Abstract
Supplementation of broiler chicken diets with resin rich in bioactive components, such as different boswellic acids, can improve the productivity, fatty acid composition, and technological parameters of produced meat. The aim of the study was to assess the effect of different levels of Boswellia serrata resin (BSR) supplementation in broiler chicken diet on fatty acid profiles in tissues and meat quality (physicochemical properties). The experimental Ross 308 broiler chickens were randomly assigned to 4 dietary treatments with 5 cages per treatment. The dietary treatments applied for 6 wk consisted of the control (C) and supplementation with 1.5 (BSR1.5), 2 (BSR2), or 2.5% (BSR2.5) of BSR resin. All the diets were isoenergetic and isonitrogenous. The BSR supplementation had a positive effect (P < 0.05) on the share of polyunsaturated fatty acid in the sum of total fatty acids in breast and drumstick muscles and abdominal fat. In addition, the following dietary parameters of the meat were improved: n-3/n-6, saturation, atherogenic and thrombogenic indices, and hypocholesterolemic/hypercholesterolemic ratio. The addition of BSR to the broiler chicken diets increased linearly (C vs. BSR, P < 0.05) the physicochemical properties of the breast and drumstick muscles: water-holding capacity and cooking losses. The color parameter a* decreased linearly (P = 0.033) in the breast muscles of the BSR-treated broiler chickens (8.6 and 7.8% C vs. BSR2 and BSR 2.5).
Key words: broiler chicken, fatty acids, meat quality, Boswellia serrata
Introduction
Dietary methods are mainly used for enhancement of poultry performance and production of high-quality meat with high nutritional and dietary values. Appropriate optimization of feed mixtures based on local feed resources (Apperson and Cherian, 2017, Grela et al., 2017), application of processed feed components in diets (Kiczorowska et al., 2016c), or addition of oil-bearing plant raw materials or their oils provides good effects (Decker and Park, 2010, Hong et al., 2012, Chowdhury et al., 2018). Still, the most popular plant supplements of feed mixtures include herbs and essential oils whose beneficial impact on the effectiveness of poultry production has already been comprehensively described in literature (Kirkpinar et al., 2014, Kiczorowska et al., 2017). New phytobiotics used in poultry production include plant raw materials that have been so far used by local communities, often for therapeutic purposes, for example, resins, bark, or leaves from locally growing trees (Nkukwana et al., 2014, Mpofu et al., 2016).
One of such plants is Boswellia serrata. This is a tree, native to the Arabian Peninsula and India, from the Burseraceae family. Several studies have shown that boswellic acids, which are the major constituents of Boswellia serrata resin (BSR), have anti-inflammatory, anticancerous, antioxidative, and hepatoprotective activities (Al-Yasiry and Kiczorowska, 2016). The available literature reports that there is a relationship between lipid metabolism in chicken organism and dietary phytobiotic supplementation (Kiczorowska et al., 2016a). However, no studies have been reported on hypolipidemic effects of BSR and its effect on the quality of poultry meat. Currently, high-quality meat is in the center of attention of poultry production. The principal focus is placed on not only the nutritional value but also on dietary value of meat, which is determined by fatty acid profiles in tissues (Apperson and Cherian, 2017, Chowdhury et al., 2018). Equally important are the physicochemical properties of poultry meat, which determine its potential to be used in the processing industry (Decker and Park, 2010). Therefore, the present study was performed to evaluate the effect of different levels of BSR supplementation in broiler chicken diet on fatty acid profiles in tissues and meat quality (physicochemical properties).
Materials and methods
The experiment was carried out after obtaining approval from the second Local Ethics Committee at the University of Life Sciences in Lublin (No. 27/2014).
Birds, Diets, and Experimental Design
Two hundred 1-day-old broiler chickens (Ross 308; Aviagen, Cracow, Malopolskie province, Poland) were randomly assigned to 4 dietary treatments with 5 cages per treatment and 5 female and 5 male birds per cage to obtain optimally averaged results. The gender was not included in the experimental observations. The experiment lasted 6 wk. The basal feed diets were made from cereal meal middlings (wheat and corn) and postextraction soybean meal as recommended (Aviagen, 2014). The broiler chickens were fed 3 types of diets: starter (0 to 21 D), grower (22 to 35 D), and finisher (36 to 42 D); the detailed composition of the diets in each stage of animal feeding is presented in Table 1. The starter diet was fed to the broiler chickens in a crumbled form and the grower and finisher diets in a granulated form. The resin was obtained from Boswellia serrata trees by incision of a bark-less trunk and left to dry in natural conditions (direct information from the seller). Fragmented natural BSR was obtained commercially (Baghdad, Iraq). The chemical composition of the resin comprised 95.34% dry matter, ash 1.59% dry matter, protein 2.65% dry matter, fat 63.88% dry matter, and 2.38% gum resin acetyl-11-keto-ß-boswellic acid (Kiczorowska et al., 2016a). The dietary treatments consisted of the control (C) and the control supplemented with 1.5 (BSR1.5), 2 (BSR2), or 2.5% (BSR2.5) of BSR. All the diets were isoenergetic and isonitrogenous. The growth performance effects of the experimental birds are presented in Table 2 (Kiczorowska et al., 2016b).
Table 1.
Dietary ingredients and the nutrient content of the experimental diets (as-fed basis).
| Item | Diets1 |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Starter (1-21 D) |
Grower (22-35 D) |
Finisher (36-42 D) |
||||||||||
| C | BSR1.5 | BSR2 | BSR2.5 | C | BSR1.5 | BSR2 | BSR2.5 | C | BSR1.5 | BSR2 | BSR2.5 | |
| Ingredients, % | ||||||||||||
| Maize | 30.0 | 30.0 | 30.0 | 30.0 | 29.0 | 29.0 | 29.0 | 29.0 | 30.0 | 30.0 | 30.0 | 30.0 |
| Wheat | 20.0 | 20.0 | 20.0 | 20.0 | 23.0 | 23.0 | 23.0 | 23.0 | 26.0 | 26.0 | 26.0 | 26.0 |
| Soybean meal (46% crude protein) | 39.47 | 39.47 | 38.97 | 38.47 | 36.76 | 37.26 | 36.76 | 36.26 | 32.13 | 32.13 | 31.63 | 31.13 |
| Boswellia serrata resin | - | 1.5 | 2.0 | 2.5 | - | 1.5 | 2.0 | 2.5 | - | 1.5 | 2.0 | 2.5 |
| Soybean oil | 6.0 | 4.5 | 4.5 | 4.5 | 7.0 | 5.0 | 5.0 | 5.0 | 8 | 6.5 | 6.5 | 6.5 |
| Dicalcium phosphate | 1.8 | 1.8 | 1.8 | 1.8 | 1.8 | 1.8 | 1.8 | 1.8 | 1.8 | 1.8 | 1.8 | 1.8 |
| Limestone | 1.2 | 1.2 | 1.2 | 1.2 | 1.0 | 1.0 | 1.0 | 1.0 | 0.7 | 0.7 | 0.7 | 0.7 |
| NaCl | 0.33 | 0.33 | 0.33 | 0.33 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| DL-Methionine2 | 0.36 | 0.36 | 0.36 | 0.36 | 0.33 | 0.33 | 0.33 | 0.33 | 0.33 | 0.33 | 0.33 | 0.33 |
| L-Lysine3 | 0.34 | 0.34 | 0.34 | 0.34 | 0.36 | 0.36 | 0.36 | 0.36 | 0.34 | 0.34 | 0.34 | 0.34 |
| Vitamin-mineral premix4 | 0.5 | 0.5 | 0.5 | 0.5 | 0.25 | 0.25 | 0.25 | 0.25 | 0.2 | 0.2 | 0.2 | 0.2 |
| Chemical composition, g/kg | ||||||||||||
| Men5, MJ/kg | 12.55 | 12.43 | 12.43 | 12.44 | 12.97 | 12.90 | 12.93 | 12.95 | 13.39 | 13.33 | 13.36 | 13.37 |
| Crude protein | 212.0 | 211.0 | 212.0 | 212.0 | 192.0 | 192.0 | 193.0 | 193.0 | 185.0 | 185.0 | 185.0 | 185.0 |
| Lys | 13.8 | 13.8 | 13.8 | 13.8 | 12.9 | 12.9 | 12.9 | 12.9 | 11.3 | 11.3 | 11.3 | 11.3 |
| Met + Cys | 10.5 | 10.5 | 10.5 | 10.5 | 9.8 | 9.8 | 9.8 | 9.8 | 9.0 | 9.0 | 9.0 | 9.0 |
| Na | 1.7 | 1.7 | 1.7 | 1.7 | 1.7 | 1.7 | 1.7 | 1.7 | 1.7 | 1.7 | 1.7 | 1.7 |
| Total calcium6 | 9.77 | 9.77 | 9.76 | 9.74 | 8.97 | 8.99 | 8.97 | 8.95 | 7.75 | 7.75 | 7.73 | 7.72 |
| Total phospohorus6 | 7.31 | 7.31 | 7.29 | 7.26 | 7.24 | 7.27 | 7.24 | 7.22 | 7.14 | 7.14 | 7.11 | 7.08 |
| Total magnesium6 | 1.85 | 1.85 | 1.84 | 1.83 | 1.79 | 1.80 | 1.79 | 1.77 | 1.69 | 1.69 | 1.68 | 1.67 |
| Total iron6, mg/kg | 424.34 | 424.34 | 423.41 | 422.48 | 413.35 | 414.28 | 413.35 | 412.42 | 395.7 | 395.7 | 394.77 | 393.84 |
| Total zinc6, mg/kg | 130.86 | 130.86 | 130.61 | 130.36 | 130.01 | 130.26 | 130.01 | 129.76 | 128.48 | 128.48 | 128.23 | 127.98 |
| Total copper6, mg/kg | 18.05 | 18.05 | 17.97 | 17.89 | 17.65 | 17.73 | 17.65 | 17.57 | 17.00 | 17.00 | 16.92 | 16.84 |
Treatments: C = control diet without Boswellia serrata reisn (BSR) supplementation; BSR1.5 = diet with 1.5% BSR supplementation; BSR2 = diet with 2.0% BSR supplementation; BSR2.5 = diet with 2.5% BSR supplementation.
