Abstract
A total of 294 male, off-sex Ross 308 chickens were offered 7 dietary treatments with crude protein (CP) contents of 210, 195, 180, and 165 g/kg. One of the four 165 g/kg diet was consistent with the higher protein diets and 3 were modified to investigate the effects of increased methionine levels, pre-pellet inclusion of whole maize, and whey protein concentrate in reduced-CP broiler diets. There were 7 replicate cages, 6 birds per cage, from 14 to 35 D post-hatch. The average feed conversion ratio (FCR) of birds offered 210, 195, 180 g/kg CP diets was 1.555 which was superior (P < 0.05) to the 1.608 FCR of their 165 g/kg counterparts. The transition from 210 to 165 g/kg (diet 4) CP diets linearly increased (P < 0.001) relative fat-pad weights from 8.64 to 14.62 g/kg. The same transition linearly increased jejunal and ileal starch digestibility coefficients (P < 0.001), metabolizable to gross energy ratios (ME:GE) ratios (P < 0.001) and nitrogen (N)-corrected apparent metabolizable energy (AMEn) (P = 0.001) but did not influence N retention. Starch:protein disappearance rate ratios increased linearly (P < 0.001) from 2.68 to 3.82 in the jejunum and from 1.76 to 2.94 in the ileum following dietary CP reductions. Ileal disappearance rate ratios were quadratically related to FCR (r = 0.486; P < 0.005) and linearly related to relative fat-pad weights (r = 0.663; P < 0.001) where both parameters were disadvantaged by widening ratios. The transition from 210 to 165 g/kg crude protein diets linearly increased the average digestibility coefficient of 17 amino acids from 0.459 to 0.594 in jejunum and from 0.744 to 0.790 in the ileum. The present study demonstrates that dietary CP can be reduced from 210 to 180 g/kg without negatively influencing broiler performance but the further reduction to 165 g/kg compromised FCR. However, the three modifications to the 165 g/kg CP diet failed to enhance broiler performance.
Key words: amino acids, broiler chickens, reduced-crude protein, starch-protein digestive dynamics
INTRODUCTION
Research into reducing CP contents of diets for broiler chickens has intensified. One prime reason for developing reduced-CP broiler diets is to lessen nitrogen (N) excretion and environmental pollution. Moreover, reductions in N excretion decrease the incidence of footpad dermatitis and enhance bird welfare (van Harn et al., 2017).
It is well documented that reduced CP broiler diets may cause negative effects on growth performance with associated increases in fat deposition (Edmonds et al., 1985; Fancher and Jensen, 1989a,b; Leeson et al., 1996; Corzo et al., 2005; Aftab et al., 2006; Siegert et al., 2016). Fancher and Jensen (1989a) offered male broiler chickens a 215 g/kg CP control diet which was compared with four 160 g/kg CP low-protein diets supplemented with different schedules of amino acids (AA) and potassium. This tangible CP reduction of 55 g/kg compromised FCR by 12.1% (2.084 vs. 1.859) and increased relative fat-pad weights by 39.6% (21.5 vs. 15.4 g/kg). Thus, inferior feed conversion efficiency and increased fat deposition epitomize the challenge of developing reduced-CP diets. Nevertheless, some researchers have shown that conservative reductions in dietary CP, accompanied by judicious inclusions of unbound AA, do not compromise growth performance. For example, van Harn et al. (2017) found that moderate reductions in dietary CP of 22 and 23 g/kg in grower and finisher broilers, respectively, did not influence weight gain in Ross 308 male broilers and FCR was significantly more efficient at 35 D post- hatch.
The objective of this study was to determine the effects of graded reductions in dietary CP from 210 to 165 g/kg with additions of notionally “essential” AA on growth performance, nutrient utilization, starch–protein digestive dynamics, apparent jejunal and ileal AA digestibility coefficients, and free AAs concentration in systemic plasma. In addition, 3 strategies were applied to the 165 g/kg diet for evaluation. Firstly, methionine and the methionine:cysteine ratio were increased given that Fatufe and Rodehutscord (2005) found the magnitude of responses to additional methionine was more pronounced in low protein (183 g/kg) than high protein (229 g/kg) broiler diets. Reduced CP diets may contain less fiber in comparison to conventional diets; therefore, the second strategy was the pre-pellet inclusion of whole maize grain to enhance gizzard functionality (Liu et al., 2015). The third strategy was to replace soybean meal with a more rapidly digested protein source in whey protein concentrate. Rapidly digestible protein may enhance FCR (Truong et al., 2017) and the digestive dynamics of a rapid protein source are more closely aligned with those of unbound AA (Liu and Selle, 2017). The hypotheses were that reductions in dietary CP would compromise growth performance but the three facilitative strategies might retrieve these losses in performance.
MATERIALS AND METHODS
Experimental Design
A total of 294 day-old, male chicks from Ross 308 breeding stock (known as “off-sex” males) were procured from a commercial hatchery and were initially offered a conventional maize–soy starter diet with 12.55 MJ/kg and 230 g/kg CP. At 14 D post-hatch, birds were individually identified (wing-tags) and allocated into bioassay cages on the basis of body-weights so that mean weights and their standard variations across all cages were nearly identical. The 7 maize–soyabean meal dietary treatments was offered to 7 replicate cages (6 birds per cage) from 14 to 35 D post-hatch. The CP of the first 4 treatments (diets 1 to 4) were decreased in a step-wise manner from 210 to 165 g/kg with increasing inclusions of unbound AA as shown in Table 1. The final 3 treatments (diets 5 to 7) constituted modifications to diet 4 to evaluate the previously described facilitative strategies.
Table 1.
Description of dietary treatments (maize–soybean meal diets, all formulated to 13.10 MJ/kg AME and 10.90 g/kg digestible lysine).
| Analysed dietary concentrations |
|||||
|---|---|---|---|---|---|
| Dietary treatment | Crude protein (g/kg) | Unbound amino acids (g/kg) | Starch (g/kg) | Crude protein (g/kg) | Starch:Protein ratio |
| 1a | 210 | 4.5 | 312 | 215 | 1.45 |
| 2 | 195 | 7.9 | 327 | 199 | 1.64 |
| 3 | 180 | 14.0 | 362 | 186 | 1.95 |
| 4 | 165 | 21.5 | 395 | 172 | 2.30 |
| 5b | 165 | 23.5 | 386 | 171 | 2.26 |
| 6c | 165 | 21.5 | 404 | 166 | 2.43 |
| 7d | 165 | 15.1 | 443 | 166 | 2.67 |
Diets 1 to 4: Reduced-CP titration assay, soybean meal decreased from 355 to 195 g/kg, maize increased from 543 to 714 g/kg.
Increased methionine (6.29 vs. 4.58 g/kg).
250 g/kg whole maize included pre-pelleting.
124 g/kg whey protein concentrate, nil soybean meal.
Bird Management
This study fully complied with specific guidelines (Project number 2016/973) approved by Research Integrity and Ethics Administration of The University of Sydney. Broilers had unlimited access to water and feed under a “23-hour-on-1-hour-off” lighting regime for the first 3 D and then under a “16-hour-on-8-hour-off” lighting regime for the remainder of the study. Room temperature was maintained at 32°C for the first week, then gradually decreased to 22 ± 1°C by the end of the third week and maintained at the same temperature until the end of the feeding study.
Diet Preparation
Soybean meal and maize were characterized by near-infrared spectroscopy (AMINONIR and AMINORED, Evonik Nutrition and Care GmgH, Hanau, Germany). Dietary treatments were formulated, as shown in Table 2, on this basis. Maize was coarsely ground (6.0 mm hammer-mill screen) prior to incorporation into the complete diets, except when 250 g/kg whole maize was incorporated into diet 6 prior to pelleting. Diets were steam-pelleted at 80°C using a Palmer PP330 pellet press (Palmer Milling Engineering, Griffith, NSW, Australia) with a conditioner residence time of 14 s. Acid insoluble ash (AIA) (Celite, Celite Corporation. Lompoc, CA) was included in diets at 15 g/kg as a dietary marker to determine starch, protein (N) and AA digestibility coefficients.
Table 2.
