Abstract
The aim of this study was to evaluate the effects of freezing diluents supplemented in three potential amines/amino acids, namely, antioxidant cysteamine (2-aminoethanethiol [AET]), ergothioneine (ERG), and serine (SER), in optimization of chicken sperm cryopreservation. The semen of 36 Pradu Hang Dum males, selected based on their motility vigor score, was frozen by a simple freezing method using nitrogen vapors and dimethylformamide (DMF). In a first experiment, a wide range of AET, ERG, and SER doses were tested. Semen quality was evaluated after incubation at 5°C or after cryopreservation in straws in the Blumberger Hahnen Sperma Verdünner (BHSV) diluent + DMF (6% v/v) with or without AET, ERG, or SER. The best targeted doses of AET, ERG, or SER were then selected for experiment 2 that was focused on cryopreserved semen. Frozen-thawed sperm quality was evaluated by different in vitro tests and by evaluation of fertility. Objective motility parameters were evaluated by computer-assisted sperm analysis. Membrane integrity, acrosome integrity, and mitochondria function were evaluated using appropriate dyes and flow cytometry. Lipid peroxide production was assessed by the thiobarbituric acid test (malondialdehyde production). Fertility obtained with frozen-thawed semen supplemented or not in AET, ERG, or SER was evaluated after artificial insemination of laying hens. ERG and AET decreased sperm lipid peroxidation and decreased fertility, even at low doses. The presence of 4 mmol of SER significantly decreased lipid peroxidation, increased the frozen-thawed sperm quality, and increased fertility after sperm cryopreservation (90% vs. control 84%, P < 0.05). In a third experiment, the use of 1 mmol of sucrose (the best result of our previous study) added to 4 mmol of SER-supplemented extender was tested. This addition allowed to the highest levels of fertility (93%). In conclusion, the addition of 4 mmol of SER in semen cryopreservation diluents decreases peroxidation and improves the efficiency of the process.
Key words: serine, semen cryopreservation, chicken, fertility
Introduction
The cryopreservation of sperm allows the conservation of genetic resources in sperm banks. This process is highly successful in many mammalian species but is still difficult in birds because of their specific adaptive reproductive process that increases their need for a long survival in the female tract with the maintenance of sperm functions (Blesbois, 2011, Blesbois, 2012). In poultry, semen cryopreservation represents the most favorite method for the in vitro conservation of avian genetic resources and preservation of rare breeds because it is non-invasive and the least expensive in vitro method available currently (Blesbois, 2011, Ehling et al., 2012, Chuaychu-noo et al., 2017, Thélie et al., 2019). Many studies have been performed to improve methods for avian sperm reproductive potential conservation after freezing-thawing. They were performed to define the best conditions of freezing to avoid cell damages and preserve fertilization ability (i.e., cooling and thawing rates, freezing methods and packaging, internal and external cryoprotectants, addition of antioxidants to semen extender) (Seigneurin and Blesbois, 1995, Chalah et al., 1999, Tselutin et al., 1999; Partyka et al., 2012, Abouelezz et al., 2015, Rakha et al., 2016, Thananurak et al., 2016, Chuaychu-noo et al., 2017, Rakha et al., 2017, Thananurak et al., 2017, Miranda et al., 2018, Thananurak et al., 2019, Thélie et al., 2019). Semen cryopreservation leads to the death of a significant proportion of sperms in all species (40–60% in the chicken) and has a critical impact on the quality of surviving gametes (Blesbois et al., 2005, Partyka et al., 2012, Najafi et al., 2014, Nguyen et al., 2015, Chuaychu-noo et al., 2017, Thananurak et al., 2017). Membrane alterations and oxidative stress are key factors involved in these alterations (Blesbois et al., 2005, Tuncer et al., 2010, Naijian et al., 2013, Chuaychu-noo et al., 2017).
Avian sperm membranes are rich in polyunsaturated fatty acids and can easily undergo lipid peroxidation (LPO) in the presence of reactive oxygen species (Fujihara and Howarth, 1978, Surai et al., 1998, Cerolini et al., 2006, Partyka et al., 2012). Reactive oxygen species are reactive molecules produced during oxygen reduction that induces peroxidation which alters the sperm functions and viability, if produced at too high concentrations (Aitken et al., 1989, De Lamirande and Gagnon, 1993, Partyka et al., 2012). Peroxidation of polyunsaturated fatty acids in the sperm cell membrane is an autocatalytic, self-propagating reaction that can cause cell dysfunctions associated with loss of the membrane function and integrity and finally leads to decreased fertilizing ability (Alvarez and Storey, 1982). As a consequence of the destructive potential of LPO, sperm membranes must be protected by a highly effective antioxidant system to prevent the peroxidative damage (Blesbois et al., 1993, Breque et al., 2003, Cerolini et al., 2006, Partyka et al., 2012). Among the extracellular factors involved in the chicken, seminal plasma is a key and complex biological fluid that modulates sperm function in the reproduction process (Blesbois and de Reviers, 1992, Santiago-Moreno et al., 2019). Its amino acid composition is expected to be involved in defending chicken sperm from peroxidation (Santiago-Moreno et al., 2019).
Although sperms contain antioxidant systems, which include different antioxidant enzymes, that is, glutathione peroxidase, superoxide dismutase, catalase, and other antioxidants such as vitamins E and C, their activity is affected by cryopreservation, which increases the intensity of LPO (Chatterjee et al., 2001, Naijian et al., 2013, Nguyen et al., 2015). Therefore, naturally occurring antioxidants may be insufficient to prevent LPO during the freeze-thaw process. The addition of antioxidants to the extender may have positive effects (Lewis et al., 1997, Naijian et al., 2013, Salmani et al., 2013, Zanganeh et al., 2013). There is a great variety of antioxidant substances, and their mechanisms of action, toxicity, and effectiveness vary enormously. Moreover, the effect of antioxidants may change depending on species, medium, and protocols (Mata-Campuzano et al., 2012a), and in some cases, their presence could be detrimental (Mata-Campuzano et al., 2012b).
Cysteamine (2-aminoethanethiol [AET]), an aminothiol antioxidant known to be an efficient scavenger of the hydroxyl radical (Ishii et al., 1981), enhances glutathione synthesis (Issels et al., 1988) and may contribute to the maintenance of the redox status in oocytes (Guerin et al., 2001). The addition of AET to the oocytes in vitro maturation medium increased glutathione (GSH) synthesis (Matos et al., 1995) and improved the embryo development in bovine (Takahashi et al., 1993, Matos et al., 1995, Matos et al., 2002, Anand et al., 2008) and increased the embryo development rates in the goat (Rodríguez-González et al., 2003). In some reports, AET improved the cryopreservation of frozen ram sperm (Bucak et al., 2007), increased motility, and decreased the number of abnormal sperm in postthaw Angora goat semen (Bucak et al., 2009). However, addition of AET to freezing extender of semen of various species generated contradictory results (Bucak et al., 2009, Tuncer et al., 2014, Najafi et al., 2014, Sariözkan et al., 2015, Akalin et al., 2016, Büyükleblebici et al., 2016, Gungor et al., 2016, Swami et al., 2017).
Ergothioneine (ERG) is an amino acid derived from histidine and present in millimolar concentrations in some tissues such as erythrocytes, kidneys, seminal fluid, and the liver (Kaneko et al., 1980). It scavenges singlet oxygen (Dahl et al., 1988), hydroxyl radicals (Akanmu et al., 1991), and peroxyl radicals (Asmus et al., 1996). ERG is the predominant sulfhydryl in human, stallion, and pig semen (Haag and McLeod, 1959). Its role is to protect sperm from oxidative stress, given the exceptionally high metabolic rate, and counteracts the deleterious effects of peroxides on sperm viability and survival during storage (Mann and Leone, 1953, Haag and McLeod, 1959). ERG improves sperm DNA integrity during short storage at 5°C and frozen-thawed motility in ram and stallion (Coutinho de Silva et al., 2008, Metcalf et al., 2008, Ҫoyan et al., 2011, Ari et al., 2012, Çoyan et al., 2012, Najafi et al., 2014).
Serine (SER), recently classified as a conditionally non-essential amino acid, plays important biological roles ranging from protein synthesis to cell signaling, the latter mostly through posttranslational modification by phosphorylation (Metcalf et al., 2008, Hunter, 2012). It is important in all SER-threonine protein kinases to transfer phosphates to the oxygen atom of a SER or threonine sidechain. SER have many other actions: It could reduce oxidative stress by regulating the expression of glutathione synthesis–related genes and by increasing glutathione concentration (Zhou et al., 2017a, Zhou et al., 2017b). SER was reported to improve glutathione peroxidase activity (Wang et al., 2016), to enhance glutathione content (Sim et al., 2015), to lower malondialdehyde concentration, and to increase concentration of superoxide dismutase, GSH-Px, and catalase in the digestive system of piglets (Zhou et al., 2018). The SER antioxidant function seems rather indirect by supporting glutathione synthesis and methionine cycle, mostly by condensing with homocysteine to synthesize cysteine and providing one-carbon units for homocysteine remethylation (Zhou et al., 2017a).
As there is no study on chicken semen cryopreservation with based extender containing amines/amino-acid antioxidant, our first aim was to examine the effects of AET, ERG, and SER, used at osmotic levels normal for semen (called here “inactive”), on the success of chicken sperm in vitro conservation. A simple sperm cryopreservation method using nitrogen vapors and dimethylformamide (DMF) cryoprotectant was used. We successively examined the effect of a wide range of AET, ERG, and SER doses on the quality of unfrozen and frozen-thawed semen in vitro (motility vigor score and membrane integrity/viability). Then, we restrained the observations to the most promising doses that we tested on frozen-thawed semen in vitro (objective motility measurements with computer-assisted sperm analysis [CASA], membrane integrity/viability, acrosome integrity, mitochondria function, lipid peroxide production) and in vivo (fertility rate) quality. In our more recent study (Thananurak et al., 2019), we showed that a sucrose (SU) supplementation of the standard freezing medium used here increased the results of fertility obtained after semen thawing. We thus wanted to know if a combination of the most successful amine/amino acid studied here and SU addition could show additive beneficial actions on sperm. We thus also tested the effect of combining SU and SER addition on the fertility obtained with frozen-thawed chicken semen.
Materials and methods
All experimental procedures used were approved by the Animal Ethics Committee of the Khon Kaen University, based on the Ethic of Animal Experimentation of National Research Council of Thailand (Approval No: 0514.1.75/22).
Chemicals
Unless otherwise indicated, all chemicals used in this study were purchased from Sigma-Aldrich (St. Louis, MO).
