Abstract
Cold stimulations during egg incubation were reported to limit the occurrence of ascites in broilers subjected to cold temperature after 14 d of age. However, data are lacking on the impacts of such strategy in case of cold temperature conditions at start. This study aimed to evaluate the effects of incubation and posthatch cold challenge on performance, breast muscle integrity, and meat processing quality in broiler chickens. Ross 308 eggs were incubated under control temperature (I0, 37.6°C) or subjected to 15°C during 30 min on day 18 and 19 of incubation (I1). Chicks from each group were reared in floor pens either at standard rearing temperature (T0), from 32°C at 0 d to 21°C at 21 d of age, or exposed to colder rearing temperature (T1), from 29°C at 0 to 21°C at 21 d of age. All birds were then kept at 21°C until slaughter (day 40), when body weights (BW), feed conversion ratio (FCR), breast muscle yield, meat processing quality, and the occurrences of meat defects, hock burns, and pododermatitis were recorded. No significant impact of incubation conditions on hatchability was observed. At day 40, BW was more under T1 than under T0 conditions, with T0 females (but not males) presenting more BW after I1 than after I0 conditions. In the whole period, T1 chickens presented lower FCR than T0 chickens and higher breast meat yields at day 40. The occurrence of white striping was more in I1T1 males than in all other groups, except for the I0T1 males. Hock burns were more frequent in I1T1 males than in all females and I0T0 males, whereas the occurrence of pododermatitis was lower in T0 males than in other groups. Despite some positive effects of I1 incubation on growth after starting under low ambient temperature, this study reveals the limits of such strategy concerning chicken health and welfare, demonstrating that early thermal environment is a major component of the quality and sustainability of chicken meat production.
Key words: broiler, incubation, meat quality, white striping, welfare
Introduction
Chicken production is expected to increase continuously (+1.8%/year) for the next decades (Alexandratos and Bruinsma, 2012) in view of the growing global demand in proteins from animal source and the efficiency of this production (Petracci and Cavani, 2012). However, the environmental, economic, and social impacts of the production systems, including meat quality and animal welfare, are criteria of concern for consumers and citizens.
It was suggested that the genetic selection of fast-growing broilers based on growth rate, feed efficiency, and breast meat yield has simultaneously increased the sensitivity of these birds to temperature variations (Piestun et al., 2008, Havenstein et al., 2003a, Havenstein et al., 2003b, Zuidhof et al., 2014). Because the thermoregulatory system of chicks is immature at hatching (Tzschentke, 2007), they remain very sensitive to postnatal cold temperatures during the first days of breeding (Collin et al., 2003, Mujahid and Furuse, 2009). Environmental conditions during the perinatal period are considered critical for later performance and fitness of chickens (Guilloteau et al., 2019). Especially, brooding chicks at low temperatures (26.7°C vs. 32.2°C) results in a decreased feed efficiency and increased mortality rate (Renwick and Washburn, 1982). Fast-growing broilers exhibit greater ascites prevalence (Druyan et al., 2007) and leg disorders (Yalçin et al., 2007, Zhang et al., 2014, Nyuiadzi et al., 2017) when they are subjected to low rearing temperatures after hatching.
Few studies showed that the exposure to cold temperature during embryogenesis reduced the prevalence of ascites and changed thermoregulatory mechanisms in the early life of chicks, helping them to cope with low rearing temperatures after hatching (Shinder et al., 2011, Akşit et al., 2013). These authors showed that it decreased the mortality rate, but it also increased the antioxidant activity of catalase in the liver of chicks at hatching (Loyau et al., 2014). Cold treatment in embryonic life can affect not only chick behavior (Bertin et al., 2018) but also performance. Van der Pol et al. (2013) showed that cold brooding temperature resulted in lower body weight (BW) than normal brooding temperature at 4 d of age. However, long-term positive effects of embryonic and postnatal cold acclimation in broilers were reported on BW (Shinder et al., 2009) and breast muscle yield (Shinder et al., 2011) when broiler chickens are reared at standard temperature. Their treatment was applied in the last phase of embryogenesis (days 18 and 19 of incubation), just before the blood concentrations of triiodothyronine, a major hormone involved in thermoregulation and hatching process, are reaching a peak (Reyns et al., 2003). The late phase of embryogenesis corresponds to the switch of embryos from the ectothermic phase to the endothermic phase when a higher ability to produce heat is acquired (Minne and Decuypere, 1984, Nichelmann and Tzschentke, 2002, Tzschentke, 2007). Cold stimulations during embryogenesis were shown to induce long-lasting effects partially limiting the detrimental effects of postnatal cold temperatures on feed conversion ratio (FCR) in males reared in cages (Nyuiadzi et al., 2017).
To ensure the sustainability of poultry meat production, not only performance but also meat processing quality and animal welfare have to be considered. Metabolic diseases, including ascites, but also muscle myopathies including white striping (WS) and wooden breast (WB) have recently become a major source of loss for poultry meat production of fast-growing broilers (Julian, 2005, Kuttappan et al., 2016). The rapid growth rate, the increase in breast meat yield, and gender are factors affecting the prevalence of WS (Petracci and Cavani, 2012, Alnahhas et al., 2016). The latter authors have pointed out genetics as the major determinant of this meat defect. To a lesser extent, environmental and management factors, including nutrition (Meloche et al., 2018), also contribute to the variance of the WS occurrence in the breast muscle (Bailey et al., 2015). However, the impacts of rearing temperatures and early life environment on this defect have been poorly investigated, except in the case of heat embryonic exposure (Clark et al., 2017).
To our knowledge, no experimentation has been carried out on the effects of cold exposure during embryogenesis on meat processing quality and muscle defects when chickens are later subjected to standard or low environmental temperature. Thus, the objective of the present study was to assess the performance and meat quality at slaughter age in broilers exposed to low or standard temperature incubation in interaction with postnatal low or standard ambient temperature, while recording the impacts of these treatments on animal performance as well as on some animal welfare criteria.
Materials and methods
All experimental procedures were approved by the Ethics Committee for Animal Experimentation Val de Loire (CEEA Val de Loire, Tours, France, N°2014111809444741 (APAFIS#70).03).
Incubation Process
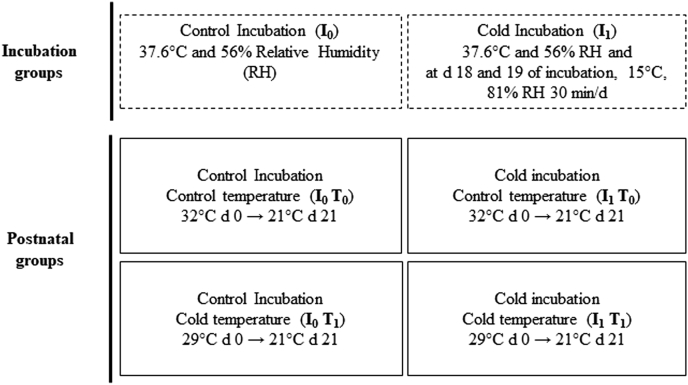
A total of 1,200 fertile Ross 308 broilers eggs with 3 d of storage from 36-wk-old breeder flock were provided from a commercial hatchery (Couvoir Perrot, Pommerit-Jaudy, France). Before incubation, average egg weight was determined (62.3 ± 0.7 g), and the eggs were randomly divided into 2 treatment groups exposed to control (I0 at 37.6°C and 56% RH) or cold incubation (I1) at PEAT INRAE Poultry Experimental Facility (2018, https://doi.org/10.15454/1.5572326250887292E12). I1 consisted in exposing 3 trays of 132 eggs to 15°C and 81% RH during 30 min on day 18 and day 19 of incubation by transferring them from the hatcher to a cold room, eggs being incubated at 37.6°C and 56% RH for the remainder of incubation (Figure 1). This treatment resulted in a minimal eggshell temperature (±SD) of 26.9°C ± 2.1°C and maximal eggshell temperature of 33.1 ± 0.6°C, measured by thermal imaging after 30 min of exposure. On day 7 and day 14 of incubation, unfertile and undeveloped eggs were eliminated after candling. At day 18 of incubation, all eggs were moved to a common hatcher set at 37.6°C and 70% RH, with only the 30 min of interruption at day 19 for the transfer of the group I1 to the cold room.
Figure 1.
Experimental design. Number of birds per treatment is reported in the Materials and Methods section.