Composition of the experimental diets.
| Feed ingredient (g/kg) | Diet 1 | Diet 2 | Diet 3 | Diet 4 | Diet 5 | Diet 6 | Diet 7 |
|---|---|---|---|---|---|---|---|
| Maize ground | 543 | 594 | 654 | 714 | 716 | 464 | 804 |
| Maize whole | 0 | 0 | 0 | 0 | 0 | 250 | 0 |
| Soybean meal | 355 | 309 | 253 | 195 | 192 | 195 | 0 |
| Whey protein concentrate | 0 | 0 | 0 | 0 | 0 | 0 | 124 |
| Soy oil | 47.2 | 38.0 | 26.8 | 14.9 | 14.1 | 14.9 | 0 |
| l- lysine HCl | 0.869 | 2.238 | 3.895 | 5.629 | 5.731 | 5.629 | 2.215 |
| d,l-methionine | 1.842 | 2.043 | 2.288 | 2.545 | 4.301 | 2.545 | 1.700 |
| l- cysteine | 0.902 | 1.090 | 1.309 | 1.560 | 1.369 | 1.560 | 0.544 |
| l- threonine | 0.931 | 1.552 | 2.305 | 3.094 | 3.142 | 3.094 | 0.941 |
| l- tryptophan | 0 | 0 | 0.138 | 0.442 | 0.460 | 0.442 | 0.010 |
| l- valine | 0.144 | 0.919 | 1.859 | 2.845 | 2.905 | 2.845 | 1.630 |
| l- arginine | 0 | 0.196 | 1.793 | 3.465 | 3.563 | 3.465 | 6.858 |
| l- isoleucine | 0 | 0.384 | 1.344 | 2.330 | 2.390 | 2.330 | 0.991 |
| l- leucine | 0 | 0 | 0 | 0.590 | 0.653 | 0.590 | 0 |
| l- histidine | 0 | 0 | 0 | 0.259 | 0.293 | 0.259 | 0.655 |
| Sodium chloride | 2.37 | 1.93 | 1.35 | 0.70 | 0.67 | 0.70 | 1.82 |
| Sodium bicarbonate | 2.24 | 2.91 | 3.78 | 4.76 | 4.81 | 4.76 | 3.33 |
| Limestone | 5.63 | 5.61 | 5.57 | 5.54 | 5.54 | 5.54 | 5.35 |
| Dicalcium phosphate | 21.72 | 22.32 | 23.06 | 23.83 | 23.88 | 23.83 | 26.88 |
| Choline chloride | 0.80 | 0.80 | 0.99 | 1.35 | 1.38 | 1.35 | 1.88 |
| Vitamin-mineral premix | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
| Celite | 15.0 | 15.0 | 15.0 | 15.0 | 15.0 | 15.0 | 15.0 |
1The vitamin–mineral premix supplied per tonne of feed: [MIU] retinol 12, cholecalciferol 5, [g] tocopherol 50, menadione 3, thiamine 3, riboflavin 9, pyridoxine 5, cobalamin 0.025, niacin 50, pantothenate 18, folate 2, biotin 0.2, copper 20, iron 40, manganese 110, cobalt 0.25, iodine 1, molybdenum 2, zinc 90, and selenium 0.3.
The nutrient specifications of the experimental diets are shown in Table 3. Standardized ileal digestible lysine concentrations remained constant at 10.9 g/kg across all dietary treatments. Energy density (AMEn) was maintained constant at 13.10 MJ/kg, except for diet 7, which was 0.3 MJ/kg higher. This necessitated a reduction in dietary lipid from 73 to 44 g/kg in diet 1 vs. diet 4. Dietary electrolyte balance (DEB) declined from 280 to 207 mEq/kg in diets 1 to 4 and the average DEB of diets 4 to 7 was 177 mEq/kg. Unbound AA inclusions increased from 4.50 g/kg in diet 1 to 21.52 g/kg in diets 4 and represented from 2.1 to 13.0% of dietary CP. The methionine plus cysteine to lysine ratio was 0.75 in all diets, bar diet 5, where the ratio was increased to 0.89. Whole maize (250 g/kg) was incorporated into diet 6 prior to steam-pelleting which was identical to diet 4 in all other respects. Whey protein concentrate (124 g/kg) was incorporated into diet 7 at the expense of soybean meal. The analyzed AA concentrations of the experimental diets are shown in Table 4 and the relevant methodology is outlined in the next section.
Table 3.
Nutrient specifications of experimental diets.
| Specification (g/kg) | Diet 1 | Diet 2 | Diet 3 | Diet 4 | Diet 5 | Diet 6 | Diet 7 |
|---|---|---|---|---|---|---|---|
| AMEn (MJ/kg) | 13.10 | 13.10 | 13.10 | 13.10 | 13.10 | 13.10 | 13.41 |
| Crude Protein | 210.0 | 195.0 | 180.0 | 165.0 | 165.0 | 165.0 | 165.0 |
| Calcium | 8.25 | 8.25 | 8.25 | 8.25 | 8.25 | 8.25 | 8.25 |
| Phosphorus | 6.94 | 6.88 | 6.79 | 6.71 | 6.70 | 6.71 | 6.24 |
| Phytate phosphorus | 2.20 | 2.11 | 2.01 | 1.90 | 1.90 | 1.90 | 1.35 |
| Nonphytate phosphorus | 4.74 | 4.77 | 4.78 | 4.81 | 4.81 | 4.81 | 4.89 |
| Chlorine | 2.50 | 2.50 | 2.50 | 2.50 | 2.50 | 2.50 | 2.50 |
| Potassium | 10.66 | 9.83 | 8.83 | 7.77 | 7.70 | 7.77 | 3.69 |
| Sodium | 1.80 | 1.80 | 1.80 | 1.80 | 1.80 | 1.80 | 1.80 |
| DEB (mEq/kg) | 280 | 259 | 233 | 207 | 205 | 192 | 102 |
| Crude fat | 73.0 | 64.8 | 54.8 | 44.0 | 43.2 | 44.0 | 28.6 |
| Crude fibre | 24.4 | 23.8 | 23.1 | 22.3 | 22.2 | 22.3 | 17.1 |
| SID1 amino acids | |||||||
| Lysine | 10.90 | 10.90 | 10.90 | 10.90 | 10.90 | 10.90 | 10.90 |
| Methionine | 4.58 | 4.58 | 4.58 | 4.58 | 6.29 | 4.58 | 4.58 |
| Cysteine | 3.60 | 3.60 | 3.60 | 3.60 | 3.40 | 3.60 | 3.60 |
| Met + Cys | 8.18 | 8.18 | 8.18 | 8.18 | 9.69 | 8.18 | 8.18 |
| Threonine | 7.63 | 7.63 | 7.63 | 7.63 | 7.63 | 7.63 | 7.63 |
| Tryptophan | 2.23 | 2.00 | 1.85 | 1.85 | 1.85 | 1.85 | 1.85 |
| Valine | 8.72 | 8.72 | 8.72 | 8.72 | 8.72 | 8.72 | 8.72 |
| Arginine | 12.88 | 11.77 | 11.77 | 11.77 | 11.77 | 11.77 | 11.77 |
| Isoleucine | 7.91 | 7.52 | 7.52 | 7.52 | 7.52 | 7.52 | 7.52 |
| Leucine | 15.21 | 14.09 | 12.73 | 11.88 | 11.88 | 11.88 | 14.84 |
| Histidine | 5.01 | 4.60 | 4.09 | 3.82 | 3.82 | 3.82 | 3.82 |
| Gly + Ser | 15.87 | 14.38 | 12.58 | 10.69 | 10.57 | 10.50 | 9.79 |
Standardized ileal digestible.
Table 4.
Analysed amino acid concentrations (as-is basis) of experimental diets.