Animals
A flock of 60 adult males of the Thai native breed, Pradu Hang Dum, was breed at the Khon Kaen University facilities. Their age ranged from 40 to 63 wk between the start and the end of the experiment. Thirty-six of these males were chosen for our experiments based on their semen capacities (vigor motility, semen production). Animals were kept individually in cages. The birds were offered commercial poultry cock breeder food (130 g/364 Kcal/D; Betagro Public Company Limited, Songkhla, Thailand) and were exposed to natural light (mean 12L:12D). Fresh water was available ad libitum throughout the experimental period.
The females were 60 in number and of a commercial layer type (ISA Brown; Betagro Public Company Limited, Songkhla, Thailand). They were 38 wk old at the beginning of the experiment. They were exposed to natural photoperiod (12L:12D) and received a laying hen diet supplemented with calcium of 110 g/308 Kcal/D. For all the animals, care was taken to ensure maximized welfare (constant care and gentle manipulations).
Semen Collection and Preparation
Semen was routinely collected twice a week, by the dorsoabdominal massage method (Burrows and Quinn, 1937). This technique respects welfare. The animals are simply caught by hand and free to go after the abdominal massage, without suffering any injury. Semen from individual cocks was collected in a 1.5-mL microtube. Semen samples were evaluated under a microscope within 15 min after collection and were selected on the basis of the following criteria: mass motility score ≥4 (score range 0–5, phase contrast microscope ×40); sperm concentration ≥ 3 × 109 sperm/mL (hemocytometer counting method); and membrane integrity/viability (SYBR-14 and propidium iodide [PI]; Live/dead sperm viability kit L7011, Invitrogen, Vista, CA) ≥ 90%. To maximize semen quality and quantity, collection was always performed by the same people, under the same conditions, at the same time, and using the massage method. Special care was taken to avoid contamination of semen with feces, urates, and transparent fluid, which decreases semen quality.
Extender Preparation and Processing
Experiment 1: Effects of a Wide Range of AET, ERG, and SER Doses
The experiments involving AET (0, 0.001, 0.002, 0.004, 0.006, 0.008, 0.01, 0.05, 0.1, and 0.5 mmol), ERG (0, 0.01, 0.02, 0.04, 0.06, 0.08, 0.1, 0.5, 1, and 5 mmol), and SER (0, 1, 2, 4, 6, 8, 10, 15, and 20 mmol) were performed. The experimental doses were chosen based on the different doses expected to act as antioxidant in the literature in another species.
Semen samples were pooled, each one assigned to one treatment. Semen aliquots were diluted 1:2 with BHSV-based diluent (5 g glucose, 2.5 g inositol, 28.5 g sodium glutamate, 0.7 g magnesium acetate tetrahydrate, 5 g potassium acetate, all of which were dissolved in 1,000 mL of double-distilled water; Schramm, 1991) supplemented with different levels of AET, ERG, and SER.
The diluent osmolality was assessed using an osmometer (FISKE Mark 3 Osmometer; FISKE Associates, Norwood, MA). The pH was assessed using a pH meter (CORITON Instrument, S.A., Spain). The dose of AET (higher than 0.01 mmol), ERG (higher than 0.1 mmol), and SER (higher than 6 mmol) showed a consistent increase in the osmolarity of the diluent (Table 1). The pH of the diluent was not affected by any concentration of the ERG and SER tested. However, the dose of AET higher than 0.01 mmol increased the pH of the diluent (Table 1).
Table 1.
Osmolality and pH of the BHSV diluent supplemented with different doses of cysteamine, ergothioneine, and serine.
| Factor | pH | Osmotic pressure (mOsm/kg) | |
|---|---|---|---|
| Control | 0 | 6.8 | 421 |
| Cysteamine (mmol) | 0.001 | 6.8 | 423 |
| 0.002 | 6.8 | 423 | |
| 0.004 | 6.8 | 423 | |
| 0.006 | 6.8 | 423 | |
| 0.008 | 6.8 | 423 | |
| 0.01 | 6.8 | 423 | |
| 0.05 | 8.8 | 447 | |
| 0.10 | 9.5 | 606 | |
| 0.50 | 9.9 | 1,407 | |
| Ergothioneine (mmol) | 0.01 | 6.8 | 421 |
| 0.02 | 6.8 | 422 | |
| 0.04 | 6.8 | 423 | |
| 0.06 | 6.8 | 423 | |
| 0.08 | 6.8 | 423 | |
| 0.1 | 6.8 | 423 | |
| 0.5 | 6.8 | 430 | |
| 1 | 6.9 | 459 | |
| 5 | 6.9 | 492 | |
| Serine (mmol) | 1 | 6.8 | 422 |
| 2 | 6.8 | 424 | |
| 4 | 6.8 | 425 | |
| 6 | 6.8 | 426 | |
| 8 | 6.8 | 431 | |
| 10 | 6.8 | 434 | |
| 15 | 6.8 | 464 | |
| 20 | 6.8 | 481 | |
Abbreviation: BHSV, Blumberger Hahnen Sperma Verdünner.
Then, the diluted semen samples were cooled down from 25°C to 5°C in 1 h (1°C per 3 min). Unfrozen semen was then equilibrated 24 h at 5°C. For frozen semen, we added DMF (Sigma Aldrich, St. Louis, MO) diluted in BHSV to each semen-extender treatment previously cooled down to 5°C (final dilution 1:3; final concentration of 6% DMF). Semen was then immediately loaded into 0.5-mL plastic straws (IMV Technologies, L'Aigle, France), sealed with polyvinylpyrrolidone powder (IMV Technologies, L'Aigle, France) and equilibrated at 5°C for 15 min. After equilibration, the filled straws were laid horizontally on a rack 11 cm above the surface of LN2 (−35°C) for 12 min, then placed 3 cm above liquid nitrogen vapor (−135°C) for 5 min and subsequently immersed in LN2 as previously described (Vongpralub et al., 2011). Semen straws were transferred to a LN2 container for storage. Sperm was thawed for 5 min in a water bath adjusted to 5°C. After thawing, the straws were quickly opened, and semen was transferred to a 1.5-mL microtube and then evaluated for various sperm functions. Sperm quality were determined in six replicates in both incubated unfrozen and frozen-thawed semen samples.
Experiment 2: Effects of Targeted Doses of AET, ERG, and SER
Semen samples were pooled and split into 10 aliquots, each one assigned to one treatment. Semen aliquots were diluted with BHSV-based extender and supplemented with different levels of AET (0.001, 0.002, and 0.004 mmol), ERG (0.01, 0.02, and 0.04 mmol), and SER (2, 4 and 6 mmol). After dilution and cooling from 25°C to 5°C for 1 h, samples were frozen as stated previously in experiment 1. Three days later, frozen semen samples were thawed. Sperm quality was determined in 12 replications.
Experiment 3: Combination Between SU and SER Added on Extender
Pooled semen was divided into 2 aliquots and extended with BHSV-based extender (control) or BHSV-based extender containing SER 4 mmol + SU 1 mmol (the best result of our previous study, Thananurak et al., 2019). Semen samples were frozen and assessed as stated previously for experiment 2.
Analysis of Vigor Motility Score
Vigor motility scores (1–5) were assessed using an arbitrary scale of 0–5 (0: no movement, 1: little movement, 2: no swirls, but prominent individual movement, 3: slower swirls and eddied, 4: medium swirls and eddied, and 5: very strong movement and rapid dark swirls). A drop of 15 μL of semen sample (semen diluted with BHSV extender; 1:3) was dropped on a slide and observed under microscope at 10× magnification.
Analysis of Membrane Integrity/Viability
Sperm membrane integrity was determined by double-fluorescent labeling technique, using SYBR-14 and PI (LIVE/DEAD Sperm Viability Kit; Invitrogen, Thermo Fisher Scientific, Waltham, MA), according to the protocol adapted from the study by Chalah et al. (1999). Briefly, each sample was diluted to a concentration of 150 × 106 sperm/mL. Portions of the diluted samples were dropped into a polystyrene round-bottom tube and 5 μL of SYBR-14 (commercial solution diluted 50-fold). Samples were mixed and incubated at room temperature for 10 min. The cells were counterstained with 5 μL of PI for 5 min and then fixed with 30 mL of 20% formaldehyde. The sperm was then evaluated under a fluorescent microscope IX71 (Olympus, Tokyo, Japan) in experiment 1 or by flow cytometry in experiment 2 (FACSCalibur; Becton Dickinson, San Jose, CA).
Analysis of the Postthaw Sperm Motion Parameters
Sperm motion parameters were evaluated in frozen-thawed sperm diluted a second time in each extender at a ratio of 1:15 using CASA (HTM-IVOS Model 10 Spermatozoa Analyzer; Hamilton Thorne Biosciences, Beverly, MA). For each sample, 2 slides (maintained at 25°C) were filled with 5 μL of diluted semen, and three fields per slide were recorded for 10 s. The instrument settings for CASA were as follows: Apply sort = 0, frames acquired = 30, frame rate = 60 Hz, minimum contrast = 25, minimum cell size 4 pixels, minimum static contrast = 15, threshold = 80.0%, average path velocity (VAP) cutoff = 5 μm/sec, prog. min. VAP = 20 μm/s, straight line velocity (VSL) cutoff = 20 μm/s, cell size = 4 pixels, cell intensity = 50, static head size = 0.72 to 8.82, static head intensity = 0.14 to 1.84, static elongation = 0 to 47, slow cell motile = yes, magnification = 1.92, video frequency = 60 frames/s, bright filed = no (i.e., no bright field), chamber depth = 20 μm, field selection mode = auto, and integration time = 1 frame. The following motility characteristics were determined: percentage of total motile sperm (MOT), progressive motile sperm (PMOT), VAP, VSL, and curvilinear velocity (VCL).
Analysis of Acrosome Integrity
Sperm acrosome integrity was assessed by fluorescein isothiocyanate–conjugated peanut agglutin (Sigma L7381). Samples of semen were diluted to a concentration 150 × 106 sperm/mL with BHSV-based extenders. Then, 300-μL aliquot from diluted semen samples were mixed with 5 μL of fluorescein isothiocyanate–conjugated peanut agglutin working solution (2 μL/mL) and incubated for 5 min in room temperature in dark. After incubation, samples were then centrifuged at 1,200 × g for 3 min. The sperm pellets were resuspended in 500 μL of BHSV-based diluent and 3 μL of PI (LIVE/DEAD Sperm Viability Kit L7011; Invitrogen, Thermo Fisher Scientific, Waltham, MA) to identify dead cells in a population (1 mg/mL) and these were added before cytometric analysis (adapted from the study by Partyka et al., 2011).
