Abstract
The objective of this study was to investigate the effects of dietary Bacillus subtilis supplementation on growth performance, jejunal lesion scores, oocyst shedding, and cytokine and tight junction protein expression in broiler chickens infected with Eimeria maxima. A total of 196 male day-old Ross 708 broilers were given a nonexperimental diet until 14 D of age. Then, all chickens were randomly assigned to one of seven dietary treatments: 2 basal diets (CON and NC); CON + virginiamycin (AB1); CON + bacitracin methylene disalicylate (BMD; AB2); CON + B. subtilis 1781 (PB1); CON + B. subtilis 747 (PB2); or CON + B. subtilis 1781 + 747 (PB3). At day 21, all chickens except those in the CON group were orally inoculated with E. maxima oocysts. At 7 D after E. maxima infection, the body weight gains of chickens fed PB2 and PB3 increased (P = 0.032) as much as those in chickens fed AB2. The body weight gain and feed efficiency of chickens fed PB2 were significantly increased (P < 0.001), and PB2 chickens showed (P = 0.005) the lowest lesion scores after E. maxima infection. Chickens fed PB2 showed (P < 0.05) lower mRNA expression of IL-1β in infected chicken groups. Chickens in the AB1, AB2, PB1, PB2, and PB3 groups showed (P < 0.05) greater mRNA expression of junctional adhesion molecule 2 in jejunal tissue, whereas occludin expression increased (P < 0.05) in the jejunal tissue of chickens fed AB2 or PB2. Dietary B. subtilis supplementation significantly improved the growth performance of young chickens to a level comparable with that induced by virginiamycin or BMD without E. maxima infection. After infection with E. maxima, dietary virginiamycin and BMD significantly enhanced the epithelial barrier integrity, and the dietary B. subtilis 747 showed significantly enhanced growth performance, intestinal immunity, and epithelial barrier integrity. Together our results indicated that certain strains of B. subtilis provide beneficial effects on the growth of young broiler chickens and have the potential to replace antibiotic growth promoters.
Key words: Bacillus subtilis, chicken, Eimeria maxima, intestinal immunity, gut health
Introduction
The United Nations estimates that there will be more than 9 billion people on the planet by the year 2050 (Roberts, 2011); thus, the world population will be 32% higher than that in 2006. In addition, the meat consumption per person per year is predicted to increase by 26% in the same period and will primarily comprise chicken consumption (FAO, 2010, OECD-FAO, 2010). Because of increasing concerns regarding antimicrobial resistance (Gadde et al., 2017b), growing consumer preference for antibiotic-free meat products will influence future directions in poultry and livestock production (Godfray et al., 2010, Shepon et al., 2018, Sander et al., 2019). As of 2017, about 40% of boiler feed in the U.S. was already antibiotic free under “No Antibiotics Ever” programs (Rennier, 2017).
Consumer awareness of antimicrobial resistance and food safety and increasing understanding of the interaction of nutrients, intestinal microbiota, and the immune system in maintaining good gut health have resulted in the limited use of antibiotic growth promoters (AGP) and anticoccidials in animal agriculture and in a paradigm shift in the use of feed additives by commercial companies (Lee et al., 2011a, Lee et al., 2011b, Yadav and Jha, 2019). Therefore, there will be an increasing need to understand how intestinal microbiota and the immune system can be modulated by dietary nutraceuticals and natural feed additives as alternatives to antibiotics in controlling enteric diseases (Ganguly, 2013, Chan et al., 2015, Gadde et al., 2017b). Notably, Eimeria spp. are the etiologic agents of avian coccidiosis, an intestinal disease responsible for an estimated annual economic loss of more than $3 billion worldwide (Lillehoj and Trout, 1996, Shirley and Lillehoj, 2012). Increasing implementation of antibiotic-free poultry production system in the U.S. is making the control of some enteric pathogens such as coccidiosis-causing Eimeria species and NE-inducing Clostridium perfringens (C. perfringens) strains challenging. Because coccidiosis is a primary risk factor for NE, it will be more desirable if alternatives to antibiotics can reduce Eimeria as well as C. perfringens. (Gallucci and Matzinger, 2001, Peek and Landman, 2011). There is currently a wide range of feed additives available through the feed industry, including acidifiers, prebiotics, probiotics, phytochemicals, enzymes, osmoregulators, nucleotides, and zinc oxide (Gadde et al., 2017b, Lin et al., 2017).
Many strains of Bacillus subtilis have been selected as probiotics on the basis of their in vitro inhibitory effects on chicken pathogenic bacteria (Fritts et al., 2000, Li et al., 2016, Nhung et al., 2017, Grant et al., 2018). Dietary supplementation with B. subtilis has been shown not only to improve growth performance and to beneficially alter the gastrointestinal microflora to decrease colonization by chicken pathogenic E. coli and C. perfringens but also to have a protective role against chicken coccidiosis (Knap et al., 2010, Lee et al., 2015). Therefore, this study was conducted to investigate the effects of dietary B. subtilis supplementation on posthatch growth, intestinal immunity, and epithelial barrier integrity in broiler chickens infected with Eimeria maxima during their early growth phase. To evaluate host immune function during coccidiosis, we also investigated growth performance, lesion scores, oocyst shedding, jejunal cytokines, and tight junction (TJ) proteins in broiler chickens infected with E. maxima.
Materials and methods
All experiments were approved by the Beltsville Agricultural Research Center Institutional Animal Care and Use Committee.
Chickens and Experimental Design
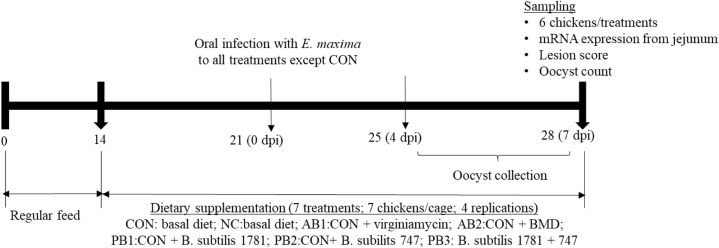
A total of 196 male day-old Ross 708 broilers were obtained from a local hatchery (Longenecker's Hatchery, Elizabethtown, PA) and were randomly housed in Petersime starter brooder cage units (Zulte, Belgium) and provided with normal feed (not the experimental diet) until they were 14 D old. All chickens were weighed and allocated to 7 dietary treatments in a randomized complete block design at 14 D of age. The dietary treatments included a basal diet based on corn and soybean meal (CON), a second basal diet similar to CON (NC), CON + virginiamycin (Phibro Animal Health, Teaneck, NJ) at 20 g/ton (22 ppm) (AB1), CON + bacitracin methylene disalicylate (BMD; Zoetis, Durham, NC) at 50 g/ton (55 ppm) (AB2), CON + B. subtilis 1781 (PB1), CON + B. subtilis 747 (PB2), and CON + B. subtilis 1781 + 747 (PB3). B. subtilis strains were obtained from Church & Dwight Co., Inc. (Waukesha, WI). The dose of B. subtilis in the treatment was a total of 1.5 × 105 CFU/g feed. For PB3 (2-strain combination), each strain composed 50% of the total CFU count (each strain at 7.5 × 104 CFU/g feed). At the beginning of the study, each treatment contained 4 cages with 7 chickens (Figure 1). Each cage was 0.65 m in width and 0.75 m in length (14 chickens/m2). All cages were kept in the same room. Each cage was considered an experimental unit. The chickens were given ad libitum access to water and feed throughout the study. Figure 1 shows the experimental schedules.
