Abstract
The present study aims to investigate the effects of supplementing broiler diets with a bioactive olive pomace extract (OE) from Olea europaea on growth performance, digestibility, gut microbiota, bile acid composition, and immune response. To this end, three hundred and six 1-day-old broiler chickens (Ross 308) were housed in floor pens (6 pens/treatment, with 17 birds/pen). Animals were fed with a standard non-medicated starter diet for 21 D, and from 22 to 42 D of age with their respective experimental diet: a negative control with no additives (Control), a positive control with 100 ppm of monensin (Monensin) and the basal diet supplemented with 750 ppm of an OE (Lucta S.A., Spain). Feed intake and growth rate were monitored weekly throughout the trial. From 21 to 42 D of age, no significant differences in feed intake were observed among dietary treatments; however, lower average daily gain and higher feed conversion ratio (P < 0.05) was observed in birds fed the Control compared to Monensin and OE groups. Performance of birds fed OE or Monensin was similar throughout the trial. The apparent ileal digestibility of crude protein was higher in birds fed Monensin than Control treatment (P < 0.05). No significant changes on bacterial composition at a family level were observed in the caeca of birds fed the experimental diets. Moreover, no significant differences on plasma and intestinal bile acid composition were observed among treatments. Birds fed the OE showed a significant decrease of IL-8 expression in the ileum (P < 0.05). Additionally, the expression of TGF-β4, and Bu-1 was significantly upregulated (P < 0.01) in broilers fed the OE and Monensin diets compared to those fed the Control. In conclusion, the inclusion of 750 ppm of a bioactive olive pomace extract from Olea europaea in broiler chicken diets improved animal growth likely as result of its anti-inflammatory properties.
Key words: broiler chicken, olive extract, performance, gut function, immune response
INTRODUCTION
Agricultural eco-innovation is based on circular economy and cradle to cradle concepts. This innovation concept aims at “zero waste” economy where new products and applications are created from raw material wastes (Mirabella et al., 2014). Spain is the largest olive oil (Olea europaea L.) producer worldwide with around 1.3 million tons that generates wastes such as olive pomace and leaves totaling annually more than 4 and 0.2 million tons, respectively (International Olive Council, 2017).
One of the ways to take advantage of the olive oil industry wastes is its use in animal feed. It has been shown that the inclusion of up to 150 g/kg of byproducts such as olive pomace and cake in the feed has no adverse effects in broiler performance (El Hachemi et al., 2007; Sayehban et al., 2016). Moreover, recent interest is being generated in the purification of the bioactive compounds (polyphenols, oleuropeoside, flavonoid, and simple phenolics) from botanicals such as the olive by-products to enhance animal health and performance (Liehr et al., 2017; Leskovec et al., 2018). In a global strategy to reduce the use of drugs in animal production, plant extracts rich in bioactive compounds with anti-microbial, antioxidant, and anti-inflammatory properties are promising alternatives to antibiotics (Niewold, 2007, 2014; Lillehoj et al., 2018). According to Niewold (2014) the positive effects of antibiotics on animal performance are directly related to their anti-inflammatory effects, which attenuate the intestinal inflammatory insults that take place under normal productive circumstances. Moreover, bioactive phytochemicals can stimulate innate immunity and might be an alternative to control coccidiosis in poultry (Lillehoj and Lee, 2012). In this regard, it has been recently suggested that olive pomace extracts might improve the intestinal health of calves and pigs (Liehr et al., 2017; Morrison et al., 2017). Also, inclusion of an olive pomace bioactive extract in diets fed to sea bream showed a positive effect on growth performance by improving liver lipid metabolism and the intestinal innate immune function (Gisbert et al., 2017).
In addition, phenolic compounds extracted from olive leafs might be beneficial to broilers through their antimicrobial activity against intestinal pathogenic bacteria (Sarica and Ürkmez, 2016). Also, phenolic compounds can stimulate or inhibit digestive enzyme activities affecting nutrient digestibility in broilers (Brenes and Roura, 2010; Leskovec et al., 2018). The stimulation of other digestive secretions such as bile acids by plant bioactives has been reported in rats (Platel and Srinivasan, 2000). Because of their potential use in poultry feeds there is a need to increase our knowledge on olive byproducts extracts and their effects on gut microbiota, nutrient digestibility, and bile acid metabolism.
The present study aimed to investigate the effects of supplementing broiler diets with an olive pomace extract from Olea europaea on animal performance, nutrient digestibility, bile acid composition, gut microbiota, and immune function.
MATERIALS AND METHODS
Housing and Experimental Animals
A feeding trial was carried out at the Polytechnic University of Madrid (UPM) experimental facilities (Agricultural Production Department, Madrid). All the experimental procedures used were approved by the Animal Ethic Committee of the Universidad Politécnica de Madrid, according with principles of care of animals in experimentation (Boletin Oficial del Estado, 53/2013, BOE, 2013).
A total of three hundred and six 1-day-oldmixed sex broiler chickens (Ross 308) were obtained from a commercial hatchery (Avimosa Group, Toledo, Spain). Chicks were assigned to 18 floor pens (1.1 m × 1.1 m) with 17 birds per pen (initial live weight 40.6 ± 0.7 g). Pens were bedded with wood shaving and provided with a hopper feeder and a bell drinker. Environmental conditions such as temperature, humidity, ventilation, and illumination were automatically controlled during the experiment, depending on the age of the birds (33°C during the first week of age and then was reduced 2°C each week until reaching 23°C at 6 wk of age). Regarding light program, chicks received 23 h light and 1 h dark for the first 7 D of life and then 18 h light and 6 h dark until the end of the experiment.
Experimental Design and Diets
All birds were raised with a standard non-medicated starter diet based on wheat and soybean meal in crumble form for the first 3 wk (Table 1). After that, 252 animals with 21 D of age (84 birds/treatment) and similar body weight (BW) were used in the feeding trial (14 birds/pen × 6 pens/treatment × 3 treatments). The design was completely randomized with 3 treatments, a negative control with no additives (Control), a positive control with 100 ppm of monensin (Monensin; Elanco Valquimia S.A.) and the basal diet supplemented with 750 ppm of an olive extract (OE, Lucta S. A.; Spain) which consisted of an olive pomace extract standardized to contain a minimum of 10% total triterpenes and 2% polyphenols. The theoretical concentration of OE (750 ppm, with 2% of polypenols and 10% of triterpenes) was similar to the concentration found in the experimental diet (722 ± 23 ppm, with 2.4 ± 0.25% of polypenols and 12.9 ± 0.54% of triterpenes). This analysis developed with ultra high performance liquid chromatography-mass spectroscopy (UPLC-MS) technique indicated that extract mixed procedure and stability of the product fitted with the expected values.