Analysis of Mitochondrial Function of Viable Sperm
Sperm mitochondrial function was determined by evaluating the mitochondrial membrane potential using fluorescence staining with the 5, 5′, 6, 6′-tetrachloro-1, 1′, 3, 3′ tetraethylbenzimidazolyl-carbocyanine iodide (JC-1; Sigma, C50390) and PI. The 1-mmol stock solution of JC-1 in DMSO was prepared. From each sample, 300 μL of sperm was stained with 2 μL of JC-1. The samples were incubated at room temperature in the dark for 8 min before cytometric analysis. Sperm emitting orange fluorescence were classified as high mitochondrial function, emitting both green and orange fluorescence as medium, and emitting only green fluorescence as low mitochondrial function (adapted from the study by Partyka et al., 2011, and Thananurak et al., 2019).
Flow Cytometry
Flow cytometric analyses were performed on a FACSCalibur (Becton Dickinson, San Jose, CA) flow cytometer with a triple filter, showing set UV-2E/C (excitation 340–380 nm and emission 435–485 nm), B-2E/C (excitation 465–495 nm and emission 515–555 nm), and G-2E/C (excitation 540–525 nm and emission 605–655 nm). The non-sperm events were gated out based on scatter properties and not analyzed. A total of 100,000 events were analyzed for each samples (adapted from the studies by Andrade et al., 2007, Partyka et al., 2011, Consiglio et al., 2013).
Production of Lipid Peroxide
The test was performed according to the procedure of Partyka et al. (2007). Sperm lipid peroxides were determined using the thiobarbituric acid reactive test, which is an indicator of malondialdehyde production. Semen aliquots of each treatment were incubated in the presence of 2.78% ferrous sulfate × 7H2O (Ajex, 0906251) and 0.1 mL of 0.22% butylated hydroxytoluene (Sigma, B1378) at 37°C for 60 min. The reaction was stopped by adding 1 mL of 35% trichloroacetic acid (Sigma, T6399) and kept on ice for 15 min. Samples were centrifuged at 7,800 × g for 15 min, and the supernatant was retained. The progress of endogenous peroxidation was followed by adding 1 mL of 0.36% thiobarbituric acid (Sigma-T550-0) to 2 mL of supernatant. The mixture was boiled for 10 min, allowed to cool, and then the production of lipid peroxide was measured by using a Carry Conc. UV-visible Spectrophotometer (Specord 250 Plus, Analytik Jena, Jena, Germany), and absorbance levels acquired by spectrometry were at 532 nm.
Artificial Insemination
For fertility test, straws were thawed in an ice water bath at 5°C for 5 min. All hens (6 hens/each treatment) were inseminated once every 14 D with frozen/thawed semen from each group at a dose of 0.4 mL (mean 400 million sperm). Artificial insemination was performed between 3:00 and 5.00 pm. Ten replications of the fertility test were carried out, and change of female, between each replication. Eggs were collected on days 2–8 after each insemination before incubation.
Fertility was determined by candling eggs on day 7 of incubation. The fertility rates were calculated with the following formulas: (Total number of fertile eggs/Total number of incubated eggs) × 100.
Statistical Analysis
Data were analyzed using the software SAS system (SAS Institute, Cary, NC). Statistical differences among various treatment means were determined by Duncan's new multiple range test, and a P ≤ 0.05 was considered to be statistically significant. All the data were checked for normal distribution by UNIVARIATE procedure and Shapiro-Wilk test, and the percentages were transformed to arcsine square root before analysis.
Results
Effect of a Wide Range of AET Doses on Vigor and Membrane Integrity of Unfrozen Stored or Cryopreserved Chicken Sperm
The effects of 0 to 0.5 mmol of AET added to BHSV-based extender for unfrozen stored semen or to BHSV + 6% DMF for frozen-thawed semen are reported on Table 2. All the higher doses tested (up to 10 mmol) severely decreased sperm viability and are not reported here (data not shown). The lowest dose tested, 0.001 mmol, significantly increased the vigor in semen stored unfrozen, but the doses higher to 0.006 decreased it (P < 0.05). Sperm membrane integrity of unfrozen semen stored for 24 h at 5°C was not affected by 0.001 to 0.004 mmol AET but was altered by the higher doses (P < 0.05).
Table 2.
Effect of the addition of cysteamine on the vigor score and membrane integrity of chicken sperm stored frozen or unfrozen.
| Cysteamine (mmol) | Vigor score (1–5) | Membrane integrity (%) | |
|---|---|---|---|
| Unfrozen semen (incubated for 24 h) | 0 | 4.34 ± 2.14b | 87.96 ± 2.44a |
| 0.001 | 4.59 ± 3.20a | 89.60 ± 3.06a | |
| 0.002 | 4.45 ± 2.15a,b | 88.62 ± 2.35a | |
| 0.004 | 4.42 ± 2.11a,b | 90.06 ± 2.12a | |
| 0.006 | 4.24 ± 2.07b | 76.70 ± 2.82b | |
| 0.008 | 3.34 ± 3.24c | 68.17 ± 2.63c | |
| 0.01 | 2.93 ± 3.12d | 47.90 ± 3.24d | |
| 0.05 | 2.04 ± 3.25e | 46.32 ± 2.58d | |
| 0.1 | 1.41 ± 2.26f | 35.64 ± 2.99e | |
| 0.5 | 0.53 ± 3.41g | 13.48 ± 3.44f | |
| Frozen-thawed semen | 0 | 3.55 ± 2.11a | 40.69 ± 4.58a,b |
| 0.001 | 3.69 ± 3.14a | 41.87 ± 3.29a | |
| 0.002 | 3.57 ± 3.08a | 42.61 ± 2.49a | |
| 0.004 | 3.69 ± 2.14a | 41.05 ± 4.83a,b | |
| 0.006 | 3.59 ± 3.07a | 39.35 ± 5.08b | |
| 0.008 | 2.19 ± 4.18b | 19.43 ± 3.95c | |
| 0.01 | 1.08 ± 3.06c | 11.99 ± 5.73d | |
| 0.05 | 0.51 ± 2.49d | 8.12 ± 4.77e | |
| 0.1 | 0 | 0 | |
| 0.5 | 0 | 0 | |
The results are expressed as means ± standard deviations. N = 6. Different letters (a–g) indicate significant differences within columns (P < 0.05).
Cryopreservation significantly decreased vigor and membrane integrity (P < 0.05). The AET doses 0.001 to 0.006 mmol did not affect vigor or membrane integrity of cryopreserved chicken sperm. All the doses tested higher than 0.006 mmol were severely harmful to cryopreserved sperm (more than 80% sperm death).
Effect of a Wide Range of ERG Doses on Vigor and Membrane Integrity of Unfrozen Stored or Cryopreserved Chicken Sperm
The effects of 0 to 10 mmol of ERG added to BHSV-based extender for unfrozen stored semen and BHSV + 6% DMF for frozen-thawed semen are reported on Table 3. The doses of ERG higher than 0.5 mmol had significant negative effects on sperm vigor score and membrane integrity of sperm stored unfrozen. The lower doses did not affect these parameters.
Table 3.
Effect of the addition of ergothioneine on unfrozen or cryopreserved chicken sperm characteristic.
| Ergothioneine (mmol) | Vigor score (1–5) | Membrane integrity (%) | |
|---|---|---|---|
| Unfrozen semen (incubated for 24 h) | 0 | 4.46 ± 3.14a | 87.24 ± 4.59a,b,c |
| 0.01 | 4.49 ± 2.18a | 88.93 ± 4.31a,b | |
| 0.02 | 4.67 ± 3.19a | 90.34 ± 3.09a | |
| 0.04 | 4.55 ± 2.23a | 90.05 ± 3.88a | |
| 0.06 | 4.54 ± 2.24a | 85.49 ± 5.94b,c | |
| 0.08 | 4.34 ± 2.16a | 85.37 ± 5.46b,c | |
| 0.1 | 4.33 ± 3.14a | 83.69 ± 3.65c | |
| 0.5 | 4.02 ± 2.54a,b | 81.81 ± 3.47c,d | |
| 1 | 3.57 ± 2.58b | 80.15 ± 3.56d | |
| 5 | 3.36 ± 3.12b | 77.63 ± 2.47e | |
| 10 | 3.21 ± 3.46b | 69.47 ± 4.18f | |
| Frozen-thawed semen | 0 | 3.42 ± 3.11a,b | 40.24 ± 3.28a,b,c |
| 0.01 | 3.67 ± 4.46a,b | 41.87 ± 2.94a | |
| 0.02 | 3.85 ± 3.12a | 41.92 ± 4.40a | |
| 0.04 | 3.79 ± 2.23a | 41.06 ± 3.74a,b | |
| 0.06 | 3.34 ± 4.60b | 39.38 ± 3.97b,c | |
| 0.08 | 2.68 ± 3.55c | 38.78 ± 2.57c | |
| 0.1 | 2.40 ± 3.42c | 35.49 ± 3.48d | |
| 0.5 | 2.01 ± 2.59c,d | 28.52 ± 4.42e | |
| 1 | 1.41 ± 3.24d | 12.46 ± 3.51f | |
| 5 | 0.32 ± 2.13e | 8.51 ± 2.47g | |
| 10 | 0.1 ± 3.36f | 2.12 ± 3.96h | |
The results are expressed as means ± standard deviations. N = 6. Different letters (a–h) indicate significant differences within columns (P < 0.05).
On the frozen-thawed semen, the doses of ERG higher than 0.06 mmol decreased the vigor score and the dose higher than 0.08 decreased the membrane integrity (P < 0.05). All the ERG doses tested higher than 0.08 mmol were severely harmful to sperm (more than 60% sperm death).
Effect of a Wide Range of SER Doses on Vigor and Membrane Integrity of Unfrozen Stored or Cryopreserved Chicken Sperm
The effects of 0 to 20 mmol SER added to BHSV-based extender for unfrozen semen and BHSV + 6% DMF of frozen-thawed semen are reported on Table 4. The doses 0.01, 0.02, 0.04, 0.06, 0.08, 0.1, 0.5, and 1 mmol were also tested but did not show any effect and were not reported here (data not shown). The vigor of sperm stored unfrozen was not affected by 1 to 15 mmol but was altered by higher doses (P < 0.05). The membrane integrity was decreased by the doses higher than 8 mmol (P < 0.05). As for the other trials, cryopreservation significantly decreased the semen in vitro quality parameters.
Table 4.