Evonik Degussa GmbH, Essen, Germany (per kilogram of 990 g Met).
Ajinomoto Eurolysine S.A.S., Amiens. France (per kilogram of 780 g Lys).
Added minerals and vitamins per kg of starter diet: Mn, 100 mg; I, 1 mg; Fe, 40 mg; Zn, 100 mg; Se, 0.15 mg; Cu, 10 mg; vitamin A, 15,000 IU; vitamin D3, 5,000 UI; vitamin E, 75 mg; vitamin K3, 4 mg; vitamin B1, 3 mg; vitamin B2, 8 mg; vitamin B6, 5 mg; vitamin B12, 0.016 mg; biotin, 0.2 mg; folic acid, 2 mg; nicotic acid, 60 mg; pantothenic acid, 18 mg; choline, 1,800 mg. Added minerals and vitamins per kg of grower diet: Mn, 100 mg; I, 1 mg; Fe, 40 mg; Zn, 100 mg; Se, 0.15 mg; Cu, 10 mg; vitamin A, 12,000 IU; vitamin D3, 5,000 UI; vitamin E, 50 mg; vitamin K3, 3 mg; vitamin B1, 2 mg; vitamin B2, 6 mg; vitamin B6, 4 mg; vitamin B12, 0.016 mg; biotin, 0.2 mg; folic acid, 1.75 mg; nicotic acid, 60 mg; pantothenic acid, 18 mg; choline, 1,600 mg. Added minerals and vitamins per kg of finisher diet: Mn, 100 mg; I, 1 mg; Fe, 40 mg; Zn, 100 mg; Se, 0.15 mg; Cu, 10 mg; vitamin A,12,000 IU; vitamin D3, 5,000 UI; vitamin E, 50 mg; vitamin K3, 2 mg; vitamin B1, 2 mg; vitamin B2, 5 mg; vitamin B6, 3 mg; vitamin B12, 0.011 mg; biotin, 0.05 mg; folic acid, 1.5 mg; nicotic acid, 35 mg; pantothenic acid, 18 mg; choline, 1,600 mg.
MEn = metabolizable energy (ME) in the diets corrected to zero nitrogen balance.
Analyzed values. Each value based on triplicate determinations.
Table 2.
The effects of experimental broiler chicken growth performance in the whole fattening period (1–42 D) (Kiczorowska et al., 2016b).
| Item | Treatment1 |
Statistical parameters |
||||||
|---|---|---|---|---|---|---|---|---|
| C | BSR1.5 | BSR2 | BSR2.5 | SEM | P value, C vs. BSR | Linear | Quadratic | |
| Total feed intake, g kg−1 | 4,221 | 4,195 | 4,128 | 4,174 | 19.45 | 0.078 | 0.278 | 0.231 |
| Daily BWG, g bird−1 | 56 | 53 | 54 | 55 | 0.18 | 0.278 | 0.167 | 0.184 |
| Final BW, g | 2,526 | 2,515 | 2,547 | 2,560 | 15.47 | 0.128 | 0.063 | 0.109 |
| FCR, kg/kg | 1.73a | 1.67a,b | 1.62a,b | 1.63b | 0.03 | 0.038 | 0.164 | 0.122 |
| Mortality of chicken, heads | 0 | 1 | 0 | 0 | - | - | - | - |
Data represent the mean of 50 broiler chickens per treatment.
a, b, cSignificantly different at P < 0.05.
Abbreviations: BW, body weight; BWG, body weight grain; FCR, feed conversion ratio; SEM, standard error of the mean.
Treatments: C = control diet without Boswellia serrata supplementation; BSR1.5 = diet with 1.5% Boswellia serrata supplementation; BSR2 = diet with 2% Boswellia serrata supplementation; BSR2.5 = diet with 2.5% Boswellia serrata supplementation.
Ten clinically healthy birds (1 hen and 1 cock per pen) were chosen at random from each treatment, and their blood was sampled for biochemical analyses. Blood was sampled in the morning before the slaughter from the ulnar vein (vena cutanea ulnaris) using 6-mL Vacutest tubes containing lithium heparin as an anticoagulant (transported at a temperature from 2 to 8°C).
Twenty birds (2 hens and 2 cocks per pen) with body weight close to the average body weight in the group were selected from every group for slaughter by decapitation. A simplified dissecting analysis was carried out, during which breast muscles, drumstick muscles, and abdominal fat were sampled (Ziołecki and Doruchowski, 1989). The tissues were weighed, and some of them were frozen at a temperature of −80°C until chemical analyses. The meat intended for quality analysis was stored at a temperature of 4°C (to 24 h) to avoid drying out.
Fatty Acid Composition and Cholesterol in Broiler Chicken Tissues
The composition of fatty acids in breast muscles, drumstick muscles, and abdominal fat was determined by means of gaseous chromatography using an INCO 505 apparatus (Prague, Czech Republic) (PN-EN ISO 5508 [Polish Standard 1996]), capillary column (length 60 m/dia. 0.25 mm), temperature of the column 220°C, injector 260°C, detector 260°C, and gas-helium (14 mL·min−1); dose per column 2 μl. The contents of fatty acids were determined using the same apparatus with an SPTM-2560 capillary column (Supelco Inc., Bellefonte, PA), length 100 m/ID 0.25 mm. The temperature of the column was 200°C, that of the injector 220°C, and of the detector 250°C, and the flow of gas (helium) was 20 cm·s−1 and dose per column 1 μl. The determinations were based on templates such as Supelco 37-Component FAME MIX and Linolenic Acid Methyl Ester Isomer Mix (Sigma-Aldrich, Poznań, Poland). Fatty acids were expressed as a percentage of total fatty acids identified (Wu et al., 2007) and grouped as saturated fatty acids (SFA) and unsaturated fatty acids (UFA): monounsaturated fatty acids (MUFA) and polyunsaturated fatty acids (PUFA).
