Abstract
Low performing dual purpose hens have different nutritional requirements compared to conventional hybrid hens. Lignocellulose is a low fermentable polymer, acting as a diet diluent and may influence physiological and digestive processes. This study investigated the effect of a 10% dietary lignocellulose dilution on the development of gastrointestinal organs, intestinal morphology, intestinal microbiota, and excreta characteristics of dual purpose hens. One-day-old female Lohmann Dual chicks were allocated to 12 pens and fed two different diets: A standard control diet (CON) and a treatment diet (LC), based on CON but diluted with 10% lignocellulose (ARBOCEL®). At 52 wk of age, gastrointestinal organs were extracted and weights determined. Colorectal tissue samples were chemically fixed and stained for histomorphological examinations. Cecal digesta samples were analyzed for bacterial metabolites and composition using gas chromatography, HPLC, photometry, and PCR. Excreta dry matter and viscosity was consistently assessed during the trial. LC-fed hens showed increased weights of the gizzard (P = 0.003), small (P < 0.001), and large intestine (P = 0.048) compared to hens fed CON. LC-fed hens had a larger colorectal villus area (P = 0.049), a higher mucosal enlargement factor of villi (P = 0.016) and crypts (P = 0.030) than CON-fed hens. The concentration of short-chain fatty acids (SCFAs) (P = 0.017) and ammonia (P = 0.013) was higher in CON-fed hens compared to LC-fed hens. Bacterial composition and activity was generally not affected by feeding the different diets. LC-fed hens had a higher excreta dry matter content than hens fed CON at 10 (P < 0.001), 17 (P < 0.001), and 22 (P = 0.002) wk of age. Correlation analyses revealed a negative relationship between the concentration of SCFAs in the cecum and the colorectal villus surface area (P < 0.01). In conclusion, the feeding of high levels of lignocellulose increased gastrointestinal organ weights and colorectal surface area in dual purpose laying hens. A higher intestinal surface area in combination with lower concentrations of SCFAs might indicate a compensatory reaction of hens fed LC enhancing the absorption of bacterial metabolites by increasing the intestinal mucosal surface.
Key words: dual purpose chicken, lignocellulose, digestive tract, gastrointestinal morphology, microbiota
INTRODUCTION
New approaches to avoid the killing of day-old male chicks of the layer type are necessary. The use of dual purpose chicken might be one possible solution using both sexes, the male for meat and the female for egg production. Studies showed that dual purpose hens fed with standard layer diets developed higher bodyweights and an increased body fat percentage accompanied with lower productivity in comparison with commercial hybrid hens (Rizzi et al., 2002; Rizzi et al., 2007; Rizzi and Chiericato, 2010; Steenfeldt and Hammershøj, 2015). From this, it can be concluded that low performing hens have different nutritional requirements compared to conventional hybrid laying hens. Recently published data showed that the feeding of an energy- and nutrient-reduced diet containing 10% lignocellulose reduced body fat content and improved laying performance in dual purpose laying hens (Röhe et al., 2019). The question arose whether a high concentration of dietary fiber might be accompanied with alterations of the chickens intestinal tract and microbiota. It is well known that the feeding of dietary fiber could affect the digestive tract development, intestinal morphology, and gut microbiota in poultry. Known changes depend on the used fiber source, inclusion level, and its chemical and physical characteristics such as particle size, solubility, and degree of lignification (Hetland and Svihus, 2001; Montagne et al., 2003; De Vries et al., 2012). The term “dietary fiber” underwent different definitions, one is that it includes any polysaccharide reaching the large intestine such as resistant starch, lignin, soluble and insoluble non-starch polysaccharides (NSP) (Montagne et al., 2003). Lignocellulose, a constituent of plant cell walls, is mainly composed of the insoluble NSP cellulose (40 to 47 wt%) and hemicellulose (25 to 35 wt%) as well as the biopolymer lignin (16 to 31 wt%) (Liu et al., 2014). Lignocellulose is the most abundant and bio-renewable biomass on earth and has gained particular attention as potential resource for sustainable production of chemicals and fuels (Zhou et al., 2011). Moreover, research has been focused on the use of lignocellulose as a dietary component for livestock and companion animals with potential effects on digestive physiology and function. Studies showed that dietary lignocellulose at low inclusion levels up to 0.8% might stimulate the development of the digestive tract in pullets and laying hens (Yokhana et al., 2015) and enhance mucosal development in broilers (Sarikhan et al., 2010; Makivic et al., 2019). In general, chickens show a low bacterial capacity to ferment insoluble NSP because they have a high feed passage rate, a short digestive tract and limited microbial cellulolytic activity in the hindgut (McNab, 1973; Carré et al., 1990; Jørgensen et al., 1996; De Vries et al., 2012; Waite and Taylor, 2014). However, it was reported that the dietary inclusion of low concentrations of lignocellulose might modulate bacterial populations and metabolites in the small and large intestine of broilers (Sarikhan et al., 2010; Bogusławska-Tryk et al., 2015; Kheravii et al., 2017; Makivic et al., 2019). Moreover, some studies showed that dietary lignocellulose at low inclusion levels might have a beneficial effect on litter quality by lowering the excreta moisture content (Farran et al., 2013; Kheravii et al., 2017; Makivic et al., 2019).
The goal of the current study was to investigate the effect of feeding diets containing high levels of lignocellulose on the development of gastrointestinal organs, intestinal histomorphology, intestinal microbiota, and excreta characteristics in dual purpose laying hens. We hypothesized that the diet dilution by 10% lignocellulose (ARBOCEL® R, J. Rettenmaier & Söhne GmbH + Co KG, Rosenberg, Germany) would have a clear impact on the digestive tract traits, the bacterial composition and activity in the hindgut, and excreta characteristics of dual purpose laying hens.
MATERIAL AND METHODS
All procedures involving handling and treatments of animals were approved by the local state office of occupational health and technical safety (Landesamt für Gesundheit und Soziales, Berlin, Germany, LaGeSo G 0171/16).
Animals, Rearing Conditions, and Experimental Diets
In total, one hundred thirty two 1-day-old female chicks of a dual purpose breed (Lohmann Dual, Lohmann Tierzucht, Cuxhaven) were randomly allocated to 12 pens. The birds were kept on litter-floor pens (Miscanthus shavings) and had ad libitum access to feed and water. The ambient temperature was adjusted as follows: for the first 2 d of age the ambient temperature was 35°C and was then gradually decreased to 19 ± 1°C by 35 d of age and maintained at a constant to the end of the experiment. The lighting regime was 24 h during the first 2 d, followed by a gradually reduction to 9 h of light per d until 17 wk and followed by an increase to 14 h of light per d until the end of the trial. Two different experimental diets were offered in mash form resulting in 6 replicates per feeding group: the basal control diet (CON) and a treatment diet (LC), based on CON but diluted with 10% lignocellulose (ARBOCEL® R, J. Rettenmaier & Söhne GmbH + Co KG, Rosenberg, Germany). The used lignocellulose source was produced by means of mechanical processing of fresh natural dried wood and had an average fiber length of 200 to 300 μm and a bulk density of 60 to 105 g/l, per supplier information. Detailed information on the feed composition, nutrient content, and feeding schedule are described in Röhe et al. (2019). Feed composition and analyzed nutrient content of a grower and a layer diet are displayed in Table 1. In addition, the feed discrete mean particle size (dMean) is indicated based on the results of the conducted dry-sieve analysis.
Table 1.