| Specification (g/kg) | Diet 1 | Diet 2 | Diet 3 | Diet 4 | Diet 5 | Diet 6 | Diet 7 |
|---|---|---|---|---|---|---|---|
| Protein (N × 6.25) | 216 | 195 | 188 | 170 | 169 | 166 | 168 |
| Arginine | 14.32 | 12.71 | 12.60 | 12.03 | 12.49 | 12.25 | 11.41 |
| Histidine | 5.63 | 5.07 | 4.56 | 4.26 | 4.16 | 4.03 | 4.04 |
| Isoleucine | 9.00 | 8.38 | 8.16 | 8.01 | 7.51 | 7.26 | 6.43 |
| Leucine | 17.22 | 16.31 | 15.01 | 14.41 | 13.66 | 13.20 | 17.35 |
| Lysine | 12.74 | 12.09 | 11.82 | 11.08 | 11.88 | 11.67 | 12.80 |
| Methionine | 4.72 | 4.62 | 4.67 | 4.25 | 6.63 | 4.77 | 4.83 |
| Phenylalanine | 11.03 | 9.92 | 8.95 | 8.01 | 7.51 | 7.26 | 6.43 |
| Threonine | 8.92 | 8.57 | 8.38 | 7.81 | 8.38 | 8.27 | 9.99 |
| Tryptophan | 2.54 | 2.27 | 2.10 | 2.02 | 2.04 | 1.97 | 2.18 |
| Valine | 10.17 | 9.85 | 9.79 | 9.32 | 9.86 | 9.67 | 10.34 |
| Alanine | 10.25 | 9.50 | 8.83 | 8.17 | 7.78 | 7.52 | 9.38 |
| Aspartic acid | 21.85 | 19.17 | 16.96 | 14.58 | 13.80 | 13.35 | 14.64 |
| Cysteine | 3.91 | 3.65 | 3.48 | 3.25 | 3.29 | 3.31 | 4.05 |
| Glutamic acid | 38.23 | 33.83 | 30.63 | 27.36 | 25.60 | 24.68 | 27.94 |
| Glycine | 8.72 | 7.77 | 6.96 | 6.14 | 5.84 | 5.71 | 4.19 |
| Proline | 12.84 | 11.41 | 10.86 | 10.03 | 9.60 | 9.07 | 11.96 |
| Serine | 10.42 | 9.28 | 8.40 | 7.40 | 7.01 | 6.74 | 7.92 |
| Total amino acids | 219.6 | 200.0 | 187.1 | 172.1 | 169.5 | 165.9 | 175.9 |
| Dry matter | 902.7 | 898.5 | 894.6 | 890.4 | 894.8 | 893.8 | 895.4 |
Data and Sample Collection, Chemical Analyses, and Calculations
Growth performance (weight gain, feed intake, FCR, and mortality/cull rates) was monitored from 14 to 35 D post-hatch. Weight gain and feed intake were determined over the entire experimental period of 21 D. The body-weights of any dead or culled birds were recorded on a daily basis to correct feed intakes on a cage basis and adjust FCR calculations. Total excreta outputs and feed intakes were recorded from 33 to 35 D post-hatch to determine parameters of nutrient utilization. The parameters included AME, ME:GE ratios, N retention, and AMEn. During the total excreta collection period water intake was monitored to calculate water:feed intake ratios. Excreta were dried in a forced-air oven at 80°C for 24 h and the gross energy (GE) of excreta and diets were determined using an adiabatic bomb calorimeter. The AME values of the diets on a dry matter basis were calculated from the following equation:
ME:GE ratios were calculated by dividing AME by the GE of the appropriate diets. N contents of diets and excreta were determined using a N analyser (Leco Corporation, St Joseph, MI) and N retentions were calculated from the following equation:
N-corrected AME (AMEn MJ/kg DM) values were calculated by correcting N retention to zero using the factor of 36.54 kJ/g N retained in the body (Hill and Anderson, 1958).
At 34 D post-hatch, systemic blood samples (brachial vein) were taken to determine free AA concentrations in plasma. Blood samples of 2.5 mL volume were taken in heparinized tubes from 3 birds at random while they had access to feed and pooled from each cage. The samples were centrifuged at 3,000 rpm for 10 min, and decanted plasma samples were then kept at −80°C prior to analysis. Concentrations of 20 proteinogenic AA in plasma taken from the brachial vein were determined using precolumn derivatization amino acid analysis with 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate (AQC; Waters AccQTag Ultra; Waters Australia PL; www.waters.com) followed by separation of the derivatives and quantification by reversed phase ultra-performance liquid chromatography (ACQUITY UPLC binary system, Waters Corporation, Milford, MA). All AA were detected by UV absorbance and this procedure is fully described in Selle et al. (2016).
At day 35, birds were euthanased by intravenous injections of 5 mL sodium pentobarbitone into the jugular vein, the abdominal cavity opened, and pH of digesta within the gizzard was determined in situ (EZDO Model 7011 pH waterproof tester, Pakistan Test Instruments. Karachi, Pakistan). The incidence of dilated proventriculi was monitored. Following their removal, gizzard, pancreas, and abdominal fat-pad weights were recorded. The small intestine was removed and digesta was gently expressed in its entirety from the distal half of both the jejunum and ileum, pooled by cage. Digesta was mixed, freeze dried, ground, and weighed to determine the apparent digestibility coefficients of starch, CP, and AA. AIA and N concentrations were determined by methods described by Siriwan et al. (1993). The concentration of starch in diets and digesta was determined by methods described by Mahasukhonthachat et al. (2010). AA concentrations of diets and digesta were determined following the procedures described in Llames and Fontaine (1994). Apparent digestibility coefficients of starch, protein (N) and AA (expressed as nutrients) were calculated from the following equation:
Starch, protein (N) and AA disappearance rates (g/bird/D) were calculated from daily feed intakes, analysed dietary concentration of nutrients and their digestibility coefficients in the relevant intestinal segment from the following equation:
Statistical Analysis
Experimental data were analysed by a one-way analyses of variance using the univariate general liner model in IBM SPSS Statistics Version 24 program (IBM Corporation. Somers, NY) and pair-wise comparisons were drawn when appropriate from the same model. Least significant differences (LSD) were calculated from the error mean square and the appopriate t value. Experimental units were the cage means and a probability level of less than 5% was considered statistically significant. Linear regressions of the CP contents of diets 1 to 4 inclusive and parameters assessed were determined and tabulated.
RESULTS
Broiler Performance
Weight gains were not influenced (P > 0.45) by dietary treatments (Table 5). However, treatments significantly influenced feed intake (P < 0.01) where reducing dietary crude protein (CP) from 210 to 165 g/kg tended to increase feed intakes linearly (r = −0.321; P = 0.096) in birds offered diets 1 to 4. Treatments significantly influenced FCR (P < 0.001) and FCR of birds offered the 165 g/kg CP diet was significantly inferior to their counterparts offered 195 and 180 g/kg CP diets by 3.14% and 4.55%, respectively. A significantly higher mortality rate of 11.9% was observed in broilers offered diets 6 and 7; whereas, the overall mortality/cull rate was an acceptable 4.77%.
Table 5.
Effect of dietary treatments on growth performance and mortality/cull rates from 14 to 35 D post-hatch.
| Growth performance |
||||
|---|---|---|---|---|
| Treatment | Weight gain (g/bird) | Feed intake (g/bird) | FCR (g/g) | Mortality and cull rate (%) |
| 1 | 1,838 | 2,882a,b | 1.569a,b,c | 0.0a |
| 2 | 1,883 | 2,932a,b | 1.559a,b | 2.4a,b |
| 3 | 1,918 | 2,949a,b | 1.538a | 0.0a |
| 4 | 1,866 | 2,999b,c | 1.608c,d,e | 4.8a,b |
| 5 | 1,845 | 2,994b,c | 1.625d,e | 2.4a,b |
| 6 | 1,886 | 3,097c | 1.642e | 11.9b |
| 7 | 1,807 | 2,808a | 1.586b,c,d | 11.9b |
| SEM | 37.277 | 50.813 | 0.0165 | 3.370 |
| Significance (P =) | 0.454 | 0.010 | <0.001 | 0.049 |
| LSD (P < 0.05) | − | 145.0 | 0.0471 | 9.62 |
| Linear effect of | r = 0.138 | r = 0.321 | r = 0.229 | r = 0.258 |
| dietary CP 1 to 4 | P = 0.484 | P = 0.096 | P = 0.242 | P = 0.185 |
Means within columns not sharing common suffixes are significantly different (P < 0.05).
The reduction in dietary CP from 210 to 165 g/kg linearly increased fat-pad weights (r = −0.673; P < 0.001) by 69.2% from 8.64 to 14.62 g/kg. The same reduction in dietary CP linearly decreased pancreas weights (r = 0.514; P < 0.01) by 12.6% and linearly decreased water intakes (r = 0.662; P < 0.001) and the water:feed intake ratio (r = 0.756; P < 0.001). The inclusion of 250 g/kg whole maize in diet 6 prior to steam-pelleting did not influence relative gizzard weights nor gizzard pH. Dietary treatments significantly influenced (P < 0.02) the incidence of dilated proventriculi which was 16.7% in birds offered the 210 g/kg CP diet but only 1.2% in birds offered the four 165 g/kg CP diets. The above results are detailed in Table 6.
Table 6.