The saturation (S/P), atherogenic (AI), and thrombogenic (TI) indices (Ulbricht and Southgate, 1991) and hypocholesterolemic/hypercholesterolemic ratio (HH) (Santos-Silva et al., 2002) were calculated as follows:
The cholesterol content in breast and drumstick muscles (Chol) was assessed with the colorimetric method using an EPOLL 20 colorimeter (Rhee et al., 1982).
Physicochemical Properties of Breast and Drumstick Muscle
Chicken carcasses were chilled with the air-blast method at a temperature of 4°C. Twenty-four hr after slaughter, pH was determined in the breast muscles and drumsticks at the depth of 0.5 cm by means of a glass electrode (Xerolyt-type, Mettler Toledo, Urdorf, Switzerland) (PN-ISO 2917 [Polish Standard 2001]). The water-holding capacity (WHC) of sampled breast and drumstick tissues was determined immediately after the slaughter according to the procedure specified by Pohja and Niinivaara (1957). Three 300-mg samples were taken from each group of muscles and placed on a 125-mm Whatman filter paper under a constant pressure of 2 kg for 5 min. The percentage share of free water in the meat was calculated based on the drip index. It was assumed that 1 cm3 of the drip corresponds to 10 mg of water.
The color of the breast and drumstick muscles was determined using a Minolta CM-2600d reflective colorimeter—light source D65, observer 10o (Konica Minolta, Japan) (Honikel, 1998).
To determine losses caused by the thermal treatment, the second part of the chicken breast muscle was heated in a water bath (temp. 90°C, for ca. 30 min until a temperature of 75 ± 2°C was achieved in the geometric center). The meat temperature was measured using a HI 98804 bayonet thermometer (Hanna Instruments, Woonsocket, RI).
To measure the shearing force, the breast muscles were cooled in air (temp. 18 to 22°C) and placed in a cold store (temp. 4 ± 2°C). After 24 h, 5 samples (1 × 1 × 5 cm) were dissected from each muscle along muscle fibers. The measurements were carried out using a strength-testing device Wick 1,120 (Zwick, Germany) equipped with a Warner-Bratzler shear element. The mean value of 5 measurements was the result of the determination.
Statistical Analysis
Each cage was used as a statistical unit. The data obtained were elaborated with the ANOVA method using one-way ANOVA (α = 0.05; P < 0.05) and calculating the mean values for the treatments () and the standard error of the mean. Linear and quadratic polynomial contrasts were used to evaluate the effects of different dietary levels of BSR. The significance of differences was determined with Statistica 13.3 software (StatSoft Inc. 2013).
Results
Fatty Acid Composition and Cholesterol in Broiler Chicken Tissues
In the pool of SFA, the breast muscle of broiler chickens in the BSR1.5, BSR2, and BSR2.5 treatments exhibited a decline in the stearic acid content (C vs. BSR diets, linear, P = 0.041) (Table 3). Among PUFA, there was an increase in the level of C20:2 acids (C vs. BSR diets, linear, P = 0.021) and C20:3 acids (C vs. BSR diets, quadratic, P = 0.048) as well as the sum of n-3 acids (C vs. BSR diets, linear, P = 0.23). These changes contributed (C vs. BSR diets, linear, P = 0.019) to improvement of the n-3/n-6 and S/P ratio. Concurrently, there was linear decrease (P < 0.05) in C20:4 in the breast muscle of BSR2 and BSR2.5 broiler treatments (respectively: by approximately 7 and 23.5%).
Table 3.
The fatty acids in the breast muscle of broiler chickens.
| Item,% sum of total fatty acids | Treatment1 |
Statistical parameters |
||||||
|---|---|---|---|---|---|---|---|---|
| C | BSR1.5 | BSR2 | BSR2.5 | SEM | P value C vs. BSR | Linear | Quadratic | |
| SFA | ||||||||
| C12:0 | 0.04 | 0.04 | 0.05 | 0.05 | <0.01 | 0.219 | 0.114 | 0.178 |
| C14:0 | 0.64 | 0.62 | 0.52 | 0.55 | 0.03 | 0.089 | 0.059 | 0.094 |
| C15:0 | 0.12a | 0.08b | 0.09b | 0.07 | <0.01 | 0.016 | 0.023 | 0.316 |
| C16:0 | 25.23 | 24.24 | 24.14 | 24.09 | 0.68 | 0.164 | 0.109 | 0.217 |
| C17:0 | 0.14 | 0.13 | 0.14 | 0.15 | 0.03 | 0.128 | 0.241 | 0.431 |
| C18:0 | 8.11a | 7.51a,b | 7.78a | 7.14 | 0.05 | 0.041 | 0.038 | 0.153 |
| C20:0 | 0.07 | 0.06 | 0.07 | 0.05 | <0.01 | 0.148 | 0.421 | 0.369 |
| ΣSFA | 34.32 | 32.68 | 32.79 | 32.73 | 0.87 | 0.073 | 0.134 | 0.196 |
| MUFA | ||||||||
| C14:1 | 0.11 | 0.10 | 0.09 | 0.13 | <0.01 | 0.157 | 0.074 | 0.298 |
| C16:1. n-9 | 4.86 | 4.70 | 4.60 | 4.49 | 0.08 | 0.213 | 0.159 | 0.076 |
| C18:1. n-7 | 2.34 | 2.18 | 2.37 | 2.44 | 0.03 | 0.109 | 0.138 | 0.247 |
| C18:1. n-9 | 33.54 | 34.18 | 34.14 | 34.24 | 0.48 | 0.324 | 0.389 | 0.315 |
| C20:1 n-9 | 2.29 | 2.33 | 2.37 | 2.14 | 0.09 | 0.064 | 0.073 | 0.092 |
| Σ MUFA | 43.14 | 43.49 | 43.57 | 43.44 | 0.78 | 0.267 | 0.097 | 0.245 |
| PUFA | ||||||||
| C18:2. n-6 | 19.98 | 21.06 | 20.75 | 21.09 | 0.64 | 0.164 | 0.119 | 0.217 |
| C18:3. n-6 | 1.09 | 1.11 | 1.23 | 1.09 | 0.06 | 0.128 | 0.236 | 0.431 |
| C20:2. n-3 | 0.06c | 0.08b,c | 0.10b | 0.23a | <0.01 | 0.021 | 0.048 | 0.178 |
| C20:3. n-3 | 0.24c | 0.35b | 0.47a | 0.51a | <0.01 | 0.048 | 0.037 | 0.169 |
| C20:4. n-6 | 1.15a | 1.20a | 1.07b | 0.88c | 0.07 | 0.019 | 0.114 | 0.043 |
| C22:4. n-6 | 0.02 | 0.03 | 0.02 | 0.03 | <0.01 | 0.187 | 0.245 | 0.324 |
| n-3 | 0.30d | 0.43c | 0.57b | 0.74a | 0.05 | 0.023 | 0.034 | 0.187 |
| n-6 | 22.24 | 23.4 | 23.07 | 23.09 | 0.45 | 0.216 | 0.053 | 0.214 |
| Σ PUFA | 22.54 | 23.83 | 23.64 | 23.83 | 0.54 | 0.264 | 0.119 | 0.367 |
| Σ UFA | 65.68 | 67.32 | 67.21 | 67.27 | 0.76 | 0.123 | 0.258 | 0.232 |
| UFA/SFA | 1.91 | 2.06 | 2.05 | 2.06 | 0.04 | 0.071 | 0.078 | 0.143 |
| MUFA/SFA | 1.26 | 1.33 | 1.33 | 1.33 | 0.07 | 0.148 | 0.437 | 0.369 |
| PUFA/SFA | 0.66 | 0.73 | 0.72 | 0.73 | <0.01 | 0.073 | 0.064 | 0.176 |
| n-3/n-6 | 0.013d | 0.018c | 0.025b | 0.032a | <0.01 | 0.019 | 0.014 | 0.178 |
| S/P | 0.52a | 0.48a,b | 0.48a,b | 0.46b | 0.03 | 0.034 | 0.048 | 0.097 |
| TI | 1.03a | 0.96a,b | 0.96a,b | 0.93b | 0.05 | 0.016 | 0.023 | 0.216 |
| AI | 0.42 | 0.40 | 0.39 | 0.39 | <0.01 | 0.164 | 0.119 | 0.361 |
| HH | 2.12 | 2.27 | 2.27 | 2.28 | 0.03 | 0.123 | 0.245 | 0.431 |
Data represent the mean of 10 broiler chickens per treatment.
a, b, cSignificantly different at P < 0.05.
