Abstract
Quercetin, the main component of flavonoids, has a wide range of biological actions. Quercetin can be made into a variety of additives for practice, because of the stable chemical structure and water-soluble derivatives. This study was intended to explore the effects of quercetin on immune function and its regulatory mechanism in Arbor Acre broiler to provide a practical basis for improving poultry immune function and figure out the optimum supplementation as functional feed additives. A total of 240 one-day-old healthy Arbor Acre broilers, similar in body weight, were randomly allotted to 4 treatments with 6 replicates, 10 broilers in each replicate and fed with diets containing quercetin at 0, 0.02, 0.04, and 0.06% for 6 wk. Blood and immune organs (spleen, thymus, and bursa) were collected from chickens at the end of the experiment. Growth performance, immune organs indexes, contents of serum immune molecules, splenic T lymphocyte proliferative responses, and expression of immune related genes were evaluated. The results showed that dietary quercetin had no significant effect (P > 0.05) on growth performance of broilers. Compared with control, 0.06% quercetin supplementation in diet significantly increased spleen index and thymus index (P < 0.05). It also increased the secretion of immune molecules including immunoglobulin A (IgA), interleukin-4 (IL-4) (P < 0.001), immunoglobulin M (IgM) (P = 0.007), complement component 4 (C4) (P = 0.001), and tumor necrosis factor-α (TNF-α) (P < 0.05). On the other hand, 0.02% quercetin supplementation significantly increased complement component 3 (C3) (P < 0.05). Additionally, both 0.04 and 0.06% quercetin supplementation significantly increased expression of TNF-α, TNF receptor associated factor-2 (TRAF-2), TNF receptor superfamily member 1B (TNFRSF1B), nuclear factor kappa-B p65 subunit (NF-κBp65), and interferon-γ (IFN-γ) mRNA (P < 0.05), and expression of NF-κB inhibitor-alpha (IκB-α) mRNA were significantly decreased (P < 0.05). Thus, quercetin improved immune function via NF-κB signaling pathway triggered by TNF-α.
Keywords: quercetin, broiler, immune organs, immune molecules, gene expression
INTRODUCTION
Poor disease resistance in commercial chicken production is the major problem which directly affects the economic benefits (Subah and Praveen, 2016; Sadiq and Mohammed, 2017; Zahoor et al., 2018; Broom and Kogut, 2019). However, the presence of various viruses, bacteria, parasites, microbial toxins, and nutrient deficiencies may lead to the emergence of immunosuppressive diseases. Immunosuppressive diseases may damage the immune organs and affect the activity of immune cells by interfering with the antigen present, inhibiting or blocking the formation of immune antibody (immunoglobulin), thus causing a reduction in the normal immune response (Sharma et al., 2000; Hoerr, 2010; Wang et al., 2014). Clinically common immunosuppressive diseases in chicken include infectious bursa disease, Marek's disease, and chicken infectious anemia (Umar et al., 2017). In these diseases, both humoral and cellular immune responses are threatened.
Quercetin is one of the most prominent dietary flavonoid which is ubiquitously present in foods including vegetables, fruit, tea, and wine as well as in countless food supplements, and it is claimed to exert beneficial health effects (Boots et al., 2008; Alfredo et al., 2019). Studies showed that quercetin has stable chemical structure and its derivative is water soluble. It can be absorbed in vivo and used for a variety of additives in production practice (Materska, 2008; Mishurov et al., 2016). The literatures showed that quercetin has many pharmacological activities, including antioxidation, anti-inflammation, antibacterial, scavenging free radicals and improving immune functions. Moreover, quercetin is nontoxic, noncarcinogenic, and harmless to the body (Bravo and Anacona, 2001; Comalada et al., 2005; Davis et al., 2008, 2009; Anand et al.,2016).
Previous studies have shown that dietary supplementation of quercetin improved the laying performance by modulating intestinal environment and liver superoxide dismutase content in laying hens (Liu et al., 2014). Particularly, animal model and human in vitro studies have shown that flavonoids alleviate inflammation by modulating the production of pro-inflammatory and anti-inflammatory molecules in cells of innate and adaptive immune system (e.g., macrophages and T cells) in response to stimuli that upregulate inflammatory processes (Kumazawa et al., 2006; Gonzalez-Gallego et al., 2010). The process of immunomodulation involves nuclear factor-kappa B (NF-κB) signaling pathway, which is activated by TNF-α (Kumazawa et al., 2006). The NF-κB family of eukaryotic transcription factors plays an important role in regulation of immune response, inflammation, and oncogenesis. Activated NF-κB is translocated to the nucleus, where it modulates the expression of immuno-related genes (Chen et al., 2001; Badr et al., 2009). However, the immune mechanism of dietary quercetin in broilers has not been fully elucidated yet. Therefore, we examined the effects of quercetin on growth performance, immune organs indexes, contents of serum immune molecules, and genes expression in immune organs to elucidate the immunomodulatory properties of dietary quercetin and mechanism of action in broilers.
MATERIALS AND METHODS
Experimental Design, Diets, and Management
Housing, management, and care of the birds confirmed to the guidelines of Animal Care and Use Committee of Northeast Agricultural University and Agricultural Animal in Agricultural Research and Teaching of Heilongjiang Province (HEI Animal Management Certificate No. 11928).
A total of 240 Arbor Acre (AA) broilers (1-day-old, healthy and similar in body weight) from a commercial company (Yinong Poultry Limited Company, Harbin, China) were purchased and randomly divided into 4 groups with 6 replicates per group and 10 broilers per replicate. The broilers were housed in wire cages (4-stacked cages, width: 52.6 cm, depth: 42.3 cm, height: 38.1 cm). A 16L: 8D lighting was provided during the 6-wk experimental period, and temperature was maintained at 32 to 34°C in the starting 3 D and decreased by 2 to 3°C per week to a final temperature of 24°C. Humidity of the experimental room varied from 60 to 65%. Experimental diets and water were available ad libitum. Chickens were inspected daily for any health-related problems.