Table 1.
Ingredients and chemical composition (%, as fed basis, unless otherwise indicated) of pre-experimental (1 to 21 D) and experimental control diet (22 to 42 D).
| Pre-experimental diet | Control diet | |
|---|---|---|
| Ingredient | ||
| Wheat (10.2% PB) | 46.9 | 35.5 |
| Soy bean meal (47% PB) | 37.3 | 30.7 |
| Barley | 6.51 | 20.5 |
| Fat1 | 5.50 | 8.00 |
| Celite | − | 2.00 |
| Dicalcium phosphate | 1.40 | 1.33 |
| Calcium carbonate | 1.15 | 0.98 |
| Vitamin and mineral premix2 | 0.30 | 0.30 |
| Sodium chloride | 0.40 | 0.35 |
| L-Lys HCl (78%) | 0.14 | 0.12 |
| DL-met (99%) | 0.26 | 0.23 |
| L-Thr (98%) | 0.05 | 0.05 |
| Etoxiquin 66% | 0.02 | 0.02 |
| Endofeed DC | 0.01 | 0.01 |
| Phyzyme XP 5000 | 0.01 | 0.01 |
| Calculated values | ||
| Dry matter | 88.3 | 89.0 |
| AMEn (Kcal/Kg) | 2,950 | 3,050 |
| Crude protein | 21.9 | 19.2 |
| Ether extract | 7.11 | 9.60 |
| Crude fiber | 3.03 | 2.90 |
| Neutral detergent fiber | 10.4 | 11.0 |
| Starch | 32.0 | 32.3 |
| Sugars | 4.75 | 4.20 |
| Ca | 1.00 | 0.90 |
| Total P | 0.61 | 0.55 |
| Digestible P | 0.36 | 0.34 |
| Na | 0.17 | 0.15 |
| Ash | 6.40 | 7.80 |
Animal fat and soybean oil blend.
Provided the following (per kilogram of diet): vitamin A (transretinyl acetate), 10,000 IU; vitamin D3 (cholecalciferol), 2,000 IU; vitamin E (all-rac-tocopherol acetate), 20 IU; vitamin K (bisulfate menadione complex), 3 mg; riboflavin, 5 mg; pantothenic acid (D-calcium pantothenate), 10 mg; nicotinic acid, 30 mg; pyridoxine (pyridoxine·HCl), 3 mg; thiamine (thiamine-mononitrate), 1 mg; vitamin B12 (cyanocobalamine), 12 μg; D-biotin, 0.15 mg; choline (choline chloride), 300 mg; folic acid, 0.5 mg; Se (Na2 SeO3), 0.1 mg; I (KI), 2.0 mg; Cu (CuSO4·H2 O), 10 mg; Fe (FeSO4·7H2 0), 30 mg; Zn (ZnO), 100 mg; Mn (MnSO4·H2 O), 100 mg; and ethoxyquin, 110 mg.
The experimental diets were formulated to have similar nutritive value (Table 1) according to FEDNA (2010) and manufactured at IRTA (Mas de Bover, Constantí, Spain). Celite was added in the feed at 2% as acid insoluble ash marker for apparent ileal digestibility (AID) determination. Animals were fed ad libitum the experimental diets as pellets with 3 mm diameter from 21 to 42 D of age.
Productive Traits and Sampling
Body weight and feed consumption were determined by pen at 21, 28, 35, and 42 D of age, to calculate the average daily gain (ADG), average daily feed intake (ADFI), and feed conversion ratio (FCR). Mortality was recorded and weighed as produced. At the end of the experiment at 42 D of age, birds were slaughtered by asphyxiation in CO2 atmosphere. To obtain enough ileal content to determine nutrient digestibility and to sample all animals in the fed state birds were deprived of food for 2 h, and then were refed under ad libitum conditions for 1.30 h to achieve as homogeneous feed intake as possible. Two birds per pen were randomly selected and sampled to run microbial, gene expression, blood and bile acid analysis. To determine the relative abundance of bacterial families approximately 1 g of caecal content was sampled, immediately placed in dry ice and stored at −80°C. For gene expression analysis around 200 mg of ileal (approximately 4 cm from the Meckel diverticulum) mucosal scrapings were sampled in RNA later (Invitrogen, Carlsbad, CA) following the manufacturer's instructions, and further stored at −80°C. Blood samples were collected from the heart using sterile syringes and needles. To obtain the plasma blood was collected into tubes containing EDTA and aprotinin (BD Vacutainer), held in ice for 30 min, centrifuged at 2,000 × g for 10 min and stored at −80°C to later analyze bile acids. Finally, 5 g of ileal content were collected and stored at −80°C to analyze bile acid concentrations.
Nutrient Apparent Ileal Digestibility
The ileal digesta from the remaining birds (10 to 12 broiler per pen) was collected as indicated in Mandalawi et al. (2014). Samples were pooled, homogenized, frozen at −20°C, and freeze-dried. Then, the dried samples were ground using a mortar and pestle to pass through a 0.5 mm screen and maintained in airtight containers at room temperature until determination of nutrient AID. The AID of dry matter (DM), organic matter (OM), crude protein (CP), ether extract (EE), and gross energy (GE) was estimated using the indigestible marker method (De Coca-Sinova et al., 2011).
Ileal Digesta Analysis
The ileal content of broilers were analyzed following the standard methods of AOAC (2000) for DM and OM (934.01), EE (920.39), total ash in muffle (942.05), and CP by combustion method (968.06) using FP-528 nitrogen analyzer (LECO, St. Joseph, MI, EE.UU). The GE was analyzed by adiabatic bomb calorimeter (PARR, 1356 model; Parr Instrument Company, Moline, IL, EE.UU). Acid insoluble ash of diets, and ileal contents were determined as indicated by De Coca-Sinova et al. (2011).
Bile Acids Analysis
Bile acids analysis was performed by UPLC-MS chromatography in an AQUITY I-Class (Waters Corp., Milford, MA) connected to a Xevo-G2 QTof MS detector. Separation was run on an ACQUITY UPLC BEH C18 1.7 mm column (2.1 × 100 mm, Waters Corp.) using water and acetonitrile as mobile phases, both containing a 0.1% of formic acid. Mass spectroscopy detection was performed in full-scan negative mode (100 to 1,200 Da). Concentration of bile acid was determined based on standard curves with QuanLynx software (Waters Corp.). Bile acids were extracted using the following methodologies and using chenodeoxycholic acid-d4 (CDCA-d4) as internal standard: lyophilized ileal digesta samples were homogenized in absence of solvent on a TissueLyzer II (QIAGEN, Hilden, Germany) and 20 mg of homogenate were extracted with 840 µL of H2 O: ACN (1:1) including internal standard, respectively. After homogenization, the mixture was centrifuged (15,000 g × 10 min, 4°C) and the supernatant diluted in H2 O: ACN (1:50) for UPLC-MS analysis. Plasma proteins were precipitated by addition of 200 µL of ACN with 5 µL internal standard to 50 µL of plasma. After centrifugation, supernatants were directly analyzed by UPLC analysis.