Figure 1.
Schematic outline of the experimental design. Dpi: days postinfection. Abberviations: CON: basal diet; NC: basal diet; AB1; diet supplemented with virginiamycin at 20 g/ton (22 ppm); AB2: diet supplemented with BMD at 50 g/ton (55 ppm); PB1: diet supplemented with B. subtilis 1781; PB2: diet supplemented with B. subtilis 747; PB3: diet supplemented with B. subtilis 1781 + 747.
Body Weight and Feed Intake Measurement
Feed additions were weighed and recorded. The feeders were shaken once per day. The chickens and feed were weighed at 21 and 28 D of age for computation of growth performance. Dead chickens were removed and weighed daily to calculate mortality and adjust the growth performance data.
Oral Infection With E. maxima
All chickens except those in the CON group were infected by oral gavage at 21 D of age with 1.0 × 104 oocysts of E. maxima Beltsville strain 41 A/chicken, as previously described (Lillehoj et al., 2016; Oh et al., 2018).
Collection of Intestinal Samples
Six chickens were randomly selected from each treatment and used for collection of intestinal samples at day 28. The chickens were euthanized by cervical dislocation, and the intestines were removed immediately. From each chicken, a small section of the jejunum without contents was collected aseptically and stored in RNAlater (Applied Biosystems, Foster City, CA) at −20°C for further use.
Coccidia Lesion Score
Lesion scores from the jejunum in chickens euthanized for sample collection at day 28 were determined on a scale from 0 (none) to 4 (high) by 4 independent observers in a blinded fashion, as previously described (Johnson and Reid, 1970).
Fecal Oocyst Shedding
Fecal oocysts were collected daily between days 25 and 28 (4 and 7 D postinfection [dpi]). Oocyst numbers were determined as previously described (Lee et al., 2011a, Lee et al., 2011b), using a McMaster chamber as per the formula: total oocysts/chicken = [oocyst count × dilution factor × (fecal sample volume/counting chamber volume)]/number of chickens per cage.
Isolation of RNA and Reverse Transcription
Total RNA was isolated from the jejunum samples stored in RNAlater by using TRIzol (Invitrogen, Carlsbad, CA) in accordance with the manufacturer's recommendations. Approximately 50 mg of jejunal tissue was homogenized in 1 mL of TRIzol using a handheld homogenizer (TissueRuptor; Qiagen Inc., Valencia, CA). Chloroform was added to the homogenized sample. The sample was centrifuged at 12,000 × g for 15 min at 4°C to allow phase separation. RNA present in the colorless upper aqueous phase was then precipitated with 100% isopropanol (Sigma-Aldrich Corp., St. Louis, MO). The RNA pellet was then washed with 75% ethanol (Sigma-Aldrich Corp.), air-dried, and resuspended in RNase-free water. The quantity of RNA was assessed using a NanoDrop (ND-1000) spectrophotometer (NanoDrop products, Wilmington, DE) according to the absorbance at 260 nm. RNA purity was evaluated as per the OD260/OD280 ratio. The eluted RNA was stored at −80°C until further use. Total RNA (1 μg) was then reverse transcribed to cDNA using a QuantiTect reverse transcription kit (Qiagen Inc., Valencia, CA). Briefly, the RNA sample was incubated with genomic DNA wipeout buffer at 42°C for 2 min to remove any genomic DNA contamination. Reverse transcription (RT) of the genomic DNA–depleted sample was then carried out by the addition of Quantiscript Reverse Transcriptase, Quantiscript RT buffer, and RT primer mix (Qiagen Inc.). The reaction was carried out in a thermal cycler (Mastercycler EP Gradient S; Eppendorf, Hauppauge, NY); the cycling conditions were 42°C for 30 min, followed by inactivation of reverse transcriptase at 95°C for 3 min. The cDNA samples were divided into aliquots and stored at −20°C.
Gene Expression Analysis by quantitative Real-Time PCR
The oligonucleotide primer sequences used for quantitative real-time PCR (qRT-PCR) are listed in Table 1. The various cytokines and intestinal TJ proteins whose differential expression was evaluated in the jejunum included IL-1β, IL-2, and IL-6; interferon (IFN)-γ; junctional adhesion molecule (JAM) 2; and occludin. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the reference gene. Amplification and detection were carried out using a Stratagene M x 3000P qPCR system (Agilent Technologies Inc., Santa Clara, CA) and RT2 SYBR Green qPCR master mix (Qiagen). Each sample was analyzed in triplicate, and nonspecific primer amplification was assessed through the inclusion of no-template controls. Standard curves were generated with log10 diluted RNA, and the levels of individual transcripts were normalized to those of GAPDH in the Q-gene program (Muller et al., 2002).
Table 1.
Quantitative real-time PCR oligonucleotide primer sequences.
| Type | Target gene | Primer sequence (5′-3′) | PCR product size (Kb) |
|---|---|---|---|
| Reference | GAPDH | F-GGTGGTGCTAAGCGTGTTAT | 264 |
| R-ACCTCTGCCATCTCTCCACA | |||
| Proinflammatory | IL-1β | F-TGGGCATCAAGGGCTACA | 244 |
| R-TCGGGTTGGTTGGTGATG | |||
| IL-6 | F-CAAGGTGACGGAGGAGGAC | 254 | |
| R-TGGCGAGGAGGGATTTCT | |||
| Th1 | IL-2 | F-TCTGGGACCACTGTATGCTCT | 256 |
| R-ACACCAGTGGGAAACAGTATCA | |||
| IFN-γ | F-AGCTGACGGTGGACCTATTATT | 259 | |
| R-GGCTTTGCGCTGGATTC | |||
| Tight junction proteins | JAM2 | F-AGCCTCAAATGGGATTGGATT | 59 |
| R-CATCAACTTGCATTCGCTTCA | |||
| Occludin | F-GAGCCCAGACTACCAAAGCAA | 68 | |
| R-GCTTGATGTGGAAGAGCTTGTTG |
Abberviations: F, forward primer; R, reverse primer.
Statistical Analysis
Data for each response were analyzed using Mixed Model (PROC MIXED) in SAS (SAS Inst. Inc., Cary NC). The design was a randomized complete block design. Each cage was considered an experimental unit. Each cage unit was the block factor. The results are given as least squares means and pooled SEM. Probability values less than 0.05 were considered significantly different. In cases in which the overall effect was significant in growth performance, means were compared in a pairwise manner (PDIFF option). For other results, the PDIFF option was used to compare significance between groups.