Effect of dietary treatments on relative gizzard weight and pH, relative pancreas and abdominal fat pad weights, incidence of dilated proventriculi at 35 D post-hatch, water intake and water:feed intake ratio from 33 to 35 D post-hatch.
| Treatment | Relative gizzard weight (g/kg) | Gizzard pH | Relative pancreas weight (g/kg) | Relative abdominal fat pad weight (g/kg) | Dilated proventriculi (%) | Water intake (g/bird) | Water:Feed intake ratio |
|---|---|---|---|---|---|---|---|
| 1 | 14.09 | 3.25 | 2.07 | 8.64a | 16.7b | 813c | 2.19d |
| 2 | 14.06 | 3.21 | 2.05 | 12.01b | 7.2a,b | 703b,c | 2.08c,d |
| 3 | 13.79 | 3.05 | 1.95 | 12.57b,c | 4.8a | 687b,c | 1.92b,c |
| 4 | 13.51 | 3.06 | 1.81 | 14.62c,d | 0.0a | 628a,b | 1.83a,b |
| 5 | 14.40 | 2.95 | 1.96 | 15.13d | 4.8a | 618a,b | 1.71a |
| 6 | 13.60 | 3.20 | 1.96 | 14.55c,d | 0.0a | 552a | 1.72a |
| 7 | 13.43 | 3.53 | 2.02 | 15.78d | 0.0a | 632a,b | 1.85a,b |
| SEM | 0.364 | 0.130 | 0.071 | 0.824 | 3.447 | 45.85 | 0.0561 |
| Significance (P =) | 0.464 | 0.070 | 0.200 | <0.001 | 0.018 | 0.010 | <0.001 |
| LSD (P < 0.05) | − | − | − | 2.352 | 9.84 | 130.9 | 0.160 |
| Linear effect of | r = 0.273 | r = 0.225 | r = 0.514 | r = −0.673 | r = 0.519 | r = 0.662 | r = 0.756 |
| dietary CP 1 to 4 | P = 0.160 | P = 0.249 | P = 0.005 | P < 0.001 | P = 0.005 | P < 0.001 | P < 0.001 |
Means within columns not sharing common suffixes are significantly different (P < 0.05).
Nutrient Utilization
The effects of dietary treatments on parameters of nutrient utilization are shown in Table 7. N retention (P > 0.25) was not influenced by treatment but highly significant responses (P < 0.001) were observed for the 3 parameters of energy utilization. AME in birds offered the 210 g/kg CP diet was 12.67 MJ/kg but this increased to an average of 13.27 MJ/kg in birds offered the four 165 g/kg CP diets. On the same basis, ME:GE ratios increased by 9.89%, from 0.748 to 0.822, and AMEn by 1.04 MJ, from 11.24 MJ/kg to 12.28 MJ/kg. Reductions in dietary CP from diets 1 to 4 linearly increased ME:GE ratios (r = −0.770; P < 0.001) and AMEn (r = −0.587; P < 0.005).
Table 7.
Effect of dietary treatments on parameters of nutrient utilization and ratio of excreta out to feed in over the total collection period from 33 to 35 D post-hatch.
| Treatment | AME (MJ/kg DM) | ME:GE ratio (MJ/MJ) | N retention (%) | AMEn (MJ/kg DM) |
|---|---|---|---|---|
| 1 | 12.67a | 0.748a | 75.18 | 11.24a |
| 2 | 12.91b | 0.769b | 71.25 | 11.66b |
| 3 | 12.93b | 0.786c | 74.98 | 11.62b |
| 4 | 12.99b | 0.802c,d | 72.43 | 11.92b,c |
| 5 | 13.03b | 0.806d | 70.17 | 11.99c |
| 6 | 13.09b | 0.804d | 65.37 | 12.19c |
| 7 | 13.96c | 0.874e | 69.35 | 13.03d |
| SEM | 0.0971 | 0.0059 | 2.9284 | 0.0113 |
| Significance (P =) | <0.001 | <0.001 | 0.253 | <0.001 |
| LSD (P < 0.05) | 0.277 | 0.0169 | − | 0.322 |
| Linear effect of | r = −0.371 | r = −0.770 | r = 0.081 | r = −0.587 |
| dietary CP 1 to 4 | P = 0.052 | P < 0.001 | P = 0.683 | P = 0.001 |
Means within columns not sharing common suffixes are significantly different (P < 0.05).
Starch–Protein Digestive Dynamics
The reduction from 210 to 165 g/kg linearly increased jejunal starch digestibility coefficients by 13.5% (0.892 vs. 0.786; P < 0.001) and ileal starch digestibility coefficients by 8.98% (0.959 vs. 0.880; P < 0.001). The corresponding increases in starch disappearance rates were 49.7% and 43.5%, respectively, and linear increases were again observed. The reduction from 210 to 165 g/kg CP linearly increased N digestibility coefficients by 30.1% (0.562 vs. 0.432; P < 0.005) in the jejunum but did not influence protein (N) disappearance rates. In comparing diets 1 and 4, there was a numerical increase of 5.53% (0.763 vs. 0.723) in ileal protein (N) digestibility but a significant decrease of 14.4% (18.46 vs. 21.56 g/bird/D; P < 0.005) in disappearance rates. Starch:protein disappearance rate ratios linearly increased (P < 0.001) from 2.68 to 3.82 in the jejunum and from 1.76 to 2.94 in the ileum across diet 1 to 4. The above results are detailed in Tables 8 and 9.
Table 8.
Effect of dietary treatments on apparent digestibility coefficients and disappearance rates (g/bird/D) of starch in distal jejunum and distal ileum at 35 D post-hatch.
| Distal jejunum |
Distal ileum |
|||
|---|---|---|---|---|
| Treatment | Digestibility | Disappearance | Digestibility | Disappearance |
| 1 | 0.786a | 33.6a | 0.880a | 37.7a |
| 2 | 0.798a | 36.1a | 0.902a,b | 40.6b |
| 3 | 0.857b | 43.6b | 0.922b,c | 46.8c |
| 4 | 0.892b,c | 50.3c | 0.959d,e | 54.1d |
| 5 | 0.884b,c | 48.6c | 0.940c,d | 51.7d |
| 6 | 0.928d | 55.2d | 0.965d,e | 57.5e |
| 7 | 0.922c,d | 54.7d | 0.979e | 58.0e |
| SEM | 0.0151 | 1.0932 | 0.0091 | 0.9164 |
| Significance (P =) | <0.001 | <0.001 | <0.001 | <0.001 |
| LSD (P < 0.05) | 0.0430 | 3.12 | 0.0259 | 2.62 |
| Linear effect of | r = −0.727 | r = −0.914 | r = −0.741 | r = −0.935 |
| dietary CP 1 to 4 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 |
Means within columns not sharing common suffixes are significantly different (P < 0.05).
Table 9.
Effect of dietary treatments on apparent digestibility coefficients and disappearance rates (g/bird/D) of protein (N) in distal jejunum and distal ileum and starch:protein disappearance rate ratios in distal jejunum and distal ileum at 35 D post-hatch.
| Distal jejunum |
Distal ileum |
Disappearance rate ratios |
||||
|---|---|---|---|---|---|---|
| Treatment | Digestibility | Disappearance | Digestibility | Disappearance | Distal jejunum | Distal ileum |
| 1 | 0.432a | 12.87 | 0.723a | 21.56d | 2.68a | 1.76a |
| 2 | 0.467a,b | 13.02 | 0.738a,b | 20.59c,d | 2.81a | 1.98b |
| 3 | 0.514b,c | 13.83 | 0.753a,b | 20.24b,c,d | 3.20a | 2.32c |
| 4 | 0.562c,d | 13.67 | 0.763a,b | 18.46a,b | 3.82b | 2.94d,e |
| 5 | 0.535b,c,d | 12.71 | 0.770a,b | 18.31a | 3.83b | 2.82d |
| 6 | 0.593d | 14.57 | 0.784b,c | 19.28a,b,c | 3.83b | 2.99e |
| 7 | 0.601d | 13.71 | 0.823c | 18.84a,b | 4.03b | 3.08e |
| SEM | 0.0267 | 0.7395 | 0.0169 | 0.5344 | 0.1840 | 0.0521 |
| Significance (P =) | <0.001 | 0.598 | <0.001 | <0.001 | <0.001 | <0.001 |
| LSD (P < 0.05) | 0.0763 | − | 0.0482 | 1.525 | 0.526 | 0.149 |
| Linear effect of | r = −0.549 | r = −0.164 | r = −0.313 | r = 0.582 | r = −0.645 | r = −0.778 |
| dietary CP 1 to 4 | P = 0.002 | P = 0.404 | P = 0.105 | P = 0.001 | P < 0.001 | P < 0.001 |
Means within columns not sharing common suffixes are significantly different at the 5% level of probability.