Gut Microbiota Analysis
The bacterial DNA extraction and sequence analysis were performed using the methodology described by Andreano et al. (2017). Briefly, bacterial DNA was isolated of caecal content samples to obtain the microbiome profile by massive sequencing of the 16S rRNA gene regions. Amplicons of the V1 to V2 16S rRNA region were amplified by PCR with F27 forward and R338 reverse primers with the sequences and conditions indicated in Andreano et al. (2017). For each amplicon, quality and concentration were analyzed using Agilent Bioanalyzer 2100 (Agilent Technologies, Santa Clara, CA). Samples were massive sequenced with the Ion 318 Chip Kit v2 (Life Technologies) under manufacturer's conditions on an Ion Torrent Personal Genome Machine (PGM).
Gene Expression Analysis
Total RNA was extracted from approximately 50 mg of ileal mucosal scraping with TRIzol reagent (Invitrogen, Carlsbad, CA, USA), disrupted with a mixer mill MM-400 (Retsch, Stuttgart, Germany) and isolated by using the GenElute Mammalian Total RNA Miniprep Kit (Sigma-Aldrich Corporation, St. Louis, MO, USA). To prevent genomic DNA contamination an “in column” DNase step was performed by using the RNAse-Free DNase Set (Quiagen, Australia). Extracted RNA yield and quality were measured by spectrophotometry (Epoch, BioTek, Winoosky, VT, USA) combined with the Take3 Micro-Volume Plate (BioTek, Santa Barbara, CA, USA) by absorbance at wavelengths of 260 and 280 nm.
Reverse transcription of around 2,400 ng of extracted RNA was performed with the SuperScript VILO Master Mix (Invitrogen, Carlsbad, CA, USA). The quantitative real-time PCR (qRT-PCR) analysis was performed in a 7300 Real Time PCR System (Applied Biosystems, Foster City, CA, USA) with already tested and published primer conditions. Following primers and PCR conditions were obtained from the literature: chicken β Actin (housekeeping) (Wang et al., 2009); ubiquitin (UB) (housekeeping) (De Boever et al., 2008); liver X receptor (LXR), carbohydrate responsive element-binding protein (ChREBP), sterol regulatory element-binding protein 1 (SREBP1), (Proszkowiec-Weglarz et al., 2009); apical sodium dependent bile acid transporter (ASBT) (Mcquaid, 2012); fatty acid binding protein 2 (FABP2) and 6 (FABP6), interleukin 6 (IL-6), claudin 1 (Claudin1), transforming growth factor beta 4 (TGF-β4), toll like receptor 4 (TLR4) and 2β (TLR-2β) (Chen et al., 2015); chicken B-cell marker chB6 (Bu-1), marker of active avian T lymphocytes (CD3γδ) (Bar-Shira et al., 2003); interleukin 8 (former CXCLi2) (Rasoli et al., 2015); interferon-gamma (IFN-γ) (Rothwell et al., 2004). Primers of chicken interleukin 2 (IL-2) (Forward: 5′-CAAGATTCATCTCGAGCTCTACACA-3′, Reverse: 5′-CCCAGGTAACACTGCAGAGTTTG-3′) were designed from the GenBank sequence with accession number AF000631.1 using Primer Express v.2 software (Applied Biosystems, Foster City, CA, USA). Samples were analyzed in triplicate using the right amount of each primer, ultra-purified water, and SYBR Green Master Mix (Applied Biosystems, Foster City, CA, USA).
Statistical Analysis
ANOVA was performed to analyze dietary effects on performance, AID, and bile acids by using the mixed-model procedure of SAS (release 9.2; SAS Institute), with diet as the fixed effect and pen as the experimental unit. Data was analyzed by Shapiro–Wilk and Levene's test to determine normality and variance homogeneity. Tables show the average values of each treatment and the standard error of the mean (SEM). When the ANOVA was significant, differences among means were separated by Tukey's test at α = 0.05.
To de-multiplex, quality-filter, and analyze the microbial raw sequencing reads QIIME 1.9.1 was utilized (Caporaso et al., 2010). Then, for taxonomy analyses, reads were clustered into operational taxonomic units (OTUs) and taxonomic assignment of representative OTUs was performed using RDP classifier (Wang et al., 2007). Representative sequences were aligned by PyNast as default in quantitative insights into microbial ecology (QIIME) pipeline (Casparoso et al., 2010) and 0.005% of total OTUs were discarded. Shannon index was assessed to analyze alpha diversity and the non-parametric Kruskal–Wallis test was applied to evaluate the statistical significance (P < 0.05).
Differences in gene expression resulting from the comparison of chickens fed Monensin and OE treatments relative to the control group were determined using a mixed-model in which a gene-specific effect and a sample-specific effect were treated as random variables and treatment was considered fixed (Steibel et al., 2009). For genes displaying efficiencies different from 2 (E ≠ 2), cycle threshold (Ct) values were adjusted according to the model described by Steibel et al. (2009). The standard error was used to recalculate the lower and upper 95% confidence intervals for each fold change.
RESULTS
Daily Gain, Feed Intake, and Feed Conversion
Growth performance data are shown in Table 2. From 28 to 35 D of age, broilers fed the OE and Monensin diets showed better FCR than those fed the Control diet (P < 0.05). During the last period, from 35 to 42 D of age, no significant differences were observed in ADFI. However, animals fed with Control showed lower ADG (P < 0.01) than those fed Monensin and OE diets resulting in a worse FCR (P < 0.001) for Control diet. In the global period, from 21 to 42 D of age, a lower ADG (P < 0.05) was observed in birds fed Control compared to Monensin and OE groups. Furthermore, no significant differences in ADFI were reported among treatments and hence broilers in Control diet showed worse FCR than animals fed the Monensin and OE (P < 0.001).
Table 2.