Results
Growth Performance
The initial body weight of chickens measured before treatment did not show significant differences (P = 0.247) among the treatment groups (Table 2). At day 21, the body weights of chickens on dietary AB1 (875 g), AB2 (888 g), PB2 (873 g), and PB3 (885 g) (P = 0.063) tended to be higher than those of chickens (827 g) fed the basal diet. As expected, infection with E. maxima decreased (P = 0.038) the body weights of chickens (average 1,050 g) at day 28 (7 dpi) regardless of dietary treatment, relative to the weights of uninfected chickens (1,175 g). From day 15 to 21 (before infection), the body weight gains in chickens fed diets supplemented with AB1 (402 g), AB2 (395 g), or PB2 (391 g) increased (P = 0.032) beyond that of CON-fed chickens (345 g) (Table 2). After infection, from day 22 to 28, chickens infected with E. maxima showed (P < 0.001) lower body weight gains (average 187 g) than uninfected chickens (346 g). However, chickens fed PB2 showed (P < 0.05) greater body weight gains (205 g) than infected chickens (169 g) fed NC (Table 2). Overall, E. maxima infection decreased (P < 0.05) the body weight gains of chickens (average 546 g) regardless of dietary treatment, as compared with those (692 g) in uninfected chickens fed CON. Chickens fed AB2 (587 g) and PB2 (580 g) showed (P < 0.05) greater body weight gain than infected chickens (493 g) fed NC (Table 2). The feed intake during the experimental period did not differ among different treatments (Table 2). Before infection, from day 14 to 21, the feed efficiency of broiler chickens was not affected (P > 0.05) by the treatments. From day 22 to 28 (after infection), the feed efficiency of infected chickens (average 0.255), regardless of dietary supplements, decreased below that of uninfected chickens (0.494) fed CON (Table 2). However, among infected chickens, chickens fed PB2 had (P < 0.05) greater feed efficiency (0.318) than infected chickens (0.267) fed NC. Over the experimental period, the feed efficiency did not differ among treatments.
Table 2.
Growth performance of chickens fed diet supplemented with antibiotics or probiotics.
| Treatments | CON | NC | AB1 | AB2 | PB1 | PB2 | PB3 | SEM | P |
|---|---|---|---|---|---|---|---|---|---|
| BW, g | |||||||||
| Initial | 488 | 493 | 471 | 492 | 513 | 496 | 492 | 10.0 | 0.247 |
| Day 21 | 827 | 845 | 875 | 888 | 842 | 873 | 885 | 14.2 | 0.063 |
| Day 28 | 1,175 | 1,008 | 1,047 | 1,079 | 1,059 | 1,058 | 1,053 | 28.5 | 0.038 |
| BWG, g | |||||||||
| Day 15 to 21 | 345c | 356b,c | 402a | 395a | 366a,b | 391a | 384a,b | 11.6 | 0.032 |
| Day 22 to 28 | 346a | 169c | 177b,c | 188b,c | 202b,c | 205b | 182b,c | 11.6 | 0.001 |
| Overall | 692a | 493c | 539b,c | 587b | 534b,c | 580b | 545b,c | 21.9 | 0.001 |
| FI, g | |||||||||
| Day 15 to 21 | 606 | 673 | 596 | 568 | 607 | 584 | 667 | 46.8 | 0.628 |
| Day 22 to 28 | 702 | 634 | 574 | 611 | 637 | 630 | 608 | 27.3 | 0.138 |
| Overall | 1,307 | 1,306 | 1,170 | 1,180 | 1,244 | 1,214 | 1,275 | 68.1 | 0.659 |
| FE | |||||||||
| Day 15 to 21 | 0.569 | 0.546 | 0.690 | 0.696 | 0.604 | 0.673 | 0.583 | 0.05 | 0.244 |
| Day 22 to 28 | 0.494a | 0.267c | 0.309b,c | 0.311b,c | 0.318b | 0.325b,c | 0.300b,c | 0.02 | 0.001 |
| Overall | 0.529 | 0.387 | 0.468 | 0.500 | 0.430 | 0.479 | 0.429 | 0.03 | 0.140 |
a–cMeans in the same row with different superscripts differ (P < 0.05).
The dose of B. subtilis strain in treatment was a total of at 1.5 × 105 CFU/g feed. For PB3 (2-strain combination), each strain composed 50% of the total CFU count (each strain represents 7.5 × 104 CFU/g feed). All chickens except those fed CON were infected by oral gavage at day 21 with 1.0 × 104 oocysts/bird of E. maxima.
Abberviations: AB1, diet supplemented with virginiamycin at 20 g/ton (22 ppm); AB2, diet supplemented with BMD at 50 g/ton (55 ppm); BW, body weight; BWG, body weight gain; CON, basal diet; FE, feed efficiency; FI, feed intake; NC, basal diet; PB1, diet supplemented with B. subtilis 1781; PB2, diet supplemented with B. subtilis 747; PB3: diet supplemented with B. subtilis 1781 + 747; P: P value; SEM: standard error of the mean.
Coccidia Lesion Score and Fecal Oocyst Shedding
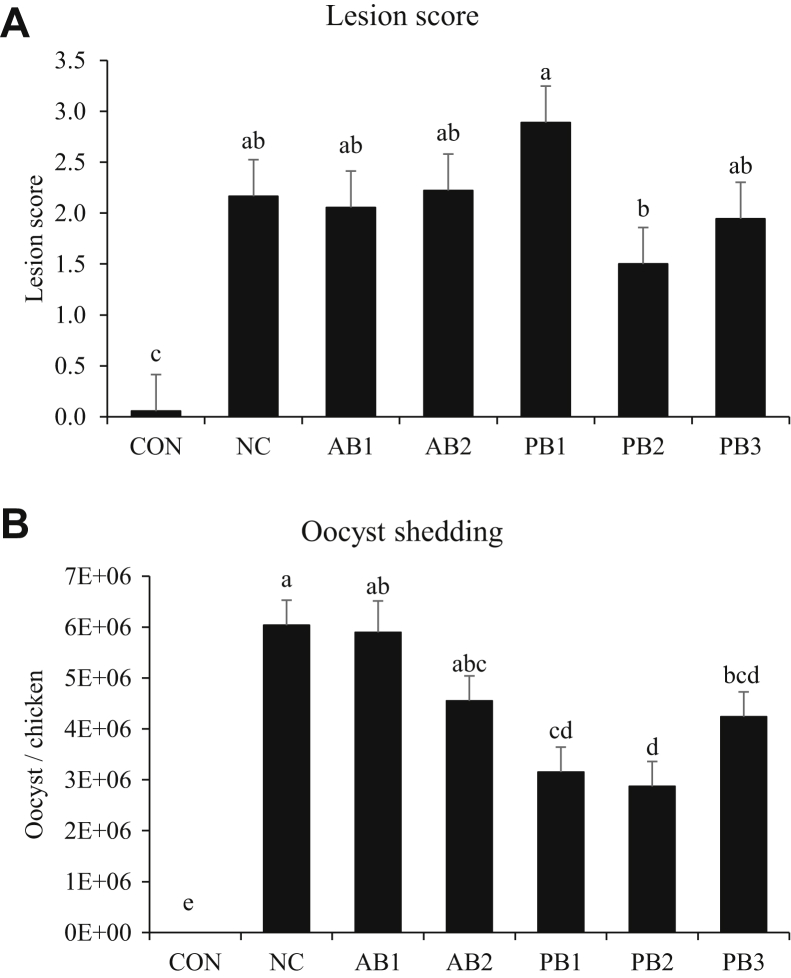
The E. maxima infection increased (P = 0.005) the lesion score in the jejunum of chickens at 7 dpi (Figure 2A). Among infected chickens, those fed PB2 had (P < 0.05) lower lesion scores (1.4) than chickens (average 2.2) fed other diets. The mean fecal oocyst shedding number per chicken is presented in Figure 2B. Chickens in the uninfected group (CON) excreted no fecal oocysts, but infection with E. maxima increased (P < 0.05) fecal oocyst shedding regardless of treatment group at 7 dpi. AB1 and AB2 did not decrease fecal oocyst appearance, whereas the fecal oocyst shedding in chickens fed a diet supplemented with PB1 (3,152,645 oocyst/chicken), PB2 (2,870,218 oocyst/chicken), and PB3 (4,236,793 oocyst/chicken) decreased (P < 0.05) below that of chickens (6,037,032 oocyst/chicken) fed NC.