Feed composition (%) and analyzed nutrient content of grower (6 to 12 wk of age) and layer diets (32 to 42 wk of age).
| CON1 |
LC1 |
|||
|---|---|---|---|---|
| Grower | Layer | Grower | Layer | |
| Ingredient (%) | ||||
| Wheat | 22.56 | 39.75 | 20.30 | 35.78 |
| Maize | 30.09 | 21.58 | 27.08 | 19.42 |
| Soybean meal. extracted | 5.00 | 5.00 | 4.50 | 4.50 |
| Rapeseed meal. extracted | 4.87 | 4.50 | 4.38 | 4.05 |
| Rapeseed expeller | 5.50 | 0.00 | 4.95 | |
| Sunflower meal. extracted | 10.00 | 9.80 | 9.00 | 8.82 |
| Triticale | 5.00 | 4.50 | 0.00 | |
| Barley | 5.00 | 4.50 | 0.00 | |
| Wheat bran | 10.00 | 9.00 | 0.00 | |
| Oat bran | 3.00 | 1.00 | 2.70 | 0.90 |
| ARBOCEL® R2 | 10.00 | 10.00 | ||
| Calcium carbonate | 1.39 | 8.51 | 1.25 | 7.66 |
| Sodium bicarbonate | 0.27 | 0.15 | 0.24 | 0.14 |
| Common salt | 0.18 | 0.23 | 0.16 | 0.21 |
| Monocalcium phosphate | 0.10 | 0.10 | 0.09 | 0.09 |
| Choline chloride | 0.05 | 0.05 | 0.05 | 0.05 |
| Premix3 | 0.301 | 0.302 | 0.27 | 0.27 |
| L-Lysin HCL | 0.48 | 0.23 | 0.43 | 0.21 |
| DL-Methionine | 0.13 | 0.11 | 0.12 | 0.10 |
| L-Threonin | 0.08 | 0.07 | 0.00 | |
| Plant oil | 1.50 | 3.19 | 1.35 | 2.87 |
| Analyzed Nutrients (g/kg) | ||||
| Crude Protein | 186 | 182 | 170 | 165 |
| Crude Fat | 39.9 | 64.4 | 35.5 | 56.1 |
| NDF | 134 | 104 | 195 | 198 |
| ADF | 67.3 | 62.1 | 154 | 120 |
| ADL | 14.6 | 20.8 | 45.2 | 37.7 |
| Starch | 430 | 372 | 375 | 326 |
| Crude Ash | 46.3 | 120 | 39.7 | 109 |
| Calcium | 6.98 | 33.5 | 6.33 | 29.3 |
| Phosphorus | 5.09 | 4.74 | 4.66 | 4.41 |
| Sodium | 1.80 | 1.76 | 1.59 | 1.97 |
| Potassium | 5.82 | 4.92 | 5.37 | 4.51 |
| Calculated | ||||
| AMEN (MJ/kg)4 | 13.15 | 12.70 | 11.65 | 11.15 |
| dMean5 | 1.47 | 1.54 | 1.36 | 1,37 |
CON = control-fed birds; LC = lignocellulose-fed birds.
J. Rettenmaier & Söhne GmbH+CO. KG, Rosenberg, Germany.
provided per kg grower (layer): 10,000 (9,000) IU vitamin A; 2,500 (2,500) IU vitamin D3; 40.0 (20.0) mg vitamin E (α-tocopherol acetate); 1.50 (2.00) mg vitamin K3; 2.50 (1.00) mg vitamin B1; 5.00 (4.00) mg vitamin B2; 25.0 (20.0) mg nicotinic acid; 3.00 (2.00) mg vitamin B6; 25.0 (25.0) μ g vitamin B12; 75 (100) μ g biotin; 8.00 (6.52) mg calcium pantothenic acid; 0.80 (0.50) mg folic acid; 80.0 (50.0) mg Zn (zinc oxide); 40.0 (5.00) mg Fe (iron carbonate); 80.0 (50.0) mg Mn (manganese oxide); 15.0 (12.0) mg Cu (copper sulfate-pentahydrate); 1.00 (1.00) mg I (calcium iodate); 0.25 (0.20) mg Se (sodium selenite).
AMEN (MJ/kg) = nitrogen-corrected apparent metabolizable energy estimated according to WPSA (1984).
dMean = discrete mean particle size (based on dry-sieve analysis) according to equation of Fritz et al. (2012).
Sampling and Analyses
Dry-Sieve Analyses
Analyses of the feed particle sizes of diets were conducted with a grower diet (6 to 12 wk of age) and a layer diet (32 to 42 wk of age). A representative 100-g sample of each diet was passed through a sieve stack situated on sieve shaker (Analysette 3, Fritsch, Idar Oberstein, Germany) for 10 min at an amplitude of 7. The sieve stack (Analysensiebe, Retsch GmbH, Haan, Germany) was composed of 9 sieves with screens of different mesh sizes (4, 2.5, 2.0, 1.6, 1.25, 1.0, 0.63, 0.40, and 0.15 mm). After the shaking process, the amount of particles retained on each screen was determined by subtracting the weight of the sieve and the retained feed from the blank weight of the sieve. The dMean was calculated as described earlier (Fritz et al., 2012).
Determination of Organ Weights
The proventriculus, gizzard, small intestine, large intestine, and the liver were extracted from the chickens carcass and subsequently intestinal content, adhering fat, and mesenteries removed. The organs were weighed, and organ-to-BW ratios calculated. The total gastrointestinal tract weight was determined by summing the single intestinal segments.
Histomorphological Analyses
Tissue sections from the colorectum were cut open longitudinally and placed on cork boards by using hedgehog spines and fixed in a 4% phosphate-buffered formaldehyde solution for 24 h. After dehydration and infiltration with solidified paraffin wax, the samples were embedded. The paraffin blocks were cut at 4 μm with a sledge microtome (Typ SM 2000 R, Leica, Nussloch, Germany). Obtained sections were mounted on glass slides. Tissue slides were stained with AB/PAS (Chroma, Waldeck, Germany) at pH 2.5 and analyzed with a light microscope (Photomicroscope III, Zeiss, Germany), which was equipped with a digital camera (DP72, Olympus, Germany). Histomorphometric parameters were measured by using an image analysis software (CellSense software, Olympus, Germany). In total, 15 vertically oriented villi and crypts per section were analyzed. The villus length (measured from the tip of the villi to the villus crypt junction) and crypt depth (defined as the depth of the invagination between adjacent villi) was measured and based on that the villus length-to-crypt depth ratio calculated. Furthermore, villus and crypt area was assessed by multiplying the individual villus respectively crypt area by the number of villi respectively crypts per 1,000 μm intestinal cross-section. In order to estimate the enlargement of the intestinal surface epithelium by villi and crypts, the mucosal enlargement factor of the villus and crypt was determined by dividing the total villus respectively crypt surface length by the length of corresponding lamina muscularis mucosae) as described earlier (Wiese et al., 2003; Rieger et al., 2015). Furthermore, the absolute number of goblet cells (total number of goblet cells per villus respectively crypt) and the relative number of goblet cells (goblet cells per 100 µ m basement membrane of villus respectively crypt) were counted. Moreover, the absolute mucin staining area (total mucin staining area per villus respectively crypt in mm2) and the relative mucin staining area (mucin staining area per villus area respectively crypt area in %) was determined for the assessment of the intestinal mucus layer thickness (Röhe et al., 2018).
Determination of Bacterial Metabolites in the Cecum Digesta
After sampling, cecal digesta was frozen in liquid nitrogen and stored at −80°C. The determination of bacterial metabolites was conducted as described by Kröger et al. (2017). Short-chain fatty acids (SCFAs) were analyzed by gas chromatography (Agilent Technologies 6890 N, auto sampler G2614A, and injection tower G2613A; Network GC Systems, Böblingen, Germany) equipped with a flame ionization detector. D- and L-lactate was measured by HPLC (Agilent 1100; Agilent Technologies, Böblingen, Germany) with a pre-column (Phenomenex C18 4.0 4.0 × 2.0 mm; Phenomenex Ltd., Aschaffenburg, Germany) and an analytical column (Phenomenex Chirex 3126 (D)-penicillamine 150 × 4.6 mm; Phenomenex Ltd.) Ammonia was analyzed calorimetrically by the Berthelot reaction in microtitration plates using a Tecan Sunrise microplate reader (Tecan Austria GmbH, Grödig, Austria).