Effect of experimental diets on broiler chickens growth performance from 21 to 42 D of age.1
| Item2 | Control | Monensin | OE | SEM3 | P- value |
|---|---|---|---|---|---|
| 21 to 28 D | |||||
| ADG (g/bird) | 103 | 103 | 100 | 2.69 | 0.77 |
| ADFI (g/bird) | 149 | 146 | 146 | 3.06 | 0.59 |
| FCR (g/g) | 1.46 | 1.42 | 1.46 | 0.014 | 0.12 |
| 28 to 35 D | |||||
| ADG (g/bird) | 105 | 107 | 108 | 2.46 | 0.60 |
| ADFI (g/bird) | 185 | 182 | 184 | 4.16 | 0.87 |
| FCR (g/g) | 1.77a | 1.71b | 1.71b | 0.018 | 0.039 |
| 35 to 42 D | |||||
| ADG (g/bird) | 77.8b | 103a | 97.2a | 4.18 | 0.002 |
| ADFI (g/bird) | 193 | 202 | 205 | 4.68 | 0.22 |
| FCR (g/g) | 2.50a | 1.98b | 2.12b | 0.083 | 0.001 |
| 21 to 42 D | |||||
| ADG (g/bird) | 95.1b | 104a | 102a | 2.03 | 0.018 |
| ADFI (g/bird) | 176 | 177 | 178 | 3.37 | 0.89 |
| FCR (g/g) | 1.91a | 1.70b | 1.76b | 0.029 | <0.001 |
Means within a column and main effect not sharing a common superscript are significantly different by Tukey's test (P ≤ 0.05).
Control, negative control with no additives; Monensin, basal diet supplemented with 100 ppm of monensin; OE, basal diet supplemented with 750 ppm of OE.
ADG, average daily gain; ADFI, average daily feed intake; FCR, feed conversion ratio.
SEM, standard error of means (n = 6 replicates with 14 birds each).
Apparent Ileal Digestibility
No significant differences on GE, OM, and EE AID were observed among treatments (Table 3). However, birds fed Monensin showed higher CP AID (P < 0.05) than those fed Control diet, and OE group showed intermediate values.
Table 3.
Effect of experimental diets on the apparent ileal digestibility (AID, %) of nutrients in broilers at 42 D of age.1
| Item | Control | Monensin | OE | SEM2 | P- value |
|---|---|---|---|---|---|
| Digestibility | |||||
| Dry matter | 65.4 | 68.6 | 66.2 | 0.99 | 0.095 |
| Gross energy | 69.5 | 72.5 | 70.2 | 1.04 | 0.13 |
| Ether extract | 83.0 | 84.2 | 82.1 | 1.54 | 0.62 |
| Organic matter | 68.5 | 71.7 | 69.2 | 1.06 | 0.12 |
| Crude protein | 72.1b | 77.8a | 73.8a,b | 1.41 | 0.035 |
Means within a column and main effect not sharing a common superscript are significantly different by Tukey's test (P ≤ 0.05).
Control, negative control with no additives; Monensin, basal diet supplemented with 100 ppm of monensin; OE, basal diet supplemented with 750 ppm of OE.
SEM, standard error of means (n = 6).
Bile Acids
No differences were observed in conjugated or unconjugated bile acid concentration in ileal digesta and plasma (Table 4). However, the sum of the total bile acids tended (P = 0.055) to be higher in birds fed the Monensin. The predominant bile acid in ileal digesta was CDCA while tauroursodeoxycholic acid (TCDCA) was the predominant in plasma.
Table 4.
Effect of experimental diets on bile acid composition in ileal contents and plasma of broilers at 42 D of age.1
| Item2 | Control | Monensin | OE | SEM3 | P- value4 |
|---|---|---|---|---|---|
| Ileal content (μmol/g of sample) | |||||
| TCA | 0.094 | 0.15 | 0.11 | 0.042 | 0.93 |
| TCDCA | 1.21 | 3.84 | 1.27 | 0.99 | 0.14 |
| AVCA | 0.17 | 0.31 | 0.23 | 0.055 | 0.19 |
| CA | 0.56 | 1.39 | 1.01 | 0.27 | 0.094 |
| CDCA | 4.61 | 9.63 | 8.07 | 2.14 | 0.12 |
| Σ Conjugated | 1.30 | 3.98 | 1.37 | 1.01 | 0.14 |
| Σ Unconjugated | 5.35 | 11.3 | 9.31 | 2.24 | 0.097 |
| Total BA | 6.65b | 15.3a | 10.7a,b | 2.89 | 0.055 |
| Plasma (nmol/mL of plasma) | |||||
| TCA | 0.41 | 0.72 | 0.77 | 0.19 | 0.37 |
| TCDCA | 14.2 | 13.6 | 14.8 | 2.59 | 0.97 |
| TLCA | 0.29 | 0.29 | 0.29 | 0.022 | 0.95 |
| CDCA | 0.31 | 0.36 | 0.64 | 0.21 | 0.24 |
| Σ Conjugated | 14.9 | 14.6 | 15.9 | 2.75 | 0.96 |
| Σ Unconjugated | 0.31 | 0.36 | 0.64 | 0.21 | 0.24 |
| Total BA | 15.2 | 14.9 | 16.5 | 2.9 | 0.72 |
Means within a column and main effect not sharing a common superscript are significantly different by Tukey's test (P ≤ 0.05).
Control, negative control with no additives; Monensin, basal diet supplemented with 100 ppm of monensin; OE, basal diet supplemented with 750 ppm of OE.
TCA, Taurocholic acid; TCDCA, Tauroursodeoxycholic acid; TLCA, Taurolithocholic acid; AVCA, Avicholic acid; CA, Cholic acid; CDCA, chenodeoxycholic acid.
SEM, standard error of means (n = 6).
P- values are from square root data transformation analysis.
Caecal Microbiota
Firmicutes was the predominant phylum in the caeca (Figure 1). No significant differences among diets were observed in relative abundance of the main bacterial families. Numerically, the most abundant families were Clostridiciaceae in Control (41.7%) and Monensin (51.1%) treatments, and Lactobacillaceae in OE (65.5%) treatment. Additionally, the diversity of caecal microbiota measured by the Shannon index was similar among treatments (Figure 2).
Figure 1.
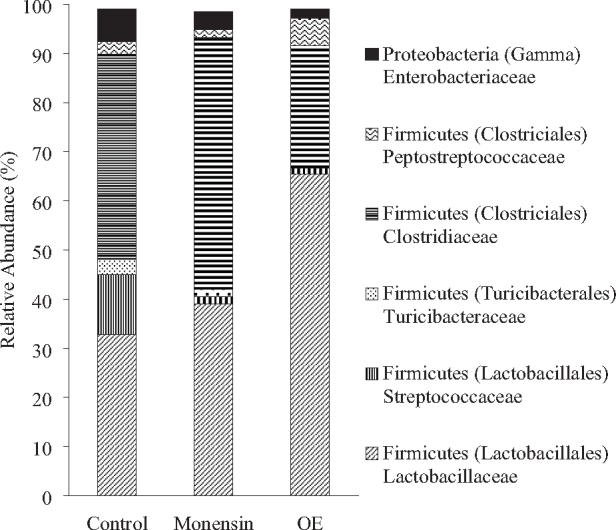
Effects of feeding broiler chicken diets supplemented with 100 ppm of monensin or 750 ppm of OE on the relative abundance of bacteria families in caecal content (n = 6). Control, negative control with no additives; Monensin, basal diet supplemented with 100 ppm of monensin; OE, basal diet supplemented with 750 ppm of OE.