Figure 2.
Lesion score and oocyst shedding of chickens fed diet supplemented with antibiotics or probiotics during infection with E. maxima. (A) Lesion score, (B) oocyst shedding. The dose of B. subtilis in treatment was 1.5 × 105 CFU/g feed. For PB3 (2-strain combination), each strain composed 50% of the total CFU count (each strain represents 7.5 × 104 CFU/g feed). All chickens except those fed CON were infected by oral gavage at day 21 with 1.0 × 104 oocysts/chicken of E. maxima. Bars with no common letter differ significantly (P < 0.05). The data were collected at day 28 (7 D postinfection) and were analyzed using Proc Mixed Procedure in SAS. Each bar represents the mean ± SEM (n = 6). Transcript levels of the cytokines were measured using quantitative RT-PCR and normalized to GAPDH transcript levels. Abberviations: CON: basal diet; NC: basal diet; AB1: diet supplemented with virginiamycin at 20 g/ton (22 ppm); AB2: diet supplemented with BMD at 50 g/ton (55 ppm); PB1: diet supplemented with B. subtilis 1781; PB2: diet supplemented with B. subtilis 747; PB3: diet supplemented with B. subtilis 1781 + 747; RT-PCR: real-time PCR.
Intestinal Transcript Levels of Proinflammatory and Th1 Cytokines
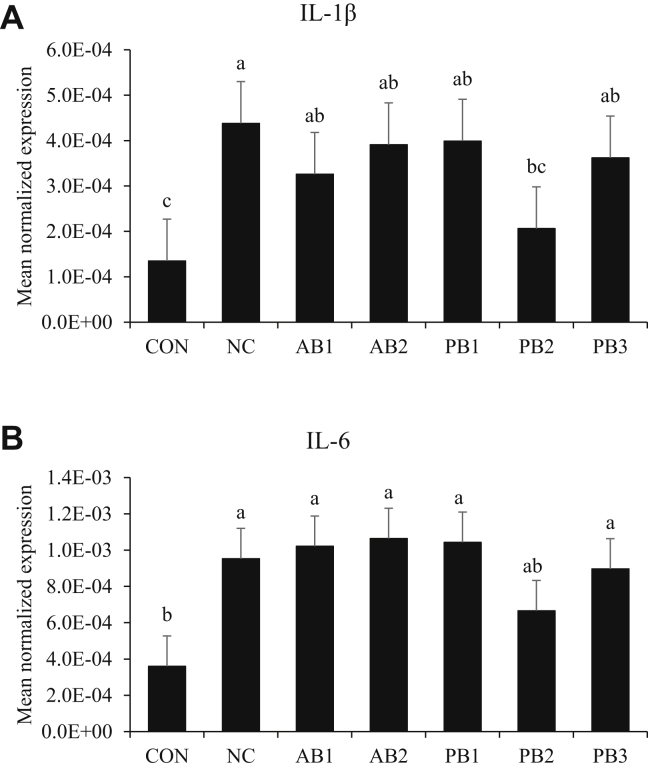
In jejunal tissue, infection with E. maxima increased (P < 0.05) the transcript levels of IL-1β (average 3.54 × 10−4; Figure 3A) and IL-6 (average 9.41 × 10−4; Figure 3B) regardless of dietary supplementation. Among treatment groups, chickens (2.06 × 10−4) fed PB2 showed lower (P < 0.05) transcript levels of IL-1β than chickens (4.38 × 10−4) fed NC.
Figure 3.
Transcripts of proinflammatory cytokines in the jejunum of chickens fed diet supplemented with antibiotics or probiotics during infection with E. maxima. (A) IL-1β, (B) IL-6. The dose of B. subtilis in treatment was 1.5 × 105 CFU/g feed. For PB3 (2-strain combination), each strain composed 50% of the total CFU count (each strain represents 7.5 × 104 CFU/g feed). All chickens except CON were infected by oral gavage at day 21 with 1.0 × 104 oocysts/bird of E. maxima. Bars with no common letter differ significantly (P < 0.05). Each bar represents the mean ± SEM (n = 6). The data were collected at day 28 (7 D postinfection) and were analyzed using Proc Mixed Procedure in SAS. Transcript levels of the cytokines were measured using quantitative RT-PCR and normalized to GAPDH transcript levels. Abberviations: CON: basal diet; NC: basal diet; AB1: diet supplemented with virginiamycin at 20 g/ton (22 ppm); AB2: diet supplemented with BMD at 50 g/ton (55 ppm); PB1: diet supplemented with B. subtilis 1781; PB2: diet supplemented with B. subtilis 747; PB3: diet supplemented with B. subtilis 0.1781 + 747.
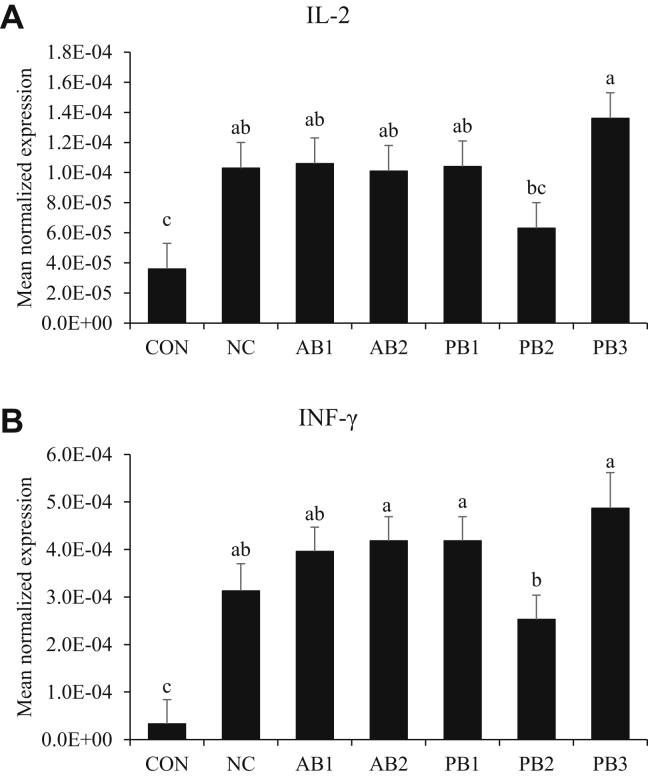
In jejunal tissue, infection with E. maxima increased (P < 0.05) the levels of IL-2 (average 1.02 × 10−4; Figure 4A) and INF-γ (average 3.8 × 10−4; Figure 4B) regardless of dietary supplementation. Among the treatment groups, chickens fed PB2 had lower transcript levels of IL-2 and INF-γ than chickens fed NC.