Hatching
Into the hatcher, the number of hatched chicks in the control (I0) and cold (I1) incubation group was recorded at 480 h of incubation, and then at 504 hours (opening of the hatcher) to calculate hatchability as the ratio of hatched chicks on fertile incubated eggs. Chicks from each treatment were weighed, wing banded, and sexed by cloacal observation. The body temperature (Tb) of chicks was measured individually at hatching (on dry chicks after opening the hatcher) by inserting an electronic thermometer (PX-TH519, Tex, Pelimex, Ingwiller, France) in the cloaca. Fifty chicks from both treatment groups were randomly selected for quality measurement, according to Tona et al. (2003). In brief, the score of 8 parameters including activity, down and appearance, retracted yolk, eyes, legs, navel area, remaining membrane, and remaining yolk on the navel was measured to determine the chick quality. Each score was summed and scored out of 100, with 100 being the highest score value for the best chick quality.
Rearing Period
Four hundred chicks from each incubation group were divided randomly into 2 groups and were transferred to 2 identical controlled rooms under standard rearing conditions T0, decreasing from 32°C at day 0 to 21°C at day 21, or under cold rearing conditions T1, consisting in continuously decreasing temperature from 29°C at day 0 to 21°C at day 21, respectively (Figure 1). From day 22 to day 40, ambient temperature was maintained at 21°C in both conditions T0 and T1. Chicks were reared in groups of same sex per condition with 4 repetitions per incubation treatment, sex, and treatment group at the rate of 23 males or 27 females from day 0 to day 21, reduced to 19 males or 22 females per group on the basis of the mean BW and standard error of the group from day 22 to day 40 to limit the rearing density in pens at slaughter age. Chicks were reared in floor pens (surface = 2.93 m2) with wood shaving litter (1.5 kg/m2). Water and pelleted feed were supplied ad libitum until slaughter age (day 40).
Performance
Chickens were weighed at the age of day 0, day 21, and day 40. Feed consumption was recorded on each floor pen from day 0 to day 21 and from day 22 to day 40. Feed conversion ratio was calculated for both periods as the feed consumption-to-BW gain ratio per pen during both periods and during the entire rearing period.
Muscle and Meat Quality Parameters
At day 40, 8 males and 8 females per floor pen per treatment group (I0T0, I0T1, I1T0, I1T1), representatives of their pen (average weight and SD), were slaughtered after 8 h of feed withdrawal in the experimental processing plant of INRAE (UE PEAT, Nouzilly, France). After evisceration, whole carcasses were stored at 4°C for 18 h until cut and deboned for meat characteristics analyses. Abdominal fat and pectoralis major and pectoralis minor (breast) muscles were weighed to calculate their yields in relation to BW. The ultimate pH (pHu) of the muscle was measured 24 h after postmortem using a portable pH meter (model 506, Crison Instruments SA, Alella, Barcelona, Spain) by inserting the electrode in the left pectoralis major muscle as described by Berri et al. (2001). The color measurements of the muscle defined by 3 components, lightness (L*), redness (a*), and yellowness (b*) values according to the CIE trichromatic (Girolami et al., 2013), were measured by using a Miniscan Spectrocolorimeter (HunterLab, Reston, VA), as described by Alnahhas et al., 2014, Alnahhas et al., 2015. The occurrence of meat quality defects (Kuttappan et al., 2012, Sihvo et al., 2014, Mudalal et al., 2015), WS, and WB was evaluated macroscopically in pectoralis major muscles on a scale of 3 classes according to Alnahhas et al. (2016). The intramuscular lipid content was calculated by near-infrared spectroscopy using a Nirflex N-500 spectrometer (Buchi, Rungis, France) from samples of the pectoralis major muscle collected at 40 d of age (Alnahhas et al., 2016). The lipid peroxidation value was determined by the quantification of thiobarbituric acid reactive substance, using the method developed by Lynch and Frei (1993). Drip loss was determined according to Berri et al. (2007). The cooking loss of the breast muscles was measured as previously described by Baéza et al. (2012) and Alnahhas et al. (2016). The average Warner-Bratzler shear force value (N/cm2) of the meat was determined for 3 samples of cooked breast muscle (1 × 1 × 3 cm) using an Instron universal testing instrument (Instron 5543, Instron S.A., Guyancourt, France), as described by Honikel (1998) and Alnahhas et al. (2016) to assess the tenderness of the meat.
Parameters Related to Chicken Welfare
At slaughter (day 40), hock burn and pododermatitis occurrences were recorded for all slaughtered animals. At the end of the experiment, the litter of each floor pen was weighed. After homogenization of the litter amount in the pen at 40 d of age, 1 kg of litter from each floor pen was sampled and analyzed for dry matter to evaluate the litter moisture depending on the incubation and postnatal treatments.
Statistical Analysis
Data were analyzed using the statistics software Statview (version 5.0, SAS Institute, Cary, NC). The hatchability results were analyzed by using a chi-square test. The effect of incubation treatment on BW and body temperature at hatching was determined by one-way ANOVA followed by the Student–Newman–Keuls test, whereas scores of chick quality were analyzed by a nonparametric Kruskal–Wallis test, because of the heterogeneity of the variance of the data set. The effects of incubation condition, rearing condition, and sex and their interactions on BW, FCR (n = 4 groups), carcass, and meat quality (breast meat yield, abdominal fat yield, shear force value, pHu, L*, a*, b*, cooking loss, drip loss, technological performance) and litter characteristics were analyzed by ANOVA followed by a Student–Newman–Keuls test. The frequencies of occurrence of pododermatitis, hock burn, WB, and WS were analyzed using the chi-square tests.
Results
Effects of Incubation and Postnatal Treatment on Hatchability, Chick Quality, and Performance
Hatchability of fertile eggs was not significantly affected by the cold incubation treatment, (P = 0.801) with hatchabilities higher than 95% in both groups in our experimental conditions (Table 1). However, at 480 h of incubation, that is, 24 h before opening the hatcher, 43% of the I0 chicks hatched, but only 36% of the I1 chicks hatched (P = 0.007). At hatching, neither the male chick percentage nor the quality of chicks from cold-incubated eggs of I1 chicks was different from that of control I0 chicks (P = 0.765 and 0.415, respectively; Table 1). Body temperature tended to be higher in I1 than in I0 chicks at hatching (P = 0.059), but BW (Table 1) was not influenced by the thermal incubation treatment (P = 0.681). There were significant interactions of incubation condition, postnatal rearing temperature, and sex on BW at 21 d (P = 0.008) and 40 d of age (P = 0.038; Table 2). At day 21, male BW were not different between the I0 and I1 groups at standard temperature T0, while male BW was higher for the I1 than for the I0 group under cold ambient temperature T1 (P < 0.05; Table 3). On the contrary, I1 females exhibited higher BW at day 21 than I0 ones at T0 (P < 0.05), whereas there were no difference observed between incubation conditions under cold ambient temperature T1. At slaughter age (day 40), BW of both males and females were greater under cold rearing temperature T1 than under standard conditions T0. In females (but not in males), under standard temperature T0, the I1 group exhibited higher slaughter BW than I0 ones (P < 0.05; Table 3). The postnatal cold temperature and the sex affected the FCR from day 0 to day 21 with higher FCR in females than in males and in chickens reared under lower ambient temperature T1 than at T0 (P < 0.001 and P < 0.001, respectively; Table 2). Between day 22 and day 40 (when ambient temperatures were maintained at 21°C for both groups T0 and T1), FCR of chickens incubated under control conditions I0 was lower in the T1 than in the T0 group, whereas it did not differ depending on the postnatal temperature in I1-incubated chickens (Table 3). During this period, females exhibited higher FCR than males (P < 0.02). On the whole period from day 0 to day 40 (Table 2), females exhibited higher FCR than males (P = 0.002), and T1 chickens presented lower FCR than T0 chickens (P = 0.007).
Table 1.
Hatchability of fertile eggs, sex ratio, quality score of chicks, and body temperature at hatch depending on incubation temperature.
| Traits2 | Incubation temperature1 |
P-value | |
|---|---|---|---|
| I0 | I1 | ||
| Rate of hatched chicks at 480 h of incubation, % of fertile eggs | 43 | 36 | 0.007 |
| Hatchability, % of fertile eggs | 95.4 | 95.7 | 0.801 |
| Male chick percentage, % | 52.8 | 51.9 | 0.765 |
| Total chick quality score3 (out of 100) | 93.3 ± 1.3 | 94.3 ± 0.5 | 0.415 |
| Body weight at hatch, g | 42.7 ± 0.1 | 42.6 ± 0.1 | 0.681 |
| Body temperature at hatch, °C | 38.8 ± 0.1 | 39.0 ± 0.1 | 0.059 |
Control incubation I0: eggs were incubated until hatch at 37.6°C and 56% RH; I1: eggs were incubated in the same conditions but exposed for 30 min to 15°C at day 18 and day 19 of incubation. Significant P values under 0.05 are highlighted in bold.