Abbreviations: AI, atherogenic indices; HH, hypocholesterolemic/hypercholesterolemic ratio; MUFA, monounsaturated fatty acids; PUFA, polyunsaturated fatty acids; SEM, standard error of the mean; SFA, saturated fatty acids; S/P, saturation indices; TI, thrombogenic indices; UFA, unsaturated fatty acids.
Treatments: C = control diet without Boswellia serrata supplementation; BSR1.5 = diet with 1.5% Boswellia serrata supplementation; BSR2 = diet with 2% Boswellia serrata supplementation; BSR2.5 = diet with 2.5% Boswellia serrata supplementation.
The BSR supplementation of broiler chicken diets modified significantly (P < 0.05) the profile of drumstick muscle fatty acids (Table 4). The addition of 1.5, 2, and 2.5% of BSR to the broiler chicken diets decreased linearly (P = 0.035) the SFA sum (C14, C16, C17, and C18) and simultaneously increased (P = 0.039) the content of the MUFA sum (C14:1; C18:1, and C20:1) and PUFA sum (P = 0.023) (C18:2, C18:3, C20:2, C20:3, C20:4, and C22:4) as well as the sum of n-3 and n-6 acids (C vs. BSR diets, linear, respectively, P = 0.019 and 0.016). The changes in the levels of SFA and PUFA in the total fatty acids of drumstick muscles improved the UFA/SFA, PUFA/SFA, and n-3/n-6 ratios, which was reflected by better dietary values of P/S, TI, AI, and HH (C vs. BSR diets, linear, P < 0.05).
Table 4.
The fatty acids in the drumstick muscle of broiler chickens (% sum of total fatty acids).
| Item, % sum of total fatty acids | Treatment1 |
Statistical parameters |
||||||
|---|---|---|---|---|---|---|---|---|
| C | BSR1.5 | BSR2 | BSR2.5 | SEM | P value C vs. BSR | Linear | Quadratic | |
| SFA | ||||||||
| C12:0 | 0.07 | 0.06 | 0.06 | 0.05 | 0.07 | 0.079 | 0.054 | 0.178 |
| C14:0 | 0.79a | 0.64b | 0.55c | 0.53c | 0.04 | 0.034 | 0.025 | 0.164 |
| C15:0 | 0.11 | 0.08 | 0.09 | 0.09 | <0.01 | 0.076 | 0.083 | 0.316 |
| C16:0 | 26.99a | 24.08b | 21.81c | 21.15c | 0.41 | 0.014 | 0.019 | 0.357 |
| C17:0 | 0.17a | 0.15b | 0.13c | 0.12c | 0.06 | 0.028 | 0.043 | 0.431 |
| C18:0 | 8.38a | 7.26a,b | 6.28 | 7.09b | 0.08 | 0.041 | 0.038 | 0.147 |
| C20:0 | 0.08 | 0.08 | 0.06 | 0.05 | <0.01 | 0.153 | 0.341 | 0.269 |
| Σ SFA | 36.59a | 32.35b | 28.98c | 29.08c | 0.78 | 0.035 | 0.044 | 0.196 |
| MUFA | ||||||||
| C14:1 | 0.17a | 0.15b | 0.16a,b | 0.14b | <0.01 | 0.027 | 0.034 | 0.278 |
| C16:1, n-9 | 6.65 | 6.53 | 6.34 | 6.12 | 0.05 | 0.168 | 0.215 | 0.164 |
| C18:1, n-7 | 2.26 | 2.33 | 2.37 | 2.49 | 0.0 | 0.278 | 0.167 | 0.314 |
| C18:1, n-9 | 37.20 | 39.65a | 40.24a | 39.32a | 0.97 | 0.048 | 0.037 | 0.279 |
| C20:1, n-9 | 0.35 | 0.41a | 0.44a | 0.43a | <0.01 | 0.027 | 0.033 | 0.154 |
| Σ MUFA | 46.63 | 49.07a | 49.55a | 48.50a,b | 0.68 | 0.039 | 0.078 | 0.241 |
| PUFA | ||||||||
| C18:2, n-6 | 16.08 | 17.34b | 19.73a | 20.14a | 0.46 | 0.034 | 0.019 | 0.367 |
| C18:3, n-6 | 0.22 | 0.54c | 0.71b | 1.12a | 0.06 | 0.028 | 0.041 | 0.334 |
| C20:2, n-3 | 0.03c | 0.08b | 0.12a,b | 0.17a | <0.01 | 0.031 | 0.028 | 0.346 |
| C20:3, n-3 | 0.15 | 0.19c | 0.24b | 0.33a | <0.01 | 0.045 | 0.017 | 0.169 |
| C20:4, n-6 | 0.22 | 0.28c | 0.46a | 0.37b | 0.07 | 0.019 | 0.044 | 0.373 |
| C22:4, n-6 | 0.08 | 0.15c | 0.21b | 0.29a | <0.01 | 0.023 | 0.018 | 0.475 |
| n-3 | 0.18 | 0.27c | 0.36b | 0.50a | 0.03 | 0.019 | 0.037 | 0.321 |
| n-6 | 16.60c | 18.31b | 21.11a | 21.92a | 0.45 | 0.016 | 0.023 | 0.516 |
| Σ PUFA | 16.78c | 18.58b | 21.47a | 22.42a | 0.04 | 0.023 | 0.019 | 0.367 |
| Σ UFA | 63.41 | 67.65 | 71.02 | 70.92 | 0.67 | 0.123 | 0.245 | 0.431 |
| UFA/SFA | 1.73 | 2.09 | 2.45a | 2.44a | 0.03 | 0.041 | 0.028 | 0.272 |
| MUFA/SFA | 1.27 | 1.52 | 1.71 | 1.67 | 0.02 | 0.148 | 0.437 | 0.369 |
| PUFA/SFA | 0.46c | 0.57b | 0.74a | 0.77a | <0.01 | 0.033 | 0.024 | 0.196 |
| n-3/n-6 | 0.011c | 0.015b | 0.017b | 0.023a | <0.01 | 0.019 | 0.014 | 0.171 |
| S/P | 0.57a | 0.47a,b | 0.40b | 0.41b | <0.01 | 0.043 | 0.038 | 0.137 |
| TI | 1.15a | 0.97b | 0.83c | 0.85c | 0.04 | 0.016 | 0.023 | 0.316 |
| AI | 0.48a | 0.39a,b | 0.34b | 0.33b | <0.01 | 0.024 | 0.019 | 0.265 |
| HH | 1.93c | 2.33b | 2.71a | 2.79a | 0.06 | 0.035 | 0.048 | 0.432 |
Data represent the mean of 10 broiler chickens per treatment.
a, b, cSignificantly different at P < 0.05.
Abbreviations: AI, atherogenic indices; HH, hypocholesterolemic/hypercholesterolemic ratio; MUFA, monounsaturated fatty acids; PUFA, polyunsaturated fatty acids; SEM, standard error of the mean; SFA, saturated fatty acids; S/P, saturation indices; TI, thrombogenic indices; UFA, unsaturated fatty acids.
Treatments: C = control diet without Boswellia serrata supplementation; BSR1.5 = diet with 1.5% Boswellia serrata supplementation; BSR2 = diet with 2% Boswellia serrata supplementation; BSR2.5 = diet with 2.5% Boswellia serrata supplementation.