Figure 4.
Transcripts of Th1 in the jejunum of chickens fed diet supplemented with antibiotics or probiotics during infection with E. maxima. (A) IL-2, (B) INF-γ. The dose of B. subtilis in treatment was 1.5 × 105 CFU/g feed. For PB3 (2-strain combination), each strain composed 50% of the total CFU count (each strain represents 7.5 × 104 CFU/g feed). All chickens except those fed CON were infected by oral gavage at day 21 with 1.0 × 104 oocysts/bird of E. maxima. Bars with no common letter differ significantly (P < 0.05). Each bar represents the mean ± SEM (n = 6). The data were collected at day 28 (7 D postinfection) and were analyzed using Proc Mixed Procedure in SAS. Transcript levels of the cytokines were measured using quantitative RT-PCR and normalized to GAPDH transcript levels. Abberviations: CON: basal diet; NC: basal diet; AB1: diet supplemented with virginiamycin at 20 g/ton (22 ppm); AB2: diet supplemented with BMD at 50 g/ton (55 ppm); PB1: diet supplemented with B. subtilis 1781; PB2: diet supplemented with B. subtilis 747; PB3: diet supplemented with B. subtilis 0.1781 + 747.
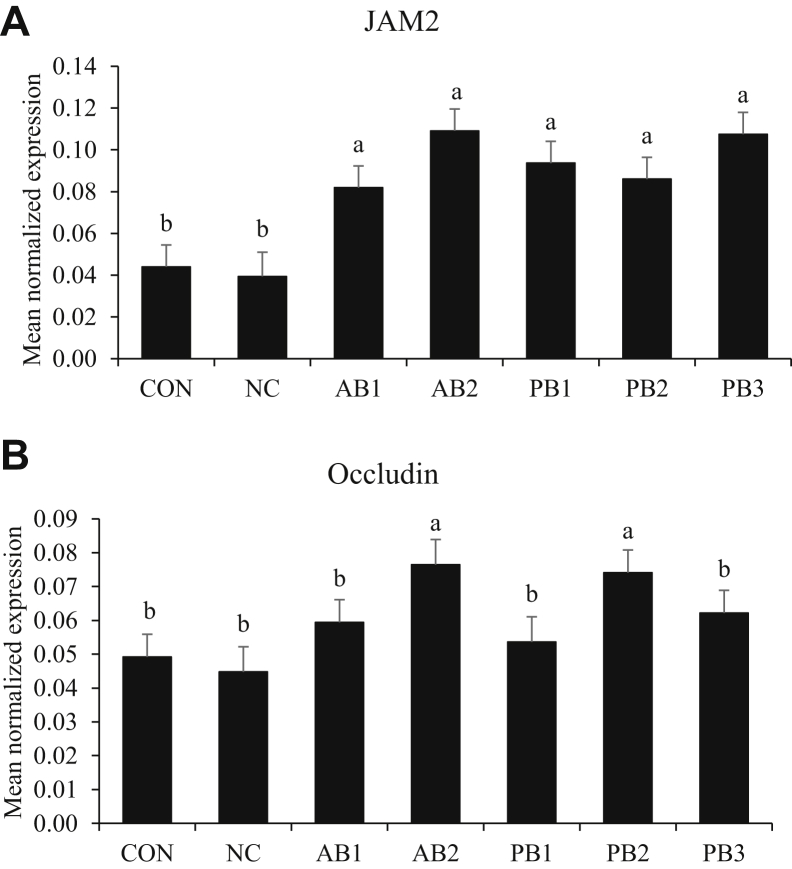
Transcript Levels of Tight Junction Proteins
In jejunal tissue, infection with E. maxima did not affect transcript levels of JAM2 (Figure 5A) and occludin (Figure 5B). Chickens fed a diet supplemented with antibiotics (AB1: 8.19 × 10−2, and AB2: 1.09 × 10−1) and probiotics (PB1: 9.37 × 10−2, PB2: 8.60 × 10−2, and PB3: 1.08 × 10−1) had greater (P < 0.05) transcript levels of JAM2 than did chickens fed both basal diets (CON: 4.40 × 10−2 and NC: 3.94 × 10−2). The transcript levels of occludin in the jejunum in chickens fed AB2 (7.65 × 10−2) and PB2 (7.41 × 10−2) were higher (P < 0.05) than those in chickens fed other diets.
Figure 5.
Transcripts of tight junction proteins in the jejunum of chickens fed diet supplemented with antibiotics or probiotics during infection with E. maxima. (A) JAM2, (B) occluding. The dose of B. subtilis in treatment was 1.5 × 105 CFU/g feed. For PB3 (2-strain combination), each strain composed 50% of the total CFU count (each strain represents 7.5 × 104 CFU/g feed). All chickens except those fed CON were infected by oral gavage at day 21 with 1.0 × 104 oocysts/bird of E. maxima. Bars with no common letter differ significantly (P < 0.05). Each bar represents the mean ± SEM (n = 6). The data were collected at day 28 (7 D postinfection) and were analyzed using Proc Mixed Procedure in SAS. Transcript levels of the tight junction proteins were measured using quantitative RT-PCR and normalized to GAPDH transcript levels. Abberviations: CON: basal diet; NC: basal diet; AB1: diet supplemented with virginiamycin at 20 g/ton (22 ppm); AB2: diet supplemented with BMD at 50 g/ton (55 ppm); PB1: diet supplemented with B. subtilis 1781; PB2: diet supplemented with B. subtilis 747; PB3: diet supplemented with B. subtilis 0.1781 + 747.
