P+H, 1500 ppm pomegranate extract + 750 ppm hydroxytyrosol + 500 ppm Harpagophytum; M, mean; SD, standard deviation; SZ, control fortified with Zn and Se meat; SZRH+P, 1000 ppm rosemary extract + 1500 ppm pomegranate extract; SZRL+GS, 1000 ppm Nutrox OS + 1500 ppm grape seed extract; SZHYT+P+H, 1500 ppm pomegranate extract + 750 ppm hydroxytyrosol + 500 ppm Harpagophytum.
By contrast, there were significant differences (P < 0.05) between samples in terms of the Se and Zn contents (mg/100 g). Samples prepared with meat from chicken broilers fed with organic forms of Zn and Se showed higher concentrations of Zn and Se than the samples made from meat enriched with inorganic Zn and Se. This fact may be related to the findings of our previous research (Martínez et al., 2018a, Martínez et al., 2018b), in which a meat emulsion prepared with chicken meat enriched with Zn and Se was evaluated in a Caco-2 cell model, when it was found that the organic forms of these minerals are more bioavailable than the inorganic forms. The highest content of Se was found in SZ with no natural extracts, while the highest concentrations of Zn were found in SZRL+GS, SZRH+P, and SZHYT+P+H. Although no mention has been found in the literature, it seems that phenolic compounds from R. officinalis, grape (V. vinifera) seed, P. granatum, hydroxytyrosol, and H. procumbens are rich in Zn, but not in Se, because their incorporation increases the Zn content but decreases the Se concentration.
The daily consumption of 100 g of chicken nuggets enriched in organic forms of Zn and Se (SZRH+P, SZRL+GS, SZHYT+P+H) would represent 6.4–9.6% of the recommended daily allowance for Zn for a healthy adult (8–12 mg/day) and 9–10% of the recommended daily allowance of Se (55–70 μg/day). It can therefore be stated that consumption of this kind of product contributes to the recommended levels of these essential minerals, as would a diet containing other products rich in Se and Zn, such as oat, mussels, mushrooms, beer yeast, or cockles.
Shelf Life Study
pH
Variations in pH are associated with food deterioration because pH values are an indicator of food stability associated with microbial growth and chemical reactions. Table 5 shows the changes in pH during the 12 mo of frozen storage. As it can be seen, there were no significant differences among samples at the same analysis times, but there were differences between months (P < 0.05). The pH values of deep-fried chicken nuggets formulated with combinations of natural extracts ranged from 6.10 to 6.64 because natural sources of phenolic compounds prevent meat oxidation and a decrease in pH, while frozen storage reduces water activity and prevents microbiological growth.
Table 5.
Results of pH values and color CIELab (M ± SD) in frozen chicken nuggets for 12 mo of storage.
| CIELab |
Storage time (months) |
||||
|---|---|---|---|---|---|
| Samples | 0 | 3 | 6 | 9 | 12 |
| pH | |||||
| C | 6.46 ± 0.03b | 6.55 ± 0.08a | 6.26 ± 0.05c | 6.14 ± 0.08d | 6.45 ± 0.09b |