The BRS addition in broiler chicken feeding induced changes (P < 0.05) only in the UFA pool in the abdominal fat (Table 5). In MUFA, there was a linear decrease in C16:1 (C vs. BSR diets, P = 0.23, ca. 19.3% C vs. BSR2.5), with a simultaneous linear increase in C20:1 (C vs. BSR diets, P = 0.034, approx. 33.3% C vs. BSR2.5). Multidirectional changes were noted in the PUFA pool as well. A higher level of C20:4 (C vs. BSR diets, linear, P = 0.019) was determined in the abdominal fat of the 2.5BSR-treated broiler chickens. The increasing BSR levels led to an increase in C18:3, C20:2, and C20:3 and the sum of n-3 fatty acids in the abdominal fat (control vs. BSR diets, linear, P < 0.05). The changes in the proportion of fatty acids improved the PUFA/SFA and n-3/n-6 ratios (control vs. BSR diets, linear, respectively, P = 0.033 and 0.019).
Table 5.
The fatty acids in the abdominal fat of broiler chickens (% sum of total fatty acids).
| Item, % sum of total fatty acids | Treatment1 |
Statistical parameters |
||||||
|---|---|---|---|---|---|---|---|---|
| C | BSR1.5 | BSR2 | BSR2.5 | SEM | P value, C vs. BSR | Linear | Quadratic | |
| SFA | ||||||||
| C12:0 | 0.06 | 0.06 | 0.05 | 0.04 | <0.01 | 0.119 | 0.234 | 0.378 |
| C14:0 | 0.64 | 0.61 | 0.58 | 0.56 | 0.04 | 0.218 | 0.109 | 0.197 |
| C15:0 | 0.08 | 0.07 | 0.07 | 0.05 | <0.01 | 0.186 | 0.083 | 0.316 |
| C16:0 | 23.2 | 23.08 | 22.8 | 22.64 | 0.51 | 0.264 | 0.119 | 0.467 |
| C17:0 | 0.08 | 0.08 | 0.09 | 0.07 | <0.01 | 0.068 | 0.148 | 0.132 |
| C18:0 | 6.19 | 6.04 | 5.77 | 5.81 | 0.06 | 0.091 | 0.072 | 0.247 |
| C20:0 | 0.04 | 0.04 | 0.05 | 0.06 | <0.01 | 0.148 | 0.457 | 0.162 |
| Σ SFA | 30.29 | 29.98 | 29.41 | 29.23 | 0.48 | 0.233 | 0.124 | 0.195 |
| MUFA | ||||||||
| C14:1 | 0.17 | 0.16 | 0.17 | 0.18 | <0.01 | 0.245 | 0.187 | 0.206 |
| C16:1, n-9 | 6.10a | 6.07a | 5.02b | 4.92b | 0.05 | 0.023 | 0.048 | 0.187 |
| C18:1, n-7 | 2.06 | 2.01 | 1.95 | 1.97 | 0.02 | 0.187 | 0.089 | 0.263 |
| C18:1, n-9 | 37.19 | 37.25 | 38.08 | 37.95 | 0.37 | 0.108 | 0.112 | 0.309 |
| C20:1, n-9 | 0.24c | 0.26b,c | 0.28b | 0.32a | <0.01 | 0.034 | 0.044 | 0.218 |
| Σ MUFA | 45.76 | 45.75 | 45.5 | 45.34 | 0.67 | 0.137 | 0.248 | 0.197 |
| PUFA | ||||||||
| C18:2, n-6 | 22.33 | 22.63 | 23.4 | 23.66 | 0.34 | 0.264 | 0.119 | 0.367 |
| C18:3, n-6 | 1.37 | 1.38 | 1.37 | 1.41 | 0.06 | 0.128 | 0.243 | 0.451 |
| C20:2, n-3 | 0.03d | 0.06c | 0.12b | 0.14a | <0.01 | 0.041 | 0.028 | 0.244 |
| C20:3, n-6 | 0.03c | 0.05b | 0.08a,b | 0.09a | <0.01 | 0.048 | 0.017 | 0.569 |
| C20:4, n-6 | 0.19a | 0.15b | 0.12a | 0.13b,c | 0.03 | 0.019 | 0.014 | 0.378 |
| n-3 | 0.03d | 0.06c | 0.12b | 0.14a | <0.01 | 0.034 | 0.046 | 0.193 |
| n-6 | 23.92 | 24.21 | 24.97 | 25.29 | 0.43 | 0.116 | 0.223 | 0.516 |
| Σ PUFA | 23.95 | 24.27 | 25.09 | 25.43 | 0.53 | 0.264 | 0.119 | 0.367 |
| Σ UFA | 69.71 | 70.02 | 70.59 | 70.77 | 0.76 | 0.128 | 0.241 | 0.332 |
| UFA/SFA | 2.30 | 2.34 | 2.40 | 2.42 | 0.03 | 0.091 | 0.075 | 0.255 |
| MUFA/SFA | 1.51 | 1.53 | 1.55 | 1.55 | 0.02 | 0.148 | 0.487 | 0.531 |
| PUFA/SFA | 0.79c | 0.81b,c | 0.85a,b | 0.87a | <0.01 | 0.033 | 0.024 | 0.196 |
| n-3/n-6 | 0.001c | 0.002 | 0.005a | 0.006a | <0.01 | 0.019 | 0.014 | 0.377 |
| S/P | 0.43 | 0.42 | 0.41 | 0.41 | <0.01 | 0.145 | 0.267 | 0.315 |
| TI | 0.64 | 0.63 | 0.61 | 0.61 | <0.01 | 0.216 | 0.183 | 0.116 |
| AI | 0.37 | 0.37 | 0.36 | 0.35 | 0.45 | 0.264 | 0.119 | 0.367 |
| HH | 2.50 | 2.53 | 2.63 | 2.66 | 0.06 | 0.121 | 0.243 | 0.259 |
Data represent the mean of 10 broiler chickens per treatment.
a, b, cSignificantly different at P < 0.05.
Abbreviations: AI, atherogenic indices; HH, hypocholesterolemic/hypercholesterolemic ratio; MUFA, monounsaturated fatty acids; PUFA, polyunsaturated fatty acids; SEM, standard error of the mean; SFA, saturated fatty acids; S/P, saturation indices; TI, thrombogenic indices; UFA, unsaturated fatty acids.
Treatments: C = control diet without Boswellia serrata supplementation; BSR1.5 = diet with 1.5% Boswellia serrata supplementation; BSR2 = diet with 2% Boswellia serrata supplementation; BSR2.5 = diet with 2.5% Boswellia serrata supplementation.
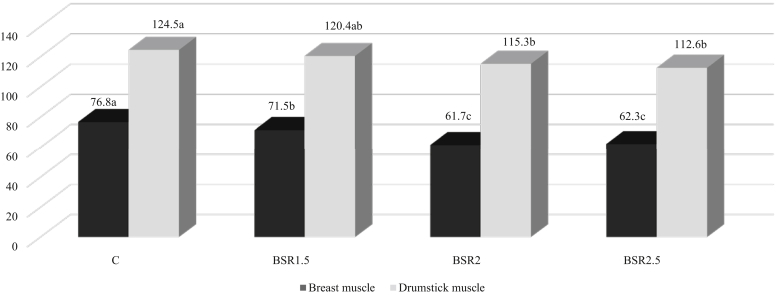
The Boswellia serrata addition to the broiler chicken feed has a linear positive effect on the cholesterol content in the breast (P = 0.019) and drumstick (P = 0.035) muscles (decrease respectively by ca. 19.6% C vs. BSR2 and 9.5% C vs. BRS 2.5) (Figure 1).
Figure 1.
Cholesterol content in broiler meat, mg 100 g-1. a, b, cSignificantly different at P < 0.05. Abbreviations: BSR, Boswellia serrata resin; C, control.
Technological and Physical Parameters of Breast and Drumstick Muscles
The BSR supplementation of chicken diets had a positive effect (control vs. BSR diets, linear, P < 0.05) on the proportion of the drumstick muscle in the chilled carcass from 17.44% in C to average 19.34% in the BSR2 and BSR2.5 treatments. There were no significant changes in the proportion of breast muscles in the chilled carcass (Table 6).
Table 6.