Corn soybean meal-based diets were formulated to meet or exceed NRC (1994) nutrient recommendations. The composition of the basal diet is shown in Table 1, with 4 levels of quercetin in feed: 0, 0.02, 0.04, and 0.06%. Quercetin dihydrate powder with 97% purity was purchased from Sigma-Aldrich Company (St. Louis, MO) and mixed with basal diet and was offered in mash form (5 mm) after grinding.
Table 1.
Composition and nutrient level of basal diet (as-fed basis).
| Items | Content (1 to 3 wk) | Content (4 to 6 wk) |
|---|---|---|
| Ingredients (%) | ||
| Corn | 57.50 | 62.30 |
| Soybean meal | 34.50 | 30.00 |
| Vegetable oil | 3.00 | 3.00 |
| Fish meal | 1.00 | 1.00 |
| Methionine | 0.20 | 0.20 |
| Dicalcium phosphate | 1.62 | 1.70 |
| Limestone | 1.55 | 1.17 |
| Sodium chloride | 0.30 | 0.30 |
| Multivitamin premix1 | 0.03 | 0.03 |
| Mineral premix1 | 0.20 | 0.20 |
| Choline | 0.10 | 0.10 |
| Total | 100.00 | 100.00 |
| Nutrient levels2 | ||
| Metabolizable energy (ME), MJ/kg | 12.33 | 12.50 |
| CP (%) | 21.75 | 19.72 |
| Total lysine (%) | 1.18 | 1.04 |
| Methionine (%) | 0.91 | 0.86 |
| Ca (%) | 1.07 | 0.96 |
| Total P (%) | 0.70 | 0.68 |
| Available P (%) | 0.46 | 0.45 |
Provided per kilogram of diet: vitamin A, 15,00 IU; vitamin D3, 3,200 IU; vitamin E, 10 IU; vitamin K, 0.5 mg; vitamin B1, 1.8 mg; vitamin B2, 3.6 mg; vitamin B6, 3.5 mg; vitamin B12, 0.01 mg; biotin, 0.15 mg; folic acid, 0.55 mg; niacin, 30 mg; pantothenic acid, 10 mg; Cu (CuSO4·5H2O), 8 mg; I (KI), 0.35 mg; Fe (FeSO4·7H2O), 80 mg; Mn (MnSO4·H2O), 60 mg; Se (NaSeO3), 0.15 mg; Zn (ZnO), 40 mg.
Based on composition of ingredients provided by NRC (1994).
Growth Performance
At 42 D of age, broilers were weighed after feed deprivation for 12 h and growth performance in terms of final body weight (BW), total feed intake (FI), and feed to weight gain ratio (F: G) per replicate (cage) were recorded weekly, and performance was calculated during 6-wk experimental period.
Collection of Samples
At 42 D of age, after feed deprivation for 12 h, 2 broilers per replicate similar in body weight were randomly selected, weighed, and killed by rapid decapitation (n = 12 per group). Blood samples from jugular vein (10 mL) were obtained and kept in ice. Immune organs namely spleen, thymus, and bursa of fabricius were dissected, washed, rinsed in saline water for splenic T lymphocyte proliferative responses, and stored in sterile tubes at −80°C for RNA extraction.
Immune Organs Indexes
Immune organs including spleen, thymus, and bursa of fabricius obtained in sterile atmosphere were weighed to calculate the immune organ index using the formula: Immune organ index = (immune organ weight in g/body weight in g) × 100.
Blood Immune Parameters
Serum was obtained after centrifuging the blood at 4°C, 3,000 × g for 15 min and stored at −20°C until analysis of hormones. Contents of IgA, IgM, C3, C4, IL-4, and TNF-α were determined using ELISA assay kit following the instructions provided by the manufacturer's (Nanjing Jiancheng Bioengineering Institute, Nanjing, Jinagsu, P. R. China).
Cell Immune Parameter
The sterile spleen was placed in a culture dish with PBS for 5 min. Then, spleen was gently torn up in 10 mL of Roswell Park Memorial Institute (RPMI) 1640 medium. Splenocyte deposit was obtained by percolating with a 200-mesh sieve and centrifugation at 1,200 × g for 6 min. Afterwards, Tris-NH4 Cl was added 3 times into splenocyte deposit and vortex oscillation, incubated at 37°C for 5 min, and centrifuged at 1,200 × g for 10 min, and the supernatant was removed. Splenocyte suspension was acquired by resuspending with 2 mL of RPMI1640 medium, and the concentration was maintained at 5 × 106/mL.
Splenocyte suspension (100 µL) was added in 96-well cell culture plates, which was divided into 2 groups with 3 replicates (samples were run in triplicates). One group contains 100 µL of RPMI1640 medium, and another includes 100 µL of ConA (10 µg/mL). 96-well cell culture plates were cultured in CO2 incubators (37°C, 5% CO2, saturation humidity) for 24 h. Then, 10 µL of methyl thiazolyl tetrazolium (MTT) (5 mg/mL) was added to each well before 4 h of termination of the culture. Cell culture plates were centrifuged, and supernatant was removed. Then, 150 µL of dimethylsulphoxide (DMSO) was injected in cell culture plates in a sterile atmosphere at room temperature for 20 min using Tecan Sunrise (Infinite 200 PRO, Austria).