| CRH+P | 6.48 ± 0.05b | 6.54 ± 0.04a | 6.36 ± 0.01c | 6.15 ± 0.02d | 6.42 ± 0.07c |
| CRL+GS | 6.50 ± 0.10b | 6.64 ± 0.02a | 6.55 ± 0.05c | 6.17 ± 0.03d | 6.40 ± 0.20c |
| CHYT+P+H | 6.49 ± 0.08b | 6.62 ± 0.05a | 6.43 ± 0.08c | 6.20 ± 0.05d | 6.46 ± 0.16c |
| SZ | 6.41 ± 0.06b | 6.56 ± 0.07a | 6.39 ± 0.05c | 6.10 ± 0.10d | 6.42 ± 0.04c |
| SZRH+P | 6.38 ± 0.04b | 6.61 ± 0.05a | 6.33 ± 0.11c | 6.11 ± 0.06d | 6.41 ± 0.02c |
| SZRL+GS | 6.54 ± 0.05b | 6.63 ± 0.09a | 6.48 ± 0.18c | 6.24 ± 0.10d | 6.45 ± 0.15c |
| SZHYT+P+H | 6.44 ± 0.02b | 6.62 ± 0.11a | 6.40 ± 0.04c | 6.19 ± 0.15d | 6.38 ± 0.06c |
| L* (lightness) | |||||
| C | 75.64 ± 2.06c | 83.62 ± 2.01a | 81.93 ± 1.98a | 84.23 ± 1.25a | 74.22 ± 1.14b |
| CRH+P | 66.83 ± 1.88c | 80.59 ± 2.15a | 82.77 ± 1.85a | 85.27 ± 1.36a | 69.84 ± 0.87b |
| CRL+GS | 62.51 ± 1.25c | 80.10 ± 1.84a | 79.86 ± 3.01a | 81.36 ± 2.05a | 65.57 ± 1.54b |
| CHYT+P+H | 64.64 ± 1.54c | 81.19 ± 1.91a | 78.94 ± 2.54a | 80.65 ± 2.47a | 69.86 ± 1.99b |
| SZ | 78.50 ± 1.78c | 82.24 ± 1.35a | 83.92 ± 1.25a | 84.74 ± 1.88a | 75.68 ± 2.30b |
| SZRH+P | 66.23 ± 2.31c | 77.47 ± 1.88a | 80.08 ± 1.86a | 80.05 ± 1.79a | 75.18 ± 3.14b |
| SZRL+GS | 64.49 ± 3.01c | 77.70 ± 2.22a | 82.47 ± 1.88a | 82.07 ± 0.98a | 73.23 ± 2.11b |
| SZHYT+P+H | 65.56 ± 2.89c | 76.03 ± 2.54a | 79.78 ± 1.96a | 79.12 ± 1.30a | 70.50 ± 1.85b |
| a* (redness) | |||||
| C | 1.64 ± 0.03a | 0.41 ± 0.01b,c | 0.55 ± 0.05b,c | 0.47 ± 0.03b,c | 1.13 ± 0.47b |
| CRH+P | 6.31 ± 1.04a | 1.77 ± 0.17b,c | 2.02 ± 0.85b,c | 0.86 ± 0.11c | 2.82 ± 1.15b |
| CRL+GS | 7.4 ± 1.07a | 1.69 ± 0.94b,c | 1.44 ± 0.34b,c | 0.16 ± 0.02c | 4.41 ± 1.99b |
| CHYT+P+H | 6.85 ± 1.17a | 1.59 ± 0.69b,c | 2.63 ± 1.02b,c | 1.91 ± 0.91c | 2.43 ± 0.87b |
| SZ | 2.98 ± 0.05a | 0.30 ± 0.01b,c | 0.73 ± 0.08b,c | 0.04 ± 0.00c | 1.03 ± 0.55b |
| SZRH+P | 6.29 ± 0.01a | 3.23 ± 1.25b,c | 2.96 ± 1.15b,c | 2.17 ± 0.88c | 3.03 ± 1.02b |
| SZRL+GS | 6.35 ± 0.15a | 1.12 ± 0.05b,c | 0.71 ± 0.22b,c | 0.93 ± 0.15c | 2.27 ± 0.77b |
| SZHYT+P+H | 5.27 ± 1.24a | 2.11 ± 0.24b,c | 2.69 ± 0.88b,c | 1.75 ± 0.79c | 2.64 ± 0.97b |
| b* (yellowness) | |||||
| C | 28.80 ± 1.15a | 21.01 ± 3.00c | 22.03 ± 1.42b,c | 20.12 ± 1.25b | 10.80 ± 0.54d |
| CRH+P | 33.90 ± 1.26a | 21.92 ± 1.87c | 21.60 ± 1.87b,c | 24.78 ± 0.86b | 15.51 ± 0.32d |
| CRL+GS | 28.89 ± 1.98a | 20.82 ± 2.03c | 22.01 ± 1.24b,c | 22.48 ± 2.74b | 14.61 ± 0.01d |
| CHYT+P+H | 33.45 ± 2.41a | 21.93 ± 1.96c | 21.88 ± 2.56b,c | 24.15 ± 2.81b | 14.12 ± 2.14d |
| SZ | 33.77 ± 1.47a | 20.09 ± 2.01c | 23.22 ± 3.05b,c | 22.44 ± 3.05b | 13.63 ± 1.24d |
| SZRH+P | 32.83 ± 1.88a | 19.25 ± 1.44c | 22.46 ± 2.87b,c | 25.91 ± 1.78b | 15.78 ± 1.01d |
| SZRL+GS | 23.49 ± 1.99a | 17.50 ± 1.87c | 20.45 ± 1.85b,c | 17.48 ± 1.45b | 12.64 ± 0.83d |
| SZHYT+P+H | 29.88 ± 2.54a | 19.15 ± 1.25c | 19.48 ± 0.76b,c | 20.92 ± 1.58b | 13.45 ± 1.02d |
Different letters among data in the same row indicate significant differences between month of analysis (P < 0.05).