Figure 2.
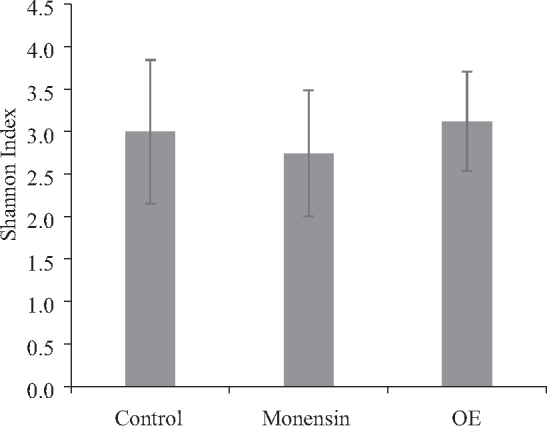
Effects of feeding broiler chicken diets supplemented with 100 ppm of monensin or 750 ppm of OE on the relative diversity of caecal microbiota (Shannon index) (n = 6). Control, negative control with no additives; Monensin, basal diet supplemented with 100 ppm of monensin; OE, basal diet supplemented with 750 ppm of OE.
Gene Expression
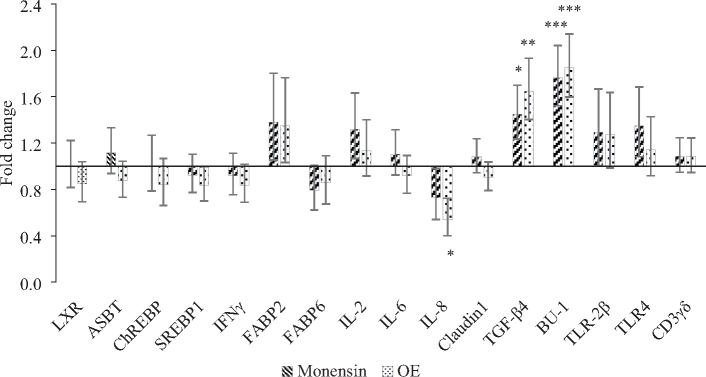
Results of gene expression in the ileum are shown in Figure 3. The expression of LXR, ASBT, REBP, SREBP1, IFNγ, FABP2, FABP6, IL-2, IL-6, Claudin1, TLR-2β, TLR4, and CD3γδ in the ileum was not affected by dietary treatments. However, the expression of IL-8 was significantly down-regulated in birds fed the OE compared to those fed Control diet (P < 0.05). In addition, the expression of TGF-β4 was significantly up-regulated in birds fed Monensin (P < 0.05) and OE treatments (P < 0.01) compared to C. The expression of Bu-1 showed a similar pattern with a significant up-regulation in Monensin (P < 0.001) and OE (P < 0.001).
Figure 3.
Effects of feeding broiler chicken diets supplemented with 100 ppm of monensin (Monensin) or 750 ppm of OE (OE) on the expression of selected genes in the ileum. Gene expression values are fold change relative to the mRNA levels in the control diet (C) set to be 1.0 (horizontal axis). Bars indicate the 95% confidence interval (Fold change up—Fold change low) (n = 12; *: P < 0.05; **: P < 0.01; ***: P < 0.001).
DISCUSSION
In the present study supplementing broiler diets with 750 ppm of OE during the grower-finisher period did not affect ADFI but improved ADG and FCR compared to a non-supplemented control. This is in agreement with Sarica and Ürmez (2016) who reported also higher final BW and better FCR in broilers fed olive leaf extracts. By contrast Leskovec et al. (2018) and King et al. (2014) were unable to detect significant differences in productive parameters after supplementing the feed and water with olive leaf and pomace extracts, respectively. However, the specified examples were performed with extracts from olive leaves (Leskovec et al., 2018) or olive pulp (King et al., 2014) rich in polyphenols, while in the present study extract contained a higher prevalence of triterpenes over the polyphenolic fraction. Contradictory results on broiler performance with other plant extracts rich in bioactive compounds have also been described (Leskovec et al., 2018) and it has been proven that not only the different botanical origin but also plant location, harvesting conditions, processing, and storage can affect the extract composition and activity (Huyghebaert et al., 2011). Therefore, the different concentration of the bioactive substances in the extracts (i.e., percentage of total triterpenes and polyphenols) and/or the route of administration (feed vs. water) might be behind the different results on performance among olive byproduct extract studies.
Plant extracts might improve broiler growth and feed efficiency by increasing nutrient digestibility as they can affect digestive enzyme function, intestinal morphology gastrointestinal tract motility, or bile acid secretion (Lee et al., 2003; Brenes and Roura, 2010; Bozkurt et al., 2016; Leskovec et al., 2018). In the present study no significant differences were observed on the AID of DM, EE, GE, and OM among dietary treatments. This is in agreement with the study of Leskovec et al. (2018) who reported no significant differences in nutrient apparent total tract digestibility coefficients among broilers fed the olive leaf extract and the no supplemented control. However, in the present study birds fed the Monenisn supplemented diet showed a better protein AID than the control but similar to those fed the OE. Recent studies support that the positive effects of Monensin on broiler growth and FCR might partially be explained by a rise in intestinal digestive enzyme activities including chymotrypsin (Bozkurt et al., 2016). Other studies have also reported improvement on broiler FCR with Monensin due to an increase of conjugated bile acids (TCA and TCDCA) and hence better dietary fat digestibility (Guban et al., 2006). Moreover, studies performed in rats have shown the ability of plant extracts rich in polyphenols to affect lipid metabolism and bile acid composition (Fotschki et al., 2017). In the present study no significant differences were observed on intestinal or plasma bile acid composition among treatments, in agreement with a lack of effect on dietary fat digestibility. Moreover, the ileal expression of genes encoding proteins involved in bile acid and lipid metabolism such as LXR, ASBT, ChREBP, SREBP1, FABP2, and FABP6 was not affected by dietary treatments. However, in the present study total bile acid concentration tended to be higher in birds fed the Monensin supplemented diet. Besides their role as physiological detergents that facilitate the absorption of lipids bile acids act as “hormone-like” molecules involved in several signaling pathways including the regulation of lipid, glucose, and energy metabolism (Li and Chiang, 2015). The composition of the bile acids pool is a balance between the primary (unconjugated) bile acids synthesized in the liver and their modifications to secondary (conjugated) bile acids in the intestine generated by the microbiome (Li and Chiang, 2015). After the transport of bile acids back to the liver via portal blood the synthesis of new primary bile acids takes place according to the physiological needs. This link between gut microbiota and hepatic bile acid synthesis might be useful to detect functional changes in the microbiome or potential changes in microbial populations impacting the animal energy metabolism. Results from this study suggest a better performance in birds fed the Monensin supplemented diets because of a better protein digestibility and higher ileal total bile acid concentrations. By contrast, our results show that the positive effects of OE on performance seem not to be due to improvement in nutrient digestibility or bile acid pool modifications.