Analyses of Bacterial Cell Concentration and Activity in the Cecum Digesta
Digesta samples were taken from the cecum, instantly frozen in liquid nitrogen and stored at −80°C. The quantification and qualification of selected representatives of the microbiota were carried out from DNA and RNA extracts in order to assess bacterial concentration and activity. The bacterial groups were examined by seven group primers: clostridial cluster I, IV, and XIVa, Lactobacillus spp., Bifidobacterium spp., the Bacteroides-Prevotella-Porphyromonas cluster and the E. coli/ Hafnia/ Shigella group (Table 2). DNA and RNA Extraction was performed with a commercial NucleoSpin® RNA Kit (REF 740955, Macherey-Nagel GmbH & Co. KG, Düren, Germany) in combination with the NucleoSpin® RNA/DNA Buffer Set (REF 740944, Macherey-Nagel GmbH & Co. KG, Düren, Germany) according to the manufacturer, except for the use of 100 mg sample. Quantification of bacterial DNA and rRNA was performed with a Stratagene MX3000p (Stratagene, Amsterdam, The Netherlands) using a commercial master mix (Brilliant II SYBR® Green QPCR Master Mix with Low ROX (Stratagene, Amsterdam, Netherlands). Primer sequences and annealing temperatures are given in Table 2. All primers were purchased from MWG Biotech (Straubing, Germany). A calibration series of PCR products with known copy numbers per ng DNA was used to calculate copy numbers/ g sample. With respect to the quantification of bacterial 16S rRNA, total RNA was transcribed into cDNA using a commercial kit (Superscript III, ThermoFisher Scientific, Berlin, Germany) and subsequently amplified as described before.
Table 2.
Primers used for quantification of bacterial 16S copy numbers in cecal contents.
| Specificity | Primer | Primer sequences (5′ to 3′) | Product (bp) | AT1 | Reference |
|---|---|---|---|---|---|
| Clostridial Cluster XIVa | g-Ccoc-F | AAATGACGGTACCTGACTAA | 440 | 60 | (Matsuki et al., 2002) |
| g-Ccoc-R | CTTTGAGTTTCATTCTTGCGAA | (Matsuki et al., 2002) | |||
| Clostridial Cluster I | CI-F1 | TACCHRAGGAGGAAGCCAC | 231 | 63 | (Song et al., 2004) |
| CI-R2 | GTTCTTCCTAATCTCTACGCAT | (Song et al., 2004) | |||
| Clostridial Cluster IV | sg-Clept-F | GCACAAGCAGTGGAGT | 239 | 60 | (Matsuki et al., 2002) |
| sg-Clept-R | CTTCCTCCGTTTTGTCAA | (Matsuki et al., 2002) | |||
| Lactobacillus spp. | Lac-1 | AGCAGTAGGGAATCTTCCA | 341 | 58 | (Walter et al., 2001) |
| Lac-2 | CACCGCTACACATGGAG | (Heilig et al., 2002) | |||
| Bifidobacterium spp. | g-BIFID-F | TCGCGTCYGGTGTGAAAG | 243 | 58 | (Rinttilä et al., 2004) |
| g-BIFID-R | CCACATCCAGCRTCCAC | (Rinttilä et al., 2004) | |||
| Bacteroides-Prevotella-Porphyromonas Cluster | BPP1 | GGTGTCGGCTTAAGTGCCAT | 140 | 55 | (Rinttilä et al., 2004) |
| BPP2 | CGGAYGTAAGGGCCGTGC | (Rinttilä et al., 2004) | |||
| E. coli/Hafnia/Shigella group | Entero-F | GTTAATACCTTTGCTCATTGA | 340 | 55 | (Malinen et al., 2003) |
| Entero-R | ACCAGGGTATCTAATCCTGTT | (Malinen et al., 2003) |
AT = annealing temperature (°C).
Analyses of Dry Matter and Viscosity in Excreta
Excreta dry matter and viscosity were measured from samples taken at different time points (at weeks 10, 17, 22, and 52 of the trial). With respect to dry matter analyses, fresh excreta was weighed into aluminum jars of known weight. Samples were dried in an incubator at 103°C and weighed again after weight constancy to detect loss of water.
Viscosity was determined by adding 10 ml of water to 5 g of excreta. Samples were continuously stirred for 30 min at 30°C, followed by centrifugation for 15 min at 1854 × g at 4°C. In total, 2 ml of the supernatant was centrifuged for further 10 min at 17500 × g. Afterwards 532 µ l of that supernatant was used for viscosity analysis (DV-II Viscometer, Brookfield Eng Labs inc., Stoughton, MA, USA).
Statistical Analyses
Statistical analyses were conducted using SPSS (version 25.0, Chicago, IL). Results are reported as means and standard error of the means (mean ± SEM). The normally distributed data were analyzed by using Students t test. Spearman correlation analyses were performed displaying correlations between the mucosal enlargement factor of colorectal villi and the relative weight of the gastrointestinal organs as well as between the concentration of SCFA in the cecum and the mucosal enlargement factor of colorectal villi. Non-normally distributed data from microbiological data was analyzed via Kruskal-Wallis test and subsequent Mann-Whitney-U test, where appropriate. Differences were considered significant at P < 0.05.
RESULTS
With respect to the particle size distribution of the experimental diets results of the dry-sieve analyses showed that the inclusion of lignocellulose led to an increase of the proportion of smaller particles resulting in a lower dMean of the LC diet compared to CON diet (Table 1). During the whole feeding trial, birds were healthy and showed no clinical evidence of disease. Results on the animal performance of dual purpose hens are display in Röhe et al. (2019).
Gastrointestinal Organ Weights
The inclusion of dietary lignocellulose affected the relative weight of gastrointestinal organs (Table 3). LC-fed hens showed increased relative weights of the gizzard (P = 0.003), small intestine (P < 0.001) and large intestine (P = 0.048) resulting in a higher weight of the total gastrointestinal organs (P = 0.002) compared to hens fed CON.
Table 3.
Impact of dietary lignocellulose on the relative weight of the gastrointestinal organs (%) and liver weight (%) of dual purpose hens.1
| Item | CON2 | LC2 | SEM3 | P-Value4 |
|---|---|---|---|---|
| Proventriculus | 0.32 | 0.35 | 0.01 | 0.264 |
| Gizzard | 2.36 | 3.51 | 0.22 | 0.003 |
| Small intestine | 1.95 | 2.29 | 0.06 | <0.001 |
| Large intestine | 0.61 | 0.74 | 0.03 | 0.048 |
| Total gastrointestinal tract | 5.25 | 6.84 | 0.30 | 0.002 |
| Liver | 2.20 | 2.45 | 0.12 | 0.313 |
Data are means of six replicate pens.
CON = control-fed birds; LC = lignocellulose-fed birds.
Results are reported as means ± SEM.
Statistical analyses are based on Students t test.
Histomorphological Analyses
LC-fed hens had a larger colorectal villus area (P = 0.049) than CON-fed hens (Table 4). The mucosal enlargement factor of villi (P = 0.016) and crypts (P = 0.030) were higher in LC-fed hens compared to those fed CON. Correlation analyses revealed that the mucosal enlargement factor of colorectal villi was positively related to the relative weight of the gastrointestinal tract (P = 0.011; Figure 1). No differences in the number of goblet cells and the detected mucin staining area of the colorectum could be detected among the feeding groups (Table 5).
Table 4.
Impact of dietary lignocellulose on the villus length (VL), crypt depth (CD), villus length-to-crypt depth ratio (VL/CD), villus area (VA), villi mucosal enlargement factor (VMEF), crypt area (CA), crypts mucosal enlargement factor (CMEV) and ratio between enlargement factors (EFV/EVC) in the colorectum of dual purpose hens.1
| Item | CON2 | LC2 | SEM3 | P-Value4 |
|---|---|---|---|---|
| VL (μ m) | 302 | 331 | 14.5 | 0.329 |
| CD (μ m) | 65.0 | 76.1 | 3.35 | 0.101 |
| VL/CD | 4.94 | 4.66 | 0.22 | 0.535 |
| VA (mm2) | 0.21 | 0.28 | 0.02 | 0.049 |
| VMEF | 3.86 | 5.52 | 0.37 | 0.016 |
| CA (mm2) | 0.036 | 0.043 | 0.002 | 0.214 |
| CMEF | 1.57 | 2.14 | 0.14 | 0.030 |
| VMEF/CMEF | 2.51 | 2.62 | 0.14 | 0.718 |
Data are means of six replicate pens.