Abbreviations: C, control; CRH+P, 1000 ppm rosemary extract + 1500 ppm pomegranate extract; CRL+GS, 1000 ppm Nutrox OS + 1500 ppm grape seed extract; CHYT+P+H, 1500 ppm pomegranate extract + 750 ppm hydroxytyrosol + 500 ppm Harpagophytum; M, mean; SD, standard deviation; SZ, control fortified with Zn and Se meat; SZRH+P, 1000 ppm rosemary extract + 1500 ppm pomegranate extract; SZRL+GS, 1000 ppm Nutrox OS + 1500 ppm grape seed extract; SZHYT+P+H, 1500 ppm pomegranate extract + 750 ppm hydroxytyrosol + 500 ppm Harpagophytum.
Teruel et al. (2015) obtained different results in chicken nuggets. During 9 mo of frozen storage, they observed no significant differences (P < 0.05) in pH values, although the initial pH values were similar. In their research, rosemary extracts were incorporated in the chicken nuggets formula, but they did not combine different sources of phenolic compound, such as RH + P, RL + GS, or HYT + P + H, as the present study does. Verma et al. (2010) and Hwang et al. (2013) also obtained different results after incorporating apple pulp and Artemisia prínceps Pamp., respectively. However, Verma et al. (2010) did not carry out a shelf life study, while Hwang et al. (2013) did so for 15 days of refrigerated storage.
Color
Table 5 shows the results obtained for CIELab measurements in all the samples during the 12 mo of frozen storage. L* (lightness), a* (redness), and b* (yellowness) showed significant differences (P < 0.05) between the months of storage (0, 3, 6, 9, and 12), but there were no differences between samples at these times.
As it can be observed, L* increased from time 0 to the third month, then remained constant, but it fell again by the 12th mo. All the values ranged from 84.74 to 62.51. On the other hand, both a* and b* decreased from month 0 to month 12. Values for a* ranged from 6.85 to 0.41, while for b*, they ranged from 33.90 to 10.80.
An analysis of these results points to no significant differences between samples (P < 0.05), although it can be observed that the samples with the lowest variations in CIELab color were the nuggets enriched in organic forms of Zn and Se, especially the sample that incorporated rosemary and pomegranate, SZRH+P, followed by SZRL+GS and SZHYT+P+H. The least stable samples in this respect were C and SZ. Thus, although incorporating organic forms of Zn and Se helps to maintain the color, it is also necessary to incorporate sources of phenolic compounds, such as rosemary, pomegranate, or hydroxytyrosol.
Other studies with chicken nuggets showed similar results regarding color (Hwang et al., 2013, Teruel et al., 2014, Teruel et al., 2015, Carvalho et al., 2018) by incorporating chia, rosemary, and even ascorbic acid with ganghwayakssuk. However, these studies were shorter than the present research, while no studies that combine feed sources of organic minerals and the addition of extracts rich in phenolic compounds have been found.
Lipid Oxidation
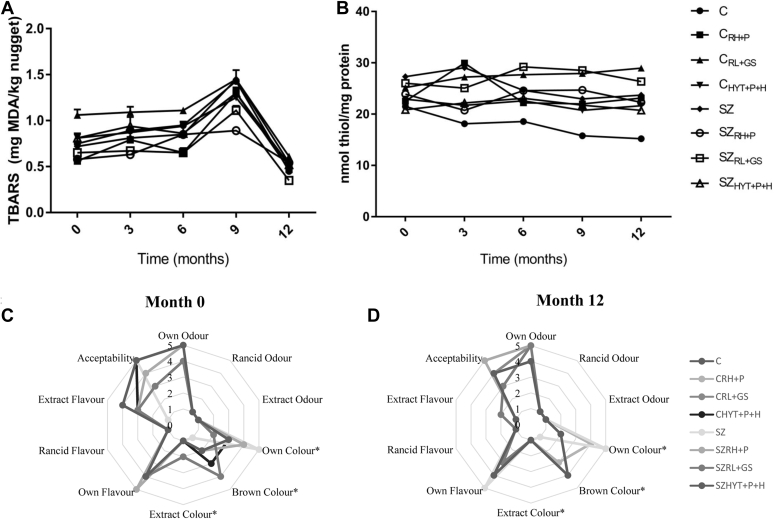
The malondialdehyde (MDA) content of the frozen nuggets is shown in Figure 1A. The TBAR values represent the aldehydes and carbonyls as secondary lipid oxidation products that alter the flavor of meat. As it can be noticed, lipid oxidation increased significantly (P < 0.05) up to 1.5 mg MDA/kg at month 9 of frozen storage in the C, SZ, and CRL+GS samples. However, when organic forms of Zn and Se were combined with rosemary and pomegranate in SZRH+P, this sample resisted lipid oxidation and showed 47% lower TBARs values than C or SZ after 12 mo of storage (P < 0.05). The decrease in lipid oxidation recorded at this time might be caused by losses in the oxidation products formed or the reaction of MDA with proteins (Maqsood and Benjakul, 2010).