Gastrointestinal microbiome plays an important role for gut health and nutrition in poultry production (Xiao et al., 2016). Phytogenics, probiotics, prebiotics, or exogenous enzymes are commonly used to modulate gut microbiome (Dibner and Richards, 2005; Oakley et al., 2014). In this context, bioactive compounds of olive pomace such as oleuropein and hydroxytyrosol are good candidates to modulate the composition of gut microbiota and enhance gut integrity (Sarica and Ürmez, 2016). It is known that triterpenes offered through the diet are only partially absorbed in the upper digestive tract (Yin et al., 2012) and are also present along the GIT tract (Lozano-Mena et al., 2016). Therefore, coating technologies are not required in this kind of extracts to reach the gastrointestinal tract and perform its activity. In the present study Firmicutes was the most abundant phylum in the broiler caeca, this is in agreement with other studies in broilers (Danzeisen et al., 2011). No significant differences were observed on caecal microbiota diversity or relative abundance of the main bacterial families among experimental treatments. This is in agreement to results obtained by Liehr et al. (2017) who observed no differences in composition and diversity of gut microbiome, at the phylum level, in pigs fed diets supplemented with an olive oil bioactive extract similar than the one used in this study. However, our results partially agree with Sarica and Ürmez (2016) who reported no changes in total aerobic bacteria but significant differences on E. coli and Lactobacilli counts in ileal samples of birds fed olive leaf extracts or a control diet with no additives. Again, different extracts origin (leaf vs. pomace) and composition (oleuropein vs. triterpenes) might potentially explain these discrepancies. On the other hand, monensin inclusion in chicken diets has been described to decrease Lactobacillus species (Guban et al., 2006; Danzeisen et al., 2011). No evidence were found that could justify these contradictory results; however, the limited number of replicates in the present study, or factors such as diet, broiler breed, the environment of the experimental farm, or technical methodologies might be behind discrepant results among microbiome studies in poultry (Danzeisen et al., 2011). Further analysis to confirm the lack of effect of the OE on bacteria at the genus or species level is needed.
Plant extracts may exert their beneficial effects on growth performance among others because of their antioxidant and/or immunomodulatory effects (Lillehoj et al., 2018). Previous studies conducted with extracts of similar composition to the one used in this study showed better animal performance related to its anti-inflammatory function rather than the antioxidant one (Gisbert et al., 2017; Liehr et al., 2017; Tedó et al., 2018). This made us explore only the potential reduction of the inflammatory status of the animals. Some plant extracts have shown immunomodulatory effects in studies in which poultry and pigs were experimentally infected (Zeng et al., 2015; Sugiharto, 2016). These effects include increase in lymphocyte proliferation, serum level antibodies, decrease of pro-inflammatory, and increase of anti-inflammatory cytokines (Zeng et al., 2015; Sugiharto, 2016). In a recent study, Liehr et al. (2017) reported a beneficial effect of an OE extract in pigs challenged with LPS on immune response by reducing the pro-inflammatory IL-1β in plasma leading also to a better animal performance. Despite inflammation is normally associated with pathogen outbreaks there might be also a moderate intestinal inflammation in response to the high energy diets used under normal productive circumstances (Niewold, 2014). Moreover, as suggested by Niewold (2014) plant extracts, like antibiotics, might increase broiler performance because of their anti-inflammatory effects at intestinal level. In the present study, the expression of intestinal IL-2, IL-6, and IFNγ was not affected by dietary means. However, birds fed the OE showed a significant decrease of IL-8 expression in the ileum. This chemokine plays an important role in the chicken inflammatory response recruiting heterophil at a local and systemic level in response to bacterial presence (Kogut, 2002). Moreover, a significant upregulation of the anti-inflammatory cytokine TGF-β4 and the B cell marker Bu-1 was observed in the ileum of birds fed the EO and Monensin diets compared to those fed the Control diet. Therefore, it is plausive that the better performance observed in birds fed the Monensin and EO diets in our study might be related to a lower intestinal inflammatory response explained by the immunosuppressant effect on IL-8 and the increase anti-inflammatory expression of TGF-β4 under non-acute inflammatory conditions.
In conclusion, the present study shows that the inclusion of 750 ppm of an olive pomace extract containing a minimum of 10% total triterpenes and 2% polyphenols positively affects growth in broiler chickens. Among the possible mechanism of action studied so far in this work improvement in performance is likely related to the extract anti-inflammatory properties. However, other possible mechanisms such as antioxidant capacity remain to be explored.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the support of Lucta S. A., Comunidad de Madrid (Spain) and European Structural and Investment Funds for Javier Herrero (Project MEDGAN-CM S2013/ABI2913).