For each parameter, values are presented as mean ± standard error. Hatchability of fertile eggs was calculated for 581 I0 and 576 I1 fertile eggs. Body weight and body temperature were measured for 548 and 50 chicks per incubation treatment, respectively.
Chick quality was calculated according to Tona et al. (2003).
Table 2.
Chicken performance and meat quality parameters and yields depending on incubation and postnatal rearing temperatures and sex at slaughter age and measured on meat cut 24 h after postmortem.1
| Factors1 | Incubation temperature2 (I) |
P-value | Postnatal temperature3 (P) |
P-value | Sex (S) |
P-value |
P-values of interactions |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I0 | I1 | T0 | T1 | Male | Female | I × P | I × S | P × S | I × P × S | ||||
| Performance and litter parameters4 | |||||||||||||
| Day 21 BW (g) | 851 ± 4 | 863 ± 4 | 0.026 | 847 ± 4 | 868 ± 4 | <0.001 | 901 ± 4 | 822 ± 4 | <0.001 | 0.708 | 0.856 | 0.513 | 0.008 |
| Day 40 BW (g) | 2,500 ± 16 | 2,537 ± 16 | 0.034 | 2,462 ± 16 | 2,574 ± 16 | <0.001 | 2,740 ± 14 | 2,337 ± 11 | <0.001 | 0.547 | 0.542 | 0.896 | 0.038 |
| Day 0–day 21 FCR (g/g) | 1.38 ± 0.01 | 1.38 ± 0.01 | 0.504 | 1.36 ± 0.00β | 1.39 ± 0.00α | <0.001 | 1.37 ± 0.00b | 1.39 ± 0.01a | <0.001 | 0.940 | 0.076 | 0.050 | 0.490 |
| Day 22–day 40 FCR (g/g) | 1.82 ± 0.02 | 1.82 ± 0.01 | 0.864 | 1.85 ± 0.01 | 1.79 ± 0.01 | <0.001 | 1.80 ± 0.01b | 1.84 ± 0.01a | 0.001 | 0.042 | 0.173 | 0.653 | 0.231 |
| Day 0–day 40 FCR (g/g) | 1.65 ± 0.01 | 1.66 ± 0.01 | 0.678 | 1.66 ± 0.01α | 1.64 ± 0.01β | 0.008 | 1.64 ± 0.01b | 1.67 ± 0.01a | 0.002 | 0.065 | 0.075 | 0.979 | 0.412 |
| Litter moisture (%) | 39.0 ± 0.7 | 38.9 ± 0.8 | 0.926 | 37.9 ± 0.7 | 40.0 ± 0.8 | 0.047 | 38.9 ± 0.8 | 39.0 ± 0.8 | 0.897 | 0.403 | 0.429 | 0.037 | 0.570 |
| Meat yields and parameters of carcass quality5 | |||||||||||||
| Breast meat yield (%) | 20.7 ± 0.1 | 20.7 ± 0.1 | 0.838 | 20.5 ± 0.1β | 20.9 ± 0.1α | 0.033 | 20.2 ± 0.1b | 21.1 ± 0.1a | <0.001 | 0.978 | 0.960 | 0.547 | 0.609 |
| Thigh yield (%) | 22.1 ± 0.1 | 22.1 ± 0.1 | 0.911 | 22.0 ± 0.0 | 22.2 ± 0.0 | 0.213 | 22.5 ± 0.0b | 21.8 ± 0.0a | <0.001 | 0.240 | 0.413 | 0.945 | 0.902 |
| Abdominal fat (%) | 2.2 ± 0.0 | 2.2 ± 0.0 | 0.236 | 2.2 ± 0.0 | 2.2 ± 0.0 | 0.155 | 2.1 ± 0.0b | 2.3 ± 0.0a | <0.001 | 0.968 | 0.861 | 0.618 | 0.461 |
| Processing yield (%) | 82.3 ± 0.5 | 82.5 ± 0.5 | 0.855 | 82.1 ± 0.6 | 82.8 ± 0.5 | 0.281 | 80.6 ± 0.5b | 83.9 ± 0.5a | <0.001 | 0.989 | 0.311 | 0.978 | 0.765 |
| Ultimate pH | 5.84 ± 0.01 | 5.83 ± 0.01 | 0.882 | 5.84 ± 0.01 | 5.83 ± 0.01 | 0.609 | 5.80 ± 0.01b | 5.87 ± 0.01a | <0.001 | 0.147 | 0.696 | 0.289 | 0.192 |
| Yellowness, b* | 10.2 ± 0.1 | 10.2 ± 0.1 | 0.733 | 10.0 ± 0.1β | 10.4 ± 0.1α | 0.014 | 10.70 ± 0.11a | 9.77 ± 0.11b | <0.001 | 0.159 | 0.277 | 0.664 | 0.302 |
| Redness, a* | –0.38 ± 0.05 | –0.39 ± 0.05 | 0.808 | –0.50 ± 0.05 | –0.27 ± 0.05 | 0.003 | –0.32 ± 0.06 | –0.45 ± 0.05 | 0.092 | 0.038 | 0.525 | 0.629 | 0.165 |
| Lightness, L* | 50.9 ± 0.2 | 50.8 ± 0.3 | 0.825 | 50.8 ± 0.3 | 50.9 ± 0.2 | 0.944 | 51.2 ± 0.3a | 50.5 ± 0.2b | 0.026 | 0.313 | 0.756 | 0.674 | 0.319 |
| Fillet intramuscular lipids (%) | 1.17 ± 0.09 | 1.12 ± 0.08 | 0.298 | 1.11 ± 0.07 | 1.18 ± 0.10 | 0.576 | 1.42 ± 0.10 | 0.92 ± 0.04 | <0.001 | 0.853 | 0.018 | 0.548 | 0.167 |
Statistical effects of sex (S), postnatal temperature (P), and incubation temperature (I) and of their interactions were analyzed by ANOVA. a, b: different letters correspond to significant differences (P < 0.05) between sexes; α, β: different letters correspond to significant differences (P < 0.05) between postnatal temperatures. Significant P values under 0.05 are highlighted in bold.
Control incubation I0: eggs were incubated until hatch at 37.6°C and 56% RH; I1: eggs were incubated in the same conditions but exposed for 30 min at 15°C at day 18 and day 19 of incubation.
Control temperature T0 from 32 C at day 0 to 21°C at day 21 or cold temperature T1 from 29°C at day 0 to 21°C at day 21, then T0 and T1 were maintained at 21°C from day 22 to day 40.
Body weights (BW) were measured for 92–108 chickens per group at day 21 and for 76–88 chickens per group at day 40, and for feed conversion ratio (FCR) and litter moisture percentage, values are presented as mean ± standard error (n = 4 per group).
Parameters were measured for 29–35 chickens, except for processing yield measured for 11–13 chickens and for fillet intramuscular lipid % measured for 6–9 chickens.
Table 3.
Performance of chickens and meat quality parameters depending on incubation and postnatal rearing temperatures and sex when at least one interaction is significant between these factors.