Expression of Immuno-Related Genes in Spleen
Gene expressions (TNF-α, TRAF-2, TNFRSF1B, NF-κB p65, IκB-α, and IFN-γ) were measured using real-time quantitative RT-PCR (7500 Real-Time PCR System, Singapore). The primer sequences for broilers (Table 2) were designed to span an intron to avoid genomic DNA contamination using Primer 5.0. Total RNA was isolated from spleen using Trizol (Invitrogen, San Diego, CA); the quantity and quality of the isolated RNA were determined as described by Wang et al. (2010). Total cDNA was synthesized following the manufacturer's instructions. Briefly, total 20 µL of reaction mixture containing 5 µL of total RNA, 4 µL of 5 × Prime Script Buffer, 1 µL of Prime Script RT Enzyme MixI, 1 µL of Oligo dT Prime (50 µmol/L), 1 µL of Random 6 mers (10 µMmoI/L), and 8 µL of RNase Free dH2 O (TaKaRa Biotechnology, Co., Ltd. Dalian, P. R. China) was incubated in the following conditions: reverse transcription at 37°C for 15 min, inactivation of reverse transcriptase at 85°C for 5 min, until temperature was decreased to 4°C. Successful cDNA synthesis was confirmed by amplifying the β-actin amplicon using PCR. cDNA was amplified using a 20 µL PCR reaction system containing 2 µL of cDNA, 10 µL of 2 × SYBR Green PCR Mix, 0.4 µL of 50 × ROX Reference DyeII (TaKaRa Biotechnology, Co., Ltd. Dalian, P. R. China), 0.8 µL of PCR Forward Primer, 0.8 µL of PCR Reverse Primer (Sangon Biological Engineering Technology & Service Co., Ltd. Shanghai, P. R. China), and 6 µL of ddH2 O. The PCR conditions were as follows: initial denaturation at 95°C for 30 s, followed by PCR reaction at 40 cycles of 95°C for 5 s and 60°C for 34 s, and melting curve analysis at 95°C for 15 s, 60°C for 2 s, and 95°C for 15 s. The PCR products were verified by electrophoresis on 1% agarose gel and DNA sequencing. Standard curves were generated using pooled cDNA from the assayed samples, and the comparative cycle threshold method (2−ΔΔCT) was used for quantifying mRNA levels (Livak and Schmittgen, 2001).
Table 2.
Sequences of primers for quantitative real-time PCR.
| Target gene1 | Primer sequences (5′→3′) | Gene Bank No. | Product size (bp) |
|---|---|---|---|
| β-Actin | F: CACCACAGCCGAGAGAGAAAT | L08165 | 135 |
| R: TGACCATCAGGGAGTTCATAGC | |||
| TNF-α | F: GAGCGTTGACTTGGCTGTC | NM_204267 | 129 |
| R: AAGCAACAACCAGCTATGCAC | |||
| TRAF-2 | F: CCAGAACACGAAAGCAAGTG | XM_015279623.1 | 112 |
| R: ATGAGGGAATGGCAGGAAG | |||
| TNFRSF1B | F: TGCCTACTCACAGCCAACTG | NM_204439.3 | 126 |
| R:AGATGCTGCTCCTCCTGTTC | |||
| NF-κBp65 | F: GTGTGAAGAAACGGGAACTG | NM_205129 | 125 |
| R: GGCACGGTTGTCATAGATGG | |||
| IκB-α | F:GGCAGATGTGAACAAGGTGA | NM_001001472.2 | 118 |
| R:TATCTGCAGGTCAGCTGTGG | |||
| IFN-γ | F:GCTCCCGATGAACGACTTGA | NM_205149 | 150 |
| R:TGTAAGATGCTGAAGAGTTCATTCG |
TNF-α = tumor necrosis factor alpha; TRAF-2 = TNF receptor associated factor-2; TNFRSF1B = TNF receptor superfamily member 1B; NF-κBp65 = nuclear factor kappa-B p65 subunit; IκB-α = NF-κB inhibitor-alpha; IFN-γ = interferon-γ.
Statistical Analysis
All the data were analyzed by 1-way analysis of variance using SPSS 22.0 statistical package for Windows (2010, SPSS Inc., Chicago, IL 60606-6307). Differences among treatments were examined using Duncan's multiple range test. Calculated ΔCt (corrected sample) = mean value of target gene-mean value of internal reference gene, ΔΔCt = ΔCt-mean value of control group. Differences with treatment means with a probability of P < 0.05 were accepted as statistically significant.
RESULTS
Growth Performance
There were no significant differences in ADG, ADFI, and F: G (P > 0.05) (Table 3).
Table 3.
Effect of quercetin on growth performance in Arbor Acre broilers.1
| Quercetin |
||||||
|---|---|---|---|---|---|---|
| Items2 | 0 | 0.02% | 0.04% | 0.06% | SEM | P-value |
| BW (kg/bird) | 3.62 | 3.25 | 3.66 | 3.57 | 0.092 | 0.163 |
| FI (kg/bird) | 7.99 | 6.32 | 7.31 | 7.61 | 0.333 | 0.385 |
| F:G (kg:kg) | 2.19 | 1.95 | 1.99 | 2.13 | 0.071 | 0.220 |
Data are means of 6 replicates per treatment.
BW = body weight; FI = feed intake; F: G = feed to weight gain ratio.
Immune Organ Indexes
Compared with control, spleen index and thymus index were significantly increased with increasing quercetin, and the best result was obtained at 0.06% quercetin supplementation (P < 0.05). However, bursa of fabricius index was not significantly affected by quercetin supplementation (P > 0.05) (Table 4).
Table 4.