REFERENCES
- Andreano S.D., Bonastre A.S., Francino O., Martí A.C., Lecchi C., Grilli G., Giovanardi D., Ceciliani F. Gastrointestinal microbial population of turkey (Meleagris gallopavo) affected by hemorrhagic enteritis virus. Poult. Sci. 2017;96:3550–3558. doi: 10.3382/ps/pex139. [DOI] [PubMed] [Google Scholar]
- AOAC International . 17th ed. AOAC Int.; Gaithersburg, MD: 2000. Official Methods of Analysis of AOAC International. [Google Scholar]
- Bar-Shira E., Sklan D., Friedman A. Establishment of immune competence in the avian GALT during the immediate post-hatch period. Dev. Comp. Immunol. 2003;27:147–157. doi: 10.1016/s0145-305x(02)00076-9. [DOI] [PubMed] [Google Scholar]
- Boletín Oficial del Estado Real Decreto por el que se establecen las normas básicas aplicables para la protección de los animales utilizados en experimentación y otros fines científicos, incluyendo la docencia. Boletín Oficial del Estado. 2013;34:11370–11421. [Google Scholar]
- Bozkurt M., Ege G., Aysul N., Akşit H., Tüzün A.E., Küçükyılmaz K., Borum A.E., Uygun M., Akşit D., Aypak S., Şimşek E., Seyrek K., Koçer B., Bintaş E., Orojpour A. Effect of anticoccidial monensin with oregano essential oil on broilers experimentally challenged with mixed Eimeria spp. Poult. Sci. 2016;95:1858–1868. doi: 10.3382/ps/pew077. [DOI] [PubMed] [Google Scholar]
- Brenes A., Roura E. Essential oils in poultry nutrition: main effects and modes of action. Anim. Feed. Sci. Technol. 2010;158:1–14. [Google Scholar]
- Caporaso J.G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F.D., Costello E.K., Fierer N., Peña A.G., Goodrich J.K., Gordon J.I., Huttley G.A., Kelley S.T., Knights D., Koenig J.E., Ley R.E., Lozupone C.A., McDonald D., Muegge B.D., Pirrung M., Reeder J., Sevinsky J.R., Turnbaugh P.J., Walters W.A., Widmann J., Yatsunenko T., Zaneveld J., Knight R. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Tellez G., Richards J.D., Escobar J. Identification of potential biomarkers for gut barrier failure in broiler chickens. Front. Vet. Sci. 2015;2:14. doi: 10.3389/fvets.2015.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danzeisen J.L., Kim H.B., Isaacson R.E., Tu Z.J., Johnson T.J. Modulations of the chicken cecal microbiome and metagenome in response to anticoccidial and growth promoter treatment. PLoS One. 2011;6 doi: 10.1371/journal.pone.0027949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Boever S., Vangestel C., De Backer P., Croubels S., Sys S.U. Identification and validation of housekeeping genes as internal control for gene expression in an intravenous LPS inflammation model in chickens. Vet. Immunol. Immunopathol. 2008;122:312–317. doi: 10.1016/j.vetimm.2007.12.002. [DOI] [PubMed] [Google Scholar]
- De Coca-Sinova A., Mateos G.G., González-Alvarado J.M., Centeno C., Lázaro R., Jiménez-Moreno E. Comparative study of two analytical procedures for the determination of acid insoluble ash for evaluation of nutrient retention in broiler diets. Span. J. Agric. Res. 2011;9:761–768. [Google Scholar]
- Dibner J.J., Richards J.D. Antibiotic growth promoters in agriculture: history and mode of action. Poult. Sci. 2005;84:634–643. doi: 10.1093/ps/84.4.634. [DOI] [PubMed] [Google Scholar]
- El Hachemi A., El Mecherfi K.E., Benzineb K., Saidi D., Kheroua O. Supplementation of olive mill wastes in broiler chicken feeding. Afr. J. Biotechnol. 2007;6:1848–1853. [Google Scholar]
- FEDNA (Fundación Española Desarrollo Nutrición Animal) In: Normas FEDNA para la Formulación de Piensos Compuestos. 3rd ed. De Blas C., Mateos G.G., Rebollar P.G., editors. Fund. Esp. Desarro. Nutri. Anim.; Madrid, Spain: 2010. [Google Scholar]
- Fotschki B., Juśkiewicz J., Jurgoński A., Rigby N., Sójka M., Kołodziejczyk K., Mackie A., Zduńczyk Z. Raspberry pomace alters cecal microbial activity and reduces secondary bile acids in rats fed a high-fat diet. J. Nutr. Biochem. 2017;46:13–20. doi: 10.1016/j.jnutbio.2017.03.004. [DOI] [PubMed] [Google Scholar]
- Gisbert E., Andree K.B., Quintela J.C., Calduch-Giner J.A., Ipharraguerre I.R., Pérez-Sánchez J. Olive oil bioactive compounds increase body weight, and improve gut health and integrity in gilthead sea bream (Sparus aurata) Br. J. Nutr. 2017;117:351–363. doi: 10.1017/S0007114517000228. [DOI] [PubMed] [Google Scholar]
- Guban J., Korver D.R., Allison G.E., Tannock G.W. Relationship of dietary antimicrobial drug administration with broiler performance, decreased population levels of Lactobacillus salivarius, and reduced bile salt deconjugation in the ileum of broiler chickens. Poult. Sci. 2006;85:2186–2194. doi: 10.1093/ps/85.12.2186. [DOI] [PubMed] [Google Scholar]
- Huyghebaert G., Ducatelle R., Van Immerseel F. An update on alternatives to antimicrobial growth promoters for broilers. Vet. J. 2011;187:182–188. doi: 10.1016/j.tvjl.2010.03.003. [DOI] [PubMed] [Google Scholar]
- International, Olive Council 2017. http://www.internationaloliveoil.org
- King A.J., Griffin J.K., Roslan F. In vivo and in vitro addition of dried olive extract in poultry. J. Agric. Food Chem. 2014;62:7915–7919. doi: 10.1021/jf4050588. [DOI] [PubMed] [Google Scholar]
- Kogut M.H. Dynamics of protective avian inflammatory response: the role of an IL-8 like cytokine in the recruitment of heterophils to the site of organ invasion by Salmonella enteritidis. Comp. Immunol. Microbiol. Infect. Dis. 2002;25:159–172. doi: 10.1016/s0147-9571(01)00035-2. [DOI] [PubMed] [Google Scholar]
- Lee K.W., Everts H., Kappert H.J., Frehner M., Losa R., Beynen A.C. Effects of dietary essential oil components on growth performance, digestive enzymes and lipid metabolism in female broiler chickens. Br. Poult. Sci. 2003;44:450–457. doi: 10.1080/0007166031000085508. [DOI] [PubMed] [Google Scholar]
- Leskovec J., Levart A., Žgur S., Jordan D., Pirman T., Salobir J., Rezar V. Effects of olive leaf and marigold extracts on the utilization of nutrients and on bone mineralization using two different oil sources in broilers. J. Poult. Sci. 2018;55:17–27. doi: 10.2141/jpsa.0170059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T., Chiang J.Y.L. Bile acids as metabolic regulators. Curr. Opin. Gastroenterol. 2015;31:159–165. doi: 10.1097/MOG.0000000000000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liehr M., Mereu A., Pastor J.J., Quintela J.C., Staats S., Rimbach G., Ipharraguerre I.R. Olive oil bioactives protect pigs against experimentally-induced chronic inflammation independently of alterations in gut microbiota. PLoS One. 2017;12 doi: 10.1371/journal.pone.0174239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillehoj H.S., Lee K.W. Immune modulation of innate immunity as alternatives-to-antibiotics strategies to mitigate the use of drugs in poultry production. Poult. Sci. 2012;91:1286–1291. doi: 10.3382/ps.2012-02374. [DOI] [PubMed] [Google Scholar]