| Sex (S) |
Males |
Females |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Postnatal temperature1 (P) |
T0 |
T1 |
T0 |
T1 |
Statistical effects3 |
||||||||||
| Incubation temperature2 (I) | I0 | I1 | I0 | I1 | I0 | I1 | I0 | I1 | I | P | S | I × P | I × S | P × S | I × P × S |
| Day 21 BW (g) | 892 ± 8b | 893 ± 9b | 896 ± 7b | 920 ± 7a | 797 ± 7d | 823 ± 7c | 835 ± 6c | 830 ± 6c | 0.026 | <0.001 | <0.001 | 0.708 | 0.856 | 0.513 | 0.008 |
| Day 40 BW (g) | 2,685 ± 31b | 2,686 ± 27b | 2,769 ± 24a | 2,818 ± 27a | 2,240 ± 18e | 2,330 ± 23d | 2,388 ± 19c | 2,390 ± 21c | 0.034 | <0.001 | <0.001 | 0.547 | 0.542 | 0.896 | 0.038 |
| Day 22–day 40 FCR (g/g) | 1.85 ± 0.03 | 1.80 ± 0.01 | 1.76 ± 0.02 | 1.78 ± 0.01 | 1.87 ± 0.01 | 1.88 ± 0.01 | 1.79 ± 0.02 | 1.82 ± 0.02 | 0.864 | <0.001 | 0.001 | 0.042 | 0.173 | 0.653 | 0.231 |
| Litter moisture (%) | 37.0 ± 1.1 | 36.4 ± 1.0 | 41.6 ± 1.3 | 40.5 ± 1.2 | 38.0 ± 1.1 | 40.1 ± 1.8 | 39.3 ± 1.6 | 38.6 ± 2.2 | 0.926 | 0.047 | 0.897 | 0.403 | 0.429 | 0.037 | 0.570 |
| Redness, a* | –0.45 ± 0.12 | –0.37 ± 0.12 | –0.22 ± 0.09 | –0.24 ± 0.13 | –0.66 ± 0.10 | –0.47 ± 0.10 | –0.16 ± 0.08 | –0.47 ± 0.08 | 0.808 | 0.003 | 0.092 | 0.038 | 0.525 | 0.629 | 0.165 |
| Fillet intramuscular lipids (%) | 1.63 ± 012 | 1.13 ± 0.09 | 1.59 ± 0.23 | 1.40 ± 0.27 | 0.80 ± 0.05 | 1.06 ± 0.10 | 0.92 ± 0.06 | 0.93 ± 0.12 | 0.298 | 0.576 | <0.001 | 0.853 | 0.018 | 0.548 | 0.167 |
For FCR and litter moisture, values are presented as mean ± standard error (n = 4 per group). a,b,c,d Different letters correspond to significant differences (P < 0.05) between treatments groups. Body weights were measured at 21 d and 40 d of age. Feed conversion ratio (FCR) was calculated between day 22 and day 40.
Abbreviation: FCR, feed conversion ratio.
Control temperature T0 from 32°C at day 0 to 21°C at day 21 or Cold temperature T1 from 29°C at day 0 to 21 C at day 21, then T0 and T1 were maintained at 21°C from day 22 to day 40. Significant P values under 0.05 are highlighted in bold.
Control incubation I0: eggs were incubated until hatch at 37.6°C and 56% RH; I1: eggs were incubated under the same conditions but exposed for 30 min at 15°C at day 18 and day 19 of incubation.
Statistical effects of sex (S), postnatal temperature (P), and incubation temperature (I) and of their interactions were analyzed by ANOVA.
Effects of Incubation, Postnatal Treatment, and Sex on Body Composition and Meat Quality Parameters
No effect of incubation conditions on breast meat yield and yellowness of the meat was observed (P = 0.838 and P = 0.733, respectively). The breast meat yields were higher under cold rearing temperature T1 (P = 0.033) than under standard temperature T0, and females showed higher percentage of fillet than males (P < 0.001; Table 2). A higher intensity of yellowness (b*) of the fillet was observed in males than in females (P < 0.001) and in chickens reared under cold temperature T1 the first 21 days than under T0 (P = 0.022; Table 3). Females exhibited lower thigh yield (P < 0.001) and lightness (L*; P = 0.026) but higher abdominal fat percentage (P < 0.001), pHu (P < 0.001), and processing yield (P < 0.001) than males (Table 2). The drip loss and the shear force of the cooked breast meat were not influenced by the incubation treatment, rearing conditions, or sex (P > 0.05, data not shown). There was a significant interaction of incubation treatment and sex on fillet intramuscular lipids of the breast (P = 0.018). The highest lipid content was observed in I0 male chickens, and the content was the lowest in both I0 and I1 female chickens (Table 3). A significant interaction of incubation treatment and postnatal condition was measured on the redness intensity (a*; P = 0.032), with a higher intensity of redness measured in the I0T1 group than in the I0T0 and I1T0 groups, the I1T1 group presenting the intermediate value (Table 3).
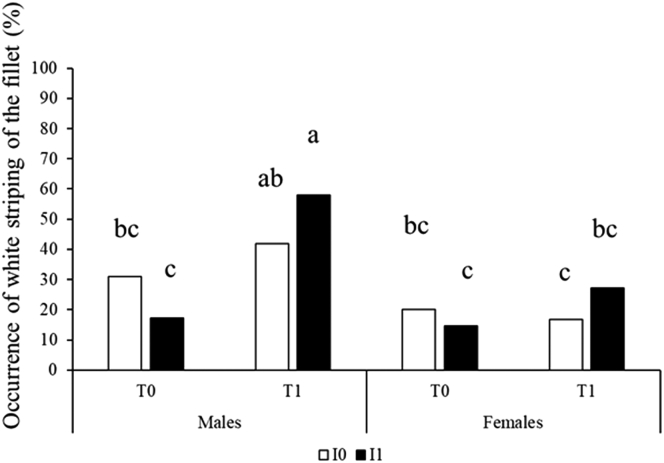
The observation of breast meat for the detection of meat defects showed no case of WB but a difference in the occurrence of WS between the experimental groups. Indeed, the occurrence of WS was higher in males from the cold-incubated and exposed I1T1 chickens (with 58% occurrence) than in all other groups except the I0T1 males (Table 3). The lowest occurrences, lower than 18%, were observed in I1T0 males and females and in I0T1 females.
Effects of Incubation and Postnatal Treatment on Chicken Welfare
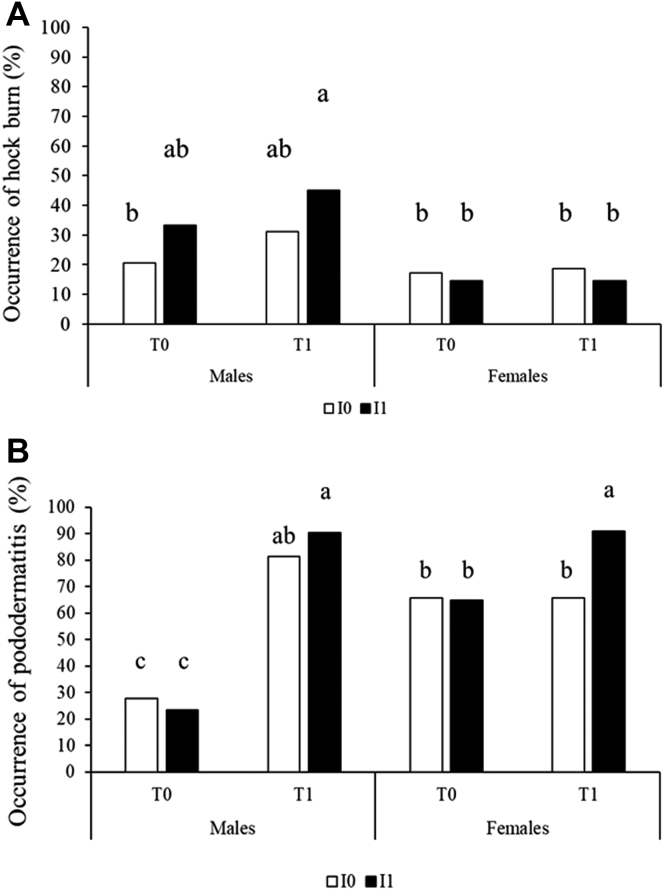
The occurrence of hock burns and pododermatitis was measured on carcasses at slaughter as parameters related to welfare troubles depending on incubating and rearing conditions and sex. Hock burns were more frequently observed in males exposed to cold temperature both during incubation and after hatching (I1T1 with 45% occurrence) than in all female and male groups incubated and reared under standard conditions (P < 0.05). The male chickens with only one cold exposure (I0T1 and I1T0 males) presented intermediate values (Figure 2). The occurrence of pododermatitis at slaughter was lower (less than 28%) in males under standard rearing conditions (in both incubation conditions) than in all other groups (Figure 3A), where occurrences were more than 64%. Higher occurrence of pododermatitis was observed in I1T1 males and females than in all other female groups (P < 0.05; Figure 3B).
Figure 2.
Effects of sex, incubation condition and postnatal rearing condition on the occurrence of white striping on the breast meat observed 24 h after slaughter at day 40. Control incubation I0 corresponds to egg incubation at 37.6°C, 56% RH. Cold incubation I1 corresponds to egg exposure for 30 min at 15°C at days 18 and 19 of incubation, eggs being incubated under the same conditions than for I0 the remaining time. Chickens were reared in floor pens either under control temperature T0 from 32°C at day 0 to 21°C at day 21 or under cold temperature T1 from 29°C at day 0 to 21°C at day 21. From day 22 to day 40, the ambient temperature in T0 and T1 was maintained at 21°C. Different letters (a, b, c) correspond to significant differences (P < 0.05) between groups.
Figure 3.