Effect of quercetin on immune organs in Arbor Acre broilers.1
| Quercetin |
SEM | P-value | ||||
|---|---|---|---|---|---|---|
| Items | 0 | 0.02% | 0.04% | 0.06% | ||
| Spleen index (%)2 | 0.93b | 0.93b | 1.06a,b | 1.17a | .0184 | 0.008 |
| Thymus index (%)3 | 2.34b | 2.62a,b | 2.96a,b | 3.28a | .0755 | 0.016 |
| Bursa of fabricius index (%)4 | 1.14 | 0.98 | 1.27 | 1.41 | .0541 | 0.117 |
Data are means of 6 replicates per treatment.
Spleen index = spleen weight/broiler weight × 100.
Thymus index = thymus weight/broiler weight × 100.
Bursa of fabricius index = bursa of fabricius weight/broiler weight × 100.
Mean values within the same row sharing a common superscript letter differ significantly (P < 0.05).
Blood Immune Molecules
IgA and C4 contents were significantly increased in dose-dependent manner (P < 0.01). IgM and IL-4 contents were significantly increased by 0.06% quercetin supplementation, compared with control (P < 0.01). On the other hand, TNF-α content was significantly increased by 0.04 and 0.06% quercetin supplementation (P < 0.05). However, C3 content was increased by 0.02% quercetin supplementation (P > 0.05) (Table 5).
Table 5.
Effect of quercetin on immune molecules of blood in Arbor Acre broilers.1
| Quercetin |
||||||
|---|---|---|---|---|---|---|
| Items2 | 0 | 0.02% | 0.04% | 0.06% | SEM | P-value |
| IgA (mg/mL) | 4.84c | 5.35b,c | 5.72b | 6.67a | 0.1737 | <0.001 |
| IgM (mg/mL) | 3.24b | 3.03b | 3.46a,b | 3.87a | 0.0964 | 0.007 |
| C3 (ng/L) | 658.72b | 802.65a | 694.32b | 689.50b | 20.36 | 0.045 |
| C4 (ng/L) | 236.86c | 275.88b,c | 307.46a,b | 336.62a | 10.50 | 0.001 |
| IL-4 (ng/L) | 139.74b | 130.07b | 131.55b | 177.37a | 5.118 | <0.001 |
| TNF-α (ng/L) | 209.18b | 248.50a,b | 285.53a | 278.73a | 10.41 | 0.026 |
Data are means of 6 replicates per treatment.
IgA = immunoglobulin A; IgM = immunoglobulin M; C3 = complement 3; C4 = complement 4; IL-4 = interleukin-4; TNF-α = tumor necrosis factor alpha.
Mean values within the same row sharing a common superscript letter differ significantly (P < 0.05).
Splenic T Lmphocyte Proliferative Responses
Splenic T lymphocyte proliferative responses (SLPR) were significantly increased with increasing quercetin supplementation (P < 0.01). However, SLPR of 0.04% quercetin supplementation was significantly higher than those of quercetin with 0.02 and 0.06% quercetin supplementation (Figure 1).
Figure 1.
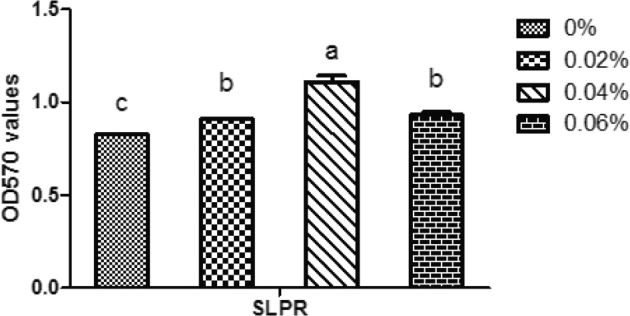
Effect of quercetin on splenic T lmphocyte proliferative responses (SLPR) in Arbor Acre broilers compared with those without quercetin. Values are means of OD570 using Tecan Sunrise. Data are presented as means ± SE (n = 12). Different superscript letters indicate significant differences among the means (P < 0.05).
Immuno-Related Genes in Spleen
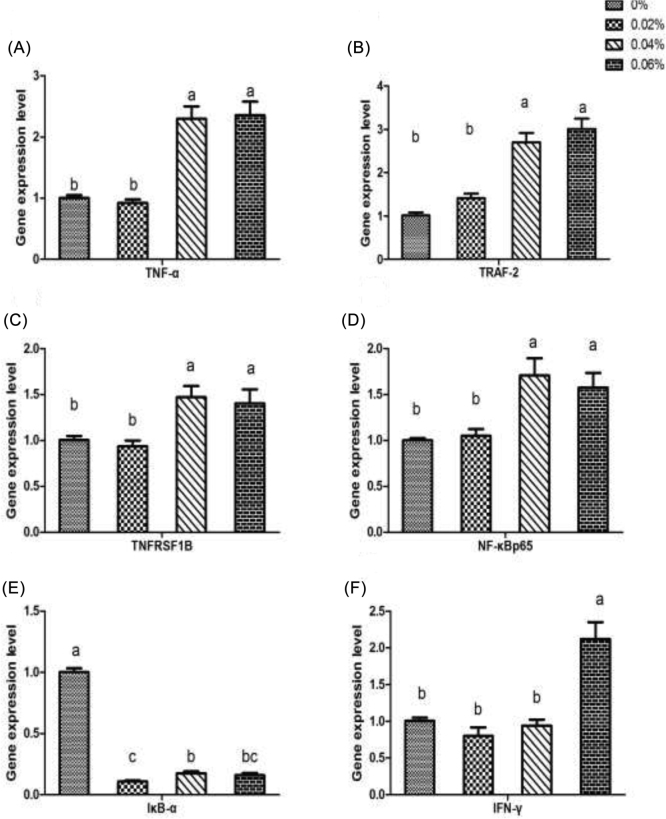
In this study, we focused on the expression of genes which are influenced by quercetin supplementation in spleen. In addition, the selected genes, based on their role in inflammation and their function in the signal pathway of NF-κB which is activated by TNF-α, are TNF-α, TRAF-2, TNFRSF1B, NF-κB p65, IκB-α, and IFN-γ.