Figure 1.
Results of lipid oxidation, TBARs (mg MDA/kg) (A); protein oxidation, thiol groups (nmol thiol/mg protein) (B); and sensory evaluation (C: at time 0 and D: at month 12) of chicken frozen nuggets for 12 mo of storage. Mean values (n=8) for each sensory descriptor by chicken nuggets. The relevant significance is reported for each descriptor (*P < 0.05). C, control; CRH+P, 1000 ppm rosemary extract + 1500 ppm pomegranate extract; CRL+GS, 1000 ppm Nutrox OS + 1500 ppm grape seed extract; CHYT+P+H, 1500 ppm pomegranate extract + 750 ppm hydroxytyrosol + 500 ppm Harpagophytum; GS, grape seed; H, Harpagophytum; HYT, hydroxytyrosol; MDA, malondialdehyde; P, pomegranate; RH, rosemary extract; RL, rosemary extract (Nutrox OS); SZ, control fortified with Zn and Se meat; SZRH+P, 1000 ppm rosemary extract + 1500 ppm pomegranate extract; SZRL+GS, 1000 ppm Nutrox OS + 1500 ppm grape seed extract; SZHYT+P+H, 1500 ppm pomegranate extract + 750 ppm hydroxytyrosol + 500 ppm Harpagophytum; TBAR, thiobarbituric acid reactive substance.
This antioxidant effect shown in samples enriched exogenously with natural extracts obtained as food industrial BPs would be due to their high phenolic content, as can be seen in Table 3. For example, the sample with the lowest MDA level combined RH with 8.10% rosmarinic acid and P with 41.38% punicalagin. In addition, the incorporation of HYT (7.16%), diterpenes from RL (5.8%), and catechins from GS (4.4%) also reduced the TBAR levels by a 25–35% compared with the values recorded in C and SZ.
Similar trends in TBAR values were observed by Hwang et al. (2013) in chicken nuggets that incorporated ganghwayakssuk (Artemisia prínceps Pamp.) in combination with ascorbic acid to increase the shelf life (15 days refrigerated storage at 4°C). While no similar results have been found in this kind of product, Nieto et al. (2017) observed the same trend in chicken sausages, which were enriched with hydroxytyrosol extracts, walnuts, and extra virgin olive oil and analyzed during 21 D of refrigerated storage.
Protein Oxidation
Protein oxidation was determined by reference to the thiol groups (see Figure 1B in which it can be seen that their concentration slightly decreases during frozen storage). Nevertheless, samples that incorporated RL and grape seed extract (CRL+GS and SZRL+GS) showed much higher concentrations of thiol groups than C and SZ. In the same way, the incorporation of organic forms of Zn and Se in SZ decreases protein oxidation compared with C because of the antioxidant capacity of the minerals. In this sense, it can be said that the combination of phenolic compound sources with Zn and Se protects against the loss of thiol groups for up to 1 y of frozen storage. All treatments with plant extracts had a higher concentration of thiol groups than the control, indicating less protein oxidation during storage. Even though no similar studies have been found, our group, Nieto et al. (2013) observed a similar protective effect against the loss of thiol groups during 9 D of chilled storage in pork patties containing sources of phenolic compounds (in this case, the essential oils of oregano, rosemary, or garlic). Jongberg et al. (2018) observed a reduction in protein oxidation in brine-injected pork loins containing ascorbate and green tea or mate extracts during chilled storage. Antioxidant compounds maintain higher levels of thiol groups, indicating less protein oxidation during storage than control nuggets without the addition of plant extracts. In fact, this might explain why CRH+P, SZRH+P, and SZRL+GS had lower levels of thiol loss at month 9 and 12 than the rest of the samples, which were also rich in phenolic compounds but had a higher molecular weight, preventing crosslinking with the protein chain.
Microbial Growth
The results of the microbiological analyses (cfu/g) made in frozen chicken nuggets over the 12 mo are shown in Table 6. As it can be seen, all the results comply with the legal limits (EC 2073/2005 for Europe; RD 474/2014 for Spain). All the samples showed <10 cfu/g of E. coli and S. aureus and no L. monocytogenes and Salmonella in 25 g at all sampling times. However, significant differences were obtained for the total viable counts (cfu/g) among different samples and months of frozen storage.
Table 6.