Effects of sex, incubation condition, and postnatal rearing condition on parameters related to welfare troubles observed on carcasses after slaughter at day 40. (A) Occurrence of hock burns. (B) Occurrence of pododermatitis. Control incubation I0 corresponds to egg incubation at 37.6°C, 56% RH. Cold incubation I1 corresponds to egg exposure for 30 min at 15°C at days 18 and 19 of incubation, eggs being incubated under the same conditions than for I0 the remaining time. Chickens were reared in floor pens either under control temperature T0 from 32°C at day 0 to 21°C at day 21 or under cold temperature T1 from 29°C at day 0 to 21°C at day 21. From day 22 to day 40, the ambient temperature in T0 and T1 was maintained at 21°C. Different letters (a, b, c) correspond to significant differences (P < 0.05) between groups.
Discussion
In an attempt for assessing the positive and negative impacts of the perinatal thermal environment of chicks, the main objective of this experiment was to study the effects of cold manipulations during incubation in male and female fast-growing chickens that were later exposed to standard or low postnatal temperature. The parameters considered consequently to these treatments relate to growth performance and feed efficiency, but also meat processing quality and defects, and animal welfare criteria. Previous experiments had permitted to compare the effects of 2 published methods of cold embryo manipulation (Shinder et al., 2011, Yalçin et al., 2012) combined to continuous or fluctuating 4°C colder or standard postnatal temperatures (Nyuiadzi et al., 2017) during the first 21 d of age in Ross 308 broilers. These studies, conducted in pens for the first two and in cages for the latter, allowed determining thermal conditions of incubation and postnatal rearing enhancing cold adaptation of chicks that had little to no impact on performance in the long term. The present experiment, realized in floor pens, confirms the positive effects of these treatments on performance parameters under normal temperature or posthatch cold temperature, however in a sex-dependent manner. However, the cumulative exposures to cold temperature both during incubation and after hatching had some detrimental effects on chicken welfare and meat quality parameters in the long term, especially in males.
Concerning hatching parameters, on the one hand, negative effect of incubation temperature I0 or I1 was observed neither on hatchability (more than 95% in both conditions) or on body temperature nor on BW of hatched chicks, as previously shown by Shinder et al. (2011) and Nyuiadzi et al. (2017). Nevertheless, cold treatment at the end of incubation slightly slowed down hatching process as demonstrated by the lower percentage of hatched chicks in the I1 than in the I0 group at 480 h of incubation. This result is not surprising because a faster hatching rate was observed after heat stimulation during embryogenesis (Piestun et al., 2008), with half percentage of hatched chicks at 480 h in heat-treated groups relative to controls, suggesting an impact of thermal treatments during incubation on hatching window. On the other hand, no significant difference was observed on chick quality between the I0 and I1 groups, contrary to what was recently observed in the study of Nyuiadzi et al. (2017), where the quality score related to the remaining membrane at the navel was lower in the I1 group than the one obtained with control incubation, probably due to a higher chick immaturity at hatching. Sex ratio was not affected by incubation treatment, whereas the male percentage at hatching was previously reported to be increased by embryonic warm stimulation during late incubation (Tzschentke and Halle, 2009).
As expected, both the sex and the postnatal temperature markedly affected growth and feed efficiency, with, at 21 d and at 40 d of age, a significant interaction between prenatal and postnatal thermal conditions and sex on the BW of the birds. At day 21, just at the end of the thermal treatments, the double cold exposure of the group I1T1 was beneficial for growth in males, whereas female growth was stimulated by in ovo or posthatch cold treatments or both. This effect persisted even after the treatment, as shown by the higher BW at slaughter age exhibited by males reared under cold conditions and all females that have experienced at least one exposition to cold temperature (in ovo and/or during rearing). The cold incubation treatment seemed particularly beneficial in terms of growth for males under cold postnatal temperature and in females under normal postnatal temperature. This may be the result of metabolic or endocrine differences between sexes in the early growth phases. In particular, there are sex-specific responses to hormonal factors or inhibitors and nutrients in the embryonic stage (Dainat et al., 1991, Kocamis et al., 1998, Bello et al., 2014) that may affect growth in the long term, supporting this hypothesis. Whatever the postnatal temperature applied, no detrimental effects of incubation condition on growth were observed. This result is consistent with the previous studies reporting positive effects of cold incubation treatments (Shinder et al., 2011) and of postnatal cold conditioning (Shahir et al., 2012) on BW at slaughter age in males subjected to ascites-inducing conditions. The positive impact of cold on BW was even clearer in the present study compared with the transient effect already observed with the same treatments applied in birds reared in cages until 21 d of age (Nyuiadzi et al., 2017). One hypothesis is that the birds exposed to cold temperature have consumed more food for sustaining lower posthatching temperatures, chickens regulating their energy intake depending on ambient temperature after hatching. However, this is expected to lower feed efficiency while increasing heat production. This was actually the case between day 0 and day 21 during the postnatal cold treatment, with higher FCR observed in T1 groups, consistent with our previous results (Nyuiadzi et al. 2017) and those of the study by Akşit et al. (2013) recorded from day 0 to day 42 in chickens issued from old flocks and postnatally exposed to cold temperatures. However, because FCR became lower from day 22 onward, especially in the I0 groups subjected to T1, broilers subjected to cold challenge during rearing were more efficient in the overall period than those reared under standard conditions, whatever the incubation treatment considered. This result supports the hypothesis that chickens subjected to slightly colder conditions during the first 3 wk of age can gain efficiency by retaining more energy and tissue when placed in standard thermal conditions afterward. This suggests that metabolic adaptation might have occurred after the cold treatments at young age, in the line of changes of the expression of metabolic enzymes previously reported by Loyau et al. (2014) in case of embryonic exposure to 1°C lower incubation temperatures.
One other remarkable outcome of the present study was the 4% higher BW observed in I1 than in I0 females reared under standard postnatal conditions, whereas BW of males remains unaffected to cold treatment during incubation. This suggests a sex-specific response of females to cold incubation temperature that would enhance their growth during the postnatal period. The underlying mechanisms explaining this sex difference remain to be elucidated.
Consistently with the higher BW measured at slaughter age, our results showed +0.4 percentage points in breast meat yield in the groups having experienced postnatal cold conditions. However, the increase in breast meat yields previously reported by Shinder et al. (2011) following the same cold incubation treatment was not observed in the present experiment. This discrepancy could be due to different environmental rearing conditions of T0 broilers in both experiments. The highest value of breast meat yield was obtained in female groups, consistent with the previous results (Collin et al., 2007). Breast meat of females also exhibited higher pHu, probably as a result of a lower muscle energy (glycogen) store (Le Bihan-Duval et al., 2008). This result is in accordance with the higher L* values observed in males than in females, this parameter having been reported to negatively correlate with pHu. Unexpected lower values of the fillet redness a* were observed in the I0T1 group than in other groups reared under standard postnatal conditions. The redness of the breast meat was previously associated with chicken behavior before slaughter and early rate of postmortem glycolysis in muscle, the most active chickens producing the most red meat (Berri et al., 2005, Chabault et al., 2012). Our study suggested a possible impact of postnatal cold on meat redness, probably in relation to a modification of the chicken metabolic rate (Collin et al., 2003) or of muscle fiber metabolic type switch toward more oxidative one, as already suggested by Ueda et al. (2004).
White striping is described as white striations parallel to muscle fibers that occur in both pectoral and thigh muscles (Kuttappan et al. 2013b) and correspond to inclusion of lipids between fibers, affecting many criteria including color, tenderness, cooked processing, and nutritional value of meat (Kuttappan et al. 2013a). In the recent study of Clark et al. (2017), broilers experiencing cyclical heat exposures during incubation were less prone to exhibit moderate to severe myopathic defects than the controls. In this case, the lower rate of myopathy was associated with lower breast meat yields. In the present study, cold stimulations during incubation (I1) did not affect the occurrence of WS under control postnatal temperature. In contrast, combining cold stimulation during incubation with cold postnatal experience (I1T1) resulted in higher scores of WS in males that were also characterized by the highest BW at day 40 compared with all T0 males and all female groups. This result points out the importance of the perinatal thermal environment on the later development of the muscle, at least in males. According to Kuttappan et al. (2012), a high growth rate of chickens over a short period leading to heavier broilers would trigger the appearance of noninfectious quality defects in broilers such as WS. This could explain the higher occurrence of WS in I1T1 males, but not the lower occurrence observed in other I1 birds. In ovo temperature alteration and rearing temperature of chicks may influence the embryonic muscle development with consequences on both metabolism and structure. Several authors have shown that embryonic heat stimulation during incubation modified adipogenic and growth factor expression, myogenesis, and cell proliferation (Halevy et al., 2006, Piestun et al., 2009, Piestun et al., 2011, Al-Musawi et al., 2012). We can hypothesize that the combined exposure to acute cold at the end of incubation (day 18 and day 19), resulting in a drop of eggshell temperature by 5°C to 11°C (data not shown) and to postnatal cold rearing temperature, may have enhanced the proliferation of adipogenic precursors and further lipid deposition in the breast muscle.