CON = control-fed birds; LC = lignocellulose-fed birds.
Results are reported as means ± SEM.
Statistical analyses are based on Students t test.
Figure 1.
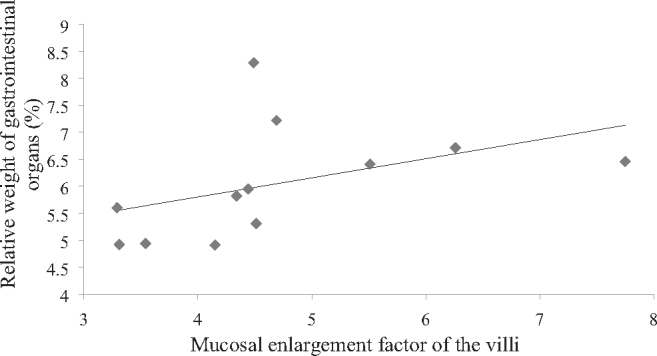
Correlation analyses of the mucosal enlargement factor of colorectal villi and the relative weight of gastrointestinal organs of dual purpose hens (Spearman coefficient: 0.699; P = 0.011).
Table 5.
Impact of dietary lignocellulose on the absolute and relative number of goblet cells as well as the absolute and relative mucin staining area in the colorectum of dual purpose hens.1
| Item | Region | CON2 | LC2 | SEM3 | P-Value4 |
|---|---|---|---|---|---|
| Absolute goblet cell number | Villus | 49.3 | 47.0 | 1.51 | 0.468 |
| Relative goblet cell number | Villus | 16.4 | 14.4 | 0.62 | 0.111 |
| Absolute goblet cell number | Crypt | 11.9 | 12.5 | 0.64 | 0.668 |
| Relative goblet cell number | Crypt | 18.2 | 16.6 | 0.85 | 0.367 |
| Absolute mucin staining area (mm2) | Villus | 0.60 | 0.80 | 0.006 | 0.102 |
| Relative mucin staining area (%) | Villus | 28.2 | 28.2 | 1.09 | 0.982 |
| Absolute mucin staining area (mm2) | Crypt | 0.012 | 0.011 | 0.001 | 0.879 |
| Relative mucin staining area (%) | Crypt | 31.6 | 26.1 | 1.54 | 0.070 |
Data are means of six replicate pens.
CON = control-fed birds; LC = lignocellulose-fed birds.
Results are reported as means ± SEM.
Statistical analyses are based on Students t test.
Bacterial Metabolites and Cell Counts
Analyses of bacterial metabolites in the cecum showed that the absolute concentration of acetic acid (P = 0.018), propionic acid (P = 0.010), n-valeric acid (P = 0.001), and the total amount of the SCFA (P = 0.017) were higher in CON-fed hens compared to those receiving LC (Table 6). With respect to the molar ratio, the proportion of SCFAs in the cecum was not influenced by feeding the different diets. The cecal concentration of ammonia was higher in CON-fed hens than in LC-fed hens (P = 0.013). Correlation analyses showed that the mucosal enlargement factor of colorectal villi (P = 0.022) was negatively related to the absolute concentration of SCFA in the cecum (Figure 2). Bacterial copy numbers of 16S rDNA as well as bacterial activity as measured via 16S rRNA were similar among the feeding groups (Table 7 and 8), except that the 16S rRNA of Lactobacillus spp. was significantly higher in CON-fed hens compared to LC-fed hens (P = 0.002).
Table 6.
Impact of dietary lignocellulose on the absolute and relative concentration of bacterial metabolites in the cecum digesta of dual purpose hens.1
| Item | CON2 | LC2 | SEM3 | P-Value4 |
|---|---|---|---|---|
| Acetic acid (μ mol/g) | 37.5 | 23.2 | 3.22 | 0.018 |
| Propionic acid (μ mol/g) | 3.79 | 2.19 | 0.34 | 0.010 |
| i-butyric acid (μ mol/g) | 0.29 | 0.34 | 0.09 | 0.759 |
| n-butyric acid (μ mol/g) | 4.83 | 2.89 | 0.53 | 0.061 |
| i-valeric acid (μ mol/g) | 0.51 | 0.28 | 0.12 | 0.355 |
| n-valeric acid (μ mol/g) | 0.61 | 0.31 | 0.05 | 0.001 |
| Total SCFA (μ mol/g) | 47.5 | 29.2 | 4.11 | 0.017 |
| Acetic acid (mol. %) | 78.8 | 80 | 0.85 | 0.530 |
| Propionic acid (mol. %) | 8.15 | 7.76 | 0.46 | 0.692 |
| i-butyric acid (mol. %) | 0.64 | 1.05 | 0.22 | 0.372 |
| n-butyric acid (mol. %) | 9.95 | 9.28 | 0.68 | 0.644 |
| i-valeric acid (mol. %) | 1.19 | 0.91 | 0.25 | 0.604 |
| n-valeric acid (mol. %) | 1.32 | 1.10 | 0.06 | 0.066 |
| D-Lactate (μ mol/g) | 0.51 | 0.57 | 0.11 | 0.813 |
| L-Lactate (μ mol/g) | 2.87 | 4.23 | 0.41 | 0.099 |
| Ammonia (μ mol/g) | 8.38 | 4.54 | 0.84 | 0.013 |
Data are means of six replicate pens.
CON = control-fed birds; LC = lignocellulose-fed birds.
Results are reported as means ± SEM.
Statistical analyses are based on Students t test.
Figure 2.
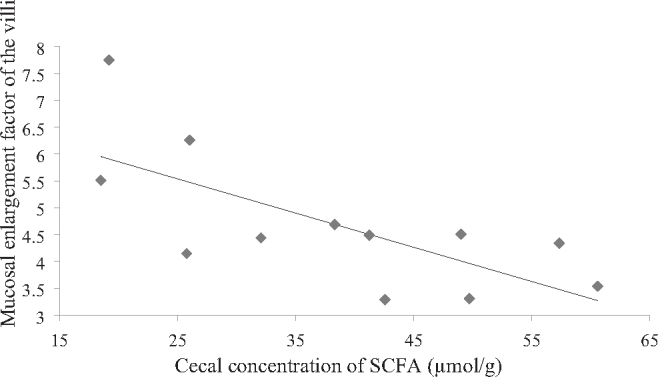
Correlation analyses of the concentration of short-chain fatty acids (SCFA) in the cecum and the mucosal enlargement factor of colorectal villi of dual purpose hens (Spearman coefficient: −0.650; P = 0.022).
Table 7.
Impact of dietary lignocellulose on bacterial cell count (log10 16S rDNA copy number/g) in cecal digesta of dual purpose hens.1
| Item | CON2 | LC2 | SEM3 | P-Value4 |
|---|---|---|---|---|
| Clostridial cluster I | 10.1 | 9.66 | 0.15 | 0.180 |
| Clostridial cluster IV | 10.3 | 10.5 | 0.05 | 0.093 |
| Clostridial cluster XIVa | 10.2 | 10.2 | 0.05 | 0.394 |
| Lactobacillus spp. | 8.48 | 8.25 | 0.07 | 0.093 |
| Bifidobacterium spp. | 8.72 | 8.98 | 0.11 | 0.485 |
| Bacteroides/Prevotella/Porphyromonas-Cluster | 9.92 | 10.1 | 0.08 | 0.310 |
| E. coli/Hafnia/Shigella group | 7.44 | 7.97 | 0.14 | 0.132 |
Data are means of six replicate pens.
CON = control-fed birds; LC = lignocellulose-fed birds.
Results are reported as means ± SEM.
Statistical analyses are based on Mann-Whitney-U-Test.