Physicochemical properties of broiler chicken meat.
| Item | Treatment1 |
Statistical parameters |
||||||
|---|---|---|---|---|---|---|---|---|
| C | BSR1.5 | BSR2 | BSR2.5 | SEM | P value, C vs. BSR | Linear | Quadratic | |
| Breast muscle | ||||||||
| Proportion in the chilled carcass, % | 20.2 | 21.9 | 22.1 | 22.4 | 0.11 | 0.134 | 0.145 | 0.201 |
| pH24 | 6.02 | 6.15 | 6.18 | 6.23 | 0.07 | 0.287 | 0.109 | 0.217 |
| WHC, % | 13.3d | 15.7c | 16.5 | 17.2a | 0.05 | 0.016 | 0.023 | 0.326 |
| Cooking losses, % | 22.5a | 18.6b | 15.4c | 15.9c | 0.27 | 0.024 | 0.019 | 0.132 |
| Shear force, N | 32.4 | 31.8 | 32.6 | 31.9 | 0.46 | 0.128 | 0.243 | 0.147 |
| Color parameters | ||||||||
| L* | 54.3 | 54.9 | 55.6 | 57.1 | 0.48 | 0.148 | 0.457 | 0.261 |
| a* | 10.4a | 9.9a,b | 9.5b | 9.6b | 0.07 | 0.033 | 0.024 | 0.196 |
| b* | 11.7 | 11.9 | 12.3 | 12.7 | 0.11 | 0.089 | 0.078 | 0.154 |
| Drumstick muscle | ||||||||
| Proportion in the chilled carcass, % | 17.44b | 17.42b | 19.41a | 19.28a | 0.21 | 0.037 | 0.042 | 0.138 |
| pH24 | 6.54 | 6.61 | 6.72 | 6.68 | 0.05 | 0.264 | 0.217 | 0.315 |
| WHC, % | 12.7c | 13.5b,c | 15.4a | 14.6b | 0.09 | 0.029 | 0.043 | 0.229 |
| Cooking losses, % | 20.8a | 17.6b | 18.4b | 17.9b | 0.21 | 0.031 | 0.025 | 0.278 |
| Shear force, N | 30.1 | 29.8 | 29.9 | 30.6 | 0.34 | 0.287 | 0.145 | 0.098 |
| Color parameters | ||||||||
| L* | 47.8 | 47.5 | 48.3 | 48.6 | 3.18 | 0.264 | 0.119 | 0.367 |
| a* | 15.6 | 15.4 | 15.9 | 16.2 | 1.24 | 0.128 | 0.248 | 0.438 |
| b* | 9.51 | 9.78 | 9.67 | 9.93 | 0.07 | 0.091 | 0.078 | 0.248 |
Data represent the mean of 10 broiler chickens per treatment.
a, b, cSignificantly different at P < 0.05.
Abbreviations: pH24, pH at 24 h postmortem; SEM, standard error of the mean; WHC, water-holding capacity.
Treatments: C = control diet without Boswellia serrata supplementation; BSR1.5 = diet with 1.5% Boswellia serrata supplementation; BSR2 = diet with 2% Boswellia serrata supplementation; BSR2.5 = diet with 2.5% Boswellia serrata supplementation.
The addition of BSR in chicken diets contributed to significant linear changes in the WHC value in the breast and drumstick muscles (respectively: P = 0.016 and 0.029; an increase to 29% BSR2.5 and 21% BSR2 vs. C) and cooking losses (respectively: P = 0.024 and 0.031; a decrease to 31.5% BSR2.5 and 15.4% BSR1.5 vs. C) (Table 6). The color parameter a* decreased linearly (P = 0.033) in the breast muscles of the BSR-treated broiler chickens (average 8.1% C vs. BSR2, BSR 2.5). No statistically significant differences were identified between the respective treatments in other physical and technological parameters (shear force and color parameters: L*, b*).
Discussion
Phytobiotics are products of plant origin extracted from medicinal plants, spices, or even mushrooms. They exert a wide impact on the organism because of their high content of bioactive compounds provided by secondary metabolism. Numerous investigations carried out on a variety of livestock animals have confirmed the multidirectional effects of application of phytobiotics in animal nutrition on improvement of health and productivity. In addition, a positive impact on the nutritional and dietary value of animal-derived products has been revealed (Al-Yasiry et al., 2017b, Kiczorowska et al., 2017). Comprehensive animal and human research has proved that BSR has antihyperglycemic and lipid-lowering effects (Zutshi et al., 1986, Singh et al., 2012). However, no such effect of Boswellia serrata on the lipid profile in the broiler blood was noted in the present study, which may be related to the form of the additive, that is, unpurified and unprocessed resin.
The article presented previously (Al-Yasiry et al., 2017a) demonstrated no effect of BSR supplementation (1.5, 2, and 2.5%) of broiler chickens diets on the dry matter content in the breast and drumstick muscle (average 26.08 and 25.96 g 100 g−1, respectively), crude ash (average 1.12 and 1.0 g 100 g−1, respectively), and crude protein (average 23.36 and 18.83 g 100 g−1, respectively). Only the supplementation with 2.5% of BSR in broiler chicken diets decreased (P < 0.05) the ether extract content by approximately 7.8% in breast muscles and by even 15% in drumstick muscle, compared with the control group. The authors emphasize that although there were no statistically significant differences in the content of nutrients in breast muscles, a negative correlation was found between their content and the level of BSR supplementation (AL-Yasiry et al., 2017a). The improvement of the dietary value of poultry meat observed in the present study resulting from the BSR supplementation can be associated with the high, more than 60% fat content in the resin. The major compound of Boswella serrata resin oil is boswellic acid (Al-Yasiry and Kiczorowska, 2016). It has strong antibacterial and immunostimulatory properties, which optimally stabilize the microbiological environment of the gastrointestinal tract (Hamidpour et al., 2015). The previous investigations reported by the authors confirmed the positive effect of BSR on the structure of intestinal villi, gastrointestinal microflora, and the health status in broiler chickens, which was reflected in improved efficiency of rearing and utilization of feed nutrients (Kiczorowska et al., 2016a). Al-Yasiry et al. (2017b) found that 3 to 4% BSR supplementation in broiler chicken diets also decreased the ether extract in breast and drumstick muscles. This resulted in reduced caloric value of breast (P < 0.041) and drumstick muscles (P < 0.038). The levels of other nutrients determined in the chicken muscles were similar to those in the control treatment. As in the present study, the authors did not observe an effect of BSR supplementation on the proportion of breast and thigh muscles in the carcass.
The addition of Boswellia serrata to the broiler chicken diets resulted in reduction (P < 0.05) of the content of SFA in chicken tissues. Compared with the control group, the biggest changes were observed in the content of C14:0, C16:0, C17:0, and C18:0 (breast muscles) and C18:0 (drumstick muscles). The literature does not provide reports on the effect of BSR on the fatty acid profile in poultry muscles. However, investigations of other phytobiotics (herbs, aromatic oils, and so forth) confirm that the bioactive compounds contained therein, including terpenes, can reduce the SFA content in the fatty acid profile (Modiry et al., 2010, Hong et al., 2012, Kirkpinar et al., 2014).
The 2% of BSR supplementation in chicken diets resulted in a 6.2% increase (P < 0.05) in the level of MUFA in the crude fat of drumstick muscles. The changes in the share of MUFA in the pool of fatty acids were mostly induced by the content of C18:1 (n-9). Similar results were obtained by Mypofu et al. (2016), who reported an increase in the oleic acid level in the drumstick muscle of broiler chickens fed with mixtures supplemented with Lippia javanica, which is very rich in terpenes. However, the improvement of the fatty acid profile observed by the authors was accompanied by an unfavorable phenomenon of an increase in the fat content in the entire broiler carcass. The use of BSR in broiler chicken mixtures, especially at the dose of 2.5%, reduced the content of crude fat from 1.03 (control) to 0.95 g 100 g−1 in the breast muscle and from 5.69 (control) to 4.84 g 100 g−1 in the drumstick muscle in the BSR treatment (Al-Yasiry et al., 2017a)
Drumstick muscles were the ones most susceptible to modification of the fatty acid profile by the BRS supplementation. The share of PUFA in total fatty acids significantly increased (P < 0.05) in these tissues of broilers fed diets with BSR supplementation. Linoleic acid is considered the main component of PUFA susceptible to growth in the muscles of poultry fed with BSR supplementation. However, the trend toward increased synthesis of PUFA in the muscle tissues of animals fed with a ration containing phytobiotics is not explicit. In the presented studies, a trend at synthesizing lower amounts of C20:4 n-6 (P = 0.019) was observed in the abdominal fat of the BSR-treated chicks. A similar effect on the fatty acid profile in poultry meat was reported by Nkukwana et al. (2014), who applied supplementation with Moringa oleifera leaves in broiler chicken mixtures at a level from 1 to 5% of dry matter intake. These leaves are characterized by strong anti-inflammatory, antioxidant, and antibacterial properties associated with the high content of terpenoids, flavonoids, and polyphenols. In addition, the authors found that the supplementation improved oxidative stability in meat.