Results of microbiological analysis (M ± SD cfu/g) in frozen chicken nuggets for 12 mo of storage.
| Microorganism | Samples | Storage time (months) |
||||
|---|---|---|---|---|---|---|
| 0 | 3 | 6 | 9 | 12 | ||
| TVC | C | 550 ± 40a,z | 2400 ± 120a yz | 6500 ± 250a xy | 8950 ± 425a wx | 10,000 ± 500a,w |
| CRH+P | 725 ± 50bc,z | 975 ± 45bc yz | 1000 ± 60bc xy | 2350 ± 115bc wx | 4500 ± 425bc,w | |
| CRL+GS | 665 ± 46c,z | 780 ± 50c yz | 1200 ± 50c xy | 1500 ± 80c wx | 2500 ± 180c,w | |
| CHYT+P+H | 615 ± 34bc,z | 900 ± 70bc yz | 1450 ± 25bc xy | 1850 ± 95bc wx | 4700 ± 200bc,w | |
| SZ | 1100 ± 90abc,z | 1200 ± 80abc yz | 3150 ± 210abc xy | 4500 ± 120abc wx | 8500 ± 350abc,w | |
| SZRH+P | 725 ± 67ab,z | 1200 ± 90ab yz | 1500 ± 200ab xy | 3200 ± 320ab wx | 4500 ± 495ab,w | |
| SZRL+GS | 220 ± 40c,z | 400 ± 50c yz | 700 ± 80c xy | 1000 ± 60c wx | 2500 ± 120c,w | |
| SZHYT+P+H | 1020 ± 98bc,z | 1475 ± 115bc yz | 2300 ± 90bc xy | 3350 ± 250bc wx | 5000 ± 290bc,w | |
| Escherichia coli | <10 | |||||
| Staphylococcus aureus | <10 | |||||
| Lysteria monocytogenes | Absence in 25 g | |||||
| Salmonella | Absence in 25 g | |||||
Different letters among data in the same column indicate significant differences between samples (P < 0.05).
Different letters among data in the same row indicate significant differences between month of analysis (P < 0.05).
Abbreviations: C, control; CRH+P, 1000 ppm rosemary extract + 1500 ppm pomegranate extract; CRL+GS, 1000 ppm Nutrox OS + 1500 ppm grape seed extract; CHYT+P+H, 1500 ppm pomegranate extract + 750 ppm hydroxytyrosol + 500 ppm Harpagophytum; M, mean; SD, standard deviation; SZ, control fortified with Zn and Se meat; SZRH+P, 1000 ppm rosemary extract + 1500 ppm pomegranate extract; SZRL+GS, 1000 ppm Nutrox OS + 1500 ppm grape seed extract; SZHYT+P+H, 1500 ppm pomegranate extract + 750 ppm hydroxytyrosol + 500 ppm Harpagophytum; TVC, total viable count.
It can be detected how the control samples, C and SZ, with inorganic and organic forms of Zn and Se, respectively, had higher TVC values than the rest of the samples that included natural extracts. Moreover, samples that incorporated RL and GS extract (CRL+GS, SZRL+GS) obtained the best results for microbiological growth (75% and 70%, respectively, less than C and SZ), followed by samples containing RH and P (CRH+P, SZRH+P), with 55% and 47% less, respectively. Finally, samples that combined HYT, P, and H (CHYT+P+H, SZHYT+P+H) showed a 53% and 41% lower TVC than the controls (C and SZ). This demonstrates that although the final counts were lower in samples incorporating organic Zn and Se, the results could be improved if phenolic compound sources were added.
Similar TVC results were obtained by Hwang et al. (2013) in chicken nuggets enriched with ganghwayakssuk and by Thomas et al. (2014 and 2016) in pork nuggets with kordoi fruit juice and bamboo shoot extract on day 0.
Sensory Analysis
Sensory analysis of chicken nuggets was carried out at 0 and 12 mo of frozen storage. The results are shown in Figure 1C and 1D.
With regard to the color values, there were significant differences (P < 0.05) between the “Own Colour”, “Brown Colour”, and “Extract Colour” results, all related with the CIELab results. The C and SZ samples obtained the highest score for “Own Colour”, at 0 and 12 mo, while the rest of samples were valued as browner (“Brown Colour”). On the other hand, rancid odor and flavor were not detected at either time, which may be related with the TBAR values that did not exceed 2 mg MDA/kg (Gray and Pearson, 1987). Therefore, “Own Odour” and “Own Flavour” were valued positively, while “Extract Flavour” was highly scored in samples with natural extracts at month 0 of analysis, although this attribute has disappeared by month 12. However, no previous research results have been found to compare this effect. It is possible that the compounds responsible for strong flavors, HYT, RH, or GS, are degraded during lengthy frozen storage because phenolic compounds react with the molecules produced by lipid and protein oxidation. This effect needs further investigation. The data regarding textural attributes are not presented because there were no significant differences between the samples and controls (C and SZ). Finally, “Acceptability” was positively valued in all the samples at month 0 as 12, so the incorporation of phenolic compounds exogenously and the minerals Zn and Se endogenously had little effect on the sensory quality compared with control samples (C and SZ).