Quercetin supplementation at 0.04 and 0.06% significantly increased the expression of TNF-α, TRAF-2, TNFRSF1B, and NF-κB p65 compared to control (P < 0.01, P < 0.05). On the contrary, we observed a significant decrease in IκB-α expression of quercetin supplementation compared to control (P < 0.01). Nevertheless, compared with control, we observed a significant increase in the expression of IFN-γ under the 0.06% quercetin supplementation (P < 0.01) (Figure 2).
Figure 2.
Effect of 3 different levels of quercetin on expression of immuno-related genes in spleen of Arbor Acre broilers compared with those without quercetin. Values are means of 6 replicates of 2 samples each. All data are presented as means ± SE. Different superscript letters indicate significant differences among the means (P < 0.05). TNF-α = tumor necrosis factor alpha; TRAF-2 = TNF receptor associated factor-2; TNFRSF1B = TNF receptor superfamily member 1B; NF-κBp65 = nuclear factor kappa-B p65 subunit; IκB-α = NF-κB inhibitor-alpha; IFN-γ = interferon-γ.
DISCUSSION
In this study, quercetin supplementation has no significant effect on growth performance in AA broilers. Several polyphenolic compounds and flavonoids including quercetin, silymarin, hesperidin, soybean isoflavone, alfalfa flavonoids, and thymol have been previously used as dietary supplements in broilers, but contradictory results were found. Quercetin is one of the major flavonoids in sea buckthorn leaves. Previous experiments in our lab showed that 0.5% sea buckthorn leaves significantly increased the average daily weight gain; however, F:G ratio was significantly decreased by 15.17% when compared with control. Therefore, 0.5% the sea buckthorn leaves is beneficial to the growth performance of AA broilers (Chen et al., 2011). Later studies indicated that the average daily intake of AA broiler was significantly decreased by 0.1% flavones of sea buckthorn leaves without affecting the growth performance (Zhao et al., 2012). Flavones of sea buckthorn fruits (FSBF) quadratically improved ADFI, ADG, and final BW (P = 0.002, P = 0.019, and P = 0.018, respectively). This result suggested that adding FSBF in to the diets improved the growth performance of broilers (Ma et al., 2015). According to the findings of Goliomytis et al. (2014), supplementation of quercetin at the levels of 0.5 and 1.0 g/kg feed had no significant effects on body weight and cumulative feed intake, but poor feed conversion ratios were obtained with increasing quercetin levels in broilers. Similar results were reported by Schiavone et al. (2007), who showed that silymarin supplementation at the dose rate of 40 and 80 ppm had no effects on growth performance of broilers. On the other hand, results of present study were not supported by the findings of Jiang et al. (2007), who reported that addition of synthetic soybean isoflavone from 10 to 80 mg/kg feed significantly increased weight gain and feed intake, while significantly decreased feed to gain ratio of chickens. Alfalfa flavonoids significantly improved average daily gain and feed to gain ratio of broiler chickens (Ouyang et al., 2016). Therefore, we speculated that the difference in results may be caused by doses and structure of various flavonoids (Espin et al., 2017).
In extensive broiler production system, chickens are continuously exposed to wide range of stresses, which decreases growth performance, disturbs/breaks immune homeostasis, and suppresses the expression of immune-related genes. Immune system is a network which is spread throughout the body and may automatically fight against foreign harmful substances. The structure of the immune system is complex, including immune organs, immune cells, and immune molecules (Work et al., 2015). Among the system, immune molecules play very important roles in regulating immune reactions. Immunoglobulins are globulins similar to antibodies that have antibody activity or chemical structure; the degree of humoral immunity is based on the immune globulin (Yasuma et al., 2016). In the primary response, IgM are first produced and are more abundant than IgG; IgA is the main Ig in external secretions (Zhang et al., 2015). In the present study, dietary quercetin enhanced humoral immune response in a dose-dependent manner. These results are in line with those of Liang et al. (2011), who reported that 10 mg/kg of flavonoids from Scutellaria baicalensis significantly increased content of IgA and IgG in broilers. Normally, the components of complement system are activated by antigen–antibody complexes to play an anti-inflammatory role in the presence of inactive precursors in plasma. Previous studies showed that total flavonoids of hippophase significantly increased the contents of serum C3 (Su et al., 2009). Our data showed that 0.02% quercetin supplementation significantly increased the contents of serum C3; on the contrary, the contents of serum C4 were significantly increased by 0.06% quercetin supplementation. In the immune responses, IL-4 plays a relevant role through a specific binding with interleukin-4 receptor, which is on the surface of target cells (LaPorte et al., 2008). The content of serum IL-4 in poultry is restricted by a series of conditions, such as breeding environment, heat stress, and inflammatory response. Previous experiments have shown that soybean isoflavones significantly increased the content of serum IL-4 and upregulated IL-4 expression by increasing DNA restriction activity of phosphatase-1 (Han et al., 2002). There are dual effects between IL-4 and TNF-α on resistance to systemic gram-negative infection (Giampietri et al., 2000). Similarly, our results showed that quercetin significantly increased the contents of serum IL-4 and TNF-α. Consequently, it revealed that quercetin significantly influenced the secretion of cytokines in AA broilers.
Reduced weight of the immune organs represents immunosuppression, while increase in weight of immune organs was a manifestation of the enhancement of immunity (Iftikhar et al., 2012). Nevertheless, quercetin did not significantly affect spleen index, thymus index, and bursa of fabricius index of Cobb chickens in former study (Hager-Theodorides et al., 2014). On the contrary, we found that 0.06% quercetin supplementation significantly increased the spleen and thymus index. The inconsistent result may be related to the dose and different breeds of broiler.