Apparent Amino Acid Digestibility Coefficients
As shown in Table 10, dietary treatments significantly influenced the digestibility of all 17 AA in the jejunum and the transition from 210 to 165 g/kg CP diets linearly increased jejunal digestibilities in each case. There was a linear increase (r = −0.556; P < 0.005) in average jejunal amino acid digestibilities. Average coefficients were increased by 32.2% (0.607 vs. 0.459) when the mean of diets 4, 5, 6, and 7 is compared with diet 1. Dietary treatments significantly influenced the digestibility of 15 AA in the ileum where glycine and serine were the two exceptions as shown in Table 11. The transition from 210 to 165 g/kg CP diets linearly increased ileal digestibilities of arginine, isoleucine, threonine, valine, and proline. When the mean of diets 4, 5, 6, and 7 is compared with diet 1, average ileal digestibility coefficients were increased by 9.14% (0.812 vs. 0.744).
Table 10.
Effect of dietary treatments on apparent digestibility coefficients of amino acids in distal jejunum in broiler chickens at 35 D post-hatch.
| Treatment | Arg | His | Ile | Leu | Lys | Met | Phe | Thr | Trp |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.575a | 0.517a | 0.416a | 0.441a | 0.500a | 0.615a | 0.490a | 0.412a | 0.392a |
| 2 | 0.590a,b | 0.530a | 0.458a,b | 0.470a | 0.530a | 0.642a | 0.509a,b | 0.458a,b | 0.400a |
| 3 | 0.646b | 0.551a,b | 0.524b | 0.506a,b | 0.584a,b | 0.687a,b | 0.538a,b,c | 0.513b,c | 0.433a |
| 4 | 0.720c | 0.621b,c | 0.624c | 0.592c | 0.665b,c | 0.738b,c | 0.598c,d | 0.586c,d | 0.538b |
| 5 | 0.729c | 0.618b,c | 0.630c | 0.581b,c | 0.669b,c | 0.839d | 0.582b,c,d | 0.629d | 0.552b |
| 6 | 0.725c | 0.613b,c | 0.635c | 0.582b,c | 0.674b,c | 0.774c,d | 0.576b,c,d | 0.622d | 0.543b |
| 7 | 0.745c | 0.646c | 0.696c | 0.716d | 0.695c | 0.778c,d | 0.621d | 0.640d | 0.613b |
| SEM | 0.0244 | 0.0252 | 0.0272 | 0.0269 | 0.0326 | 0.0253 | 0.0258 | 0.0278 | 0.0326 |
| Significance (P =) | <0.001 | 0.002 | <0.001 | <0.001 | <0.001 | <0.001 | 0.007 | <0.001 | <0.001 |
| LSD (P < 0.05) | 0.0696 | 0.0718 | 0.0776 | 0.0774 | 0.0933 | 0.0721 | 0.0736 | 0.0793 | 0.0931 |
| Linear effect of | r = −0.621 | r = −0.475 | r = −0.700 | r = −0.581 | r = −0.540 | r = −0.528 | r = −0.514 | r = −0.625 | r = −0.492 |
| dietary CP 1 to 4 | P < 0.001 | P = 0.011 | P < 0.001 | P = 0.001 | P = 0.003 | P = 0.004 | P = 0.005 | P < 0.001 | P = 0.008 |
| Treatment | Val | Ala | Asp | Cys | Glu | Gly | Pro | Ser | Average |
| 1 | 0.407a | 0.409a | 0.457a | 0.370a | 0.555a | 0.365a,b | 0.490a | 0.384a | 0.459a |
| 2 | 0.468a,b | 0.435a | 0.477a,b | 0.402a | 0.578a | 0.386a,b | 0.504a,b | 0.428a,b | 0.486a |
| 3 | 0.539b | 0.477a,b | 0.494a,b | 0.442a,b | 0.600a,b | 0.406b | 0.552b | 0.452a,b,c | 0.526a,b |
| 4 | 0.618c | 0.553b | 0.549b,c | 0.482b,c | 0.648c | 0.464b | 0.594c,d | 0.503c | 0.594b,c |
| 5 | 0648c | 0.541b | 0.527a,b | 0.521c | 0.629b,c | 0.442b | 0.583c | 0.490c | 0.602b,c |
| 6 | 0.645c | 0.541b | 0.519a,b | 0.523c | 0.626b,c | 0.440b | 0.589c | 0.481c | 0.595b,c |
| 7 | 0.676c | 0.643c | 0.611c | 0.606d | 0.675c | 0.299a | 0.649d | 0.537c | 0.638c |
| SEM | 0.0272 | 0.0301 | 0.0280 | 0.0258 | 0.0223 | 0.0362 | 0.0194 | 0.0319 | 0.0268 |
| Significance (P =) | <0.001 | <0.001 | 0.009 | 0.005 | 0.007 | 0.041 | 0.003 | 0.007 | 0.005 |
| LSD (P < 0.05) | 0.0777 | 0.0859 | 0.0798 | 0.0738 | 0.0637 | 0.1032 | 0.0554 | 0.091 | 0.0765 |
| Linear effect of | r = −0.711 | r = −0.532 | r = −0.396 | r = −0.508 | r = −0.491 | r = −0.374 | r = −0.617 | r = −0.434 | r = −0.556 |
| dietary CP 1 to 4 | P < 0.001 | P = 0.004 | P = 0.036 | P = 0.006 | P = 0.008 | P = 0.050 | P < 0.001 | P = 0.021 | P = 0.002 |
Means within columns not sharing common suffixes are significantly different at the 5% level of probability.
Table 11.
Effect of dietary treatments on apparent digestibility coefficients of amino acids in distal ileum in broiler chickens at 35 D post-hatch.
| Treatment | Arg | His | Ile | Leu | Lys | Met | Phe | Thr | Trp |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.819a | 0.781a | 0.731a | 0.744a | 0.784a | 0.844a | 0.771a | 0.699a | 0.728a |
| 2 | 0.822a | 0.786a,b | 0.750a | 0.754a | 0.793a | 0.846a | 0.773a | 0.720a,b | 0.728a |
| 3 | 0.844a,b | 0.794a,b | 0.775a,b | 0.767a,b | 0.815a,b | 0.871a,b | 0.782a | 0.748b | 0.751a,b |
| 4 | 0.862b | 0.813a,b | 0.806b,c | 0.798b | 0.830a,b,c | 0.878a,b,c | 0.802a | 0.766b,c | 0.769a,b |
| 5 | 0.876b,c | 0.821a,b | 0.817b,c | 0.798b | 0.856b,c | 0.928d | 0.803a | 0.796c,d | 0.786b |
| 6 | 0.877b,c | 0.824b,c | 0.826c | 0.809b | 0.847b,c | 0.903b,c,d | 0.808a | 0.801c,d | 0.790b |
| 7 | 0.899c | 0.864c | 0.884d | 0.902c | 0.874c | 0.916c,d | 0.868b | 0.821d | 0.850c |
| SEM | 0.0120 | 0.0146 | 0.0161 | 0.0168 | 0.0162 | 0.0133 | 0.0158 | 0.0163 | 0.0177 |
| Significance (P =) | <0.001 | 0.004 | <0.001 | <0.001 | 0.002 | <0.001 | 0.001 | <0.001 | <0.001 |
| LSD (P < 0.05) | 0.0341 | 0.0417 | 0.0460 | 0.0479 | 0.0462 | 0.0382 | 0.0450 | 0.0465 | 0.0505 |
| Linear effect of | r = −0.432 | r = −0.258 | r = −0.506 | r = −0.357 | r = −0.358 | r = −0.340 | r = −0.244 | r = −0.469 | r = −0.304 |
| dietary CP 1 to 4 | P = 0.022 | P = 0.185 | P = 0.006 | P = 0.062 | P = 0.062 | P = 0.077 | P = 0.210 | P = 0.012 | P = 0.116 |
| Treatment | Val | Ala | Asp | Cys | Glu | Gly | Pro | Ser | Average |
| 1 | 0.724a | 0.705a | 0.732a | 0.665a | 0.791a | 0.686 | 0.741a | 0.706 | 0.744a |
| 2 | 0.753a,b | 0.714a,b | 0.739a | 0.687a | 0.799a | 0.699 | 0.740a | 0.721 | 0.754a,b |
| 3 | 0.780b,c | 0.729a,b | 0.743a | 0.713b,c | 0.807a | 0.704 | 0.767a,b | 0.734 | 0.772a,b,c |
| 4 | 0.802c,d | 0.755a,b | 0.752a | 0.732b,c,d | 0.822a | 0.707 | 0.789b | 0.739 | 0.790b,c |
| 5 | 0.826d,e | 0.758a,b | 0.750a | 0.752c,d | 0.819a | 0.711 | 0.790b | 0.744 | 0.802c |
| 6 | 0.830d,e | 0.766b | 0.755a | 0.768d | 0.825a | 0.714 | 0.797b | 0.746 | 0.805c |
| 7 | 0.866e | 00842c | 0.845b | 0.839e | 0.876b | 0.702 | 0.857c | 0.787 | 0.852d |
| SEM | 0.0156 | 0.0213 | 0.0155 | 0.0163 | 0.0137 | 0.0201 | 0.0144 | 0.0171 | 0.0156 |
| Significance (P =) | <0.001 | 0.001 | <0.001 | <0.001 | 0.002 | 0.968 | <0.011 | 0.065 | <0.001 |
| LSD (P < 0.05) | 0.0447 | 0.0608 | 0.0443 | 0.0465 | 0.0391 | − | 0.0412 | − | 0.0444 |
| Linear effect of | r = −0.534 | r = −0.294 | r = −0.160 | r = −0.455 | r = −0.275 | r = −0.147 | r = −0.398 | r = −0.245 | r = −0.355 |
| dietary CP 1 to 4 | P = 0.003 | P = 0.130 | P = 0.415 | P = 0.015 | P = 0.156 | P = 0.456 | P = 0.036 | P = 0.209 | P = 0.064 |
Means within columns not sharing common suffixes are significantly different at the 5% level of probability.