Discussion
This study was conducted to investigate the effects of B. subtilis 1781 and 747 on growth performance, intestinal immunity, and epithelial barrier integrity in broiler chickens infected with E. maxima compared with antibiotics. The doses of B. subtilis 1781 and 747 used in this study were based on the recommended level of Bacillus-based probiotics for the poultry industry and would cost approximately $2 per ton of feed for use under commercial conditions (Gadde et al., 2017b). This study included virginiamycin and BMD, 2 well-established AGP widely used in the poultry industry. The growth-promoting effects of these 2 antibiotics as AGP have already been demonstrated in numerous studies (Combs and Bossard, 1963, Miles et al., 1984, Engberg et al., 2000, Gadde et al., 2018). In the present study, the effects of AGP was validated, and AGP improved body weight gain in chickens fed a diet supplemented with virginiamycin and BMD for 14 to 21 D during the noninfection period. Chickens fed a diet supplemented with B. subtilis 1781, 747, or a combination of 1781 and 747 also showed greater body weight gain than did chickens fed a basal diet. Notably, the degree of improvement in body weight gain in chickens fed a diet supplemented with these B. subtilis strains was not different from that of chickens fed a diet supplemented with virginiamycin or BMD. This finding is largely consistent with those of Gadde et al. (2017a), who have reported that chickens fed a diet supplemented with B. subtilis 1781 grow better than chickens fed a basal diet. In addition, the beneficial growth performance of noninfected chickens fed a diet supplemented with B. subtilis has already been documented (Lee et al., 2010, Aliakbarpour et al., 2012, Jeong and Kim, 2014).
Eimeria spp. contributes to an estimated $3 billion annual loss worldwide, and 7 distinct species infect avian intestinal mucosa (Lillehoj and Trout, 1996, Shirley and Lillehoj, 2012). In the present study, infection with E. maxima significantly decreased the body weight gain of chickens below that of noninfected chickens. Virginiamycin and BMD supplementation was not efficacious against coccidiosis because they are not anticoccidial medications. The results of this work thus showed beneficial effects of dietary B. subtilis supplementation to young chickens infected with E. maxima through its action on innate immunity by decreasing proinflammatory response and enhancing gut integrity by reducing intestinal damages caused by coccidiosis. In chickens that were fed B. subtilis–supplemented diet, the body weight gains of infected chickens fed B. subtilis 747 improved beyond that of infected chickens on a basal diet, whereas B. subtilis 1781 or a combination of 1781 + 747 did not affect body weight gain in infected chickens. Lee et al. (2015) have also demonstrated that only two of nine tested B. subtilis strains improved body weight gain in chickens infected with E. maxima. The beneficial effect on body weight gain was effective in improving feed efficiency in infected chickens fed a diet supplemented with B. subtilis 747. The reasons why certain strains of B. subtilis show beneficial effects on coccidiosis-infected chickens need further studies.
The body weight gain and lesion score are commonly used as clinical measurements for evaluating the severity of coccidiosis (Zhu et al., 2000). In the present study, chickens infected with E. maxima exhibited high lesion scores, thus indicating severe extensive destruction of the gut epithelium in the area of Meckel's diverticulum, whereas infected chickens fed a diet supplemented with B. subtilis 747 showed lower lesion scores at 7 dpi. Infection with E. maxima increased the fecal oocyst output of chickens; however, B. subtilis 747 supplementation markedly decreased the fecal oocyst output. Therefore, in infected chickens, the growth-promoting effect of B. subtilis 747 supplementation was supported by the results of the lesion score and oocyst shedding.
Eimeria infection activates chickens' innate and acquired immune response, which involves the secretion of various chemokines and cytokines (Lillehoj, 1998). Cytokines, small immune-regulatory peptides aid in cell-to-cell communication during immune responses. IL-1β is an important proinflammatory cytokine that is produced mainly by activated macrophages and plays an important role in the innate immune responses through recruitment of inflammatory cells (Hong et al., 2006). IL-6, produced by T cells, monocytes, and macrophages, functions as both a proinflammatory and anti-inflammatory cytokine, and also promotes Th17-cell differentiation (Waititu et al., 2014). Increased IL-6 expression has also been proposed to aid in defining populations of heterophils that are more capable of responding to and eliminating pathogens (Swaggerty et al., 2004, Hong et al., 2006). In the present study, chickens infected with E. maxima showed increased expression of IL-1β and IL-6 at 7 dpi, regardless of antibiotic or probiotic supplementation. Among the infected chickens, B. subtilis 747 decreased the expression of IL-1β and IL-6. In addition to the changes in expression of various proinflammatory cytokines, this study also investigated the alterations in IL-2 and IFN-γ levels. Chickens infected with E. maxima showed increased expression of IL-2 and INF-γ, regardless of antibiotic or probiotic supplementation. Among the infected chickens, IL-2 and INF-γ expressions were downregulated in chickens fed diets supplemented with B. subtilis 747. If an immune response occurs, cytokines or chemokines are released in sufficient amounts to suppress the immune responses (Klasing, 2007). Klasing, (2007) has reported that a cytokine storm induces metabolic changes, including increased protein degradation in skeletal muscle, thereby diverting nutrients from the muscle and other tissues, so that they are made available for the increased demands of leukocytes and the production of protective proteins. Ultimately, these responses decrease growth performance and directly influence the success of poultry production. In practice, under equalized feed intake, a vigorous acute-phase immune response in chickens has been estimated to account for approximately 10% of nutrient use (Klasing, 2007). Jiang et al., (2010) have reported that lipopolysaccharide-challenged chickens (1 mg lipopolysaccharide per kg of body weight at 14, 16, 18, and 20 D of age) show a 22% decrease in body weight gain during challenge; 59% of the loss is accounted for by decreased feed intake, and the remaining 41% is attributed to immune response–related factors (Broom and Kogut, 2018).
Many factors related to disease and stress can damage intestinal epithelial integrity, thus decreasing nutrient absorption, increasing pathogenic invasion and inflammatory disease, and consequently decreasing growth performance (Yegani and Korver, 2008). Therefore, the intestinal epithelium serves as a physical barrier against invading pathogens and intraluminal toxins (Ulluwishewa et al., 2011, Song et al., 2014). It is composed of a single layer of columnar epithelial cells that are tightly bound by intercellular junctional complexes. These junctional complexes maintain the integrity of the epithelial barrier by regulating paracellular permeability and are composed of TJs, gap junctions, adherens junctions, and desmosomes (Gadde et al., 2017a). Tight junctions include 4 integral transmembrane proteins (occludin, claudin, JAM, and tricellulin) that interact with cytosolic scaffold proteins, which in turn bind the actin cytoskeleton (Ulluwishewa et al., 2011, Lee et al., 2015). Junctional adhesion molecule-2 and occludin play important roles in the assembly and maintenance of TJs and the regulation of intestinal permeability, as evidenced by increased paracellular permeability to macromolecules in knockout mice (Al-Sadi et al., 2011, Lee et al., 2015). In the present study, the expression of JAM2 was elevated in all the supplemented groups regardless of infection with E. maxima, and occludin was elevated in the chickens fed a diet supplemented with BMD and B. subtilis 747. Gadde et al. (2017a) have suggested that increased TJ protein expression in chickens fed a diet supplemented with probiotics improves intestinal barrier function and provides optimal gut health.
Overall, dietary B. subtilis supplementation significantly improved the growth performance of noninfected chickens during the posthatch growth period similar to AGP supplementation. After infection with E. maxima, dietary virginiamycin and BMD supplementation enhanced epithelial barrier integrity, whereas B. subtilis 747 improved the growth performance, intestinal immunity, and epithelial barrier integrity of chickens in this study. Together, our results indicated that dietary B. subtilis supplementation has the potential to replace antibiotics fed to broiler chickens.