Effect of the addition of serine on unfrozen or frozen-thawed chicken sperm characteristic.
| Serine (mmol) | Vigor score (1–5) | Membrane integrity (%) | |
|---|---|---|---|
| Unfrozen semen (incubated for 24 h) | 0 | 4.52 ± 3.08a,b,c | 88.49 ± 5.00a,b |
| 1 | 4.41 ± 4.10b,c | 86.48 ± 3.37b,c | |
| 2 | 4.66 ± 2.37a | 89.55 ± 2.48a,b | |
| 4 | 4.71 ± 3.11a | 92.31 ± 4.17a | |
| 6 | 4.55 ± 2.53a,b | 87.48 ± 4.14b | |
| 8 | 4.43 ± 4.21b,c | 85.63 ± 4.66b,c | |
| 10 | 4.33 ± 3.42c | 82.98 ± 4.05c | |
| 15 | 4.32 ± 3.04c | 78.98 ± 3.57d | |
| 20 | 4.11 ± 4.22d | 73.44 ± 3.04e | |
| Frozen-thawed semen | 0 | 3.53 ± 4.27b | 39.21 ± 3.25b,c |
| 1 | 3.45 ± 5.06b | 39.41 ± 3.89b,c | |
| 2 | 3.65 ± 3.20a,b | 40.78 ± 3.21b | |
| 4 | 3.84 ± 2.54a | 44.35 ± 4.03a | |
| 6 | 3.49 ± 4.07b | 41.23 ± 2.32b | |
| 8 | 3.12 ± 3.65c | 37.56 ± 3.50c | |
| 10 | 3.13 ± 4.14c | 35.10 ± 3.85d | |
| 15 | 3.01 ± 3.45c | 29.20 ± 2.87e | |
| 20 | 2.89 ± 3.16d | 22.14 ± 3.26f | |
The results are expressed as means ± standard deviations. N = 6. Different letters (a–f) indicate significant differences within columns (P < 0.05).
For the frozen-thawed semen, the dose 4 mmol increased the vigor score and membrane integrity (P < 0.05). The doses higher than 6 mmol significantly decreased the vigor, and the dose of 10 mmol decreased also the membrane integrity (P < 0.05).
Effect of Targeted Doses of AET, ERG, and SER on Motility Parameters of Frozen-Thawed Chicken Sperm
Table 5 shows the motility parameters analyzed by CASA of chicken semen frozen-thawed with different targeted levels of AET, ERG, and SER. Addition of AET (0.001 and 0.004 mmol), ERG (0.01 to 0.04 mmol), and SER 2 mmol decreased MOT, unlike SER 4 mmol that significantly increased MOT (P < 0.05). The PMOT parameter was not affected by any treatment. The VAP parameter was significantly decreased by 2 mmol SER (P < 0.05). The VSL parameter was increased by 0.001 and 0.002 mmol AET, by 0.02 and 004 mmol ERG, and by 4 mmol SER, which showed VSL was significantly higher than that in all the other treatments (P < 0.05). The doses of 0.001 and 0.004 mmol AET, 0.01 mmol ERG, and 2 mmol SER decreased the VCL parameter (P < 0.05).
Table 5.
Effect of different concentrations of cysteamine, ergothioneine, and serine on the MOT, PMOT, VAP, VSL, and VCL of frozen-thawed semen.
| Factor | MOT (%) | PMOT (%) | VAP (μm/s) | VSL (μm/s) | VCL (μm/s) | |
|---|---|---|---|---|---|---|
| Control | 0 | 61.08 ± 4.78a,b | 23.92 ± 2.08 | 63.79 ± 3.38a,b | 47.76 ± 3.26c | 111.60 ± 2.92a |
| Cysteamine (mmol) | 0.001 | 57.50 ± 4.64c,d | 24.50 ± 3.10 | 61.04 ± 3.60b | 49.80 ± 4.78b | 104.12 ± 4.49b,c |
| 0.002 | 60.08 ± 5.94b | 25.00 ± 2.04 | 64.46 ± 2.26a,b | 49.18 ± 4.93b | 109.82 ± 4.79a,b | |
| 0.004 | 57.08 ± 3.67d | 24.16 ± 3.03 | 64.24 ± 4.92a,b | 46.54 ± 4.03c | 108.84 ± 3.86b | |
| Ergothioneine (mmol) | 0.01 | 56.83 ± 4.97d,e | 24.08 ± 3.08 | 61.82 ± 3.02b | 46.00 ± 3.20c | 105.74 ± 4.69b,c |
| 0.02 | 56.50 ± 4.64e | 25.16 ± 2.65 | 63.80 ± 3.35a,b | 49.30 ± 4.48b | 110.62 ± 5.79a,b | |
| 0.04 | 57.58 ± 5.61c,d | 24.66 ± 2.90 | 64.34 ± 4.35a,b | 49.01 ± 3.51b | 111.06 ± 5.23a | |
| Serine (mmol) | 2 | 58.00 ± 4.37c | 25.58 ± 4.50 | 56.72 ± 2.62c | 44.38 ± 3.36d | 103.35 ± 4.24c |
| 4 | 62.66 ± 5.33a | 25.85 ± 3.39 | 66.98 ± 3.58a | 52.56 ± 5.30a | 112.46 ± 5.90a | |
| 6 | 60.33 ± 4.33b | 26.55 ± 2.87 | 62.91 ± 3.94b | 48.25 ± 4.89b,c | 109.49 ± 5.83a,b | |
The results are expressed as means ± standard deviations. N = 12. Different letters (a–e) within columns indicate significant differences (P < 0.05).
Abbreviations: MOT (%), percentage of total motile sperm; PMOT (%), percentage of sperm with progressive motility; VAP (μm/s), percentage of velocity average path; VCL (μm/s), percentage of velocity curvilinear; VSL (μm/s), percentage of velocity straight line.
Effect of Targeted Doses of AET, ERG, and SER on Membrane Integrity, Acrosome Integrity, Mitochondria Function, and Lipid Peroxide Production of Frozen-Thawed Chicken Sperm
Table 6 shows the effects of different doses of AET, ERG, and SER on membrane integrity, acrosome integrity, mitochondrial membrane potential, and levels of malondialdehyde production. The different doses of AET, ERG, and SER did not affect sperm membrane integrity. The acrosome integrity was negatively affected by 0.002 and 0.004 mmol AET and by 0.02 mmol ERG, unlike 4 mmol SER that reduced the decrease of acrosome integrity (P < 0.05) due to cryopreservation and 6 mmol that did not affect this parameter. The mitochondrial membrane potential was increased by 4 and 6 mmol SER; 4 mmol was the most efficient treatment, followed by 6 mmol SER (P < 0.05). Only 0.001 mmol AET decreased the sperm mitochondrial membrane potential (P < 0.05).
Table 6.
Effects of different concentrations of cysteamine, ergothioneine, and serine supplementation of diluent on membrane integrity, acrosome integrity, functional mitochondria, and malondialdehyde of frozen-thawed chicken sperm.
| Factor | Membrane integrity (%) | Acrosome integrity (%) | Functional mitochondria (%) | MDA (μm/mL/150 × 106 spz) | |
|---|---|---|---|---|---|
| Control | 0 | 40.49 ± 3.00 | 36.88 ± 3.17b,c | 49.81 ± 3.25c | 1.98 ± 0.14a |
| Cysteamine (mmol) | 0.001 | 42.34 ± 3.97 | 35.49 ± 4.11c,d,e | 49.73 ± 2.80d | 1.40 ± 0.17b |
| 0.002 | 42.81 ± 4.61 | 34.26 ± 4.17e | 49.53 ± 3.51c | 1.39 ± 0.28b | |
| 0.004 | 41.93 ± 5.16 | 34.08 ± 3.88e | 48.59 ± 2.45c,d | 1.39 ± 0.15b | |
| Ergothioneine (mmol) | 0.01 | 40.17 ± 3.99 | 36.33 ± 3.97b,c,d | 50.06 ± 2.03c | 1.31 ± 0.19b |
| 0.02 | 41.75 ± 4.09 | 35.01 ± 4.10d,e | 49.65 ± 3.66c | 1.29 ± 0.26b | |
| 0.04 | 43.02 ± 4.33 | 36.24 ± 4.07c,d | 49.94 ± 4.35c | 1.36 ± 0.21b | |
| Serine (mmol) | 2 | 41.34 ± 3.37 | 37.21 ± 3.38b,c | 49.89 ± 5.64c | 1.39 ± 0.13b |
| 4 | 43.34 ± 4.29 | 40.99 ± 4.21a | 56.32 ± 3.89a | 1.38 ± 0.22b | |
| 6 | 41.56 ± 5.07 | 38.04 ± 5.86b | 51.78 ± 4.40b | 1.35 ± 0.15b | |
The results are expressed as means ± standard deviations. N = 12. Different letters (a–e) within columns indicate significant differences (P < 0.05).
Abbreviation: MDA, malondialdehyde.
All the AET, ERG, and SER doses tested decreased the levels of malondialdehyde production measured by thiobarbituric acid reactive test (P < 0.05).
Effect of Targeted Doses of AET, ERG, and SER on Fertility Obtained With Frozen-Thawed Chicken Sperm
The results of fertility rate after insemination with frozen-thawed sperm are shown in Table 7. Fertility levels of the eggs collected from hens inseminated with frozen-thawed semen containing 4 mmol SER were significantly higher (90%) than those in the control BHSV extender (84%) and in other treatments (P < 0.05). The higher dose of SER, 6 mmol, did not show significant difference in fertility with the control BHSV, but the dose 2 mmol showed significantly lower results than the control even if very close to the result obtained with 6 mmol SER. All the doses of AET and ERG showed fertility results severely lower than the control and significantly lower than any SER dose tested here (P < 0.05).
Table 7.
Effects of different concentrations of cysteamine, ergothioneine, and serine supplementation of diluent on fertility obtained with frozen-thawed chicken semen.
| Factor | No. of fertile eggs | No. of incubated eggs | Fertility (% fertile/incubated eggs) | |
|---|---|---|---|---|
| Control | 0 | 338 | 398 | 84.69 ± 5.11b |
| Cysteamine (mmol) | 0.001 | 261 | 384 | 67.89 ± 4.49d,e |
| 0.002 | 276 | 396 | 69.86 ± 5.65d | |
| 0.004 | 241 | 377 | 63.84 ± 4.37e | |
| Ergothioneine (mmol) | 0.01 | 259 | 390 | 66.27 ± 4.18d,e |
| 0.02 | 242 | 369 | 65.52 ± 3.34d,e | |
| 0.04 | 256 | 382 | 66.78 ± 3.74d,e | |
| Serine (mmol) | 2 | 316 | 394 | 80.01 ± 5.26c |
| 4 | 356 | 391 | 90.91 ± 3.81a | |
| 6 | 311 | 381 | 81.40 ± 5.61b,c | |
The results are expressed as means ± standard deviations. N = 10. Different letters (a–e) within columns indicate significant differences (P < 0.05).