Lymphocytes respond to antigen challenge by proliferating and expanding the antigen-specific lymphocyte clones, and producing lymphokines, thus amplifying immune responses (Singh et al., 2016). Therefore, it is an important index to evaluate cellular immunity. In this study, quercetin significantly increased the splenic lymphocyte proliferative response. It suggested that quercetin can promote the proliferation of splenic lymphocytes, thereby improving cellular immune function.
NF-κB plays a fundamental role in the adaptive immune response and is involved in the regulation of embryonic development, lymphopoiesis, and osteogenesis (Karin and Benneriah, 2000; Li and Verma, 2002). The NF-κB pathway converges with the classical pathway at IκB kinase complex activation. Resulting cytokine expression may activate NF-κB in a positive feed forward loop (Hellweg, 2015). Previous studies of TNF-α signaling suggested that it may independently activate the NF-κB pathway and diverge early in the TNF-α signaling cascade (Antwerp et al., 1996). TNFR2 is the receptor of TNF-α, and the structure has the base sequence of TRAF; thereby, it may combine with TRAF to activate NF-κB pathway, induce gene transcription, and promote cell survival, proliferation, and differentiation (Depuydt et al., 2005). TNFRSF is a protein superfamily of cytokine receptors characterized by binding TNFs via an extracellular cysteine-rich domain (Locksley et al., 2001; Hehlgans and Pfeffer, 2005). With the exception of nerve growth factor, all TNFs are homologous to the archetypal TNF-α (Gravestein and Borst, 1998). In addition, most TNF receptors require specific adaptor proteins such as TRADD, TRAF, RIP, and FADD for downstream signaling. The transcription factor NF-κB is composed of 2 subgroups, including 5 proteins (p105, p50, p100, p52, and p65). In the absence of TNF stimulation, the NF-κB is associated with its inhibitory subbase I kappa B (IκB) in the cytoplasm (Albert and Baldwin, 2003). In the absence of IκB, the NF-κB signaling pathway was activated, anti-inflammatory cytokine (IFN-γ) was produced, and the expression of genes in upstream or downstream was increased. It has also been previously shown that the structure of the flavonoids affects NF-κB activation (Shin et al., 2011). Our study showed that quercetin significantly increased expressions of TNF-α, TRAF-2, TNFRSF1B, NF-κBp65, and IFN-γ. On the contrary, quercetin significantly decreased IκB-α expression. Considered together, our data indicated that decreased IκB-α resulting from NF-κB signaling pathway activated by quercetin, thus promoting the cytokine production and facilitating downstream signaling NF-κB translocation to nucleus.
This study demonstrated that quercetin regulated the immune response by increasing secretion of cytokines, and promoting development of immune organs and the formation of splenic lymphocytes. The result revealed that dietary supplementation of quercetin improved the immune function in AA broilers via activating the NF-κB signaling pathway.
ACKNOWLEDGMENTS
The authors thank the National Natural Science Foundation of China (31872377).
REFERENCES
- Albert S., Baldwin J. The NF-κB and IκB proteins: new discoveries and insights. Annu. Rev. Immunol. 2003;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- Alfredo F.Q., Laskibar I.M., Aguirre L., Zorita S.G., Gonzalez M., Portillo M.P. Effects of quercetin on mitochondriogenesis in skeletal muscle: consequences for physical endurance and glycemic control. Nutr. Skeletal Muscle. 2019;28:485–496. [Google Scholar]
- Anand D.A.V., Arulmoli R., Parasuraman S. Overviews of biological importance of quercetin: a bioactive flavonoid. Phcog. Rev. 2016;10:84–89. doi: 10.4103/0973-7847.194044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antwerp D.J.V., Martin S.J., Kafri T., Green D.R., Vermat I.M. Suppression of TNF-a-induced apoptosis by NF-KB. Science. 1996;274:787–789. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- Badr C., Niers J.M., Tjon-Kon-Fat L.A., Noske D.P., Wurdinger T., Tannous B.A. Real-time monitoring of NF-kappaB activity in cultured cells and in animal models. Mol. Imaging. 2009;8:278–290. [PMC free article] [PubMed] [Google Scholar]
- Boots A.W., Haenen G.R.M.M., Bast A. Health effects of quercetin: from antioxidant to nutraceutical. Eur. J. Pharmacol. 2008;585:325–337. doi: 10.1016/j.ejphar.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Bravo A., Anacona J.R. Metal complexes of the flavonoid quercetin: antibacterial properties. Transit. Metal Chem. 2001;26:20–23. [Google Scholar]
- Broom L.J., Kogut M.H. Deciphering desirable immune responses from disease models with resistant and susceptible chickens. Poult. Sci. 2019;98:1634–1642. doi: 10.3382/ps/pey535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F., Castranova V., Shi X. New insights into the role of nuclear factor-κB in cell growth regulation. Am. J. Pathol. 2001;159:387–397. doi: 10.1016/s0002-9440(10)61708-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Zhao W., Liu H.N., Su J., Wang P.Z., Zhang Z.H., Li Y. Effect of sea buckthorn leaves on growth performance and calcium metabolism in Arbor Acres broilers. J. Northeast Agric. Uni. 2011;42:19–24. [Google Scholar]