Unexpectedly, the higher WS scores observed in the I1T1 male group was not associated with higher values of intramuscular fat content (IMF). Indeed, it had been previously reported that chickens with moderate to severe WS scores highlighted higher IMF percentage than nonaffected chickens (Alnahhas et al., 2016). However, we showed low IMF percentage in breast muscles of females compared with males, in accordance with previous results of Zerehdaran et al. (2004). The authors related this difference to the lower BW or muscle fatty acid metabolism of females. Consistently with variations of IMF, in which the liposoluble pigments accumulate, meat of males appeared more yellow than that of females. The meat of broilers that experienced cold postnatal conditions T1 also appeared more yellow compared with the control T0. Altogether, these results highlighted the role of early-life thermal experience in determining breast muscle development and meat quality, especially in male broilers exhibiting a high growth rate.
Beyond the observed effects on the quality of the meat, our study indicates that cold challenge in ovo and/or after hatch negatively affects animal welfare evaluated through the occurrence of hock burns and pododermatitis at slaughter. The combined cold treatments I1T1 increased the occurrence of hock burns in males and of pododermatitis in both sexes, whereas under standard postnatal temperature, these welfare parameters were not affected by incubation temperature. It is likely that increase in hock burns was in part due to the increase in BW observed in I1T1 males at 42 d. Indeed, Baéza et al. (2012) demonstrated a negative impact of increased age and BW on these types of injuries in relation to the lower walking ability measured in broilers. However, the higher BW cannot explain by itself the higher occurrence of pododermatitis observed in both I1T1 males and females than in other groups, except I0T1. Variations in the occurrence of pododermatitis between groups may also be influenced by environmental rearing conditions such as litter moisture, which was significantly greater in T1 than in T0 male pens, and intermediate in all female pens (data not shown). Indeed, low litter moisture percentages, for example, in case of low crude protein content of the diet (Li et al., 2018), resulted in a lower rate of footpad dermatitis in broilers or in turkeys (Mayne et al., 2007, Tullo et al., 2017). It is therefore possible that the lower posthatching temperature and the higher animal density of female pens (22 vs. 19 for males to account for the BW differences between sexes from 22 d to 40 d of age) have increased litter moisture and hence pododermatitis occurrence in T1 males and all female groups. This might be explained by higher water consumption in less efficient birds in this group, which remains to be explored, or by the less drying of the litter due to the lower ambient temperature. These observations are also consistent with our previous study in which a higher occurrence of leg problems was reported in birds reared in cages at low posthatching temperature (28°C instead of 33°C at day 0, Nyuiadzi et al., 2017). It is also in accordance with the recent work of Steenfeldt et al. (2019), showing that cold temperature (15°C) during the second half of the growth period resulted in a colder and wetter litter, resulting in more footpad pododermatitis than standard temperature (21°C) in fast-growing chickens. Nevertheless, the present study pointed to potential limitations of short and acute cold exposures at the end of incubation when chickens were later reared under postnatal cold temperatures. These limitations could be relative to the timing, intensity, homogeneity, and duration of the treatment, possibly favoring the chicken muscle growth at the detriment of other health-related biological functions.
Based on these results, it appears that short cold stimulations at the end of incubation has positive effects on growth in females and no deleterious effects on feed efficiency, muscle WS, and pododermatitis under normal postnatal temperatures. Despite the positive effects of cold stimulations during embryonic incubation combined to low temperature at start on performance and feed efficiency in broiler chickens, the present study also revealed the limits of such a strategy to improve bird-adaptive capacities to the cold environment. Indeed, thermal manipulation during embryogenesis and the rearing period has altered some aspects of welfare and meat quality, with a recrudescence of hock burns, pododermatitis, and WS defects in males exposed to cold temperature both before and after hatching. Our results also confirmed that early management affects in the long term both animal welfare and product quality, 2 important components of the image and competitiveness of the poultry production systems. It is now necessary to understand the cellular, molecular, and pathological disturbances involved in the observed changes to propose early management strategies allowing efficient and sustainable production systems, limiting current meat defects in broilers and improving welfare.
Acknowledgments
D.N. is funded by West Africa Agricultural Productivity Program from Togo (WAAPP-Togo) and CERSA (Centre d’Excellence Régional sur les Sciences Aviaires, Lomé University) for realizing her PhD. This study was funded by INRAE (Department Animal Physiology and Livestock Systems, Eval_Adapt project) and carried out within UMT framework Integrative Biology Research and Development (BIRD) associating ITAVI and INRAE on applied research projects. The authors thank F. Mercerand, J. Delaveau, C. Rat, I. Grimaud-Jottreau, H. Rigoreau, and C. Le Bourhis from PEAT, INRAE, 37380, Nouzilly, France, for helpful animal care, S. Crochet, N. Couroussé, E. Cailleau-Audouin, T. Bordeau, S.-A. David, M. Couty, E. Baéza, and A. Jacques from BOA, INRAE, Université de Tours, 37380 Nouzilly, France, and C. Souchet from ITAVI, 37380 Nouzilly, France, for their skilled technical assistance. The authors are grateful to C. Leterrier (UMR PRC, INRAE, CNRS, IFCE, Université F. Rabelais de Tours, 37380 Nouzilly, France) for helpful discussions.
References
- Akşit M., Yalçin S., Siegel P.B., Yenisey Ç, Özdemir D., Özkan S. Broilers respond to cooler ambient temperatures after temperature acclimation during incubation and early postnatal age. J. Appl. Poult. Res. 2013;22:298–307. [Google Scholar]
- Alexandratos N., Bruinsma J. World agriculture towards 2030/2050. Land Use Policy. 2012;20:375. [Google Scholar]
- Al-Musawi S.L., Stickland N.C., Bayol S.A.M. In ovo temperature manipulation differentially influences limb musculoskeletal development in two lines of chick embryos selected for divergent growth rates. J. Exp. Biol. 2012;215:1594–1604. doi: 10.1242/jeb.068791. [DOI] [PubMed] [Google Scholar]
- Alnahhas N., Berri C., Boulay M., Baéza E., Jego Y., Baumard Y., Chabault M., Le Bihan-Duval E. Selecting broiler chickens for ultimate pH of breast muscle: analysis of divergent selection experiment and phenotypic consequences on meat quality, growth and body composition traits. J. Anim. Sci. 2014;92:3816–3824. doi: 10.2527/jas.2014-7597. [DOI] [PubMed] [Google Scholar]
- Alnahhas N., Le Bihan-Duval E., Baéza E., Chabault M., Chartrin P., Bordeau T., Cailleau-Audouin E., Meteau K., Berri C. Impact of divergent selection for ultimate pH of pectoralis major muscle on biochemical, histological, and sensorial attributes of broiler meat. J. Anim. Sci. 2015;93:4524–4531. doi: 10.2527/jas.2015-9100. [DOI] [PubMed] [Google Scholar]