Effects of the Combination of SU and SER Added to the Diluent on the Sperm Motility Parameters of Frozen-Thawed Chicken Semen
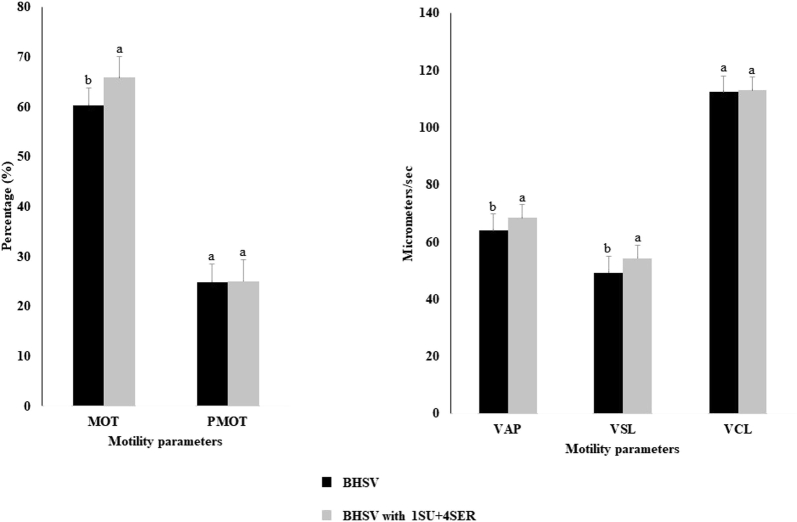
The MOT, VAP, and VSL obtained with 1 mmol SU and 4 mmol SER were significantly higher than the control (BHSV-based extender) (65.85 ± 4.32 vs. 60.31 ± 3.56, 68.53 ± 4.51 vs. 64.05 ± 3.88, and 54.41 ± 3.13 vs. 49.32 ± 5.71, respectively) (P < 0.05) and are presented in Figure 1. The PMOT and VCL were not affected by the combination SU + SER.
Figure 1.
Effect of the combination of sucrose and serine supplementation in the diluent on the sperm motility parameters of frozen-thawed semen. Results are expressed as fold change relative to the Blumberger Hahnen Sperma Verdünner (BHSV) and BHSV with 1 mmol sucrose and 4 mmol serine (1SU + 4SER) (mean ± standard deviation). Statistical differences are represented by letters a and b. Bars with the different superscript letter of each parameter are significantly different. N = 6. Abbreviations: MOT (%), percentage of total motile sperm; PMOT (%), percentage of sperm with progressive motility; VAP (μm/s), percentage of velocity average path; VCL (μm/s), percentage of velocity curvilinear; VSL (μm/s), percentage of velocity straight line. P < 0.05.
Effects of the Combination of SU and SER Added to the Diluent on Membrane Integrity, Acrosome Integrity, Mitochondria Function, and Lipid Peroxide Production of Frozen-Thawed Chicken Semen
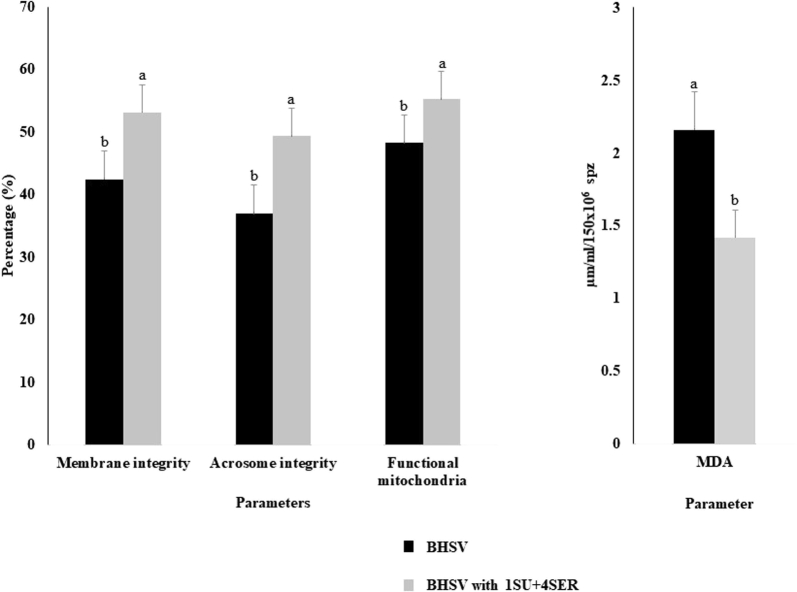
The treatment with 1 mmol SU and 4 mmol SER showed significantly higher (P < 0.05) membrane integrity, acrosome integrity, and mitochondria function than the sample with BHSV-based extender (control) (53.11 ± 4.48 vs. 42.41 ± 3.45, 49.32 ± 3.82 vs. 36.92 ± 4.57, and 55.23 ± 3.44 vs. 48.22 ± 3.36, respectively), data are presented in Figure 2. Likewise, the treatment of 1 mmol SU and 4 mmol SER decreased the lipid peroxide production evaluated with malondialdehyde (1.42 ± 0.19 vs. 2.16 ± 0.26) (P < 0.05).
Figure 2.
Effect of the combination of sucrose and serine supplementation in the diluent on the membrane integrity, acrosome integrity, functional mitochondria, and malondialdehyde of frozen-thawed chicken sperm. Results are expressed as fold change relative to the Blumberger Hahnen Sperma Verdünner (BHSV) and BHSV with 1 mmol sucrose and 4 mmol serine (1SU + 4SER) (mean ± standard deviation). Statistical differences are represented by letters a and b. Bars with the different superscript letter of each parameter are significantly different. N = 6. Abbreviation: MDA, malondialdehyde. P < 0.05.
Effects of the Combination of SU and SER Added to the Diluent on Fertility Rate Obtained With Frozen-Thawed Chicken Sperm
The data related to the number of eggs and fertility rate after insemination with frozen-thawed semen supplemented with 1 mmol SU and 4 mmol SER compared with the control (BHSV-based extender) are shown in Table 8. The combination of 1 mmol SU and 4 mmol SER treatment show significantly higher fertility (93%) than the control (85%) (P < 0.05).
Table 8.
Effect of the combination of SU and SER supplementation in the diluent on the fertility obtained with frozen-thawed chicken sperm.
| Factor | No. of fertile eggs | No. of incubated eggs | Fertility (% fertile/incubated eggs) |
|---|---|---|---|
| Control | 198 | 230 | 85.87 ± 4.93b |
| 1Su + 4Se | 213 | 228 | 93.03 ± 3.67a |
The results are expressed as means ± standard deviations. N = 6. Different letters (a, b) within columns indicate significant differences (P < 0.05).
Abbreviations: 1Su + 4Se, 1 mmol sucrose and 4 mmol serine; SER, serine; SU, sucrose.
Discussion
The present study was undertaken to obtain a suitably modified semen extender for improving the quality of the cryopreserved semen of chicken and the fertility rate after artificial insemination under field condition. This study was mainly performed to investigate which antioxidant amine or amino acids—AET, ERG, or SER—would provide the most effective protection against oxidative damages during the freeze-thaw process of chicken sperm and to observe the potential consequences on sperm quality after cryopreservation. Indeed, cryopreservation include huge decrease in temperature at freezing, then increase in temperature at thawing, and concomitant osmotic shocks that enhance oxidative stress and LPO, which irreversibly damage the sperm membranes and organelles (Aitken et al., 1989, Aitken et al., 1998, De Lamirande and Gagnon, 1992, Maxwell and Watson, 1996). The consequences are changes in membrane fluidity, reduction in sperm viability, motility, metabolism efficiency, ability to undergo acrosome reaction, and finally fertilizing ability in all species including the chicken (Nguyen et al., 2015).
We show that AET, ERG, and SER effectively decrease the malondialdehyde production that reflects LPO production in cryopreserved sperm. However, the effects on sperm quality are contrasted and depend on the component tested. None of them affect sperm membrane integrity or the proportion of PMOT, but they affect different parameters such as motility criteria, acrosome integrity, and mitochondrial membrane potential. Finally, only SER 4 mmol shows a clear positive effect on fertility. The AET and ERG showed toxic effects on sperm at the dose tested for SER. Even if AET and ERG were used at much lower concentrations than SER, they affected negatively the fertility rate obtained with frozen-thawed semen.
Different amines/amino acids are expected to protect cells toward environmental injuries (Shahsavari et al., 2018). These properties may include scavenging the free radicals, a protection method of the cell membrane against osmotic shocks or specific interactions with membrane receptors. Some organisms are known to accumulate specific amino acids in response to cold shock.
SER is one of the main free amino acids present in chicken seminal plasma (Santiago-Moreno et al., 2019). Its amount depends on the breed, but it is the second most abundant amino acid in the seminal plasma of many of them, after glutamic acid. SER is an amino acid that contains a hydroxyl group and forms a polar residue that may be phosphorylated, a reason why it is an important actor of SER-threonine kinases. Its indirect antioxidant properties have also been described in somatic functions (Zhou et al., 2017a, Zhou et al., 2017b, 2018). SER was shown to exert cryoprotective action on human endothelial cells (Maralani et al., 2012). However, SER had never been used to protect sperm during the cryopreservation process before the present study. A study by Santiago-Moreno et al. (2019) showed that the abundance of SER in the chicken seminal plasma was positively correlated with the conservation of DNA integrity in cryopreserved semen, which is an indication of a beneficial effect of SER in some circumstances. In our study, the dose of 4 mmol SER was efficient in decreasing the LPO indicator malondialdehyde but was also efficient in increasing cryopreserved sperm vigor, VCL, membrane and acrosome integrity, mitochondrial membrane potential, so all the indicators of in vitro semen quality, and finally also the fertilizing ability of frozen-thawed sperm. The positive effects of 4 mmol SER do not show a classical dose-response curve because the lower dose of 2 mmol and the higher dose of 6 mmol did not show the same optimized effect. As a very wide range of SER doses were tested in vitro in our study, with repeatable results, and because a large number of repetitions were made for the fertility test (10 repeated experiments for fertility measurements with regular changes of females/treatment), these results are sure. However, we do not provide a total explanation of them. We however propose that the antioxidant effect of SER may pass by pathways that include malondialdehyde production and also by other pathways and that fragile balances in cellular oxidations assures that the efficient and positive effects of SER occurs at a narrow range of concentration. Maralani et al. (2012) suggested that the cryoprotective antioxidant effect of SER raised also from the SER-induced elevation of the crucial antioxidant factors Nrf2, HO-1, and NO. This is still an active hypothesis.