- Comalada M., Camuesco D., Sierra S., Ballester I., Xaus J., Galvez J., Zarzuelo A. In vivo quercetin anti-inflammatory effect involves release of quercetin, which inhibits inflammation through down-regulation of the NF-kappa B pathway. Eur. J. Immunol. 2005;35:584–592. doi: 10.1002/eji.200425778. [DOI] [PubMed] [Google Scholar]
- Davis J.M., Murphy E.A., McClellan J.L., Carmichael M.D., Gangemi J.D. Quercetin reduces susceptibility to influenza infection following stressful exercise. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;295:R505–R509. doi: 10.1152/ajpregu.90319.2008. [DOI] [PubMed] [Google Scholar]
- Davis J.M., Murphy E.A., Carmichael M.D., Davis B. Quercetin increases brain and muscle mitochondrial biogenesis and exercise tolerance. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009;296:R1071–R1077. doi: 10.1152/ajpregu.90925.2008. [DOI] [PubMed] [Google Scholar]
- Depuydt B., Van L.G., Vandenabeele P., Declercq W. Induction of apoptosis by TNF receptor 2 in a T-cell hybridoma is FADD dependent and blocked by caspase-8 inhibitors. J. Cell Sci. 2005;118:497–504. doi: 10.1242/jcs.01640. [DOI] [PubMed] [Google Scholar]
- Espin J.C., Gonzalez-Sarrias A., Tomas-Barberan F.A. The gut microbiota: a key factor in the therapeutic effects of (poly) phenols. Biochem. Pharmacol. 2017;139:82–93. doi: 10.1016/j.bcp.2017.04.033. [DOI] [PubMed] [Google Scholar]
- Giampietri A., Grohmann U., Vacca C., Fioretti M.C., Puccetti P., Campanile F. Dual effect of IL-4 on resistance to systemic gram-negative infection and production of TNF-alpha. Cytokine. 2000;12:417–421. doi: 10.1006/cyto.1999.0576. [DOI] [PubMed] [Google Scholar]
- Goliomytis M., Tsoureki D., Simitzis P.E., Charismiadou M.A., Hager-Theodorides A.L., Deligeorgis S.G. The effects of quercetin dietary supplementation on broiler growth performance, meat quality and oxidative stability. Poult. Sci. 2014;93:1957–1962. doi: 10.3382/ps.2013-03585. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Gallego J., Garcia-Mediavilla M.V., Sanchez-Campos S., Tunon M.J. Fruit polyphenols, immunity and inflammation. Brit. J. Nutr. 2010;3:15–27. doi: 10.1017/S0007114510003910. [DOI] [PubMed] [Google Scholar]
- Gravestein L.A., Borst J. Tumor necrosis factor receptor family members in the immune system. Semin. Immunol. 1998;10:423–434. doi: 10.1006/smim.1998.0144. [DOI] [PubMed] [Google Scholar]
- Hager-Theodorides A.L., Goliomytis M., Delis S., Deligeorgis S. Effects of dietary supplementation with quercetin on broiler immunological characteristics. Anim. Feed Sci. Tech. 2014;198:224–230. [Google Scholar]
- Han D., Denison M.S., Tachibana H., Yamada K. Effects of estrogenic compounds on immunoglobulin production by mouse splenocytes. Biol. Pharm. Bull. 2002;25:1263–1267. doi: 10.1248/bpb.25.1263. [DOI] [PubMed] [Google Scholar]
- Hehlgans T., Pfeffer K. The intriguing biology of the tumour necrosis factor/tumour necrosis factor receptor superfamily: players, rules and the games. Immunology. 2005;115:1–20. doi: 10.1111/j.1365-2567.2005.02143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellweg C.E. The nuclear factor κB pathway: a link to the immune system in the radiation response. Cancer Lett. 2015;368:275–289. doi: 10.1016/j.canlet.2015.02.019. [DOI] [PubMed] [Google Scholar]
- Hoerr F.J. Clinical aspects of immunosuppression in poultry. Avian Dis. 2010;54:2–15. doi: 10.1637/8909-043009-Review.1. [DOI] [PubMed] [Google Scholar]
- Iftikhar H., Mahmood M.S., Arshad M.I., Akhtar M., Mahmood F., Rafique A. Immune system dysfunction in broiler chickens experimentally inoculated with fowl adenovirus serotype-4 associated with inclusion body hepatitis hydropericardium syndrome. Turk. J. Vet. Anim. Sci. 2012;36:223–230. [Google Scholar]
- Jiang Z.Y., Jiang S.Q., Lin Y.C., Xi P.B., Yu D.Q., Wu T.X. Effects of soybean isoflavone on growth performance, meat quality and antioxidation in male broilers. Poult. Sci. 2007;86:356–1362. doi: 10.1093/ps/86.7.1356. [DOI] [PubMed] [Google Scholar]
- Karin M., Benneriah Y. Phosphorylation meets ubiquitination: the control of NF-κB activity. Annu. Rev. Immunol. 2003;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- Kumazawa Y., Kawaguchi K., Takimoto H. Immunomodulating effects of flavonoids on acute and chronic inflammatory responses caused by tumor necrosis factor alpha. Curr. Pharm. Design. 2006;12:4271–4279. doi: 10.2174/138161206778743565. [DOI] [PubMed] [Google Scholar]
- LaPorte S.L., Juo Z.S., Vaclavikova J., Colf L.A., Qi X., Heller N.M., Keegan A.D., Garcia K.C. Molecular and structural basis of cytokine receptor pleiotropy in the interleukin-4/13 system. Cell. 2008;132:259–272. doi: 10.1016/j.cell.2007.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Verma I.M. NF-kappaB regulation in the immune system. Nat. Rev. Immunol. 2002;2:725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- Liang Y., Ren C.C., Jiang N., Teng Z.C., Bi H.M., Jin D. Effect of flavonoids from scutellaria baicalensis on growth performance and immune function of broilers. Chin. J. Anim. Nutr. 2011;23:1409–1414. [Google Scholar]