Table 8.
Impact of dietary lignocellulose on the bacterial activity (log10 copy number 16S rRNA/g) in cecal digesta of dual purpose hens.1
| Item | CON2 | LC2 | SEM3 | P-Value4 |
|---|---|---|---|---|
| Clostridial cluster I | 10.2 | 9.79 | 0.15 | 0.093 |
| Clostridial cluster IV | 12.8 | 12.8 | 0.05 | 0.818 |
| Clostridial cluster XIVa | 13.4 | 13.6 | 0.06 | 0.132 |
| Lactobacillus spp. | 10.2 | 9.58 | 0.11 | 0.002 |
| Bifidobacterium spp. | 9.87 | 10.0 | 0.11 | 0.589 |
| Bacteroides/Prevotella/Porphyromonas Cluster | 11.7 | 11.35 | 0.08 | 0.937 |
| E. coli/Hafnia/Shigella group | 6.95 | 7.15 | 0.13 | 0.905 |
Data are means of six replicate pens.
CON = control-fed birds; LC = lignocellulose-fed birds.
Results are reported as means ± SEM.
Statistical analyses are based on Mann-Whitney-U-Test.
Excreta Dry Matter and Viscosity
Analyses of the excreta dry matter content showed that LC-fed hens had a lower excreta water content than hens fed CON at 10 (P < 0.001), 17 (P < 0.001), and 22 (P = 0.002) wk of age while viscosity of excreta samples of both feeding groups were comparable during the trial (Table 9).
Table 9.
Impact of dietary lignocellulose on the excreta dry matter (DM) and -viscosity of dual purpose hens at different time points of the trial.1
| Weeks of age | CON2 | LC2 | SEM3 | P-Value4 |
|---|---|---|---|---|
| Excreta DM (%) | ||||
| Week 10 | 19.5 | 22.7 | 0.53 | <0.001 |
| Week 17 | 22.1 | 25.5 | 0.59 | <0.001 |
| Week 22 | 21.1 | 25.5 | 0.85 | 0.002 |
| Week 52 | 22.1 | 22.7 | 0.50 | 0.596 |
| Viscosity (mPas) | ||||
| Week 10 | 2.05 | 1.76 | 0.13 | 0.287 |
| Week 17 | 1.55 | 1.35 | 0.06 | 0.095 |
| Week 22 | 1.28 | 1.30 | 0.05 | 0.815 |
| Week 52 | 1.33 | 1.36 | 0.07 | 0.862 |
Data are means of six replicate pens.
CON = control-fed birds; LC = lignocellulose-fed birds.
Results are reported as means ± SEM.
Statistical analyses are based on Students t test.
DISCUSSION
The bodyweight and body fat percentage of dual purpose hens was reduced by feeding a nutrient reduced diet containing a 10% lignocellulose which was accompanied with an increased laying performance (Röhe et al. 2019). The aim of the current study was to examine whether the dietary addition of high levels of lignocellulose might also influence gastrointestinal organ weights, intestinal morphology and microbiota as well as excreta characteristics of dual purpose laying hens.
Gizzard Development
The results showed that hens fed with lignocellulose had increased weights of the gizzard compared to those fed the basal diet. Several studies investigated the effect of dietary fiber on the gizzard development of chicken (Jørgensen et al., 1996; Hetland and Svihus, 2001; González-Alvarado et al., 2007; Jiménez-Moreno et al., 2009). However, only a few studies used the non-fermentable fiber source lignocellulose as feed ingredient. In line with the results of the present study, increased relative weights of the gizzard were observed in pullets fed diets containing 1% lignocellulose (ARBOCEL® RC FINE) over a period of 10 wk (Yokhana et al., 2015). Similarly, laying hens, aged 31 wk, developed heavier gizzards when fed diets diluted with 0.8% lignocellulose (ARBOCEL® RC FINE) compared to hens fed a control diet after 12 wk of feeding (Yokhana et al., 2015). Interestingly, no effects on gizzard weight development were found in younger hens fed those diets for a shorter period of 3, 6, and 9 wk (Yokhana et al., 2015) which suggests that the length of the feeding period of lignocellulose might be relevant for the development of effects in the digestive tract. In contrast, feeding diets supplemented with 0.4 or 0.6% lignocellulose (ARBOCEL® R) did not affect gizzard weights in 42 d old broilers (Makivic et al., 2019). In another study, relative gizzard weights of broilers were also not influenced by feeding diets supplemented with 1 or 2% lignocellulose (OptiCell®, Agromed Austria GmbH) over a period of 35 d (Kheravii et al., 2017).
Several studies showed that the feeding of dietary fiber or so-called “structural components” could stimulate gizzard development in chickens. The feeding of mostly insoluble NSP sources, such as hulls of pea, oat, and soy or wood shavings, increased the gizzard weight of broilers (Jørgensen et al., 1996; González-Alvarado et al., 2007; Amerah et al., 2009; Jiménez-Moreno et al., 2009). Furthermore, insoluble NSP stimulated the gizzard function as indicated by a lower gizzard digesta pH (González-Alvarado et al., 2007; Jiménez-Moreno et al., 2009; Jiménez-Moreno et al., 2011; Makivic et al., 2019). An increase of dietary fiber was often accompanied with an increase in the proportion of coarser particles in the diets (Amerah et al., 2009; Jiménez-Moreno et al., 2009). It is well known that the feeding of coarsely ground as well as mash diets can increase the relative gizzard weights of broilers and laying hens compared to feeding finer particles and thermally processed diets (Engberg et al., 2002; Peron et al., 2005; Amerah et al., 2007; Rougière et al., 2009; Röhe et al., 2014). Consequently, it might be difficult to distinguish between the effect of fiber inclusion and that of the feed particle size. In the present study, the inclusion of lignocellulose did not increase the proportion of coarser particles in the diet, but on the contrary increased the fraction of smaller feed particles. Thus, observed effects regarding an enhanced gizzard development seem to be not connected with an increase in feed particle size. Independent of the particle size, fiber particles are harder to grind and thus accumulate in the gizzard lumen (Hetland et al., 2003), which in turn might stimulate organ development and function (Hetland et al., 2005; Mateos et al., 2012). Moreover, as average daily feed intake of LC-fed hens was higher during the laying period (Röhe et al., 2019), it would be also conceivable that an increased dry matter intake stimulated gizzard activity leading to an increased gizzard weight.
Intestinal Tract and Histomorphology
In the present study, relative weights of the small and large intestine were higher in lignocellulose fed hens compared to those receiving the control diet. Similarly, pullets showed increased small intestinal weights after feeding diets containing 1% lignocellulose (ARBOCEL® RC FINE) over a period of 10 wk compared to pullets fed the control (Yokhana et al., 2015). By contrast, feeding diets supplemented with 0.4 or 0.6% lignocellulose (ARBOCEL® R) did not affect relative weights of the intestine in 42 d old broilers (Makivic et al., 2019). Several studies showed that dietary inclusion of insoluble fiber sources such as oat hulls and sawdust was accompanied with an increase in length and weight of the small and large intestine in chicken (Welch et al., 1988; Jørgensen et al., 1996; Hetland and Svihus, 2001; Sklan et al., 2003; Oke and Oke, 2007; Jiménez-Moreno et al., 2009). In general, it is suggested that an increase in the intestinal size and length but also an enlargement of the intestinal mucosa contributes to a higher intestinal weight (Uni et al., 2003). In this study, correlation analyses proved that an increase of the gastrointestinal weight was related to the enlargement of the intestinal mucosa.