The simple aminothiol AET is a natural antioxidant derived from the amino acid cysteine. AET is also a key component of coenzyme A (Co A) because its thiol is involved in the interactions of Co A with carboxyl groups, leading to Co A actions in fatty acids metabolism, Krebs cycle, and sugar and protein metabolism (Theodoulou et al., 2014).
AET, as antioxidant supplement in in vitro media, has been reported to have a positive action on gametes and embryos. It contributed to the maintenance of the redox status in oocytes (Guerin et al., 2001). The addition of AET to the oocyte in vitro maturation medium increased GSH synthesis (Matos et al., 1995) and improved the embryo development in bovines (Takahashi et al., 1993, Matos et al., 1995, Matos et al., 2002, Anand et al., 2008). Higher embryo developments were observed when AET was added to the maturation medium of goat oocytes (Rodríguez-González et al., 2003). AET was reported to provide an effective protection against oxidative damage during the cryopreservation of sperm (Najafi et al., 2014). In some reports, AET improved the cryopreservation of frozen ram sperm (Bucak et al., 2007) and led to higher rates of motility and lower rates of abnormal sperm of postthaw Angora goat semen (Bucak et al., 2009). In contrast, AET showed negative effects on sperm kinetics and motility parameters in bull semen (Tuncer et al., 2014, Büyükleblebici et al., 2016, Swami et al., 2017). In our study, only very low doses of AET supplementation in the diluent of sperm were compatible with the maintenance of membrane integrity/viability (less than 0.008 mmol). The dose 0.001 to 0.006 mmol AET showed contrasted effects on in vitro quality parameters of sperm, except for the acrosome integrity that was systematically decreased at any dose despite the positive effect of AET on malondialdehyde production observed in our study. Finally, even the lower AET doses decreased the fertility obtained with frozen-thawed semen. We thus definitively suggest that supplementation of chicken sperm storage diluent in AET is not recommended despite its positive action on LPO. The extracellular addition of this aminothiol, key point of many intracellular metabolism functions, seems to disrupt too much of the chicken sperm that were already highly stressed by the cryopreservation process.
ERG is an amino acid derived from histidine. It is a powerful scavenger of hydroxyl radical (•OH) and inhibitor of iron or copper ion-dependent generation of •OH from hydrogen peroxide (H2O2) (Akanmu et al., 1991). ERG is an important component of seminal plasma in many mammals where it is expected to protect sperm from oxidative stress, to counteract the effects of peroxides on sperm viability, and to enhance the viability of sperm during storage (Mann and Leone, 1953; Haag and McLeod, 1959). Addition of ERG in the diluent improved the post-thawed motility in ram (Ҫoyan et al., 2011, Ari et al., 2012, Çoyan et al., 2012, Najafi et al., 2014) and stallion (Coutinho de Silva et al., 2008, Metcalf et al., 2008). However, some reports failed to show the antioxidant action of ERG on goat semen conservation (Bucak et al., 2007, Bucak et al., 2009, Ҫoyan et al., 2011). Unlike mammals, ERG does not make part of the main seminal plasma amino acids of chicken seminal plasma (Santiago-Moreno et al., 2019).
In our study, the toxic effect of ERG appeared at higher doses than that of AET, but at lower doses than that of SER. Doses lower than 0.08 mmol did not alter membrane integrity/viability. The doses 0.01 to 0.04 mmol showed a clear positive effect of the reduction of production of malondialdehyde but contrasted effects on in vitro quality sperm parameters. Finally, 0.01 to 0.04 mmol ERG showed a negative effect on fertility obtained with chicken frozen-thawed semen. Despite the positive action of ERG on malondialdehyde production by cryopreserved chicken sperm, we thus do not recommend its addition in chicken sperm diluents.
In a previous study, we demonstrated that the external cryoprotectant SU, used at low concentration that does not increase the osmolality, is a successful additive to chicken semen cryopreservation medium (Thananurak et al., 2019). We now show that the combination SU + SER maintains the positive effect of SU and that of SER on sperm cryopreservation because they yield an overall higher quality of the thawed samples than the control and also because the level of fertility obtained with this combination seems higher than that with the supplementation of SU or SER alone. We hypothesize that the combination SU + SER gives an added advantage to increase the fertility obtained with frozen-thawed semen. However, this need confirmation because, here, the differences are not significant. Clearly, we expect that SU and SER do not have the same action on sperm, but if each of them contribute to improve the sperm conservation, they may show a benefit when they are together in the same semen diluent.
In conclusion, the success of efficient utilization of semen cryopreservation is multifactorial. In the chicken, it must combine careful breeding of the animals, specific attention to their welfare, good initial semen quality, and an efficient method of cryopreservation. The present study shows that semen cryopreservation of a Thai local breed raised in careful and good conditions is successfully developed by the use of a combination of a simple freeze-thaw process and the addition of SER 4 mmol in the BHSV diluent. This success could be potentiated by a combination of SU and SER in the freezing extender in addition to an internal cryoprotectant to increase chicken semen freezability.
Acknowledgments
This study was financially supported by Thailand Research Fund (TRF) under Research and Researcher for Industries (RRI) (PHD59I0025) and Thailand Research Fund (TRF) contract grant IGR5980010. The authors would like to thank the French National Institute of Agronomic Research (INRA), the Research and Development Network Center for Animal Breeding (Native Chicken), and the Department of Animal Science, Faculty of Agriculture, Khon Kaen University, for supporting animal experiments. They also thank Isabelle Grasseau (INRA) for her detailed instruction in the techniques of semen quality evaluation. The authors are also grateful to the Institute of Agricultural Technology, Suranaree University of Technology, and Northeast Agriculture Research Center for semen quality evaluation equipment. The authors declare no conflicts of interest.
References
- Abouelezz F.M., Castano C., Toledano-Diaz A., Esteso M.C., Lopez-Sebastian A., Campo J.L., Santiogo-Moreno J. Effect of the interaction between cryoprotectant concentration and cryopreservation methods on frozen/thawed chicken sperm variables. Reprod. Domest. Anim. 2015;50:135–141. doi: 10.1111/rda.12464. [DOI] [PubMed] [Google Scholar]
- Akalin P.P., Bucak M.N., Güngör Ş., Başpinar N., Çoyan K., Dursun Ş., Pınar ĺ.L.ĺ., Aksoy A., Karaşör Ö.F., Sariözkan S., Deniz Y.E. N.ĺ. Influence of lycopene and cysteamine on sperm and oxidative strees parameters during liquid storage of ram semen at 5 °C. Small Rumin. Res. 2016;137:117–123. [Google Scholar]
- Akanmu D., Cecchini R., Aruoma O.L., Halliwell B. The antioxidant action of ergothioneine. Arch. Biochem. Biophys. 1991;288:10–16. doi: 10.1016/0003-9861(91)90158-f. [DOI] [PubMed] [Google Scholar]
- Alvarez J.G., Storey B.T. Spontaneous lipid peroxidation in rabbit epididymal spermatozoa: its effect on sperm motility. Biol. Reprod. 1982;27:1102–1108. doi: 10.1095/biolreprod27.5.1102. [DOI] [PubMed] [Google Scholar]
- Aitken R.J., Clarkson J.S., Fishel S. Generation of reactive oxygen species, lipid peroxidation and human sperm function. Biol. Reprod. 1989;41:183–197. doi: 10.1095/biolreprod41.1.183. [DOI] [PubMed] [Google Scholar]
- Aitken R.J., Gordon E., Harkiss D., Twigg J.P., Milne P., Jennings Z., Irvine D.S. Relative impact of oxidative stress on the functional competence and genomic integrity of human spermatozoa. Biol. Reprod. 1998;59:1037–1046. doi: 10.1095/biolreprod59.5.1037. [DOI] [PubMed] [Google Scholar]
- Anand T., Kumar D., Chauhan M.S., Manik R.S., Palta P. Cysteamine supplementation of in vitro maturation and/or culture media promote in vitro development of buffalo (Bubalus bulalis) embryos. Reprod. Fertil. Dev. 2008;20:253–257. doi: 10.1071/rd07167. [DOI] [PubMed] [Google Scholar]
- Andrade A.F.C., Arruda R.P., Celeghini E.C.C., Nascimento J., Martins S.M.M.K., Rephael C.F., Moretti A.S. Fluorescent stain method for the simultaneous determination of mitochondrial potential and integrity of plasma and acrosome membranes in boar sperm. Reprod. Domest. Anim. 2007;42:190–194. doi: 10.1111/j.1439-0531.2006.00751.x. [DOI] [PubMed] [Google Scholar]
- Ari U.C., Kulaksiz R., Ozturkler Y., Yildiz S., Lehimcioglu N.C. Effect of L-(+)- ergothioneine (EGT) on freezability of ram semen. Int. J. Anim. Vet. Adv. 2012;4:378–383. [Google Scholar]
- Asmus K., Bensasson R., Bernier J., Houssin R., Land E. One-electron oxidation of ergothioneine and analogues investigated by pulse radiolysis: redox reactive involving ergothioneine and vitamin C. Biochem. J. 1996;315:625–629. doi: 10.1042/bj3150625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blesbois E. Freezing avian semen. Avian Bio. Res. 2011;4:52–58. [Google Scholar]