Acknowledgments
This work was supported by ARS CRIS Project 8042-32000-107-00D.
References
- Aliakbarpour H.R., Chamani M., Rahimi G., Sadeghi A.A., Qujeq D. The Bacillus subtilis and lactic acid bacteria probiotics influences intestinal mucin gene expression, histomorphology and growth performance in broilers. Asian Austral. J. Anim. 2012;25:1285–1293. doi: 10.5713/ajas.2012.12110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Sadi R., Khatib K., Guo S., Ye D., Youssef M., Ma T. Occludin regulates macromolecule flux across the intestinal epithelial tight junction barrier. Am. J. Physiol. Gastrointest. Liver Physiol. 2011;300:G1054–G1064. doi: 10.1152/ajpgi.00055.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broom L.J., Kogut M.H. Inflammation: friend or foe for animal production? Poult. Sci. 2018;97:510–514. doi: 10.3382/ps/pex314. [DOI] [PubMed] [Google Scholar]
- Chan G., Guthrie A., Sivaramalingam T., Wilson J., Vancraeynest D., Moody R., Clark S. A framework for assessing the efficacy of antimicrobials in the control of necrotic enteritis in broiler chickens. J. Appl. Poult. Res. 2015;24:246–256. [Google Scholar]
- Combs G.F., Bossard E.H. Comparison of growth response of chicks to virginiamycin and other antibiotics. Poult. Sci. 1963;42:681–685. [Google Scholar]
- Engberg R.M., Hedemann M.S., Leser T.D., Jensen B.B. Effect of zinc bacitracin and salinomycin on intestinal microflora and performance of broilers. Poult. Sci. 2000;79:1311–1319. doi: 10.1093/ps/79.9.1311. [DOI] [PubMed] [Google Scholar]
- FAO . United Nations Food and Agriculture Organization; Rome, Italy: 2010. Global Forest Resources Assessment 2010. [Google Scholar]
- Fritts C.A., Kersey J.H., Motl M.A., Kroger E.C., Yan F., Si J., Jiang Q., Campos M.M., Waldroup A.L., Waldroup P.W. Bacillus subtilis C-3102 (Calsporin) improves live performance and microbiological status of broiler chickens. J. Appl. Poult. Res. 2000;9:149–155. [Google Scholar]
- Gadde U.D., Oh S.T., Lee Y.S., Davis E., Zimmerman N., Rehberger T., Lillehoj H.S. The effects of direct-fed microbial supplementation, as an alternative to antibiotics, on growth performance, intestinal immune status, and epithelial barrier gene expression in broiler chickens. Probiotics Antimicrob. Proteins. 2017;9:397–405. doi: 10.1007/s12602-017-9275-9. [DOI] [PubMed] [Google Scholar]
- Gadde U., Kim W.H., Oh S.T., Lillehoj H.S. Alternatives to antibiotics for maximizing growth performance and feed efficiency in poultry: a review. Anim. Health Res. Rev. 2017;18:26–45. doi: 10.1017/S1466252316000207. [DOI] [PubMed] [Google Scholar]
- Gadde U.D., Oh S., Lillehoj H.S., Lillehoj E.P. Antibiotic growth promoters virginiamycin and bacitracin methylene disalicylate alter the chicken intestinal metabolome. Sci. Rep. 2018;8:3592. doi: 10.1038/s41598-018-22004-6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Gallucci S., Matzinger P. Danger signals: SOS to the immune system. Curr. Opin. Immunol. 2001;13:114–119. doi: 10.1016/s0952-7915(00)00191-6. [DOI] [PubMed] [Google Scholar]
- Ganguly S. Review article nutraceutical and pharmaceutical implication of prebiotics in livestock and poultry feed. Bull. Pharm. Res. 2013;3:71–77. [Google Scholar]
- Godfray H.C., Beddington J.R., Crute I.R., Haddad L., Lawrence D., Muir J.F., Pretty J., Robinson S., Thomas S.M., Toulmin C. Food Security: the challenge of feeding 9 billion people. Science. 2010;327:812–818. doi: 10.1126/science.1185383. [DOI] [PubMed] [Google Scholar]
- Grant A., Gay C.G., Lillehoj H.S. Bacillus spp. as direct-fed microbial antibiotic alternatives to enhance growth, immunity, and gut health in poultry. Avian Pathol. 2018;47:339–351. doi: 10.1080/03079457.2018.1464117. [DOI] [PubMed] [Google Scholar]
- Hong Y.H., Lillehoj H.S., Lillehoj E.P., Lee S.H. Changes in immune-related gene expression and intestinal lymphocyte subpopulations following Eimeria maxima infection of chickens. Vet. Immunol. Immunopathol. 2006;114:259–272. doi: 10.1016/j.vetimm.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Jeong J.S., Kim I.H. Effect of Bacillus subtilis C-3102 spores as a probiotic feed supplement on growth performance, noxious gas emission, and intestinal microflora in broilers. Poult. Sci. 2014;93:3097–3103. doi: 10.3382/ps.2014-04086. [DOI] [PubMed] [Google Scholar]
- Jiang Z., Schatzmayr G., Mohnl M., Applegate T.J. Net effect of an acute phase response-Partial alleviation with probiotic supplementation. Poult. Sci. 2010;89:28–33. doi: 10.3382/ps.2009-00464. [DOI] [PubMed] [Google Scholar]
- Johnson J., Reid W.M. Anticoccidial drugs:lesion scoring techniques in battery and floor-pen experiments with chickens. Exp. Parasitol. 1970;28:30–36. doi: 10.1016/0014-4894(70)90063-9. [DOI] [PubMed] [Google Scholar]
- Klasing K. Nutrition and the immune system. Br. Poult. Sci. 2007;48:525–537. doi: 10.1080/00071660701671336. [DOI] [PubMed] [Google Scholar]
- Knap I., Lund B., Kehlet A.B., Hofacre C., Mathis G. Bacillus licheniformis prevents necrotic enteritis in broiler chickens. Avian Dis. 2010;54:931–935. doi: 10.1637/9106-101509-ResNote.1. [DOI] [PubMed] [Google Scholar]
- Lee K.W., Kyung D., Lillehoj H.S., Jang S.I., Lee S.H. Immune modulation by Bacillus subtilis-based direct-fed microbials in commercial broiler chickens. Anim. Feed Sci. Tech. 2015;200:76–85. [Google Scholar]
- Lee K.W., Li G., Lillehoj H.S., Lee S.H., Jang S.I., Babu U.S., Lillehoj E.P., Neumann A.P., Siragusa G.R. Bacillus subtilis-based direct-fed microbials augment macrophage function in broiler chickens. Res. Vet. Sci. 2011;91:e87–e91. doi: 10.1016/j.rvsc.2011.01.018. [DOI] [PubMed] [Google Scholar]
- Lee K.W., Lillehoj H.S., Jang S.I., Li G.X., Bautista D.A., Phillips K., Ritter D., Lillehoj E.P., Siragusa G.R. Effects of coccidiosis control programs on antibody levels against selected pathogens and serum nitric oxide levels in broiler chickens. J. Appl. Poult. Res. 2011;20:143–152. [Google Scholar]