Free Amino Acid Concentrations in Systemic Plasma
Dietary treatments significantly influenced free AA concentrations of 12 AA but not tryptophan, alanine, aspartic acid, asparagine, and glutamic acid as shown in Table 12. When diets 1 and 4 are compared, there were significant decreases in concentrations of arginine (33.9%), histidine (49.6%), leucine (30.6%), phenylalanine (23.1%), glycine (30.4%), serine (19.3%), and tyrosine (29.3%) where percentage reductions are shown in parentheses. Alternatively, there were significant increases in concentrations of lysine (49.6%; P = 0.023), threonine (65.9%; P < 0.001), and methionine (24.3%; P = 0.056) closely approached significance on the basis of a pair-wise comparison. The transition from 210 to 165 g/kg CP diets linearly increased concentrations of lysine, methionine, and threonine but linearly decreased concentrations of arginine, histidine, leucine, phenylalanine, aspartic acid, glycine, serine, and tyrosine.
Table 12.
Effect of dietary treatments on plasma concentrations of free amino acids (μmol/L) in the systemic circulation (brachial vein) at 34 D post-hatch.
| Treatment | Arg | His | Ile | Leu | Lys | Met | Phe | Thr | Trp |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 694c | 113c | 138b | 265c | 427a,b | 70a,b | 156c | 619a,b | 79 |
| 2 | 497b | 79b | 104a | 210b | 399a | 58a | 135b,c | 514a | 69 |
| 3 | 553b,c | 65a,b | 130b | 208a,b | 591b,c | 79b | 133b | 799b | 72 |
| 4 | 459a,b | 57a | 127b | 184a | 639c | 87b,c | 120a,b | 1,027c | 70 |
| 5 | 449a,b | 64a,b | 135b | 195a,b | 706c | 160d | 128a,b | 1,123c | 73 |
| 6 | 539b,c | 70a,b | 143b,c | 198a,b | 671c | 101c | 123a,b | 1,395d | 79 |
| 7 | 315a | 71a,b | 160c | 286c | 977d | 103c | 108a | 2,387e | 79 |
| SEM | 63.65 | 5.465 | 6.543 | 8.994 | 63.08 | 6.015 | 7.551 | 69.52 | 3.551 |
| Significance (P =) | 0.009 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.004 | <0.001 | 0.186 |
| LSD (P < 0.05) | 181.6 | 15.6 | 18.7 | 25.4 | 180.0 | 17.2 | 21.5 | 198.4 | − |
| Linear effect of | r = 0.374 | r = 0.787 | r = 0.038 | r = 0.723 | r = −0.535 | r = −0.443 | r = 0.461 | r = −0.687 | r = 0.275 |
| dietary CP 1 to 4 | P = 0.050 | P < 0.001 | P = 0.848 | P < 0.001 | P = 0.003 | P = 0.018 | P = 0.014 | P < 0.001 | P = 0.157 |
| Treatment | Val | Ala | Asp | Asn | Glu | Gly | Ser | Tyr | Total |
| 1 | 253a,b | 1,129 | 190 | 97 | 312 | 769c | 662b,c | 232c | 6,203a,b |
| 2 | 216a | 1,129 | 177 | 50 | 323 | 646b | 593a,b | 193b | 5,395a |
| 3 | 276b,c | 1,144 | 153 | 89 | 308 | 668b | 594a,b | 167b | 6,030a,b |
| 4 | 281b,c | 1,207 | 127 | 92 | 303 | 535a | 534a | 164b | 6,011a,b |
| 5 | 307c,d | 1,139 | 142 | 84 | 309 | 516a | 576a,b | 172b | 6,278a,b |
| 6 | 328d | 1,341 | 144 | 92 | 314 | 561a | 565a | 178b | 6,842b |
| 7 | 303c,d | 1,297 | 144 | 120 | 341 | 590a,b | 696c | 124a | 8,093c |
| SEM | 15.26 | 77.68 | 16.10 | 18.13 | 17.68 | 27.90 | 31.41 | 11.24 | 241.7 |
| Significance (P =) | <0.001 | 0.291 | 0.112 | 0.285 | 0.777 | <0.001 | 0.011 | <0.001 | <0.001 |
| LSD (P < 0.05) | 43.6 | − | − | − | − | 79.6 | 89.8 | 32.1 | 975.7 |
| Linear effect of | r = −0.344 | r = −0.138 | r = 0.502 | r = −0.062 | r = 0.122 | r = 0.658 | r = 0.502 | r = 0.592 | r = −0.011 |
| dietary CP 1 to 4 | P = 0.073 | P = 0.484 | P = 0.007 | P = 0.753 | P = 0.538 | P < 0.001 | P = 0.007 | P = 0.001 | P = 0.957 |
Means within columns not sharing common suffixes are significantly different at the 5% level of probability.
DISCUSSION
Overall growth performance in the present study exceeded 2014 Ross 308 performance objectives for weight gain (1,863 vs. 1,795 g/bird) and FCR (1.590 vs. 1.652). Birds offered the 180 g/kg CP diet were numerically the best performed with a 1,918 g/bird weight gain and 1.538 FCR. The incidence of dilated proventriculi linearly declined in birds offered decreasing CP diets 1 to 4 from 16.7% to zero. Proventricular dilation disease is a transmissible condition in broilers caused by a Borna virus which negatively impacts on broiler performance (Dormitorio et al., 2007; Honkavuori et al., 2008). The overall 4.77% mortality/cull rate was acceptable but there were significant differences between treatments as losses in birds offered dietary treatments 6 and 7 were more pronounced. These diets were different in that they contained whole maize or whey protein, respectively, but this does not appear to account for the higher mortality rates.
Reductions in dietary CP from 210 g/kg to 180 g/kg did not adversely influence growth performance; indeed, birds offered 180 g/kg CP diet numerically outperformed their 210 g/kg CP counterparts. However, the further reduction from 180 to 165 g/kg CP significantly compromised FCR by 4.55% (1.608 vs. 1.538) on the basis of a pair-wise comparison, which was accompanied by a 16.3% numerical increase (14.62 vs. 12.57 g/kg) in relative fat-pad weights. The differences between the 180 and 165 g/kg CP diets appear modest but FCR was tangibly compromised. Clearly, the challenge to the successful development of reduced CP diets is the identification of the factors responsible for compromised FCR generated by CP reductions.
There was a linear increase in relative fat-pad weights of 69.2% (14.62 vs. 8.64 g/kg) following graded reductions in dietary CP from 210 to 165 g/kg. Heavier relative fat-pad weights may stem from increases in net energy in reduced-CP diets as it follows that there would be less heat increment from intact protein digestion from reduced CP diets. Leeson et al. (1996) demonstrated that both increased dietary energy and decreased dietary protein generated heavier fat-pads and similar findings were observed in more recent studies (Bregendahl et al., 2002; Srilatha et al., 2018).
Dietary treatments did not influence N retention but reductions in dietary CP significantly enhanced energy utilization as shown by increased ME:GE ratios This can be attributed at least in part to increases in starch digestibility and disappearance rates generated by dietary CP reductions. In addition, the energy density of a feedstuff may not remain constant and an AMEn value of 37.0 MJ/kg was ascribed for soybean oil in the present study. However, increasing energy values of lipid as its dietary inclusion decreases have been reported. Leeson and Summers (2005) found that the energy value of lipid increased from to 34.56 to 41.42 MJ/kg AMEn when added dietary lipid was decreased from 40 to 5 g/kg.