- Liu H.N., Liu Y., Hu L.L., Suo Y.L., Zhang L., Jin F., Feng X.A., Teng N., Li Y. Effects of dietary supplementation of quercetin on performance, egg quality, cecal microflora populations, and antioxidant status in laying hens. Poult. Sci. 2014;93:347–353. doi: 10.3382/ps.2013-03225. [DOI] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Locksley R.M., Killeen N., Lenardo M.J. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104:487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- Ma J.S., Chang W.H., Liu G.H., Zhang S., Zheng A.J., Li Y., Xie Q., Liu Z.Y., Cai H.Y. Effects of flavones of sea buckthorn fruits on growth performance, carcass quality, fat deposition and lipometabolism for broilers. Poult. Sci. 2015;94:2641–2649. doi: 10.3382/ps/pev250. [DOI] [PubMed] [Google Scholar]
- Materska M. Quercetin and its derivstives: chemical structure and bioactivity-a review. Pol. J. Food Nutr. Sci. 2008;5:407–413. [Google Scholar]
- Mishurov D.A., Voronkin A.A., Roshal A.D. Synthesis, molecular structure and optical properties of glycidyl derivatives of quercetin. Struct. Chem. 2016;27:285–294. [Google Scholar]
- National Research Council (NRC) 9th rev. ed. Natl. Acad. Press; Washington, DC: 1994. Nutrient Requirements of Poultry. [Google Scholar]
- Ouyang K., Mingsheng X., Yan J., Wenjun W. Effects of alfalfa flavonoids on broiler performance, meat quality and gene expression. Can. J. Anim. Sci. 2016;96:332–341. [Google Scholar]
- Sadiq M.B., Mohammed B.R. The economic impact of some important viral diseases affecting the poultry industry in Abuja, Nigeria. Sokoto J. Vet. Sci. 2017;15:7–17. [Google Scholar]
- Schiavone A., Righi F., Quarantelli S., Bruni R., Serventi P., Fusari A. Use of Silybum marianum fruit extract in broiler chicken nutrition: Influence on performance and meat quality. J. Anim. Physiol. Anim. Nutr. 2007;91:256–262. doi: 10.1111/j.1439-0396.2007.00701.x. [DOI] [PubMed] [Google Scholar]
- Sharma J.M., Kim I.J., Rautenschlein S., Yeh H.Y. Infectious bursal disease virus of chickens: pathogenesis and immunosuppression. Dev. Comp. Immunol. 2000;24:223–235. doi: 10.1016/s0145-305x(99)00074-9. [DOI] [PubMed] [Google Scholar]
- Shin S.Y., Woo Y., Hyun J., Yong Y., Koh D., Lee Y.H., Lim Y. Relationship between the structures of flavonoids and their NF-κB-dependent transcriptional activities. Bioorg. Med. Chem. Lett. 2011;21:6036–6041. doi: 10.1016/j.bmcl.2011.08.077. [DOI] [PubMed] [Google Scholar]
- Singh V.K., Dwivedi P., Chaudhary B.R., Singh R. Gymnemic acid stimulates in vitro splenic lymphocyte proliferation. Phytother. Res. 2016;30:341–344. doi: 10.1002/ptr.5514. [DOI] [PubMed] [Google Scholar]
- Su J., Li Y., Chen X., Wang P.Z., Zhang W., Zhang Z.H. Effects of total flavonoids of hippophase on hormones and immunological function of AA broilers suffered heat stress. Feed Industry. 2009;30:22–25. [Google Scholar]
- Subah G., Praveen P.K. Economically important poultry diseases of worldwide concern: a brief review. Int. J. Phar. Biomed. Res. 2016;3:1–3. [Google Scholar]
- Umar S., Munir M.T., Ahsan U., Raza I., Chowdhury M.R., Ahmed Z., Shah M.A.A. Immunosuppressive interactions of viral diseases in poultry. Worlds Poult. Sci. J. 2017;73:121–135. [Google Scholar]
- Wang S., Teng Q., Jia L., Sun X., Wu Y., Zhou J. Infectious bursal disease virus influences the transcription of chicken γc and γc family cytokines during Infection. Plos One. 2014;9 doi: 10.1371/journal.pone.0084503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D.F., Zhang N.Y., Peng Y.Z., Qi D.S. Interaction of zearalenone and soybean isoflavone on the development of reproductive organs, reproductive hormones and estrogen receptor expression in prepubertal gilts. Anim. Reprod. Sci. 2010;122:317–323. doi: 10.1016/j.anireprosci.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Work K.A., Gibbs M.A., Friedman E.J. The immune system game. Am. Biol. Teach. 2015;77:382–390. [Google Scholar]
- Yasuma R., Cicatiello V., Mizutani T., Tudisco L., Kim Y. Intravenous immune globulin suppresses angiogenesis in mice and humans. Signal Transduct. Target Ther. 2016;1:15002–15009. doi: 10.1038/sigtrans.2015.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahoor I., Ghayas A., Bashir A. Genetics and genomics of susceptibility and immune response to necrotic enteritis in chicken: a review. Mol. Biol. Rep. 2018;45:31–37. doi: 10.1007/s11033-017-4138-8. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Eicher S.D., Applegate T.J. Development of intestinal mucin 2, IgA, and polymeric Ig receptor expressions in broiler chickens and Pekin ducks. Poult. Sci. 2015;94:172–180. doi: 10.3382/ps/peu064. [DOI] [PubMed] [Google Scholar]
- Zhao W., Chen X., Liu H.N., Ouyang W.W., Wang M.H., Li Y. Effects of flavones from sea buckthorn leaves on growth performance and carcass quality of broilers. Chin. J. Anim. Nutr. 2012;24:117–123. [Google Scholar]