These results can be compared with those of previous research. For example, Banerjee et al. (2012) showed that the incorporation of broccoli extract did not affect goat meat nuggets stored refrigerated for 16 days. However, chicken with its mild flavor, compared with goat meat, allows strong spice flavors to be more noticeable, with negative effects on chicken nugget flavor. Radha et al. (2014) found that Syzygium aromaticum, Cinnamomum cassia, Origanum vulgare, and Brassica nigra extracts negatively affected the sensory quality. Similarly, the addition of rosemary extracts at 300 – 900 ppm to chicken nuggets had the same effect, decreasing the sensory quality of the product (Teruel et al. 2015). In contrast, Carvalho et al. (2018) obtained chicken nuggets with good sensory quality after incorporating chia (Salvia hispánica L.) flour, although no herbs or spices with strong flavor were added.
Conclusions
The present study showed that the addition of phenolic compounds in the form of natural extracts from seeds, herbs, and fruits, together with organic forms of Zn and Se, can retard microbial growth, reduce protein and lipid oxidation, maintain the sensory quality, and extend the shelf life of chicken nuggets during 1 y of frozen storage. In particular, the SZRH+P and SZRL+GS samples were significantly more resistant to oxidative reactions and microbial growth than control samples. The chicken nugget samples enriched with minerals did not show significant differences in pH, color (CIELab), or proximate composition. Therefore, the incorporation of minerals in animal feed combined with the use of natural antioxidant sources may be considered a good way to replace synthetic additives in meat products and a further step toward obtaining “C. label” meat products. Owing to the health benefits of these extracts as a source of antioxidants, their application in the meat industry is to be recommended to substitute or reduce the concentration of synthetic additives currently used.
References
- AESAN/MARM . Ministerio de Agricultura, Alimentación y Medio Ambiente; 2019. Encuesta Nacional de Ingesta Dietética (ENIDE). Agencia Española de Seguridad Alimentaria y Nutrición. [Google Scholar]
- Alu'datt M.H., Rababah T., Alhamad M.N., Gammoh S., Al-Mahasneh M.A., Tranchant C.C., Rawshdeh M. Chapter 15 – Pharmaceutical Nutraceutical and Therapeutic properties of selected wild medicinal plants: thyme, spearmint and Rosemary. In: Grumezescu A.M., Holban A.M., editors. Therapeutic, Probiotic and Unventional Food. Academic Press; 2018. pp. 275–290. [DOI] [Google Scholar]
- AOAC . 17th ed. Association of Official Analyticial Chemistry; Maryland, USA: 2012. Official Methods of Analysis of AOAC International. [Google Scholar]
- Banerjee R., Verma A.K., Das A.K., Rajkumar V., Shewalkar A.A., Narkhede H.P. Antioxidant effects of broccoli poder extract in goat meat nuggets. Meat. Sci. 2012;91:179–184. doi: 10.1016/j.meatsci.2012.01.016. [DOI] [PubMed] [Google Scholar]
- Carvalho J., Sichetti P.E., Alves M., Rodrigues I., Slaoui O., Da Costa C.E., Trindade M.A. Omega-3- and fibre-enriched chicken nuggets by replacement of chicken skin with chia (Salvia hispánica L.) flour. LWT- Food Scie. Technol. 2018;90:283–289. [Google Scholar]
- BEDCA/AESAN Spanish food composition database (BEDCA) 2019. http://www.bedca.net/bdpub/
- Biswas A.K., Keshri R.C., Bisht G.S. Effect of enrobing and antioxidants on quality characteristics of precooked pork patties under chilled and frozen storage conditions. Meat Sci. 2004;66:733–741. doi: 10.1016/j.meatsci.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Cosansu S., Juneja V.K. Growth of Clostridium perfringens in sous vide cooked ground beef with added grape seed extract. Meat Sci. 2018;143:252–256. doi: 10.1016/j.meatsci.2018.05.013. [DOI] [PubMed] [Google Scholar]
- Delles R.M., Xiong Y.L., True A.D., Ao T., Dawson K.A. Dietary antioxidant supplementation enhances lipid and protein oxidative stability of chicken broiler meat through promotion of antioxidant enzyme activity. Poult. Sci. 2014;93(6):1561–1570. doi: 10.3382/ps.2013-03682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estévez M., Parra V., Villaverde A., Utrera M. Oxidación de proteínas cárnicas (II): estrategias antioxidants. Eurocarne. 2012;212:88–94. [Google Scholar]
- Gray J.L., Pearson A.M. Rancidity and warmed-over flavor. In: Pearson A.M., Dutson T.R., editors. Advances in Meat Research. Van Nostrand; New York: 1987. pp. 221–269. [Google Scholar]
- Gustavsson J., Cederberg C., Sonesson U., Van Otterdijk R., Meybeck A. Food and agriculture organization of the United Nations; Rome, Italy: 2011. Global Food Losses and Food Waste. [Google Scholar]
- Hwang K.E., Choi Y.S., Choi S.M., Kim H.W., Choi J.H., Lee M.A., Kim C.J. Antioxidant action of ganghwayakssuk (Artemisa princeps Pamp.) in combination with ascorbic acid to increase the shelf life in raw and deep fried chicken nuggets. Meat Sci. 2013;95:593–602. doi: 10.1016/j.meatsci.2013.05.035. [DOI] [PubMed] [Google Scholar]
- International commission on microbiological specifications for foods – ICMSF. (2000). Microorganisms in foods I. 2º Edición.