The general case for the relevance of starch-protein digestive dynamics to broiler performance has been advanced (Liu et al., 2015; Selle and Liu, 2019). However, with reduced-CP diets specifically, dietary starch:protein ratios automatically expand as maize is increased at the expense of soybean meal in order to reduce dietary CP. For example, analysed dietary starch:protein ratios increased from 1.45 in the 210 g/kg CP diet to 2.30 in the 165 g/kg CP diet in the present study (Table 1). The transition from 210 to 165 g/kg CP diets linearly increased starch digestion in the jejunum and ileum by 13.5% and 8.98%, respectively. These outcomes are consistent with maize starch being a readily digestible energy source in poultry (Moran, 1982). The pronounced increases in starch disappearance rates were a consequence of increased dietary concentrations coupled with higher digestibility coefficients.
On the other hand, jejunal protein (N) disappearance rates were not influenced by treatment but ileal protein (N) disappearance rates linearly declined following the transition from 210 to 165 g/kg CP diets by 14.4% (18.46 vs. 21.56 g/bird/D). Consequently, starch:protein disappearance rate ratios increased by 42.5% in the jejunum and by 67.0% on the ileum in birds offered the 165 g/kg CP in comparison to their 210 g/kg CP counterparts. The relevance of starch:protein disappearance rate ratios to broiler performance has been demonstrated by Sydenham et al. (2017). In the present study there was a quadratic relationship (r = 0.486; P < 0.005) between ileal starch:protein disappearance rate ratios and FCR across all treatments as shown in Figure 1. It may be predicted from the regression equation that a disappearance rate ratio of 2.01 would support the minimum FCR of 1.560. Moreover, ileal starch:protein disappearance rate ratios were linearly related to relative fat-pad weights and fat-pad weights, in turn, were linearly related to FCR. Thus, FCR is compromised and fat deposition increased with expanding ratios.
Figure 1.
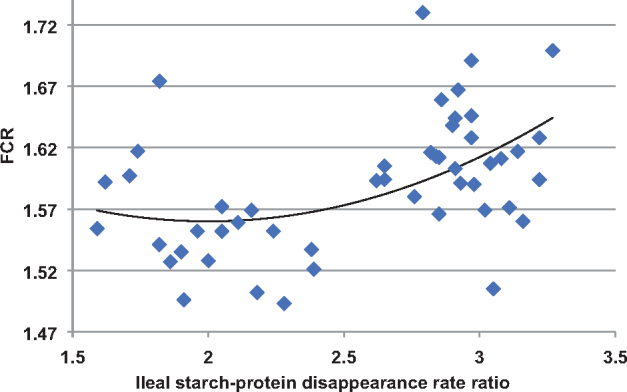
The quadratic relationship (r = 0.486; P < 0.005) between ileal starch:protein disappearance rate ratios and FCR where y(FCR) = 1.769 + 0.052*ratio2 −0.209*ratio.
Scant attention has been paid to the impacts on apparent amino acid digestibility coefficients in the quest to develop reduced-CP diets as the study by Awad et al. (2016) is the only relevant paper identified. There was a significant increase in average ileal amino acid digestibility coefficients of 6.18% (0.790 vs. 0.744) in birds offered the 165 g/kg CP diet in comparison to the 210 g/kg diet and the linear increase approached significance. The 6.18% increase in ileal amino acid digestibility was similar to that reported by Awad et al. (2016). In the present study, significant increases in ileal digestibility were confined to arginine, isoleucine, leucine, threonine, valine, cysteine, and proline when diets 1 and 4 are compared. With the exception of proline, all these AA were present as unbound AA at varying concentrations in diet 4.
The significant increases in amino acid digestibility coefficients in the jejunum were more pronounced. There was a significant increase in average jejunal amino acid digestibility coefficients of 29.4% (0.594 vs. 0.459) when the 165 g/kg and 210 g/kg CP diets are compared with a linear increase (r = −0.556; P < 0.005) in diets 1 to 4. The more pronounced responses in the jejunum may be attributed partially to the notional 100% digestibility of unbound AA and the likelihood that unbound AA were rapidly and completely absorbed in the jejunum (Wu, 2009). Phenylalanine, alanine, aspartic acid, glutamic acid, glycine, proline, and serine were present in both diets as only protein-bound AA in diets 1 to 4. The mean jejunal digestibilities of these 7 protein-bound AA increased by 24.0%, (0.558 vs. 0.450) in diet 4 relative to diet 1. In contrast, average jejunal digestibilities of 10 AA that were present in both unbound and protein-bound forms increased by 32.9% (0.618 vs. 0.465). The most pronounced increases were observed for isoleucine (50.0%) and valine (51.8%). Diet 4 contained 2.330 g/kg unbound isoleucine which represented 29.9% of its analysed concentration and 2.845 g/kg valine or 30.5% of its analysed concentration. Broiler diets are formulated on the basis of ileal amino acid digestibilities, so it could be expected that jejunal digestibilities in reduced-CP diets would be higher for AA with relatively large proportions of unbound as opposed to protein-bound components.
Free concentrations of lysine, methionine, and threonine in systemic plasma were linearly increased pursuant to the 210 to 165 g/kg reduction in CP. However, there were notable increases in the unbound proportions of their analysed dietary concentrations as CP reduced: lysine from 5.3 to 40%, methionine from 39 to 60%, and threonine from 10 to 40%. In contrast, free concentrations of arginine, leucine, phenylalanine, aspartic acid, glycine, serine, and tyrosine linearly decreased. These linear decreases were largely a consequence of reduced concentrations in the 165 g/kg CP diet of the relevant AA and the majority was present only as protein- bound AA in the experimental diets. The pronounced 65.9% increase in free threonine concentrations in systemic plasma when the 210 to 165 g/kg diets are compared is noteworthy but not without precedent. Fancher and Jensen (1989b) offered female broilers 183 g/kg or 159 g/kg CP diets which both contained 7.2 g/kg threonine. This reduction in CP resulted in an increase of 86.6% (1,635 vs. 876 nmol/L) in free threonine concentrations in systemic plasma at 42 D post-hatch possible explanation for the pronounced increases in concentrations of free threonine in systemic plasma in both the present study and the Fancher and Jensen (1989b) study is that dietary CP reductions down-regulate threonine dehydrogenase activity (Keene and Austic, 2001). Therefore, further research into the regulation of hepatic threonine dehydrogenase activity in the context of reduced-crude protein broiler diets is certainly needed.
The potential of three strategies to facilitate reductions in dietary CP was investigated in the present study. In the first, dietary sulfur-containing AA were formulated to a ratio of 1.27 methionine:cysteine, with the exception of diet 5 where the ratio was set at 1.85. The increase in the methionine:cysteine ratio and concomitant small decrease in cysteine had 2 objectives, firstly to determine the broiler response to increased methionine in reduced CP diets and secondly, to determine if a wider methionine:cysteine ratio had any effect on the assessed parameters. However, increasing methionine concentrations and ratios in reduced CP diets did not affect broiler performance in the present study.
Whole maize (250 g/kg) was incorporated into diet 6 prior to steam-pelleting as the second facilitative strategy. However, pre-pellet whole maize addition failed to increase relative gizzard weights (13.60 vs. 13.51 g/kg); consequently, it is not possible to draw any conclusions.
In the third facilitative strategy, whey protein concentrate replaced soybean meal in Diet 7, to provide a more rapidly digested source of protein. Given the relevance of starch–protein digestive dynamics (Liu and Selle, 2017) and that unbound AA are rapidly absorbed, there is the possibility that unbound AA would be better utilized with the provision of more rapidly absorbed protein-bound AA. However, the addition of whey protein generated a 6.37% (2,808 vs. 2,999 g/bird) decrease in feed intake, increased average ileal amino acid digestibility coefficients by 10.5% (0.850 vs. 0.769) but did not influence weight gain or FCR when diets 4 and 7 were compared.
In conclusion, the present study indicates that CP can be reduced by 30 g/kg from 210 to 180 g/kg without negatively influencing broiler performance. However, the further reduction from 180 to 165 g/kg CP compromised FCR, although concentrations of notionally “essential” AA were maintained. There is the possibility that the 165 g/kg CP diets may have been deficient in certain “non-essential” AA, including glycine and serine (Dean et al., 2006). The reduction from 210 to 165 g/kg CP enhanced energy utilization which was probably largely a consequence of increased starch digestibility coefficients coupled with increases in dietary starch levels as dietary CP was reduced. Dietary CP reductions widened starch-protein disappearance rate ratios, which were related to both poorer FCR and heavier relative fat-pad weights. AA digestibility coefficients were enhanced by dietary CP reductions, especially in the jejunum, where the increasing quantities of unbound AA relative to protein-bound AA appeared to be a major contributing factor. Again, the ratio of unbound to protein-bound AA appeared to trigger the divergent outcomes in free AA concentrations in systemic plasma. These imbalances may be a restraint on bird performance in the context of reduced-CP diets and clearly further research is required if reduced-CP diets are to be successfully developed.