Furthermore, the results showed that dietary lignocellulose inclusion enhanced the mucosal development of the large intestine indicated by a greater villus area and a higher villus and crypt mucosal enlargement factor in the colorectum. However, the number of mucus producing goblet cells and the relative mucin staining area were not affected by feeding lignocellulose. Studies on the effect of feeding lignocellulose on intestinal histomorphology and intestinal mucus production are scarce but are generally in line with the results of this study. Broilers fed 0.6% lignocellulose (ARBOCEL® R) at the expense of soybean meal showed an increased villus height and width as well as crypt depth in the duodenum, jejunum and ileum compared to those receiving the control diet (Makivic et al., 2019). Similarly, an increase of dietary insoluble fiber by adding lignocellulose up to 0.75% (ARBOCEL®) led to an increase of the villus height and crypt depth in the ileum of broilers after 42 d of feeding (Sarikhan et al., 2010). The dietary inclusion of 1.25% lignin resulted in an increased jejunal villus length in 42-day-old broilers while a higher inclusion level of 2.5% lignin (Alcell®, Alcell Technologies Inc., Canada) decreased villus length (Baurhoo et al., 2007). In the same study, the number of jejunal goblet cells per villus were not affected by feeding the different lignin inclusion levels (Baurhoo et al., 2007). Apart from lignocellulose or lignin as fiber source, only few studies exist investigating the effect of other insoluble fiber sources on intestinal morphology in chickens providing contradictory results. An elevation of the dietary crude fiber content from 1.61 to 4.44% by adding pea hulls, mainly consisting of insoluble fiber, tended to reduce linearly villus height and significantly lowered crypt depth in the jejunum of broilers (Jiménez-Moreno et al., 2011). In contrast to that but in line with our findings, the jejunal villus height and the villus surface area of 98-day-old turkeys increased as the concentration of dietary crude fiber was heightened from 3 to 9% (Sklan et al., 2003). It was speculated that an increase in the digestive tract weight accompanied with an enlargement of the intestinal mucosa displays a compensatory reaction of chickens due to the feeding of high fiber, low nutrient diets (Bedford, 2000). Thus, nutrient absorption might be enhanced by increasing the digestive capacity (Brenes et al., 1993; Bedford, 2000). On the other hand, Amerah et al. (2009) reported a decrease in weight and length of the small intestine of broilers fed increasing levels of dietary fiber. It was suggested that the lower nutrient density in the intestine of birds fed diets containing insoluble fiber might reduce the intestinal surface area although histomorphometric parameters were not determined (Amerah et al., 2009).
Bacterial Metabolites and Microbiota
The hypothesis of Bedford (2000) that chickens fed with nutrient reduced diets enhance the absorption of nutrients by increasing the intestinal mucosal surface area is supported by the results of this study. Hens fed the nutrient reduced diet had lower cecal concentrations of SCFAs, particularly lower levels of acetic acid, propionic acid and n-valeric acid, compared to hens fed with the control diet. Coincidentally, those hens had a higher colorectal mucosal enlargement factor, a histomorphometric parameter reflecting the mucosal surface (Wiese et al., 2003). This might indicate a compensatory reaction of birds fed lignocellulose enhancing the absorption of bacterial metabolites by developing a higher intestinal mucosal surface area. Accordingly, correlation analyses in this study have shown that the concentration of SCFAs in the cecum of hens was negatively correlated with the colorectal mucosal enlargement factor of the villi, in other words: the lower the concentration of SCFA in the gut lumen, the higher the absorptive villus surface area. Thus, the hypothesis on a compensatory reaction to increase resorption of energy yielding SCFA may hold true.
In chickens, the bacterial fermentation of insoluble fiber sources and lignified material such as lignocellulose is low (McNab, 1973; Carré et al., 1990; Jørgensen et al., 1996; Montagne et al., 2003; De Vries et al., 2012; Waite and Taylor, 2014). Lower concentrations of SCFAs and ammonia were also detected in the cecum of LC-fed hens compared to those fed CON suggesting a diet dilution effect of the lignocellulose inclusion. However, the results also showed that generally neither the number nor the activity of detected bacterial populations differed between hens of both feeding groups, which favors the idea that the reduced SCFA concentrations are due to an increase in the villus surface accompanied with a higher SCFA absorption. Uniquely, the activity of Lactobacillus spp. was significantly higher in CON-fed hens compared to LC-fed hens. There is a lack of studies investigating the impact of feeding lignocellulose on the microbiota in laying hens. Some studies on broilers showed that the feeding of lower inclusion levels of lignocellulose might modulate bacterial populations and metabolites in the small and large intestine (Sarikhan et al., 2010; Bogusławska-Tryk et al., 2015; Kheravii et al., 2017; Makivic et al., 2019). The feeding of broilers with diets containing lignocellulose up to 1% (ARBOCEL® RC) increased counts of Lactobacillus spp. in the ileum and Bifidobacterium spp. in the ileum and caeca, and decreased counts of ileal and cecal Escherichia coli and Clostridium spp. (Bogusławska-Tryk et al., 2015). The concentration of ileal and cecal SCFAs was higher in broilers fed 0.5% lignocellulose compared to those fed the control diet. Interestingly, a higher dietary inclusion level of 1% lignocellulose showed no effect on cecal SCFAs concentration (Bogusławska-Tryk et al., 2015). As lignocellulose was included in the diet as an expense of wheat it could be speculated that observed effects on intestinal microbiota and metabolites could be also attributed to a varying nutrient composition of the feed. Diets diluted with 1 and 2% lignocellulose had in general no effect on detected bacteria counts except that Clostridium spp. counts were reduced in the cecum of broilers fed 2% lignocellulose (OptiCell®) (Kheravii et al., 2017).
Excreta Characteristics
Analyses of the excreta revealed that hens fed LC had generally a higher dry matter content than hens fed CON, although hens of both feeding groups showed a comparable excreta dry matter content at 52 wk of age. Few studies display that dietary lignocellulose inclusion might have a positive effect on litter quality observed during broiler trials. Broilers fed with diets supplemented with 0.6, 0.8, 1, and 2% lignocellulose had a lower moisture content in the litter compared to broilers fed control diets (Farran et al., 2013; Kheravii et al., 2017; Makivic et al., 2019). It was hypothesized that the dietary inclusion of lignocellulose might increase digesta retention time and water holding capacity, which might enhance the water absorption in the digestive tract and thus elevates the excreta dry matter content (Kheravii et al., 2017). With respect to the excreta viscosity, differences could be not observed between LC- and CON-fed hens. It is well known that dietary soluble fiber can increase gut viscosity resulting in a reduced feed passage rate while an opposite effect is supposed for dietary insoluble fiber (Van der Klis and Van Voorst, 1993; Almirall and Esteve-Garcia, 1994; Choct et al., 1996).
In conclusion, the results of this study show that feeding of high levels of lignocellulose increased the weights of gastrointestinal organs of dual purpose laying hens, which was accompanied with the development of an increased colorectal mucosal surface. The amount of cecal SCFAs and ammonia was reduced in lignocellulose-fed hens compared to those fed the basal diet. Interestingly, the concentration of SCFAs in the cecum of hens was negatively correlated with the colorectal villus surface. This might indicate a compensatory reaction of birds fed lignocellulose enhancing the absorption of energy yielding bacterial metabolites by increasing the intestinal mucosal surface. In order to prove this, further studies are needed including experiments in metabolism cages investigating the energy requirements for maintenance and production of dual purpose hens in relation to feeding diets with varying energy- and nutrient levels. Cecal microbial composition and activity was generally not influenced by feeding the different diets supporting the hypothesis that the mucosal absorption rate of cecal SCFAs was increased in lignocellulose-fed hens due to an increased mucosal surface area. Moreover, a lower excreta moisture content could be detected in lignocellulose fed hens, which might have positive effects on litter quality under practical conditions. In connection with recently published data on animal performance and body composition (Röhe et al., 2019) results of this study suggest that the feeding of nutrient reduced diets containing high levels of fibre might be an interesting possibility to feed dual purpose chickens, maintain animal health and simultaneously improve economic viability.
ACKNOWLEDGEMENTS
This work was supported by funds of the German Government's Special Purpose Fund held at Landwirtschaftliche Rentenbank (grant number 28RZ372049).