- Lillehoj H., Liu Y., Calsamiglia S., Fernandez-Miyakawa M.E., Chi F., Cravens R.L., Oh S., Gay C.G. Phytochemicals as antibiotic alternatives to promote growth and enhance host health. Vet. Res. 2018;49:76. doi: 10.1186/s13567-018-0562-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano-Mena G., Sánchez-Gonzalez M., Parra A., Juan M.E., Planas J.M. Identification of gut-derived metabolites of maslinic acid, a bioactive compound from Olea europaea L. Mol. Nutr. Food Res. 2016;60:2053–2064. doi: 10.1002/mnfr.201501060. [DOI] [PubMed] [Google Scholar]
- Mandalawi H.A., Kimiaeitalab M.V., Obregon V., Menoyo D., Mateos G.G. Influence of source and level of glycerin in the diet on growth performance, liver characteristics, and nutrient digestibility in broilers from hatching to 21 days of age. Poult. Sci. 2014;93:2855–2863. doi: 10.3382/ps.2014-04156. [DOI] [PubMed] [Google Scholar]
- Mcquaid R. McGrill Univ.; Montreal, Canada: 2012. Characterization of the ileal lipid binding protein (FABP6) in tissues involved in bile acid and steroid metabolism in poultry. PhD Diss. [Google Scholar]
- Mirabella N., Castellani V., Sala S. Current options for the valorization of food manufacturing waste: a review. J. Clean. Prod. 2014;65:28–41. [Google Scholar]
- Morrison S.Y., Pastor J.J., Quintela J.C., Holst J.J., Hartmann B., Drackley J.K., Ipharraguerre I.R. Short communication: promotion of GLP-2 secretion in dairy calves with a bioactive extract from Olea europea. J. Dairy Sci. 2017;100:1940–1945. doi: 10.3168/jds.2016-11810. [DOI] [PubMed] [Google Scholar]
- Niewold T.A. The non-antibiotic anti-inflammatory effect of antimicrobial growth promoters, the real mode of action? A hypothesis. Poult. Sci. 2007;86:605–609. doi: 10.1093/ps/86.4.605. [DOI] [PubMed] [Google Scholar]
- Niewold T.A. Why anti-inflammatory compounds are the solution for the problem with in feed antibiotics. Qual. Assur. Saf. Crop. Foods. 2014;6:119–122. [Google Scholar]
- Oakley B.B., Lillehoj H.S., Kogut M.H., Kim W.K., Maurer J.J., Pedroso A., Lee M.D., Collett S.R., Johnson T.J., Cox N.A. The chicken gastrointestinal microbiome. FEMS Microbiol. Lett. 2014;360:100–112. doi: 10.1111/1574-6968.12608. [DOI] [PubMed] [Google Scholar]
- Platel K., Srinivasan K. Stimulatory influence of select spices on bile secretion in rats. Nutr. Res. 2000;20:1493–1503. [Google Scholar]
- Proszkowiec-Weglarz M., Richards M.P., Humphrey B.D., Rosebrough R.W., McMurtry J.P. AMP-activated protein kinase and carbohydrate response element binding protein: a study of two potential regulatory factors in the hepatic lipogenic program of broiler chickens. Comp. Biochem. Physiol. B, Biochem. Mol. Biol. 2009;154:68–79. doi: 10.1016/j.cbpb.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Rasoli M., Yeap S.K., Tan S.W., Roohani K., Kristeen-Teo Y.W., Alitheen N.B., Rahaman Y.A., Aini I., Bejo M.H., Kaiser P., Omar A.R. Differential modulation of immune response and cytokine profiles in the bursae and spleen of chickens infected with very virulent infectious bursal disease virus. BMC Vet. Res. 2015;11:75. doi: 10.1186/s12917-015-0377-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell L., Young J.R., Zoorob R., Whittaker C.A., Hesketh P., Archer A., Smith A.L., Kaiser P. Cloning and characterization of chicken IL-10 and its role in the immune response to Eimeria maxima. J. Immunol. 2004;173:2675–2682. doi: 10.4049/jimmunol.173.4.2675. [DOI] [PubMed] [Google Scholar]
- Sarica S., Ürkmez D. The use of grape seed-, olive leaf- and pomegranate peel-extracts as alternative natural antimicrobial feed additives in broiler diets. Eurp. Poult. Sci. 2016;80:1–13. [Google Scholar]
- Sayehban P., Seidavi A., Dadashbeiki M., Ghorbani A., Araújo W.A.G., Albino L.F.T. Effects of different levels of two types of olive pulp with or without exogenous enzyme supplementation on broiler performance and economic parameters. Rev. Bras. Cienc. Avic. 2016;18:489–500. [Google Scholar]
- Steibel J.P., Poletto R., Coussens P.M., Rosa G.J. A powerful and flexible linear mixed model framework for the analysis of relative quantification RT-PCR data. Genomics. 2009;94:146–152. doi: 10.1016/j.ygeno.2009.04.008. [DOI] [PubMed] [Google Scholar]
- Sugiharto S. Role of nutraceuticals in gut health and growth performance of poultry. J. Saudi Soc. Agric. Sci. 2016;15:99–111. [Google Scholar]
- Tedó G., Martínez A., Muller C., Reina M., Blanch M., Pastor J.J. Targeting gut inflammation in pigs with the use of olive fruits bioactives positively influences pig performance. Proc. 14th Internat. Symp. Digest. Physiol. Pigs. 2018;9:262. (Abstr.). [Google Scholar]
- Wang Q., Garrity G.M., Tiedje J.M., Cole J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007;73:5261–5267. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P.H., Ko Y.H., Chin H.J., Hsu C., Ding S.T., Chen C.Y. The effect of feed restriction on expression of hepatic lipogenic genes in broiler chickens and the function of SREBP1. Comp. Biochem. Physiol. B. Biochem. Mol. Biol. 2009;153:327–331. doi: 10.1016/j.cbpb.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Xiao Y., Xiang Y., Zhou W., Chen J., Li K., Yang H. Microbial community mapping in intestinal tract of broiler chicken. Poult. Sci. 2016;96:1387–1393. doi: 10.3382/ps/pew372. [DOI] [PubMed] [Google Scholar]
- Yin M.C., Lin M.C., Mong M.C., Lin C.Y. Bioavailability, distribution, and antioxidative effects of selected triterpenes in mice. J. Agric. Food Chem. 2012;60:7697–77101. doi: 10.1021/jf302529x. [DOI] [PubMed] [Google Scholar]
- Zeng Z., Zhang S., Wang H., Piao X. Essential oil and aromatic plants as feed additives in non-ruminant nutrition. J. Anim. Sci. Biotechnol. 2015;6:1–10. doi: 10.1186/s40104-015-0004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]