- International Standards Organization – ISO . The International Organization of Standardization; Geneva, Switzerland: 1999. Meat and Meat Products – Measurements of pH (Reference Method), ISO 2917. [Google Scholar]
- International Standards Organization – ISO . The International Organization of Standardization; Geneva, Switzerland: 2012. Sensory Analysis – General Guidance for the Selection, Training and Monitoring of Assessors, ISO 8586. [Google Scholar]
- Jongberg S., Torngren M.A., Skibsted L.H. Brine-injected porl loins added ascorbate or extracts of green tea or maté during chill-storage in high-oxygen modified atmosphere. Medicines. 2018;5 doi: 10.3390/medicines5010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khwairakpam A.D., Bordoloi D., Thakur K.K., Monisha J., Arfuso F., Sethi G., Mishra S., Kumar A.P., Kunnumakkara A.B. Possible use of Punica gratum (Pomegranate) in cáncer therapy. Pharmacol. Res. 2018;133:53–64. doi: 10.1016/j.phrs.2018.04.021. [DOI] [PubMed] [Google Scholar]
- Lytou A.E., Nychas G.J.E., Panagou E.Z. Effect of pomegranate based marinades on the microbiological, chemical and sensory quality of chicken meat. A metabolomics approach. Int. J. Food Microbiol. 2018;267:42–53. doi: 10.1016/j.ijfoodmicro.2017.12.023. [DOI] [PubMed] [Google Scholar]
- Maqsood S., Benjakul S. Synergistic effect of tannic acid and modified atmospheric packaging on the prevention of lipid oxidation and quality losses of refrigerated striped catfish slices. Food Chem. 2010;121:29–38. [Google Scholar]
- Martinez J., Nieto G., Castillo J., Ros G. Influence of in vitro gastrointestinaldigestion and/or grape seed extract addition on antioxidant capacity of meat emul-sions. Lebensmittel-Wissenschaft und-Technologie. 2014;59:834–840. doi: 10.1016/j.lwt.2014.07.048. [DOI] [Google Scholar]
- Martínez L., Ros G., Nieto G. Hydroxytyrosol: health benefits and use as functional ingredient in meat. Medicines. 2018;5:1–13. doi: 10.3390/medicines5010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez L., Ros G., Nieto G. Fe, Zn and Se bioavailability in chicken meat emulsions enriched with minerals, hydroxytyrosol and extra virgin olive oil as measured by Caco-2 cell model. Nutrients. 2018;10 doi: 10.3390/nu10080969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWangi N., Chen W., Vermaak I., Viljoen A.M., Gericke N. Deviĺs claw-A review of the ethnobotany, phytochemistry and biological activity of Harpagophytum procumbens. J. Ethnopharmacol. 2012;143:755–771. doi: 10.1016/j.jep.2012.08.013. [DOI] [PubMed] [Google Scholar]
- Mora L., Reig M., Toldrá F. Bioactive peptides generated from meat industry by-products. Food Res. Int. 2014;65:344–349. [Google Scholar]
- Naveena B.M., Sen A.R., Kingsly R.P., Singh D.B., Kondaiah N. Antioxidant activity of pomegranate rind poder extract in cooked chicken patties. J. Food Sci.Tecnhnol. 2008;43:1807–1812. [Google Scholar]
- Nieto G., Bañon S., Garrido M.D. Incorporation of thyme leaves in the diet of pregnant and lactating ewes: effect on the fatty acid profile of lamb. Small Rum. Res. 2012;105:140–147. [Google Scholar]
- Nieto G., Bañon S., Garrido M.D. Administration of distillate thyme leaves into the diet of Segureña ewes: effect on lamb meat quality. Animal. 2012;6:2048–2056. doi: 10.1017/S1751731112001012. [DOI] [PubMed] [Google Scholar]
- Nieto G. Incorporation of by-products of rosemary and thyme in the diet of ewes: effect on the fatty acid profile of lamb. Eur. Food Res. Technol. 2013;236:379–389. [Google Scholar]