The supplementation of BRS in broiler diets, especially at the levels of 2 and 2.5% resulted in a decrease in the n-3/n-6 ratio, on average by 11% in the breast muscles, 2.5- and almost 4-fold in the drumsticks, and 5- to 6-fold in the abdominal fat (P < 0.05). This was primarily caused by the higher level of health-enhancing n-3 fatty acids, that is, C20:2. Studies of the effect of terpene-rich phytobiotics applied in poultry diets emphasize the favorable dietary modification of meat lipid parameters. These changes are mainly found in C20 acids with a slight modification in the linolenic acid content, which can be explained by the role of C18:3 in the metabolism of longer chain n-3 fatty acid (Nkukwana et al., 2014, Mpofu et al., 2016, Chowdhury et al., 2018).
The proportion of n-6 in the n-3 and n-6 acid ratio in poultry meat is normally high. This is undesirable in human nutrition, as fatty acids from the n-6 family that are metabolized to arachidonic acid and then to eicosanoids can be intermediaries in transformations that are adverse to health (Decker and Park, 2010). There are many reports on the possibility of increasing the n-3/n-6 ratio in fat deposits, in particular by the nutritional use of various sources of plant fats, which are rich in UFA (Ciurescu et al., 2016, Apperson and Cherian, 2017). The introduction of BSR into feed mixes makes it possible to control the fatty acid composition of muscles and improve the PUFA to SFA ratio. The tissues of broiler chickens from the BSR2 and BSR2.5 treatments were characterized by lower AI and TI and a higher HH ratio, which indicates reduced atherogenicity of the meat and fat. A beneficial effect of the application of BSR in broiler chicken nutrition was reflected in the reduction of cholesterol level, especially in the breast muscles. Prabakar et al. (2016) confirm that the antioxidant activity and hypolipidemic properties of phytobiotics could be involved in improvement of poultry meat. The hypolipidemic properties of natural additives in broiler nutrition can be used for production of lean meat with a lower cholesterol level (Hong et al., 2012, Kirkpinar et al., 2014, Mpofu et al., 2016). In the previous work, the authors showed a positive effect of 2 and 2.5% BSR supplementation of broiler chicken diets on the proportion of the analyzed muscles in the carcass. Significantly higher content was noted in the case of the drumstick muscle, that is, even up to 10.5% in comparison with the control treatment. The full slaughter analysis presented in the previous publication (Al-Yasiry et al. 2018) did not confirm the effect of this factor on the total slaughter performance, which was estimated at a mean level of 73.68% (for the BSR treatments and control). In turn, the addition of 2 and 2.5% BSR in chicken diets (P < 0.05) resulted in an increase in the content of total muscle (41.51 and 41.68%, respectively) and a decrease in the abdominal fat content (BSR 2.26 and 2.30%, respectively) compared with the control treatment (C 37.64 and 2.81%, respectively).
The breast and drumstick muscles of the BSR-treated broiler chicken exhibited an increase in WHC and a decrease in cooking losses. These results are supported by studies conducted on broiler chickens receiving supplementation with phytobiotics (Kirkpinar et al., 2014, Nkukwana et al., 2014). Contrasting results were reported by Sukoco et al. (2015) on their investigations on broiler chicken fed with a mixture with noni leaf extract (Morinda citrifolia L.). Besides beneficial production effects, there was no influence of the phytobiotic addition on the technological parameters of meat. WHC is a criterion of the technological quality of meat referred to as forced leakage of meat juice. WHC, tenderness, and color largely depend on acidity. At a higher pH, meat usually has a greater capacity to bind and retain water during thermal processing (Fanatico et al., 2007). In the present study, a tendency toward increasing the pH value was only observed, which may have contributed to the improvement of WHC. High WHC accompanied by lower thermal losses makes the meat a good raw material not just for culinary purposes. The color is one of the most important indicators of the quality of poultry meat with high relevance for consumers. It is also an important determinant of the technological suitability of meat as a raw material intended directly for sale. The breast muscles of the chickens fed with Boswellia serrata were characterized by a lighter color than that in the control chickens, as shown by the higher values of the L * color component and the lower values of the a * component. Meat with pale pink or even white color is more readily chosen by consumers who consider it fresh, tender, and juicy (Wideman et al., 2016).
Conclusions
The PUFA share in the sum of total fatty acids in the breast and drumstick muscles and abdominal fat were positively influenced by the 2 and 2.5% Boswellia serrata supplementation, improving the dietary meat parameters (n-3/n-6, S/P, TI, AI, and HH). The addition of BSR to the broiler chicken diets increased linearly the technological breast and drumstick parameters (WHC, cooking losses). The results obtained indicate the validity of further investigations using the 2 and 2.5 of BSR, which is likely to enhance its favorable effect.
Acknowledgments
The study was conducted under the scientific program of Institute Animal Nutrition and Bromatology, University of Life Sciences in Lublin (Polnad), Ministry of High Education in Iraq (No. IRQ/02/2014), and Greenland Technologia EM sp. z o.o. (Poland). No potential conflicts of interest are reported by the authors.
Contributor Information
Bożena Kiczorowska, Email: bozena.kiczorowska@up.lublin.pl.
Wioletta Samolińska, Email: wioletta.samolinska@up.lublin.pl.