- Lee K.W., Lee S.H., Lillehoj H.S., Li G.X., Jang S.I., Babu U.S., Park M.S., Kim D.K., Lillehoj E.P., Neumann A.P., Rehberger T.G., Siragusa G.R. Effects of direct-fed microbials on growth performance, gut morphometry, and immune characteristics in broiler chickens. Poult. Sci. 2010;89:203–216. doi: 10.3382/ps.2009-00418. [DOI] [PubMed] [Google Scholar]
- Li Y., Xu Q., Huang Z., Lv L., Liu X., Yin C., Yan H., Yuan J. Effect of Bacillus subtilis CGMCC 1.1086 on the growth performance and intestinal microbiota of broilers. J. Appl. Microbiol. 2016;120:195–204. doi: 10.1111/jam.12972. [DOI] [PubMed] [Google Scholar]
- Lillehoj H.S. Role of T lymphocytes and cytokines in coccidiosis. Int. J. Parasitol. 1998;28:1071–1081. doi: 10.1016/s0020-7519(98)00075-7. [DOI] [PubMed] [Google Scholar]
- Lillehoj H.S., Lee S.H., Park S.S., Jeong M., Lim Y., Mathis G.F., Lumpkins B., Chi F., Ching C., Cravens R.L. Calcium montmorillonite-based dietary supplement attenuates necrotic enteritis induced by Eimeria maxima and Clostridium perfringens in broilers. J. Poult. Sci. 2016;53:329–340. doi: 10.2141/jpsa.0150182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillehoj H.S., Trout J.M. Avian gut-associated lymphoid tissues and intestinal immune responses to Eimeria parasites. Clin. Microbiol. Rev. 1996;9:349–360. doi: 10.1128/cmr.9.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y., Xu S., Zeng D., Ni X., Zhou M., Zeng Y., Wang H., Zhou Y., Zhu H., Pan K., Li G. Disruption in the cecal microbiota of chickens challenged with Clostridium perfringens and other factors was alleviated by Bacillus licheniformis supplementation. Plos One. 2017;12:e0182426. doi: 10.1371/journal.pone.0182426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles R.D., Janky D.M., Harms R.H. Virginiamycin and broiler performance. Poult. Sci. 1984;63:1218–1221. doi: 10.3382/ps.0631218. [DOI] [PubMed] [Google Scholar]
- Muller P., Janovjak H., Miserez A., Dobbie Z. Processing of gene expression data generated by quantitative real-time RT PCR (vol 32, pg 1378,2002) BioTechniques. 2002;33:514. [PubMed] [Google Scholar]
- Nhung N.T., Chansiripornchai N., Carrique-Mas J.J. Antimicrobial resistance in bacterial poultry pathogens: a review. Front. Vet. Sci. 2017;4:126. doi: 10.3389/fvets.2017.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OECD-FAO . OECD-FAO; Rome, Italy: 2010. OECD-FAO Agricultural Outlook 2010–2019. [Google Scholar]
- Oh S.T., Gadde U.D., Bravo D., Lillehoj E.P., Lillehoj H.S. Growth-promoting and antioxidant effects of magnolia bark extract in chickens uninfected or co-Infected with Clostridium perfringens and Eimeria maxima as an experimental model of necrotic enteritis. Curr. Dev. Nutr. 2018;2:nzy009. doi: 10.1093/cdn/nzy009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peek H.W., Landman W.J.M. Coccidiosis in poultry: anticoccidial products, vaccines and other prevention strategies. Vet. Q. 2011;31:143–161. doi: 10.1080/01652176.2011.605247. [DOI] [PubMed] [Google Scholar]
- Rennier G. Poultry health today. 2017. https://poultryhealthtoday.com/rennier-nae-programs-represented-40-of-us-broiler-feeds-in-2017/ Accessed April 2018.
- Roberts S. 9 billion? Science. 2011;333:540–543. doi: 10.1126/science.333.6042.540. [DOI] [PubMed] [Google Scholar]
- Sander V.A., Corigliano M.G., Clemente M. Promising plant-derived adjuvants in the development of coccidial vaccines. Front. Vet. Sci. 2019;6:20. doi: 10.3389/fvets.2019.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepon A., Eshel G., Noor E., Milo R. The opportunity cost of animal based diets exceeds all food losses. Proc. Natl. Acad. Sci. 2018;115:3804–3809. doi: 10.1073/pnas.1713820115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirley M.W., Lillehoj H.S. The long view: a selective review of 40 years of coccidiosis research. Avian Pathol. 2012;41:111–121. doi: 10.1080/03079457.2012.666338. [DOI] [PubMed] [Google Scholar]
- Song J., Xiao K., Ke Y.L., Jiao L.F., Hu C.H., Diao Q.Y., Shi B., Zhou X.T. Effect of probiotic mixture on intestinal microflora, morphology, and barrier integrity of broilers subjected to heat stress. Poult. Sci. 2014;93:581–588. doi: 10.3382/ps.2013-03455. [DOI] [PubMed] [Google Scholar]
- Swaggerty C.L., Kogut M.H., Ferro P.J., Rothwell L., Pevzner I.Y., Kaiser P. Differential cytokine mRNA expression in heterophils isolated from Salmonella-resistant and -susceptible chickens. Immunology. 2004;113:139–148. doi: 10.1111/j.1365-2567.2004.01939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulluwishewa D., Anderson A.C., McNabb W.C., Moughan P.J., Well J.M., Roy N.C. Regulation of tight junction permeability by intestinal bacteria and dietary components. J. Nutr. 2011;141:769–776. doi: 10.3945/jn.110.135657. [DOI] [PubMed] [Google Scholar]
- Waititu S.M., Yitbarek A., Matini E., Echeverry H., Kiarie E., Rodriguez-Lecompte J.C., Nyachoti C.M. Effect of supplementing direct-fed microbials on broiler performance, nutrient digestibilities, and immune responses. Poult. Sci. 2014;93:625–635. doi: 10.3382/ps.2013-03575. [DOI] [PubMed] [Google Scholar]
- Yadav S., Jha R. Strategies to modulate the intestinal microbiota and their effects on nutrient utilization, performance, and health of poultry. J. Anim. Sci. Biotechno. 2019;10:2. doi: 10.1186/s40104-018-0310-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yegani M., Korver D.R. Factors affecting intestinal health in poultry. Poult. Sci. 2008;87:2052–2063. doi: 10.3382/ps.2008-00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J.J., Lillehoj H.S., Allen P.C., Yun C.H., Pollock D., Sadjadi M., Emara M.G. Analysis of disease resistance-associated parameters in broiler chickens challenged with Eimeria maxima. Poult. Sci. 2000;79:619–625. doi: 10.1093/ps/79.5.619. [DOI] [PubMed] [Google Scholar]