- Blesbois E. Biological features of the avian male gamete and their application to biotechnology of conservation. J. Poult. Sci. 2012;49:141–149. [Google Scholar]
- Blesbois E., de Reviers M. Effect of different fractions of seminal plasma on the fertilizing ability of fowl spermatozoa stored in vitro. J. Reprod. Fertil. 1992;95:263–268. doi: 10.1530/jrf.0.0950263. [DOI] [PubMed] [Google Scholar]
- Blesbois E., Grasseau I., Blum J.C. Effects of vitamin E on fowl semen storage at 4 degrees C. Theriogenology. 1993;39:771–779. doi: 10.1016/0093-691x(93)90260-c. [DOI] [PubMed] [Google Scholar]
- Blesbois E., Grasseau I., Seigneurin F. Membrane fluidity and the ability of domestic bird spermatozoa to service cryopreservation. Reproduction. 2005;129:371–378. doi: 10.1530/rep.1.00454. [DOI] [PubMed] [Google Scholar]
- Breque C., Surai P., Brillard J.P. Roles of antioxidants on prolonged storage of avian spermatozoa in vivo and in vitro. Mol. Reprod. Dev. 2003;66:314–323. doi: 10.1002/mrd.10347. [DOI] [PubMed] [Google Scholar]
- Bucak M.N., Atessahin A., Varisli O., Yuce A., Tekin N., Akcay A. The influence of trehalose, taurine, cysteamine and hyaluronan on ram semen: microscopic and oxidative stress parameters after freeze-thawing process. Theriogenology. 2007;67:1060–1067. doi: 10.1016/j.theriogenology.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Bucak M.N., Sariözkan S., Tuncer P.B., Ulutas P.A., Akçadağ H.A. Effects of antioxidants on microscopic semen parameters, lipid peroxidation and antioxidant activities in Angora goat semen following cryopreservation. Small Rumin. Res. 2009;81:90–95. [Google Scholar]
- Burrows W.H., Quinn J.P. The collection of spermatozoa from the domestic fowl and Turkey. Poult. Sci. 1937;16:19–24. [Google Scholar]
- Büyükleblebici O., Büyükleblebici S., Tasdemir U., Tuncer P.B. The effects of different antioxidants on post-thaw microscopic and oxidative stress parameters in the cryopreservation of Brown-Swiss Bull semen. Kafkas Universitesi Veteriner Fakultesi Dergisi. 2016;22:101–107. [Google Scholar]
- Cerolini S., Zainiboni L., Maldjian A., Gliozzi T. Effect of docosahexaenic acid and α-tocopherol enrichment in chicken sperm on semen quality, sperm lipid composition and susceptibility on peroxidation. Theriogenology. 2006;66:877–886. doi: 10.1016/j.theriogenology.2006.02.022. [DOI] [PubMed] [Google Scholar]
- Chalah T., Seigneurin F., Blesbois E., Brillard J.P. In vitro comparison of fowl sperm viability in ejaculates frozen by three different techniques and relationship with subsequent fertility in vivo. Cryobiology. 1999;39:185–191. doi: 10.1006/cryo.1999.2201. [DOI] [PubMed] [Google Scholar]
- Chatterjee S., de Lamirande E., Gagnon C. Cryopreservation alters membrane sulfhydryl status of bull spermatozoa: protection by oxidized glutathione. Mol. Reprod. Dev. 2001;60:498–506. doi: 10.1002/mrd.1115. [DOI] [PubMed] [Google Scholar]
- Chuaychu-noo N., Thananurak P., Chankitisakul V., Vongpralub T. Supplementing rooster sperm with cholesterol-loaded-cyclodextrin improves fertility after cryopreservation. Cryobiology. 2017;74:8–12. doi: 10.1016/j.cryobiol.2016.12.012. [DOI] [PubMed] [Google Scholar]
- Consiglio A.L., Meucci A., Cremonesi F. Fluorescent multiple staining and CASA system to assess boar sperm viability and membranes integrity in short and long-term extenders. Open Vet. J. 2013;3:21–35. [PMC free article] [PubMed] [Google Scholar]
- Coutinho de Silva M., Ferreira H., Johnson A. Effects of Tempol and L- ergothioneine on motility parameters of cryopreserved stallion sperm. Anim. Repord. Sci. 2008;107:317–318. [Google Scholar]
- Çoyan K., Baspinar N., Bucak M.N., Akalin P.P. Effects of cysteine and ergothioneine on post-thawed Merino ram sperm. Cryobiology. 2011;63:1–6. doi: 10.1016/j.cryobiol.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Çoyan K., Bucak M.N., Başpinar N., Taşpinar M., Aydos S. Ergothioneine attenuates the DNA damage of post-thawed Merino ram sperm. Small Rumin. Res. 2012;106:165–167. [Google Scholar]
- Dahl T.A., Midden W.R., Hartman P.E. Some prevalent biomolecules as defenses against singlet oxygen damage. Photochem. Photobiol. 1988;47:357–362. doi: 10.1111/j.1751-1097.1988.tb02737.x. [DOI] [PubMed] [Google Scholar]
- De Lamirande E., Gagnon C. Reactive oxygen species and human permatozoa, Effects on the motility of intact spermatozoa and on sperm axonemes. J. Androl. 1992;13:368–378. [PubMed] [Google Scholar]
- De Lamirande E., Gagnon C. A positive role for superoxide anions in triggering hyperactivation and capacitation of human spermatozoa. Int. J. Androl. 1993;16:21–25. doi: 10.1111/j.1365-2605.1993.tb01148.x. [DOI] [PubMed] [Google Scholar]
- Ehling C., Taylor U., Baulain U., Weigend S., Henning M., Rath D. Cryopreservation of semen from genetic resource chicken lines. Agric. For. Res. 2012;62:151–158. [Google Scholar]
- Fujihara N., Howarth B. Lipid peroxidation in fowl spermatozoa. Poult. Sci. 1978;57:1766–1768. doi: 10.3382/ps.0571766. [DOI] [PubMed] [Google Scholar]
- Guerin P., Mouatassim S.E., Menezo Y. Oxidative stress and protection against reactive oxygen species in the pre-implantation embryo and its surrounding. Hum. Reprod. Update. 2001;7:175–189. doi: 10.1093/humupd/7.2.175. [DOI] [PubMed] [Google Scholar]
- Gungor S., Aksoy A., Yeni D., Avdatek F. Combination of cysteamine and lipoic acid improves the post-thawed bull sperm parameters. Kocatepe Vet. J. 2016;9:87–95. [Google Scholar]
- Haag F.M., McLeod J. Relationship between no protein sulfhdryl concentration of seminal fluid and motility of spermatozoa in man. J. Appl. Physiol. 1959;14:27–30. doi: 10.1152/jappl.1959.14.1.27. [DOI] [PubMed] [Google Scholar]
- Hunter T. Why nature chose phosphate to modify protein. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2012;367:2513–2516. doi: 10.1098/rstb.2012.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii T., Bannai S., Sugita Y. Mechanism of growth stimulation of L1210 cells by 2-meceptoethanol in vitro: role of the mixed disulfide of 2-merceptoethanol and cysteine. J. Biol. Chem. 1981;256:12387–12392. [PubMed] [Google Scholar]
- Issels R.D., Nagele A., Eckert K.G., Wilmanns W. Promotion of cysteine uptake and its utilization for glutathione biosynthesis induced by cysteamine and N-acetil- cysteine. Biochem. Pharmacol. 1988;37:881–888. doi: 10.1016/0006-2952(88)90176-1. [DOI] [PubMed] [Google Scholar]
- Kaneko I., Takeuchi Y., Yamaoka Y., Tanaka Y., Fukada T., Fukumori Y., Mayumi T., Hama T. Quantitative determination of ergothioneine in plasma and tissue by TLC- densitometry. Chem. Pharm. Bull. 1980;28:3093–3097. doi: 10.1248/cpb.28.3093. [DOI] [PubMed] [Google Scholar]
- Lewis S.E.M., Sterling E.S.L., Young I.S., Thompson W. Comparison of individual antioxidants of sperm and seminal plasma in fertile and infertile men. Fertil. Steril. 1997;67:142–147. doi: 10.1016/s0015-0282(97)81871-7. [DOI] [PubMed] [Google Scholar]
- Mann T., Leone E. Studies on the metabolism of semen 8: L-ergothioneine content in normal constituent of boar seminal plasma. Biochem. J. 1953;53:140–148. doi: 10.1042/bj0530140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maralani M.N., Movahedian A., Javanmaed S.H. Antioxidant and cryoprotective effects of L-serine on human endothelial cells. Res. Pharm. Sci. 2012;7:209–215. [PMC free article] [PubMed] [Google Scholar]
- Matos D.G., Furnus C.C., Moses D.F., Baldassarre H. Effect of cysteamine on glutathione levels and developmental capacity of bovine oocyte matured in vitro. Mol. Reprod. Dev. 1995;42:432–436. doi: 10.1002/mrd.1080420409. [DOI] [PubMed] [Google Scholar]
- Matos D.G., Herrera C., Conrtvrindt R., Smitz J., Van Soom A., Nogueira D. Cysteamine supplementation during in vitro maturation and embryo culture: a useful tool for increasing the efficiency of bovine in vitro embryo production. Mol. Reprod. Dev. 2002;62:203–209. doi: 10.1002/mrd.10087. [DOI] [PubMed] [Google Scholar]
- Maxwell W.M.C., Watson P.F. Recent progress in the preservation of ram semen. Anim. Reprod. Sci. 1996;84:121–133. [Google Scholar]
- Metcalf E.S., Dideon B.A., Blehr R., Schlimagen T., Bartend W., Varner D.D., Teague S.R., Hausman M.S. Effect of DMSO and L-Ergothioneine on post-thaw semen parameters in stallions: preliminary results. Anim. Reprod. Sci. 2008;107:332–333. [Google Scholar]
- Mata-Campuzano M., Άlvarez-Rodríguez M., de Olmo E., Fernández-Santos M., Garde J., Martinez-Pastor F. Quality, oxidative markers and DNA damage (DNA) fragmentation of red deer thawed spermatozoa after incubation at 37 °C in presence of several antioxidants. Theriogenology. 2012;78:1005–1019. doi: 10.1016/j.theriogenology.2011.12.018. [DOI] [PubMed] [Google Scholar]
- Mata-Campuzano M., Άlvarez-Rodríguez M., Alvarez, Anel L., de Paz P., Garde J., Martínez-Pastor F. Effect of several antioxidants on thawed ram spermatozoa submitted to 37 °C up to four hours. Reprod. Domest. Anim. 2012;47:907–914. doi: 10.1111/j.1439-0531.2012.01990.x. [DOI] [PubMed] [Google Scholar]
- Miranda M., Kulíkovà B., Vašíček J., Olexikova L., Laffaldano N., Chrenek P. Effect of cryoprotectants and thawing temperatures on chicken sperm quality. Reprod. Dom. Anim. 2018;53:93–100. doi: 10.1111/rda.13070. [DOI] [PubMed] [Google Scholar]