- Nieto G., Jongberg S., Andersen M.L., Skibsted L.H. Thiol oxidation and protein cross-link formation during chill storage of pork patties added essential oil of oregano, rosemary or garlic. Meat Sci. 2013;95:177–184. doi: 10.1016/j.meatsci.2013.05.016. [DOI] [PubMed] [Google Scholar]
- Nieto G., Martínez L., Ros G. Hydroxytyrosol extracts, olive oil and walnuts as functional components in chicken sausages. J. Sci. Food Agric. 2017 doi: 10.1002/jsfa.8240. [DOI] [PubMed] [Google Scholar]
- Nowshehri J.A., Bhat Z.A., Shah M.Y. Blessings in disguise: bio-functional benefits of grape seed extracts. Food Res. Int. 2015;77:333–348. [Google Scholar]
- Oropeza-Moe M., Wisløff H., Bernhoft A. Selenium deficiency associated porcine and human cardiomyopathies. J. Trace. Elem. Med. Biol. 2015;31:148–156. doi: 10.1016/j.jtemb.2014.09.011. [DOI] [PubMed] [Google Scholar]
- Pradal D., Vauchel P., Decossin S., Dhulster P., Dimitrov K. Integrated extraction-adsorption process for selective recovery of antioxidant phenolics from food industry by-product. Chem. Eng. Process. – Process Intensification. 2018;127:83–92. [Google Scholar]
- Radha K., Babuskin S., Ashagu P., Sasikala M., Sabina K., Archana G., Sivarajan M., Sukumar M. Antiomicrobial and antioxidant effects of spice extracts on the shelf life extension of raw chicken meat. Int. J. Food Microbiol. 2014;171:32–40. doi: 10.1016/j.ijfoodmicro.2013.11.011. [DOI] [PubMed] [Google Scholar]
- Ragni M., Vicenti A., Melodia L., Marsico G. Use of grape seed flour in feed for lambs and effects on performance and meat quality. APCBEE Proced. 2014;8:59–64. [Google Scholar]
- Smeti S., Hajji H., Mekki I., Mahouachi M., Atti N. Effects of dose and administration form of Rosemary essential oils on meat quality and fatty acid profile of lamb. Small Ruminant Res. 2018;148:62–68. [Google Scholar]
- Stojanovi-Radic Z., Pejcic M., Jokovi N., Jokanovic M., Ivic M., Sojic B., Skaljac S., Stojanovic P., Mihajilov-Krstev T. Inhibition of Salmonella Enteritidis growth and storage stability in chicken meat treated with basil and Rosemary essential oils alone or in combination. Food Control. 2018;90:332–343. [Google Scholar]
- Teruel M.R., García-Segovia P., Martínez-Monzó J., Linares M.B., Garrido M.D. Use of vaccum-frying in chicken nugget processing. Innov. Food Sci. Emerg. Technol. 2014;26:482–489. [Google Scholar]
- Teruel M.R., Garrido M.D., Espinosa M.C., Linares M.B. Effect of different format-solvent rosemary extracts (Rosmarinus officinalis L.) on frozen chicken nuggets quality. Food Chem. 2015;172:40–46. doi: 10.1016/j.foodchem.2014.09.018. [DOI] [PubMed] [Google Scholar]
- Thomas R., Jebin N., Barman K., Das A. Quality and shelf life evaluation of pork nuggets incorporated with fermented bamboo shoot (Bambusa polymorpha) mince. Meat Sci. 2014;96:1210–1218. doi: 10.1016/j.meatsci.2013.10.035. [DOI] [PubMed] [Google Scholar]
- Thomas R., Jebin N., Saha R., Sarma D.K. Antioxidant and antimicrobial effects of kordoi (Averrhoa carambola) fruit juice and bamboo (Bambusa polymorpha) shoot extract in pork nuggets. Food Chem. 2016;190:41–49. doi: 10.1016/j.foodchem.2015.05.070. [DOI] [PubMed] [Google Scholar]
- Verma A.K., Sharma B.D., Banerkee R. Effect of sodium chloride replacement and apple pulp inclusión on the physico-chemical, textural and sensory properties of low fat chicken nuggets. LWT-Food Sci. Technol. 2010;43:715–719. [Google Scholar]
- Wang L.L., Xiong Y.L. Inhibition of lipid oxidation in cooked beef patties by hydrolyzed potato protein is related to its reducing and radical scavenging ability. J. Agric. Food Chem. 2005;53:9186–9192. doi: 10.1021/jf051213g. [DOI] [PubMed] [Google Scholar]