References
- Al-Yasiry A.R.M., Kiczorowska B., Samolińska W., Kowalczuk-Vasilev E. Growth performance, digestibility, hematology, biochemistry, and same humoral immunity blood parameters of broiler chickens fed different levels of olibanum (Boswellia serrata) Anim. Prod. Sci. 2018;58:1885–1891. [Google Scholar]
- Al-Yasiry A.R.M., Kiczorowska B., Samolińska W. Effect of Boswellia serrata resin supplementation on basic chemical and mineral element composition in the muscles and liver of broiler chickens. Biol. Trace Elem. Res. 2017;179:294–303. doi: 10.1007/s12011-017-0966-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Yasiry A.R.M., Kiczorowska B., Samolińska W. The nutritional value and content of mineral elements in meat of broiler chicken feed diets supplemented with Boswellia serrata. J. Elem. 2017;22:1027–1037. [Google Scholar]
- Al-Yasiry A.R.M., Kiczorowska B. Frankincense – therapeutic properties. Postepy. Hig. Med. Dosw. 2016;70:380–391. doi: 10.5604/17322693.1200553. [DOI] [PubMed] [Google Scholar]
- Apperson K.D., Cherian G. Effect of whole flax seed and carbohydrase enzymes on gastrointestinal morphology, muscle fatty acids, and production performance in broiler chickens. Poult. Sci. 2017;96:1228–1234. doi: 10.3382/ps/pew371. [DOI] [PubMed] [Google Scholar]
- Aviagen . 2014. Ross 308 Broiler: Nutrition Specifications.http://en.aviagen.com/assets/Tech_Center/Ross_Broiler/Ross308BroilerNutritionSpecs2014-EN.pdf Accessed April 2016. [Google Scholar]
- Chowdhury S., Mandal G.P., Patra A.K. Different essential oils in diets of chickens: 1. Growth performance, nutrient utilisation, nitrogen excretion, carcass traits and chemical composition of meat. Anim. Feed Sci. Technol. 2018;236:86–97. [Google Scholar]
- Ciurescu G., Ropota M., Toncea I., Habeanu M. Camelia (Camelina sativa L. Crantz variety) oil and seeds as n-3 fatty acids rich products in broiler diets and its effects on performance, meat fatty acid composition, immune tissue weights, and plasma metabolic profile. J. Agric. Sci. Technol. 2016;18:315–326. [Google Scholar]
- Decker E.A., Park Y. Healthier meat products as functional foods. Meat Sci. 2010;86:49–55. doi: 10.1016/j.meatsci.2010.04.021. [DOI] [PubMed] [Google Scholar]
- Fanatico A.C., Pillai P.B., Emmert J.L., Owens C.M. Meat quality of slow- and fast-growing chicken genotypes fed low-nutrient or standard diets and raised indoors or with outdoor access. Poult. Sci. 2007;86:2245–2255. doi: 10.1093/ps/86.10.2245. [DOI] [PubMed] [Google Scholar]
- Grela E.R., Samolińska W., Kiczorowska B., Klebaniu R., Kiczorowski P. Content of minerals, fatty acids and their correlation with phytochemical compounds and antioxidant activity of Leguminous seeds. Biol. Trace Elem. Res. 2017;180:338–348. doi: 10.1007/s12011-017-1005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamidpour S.H., Hamidpour M., Shahlari M., Hamidpour R. Chemistry, pharmacology and medicinal property of Frankincense (Boswellia Species): from the selection of Traditional applications to the novel phytotherapy for the prevention and treatment of Serious diseases. GJMR. 2015;15:1–9. doi: 10.4103/2225-4110.119723. https://globaljournals.org/GJMR_Volume15/3-Chemistry-Pharmacology-and-Medicinal.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong J.C., Steiner T., Aufy T.A., Lien T.F. Effects of supplemental essential oil on growth performance, lipid metabolites and immunity, intestinal characteristics, microbiota and carcass traits in broilers. Livest Sci. 2012;144:253–262. [Google Scholar]
- Honikel K.O. Reference methods for the assessment of physical characteristics of meat. Meat Sci. 1998;49:447–457. doi: 10.1016/s0309-1740(98)00034-5. [DOI] [PubMed] [Google Scholar]
- Kiczorowska B., Al-Yasiry A.R.M., Samolińska W., Marek A., Pyzik E. The effect of dietary supplementation of the broiler chicken diet with Boswellia serrata resin on growth performance, digestibility, and gastrointestinal characteristics, morphology, and microbiota. Livest Sci. 2016;191:117–124. [Google Scholar]
- Kiczorowska B., Samolińska W., Al-Yasiry A.R.M., Kowalczyk-Pecka D. Effect of supplementation of mixtures for broiler chickens with Boswellia serrata on the condition of the gastrointestinal tract and rearing efficiency. Ann. Anim. Sci. 2016;16:835–849. [Google Scholar]
- Kiczorowska B., Samolińska W., Al-Yasiry A.R.M., Kiczorowski P., Winiarska-Mieczan A. The natural feed additives as immunostimulants in monogastric animal nutrition – a review. Ann. Anim. Sci. 2017;17:1–21. [Google Scholar]
- Kiczorowska B., Samolińska W., Andrejko D. Effect of micronized pea seeds (Pisum sativum L.) as a substitute of soybean meal on tissue fatty acid composition and quality of broiler chicken meat. Anim. Sci. J. 2016;87:1396–1406. doi: 10.1111/asj.12592. [DOI] [PubMed] [Google Scholar]
- Kirkpinar F., Ünlü H.B., Serdaroğlu M., Turp G.Y. Effects of dietary oregano and garlic essential oils on carcass characteristics, meat composition, colour, pH and sensory quality of broiler meat. Br. Poult. Sci. 2014;55:157–166. doi: 10.1080/00071668.2013.879980. [DOI] [PubMed] [Google Scholar]
- Modiry A., Nobakht A., Mehmannavaz Y. Investigation the effects using different mixtures of Nettle (Urtica dioica), Menta pulagum (Oreganum vulgare) and Zizaphora (Thymyus vulgaris) on performance and carcass traits of broilers. Proc. 4th Iranian Congress Animal Science. 2010:252–254. [Google Scholar]
- Mpofu D.A., Marume U., Mlambo V., Hugo A. The effects of Lippia javanica dietary inclusion on growth performance, carcass characteristics and fatty acid profiles of broiler chickens. Anim. Nutr. 2016;2:160–167. doi: 10.1016/j.aninu.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nkukwana T.T., Muchenje V., Masika P.J., Hoffman L.C., Dzama K., Descalzo A.M. Fatty acid composition and oxidative stability of breast meat from broiler chickens supplemented with Moringa oleifera leaf meal over a period of refrigeration. Food Chem. 2014;142:255–261. doi: 10.1016/j.foodchem.2013.07.059. [DOI] [PubMed] [Google Scholar]
- Polish Standard, PN-EN ISO 5508 . Polish Committee for Standardization; Warsaw (Poland): 1996. Oils and Vegetable and Animal Fats. The Analysis of Fatty Acid Methyl Esters by Gas Chromatography (In Polish) [Google Scholar]
- Polish Standard, PN-ISO 2917 . Polish Committee for Standardization; Warsaw (Poland): 2001. Meat and Meat Products. PH Measurement (In Polish) [Google Scholar]
- Pohja M.S., Niinivaara F.P. Die Bestimmung der Wasserbindung des Fleisches mittels der Konstantdrückmethode. Fleischwirtschaft. 1957;9:193–195. [Google Scholar]
- Prabakar G., Gopi M., Karthik K., Shanmuganathan S., Kirubakaran A., Pavulraj S. Phytobiotics: could the greens inflate the poultry production. Asian J. Anim. Vet. Adv. 2016;11:383–392. [Google Scholar]
- Rhee K.S., Dutson T.R., Smith G.C., Hostetler R.L., Reiser R. Cholesterol content of raw and cooked beef longissimus muscles with different degrees of marbling. J. Food Sci. 1982;47:716–719. [Google Scholar]
- Santos-Silva J., Bessa R.J.B., Santos-Silva F. Effect of genotype, feeding system and slaughter weight on the quality of light lambs: II Fatty acid composition of meat. Livest Prod. Sci. 2002;77:187–194. [Google Scholar]
- Singh P., Chacko K.M., Aggarwal M.L., Bhat B., Khandal R.K., Sultana S., Kuruvilla B.T. A-90 day gavage safety assessment of Boswellia serrata in rats. Toxicol. Int. 2012;19:273–278. doi: 10.4103/0971-6580.103668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- StatSoft Inc. 2013. STATISTICA (data analysis software system). version 13.3. www.statsoft.com.
- Sukoco A., Widodo E., Thohari I. Effect of noni leaves extract (Morinda citrifolia L.) supplementation in feed on physical quality of broiler breast meat. Res. J. Life Sci. 2015;2:77–83. [Google Scholar]
- Ulbricht T.L., Southgate D.A. Coronary heart disease: seven dietary factors. Lancet. 1991;338:985–992. doi: 10.1016/0140-6736(91)91846-m. [DOI] [PubMed] [Google Scholar]
- Wideman N., O'Bryan C.A., Crandall P.G. Factors affecting poultry meat colour and consumer preferences - a review. World Poult. Sci J. 2016;72:353–366. [Google Scholar]
- Wu H.-Q., Huang X.-L., Lin X.-S., Huang F., Zhu Z.-X., Ma Y.-F. Chromatographic retention time rule and mass spectrometric fragmentation rule of fatty acids and its. Chin. J. Anal. Chem. 2007;35:998–1003. [Google Scholar]
- Ziołecki J., Doruchowski W. COBRD Publishing; Poznań, Poland: 1989. The Method of Assessment of Slaughter Poultry (In Polish) [Google Scholar]
- Zutshi U., Rao P.G., Ravi S., Singh G.B., Surjeet S., Atal C.K. Mechanism of cholesterol lowering effect of Salai guggal ex. Boswellia serrata roxb. Indian J. Pharmacol. 1986;18:182–183. [Google Scholar]