- Alnahhas N., Berri C., Chabault M., Chartrin P., Boulay M., Bourin M.C., Le Bihan-Duval E. Genetic parameters of white striping in relation to body weight, carcass composition, and meat quality traits in two broiler lines divergently selected for the ultimate pH of the pectoralis major muscle. BMC Genet. 2016;17:1–9. doi: 10.1186/s12863-016-0369-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baéza E., Arnould C., Jlali M., Chartrin P., Gigaud V., Mercerand F., Durand C., Méteau K., le Bihan-Duval E., Berri C. Influence of increasing slaughter age of chickens on meat quality, welfare, and technical and economic results. J. Anim. Sci. 2012;90:2003–2013. doi: 10.2527/jas.2011-4192. [DOI] [PubMed] [Google Scholar]
- Bailey R.A., Watson K.A., Bilgili S.F., Avendano S. The genetic basis of pectoralis major myopathies in modern broiler chicken lines. Poult. Sci. 2015;94:2870–2879. doi: 10.3382/ps/pev304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello A., Hester P.Y., Gerard P.D., Zhai W., Peebles E.D. Effects of commercial in ovo injection of 25-hydroxycholecalciferol on bone development and mineralization in male and female broilers. Poult. Sci. 2014;93:2734–2739. doi: 10.3382/ps.2014-03981. [DOI] [PubMed] [Google Scholar]
- Berri C., Wacrenier N., Millet N., Le Bihan-Duval E. Effect of selection for improved body composition on muscle and meat characteristics of broilers from experimental and commercial lines. Poult Sci. 2001;80:833–838. doi: 10.1093/ps/80.7.833. [DOI] [PubMed] [Google Scholar]
- Berri C., Debut M., Santé-Lhoutellier V., Arnould C., Boutten B., Sellier N., Baéza E., Jehl N., Jégo Y., Duclos M.J., Le Bihan-Duval E. Variations in chicken breast meat quality: implications of struggle and muscle glycogen content at death. Br. Poult. Sci. 2005;46:572–579. doi: 10.1080/00071660500303099. [DOI] [PubMed] [Google Scholar]
- Berri C., Le Bihan-Duval E., Debut M., Santé-Lhoutellier V., Baéza E., Gigaud V., Jégo Y., Duclos M.J. Consequence of muscle hypertrophy on characteristics of Pectoralis major muscle and breast meat quality of broiler chickens. J. Anim. Sci. 2007;85:2005–2011. doi: 10.2527/jas.2006-398. [DOI] [PubMed] [Google Scholar]
- Bertin A., Calandreau L., Meurisse M., Georgelin M., Palme R., Lumineau S., Houdelier C., Darmaillacq A.-S., Dickel L., Colson V., Cornilleau F., Rat C., Delaveau J., Arnould C. Incubation temperature affects the expression of young precocial birds’ fear-related behaviours and neuroendocrine correlates. Sci. Rep. 2018;8:1857. doi: 10.1038/s41598-018-20319-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabault M., Baéza E., Gigaud V., Chartrin P., Chapuis H., Boulay M., Arnould C., D'Abbadie F., Berri C., Le Bihan-Duval E. Analysis of a slow-growing line reveals wide genetic variability of carcass and meat quality-related traits. BMC Genet. 2012;13:90. doi: 10.1186/1471-2156-13-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D.L., Walter K.G., Velleman S.G. Incubation temperature and time of hatch impact broiler muscle growth and morphology. Poult. Sci. 2017;96:4085–4095. doi: 10.3382/ps/pex202. [DOI] [PubMed] [Google Scholar]
- Collin A., Buyse J., van As P., Darras V.M., Malheiros R.D., Moraes V.M., Reyns G.E., Taouis M., Decuypere E. Cold-induced enhancement of avian uncoupling protein expression, heat production, and triiodothyronine concentrations in broiler chicks. Gen. Comp. Endocrinol. 2003;130:70–77. doi: 10.1016/s0016-6480(02)00571-3. [DOI] [PubMed] [Google Scholar]
- Collin A., Berri C., Tesseraud S., Rodón F.E., Skiba-Cassy S., Crochet S., Duclos M.J., Rideau N., Tona K., Buyse J., Bruggeman V., Decuypere E., Picard M., Yahav S. Effects of thermal manipulation during early and late embryogenesis on thermotolerance and breast muscle characteristics in broiler chickens. Poult. Sci. 2007;86:795–800. doi: 10.1093/ps/86.5.795. [DOI] [PubMed] [Google Scholar]
- Dainat J., Saleh L., Bressot C., Marger L., Bacou F., Vigneron P. Effects of thyroid state alterations in ovo on the plasma levels of thyroid hormones and on the populations of fibers in the plantaris muscle of male and female chickens. Reprod. Nutr. Dev. 1991;31:703–716. doi: 10.1051/rnd:19910610. [DOI] [PubMed] [Google Scholar]
- Druyan S., Ben-David A., Cahaner A. Development of ascites-resistant and ascites-susceptible broiler lines. Poult. Sci. 2007;86:811–822. doi: 10.1093/ps/86.5.811. [DOI] [PubMed] [Google Scholar]
- Girolami A., Napolitano F., Faraone D., Braghieri A. Measurement of meat color using a computer vision system. Meat Sci. 2013;93:111–118. doi: 10.1016/j.meatsci.2012.08.010. [DOI] [PubMed] [Google Scholar]
- Guilloteau L.A., Collin A., Koch A., Leterrier C. Spontaneous intake and long-term effects of essential oils after a negative postnatal experience in chicks. Front. Vet. Sci. 2019;6:72. doi: 10.3389/fvets.2019.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halevy O., Yahav S., Rozenboim I. Enhancement of meat production by environmental manipulations in embryo and young broilers. World's Poult. Sci. J. 2006;62:485–497. [Google Scholar]
- Havenstein G., Ferket P., Qureshi M. Growth, livability, and feed conversion of 1957 versus 2001 broilers when fed representative 1957 and 2001 broiler diets. Poult. Sci. 2003;82:1500–1508. doi: 10.1093/ps/82.10.1500. [DOI] [PubMed] [Google Scholar]
- Havenstein G., Ferket P., Qureshi M. Carcass composition and yield of 1957 versus 2001 broilers when fed representative 1957 and 2001 broiler diets. Poult. Sci. 2003;82:1509–1518. doi: 10.1093/ps/82.10.1509. [DOI] [PubMed] [Google Scholar]
- Honikel K.O. Reference methods for the assessment of physical characteristics of meat. Meat Sci. 1998;49:447–457. doi: 10.1016/s0309-1740(98)00034-5. [DOI] [PubMed] [Google Scholar]
- Julian R.J. Production and growth related disorders and other metabolic diseases of poultry - a review. Vet. J. 2005;169:350–369. doi: 10.1016/j.tvjl.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Kocamis H., Kirkpatrick-Keller D.C., Klandorf H., Killefer J. In ovo administration of recombinant human insulin-like growth factor-I alters postnatal growth and development of the broiler chicken. Poult. Sci. 1998;77:1913–1919. doi: 10.1093/ps/77.12.1913. [DOI] [PubMed] [Google Scholar]
- Kuttappan V.A., Brewer V.B., Apple J.K., Waldroup P.W., Owens C.M. Influence of growth rate on the occurrence of white striping in broiler breast fillets. Poult. Sci. 2012;91:2677–2685. doi: 10.3382/ps.2012-02259. [DOI] [PubMed] [Google Scholar]
- Kuttappan V.A., Brewer V.B., Mauromoustakos A., McKee S.R., Emmert J.L., Meullenet J.F., Owens C.M. Estimation of factors associated with the occurrence of white striping in broiler breast fillets. Poult. Sci. 2013;92:811–819. doi: 10.3382/ps.2012-02506. [DOI] [PubMed] [Google Scholar]
- Kuttappan V.A., Shivaprasad H.L., Shaw D.P., Valentine B.A., Hargis B.M., Clark F.D., McKee S.R., Owens C.M. Pathological changes associated with white striping in broiler breast muscles. Poult. Sci. 2013;92:331–338. doi: 10.3382/ps.2012-02646. [DOI] [PubMed] [Google Scholar]
- Kuttappan V.A., Hargis B.M., Owens C.M. White striping and woody breast myopathies in the modern poultry industry: a review. Poult. Sci. 2016;95:2724–2733. doi: 10.3382/ps/pew216. [DOI] [PubMed] [Google Scholar]
- Le Bihan-Duval E., Debut M., Berri C.M., Sellier N., Santé-Lhoutellier V., Jégo Y., Beaumont C. Chicken meat quality: genetic variability and relationship with growth and muscle characteristics. BMC Genet. 2008;9:53. doi: 10.1186/1471-2156-9-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Lesuisse J., Schallier S., Clímaco W., Wang Y., Bautil A., Everaert N., Buyse J. The effects of a reduced balanced protein diet on litter moisture, pododermatitis and feather condition of female broiler breeders over three generations. Animal. 2018;12:1493–1500. doi: 10.1017/S1751731117002786. [DOI] [PubMed] [Google Scholar]