REFERENCES
- Almirall M., Esteve-Garcia E. Rate of passage of barley diets with chromium oxide: Influence of age and poultry strain and effect of β-glucanase supplementation. Poult. Sci. 1994;73:1433–1440. doi: 10.3382/ps.0731433. [DOI] [PubMed] [Google Scholar]
- Amerah A.M., Lentle R.G., Ravindran V. Influence of feed form on gizzard morphology and particle size spectra of duodenal digesta in broiler chickens. J. Poult. Sci. 2007;44:175–181. [Google Scholar]
- Amerah A.M., Ravindran V., Lentle R.G. Influence of insoluble fibre and whole wheat inclusion on the performance, digestive tract development and ileal microbiota profile of broiler chickens. Br. Poult. Sci. 2009;50:366–375. doi: 10.1080/00071660902865901. [DOI] [PubMed] [Google Scholar]
- Baurhoo B., Phillip L., Ruiz-Feria C.A. Effects of purified lignin and mannan oligosaccharides on intestinal integrity and microbial populations in the ceca and litter of broiler chickens. Poult. Sci. 2007;86:1070–1078. doi: 10.1093/ps/86.6.1070. [DOI] [PubMed] [Google Scholar]
- Bedford M. Removal of antibiotic growth promoters from poultry diets: implications and strategies to minimise subsequent problems. Worlds Poult. Sci. J. 2000;56:347–365. [Google Scholar]
- Bogusławska-Tryk M., Szymeczko R., Piotrowska A., Burlikowska K., Śliżewska K. Ileal and cecal microbial population and short-chain fatty acid profile in broiler chickens fed diets supplemented with lignocellulose. Pak. Vet. J. 2015;35:212–216. [Google Scholar]
- Brenes A., Smith M., Guenter W., Marquardt R.R. Effect of enzyme supplementation on the performance and digestive tract size of broiler chickens fed wheat-and barley-based diets. Poult. Sci. 1993;72:1731–1739. doi: 10.3382/ps.0721731. [DOI] [PubMed] [Google Scholar]
- Carré B., Derouet L., Leclercq B. The digestibility of cell-wall polysaccharides from wheat (bran or whole grain), soybean meal, and white lupin meal in cockerels, muscovy ducks, and rats. Poult. Sci. 1990;69:623–633. doi: 10.3382/ps.0690623. [DOI] [PubMed] [Google Scholar]
- Choct M., Hughes R.J., Wang J., Bedford M.R., Morgan A.J., Annison G. Increased small intestinal fermentation is partly responsible for the anti-nutritive activity of non-starch polysaccharides in chickens. Br. Poult. Sci. 1996;37:609–621. doi: 10.1080/00071669608417891. [DOI] [PubMed] [Google Scholar]
- De Vries S., Pustjens A.M., Schols H.A., Hendriks W.H., Gerrits W.J.J. Improving digestive utilization of fiber-rich feedstuffs in pigs and poultry by processing and enzyme technologies: a review. Anim. Feed Sci. Technol. 2012;178:123–138. [Google Scholar]
- Engberg R.M., Hedemann M.S., Jensen B.B. The influence of grinding and pelleting of feed on the microbial composition and activity in the digestive tract of broiler chickens. Br. Poult. Sci. 2002;43:569–579. doi: 10.1080/0007166022000004480. [DOI] [PubMed] [Google Scholar]
- Farran M. T., Pietsch M., Chabrillat T. 2013. Effect of lignocellulose on the litter quality and the ready to cook carcass yield of male broilers. Actes des 10èmes Journées de la Recherche Avicole et Palmipèdes à Foie Gras du 26 au 28 mars, 2013, La Rochelle, France:917–921.
- Fritz J., Streich W.J., Schwarm A., Clauss M. Condensing results of wet sieving analyses into a single data: a comparison of methods for particle size description. J. Anim. Physiol. Anim. Nutr. 2012;96:783–797. doi: 10.1111/j.1439-0396.2011.01183.x. [DOI] [PubMed] [Google Scholar]
- González-Alvarado J.M., Jiménez-Moreno E., Lázaro R., Mateos G.G. Effect of type of cereal, heat processing of the cereal, and inclusion of fiber in the diet on productive performance and digestive traits of broilers. Poult. Sci. 2007;86:1705–1715. doi: 10.1093/ps/86.8.1705. [DOI] [PubMed] [Google Scholar]
- Heilig H.G.H.J., Zoetendal E.G., Vaughan E.E., Marteau P., Akkermans A.D.L., de Vos W.M. Molecular diversity of Lactobacillus spp. and other lactic acid bacteria in the human intestine as determined by specific amplification of 16S ribosomal DNA. Appl. Environ. Microbiol. 2002;68:114–123. doi: 10.1128/AEM.68.1.114-123.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetland H., Svihus B. Effect of oat hulls on performance, gut capacity and feed passage time in broiler chickens. Br. Poult. Sci. 2001;42:354–361. doi: 10.1080/00071660120055331. [DOI] [PubMed] [Google Scholar]
- Hetland H., Svihus B., Krogdahl A. Effects of oat hulls and wood shavings on digestion in broilers and layers fed diets based on whole or ground wheat. Br. Poult. Sci. 2003;44:275–282. doi: 10.1080/0007166031000124595. [DOI] [PubMed] [Google Scholar]
- Hetland H., Svihus B., Choct M. Role of insoluble fiber on gizzard activity in layers. J. Appl. Poult. Res. 2005;14:38–46. [Google Scholar]
- Jiménez-Moreno E., González-Alvarado J.M., González-Serrano A., Lázaro R., Mateos G.G. Effect of dietary fiber and fat on performance and digestive traits of broilers from one to twenty-one days of age. Poult. Sci. 2009;88:2562–2574. doi: 10.3382/ps.2009-00179. [DOI] [PubMed] [Google Scholar]
- Jiménez-Moreno E., Chamorro S., Frikha M., Safaa H.M., Lázaro R., Mateos G.G. Effects of increasing levels of pea hulls in the diet on productive performance, development of the gastrointestinal tract, and nutrient retention of broilers from one to eighteen days of age. Anim. Feed Sci. Technol. 2011;168:100–112. [Google Scholar]
- Jørgensen H., Zhao X.Q., Knudsen K.E.B., Eggum B.O. The influence of dietary fibre source and level on the development of the gastrointestinal tract, digestibility and energy metabolism in broiler chickens. Br. J. Nutr. 1996;75:379–395. doi: 10.1079/bjn19960141. [DOI] [PubMed] [Google Scholar]
- Kheravii S.K., Swick R.A., Choct M., Wu S.B. Coarse particle inclusion and lignocellulose-rich fiber addition in feed benefit performance and health of broiler chickens. Poult. Sci. 2017;96:3272–3281. doi: 10.3382/ps/pex123. [DOI] [PubMed] [Google Scholar]
- Kröger S., Vahjen W., Zentek J. Influence of lignocellulose and low or high levels of sugar beet pulp on nutrient digestibility and the fecal microbiota in dogs. J. Anim. Sci. 2017;95:1598–1605. doi: 10.2527/jas.2016.0873. [DOI] [PubMed] [Google Scholar]
- Liu C., Wang H., Karim A.M., Sun J., Wang Y. Catalytic fast pyrolysis of lignocellulosic biomass. Chem. Soc. Rev. 2014;43:7594–7623. doi: 10.1039/c3cs60414d. [DOI] [PubMed] [Google Scholar]
- Makivic L., Glisic M., Boskovic M., Djordjevic J., Markovic R., Baltic M., Sefer D. Performances, ileal and cecal microbial populations and histological characteristics in broilers fed diets supplemented with lignocellulose. Kafkas Univ. Vet. Fak. Derg. 2019;25:83–91. [Google Scholar]
- Malinen E., Kassinen A., Rinttilä T., Palva A. Comparison of real-time PCR with SYBR Green I or 5′-nuclease assays and dot-blot hybridization with rDNA-targeted oligonucleotide probes in quantification of selected faecal bacteria. Microbiology. 2003;149:269–277. doi: 10.1099/mic.0.25975-0. [DOI] [PubMed] [Google Scholar]
- Mateos G.G., Jiménez-Moreno E., Serrano M.P., Lázaro R.P. Poultry response to high levels of dietary fiber sources varying in physical and chemical characteristics. J. Appl. Poult. Res. 2012;21:156–174. [Google Scholar]