ACKNOWLEDGEMENTS
The authors would like to acknowledge Preethi Ramesh and her colleagues in the Evonik AMINOLab in Singapore for their analyses of amino acid concentrations in diets and digesta. The authors would also like to thank the indefatigable Joy Gill and her technical team in the Poultry Research Foundation.
REFERENCES
- Aftab U., Ashraf M., Jiang Z. Low protein diets for broilers. World Poult. Sci. J. 2006;62:688–701. [Google Scholar]
- Awad E.A., Zulkifli I., Farjam A.S., Chwen L.T., Hossain M.A., Aljoubori A. Effect of low-protein diet, gender and age on the apparent ileal amino acid digestibilities in broiler chickens raised under hot-humid tropical condition. Indian J. Anim. Sci. 2016;86:696–701. [Google Scholar]
- Bregendahl K., Sell J.L., Zimmerman D.R. Effect of low-protein diets on growth performance and body composition of broiler chicks. Poult. Sci. 2002;81:1156–1167. doi: 10.1093/ps/81.8.1156. [DOI] [PubMed] [Google Scholar]
- Corzo A., Fritts C.A., Kidd M.T., Kerr B.J. Response of broiler chicks to essential and non-essential amino acid supplementation of low crude protein diets. Anim. Feed Sci. Technol. 2005;118:319–327. [Google Scholar]
- Dean D.W., Bidner T.D., Southern L.L. Glycine supplementation to low protein, amino acid-supplemented diets supports optimal performance of broiler chicks. Poult. Sci. 2006;85:288–296. doi: 10.1093/ps/85.2.288. [DOI] [PubMed] [Google Scholar]
- Dormitorio T.V., Giambrone J.J., Hoerr F.J. Transmissable proventriculitis in broilers. Avian Path. 2007;36:87–91. doi: 10.1080/03079450601142588. [DOI] [PubMed] [Google Scholar]
- Edmonds M.S., Parsons C.M., Baker D.H. Limiting amino acids in low-protein corn-soybean meal diets fed to growing chicks. Poult. Sci. 1985;64:1519–1526. [Google Scholar]
- Fancher B.I., Jensen L.S. Male broiler performance during the starting and growing periods as affected by dietary protein, essential amino acids and potassium levels. Poult. Sci. 1989;68:1385–1395. doi: 10.3382/ps.0681385. [DOI] [PubMed] [Google Scholar]
- Fancher B.I., Jensen L.S. Dietary protein levels and essential amino acid content: influence upon female broiler performance during the growing period. Poult. Sci. 1989;68:897–908. doi: 10.3382/ps.0680897. [DOI] [PubMed] [Google Scholar]
- Fatufe A., Rodehutscord M. Growth, body composition, and marginal efficiency of methionine utilization are affected by nonessential amino acid nitrogen supplementation in male broiler chicken. Poult. Sci. 2005;84:1584–1592. doi: 10.1093/ps/84.10.1584. [DOI] [PubMed] [Google Scholar]
- Hill F.W., Anderson D.L. Comparison of metabolizable energy and productive energy determinations with growing chicks. J. Nutr. 1958;64:587–603. doi: 10.1093/jn/64.4.587. [DOI] [PubMed] [Google Scholar]
- Honkavuori K.S., Shivaprasad H.L., Williams B.L., Quan P.L., Hornig M., Street C., Palacios G., Hutchinson S.K., Franca M., Egholm M., Briese T., Lipkin W.I. Novel Borna virus in Psittacine birds with Proventricular Dilation Disease. Emerg. Infect. Dis. 2008;14:1883–1886. doi: 10.3201/eid1412.080984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene J.C., Austic R.E. Dietary supplements of mixtures of indispensable amino acids lacking threonine, phenylalanine or histidine increase the activity of hepatic threonine dehydrogenase, phenylalanine hydroxylase or histidase, respectively, and prevent growth depressions in chicks caused by dietary excesses of threonine, phenylalanine, or histidine. J. Nutr. Biochem. 2001;12:274–284. doi: 10.1016/s0955-2863(01)00139-5. [DOI] [PubMed] [Google Scholar]
- Leeson S., Caston L., Summers J.D. Broiler response to diet energy. Poult. Sci. 1996;75:529–535. doi: 10.3382/ps.0750529. [DOI] [PubMed] [Google Scholar]
- Leeson S., Summers J.D. 3rd ed. University Books; Guelph, Ontario, Canada: 2005. Commercial Poultry Nutrition. [Google Scholar]
- Liu S.Y., Truong H.H., Selle P.H. Whole grain feeding for chicken-meat production: possible mechanisms driving enhanced energy utilisation and feed conversion. Anim. Prod. Sci. 2015;55:559–572. [Google Scholar]
- Liu S.Y., Selle P.H. Starch and protein digestive dynamics in low-protein diets supplemented with crystalline amino acids. Anim. Prod. Sci. 2017;57:2250–2256. [Google Scholar]
- Llames C.R., Fontaine J. Determination of amino acids in feeds. Collaborative Study. J. AOAC Int. 1994;77:1362–1402. [Google Scholar]
- Mahasukhonthachat K., Sopade P.A., Gidley M.J. Kinetics of starch digestion and functional properties of twin-screw extruded sorghum. J. Cereal Sci. 2010;51:392–401. [Google Scholar]
- Moran E.T. Starch digestion in fowl. Poult. Sci. 1982;61:1257–1267. doi: 10.3382/ps.0611257. [DOI] [PubMed] [Google Scholar]
- Selle P.H., Truong H.H., McQuade L.R., Moss A.F., Liu S.Y. Reducing agent and exogenous protease additions, individually and in combination, to wheat- and sorghum-based diets interactively influence parameters of nutrient utilisation and digestive dynamics in broiler chickens. Anim. Nutr. 2016;2:303–311. doi: 10.1016/j.aninu.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selle P.H., Liu S.Y. The relevance of starch and protein digestive dynamics in poultry. J. Appl. Poult. Res. 2019;28:531–545. [Google Scholar]
- Siegert W., Wild K.J., Schollenberger M., Helmbrecht A., Rodehutscord M. Effect of glycine supplementation in low protein diets with amino acids from soy protein isolate or free amino acids on broiler growth and nitrogen utilisation. Brit. Poult. Sci. 2016;57:424–434. doi: 10.1080/00071668.2016.1163523. [DOI] [PubMed] [Google Scholar]
- Siriwan P., Bryden W.L., Mollah Y., Annison E.F. Measurement of endogenous amino acid losses in poultry. Brit. Poult. Sci. 1993;34:939–949. doi: 10.1080/00071669308417654. [DOI] [PubMed] [Google Scholar]
- Srilatha T., Reddy V.R., Preetam V.C., Rao S.V.R., Reddy Y.R. Effect of different levels of dietary crude protein on the growth performance and carcass characteristics of commercial broilers at different phases of growth. Indian J. Anim. Res. 2018;52:559–563. [Google Scholar]
- Sydenham C.J., Truong H.H., Moss A.F., Selle P.H., Liu S.Y. Fishmeal and maize starch inclusions in sorghum-soybean meal diets generate different responses in growth performance, nutrient utilisation, starch and protein digestive dynamics of broiler chickens. Anim. Feed Sci. Technol. 2017;227:32–41. [Google Scholar]
- Truong H.H., Chrystal P.V., Moss A.F., Selle P.H., Liu S.Y. Rapid protein disappearance rates along the small intestine advantage poultry performance and influence the post-enteral availability of amino acids. Brit. J. Nutr. 2017;118:1031–1042. doi: 10.1017/S0007114517003257. [DOI] [PubMed] [Google Scholar]
- Van Harn J., Dijkslag M.A., Van Krimpen M. Proc. 21st Euro. Symp. Poult. Nutr. World's Poultry Science Association; Spain: 2017. Effect of low dietary protein levels on performance, litter quality and footpad lesions in broilers; p. 185. [Google Scholar]
- Wu G.Y. Amino acids: metabolism, functions, and nutrition. Amino Acids. 2009;37:1–17. doi: 10.1007/s00726-009-0269-0. [DOI] [PubMed] [Google Scholar]