- Najafi A., Kia H.D., Mohammadi H., Najafi M.H., Zaynab Z., Sharafi M., Martinez- Postor F., Adeldust H. Different concentration of cysteamine and ergothioneine improve microscopic and oxidative parameters in ram semen frozen with a soybean lecithin extender. Cryobiology. 2014;69:68–73. doi: 10.1016/j.cryobiol.2014.05.004. [DOI] [PubMed] [Google Scholar]
- Naijian H.R., Kohram H., Shahneh A.Z., Sharafi M. Effects of different concentrations of BSA on microscopic and oxidative parameters of Mahabadi goat semen following the following the freeze-thaw process. Cryobiology. 2013;66:151–155. doi: 10.1016/j.cryobiol.2012.12.010. [DOI] [PubMed] [Google Scholar]
- Nguyen T.M., Seigneurin F., Froment P., Combarnous Y., Blesbois E. The 5’- AMP-activated protein kinase (AMPK) is involved in the augmentation of antioxidant defenses in cryopreserved chicken semen. PLoS One. 2015;10:e0134420. doi: 10.1371/journal.pone.0134420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partyka A., Jerysz A., Pokorny P. Lipid peroxidation in fresh and storage semen of Green-legged partridge. EJPAU. 2007;10 http://www.ejpau.media.pl/volume10/issue2/art- 08.html [Google Scholar]
- Partyka A., Łukaszewicz E., Nizanski W., Twardon J. Detection of lipid peroxidation in frozen-thawed avian spermatozoa using C11-BODIPY581/591. Theriogenology. 2011;75:1623–1629. doi: 10.1016/j.theriogenology.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Partyka A., Łukaszewicz E., Niżański W. Lipid peroxidation and antioxidant enzymes activity in avian semen. Anim. Reprod. Sci. 2012;134:184–190. doi: 10.1016/j.anireprosci.2012.07.007. [DOI] [PubMed] [Google Scholar]
- Rakha B.A., Ansari M.S., Hussain H., Blesbois E. Cryopreservation of Indian red jungle fowl (Gallus gallus murghi) semen. Anim. Reprod. Sci. 2016;174:45–55. doi: 10.1016/j.anireprosci.2016.09.004. [DOI] [PubMed] [Google Scholar]
- Rakha B.A., Ansari M.S., Hussain I., Akhter S., Santiago-Moreno J., Blesbois E. Cryopreservation of Indian red jungle fowl (Gallus gallus murghi) semen with polyvinylpyrrolidone. Cryobiology. 2017;78:27–33. doi: 10.1016/j.cryobiol.2017.07.006. [DOI] [PubMed] [Google Scholar]
- Rodríguez-González E., López-Bejar M., Mertens M.J., Paramio M.T. Effects on in vitro embryo development and intracellular glutathione content of the presence of thiol compounds during maturation of prepubertal goat oocytes. Mol. Reprod. Dev. 2003;65:446–453. doi: 10.1002/mrd.10316. [DOI] [PubMed] [Google Scholar]
- Salmani H., Nabi M.M., Vaseghi-Dodaran H., Rahman M.B., Mohammadi- Sangcheshmeh A., Shakeri M., Towhidi A., Shahneh A.Z., Zhandi M. Effect of glutathione in soybean lecithin-based semen extender on goat semen quality after freeze-thawing. Small Rumin. Res. 2013;112:123–127. [Google Scholar]
- Santiago-Moreno J., Bernal B., Pérez-Cerezales S., Castano C., Toledano-Díaz A., Esteso M.C., Gutiérrez-Adán A., López-Sebastiàn A., Gil M.G., Woelders H., Blesbois E. Seminal plasma amino acid profile in different breeds of chicken: role of seminal plasma on sperm cryoresistance. PLoS One. 2019;14:e0209910. doi: 10.1371/journal.pone.0209910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sariözkan S., Tuncer P.B., Buyukleblebici S., Bucak M.N., Canturk F., Eken A. Antioxidative effects of cysteamine, hyaluronan and fetuin on post-thaw semen quality, DNA integrity and oxidative stress parameters in the Brown Swiss bull. Andrologia. 2015;47:138–147. doi: 10.1111/and.12236. [DOI] [PubMed] [Google Scholar]
- Schramm G.P. Suitability of different antifreeze agents fr cryoprotection of cock sperm. Eignung Verschiedener Gefrierschutzstoffe Zur Kryoprotektion von Hahnensperma. Monatsh. Veterinäermedizin. 1991;46:438–440. [Google Scholar]
- Seigneurin F., Blesbois E. Effects of the freezing rate on viability and fertility of frozen-thawed fowl spermatozoa. Theriogenology. 1995;43:1351–1358. [Google Scholar]
- Shahsavari S., McNamara C., Sylvester M., Bromley E., Joslin S., Lu B.Y., Fang S. An amine protecting group deprotectable under nearly neutral oxidative conditions. Beilstein J. Org. Chem. 2018;14:1750–1757. doi: 10.3762/bjoc.14.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim W.C., Yin H.Q., Choi H.S., Choi Y.J., Kwak H.C., Kim S.K., Lee B.H. L- serine supplementation attenuates alcoholic fatty liver by enhancing homocysteamine metabolism in mice and rats. J. Nutr. 2015;145:260–267. doi: 10.3945/jn.114.199711. [DOI] [PubMed] [Google Scholar]
- Surai P.E., Blesbois E., Grasseau I., Chalah T., Brillard J.P., Wishart G.J., Cerolini S., Sparks N.H. Fatty acid composition, glutathione peroxidase and superoxide dismutase activity and total antioxidant activity of avian semen. Comp. Biochem. Physiol. B. Biochem. Mol. Biol. 1998;120:527–533. doi: 10.1016/s0305-0491(98)10039-1. [DOI] [PubMed] [Google Scholar]
- Swami D.S., Kumar P., Malik P.K., Saini M., Kumar D., Jan M.H. Cysteamine supplementation revealed detrimental effect on cryosurvival of buffalo sperm based on computer-assisted semen analysis and oxidative parameters. Anim. Reprod. Sci. 2017;177:56–64. doi: 10.1016/j.anireprosci.2016.12.006. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Nagai T., Hamino S., Kuwayama M., Okamura N., Okano A. Effect of thiol compound on in vitro development and intracellular GSH content of bovine embryos. Biol. Reprod. 1993;49:228–232. doi: 10.1095/biolreprod49.2.228. [DOI] [PubMed] [Google Scholar]
- Thananurak P., Vongpralub T., Sittikasamkit C., Sakwiwatkul K. Optimization of trehalose concentration in semen freezing extender in Thai native chicken semen. Thai J. Vet. Med. 2016;46:287–294. [Google Scholar]
- Thananurak P., Chuaychu-noo N., Vongpralub T. Freezability and fertility of Thai native chicken semen in different diluents. Thai J. Vet. Med. 2017;47:551–556. [Google Scholar]
- Thananurak P., Chuaychu-noo N., Thélie A., Phasuk Y., Vongpralub T., Blesbois E. Sucrose increase the quality and fertilizing ability of cryopreserved chicken semen in contrast to raffinose. Poult. Sci. 2019;98:4161–4171. doi: 10.3382/ps/pez196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thélie A., Bailliard A., Seigneurin F., Zerjal T., Boichard M., Blesbois E. Chicken semen cryopreservation and use for the restoration of rare genetic resources. Poult. Sci. 2019;98:447–455. doi: 10.3382/ps/pey360. [DOI] [PubMed] [Google Scholar]
- Theodoulou F.L., Sibon O.C., Jackowski S., Gout I. Coenzyme A and its derivatives: renaissance of a textbook classic. Biochem. Soc. Trans. 2014;42:1025–1032. doi: 10.1042/BST20140176. [DOI] [PubMed] [Google Scholar]
- Tselutin K., Seigneurin F., Blesbois E. Comparison of cryoprotectants and methods of cryopreservation of fowl spermatozoa. Poult. Sci. 1999;78:586–590. doi: 10.1093/ps/78.4.586. [DOI] [PubMed] [Google Scholar]
- Tuncer P.B., Buyukleblebici S., Ekan A., Tasdemir U., Durmaz E., Buyukleblebici O., Coskun E. Comparison of cryopeotective effects of lycopene and cysteamine in different cryoprotectants on bull semen and fertility results. Reprod. Domest. Anim. 2014;49:746–752. doi: 10.1111/rda.12359. [DOI] [PubMed] [Google Scholar]
- Tuncer P.B., Bucak M.N., Sariözkan S., Sakin F., Yeni D., Ciğerci I.H., Ateşşahin A., Gündoğan M., Büyükleblebici O. The effect of reffinose and methionine on frozen/thawed Angora buck (Capra hircus ancryrensis) semen quality, lipid peroxidation and antioxidant enzyme activity. Cryobiology. 2010;61:89–93. doi: 10.1016/j.cryobiol.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Vongpralub T., Utha A., Pongpeng J., Sonseeda P., Yindee W. Proceedings of the 3rd International Conference on Sustainable Animal Agriculture for Developing Countries (SAADC2011) Nakhon Ratchasima; Thailand: 2011. A comparison of simple vapour method and programmable freezer on motility and fertility of frozen Thai native chicken semen; pp. 649–652. [Google Scholar]
- Wang Q., Sun L.C., Liu Y.Q., Lu J.X., Han F., Huang Z.W. The synergistic effect of serine with selenocompounds on the expression of SelP and GPx in HepG2 cells. Biol. Trace. Elem. Res. 2016;173:291–296. doi: 10.1007/s12011-016-0665-8. [DOI] [PubMed] [Google Scholar]
- Zanganeh Z., Zhandi M., Zare-Shahneh A., Najafi A., Nabi M.M., Mohammadi-Sangcheshmeh A. Dose rosemary aqueous extract improve buck semen cryopreservation? Small Rumin. Res. 2013;114:120–125. [Google Scholar]
- Zhou X., Hu L., Wu C., Zhang Y., Wu X., Yin Y. Serine alleviates oxidative stress via supporting glutathione synthesis and methionine cycle in mice. Mol. Nutr. Food Res. 2017;61 doi: 10.1002/mnfr.201700262. [DOI] [PubMed] [Google Scholar]
- Zhou X., He L., Zuo S., Zhang Y., Wan D., Long C., Huang P., Wu X., Wu C., Liu G., Yin Y. Serine prevented high-fat diet-induced oxidative stress by activating AMPK and epigenetically modulating the expression of glutathione synthesis-related genes. Biochem. Biophys. Acta. 2017;1867:488–498. doi: 10.1016/j.bbadis.2017.11.009. [DOI] [PubMed] [Google Scholar]
- Zhou X., Zhang Y., Wu X., Wan D., Yin Y. Effects of dietary serine supplementation on intestinal integrity, inflammation and oxidative status in early- weaned piglets. Cell Physiol. Biochem. 2018;48:993–1002. doi: 10.1159/000491967. [DOI] [PubMed] [Google Scholar]