- Loyau T., Collin A., Yenisey C., Crochet S., Siegel P.B., Akşit M., Yalçin S. Exposure of embryos to cyclically cold incubation temperatures durably affects energy metabolism and antioxidant pathways in broiler chickens. Poult. Sci. 2014;93:2078–2086. doi: 10.3382/ps.2014-03881. [DOI] [PubMed] [Google Scholar]
- Lynch S.M., Frei B. Mechanisms of copper- and iron-dependent oxidative modification of human low density lipoprotein. J. Lipid Res. 1993;34:1745–1753. [PubMed] [Google Scholar]
- Mayne R.K., Else R.W., Hocking P.M. High litter moisture alone is sufficient to cause footpad dermatitis in growing turkeys. Br. Poult. Sci. 2007;48:538–545. doi: 10.1080/00071660701573045. [DOI] [PubMed] [Google Scholar]
- Meloche K.J., Fancher B.I., Emmerson D.A., Bilgili S.F., Dozier W.A., 3rd Effects of quantitative nutrient allocation on myopathies of the Pectoralis major muscles in broiler chickens at 32, 43, and 50 days of age. Poult. Sci. 2018;97:1786–1793. doi: 10.3382/ps/pex453. [DOI] [PubMed] [Google Scholar]
- Minne B., Decuypere E. Effects of late prenatal temperatures on some thermoregulatory aspects in young chickens. Arch. Exp. Veterinarmed. 1984;38:374–383. [PubMed] [Google Scholar]
- Mudalal S., Lorenzi M., Soglia F., Cavani C., Petracci M. Implications of white striping and wooden breast abnormalities on quality traits of raw and marinated chicken meat. Animal. 2015;9:728–734. doi: 10.1017/S175173111400295X. [DOI] [PubMed] [Google Scholar]
- Mujahid A., Furuse M. Oxidative damage in different tissues of neonatal chicks exposed to low environmental temperature. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 2009;152:604–608. doi: 10.1016/j.cbpa.2009.01.011. [DOI] [PubMed] [Google Scholar]
- Nichelmann M., Tzschentke B. Ontogeny of thermoregulation in precocial birds. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 2002;131:751–763. doi: 10.1016/s1095-6433(02)00013-2. [DOI] [PubMed] [Google Scholar]
- Nyuiadzi D., Travel A., Méda B., Berri C., Guilloteau L.A., Coustham V., Wang Y., Tona J.K., Collin A. Effect of low incubation temperature and low ambient temperature until 21 days of age on performance and body temperature in fast-growing chickens. Poult. Sci. 2017;96:4261–4269. doi: 10.3382/ps/pex264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petracci M., Cavani C. Muscle growth and poultry meat quality issues. Nutrients. 2012;4:1–12. doi: 10.3390/nu4010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piestun Y., Shinder D., Ruzal M., Halevy O., Brake J., Yahav S. Thermal manipulations during broiler embryogenesis: effect on the acquisition of thermotolerance. Poult. Sci. 2008;87:1516–1525. doi: 10.3382/ps.2008-00030. [DOI] [PubMed] [Google Scholar]
- Piestun Y., Halevy O., Yahav S. Thermal manipulations of broiler embryos - the effect on thermoregulation and development during embryogenesis. Poult. Sci. 2009;88:2677–2688. doi: 10.3382/ps.2009-00231. [DOI] [PubMed] [Google Scholar]
- Piestun Y., Halevy O., Shinder D., Ruzal M., Druyan S., Yahav S. Thermal manipulations during broiler embryogenesis improves post-hatch performance under hot conditions. J. Therm. Biol. 2011;36:469–474. [Google Scholar]
- Renwick G.M., Washburn K.W. Adaptation of chickens to cool temperature brooding. Poult. Sci. 1982;61:1279–1289. doi: 10.3382/ps.0611279. [DOI] [PubMed] [Google Scholar]
- Reyns G.E., Venken K., Morreale de Escobar G., Kühn E.R., Darras V.M. Dynamics and regulation of intracellular thyroid hormone concentrations in embryonic chicken liver, kidney, brain, and blood. Gen. Comp. Endocrinol. 2003;134:80–87. doi: 10.1016/s0016-6480(03)00220-x. [DOI] [PubMed] [Google Scholar]
- Shahir M.H., Dilmagani S., Tzschentke B. Early-age cold conditioning of broilers: effects of timing and temperature. Br. Poult. Sci. 2012;53:538–544. doi: 10.1080/00071668.2012.719604. [DOI] [PubMed] [Google Scholar]
- Shinder D., Rusal M., Giloh M., Yahav S. Effect of repetitive acute cold exposures during the last phase of broiler embryogenesis on cold resistance through the life span 1. Poult. Sci. 2009;88:636–646. doi: 10.3382/ps.2008-00213. [DOI] [PubMed] [Google Scholar]
- Shinder D., Ruzal M., Giloh M., Druyan S., Piestun Y., Yahav S. Improvement of cold resistance and performance of broilers by acute cold exposure during late embryogenesis 1. Poult. Sci. 2011;90:633–641. doi: 10.3382/ps.2010-01089. [DOI] [PubMed] [Google Scholar]
- Sihvo H.K., Immonen K., Puolanne E. Myodegeneration with fibrosis and regeneration in the Pectoralis major muscle of broilers. Vet. Pathol. 2014;51:619–623. doi: 10.1177/0300985813497488. [DOI] [PubMed] [Google Scholar]
- Steenfeldt S., Sørensen P., Nielsen B.L. Effects of choice feeding and lower ambient temperature on feed intake, growth, foot health, and panting of fast- and slow-growing broiler strains. Poult. Sci. 2019;98:503–513. doi: 10.3382/ps/pey323. [DOI] [PubMed] [Google Scholar]
- Tona K., Bamelis F., De Ketelaere B., Bruggeman V., Moraes V.M.B., Buyse J., Onagbesan O., Decuypere E. Effects of egg storage time on spread of hatch, chick quality, and chick juvenile growth. Poult. Sci. 2003;82:736–741. doi: 10.1093/ps/82.5.736. [DOI] [PubMed] [Google Scholar]
- Tullo E., Fontana I., Peña Fernandez A., Vranken E., Norton T., Berckmans D., Guarino M. Association between environmental predisposing risk factors and leg disorders in broiler chickens. J. Anim. Sci. 2017;95:1512–1520. doi: 10.2527/jas.2016.1257. [DOI] [PubMed] [Google Scholar]
- Tzschentke B. Attainment of thermoregulation as affected by environmental factors. Poult. Sci. 2007;86:1025–1036. doi: 10.1093/ps/86.5.1025. [DOI] [PubMed] [Google Scholar]
- Tzschentke B., Halle I. Influence of temperature stimulation during the last 4 days of incubation on secondary sex ratio and later performance in male and female broiler chicks. Br. Poult. Sci. 2009;50:634–640. doi: 10.1080/00071660903186570. [DOI] [PubMed] [Google Scholar]
- Ueda M., Watanabe K., Sato K., Akiba Y., Toyomizu M. Possible role for avPGC-1alpha in the control of expression of fiber type, along with avUCP and avANT mRNAs in the skeletal muscles of cold-exposed chickens. FEBS Lett. 2004;579:11–17. doi: 10.1016/j.febslet.2004.11.039. [DOI] [PubMed] [Google Scholar]
- Van der Pol C.W., van Roovert-Reijrink I.A.M., Maatjens C.M., van den Brand H., Molenaar R. Effect of relative humidity during incubation at a set eggshell temperature and brooding temperature posthatch on embryonic mortality and chick quality. Poult. Sci. 2013;92:2145–2155. doi: 10.3382/ps.2013-03006. [DOI] [PubMed] [Google Scholar]
- Yalçin S., Molayo H.B., Baka M., Genin O., Pines M. Effect of temperature during the incubation period on tibial growth plate chondrocyte differentiation and the incidence of tibial dyschondroplasia. Poult. Sci. 2007;86:1772–1783. doi: 10.1093/ps/86.8.1772. [DOI] [PubMed] [Google Scholar]
- Yalçin S., Özkan S., Siegel P., Yenisey Ç., Akşit M. Manipulation of incubation temperatures to increase cold resistance of broilers: influence on embryo development, organ weights, hormones and body composition. J. Poult. Sci. 2012;49:133–139. [Google Scholar]
- Zerehdaran S., Vereijken A.L.J., van Arendonk J.A.M., van der Waaij E.H. Estimation of genetic parameters for fat deposition and carcass traits in broilers. Poult. Sci. 2004;83:521–525. doi: 10.1093/ps/83.4.521. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Bi M., Yao H., Fu J., Li S., Xu S. Effect of cold stress on expression of AMPKalpha-PPARalpha pathway and inflammation genes. Avian Dis. 2014;58:415–426. doi: 10.1637/10763-010814-Reg.1. [DOI] [PubMed] [Google Scholar]
- Zuidhof M.J., Schneider B.L., Carney V.L., Korver D.R., Robinson F.E. Growth, efficiency, and yield of commercial broilers from 1957, 1978, and 2005. Poult. Sci. 2014;93:2970–2982. doi: 10.3382/ps.2014-04291. [DOI] [PMC free article] [PubMed] [Google Scholar]