- Matsuki T., Watanabe K., Fujimoto J., Miyamoto Y., Takada T., Matsumoto K., Oyaizu H., Tanaka R. Development of 16S rRNA-gene-targeted group-specific primers for the detection and identification of predominant bacteria in human feces. Appl. Environ. Microbiol. 2002;68:5445–5451. doi: 10.1128/AEM.68.11.5445-5451.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNab J.M. The avian caeca: a review. Worlds Poult. Sci. J. 1973;29:251–263. [Google Scholar]
- Montagne L., Pluske J.R., Hampson D.J. A review of interactions between dietary fibre and the intestinal mucosa, and their consequences on digestive health in young non-ruminant animals. Anim. Feed Sci. Technol. 2003;108:95–117. [Google Scholar]
- Oke D.B., Oke M.O. Effects of feeding graded levels of sawdust obtained from Daniellia ogea tree on the performance and carcass characteristics of broiler chickens. Res. J. Poult. Sci. 2007;1:12–15. [Google Scholar]
- Peron A., Bastianelli D., Oury F.X., Gomez J., Carre B. Effects of food deprivation and particle size of ground wheat on digestibility of food components in broilers fed on a pelleted diet. Br. Poult. Sci. 2005;46:223–230. doi: 10.1080/00071660500066142. [DOI] [PubMed] [Google Scholar]
- Rieger J., Janczyk P., Hünigen H., Neumann K., Plendl J. Intraepithelial lymphocyte numbers and histomorphological parameters in the porcine gut after Enterococcus faecium NCIMB 10415 feeding in a Salmonella Typhimurium challenge. Vet. Immunol. Immunopathol. 2015;164:40–50. doi: 10.1016/j.vetimm.2014.12.013. [DOI] [PubMed] [Google Scholar]
- Rinttilä T., Kassinen A., Malinen E., Krogius L., Palva A. Development of an extensive set of 16S rDNA-targeted primers for quantification of pathogenic and indigenous bacteria in faecal samples by real-time PCR. J. Appl. Microbiol. 2004;97:1166–1177. doi: 10.1111/j.1365-2672.2004.02409.x. [DOI] [PubMed] [Google Scholar]
- Rizzi C., Chiericato G.M., Baruchello M. Prestazioni produttive di galline ovaiole appartenenti a due ibridi commerciali ea due razze autoctone allevate con metodo biologico. Riv. Avicoltura. 2002;6:39–41. [Google Scholar]
- Rizzi C., Marangon A., Chiericato G.M. Effect of genotype on slaughtering performance and meat physical and sensory characteristics of organic laying hens. Poult. Sci. 2007;86:128–135. doi: 10.1093/ps/86.1.128. [DOI] [PubMed] [Google Scholar]
- Rizzi C., Chiericato G.M. Chemical composition of meat and egg yolk of hybrid and Italian breed hens reared using an organic production system. Poult. Sci. 2010;89:1239–1251. doi: 10.3382/ps.2008-00045. [DOI] [PubMed] [Google Scholar]
- Röhe I., Ruhnke I., Knorr F., Mader A., Goodarzi Boroojeni F., Löwe R., Zentek J. Effects of grinding method, particle size, and physical form of the diet on gastrointestinal morphology and jejunal glucose transport in laying hens. Poult. Sci. 2014;93:2060–2068. doi: 10.3382/ps.2013-03783. [DOI] [PubMed] [Google Scholar]
- Röhe I., Hüttner F.J., Plendl J., Drewes B., Zentek J. Comparison of different histological protocols for the preservation and quantification of the intestinal mucus layer in pigs. Eur. J. Histochem. 2018;62 doi: 10.4081/ejh.2018.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röhe I., Urban J., Dijkslag A., te Paske J., Zentek J. Impact of an energy-and nutrient-reduced diet containing 10% lignocellulose on animal performance, body composition and egg quality of dual purpose laying hens. Arch. Anim. Nutr. 2019;73:1–17. doi: 10.1080/1745039X.2018.1551950. [DOI] [PubMed] [Google Scholar]
- Rougière N., Gomez J., Mignon-Grasteau S., Carré B. Effects of diet particle size on digestive parameters in D+ and D− genetic chicken lines selected for divergent digestion efficiency. Poult. Sci. 2009;88:1206–1215. doi: 10.3382/ps.2008-00408. [DOI] [PubMed] [Google Scholar]
- Sarikhan M., Shahryar H.A., Gholizadeh B., Hosseinzadeh M.H., Beheshti B., Mahmoodnejad A. Effects of insoluble fiber on growth performance, carcass traits and ileum morphological parameters on broiler chick males. Int. J. Agric. Biol. 2010;12:531–536. [Google Scholar]
- Sklan D., Smirnov A., Plavnik I. The effect of dietary fibre on the small intestines and apparent digestion in the turkey. Br. Poult. Sci. 2003;44:735–740. doi: 10.1080/00071660310001643750. [DOI] [PubMed] [Google Scholar]
- Song Y., Liu C., Finegold S.M. Real-time PCR quantitation of clostridia in feces of autistic children. Appl. Environ. Microbiol. 2004;70:6459–6465. doi: 10.1128/AEM.70.11.6459-6465.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenfeldt S., Hammershøj M. Organic egg production. I: Effects of different dietary protein contents and forage material on organic egg production, nitrogen and mineral retention and total tract digestibility of nutrients of two hen genotypes. Anim. Feed Sci. Technol. 2015;209:186–201. [Google Scholar]
- Uni Z., Smirnov A., Sklan D. Pre- and posthatch development of goblet cells in the broiler small intestine: effect of delayed access to feed. Poult. Sci. 2003;82:320–327. doi: 10.1093/ps/82.2.320. [DOI] [PubMed] [Google Scholar]
- Van der Klis J.D., Van Voorst A. The effect of carboxy methyl cellulose (a soluble polysaccharide) on the rate of marker excretion from the gastrointestinal tract of broilers. Poult. Sci. 1993;72:503–512. doi: 10.1080/00071669308417658. [DOI] [PubMed] [Google Scholar]
- Waite D.W., Taylor M.W. Characterizing the avian gut microbiota: membership, driving influences, and potential function. Front. Microbiol. 2014;5:223. doi: 10.3389/fmicb.2014.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter J., Hertel C., Tannock G.W., Lis C.M., Munro K., Hammes W.P. Detection of Lactobacillus, Pediococcus, Leuconostoc, and Weissella species in human feces by using group-specific PCR primers and denaturing gradient gel electrophoresis. Appl. Environ. Microbiol. 2001;67:2578–2585. doi: 10.1128/AEM.67.6.2578-2585.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch R.W., Peterson D.M., Schramka B. Hypocholesterolemic and gastrointestinal effects of oat bran fractions in chicks. Nutr. Rep. Int. 1988;38:551–561. [Google Scholar]
- Wiese F., Simon O., Weyrauch K.D. Morphology of the small intestine of weaned piglets and a novel method for morphometric evaluation. Anat. Histol. Embryol. 2003;32:102–109. doi: 10.1046/j.1439-0264.2003.00430.x. [DOI] [PubMed] [Google Scholar]
- World's Poultry Science Association The prediction of apparent metabolizable energy values for poultry in compound feeds. World's Poult. Sci. J. 1984;40:181–182. [Google Scholar]
- Yokhana J.S., Parkinson G., Frankel T.L. Effect of insoluble fiber supplementation applied at different ages on digestive organ weight and digestive enzymes of layer-strain poultry. Poult. Sci. 2015;95:550–559. doi: 10.3382/ps/pev336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C.H., Xia X., Lin C.X., Tong D.S., Beltramini J. Catalytic conversion of lignocellulosic biomass to fine chemicals and fuels. Chem. Soc. Rev. 2011;40:5588–5617. doi: 10.1039/c1cs15124j. [DOI] [PubMed] [Google Scholar]


